Abstract
In the past decade, novel targeted therapy approaches, such as BTK inhibitors and Bcl2 blockers, and innovative treatments that regulate the immune response against cancer cells, such as monoclonal antibodies, CAR-T cell therapy, and immunomodulatory molecules, have been established to provide support for the treatment of patients. However, drug resistance development and relapse are still major challenges in CLL treatment. Several studies revealed that non-coding RNAs have a main role in the development and progression of CLL. Specifically, microRNAs (miRs) and tRNA-derived small-RNAs (tsRNAs) were shown to be outstanding biomarkers that can be used to diagnose and monitor the disease and to possibly anticipate drug resistance and relapse, thus supporting physicians in the selection of treatment regimens tailored to the patient needs. In this review, we will summarize the most recent discoveries in the field of targeted therapy and immunotherapy for CLL and discuss the role of ncRNAs in the development of novel drugs and combination regimens for CLL patients.
Keywords: CLL, mir-15/16, venetoclax, targeted therapy, immunotherapy
1. Chronic Lymphocytic Leukemia
Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in western countries, typically diagnosed in the elderly, with a higher prevalence in males and individuals with a family history of CLL [1]. CLL occurs in two forms, indolent or aggressive, and is characterized by the clonal proliferation of CD-5-positive B-lymphocytes that accumulate in the bloodstream, bone marrow, lymph nodes, and spleen [2]. Most patients with an indolent disease survive for several years without treatment, showing mild symptoms. Aggressive CLL, instead, is lethal if not treated and the prognosis is often poor [3]. Two staging methods for CLL are used: Rai [4] and Binet [5] systems. The Rai classification is based on parameters, such as lymphocytosis, enlarged lymph nodes, splenomegaly, hepatomegaly, anemia, and thrombocytopenia. The Binet staging is based on the presence of anemia or thrombocytopenia and the number of areas involved, defined by the presence of enlarged lymph nodes or organomegaly. Both systems are used to select patients for treatment and clinical trials and to evaluate the progression of the disease [6]. CLL diagnosis is also based on the lymphocytes’ immunophenotype and CLL cells typically express CD5, CD19, and CD23 antigens. Other factors involved in CLL diagnosis and prognosis are the expression of CD38, zeta-chain-associated protein kinase 70 (ZAP-70), and mutational status of the immunoglobulin heavy chain variable region genes (IgVH) [6]. Both indolent and aggressive forms show the clonal expansion of CD5-positive B-cells [7]. However, aggressive CLLs show high ZAP-70 expression and unmutated IgVH while indolent CLLs show low ZAP-70 and mutated IgVH. Genomic aberrations are present in more than 80% of CLL cases [8]. The most common chromosomal abnormalities detectable by cytogenetic include deletion at 13q14.3 (~55%), deletion at 11q22 (~25%), trisomy of chromosome 12 (~10%–20%), and deletion of chromosome 17p13 (~5%–8%) [9,10,11]. The identification of the minimal deleted region (MDR) on the short arm of chromosome 13 in CLL patients was the first indication that the leukemic transformation is initiated by a defect in the apoptotic mechanism driven by the loss of a non-coding RNA gene cluster [12]. Indeed, the 13q14.3 MDR contains the gene cluster encoding for miR-15a/miR-16-1 [13], which regulates the B-cell lymphoma 2 gene (BCL2), a crucial player for apoptosis initiation [14]. Loss of miR-15a/miR-16-1 prompts overexpression of BCL2, which inhibits apoptosis, leading to CLL cell accumulation [15]. Furthermore, miR-15a/miR-16-1 also target another gene involved in cell growth, the tyrosine-protein kinase transmembrane receptor 1 (ROR1), a surface antigen that binds Wnt5a, activating the non-canonical pathway to induce cell proliferation [16]. In this scenario, additional genomic aberration can arise, and indeed, most patients with 11q22 or 17p13 deletions also show the 13q14 deletion. The MDR of chromosome 11 includes the ataxia-telangiectasia mutated gene (ATM), involved in the response to DNA double-strand breaks [17]. Similarly, in 17p13-deleted CLL cells, another key regulator of the cell cycle and response to DNA damage is lost: TP53. The deletions affecting chromosomes 11 and 17 are mostly associated with aggressive disease while the presence of the 13q deletion as a sole abnormality is associated with an indolent presentation [10]. In addition, genetic mutations of regulatory genes, such as TP53 [18], NOTCH1 [19], and ATM [20], and aberrant expression of other microRNAs are associated with CLL pathogenesis, drug resistance development, and relapse [21].
2. Non-coding RNAs in CLL
In humans, only 1.5% of the genome encodes for proteins [22]. However, ~90% of the total genome is actively transcribed [23]. Most transcripts are non-coding RNAs (ncRNAs) and have a role in various biological processes [24,25]. Infrastructural ncRNAs, such as tRNA and rRNA, are components of the translational machinery [26], while regulatory ncRNAs modulate gene expression [24]. NcRNAs are divided into two categories based on their length: Long ncRNAs (lncRNAs > than 200bp), and small non-coding RNAs (sncRNAs < than 200 bp), including microRNAs (miRNAs), Piwi-interacting RNAs (piRNAs) circRNA, and tRNA fragments. SncRNAs expression is tissue-specific, and these molecules are important regulators of gene expression both at a pre- and post-transcriptional level [27,28] and the first evidence that sncRNAs have a key role in cancer was described in 2002 [13]. This study showed that the microRNA cluster miR-15a/miR-16-1 is located in the MDR of 13q14.3 observed in most CLL cases. Since then, miRNAs have been widely studied in cancer. MiRNAs are 18–28 ribonucleotide ncRNAs that regulate gene expression by promoting mRNA degradation or by inhibiting mRNA translation [29]. In 2005, a signature of microRNAs was associated with CLL prognosis [30], and in the same year, miR-15a/miR-16-1 loss was identified as a driver event in CLL onset [31]. In that study, Cimmino et al. demonstrated that miR-15a/miR-16-1 targets BCL2, a key gene involved in the regulation of apoptosis [15,31]. Several years later, it was revealed that miR-15a/miR-16-1 also targets ROR1, the receptor for Wnt5a that initiates the Wnt non-canonical growth signaling pathway [32]. Mutations and microdeletion leading to loss of function of miR-15a/miR-16-1 were also found not only in CLL (~90%) but in other cancers as well [33,34]. Following those discoveries, several studies identified other dysregulated miRNAs in CLL [21]. In 2006, miR-29 was found downregulated in aggressive CLL when compared to indolent CLL [35]. However, in 2010, miR-29a and miR-29b were found overexpressed in indolent CLL when compared to normal CD19+ B-cells [36]. Interestingly, miR-29b targets the oncogene T-cell leukemia/lymphoma 1 (TCL1) whose overexpression leads to aggressive CLL [35]. Thus, dysregulation of miR-29 alone is not sufficient to develop an aggressive disease, but since miR-29 targets TCL1 [37], its downregulation in aggressive CLL may contribute to the overexpression of TCL1, leading to the development of an aggressive disease [36]. In 2007, Auer et al. indicated that the miR-34b/c cluster is deleted in 11q-CLL [38], and in 2008, Lehmann et al. described an 11q- CLL case where an additional microdeletion was affecting the remaining allele of miR-34b/c [39]. Later, the same group showed that the remaining allele of miR-34b/c is often epigenetically silenced in most 11q- CLL cases [40]. Interestingly, miR-34a, residing on chromosome 1, is dysregulated in many cancers [41], and frequently downregulated in fludarabine-refractory CLLs [42]. Thus, in CLL, miR-34b/c are mostly lost or epigenetically silenced. Furthermore, miR-34a and miR-34b/c are involved with miR-15a/miR-16-1 and TP53 in a feedback loop that explains the indolent presentation of 13q- when compared to 11q- aggressive CLL [43]. Indeed, TP53 positively regulates both miR-34a and miR-34b/c, and the loss of miR-34 expression is associated with resistance against apoptosis induced by TP53-activating agents [44]. Interestingly, miR-34a targets AXL [45], encoding for a receptor tyrosine involved in cell proliferation and survival [46]. Activation of TP53, in CLL cells with functional TP53, inhibits AXL expression by activating miR-34a transcription. In contrast, CLL B-cells with a non-functional TP53 show high levels of AXL because miR-34a is not transactivated. As a result, CLL B-cells with 17p13 deletion express higher levels of AXL when compared to those with no 17p deletion. Thus, AXL is an attractive therapeutic target in CLL patients with 17p13 deletion and has the potential to be an effective therapeutic target in CLL B-cells regardless of the TP53 and miR34 status [45]. Remarkably, the downregulation of miR-34a was recently also associated with Richter’s syndrome [47]. Altogether, these data suggest that a compound mimicking miR-34a or miR-34b/c could be studied for CLL treatment. In 2009, Calin et al. found that at least 50% of microRNA genes are mutated and located near fragile sites, deleted regions, or common breakpoints, again indicating that miRNAs may be valuable tools for diagnostic and treatment purposes [48]. In the same year, a karyotype-specific microRNA signature was described in CLL patients [49], and the mechanism for the activation of the vascular endothelial growth factor (VEGF)-based autocrine pathway in CLL B-cells was elucidated, involving miR-92-1 (also known as miR-92a-3p), which is overexpressed in CLL B-cells. In this report, miR-92-1 was found to target the Von Hippel–Lindau transcript (pVHL) that, in turn, is responsible for HIF-1α degradation [50]. The accumulation of HIF-1α facilitates the formation of an active complex at the VEGF promoter to induce its expression and secretion [50]. CLL B-cells express VEGF receptors and respond to VEGF stimuli by upregulating the myeloid cell leukemia 1 gene (MCL1) and the X-linked inhibitor of apoptosis protein XIAP. Thus, miR-92-1 overexpression enhances the VEGF autocrine pathway for cell survivorship, suggesting that VEGF inhibition may be a promising new therapeutic approach in CLL [51,52]. In 2011, miR-181b expression was studied in CLL patients during disease progression. This report showed that in sequential samples taken from CLL patients with a progressive disease, the expression of miR-181b decreases from the indolent to the aggressive stage, whereas sequential samples taken from patients with a stable indolent disease show a steady expression of miR-181b, suggesting a diagnostic value for this microRNA [53]. Later on, miR-181b was shown to target BCL2 and TCL1 genes [35,54,55], and high miR-181a/b expression was associated with better response to chemotherapy [56]. In 2012, Tili et al. described the downregulation of miR-125b in CLL, mapping on to chromosome 11q24 near the epicenter of the deleted region in 11q- CLLs [57]. However, a subsequent study showed that the miR-125 family is implicated in a wide variety of cancers as either repressors or promoters and that upregulation of miR-125b in other hematological malignancies is associated to disease progression [58]. In addition, miR-125a targets TP53 [59], and very recently, miR-125a upregulation was also found to be associated to Richter’s syndrome along with miR-34a downregulation [47]. These conflicting data indicate that miR-125a and miR-125b may have a similar role in CLL development as miR-29 and further studies are needed to evaluate the role of these microRNAs in CLL. In 2013, miR-155 overexpression was found associated with CLL [60]. This report indicates that miR-155 levels gradually increase as normal B-cells progress to monoclonal B-cell lymphocytosis and to CLL, and pre-treatment plasma levels of miR-155 were found to be lower in patients who experienced a complete response when compared to those that did not. In 2014, miR-155 overexpression was associated with aggressive CLL [61]. The expression of miR-155 is positively regulated by environmental factors, such as BAFF signaling, and its activation can enhance the B-cell receptor (BCR) signaling, promoting proliferation in cancer cells [61]. Additionally, miR-155 was also associated to aneuploidy and early cancer transformation, indicating that its overexpression is an early event in disease onset [62] and that this microRNA could represent a valuable target for therapy. In the same year, miR-150 was found to be involved in the BCR signaling regulation in CLL by regulating GAB1 and FOXP1 [63].
Lastly, the dysregulation of tRNA-derived small non-coding RNAs (tsRNAs) was very recently described in CLL and other cancers. In 2015, miR-3676 was found to be downregulated in all types of CLL and co-deleted with TP53 in 17p- CLL [64]. Additionally, TCL1 is a confirmed target of miR-3676, suggesting that this molecule has a key role in the development of aggressive CLL. Later on, it was revealed that miR-3676 is a tsRNA, generated during the tRNA maturation process [65]. Interestingly, tsRNAs can interact with both Ago and Piwi proteins, affecting the regulation of gene expression at both pre- and post-transcriptional levels [65,66]. In 2017, the expression of tsRNAs in cancers was studied and found to be dysregulated in several types of malignancies [65]. In 2019, tsRNAs and other types of tRNA fragment were found dysregulated in CLLs, thus possibly representing additional diagnostic tools. More research is warranted to evaluated tsRNAs targets as possible targets for the new therapeutic strategies [67].
The dysregulation of different types of sncRNAs in cancer and their role of in the fine tuning of genes and pathways that can be targeted by specific anti-cancer drugs, is crucial. This discovery opened two new fields of study: the identification of specific sncRNAs signatures in cancer to use as diagnostic tools, and that identification of their targets to evaluate as targets for the development of new therapeutic strategies. Indeed, the discovery that altered expression of sncRNAs is associated with the dysregulation of genes and pathways with a key role in CLL onset, progression, and drug resistance, led to the development of several novel compounds to treat CLL and possibly other malignancies, by targeting cancer-specific molecules involved in apoptosis and cell growth [32]. Some of these compounds are small molecules that target proteins essential for cancer cell survival. Other compounds are immunotherapeutic agents such as monoclonal antibodies against specific cancer markers. Both targeted and immunotherapeutic strategies are nowadays at the forefront of cancer treatment.
3. NcRNAs, Tumor Microenvironment, and Extracellular Vesicles in CLL
Extracellular vesicles (EVs) are lipid bi-layered particles naturally released into the extracellular environment by many cells, including cancer cells, and carrying a cargo of several molecules from the parent cell. EVs have several biological functions, including the transfer of functional proteins and ncRNA. EVs can be taken up from recipient cells in several ways: receptor–ligand interactions, fusion with the target cell membrane, or internalization by endocytosis [68]. It has been suggested that cancer cell-derived EVs may stimulate the tumor microenvironment (TME) to support tumor growth and spread, and that microRNAs transferred via EVs may target specific pathways that induce a prometastatic inflammatory response [69]. In turn, tumor-associated macrophages can act as nurse-like cells (NLC) and prevent CLL B-cells from apoptosis [70,71]. Thus, since then, circulating microRNAs in body fluids have been proposed as new biomarkers and possible targets for the development of new therapy delivery systems.
In 2013, Umezu et al. showed that EV miRNAs have a key role in leukemia-endothelial cells’ crosstalk [72]. In 2015, a study was carried out to characterize CLL-derived EVs, revealing that the α-IgM-driven activation of the BCR induces CLL B-cells to release EVs, whereas BCR inactivation via ibrutinib inhibits α-IgM-stimulated EV release. The same study also showed a microRNA profiling of the plasma CLL-EVs, identifying a signature that includes the miR-29 family, miR-150, and miR-155, the expression of which increases with BCR activation [73]. In addition, cellular and serum levels of miR-150 were associated with the opposite clinical prognoses and suggested as a molecular prognostic factor in CLL progression [74].
Several additional major components of the TME were described in the last few years, including myeloid-derived suppressor cells (MDSCs), the activation of which in CLL suppresses T-cell responses. MDSCs are generated in the bone marrow and, in tumor-bearing hosts, migrate to the tumor site to support the establishment of the TME [75]. Bruns et al. showed that miR-155 delivered by CLL-EV to MDSC for induction can be disrupted by vitamin D [76].
