Notes
Editorial note
This review has been superseded by Cochrane Reviews: 'Antithrombotics after infra‐inguinal bypass grafting' (https://doi.org/10.1002/14651858.CD015141) and 'Antithrombotics after infra‐inguinal peripheral endovascular treatment' (https://doi.org/10.1002/14651858.CD015142).
Abstract
Background
Peripheral arterial disease (PAD) may cause occlusions (blockages) in the main arteries of lower limbs. One treatment option is bypass surgery using autologous (the patient's own tissue) vein graft or prosthetic (artificial) graft. A number of factors influence occlusion rates in these patients, including the material used. To prevent graft occlusion patients are usually treated with antiplatelet, antithrombotic drugs, or a combination of both.
Objectives
To determine the effects of antiplatelet agents for the prevention of thrombosis in people with lower limb atherosclerosis who were undergoing femoropopliteal or femorodistal bypass grafting. Outcomes included the overall success of therapy (graft patency and limb salvage rates) and complications of treatment.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched June 2014) and the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 5). We sought additional trials through screening the reference lists of relevant papers.
Selection criteria
Two review authors, RB and AL, independently reviewed studies found in the search and evaluated them based on the inclusion and exclusion criteria, resolving disagreements through discussion.
Data collection and analysis
RB and AL independently extracted details of the selected studies for the update. We compared the treatment and control groups for important prognostic factors and differences described. If any data were unavailable, we sought further information from study authors. We synthesised data by comparing group results. We addressed unit of analysis issues by subgroup analysis.
Main results
We include 16 studies with 5683 randomised participants. Nine different treatment groups were evaluated: aspirin (ASA) or aspirin and dipyridamole (ASA/DIP) versus placebo or nothing (six studies); ASA or ASA/DIP versus pentoxifylline (two studies); ASA/DIP versus indobufen (one study); ASA or ASA/DIP versus vitamin K antagonists (two studies); ASA/DIP versus low molecular weight heparin (one study); ticlopidine versus placebo (one study); ASA versus prostaglandin E1 (one study); ASA versus naftidrofuryl (one study); and clopidogrel and ASA versus ASA alone (one study). The treatment comparisons were evaluated separately, and, where possible, we performed subgroup analysis for venous grafts and prosthetic grafts and at different follow‐up time points. The quality of evidence was low to moderate as many of the treatment comparisons had very few studies to contribute data, several of the included studies had unit of analysis issues, the treatment dosages varied between studies, and data for many outcomes important to this review were not given in any of the studies, or differed greatly between studies. Overall study quality was moderate, with the largest problem being that the majority of studies did not describe their methods of randomisation, allocation concealment or blinding of outcome assessors, leading to risk ratings of 'unclear'. The other main issue with study quality was studies not blinding participants or personnel.
The treatment comparison with the most number of included studies, which allowed for robust conclusions, was that of aspirin (ASA) or ASA and dipyridamole (ASA/DIP) versus placebo or nothing, covered by six studies. For this treatment group, there was improved graft patency in the ASA or ASA/DIP treatment group, odds ratio (OR) 0.42 (95% confidence interval (CI) 0.22 to 0.83; P = 0.01; 952 participants). This effect was not seen for venous grafts alone at any of the time points, but was observed for all time points in prosthetic grafts, including the final time point of 12 months (OR 0.19, 95% CI 0.10 to 0.36; P < 0.00001; 222 participants). Only a single study evaluated secondary patency, for which there was no difference between treatment groups. For the comparison ASA or ASA/DIP versus placebo or nothing there was no difference for any of the side effects, including general, gastrointestinal, bleeding and wound/graft infection. Amputations, cardiovascular events and mortality were also similar between the treatment groups. The comparison of ASA or ASA/DIP versus vitamin K antagonists included two studies, one of which was very large, with over 2000 participants. There were no differences between treatment for primary graft patency at three, six, 12 or 24 months, and there was also no evidence of a difference for limb amputation, cardiovascular events or mortality. One large study (851 participants) evaluated clopidogrel and ASA versus ASA alone, and for all grafts there was no evidence of a difference of primary patency at 24 months. There was evidence of increased total bleeding in the clopidogrel and ASA group (OR 2.65, 95% CI 1.69 to 4.15) from an increase in mild (OR 2.34, 95% CI 1.37 to 4.00), and moderate bleeding (OR 4.13, 95% CI 1.37 to 12.45), but no difference in severe or fatal bleeding. There was no difference between the treatment groups for limb amputation or mortality. For the remaining treatment comparisons there is not currently enough evidence to draw any robust conclusions about the efficacy or safety of the treatment on graft patency after peripheral bypass.
Authors' conclusions
Antiplatelet therapy with aspirin or with aspirin plus dipyridamole had a beneficial effect on primary patency of peripheral bypass grafts compared to placebo or no treatment. This effect was not evident when evaluating venous grafts alone, but antiplatelet therapy did have a beneficial effect on patency in those who had prosthetic grafts. There was no evidence of differences in side effects (including general, gastrointestinal, bleeding or infection), amputation, cardiovascular events or mortality between the treatment groups. However, the number of participants included in this analysis might be too small to detect a statistically significant effect for side effects, amputation, cardiovascular morbidity or mortality. We found no difference in primary graft patency when aspirin or aspirin with dipyridamole was compared to a vitamin K antagonist or when clopidogrel with aspirin was compared to aspirin alone. However, there was evidence of increase bleeding in the clopidogrel with aspirin group for the latter comparison. The remaining six treatment comparisons did not include enough data to draw any robust conclusions about their efficacy or safety at this time.
Plain language summary
Antiplatelet agents for preventing failure of peripheral arterial grafts
Background
Symptomatic peripheral arterial disease in people with atherosclerosis can present as intermittent claudication, disabling pain on walking, or as critical limb ischaemia with pain at rest, ulceration, gangrene and the risk of losing a leg. One treatment option is to implant a graft or makeshift blood vessel to bypass a blockage in the main artery of the thigh. Using a section of the vein from the patient's leg is often better than artificial or prosthetic materials such as Dacron or polytetrafluoroethylene, which take up platelets that could lead to clotting that could block the graft. Other factors affecting the patency of the graft (how long the bypass remains open) include length of the bypass, site where the graft connects to the existing artery and blood flow out of the graft. Stenosis (narrowing) of the graft most frequently occurs at the surgical connections because of hyperplasia, or an increase in the number of smooth muscle cells, into the inner layer of the vessel, often followed by the formation of a thrombosis (clot) at the stenotic site.
Study characteristics
We include 16 studies in this review with 5683 randomised participants. Nine different treatment groups were evaluated: Aspirin (ASA) or aspirin and dipyridamole (ASA/DIP) versus placebo or nothing (six studies); ASA or ASA/DIP versus pentoxifylline (two studies); ASA/DIP versus indobufen (one study); ASA or ASA/DIP versus vitamin K antagonists (two studies); ASA/DIP versus low molecular weight heparin (one study); ticlopidine versus placebo (one study); ASA versus prostaglandin E1 (one study); ASA versus naftidrofuryl (one study); and clopidogrel and ASA versus ASA alone (one study). We evaluated the different treatment comparisons separately, and, where possible, we evaluated separately those participants who received different types of grafts, venous or prosthetic.
Quality of the evidence
The quality of evidence from the review was low to moderate, as there were few studies to provide evidence for the different comparisons; several of the included studies randomised and analysed participants in a way that could introduce bias; and many of the prespecified outcomes of the review were not addressed within the studies, or were reported on in different ways between studies. Also, the treatment dosages varied between studies. Overall study quality was moderate, with the largest problem being that the majority of studies did not described their methods of randomisation or blinding of those that evaluated the outcomes. The other main issue with study quality was not blinding participants or personnel to the treatment received.
Key results
The comparison of ASA or ASA and dipyridamole (ASA/DIP) versus placebo or nothing, included the most studies (six), which allowed for robust analysis. For this treatment group, there was improved graft patency in the ASA or ASA/DIP treatment group. There was an improvement in those that received prosthetic grafts, but not in those that received venous grafts. Only a single study evaluated secondary patency, for which there was no difference between treatment groups. For this comparison there was no difference for any of the side effects, including general, gastrointestinal, bleeding and wound/graft infection. Amputations, cardiovascular events and death from any cause were also similar between the treatment groups. The comparison of ASA or ASA/DIP versus vitamin K antagonists included two studies, one of which was very large, with over 2000 participants. There were no differences between treatment for primary graft patency at three, six, 12 or 24 months, and there was also no evidence of a difference for limb amputation, cardiovascular events or mortality. One large study evaluated clopidogrel and ASA versus ASA alone, and for all grafts (including prosthetic and venous grafts) there was no evidence of a difference of primary patency at 24 months. There was evidence of increased total bleeding in the clopidogrel and ASA group, from an increase in mild and moderate bleeding, but there was no difference in severe or fatal bleeding. There was no difference between the treatment groups for limb amputation or death from any cause. For the remaining treatment comparisons there is not currently enough evidence to draw any robust conclusions about the efficacy or safety of the treatment on graft patency after peripheral bypass.
Background
Description of the condition
Symptomatic, chronic peripheral arterial disease (PAD) of the lower extremities may present either as intermittent claudication (IC), pain on walking, or as critical limb ischaemia (CLI), which is a more progressive stage with pain at rest, ulceration and gangrene (Becker 2011). The implantation of a femoropopliteal or femorodistal bypass graft (a makeshift blood vessel used to bypass a blockage in the main artery of the thigh) is one treatment option for people who are at risk of losing a limb or whose walking ability is greatly impaired because of the disease. By placing a graft in the groin area (an infrainguinal graft) the blocked arterial segment is bypassed, thereby improving blood flow in the limb. This then relieves the symptoms of claudication or rest pain and decreases the potential for amputation due to ulceration and gangrene, termed limb salvage.
