Abstract
In Ghana, gap-junction protein β 2 (GJB2) variants account for about 25.9% of familial hearing impairment (HI) cases. The GJB2-p.Arg143Trp (NM_004004.6:c.427C>T/OMIM: 121011.0009/rs80338948) variant remains the most frequent variant associated with congenital HI in Ghana, but has not yet been investigated in clinical practice. We therefore sought to design a rapid and cost-effective test to detect this variant. We sampled 20 hearing-impaired and 10 normal hearing family members from 8 families segregating autosomal recessive non syndromic HI. In addition, a total of 111 unrelated isolated individuals with HI were selected, as well as 50 normal hearing control participants. A restriction fragment length polymorphism (RFLP) test was designed, using the restriction enzyme NciI optimized and validated with Sanger sequencing, for rapid genotyping of the common GJB2-p.Arg143Trp variant. All hearing-impaired participants from 7/8 families were homozygous positive for the GJB2-p.Arg143Trp mutation using the NciI-RFLP test, which was confirmed with Sanger sequencing. The investigation of 111 individuals with isolated non-syndromic HI that were previously Sanger sequenced found that the sensitivity of the GJB2-p.Arg143Trp NciI-RFLP testing was 100%. All the 50 control subjects with normal hearing were found to be negative for the variant. Although the test is extremely valuable, it is not 100% specific because it cannot differentiate between other mutations at the recognition site of the restriction enzyme. The GJB2-p.Arg143Trp NciI-RFLP-based diagnostic test had a high sensitivity for genotyping the most common GJB2 pathogenic and founder variant (p.Arg143Trp) within the Ghanaian populations. We recommend the adoption and implementation of this test for hearing impairment genetic clinical investigations to complement the newborn hearing screening program in Ghana. The present study is a practical case scenario of enhancing genetic medicine in Africa.
Keywords: hearing impairment, GJB2-p.R143W, NciI-RFLP, rapid diagnostic test, Ghana
1. Introduction
Globally, the most prevailing sensorineural disorder is hearing impairment (HI) [1], which accounts for about 466 million people worldwide [2]. According to the World Health Organization fact sheet, an estimate of 900 million people will be living with the condition by the year 2050 [2]. Over 119 genes [3] with more than 1000 mutations have been associated with hearing impairment of varied degrees in different populations [1]. Gap-junction protein β 2 (GJB2) and gap-junction protein β 6 (GJB6) are the most common genes associated with the condition globally, with high prevalence reported in the European and Asian populations. However, recent data including the use of mouse models has indicated that mutations in the coding region of the GJB6 gene do not result in hearing impairment. The large genomic deletions in GJB6, especially GJB6-D13S1830, alters a cis-acting element and subsequently abolishes the expression of the cis-GJB2 allele [4,5]. Thus, the GJB6 gene itself plays no role in the development of hearing impairment but the surrounding sequences consisting of the cis-acting elements are responsible for the development of hearing impairment [5,6]. Nevertheless, in most African populations, GJB2 and GJB6 variants are rarely implicated in hearing impairment [7,8] with some GJB2 cases found in Morocco [9,10], Sudan, and Kenya [11], yet an exceptionally high prevalence is found in Ghana [12,13,14]. Indeed, in Ghana, GJB2 mutation (p.Arg143Trp) in the homozygous state accounts for 25.9% of cases in families segregating non-syndromic HI, as well as 7.9% of non-familial non-syndromic congenital HI cases (Adadey et al., 2019). This Ghanaian exception, in the African context, is predominantly due to a GJB2 founder mutation (p.Arg143Trp), which was first reported in a village known as “the deaf village”, Adamorobe [13]. Adamorobe is a village located in the Eastern Region of Ghana and known to have a high hereditary hearing impairment incidence [15]. As of 2012, 41 people living with deafness were recorded among a population of 3500 in Adamorobe [16]. In this village, both the hearing and the deaf citizens interact and live together in one society.
The exceptionally high proportion of GJB2 (p.Arg143Trp) variant in Ghana has created the need to develop a simple tool for testing in order to support appropriate informed counselling and planning for appropriate interventions. To develop molecular diagnostic tools for screening non-syndromic HI, there is a need for utilizing population and ethnic specific genetic markers due to the ethnically diverse nature of hearing impairment genes [17,18]. Recent clinical genetic testing efforts are centered around targeted genomic enrichment and/or massive parallel sequencing [18,19,20]. There are some efforts to develop polymerase chain reaction (PCR)-based diagnostic tools for screening for hearing impairment; however, most of these tools are in combination with DNA sequencing technologies [21,22,23], which are not easily implementable in low-income countries. To develop cheaper but effective diagnostic tools, mutations specific to populations have been considered, especially in populations where GJB2 is prevalent. Specific genetic tests have been developed for carrier testing and prenatal diagnoses for GJB2-35delG variant in Caucasian populations [24,25]. In this study, we sought to design a restriction fragment length polymorphism test for GJB2-p.Arg143Trp genotyping in Ghana.
