Abstract
This report details an unusual clinical presentation of suspected acute pancreatitis that occurred after honeybee envenomation in a dog. A 13-year-old spayed female dog was presented for further evaluation of vomiting 3 days after honeybee envenomation. Abdominal ultrasound, fine-needle aspirate cytology, and blood analysis were used to establish the diagnosis. The dog recovered following supportive care. While bee envenomation induced acute pancreatitis has been reported in human literature, to the authors’ knowledge, this is the first reported case in a dog in which pancreatitis ensued soon after envenomation.
Key clinical message:
This report describes a case of a dog with honeybee envenomation that subsequently developed acute pancreatitis. Given the widespread presence of the honeybee across the globe, while rare, it is important that clinicians are aware of potential envenomation sequelae such as acute pancreatitis.
Résumé
Suspicion de pancréatite aigüe chez un chien à la suite d’une envenimation par des abeilles. Le présent rapport donne les détails d’une présentation clinique inhabituelle d’une suspicion de pancréatite aigüe qui est survenue à la suite d’une envenimation par des abeilles chez un chien. Une chienne stérilisée âgée de 13 ans fut présentée pour évaluation plus poussée de vomissements 3 jours après envenimation par des abeilles. Une échographie abdominale, une cytologie d’aspiration à l’aiguille fine et une analyse sanguine furent utilisées afin d’établir le diagnostic. La chienne a récupéré à la suite d’un traitement de support. Alors que l’envenimation par les abeilles induisant une pancréatite aigüe a été rapportée dans la littérature humaine, à la connaissance de l’auteur ceci est le premier cas rapporté chez le chien dans lequel une pancréatite est apparue peu de temps après l’envenimation.
Message clinique clé:
Ce rapport décrit le cas d’un chien avec envenimation par des abeilles qui développa subséquemment une pancréatite aigüe. Étant donné la présence répandue des abeilles à travers le globe, bien que rare, il est important que les cliniciens soient au fait des séquelles potentielles d’une envenimation telle qu’une pancréatite aigüe.
(Traduit par Dr Serge Messier)
Hypersensitivity reactions to hymenopteran stings are diverse and can range from mild local abnormalities to severe life-threatening anaphylactic reactions. Intravascular hemolysis, acute kidney injury, pulmonary edema, cerebral edema, multiple organ dysfunction, and pancreatitis are all reported systemic reactions in humans (1,2). While the human literature shows multiple stings are often necessary to cause such severe systemic effects, there are reports of single stings resulting in multiple organ dysfunction (1–5). Adverse effects including acute kidney injury have recently been reported in the dog (6).
Bees, aculeate wasps, yellow jackets, hornets, and fire ants are venomous insects that are part of the insect order Hymenoptera, which contains over 92 000 species (7). Bee venom contains histamine, dopamine, phospholipase A1 (PLA1), phospholipase A2 (PLA2), hyaluronidase, and toxic peptides such as the hemolysin, mellitin, mast cell degranulators, and neurotoxin apamin (8,9). Phospholipases are active components of several animal species’ venoms as well as mammalian pancreatic secretion (3). Reports of pancreatitis after bee and wasp stings are rare in human medicine, being documented only 6 times in the literature (1–5). To the authors’ knowledge, this is the first reported case in the veterinary literature in which acute pancreatitis is associated with hymenopteran envenomation in a dog. While it is difficult to prove that hymenopteran venom directly causes pancreatitis, it is important that clinicians are aware of this possible sequela.
Case description
A 13-year-old spayed female husky mixed breed dog was presented to the emergency service for vomiting and suspected acute pancreatitis as a referral by her primary veterinarian. The dog was reportedly normal until the owner witnessed the dog in the back yard being stung by a honeybee, an event that was soon followed by rapidly developing facial and lingual swelling. The dog was tachypneic and weak on initial examination. A sting site was identified on the left side of the tongue by the primary veterinarian. The dog was excessively licking, which was thought to have dislodged the stinger. The dog was treated with dexamethasone (Aspen Dexasone; Sparhawk Laboratories, Lenexa, Kansas, USA), 0.4 mg/kg body weight (BW), IM, once, diphenhydramine (Diphenhydramine HCl Injection; Baxter Healthcare, Deerfield, Illinois, USA), 0.8 mg/kg BW, IM, once. The swelling markedly decreased after this treatment.