Lastly, it is interesting to notice that even though the miRNA cargo in EVs largely represents the cell of origin, selective enrichment for specific microRNAs can occur, as observed for miR-202-3p in CLL [77]. Since a tumor suppressor role of miR-202-3p was suggested by a previous study showing that this miR is downregulated in follicular lymphoma [78], it was suggested that the compartmentalization in EVs of miR-202-3p in CLL may represent a system to remove a tumor suppressor. Indeed, in CLL, the release of miR-202-3p results in a decrease of its anti-tumorigenic effect within malignant cells.
Thus, CLL-EVs may influence the disease behavior by affecting both the donor and the recipient cells, as also described by Paggetti et al. [79]. In this report, it is described how CLL EVs taken up by endothelial cells increases angiogenesis and promotes disease progression by inducing the surrounding stromal cells to acquire features of cancer-associated fibroblasts. For these reasons, EVs are currently under investigation as potential drug delivery systems [80,81].
4. Treatment Options for CLL
CLL patients are selected for treatment when showing an aggressive symptomatic disease. Chemotherapy alone is no longer used for CLL treatment. However, chemotherapeutic agents are still administered to CLL patients in combination with immunotherapeutic agents and, less frequently, with targeted therapy. Examples of chemotherapy agents used in CLL are purine analogs, such as fludarabine, pentostatin, or cladribine; alkylating agents, such as cyclophosphamide, bendamustine or chlorambucil; and corticosteroids [82]. Unfortunately, the cytotoxicity of chemotherapeutic agents is not cancer specific, and thus these compounds damage normal cells, inducing severe side effects. Therefore, newer treatment approaches based on drugs targeting cancer-specific molecules, are being evaluated to provide better response and milder side effects. Targeted therapy can be administrated alone or in combination with chemotherapy and the recent approval of a wide array of exceptionally efficient targeted therapeutic agents led to a reduction in the use of chemoimmunotherapy regimens (FDA approved drugs and clinical trials are reported in Table 1).
Table 1.
Therapeutic approaches for CLL treatment (FDA approved and ongoing clinical trials).
| Standard FDA Approved Approaches for CLL Treatment | Type of Therapy/ Clinical Trial Code |
Reference |
|---|---|---|
| Acalabrutinib | Targeted therapy (BTK) NCT02029443 |
[94] |
| BR (bendamustine, rituximab) |
Chemo-Immunotherapy NCT02381899 |
[196] |
| CG (Chlorambucil, obinutuzumab) |
Chemo-Immunotherapy Approved for first-line therapy NCT01010061 |
[197] |
| FCR (fludarabine, cyclophosphamide, rituximab) |
Chemo-Immunotherapy NCT00090051 |
[132] |
| FR (fludarabine, rituximab) |
Chemo-Immunotherapy Approved for first-line therapy NCT00860457 |
[198] |
| Ibrutinib | Targeted therapy (BTK) Approved for first-line therapy NCT02801578 |
[91] |
| Ibrutinib/obinutuzumab | Chemo-Immunotherapy NCT02537613 |
[199] |
| Ofatumumab/chlorambucil | Chemo-Immunotherapy NCT00748189 |
[138] |
| PCR (pentostatin, cyclophosphamide, and rituximab) |
Chemo-Immunotherapy Approved for first-line therapy NCT00049413 |
[200] |
| Rituximab/chlorambucil | Chemo-Immunotherapy Approved for first-line therapy NCT00532129 |
[201] |
| Rituximab/human hyaluronidase | Chemo-Immunotherapy NCT03467867 |
[202] |
| Venetoclax | Targeted therapy (Bcl2) NCT01328626 |
[118] |
Targeted therapies are designed to target proteins expressed by cancer cells and are essential for their survival but not for that of normal cells. The most commonly used targeted therapy approaches for CLL treatment are tyrosine kinase inhibitors (such as BTK and PI3K inhibitors) and Bcl2 blockers (such as venetoclax). Immunotherapy is designed to stimulate the immune system to target cancer cells. Immunotherapy options currently available for CLL are mainly represented by monoclonal antibodies (often used in combination with chemotherapy), and the more recently developed adoptive cell therapy, such as chimeric antigen receptor T-cell (CAR T-cell). Studies on checkpoint inhibitors’ and immunomodulators’ efficacy in CLL treatment are also ongoing.
Targeted therapy and immunotherapy can be used alone or in combination with chemotherapy. Although highly effective, chemo-targeted therapy and chemo-immunotherapy have side effects and do not always induce a complete remission (CR) without minimal residual disease. Furthermore, patients can develop drug resistance and relapse [83] as a consequence of the selective pressure provided by the therapeutic agent on initially undetectable subclones [84]. Studies on the intratumoral population evolution are ongoing [85]; however, our understanding of the tumor sub-clonal distributions and response to therapy remains limited and it is still not possible to anticipate which clone will progress [86]. Thus, numerous clinical trials are evaluating targeted-immuno combination therapy to provide more tailored and efficient approaches. In the next sections, we will focus on the most recent targeted and immunotherapeutic strategies for the treatment of CLL.
4.1. Targeted Therapy for CLL
The National Cancer Institute defines targeted therapies as treatments designed to interfere with the activity of molecular targets associated specifically with cancer cells. In this section, we will describe the newest targeted agents for the treatment of CLL. Table 2 shows the most recent clinical trials of the therapeutic strategies discussed in this review.
Table 2.
Ongoing clinical trials for novel therapeutic approaches of CLL updated January 2020.
| Clinical Trial ID | Treatment | Phase | Status | Date of Start | Reference for Results |
|---|---|---|---|---|---|
| NCT00060372 | Ipilimumab | I | Completed | 04/2003 | |
| NCT00108108 | Lucatumumab | I/II | Terminated | 04/2005 | [157] |
| NCT00285103 | SPC2996 | I/II | Completed | 06/2005 | [203] |
| NCT00511043 | PTK787 (vatalanib) | II | Terminated | 11/2005 | |
| NCT00602459 | Lenalidomide combined with fludarabine and rituximab | II | Completed | 01/2008 | [192] |
| NCT00738829 | Lenalidomide combined with fludarabine and rituximab (dose escalation) | I/II | Completed | 10/2008 | [193] |
| NCT00774345 | Lenalidomide as maintenance therapy for CLL | III | Active | 01/2009 | [204] |
| NCT01029366 | Autologous CART19 | I | Completed | 03/2010 | [171] |
| NCT01161511 | XmAb5574 | I | Completed | 10/2010 | [142] |
| NCT01188681 | Otlertuzumab in combination with bendamustine | I/II | Completed | 10/2010 | [151] |
| NCT01361334 | Pazopanib | II | Completed | 06/2011 | [205] |
| NCT01400685 | Lenalidomide as first line with bendamustine and rituximab | I | Completed | 07/2011 | |
| NCT01466153 | Inebilizumab in combination with bendamustine or rituximab | II | Completed | 02/2012 | [206] |
| NCT01569295 | Idelalisib in Combination With Bendamustine and Rituximab | III | Completed | 06/2012 | [106] |
| NCT01699152 | TG02 | I | Completed | 09/2012 | |
| NCT01747486 | Autologous CART19 (dose optimization) | II | Completed | 02/2013 | [207] |
| NCT01829971 | miR-RX34 liposomal injection | I | Terminated | 04/2013 | |
| NCT02005289 | MOR00208 in combination with lenalidomide | II | Active | 12/2013 | |
| NCT02137889 | Ianalumab | I | Terminated | 07/2012 | |
| NCT02222688 | Cirmtuzumab | I | Completed | 10/2014 | |
| NCT02242942 | Obinutuzumab in combination with venetoclax, and obinutuzumab and chlorambucil | III | Active | 12/2014 | [133] |
| NCT02254772 | Ipilimumab with SD-101 and radiation therapy | I/II | Completed | 09/2014 | [208] |
| NCT02329847 | Nivolumab with ibrutinib | I/II | Active | 03/2015 | [178] |
| NCT02332980 | Pembrolizumab in combination with idelalisib or ibrutinib | II | Recruiting | 02/2015 | [176] |
| NCT02406742 | CC-122 combined with ibrutinib and obinutuzumab | I/II | Active | 09/2015 | |
| NCT02420912 | Nivolumab and ibrutinib | II | Active | 06/2015 | |
| NCT02500407 | BTCT4465A (Mosunetuzumab) as a single agent and combined with Atezolizumab | I | Recruiting | ||
| NCT02535286 | Ublituximab in combination with umbralisib | I/II | Recruiting | 09/2015 | |
| NCT02580552 | MRG-106 | I | Recruiting | 02/2016 | |
| NCT02640209 | CART19 with ibrutinib | --- | Active | 12/2015 | |
| NCT02706392 | ROR1-specific CART-cells | I | Recruiting | 03/2016 | |
| NTC02733042 | Durvalumab in combinations with lenalidomide, rituximab, ibrutinib, and bendamustine | I/II | Active | 03/2016 | |
| NCT02742090 | Duvelisib | II | Active | 04/2016 | [109] |
| NCT02846623 | Atezolizumab in combination with obinutuzumab and venetoclax | II | Recruiting | 01/2017 | |
| NCT02910583 | Ibrutinib plus venetoclax | II | Active | 10/2016 | |
| NCT02953509 | Hu5F9-G4 in Combination with Rituximab | I/II | Recruiting | 11/2016 | [209] |
| NCT02968563 | Tirabrutinib and Idelalisib with and Without Obinutuzumab | II | Active | 12/2016 | |
| NCT03037645 | Vecabrutinib | I/II | Recruiting | 04/2017 | |
| NCT03056339 | CAR-NK | I/II | Recruiting | 06/2017 | |
| NCT03088878 | Cirmtuzumab in combination with ibrutinib | I/II | Recruiting | 01/2018 | |
| NCT03162536 | ARQ-531 | I/II | Recruiting | 07/2017 | |
| NCT03218683 | AZD5991 with or without venetoclax | I | Recruiting | 10/2017 | [127] |
| NCT03336333 | BGB-3111 (zanubrutinib)with Bendamustine plus Rituximab | III | Recruiting | 11/2017 | |
| NCT03400176 | Ianalumab with ibrutinib | I | Recruiting | 04/2018 | |
| NCT03447808 | Daratumumab with ibrutinib | I | Recruiting | 07/2018 | |
| NCT03454165 | BNC105P in combination with ibrutinib | I | Recruiting | 03/2018 | |
| NCT03572634 | TP-0903 | I/II | Recruiting | 06/2019 | |
| NCT03734016 | Zanubrutinib (BGB-3111) versus Ibrutinib | III | Active | 11/2018 | |
| NCT03739554 | CYC065 and venetoclax | I | Recruiting | 01/2019 | |
| NCT03740529 | LOXO-305 | I/II | Recruiting | 11/2018 | |
| NCT03823365 | Blinatumomab | I | Recruiting | 12/2018 | |
| NCT03824483 | Zanubrutinib, obinutuzumab, and venetoclax | II | Recruiting | 02/2019 | |
| NCT04116437 | Zanubrutinib (BGB-3111) | II | Recruiting | 10/2019 |
4.1.1. Bruton’s Tyrosine Kinase Inhibitors (BTKis)
The Bruton’s tyrosine kinase (BTK) is a key component of the BCR signaling. BTK expression is upregulated in CLL cells and targeting BTK leads to cytotoxicity, inhibition of proliferation/cell migration, and disruption of cytokine/chemokine signaling [87]. Ibrutinib was the first BTK inhibitor approved by the Food and Drug Administration (FDA) for CLL treatment in 2014 [88,89] and it is used for frontline therapy of newly diagnosed CLL patients who require immediate treatment [90], and for relapsed/refractory CLL [91]. Ibrutinib binds to BTK, blocking the BCR signaling, inducing apoptosis, and preventing CLL cells from responding to microenvironment stimuli of survival [92]. Albeit effective, ibrutinib treatment has unique toxicity that can be explained by off-target effects that inhibit other tyrosine kinases [93]. Thus, second-generation BTK inhibitors were designed to reduce toxicity/off-target effects. Among these, acalabrutinib, approved by FDA as monotherapy for CLL in November 2019, offers a higher selectivity in the inhibition of BTK, less adverse effects, and improved efficacy in 17p-deleted cases [94,95] (NCT02029443). Zanubrutinib, approved by the FDA in November 2019, and tirabrutinib are currently under investigation. Zanubrutinib as a single agent is being assessed in CLL/SLL patients intolerant to prior treatment with ibrutinib (NCT04116437) and in comparison with ibrutinib in patients with relapsed/refractory CLL (NCT03734016). The combination of zanubrutinib with obinutuzumab (an anti-CD20 described in Section 4.2.1) plus venetoclax (NCT03824483) or with bendamustine plus rituximab (an anti-CD20 described in Section 4.2.1) are also being evaluated in patients with previously untreated CLL or SLL (NCT03336333). Tirabrutinib is currently under investigation in four active clinical trials for the treatment of B-cell malignancies, and one of them is specifically designed to evaluate safety and efficacy when used in combination with idelalisib (a PI3K inhibitor described in Section 4.1.2) with or without obinutuzumab (NCT02968563).
Acalabrutinib, zanubrutinib, and tirabrutinib limit off-target toxicity but do not overcome the development of ibrutinib resistance, often arising as a consequence of the selection of BTK-mutant clones. Specifically, mutations affecting the C481 residue impair ibrutinib affinity for BTK [96,97]. Thus, the reversible BTKis vecabrutinib (NCT03037645) and LOXO-305 (NCT03740529) were designed to target both the wild-type BTK and the mutated BTK-C481S forms. In addition, mutations of PLCγ2 are also associated with ibrutinib resistance [98]. Interestingly, preclinical studies showed that the non-selective reversible BTKi ARQ-531 (NCT03162536) binds BTK without interacting with the C481 residue and also inhibits kinases involved in the downstream BCR signaling, thus possibly retaining its activity despite mutations within PLCγ2 [99].
4.1.2. Phosphoinositide 3-Kinase Inhibitors (PI3Ki)
The phosphoinositide 3-kinase (PI3K) signaling pathway regulates cell proliferation and is often altered in CLLs and other cancers [100]. PI3Ks are implicated in cell signaling (class I, II) and membrane trafficking (class II, III). Class I PI3Ks are the most involved in cancer and four isoforms can be identified (α, β, δ, or γ) [101]. In CLL, activation of PI3K-δ and PI3K-γ is a consequence of BCR stimulation, which leads to inhibition of apoptosis and cell survival [102,103,104]. Idelalisib, used for the treatment of relapsed CLL, targets PI3K-δ, induces apoptosis, and prevents the proliferation of CLL cells. Additionally, idelalisib inhibits cell signaling pathways involved in trafficking and homing of B-cells to the lymph nodes and bone marrow [105]. In combination with bendamustine/rituximab therapy, idelalisib triggered a better response in recurrent CLL (NCT01569295). However, this treatment frequently induced adverse effects [106]. Duvelisib, an inhibitor of PI3K-δ and PI3K-γ approved in 2018 for the treatment of relapsed/refractory CLL and small lymphocytic lymphoma (SLL) [107], was designed to affect the TME. Unfortunately, duvelisib showed serious side effects and it is only administered to patients who did not achieve a satisfactory response after at least two systemic therapies [108]. Finally, umbralisib is a PI3K-δ and casein kinase 1 epsilon (CK1-ε) inhibitor. CK1-ε is a regulator of protein translation and it is crucial for activating non-canonical Wnt5a signaling. Interestingly, CK1-ε modulates T-cell activity, reducing the adverse events observed with previous PI3K inhibitors. Indeed, umbralisib shows improved efficacy in non-Hodgkin lymphoma, with a more favorable safety profile [109], and is currently being evaluated for treatment of CLL (NCT02742090). Additionally, a clinical trial is evaluating the umbralisib–ublituximab (an anti-CD20 monoclonal antibody described in Section 4.2.1) combination in patients with relapsed/refractory CLL or Richter’s syndrome (NCT02535286), indicating that this drug may be used in the most advanced and aggressive stages of the disease.
