Abstract
Background
Increased body temperatures are common in patients with acute stroke and are associated with poor outcome. In animal models of focal cerebral ischaemia, temperature‐lowering therapy reduces infarct volume. In patients with acute stroke, lowering temperature may therefore improve outcome. This is an update of a Cochrane review first published in 1999.
Objectives
To assess the effects of pharmacological and physical strategies to reduce body or brain temperature in patients with acute stroke.
Search methods
We searched the Cochrane Stroke Group trials register (last searched December 2007). In addition, we searched MEDLINE and EMBASE (January 1998 to December 2007). We scanned references and contacted authors of included trials. For the previous version of this review, the authors contacted pharmaceutical companies and manufactures of cooling equipment in this field.
Selection criteria
We considered all completed randomised or non‐randomised controlled clinical trials, published or unpublished, where pharmacological or physical strategies or both to reduce temperature were applied in patients with acute ischaemic stroke or intracerebral haemorrhage. Outcome measures were death or dependency (modified Rankin Scale score ≥ 3) at the end of follow up, and adverse effects.
Data collection and analysis
Two review authors independently applied the inclusion criteria, assessed trial quality, and extracted and cross‐checked the data.
Main results
We included five pharmacological temperature reduction trials and three physical cooling trials involving a total of 423 participants. We found no statistically significant effect of pharmacological or physical temperature‐lowering therapy in reducing the risk of death or dependency (odds ratio (OR) 0.9, 95% confidence interval (CI) 0.6 to 1.4) or death (OR 0.9, 95% CI 0.5 to 1.5). Both interventions were associated with a non‐significant increase in the occurrence of infections.
Authors' conclusions
There is currently no evidence from randomised trials to support routine use of physical or pharmacological strategies to reduce temperature in patients with acute stroke. Large randomised clinical trials are needed to study the effect of such strategies.
Plain language summary
Cooling therapy for acute stroke
Stroke is a life‐threatening event in which part of the brain stops functioning properly, because it either does not receive blood and oxygen or it is damaged by bleeding from a ruptured blood vessel. Interventions to reduce temperature may protect brain tissue from damage during stroke. Previous studies have shown that patients with a lower body temperature at the time of stroke have a better outcome than those with a higher body temperature. To reduce death or disability, temperature‐lowering therapy is used in open‐heart surgery, after cardiac arrest and in babies who may have suffered from a lack of oxygen at birth. By contrast, the therapeutic effect of temperature‐lowering therapy in patients with traumatic brain injury is less promising. Besides its potential beneficial effects, temperature‐lowering therapy may have adverse effects including chest infection, venous thrombosis or cardiac arrhythmias. This review aimed to assess the potential benefits and risks of temperature‐lowering therapy in patients with acute stroke. All studies that compared the use of physical or pharmacological temperature‐lowering therapies on acute stroke with usual medical management in acute stroke patients were considered. Physical temperature‐lowering techniques included cooling blankets, cooling fluids, cooling helmets and other devices. Pharmacological temperature‐lowering interventions included drugs used to reduce temperature. The results of the five included pharmacological and three physical temperature reduction trials, involving 423 participants with acute stroke, do not indicate a clinical benefit or harm. Both interventions were associated with a slight increase in the occurrence of infections, but this was not statistically significant. A clinically significant effect of temperature‐lowering therapy on outcome after stroke was not demonstrated, but cannot be ruled out. Large clinical trials are therefore needed to assess the effect of temperature‐lowering therapies in acute stroke.
Background
Stroke is the second cause of death worldwide and the second cause of disability in high‐income countries. As stroke incidence rises exponentially with age, the ageing of the world's population will increase its socio‐economic impact (Lopez 2006). This calls for effective treatments that are easy to administer and also cost‐effective. Unfortunately, treatment of ischaemic stroke and intracerebral haemorrhage has remained unsatisfactory. In patients with ischaemic stroke, aspirin administered within 48 hours after the onset of symptoms reduces the risk of disability or death by only 1% (Sandercock 2008). Intravenous thrombolysis with recombinant tissue‐plasminogen activator (rt‐PA) within three hours of symptom onset increases the absolute probability of a good outcome by 10% (Wardlaw 2003). However, intravenous thrombolysis is only available for a limited number of patients, mainly because of the current time window to treatment of three hours. Intra‐arterial thrombolysis with recombinant pro‐urokinase within six hours of stroke onset results in a similar improvement of functional outcome, but the procedures required to deliver the thrombolytic agent to the site of vascular occlusion take more precious time and have their own complications (Furlan 1999). No other pharmacological treatment modalities have been proven effective in acute ischaemic stroke (Van der Worp 2007b).
One approach to develop new stroke treatment strategies is to influence physiologic parameters that have been proven to be related to outcome. One of these factors is body temperature. Body temperature is increased in 4% to 25 % of patients with acute ischaemic stroke within the first six hours after symptom onset (Azzimondi 1998; Boysen 2001; Castillo 1998; Reith 1996). The pathophysiology of this increase in body temperature is not completely understood. On the one hand, increased body temperature may be a natural consequence of brain infarction. However, animal studies have suggested that increased body temperatures may increase the damage induced by cerebral ischaemia (Chopp 1998; Dietrich 1990; Globus 1995; Henker 1998; Huang 1999). Observational studies in patients with acute stroke have shown an association between increased body temperature and poor outcome (Azzimondi 1998; Boysen 2001; Castillo 1998; Reith 1996). This association may be limited to the first 12 to 24 hours from stroke onset (Jorgensen 1996; Reith 1996). These studies therefore suggest that control of body temperature and prevention of fever may improve functional outcome after stroke.
In a systematic review of animal studies, therapeutic hypothermia reduced infarct size by 44% (95% confidence interval (CI) 40 to 47%). Efficacy was highest with temperature reduction to lower temperatures (≤ 31 °C), when treatment was started before or at the onset of ischaemia, and in temporary rather than permanent ischaemia models. However, a reduction in infarct volume by about one third was also observed with temperature reduction to 35 °C, with initiation of treatment between 90 and 180 minutes, and in permanent ischaemia models (Van der Worp 2007a). The effects of hypothermia on functional outcome were broadly similar (Van der Worp 2007a). This suggests that temperature‐lowering therapy might be efficacious under conditions that are achievable for large numbers of patients with ischaemic stroke.
Profound hypothermia has been applied for many years in open‐heart surgery to counter the effects of cerebral hypoxia (Chyatte 1989; Saccani 1992). Furthermore, mild therapeutic hypothermia improves functional outcome and survival in patients resuscitated after cardiac arrest (Bernard 2002; Holzer 2005) and in infants with moderate or severe hypoxic‐ischaemic encephalopathy (Shankaran 2005). By contrast, the therapeutic effect of hypothermia in patients with traumatic brain injury is less promising (Alderson 2004).
This systematic review aims to assess the relation between interventions to reduce body or brain temperature and functional outcome in patients with acute stroke and to determine whether there is any clear evidence that reduction in temperature of any kind is beneficial, or whether the intervention is sufficiently promising to merit further trials. Several studies have suggested that temperature‐lowering therapy may be associated with an increased risk of infections, cardiac arrhythmias, haemorrhagic transformation of infarcts, and venous thrombosis (De Georgia 2004; Krieger 2001; Schwab 1998). In this review, we will specifically address this issue.
This is an update of a Cochrane review first published in 1999.
Objectives
To determine whether temperature‐lowering therapy is effective and safe in patients with acute ischaemic stroke or intracerebral haemorrhage. We wished to determine whether temperature‐lowering therapy: (1) reduces the risk of dependency or death at follow up in patients with acute stroke; (2) increases the risk of haemorrhagic transformation of the infarct in patients with acute ischaemic stroke, the risk of re bleeding in patients with primary intracerebral haemorrhage, and the risk of major extracranial haemorrhage in all patients with acute stroke; (3) affects the risk of infections and other complications like cardiac arrhythmias, hypotension, and venous thrombosis in patients with acute stroke.
Methods
Criteria for considering studies for this review
Types of studies
We searched for all randomised controlled trials (RCT) and controlled clinical trials (CCT) of temperature‐lowering therapy versus control (placebo or open label).
Types of participants
Trials including patients over 18 years of age within 24 hours of a cerebral infarction or primary intracerebral haemorrhage were eligible.
Types of interventions
Temperature‐lowering therapy
(1) Physical: fluid‐filled cooling blanket, ice water lavage, cold infusions, forced air, cooling helmets, endovascular cooling, and other methods (2) Pharmacological: non‐steroidal anti‐inflammatory or antipyretic drugs (3) Any combination of pharmacological and physical temperature‐lowering therapy The temperature‐lowering therapy had to be started within 24 hours after symptom onset, because prognostic studies indicated that the association with functional outcome might be limited to body temperature measured in the first 12 to 24 hours from stroke onset.
Types of outcome measures
Primary outcome measure
Poor functional outcome at the end of follow up, mostly one to three months after stroke onset. This was defined as death or dependency measured by the modified Rankin scale (mRS), Barthel index (BI), or another method assessing dependency in activities of daily living. Poor functional outcome is the most important measure of outcome since the aim of treatment should not only be to prevent death but also to prevent disability and dependency in survivors.
