Abstract
Atypical chest pain and diabetic autonomic neuropathy attract less clinical attention, leading to underdiagnosis and delayed treatment. To evaluate the long-term clinical impact of atypical chest pain and diabetes mellitus (DM), we categorized 11,159 patients with acute myocardial infarction (AMI) from the Korea AMI-National Institutes of Health between November 2011 and December 2015 into four groups (atypical DM, atypical non-DM, typical DM, and typical non-DM). The primary endpoint was defined as patient-oriented composite endpoint (POCE) at 2 years including all-cause death, any myocardial infarction (MI), and any revascularization. Patients with atypical chest pain showed higher 2-year mortality than those with typical chest pain in both DM (29.5% vs. 11.4%, p < 0.0001) and non-DM (20.4% vs. 6.3%, p < 0.0001) groups. The atypical DM group had the highest risks of POCE (hazard ratio (HR) 1.76, 95% confidence interval (CI) 1.48–2.10), all-cause death (HR 2.23, 95% CI 1.80–2.76) and any MI (HR 2.34, 95% CI 1.51–3.64) in the adjusted model. In conclusion, atypical chest pain was significantly associated with mortality in patients with AMI. Among four groups, the atypical DM group showed the worst clinical outcomes at 2 years. Application of rapid rule in/out AMI protocols would be beneficial to improve clinical outcomes.
Keywords: chest pain, diabetes, myocardial infarction
1. Introduction
Chest pain is one of the cardinal symptoms that warrants a visit to the emergency department (ED) [1]. Among patients with chest pain, those with pain of cardiac origin consist of a quarter to a third of the total burden [1,2]. It is challenging to determine whether chest pain is related to acute coronary syndrome (ACS). Careful history taking is the first step to distinguish high-risk patients, reduce overcrowding of ED, and prevent unnecessary examinations to control medical expenditure [3,4]. The atypical features of chest pain of non-cardiac origin are considered less urgent and tend to be overlooked and underestimated. Consequently, delayed diagnosis and treatment of these patients, who were eventually diagnosed with acute myocardial infarction (AMI), have known to be associated with unfavorable clinical outcomes [2,5,6,7].
Diabetes mellitus (DM) is considered to have a risk equivalent to that of coronary heart disease, and global prevalence is gradually increasing [8,9]. Diabetic cardiovascular autonomic neuropathy contributes to inappropriate perception of chest pain [10]. A previous study including patients with suspected stable angina showed that the incidence rate of coronary events was higher among patients with typical chest pain, especially among diabetic patients, than that among patients with atypical chest pain [11]. Interestingly, atypical patients with diabetes experienced more coronary events than those without diabetes. Although the recent improvement in optimal medical treatment and device therapy has reduced cardiovascular events, better clinical outcomes of atypical chest pain in patients with AMI are still not established. Therefore, this study aimed to assess the long-term impact of atypical chest pain in AMI patients with or without DM.
2. Methods
2.1. Study Population
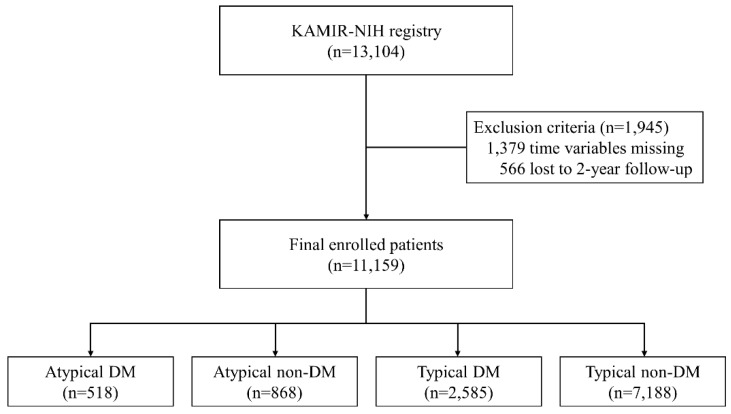
The Korea Acute Myocardial Infarction-National Institutes of Health registry (KAMIR-NIH) is a prospective, multicenter, nationwide observational cohort study (cris.nih.go.kr identifier: KCT-0000863). Patients diagnosed with AMI in 20 tertiary hospitals from November 2011 to December 2015 were enrolled in the study. The details of this study have been published previously [12]. This study was approved by the institutional review board of Chonnam National University Hospital (CNUH-2011-172) on 27 October 2011. The Institutional Review Board at each participating hospital approved the study protocol. Patients provided written informed consent to participate in this study. Among 13,104 patients, 1945 with missing values for symptom onset time, hospital arrival time, Thrombolysis In Myocardial Infarction (TIMI) flow grade, and 2-year follow-up loss were excluded (Figure 1). A total of 11,159 patients were categorized into four groups according to the presenting symptoms and history of diabetes (atypical DM, atypical non-DM, typical DM, and typical non-DM).
Figure 1.
Study flowchart. KAMIR-NIH, Korea Acute Myocardial Infarction-National Institute of Health; DM, diabetes mellitus.
2.2. Definition and Data Collection
Diagnosis of AMI was based on the presenting symptoms, serial ECG changes suggesting myocardial infarction (MI), and an increase in cardiac biomarkers, especially cardiac troponins above the 99th percentile of the upper reference limit. ST-segment elevation myocardial infarction (STEMI) was defined as new ST-segment elevation in more than two contiguous leads, measuring > 0.2 mV in leads V 1–3 or 0.1 mV in other leads, or a new left bundle branch block on 12-lead ECG with at least one positive cardiac troponin T or I. Non-STEMI (NSTEMI) was defined as at least one positive cardiac biomarker without ST-segment elevation. Chronic kidney disease (CKD) was defined as eGFR < 60 mL/min/1.73 m2. The features of chest pain were classified as typical or atypical chest pain according to the guidelines for the management of patients with non-ST-elevation acute coronary syndromes [13].
Data were recorded using a Web-based case report form in the Clinical Data Management System (iCReaT) of the Korea NIH by the physicians and trained coordinators. Demographic and baseline characteristics, including cardiovascular risk factors, other comorbidities, initial presenting symptoms, time of presentation, hospital arrival time, balloon time, and laboratory findings were collected. Angiographic findings and details of procedural characteristics, echocardiographic results, and medications were recorded. Treatment strategy and prescription of medications were based on the physician’s discretion. Patients were treated with the standard of care according to the guidelines. Patients were followed up through out-patient clinic visits or by telephonic interviews. Death, MI, and repeat revascularization were assessed at discharge and during the clinical follow-up period.