4.1.3. Bcl2 Blockers
The B cell lymphoma 2 (Bcl2) family proteins are key regulators of the apoptotic processes [110,111]. Bcl2 is an important inhibitor of apoptosis and, in follicular B-cell lymphoma is overexpressed as a consequence of a translocation that place the BCL2 gene under the control of the immunoglobulin heavy chain locus at the breakpoints of t(14;18) [14]. In CLL, Bcl2 overexpression has been associated to the loss of miR-15a/miR-16-1, a driver event in CLL onset [13,31,112,113]. These observations led scientists to design a drug that could target Bcl2 [114]. In 2005 the first inhibitor of Bcl2, ABT-737, was designed. ABT-737 is not bioavailable [115], and thus an orally-available derivative navitoclax was developed [116]. Unfortunately, navitoclax also inhibits Bcl-XL which is essential for platelet survival and clinical trials indicated that this drug causes thrombocytopenia [117]. Thus, a very specific Bcl2 inhibitor was generated: venetoclax. The efficacy of venetoclax in targeting CLL cells is outstanding. Venetoclax induces complete remission (CR) in most CLL patients, even though a single-agent treatment may not eradicate the minimal residual disease [118]. Venetoclax induces such strong apoptotic response in CLL cells that patients need to be closely monitored for tumor lysis syndrome during the initial phases of the therapy [119]. Thus venetoclax treatment starts with a low dose that is increased in time (NCT01328626) [120]. Because of its efficiency in inducing CR, venetoclax was approved by the FDA for the treatment of patients with relapsed/refractory CLL in 2016 [121]. In May 2019, venetoclax was approved for first-line treatment of CLL and SLL in adults, and additional clinical trials of combination therapy with ibrutinib or obinutuzumab, are currently ongoing (NCT02910583) (NCT02242942).
4.1.4. Other Targets: CDK Inhibitors, Mcl1 Inhibitors, Axl Inhibitors
CDKs are involved in transcription regulation, mRNA processing, and cell differentiation, and alteration of cyclin-dependent kinase activity is common in several cancers, including CLL [122]. CDKs bind to cyclins to form cyclin-CDK complexes, which are targeted by CDK-inhibitors. Among the most promising CDK inhibitors, CYC065, targeting CDK 2/5/9, is currently studied for relapsed/refractory CLLs in combination with venetoclax (NCT03739554). TG02, a CDK9 inhibitor, leads to the depletion of survival proteins as Mcl1, resulting in p53-independent apoptosis [123], and a clinical trial for CLL patients resistant to ibrutinib treatment is ongoing (NCT01699152).
Myeloid cell leukemia 1 gene (MCL1) is an anti-apoptotic member of the BCL2 family and its overexpression is often associated with drug resistance and relapse in multiple myeloma patients [124]. Interestingly, overexpression of MCL1 is also associated with venetoclax resistance [125]. Therefore, new drugs are being developed for CLL treatment to target MCL1 in combination with the Bcl2 inhibitor. Voruciclib, a CDK9/Mcl1 inhibitor, is under examination for FDA approval as in vitro treatment of voruciclib combined with venetoclax, showed induction of apoptosis of CLL cell models and growth arrest in high risk diffuse large B-cells lymphoma models [126]. AZD5991, a highly selective anti-Mcl1, is currently in clinical development. In vitro studies showed that AZD5991 induces apoptosis [127], and a clinical trial for relapsed/refractory hematological malignancies, including CLL, is ongoing (NCT03218683).
As previously mentioned, Axl inhibition induces apoptosis in CLL B-cells with 17p13 deletion and thus several Axl-inhibitors are being developed [46]. In a recent report, TP-0903 treatment effectively reduced Axl phosphorylation and lowered the expression levels of Mcl-1 in ibrutinib exposed CLL B-cells from patients. TP-0903 was found very effective at inducing apoptosis in CLL B-cells from ibrutinib-exposed patients supporting the use of this drug in relapsed/refractory CLL [128]. Thus, an oral formulation of TP-0903 is currently under evaluation in patients with previously treated CLL (NCT03572634).
4.2. Immunotherapy for CLL
A remarkable improvement in the treatment approach to CLL was provided by the development of immunotherapy strategies that modulate the immune system response of patients. In this section, we will describe the most recent advances and effective approaches available for CLL patients.
4.2.1. Monoclonal Antibodies
Monoclonal antibodies designed for CLL treatment are grouped according to the targeted protein. The most relevant are monoclonal antibodies directed against CD20, CD19, CD52, CD37, CD38, CD40, BAFF-R and ROR1. Monoclonal antibodies trigger cancer cell death by either activating the antibody-dependent cell-mediated cytotoxicity (ADCC), the antibody-dependent cellular phagocytosis (ADCP), the complement-dependent cell lysis (CDCL) processes [129], the cytotoxic T-lymphocyte response (CTL) or the Helper T lymphocyte response (HTL) [130]. In ADCC, an effector cell (usually a natural killer cell) lyses the target cell whose membrane-surface antigens are bound by specific antibodies. In ADCP, macrophages destroy the targeted cells by phagocytosis. In CDCL, an antibody-coated target cell recruits and activates components of the complement cascade to form a Membrane Attack Complex (MAC) that prompt to cell lysis. In CTL and HTL T-lymphocytes induce or promote the apoptotic death of the target cells.
Anti-CD20: The most frequently used monoclonal antibodies for CLL treatment are targeting CD20, a marker of normal pre-B and mature B lymphocytes. Rituximab was the first monoclonal antibody approved for the treatment of CLLs [129], introduced in 1998 for frontline therapy [131]. After binding to CD20, rituximab triggers a host cytotoxic immune response against CD20-positive cells. Rituximab is often used in combination with chemotherapy as part of the fludarabine-cyclophosphamide-rituximab (FCR) regimen [132]. Obinutuzumab induce ADCC and caspase-independent apoptosis and a clinical trial for combination treatment with venetoclax showed a significant improvement when compared to the previous combination with chlorambucil [133]. Ofatumumab targets a unique epitope of CD20 [134] and induces a strong response against low-CD20 expressing CLL when compared to rituximab [135] or obinutuzumab [136] triggering CDCL and ADCC [137]. Ofatumumab in combination with chlorambucil is indicated for first-line treatment of CLL patients for whom fludarabine-based therapy is inappropriate [138,139]. Ublituximab is showing promising results in comparison to rituximab [140], and is under evaluation for combination immunotherapy with the anti-PI3K umbralisib to treat relapsed/refractory CLL and Richter’s syndrome (NCT02535286). Lastly, mosunetuzumab is an anti-CD20/anti-CD3 bispecific antibody with two antigen-recognition sites, one for CD20, expressed on the surface of B-cells, and one for the CD3 expressed on the surface of T-cells. Upon administration, mosunetuzumab binds to both T-cells (cytotoxic and helper T-cells) and CD20-expressing tumor B-cells resulting in CTL and HTL response against CD20-expressing tumor B-cells. This drug has entered a clinical trial for evaluation of safety and pharmacokinetic as a single agent and combined with atezolizumab (an anti-PD-L1 described in Section 4.2.3) for treatment of Non-Hodgkin’s Lymphoma (NHL) and CLL (NCT02500407).
Anti-CD19: CD19 is expressed during B-cell development [141]. Inebilizumab [115], MOR208 (formerly known as XmAb5574) [142,143], blinatumomab [144] and MDX-1342 [145] are the main anti-CD19 monoclonal antibodies currently under evaluation for CLL treatment. Inebilizumab [146] induces both CTL response and ADCC [147]. A clinical trial evaluated the combination of inebilizumab with bendamustine or rituximab for the treatment of relapsed/refractory CLL (NCT01466153), showing similar efficacy and acceptable safety profile (PMC3990958). MOR208 induces ADCC and ADCP and was well tolerated in relapsed/refractory CLL (NCT01161511) [142]. MOR208 is also under evaluation in several clinical trials for the treatment of B cell lymphomas, and a combination treatment with lenalidomide (an immunomodulatory agent described in Section 4.2.4) is being assessed in patients with relapsed/refractory CLL, SLL or prolymphocytic leukemia (PLL) (NCT02005289). The most interesting, blinatumomab is an anti-CD19/anti-CD3 bispecific monoclonal antibody recognizing the CD19, expressed on the B-cells, and the CD3 expressed on the T-cells (cytotoxic and helper T-cells). This antibody brings CD19-expressing tumor B-cells and CD-3 expressing T-lymphocytes together, resulting in CTL- and HTL-mediated cell death of CD19-expressing B-lymphocytes [144]. In vitro experiments showed that blinatumomab induces faster CLL death that ibrutinib [148], thus this drug is being evaluated for use in non-Hodgkin lymphomas and CLL (NCT03823365).
Anti-CD52, Anti-CD37, Anti-CD38, and Anti-CD40: CD52 is expressed by B- and T- lymphocytes, granulocytes, monocytes, macrophages, Natural Killer and dendritic cells [149]. Alemtuzumab induces lysis of all CD52+ cells, thus targeting many components of the immune system and inducing severe side effects. For this reason, alemtuzumab was withdrawal from the market [11]. CD37 is expressed on B-cells and, to a lesser extent, on T-cells and myeloid cells. In vitro experiments on CLL cells showed that CD37 targeting has a potential immunomodulatory role in CLL [150]. Otlertuzumab induces apoptosis of malignant B-cells and ADCC without damaging T-cells and, in combination with bendamustine, increases the response rate and prolongs the progression-free survival of relapsed/refractory CLL patients (NCT01188681) [151]. CD38 is expressed on B lymphocytes and other hematopoietic cells [152], and its stimulation enhanced BCR-signaling inducing cellular proliferation [153,154]. Recently, daratumumab, an anti-CD38 approved for multiple myeloma, was evaluated for CLL treatment. Preliminary data showed that, in combination with ibrutinib, daratumumab induces direct apoptosis and enhances ibrutinib activity [155] thus, a daratumumab-ibrutinib clinical trial was very recently started (NCT03447808) to evaluate this treatment regimen in patients with symptomatic CLL. CD40 is a member of the tumor necrosis factor (TNF) receptor superfamily expressed by 90–100% of CLL cells [156]. Lucatumumab, an anti-CD40 antibody, mediates ADCC and is well tolerated. However, minimal single-agent activity was reported [157] (NCT00108108).
Anti-BAFF-R: BAFF is a member of the TNF superfamily that supports normal B-cell development and proliferation signaling [158] and the BAFF-receptor (BAFF-R) is often overexpressed in CLL [159]. The CLL microenvironment supports the survival of CLL B-cells by providing pro-proliferation factors and ligands, including BAFF [160]. BAFF/BAFF-R pathway is often over-activated in CLL regardless of treatment [161]. Interestingly, BAFF-driven activation of CLL prompts overexpression of miR-155 which enhances the responsiveness of CLL cells to BCR signaling and promotes the proliferation of cancer cells [61]. Thus, ianalumab a humanized antibody targeting BAFF-R, was developed. Ianalumab induces ADCC-mediated depletion of B–cells and blocks the BAFF signaling that drives B-cell differentiation, proliferation and survival [161]. Additionally, this drug induces NK response against the targeted cells, and in vitro experiments showed superior ADCC induction when compared with CD20- and CD52-directed antibodies [161]. Ianalumab tolerability was assessed in relapsed/refractory CLL patients (NCT02137889), and is currently under evaluation for combinatory therapy with ibrutinib (NCT03400176).
Anti-ROR1: ROR1 is a receptor of Wnt5a, widely expressed during embryogenesis, but not in normal adult tissues [162]. Recently, Dr. Kipps groups showed that ROR1 is highly expressed in most cancer cells, including CLL [163]. In CLL cells, ROR1 acts as a Wnt5a receptor to activate the non-canonical Wnt5 pathway signal that leads to proliferation [164]. Higher expression of ROR1 in CLL is associated with aggressive disease and poor prognosis [163] and recent studies have shown that, along with BCL2, ROR1 is a target of miR-15a/miR-16-1, often deleted in CLL [165]. These observations provided the rationale for developing a monoclonal antibody targeting ROR1. Cirmtuzumab is a recently developed humanized monoclonal antibody that targets the extracellular domain of ROR1, blocking the ROR1-mediated signaling and preventing tumor cell proliferation. In a phase 1 clinical trial on relapsed/refractory CLL (NCT02222688), cirmtuzumab was able to inhibit CLL stemness gene expression signatures. The treatment was well tolerated and effective at inhibiting ROR1 signaling. Moreover, a significant reduction of the lymphocyte count was observed, and some patients showed a reduction of CLL cell infiltration. However, no patient showed complete response, indicating that cirmtuzumab may be more effective when used in combination with other drugs [166]. Thus, a clinical trial to evaluate the safety of cirmtuzumab in combination with ibrutinib was started and proved to be well-tolerated and effective, with few cases of complete response (NCT03088878). Since in vitro experiments showed that cirmtuzumab enhances the venetoclax ADCC activity, a clinical trial to evaluate a combination of cirmtuzumab with venetoclax to treat CLL patients is warranted [32]. Both venetoclax and cirmtuzumab were designed to target the genes that are overexpressed in CLL as a consequence of miR-15a/miR-16-1 loss, thus, the efficiency of this combination therapy provides an additional remarkable indication that microRNAs studies are essential for the development of more effective therapeutic approaches.
4.2.2. CAR-T Cells
Chimeric antigen receptor T therapy (CAR-T) is a type of treatment in which T-cells are withdrawn from the patient (autologous) or from a healthy donor (allogeneic), genetically engineered to express an artificial T-cell receptor designed to bind to a specific antigen on cancer cells, and then infused in the patient. Once re-injected, the CAR-T cells multiply in the bloodstream, generating a population of T lymphocytes that can recognize target cancer cells without Major Histocompatibility Complex (MHC) restriction, and destroy them through cytotoxic effector mechanisms [167]. Indeed, the chimeric receptors combine both antigen-binding and T-cell activating functions into a single receptor. Thus, CAR-T cells destroy the target cells by increasing both cytotoxicity and secretion of cytokines, interleukins and growth factors.
The first approved CAR-T cell approach was based on CD19 antigen targeting. Since CD19 is a marker of B-cell malignancies, CAR-T treatment against CD19 (CTL109 or CART19) has been tested in CLL, acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL) [168,169]. To determine the optimal dose and safety of autologous CART19, a dose optimization (NCT01747486) and a safety-efficacy-cellular kinetics studies were carried on (NCT01029366) showing complete and durable response in about 50% of patients [169,170,171]. Thus, clinical trials were initiated to evaluate CAR-T cells strategy in combination with targeted therapy for the treatment of relapsed/refractory CLLs, such as a CART19-ibrutinib combination, currently under evaluation (NCT02640209). In addition, a clinical trial is currently evaluating the use of ROR1-specific (CAR) T-cells, to treat patients with advanced ROR1 positive malignancies. In this study, T-cells are engineered to specifically recognize and kill ROR1-expressing cancer cells and infused to the patient after conventional therapy (NCT02706392). Lastly, new approaches using engineered Natural Killer cell (CAR-NK) are under investigation for the treatment of CLL (NCT03056339) [172].
