Abstract
Gastric carcinoma is not commonly reported in dogs. There is an increased risk, however, in certain breeds such as the Belgian Tervuren. Review of the Veterinary Medical Database (VMDB) established an increase in risk for gastric carcinoma in the chow chow breed. In 106 chow chow dogs signs commenced, on average, 3 weeks before definitive diagnosis. The most common clinical signs were vomiting, loss of appetite, diarrhea, and melena. Most affected dogs were euthanized, without treatment, within 2 weeks of diagnosis. Two dogs which were treated aggressively (surgery and chemotherapy) survived a considerably longer time (12 and 36 months). Histologically, these chow chow dogs comprised a similar histologic type as familial gastric carcinoma in humans; diffuse-type carcinoma that was enriched in the signet ring and mucinous variants. Understanding the pathogenesis of diffuse gastric carcinoma in the chow chow dog may provide insight into the biology of this aggressive cancer in humans.
Résumé
Risques et caractéristiques d’un carcinome gastrique chez le chien de race chow-chow. Le carcinome gastrique n’est par rapporté fréquemment chez les chiens. Il y a toutefois une augmentation du risque chez certaines espèces telle que le Tervuren belge. Une revue de la base de données Veterinary Medical Database (VMDB) a établi une augmentation dans le risque pour le carcinome gastrique chez la race chow chow. Chez 106 chiens chow chow les signes débutèrent, en moyenne, 3 semaines avant le diagnostic définitif. Les signes cliniques les plus fréquents étaient vomissement, perte d’appétit, diarrhée et méléna. La plupart des chiens affectés furent euthanasiés, sans traitement, à l’intérieur de 2 semaines du diagnostic. Deux chiens furent traités de manière agressive (chirurgie et chimiothérapie) ont survécu beaucoup plus longtemps (12 et 36 mois). Histologiquement, ces chiens chow chow comprennent un type histologique similaire aux carcinomes gastrique familiaux chez les humains; le carcinome de type-diffus qui s’est développé dans les variants de cellules en bague à chatons et mucineux. Comprendre la pathogénie du carcinome gastrique diffus chez le chien chow chow pourrait fournir des informations sur la biologie de ce cancer agressif chez l’humain.
(Traduit par Dr Serge Messier)
Introduction
While gastric cancer in humans is among the leading causes of cancer death worldwide, gastric cancer is uncommon in the dog, accounting for less than 1% of neoplastic diagnoses (1–5). Most canine gastric tumors have been identified as gastric carcinomas but other, less common tumors (listed in order of most frequently reported), include lymphosarcoma, mast cell tumor, leiomyosarcoma, leiomyoma, extramedullary plasmacytoma, gastrointestinal stromal tumor (GIST), and fibrosarcoma (6–12). Despite its reported infrequency in the canine population in general, several breeds have been reported to have a higher incidence of gastric carcinoma including the Belgian shepherd (Groenendeal and Tervuren), Dutch shepherd, Norwegian lundehund, Bouvier des Flandres, collie, and standard poodle (3,13–15). There are also case reports of gastric cancer in association with Ménétrier’s disease in a West Highland white terrier (16) and a family of cairn terriers (17). The high incidence in these specific breeds suggests a hereditary component to canine gastric carcinoma in the dog. However, most clinical or histologic descriptions of dogs with gastric carcinoma include relatively small numbers of animals from which it can be difficult to establish a clinical phenotype and investigate the pathogenesis of the disease.
The first report suggesting a predisposition in the chow chow breed described ultrasonographic findings consistent with gastric cancer in 16 dogs, 4 of which were chow chow (18). Other reports in the United States and Europe have also supported the authors’ hypothesis (18,19). Given the poor prognosis for most dogs with gastric carcinoma, understanding the breed-associated pathogenesis could lead to prevention strategies, techniques for early diagnosis, and more effective treatment. In an effort to describe gastric carcinoma in this breed, the aims of the study were to determine the risk ratio for this disease in dogs in North America and then establish a canine gastric cancer tissue repository and database to permit the clinical and histologic characterization of the disease.