Secondary outcome measures
(1) Death from all causes during the treatment period and the whole follow‐up period (2) Mean body temperature 24 hours after start of treatment (3) Intracranial and extracranial haemorrhages or haemorrhagic transformation of infarctions (both symptomatic and asymptomatic, as assessed with computerised tomography (CT) or magnetic resonance imaging (MRI) (4) Infections (5) Other complications (e.g. cardiac arrhythmias, venous thrombosis, and hypotension during treatment)
Search methods for identification of studies
See: 'Specialized register' section of Cochrane Stroke Group
We searched the Cochrane Stroke Group Trials Register, which was last searched by the review Group Co‐ordinator in November 2007. In addition, we searched MEDLINE (1966 to December 2007) (Appendix 1) and EMBASE (1980 to September 2006) (Appendix 2). No language restrictions were applied. We obtained translations of non‐English language study reports. We excluded animal studies. We checked citations in the reference lists from publications identified. We discussed unpublished (or published) work on temperature‐lowering therapy in acute stroke with relevant investigators during meetings.
For the previous version of this review, published in 1999, the authors contacted the following pharmaceutical companies: Bayer (Aspirin), Smithkline Beecham (Paracetamol, Nabumetone), Parke‐Daves (Meclofenamate sodium), Upsamedica (Niflumic acid), Diamant (Tiaprofenic acid), Sigma (Tolfenamic acid), Medibial (Acemetacine), Jansen‐Cilag (Naproxen), Searle / Geig (Diclofenac), Wyeth (Etodolac. Fentiazac), Bial (Etofenamato), Basi (Fenbufen), Helsinn (Fentiazac, Nimesulid), Knoll (Flurbiprofen, Ibuprofen), Upjohn (Ibuprofen), Merck, Sharp & Dohm (Indomethacin, Sulindac), Alter (ketoprofen), Byk (Lonazolac), Pfizer (Piroxicam), Roche (Tenoxicam), Delta (Proglumetacina). They also contacted Cincinnati Sub‐Zero Products, Inc, and Helsinn Healthcare, manufacturers of cooling equipment. This contact was not renewed for this update.
Data collection and analysis
Two review authors independently searched for RCTs and CCTs. We resolved disagreement through discussion. The same two review authors assessed the methodological quality of each identified trial. MC Tseng translated those trial reports published in Chinese. We did not use a scoring system to assess the quality of each included trial, but for each one we collected the following information.
Method of randomisation (including concealment of allocation)
Blinding (care provider, patient, outcome assessment)
Number lost to follow up
Whether the trial data were analysed according to intention to treat
We assessed allocation concealment by four categories: A (adequate); B (unclear); C (inadequate); D (not used).
The same two review authors extracted and cross‐checked the data. We discussed any discrepancies. We collected the following data.
Age
Sex
Stroke type
Time since onset to start of treatment
Type of dosing procedure and route of administration
Type of physical temperature reduction method
Duration of temperature‐lowering therapy
Details of control intervention
Type of temperature measurement
Reduction in temperature
For dichotomous outcomes, we calculated a weighted estimate of the treatment effects across trials (odds ratio (OR)) using a fixed‐effect model. Where continuous scales of measurement were used to assess the effects of treatment, we used the mean difference (MD).
We tested heterogeneity between trial results using the Cochrane Q statistic and the I‐squared (I2) statistic (percentage of total variation across studies due to heterogeneity). If we found substantial heterogeneity on efficacy analysis, our intention was to explore heterogeneity with comparison of trials with quality rating A with trials with quality rating B or C.
We analysed the effects of pharmacological temperature‐lowering therapy and physical temperature‐lowering therapy separately and together. We planned to perform analyses for all strokes combined and for ischaemic stroke and intracerebral haemorrhages separately.
We carried out subgroup analyses for duration of therapy and type of intervention. We carried out all analyses according to the intention‐to‐treat principle. If trials had two intervention groups, we divided the number of patients with poor outcome or adverse events and the total number of patients in the control group by two in order to avoid multiple comparisons using the same subset of patients.
If the published information did not allow an intention‐to‐treat analysis, we contacted the authors to get as complete follow up as possible on all randomised patients for the originally proposed period of follow up. If the modified Rankin Score (mRS) or Barthel Index (BI) scores were not available, we contacted the investigators for additional data.
Results
Description of studies
We identified a total of 25 studies (completed trials, ongoing trials and trials awaiting assessment) by December 2007. We excluded 13 trials for various reasons (seeCharacteristics of excluded studies). We included five pharmacological temperature reduction trials and three physical temperature reduction trials involving a total of 423 patients (seeCharacteristics of included studies). We are aware of one ongoing pharmacological temperature reduction trial (van Breda 2005), two ongoing physical temperature reduction trials (Guluma 2006; Takasato 2000) and one physical temperature reduction trial awaiting assessment (Weber 2004), which are potentially relevant for a future update of this review (seeCharacteristics of ongoing studies and Characteristics of studies awaiting classification). One of the five included pharmacological temperature reduction trials (Castillo 2003) was stopped prematurely because of low accrual rates, and has not been published. All studies were performed in a national setting.
Patients' characteristics
The methods used in the eight included trials are summarised in Characteristics of included studies. Variability in patients' characteristics between the studies was generally low. However, there appeared to be a difference in stroke severity between the pharmacological temperature reduction trials and physical temperature reduction trials. The numbers of trial participants ranged from 42 to 76 in the pharmacological temperature reduction studies and from 19 to 77 in the physical temperature reduction studies. Imaging with CT or MRI was performed before randomisation in all patients. One physical temperature reduction study and one pharmacological temperature reduction study included both ischaemic stroke patients and patients with intracerebral haemorrhage (Kammersgaard 2000; Kasner 2002). In all other studies only patients with ischaemic stroke were included.
Types of interventions
The list below describes the pharmacological and physical strategies to reduce temperature applied and the total number of participants included in the studies.
Pharmacological temperature reduction studies
Dippel 2001a: paracetamol 1000 mg or paracetamol 500 mg, both as a suppository, six times daily for five days (75 participants) Koennecke 2001: paracetamol 1000 mg orally, four times daily for five days (44 participants) Kasner 2002: a total dose of 3900 mg oral paracetamol over 24 hours (39 participants) Dippel 2003a: paracetamol 1000 mg or ibuprofen 400 mg orally or as a suppository, six times daily for five days (75 participants) Castillo 2003: metamizole 2000 mg, three times daily for three days (60 participants)
Physical temperature reduction studies
Kammersgaard 2000: surface cooling with forced air for six hours (73 participants) Krieger 2001: cooling blanket, ice water, and whole‐body ice rubs for 12 to 72 hours (19 participants) De Georgia 2004: endovascular cooling for 24 hours (40 participants)
Time window for inclusion
All pharmacological temperature reduction studies included patients within 24 hours of stroke onset. The maximum time interval allowed between stroke onset and start of physical temperature reduction varied from five hours to 24 hours.
Type of temperature measurement
In three trials, temperatures were measured by both rectal and tympanic thermometry (Dippel 2001a; Dippel 2003a; Kammersgaard 2000). Two trials measured temperature using a tympanic thermometer (Castillo 2003; Koennecke 2001) In another trial, temperature was measured in the oesophagus in the intervention group, whereas bladder or rectal temperatures were monitored in the control group (De Georgia 2004). Two trials assessed temperature in the bladder (Kasner 2002; Krieger 2001). No trials measured brain temperature.
Risk of bias in included studies
The methods used in the eight included trials are summarised in Characteristics of included studies.
Blinding
Three of the five included pharmacological temperature reduction studies were double blind (Castillo 2003; Dippel 2001a; Dippel 2003a). One study had a single‐blind design: only participants were blinded to treatment (Koennecke 2001). In one study, blinding was handled differently at the two participating centres: at one site, the study was performed in a double‐blinded fashion, whereas investigators were not blinded to treatment at the other site (Kasner 2002). None of the physical temperature reduction trials were blinded or used a blinded outcome assessment.
Randomisation and type of allocation concealment
All pharmacological temperature reduction trials were randomised. The method of randomisation was stated in two of the five included trials (Dippel 2001a; Dippel 2003a). In those two, computer‐generated random numbers were used. One of the three physical temperature reduction trials was randomised and used sealed envelopes (De Georgia 2004). There were no quasi‐randomised trials. One controlled clinical trial matched patients by age, sex, and stroke severity (Kammersgaard 2000) and another controlled clinical trial allocated therapy according to availability (Krieger 2001).
Outcome measures
In all pharmacological temperature reduction studies, the final outcome assessment was at one month. Outcome measures were assessed at various times across the physical temperature reduction trials, ranging from one month to six months. For our primary outcome, we contacted the authors of included trials to provide individual patient data. For all pharmacological temperature reduction trials and for two of the three physical temperature reduction trials, it was possible to obtain outcome assessments according to a dichotomisation of the mRS, i.e. a score of 0 to 2 indicating good outcome versus a score of 3 to 6 indicating poor outcome, defined as dependency or death. All pharmacological and physical temperature reduction studies evaluated the occurrence of death.