4.2.3. Immune Checkpoint Inhibitors: Anti-PD1/PD-L1, Anti-CTLA-4, and Anti-CD47-SIRPα Monoclonal Antibodies
Anti-PD1/PD-L1: An escaping mechanism adopted by cancer cells to suppress host T-cell responses is mediated by the surface protein programmed death-ligand 1 (PD-L1/CD274). In normal conditions PD-L1, expressed on dendritic cells or macrophages, interacts with its receptor PD-1, expressed on activated T-cells, to halt T-cell response and minimize the possibility of chronic autoimmune inflammation [173]. However, in cancer, the PD-1/PD-L1 pathway represents a resistance mechanism for malignant cells to avoid endogenous immune anti-tumor activity. PD-L1 is over-expressed on tumor cells (or on cells of the TME) and by binding to PD-1 receptors on activated T-cells, inhibit their cytotoxic activity [174]. Monoclonal antibodies have been developed to target PD-L1 such as atezolizumab and durvalumab, and PD-1 such as nivolumab and pembrolizumab [175]. Since the efficiency of the immune checkpoint blockade with monoclonal antibodies in solid cancer treatment is remarkable, clinical trials were started to evaluate their use for the treatment of CLL. Atezolizumab is under evaluation for combination with obinutuzumab and venetoclax in relapsed/refractory CLL, SLL, or patients with Richter’s syndrome (NCT02846623), while durvalumab is being tested in combinations with lenalidomide, rituximab, ibrutinib and bendamustine (NCT02733042). Pembrolizumab is under evaluation for the treatment of relapsed/refractory CLL and Richter’s syndrome after ibrutinib treatment. Pembrolizumab induced response in 44% of the patients with Richter’s syndrome with a satisfactory safety profile. However, single-agent pembrolizumab does not have significant therapeutic activity in relapsed/refractory CLL [176]. Therefore, combination with other drugs such as idelalisib or ibrutinib may be more efficient (NCT02332980), and a preliminary study for umbralisib, ublituximab and pembrolizumab combination is showing promising results (NCT02535286). Nivolumab showed an encouraging synergy with ibrutinib in an in-vitro experiment [177]. In addition, the results from a phase 1/2 study to evaluate safety, pharmacokinetics, pharmacodynamics and efficacy of the combination of ibrutinib with nivolumab in hematologic malignancies, indicated that this combinatory treatment was particularly beneficial for patients with Richter’s transformation [178] (NCT02329847). An additional active clinical trial (NCT02420912) is evaluating this combination therapy in patients with relapsed/refractory, high-risk untreated CLL, SLL, and Richter’s syndrome. Notwithstanding the promising results, none of these PD-1/PDL-1 inhibitor drugs have been yet approved for CLL treatment.
Anti-CTLA-4: The cytotoxic T lymphocyte-associated antigen 4 (CTLA-4 or CD152) is expressed on normal circulating T-cells, where it acts as a negative regulator to downregulate immune responses [179]. CTLA-4 deficiencies are associated with autoimmune diseases and CTLA-4 agonists are currently used a potential therapy for autoimmune diseases to reduce immune activity [180]. Conversely, blocking CTLA-4 to enhance the immune response toward cancer cells may provide therapeutic benefits for patients [181]. Interestingly, CTLA-4 is expressed in CLL where its activation is associated to increase of survival and proliferation [182]. Ipilimumab was tested in clinical trials accruing patients with several malignancies, including CLL, and showed durable clinical responses in a relatively low proportion of patients (NCT00060372) [183]. In a recently completed clinical trial, a combination of ipilimumab with the toll-like receptor 9 agonist SD-101 and radiation therapy to treat patients with recurrent low-grade B-cell lymphoma showed a partial response in only one patient out of seven, but later had progressive disease (NCT02254772). Thus, further studies are warranted to evaluate this strategy for the treatment of B cell malignancies.
Anti-CD47: CD47 is a cell surface protein with key roles in several cell processes, including apoptosis and phagocytosis. Physiologically, CD47 functions as a marker of “self” by binding to SIRPα on the surface of circulating macrophages to deliver an inhibitory “don’t eat me” signal [184]. CD47 was found overexpressed in leukemia cells as a mechanism of immune evasion [185]. Thus, blocking CD47 may render the cancer cells vulnerable to phagocytosis. Interestingly, the use of specific monoclonal antibodies to bind the CD47 on the surface of CLL cells was shown to also induce caspase-independent cell death [186]. Thus, blocking CD47 may induce both innate and adaptive immune systems to attack the cancer cell. Clinical trials are ongoing to verify the safety of humanized anti-CD47 antibodies [187,188] and among these, Hu5F9-G4, is currently under investigation for the treatment of relapsed/refractory B-cell Non-Hodgkin’s Lymphoma in combination therapy with rituximab (NCT02953509).
4.2.4. Immunomodulatory Drugs (IMiDs)
Immunomodulatory drugs are molecules that boost the immune system response against cancer. The main IMiDs currently under investigation for CLL treatment are Lenalidomide and BNC105P. Lenalidomide induces a reduction of PD-L1 expression on the surface of CLL cells, activates NK-cell response, and restores the cancer-targeting functions of T cell in CLL patients [189,190,191]. In a clinical trial for patients with symptomatic CLL (NCT00602459), lenalidomide was combined with fludarabine and rituximab. Results showed that a lenalidomide consolidation after chemoimmunotherapy is tolerated and extends progression-free survival and overall survival [192]. Thus, clinical trials are ongoing to test the safety of lenalidomide as maintenance therapy for CLL patients after chemotherapy (NCT00774345), and its use as first-line treatment in combinations with bendamustine and rituximab (NCT01400685). Unfortunately, another trial showed elevated toxicity and was stopped (NCT00738829) [193]. A derived of lenalidomide, CC-122, that promotes the degradation of the transcription factors Aiolos and Ikaros, showed effective anti-proliferative, anti-angiogenic and immunomodulatory activities in B-cell lymphoma [194]. Thus a clinical trial to test its activity in CLL when combined with ibrutinib and obinutuzumab was started (NCT02406742). Lastly, BNC105P causes occlusion of tumor vasculature resulting in hypoxia-driven tumor cell necrosis, and thus is defined as “Vascular Disrupting Agent”. BNC105P is converted to BNC105 in activated endothelial cells where it inhibits tubulin polymerization, resulting in a blockage of mitotic spindle formation, cell cycle arrest, and disruption of the tumor vasculature [195]. Interestingly, ibrutinib inhibits the pro-survival BCR signaling of CLL cells in the stromal niche, resulting in their release to the bloodstream. Since ibrutinib-induced lymphocytosis can linger for more than a year, it is possible that CLL cells do not die once they exit the lymph nodes. Furthermore, if the administration of ibrutinib is stopped, the CLL cells return to the lymph nodes. For these reasons ibrutinib efficiency may be increased if combined with a vascular disrupting agent such as BNC105P that would eradicate the CLL cells from the bloodstream, thus prevent them from returning to the lymph node niche. A clinical trial for the use of BNC105P in combination with ibrutinib for the treatment of CLL is currently recruiting (NCT03454165) patients with relapsed/refractory CLL.
5. Role of Non-Coding RNAs in Targeted and Immunotherapeutic Strategies for CLL
Target therapy and immunotherapy are showing promising results for the treatment of CLL and other malignancies. However, relapse is often observed as a consequence of the selection of a drug-resistant clone. In addition, cancer cells can develop drug-escaping mechanisms when exposed to single-agent treatment strategies and become resistant, leading to relapse. This observation suggests that early initiation of combinatory treatment may be more efficient in eradicating the disease and that the development of sensitive and accurate detection systems for swift identification of an emerging resistant clone is essential [181]. Several studies showed that signatures of ncRNAs can be used to monitor the progression of CLL and cancer in general. Specifically, microRNAs showed significant practical applications for drug development [15]. In this section, we will discuss their role in the development of novel drugs.
The best example of the key role of non-coding RNAs in the development of new drugs for cancer treatment is provided by the venetoclax, a powerful, specific and well tolerated drug that compensates for the lack of miR-15a/miR-16-1 targeting of BCL2 in CLL. Shortly after, cirmtuzumab was developed to compensate for the lack of miR-15a/miR-16-1 targeting of ROR1 in CLL. The mechanism of action of these drugs substantiates the essential role of miRNAs in cancer and endorses the study of the molecular profiling of cancer for the identification of more specific targets and the development of less toxic drugs. Interestingly, these drugs could be used in combination to target different pathways in CLL that are dysregulated as a consequence of the same driver event, the loss of miR-15a/miR-16-1, as shown by the synergistic effect observed when treating CLL cells from patients [32]. Based on these evidences, leukemic cells with low miR-15a/miR-16-1 expression, and therefore overexpressing of BCL2 and ROR1, would be more sensitive to combinatory therapy with venetoclax and cirmtuzumab. Remarkably, antisense oligonucleotides against BCL2 such as SPC2996, have also been tested in a clinical trial to inhibit BCL2 in CLL (NCT00285103) [203], and ROR1-CART cells are being evaluated for treatment (NCT02706392), further supporting the key role of BCL2 and ROR1 dysregulation in this disease, driven by the loss of miR-15a/miR-16-1.
Another indication of the pivotal role of microRNAs in the development of novel drugs is provided by the development of the monoclonal antibody ianalumab, an anti-BAFF-L which could exert its anti-cancer activity by reducing the expression of miR-155. MiR-155 overexpression has oncogenic activity in CLL, and its dysregulation can be triggered by microenvironment signals such as BAFF binding to its receptor BAFF-L on the CLL cell surface [61]. Interestingly, recent observations from preclinical models suggest that the transfer of miR-155 via EVs may also contribute to CLL-mediated myeloid-derived suppressor cells (MDSC) induction. MDSC have a key role in the immune suppressive networks in the TME, and the modulation of the TME is under evaluation as a possible strategy for cancer treatment [75,76]. In addition, an inhibitor of miR-155, (MRG-106), was developed for the treatment of blood cancers (www.miRagen.com) and it is currently under investigation for safety and tolerability in CLL patients (NCT02580552). Indeed, the generation of synthetic molecules such as miRNA mimics and anti-miRNA molecules for cancer treatment is a very novel approach, and some of these have entered clinical trials, further supporting the outstanding potential of microRNAs for both diagnostic and treatment purposes. The use of ncRNAs as therapeutic agents, however, requires a very different delivery approach when compared to other types of drugs such as small molecules or antibodies. Indeed, small non-coding RNAs need to be delivered to the target cells by a carrier. Liposomal nanoparticles have been used for this purpose. For instance, a miR-34a mimic carried by liposomal nanoparticles (MRX34), was tested in a phase I clinical trial in 2013, in patients with primary liver cancer [210] (NCT01829971). Although promising, this study was halted in 2016 because of multiple adverse events. However, other in vitro experiments showed that miR-34a restoration can induce cell-cycle arrest and apoptosis [211]. In addition, miR-34a targets NOTCH1, E2F1 and B-MYB, which promote CLL cell proliferation [212], regulates the NF-κβ signaling in T-cells [213] and has a key role in the immune system response in cancer [214]. Thus therapeutic strategy involving the administration of miR-34a mimics in CLL may warrant further studies. Additionally, miR-34a downregulation could be a marker for Richter’s syndrome, indicating that its restoration may be beneficial in the treatment of this particularly aggressive disease. Lastly in silico analysis suggested that miR-34a targets PD-L1, indicating a role for this microRNA in the regulation of the TME and a possible application as an immunotherapeutic agent [215,216].
MiR-181b is also under investigation as a therapeutic agent. MiR-181b targets BCL2, MCL1 and TCL1 genes in CLL [35,54,55] and thus its potential as a therapeutic agent was tested in a CLL mouse model. In this study, an in vivo transfection reagent (in vivo-jetPEI®) was used to deliver the synthetic miR-181b mimic in Eµ-TCL1 transgenic CLL mouse model. A significant reduction in the leukemic progression and increase survival of the study cohort was recorded indicating that miR-181b could potentially be used to reduce the expansion of CLL B-cells in patients [217]. In addition, miR-29 [218] and miR-125a [219] inhibitors and miRNA mimics are also under investigation for the treatment of different diseases (www.miRagen.com). However, the regulation of miR-29 and miR-125 expression in CLL is extremely complex end thus no study has yet evaluated these microRNA as a therapeutic agent for CLL treatment.
Recent studies have suggested that a potential approach for the delivery of small non-coding RNAs in cancer may be the use of EVs [81]. Indeed, sncRNAs can be transferred between cells via EVs, and have different functions [220]. For instance, in vitro experiments showed that EVs from bone marrow stromal cells can rescue CLL B-cells from apoptosis, enhance their migration and induce drug resistance [221]. Thus, the EV-mediated transfer of ncRNAs between cells is currently under investigation [68]. Interestingly, EVs can be artificially designed to deliver their cargo in specific tissues [222] and can be loaded with ncRNAs and other molecules with therapeutic activity [81]. EVs are an intriguing component of TME signaling, and can amplify oncogenic pathways in cancer cells to promote tumor progression, spread, and therapy resistance. Indeed, EVs may be used not only to target cancer cells directly, but also to affect the TME to hinder nursing-like cells from supporting the growth and spread of the tumor, and to prevent the development of resistance [223,224]. Unfortunately, despite the emerging role of EVs as drug delivery systems, safety aspects need to be overcome before introducing this technology to clinical studies [225]. Nonetheless, the study of EVs and TME led to the identification of other potential targets for therapy. Indeed, while studying the ability of leukemia cells to communicate with endothelial cells, Umezu et al. discovered that miR-92a-3p is selectively transferred from donor cells to endothelial cells via EVs [72]. More recently, a study on colon cancer showed that EVs containing miR-92a-3p can facilitate the endothelial-mesenchymal transition and the angiogenesis process affecting the TME [226]. As mentioned in Section 2, the same molecule is involved in a mechanism of activation of the VEGF-based autocrine pathway in CLL B-cells which leads to disease progression and is associated with poor prognosis in CLL [50,51,227]. These observations indicated that an anti-VEFG approach may be therapeutically valid. Thus, in vitro experiment were carried out to evaluate the efficacy of VEGF inhibitors such as vatalanib and pazopanib in the treatment of CLL [52] and, shortly after, clinical trials for vatalanib (NCT00511043), and for pazopanib (NCT01361334), were started. Unfortunately, the response to pazopanib was limited, showing short term efficacy and low percentage of partial response. Thus, further trials for this drug were discouraged at least as monotherapy for AML [205]. Vatalanib also showed low response rates and concerns for toxicities with high doses [228]. For these reasons, the use of anti-angiogenetic drugs remains widely overlooked in CLL.
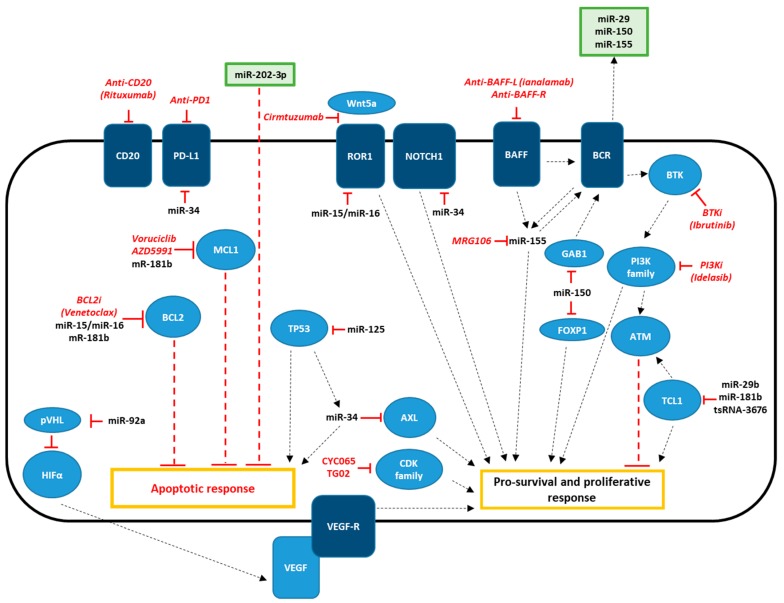
A simplified scheme of the interactions between microRNAs, their target, and the most common therapeutic agents used in CLL, is reported in Figure 1.
Figure 1.
Schematic diagram of effectors, ncRNAs, and drugs involved in CLL therapy. NcRNAs are indicated in black, therapeutic agents are indicated in red. Surface receptors are indicated in dark blue and other effectors are indicated in light blue. Extracellular vesicles are indicated in green.