2. Materials and Methods
2.1. Ethical Approvals
The study was performed in accordance with the Declaration of Helsinki. Ethical approval for the study was obtained from the Noguchi Memorial Institute for Medical Research Institutional Review Board (NMIMR-IRB CPN 006/16-17) and the University of Cape Town’s Faculty of Health Sciences’ Human Research Ethics Committee (HREC 104/2018). Written and signed informed consent was obtained from all participants who were 21 years of age or older, and from parents or guardians in cases of minors, with verbal assent from participants, including permission to publish photographs.
2.2. Study Participants
Congenital hearing-impaired patients were recruited from schools for the deaf and from the Adamorobe community following procedures reported previously [12]. Briefly, all participants’ details, as well as their personal and family histories, were obtained; medical records were reviewed by a medical geneticist and an ear, nose, and throat (ENT) specialist when possible; and relevant data were extracted, including three-generation pedigrees and perinatal histories, using a structured questionnaire to query possible environmental causes of hearing impairment. A general systemic and otological examination and audiological evaluation were performed, including a pure tone audiometric test, following the recommendation number 02/1 of the Bureau International d’Audiophonologie (BIAP), Belgium, to classify hearing levels [26,27]. The audiometric tests were conducted using KUDUwave portable audiometer (KUDUwave, Johannesburg, South Africa) in a quiet room. In bilateral octaves, the air conduction thresholds were from 250 HZ through to 8000 HZ and the bone conduction from 250 HZ through to 4000 HZ. The pure tone average was determined using thresholds at 500, 1000, 2000, and 4000 HZ.
The study participants were categorized into three groups: (1) deaf community-based familial cases, (2) nation-wide isolated/non-familial cases, and (3) control individuals without a personal or family history of HI. The first group was made of families segregating HI, with at least two affected individuals and with evidence of non-environmental causes. In this group, 30 study participants from 8 families segregating hearing impairment were recruited from the Adamorobe community in the Eastern Region of Ghana. Out of the 30 participants, 20 were hearing-impaired and 10 participants had normal hearing. Apart from the families with putative genetic etiology of hearing loss, an additional family was found to have a putative environmental etiology of the condition and was excluded from the study. The second group of participants was made up of 111 isolated/non-familial cases of unrelated probands with putative genetic causes of hearing impairment and were recruited from 6 schools for the deaf across Ghana. All the affected individuals (familial and isolated cases) considered for the study had congenital non syndromic HI. The third group (the control group) was made of 50 normal hearing participants that were randomly recruited nationwide from the Ghanaian population.
2.3. Molecular Analyses
DNA extraction: Venous blood was collected from each participant and DNA was extracted from the blood samples using a QIAamp DNA Blood Maxi Kit (Qiagen, Germantown, MD, USA) in the Laboratory of West African Centre for Cell Biology of Infectious Pathogens (WACCBIP), University of Ghana, Accra, Ghana.
Polymerase chain reaction (PCR) and Sanger sequencing: At the Division of Human Genetics, University of Cape Town, specific primers (Table S1) were used to amplify the coding regions of GJB2 (exon 2) and GJB6, as described by Bosch et al. in 2014. The annealing and extension temperatures for the PCR were 60 °C and 70 °C for 30 s and 1 min, respectively. The PCR amplicons were Sanger sequenced as described by Bosch et al. [28] using an ABI 3130XL Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). Screening for del(GJB6-D13S1830) was performed as previously described, using primers and methods by del Castillo et al. [29].
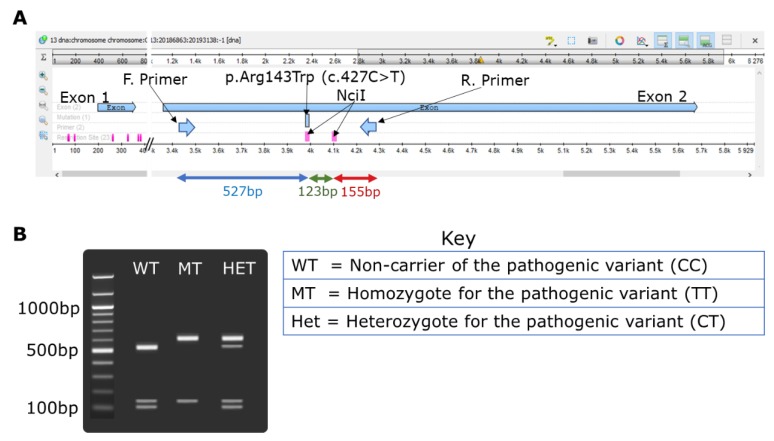
Restriction fragment length polymorphism (RFLP) technique: The p.Arg143Trp variant in the GJB2 gene was investigated using RFLP technique designed as follows. GJB2-specific primers [28] were used to amplify exon 2 of the gene where the p.Arg143Trp variant is located. Carefully selected restriction enzyme NciI (supplied by New England Biolabs Inc., Massachusetts, MA, USA, through Inqaba biotec, Pretoria, South Africa) with the recognition site “CCSGG” was used to digest the PCR amplicons. The gene layout and the GJB2-p.Arg143Trp NciI-RFLP design including the cut sites is illustrated in Figure 1. The RFLP reaction consisted of 15 μL of the PCR product, 2 μL of 10X buffer, 0.25 μL of an NciI enzyme (20,000 units/mL), and 2.75 μL of nuclease-free water. The restriction reaction mixture was incubated overnight at 37 °C. The digested products were resolved on 2% agarose gel for 1.5 h. The accuracy, sensitivity, and specificity of the RFLP test was determined as described by Baratloo et al. [30] using sequencing as the gold standard.