Losses to follow up and intention‐to‐treat analyses
In two trials, one participant was lost to follow up (Dippel 2001a; Dippel 2003a). The other pharmacological and physical temperature reduction trials reported no losses to follow up. Intention‐to‐treat analyses were performed in three of the five pharmacological temperature reduction trials (Castillo 2003; Dippel 2001a; Dippel 2003a) and in two of the three physical temperature reduction trials (De Georgia 2004; Kammersgaard 2000). For the other trials, the authors were contacted. Complete follow up of all participants for the originally proposed period of follow up were obtained.
Effects of interventions
We extracted data from five pharmacological temperature and three physical temperature reduction trials. We analysed the effects of pharmacological temperature lowering therapies and physical body temperature lowering therapies both separately and together.
As only one physical temperature reduction trial (Kammersgaard 2000) and one pharmacological temperature reduction trial (Kasner 2002) included both patients with intracerebral haemorrhages and ischaemic stroke and all other studies only included patients with ischaemic stroke, we did not perform subgroup analyses for ischaemic stroke and intracerebral haemorrhage.
Two included pharmacological temperature reduction trials had two intervention groups (Dippel 2001a; Dippel 2003a). For those two studies, we divided the number of participants with poor outcome or adverse effects and the total number of participants in the control group by two.
Primary outcome measure
Poor outcome (death or dependency)
All of the five pharmacological temperature reduction studies and two of the three physical temperature reduction trials reported relevant outcome measures on death and dependency (De Georgia 2004; Krieger 2001). A pooled analysis of the temperature reduction trials showed no significant difference between active treatment and control in the proportion of patients who were dead or dependent (score on the mRS ≥ 3) at final follow up (OR 0.9, 95% CI 0.6 to 1.4 ) (Analysis 1.1). We did not detect heterogeneity in the effects of pharmacological temperature reduction studies and physical reduction studies (I2 = 0%). We found high levels of heterogeneity between the two physical temperature reduction trials (I2 = 76%).
1.1. Analysis.
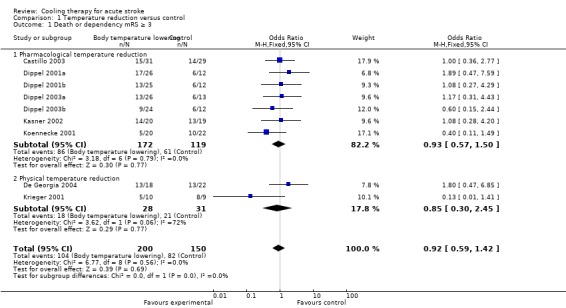
Comparison 1 Temperature reduction versus control, Outcome 1 Death or dependency mRS ≥ 3.
Secondary outcome measures
Death
All pharmacological and physical temperature reduction studies reported on the occurrence of death. We found no statistically significant effect of temperature reduction on the risk of death (OR 0.9, 95% CI 0.5 to 1.5) (Analysis 1.2). We did not detect heterogeneity in the effect of pharmacological temperature reduction studies and physical temperature reduction studies (I2 = 0%).
1.2. Analysis.
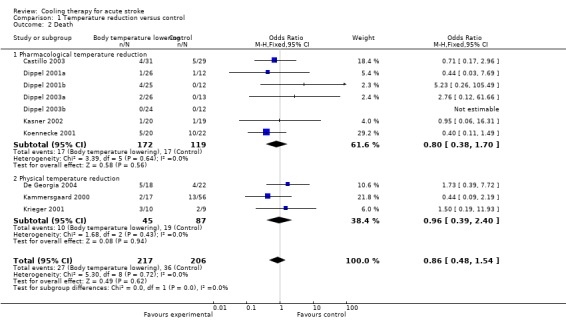
Comparison 1 Temperature reduction versus control, Outcome 2 Death.
Mean temperature at 24 hours of treatment
Data on temperature measurement were available in three of the five pharmacological temperature reduction trials and in one physical temperature reduction trial (De Georgia 2004). A pooled analysis of the pharmacological temperature reduction trials showed that the mean body temperature at 24 hours after start of treatment was 0.2 °C lower in the active treatment group than in the control group (‐0.2 °C 95% CI ‐0.3 to ‐0.2 °C) (Analysis 4.1).
4.1. Analysis.
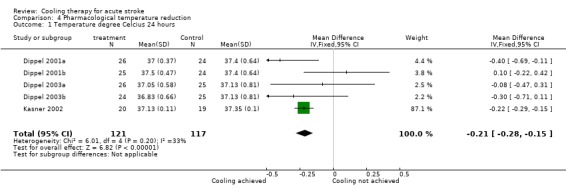
Comparison 4 Pharmacological temperature reduction, Outcome 1 Temperature degree Celcius 24 hours.
Haemorrhagic transformation of infarcts and other intracranial haemorrhages
One pharmacological temperature reduction study reported a symptomatic haemorrhagic transformation of an infarct in the active treatment group (Kasner 2002). In the group on active treatment of one of the physical temperature reduction studies, two participants developed symptomatic haemorrhagic transformation (De Georgia 2004). Another physical temperature reduction study reported one participant with an intracerebral haematoma during treatment (Krieger 2001). No extracranial haemorrhages were reported. None of the trials were terminated prematurely because of these side effects.
Infections
In both the physical and pharmacological temperature reduction trials, more infections were reported in the active treatment group, but a pooled analysis indicated no significant difference in the risk of infections in the active treatment group as compared with the control group (OR 1.5, 95% CI 0.8 to 2.6) (Analysis 2.1). There was no significant heterogeneity across the studies for this outcome (I2 = 0). None of the trials were terminated prematurely because of infections.
2.1. Analysis.
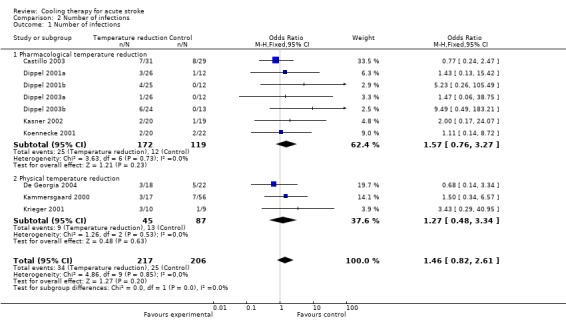
Comparison 2 Number of infections, Outcome 1 Number of infections.
Other side effects
Other side effects of temperature‐lowering therapy were evaluated in all pharmacological and physical temperature reduction trials. One trial reported cardiac arrhythmias in three of the 20 participants in the intervention group and in two of the 20 participants in the control group (De Georgia 2004). Hypotension was more frequently reported in the intervention group in one physical temperature reduction study (four of 10 participants in the intervention group versus one of nine participants in the control group) (Krieger 2001). Deep venous thrombosis was reported as being more frequent in the temperature‐lowering therapy group in one trial (seven of 20 participants in the intervention group versus four of 20 participants in the control group) (De Georgia 2004). None of these events resulted in termination of the trial.
Subgroup analyses pharmacological temperature reduction studies
We compared the results of the pharmacological temperature reduction trials using different types of agents (paracetamol, metamizole, and ibuprofen) and those with different duration of treatment (24 hours, three days and five days). There was no evidence of difference in treatment effect among the different subgroups.
Subgroup analyses physical temperature reduction studies based on quality
As we found substantial heterogeneity in the efficacy analysis of the physical temperature reduction trials, we performed a subgroup analysis based on trial quality (trial quality A versus C). No clear difference in death and or dependency was present between the randomised physical temperature reduction trial and the controlled physical temperature reduction trial.
Discussion
In this review, we analysed the available randomised and non‐randomised controlled trials of temperature‐lowering therapy in acute ischaemic stroke and intracerebral haemorrhage. We identified five randomised pharmacological temperature reduction studies, one randomised physical temperature reduction study, and two non‐randomised controlled physical temperature reduction studies. The authors of the previous version of this Cochrane review did not identify any randomised pharmacological or physical temperature reduction trials.
The studies included in this review provide no evidence that either pharmacological or physical temperature‐lowering therapy in the acute phase of stroke decreases the risk of death or dependency. Several factors may explain the observed lack of treatment effect.
First of all, the total number of participants included in these studies was far too small and the interventions too heterogeneous for definitive conclusions. Moreover, all studies were designed to test safety and feasibility, and allowed rather long time periods between stroke onset and start of treatment, which may lower the likelihood of observing an effect of treatment.
Secondly, in the pharmacological temperature reduction studies, the mean body temperature at 24 hours after the start of treatment was 0.2 °C lower in the treatment group than in the control group (95% CI ‐0.3 to ‐0.2 °C). This small effect may seem insignificant, but may prove to be clinically relevant as in an observational study the relative risk of poor outcome rose by a factor of 2.2 (95% CI: 1.4 to 3.5) with each °C increase in body temperature (Reith 1996). Data on body temperature during physical temperature reduction trials were insufficient for a pooled analysis.
Several other methodological issues such as the method of measuring body or brain temperature and the timing, size and duration of the temperature reduction, may turn out to be important determinants of treatment effect, but they remain as yet unresolved in this review.