The patency rate for femoropopliteal and femorodistal grafts, or the number of bypasses remaining open after a certain period of time, depends on several risk factors. These include whether or not the patient has acute lower limb ischaemia (Baril 2013), graft material, length of the bypass, site of the distal anastomosis (surgical connection of the graft to the existing artery), outflow conditions in the calf (flow of blood out of the graft) and female gender (Cooper 1990; Tangelder 2000). Autologous saphenous vein (using a vein from the patient's calf or thigh or both) is superior to prosthetic (artificial) materials such as Dacron or polytetrafluoroethylene (PTFE) (Rutherford 1988). Where the distal anastomosis is above the knee there is a lower risk of graft failure, and patients with two or three patent calf arteries have a better outcome than those with only one patent artery. Graft failure most frequently occurs at the site of either the distal or proximal anastomosis, when smooth muscle cells of the medial (middle) layer of the vessel wall grow into the intimal (inner) layer, which is known as intimal hyperplasia. This in turn causes the diameter of the perfused graft to become smaller, known as stenosis. When blood flow through the vessel is significantly reduced intermittent claudication may be experienced. Graft occlusion (closure) can occur by formation of a thrombosis (clot) at the stenotic site. If blood flow in the failed graft cannot be restored and further bypass surgery is not possible, then blood flow may be so poor that the limb cannot remain viable and amputation is required. Successful prevention of graft failure and thus the need for surgical re‐intervention is of major clinical and economic importance. Occlusion rates one year after the operation vary between 15% and 75% depending on the various risk factors described above (Abbott 1997; Consensus 1991). In addition, patients with lower limb atherosclerosis (progressive hardening of the arteries) frequently experience increased platelet aggregation, leading to clot formation. Moreover, the body's physiological stress response to surgery is a prothrombotic state (where the body is preparing to form a blood clot).
Description of the intervention
Antiplatelet treatment is used to prevent formation of blood clots by preventing platelet aggregation (NICE 2013). Antiplatelet agents work by improving blood rheology and also by suppressing the advancement of in‐ and outflow atherosclerosis (reducing the rate of development of stenosis) as well as coronary artery atherosclerosis, indirectly optimising cardiac function and circulation.
For patients receiving bypass surgery, the intensity of platelet uptake by the graft material has been shown to be inversely related to graft patency at one year, indicating the need for antiplatelet intervention. In animal experiments, antiplatelet drug administration started before bypass surgery has been shown to increase patency in prosthetic grafts when compared to no treatment (Fujitani 1988).
To prevent graft occlusion patients are usually treated with either an antiplatelet or an anticoagulant drug, or a combination of both. It is not known which regimen is best to prevent infrainguinal graft occlusion. The aim of this review was to evaluate whether antiplatelet treatment improves graft patency, limb salvage and survival in patients with chronic PAD who are undergoing infrainguinal bypass surgery.
Why it is important to do this review
Another Cochrane review was recently undertaken to also evaluate the prevention of thrombosis after infrainguinal arterial bypass, but this review focused on the use of antithrombotic therapies (Geraghty 2011). Overall their findings were inconclusive, with a possible benefit of antithrombotics for autologous vein grafts. Also, there may be higher economic costs and adverse haemorrhagic events associated with the use of anticoagulants compared with antiplatelet therapy. If the efficacy of antiplatelets is acceptable then it may be a more economical alternative to anticoagulant therapy following bypass graft surgery in the legs.
Objectives
To determine the effects of antiplatelet agents for the prevention of thrombosis in patients with lower limb atherosclerosis who were undergoing femoropopliteal or femorodistal bypass grafting. Outcomes include the overall success of therapy (graft patency and limb salvage rates) and complications of treatment.
Methods
Criteria for considering studies for this review
Types of studies
Trials in which participants were randomly allocated to receive either antiplatelet therapy versus placebo, one antiplatelet regimen versus another or antiplatelet therapy versus an alternative treatment. We include trials using alternation (allocation of treatment alternating between two interventions) and consider them as quasi‐randomised clinical trials (qRCTs).
Types of participants
All people undergoing femoropopliteal or femorodistal bypass grafting for the treatment of intermittent claudication and critical limb ischaemia. We excluded people undergoing bypass surgery for trauma.
Types of interventions
Antiplatelet therapy versus placebo, one antiplatelet regimen versus another, or antiplatelet therapy versus an alternative treatment. We excluded studies that included the same antiplatelet agent in both treatment groups, unless another antiplatelet was also used, but in only one treatment arm. We recorded the type of therapy, dosage, time of starting compared to surgery (pre‐ or postoperatively) and duration of the therapy.
Types of outcome measures
Primary outcomes
(1) Primary graft patency: patency rates after surgery with no further intervention, as determined by clinical examination, measurement of the ankle‐brachial pressure index (ABPI), duplex ultrasonography, angiography. (2) Assisted primary patency: patency rates after intervention to improve blood flow in a graft which has not occluded.
We analysed primary patency and primary assisted patency for all grafts and for venous or prosthetic (artificial) grafts separately.
Secondary outcomes
Secondary graft patency: patency rates following secondary intervention to restore blood flow to the graft
Objective assessment of lower limb blood flow: ABPI, exercise tolerance test
Side effects of treatment and complications
Limb salvage rate: survival rates with limb intact (or limb amputation)
Incidence of other cardiovascular events and mortality
Participants' quality of life
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched June 2014) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2014, Issue 5, part of The Cochrane Library (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library.
Searching other resources
We scanned reference lists of studies and reviews identified by the search for relevant studies.
Data collection and analysis
Selection of studies
In this update, Rachel Bedenis (RB) and Anne Lethaby (AL) independently selected trials for inclusion or exclusion, resolving disagreements by discussion between them.
Data extraction and management
For newly‐included studies RB and AL independently extracted the number of participants originally allocated to each treatment group and performed an intention‐to‐treat analysis. Data collection on each trial consisted of: inclusion and exclusion criteria; participant details (age, gender, co‐morbidity); severity of arterial occlusive disease (as determined by the ABPI and the European Consensus (Consensus 1991) definition of critical limb ischaemia); type of graft (autologous vein, prosthetic, human umbilical vein, composite graft); level of proximal graft anastomosis (common superficial femoral artery) and distal anastomosis (above‐knee popliteal, below‐knee popliteal, distal arteries); type of antiplatelet therapy used (dose, commencement of therapy relative to surgery, duration of therapy, compliance); and outcome (as mentioned in the section 'Criteria for considering studies for this review'). We compared the treatment and control groups for important prognostic factors. If any of the above data were not available, we sought further information from the authors.
Assessment of risk of bias in included studies
RB and AL independently assessed the methodological quality of included trials, using the 'Risk of bias' tool from The Cochrane Collaboration (Higgins 2011). We assessed the following five domains: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel and blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other potential sources of bias. We classified the domains as being at low risk of bias, high risk of bias or unclear risk of bias according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The two review authors assessing bias resolved disagreements by discussion.
Measures of treatment effect
We extracted dichotomous data and transformed them into odds ratios (ORs) with 95% confidence intervals (CIs) in the meta‐analysis.
Unit of analysis issues
The majority of the included studies use individual participants as the unit of analysis, although four studies were not consistent in this method (Clyne 1987; Donaldson 1985; Kohler 1984; Lucas 1984). Kohler 1984 reported on grafts rather than participants: 14 participants were re‐entered and re‐randomised after graft failure; participants with bilateral reconstructions were followed for patency of both grafts but were randomised only after the first reconstruction. There were 102 grafts in 88 participants in this study. In Donaldson 1985, the aspirin group recorded 35 grafts in 33 participants and the placebo group recorded 38 grafts in 32 participants. These additional grafts were due to bilateral grafts. In Clyne 1987, 148 grafts were undertaken in 140 participants. Lucas 1984 examined a mixture of single and multiple bypasses, using different materials, and also included thromboendarterectomy; there were considerable sources of heterogeneity within this study. The remaining papers appeared to report on one graft per individual.
For the meta‐analyses, we used the number of participants randomised where possible and not the number of grafts, in order to maintain consistency with the majority of included studies. We have included the four studies using grafts as the unit of analysis (Clyne 1987; Donaldson 1985; Kohler 1984; Lucas 1984) in the meta‐analysis, but we have excluded them from a subsequent sensitivity analysis, to assess the influence these studies may have on the overall results.
Dealing with missing data
Where data were missing, we contacted study authors for more information. Where possible, we performed analyses on an intention‐to‐treat basis.
Assessment of heterogeneity
To test for heterogeneity, we used the I² statistic (Higgins 2003). Where heterogeneity was high (I² > 50%), we used a random‐effects model for data synthesis.
Assessment of reporting biases
We obtained data from full papers, conference abstracts and unpublished sources, where found. If at least ten studies were included in a meta‐analysis, we constructed a funnel plot to look at issues around publication bias.
Data synthesis
Where possible, we calculated the number of events occurring within the sample for each of the outcomes. We generated ORs with 95% CIs to evaluate the effect of treatment, using a fixed‐effect model. Where heterogeneity was high (I² > 50%) we used a random‐effects model for data synthesis.
Subgroup analysis and investigation of heterogeneity
We subgrouped data for graft patency at follow‐up (that is, at 1, 3, 6, 9, 12 and 24 months, where data were available) and for whether the graft was venous or prosthetic.
Sensitivity analysis
We performed sensitivity analysis to evaluate the effect of studies with unit of analysis concerns. Please see Unit of analysis issues for more detail.
Results
Description of studies
Results of the search
See Figure 1 for study flow diagram.
1.
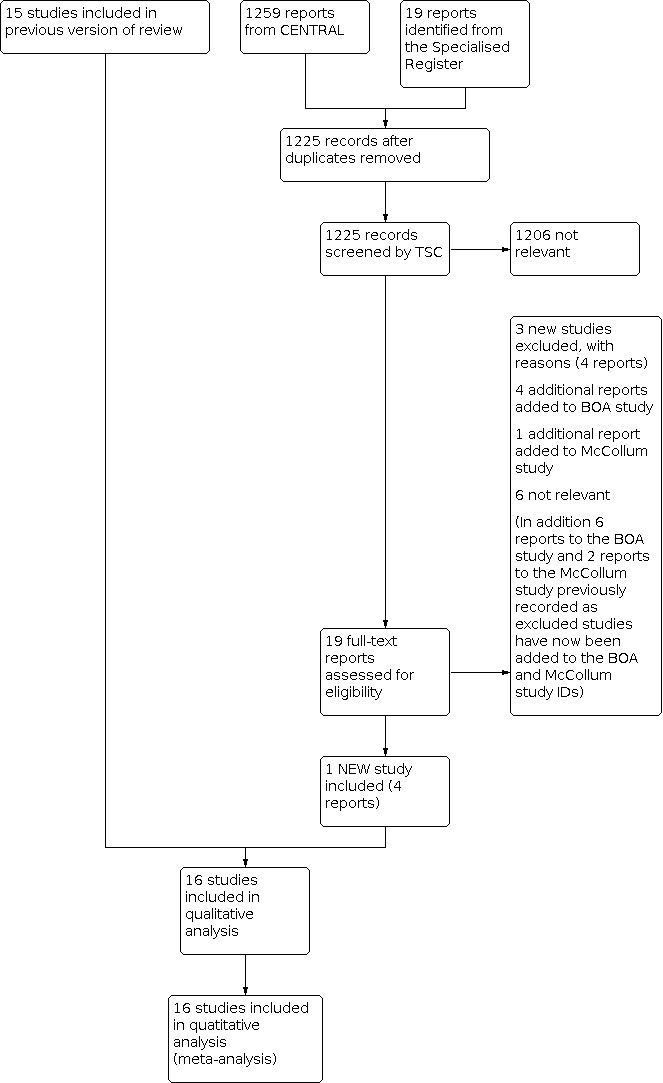
Study flow diagram.
For the current update of the review, we considered 19 reports of 12 studies retrieved by the search. We included one additional study with four reports (CASPAR 2010). We deemed six studies with six citations not to be relevant, and we added another three studies with four reports to the excluded studies (Burdess 2010; EUCTR2007 2008; Monaco 2012). We added five new reports of two studies to previously included studies (BOA 2000; McCollum 1991) and eight previously excluded reports to two included studies (BOA 2000; McCollum 1991).
Included studies
For this update there was one additional study included (CASPAR 2010) making a total of 16 studies (Becquemin 1997; BOA 2000; CASPAR 2010; Clyne 1987; D'Addato 1992; Donaldson 1985; Edmondson 1994; Goldman 1984; Green 1982; Gruss 1991; Kohler 1984; Lucas 1984; McCollum 1991; Noppeney 1988; Raithel 1987; Schneider 1979) which were included in this analysis. See Characteristics of included studies for full details.
We conducted comparisons of the following interventions:
Aspirin (ASA) or aspirin and dipyridamole (ASA/DIP) versus placebo or nothing (Clyne 1987; Donaldson 1985; Goldman 1984; Green 1982; Kohler 1984; McCollum 1991)
ASA or ASA/DIP versus pentoxifylline (PTX) (Lucas 1984; Raithel 1987)
ASA/DIP versus indobufen (IND), a reversible cyclo‐oxygenase inhibitor (D'Addato 1992)
ASA or ASA/DIP versus vitamin K antagonists (VKA) (BOA 2000; Schneider 1979)
ASA/DIP versus low molecular weight heparin (LMWH) (Edmondson 1994)
Ticlopidine (TIC) versus placebo (Becquemin 1997)
ASA versus prostaglandin E1 (Gruss 1991)
ASA versus naftidrofuryl (Noppeney 1988)
Clopidogrel and ASA versus ASA alone (CASPAR 2010)
Details of the study designs are shown in the table 'Characteristics of included studies' and in Table 1.
1. Patient characteristics.
| Study ID | % CLI | % below knee | % vein | % 1‐vessel run‐off | dose | preoperative start of treatment | treatment duration | randomisation | ABI pre‐op to postop |
| Becquemin 1997 | > 70 | nd | 100 | nd | TIC 250 mg vs placebo | between the 3rd and 14th post‐op day | 24 months | post‐op | nd |
| BOA 2000 | 50 | 52 | 58.5 | nd | ASA 80 mg vs coumarin INR 3.0 ‐ 4.5 reached in 50% | within 5 days until 1 month post‐op | 21 months | before surgery | nd |
| CASPAR 2010 | 66 | 100 | 70 | nd | ASA 75 ‐ 100 mg plus clopidogrel 75 mg vs ASA 75 ‐ 100 mg plus placebo | 2 to 4 days post‐op | 24 months | post‐op | nd |
| Clyne 1987 | 70 | 85 | 63 | 24 | ASA 2 x 300 mg, DIP 2 x 200 mg | 48 hours pre‐op | 6 weeks | before surgery | 0.38 to 0.78 |
| D'Addato 1992 | 70 | 25 | 0 | 56 | ASA 900 mg plus DIP 225 mg vs 400 mg IND | 48 h | 12 months | before surgery | nd |
| Donaldson 1985 | 0 | 32 | 0 | 37 | ASA 3 x 330 mg plus DIP 75 mg | Evening prior to surgery | 12 months | before surgery | 0.62 to nd |
| Edmondson 1994 | 46 | 31 | 27 | 20 | 3 x 300 mg ASA plus 3 x 100 mg DIP versus 2500 IU LMWH | LMWH 2 hours pre‐op; ASA/DIP 1 week post‐op | 3 months | post‐op | nd |
| Goldman 1984 | 80 | 47 | 0 | nd | ASA 3 x 300 mg plus DIP 3 x 75 mg | 2 days ASA/DIP | 12 months | before surgery | nd |
| Green 1982 | 82 | 47 | 0 | nd | ASA 3 x 325 mg, or ASA 3 x 325 mg plus DIP 3 x 75 mg | 2 days ASA/DIP | 12 months | before surgery | 0.42 to 1.01 |
| Gruss 1991 | nd | nd | 100 | nd | 3 x 0.5 g ASA plus 15,000 IU heparin versus PGE1, 0.2 ng per kg body weight/min plus 15,000 IU heparin | post‐op | 10 days | nd | nd |
| Kohler 1984 | 76 | 52 | 69 | nd | ASA 3 x 325 mg plus DIP 3 x 75 mg | first post‐op day | 24 months | before surgery | nd |
| Lucas 1984 | 55 | nd | 27 | nd | ASA 1050 mg, DIP 150 mg or 1200 mg PTX | pre‐op | 6 months | before surgery | 0.4 |
| McCollum 1991 | 60 | 59 | 100 | nd; vein < 4 mm:38 | ASA 2 x 300 mg plus DIP 2 x 150 mg | 2 days ASA/DIP | continuing indefinitely | before surgery | 0.47 to nd |
| Noppeney 1988 | nd | nd | nd | nd | ASA 1500 mg vs naftidrofuryl 600 mg daily orally | day 1 post‐op | 12 months | before surgery | nd |
| Raithel 1987 | 95 | nd | 0 | 56 | ASA 1500 mg vs PTX 1200 mg | 48 h | 12 months | before surgery | nd |
| Schneider 1979 | nd | nd | 100 | nd | ASA 1000 mg, or ASA 1000 mg plus DIP 225 mg vs coumarin | post‐op | 24 months | post‐op | nd |
ASA: Aspirin CLI: critical limb ischaemia DIP: dipyridamole h: hour IU: international unit LMWH: low molecular weight heparin mg: milligram nd: no data ng: nanogram PGE1: prostaglandin E1 PTX: pentoxifylline TIC: ticlopidine vs: versus
Aspirin (ASA) or aspirin and dipyridamole (ASA/DIP) versus placebo or nothing
In all six trials study medication was started prior to bypass surgery, mostly 48 hours before the operation (Clyne 1987; Donaldson 1985; Goldman 1984; Green 1982; Kohler 1984; McCollum 1991). Doses ranged from 300 mg to 325 mg ASA and 75 mg DIP, given two and three times daily. Participants were randomised before surgery. Duration of treatment was six weeks in the Clyne 1987 trial but at least 12 to 24 months in the other trials. The percentage of participants with critical leg ischaemia (CLI) ranged from 60% to 80%, with the exception of Donaldson 1985 which only included participants with disabling intermittent claudication.
Aspirin (ASA) or aspirin and dipyridamole (ASA/DIP) versus pentoxifylline (PTX)
Two studies were included, comparing 1000 mg or 1500 mg ASA to 1200 mg PTX daily (Lucas 1984; Raithel 1987). Participant characteristics and graft type were similar in both trials. While Raithel 1987's participants all received prosthetic grafts, Lucas 1984 also included participants with venous grafts. More than 90% of Raithel 1987's participants had CLI compared to only 55% in the Lucas 1984 trial.
Aspirin and dipyridamole (ASA/DIP) versus indobufen (IND)
We found only one multicentre double‐blind randomised clinical trial including participants operated on for a prosthetic polytetrafluoroethylene (PTFE) femoropopliteal graft for inclusion (D'Addato 1992).
Aspirin and dipyridamole (ASA/DIP) versus vitamin K antagonists (VKA)
We include two trials in this category (BOA 2000; Schneider 1979), comparing ASA or ASA and DIP with VKA. The Dutch BOA 2000 trial included a much higher number of participants than the Schneider 1979 trial; prosthetic grafts were included in the BOA 2000 trial. It should be noted that participants receiving ASA in the BOA 2000 trial were treated with a dose of only 80 mg, while all other trials included in this review compared doses of 600 mg to 1500 mg daily, sometimes with additional DIP.
Aspirin and dipyridamole (ASA/DIP) versus low molecular weight heparin (LMWH)
We include one open, randomised clinical trial for comparison of LMWH (Fragmin) and ASA and DIP (Edmondson 1994). Participants were assessed for their clinical outcomes on the seventh postoperative day.
Ticlopidine (TIC) versus nothing
Only one double‐blind trial (Becquemin 1997) could be included for analysis, which randomised 243 participants after surgery. All participants received autologous saphenous vein grafts.
Aspirin versus prostaglandin E1 (PGE1)
One open, randomised clinical trial compared ASA to PGE1 on a background of 15,000 IU of heparin for early graft occlusion, within three days postoperatively, in 100 participants who all received autologous saphenous vein grafts (Gruss 1991).
Aspirin versus naftidrofuryl
We could consider only one trial in the analysis that compared ASA to naftidrofuryl (Noppeney 1988). This study, which commenced on postoperative day one, randomised 99 participants who all had prosthetic PTFE grafts.
Clopidogrel and ASA (Clopidogrel/ASA) versus ASA alone
A single trial evaluated the comparison between clopidogrel with ASA versus ASA alone (CASPAR 2010), which was a large trial of 851 randomised, receiving a mixture of venous and prosthetic grafts.
Excluded studies
For this update we excluded an additional three studies (Burdess 2010; EUCTR2007 2008; Monaco 2012), making a total of 20 excluded studies (Böhmig 1995; Burdess 2010; DeWeese 1971; Ehersmann 1977; EUCTR2007 2008; Gorter 2001; Harjola 1981; Jivegard 2005; Johnson 2002; Johnson 2004; Kibbe 2002; Lassila 1991; Monaco 2012; Nevelsteen 1991; Raithel 1987b; Reichle 1979; Rosenthal 1987; Satiani 1985; Shionoya 1990; Smout 2004).The reasons for exclusion varied and include, among others: lack of a control group, not a randomised controlled trial, a heterogeneous cohort of participants, intervention not within the scope of the review, and too little information to determine inclusion.
See Characteristics of excluded studies for full descriptions of excluded studies.
Risk of bias in included studies
See Figure 2and Figure 3 for 'Risk of bias' evaluation.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation methods were adequately described in six studies (Becquemin 1997; BOA 2000; CASPAR 2010; Edmondson 1994; Kohler 1984; Lucas 1984). The remaining 10 studies did not sufficiently describe how they generated their random sequence, leading to a rating of 'unclear' (Clyne 1987; D'Addato 1992; Donaldson 1985; Goldman 1984; Green 1982; Gruss 1991; McCollum 1991; Noppeney 1988; Raithel 1987; Schneider 1979).
Six studies described allocation concealment methods that were low‐risk (BOA 2000; CASPAR 2010; Edmondson 1994; Green 1982; Lucas 1984; McCollum 1991), while the remaining 10 studies did not describe their methods adequately (Becquemin 1997; Clyne 1987; D'Addato 1992; Donaldson 1985; Goldman 1984; Gruss 1991; Kohler 1984; Noppeney 1988; Raithel 1987; Schneider 1979).
Blinding
Blinding of participants and personnel was adequate in seven studies (Becquemin 1997; CASPAR 2010; Donaldson 1985; Goldman 1984; Green 1982; Kohler 1984; McCollum 1991). Six studies were unblinded, leading to a high risk of performance bias (BOA 2000; Clyne 1987; Edmondson 1994; Gruss 1991; Noppeney 1988; Raithel 1987). The remaining three studies mentioned blinding but did not adequately describe their methods (D'Addato 1992; Lucas 1984; Schneider 1979), and are rated at unclear risk.
Blinding of outcome assessors was only described in two studies (Becquemin 1997; CASPAR 2010), while the remaining were at unclear risk of detection bias, with no description of assessor blinding.
Incomplete outcome data
The majority of studies had no issues with attrition bias, but five studies were at unclear risk, with Donaldson 1985 having a higher rate of drop‐outs in the intervention arm, Gruss 1991 not reporting drop‐outs or withdrawals, Kohler 1984 inconsistently reporting drop‐outs between treatment groups, Raithel 1987 only reporting participants available for follow‐up but not the number initially randomised, and Schneider 1979 not having enough information for us to adequately assess whether attrition bias was a problem.
Selective reporting
Selective reporting was a concern in three studies, which we evaluated as 'unclear' (Green 1982; Gruss 1991; Schneider 1979). For Green 1982 adverse events were not clearly reported, and for both Gruss 1991 and Schneider 1979 there was not enough information in the text to determine the risk of reporting bias. All remaining studies were at low risk of reporting bias.
Other potential sources of bias
Other bias was a concern in five studies (Clyne 1987; Donaldson 1985; Goldman 1984; McCollum 1991; Raithel 1987), with the rest at low risk of other bias. For Clyne 1987 and Donaldson 1985 all the data were presented by number of bypasses and not by the number of participants. Goldman 1984, McCollum 1991, and Raithel 1987 had differences in the treatment groups at baseline, and McCollum 1991 may be at risk of being underpowered, as the study power calculation required a study population of 800 participants to detect a 10% reduction in occlusion rate, but only 549 participants were randomised.
Effects of interventions
For the outcome 'primary patency' the numbers recorded for analyses were actually the number of grafts that occluded during the specified time period as reported by the trialists. We used these numbers due to the properties of odds ratios (ORs), and we found this method more straightforward to interpret. Both patency and occlusion are used to describe the treatment outcomes within the included studies and within this review, which should be kept in mind when interpreting the presented data.
Analysis for secondary graft patency, side effects of treatment and complications, limb amputation, cardiovascular events and mortality are only described when sufficient data were available. Otherwise, these outcomes are not mentioned.
None of the included studies reported on assisted primary patency, objective assessment of lower limb blood flow or participant quality of life.
None of the meta‐analyses included 10 or more studies, so we could not construct funnel plots to evaluate possible publication bias.
Aspirin (ASA) or aspirin and dipyridamole (ASA/DIP) versus nothing or placebo
Primary graft patency
The effect of ASA or ASA/DIP on infrainguinal bypass patency was assessed in six trials (Clyne 1987; Donaldson 1985; Goldman 1984; Green 1982; Kohler 1984; McCollum 1991). The OR from the random‐effects model for primary occlusion at 12 months for all grafts was 0.42 (95% confidence interval (CI) 0.22 to 0.83; P = 0.01, I² = 72%, participants = 952), showing a positive effect of ASA on infrainguinal grafts at one year (Analysis 1.1). Three of these studies had unit of analysis issues, with multiple grafts or re‐randomisation of participants (Clyne 1987; Donaldson 1985; Kohler 1984). The association becomes attenuated when these studies are excluded (OR 0.36, 95% CI 0.13 to 0.99; P = 0.05, participants = 651).
1.1. Analysis.