6. Conclusions
Altered gene expression in cancer cells is often associated with small non-coding RNA dysregulation. This groundbreaking discovery provided the foundation for the outstanding progress made in the development of novel, more effective drugs for cancer treatment. In CLL, the alteration of miRNA profiles led to the development of Bcl2 inhibitors and anti-ROR1 antibodies, and more targets are currently being studied not only for CLL but also for other malignancies. Altogether, these data indicate that the study of non-coding RNA’s dysregulation in cancer has a pivotal role in the development of novel diagnostic tools and treatment strategies to improve the quality of life of patients.
Author Contributions
Conceptualization, V.B.; writing—original draft preparation, V.B. and F.P.; writing—review and editing, V.B. and F.P.; visualization, F.P.; supervision, V.B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.DeSantis C.E., Lin C.C., Mariotto A.B., Siegel R.L., Stein K.D., Kramer J.L., Alteri R., Robbins A.S., Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Pekarsky Y., Zanesi N., Croce C.M. Molecular basis of CLL. Semin. Cancer Biol. 2010;20:370–376. doi: 10.1016/j.semcancer.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalil N., Cheson B.D. Chronic lymphocytic leukemia. Oncologist. 1999;4:352–369. doi: 10.1634/theoncologist.4-5-352. [DOI] [PubMed] [Google Scholar]
- 4.Rai K.R., Jain P. Advances in the Clinical Staging of Chronic Lymphocytic Leukemia. Clin. Chem. 2011;57:1771–1772. doi: 10.1373/clinchem.2010.159004. [DOI] [PubMed] [Google Scholar]
- 5.Binet J.L., Auquier A., Dighiero G., Chastang C., Piguet H., Goasguen J., Vaugier G., Potron G., Colona P., Oberling F., et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::AID-CNCR2820480131>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Hallek M., Cheson B.D., Catovsky D., Caligaris-Cappio F., Dighiero G., Döhner H., Hillmen P., Keating M.J., Montserrat E., Rai K.R., et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute–Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaseb K., Purrahman D., Shahrabi S., Ghanavat M., Rezaeean H., Saki N. Prognostic significance of aberrant CD5 expression in B-cell leukemia. Oncol. Rev. 2019;13:400. doi: 10.4081/oncol.2019.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rassenti L.Z., Jain S., Keating M.J., Wierda W.G., Grever M.R., Byrd J.C., Kay N.E., Brown J.R., Gribben J.G., Neuberg D.S., et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–1930. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelmann J., Holzmann K., Miller F., Winkler D., Bühler A., Zenz T., Bullinger L., Kühn M.W.M., Gerhardinger A., Bloehdorn J., et al. High-resolution genomic profiling of chronic lymphocytic leukemia reveals new recurrent genomic alterations. Blood. 2012;120:4783–4794. doi: 10.1182/blood-2012-04-423517. [DOI] [PubMed] [Google Scholar]
- 10.Döhner H., Stilgenbauer S., Benner A., Leupolt E., Kröber A., Bullinger L., Döhner K., Bentz M., Lichter P. Genomic aberrations and survival in chronic lymphocytic leukemia. N. Engl. J. Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 11.Hallek M. Chronic lymphocytic leukemia: 2015 Update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2015;90:446–460. doi: 10.1002/ajh.23979. [DOI] [PubMed] [Google Scholar]
- 12.Calin G.A., Croce C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 13.Calin G.A., Dumitru C.D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzifi F., Economopoulou C., Gourgiotis D., Ardavanis A., Papageorgiou S., Scorilas A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012;2012:524308. doi: 10.1155/2012/524308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pekarsky Y., Croce C.M. Role of miR-15/16 in CLL. Cell Death Differ. 2015;22:6–11. doi: 10.1038/cdd.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borcherding N., Kusner D., Liu G.-H., Zhang W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell. 2014;5:496–502. doi: 10.1007/s13238-014-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savitsky K., Sfez S., Tagle D.A., Ziv Y., Sartiel A., Collins F.S., Shiloh Y., Rotman G. The complete sequence of the coding region of the ATM gene reveals similarity to cell cycle regulators in different species. Hum. Mol. Genet. 1995;4:2025–2032. doi: 10.1093/hmg/4.11.2025. [DOI] [PubMed] [Google Scholar]
- 18.Zenz T., Eichhorst B., Busch R., Denzel T., Häbe S., Winkler D., Bühler A., Edelmann J., Bergmann M., Hopfinger G., et al. TP53 mutation and survival in chronic lymphocytic leukemia. J. Clin. Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 19.Rosati E., Baldoni S., De Falco F., Del Papa B., Dorillo E., Rompietti C., Albi E., Falzetti F., Di Ianni M., Sportoletti P. NOTCH1 Aberrations in Chronic Lymphocytic Leukemia. Front. Oncol. 2018;8:229. doi: 10.3389/fonc.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffner C., Stilgenbauer S., Rappold G.A., Döhner H., Lichter P. Somatic ATM mutations indicate a pathogenic role of ATM in B-cell chronic lymphocytic leukemia. Blood. 1999;94:748–753. doi: 10.1182/blood.V94.2.748. [DOI] [PubMed] [Google Scholar]
- 21.Calin G.A., Cimmino A., Fabbri M., Ferracin M., Wojcik S.E., Shimizu M., Taccioli C., Zanesi N., Garzon R., Aqeilan R.I., et al. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc. Natl. Acad. Sci. USA. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lander E.S., Linton L.M., Birren B., Nusbaum C., Zody M.C., Baldwin J., Devon K., Dewar K., Doyle M., FitzHugh W., et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 23.Birney E., Stamatoyannopoulos J.A., Dutta A., Guigó R., Gingeras T.R., Margulies E.H., Weng Z., Snyder M., Dermitzakis E.T., et al. ENCODE Project Consortium Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799–816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteller M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 25.Ponting C.P., Oliver P.L., Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136:629–641. doi: 10.1016/j.cell.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Bastide A., David A. Interaction of rRNA with mRNA and tRNA in Translating Mammalian Ribosome: Functional Implications in Health and Disease. Biomolecules. 2018;8:100. doi: 10.3390/biom8040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balatti V., Pekarsky Y., Croce C.M. Role of the tRNA-Derived Small RNAs in Cancer: New Potential Biomarkers and Target for Therapy. Adv. Cancer Res. 2017;135:173–187. doi: 10.1016/bs.acr.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Slack F.J., Chinnaiyan A.M. The Role of Non-coding RNAs in Oncology. Cell. 2019;179:1033–1055. doi: 10.1016/j.cell.2019.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 30.Calin G.A., Ferracin M., Cimmino A., Di Leva G., Shimizu M., Wojcik S.E., Iorio M.V., Visone R., Sever N.I., Fabbri M., et al. A MicroRNA Signature Associated with Prognosis and Progression in Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2005;353:1793–1801. doi: 10.1056/NEJMoa050995. [DOI] [PubMed] [Google Scholar]
- 31.Cimmino A., Calin G.A., Fabbri M., Iorio M.V., Ferracin M., Shimizu M., Wojcik S.E., Aqeilan R.I., Zupo S., Dono M., et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rassenti L.Z., Balatti V., Ghia E.M., Palamarchuk A., Tomasello L., Fadda P., Pekarsky Y., Widhopf G.F., Kipps T.J., Croce C.M. MicroRNA dysregulation to identify therapeutic target combinations for chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2017;114:10731–10736. doi: 10.1073/pnas.1708264114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aqeilan R.I., Calin G.A., Croce C.M. miR-15a and miR-16-1 in cancer: Discovery, function and future perspectives. Cell Death Differ. 2010;17:215–220. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- 34.Liu T., Xu Z., Ou D., Liu J., Zhang J. The miR-15a/16 gene cluster in human cancer: A systematic review. J. Cell Physiol. 2019;234:5496–5506. doi: 10.1002/jcp.27342. [DOI] [PubMed] [Google Scholar]
- 35.Pekarsky Y., Santanam U., Cimmino A., Palamarchuk A., Efanov A., Maximov V., Volinia S., Alder H.-G., Liu C., Rassenti L., et al. Tcl1 Expression in Chronic Lymphocytic Leukemia Is Regulated by miR-29 and miR-181. Cancer Res. 2006;66:11590–11593. doi: 10.1158/0008-5472.CAN-06-3613. [DOI] [PubMed] [Google Scholar]
- 36.Pekarsky Y., Croce C.M. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget. 2010;1:224–227. doi: 10.18632/oncotarget.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mott J.L., Kobayashi S., Bronk S.F., Gores G.J. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Auer R.L., Riaz S., Cotter F.E. The 13q and 11q B-cell chronic lymphocytic leukaemia-associated regions derive from a common ancestral region in the zebrafish. Br. J. Haematol. 2007;137:443–453. doi: 10.1111/j.1365-2141.2007.06600.x. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann S., Ogawa S., Raynaud S.D., Sanada M., Nannya Y., Ticchioni M., Bastard C., Kawamata N., Phillip Koeffler H. Molecular allelokaryotyping of early-stage, untreated chronic lymphocytic leukemia. Cancer. 2008;112:1296–1305. doi: 10.1002/cncr.23270. [DOI] [PubMed] [Google Scholar]
- 40.Deneberg S., Kanduri M., Ali D., Bengtzen S., Karimi M., Qu Y., Kimby E., Mansouri L., Rosenquist R., Lennartsson A., et al. microRNA-34b/con chromosome 11q23 is aberrantly methylated in chronic lymphocytic leukemia. Epigenetics. 2014;9:910–917. doi: 10.4161/epi.28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X.J., Ren Z.J., Tang J.H. MicroRNA-34a: A potential therapeutic target in human cancer. Cell Death Dis. 2014;5:e1327. doi: 10.1038/cddis.2014.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferracin M., Zagatti B., Rizzotto L., Cavazzini F., Veronese A., Ciccone M., Saccenti E., Lupini L., Grilli A., De Angeli C., et al. MicroRNAs involvement in fludarabine refractory chronic lymphocytic leukemia. Mol. Cancer. 2010;9:123. doi: 10.1186/1476-4598-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fabbri M. Association of a MicroRNA/TP53 Feedback Circuitry With Pathogenesis and Outcome of B-Cell Chronic Lymphocytic Leukemia. JAMA. 2011;305:59. doi: 10.1001/jama.2010.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ. 2010;17:193–199. doi: 10.1038/cdd.2009.56. [DOI] [PubMed] [Google Scholar]
- 45.Boysen J., Sinha S., Price-Troska T., Warner S.L., Bearss D.J., Viswanatha D., Shanafelt T.D., Kay N.E., Ghosh A.K. The tumor suppressor axis p53/miR-34a regulates Axl expression in B-cell chronic lymphocytic leukemia: Implications for therapy in p53-defective CLL patients. Leukemia. 2014;28:451–455. doi: 10.1038/leu.2013.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu X., Liu X., Koul S., Lee C.Y., Zhang Z., Halmos B. AXL kinase as a novel target for cancer therapy. Oncotarget. 2014;5:9546–9563. doi: 10.18632/oncotarget.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balatti V., Tomasello L., Rassenti L.Z., Veneziano D., Nigita G., Wang H.-Y., Thorson J.A., Kipps T.J., Pekarsky Y., Croce C.M. miR-125a and miR-34a expression predicts Richter syndrome in chronic lymphocytic leukemia patients. Blood. 2018;132:2179–2182. doi: 10.1182/blood-2018-04-845115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calin G.A., Sevignani C., Dumitru C.D., Hyslop T., Noch E., Yendamuri S., Shimizu M., Rattan S., Bullrich F., Negrini M., et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. USA. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visone R., Rassenti L.Z., Veronese A., Taccioli C., Costinean S., Aguda B.D., Volinia S., Ferracin M., Palatini J., Balatti V., et al. Karyotype-specific microRNA signature in chronic lymphocytic leukemia. Blood. 2009;114:3872–3879. doi: 10.1182/blood-2009-06-229211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh A.K., Shanafelt T.D., Cimmino A., Taccioli C., Volinia S., Liu C.-G., Calin G.A., Croce C.M., Chan D.A., Giaccia A.J., et al. Aberrant regulation of pVHL levels by microRNA promotes the HIF/VEGF axis in CLL B cells. Blood. 2009;113:5568–5574. doi: 10.1182/blood-2008-10-185686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kay N.E., Bone N.D., Tschumper R.C., Howell K.H., Geyer S.M., Dewald G.W., Hanson C.A., Jelinek D.F. B-CLL cells are capable of synthesis and secretion of both pro- and anti-angiogenic molecules. Leukemia. 2002;16:911–919. doi: 10.1038/sj.leu.2402467. [DOI] [PubMed] [Google Scholar]
- 52.Paesler J., Gehrke I., Gandhirajan R.K., Filipovich A., Hertweck M., Erdfelder F., Uhrmacher S., Poll-Wolbeck S.J., Hallek M.-A., Kreuzer K. The Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors Vatalanib and Pazopanib Potently Induce Apoptosis in Chronic Lymphocytic Leukemia Cells In vitro and In vivo. Clin. Cancer Res. 2010;16:3390–3398. doi: 10.1158/1078-0432.CCR-10-0232. [DOI] [PubMed] [Google Scholar]
- 53.Visone R., Veronese A., Rassenti L.Z., Balatti V., Pearl D.K., Acunzo M., Volinia S., Taccioli C., Kipps T.J., Croce C.M. miR-181b is a biomarker of disease progression in chronic lymphocytic leukemia. Blood. 2011;118:3072–3079. doi: 10.1182/blood-2011-01-333484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ouyang Y.-B., Lu Y., Yue S., Giffard R.G. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion. 2012;12:213–219. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X., Yu J., Zhao C., Ren H., Yuan Z., Zhang B., Zhuang J., Wang J., Feng B. MiR-181b-5p modulates chemosensitivity of glioma cells to temozolomide by targeting Bcl-2. Biomed Pharm. 2019;109:2192–2202. doi: 10.1016/j.biopha.2018.11.074. [DOI] [PubMed] [Google Scholar]
- 56.Zhu D.-X., Zhu W., Fang C., Fan L., Zou Z.-J., Wang Y.-H., Liu P., Hong M., Miao K.-R., Liu P., et al. miR-181a/b significantly enhances drug sensitivity in chronic lymphocytic leukemia cells via targeting multiple anti-apoptosis genes. Carcinogenesis. 2012;33:1294–1301. doi: 10.1093/carcin/bgs179. [DOI] [PubMed] [Google Scholar]