Figure 1.
NciI restriction fragment polymorphism investigations for gap-junction protein β 2 (GJB2)-p.Arg143Trp (c.427C > T rs80338948) variant. (A) Unipro UGENE [31] map of GJB2 exon 2 showing the primer binding sites (F. primer and R. primer) and the restriction sites (CCSGG) for the restriction enzyme NciI and the resulting DNA fragments. (B) Expected gel electrophoresis result.
2.4. Data Analysis
Data from the study was inputted into Microsoft Excel and analyzed with GraphPad Prism version 6. One-way analysis of variance (ANOVA) was used to determine the differences between the mean hearing measurements (pure tone average) of the different GJB2-p.Arg143Trp genotypes. Tukey’s multiple comparisons test was used to compare between the GJB2-p.Arg143Trp genotypes. The specificity and sensitivity of the RFLP test were calculated as described by Schrauwen et al. [23].
3. Results
3.1. Selected Families Segregating Hearing Impairment from Adamorobe Village, Ghana
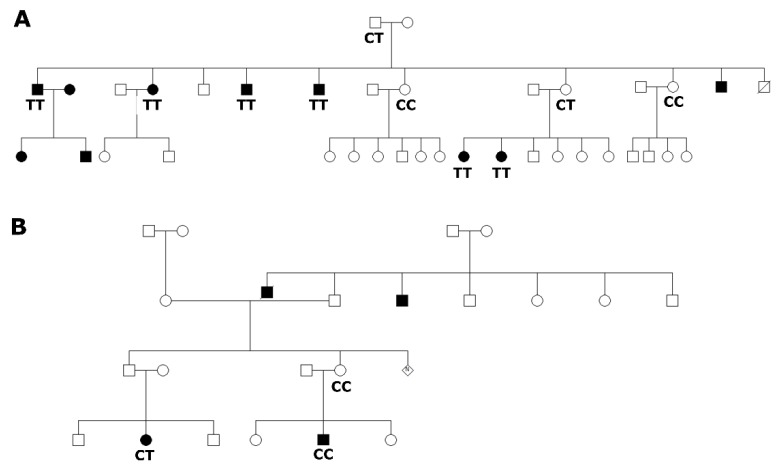
In this study, 8 families from Adamorobe were found to have 2 or more family members living with hearing impairment (Figure 2), from which 20 congenital deaf and 10 normal hearing family members were identified. Audiological assessment of the participants from Adamorobe revealed that all the hearing-impaired patients had profound sensorineural HI. The unaffected family members without the homozygous mutant (TT) genotype had normal-to-moderate hearing impairment.
Figure 2.
Pedigrees and genotypes of familial cases from Adamorobe. (A) Representative pedigree of families that segregate GJB2-p.Arg143Trp (c.427C > T rs80338948) variant with hearing impairment. (B) Pedigree of a family that did not segregate GJB2-p.Arg143Trp variant with the phenotype.
3.2. Restriction Fragment Polymorphism Design for GJB2-p.Arg143Trp
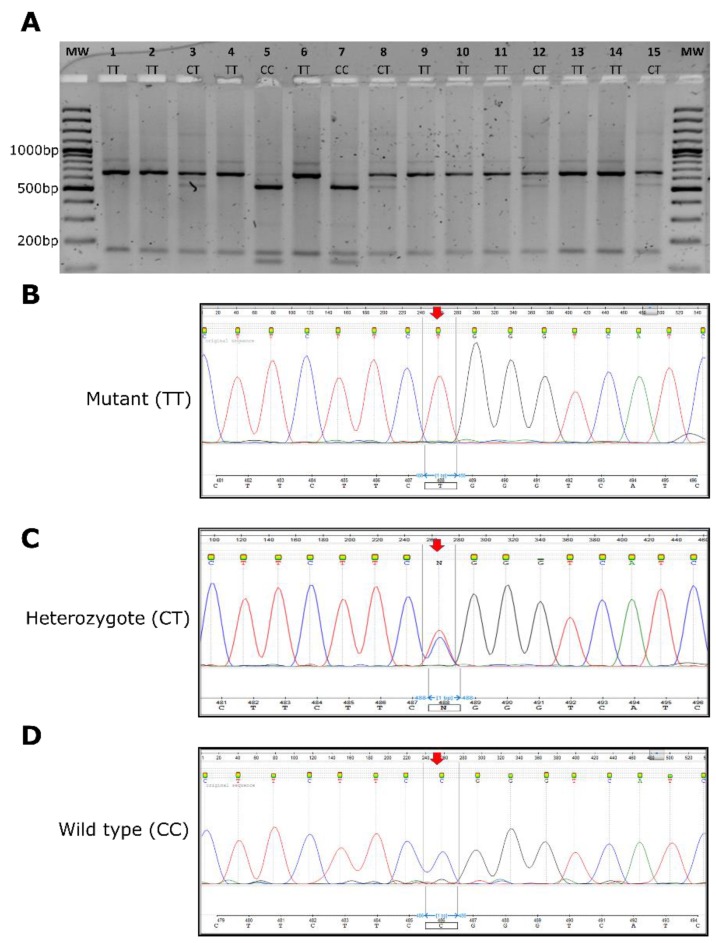
The target region of GJB2-p.Arg143Trp variant was PCR amplified for each participant (Figure S1). The NciI restriction enzyme had two restriction sites on the DNA amplified (Figure 1A) and cleaves the PCR amplicons of the wildtype (CC genotype) samples to give three products of the lengths 527, 123, and 155 bp. The NciI restriction digest of the homozygous mutant (TT) produced two fragments of the lengths 600 and 155 bp, with the enzyme cutting only once. The heterozygous carriers (CT) yielded four different fragments (600, 527, 123, and 155 bp) (Figure 1B). The NciI enzyme cleaved the PCR product in any of the above circumstances; this served as an internal control, and hence an invalid test was when there was no cleavage. The GJB2-p.Arg143Trp NciI-RFLP genotyping results of 20 selected samples from Adamorobe were validated using Sanger sequencing (Figure 3).
Figure 3.