Comparison 1: ASA or ASA/DIP vs placebo or nothing, all grafts, Outcome 1: Primary graft patency at 12 months
When we performed the analysis for venous grafts alone, there was no difference in primary graft patency between the treatment groups at one, three and six months (OR 0.76, 95% CI 0.26 to 2.25, P = 0.62; OR 0.85, 95% CI 0.54 to 1.35, P = 0.50; and OR 0.88, 95% CI 0.59 to 1.31; P = 0.53, respectively, participants = 642 for all) (all fixed‐effect analyses) (Analysis 2.1; Analysis 2.2; Analysis 2.3). At 12 months post‐operation there was a possible increase in occlusion in the control group (OR 0.69, 95% CI 0.48 to 0.99; P = 0.05, participants = 642) (fixed‐effect), but this was not seen at 24 months in the random‐effects model analysis, (OR 1.03; 95% CI 0.32 to 3.28; P = 0.96, I² = 79%, participants = 620) (Analysis 2.4; Analysis 2.5).
2.1. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 1: Primary graft patency, venous grafts, 1 month
2.2. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 2: Primary graft patency, venous grafts, 3 months
2.3. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 3: Primary graft patency, venous grafts, 6 months
2.4. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 4: Primary graft patency, venous grafts, 12 months
2.5. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 5: Primary graft patency, venous grafts, 24 months
Analysis for prosthetic grafts, however, showed a much stronger positive effect of ASA on primary patency as calculated from four studies (Clyne 1987; Donaldson 1985; Goldman 1984; Green 1982): ORs of 0.14 (95% CI 0.04 to 0.51; P = 0.003, participants = 157) at one month; 0.31 (95% CI 0.14 to 0.66; P = 0.003, participants = 222) at three months; 0.21 (95% CI 0.11 to 0.41; P < 0.00001, participants = 222) at six months; and 0.19 (95% CI 0.10 to 0.36, participants = 222) at 12 months (Analysis 2.6; Analysis 2.7; Analysis 2.8; Analysis 2.10). Primary patency at nine months was only evaluated in a single study, with an OR of 0.17 (95% CI 0.04 to 0.67, participants = 65) (Analysis 2.9).
2.6. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 6: Primary graft patency, prosthetic grafts, 1 month
2.7. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 7: Primary graft patency, prosthetic grafts, 3 months
2.8. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 8: Primary graft patency, prosthetic grafts, 6 months
2.10. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 10: Primary graft patency, prosthetic grafts, 12 months
2.9. Analysis.