In all studies except one, body temperature was assessed with a tympanic, rectal or a bladder thermometer. One study monitored oesophageal temperature. No studies determined brain temperature. A study of eight patients with traumatic brain injury studied the correlation between temperatures measured rectally or in the bladder and those measured in the brain (Henker 1998). Brain temperature was found to be 1 °C to 2 °C higher than rectal and bladder temperatures. Differences were largest when the rectal or bladder temperatures were higher than 38 °C. As temperatures in all included trials were assessed with rectal, bladder, or tympanic thermometry, the brain temperature in these patients may have been underestimated, and the body temperature reduction may not be representative for brain temperature reduction. On the other hand, all clinical prognostic studies and most animal experiments have suggested a benefit of temperature reduction reported body temperatures and not brain temperatures. In addition, in another longitudinal study of 20 patients with traumatic brain injury or subarachnoid haemorrhage and fever the mean difference between body and brain temperature was only 0.3 °C (SD 0.3 °C) (Rossi 2001).
In ischaemic stroke, temperature‐lowering therapy may reduce tissue damage via several mechanisms, including protection of the blood‐brain barrier, suppression of the release of excitatory amino acids and free radicals, lowering the cerebral metabolic rate, and anti‐inflammatory actions (Chopp 1998; Dietrich 1990; Dietrich 1991; Globus 1995; Henker 1998; Huang 1999). The mechanisms underlying a possible benefit of temperature‐lowering therapy in intracerebral haemorrhage are less clear.
However, observational studies have shown an association between increased body temperatures and poor outcome both in ischaemic stroke patients and in patients with intracerebral haemorrhage (Azzimondi 1998; Boysen 2001; Schwartz 2000). As only one physical temperature reduction trial and one pharmacological temperature reduction trial included patients with intracerebral haemorrhage and patients with ischaemic stroke and all other studies included patients with ischaemic stroke only, we did not perform subgroup analysis for ischaemic stroke and intracerebral haemorrhage.
Due to the lack of sufficient data, no conclusion can be drawn on the maximum time window to treatment, the optimal target temperature, and duration of temperature‐lowering therapy.
Animal studies have suggested that although the efficacy of temperature‐lowering therapy is largest when started before or at the onset of ischaemia, there is still substantial benefit when this is delayed for up to six hours (Van der Worp 2007a). On the other hand, observational studies have suggested that increased body temperatures measured up to 24 hours after stroke onset are associated with poor outcome. (Azzimondi 1998; Boysen 2001; Castillo 1998; Jorgensen 1996; Reith 1996).
Similar uncertainty exists on the optimal duration of treatment. In animal models of focal cerebral ischaemia, pathophysiological processes exert their deleterious effects over various time courses, extending from the first hours to several days after vessel occlusion (Dirnagl 1999). Such observations may imply that temperature‐lowering therapy should be more efficacious if prolonged. On the other hand, longer durations of treatment were not associated with improved outcomes in a meta‐analysis of hypothermia in animal models of focal cerebral ischaemia, and the risk of side effects such as infections may increase with longer durations of hypothermia (Van der Worp 2007a). In clinical trials of cardiac arrest, temperature‐lowering therapy has been proven efficacious if maintained for 12 or 24 hours (Bernard 2002; Holzer 2005).
In addition, the optimal target temperature in acute stroke is under debate. In animal studies of focal cerebral ischaemia, efficacy was highest with temperatures below 32 °C, but infarct volume was still reduced by about one‐third after cooling to 35 °C (Van der Worp 2007a). In the above‐mentioned cardiac arrest trials, the target temperature was 32 °C to 34 °C (Bernard 2002; Holzer 2005). However, for comfort, monitoring, and the prevention of shivering, temperature‐lowering therapy to such levels generally requires sedation, mechanical ventilation, and therefore admission to an intensive care unit. Given the limited availability of intensive care beds in most countries, cooling to temperatures around 33 °C is therefore highly impractical, even in the setting of a clinical trial. In addition, sedation and mechanical ventilation may increase the risk of side effects.
Even when temperature reduction leads to preservation and recovery of brain tissue, its beneficial effect may be offset by adverse events. All pharmacological and physical temperature reduction studies included in this review reported on the occurrence of infections. In these trials, temperature reduction did not lead to a statistically significant increase in the incidence of infections.Two physical temperature reduction trials and one pharmacological temperature reduction trial reported symptomatic haemorrhagic transformation of an infarct or intracranial haemorrhage in the active treatment group, but numbers are too small for definitive conclusions. None of the trials were terminated prematurely because of these side effects or other side effects.
Trials of physical interventions to lower temperature seem to be of lower methodological quality than pharmacological temperature reduction trials. This can partly be explained by the nature of the intervention, which prohibits a double‐blind design. However, in most intervention studies, blind assessment of outcome and allocation concealment and a proper randomisation procedure should be feasible.
We need further studies of temperature reduction in acute stroke that explore the optimal cooling strategy, intensity, and duration in terms of feasibility, safety, and, ultimately, efficacy. Based on this review we can make several recommendations to improve the quality of new intervention studies of temperature reduction in acute stroke. The next generation of trials should measure body core temperature in all treated patients, should systematically assess and report adverse events, should use proper treatment allocation schemes and be properly blinded, at least for the assessment of the primary outcome.
In conclusion, the six randomised and two controlled temperature reduction studies included in this review do not provide sufficient evidence for routine use of strategies to reduce temperature in patients with acute stroke. Large randomised clinical trials are needed to study the effect of both physical and pharmacological temperature reduction strategies.
Authors' conclusions
Implications for practice.
Routine application of pharmacological or physical temperature reduction therapy for acute stroke cannot be recommended at present.
Implications for research.
Increased body temperatures following stroke are associated with poor outcome. Animal studies have shown that temperature reduction may reduce brain tissue damage incurred by focal cerebral ischaemia. In man, temperature‐lowering therapy has been proven effective in preventing death and dependency in patients who were resuscitated after cardiac arrest. These findings suggest that therapeutic hypothermia may also improve outcome in patients with acute stroke. Hence, further large randomised clinical trails are needed to study the safety, optimal duration and the effectiveness of both physical and medical temperature reduction in patients with acute stroke. Attention should be paid to trial quality issues, including randomisation, blind outcome assessment, relevant intervention contrast and relevant outcome measures. In addition, it is important that these trials should pay attention to the occurrence of infections and to the recording of temperature.
What's new
| Date | Event | Description |
|---|---|---|
| 3 April 2008 | Amended | Converted to new review format. |
| 13 February 2008 | New search has been performed | We updated the searches between November and December 2007. The previous version of the review considered all studies where temperature‐lowering therapy was applied within two weeks of stroke onset. For the current version, temperature‐lowering therapy had to be started within 24 hours after symptom onset. The previous version of this review had no included trials. In this update we have included eight trials with 423 participants. The review has been updated and edited extensively throughout; the conclusions have not changed. |
| 13 February 2008 | New citation required but conclusions have not changed | Changes to authorship. |
Acknowledgements
We would like to thank the authors of included trials who provided individual patient data. We would also like to thank the authors of the previous version of this review. We are grateful to the Review Group Co‐ordinator Hazel Fraser, Brenda Thomas and Peter Sandercock for their comments on this review.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE (Ovid)
1. exp cerebrovascular disorders/ 2. (Stroke$ or poststroke$ or cva$).tw. 3. (cerebrovascular$ or cerebral vascular).tw. 4. (cerebral or cerebellar or brainstem or vertebrobasilar or brain).tw. 5. (Infarct$ or isch?emi$ or thrombo$ or apoplexy or emboli$).tw. 6. 4 AND 5 7. (cerebral or intracerebral or intracranial or brain or brainstem or cerebellar or vertebrobasilar).tw. 8. (haemorrhage$ or hemorrhag$ or haematoma or hematoma or bleeds).tw. 9. 7 or 8 10. 1 or 2 or 3 or 6 or 9. 11. body temperature/ 12. temperature/ or cold/ 13. hypothermia/ or hypothermia, induced/ or cryotherapy/ or fever/ 14. (fever adj5 reduc$).tw. 15. (hypotherm$ or cold$ or cool$ or temperature$ or antipyretic).tw. 16. or/11‐15 17. 10 and 16 18. limit 17 to human
Appendix 2. EMBASE search strategy
EMBASE (OVID)
1. exp cerebrovascular disorders/ 2. (stroke$ or poststroke$ or cva$).tw. 3. (cerebrovascular$ or cerebral vascular).tw. 4. (cerebral or cerebellar or brainstem or vertebrobasilar or brain).tw. 5. (Infarct$ or isch?emi$ or thrombo$ or apoplexy or emboli$).tw. 6. 4 AND 5 7. (cerebral or intracerebral or intracranial or brain or brainstem or cerebellar or vertebrobasilar).tw. 8. (haemorrhage$ or hemorrhag$ or haematoma or hematoma or bleed$).tw. 9. 7 or 8 10. 1 or 2 or 3 or 6 or 9 11. temperature/ or skin temperature/ or exp body temperature 12. low temperature procedures/ or body temperature monitoring/ 13. cooling/ or cooling water/ 14. cold/ or cold air/ or cold exposure/ or cryotherapy/ or fever/ 15. hypothermia/ or induced hypothermia/ or profound induced hypothermia 16. (fever adj5 reduc$).tw. 17. (hypotherm$ or cold$ or cool$ or temperature$ or antipyretic).tw. 18. or/11‐17 19. 10 and 18 20. limit 19 to human
Data and analyses
Comparison 1. Temperature reduction versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency mRS ≥ 3 | 9 | 350 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.42] |
| 1.1 Pharmacological temperature reduction | 7 | 291 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.57, 1.50] |
| 1.2 Physical temperature reduction | 2 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.30, 2.45] |
| 2 Death | 10 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.48, 1.54] |
| 2.1 Pharmacological temperature reduction | 7 | 291 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.38, 1.70] |
| 2.2 Physical temperature reduction | 3 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.39, 2.40] |
Comparison 2. Number of infections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of infections | 10 | 423 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.82, 2.61] |
| 1.1 Pharmacological temperature reduction | 7 | 291 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.76, 3.27] |
| 1.2 Physical temperature reduction | 3 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.48, 3.34] |
Comparison 3. Physical temperature reduction randomised vs controlled.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency mRS ≥ 3 | 2 | 59 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.30, 2.45] |
| 1.1 Randomised | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.47, 6.85] |
| 1.2 Controlled | 1 | 19 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 1.41] |
| 2 Death | 3 | 132 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.39, 2.40] |
| 2.1 Randomised | 1 | 40 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.39, 7.72] |
| 2.2 Controlled | 2 | 92 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.20, 2.24] |