2.3. Study Outcomes
The primary endpoint was defined as patient-oriented composite endpoint (POCE) at 2 years including all-cause death, any MI, and any revascularization. The secondary endpoint was a device-oriented composite endpoint (DOCE) at 2 years including cardiac death, target vessel MI, and target lesion revascularization (TLR). Other secondary endpoints included individual components of POCE and DOCE. Death was considered as cardiac death if the apparent non-cardiac cause of death did not exist. Target vessel MI was defined as the presence of ischemic symptoms or ECG changes with an elevation of cardiac biomarker above the upper limit of normal related to a previously treated target vessel. TLR was defined as repeated revascularization of the target lesion.
2.4. Statistical Analysis
Continuous variables are expressed as mean ± SD or median (interquartile range) and assessed using the Wilcoxon signed-rank test or two-sample t-test. Categorical variables are expressed as frequencies (percentage) and compared with the chi-square test or Fisher’s exact test. Two-year Kaplan-Meier survival curves of four groups were compared using the log-rank test. The hazard ratios of study outcomes were evaluated using Cox proportional hazards models in the unadjusted and adjusted models. Multivariable-adjust proportional hazard model included age, sex, body mass index, hypertension, chronic kidney disease, dyslipidemia, current smoker, clinical diagnosis, systolic blood pressure, heart rate, Killip class ≥ 2, symptom to door time, and door to balloon time. Potential effect modification by sex, age (≥75 years), diabetes, CKD, clinical diagnosis, multivessel disease, and left ventricular ejection fraction (≥40%) was evaluated through the stratified analysis and interaction testing using a likelihood ratio test. Statistical analyses were performed using SAS 9.4 version (SAS Institute, Cary, NC, USA). Statistical significance was considered when p < 0.05.
3. Results
3.1. Baseline Characteristics
Among 11,161 patients, 1386 (12.4%) patients had atypical chest pain and 3103 (27.8%) had DM. More patients with atypical chest pain were females and older in both DM and non-DM groups (Table 1). Those with atypical chest pain had more cardiovascular risk factors, such as hypertension, CKD, previous history of MI, and old cerebrovascular accident. Dyspnea was more frequently associated with atypical chest pain. A higher proportion of NSTEMI, Killip class ≥ 2, lower left ventricular ejection fraction, longer symptom to door time, and longer door to balloon time was observed in patients with atypical chest pain. Beta-blockers, renin-angiotensin system inhibitors, and statins were less frequently prescribed in patients with atypical chest pain, regardless of diabetic status.
Table 1.
Baseline characteristics.
| Total n = 11,161 |
Diabetic Patients | Non-Diabetic Patients | |||||
|---|---|---|---|---|---|---|---|
| Atypical n = 518 |
Typical n = 2585 |
p-Value | Atypical n = 868 |
Typical n = 7188 |
p-Value | ||
| Age (year) | 63.6 ± 12.5 | 68.5 ± 10.7 | 65.2 ± 11.2 | <0.0001 | 68.4 ± 12.8 | 62.0 ± 12.7 | <0.0001 |
| Male | 8812 (75.2) | 328 (63.3) | 1822 (70.5) | 0.0013 | 595 (68.6) | 5646 (78.6) | <0.0001 |
| Height (cm) | 164.4 ± 11.3 | 161.9 ± 11.4 | 163.5 ± 12.1 | 0.0062 | 162.8 ± 9.0 | 165.1 ± 11.2 | <0.0001 |
| Weight (kg) | 65.7 ± 12.1 | 62.2 ± 12.0 | 65.8 ± 11.6 | <0.0001 | 61.6 ± 12.2 | 66.4 ± 12.1 | <0.0001 |
| BMI (kg/m2) | 24.1 ± 3.3 | 23.5 ± 3.6 | 24.3 ± 3.3 | <0.0001 | 23.1 ± 3.5 | 24.2 ± 3.3 | <0.0001 |
| Medical history | |||||||
| Hypertension | 5894 (50.3) | 371 (71.6) | 1719 (66.5) | 0.0232 | 429 (49.4) | 3095 (43.1) | 0.0004 |
| CKD | 2566 (21.9) | 294 (56.8) | 812 (31.4) | <0.0001 | 298 (34.3) | 1018 (14.2) | <0.0001 |
| Dyslipidemia | 1330 (11.3) | 49 (9.5) | 417 (16.1) | 0.0001 | 52 (6.0) | 753 (10.5) | <0.0001 |
| Previous MI | 824 (7.0) | 72 (13.9) | 252 (9.8) | 0.0048 | 52 (6.0) | 401 (5.6) | 0.6187 |
| Old CVA | 777 (6.6) | 72 (13.9) | 234 (9.1) | 0.0007 | 83 (9.6) | 355 (4.9) | <0.0001 |