After the event, the owner reported that the dog never reverted to normal as evidenced by signs of progressive depression, inactivity, and decreased appetite. Vomiting ensued 3 d later and the dog was returned to the primary veterinarian. Biochemical analysis was done on withdrawn blood samples (Catalyst One; IDEXX Laboratories, Westbrook, Maine, USA), and the results showed elevated amylase and lipase activities, alkaline phosphatase, and liver transaminase enzymes (Table 1). The dog was hospitalized and treated with famotidine (Famotidine; West-Ward, Eatontown, New Jersey, USA), 0.2 mg/kg BW, IV, q8h, maropitant citrate (Cerenia; Pfizer Animal Health, New York, New York, USA), 1.2 mg/kg BW, IV, q24h, ampicillin (Ampicillin; Hanford Manufacturing, Syracuse, New York, USA), 20 mg/kg BW, IV, q12h, and lactated Ringer’s solution (Vetivex; Dechra Veterinary Products, Overland Park, Kansas, USA), 80 mL/kg BW per day, IV. After 4 days of hospitalization, the dog was referred to a tertiary care hospital due to persistent clinical signs.
Table 1.
Serum chemistry results on a 13-year-old dog with acute pancreatitis following honeybee envenomation and recovery.
| Biochemistry value | Day 3 after envenomation | Day 20 after envenomation | Reference range |
|---|---|---|---|
| BUN (mmol/L) | 14.3 | 5.0 | 2.5 to 9.6 |
| Creatinine (μmol/L) | 140 | 110 | 40 to 160 |
| Phosphorus (mmol/L) | 2.5 | 1.5 | 0.8 to 2.2 |
| Albumin (g/L) | 31 | 28 | 22 to 39 |
| Globulins (g/L) | 46 | 40 | 25 to 45 |
| Alkaline phosphatase (μkat/L) | 27.7 | 33.0 | 0.4 to 3.5 |
| Cholesterol (mmol/L) | 9.1 | 6.9 | 2.9 to 8.3 |
| Amylase (U/L) | > 2500 | 796 | 500 to 1500 |
| Lipase (U/L) | 4726 | 4350 | 200 to 1800 |
BUN — blood urea nitrogen.
On arrival, the dog was ataxic and had a temperature of 39.4°C, the pulse and respiration rates were 90 beats/min and 12 breaths/min, respectively. The abdomen was painful on palpation, and the dog appeared grossly uncomfortable as evidenced by stiff posturing. There were no localizing neurological abnormalities to explain the cause of the ataxia which was subsequently attributed to abdominal pain and suspected chronic coxo-femoral osteoarthritis.