Materials and methods
Risk of gastric carcinoma
The veterinary medicine database (VMDB) established in 1964 is a resource that contains coded medical record data from North American veterinary schools (20). Initially, to determine the relative frequency of gastric carcinoma in various canine breeds, data were obtained from the VMDB for the 21-year period between January 1, 1983 and December 31, 2003. The frequency of the gastric carcinoma diagnosis for each breed was compared to the frequency of gastric carcinoma in all dogs and the risk ratio was determined for these breeds. Once an increased risk was identified in the chow chow breed a more extensive search was performed to target the prevalence of gastric carcinoma in the chow chow breed within this time period. For this search, the total number of chow chow dogs, the number of dogs diagnosed with gastric carcinoma, and the number of chow chow dogs diagnosed with gastric carcinoma were investigated.
Evaluation of clinical phenotype
A canine gastric cancer database and repository was established by one of the authors to permit the study of canine gastric carcinoma which couples basic clinical data (age, gender, breed, clinical signs, survival time, diagnosis, and method of diagnosis) with biologic specimens. This repository searched for dogs which were included if they were American Kennel Club (AKC) registered chow chow dogs with a diagnosis of gastric carcinoma and were entered in the database before April 1, 2010. A total of 106 cases fit these criteria. Information abstracted from repository records included gender, age at diagnosis, presenting clinical signs, duration of clinical signs, age at death, location of tumor, and method of diagnosis and treatment. In cases of incomplete data, additional information was gathered by contacting the managing veterinarian and/or the owner when possible. Continuous numerical variables were tested for normality using the Kolmogorov-Smirnov test. If the reported variable did not deviate significantly from a normal distribution, then it was described using mean ± standard deviation (SD). For variables that were not normally distributed, median and range were reported.
A cohort of chow chow dogs collected as controls for the repository were used to compare gender distribution between affected and unaffected dogs. Control dogs were AKC registered chow chow dogs that had no clinical signs of gastric carcinoma, regardless of gender. Clinical signs included vomiting, diarrhea, lethargy, inappetence, and melena. The distribution of male versus female and sexually intact versus altered dogs were compared between animals affected and unaffected by gastric cancer using a Fisher’s exact test with significance set at P < 0.05.
Histologic classification
The Lauren (21) and the World Health Organization (22) classification schemes were used to classify hematoxylin and eosin (H&E) stained sections. While all dogs included in the clinical evaluation had a histopathologic diagnosis of gastric carcinoma, the tumors were not systematically classified. Tissue from the repository was reviewed for 18 chow chow dogs; 10 additional previously diagnosed gastric carcinomas from dogs of other breeds were reviewed solely for histologic comparison to the chow chow dogs.
Results
Risk of gastric carcinoma in dogs in North America
To determine the risk of gastric carcinomas within the period from 1983 through 2003, available coded medical records for 932 172 dogs were reviewed through the Veterinary Medicine Database (VMDB). A search of these data identified 568 cases diagnosed with gastric carcinoma (prevalence = 0.06%). One hundred and three gastric carcinoma cases occurred in mixed breed dogs and the rest (n = 465) occurred in purebred dogs representing 77 breeds. There was a small, statistically significant, increase in the odds for gastric carcinoma diagnosis in a purebred dog relative to a mixed breed dog (OR = 1.37; 95% CI: 1.11% to 1.69%). Further analysis was performed for breeds in which at least 5 individuals were diagnosed with gastric carcinoma. The frequencies of occurrence and ORs for the diagnosis of gastric carcinoma for the 28 breed classifications meeting this criterion are summarized in Table 1. These data indicate that 3 breeds, including the American cocker spaniel, German shepherd, and the Labrador retriever, as well as dogs designated as mixed breed had a statistically significant decreased risk for developing gastric carcinoma. However, 12 breeds including the chow chow, which is the focus of this paper, had statistically significant increased odds for the diagnosis of gastric carcinoma with ORs ranging between 2.4 and 14. The highest OR was in the chow chow breed.
Table 1.