| Current smoker | 4713 (40.2) | 135 (26.1) | 852 (33.0) | 0.0021 | 279 (32.1) | 3211 (44.7) | <0.0001 |
| Associated dyspnea | 2630 (22.4) | 266 (51.4) | 545 (21.1) | <0.0001 | 373 (43.0) | 1298 (18.1) | <0.0001 |
| Clinical Diagnosis | |||||||
| STEMI | 6078 (51.8) | 165 (31.9) | 1273 (49.3) | <0.0001 | 329 (37.9) | 4016 (55.8) | <0.0001 |
| NSTEMI | 5646 (48.2) | 353 (68.2) | 1312 (50.8) | 539 (62.1) | 3171 (44.1) | ||
| Systolic BP (mmHg) | 129.9 ± 30.1 | 124.9 ± 33.4 | 130.4 ± 29.6 | 0.0006 | 124.1±33.9 | 130.8 ± 29.4 | <0.0001 |
| Diastolic BP (mmHg) | 78.7 ± 18.5 | 74.5 ± 20.5 | 77.7 ± 17.4 | 0.0008 | 75.4 ± 21.2 | 79.7 ± 18.3 | <0.0001 |
| HR, bpm | 78.1 ± 19.3 | 85.7 ± 24.6 | 80.0 ± 19.2 | <0.0001 | 80.8 ± 25.1 | 76.5 ± 17.9 | <0.0001 |
| Hemoglobin (g/dL) | 13.9 ± 2.1 | 12.0 ± 2.4 | 13.4 ± 2.1 | <0001 | 13.1 ± 2.3 | 14.3 ± 1.9 | <0001 |
| Glucose (mg/dL) | 169.7 ± 81.0 | 245.0 ± 123.8 | 223.1 ± 99.1 | 0.0002 | 157.7 ± 70.4 | 146.3 ± 53.9 | <0001 |
| Creatinine (mg/dL) | 1.1 ± 1.1 | 2.0 ± 2.1 | 1.3 ± 1.4 | <0001 | 1.3 ± 1.4 | 1.0 ± 0.8 | <0001 |
| Peak CK-MB (ng/mL) | 118.80 ± 170.90 | 62.11 ± 108.80 | 97.32 ± 134.30 | <0001 | 100.30 ± 164.30 | 132.80 ± 184.60 | <0001 |
| Peak Troponin I (ng/mL) | 50.58 ± 109.70 | 32.31 ± 59.37 | 49.86 ± 89.21 | <0001 | 45.77 ± 127.60 | 52.90 ± 116.80 | 0.1337 |
| Total cholesterol (mg/dL) | 179.4 ± 45.7 | 158.3 ± 46.3 | 167.2 ± 46.2 | 0.0001 | 175.1 ± 45.9 | 185.8 ± 44.0 | <0001 |
| Triglyceride (mg/dL) | 136.1 ± 119.4 | 118.2 ± 70.5 | 142.1 ± 115.0 | <0001 | 109.7 ± 68.1 | 138.3 ± 127.4 | <0001 |
| HDL-cholesterol (mg/dL) | 42.6 ± 11.7 | 39.0 ± 12.7 | 41.1 ± 11.6 | 0.0010 | 42.6 ± 12.6 | 43.4 ± 11.4 | 0.1126 |
| LDL-cholesterol (mg/dL) | 113.1 ± 39.2 | 94.6 ± 39.0 | 101.9 ± 38.3 | 0.0004 | 111.0 ± 40.6 | 118.5 ± 38.2 | <0001 |
| Killip class | <0.0001 | <0.0001 | |||||
| 1 | 9280 (79.2) | 248 (48.0) | 1993 (77.1) | 521 (60.0) | 6054 (84.2) | ||
| 2 | 959 (8.2) | 67 (13.0) | 241 (9.3) | 105 (12.1) | 509 (7.1) | ||
| 3 | 789 (6.7) | 124 (24.0) | 192 (7.4) | 142 (16.4) | 291 (4.1) | ||
| 4 | 696 (5.9) | 78 (15.1) | 159 (6.2) | 100 (11.5) | 334 (4.7) | ||
| Killip class ≥2 | 2445 (20.9) | 270 (52.1) | 592 (22.9) | <0.0001 | 347 (40.0) | 1134 (15.8) | <0.0001 |
| LVEF, % | 52.0 ± 10.9 | 46.3±13.0 | 51.1 ± 11.4 | <0.0001 | 49.6 ± 11.5 | 53.0 ± 10.2 | <0.0001 |
| STD time (minute) | 216.0 (87.0, 749.5) | 408.5 (92.0, 2880.0) | 238.0 (100.5, 822.0) | <0.0001 | 361.0 (112.0, 1448.0) | 194.0 (80.0, 597.0) | <0.0001 |
| DTB time (minute) | 104.0 (58.0, 861.0) | 401.5 (96.0, 1388.0) | 122.0 (59.00, 922.0) | <0.0001 | 233.5 (75.0, 1125.0) | 85.0 (55.0, 728.0) | <0.0001 |
| Discharge medication | |||||||
| Aspirin | 11,698 (99.8) | 511 (98.7) | 2579 (99.8) | 0.0024 | 866 (99.8) | 7176 (99.8) | 0.6572 |
| P2Y12 inhibitor | 11,108 (99.5) | 511 (98.7) | 2575 (99.6) | 0.0146 | 863 (99.4) | 7159 (99.6) | 0.4068 |
| CCB | 651 (5.8) | 53 (10.2) | 174 (6.7) | 0.0052 | 53 (6.1) | 371 (5.2) | 0.2391 |
| Beta blocker | 9355 (83.8) | 388 (74.9) | 2190 (84.7) | <0.0001 | 655 (75.5) | 6122 (85.2) | <0.0001 |
| RAS inhibitor | 8882 (79.6) | 380 (73.4) | 2050 (79.3) | 0.0027 | 640 (73.7) | 5812 (80.9) | <0.0001 |
| Statin | 10,277 (92.1) | 405 (78.2) | 2342 (90.6) | <0.0001 | 748 (86.2) | 6782 (94.4) | <0.0001 |
Values are n (%), mean ± SD or median (interquartile range); BMI, body mass index; CKD, chronic kidney disease; MI, myocardial infarction; CVA, cerebrovascular accident; STEMI, ST segment elevation myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; BP, blood pressure; HR, heart rate; CK-MB, creatinine kinase-myocardial band; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; STD, symptom to door; DTB, door to balloon; CCB, calcium channel blocker; RAS, renin angiotensin system.
3.2. Angiographic and Procedural Characteristics
Patients with atypical chest pain showed more prevalence of multivessel disease in both DM and non-DM groups (Table 2). No differences were seen in the lesion type and treatment strategy between the groups. Post TIMI 3 flow was achieved more often among patients with typical chest pain.
Table 2.