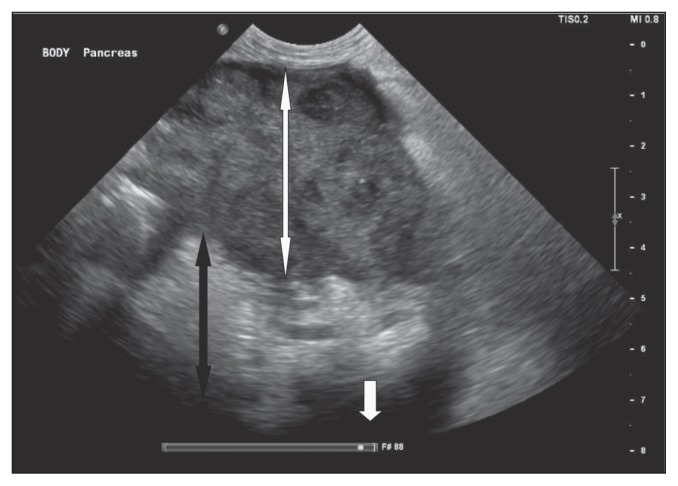
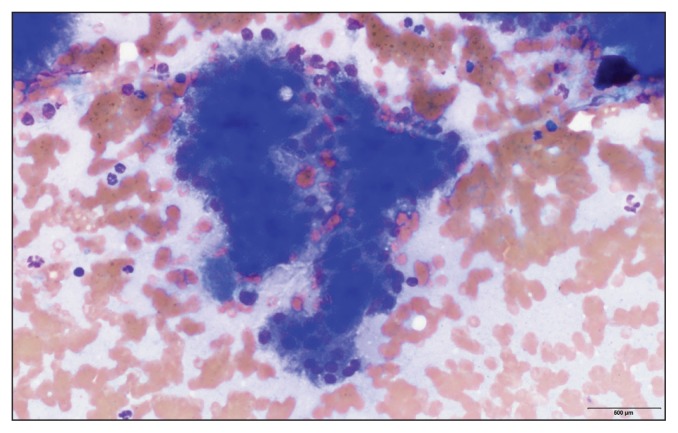
An abdominal ultrasound examination showed an enlarged pancreas measuring up to 5 cm in width with diffusely hypoechoic parenchyma (Figure 1). The peripancreatic fat was hyperechoic and hyperattenuating. A moderate amount of anechoic fluid was present throughout the peritoneal cavity. Additional findings included hepatomegaly, bilateral adrenal nodules (1.7 cm diameter each), and bilateral degenerative renal changes. Pancreatitis was suspected based on the ultra-sonographic findings. Cytology of the peritoneal fluid obtained by ultrasound-guided fine-needle aspiration showed a non-septic exudate with numerous red blood cells and degenerate neutrophils without any evidence of infectious organisms or neoplastic cells. A fine-needle aspirate of the pancreas was obtained, and the cytological evaluation, which was returned the next day, indicated mildly atypical pancreatic epithelium with mild neutrophilic inflammation compatible with acute pancreatitis (Figure 2). No infectious organisms or neoplastic cells were identified.
Figure 1.
Abdominal ultrasound showing an enlarged hypoechoic pancreas (white double arrowhead) with surrounding hyperechoic saponification (black double arrowhead) and free fluid (single white arrowhead).
Figure 2.
Cytology sample from fine-needle aspiration of the pancreas in Figure 1 showing red blood cells, neutrophils, and mildly atypical pancreatic epithelial cells compatible with pancreatic inflammation (magnification 100×).
The dog was treated supportively with parenteral lidocaine (VetOne Lidocaine 2%; MWI, Boise, Idaho, USA), 25 μg/kg BW per min and ketamine (Ketaject; Phoenix Pharmaceuticals, Burlingame, California, USA), 3 to 5 μg/kg BW per min continuous rate infusions titrated to effect. Enteral feeding via a nasogastric tube with Jevity (Jevity 1.5 Cal; Abbott Laboratories, Columbus, Ohio, USA) was titrated from 1/4 resting energy rate up to full resting energy rate, and intravenous lactated Ringer’s solution (Baxter), was initially given at 90 mL/kg BW per day, then titrated to 60 mL/kg BW per day, and then weaned over 4 d. Hypertension with an average reading of 220 mmHg was found on the first day of hospitalization, prompting the administration of anti-hypertensive drugs [amlodipine (Amlodipine Besylate; Cipla, Sunrise, Florida, USA), 0.18 mg/kg BW, PO, q12h and enalapril (Enalapril Maleate; Wockhardt, Aurangabad, Maharashtra, India), 0.18 mg/kg BW, PO, q12h]. Maropitant citrate (Cerenia; Pfizer Animal Health), 1 mg/kg BW, IV, q24h, was given for nausea and ampicillin-sulbactam (Unasyn; Pfizer Animal Health), 30 mg/kg BW, IV, q8h, was given until the cytology results showed no infectious organisms; after which the antibiotic was discontinued. The ketamine and lidocaine medications were discontinued after 4 days when the dog began eating, and the ataxia resolved on day 5 before discharge.