GJB2-p.Arg143Trp screening. (A) Representative gel of NciI-restriction fragment polymorphism (RFLP) test used to screen samples for GJB2-p.Arg143Trp variant. (B–D) Representative chromatograms of Sanger sequences validating the p.Arg143Trp NciI-RFLP results.
3.3. GJB2-p.Arg143Trp NciI-Restriction Fragment Polymorphism Investigations
The molecular analysis using GJB2-p.Arg143Trp NciI-RFLP test identified 18 out of the 20 hearing-impaired patients, from 7/8 families, to be homozygous for the p.Arg143Trp (TT) variant. In the eighth family were two individuals affected by HI, one was heterozygous (CT) and the other had the CC genotype (Figure 2B). In order to exclude GJB6-related HI in this family, we investigated variants in GJB6, and no variant was found. No other participant had a variant in the GJB6 gene, (n = 20).
Seven (7) out of the 10 family members without hearing impairment were heterozygous (CT), thus having the p.Arg143Arg/p.Arg143Trp variant, while the rest had the p.Arg143 variant (Figure 3A).
A total of 111 individuals with non-familial isolated non-syndromic HI, whose samples were previously Sanger sequenced for GJB2 variants [12], were analyzed using the developed GJB2-p.Arg143Trp NciI-RFLP test. Table 1 illustrates that the GJB2-p.Arg143Trp NciI-RFLP test was found to have 100% sensitivity compared to Sanger sequencing as the gold standard. To examine the clinical applicability of the test, 50 control participants with normal hearing were screened and found negative for the GJB2-p.Arg143Trp variant.
Table 1.
Validation of GJB2-p.Arg143Trp NciI-restriction fragment polymorphism tests with Sanger sequencing.
| Familial Cases from Adamorobe | ||||
| Sanger Sequencing | ||||
| Genotype | TT | CT | CC | |
| GJB2-p.Arg143Trp NciI-RFLP | TT | 12 | 0 | 0 |
| CT | 0 | 6 | 0 | |
| CC | 0 | 0 | 2 | |
| Nation-Wide Isolated/Non-Familial Cases | ||||
| Sanger Sequencing | ||||
| Genotype | TT | CT | CC | |
| GJB2-p.Arg143Trp NciI-RFLP | TT | 6 | 0 | 0 |
| CT | 0 | 1 | 0 | |
| CC | 0 | 0 | 104 | |
The mutant, heterozygote, and wild type are represented by TT, CT, and CC, respectively.
3.4. Genotype to Phenotype Correlations
On the basis of GJB2-p.Arg143Trp genotypic classification of the familial cases from Adamorobe, the pure tone average of homozygous mutant (TT) ranged from 97 to 108 dB with a mean of 105.4 and 107.3 dB in the left and right ears, respectively. The pure tone average range for the heterozygote (CT) was from 17 to 108 dB, with a mean of 43.6 and 40.6 dB in the left and right ears, respectively. The range for the homozygous CC genotype (p.Arg143Arg) was from 18 to 108 dB, with the mean 53 and 46.5 dB in the left and right ears, respectively. There was a statistically significant difference between the audiometric measurements of the TT and CT genotypes in both ears. Similarly, in both ears, there was a statistically significant difference between the TT and CC genotypes (Figure 4).
Figure 4.