- 57.Tili E., Michaille J.-J., Luo Z., Volinia S., Rassenti L.Z., Kipps T.J., Croce C.M. The down-regulation of miR-125b in chronic lymphocytic leukemias leads to metabolic adaptation of cells to a transformed state. Blood. 2012;120:2631–2638. doi: 10.1182/blood-2012-03-415737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y.-M., Lin K.-Y., Chen Y.-Q. Diverse functions of miR-125 family in different cell contexts. J. Hematol. Oncol. 2013;6:6. doi: 10.1186/1756-8722-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y., Gao J.-S., Tang X., Tucker L.D., Quesenberry P., Rigoutsos I., Ramratnam B. MicroRNA 125a and its regulation of the p53 tumor suppressor gene. FEBS Lett. 2009;583:3725–3730. doi: 10.1016/j.febslet.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrajoli A., Shanafelt T.D., Ivan C., Shimizu M., Rabe K.G., Nouraee N., Ikuo M., Ghosh A.K., Lerner S., Rassenti L.Z., et al. Prognostic value of miR-155 in individuals with monoclonal B-cell lymphocytosis and patients with B chronic lymphocytic leukemia. Blood. 2013;122:1891–1899. doi: 10.1182/blood-2013-01-478222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui B., Chen L., Zhang S., Mraz M., Fecteau J.-F., Yu J., Ghia E.M., Zhang L., Bao L., Rassenti L.Z., et al. MicroRNA-155 influences B-cell receptor signaling and associates with aggressive disease in chronic lymphocytic leukemia. Blood. 2014;124:546–554. doi: 10.1182/blood-2014-03-559690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pagotto S., Veronese A., Soranno A., Lanuti P., Di Marco M., Russo M.V., Ramassone A., Marchisio M., Simeone P., Guanciali Franchi P.E., et al. Hsa-miR-155-5p drives aneuploidy at early stages of cellular transformation. Oncotarget. 2018;9:13036–13047. doi: 10.18632/oncotarget.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mraz M., Chen L., Rassenti L.Z., Ghia E.M., Li H., Jepsen K., Smith E.N., Messer K., Frazer K.A., Kipps T.J. miR-150 influences B-cell receptor signaling in chronic lymphocytic leukemia by regulating expression of GAB1 and FOXP1. Blood. 2014;124:84–95. doi: 10.1182/blood-2013-09-527234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Balatti V., Rizzotto L., Miller C., Palamarchuk A., Fadda P., Pandolfo R., Rassenti L.Z., Hertlein E., Ruppert A.S., Lozanski A., et al. TCL1 targeting miR-3676 is codeleted with tumor protein p53 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2015;112:2169–2174. doi: 10.1073/pnas.1500010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pekarsky Y., Balatti V., Palamarchuk A., Rizzotto L., Veneziano D., Nigita G., Rassenti L.Z., Pass H.I., Kipps T.J., Liu C.-G., et al. Dysregulation of a family of short noncoding RNAs, tsRNAs, in human cancer. Proc. Natl. Acad. Sci. USA. 2016;113:5071–5076. doi: 10.1073/pnas.1604266113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balatti V., Nigita G., Veneziano D., Drusco A., Stein G.S., Messier T.L., Farina N.H., Lian J.B., Tomasello L., Liu C.-G., et al. tsRNA signatures in cancer. Proc. Natl. Acad. Sci. USA. 2017;114:8071–8076. doi: 10.1073/pnas.1706908114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veneziano D., Tomasello L., Balatti V., Palamarchuk A., Rassenti L.Z., Kipps T.J., Pekarsky Y., Croce C.M. Dysregulation of different classes of tRNA fragments in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA. 2019;116:24252–24258. doi: 10.1073/pnas.1913695116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 69.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burger J.A., Tsukada N., Burger M., Zvaifler N.J., Dell’Aquila M., Kipps T.J. Blood-derived nurse-like cells protect chronic lymphocytic leukemia B cells from spontaneous apoptosis through stromal cell–derived factor-1. Blood. 2000;96:2655–2663. doi: 10.1182/blood.V96.8.2655. [DOI] [PubMed] [Google Scholar]
- 71.Jia L., Clear A., Liu F.-T., Matthews J., Uddin N., McCarthy A., Hoxha E., Durance C., Iqbal S., Gribben J.G. Extracellular HMGB1 promotes differentiation of nurse-like cells in chronic lymphocytic leukemia. Blood. 2014;123:1709–1719. doi: 10.1182/blood-2013-10-529610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Umezu T., Ohyashiki K., Kuroda M., Ohyashiki J.H. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32:2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 73.Yeh Y.-Y., Ozer H.G., Lehman A.M., Maddocks K., Yu L., Johnson A.J., Byrd J.C. Characterization of CLL exosomes reveals a distinct microRNA signature and enhanced secretion by activation of BCR signaling. Blood. 2015;125:3297–3305. doi: 10.1182/blood-2014-12-618470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stamatopoulos B., Van Damme M., Crompot E., Dessars B., El Housni H., Mineur P., Meuleman N., Bron D., Lagneaux L. Opposite Prognostic Significance of Cellular and Serum Circulating MicroRNA-150 in Patients with Chronic Lymphocytic Leukemia. Mol. Med. 2015;21:123–133. doi: 10.2119/molmed.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruns H., Böttcher M., Qorraj M., Fabri M., Jitschin S., Dindorf J., Busch L., Jitschin R., Mackensen A., Mougiakakos D. CLL-cell-mediated MDSC induction by exosomal miR-155 transfer is disrupted by vitamin D. Leukemia. 2017;31:985–988. doi: 10.1038/leu.2016.378. [DOI] [PubMed] [Google Scholar]
- 77.Farahani M., Rubbi C., Liu L., Slupsky J.R., Kalakonda N. CLL Exosomes Modulate the Transcriptome and Behaviour of Recipient Stromal Cells and Are Selectively Enriched in miR-202-3p. PLoS ONE. 2015;10:e0141429. doi: 10.1371/journal.pone.0141429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoffman A.E., Liu R., Fu A., Zheng T., Slack F., Zhu Y. Targetome Profiling, Pathway Analysis and Genetic Association Study Implicate miR-202 in Lymphomagenesis. Cancer Epidemiol. Biomark. Prev. 2013;22:327–336. doi: 10.1158/1055-9965.EPI-12-1131-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paggetti J., Haderk F., Seiffert M., Janji B., Distler U., Ammerlaan W., Kim Y.J., Adam J., Lichter P., Solary E., et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126:1106–1117. doi: 10.1182/blood-2014-12-618025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Surman M., Drożdż A., Stępień E., Przybyło M. Extracellular Vesicles as Drug Delivery Systems - Methods of Production and Potential Therapeutic Applications. Curr. Pharm. Des. 2019;25:132–154. doi: 10.2174/1381612825666190306153318. [DOI] [PubMed] [Google Scholar]
- 81.Lamichhane T.N., Jay S.M. Production of Extracellular Vesicles Loaded with Therapeutic Cargo. Methods Mol. Biol. 2018;1831:37–47. doi: 10.1007/978-1-4939-8661-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flinn I.W., Neuberg D.S., Grever M.R., Dewald G.W., Bennett J.M., Paietta E.M., Hussein M.A., Appelbaum F.R., Larson R.A., Moore D.F., et al. Phase III Trial of Fludarabine Plus Cyclophosphamide Compared With Fludarabine for Patients With Previously Untreated Chronic Lymphocytic Leukemia: US Intergroup Trial E2997. J. Clin. Oncol. 2007;25:793–798. doi: 10.1200/JCO.2006.08.0762. [DOI] [PubMed] [Google Scholar]
- 83.Woyach J.A., Johnson A.J. Targeted therapies in CLL: Mechanisms of resistance and strategies for management. Blood. 2015;126:471–477. doi: 10.1182/blood-2015-03-585075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nowell P. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 85.Greaves M., Maley C.C. Clonal evolution in cancer. Nature. 2012;481:306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leeksma A.C., Taylor J., Wu B., Gardner J.R., He J., Nahas M., Gonen M., Alemayehu W.G., te Raa D., Walther T., et al. Clonal diversity predicts adverse outcome in chronic lymphocytic leukemia. Leukemia. 2019;33:390–402. doi: 10.1038/s41375-018-0215-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Singh S.P., Dammeijer F., Hendriks R.W. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol. Cancer. 2018;17 doi: 10.1186/s12943-018-0779-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis R.E., Eric Davis R., Ngo V.N., Lenz G., Tolar P., Young R.M., Romesser P.B., Kohlhammer H., Lamy L., Zhao H., et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Honigberg L.A., Smith A.M., Sirisawad M., Verner E., Loury D., Chang B., Li S., Pan Z., Thamm D.H., Miller R.A., et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA. 2010;107:13075–13080. doi: 10.1073/pnas.1004594107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Itchaki G., Brown J.R. Experience with ibrutinib for first-line use in patients with chronic lymphocytic leukemia. Ther. Adv. Hematol. 2018;9:3–19. doi: 10.1177/2040620717741861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Byrd J.C., Furman R.R., Coutre S.E., Flinn I.W., Burger J.A., Blum K.A., Grant B., Sharman J.P., Coleman M., Wierda W.G., et al. Targeting BTK with Ibrutinib in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herman S.E.M., Gordon A.L., Hertlein E., Ramanunni A., Zhang X., Jaglowski S., Flynn J., Jones J., Blum K.A., Buggy J.J., et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117:6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Owen C., Berinstein N.L., Christofides A., Sehn L.H. Review of Bruton tyrosine kinase inhibitors for the treatment of relapsed or refractory mantle cell lymphoma. Curr. Oncol. 2019;26:e233–e240. doi: 10.3747/co.26.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Byrd J.C., Harrington B., O’Brien S., Jones J.A., Schuh A., Devereux S., Chaves J., Wierda W.G., Awan F.T., Brown J.R., et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016;374:323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Awan F.T., Schuh A., Brown J.R., Furman R.R., Pagel J.M., Hillmen P., Stephens D.M., Woyach J., Bibikova E., Charuworn P., et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019;3:1553–1562. doi: 10.1182/bloodadvances.2018030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maddocks K.J., Ruppert A.S., Lozanski G., Heerema N.A., Zhao W., Abruzzo L., Lozanski A., Davis M., Gordon A., Smith L.L., et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jain P., Keating M., Wierda W., Estrov Z., Ferrajoli A., Jain N., George B., James D., Kantarjian H., Burger J., et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125:2062–2067. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walliser C., Hermkes E., Schade A., Wiese S., Deinzer J., Zapatka M., Désiré L., Mertens D., Stilgenbauer S., Gierschik P. The Phospholipase Cγ2Mutants R665W and L845F Identified in Ibrutinib-resistant Chronic Lymphocytic Leukemia Patients Are Hypersensitive to the Rho GTPase Rac2 Protein. J. Biol. Chem. 2016;291:22136–22148. doi: 10.1074/jbc.M116.746842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bond D.A., Woyach J.A. Targeting BTK in CLL: Beyond Ibrutinib. Curr. Hematol. Malig. Rep. 2019;14:197–205. doi: 10.1007/s11899-019-00512-0. [DOI] [PubMed] [Google Scholar]
- 100.Bunney T.D., Katan M. Phosphoinositide signalling in cancer: Beyond PI3K and PTEN. Nat. Rev. Cancer. 2010;10:342–352. doi: 10.1038/nrc2842. [DOI] [PubMed] [Google Scholar]
- 101.Jean S., Kiger A.A. Classes of phosphoinositide 3-kinases at a glance. J. Cell Sci. 2014;127:923–928. doi: 10.1242/jcs.093773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chang J.E., Kahl B.S. PI3-Kinase Inhibitors in Chronic Lymphocytic Leukemia. Curr. Hematol. Malig. Rep. 2014;9:33–43. doi: 10.1007/s11899-013-0189-7. [DOI] [PubMed] [Google Scholar]
- 103.Vanhaesebroeck B., Welham M.J., Kotani K., Stein R., Warne P.H., Zvelebil M.J., Higashi K., Volinia S., Downward J., Waterfield M.D. p110, a novel phosphoinositide 3-kinase in leukocytes. Proc. Natl. Acad. Sci. USA. 1997;94:4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ali A.Y., Wu X., Eissa N., Hou S., Ghia J.-E., Murooka T.T., Banerji V., Johnston J.B., Lin F., Gibson S.B., et al. Distinct roles for phosphoinositide 3-kinases γ and δ in malignant B cell migration. Leukemia. 2018;32:1958–1969. doi: 10.1038/s41375-018-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoellenriegel J., Meadows S.A., Sivina M., Wierda W.G., Kantarjian H., Keating M.J., Giese N., O’Brien S., Yu A., Miller L.L., et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zelenetz A.D., Barrientos J.C., Brown J.R., Coiffier B., Delgado J., Egyed M., Ghia P., Illés Á., Jurczak W., Marlton P., et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: Interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18:297–311. doi: 10.1016/S1470-2045(16)30671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rodrigues D.A., Sagrillo F.S., Fraga C.A.M. Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases. Pharmaceuticals. 2019;12:69. doi: 10.3390/ph12020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Patel K., Danilov A.V., Pagel J.M. Duvelisib for CLL/SLL and follicular non-Hodgkin lymphoma. Blood. 2019;134:1573–1577. doi: 10.1182/blood.2019001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mato A.R., Nabhan C., Barr P.M., Ujjani C.S., Hill B.T., Lamanna N., Skarbnik A.P., Howlett C., Pu J.J., Sehgal A.R., et al. Outcomes of CLL patients treated with sequential kinase inhibitor therapy: A real world experience. Blood. 2016;128:2199–2205. doi: 10.1182/blood-2016-05-716977. [DOI] [PubMed] [Google Scholar]
- 110.Reed J.C., Miyashita T., Krajewski S., Takayama S., Aime-Sempe C., Kitada S., Sato T., Wang H.-G., Harigai M., Hanada M., et al. Bcl-2 family proteins and the regulation of programmed cell death in leukemia and lymphoma. Cancer Treat. Res. 1996;84:31–72. doi: 10.1007/978-1-4613-1261-1_3. [DOI] [PubMed] [Google Scholar]
- 111.Chao D.T., Korsmeyer S.J. BCL-2 FAMILY: Regulators of Cell Death. Annu. Rev. Immunol. 1998;16:395–419. doi: 10.1146/annurev.immunol.16.1.395. [DOI] [PubMed] [Google Scholar]
- 112.Balatti V., Acunzo M., Pekarky Y., Croce C.M. Novel Mechanisms of Regulation of miRNAs in CLL. Trends Cancer. 2016;2:134–143. doi: 10.1016/j.trecan.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rampazzo E., Bojnik E., Trentin L., Bonaldi L., Del Bianco P., Frezzato F., Visentin A., Facco M., Semenzato G., De Rossi A. Role of miR-15a/miR-16-1 and the TP53 axis in regulating telomerase expression in chronic lymphocytic leukemia. Haematologica. 2017;102:e253–e256. doi: 10.3324/haematol.2016.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pekarsky Y., Balatti V., Croce C.M. BCL2 and miR-15/16: From gene discovery to treatment. Cell Death Differ. 2018;25:21–26. doi: 10.1038/cdd.2017.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oltersdorf T., Elmore S.W., Shoemaker A.R., Armstrong R.C., Augeri D.J., Belli B.A., Bruncko M., Deckwerth T.L., Dinges J., Hajduk P.J., et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 116.Tse C., Shoemaker A.R., Adickes J., Anderson M.G., Chen J., Jin S., Johnson E.F., Marsh K.C., Mitten M.J., Nimmer P., et al. ABT-263: A Potent and Orally Bioavailable Bcl-2 Family Inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 117.Roberts A.W., Seymour J.F., Brown J.R., Wierda W.G., Kipps T.J., Khaw S.L., Carney D.A., He S.Z., Huang D.C.S., Xiong H., et al. Substantial Susceptibility of Chronic Lymphocytic Leukemia to BCL2 Inhibition: Results of a Phase I Study of Navitoclax in Patients With Relapsed or Refractory Disease. J. Clin. Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Souers A.J., Leverson J.D., Boghaert E.R., Ackler S.L., Catron N.D., Chen J., Dayton B.D., Ding H., Enschede S.H., Fairbrother W.J., et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 119.Cheson B.D., Enschede S.H., Cerri E., Desai M., Potluri J., Lamanna N., Tam C. Tumor Lysis Syndrome in Chronic Lymphocytic Leukemia with Novel Targeted Agents. Oncologist. 2017;22:1283–1291. doi: 10.1634/theoncologist.2017-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roberts A.W., Davids M.S., Pagel J.M., Kahl B.S., Puvvada S.D., Gerecitano J.F., Kipps T.J., Anderson M.A., Brown J.R., Gressick L., et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Deeks E.D. Venetoclax: First Global Approval. Drugs. 2016;76:979–987. doi: 10.1007/s40265-016-0596-x. [DOI] [PubMed] [Google Scholar]