Breed-associated risk for the development of gastric carcinoma.
| Breed | Frequency (%) | OR | [95% CI] | |
|---|---|---|---|---|
| * | American cocker spaniel | 0.01 | 0.21 | [0.09% to 0.49%] |
| * | German shepherd | 0.02 | 0.32 | [0.16% to 0.61%] |
| Shetland sheepdog | 0.03 | 0.53 | [0.22% to 1.3%] | |
| * | Labrador retriever | 0.03 | 0.53 | [0.35% to 0.80%] |
| Yorkshire terrier | 0.04 | 0.64 | [0.26% to 1.5%] | |
| * | Mixed breed | 0.05 | 0.73 | [0.59% to 0.91%] |
| Poodle, miniature | 0.05 | 0.78 | [0.42% to 1.5%] | |
| Schnauzer, miniature | 0.05 | 0.80 | [0.40% to 1.6%] | |
| Golden retriever | 0.06 | 0.92 | [0.62% to 1.4%] | |
| Boston terrier | 0.07 | 1.2 | [0.49% to 2.9%] | |
| English springer spaniel | 0.07 | 1.2 | [0.62% to 2.3%] | |
| Lhasa apso | 0.07 | 1.2 | [0.57% to 2.6%] | |
| Collie | 0.08 | 1.3 | [0.68% to 2.4%] | |
| Greyhound | 0.09 | 1.4 | [0.60% to 3.5%] | |
| Siberian husky | 0.09 | 1.5 | [0.75% to 2.8%] | |
| Shih tzu | 0.10 | 1.6 | [0.92% to 2.8%] | |
| Border collie | 0.12 | 2.0 | [0.88% to 4.4%] | |
| ** | Samoyed | 0.14 | 2.4 | [1.2% to 4.6%] |
| ** | Cairn terrier | 0.19 | 3.1 | [1.4% to 6.9%] |
| ** | West Highland white terrier | 0.21 | 3.4 | [1.9% to 6.1%] |
| ** | Basset hound | 0.22 | 3.6 | [2.2% to 5.9%] |
| ** | Poodle, standard | 0.23 | 4.0 | [2.6% to 6.1%] |
| ** | Irish setter | 0.30 | 5.1 | [3.3% to 8.1%] |
| ** | Norwegian elkhound | 0.41 | 6.9 | [3.4% to 14%] |
| ** | Keeshond | 0.44 | 7.4 | [4.2% to 13%] |
| ** | Bouvier des Flandres | 0.66 | 11 | [6.6% to 19%] |
| ** | Belgian herding dogs | 0.74 | 12 | [6.8% to 23%] |
| ** | Scottish terrier | 0.74 | 13 | [9.0% to 19%] |
| ** | Chow chow | 0.75 | 14 | [11% to 18%] |
The VMDB database was searched to identify dogs with a diagnosis of gastric carcinoma between 1983 and 2003. For dog breeds with at least 5 affected animals, the frequency of the diagnosis of gastric cancer (Frequency), the odds ratio (OR) for the diagnosis of gastric cancer, and the 95% confidence interval [95% CI] were calculated. Breed designations with statistically significant decrease or increase in risk are indicated by * and **, respectively.
To determine whether there has been any change in the diagnosis of gastric carcinoma over time, we searched the VMDB for 3 different time intervals namely 1964 (the start of the database) through 1979, 1980 through 1989, and 1990 through 1999. For each time interval, data were available for 524 210 (1964 to 1979), 559 115 (1980 to 1989), and 420 257 (1990 to 1999) individual dogs. The number of dogs classified as chow chow for the same time periods were 995 (1964 to 1979), 4175 (1980 to 1989), and 3913 (1990 to 1999). The frequency of gastric cancer diagnosis in the dog population and in the chow chow population, as well as the OR for the diagnosis in chow chow dogs are shown in Table 2. The prevalence of gastric cancer increased 3-fold in all dogs between the earliest and the more recent time periods. During the same time periods the prevalence of gastric cancer in the chow chow breed increased nearly 10-fold.
Table 2.