Comparison 2: ASA or ASA/DIP vs placebo or nothing, subgroups, Outcome 9: Primary graft patency, prosthetic grafts, 9 months
Removal of the trials associated with unit of analysis issues meant that we could not conduct data analysis for venous grafts at one, three, six,12, and 24 months as only one study remained. For prosthetic grafts, the OR was 0.25 (95% CI 0.05 to 1.30; P = 0.10, participants = 102) at one month; 0.30 (95% CI 0.09 to 1.01; P = 0.05, participants = 102) at three months; 0.31 (95% CI 0.13 to 0.74; P = 0.008, participants = 102) at six months; and 0.23 (95% CI 0.10 to 0.54; P = 0.0008, participants = 102) at 12 months, all fixed‐effect models. For all comparisons the overall effect was reduced, and for months one and three the association no longer favoured ASA or ASA/DIP.
Secondary graft patency
McCollum 1991 reported on secondary patency of the failed grafts, but the time frame of the reported cases is unclear. The study reported that of the 172 failed grafts (86 in each treatment group), eight in the ASA/DIP group and four in the placebo group were restored by re‐operation but it is unclear if all failed grafts were re‐operated. McCollum 1991 also reported that the cumulative secondary patency rate for the ASA/DIP group was 80% at one year, 73% at two years and 63% at three years, and 74%, 64% and 61% respectively for the placebo group. Because the number of participants that were included to derive these values was unclear, we cannot use them in meta‐analysis.
Side effects of treatment and complications
Side effects were reported in five studies (Clyne 1987; Donaldson 1985; Green 1982; Kohler 1984; McCollum 1991) and the fixed‐effect model found an OR of 1.55 (95% CI 1.00 to 2.41; P = 0.05, participants = 913) for general side effects (Analysis 1.2). However, when Clyne 1987, Donaldson 1985 and Kohler 1984 were removed due to unit of analysis issues the association was attenuated with an OR of 1.34 (95% CI 0.83 to 2.16; P = 0.23, participants = 598). Six studies evaluated side effects specific to the gastrointestinal tract (Clyne 1987; Donaldson 1985; Goldman 1984; Green 1982; Kohler 1984; McCollum 1991), and found no differences between the two treatment groups (OR 1.44, 95% CI 0.92 to 2.24; P = 0.11, participants = 952) in the fixed‐effect model. When Clyne 1987Donaldson 1985 and Kohler 1984 were removed due to unit of analysis issues there was very little change in the outcome (OR 1.38, 95% CI 0.87 to 2.21; P = 0.17, participants = 651).
1.2. Analysis.