Audiological characterization of hearing-impaired participants from the deaf community of Adamorobe. (A) Left ear and (B) right ear pure tone average of participants according to their GJB2-p.Arg143Trp genotypes. The age range of the genotypes TT (n = 17), CT (n = 6), and CC (n = 4) were 9 to 80 years, 23 to 66 years, and 11 to 63 years, respectively. p-values less than 0.05 were considered significant. p-values less than 0.0001 and 0.001 are represented by (****) and (***), respectively.
4. Discussion
This study designed a restriction fragment length polymorphism (RFLP) test for effective screening of GJB2-p.Arg143Trp (rs80338948). The GJB2-p.Arg143Trp variant results from a pathogenic point mutation (c.427C > T) in the exon 2 of the connexin 26 gene on chromosome 13 [13,14]. The drive for an efficient and cost-effective test was from the fact that the founder mutation, GJB2-p.Arg143Trp, is the most common variant associated with hearing impairment in Ghana [12,13,14].
The use of next generation sequencing (NGS) has been proposed as the best tool for the discovery of hearing impairment genes [32], especially in Africa because of the high diversity within the African population [33,34]. Due to ethical and social challenges, NGS needs to be carefully considered in clinical practice [35]. In developing countries, the clinical use of NGS is still a major challenge because of the associated high cost of the equipment and the computational challenges posed by the approach [36]. However, there were some attempts to develop relatively simple, low cost, and population-specific screening approaches for some of the major hearing impairment gene mutations [37,38,39].
For the first time, we designed and tested the effectiveness of RFLP, using the NciI enzyme, to screen for the founder mutation (GJB2-p.Arg143Trp) in Ghana. Accuracy, sensitivity, specificity, and predictive values are critical parameters considered for the clinical use of a test [30,40]. Our GJB2-p.Arg143Trp NciI-RFLP test had good positive and negative predictive values for genotyping of the GJB2-p.Arg143Trp variant in the Adamorobe participants from Ghana, and also in a nationwide sample of unrelated affected individuals. Nevertheless, the test cannot differentiate between other variants within the recognition site of the restriction enzyme; hence, similar results would be obtained for the following pathogenic mutations: p.Phe142Leu (c.426C > A), p.Y142del (c.424_426delTTC), and p.Arg143Gln (c.428G > A). To confirm the specific mutation at the enzyme restriction site, Sanger sequencing would be needed. However, the high prevalence of the GJB2-p.Arg143Trp variant within the Ghanaian population makes the NciI-RFLP test relevant. A 100% sensitivity was obtained for the GJB2-p.Arg143Trp NciI-RFLP test when compared with the gold standard, Sanger sequencing. Although a single gene test for hearing impairment is inefficient for many populations [39], the aforementioned qualities of the test would enable it to be used as a first-line diagnosis for hearing impairment genetics in the newborn hearing screening program, as well as for prenatal testing. The GJB2-p.Arg143Trp NciI-RFLP test would therefore be of great clinical value in Ghana.
The GJB2-p.Arg143Trp NciI-RFLP test identified the founder mutation in the eight Adamorobe families investigated. In all the families, the mutation segregated with the phenotype, and all affected individuals reported a homozygous variant (TT genotype), except in one family where one affected individual was heterozygous (CT) and the other without any variant (CC), suggesting that there are other genes still to be discovered to explain the HI in this family. Similar to a family from Japan [37], the heterozygous GJB2-p.Arg143Trp variant in the above family did not segregate with the HI phenotype (Figure 2B). Variants in the GJB6 gene are no longer considered as causes of hearing impairment. However, the presence of GJB6 variants affecting the cis-acting element upstream of both GJB6 and GJB2 in association with variants in GJB2 (digenic inheritance) are now known to be pathogenic through the modification of GJB2 expression [5,6]. Hence, we sought to exclude any GJB6 variant that might disrupt the cis-acting element. We therefore investigated GJB6 variants in particular; GJB6-D13S1830 and no GJB6 pathogenic variant was identified in this family. Hence, we propose the use of whole exome sequencing (WES) in future, or targeted panel sequencing, which has been shown to be efficient in Cameroonian families [33], to further investigate this family, as well as any other family that is specifically negative for the GJB2-p.Arg143Trp variant in Ghana.
GJB2-p.Arg143Trp variant is known to be associated with profound HI [13,14,37]. The audiometric characterization of the GJB2-p.Arg143Trp homozygous individuals showed that they had profound HI. Previous studies by Brobby et al. from the same village indicated that GJB2-p.Arg143Trp homozygous individuals express profound hearing impairment [13]. Similar to the previous report [13], we found that there was no significant difference between the average hearing levels of the CT (heterozygote for the pathogenic variant) and the CC (non-carrier of the pathogenic variant) genotypes. Our results and previous reports confirmed the autosomal recessive mode of inheritance of GJB2-p.Arg143Trp [12,13,14].
5. Conclusions
We developed a rapid and cost-effective NciI-RFLP test for the GJB2-p.Arg143Trp founder mutation in Ghana. The GJB2-p.Arg143Trp NciI-RFLP test had 100% sensitivity when compared with Sanger sequencing, the gold standard. We therefore propose that testing for GJB2-p.Arg143Trp variant using the NciI-RFLP test should be implemented as part of the newborn hearing screening program in Ghana, a practical case scenario of enhancing genetic medicine in Africa.