Angiographic and procedural characteristics.
| Total n = 11,161 |
Diabetic Patients | Non-Diabetic Patients | |||||
|---|---|---|---|---|---|---|---|
| Atypical n = 518 |
Typical n = 2585 |
p-Value | Atypical n = 868 |
Typical n = 7188 |
p-Value | ||
| Disease extent | 0.0020 | <0.0001 | |||||
| 1-vessel disease | 5475 (49.1) | 175 (33.8) | 1054 (40.8) | 401 (46.2) | 3845 (53.5) | ||
| 2-vessel disease | 3812 (34.2) | 201 (38.8) | 978 (37.8) | 305 (35.1) | 2328 (32.4) | ||
| 3-vessel disease | 1872 (16.8) | 142 (27.4) | 553 (21.4) | 162 (18.7) | 1015 (14.1) | ||
| Multivessel disease | 5684 (50.9) | 343 (66.2) | 1531 (59.2) | 0.0030 | 467 (53.8) | 3343 (46.5) | <0.0001 |
| Culprit lesion | 0.0367 | 0.0003 | |||||
| Left main | 264 (2.4) | 24 (4.6) | 70 (2.7) | 27 (3.1) | 143 (2.0) | ||
| LAD | 5208 (46.7) | 235 (45.4) | 1174 (45.4) | 357 (41.1) | 3442 (47.9) | ||
| LCX | 1950 (17.5) | 73 (14.1) | 452 (17.5) | 157 (18.1) | 1268 (17.6) | ||
| RCA | 3737 (33.5) | 186 (35.9) | 889 (34.4) | 327 (37.7) | 2335 (32.5) | ||
| Lesion type | 0.0521 | 0.1673 | |||||
| A | 148 (1.3) | 12 (2.3) | 25 (1.0) | 10 (1.2) | 101 (1.4) | ||
| B1 | 1337 (12.0) | 66 (12.7) | 308 (11.9) | 103 (11.2) | 860 (12.0) | ||
| B2 | 4180 (37.5) | 197 (38.0) | 961 (37.2) | 299 (34.5) | 2723 (37.9) | ||
| C | 5494 (49.2) | 243 (46.9) | 1291 (49.9) | 456 (52.5) | 3504 (48.8) | ||
| Lesion treatment | 0.9000 | 0.4315 | |||||
| Stent | 10,343 (93.1) | 473 (91.8) | 2372 (92.0) | 800 (92.9) | 6698 (93.6) | ||
| Balloon angioplasty | 766 (6.9) | 42 (8.2) | 206 (8.0) | 61 (7.1) | 457 (6.4) | ||
| Total number of stents | 1.5 ± 0.8 | 1.6 ± 0.9 | 1.6 ± 0.8 | 0.0745 | 1.5 ± 0.8 | 1.4 ± 0.7 | 0.0030 |
| GP IIb/IIIa inhibitor | 1720 (15.4) | 51 (9.9) | 355 (13.7) | 0.0166 | 109 (12.6) | 1205 (16.8) | 0.0015 |
| Thrombolysis | 2790 (25.0) | 62 (12.0) | 582 (22.5) | <0.0001 | 143 (16.5) | 2003 (27.9) | <0.0001 |
| Pre TIMI | 0.0062 | 0.0117 | |||||
| 0 | 5258 (47.1) | 172 (33.2) | 1066 (41.2) | 387 (44.6) | 3633 (50.5) | ||
| 1 | 1232 (11.0) | 77 (14.9) | 322 (12.5) | 100 (11.5) | 733 (10.2) | ||
| 2 | 1739 (15.6) | 109 (21.0) | 457 (17.7) | 140 (16.1) | 1033 (14.4) | ||
| 3 | 2930 (26.3) | 160 (30.9) | 740 (28.6) | 241 (27.8) | 1789 (24.9) | ||
| Pre TIMI 0, 1 | 6490 (58.2) | 249 (48.1) | 1388 (53.7) | 0.0193 | 487 (56.1) | 4366 (60.7) | 0.0084 |
| Pre TIMI 3 | 2930 (26.3) | 160 (30.9) | 740 (28.6) | 0.3006 | 241 (27.8) | 1789 (24.9) | 0.0652 |
| Post TIMI | 0.0028 | 0.0256 | |||||
| 0 | 43 (0.4) | 4 (0.8) | 8 (0.3) | 8 (0.9) | 23 (0.3) | ||
| 1 | 48 (0.4) | 8 (1.5) | 8 (0.3) | 5 (0.6) | 27 (0.4) | ||
| 2 | 301 (2.7) | 17(3.28) | 72 (2.79) | 28 (3.2) | 184 (2.6) | ||
| 3 | 10,767 (96.5) | 489 (94.4) | 2497 (96.6) | 827 (95.3) | 6954 (96.7) | ||
| Post TIMI 0, 1 | 91 (0.8) | 12 (2.3) | 16 (0.6) | 0.0009 | 13 (1.5) | 50 (0.7) | 0.0113 |
| Post TIMI 3 | 10,767 (96.5) | 489 (94.4) | 2497 (96.6) | 0.0167 | 827 (95.3) | 6954 (96.7) | 0.0244 |
Values are n (%). LAD, left anterior descending; LCX, left circumflex; RCA, right coronary artery; GP, glycoprotein; TIMI, Thrombolysis in Myocardial Infarction.
3.3. Clinical Outcomes
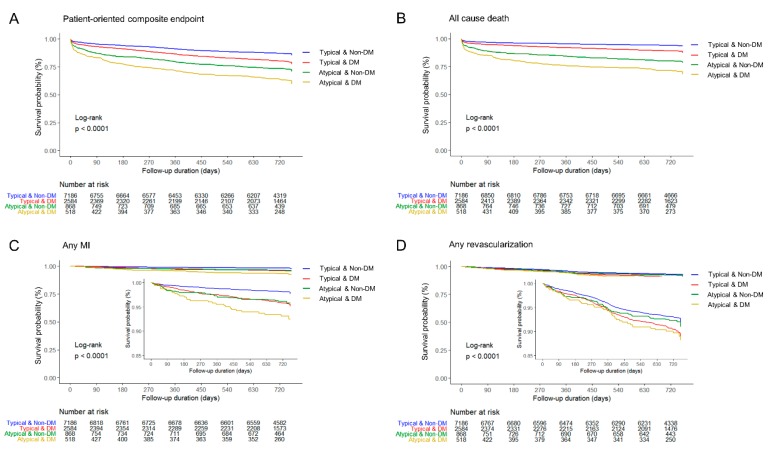
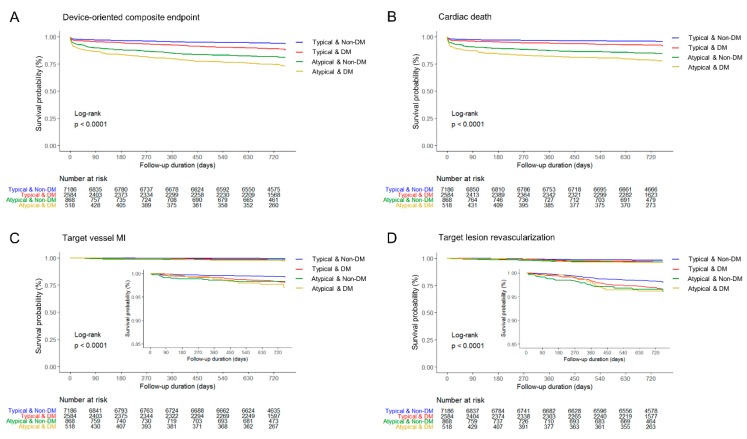