- 122.Malumbres M. Cyclin-dependent kinases. Genome Biol. 2014;15:122. doi: 10.1186/gb4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goh K.C., Novotny-Diermayr V., Hart S., Ong L.C., Loh Y.K., Cheong A., Tan Y.C., Hu C., Jayaraman R., William A.D., et al. TG02, a novel oral multi-kinase inhibitor of CDKs, JAK2 and FLT3 with potent anti-leukemic properties. Leukemia. 2012;26:236–243. doi: 10.1038/leu.2011.218. [DOI] [PubMed] [Google Scholar]
- 124.Craig R.W., Jabs E.W., Zhou P., Kozopas K.M., Hawkins A.L., Rochelle J.M., Seldin M.F., Griffins C.A. Human and Mouse Chromosomal Mapping of the Myeloid Cell Leukemia-1 Gene: MCL1 Maps to Human Chromosome 1q21, a Region That Is Frequently Altered in Preneoplastic and Neoplastic Disease. Genomics. 1994;23:457–463. doi: 10.1006/geno.1994.1523. [DOI] [PubMed] [Google Scholar]
- 125.Choudhary G.S., Al-Harbi S., Mazumder S., Hill B.T., Smith M.R., Bodo J., Hsi E.D., Almasan A. MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies. Cell Death Dis. 2015;6:e1593. doi: 10.1038/cddis.2014.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dey J., Deckwerth T.L., Kerwin W.S., Casalini J.R., Merrell A.J., Grenley M.O., Burns C., Ditzler S.H., Dixon C.P., Beirne E., et al. Voruciclib, a clinical stage oral CDK9 inhibitor, represses MCL-1 and sensitizes high-risk Diffuse Large B-cell Lymphoma to BCL2 inhibition. Sci. Rep. 2017;7:18007. doi: 10.1038/s41598-017-18368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tron A.E., Belmonte M.A., Adam A., Aquila B.M., Boise L.H., Chiarparin E., Cidado J., Embrey K.J., Gangl E., Gibbons F.D., et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 2018;9:5341. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sinha S., Boysen J.C., Chaffee K.G., Kabat B.F., Slager S.L., Parikh S.A., Secreto C.R., Call T., Shanafelt T.D., Leis J.F., et al. Chronic lymphocytic leukemia cells from ibrutinib treated patients are sensitive to Axl receptor tyrosine kinase inhibitor therapy. Oncotarget. 2018;9:37173–37184. doi: 10.18632/oncotarget.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jaglowski S.M., Alinari L., Lapalombella R., Muthusamy N., Byrd J.C. The clinical application of monoclonal antibodies in chronic lymphocytic leukemia. Blood. 2010;116:3705–3714. doi: 10.1182/blood-2010-04-001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maher J., Davies E.T. Targeting cytotoxic T lymphocytes for cancer immunotherapy. Br. J. Cancer. 2004;91:817–821. doi: 10.1038/sj.bjc.6602022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coiffier B., Haioun C., Ketterer N., Engert A., Tilly H., Ma D., Johnson P., Lister A., Feuring-Buske M., Radford J.A., et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 132.Hallek M., Fischer K., Fingerle-Rowson G., Fink A.M., Busch R., Mayer J., Hensel M., Hopfinger G., Hess G., von Grünhagen U., et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 133.Fischer K., Al-Sawaf O., Bahlo J., Fink A.-M., Tandon M., Dixon M., Robrecht S., Warburton S., Humphrey K., Samoylova O., et al. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2019;380:2225–2236. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 134.Castillo J., Perez K. The role of ofatumumab in the treatment of chronic lymphocytic leukemia resistant to previous therapies. J. Blood Med. 2010;1:1–8. doi: 10.2147/JBM.S7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Teeling J.L., Mackus W.J.M., Luus J.J., van den Brakel J.H.N., Beers S.A., French R.R., van Meerten T., Ebeling S., Vink T., Slootstra J.W., et al. The Biological Activity of Human CD20 Monoclonal Antibodies Is Linked to Unique Epitopes on CD20. J. Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 136.Bologna L., Gotti E., Manganini M., Rambaldi A., Intermesoli T., Introna M., Golay J. Mechanism of Action of Type II, Glycoengineered, Anti-CD20 Monoclonal Antibody GA101 in B-Chronic Lymphocytic Leukemia Whole Blood Assays in Comparison with Rituximab and Alemtuzumab. J. Immunol. 2011;186:3762–3769. doi: 10.4049/jimmunol.1000303. [DOI] [PubMed] [Google Scholar]
- 137.Coiffier B., Lepretre S., Pedersen L.M., Gadeberg O., Fredriksen H., van Oers M.H.J., Wooldridge J., Kloczko J., Holowiecki J., Hellmann A., et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: A phase 1-2 study. Blood. 2008;111:1094–1100. doi: 10.1182/blood-2007-09-111781. [DOI] [PubMed] [Google Scholar]
- 138.Hillmen P., Robak T., Janssens A., Govind Babu K., Kloczko J., Grosicki S., Doubek M., Panagiotidis P., Kimby E., Schuh A., et al. Chlorambucil plus ofatumumab versus chlorambucil alone in previously untreated patients with chronic lymphocytic leukaemia (COMPLEMENT 1): A randomised, multicentre, open-label phase 3 trial. Lancet. 2015;385:1873–1883. doi: 10.1016/S0140-6736(15)60027-7. [DOI] [PubMed] [Google Scholar]
- 139.Laurenti L., Innocenti I., Autore F., Sica S., Efremov D. New developments in the management of chronic lymphocytic leukemia: Role of ofatumumab. Onco Targets Ther. 2016;9:421–429. doi: 10.2147/OTT.S72845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sawas A., Farber C.M., Schreeder M.T., Khalil M.Y., Mahadevan D., Deng C., Amengual J.E., Nikolinakos P.G., Kolesar J.M., Kuhn J.G., et al. A phase 1/2 trial of ublituximab, a novel anti-CD20 monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma or chronic lymphocytic leukaemia previously exposed to rituximab. Br. J. Haematol. 2017;177:243–253. doi: 10.1111/bjh.14534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tedder T.F., Inaoki M., Sato S. The CD19–CD21 Complex Regulates Signal Transduction Thresholds Governing Humoral Immunity and Autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/S1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- 142.Woyach J.A., Awan F., Flinn I.W., Berdeja J.G., Wiley E., Mansoor S., Huang Y., Lozanski G., Foster P.A., Byrd J.C. A phase 1 trial of the Fc-engineered CD19 antibody XmAb5574 (MOR00208) demonstrates safety and preliminary efficacy in relapsed CLL. Blood. 2014;124:3553–3560. doi: 10.1182/blood-2014-08-593269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaplon H., Reichert J.M. Antibodies to watch in 2019. mAbs. 2019;11:219–238. doi: 10.1080/19420862.2018.1556465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wong R., Pepper C., Brennan P., Nagorsen D., Man S., Fegan C. Blinatumomab induces autologous T-cell killing of chronic lymphocytic leukemia cells. Haematologica. 2013;98:1930–1938. doi: 10.3324/haematol.2012.082248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cardarelli P.M., Rao-Naik C., Chen S., Huang H., Pham A., Moldovan-Loomis M.-C., Pan C., Preston B., Passmore D., Liu J., et al. A nonfucosylated human antibody to CD19 with potent B-cell depletive activity for therapy of B-cell malignancies. Cancer Immunol. Immunother. 2010;59:257–265. doi: 10.1007/s00262-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Herbst R., Wang Y., Gallagher S., Mittereder N., Kuta E., Damschroder M., Woods R., Rowe D.C., Cheng L., Cook K., et al. B-Cell Depletion In Vitro and In Vivo with an Afucosylated Anti-CD19 Antibody. J. Pharmacol. Exp. Ther. 2010;335:213–222. doi: 10.1124/jpet.110.168062. [DOI] [PubMed] [Google Scholar]
- 147.Awan F.T., Lapalombella R., Trotta R., Butchar J.P., Yu B., Benson D.M., Roda J.M., Cheney C., Mo X., Lehman A., et al. CD19 targeting of chronic lymphocytic leukemia with a novel Fc-domain–engineered monoclonal antibody. Blood. 2010;115:1204–1213. doi: 10.1182/blood-2009-06-229039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Robinson H.R., Qi J., Cook E.M., Nichols C., Dadashian E.L., Underbayev C., Herman S.E.M., Saba N.S., Keyvanfar K., Sun C., et al. A CD19/CD3 bispecific antibody for effective immunotherapy of chronic lymphocytic leukemia in the ibrutinib era. Blood. 2018;132:521–532. doi: 10.1182/blood-2018-02-830992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Golay J., Manganini M., Rambaldi A., Introna M. Effect of alemtuzumab on neoplastic B cells. Haematologica. 2004;89:1476–1483. [PubMed] [Google Scholar]
- 150.Zhao X., Lapalombella R., Joshi T., Cheney C., Gowda A., Hayden-Ledbetter M.S., Baum P.R., Lin T.S., Jarjoura D., Lehman A., et al. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110:2569–2577. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Robak T., Hellmann A., Kloczko J., Loscertales J., Lech-Maranda E., Pagel J.M., Mato A., Byrd J.C., Awan F.T., Hebart H., et al. Randomized phase 2 study of otlertuzumab and bendamustineversusbendamustine in patients with relapsed chronic lymphocytic leukaemia. Br. J. Haematol. 2017;176:618–628. doi: 10.1111/bjh.14464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Malavasi F., Deaglio S., Funaro A., Ferrero E., Horenstein A.L., Ortolan E., Vaisitti T., Aydin S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- 153.Funaro A., De Monte L.B., Dianzani U., Forni M., Malavasi F. Human CD38 is associated to distinct molecules which mediate transmembrane signaling in different lineages. Eur. J. Immunol. 1993;23:2407–2411. doi: 10.1002/eji.1830231005. [DOI] [PubMed] [Google Scholar]
- 154.Dürig J., Naschar M., Schmücker U., Renzing-Köhler K., Hölter T., Hüttmann A., Dührsen U. CD38 expression is an important prognostic marker in chronic lymphocytic leukaemia. Leukemia. 2002;16:30–35. doi: 10.1038/sj.leu.2402339. [DOI] [PubMed] [Google Scholar]
- 155.Manna A., Aulakh S., Jani P., Ahmed S., Akhtar S., Coignet M., Heckman M., Meghji Z., Bhatia K., Sharma A., et al. Targeting CD38 Enhances the Antileukemic Activity of Ibrutinib in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2019;25:3974–3985. doi: 10.1158/1078-0432.CCR-18-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hulkkonen J., Vilpo L., Hurme M., Vilpo J. Surface antigen expression in chronic lymphocytic leukemia: Clustering analysis, interrelationships and effects of chromosomal abnormalities. Leukemia. 2002;16:178–185. doi: 10.1038/sj.leu.2402363. [DOI] [PubMed] [Google Scholar]
- 157.Byrd J.C., Kipps T.J., Flinn I.W., Cooper M., Odenike O., Bendiske J., Rediske J., Bilic S., Dey J., Baeck J., et al. Phase I study of the anti-CD40 humanized monoclonal antibody lucatumumab (HCD122) in relapsed chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53:2136–2142. doi: 10.3109/10428194.2012.681655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Schneider P., MacKay F., Steiner V., Hofmann K., Bodmer J.-L., Holler N., Ambrose C., Lawton P., Bixler S., Acha-Orbea H., et al. BAFF, a Novel Ligand of the Tumor Necrosis Factor Family, Stimulates B Cell Growth. J. Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rodig S.J., Shahsafaei A., Li B., Mackay C.R., Dorfman D.M. BAFF-R, the major B cell–activating factor receptor, is expressed on most mature B cells and B-cell lymphoproliferative disorders. Hum. Pathol. 2005;36:1113–1119. doi: 10.1016/j.humpath.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 160.Burger J.A., Ghia P., Rosenwald A., Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: A target for new treatment strategies. Blood. 2009;114:3367–3375. doi: 10.1182/blood-2009-06-225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McWilliams E.M., Lucas C.R., Chen T., Harrington B.K., Wasmuth R., Campbell A., Rogers K.A., Cheney C.M., Mo X., Andritsos L.A., et al. Anti–BAFF-R antibody VAY-736 demonstrates promising preclinical activity in CLL and enhances effectiveness of ibrutinib. Blood Adv. 2019;3:447–460. doi: 10.1182/bloodadvances.2018025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Broome H.E., Elizabeth Broome H., Rassenti L.Z., Wang H.-Y., Meyer L.M., Kipps T.J. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leukemia Res. 2011;35:1390–1394. doi: 10.1016/j.leukres.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cui B., Ghia E.M., Chen L., Rassenti L.Z., DeBoever C., Widhopf G.F., Yu J., Neuberg D.S., Wierda W.G., Rai K.R., et al. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood. 2016;128:2931–2940. doi: 10.1182/blood-2016-04-712562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.DaneshManesh A.H., Mikaelsson E., Jeddi-Tehrani M., Bayat A.A., Ghods R., Ostadkarampour M., Akhondi M., Lagercrantz S., Larsson C., Österborg A., et al. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int. J. Cancer. 2008;123:1190–1195. doi: 10.1002/ijc.23587. [DOI] [PubMed] [Google Scholar]
- 165.Balatti V., Croce C.M. MicroRNA dysregulation and multi-targeted therapy for cancer treatment. Adv. Biol. Regul. 2019;2019:100669. doi: 10.1016/j.jbior.2019.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Choi M.Y., Widhopf G.F., Ghia E.M., Kidwell R.L., Hasan M.K., Yu J., Rassenti L.Z., Chen L., Chen Y., Pittman E., et al. Phase I Trial: Cirmtuzumab Inhibits ROR1 Signaling and Stemness Signatures in Patients with Chronic Lymphocytic Leukemia. Cell Stem Cell. 2018;22:951–959. doi: 10.1016/j.stem.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.June C.H., O’Connor R.S., Kawalekar O.U., Ghassemi S., Milone M.C. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–1365. doi: 10.1126/science.aar6711. [DOI] [PubMed] [Google Scholar]
- 168.Porter D.L., Levine B.L., Kalos M., Bagg A., June C.H. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Porter D.L., Hwang W.-T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V., et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015 doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van Bruggen J.A.C., Martens A.W.J., Fraietta J.A., Hofland T., Tonino S.H., Eldering E., Levin M.-D., Siska P.J., Endstra S., Rathmell J.C., et al. Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8 T cells and impede CAR T-cell efficacy. Blood. 2019;134:44–58. doi: 10.1182/blood.2018885863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F., et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kloess S., Kretschmer A., Stahl L., Fricke S., Koehl U. CAR-Expressing Natural Killer Cells for Cancer Retargeting. Transfus. Med. Hemother. 2019;46:4–13. doi: 10.1159/000495771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qin W., Hu L., Zhang X., Jiang S., Li J., Zhang Z., Wang X. The Diverse Function of PD-1/PD-L Pathway Beyond Cancer. Front. Immunol. 2019;10:2298. doi: 10.3389/fimmu.2019.02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Greaves P., Gribben J.G. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121:734–744. doi: 10.1182/blood-2012-10-385591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee H., Lee S., Heo Y.-S. Molecular Interactions of Antibody Drugs Targeting PD-1, PD-L1, and CTLA-4 in Immuno-Oncology. Molecules. 2019;24:1190. doi: 10.3390/molecules24061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ding W., LaPlant B.R., Call T.G., Parikh S.A., Leis J.F., He R., Shanafelt T.D., Sinha S., Le-Rademacher J., Feldman A.L., et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129:3419–3427. doi: 10.1182/blood-2017-02-765685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sagiv-Barfi I., Kohrt H.E.K., Czerwinski D.K., Ng P.P., Chang B.Y., Levy R. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc. Natl. Acad. Sci. USA. 2015;112:E966–E972. doi: 10.1073/pnas.1500712112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Younes A., Brody J., Carpio C., Lopez-Guillermo A., Ben-Yehuda D., Ferhanoglu B., Nagler A., Ozcan M., Avivi I., Bosch F., et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: A phase 1/2a study. Lancet Haematol. 2019;6:e67–e78. doi: 10.1016/S2352-3026(18)30217-5. [DOI] [PubMed] [Google Scholar]