Comparison 1: ASA or ASA/DIP vs placebo or nothing, all grafts, Outcome 2: Side effects and complications
Major bleeding was similar between the treatment groups for two trials (Green 1982; McCollum 1991) (OR 1.88, 95% CI 0.85 to 4.16; P = 0.12, participants = 598) in the fixed‐effect model. Minor bleeding was only evaluated in one study (Clyne 1987), with an OR of 0.73 (95% CI 0.31 to 1.70, participants = 148). Wound or graft infection was only evaluated in McCollum 1991, with similar events in both treatment groups (OR 1.07, 95% CI 0.67 to 1.71, participants = 549).
Limb amputation
Clyne 1987 reported amputations with an OR of 0.55 (95% CI 0.21 to 1.44, grafts = 148), but it should be noted this was one of the studies with multiple grafts in some of the participants (148 grafts in 140 participants) (Analysis 1.3).
1.3. Analysis.

Comparison 1: ASA or ASA/DIP vs placebo or nothing, all grafts, Outcome 3: Limb amputation
Cardiovascular events and mortality
Analysis of cardiovascular events was performed in four trials (Clyne 1987; Donaldson 1985; Green 1982; McCollum 1991) and the random‐effects model found an OR of 1.27 (95% CI 0.43 to 3.80; P = 0.66, I² = 52%, participants = 811) (Analysis 1.4). When Clyne 1987 and Donaldson 1985 were removed for unit of analysis issues, the association was statistically significant, in favour of ASA or ASA/DIP (OR 0.58, 95% CI 0.37 to 0.91; P = 0.02, participants = 598), although it should be noted that McCollum 1991 then comprises 96% of the analysis.
1.4. Analysis.