Prevalence of canine gastric cancer diagnosis over time.
| Prevalence of gastric cancer diagnosis | |||
|---|---|---|---|
|
| |||
| Time period | In all dogs | In chow chow dogs | OR (95% CI) |
| 1964 to 1979 | 0.02% | 0.10% | 5.2 (0.72% to 37%) |
| 1980 to 1989 | 0.05% | 0.36% | 7.4 (4.4% to 12%)* |
| 1990 to 1999 | 0.06% | 0.95% | 16 (12% to 23%)* |
The VMDB database was searched to identify dogs with a diagnosis of gastric carcinoma. The frequency of gastric cancer was computed by dividing the number of individual dogs with a gastric carcinoma diagnosis by the total number of individuals dogs in the time period indicated. The frequency and OR (odds ratio) were also calculated for chow chow dogs.
Statistically significant increase in risk.
Clinical characteristics
One hundred and six chow chow dogs met the inclusion criteria. In 102 cases (96.2%) gender of the chow chow dog was clearly recorded. Of those reported 46 (45.1%) were male and 56 (54.9%) were female with 25 and 31 sexually altered, respectively. There was no significant difference between the distribution of males and females compared to a set of chow chow dogs collected as controls. Information about the timing of neutering (e.g., as juvenile or adult) was not readily available, precluding any further analysis of the role of neutering in disease risk. Age at diagnosis was reported in 88/106 of these dogs (83%) and ranged from 5 to 15 y (mean: 10 ± 2.2 y).
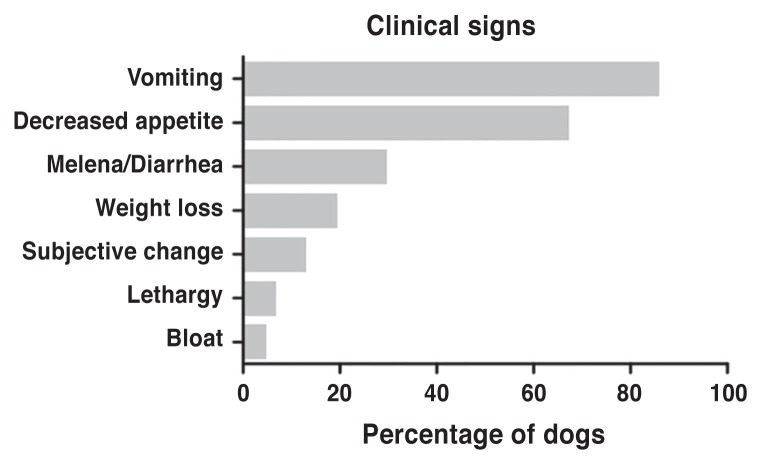
Clinical signs were specifically reported for 48 cases (Figure 1). Most of the dogs (41 of 48) were reported to have more than 1 clinical sign. The mean time interval between onset of clinical signs and diagnosis was 6 wk (median: of 3 wk), but ranged between 4 d and 28 wk.
Figure 1.
Clinical signs of gastric carcinoma.
For 61 dogs, there was no indication of premortem diagnostic evaluation. The remaining 45 dogs underwent premortem evaluation to diagnose and stage the patient, including abdominal sonography, endoscopy, or abdominal exploratory surgery. Abdominal sonography identified a gastric mass in 8 cases (17.8%), 5 of which were confirmed with cytology and 3 of which were confirmed at necropsy. Nine cases (20%) were diagnosed with endoscopic biopsy. Surgical exploratory laparotomy with biopsy was the primary diagnostic method for 28 (62.2%) of the cases.
Location of the tumor was noted in 69 (65.1%) cases. Masses occurred from the cardia to the pylorus. The 2 most common sites for the tumor were diffuse infiltration of the entire stomach and the pylorus. These occurred in 52 (75.4%) and 9 (13.0%) cases, respectively. The remaining tumors occurred in the lesser curvature (n = 3, 4.3%), greater curvature (n = 2, 2.9%), fundus (n = 2, 2.9%) and the cardia (n = 1, 1.5%). Metastatic disease identified through exploratory surgery, ultrasonography, or necropsy was reported in 7 cases. The most common location was lymph node (n = 4), followed by the small intestine (n = 2) and liver (n = 1).