The dog was discharged on day 6 with amlodipine (Amlodipine Besylate; Cipla), 0.18 mg/kg BW, PO, q12h, enalapril (Enalapril Maleate; Wockhardt), 0.18 mg/kg BW, PO, q12h, and maropitant citrate (Cerenia; Pfizer Animal Health), 2 mg/kg BW, PO, q24. During follow-up at 2 d and again 2 wk after discharge, the owner reported that the dog had done well at home and had completely recovered. Repeat blood analysis performed 2 wk later (Catalyst One; IDEXX Laboratories) by the primary veterinarian showed significantly decreased serum amylase and slightly decreased lipase concentrations (Table 1). A urinalysis showed appropriately concentrated urine (specific gravity of 1.030), normal sediment analysis, and was unremarkable apart from proteinuria. A urine protein-creatinine ratio (UPC) was 4.3 (reference value < 0.5). Enalapril was continued for proteinuria, but amlodipine was discontinued due to resolution of hypertension with repeated measurements of 120 to 140 mmHg after discontinuation.
Discussion
Hymenoptera envenomation occurs worldwide and while the episodes typically cause local pain and cutaneous wheal formation, they can also cause life-threatening systemic Type 1 hypersensitivity and other severe inflammatory reactions. This report describes the case of a dog that experienced acute pancreatitis subsequent to honeybee envenomation. This is a rare phenomenon documented in humans and this report outlines the first suspected case in a dog (1–5).
Diagnosis of pancreatitis in the absence of confirmatory histopathology is challenging. Since obtaining histopathology is usually invasive, several other diagnostic tests are often used to support a diagnosis of pancreatitis. The significance of the elevation of amylase and lipase activities in this case is difficult to interpret. Unfortunately, canine pancreas-specific lipase test, abdominal ultrasound, and fine-needle aspirate cytology were not available at the referring veterinary hospital; this may have proved more useful towards suggesting the pancreas as the source of the clinical signs prior to referral. Histopathology is the gold standard diagnosis for acute pancreatitis, but it is not often performed due to its invasiveness (10). While enzyme-linked immunosorbent assay (ELISA) for canine pancreas-specific lipase tests are commonly used in practice, these tests can have up to a 40% false positive rate in the diagnosis of acute pancreatitis in dogs presenting with an acute abdomen, with pancreatic inflammation being secondary to other primary abdominal pathology (11). While a final diagnosis of pancreatitis was made 7 d after the bee sting, which leaves room for the possibility of a different underlying cause, the dog exhibited the same persistent clinical signs at the primary veterinarian as at the referral hospital. This assumes that continuation of the same underlying disease process is more likely than 2 separate diseases. While exacerbation of underlying chronic pancreatitis or glomerulonephritis is also possible, this patient had no history of gastrointestinal or renal disease. The hyperlipasemia on day 20 might indicate that the dog developed indolent pancreatitis after the initial presentation, or perhaps had subclinical disease before the envenomation.
Controversy exists over the role of glucocorticoids in pancreatitis. Anti-inflammatory dosed steroids are frequently used to treat anaphylactic reactions. While there are a few case reports associating steroids as an underlying cause of acute pancreatitis, other prospective studies do not show this correlation (12–14). A recent non-blinded, non-randomized study showed faster clinical improvement and decrease in C-reactive protein levels in dogs treated with prednisolone for acute pancreatitis (15). Dexamethasone has been shown to increase serum lipase activity without any histologic damage to the pancreas (16). Biochemical changes as a result of steroids in addition to the challenge of diagnosing pancreatitis without histopathology may contribute to this controversy. While steroids could theoretically have altered the clinical course of this dog’s disease, the role of glucocorticoids continues to be a debatable topic as the cause of acute pancreatitis.