Comparison 1: ASA or ASA/DIP vs placebo or nothing, all grafts, Outcome 4: Cardiovascular events
The OR for postoperative death for the fixed‐effect model, as evaluated in four studies (Clyne 1987; Goldman 1984; Green 1982; McCollum 1991) was 0.84 (95% CI 0.56 to 1.26; P = 0.41, participants = 799) (Analysis 1.5). When Clyne 1987 was removed for sensitivity analysis due to unit of analysis issues the resulting OR was 0.77 (95% CI 0.49 to 1.21; P = 0.26, participants = 651). Again McCollum 1991 accounted for a large majority of the weight.
1.5. Analysis.

Comparison 1: ASA or ASA/DIP vs placebo or nothing, all grafts, Outcome 5: Mortality
Aspirin (ASA) or aspirin and dipyridamole (ASA/DIP) versus pentoxifylline (PTX)
Primary graft patency
The effect of PTX on graft patency when compared to ASA or ASA/DIP treatment could only be evaluated in a formal analysis from two RCTs (Lucas 1984; Raithel 1987) at six months post‐operation. The fixed‐effect model had an OR of 1.32 (95% CI 0.56 to 3.11; P = 0.52, participants = 151) showing no difference between the treatment groups (Analysis 3.3). This analysis should be considered with caution as Lucas 1984 involved multiple grafts and there appeared to be substantial heterogeneity among the sample population. At other time points, only Raithel 1987 provided raw data and showed a similar effect of both drugs on graft patency: OR 2.04 (95% CI 0.18 to 23.07, participants = 118) at one month; OR 1.00 (95% CI 0.27 to 3.65, participants = 118) at three months; and OR 0.91 (95% CI 0.38 to 2.15, participants = 118) at 12 months (Analysis 3.1; Analysis 3.2; Analysis 3.4).
3.3. Analysis.

Comparison 3: ASA or ASA/DIP versus pentoxifylline (PTX), all grafts, Outcome 3: Primary graft patency, 6 months
3.1. Analysis.

Comparison 3: ASA or ASA/DIP versus pentoxifylline (PTX), all grafts, Outcome 1: Primary graft patency, 1 month
3.2. Analysis.

Comparison 3: ASA or ASA/DIP versus pentoxifylline (PTX), all grafts, Outcome 2: Primary graft patency, 3 months
3.4. Analysis.

Comparison 3: ASA or ASA/DIP versus pentoxifylline (PTX), all grafts, Outcome 4: Primary graft patency, 12 months
Side effects of treatment and complications
Raithel 1987 reported on side effects that included gastric intolerance, gastric haemorrhage and vertigo, with ORs of 18.04 (95% CI 5.07 to 64.17), 3.11 (95% CI 0.31 to 30.77) and 0.48 (95% CI 0.08 to 2.74) respectively, all with 118 participants (Analysis 3.5). It should be noted that side effects were extrapolated from graphs and therefore may not be completely accurate.
3.5. Analysis.

Comparison 3: ASA or ASA/DIP versus pentoxifylline (PTX), all grafts, Outcome 5: Side effects
Limb amputation
Amputation was also only evaluated in the Raithel 1987 study, with an OR of 0.38 (95% CI 0.07 to 2.04, participants = 118) (Analysis 3.6).
3.6. Analysis.

Comparison 3: ASA or ASA/DIP versus pentoxifylline (PTX), all grafts, Outcome 6: Limb amputation
Cardiovascular events and mortality
Reported only in Raithel 1987, there were six deaths in the ASA group and three in the PTX group, with an OR of 2.11 (95% CI 0.50 to 8.88, participants = 118) (Analysis 3.7). The authors reported that eight of the deaths were from myocardial infarction and one from cardiac failure, but they did not detail which treatment groups they came from.
3.7. Analysis.

Comparison 3: ASA or ASA/DIP versus pentoxifylline (PTX), all grafts, Outcome 7: Mortality
Aspirin and dipyridamole (ASA/DIP) versus indobufen (IND)
Primary graft patency
The one eligible RCT (D'Addato 1992) compared ASA/DIP to IND in receiving infrainguinal prosthetic PTFE grafts. The study had an OR of 1.67 (95% CI 0.51 to 5.44, participants = 113) for primary patency at three months, OR 1.60 (95% CI 0.60 to 4.27, participants = 113) at six months, OR 1.26 (95% CI 0.56 to 2.85, participants = 113) at nine months and OR 1.34 (95% CI 0.61 to 2.93, participants = 113) at 12 months (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4).
4.1. Analysis.

Comparison 4: ASA/DIP versus indobufen, prosthetic grafts, Outcome 1: Primary graft patency, 3 months
4.2. Analysis.

Comparison 4: ASA/DIP versus indobufen, prosthetic grafts, Outcome 2: Primary graft patency, 6 months
4.3. Analysis.

Comparison 4: ASA/DIP versus indobufen, prosthetic grafts, Outcome 3: Primary graft patency, 9 months
4.4. Analysis.

Comparison 4: ASA/DIP versus indobufen, prosthetic grafts, Outcome 4: Primary graft patency, 12 months
Aspirin and dipyridamole (ASA/DIP) versus vitamin K antagonists (VKA)
Primary graft patency
This was reported in two trials (BOA 2000; Schneider 1979). Primary graft patency for all grafts showed no difference for coumarin versus aspirin, irrespective of time point: OR 0.88 (95% CI 0.68 to 1.14; P = 0.34, I² = 0%) at three months, OR 0.72 (95% CI 0.27 to 1.96; P = 0.52, I² = 61%) at six months, OR 0.68 (95% CI 0.27 to 1.69; P = 0.40, I² = 67%) at 12 months; and OR 0.64 (95% CI 0.25 to 1.63; P = 0.36, I² = 74%) at 24 months, with 2781 participants included in each outcome (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4). We used a fixed‐effect model at three months, and random‐effects models for months six, 12 and 24, due to high heterogeneity.
5.1. Analysis.

Comparison 5: ASA or ASA/DIP versus vitamin K antagonists, all grafts, Outcome 1: Primary graft patency, 3 months
5.2. Analysis.

Comparison 5: ASA or ASA/DIP versus vitamin K antagonists, all grafts, Outcome 2: Primary graft patency, 6 months
5.3. Analysis.

Comparison 5: ASA or ASA/DIP versus vitamin K antagonists, all grafts, Outcome 3: Primary graft patency, 12 months
5.4. Analysis.

Comparison 5: ASA or ASA/DIP versus vitamin K antagonists, all grafts, Outcome 4: Primary graft patency, 24 months
Side effects of treatment and complications
In the BOA 2000 trial, haemorrhage necessitating hospital admission was reported for 119 (9%) participants in the coumarin group and 59 (4.5%) in the aspirin group. A total of 16 (1.2%) participants died from fatal bleeding in the coumarin group and 12 (0.9%) participants in the aspirin group. In Schneider 1979 the adverse effects reported were: two participants (0.6%) who stopped coumarin treatment because of bleeding complications and 13 participants (21%) who stopped aspirin for differing reasons.
Limb amputation
The two trials (BOA 2000; Schneider 1979), did not report data on limb salvage or survival that was suitable for formal meta‐analysis. However, in BOA 2000 limb amputation had to be performed in a similar number of participants in each treatment group, with an OR of 0.99 (95% CI 0.75 to 1.30, participants = 2690) (Analysis 5.5).
5.5. Analysis.