Most patients (43 of 63, 68.3%) were euthanized within 2 wk of diagnosis and only 6 dogs in total were treated. Two of the 6 dogs received 1 chemotherapy treatment alone (doxorubicin and carboplatin) and survived no more than 21 d. Two patients underwent surgery alone; they were euthanized 10 d after surgery. Two of the six dogs had long-term survival: 1 was treated with a Bilroth I surgery and doxorubicin (6 treatments) and the other was treated with surgical excision alone. The survival times for these 2 patients were 1 y and 3 y, respectively. In the dog treated with surgery alone, the mass was described as focal and polypoid.
Histologic classification
Formalin-fixed paraffin-embedded gastric specimens were histologically reviewed for dogs of the following breeds: 18 chow chow, 7 Belgian shepherd dogs (3 Groenendeal, 4 Tervuren), 1 Boston terrier, one keeshond, and 1 Norwegian elkhound (Table 3). The Lauren classification system which has been used since the 1960’s in characterizing gastric carcinoma into diffuse and intestinal type cancers, was applied to these canine samples (21). All samples were classified as diffuse type tumors.
Table 3.
Classification of canine gastric carcinoma based on World Health Organization criteria.
| Case | Breed | WHO Classification | |||
|---|---|---|---|---|---|
|
| |||||
| Papillary | Tubular | Mucinous | Poorly cohesive/signet ring | ||
| 1 | Chow chow | X | |||
| 2 | Chow chow | X | X | X | |
| 3 | Chow chow | X | |||
| 4 | Chow chow | X | |||
| 5 | Chow chow | X | X | ||
| 6 | Chow chow | X | X | ||
| 7 | Chow chow | X | X | X | |
| 8 | Chow chow | X | X | X | |
| 9 | Chow chow | X | |||
| 10 | Chow chow | X | |||
| 11 | Chow chow | X | |||
| 12 | Chow chow | X | X | ||
| 13 | Chow chow | X | |||
| 14 | Chow chow | X | X | X | |
| 15 | Chow chow | X | X | ||
| 16 | Chow chow | X | X | ||
| 17 | Chow chow | X | |||
| 18 | Chow chow | X | X | ||
| 19 | Groenendael | X | X | ||
| 20 | Groenendael | X | X | ||
| 21 | Groenendael | X | |||
| 22 | Tervuren | X | X | X | |
| 23 | Tervuren | X | |||
| 24 | Tervuren | X | X | ||
| 25 | Tervuren | X | |||
| 26 | Boston terrier | X | |||
| 27 | Keeshond | X | |||
| 28 | Norwegian elkhound | X | |||
The WHO classification system divides carcinomas based on cellular differentiation into papillary, tubular, mucinous, and poorly cohesive (also encompassing signet ring differentiation) (Table 3). While 50% of the tumors (14 of 28) exhibited mixed differentiation with more than 1 pattern, papillary differentiation was not observed in any of the tumors. Most tumors (21 of 28; 75%) exhibited signet ring/poorly cohesive morphology in at least portions of the tumor. There was no difference in the occurrence of signet ring morphology between tumors from chow chow dogs compared to those from other breeds (P = 0.36), nor was there any clear difference in the occurrence of tubular morphology between chow chow dogs and other dogs (P = 0.70). However, tumors from chow chow dogs were particularly enriched with mucinous morphology compared to tumors from other dogs (chow chow: 13 of 18, 72%; others: 3 of 10, 30%; P = 0.05). There were 2 tumors that exhibited only mucinous morphology and both of these occurred in chow chow dogs.