3.1. Analysis.
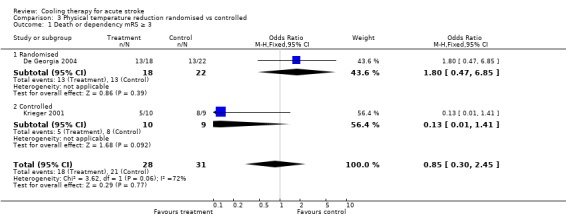
Comparison 3 Physical temperature reduction randomised vs controlled, Outcome 1 Death or dependency mRS ≥ 3.
3.2. Analysis.
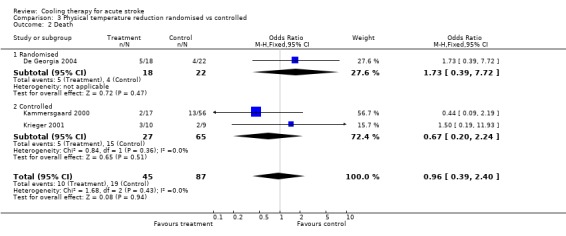
Comparison 3 Physical temperature reduction randomised vs controlled, Outcome 2 Death.
Comparison 4. Pharmacological temperature reduction.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Temperature degree Celcius 24 hours | 5 | 238 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.28, ‐0.15] |
Comparison 5. Pharmacological cooling by duration of therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency | 7 | 291 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.57, 1.50] |
| 1.1 Treatment during 24 hours | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.28, 4.20] |
| 1.2 Treatment during 3 days | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.36, 2.77] |
| 1.3 Treatment during 5 days | 5 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.48, 1.60] |
| 2 Death | 7 | 291 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.45, 3.02] |
| 2.1 Treatment during 24 hours | 1 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.06, 16.31] |
| 2.2 Treatment during 3 days | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.17, 2.96] |
| 2.3 Treatment during 5 days | 5 | 192 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.43, 10.07] |
5.1. Analysis.
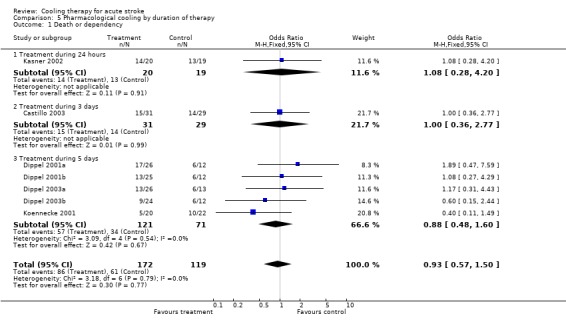
Comparison 5 Pharmacological cooling by duration of therapy, Outcome 1 Death or dependency.
5.2. Analysis.
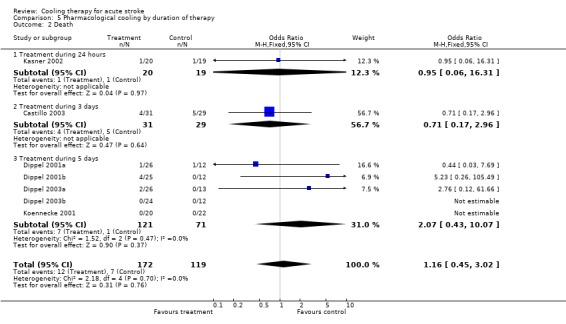
Comparison 5 Pharmacological cooling by duration of therapy, Outcome 2 Death.
Comparison 6. Pharmacological cooling by type of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependency mRS ≥ 3 | 7 | 280 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.54, 1.48] |
| 1.1 Paracetamol | 5 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.48, 1.75] |
| 1.2 Metamizol | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.36, 2.77] |
| 1.3 Ibuprofen | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.44] |
| 2 Death | 7 | 291 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.45, 3.02] |
| 2.1 Paracetamol | 5 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.45, 6.82] |
| 2.2 Metamizol | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.17, 2.96] |
| 2.3 Ibuprofen | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
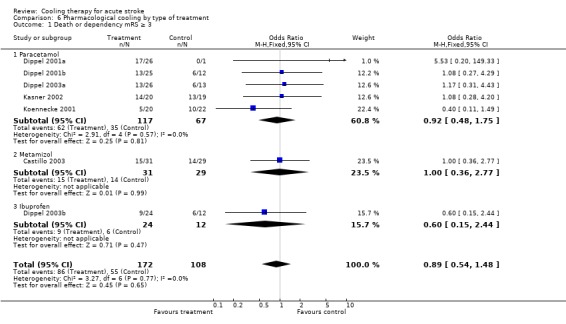
Comparison 6 Pharmacological cooling by type of treatment, Outcome 1 Death or dependency mRS ≥ 3.
6.2. Analysis.
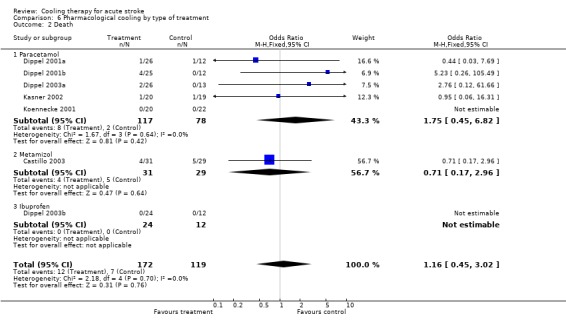
Comparison 6 Pharmacological cooling by type of treatment, Outcome 2 Death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Castillo 2003.
| Methods | Randomised, method not stated Blinding: double‐blind Placebo‐controlled trial Losses to follow up: 0 Intention to treat: yes | |
| Participants | Patients with acute ischaemic stroke ≤ 24 hours of symptom onset Admission body temperature between 37 and 38 degrees 60 participants Mean age (SD) metamizole 71 years (10 years), placebo 70 years (11 years) 24 male (40%) Stroke severity: (SSS) mean (SD) metamizole 27.2 (8.6), placebo 28.9 (10.5) Body temperature was measured using a tympanic thermometer | |
| Interventions | Metamizole 2 g 3 times daily Control: placebo Duration: 3 days | |
| Outcomes | Death or dependency (mRS ≥ 3) at 1 month Death at 1 month Infections Other side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
De Georgia 2004.
| Methods | Randomised, using sealed envelopes Blinding: none Controlled Losses to follow up: none Intention to treat: yes | |
| Participants | Patients with acute ischaemic anterior circulation stroke < 24 hours of symptom onset 40 participants Mean age (SD) endovascular cooling 61 years (12 years), control 67 years (13 years) 19 male (48%) Stroke severity: (NIHSS) mean (SD) treatment 16 (4), control 14 (5) In the intervention group oesophageal temperatures were monitored Bladder or rectal temperatures were monitored in the control group | |
| Interventions | Endovascular cooling using the Reprieve Endovascular Temperature Management System Control: standard treatment Duration: 24 hours | |
| Outcomes | Body temperature Death or dependency (mRS ≥ 3) at 1 month Death at 1 month Intracranial/extracranial haemorrhage/haemorrhagic transformation of infarction Infections Other side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A‐ adequate |
Dippel 2001a.