Acknowledgments
Samuel Mawuli Adadey is supported by WACCBIP DELTAS PhD fellowship and Africa Regional International Staff/Student Exchange (ARISE) II mobility fund, and Darius Quansah is supported by the WACCBIP Africa Centre of Excellence (ACE) Masters fellowship. We are grateful to all the parents, patients, control participants, and the staff of the schools for the deaf for their support during the recruitment process.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/2/132/s1, Figure S1: Representative agarose gel picture of GJB2 exon 2 PCR products, Table S1: Primer sequencing for GJB2 and GJB6 coding region amplification.
Author Contributions
Conceptualization, A.W., G.A.A., G.K.A., and S.M.A.; methodology, S.M.A., E.T.W., E.T.A., and D.Q.; validation, A.W., G.A.A., G.K.A., and O.Q.; formal analysis, S.M.A., E.T.W., E.T.A., and D.Q.; resources, A.W., G.A.A., G.K.A., A.A.-P., and O.Q.; writing—original draft preparation, S.M.A.; writing—review and editing, S.M.A., E.T.W., E.T.A., D.Q., A.A.-P., O.Q., G.K.A., G.A.A., and A.W.; supervision, A.W., G.A.A., G.K.A., and O.Q.; funding acquisition, A.W. and G.A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the World Bank African Centres of Excellence grant (ACE02-WACCBIP: Awandare) and a Developing Excellence in Leadership, Training and Science Initiative (DELTAS) Africa grant (DEL-15-007: Awandare). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)’s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa’s Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107755/Z/15/Z: to G.A.A. and A.W.) and the U.K. government; the National Institutes of Health (NIH), USA, grant number U01-HG-009716 to AW; and the African Academy of Science/Wellcome Trust, grant number H3A/18/001 to A.W. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rudman J.R., Kabahuma R.I., Bressler S.E., Feng Y., Blanton S.H., Yan D., Liu X.-Z. The genetic basis of deafness in populations of African descent. J. Genet. Genom. 2017;44:285–294. doi: 10.1016/j.jgg.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 2.WHO Deafness and Hearing Loss. [(accessed on 10 August 2019)]; Available online: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.
- 3.Van Camp G., Smith R. Hereditary Hearing Loss Homepage. [(accessed on 9 September 2019)]; Available online: https://hereditaryhearingloss.org/
- 4.Ahmad S., Tang W., Chang Q., Qu Y., Hibshman J., Li Y., Söhl G., Willecke K., Chen P., Lin X. Restoration of connexin26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc. Natl. Acad. Sci. USA. 2007;104:1337–1341. doi: 10.1073/pnas.0606855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Paris J., Schrijver I. The digenic hypothesis unraveled: The GJB6 del(GJB6-D13S1830) mutation causes allele-specific loss of GJB2 expression in cis. Biochem. Biophys. Res. Commun. 2009;389:354–359. doi: 10.1016/j.bbrc.2009.08.152. [DOI] [PubMed] [Google Scholar]
- 6.Distefano M.T., On Behalf of the ClinGen Hearing Loss Clinical Domain Working Group. Hemphill S.E., Oza A.M., Siegert R.K., Grant A.R., Hughes M.Y., Cushman B.J., Azaiez H., Booth K.T., et al. ClinGen expert clinical validity curation of 164 hearing loss gene–disease pairs. Genet. Med. 2019;21:2239–2247. doi: 10.1038/s41436-019-0487-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wonkam A. Letter to the editor regarding “GJB2, GJB6 or GJA1 genes should not be investigated in routine in non syndromic deafness in people of sub-Saharan African descent”. Int. J. Pediatr. Otorhinolaryngol. 2015;79:632–633. doi: 10.1016/j.ijporl.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Wonkam A., Bosch J., Noubiap J.J.N., Lebeko K., Makubalo N., Dandara C. No evidence for clinical utility in investigating the connexin genes GJB2, GJB6 and GJA1 in non-syndromic hearing loss in black Africans. S. Afr. Med. J. 2015;105:23–26. doi: 10.7196/SAMJ.8814. [DOI] [PubMed] [Google Scholar]
- 9.Gazzaz B., Weil M., Raïs L., Akhyat O., Azeddoug H., Nadifi S. Autosomal recessive and sporadic deafness in Morocco: High frequency of the 35delG GJB2 mutation and absence of the 342-kb GJB6 variant. Hear. Res. 2005;210:80–84. doi: 10.1016/j.heares.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Ratbi I., Hajji S., Ouldim K., Aboussair N., Feldmann D., Sefiani A. The mutation 35delG of the gene of the connexin 26 is a frequent cause of autosomal-recessive non-syndromic hearing loss in Morocco. Arch. Pediatr. 2007;14:450–453. doi: 10.1016/j.arcped.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Gasmelseed N.M., Schmidt M., Magzoub M.M., Macharia M., Elmustafa O.M., Ototo B., Winkler E., Ruge G., Horstmann R.D., Meyer C.G. Low frequency of deafness-associatedGJB2 variants in Kenya and Sudan and novelGJB2 variants. Hum. Mutat. 2004;23:206–207. doi: 10.1002/humu.9216. [DOI] [PubMed] [Google Scholar]