Discussion
Our findings are consistent with reports from Europe suggesting an increase in prevalence of gastric carcinoma in a number of breeds of dogs including chow chow and Belgian shepherd (3,15,19). In addition, the current study showed an increased risk in some breeds not previously documented. For instance, we report that terrier breeds including the West Highland white terrier, Scottish terrier, and cairn terrier have an increased risk. Given the reports of Ménétrier’s disease and associated carcinomas in these breeds, the terrier predisposition for gastric cancer may be distinct mechanistically from that of other breeds (17,23). The increased risk in individual breeds supports the theory that gastric cancer is a heritable disease in the dog (24). In humans, most gastric carcinomas are considered sporadic, although hereditary forms of gastric cancer are thought to occur in about 10% of cases (25). Germline mutations predisposing gastric cancer include those associated with the E-cadherin gene, CDH1, and others (25). In humans there is a correlation between E-cadherin expression and the grade of tumor differentiation, Lauren histologic type, and WHO classification. The diffuse histologic type is more often associated with the genetic abnormalities in humans (25). Interestingly, certain features of human hereditary gastric carcinoma including a predominance of the diffuse (Lauren classification) histologic type, was also observed in the chow chow dogs (26). Whether or not the germline mutations play a role in the canine population has yet to be established. Helicobacter pylori infection is a leading cause of sporadic gastric carcinoma in humans, which is typically intestinal type and occurs most frequently in men (26,27). It is unlikely that H. pylori specifically has a role in canine gastric cancer since natural infection with this organism appears to be rare in dogs and it induces only mild inflammatory reactions with experimental infection (27–30). However, dogs are commonly affected by other gastric Helicobacter (29,31). It is possible that inflammation, microbial induced or otherwise, contributes to canine gastric carcinogenesis. There is also evidence in humans that particular genetic variations in immunologic genes can cooperate with H. pylori in gastric cancer pathogenesis (32).
Gastric cancer in dogs is diagnosed with increasing frequency likely due to several factors. Amorim et al (33) hypothesized that gastric cancer diagnosis may be due to increased availability and development of accurate diagnostic techniques, such as endoscopy. Moreover, the pet’s increasing role as a family member may also contribute to the increased frequency of diagnosis (34). Given the breed associations, it is also possible that breeding practices may have contributed to an increasing risk.
Despite the increased frequency of diagnosis in dogs, gastric cancer remains a diagnostic challenge. Humans with gastric cancer often report subtle and nonspecific clinical symptoms including fatigue, bloating after eating, and feeling full after meals. The early clinical signs of human gastric cancer would likely be overlooked in dogs. Given our data regarding breed predisposition, it would be prudent for veterinarians to pursue more advanced diagnostics in dogs that belong to breeds at high risk of gastric carcinoma and are presented with vague signs.
The clinical signs observed in dogs are analogous to what are termed “alarm” symptoms in humans including dysphagia, anorexia, weight loss, gastrointestinal bleeding, and vomiting. When such signs are present, endoscopy is warranted. However, these alarm symptoms are not always present, and are only reported in 56% to 86% of human patients (27,35–37). In humans such signs are often seen as a negative prognostic indicator.
The canine gastric cancer repository should enable large-scale characterization of the molecular landscape of canine gastric carcinoma. Due to the aggressive nature and negative impact in humans, gastric carcinoma was one of several malignancies selected by the cancer genome atlas project (TCGA) for complete genomic analysis of large sets of human tumors. The TCGA data demonstrate that human gastric carcinoma is comprised of distinct molecular subtypes (1). It would be useful to compare the molecular characteristics of canine and human gastric carcinoma using our specimens. This might assist in developing diagnostic aids for the dog and for identifying targets.