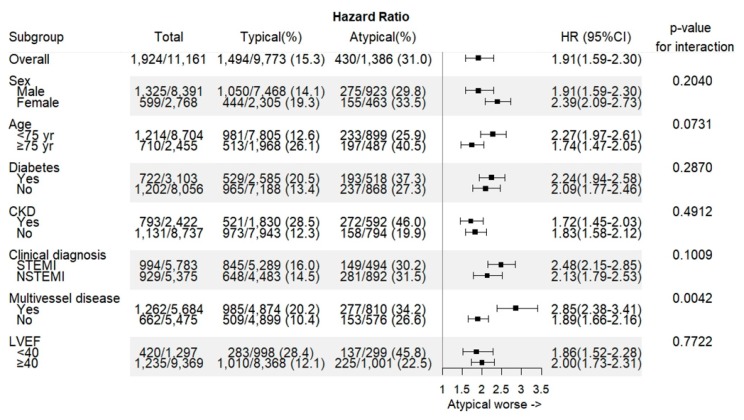
Median follow-up duration was 730 days (interquartile range 687–759 days). Overall in-hospital death occurred in 388 (3.5%) patients (Table 3). In-hospital death was significantly higher in patients with atypical chest pain among both DM and non-DM groups (11.6% vs. 3.8%, p < 0.0001; 7.8% vs. 2.3%, p < 0.0001, respectively). The incidences of POCE and all-cause death at 2-year were 17.2% and 9.7%, respectively. The 2-year rate of POCE and DOCE were about twice higher among patients with atypical pain than among those with typical chest pain. All-cause death and cardiac death rate were the main contributing factors of the difference. The 2-year cumulative incidence of all-cause death was highest in the atypical DM group (29.5%) among the four groups. In the logistic regression analysis, patients in the atypical DM group were at the highest risk of POCE (hazard ratio (HR) 1.76, 95% confidence interval (CI) 1.48–2.10), all-cause death (HR 2.23, 95% CI 1.80–2.76), and any MI (HR 2.34, 95% CI 1.51–3.64) in adjusted models (Table 4), as well as DOCE (HR 2.16, 95% CI 1.72–2.72), cardiac death (HR 2.27, 95% CI 1.75–2.94), and target vessel MI (HR 2.17, 95% CI 1.05–4.49). All estimated HRs for clinical endpoints were statistically significant (p for trend < 0.0001) among the four groups. The cumulative incidences of primary and secondary clinical endpoints were significantly different (log-rank p < 0.0001) (Figure 2 and Figure 3). Atypical chest pain was consistently worse in the subgroup analysis of interest for POCE (Figure 4). Patients with multivessel disease were associated with worse clinical outcomes (p-value for interaction = 0.0042).
Table 3.
In-hospital death and 2-year clinical outcomes.
| Total n = 11,161 |
Diabetic Patients | Non-Diabetic Patients | |||||
|---|---|---|---|---|---|---|---|
| Atypical n = 518 |
Typical n = 2585 |
p-Value | Atypical n = 868 |
Typical n = 7188 |
p-Value | ||
| In-hospital death | 388 (3.5) | 60 (11.6) | 98 (3.8) | <0.0001 | 67 (7.8) | 163 (2.3) | <0.0001 |
| Cardiac death | 329 (3.0) | 50 (9.7) | 79 (3.1) | <0.0001 | 56 (6.5) | 144 (2.0) | <0.0001 |
| Non-cardiac death | 59 (0.5) | 10 (1.9) | 19 (0.7) | 0.0200 | 11 (1.3) | 19 (0.3) | 0.0002 |
| Two-year clinical outcomes | |||||||
| POCE | 1924 (17.2) | 193 (37.3) | 529 (20.5) | <0.0001 | 237 (27.3) | 965 (13.4) | <0.0001 |
| All-cause of death | 1077 (9.7) | 153 (29.5) | 294 (11.4) | <0.0001 | 177 (20.4) | 453 (6.3) | <0.0001 |
| Any MI | 300 (2.7) | 29 (5.6) | 104 (4.0) | 0.1062 | 30 (3.5) | 137 (1.9) | 0.0025 |
| Any revascularization | 836 (7.5) | 43 (8.3) | 238 (9.2) | 0.5120 | 60 (6.9) | 495 (6.9) | 0.9772 |
| DOCE | 1020 (9.1) | 129 (24.9) | 292 (11.3) | <0.0001 | 159 (18.3) | 440 (6.1) | <0.0001 |
| Cardiac death | 755 (6.8) | 109 (21.0) | 206 (8.0) | <0.0001 | 130 (15.0) | 310 (4.3) | <0.0001 |
| Target vessel MI | 117 (1.1) | 10 (1.9) | 43 (1.7) | 0.6685 | 13 (1.5) | 51 (0.7) | 0.0135 |
| TLR | 246 (2.2) | 15 (2.9) | 78 (3.0) | 0.8822 | 27 (3.1) | 126 (1.8) | 0.0056 |
POCE, patient-oriented composite endpoint; MI, myocardial infarction; DOCO, device-oriented composite endpoint; TLR, target lesion revascularization.
Table 4.