- 179.Mittal A.K., Chaturvedi N.K., Rohlfsen R.A., Gupta P., Joshi A.D., Hegde G.V., Gregory Bociek R., Joshi S.S. Role of CTLA4 in the Proliferation and Survival of Chronic Lymphocytic Leukemia. PLoS ONE. 2013;8:e70352. doi: 10.1371/journal.pone.0070352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Schubert D., Bode C., Kenefeck R., Hou T.Z., Wing J.B., Kennedy A., Bulashevska A., Petersen B.-S., Schäffer A.A., Grüning B.A., et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014;20:1410–1416. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Syn N.L., Teng M.W.L., Mok T.S.K., Soo R.A. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 2017;18:e731–e741. doi: 10.1016/S1470-2045(17)30607-1. [DOI] [PubMed] [Google Scholar]
- 182.Ciszak L., Frydecka I., Wolowiec D., Szteblich A., Kosmaczewska A. Patients with chronic lymphocytic leukaemia (CLL) differ in the pattern of CTLA-4 expression on CLL cells: The possible implications for immunotherapy with CTLA-4 blocking antibody. Tumour. Biol. 2016;37:4143–4157. doi: 10.1007/s13277-015-4217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bashey A., Medina B., Corringham S., Pasek M., Carrier E., Vrooman L., Lowy I., Solomon S.R., Morris L.E., Kent Holland H., et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1581–1588. doi: 10.1182/blood-2008-07-168468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Russ A., Hua A.B., Montfort W.R., Rahman B., Riaz I.B., Khalid M.U., Carew J.S., Nawrocki S.T., Persky D., Anwer F. Blocking “don’t eat me” signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018;32:480–489. doi: 10.1016/j.blre.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jaiswal S., Jamieson C.H.M., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mateo V., Lagneaux L., Bron D., Biron G., Armant M., Delespesse G., Sarfati M. CD47 ligation induces caspase-independent cell death in chronic lymphocytic leukemia. Nat. Med. 1999;5:1277–1284. doi: 10.1038/15233. [DOI] [PubMed] [Google Scholar]
- 187.Sikic B.I., Narayanan S., Dimitrios Colevas A., Padda S.K., Fisher G.A., Supan D., Wakelee H.A., Aoki R., Pegram M.D., Villalobos V.M., et al. A first-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 2019;37:946–953. doi: 10.1200/JCO.18.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Andrechak J.C., Dooling L.J., Discher D.E. The macrophage checkpoint CD47 : SIRPα for recognition of “self” cells: From clinical trials of blocking antibodies to mechanobiological fundamentals. Philos. Trans. R. Soc. Lond B Biol. Sci. 2019;374:20180217. doi: 10.1098/rstb.2018.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kater A.P., Tonino S.H., Egle A., Ramsay A.G. How does lenalidomide target the chronic lymphocytic leukemia microenvironment? Blood. 2014;124:2184–2189. doi: 10.1182/blood-2014-05-578286. [DOI] [PubMed] [Google Scholar]
- 190.Ramsay A.G., Clear A.J., Fatah R., Gribben J.G. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: Establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412–1421. doi: 10.1182/blood-2012-02-411678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Giuliani M., Janji B., Berchem G. Activation of NK cells and disruption of PD-L1/PD-1 axis: Two different ways for lenalidomide to block myeloma progression. Oncotarget. 2017;8:24031–24044. doi: 10.18632/oncotarget.15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Byrd J.C., Ruppert A.S., Heerema N.A., Halvorson A.E., Hoke E., Smith M.R., Godwin J.E., Couban S., Fehniger T.A., Thirman M.J., et al. Lenalidomide consolidation benefits patients with CLL receiving chemoimmunotherapy: Results for CALGB 10404 (Alliance) Blood Adv. 2018;2:1705–1718. doi: 10.1182/bloodadvances.2017015396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Egle A., Steurer M., Melchardt T., Weiss L., Gassner F.J., Zaborsky N., Geisberger R., Catakovic K., Hartmann T.N., Pleyer L., et al. Fludarabine and rituximab with escalating doses of lenalidomide followed by lenalidomide/rituximab maintenance in previously untreated chronic lymphocytic leukaemia (CLL): The REVLIRIT CLL-5 AGMT phase I/II study. Ann. Hematol. 2018;97:1825–1839. doi: 10.1007/s00277-018-3380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rasco D.W., Papadopoulos K.P., Pourdehnad M., Gandhi A.K., Hagner P.R., Li Y., Wei X., Chopra R., Hege K., DiMartino J., et al. A First-in-Human Study of Novel Cereblon Modulator Avadomide (CC-122) in Advanced Malignancies. Clin. Cancer Res. 2019;25:90–98. doi: 10.1158/1078-0432.CCR-18-1203. [DOI] [PubMed] [Google Scholar]
- 195.Kremmidiotis G., Leske A.F., Lavranos T.C., Beaumont D., Gasic J., Hall A., O’Callaghan M., Matthews C.A., Flynn B. BNC105: A Novel Tubulin Polymerization Inhibitor That Selectively Disrupts Tumor Vasculature and Displays Single-Agent Antitumor Efficacy. Mol. Cancer Ther. 2010;9:1562–1573. doi: 10.1158/1535-7163.MCT-09-0815. [DOI] [PubMed] [Google Scholar]
- 196.Eichhorst B., Fink A.-M., Bahlo J., Busch R., Kovacs G., Maurer C., Lange E., Köppler H., Kiehl M., Sökler M., et al. First-line chemoimmunotherapy with bendamustine and rituximab versus fludarabine, cyclophosphamide, and rituximab in patients with advanced chronic lymphocytic leukaemia (CLL10): An international, open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2016;17:928–942. doi: 10.1016/S1470-2045(16)30051-1. [DOI] [PubMed] [Google Scholar]
- 197.Goede V., Fischer K., Busch R., Engelke A., Eichhorst B., Wendtner C.M., Chagorova T., de la Serna J., Dilhuydy M.-S., Illmer T., et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 198.Byrd J.C. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: Results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 199.Moreno C., Greil R., Demirkan F., Tedeschi A., Anz B., Larratt L., Simkovic M., Samoilova O., Novak J., Ben-Yehuda D., et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:43–56. doi: 10.1016/S1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 200.Lamanna N., Kalaycio M., Maslak P., Jurcic J.G., Heaney M., Brentjens R., Zelenetz A.D., Horgan D., Gencarelli A., Panageas K.S., et al. Pentostatin, Cyclophosphamide, and Rituximab Is an Active, Well-Tolerated Regimen for Patients With Previously Treated Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2006;24:1575–1581. doi: 10.1200/JCO.2005.04.3836. [DOI] [PubMed] [Google Scholar]
- 201.Hillmen P., Gribben J.G., Follows G.A., Milligan D., Sayala H.A., Moreton P., Oscier D.G., Dearden C.E., Kennedy D.B., Pettitt A.R., et al. Rituximab Plus Chlorambucil As First-Line Treatment for Chronic Lymphocytic Leukemia: Final Analysis of an Open-Label Phase II Study. J. Clin. Oncol. 2014;32:1236–1241. doi: 10.1200/JCO.2013.49.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hill S.L., Davies A. Subcutaneous rituximab with recombinant human hyaluronidase in the treatment of non-Hodgkin lymphoma and chronic lymphocytic leukemia. Future Oncol. 2018;14:1691–1699. doi: 10.2217/fon-2017-0574. [DOI] [PubMed] [Google Scholar]
- 203.Dürig J., Dührsen U., Klein-Hitpass L., Worm J., Rode Hansen J.B., Ørum H., Wissenbach M. The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia. Leukemia. 2011;25:638–647. doi: 10.1038/leu.2010.322. [DOI] [PubMed] [Google Scholar]
- 204.Chanan-Khan A.A., Zaritskey A., Egyed M., Vokurka S., Semochkin S., Schuh A., Kassis J., Simpson D., Zhang J., Purse B., et al. Lenalidomide maintenance therapy in previously treated chronic lymphocytic leukaemia (CONTINUUM): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Haematol. 2017;4:e534–e543. doi: 10.1016/S2352-3026(17)30168-0. [DOI] [PubMed] [Google Scholar]
- 205.Kessler T., Koschmieder S., Schliemann C., Crysandt M., Mikesch J.-H., von Stillfried S., Stelljes M., Pohlen M., Lenz G., Kirsch A., et al. Phase II clinical trial of pazopanib in patients with acute myeloid leukemia (AML), relapsed or refractory or at initial diagnosis without an intensive treatment option (PazoAML) Ann. Hematol. 2019;98:1393–1401. doi: 10.1007/s00277-019-03651-9. [DOI] [PubMed] [Google Scholar]
- 206.Forero A., Hamadani M., Kipps T., Fanale M., Cuneo A., Perez de Oteyza J., Gladstone D., Andre M., Bellam N., Goswami T., et al. Safety and disease response to MEDI-551, an anti-CD19 antibody, in chronic lymphocytic leukemia patients previously treated with rituximab. J. Immunother. Cancer. 2013;1:43. doi: 10.1186/2051-1426-1-S1-P43. [DOI] [Google Scholar]
- 207.Fraietta J.A., Beckwith K.A., Patel P.R., Ruella M., Zheng Z., Barrett D.M., Lacey S.F., Melenhorst J.J., McGettigan S.E., Cook D.R., et al. Ibrutinib enhances chimeric antigen receptor T-cell engraftment and efficacy in leukemia. Blood. 2016;127:1117–1127. doi: 10.1182/blood-2015-11-679134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cheson B.D., Horning S.J., Coiffier B., Shipp M.A., Fisher R.I., Connors J.M., Andrew Lister T., Vose J., Grillo-López A., Hagenbeek A., et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphomas. J. Clin. Oncol. 1999;17:1244. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 209.Advani R., Flinn I., Popplewell L., Forero A., Bartlett N.L., Ghosh N., Kline J., Roschewski M., LaCasce A., Collins G.P., et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 2018;379:1711–1721. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Beg M.S., Brenner A.J., Sachdev J., Borad M., Kang Y.-K., Stoudemire J., Smith S., Bader A.G., Kim S., Hong D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs. 2017;35:180–188. doi: 10.1007/s10637-016-0407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kasinski A.L., Slack F.J. miRNA-34 Prevents Cancer Initiation and Progression in a Therapeutically Resistant K-ras and p53-Induced Mouse Model of Lung Adenocarcinoma. Cancer Res. 2012;72:5576–5587. doi: 10.1158/0008-5472.CAN-12-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zauli G., Voltan R., di Iasio M.G., Bosco R., Melloni E., Sana M.E., Secchiero P. miR-34a Induces the Downregulation of Both E2F1 and B-Myb Oncogenes in Leukemic Cells. Clin. Cancer Res. 2011;17:2712–2724. doi: 10.1158/1078-0432.CCR-10-3244. [DOI] [PubMed] [Google Scholar]
- 213.Hart M., Walch-Rückheim B., Friedmann K.S., Rheinheimer S., Tänzer T., Glombitza B., Sester M., Lenhof H.-P., Hoth M., Schwarz E.C., et al. miR-34a: A new player in the regulation of T cell function by modulation of NF-κB signaling. Cell Death Dis. 2019;10:46. doi: 10.1038/s41419-018-1295-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hart M., Walch-Rückheim B., Krammes L., Kehl T., Rheinheimer S., Tänzer T., Glombitza B., Sester M., Lenhof H.-P., Keller A., et al. miR-34a as hub of T cell regulation networks. J. Immunother. Cancer. 2019;7:187. doi: 10.1186/s40425-019-0670-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang X., Li J., Dong K., Lin F., Long M., Ouyang Y., Wei J., Chen X., Weng Y., He T., et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal. 2015;27:443–452. doi: 10.1016/j.cellsig.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 216.Yang N., Zhu S., Lv X., Qiao Y., Liu Y.-J., Chen J. MicroRNAs: Pleiotropic Regulators in the Tumor Microenvironment. Front. Immunol. 2018;9:2491. doi: 10.3389/fimmu.2018.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bresin A., Callegari E., D’Abundo L., Cattani C., Bassi C., Zagatti B., Grazia Narducci M., Caprini E., Pekarsky Y., Croce C.M., et al. miR-181b as a therapeutic agent for chronic lymphocytic leukemia in the Eµ-TCL1 mouse model. Oncotarget. 2015;6:19807–19818. doi: 10.18632/oncotarget.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gallant-Behm C.L., Piper J., Lynch J.M., Seto A.G., Hong S.J., Mustoe T.A., Maari C., Pestano L.A., Dalby C.M., Jackson A.L., et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019;139:1073–1081. doi: 10.1016/j.jid.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 219.Misso G., Zarone M.R., Lombardi A., Grimaldi A., Cossu A.M., Ferri C., Russo M., Vuoso D.C., Luce A., Kawasaki H., et al. miR-125b Upregulates miR-34a and Sequentially Activates Stress Adaption and Cell Death Mechanisms in Multiple Myeloma. Mol. Ther. Nucleic Acids. 2019;16:391–406. doi: 10.1016/j.omtn.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhao W., Liu Y., Zhang C., Duan C. Multiple Roles of Exosomal Long Noncoding RNAs in Cancers. Biomed Res. Int. 2019;2019:1460572. doi: 10.1155/2019/1460572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Crompot E., Van Damme M., Pieters K., Vermeersch M., Perez-Morga D., Mineur P., Maerevoet M., Meuleman N., Bron D., Lagneaux L., et al. Extracellular vesicles of bone marrow stromal cells rescue chronic lymphocytic leukemia B cells from apoptosis, enhance their migration and induce gene expression modifications. Haematologica. 2017;102:1594–1604. doi: 10.3324/haematol.2016.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Armstrong J.P.K., Stevens M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018;130:12–16. doi: 10.1016/j.addr.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li I., Nabet B.Y. Exosomes in the tumor microenvironment as mediators of cancer therapy resistance. Mol. Cancer. 2019;18:32. doi: 10.1186/s12943-019-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Steinbichler T.B., Dudás J., Skvortsov S., Ganswindt U., Riechelmann H., Skvortsova I.-I. Therapy resistance mediated by exosomes. Mol. Cancer. 2019;18:58. doi: 10.1186/s12943-019-0970-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fuster-Matanzo A., Gessler F., Leonardi T., Iraci N., Pluchino S. Acellular approaches for regenerative medicine: On the verge of clinical trials with extracellular membrane vesicles? Stem. Cell Res. Ther. 2015;6:227. doi: 10.1186/s13287-015-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yamada N.O., Heishima K., Akao Y., Senda T. Extracellular Vesicles Containing MicroRNA-92a-3p Facilitate Partial Endothelial-Mesenchymal Transition and Angiogenesis in Endothelial Cells. Int. J. Mol. Sci. 2019;20:4406. doi: 10.3390/ijms20184406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Palma L.M.A., Gehrke I., Kreuzer K.-A. Angiogenic factors in chronic lymphocytic leukaemia (CLL): Where do we stand? Crit. Rev. Oncol. Hematol. 2015;93:225–236. doi: 10.1016/j.critrevonc.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 228.Brander D., Rizzieri D., Gockerman J., Diehl L., Shea T.C., Decastro C., Moore J.O., Beaven A. Phase II open label study of the oral vascular endothelial growth factor-receptor inhibitor PTK787/ZK222584 (vatalanib) in adult patients with refractory or relapsed diffuse large B-cell lymphoma. Leuk Lymphoma. 2013;54:2627–2630. doi: 10.3109/10428194.2013.784969. [DOI] [PMC free article] [PubMed] [Google Scholar]