In conclusion, while gastric cancer is uncommon in the dog population as a whole, dogs of certain breeds, including the chow chow have a significantly increased odds of this disease. While gastric carcinoma is considered low on the list of differential diagnoses for dogs exhibiting nonspecific signs, it would be warranted to consider a more aggressive diagnostic approach in dogs that belong to breeds at high risk. Gastric cancer in the chow chow dog is similar to hereditary diffuse gastric carcinoma in humans in many ways. Understanding the pathogenesis of canine gastric cancer may provide an opportunity to understand the diffuse variant of gastric cancer that is particularly aggressive in humans. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Head KW. Tumors of the lower alimentary tract. Bull World Health Organ. 1976;53:167–186. [PMC free article] [PubMed] [Google Scholar]
- 3.Seim-Wikse T, Jorundsson E, Nodtvedt A, et al. Breed predisposition to canine gastric carcinoma — A study based on the Norwegian canine cancer register. Acta Vet Scan. 2013;55:25. doi: 10.1186/1751-0147-55-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hugen S, Thomas RE, German AJ, Burgener IA, Mandigers PJ. Gastric carcinoma in canines and humans, a review. Vet Comp Oncol. 2016;15:692–705. doi: 10.1111/vco.12249. [DOI] [PubMed] [Google Scholar]
- 5.Patnaik AK, Hurvitz AI, Johnson GF. Canine gastric adenocarcinoma. Vet Pathol. 1978;15:600–607. doi: 10.1177/030098587801500503. [DOI] [PubMed] [Google Scholar]
- 6.Culbertson R, Branam JE, Rosenblatt LS. Esophageal/gastric leiomyoma in the laboratory Beagle. J Am Vet Med Assoc. 1983;183:1168–1171. [PubMed] [Google Scholar]
- 7.Swann HM, Holt DE. Canine gastric adenocarcinoma and leiomyosarcoma: A retrospective study of 21 cases (1986–1999) and literature review. J Am Anim Hosp Assoc. 2002;38:157–164. doi: 10.5326/0380157. [DOI] [PubMed] [Google Scholar]
- 8.Frost D, Lasota J, Miettinen M. Gastrointestinal stromal tumors and leiomyomas in the dog: A histopathologic, immunohistochemical, and molecular genetic study of 50 cases. Vet Pathol. 2003;40:42–54. doi: 10.1354/vp.40-1-42. [DOI] [PubMed] [Google Scholar]
- 9.Ozaki K, Yamagami T, Nomura K, Narama I. Mast cell tumors of the gastrointestinal tract in 39 dogs. Vet Pathol. 2002;39:557–564. doi: 10.1354/vp.39-5-557. [DOI] [PubMed] [Google Scholar]
- 10.Brunnert SR, Dee LA, Herron AJ, Altman NH. Gastric extramedullary plasmacytoma in a dog. J Am Vet Med Assoc. 1992;200:1501–1502. [PubMed] [Google Scholar]
- 11.MacEwen EG, Patnaik AK, Johnson GF, Hurvitz AI, Erlandson RA, Lieberman PH. Extramedullary plasmacytoma of the gastrointestinal tract in two dogs. J Am Vet Med Assoc. 1984;184:1396–1398. [PubMed] [Google Scholar]
- 12.Willard MD. Alimentary neoplasia in geriatric dogs and cats. Vet Clin North Am Small Anim Pract. 2012;42:693–706. doi: 10.1016/j.cvsm.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Fonda D, Gualtieri M, Scanziani E. Gastric carcinoma in the dog — A clinicopathological study of 11 cases. J Small Anim Pract. 1989;30:353–360. [Google Scholar]
- 14.Qvigstad G, Kolbjornsen O, Skancke E, Waldum HL. Gastric neuroendocrine carcinoma associated with atrophic gastritis in the Norwegian lundehund. J Comp Pathol. 2008;139:194–201. doi: 10.1016/j.jcpa.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Scanziani E, Giusti AM, Gualtieri M, Fonda D. Gastric carcinoma in the Belgian shepherd dog. J Small Anim Pract. 1991;32:465–469. [Google Scholar]
- 16.Lecoindre P, Bystricka M, Chevallier M, Peyron C. Gastric carcinoma associated with Ménétrier’s-like disease in a West Highland white terrier. J Small Anim Pract. 2012;53:714–718. doi: 10.1111/j.1748-5827.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- 17.Munday JS, Aberdein D, Cullen GD, French AF. Ménétrier disease and gastric adenocarcinoma in 3 Cairn terrier littermates. Vet Pathol. 2012;49:1028–1031. doi: 10.1177/0300985812439076. [DOI] [PubMed] [Google Scholar]