| Methods | Randomised, computer‐generated random numbers Blinding: double blind Placebo controlled Losses to follow up: 0 | |
| Participants | Patients with acute ischaemic anterior circulation stroke < 24 hours of symptom onset 50 participants (high dose 26, placebo 24) Mean age ( SD): high dose paracetamol 69 years (13 years), placebo 68 years (15 years) 28 male (56%) Stroke severity: (NIHSS) mean (SD) high dose paracetamol 8.8 (5.6), placebo 8.8 (5.4) Body temperature was measured by both tympanic and rectal thermometers | |
| Interventions | Paracetamol 1000 mg suppository 6 times daily Control: placebo Duration: 5 days | |
| Outcomes | Body temperature Death or dependency (mRS ≥ 3) at 1 month Death at 1 month Intracranial/extracranial haemorrhage/haemorrhagic transformation of infarction Infections Other side effects: deep venous thrombosis, cardiac arrhythmias | |
| Notes | Two intervention groups: we divided the number of participants with poor outcome and the total number of participants in the control group by 2 in order to avoid multiple comparisons using the same subset of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dippel 2001b.
| Methods | Randomised, computer‐generated random numbers Blinding: double blind Placebo controlled Losses to follow up: 1 Intention to treat: yes | |
| Participants | Patients with acute ischaemic anterior circulation stroke < 24 hours of symptom onset 49 patients (medium dose 25, placebo 24) Mean age ( SD) medium dose paracetamol 74 years (14 years), placebo 68 years (15 years) 30 male (61%) Stroke severity: (NIHSS) mean (SD), medium dose paracetamol 10.0 (8.2), placebo 8.8 (5.4) Body temperature was measured by both tympanic and rectal thermometers | |
| Interventions | Paracetamol 500 mg suppository 6 times daily Control: placebo Duration: 5 days | |
| Outcomes | Body temperature Death or dependency (mRS ≥ 3) at 1 month Death at 1 month Intracranial/extracranial haemorrhage/haemorrhagic transformation of infarction Infections Other side effects: deep venous thrombosis and cardiac arrhythmias | |
| Notes | Two intervention groups: we divided the number of participants with poor outcome and the total number of participants in the control group by 2 in order to avoid multiple comparisons using the same subset of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dippel 2003a.
| Methods | Randomised, computer‐generated random numbers Blinding: double blind Placebo‐controlled trial Losses to follow up: 1 Intention to treat: yes | |
| Participants | Patients with acute ischaemic anterior circulation stroke < 24 hours of symptom onset 51 participants (paracetamol 26, placebo 25) Mean age (SD) paracetamol 69 years (16 years), placebo 65 years (10 years) 33 male (65%) Stroke severity: (NIHSS) mean (SD) paracetamol 18 (14), placebo 14 (11) Body temperature was measured by both tympanic and rectal thermometers | |
| Interventions | Paracetamol 1000 mg 6 times daily Control: placebo Duration: 5 days | |
| Outcomes | Body temperature Poor outcome (mRS ≥ 3) at 1 month Mortality at 1 month Intracranial/extracranial haemorrhage/haemorrhagic transformation of infarction Infections Other side effects: deep venous thrombosis, cardiac arrhythmias | |
| Notes | Two intervention groups: we divided the number of participants with poor outcome and the total number of participants in the control group by 2 in order to avoid multiple comparisons using the same subset of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Dippel 2003b.
| Methods | Randomised, computer‐generated random numbers Blinding: double blind Placebo‐controlled trial Losses to follow up: 0 Intention to treat: yes | |
| Participants | Patients with acute ischaemic anterior circulation stroke < 24 hours since onset 49 participants (ibuprofen 24, placebo 25) Mean age (SD) ibuprofen 67 years (15 years), placebo 65 years (10 years) 49 male (65%) Stroke severity: (NIHSS) mean (SD) ibuprofen 12 (10), placebo 14 (11) | |
| Interventions | Ibuprofen 400 mg 6 times daily Control: placebo Duration: 5 days | |
| Outcomes | Body temperature Death or dependency (mRS ≥ 3) at 1 month Death at 1 month Intracranial/extracranial haemorrhage/haemorrhagic transformation of infarction Infections Other side effects: deep venous thrombosis, cardiac arrhythmias | |
| Notes | Two intervention groups: we divided the number of participants with poor outcome and the total number of participants in the control group by 2 in order to avoid multiple comparisons using the same subset of participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Kammersgaard 2000.
| Methods | Cohort study with historical controls Blinding: none Losses to follow up: none Intention to treat: yes | |
| Participants | Patients with a clinical diagnosis of acute ischaemic and haemorrhagic stroke < 12 hours since onset 73 participants Mean age (SD) surface cooling 69 years (16 years), control 70 years (10 years) 55 male (75%) Stroke type: ischaemic stroke 60%, haemorrhagic stroke 40% Stroke severity: (SSS) mean (SD) surface cooling 26 (12), control 28 (12) Body temperature was measured by both tympanic and rectal thermometers | |
| Interventions | Surface cooling by using the 'forced air' method, with the Bair Hugger Model 600 Polar Air Control: none Duration: 6 hours | |
| Outcomes | Death or dependency SSS (median) at 6 months Death at 6 months Infections | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kasner 2002.
| Methods | Randomised, method not stated Blinding: partly double blind (1 centre) partly not blinded (1 centre) Partly placebo controlled (1 centre), partly open label (1 centre) Losses to follow up: unknown Intention to treat: unknown | |
| Participants | Patients with acute ischaemic or haemorrhagic stroke < 24 hours since onset 39 participants Mean age (SD) treatment 70 years (13 years), control 67 years (18 years) 19 male (41%) Stroke type: ischaemic stroke 85%, haemorrhagic stroke 15% Stroke severity: (NIHSS) median (range) treatment 11 (5 to 23), control 9 (5 to 23) Body temperature was measured in the bladder | |
| Interventions | Paracetamol 3900 mg daily Control: 1 centre placebo, 1 centre avoid paracetamol Duration: 24 hours | |
| Outcomes | Body temperature Death or dependency (mRS ≥ 3) at 1 month Death at 1 month Intracranial/extracranial haemorrhage/haemorrhagic transformation of infarction Infections Other side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Koennecke 2001.
| Methods | Randomised, method not stated Placebo‐controlled trial Blinding: double blind Losses to follow up: none Intention to treat: unknown | |
| Participants | Patients with a clinical diagnosis of acute ischaemic stroke 44 participants Mean age (SD) paracetamol 69 years (13 years), placebo 68 years (15 years) 42 male (56%) Stroke severity: (NIHSS) paracetamol 8.8 (5.6), placebo 8.8 (5.4) Body temperature was measured using a tympanic thermometer | |
| Interventions | Paracetamol 1g, 4 times daily Control: placebo Duration: 5 days | |
| Outcomes | Death or dependency (mRS ≥ 3) at 1 month Death at 1 month Infections Other side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ unclear |
Krieger 2001.
| Methods | Eligible patients screened during the study period who were not enrolled served as concurrent controls Blinding: none Losses to follow up: none Intention to treat: unknown | |
| Participants | Patients with a clinical diagnosis of acute ischaemic middle cerebral artery territory stroke Eligible for intravenous thrombolysis or intra‐arterial thrombolysis/thrombectomy and the initiation of moderate hypothermia within 5 hours of symptom onset (for patients treated with intravenous thrombolysis) or 8 hours after symptom onset (for patients treated with intra‐arterial thrombolysis) Mean age (SD) cooling blanket 71.1 years (14.3 years), control 68.2 years (12.3 years) 19 participants 10 male (53%) Stroke severity: (NIHSS) mean (SD) cooling blanket 19.8 (3.3), control 19.6 (2.6) Body temperature was measured in the bladder | |
| Interventions | Cooling blanket (Aquamatic K‐Thermia EC600); ice water and whole body alcohol rubs were applied concurrently Control: none Duration: 12 to 72 hours | |
| Outcomes | Body temperature Death or dependency (mRS ≥ 3) at 3 months Death at 3 months Intracranial/extracranial haemorrhage/haemorrhagic transformation of infarction Infections Other side effects: cardiac arrhythmias and hypotension | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
SD: standard deviation SSS: Scandinavian Stroke Scale mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Diringer 2004 | No distinction between patients with stroke and other acute central nervous system disease is possible Study population has not been stratified for trauma, stroke, subarachnoid haemorrhages and other diseases |
| Els 2005 | No relevant outcome measures |
| Feng 2002 | No relevant outcome measures |
| Georgiadis 2002 | No relevant intervention contrast |
| Hua 2004 | No relevant outcome measures |
| Jian 2003 | The study was retracted and it was not controlled |
| Lyden 2005 | Uncontrolled |
| Mayer 2001 | Only about a quarter of the participants had ischaemic stroke and the results for these participants cannot be differentiated from those for others |
| Mayer 2004 | Only about a quarter of the participants had ischaemic stroke and the results for these participants cannot be differentiated from those for others |
| Meijer 2001 | No relevant outcome measures |
| Su 2004 | No relevant outcome measures |
| Wang 2004 | No relevant outcome measures |
| Wang 2005 | No relevant outcome measures |
| Zhou 2003 | No relevant outcome measures |
Characteristics of studies awaiting assessment [ordered by study ID]
Weber 2004.
| Methods | Randomised, blinded, controlled |
| Participants | All stroke patients within 6 hours of symptom onset |
| Interventions | Surface cooling Target temperature 35 °C |
| Outcomes | Functional outcome Death |
| Notes | 80 participants were included in this trial |
Characteristics of ongoing studies [ordered by study ID]
Guluma 2006.