Hazard ratio estimates from logistic regression analysis of the associations for 2-year clinical outcomes.
| Unadjusted Models | Adjusted Models | |||
|---|---|---|---|---|
| HR(95% CI) | p for Trend | HR (95% CI) | p for Trend | |
| POCE | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 1.58 (1.43–1.76) | 1.27 (1.13–1.42) | ||
| Atypical & Non-DM | 2.24 (1.94–2.58) | 1.43 (1.22–1.68) | ||
| Atypical & DM | 3.30 (2.83–3.85) | 1.76 (1.47–2.10) | ||
| All-cause of death | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 1.85 (1.60–2.14) | 1.44 (1.23–1.69) | ||
| Atypical & Non-DM | 3.50 (2.94–4.16) | 1.62 (1.33–1.97) | ||
| Atypical & DM | 5.36(4.46-6.44) | 2.23 (1.80–2.77) | ||
| Any MI | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 2.20 (1.70–2.84) | 1.79 (1.37–2.34) | ||
| Atypical & Non-DM | 2.03 (1.37–3.02) | 1.67 (1.11–2.53) | ||
| Atypical & DM | 3.60 (2.41–5.37) | 2.34 (1.51–3.63) | ||
| Any revascularization | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 1.40 (1.20–1.63) | 1.25 (1.06–1.48) | ||
| Atypical & Non-DM | 1.13 (0.86–1.47) | 1.11 (0.84–1.46) | ||
| Atypical & DM | 1.50 (1.10–2.05) | 1.34 (0.96–1.86) | ||
| DOCE | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 1.90 (1.64–2.20) | 1.48 (1.26–1.74) | ||
| Atypical & Non-DM | 3.26 (2.72–3.91) | 1.76 (1.44–2.17) | ||
| Atypical & DM | 4.70 (3.87–5.72) | 2.16 (1.72–2.72) | ||
| Cardiac death | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 1.88 (1.58–2.25) | 1.49 (1.22–1.81) | ||
| Atypical & Non-DM | 3.71 (3.02–4.55) | 1.70 (1.34–2.15) | ||
| Atypical & DM | 5.46 (4.39–6.80) | 2.27 (1.75–2.94) | ||
| Target vessel MI | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 2.43 (1.62–3.65) | 1.97 (1.28–3.01) | ||
| Atypical & Non-DM | 2.37 (1.29–4.35) | 1.92 (1.02–3.63) | ||
| Atypical & DM | 3.30 (1.68–6.51) | 2.17 (1.05–4.48) | ||
| TLR | <0.0001 | <0.0001 | ||
| Typical & Non-DM | 1 | 1 | ||
| Typical & DM | 1.79 (1.35–2.38) | 1.51 (1.12–2.03) | ||
| Atypical & Non-DM | 2.01 (1.32–3.04) | 1.79 (1.15–2.77) | ||
| Atypical & DM | 2.05 (1.20–3.50) | 1.55 (0.88–2.73) | ||
HR, hazard ratio; CI, confidence interval; POCE, patient-oriented composite endpoint; MI, myocardial infarction; DOCO, device-oriented composite endpoint; TLR, target lesion revascularization; DM, diabetes mellitus. Model adjusted for age, sex, body mass index, hypertension, chronic kidney disease, dyslipidemia, current smoker, clinical diagnosis, systolic blood pressure, heart rate, Killip class ≥2, symptom to door time, door to balloon time.
Figure 2.
Two-year patient-oriented clinical endpoint and individual clinical outcomes. (A) Patient-oriented composite endpoint, (B) All-cause death, (C) Any myocardial infarction, and (D) Any revascularization. DM, diabetes mellitus; MI, myocardial infarction.
Figure 3.
Two-year device-oriented clinical endpoint and individual clinical outcomes. (A) Device-oriented composite endpoint, (B) Cardiac death, (C) Target vessel myocardial infarction, and (D) Target lesion revascularization.DM, diabetes mellitus; MI, myocardial infarction.
Figure 4.
The primary outcome in the subgroup of interest. CKD, chronic kidney disease; STEMI, ST-segment elevation myocardial infarction; LVEF, left ventricular ejection fraction.
4. Discussion
The main purpose of this study was to evaluate the long-term clinical impact of atypical chest pain among patients with AMI. The key findings were as follows:
Patients with atypical chest pain were older, affected more females, had more comorbidities, delayed admission, and delayed treatment.
Patients with atypical chest pain showed higher in-hospital death and long-term mortality irrespective of diabetes history.
The coexistence of atypical chest pain and DM was at the highest risk of cardiovascular events.
To differentiate typical chest pain from atypical or noncardiac origin is challenging, because the value of chest pain characteristics is limited. Although typical features of chest pain are well described [3,13], a previous study demonstrated that typical symptoms, such as left anterior chest pain and resting pain or radiating pain to the left arm had no value in the diagnosis of AMI. In contrast, atypical symptoms, including radiating pain to the right arm or both arms, vomiting, and observed sweating were more likely to help diagnose AMI [4]. In the extensive review of studies between 1970 and 2005, radiation to right arm or shoulder showed higher likelihood ratio of 4.7 (95% CI 1.9–12) for the diagnosis of AMI than did radiation to the left arm (HR 2.3, 95% CI 1.7–3.1) [14]. Therefore, the likelihood of AMI should not only be based upon history taking but also on a combination of cardiac biomarkers, electrocardiogram, and imaging study [14,15].
Several studies reported the in-hospital mortality for patients with atypical chest pain in the ACS setting. In the National Registry of Myocardial Infarction 2 (NRMI-2) including 1674 hospitals in the United States from June 1994 to March 1998, 142,445 (33%) of 434,877 patients diagnosed with MI complained of no chest pain on presentation. The rate of in-hospital mortality was 23.3% among patients without chest pain compared with 9.3% among those with chest pain [5]. In NRMI-2 registry, 25.3% patients without chest pain and 74.0% patients with chest pain received thrombolysis or primary angioplasty. The Global Registry of Acute Coronary Events (GRACE) study involving 14 countries enrolled 20,881 ACS patients [6]. Patients with atypical symptoms experienced higher in-hospital death (13%) than those with typical symptoms (4.3%). The Gulf Registry of Acute Coronary Events (Gulf RACE) with 6704 ACS patients from six Middle Eastern countries also demonstrated that the absence of typical chest pain was a significant predictor of in-hospital mortality (odds ratio 2.0, 95% CI 1.29–2.75) [16]. Considering the temporal trends of in-hospital mortality that gradually decreased from 4.8% to 3.8% between 2005 and 2018 in KAMIR registry [17], the in-hospital mortality rate (9.2%) in patients with atypical chest pain is still very high, despite patients undergoing treatment with newer drugs and devices than those in the previous decade of NRMI-2 and GRACE study.