- 18.Penninck DG, Moore AS, Gliatto J. Ultrasonography of canine gastric epithelial neoplasia. Vet Radiol Ultrasound. 1998;39:342–348. doi: 10.1111/j.1740-8261.1998.tb01618.x. [DOI] [PubMed] [Google Scholar]
- 19.von Babo V, Eberle N, Mischke R, et al. Canine non-hematopoietic gastric neoplasia. Epidemiologic and diagnostic characteristics in 38 dogs with post-surgical outcome of five cases. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2012;40:243–249. [PubMed] [Google Scholar]
- 20.Veterinary Medicine Database. c2012. [Last accessed February 12, 2020]. Available from: https://www.vmdb.org/
- 21.Lauren P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 22.Lauwers GY, Carneiro DY, Graham DY, Curado MP. Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumors on the Digestive System. 4th ed. Lyon, France: IARC Pr; 2010. pp. 48–58. [Google Scholar]
- 23.Lecoindre P, Bystricka M, Chevallier M, Peyron C. Gastric carcinoma associated with Ménétrier’s-like disease in a West Highland white terrier. J Small Anim Pract. 2012;53:714–718. doi: 10.1111/j.1748-5827.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- 24.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary diffuse gastric cancer syndrome: Cdh1 mutations and beyond. JAMA Oncol. 2015;1:23–32. doi: 10.1001/jamaoncol.2014.168. [DOI] [PubMed] [Google Scholar]
- 25.Hu B, Nassim EH, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J Gastrointest Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med. 2011;17:380–388. doi: 10.1016/j.molmed.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017;19(8):36. doi: 10.1007/s11894-017-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radin MJ, Eaton KA, Krakowka S, et al. Helicobacter pylori gastric infection in gnotobiotic beagle dogs. Infect Immun. 1990;58:2606–2612. doi: 10.1128/iai.58.8.2606-2612.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kubota-Aizawa S, Ohno K, Fukushima K, et al. Epidemiological study of gastric Helicobacter spp. in dogs with gastrointestinal disease in Japan and diversity of Helicobacter heilmannii sensu stricto. Vet J. 2017;225:56–62. doi: 10.1016/j.tvjl.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Simpson KW, Strauss-Ayali D, McDonough PL, Chang YF, Valentine BA. Gastric function in dogs with naturally acquired gastric Helicobacter spp. infection. J Vet Intern Med. 1999;13:507–515. doi: 10.1892/0891-6640(1999)013<0507:gfidwn>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Simpson K, Neiger R, DeNovo R, Sherding R. The relationship of Helicobacter spp. infection to gastric disease in dogs and cats. J Vet Intern Med. 2000;14:223–227. [PubMed] [Google Scholar]
- 32.Peleteiro B, Lunet N, Carrilho C, et al. Association between cytokine gene polymorphisms and gastric precancerous lesions: Systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19:762–776. doi: 10.1158/1055-9965.EPI-09-0917. [DOI] [PubMed] [Google Scholar]
- 33.Amorim I, Taulescu MA, Day MJ, et al. Canine gastric pathology: A review. J Comp Pathol. 2016;154:9–37. doi: 10.1016/j.jcpa.2015.10.181. [DOI] [PubMed] [Google Scholar]
- 34.American Veterinary Medical Association c2019 AVMA. [Last accessed February 12, 2020]. Available from: https://www.avma.org/News/JAVMANews/Pages/190115a.aspx.
- 35.Fitzgerald RC, Caldas C. Clinical Implications of E-Cadherin associated hereditary diffuse gastric carcinoma. Gut. 2004;54:775–778. doi: 10.1136/gut.2003.022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van der Post RS, Vogelaar I, Carnerio F, et al. Hereditary diffuse gastric cancer: Updated clinical guidelines with an emphasis on gremlin CDH1 mutation carriers. J Med Genet. 2015;52:361–374. doi: 10.1136/jmedgenet-2015-103094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mi EZ, Mi EZ, di Pietro M, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutations status. Gastrointest Endosc. 2016;87:408–417. doi: 10.1016/j.gie.2017.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]