| Trial name or title | ICTUS‐L |
| Methods | Randomised, blinded, controlled |
| Participants | Acute ischaemic stroke patients presenting within 6 hours of onset |
| Interventions | Endovascular temperature reduction + tPA versus tPA alone |
| Outcomes | Death at 3 months Functional outcome (NIHSS at 24 hours, mRS and NIHSS at 30 days) Side effects Mean temperature |
| Starting date | 2006 |
| Contact information | Principal investigator: Lyden P, email: plyden@ucsd.edu |
| Notes |
Takasato 2000.
| Trial name or title | Effect of local thrombolysis/brain hypothermia treatment for acute severe cerebral infarction |
| Methods | Unknown |
| Participants | Acute ischaemic stroke patients with GCS 8‐9 |
| Interventions | Pro‐urokinase intravenously whilst thrombus broken up with microcatheter and brain hypothermia treatment versus pro‐urokinase intravenously whilst thrombus broken up with microcatheter |
| Outcomes | Unknown |
| Starting date | 2000 |
| Contact information | Takasato Y, no contact information |
| Notes | Not registered in the International Stroke Register |
van Breda 2005.
| Trial name or title | PAIS: Paracetamol (acetaminophen) in stroke |
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled trial |
| Participants | Acute stroke patients Inclusion ≤ 12 hours from stroke onset |
| Interventions | Paracetamol, daily dose 6 g or placebo |
| Outcomes | mRS at 3 months BI at 3 months, EuroQol‐5D and body temperature at 24 hours from start of treatment The primary effect estimate is the odds ratio of improvement on the mRS according to the sliding dichotomy approach Secondary effect analyses are an estimate of the odds ratio for improvement assessed by means of ordinal logistic regression analysis, and the dichotomized mRS (≤ 2: good outcome, > 2: poor outcome) |
| Starting date | 2003 |
| Contact information | Heleen den Hertog, email: m.denhertog@erasmusmc.nl |
| Notes | 1383 participants were included Follow up will be completed on 1 August 2008. |
BI: Barthel Index mRS: modified Rankin Scale tPA: tissue plasminogen activator
Differences between protocol and review
The previous version of the review considered all studies where temperature‐lowering therapy was applied within two weeks of stroke onset. For the current version, temperature‐lowering therapy had to be started within 24 hours after symptom onset.
Contributions of authors
Search for RCTs and CCT: Heleen M den Hertog and Diederik WJ Dippel Assessment methodological quality of each identified trial: Heleen M den Hertog, Diederik WJ Dippel and H Bart van der Worp Translation and assessment of Chinese trials: Mei‐Chung Tseng Data extraction: Heleen M den Hertog, Diederik WJ Dippel and H Bart van der Worp Contacting the investigators for additional data: Heleen M den Hertog and Diederik WJ Dippel Analyses: Heleen M den Hertog and Diederik WJ Dippel Text review: Heleen M den Hertog, Diederik WJ Dippel, and H Bart van der Worp
Declarations of interest
Heleen M den Hertog is the study co‐ordinator of the ongoing PAIS trial. Dr Diederik WJ Dippel and Dr H Bart van der Worp were principal investigators of the PAPAS and PISA trials included in this review and of the ongoing PAIS trial. All the analyses and the interpretations reflect the opinions of the authors. No commercial organisation or other party was involved in the analysis or interpretation of data or in writing this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Castillo 2003 {unpublished data only}
- Castillo J, Ortiz P, Ruiz J, Puerta JL. A randomized multicentre double blind clinical trial of metamizole 2g/8h, iv ampoules versus placebo every 8h iv ampoules, administered during 3 days consecutively as antithermic therapy in the acute phase of ischemic stroke. A 30 day study (ATIS study). Unpublished.
De Georgia 2004 {published and unpublished data}
- Georgia MA, Krieger DW, Abou‐Chebl A, Devlin TG, Jauss M, Davis SM, et al. Cooling for acute ischemic stroke brain damage (COOLAID). Neurology 2004;63:312‐7. [DOI] [PubMed] [Google Scholar]
Dippel 2001a {published and unpublished data}
- Dippel DWJ, Breda EJ, Gemert HMA, Worp HB, Meijer RJ, Kappelle LJ, et al. Effect of paracetamol (acetaminophen) on body temperature in acute ischemic stroke: a double blind, randomized phase II clinical trial. Stroke 2001;32:1607‐12. [DOI] [PubMed] [Google Scholar]
Dippel 2001b {published and unpublished data}
- Dippel DWJ, Breda EJ, Gemert HMA, Worp HB, Meijer RJ, Kappelle LJ, et al. Effect of paracetamol (acetaminophen) on body temperature in acute ischemic stroke: a double blind, randomized phase II clinical trial. Stroke 2001;32:1607‐12. [DOI] [PubMed] [Google Scholar]
Dippel 2003a {published data only}
- Dippel DWJ, Breda EJ, Worp HB, Gemert HMA, Meijer RJ, Kapelle LJ, et al. Effect of paracetamol (acetaminophen) and ibuprofen on body temperature in acute ischemic stroke PISA, a phase II double blind, randomized, placebo controlled trial. BMC Cardiovascular Disorders 2003;27:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dippel 2003b {published and unpublished data}
- Dippel DWJ, Breda EJ, Worp HB, Gemert HMA, Meijer RJ, Kapelle LJ, et al. Effect of paracetamol (acetaminophen) and ibuprofen on body temperature in acute ischemic stroke PISA, a phase II double blind, randomized, placebo controlled trial. BMC Cardiovascular Diseases 2003;27:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kammersgaard 2000 {published and unpublished data}
- Kammersgaard LP, Rasmussen BH, Jorgensen HS, Reith J, Weber U, Olsen TS. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: a case‐control study: the Copenhagen Stroke Study. Stroke 2000;31:2251‐6. [DOI] [PubMed] [Google Scholar]
Kasner 2002 {published and unpublished data}
- Kasner SE, Wein T, Piriyawat P, Villar‐Cordova CE, Chalela JA, Krieger DW, et al. Acetaminophen for altering body temperature in acute stroke: a randomized clinical trial. Stroke 2002;33:130‐5. [DOI] [PubMed] [Google Scholar]
Koennecke 2001 {published data only}
- Koennecke HC, Leistner S. Prophylactic antipyretic treatment with acetaminophen in acute ischemic stroke: a pilot study. Neurology 2001;57:2301‐3. [DOI] [PubMed] [Google Scholar]
Krieger 2001 {published data only}
- Krieger DW, Georgia MA, Abou‐Chebl A, Andrefsky JC, Sila CA, Katzan IL, et al. Cooling for acute ischemic brain damage (cool aid): an open pilot study of induced hypothermia in acute ischemic stroke. Stroke 2001;32:1847‐54. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Diringer 2004 {published data only}
- Diringer MN. Treatment of fever in the neurologic intensive care unit with a catheter‐based heat exchange system. Critical Care Medicine 2004;32:559‐64. [DOI] [PubMed] [Google Scholar]
Els 2005 {published data only}
- Els T, Oehm E, Voigt S, Klisch J Hetzel A, Kassubek J. Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovascular Diseases 2006;21:79‐85. [DOI] [PubMed] [Google Scholar]
Feng 2002 {published data only}
- Feng H, Shi D, Wang D, Xin X, Feng L, Zhang Y, et al. Effect of local mild hypothermia on treatment of acute intracerebral hemorrhage, a clinical study. Zhonghua Yi Xue Za Zhi 2002;82:1622‐4. [PubMed] [Google Scholar]
Georgiadis 2002 {published data only}
- Georgiadis D, Schwarz S, Aschoff A, Schwab S. Hemicraniectomy and moderate hypothermia in patients with severe ischemic stroke. Stroke 2002;33:1584‐8. [DOI] [PubMed] [Google Scholar]
Hua 2004 {published data only}
- Hua T, Luo H, He XY, Li XG, Zhang ZL, Li ZX. Effects of mild hypothermia therapy on neurological function, serum superoxide dismutase and malondialdehyde in patients with acute cerebral infarction of big area. Zhongguo Linchuang Kangfu 2004;8:4504‐5. [Google Scholar]
Jian 2003 {published data only}
- Jian S, Yongming Q, Zhihua C, Yan C. Feasibility and safety of moderate hypothermia after acute ischemic stroke. International Journal of Developmental Neuroscience 2003;21:353‐6. [DOI] [PubMed] [Google Scholar]
Lyden 2005 {published data only}
- Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al‐Sanani F, et al. Intravascular Cooling in the Treatment of Stroke (ICTuS): early clinical experience. Journal of Stroke and Cerebrovascular Diseases 2005;14:107‐14. [DOI] [PubMed] [Google Scholar]
Mayer 2001 {published data only}
- Mayer S, Commichau C, Scarmeas N, Presciutti M, Bates J, Copeland D. Clinical trial of an air‐circulating cooling blanket for fever control in critically ill neurologic patients. Neurology 2001;56:286‐7. [DOI] [PubMed] [Google Scholar]
Mayer 2004 {published data only}
- Mayer SA, Kowalski RG, Presciutti M, Ostapkovich ND, McGann E, Fitzsimmons BF, et al. Clinical trial of a novel surface cooling system for fever control in neurocritical care patients. Critical Care Medicine 2004;32:2508‐15. [DOI] [PubMed] [Google Scholar]
Meijer 2001 {published data only}
- Meijer RJ, Visser H, Koudstaal PJ, Dippel DWJ. Lowering body temperature in acute ischemic stroke without artificial ventilation and heavy sedation: a feasibility study. Journal of Stroke and Cerebrovascular Diseases 2001;10:157‐60. [DOI] [PubMed] [Google Scholar]
Su 2004 {published data only}
- Su ZQ, Wang Y, Zhao QJ, Sun XY, Yang HY, Wang DS. Recent effect of local mild hypothermia for improving neurological deficits in patients with cerebral hemorrhage. Zhongguo Linchuang Kangfu 2004;8:1816‐7. [Google Scholar]
Wang 2004 {published data only}
- Wang H, Olivero W, Lanzino G, Elkins W, Rose J, Honings D, et al. Rapid and selective cerebral hypothermia achieved using a cooling helmet. Journal of Neurosurgery 2004;100:272‐7. [DOI] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang XL, Wang SM, Zhi DS, Zhang S. Mild hypothermia on patients with massive hemispheric infarction. Chinese Journal of Neurology 2005;38:255‐7. [Google Scholar]
Zhou 2003 {published data only}
- Zhou J, Wang J. The protective effect of subhypothermia on brain of patient with ischemic cerebrosvascular disease on different time. Zhongguo Linchuang Kangfu 2003;7:139. [Google Scholar]
References to studies awaiting assessment
Weber 2004 {published data only (unpublished sought but not used)}
- Weber UJ. NOCSS: the Nordic Cooling Stroke Study. International Stroke Register Last updated on 5/25/2004.