There is a lack of evidence on whether atypical chest pain affects long-term clinical outcomes. SWEDEHEART registry included 172,981 AMI patients between 1996 and 2010 [7]. Patients without chest pain were defined as those not having chest pain, including dyspnea and non-specific symptoms. The 5-year mortality in patients without chest pain aged < 65 years was 25.5% compared with 7.9% in those with chest pain. Among patients aged ≥ 65 years, the mortality rates were 57.7% in patients without chest pain and 34% in those with chest pain during 5-year follow-up. In this registry, a total of 40.1% of patients (67,700/168,981) received either percutaneous coronary intervention or coronary artery bypass graft. Of all the patients without chest pain, only 19.3% (4240/21,991) received cardiac intervention. In MONICA/KORA MI registry (population-based registry) from Augsburg, Germany consisting of 1646 patients (1231 men and 415 women aged 25 to 74 years) during median 4.1 years follow-up (interquartile range 15 years), the absence of chest symptoms significantly increased the risk of mortality (HR 1.85, 95% CI 1.13–3.03) [18]. One of the main limitations of this study was that a large number of patients (45.9%) were excluded because of impossible interview (n = 660) and incomplete data (n = 366) among 2672 patients. Although our study has a relatively shorter follow-up period than did the SWEDEHEART registry and MONICA/KORA MI registry, the strength of this study is that all the patients were treated with percutaneous coronary intervention, and most clinical events were clearly identified. In addition, hard endpoints, such as death and MI were monitored and captured by the government insurance system.
In this study, the combination of atypical chest pain and diabetes history showed the highest incidence of cardiovascular events. These worst outcomes can be explained by the adverse effect of diabetes itself and diabetic autonomic neuropathy. Approximately one-third of type 2 diabetic patients are known to experience serious cardiovascular complications, such as coronary heart disease, MI, and stroke [19,20]. Further, the mortality risk of a patient with a history of DM was comparable to that of a patient with a history of MI or stroke [21]. Meanwhile, diabetic autonomic neuropathy is one of the common complications, resulting in inappropriate perception of pain [22]. Meta-analysis has found that diabetic cardiovascular autonomic neuropathy with ≥2 abnormalities was associated with a 3.45-fold increase in the risk of mortality (95% CI 2.66–4.47) [23]. Accordingly, optimal management of DM and related complications is a major goal to reduce future cardiovascular events.
Modifiable cardiovascular risk factors are substantial targets to reduce mortality. Data from the National Health and Nutrition Examination Survey and Behavioral Risk Factor Surveillance System in the U.S. demonstrated that one third to half of diabetic patients did not reach the target goal of blood sugar level, optimal blood pressure, or low-density lipoprotein (LDL)-cholesterol level [24]. Intensive glycemic control in patients with cardiovascular autonomic neuropathy at baseline had no effect on reducing mortality compared with standard treatment in the ACCORD trial [25]. In contrast, well-treated diabetic patients who maintained target ranges of five risk factors including glycated hemoglobin, LCL-cholesterol, albuminuria, smoking, and blood pressure, had little or no excess risk regarding death, MI, or stroke during median follow-up of 5.7 years [26]. From this point of view, atypical chest pain in diabetic patients could be a potential target to improve clinical outcomes.
Fourth universal definition of MI emphasizes that the use of high-sensitivity cardiac troponin assays is beneficial to rule in/out myocardial injury and to define MI with specific subtypes [15]. Although the diagnostic role of cardiac troponin was established, the detection of a rise and/or fall in cardiac troponin level still needs several hours, resulting in delayed diagnosis of MI in patients with atypical chest pain. Early echocardiographic evaluation combined with troponin assay would be helpful to discriminate vague symptoms. Further detailed assessment strategy related to atypical chest pain is needed. Besides, promising cardiac biomarkers are awaited for early diagnosis of AMI [27].
Limitations
This study has several limitations. First, this is a prospective, observational multicenter registry, not a randomized controlled design. However, it is difficult to randomize therapeutic strategy according to the presenting symptom because there may be an ethical problem to delay interventional treatment. Second, only 20 tertiary hospitals participated in this registry, and this would not represent the overall population in our country. Third, the duration of diabetes and detailed medications were not analyzed in this study. Fourth, propensity score-matched analysis was not considered because most of the baseline characteristics were heterogeneous between the typical and atypical chest pain groups.
5. Conclusions
Atypical chest pain was significantly associated with mortality among patients with AMI. Among four groups, atypical DM group showed the worst 2-year clinical outcomes regarding POCE, all-cause death, and any MI at 2 years. Application of rapid rule in/out AMI protocols would be beneficial to improve clinical outcomes in patients with atypical chest pain.
Acknowledgments
The authors thank all of the KAMIR-NIH registry investigators. We thank clinical research nurses Sunhui Han, Jungja Woo, Hae Jung An, and Sunmi Shin for their help and support.
Author Contributions
Investigation, Y.J.Y., S.G.A., M.-S.A., J.-Y.K., B.-S.Y., S.-H.L., J.H.K., M.H.J., J.-S.P., S.C.C., S.H.H., M.-C.C., S.W.R., K.S.C., J.K.C., D.-J.C., I.W.S., S.K.O., J.Y.H.; data analysis and interpretation, J.-W.S., J.S.M., D.R.K.; writing—original draft preparation, J.-W.L.; review and editing, S.J.L., J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a fund (2016-ER6304-02) by Research of Korea Centers for Disease Control and Prevention. The electronic CRF development and data management for this study were performed using iCReaT (internet-based Clinical Research and Trial management system), a data management system established by Centers for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (iCReaT Study No. C110016).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Lee T.H., Goldman L. Evaluation of the Patient with Acute Chest Pain. N. Engl. J. Med. 2000;342:1187–1195. doi: 10.1056/NEJM200004203421607. [DOI] [PubMed] [Google Scholar]