- 12.Adadey S.M., Manyisa N., Mnika K., De Kock C., Nembaware V., Quaye O., Amedofu G.K., Awandare G.A., Wonkam A. GJB2 and GJB6 Mutations in Non-Syndromic Childhood Hearing Impairment in Ghana. Front. Genet. 2019;10:841. doi: 10.3389/fgene.2019.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brobby G.W., Horstmann R.D., Müller-Myhsok B. Connexin 26 R143W Mutation Associated with Recessive Nonsyndromic Sensorineural Deafness in Africa. N. Engl. J. Med. 1998;338:548–550. doi: 10.1056/NEJM199802193380813. [DOI] [PubMed] [Google Scholar]
- 14.Hamelmann C., Amedofu G.K., Albrecht K., Muntau B., Gelhaus A., Brobby G.W., Horstmann R.D. Pattern of connexin 26 (GJB2) mutations causing sensorineural hearing impairment in Ghana. Hum. Mutat. 2001;18:84–85. doi: 10.1002/humu.1156. [DOI] [PubMed] [Google Scholar]
- 15.Nyst V.A.S. A Descriptive Analysis of Adamorobe Sign Language (Ghana) LOT; Utrecht, The Netherlands: 2007. [Google Scholar]
- 16.Kusters A. Village Sign Languages: Anthropological and Linguistic Insights. Ishara Press & Mouton; UK: 2012. [(accessed on 22 January 2020)]. Being a deaf white anthropologist in Adamorobe: Some ethical and methodological issues; pp. 27–52. Available online: https://www.degruyter.com/downloadpdf/books/9781614511496/9781614511496.27/9781614511496.27.pdf. [Google Scholar]
- 17.de Freitas Cordeiro-Silva M., Barbosa A., Santiago M., Provetti M., Dettogni R.S., Tovar T.T., Rabbi-Bortolini E., Louro I.D. Mutation analysis of GJB2 and GJB6 genes in Southeastern Brazilians with hereditary nonsyndromic deafness. Mol. Biol. Rep. 2011;38:1309–1313. doi: 10.1007/s11033-010-0231-y. [DOI] [PubMed] [Google Scholar]
- 18.Sloan-Heggen C.M., Bierer A.O., Shearer A.E., Kolbe D.L., Nishimura C.J., Frees K.L., Ephraim S.S., Shibata S.B., Booth K.T., Campbell C.A., et al. Comprehensive genetic testing in the clinical evaluation of 1119 patients with hearing loss. Qual. Life Res. 2016;135:441–450. doi: 10.1007/s00439-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shearer A.E., DeLuca A.P., Hildebrand M.S., Taylor K.R., Gurrola J., Scherer S., Scheetz T.E., Smith R.J.H. Comprehensive genetic testing for hereditary hearing loss using massively parallel sequencing. Proc. Natl. Acad. Sci. USA. 2010;107:21104–21109. doi: 10.1073/pnas.1012989107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu X., Guo L., Ji H., Sun S., Chai R., Wang L., Li H. Genetic testing for sporadic hearing loss using targeted massively parallel sequencing identifies 10 novel mutations. Clin. Genet. 2015;87:588–593. doi: 10.1111/cge.12431. [DOI] [PubMed] [Google Scholar]
- 21.Tayoun A.N.A., Mason-Suares H., Frisella A.L., Bowser M., Duffy E., Mahanta L., Funke B., Rehm H.L., Amr S.S. Targeted droplet-digital PCR as a tool for novel deletion discovery at the DFNB1 locus. Hum. Mutat. 2016;37:119–126. doi: 10.1002/humu.22912. [DOI] [PubMed] [Google Scholar]
- 22.Schade G., Kothe C., Ruge G., Hess M., Meyer C.G. Non-invasive screening for GJB2 mutations in buccal smears for the diagnosis of inherited hearing impairment. Laryngo-rhino-Otol. 2003;82:397–401. doi: 10.1055/s-2003-40538. [DOI] [PubMed] [Google Scholar]
- 23.Schrauwen I., Sommen M., Corneveaux J.J., Reiman R.A., Hackett N.J., Claes C., Claes K., Bitner-Glindzicz M., Coucke P., Van Camp G. A sensitive and specific diagnostic test for hearing loss using a microdroplet PCR-based approach and next generation sequencing. Am. J. Med. Genet. Part A. 2013;161:145–152. doi: 10.1002/ajmg.a.35737. [DOI] [PubMed] [Google Scholar]
- 24.Antoniadi T., Pampanos A., Petersen M.B. Prenatal diagnosis of prelingual deafness: Carrier testing and prenatal diagnosis of the common GJB2 35delG mutation. Prenat. Diagn. 2001;21:10–13. doi: 10.1002/1097-0223(200101)21:1<10::AID-PD968>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Lucotte G., Bathelier C., Champenois T. PCR test for diagnosis of the common GJB2 (connexin 26) 35delG mutation on dried blood spots and determination of the carrier frequency in France. Mol. Cell. Probes. 2001;15:57–59. doi: 10.1006/mcpr.2000.0335. [DOI] [PubMed] [Google Scholar]
- 26.BIAP Classification Audiométrique Des Déficiences Auditives. [(accessed on 25 February 2019)]; Available online: http://www.biap.org/index.php?option=com_content&view=article&id=5%3Arecommandation-biap-021-bis&catid=65%3Act-2-classification-des-surdites&Itemid=19&lang=en.