References to ongoing studies
Guluma 2006 {published data only (unpublished sought but not used)}
- Guluma KZ, Hemmen TM, Olsen SE, Rapp KS, Lyden PD. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS‐L). Stroke 2006;37:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takasato 2000 {unpublished data only}
- Takasato Y, Masaoka H, Wakimoto H, Naoe N, Saigusa K, Nagai M, et al. Combined local‐intraarterial thrombolysis/brain hypothermia in acute occlusion of cerebral main trunk arteries. Journal of Stroke and Cerebrovascular Diseases 2000;9:63‐4. [Google Scholar]
van Breda 2005 {published data only}
- Breda EJ, Worp HB, Gemert HM, Algra A, Kappelle LJ, Gijn J, et al. PAIS: paracetamol (acetaminophen) in stroke; protocol for a randomized, double blind clinical trial. BMC Cardiovascular Disorders 2005;5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Gadkary C, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD001048. DOI: 10.1002/14651858.CD001048.pub2] [DOI] [PubMed] [Google Scholar]
Azzimondi 1998
- Azzimondi G, Bassein L, Nonino F, Fiorani L, Vignatelli L, Re G, et al. Fever in acute stroke worsens prognosis. A prospective study. Stroke 1998;26(11):2040‐3. [DOI] [PubMed] [Google Scholar]
Bernard 2002
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out‐of‐hospital cardiac arrest with induced hypothermia. New England Journal Medicine 2002;346:557‐63. [DOI] [PubMed] [Google Scholar]
Boysen 2001
- Boysen G, Christensen H. Stroke severity determines body temperature in acute stroke. Stroke 2001;32:413‐7. [DOI] [PubMed] [Google Scholar]
Castillo 1998
- Castillo J, Davalos A, Marrugat J, Noya M. Timing for fever‐related brain damage in acute ischemic stroke. Stroke 1998;29(12):2455‐60. [DOI] [PubMed] [Google Scholar]
Chopp 1998
- Chopp M, Welch KM, Tidwell CD, Knight R, Helpern JA. Effect of mild hyperthermia on recovery of metabolic function after global cerebral ischemia in cats. Stroke 1998;19:1521‐5. [DOI] [PubMed] [Google Scholar]
Chyatte 1989
- Chyatte D, Elefteriades J, Kim B. Profound hypothermia and circulatory arrest for aneurysm surgery. Case report. Journal of Neurosurgery 1989;70(3):489‐91. [DOI] [PubMed] [Google Scholar]
Dietrich 1990
- Dietrich WD, Busto R, Halley M, Valdes I. The importance of brain temperature in alterations of the blood‐brain barrier following cerebral ischemia. Journal of Neuropathology and Experimental Neurology 1990;49(5):486‐97. [DOI] [PubMed] [Google Scholar]
Dietrich 1991
- Dietrich WD, Halley M, Valdes I, Busto R. Interrelationships between increased vascular permeability and acute neuronal damage following temperature‐controlled brain ischemia in rats. Acta Neuropathology 1991;81(6):615‐25. [DOI] [PubMed] [Google Scholar]
Dirnagl 1999
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends in Neuroscience 1999;22:291‐7. [DOI] [PubMed] [Google Scholar]
Furlan 1999
- Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. Journal of the American Medical Association 1999;282:2003‐11. [DOI] [PubMed] [Google Scholar]
Globus 1995
- Globus MY, Busto R, Lin B, Schnippering H, Ginsberg MD. Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. Journal of Neurochemistry 1995;65(3):1250‐6. [DOI] [PubMed] [Google Scholar]
Henker 1998
- Henker RA, Brown SD, Marion DW. Comparison of brain temperature with bladder and rectal temperatures in adults with severe head injury. Neurosurgery 1998;42:1071‐5. [DOI] [PubMed] [Google Scholar]
Holzer 2005
- Holzer M, Bernard SA, Hachimi‐Idrissi S, Roine RO, Sterz F, Mullner M, et al. Hypothermia for neuroprotection after cardiac arrest: systematic review and individual patient data meta‐analysis. Critical Care Medicine 2005;33(2):414‐8. [DOI] [PubMed] [Google Scholar]
Huang 1999
- Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM. Biphasic opening of the blood‐brain barrier following transient focal ischemia: effects of hypothermia. Canadian Journal of Neurological Sciences 1999;26(4):298‐304. [DOI] [PubMed] [Google Scholar]
Jorgensen 1996
- Jorgensen HS, Reith J, Pedersen PM, Nakayama H, Olsen TS. Body temperature and outcome in stroke patients. Lancet 1996;348:193. [DOI] [PubMed] [Google Scholar]
Lopez 2006
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 2006;367:1747‐57. [DOI] [PubMed] [Google Scholar]
Reith 1996
- Reith J, Jorgensen HS, Pedersen PM, Nakayama H, Raaschou HO, Jeppesen LL, et al. Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome. Lancet 1996;347(8999):422‐5. [DOI] [PubMed] [Google Scholar]
Rossi 2001
- Rossi S, Zanier ER, Mauri I, Columbo A, Stocchetti N. Brain temperature, body core temperature, and intracranial pressure in acute cerebral damage. Journal of Neurology, Neurosurgery and Psychiatry 2001;71:448‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saccani 1992
- Saccani S, Beghi C, Fragnito C, Barboso G, Fesani F. Carotid endarterectomy under hypothermic extracorporeal circulation: a method of brain protection for special patients. Journal of Cardiovascular Surgery 1992;33(3):311‐4. [PubMed] [Google Scholar]
Sandercock 2008
- Sandercock P, Counsell C, Gubitz G, Tseng MC. Antiplatelet therapy for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2008, Issue 3. [Art. No.: CD000029. DOI: 10.1002/14651858.CD000029.pub2] [DOI] [PubMed] [Google Scholar]
Schwab 1998
- Schwab S, Schwarz S, Spranger M, Keller E, Bertram M, Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke 1998;29:2461‐6. [DOI] [PubMed] [Google Scholar]
Schwartz 2000
- Schwarz S, Hafner K, Aschoff A, Schwab S. Incidence and prognostic significance of fever following intracerebral hemorrhage. Neurology 2000;54(2):354‐61. [DOI] [PubMed] [Google Scholar]
Shankaran 2005
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole‐body hypothermia for neonates with hypoxic‐ischemic encephalopathy. New England Journal of Medicine 2005;353:1574‐84. [DOI] [PubMed] [Google Scholar]
Van der Worp 2007a
- Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta‐analysis. Brain 2007;130:3063‐74. [DOI] [PubMed] [Google Scholar]
Van der Worp 2007b
- Worp HB, Gijn J. Clinical practice. Acute ischemic stroke. New England Journal of Medicine 2007;357:572‐9. [DOI] [PubMed] [Google Scholar]
Wardlaw 2003
- Wardlaw JM, Zoppo G, Yamaguchi T, Berge E. Thrombolysis for acute ischaemic stroke. Cochrane Database of Systematic Reviews 2003, Issue 3. [Art. No.: CD000213. DOI: 10.1002/14651858.CD000213] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Correia 1999
- Correia M, Silva M, Veloso M. Cooling therapy for acute stroke. Cochrane Database of Systematic Reviews 1999, Issue 4. [Art. No.: CD001247. DOI: 10.1002/14651858.CD001247] [DOI] [PubMed] [Google Scholar]