Hypotension from the suspected initial anaphylactic reaction could have caused ischemia to the pancreas in this dog (7). Acute pancreatitis could also be one of the adverse effects caused directly by the venom, as described in human literature that there can be a delayed appearance for as long as 4 d after the envenomation (4). It is suspected that PLA2 can have direct damaging effect on pancreatic acinar cells, which is compounded by the PLA2 released by infiltrating neutrophilic granulocytes, macrophages, and platelets into the pancreas during acute pancreatitis (17). These can cause leakage of lysosomal enzymes into the pancreatic periacinar tissue causing the premature activation of the zymogens within the parenchyma (3). Additionally, PLA1 can also contribute to IgE immunological inflammatory reactions to hymenopteran venom (9). Literature on humans has shown that the PLA2 concentration may correlate with disease severity and prognosis (3,17).
The additional finding of hypertension in this dog is interesting in light of 2 case reports in humans describing bee sting associated hypertension (3,5). While this dog did not have a history of hypertension, it was diagnosed with this abnormality during its hospitalization. The hypertension associated with bee stings in humans has been explained by the presence of vasopressor amines such as noradrenaline and dopamine found in the honeybee venom (18,19). In this case, renal changes and abdominal pain likely contributed to the elevation of blood pressure. The presence of bilateral adrenal nodules would have required a follow-up medical evaluation to assess the possibility of the dog having pheochromocytomas or other adrenocortical neoplastic processes as the cause of the hypertension. Another possible cause of hypertension was fluid overload. The dog in this case report did not show signs of fluid overload and his body weight ranged from a low of 26.6 kg to a high of 27.4 kg during hospitalization.
Decompensation of chronic kidney disease or immunemediated glomerulonephropathy from pancreatitis-induced systemic inflammatory response syndrome should be considered in the proposed pathogenesis of the hypertension. This dog had bilateral ultrasonographic changes to the kidneys consistent with chronic kidney disease. A urinalysis showed appropriate concentration with a urine specific gravity of 1.030 but did show proteinuria. Given the urinalysis was obtained 9 d after envenomation, antigen-antibody complexes could have formed in the glomeruli, also explaining the proteinuria. When the patient was still hypertensive 2 wk following discharge, a urinary protein-creatinine ratio (UPC) was done showing an increase to 4.3 (reference range: < 0.5). A report of a human envenomated by a bee describes an immune-mediated membranoproliferative glomerulonephritis based on histopathology that was suspected to be due to direct nephrotoxicity caused by the toxic active amines in hymenopteran venom (20). Due to lack of a urinalysis before the event, it is unknown whether exacerbation of chronic kidney disease or immune-mediated glomerular nephropathy ultimately caused the proteinuria and hypertension in this dog.
In conclusion, this dog had a complex clinical picture involving an immediate hypersensitivity reaction, hypertension, and acute pancreatitis that occurred after bee envenomation. Although rare, given the widespread presence of the honeybee around the globe, it is important that clinicians be aware of potential envenomation sequelae such as acute pancreatitis. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Sharma N, Balamurugeshan PK, Sharma A. Acute pancreatitis and acute renal failure following multiple hornet stings. J Venom Anim Toxins Incl Trop Dis. 2006;12:310–314. [Google Scholar]