- 27.Wonkam A., Noubiap J.J.N., Djomou F., Fieggen K., Njock R., Toure G.B. Aetiology of childhood hearing loss in Cameroon (sub-Saharan Africa) Eur. J. Med. Genet. 2013;56:20–25. doi: 10.1016/j.ejmg.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Bosch J., Noubiap J.J.N., Dandara C., Makubalo N., Wright G., Entfellner J.-B.D., Tiffin N., Wonkam A. Sequencing of GJB2 in Cameroonians and Black South Africans and comparison to 1000 Genomes Project Data Support Need to Revise Strategy for Discovery of Nonsyndromic Deafness Genes in Africans. OMICS. 2014;18:705–710. doi: 10.1089/omi.2014.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del Castillo I., Villamar M., Moreno-Pelayo M.A., Del Castillo F.J., Álvarez A., Tellería D., Menendez I., Moreno F. A Deletion Involving the Connexin 30 Gene in Nonsyndromic Hearing Impairment. N. Engl. J. Med. 2002;346:243–249. doi: 10.1056/NEJMoa012052. [DOI] [PubMed] [Google Scholar]
- 30.Baratloo A., Hosseini M., Negida A., El Ashal G. Part 1: Simple Definition and Calculation of Accuracy, Sensitivity and Specificity. Arch. Acad. Emerg. Med. 2015;3:48–49. [PMC free article] [PubMed] [Google Scholar]
- 31.Okonechnikov K.G.O., Fursov M. The_UGENE_Team Unipro UGENE: A Unified Bioinformatics Toolkit, Version 33. Bioinformatics. 2012;28:1166–1167. doi: 10.1093/bioinformatics/bts091. [DOI] [PubMed] [Google Scholar]
- 32.Gao X., Dai P. Impact of next-generation sequencing on molecular diagnosis of inherited non-syndromic hearing loss. J. Otol. 2014;9:122–125. doi: 10.1016/j.joto.2014.11.003. [DOI] [Google Scholar]
- 33.Lebeko K., Bosch J., Noubiap J.J.N., Dandara C., Wonkam A. Genetics of hearing loss in Africans: Use of next generation sequencing is the best way forward. Pan Afr. Med. J. 2015;20:383. doi: 10.11604/pamj.2015.20.383.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lebeko K., Sloan-Heggen C.M., Noubiap J.J.N., Dandara C., Kolbe D.L., Ephraim S.S., Booth K.T., Azaiez H., Santos-Cortez R.L.P., Leal S.M., et al. Targeted genomic enrichment and massively parallel sequencing identifies novel nonsyndromic hearing impairment pathogenic variants in Cameroonian families. Clin. Genet. 2016;90:288–290. doi: 10.1111/cge.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto Y.M., Elliott P.M., Arbustini E., Adler Y., Anastasakis A., Böhm M., Duboc D., Gimeno J., De Groote P., Imazio M., et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: A position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 2016;37:1850–1858. doi: 10.1093/eurheartj/ehv727. [DOI] [PubMed] [Google Scholar]
- 36.Calistri A., Palù G. Editorial Commentary: Unbiased Next-Generation Sequencing and New Pathogen Discovery: Undeniable Advantages and Still-Existing Drawbacks. Oxford University Press; Oxford, UK: 2015. [DOI] [PubMed] [Google Scholar]
- 37.Abe S., Nishio S.-Y., Yokota Y., Moteki H., Kumakawa K., Usami S.-I. Diagnostic pitfalls for GJB2-related hearing loss: A novel deletion detected by Array-CGH analysis in a Japanese patient with congenital profound hearing loss. Clin. Case Rep. 2018;6:2111–2116. doi: 10.1002/ccr3.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown K.K., Rehm H.L. Molecular Diagnosis of Hearing Loss. Curr. Protoc. Hum. Genet. 2012 doi: 10.1002/0471142905.hg0916s72. [DOI] [PubMed] [Google Scholar]
- 39.Yan D., Xiang G.X., Chai X.P., Qing J., Shang H.Q., Zou B., Mittal R., Shen J., Smith R.J.H., Fan Y.S., et al. Screening of deafness-causing DNA variants that are common in patients of European ancestry using a microarray-based approach. PLoS ONE. 2017;12:e0169219. doi: 10.1371/journal.pone.0169219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Šimundić A.-M. Measures of diagnostic accuracy: Basic definitions. Ejifcc. 2009;19:203–211. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.