- 2.Hermann L.K., Weingart S.D., Yoon Y.M., Genes N.G., Nelson B.P., Shearer P.L., Duvall W.L., Henzlova M.J. Comparison of Frequency of Inducible Myocardial Ischemia in Patients Presenting to Emergency Department with Typical Versus Atypical or Nonanginal Chest Pain. Am. J. Cardiol. 2010;105:1561–1564. doi: 10.1016/j.amjcard.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Abrams J. Chronic Stable Angina. N. Engl. J. Med. 2005;352:2524–2533. doi: 10.1056/NEJMcp042317. [DOI] [PubMed] [Google Scholar]
- 4.Body R., Carley S., Wibberley C., McDowell G., Ferguson J., Mackway-Jones K. The value of symptoms and signs in the emergent diagnosis of acute coronary syndromes. Resuscitation. 2010;81:281–286. doi: 10.1016/j.resuscitation.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 5.Canto J.G., Shlipak M.G., Rogers W.J., Malmgren J.A., Frederick P.D., Lambrew C.T., Ornato J.P., Barron H.V., Kiefe C.I. Prevalence, Clinical Characteristics, and Mortality Among Patients With Myocardial Infarction Presenting Without Chest Pain. JAMA. 2000;283:3223–3229. doi: 10.1001/jama.283.24.3223. [DOI] [PubMed] [Google Scholar]
- 6.Brieger D., Eagle K.A., Goodman S.G., Steg P.G., Budaj A., White K., Montalescot G., GRACE Investigators Acute Coronary Syndromes Without Chest Pain, An Underdiagnosed and Undertreated High-Risk Group: Insights From The Global Registry of Acute Coronary Events. Chest. 2004;126:461–469. doi: 10.1378/chest.126.2.461. [DOI] [PubMed] [Google Scholar]
- 7.Bjorck L., Nielsen S., Jernberg T., Zverkova-Sandstrom T., Giang K.W., Rosengren A. Absence of chest pain and long-term mortality in patients with acute myocardial infarction. Open Heart. 2018;5 doi: 10.1136/openhrt-2018-000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Expert Panel on Detection. Evaluation and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 9.Newman J.D., Rockman C.B., Kosiborod M., Guo Y., Zhong H., Weintraub H.S., Schwartzbard A.Z., Adelman M.A., Berger J.S. Diabetes mellitus is a coronary heart disease risk equivalent for peripheral vascular disease. Am. Heart J. 2017;184:114–120. doi: 10.1016/j.ahj.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acharya D.U., Shekhar Y.C., Aggarwal A., Anand I.S. Lack of pain during myocardial infarction in diabetics—Is autonomic dysfunction responsible? Am. J. Cardiol. 1991;68:793–796. doi: 10.1016/0002-9149(91)90657-7. [DOI] [PubMed] [Google Scholar]
- 11.Junghans C., Sekhri N., Zaman M.J., Hemingway H., Feder G.S., Timmis A. Atypical chest pain in diabetic patients with suspected stable angina: impact on diagnosis and coronary outcomes. Eur. Heart J. Qual. Care Clin. Outcomes. 2015;1:37–43. doi: 10.1093/ehjqcco/qcv003. [DOI] [PubMed] [Google Scholar]
- 12.Kim J.H., Chae S.C., Oh D.J., Kim H.S., Kim Y.J., Ahn Y., Cho M.C., Kim C.J., Yoon J.H., Park H.Y., et al. Korea Acute Myocardial Infarction-National Institutes of Health Registry Investigators. Multicenter Cohort Study of Acute Myocardial Infarction in Korea—Interim Analysis of the Korea Acute Myocardial Infarction Registry-National Institutes of Health Registry. Circ. J. 2016;80:1427–1436. doi: 10.1253/circj.CJ-16-0061. [DOI] [PubMed] [Google Scholar]
- 13.Amsterdam E.A., Wenger N.K., Brindis R.G., Casey D.E., Jr., Ganiats T.G., Holmes D.R., Jr., Jaffe A.S., Jneid H., Kelly R.F., Kontos M.C., et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: Executive Summary. J. Am. Coll. Cardiol. 2014;64:e139–e228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Swap C.J., Nagurney J.T. Value and Limitations of Chest Pain History in the Evaluation of Patients With Suspected Acute Coronary Syndromes. JAMA. 2005;294:2623–2629. doi: 10.1001/jama.294.20.2623. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Mickley H., Crea F., Van De Werf F. Fourth universal definition of myocardial infarction (2018) Eur. Heart. J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 16.El-Menyar A., Zubaid M., Sulaiman K., AlMahmeed W., Singh R., Alsheikh-Ali A.A., Al Suwaidi J., Gulf Registry of Acute Coronary Events (Gulf RACE) Investigators Atypical presentation of acute coronary syndrome: a significant independent predictor of in-hospital mortality. J. Cardiol. 2011;57:165–171. doi: 10.1016/j.jjcc.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y., Ahn Y., Cho M.C., Kim C.J., Kim Y.J., Jeong M.H. Current status of acute myocardial infarction in Korea. Korean J. Intern. Med. 2019;34:1–10. doi: 10.3904/kjim.2018.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchberger I., Heier M., Kuch B., von Scheidt W., Meisinger C. Presenting symptoms of myocardial infarction predict short- and long-term mortality: The MONICA/KORA Myocardial Infarction Registry. Am. Heart. J. 2012;164:856–861. doi: 10.1016/j.ahj.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Einarson T.R., Acs A., Ludwig C., Panton U.H. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018;17:83. doi: 10.1186/s12933-018-0728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckman J.A., Paneni F., Cosentino F., Creager M.A. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Eur. Heart J. 2013;34:2444–2452. doi: 10.1093/eurheartj/eht142. [DOI] [PubMed] [Google Scholar]
- 21.Emerging Risk Factors Collaboration Association of Cardiometabolic Multimorbidity with Mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinik A.I., Maser R.E., Mitchell B.D., Freeman R. Diabetic Autonomic Neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 23.Maser R.E., Mitchell B.D., Vinik A.I., Freeman R. The Association Between Cardiovascular Autonomic Neuropathy and Mortality in Individuals With Diabetes. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 24.Ali M.K., Bullard K.M., Saaddine J.B., Cowie C.C., Imperatore G., Gregg E.W. Achievement of Goals in U.S. Diabetes Care, 1999–2010. N. Engl. J. Med. 2013;368:1613–1624. doi: 10.1056/NEJMsa1213829. [DOI] [PubMed] [Google Scholar]
- 25.Pop-Busui R., Evans G.W., Gerstein H.C., Fonseca V., Fleg J.L., Hoogwerf B.J., Genuth S., Grimm R.H., Corson M.A., Prineas R., et al. Effects of Cardiac Autonomic Dysfunction on Mortality Risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Diabetes Care. 2010;33:1578–1584. doi: 10.2337/dc10-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawshani A., Rawshani A., Franzén S., Sattar N., Eliasson B., Svensson A.M., Zethelius B., Miftaraj M., McGuire D.K., Rosengren A., et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 27.Aydin S., Ugur K., Aydin S., Sahin I., Yardim M. Biomarkers in acute myocardial infarction: current perspectives. Vasc. Health Risk Manag. 2019;15:1–10. doi: 10.2147/VHRM.S166157. [DOI] [PMC free article] [PubMed] [Google Scholar]