- 2.Azad C, Parmar VR, Jat KR. Unusual fatal multiple-organ dysfunction and pancreatitis induced by a single wasp sting. J Venom Anim Toxins Incl Trop Dis. 2011;17:108–110. [Google Scholar]
- 3.Daisley H. Acute haemorrhagic pancreatitis following multiple stings by Africanized bees in Trinidad. Trans R Soc Trop Med Hyg. 1998;92:71–72. doi: 10.1016/s0035-9203(98)90960-9. [DOI] [PubMed] [Google Scholar]
- 4.Umakanth M. Acute pancreatitis following multiple wasps stung. SJMPS. 2017;3:441–443. [Google Scholar]
- 5.Yang SH, Song YH, Kim TH, et al. Acute pancreatitis and rhabdomyolysis with acute kidney injury following multiple wasp stings. Case Rep Nephrol. 2017;2017 doi: 10.1155/2017/8596981. 8596981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley GJ, Corrie C, Bandt C, Schaer M. Kidney injury in a dog following bee sting-associated anaphylaxis. Can Vet J. 2017;58:256–269. [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt JO. Clinical consequences of toxic envenomations by Hymenoptera. Toxicon. 2018;150:96–104. doi: 10.1016/j.toxicon.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Ellenhorn MJ, Barceloux DG. Envenomations from bites and stings. New York, New York: Elsevier; 1998. pp. 1133–1141. [Google Scholar]
- 9.Santos LD, Santos KS, Souza BM, et al. Purification, sequencing and structural characterization of the phospholipase A1 from the venom of the social wasp Polybiapaulista (Hymenoptera, Vespidae) Toxicon. 2007;50:923–937. doi: 10.1016/j.toxicon.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Xenoulis PG. Diagnosis of pancreatitis in dogs and cats. J Small Anim Pract. 2015;56:13–26. doi: 10.1111/jsap.12274. [DOI] [PubMed] [Google Scholar]
- 11.Haworth MD, Hosgood G, Swindells KL, Mansfield CS. Diagnostic accuracy of the SNAP and Spec canine pancreatic lipase tests for pancreatitis in dogs presenting with clinical signs of acute abdominal disease. J Vet Crit Care. 2014;24:135–143. doi: 10.1111/vec.12158. [DOI] [PubMed] [Google Scholar]
- 12.Fittschen C, Bellamy JEC. Prednisone treatment alters the serum amylase and lipase activities in normal dogs without causing pancreatitis. Can J Comp Med. 1984;48:136–140. [PMC free article] [PubMed] [Google Scholar]
- 13.Moriello KA, Bowne D, Meyer DJ. Acute pancreatitis in two dogs given azathioprine and prednisone. J Am Vet Med Assoc. 1987;191:695–696. [PubMed] [Google Scholar]
- 14.Kimura T, Zuidema GD, Cameron JL. Steroid administration and acute pancreatitis: Studies with an isolated, perfused canine pancreas. Surgery. 1979;85:520–524. [PubMed] [Google Scholar]
- 15.Okanishi H, Nagata T, Nakane S, Watari T. Comparison of initial treatment with and without corticosteroids for suspected acute pancreatitis in dogs. J Small Anim Pract. 2019;60:298–304. doi: 10.1111/jsap.12994. [DOI] [PubMed] [Google Scholar]
- 16.Parent J. Effects of dexamethasone on pancreatic tissue and on serum amylase and lipase activities in dogs. J Am Vet Med Assoc. 1982;180:743–746. [PubMed] [Google Scholar]
- 17.Aufenanger J, Samman M, Quintel M, Fassbender K, Zimmer W, Bertsch T. Pancreatic phospholipase A2 activity in acute pancreatitis: A prognostic marker for early identification of patients at risk. Clin Chem Lab Med. 2002;40:293–297. doi: 10.1515/CCLM.2002.046. [DOI] [PubMed] [Google Scholar]
- 18.Komi DEA, Shafaghat F, Zwiener RD. Immunology of bee venom. Clin Rev Allergy Immunol. 2018;54:386–396. doi: 10.1007/s12016-017-8597-4. [DOI] [PubMed] [Google Scholar]
- 19.Banks BEC, Hanson JM, Sinclair NM. The isolation and identification of noradrenaline and dopamine from the venom of the honey bee, Apismellifica. Toxicon. 1976;14:117–125. doi: 10.1016/0041-0101(76)90101-x. [DOI] [PubMed] [Google Scholar]
- 20.Pai S, Harischandra P, Jagadeesh T. Immune-mediated membranoproliferative glomerulonephritis precipitated by a bee sting: A case report. J Integr Nephrol Adrol. 2015;2:67–68. [Google Scholar]