Comparison 5: ASA or ASA/DIP versus vitamin K antagonists, all grafts, Outcome 5: Limb amputation
Cardiovascular events and mortality
Only BOA 2000 reported on cardiovascular events and mortality for this treatment comparison. Myocardial infarction and stroke were similar for both treatment groups, with slightly higher occurrences in the ASA/DIP group: OR 1.45 (95% CI 0.90 to 2.34) and OR 1.34 (95% CI 0.86 to 2.09) respectively; participants = 2690 (Analysis 5.6). Death from all causes, after two years, was similar in both treatment groups, with an OR of 1.02 (95% CI 0.83 to 1.26, participants = 2690) (Analysis 5.7).
5.6. Analysis.

Comparison 5: ASA or ASA/DIP versus vitamin K antagonists, all grafts, Outcome 6: Cardiovascular events
5.7. Analysis.

Comparison 5: ASA or ASA/DIP versus vitamin K antagonists, all grafts, Outcome 7: Mortality
Aspirin and dipyridamole (ASA/DIP) versus low molecular weight heparin (LMWH)
Primary graft patency
In Edmondson 1994 primary patency was measured at six and 12 months with little difference between the treatment groups: OR 1.69 (95% CI 0.78 to 3.65, participants = 200), and OR 1.19 (95% CI 0.66 to 2.15, participants = 200), respectively (Analysis 6.1; Analysis 6.2).
6.1. Analysis.

Comparison 6: ASA/DIP versus LMWH, all grafts, Outcome 1: Primary graft patency, 6 months
6.2. Analysis.

Comparison 6: ASA/DIP versus LMWH, all grafts, Outcome 2: Primary graft patency, 12 months
Side effects of treatment and complications
No major bleedings or adverse events occurred.
Cardiovascular events and mortality
There were more deaths in the LMWH treatment group compared to the ASA/DIP group: OR 0.18 (95% CI 0.04 to 0.86, participants = 200) (Analysis 6.3).
6.3. Analysis.

Comparison 6: ASA/DIP versus LMWH, all grafts, Outcome 3: Mortality
Ticlopidine (TIC) versus nothing
Primary graft patency
Intention‐to‐treat analysis of one trial (Becquemin 1997) involving participants undergoing bypass with venous grafts showed no difference in primary patency at one month between the treatment groups: OR 3.00 (95% CI 0.12 to 74.37, participants = 243), with a wide confidence interval as only one event occurred by this time point (Analysis 7.1). However, primary patency at six, 12 and 24 months showed increased patency in the TIC treatment group: OR 0.26 (95% CI 0.11 to 0.63, participants = 243) at six months; OR 0.38 (95% CI 0.19 to 0.75, participants = 243) at 12 months; and OR 0.37 (95% CI 0.21 to 0.67, participants = 243) at 24 months (Analysis 7.2; Analysis 7.3; Analysis 7.4).
7.1. Analysis.

Comparison 7: Ticlopidine versus placebo, venous grafts, Outcome 1: Primary graft patency, 1 month
7.2. Analysis.

Comparison 7: Ticlopidine versus placebo, venous grafts, Outcome 2: Primary graft patency, 6 months
7.3. Analysis.

Comparison 7: Ticlopidine versus placebo, venous grafts, Outcome 3: Primary graft patency, 12 months
7.4. Analysis.

Comparison 7: Ticlopidine versus placebo, venous grafts, Outcome 4: Primary graft patency, 24 months
Aspirin versus prostaglandin E1 (PGE1)
Primary graft patency
One study (Gruss 1991) reported on the drug comparison of aspirin to PGE1 in participants receiving autologous venous grafts. Early occlusion, within the first three postoperative days, had few events, with an OR of 3.91 (95% CI 0.77 to 19.83, participants = 100) (Analysis 8.1). Longer‐term patency was not reported.
8.1. Analysis.

Comparison 8: ASA versus prostaglandin (PGE1), venous grafts, Outcome 1: Early occlusion
Aspirin versus naftidrofuryl
Primary graft patency
One study (Noppeney 1988) reported graft occlusion in seven of the 50 participants administered naftidrofuryl compared to 10 of the 49 given ASA, with an OR of 1.58 (95% CI 0.55 to 4.54, participants = 99) (Analysis 9.1).
9.1. Analysis.

Comparison 9: ASA versus naftidrofuryl, Outcome 1: Primary graft patency at 12 months
Side effects of treatment and complications
More general side effects were experienced by the ASA group compared to naftidrofuryl: OR 13.86 (95% CI 3.80 to 50.60, participants = 99). Gastrointestinal side effects were also more common in the aspirin group: OR 28.45 (95% CI 3.61 to 223.97, participants = 99). Only two participants reported vertigo, both in the naftidrofuryl treatment group: OR 0.20 (95% CI 0.01 to 4.19, participants = 99). Stomach bleeding was reported in five participants, all in the aspirin treatment group: OR 12.48 (95% CI 0.67 to 232.14, participants = 99) (Analysis 9.2).
9.2. Analysis.

Comparison 9: ASA versus naftidrofuryl, Outcome 2: Side effects
Cardiovascular events and mortality
Deaths occurred in both treatment groups, with an OR of 2.73 (95% CI 0.50 to 14.78, participants = 99) (Analysis 9.3).
9.3. Analysis.

Comparison 9: ASA versus naftidrofuryl, Outcome 3: Mortality
Clopidogrel and ASA (clopidogrel/ASA) versus ASA alone
CASPAR 2010 was the only study to compare clopidogrel/ASA to ASA alone. Outcomes were reported at 24 months as a whole population and split by subgroups based on whether participants received venous or prosthetic grafts.
Primary graft patency
Primary graft patency for all participants was similar between both treatment groups, OR 0.95 (95% CI 0.69 to 1.31, participants = 851) Analysis 10.1. There were more cases of occlusion in the clopidogrel/ASA group, compared to ASA alone, when only venous grafts were included: OR 1.47 (95% CI 0.93 to 2.31, participants = 598), but higher occlusion rates in the ASA alone group for prosthetic grafts: OR 0.53 (95% CI 0.32 to 0.88, participants = 253) (Analysis 11.1; Analysis 11.2).
10.1. Analysis.

Comparison 10: Clopidogrel and ASA versus ASA alone, all grafts, Outcome 1: Primary graft patency at 24 months
11.1. Analysis.

Comparison 11: Clopidogrel and ASA versus ASA alone, subgroups, Outcome 1: Primary graft patency, venous grafts, 24 months
11.2. Analysis.

Comparison 11: Clopidogrel and ASA versus ASA alone, subgroups, Outcome 2: Primary graft patency, prosthetic grafts, 24 months
Side effects of treatment and complications
Total cases of bleeding were fewer in the ASA alone group: OR 2.65 (95% CI 1.69 to 4.15, participants = 851), as were mild bleeding: OR 2.34 (95% CI 1.37 to 4.00, participants = 851) and moderate bleeding: OR 4.13 (95% CI 1.37 to 12.45, participants = 851). Cases of severe bleeding and fatal bleeding were few, and similar between the treatment groups: OR 1.82 (95% CI 0.61 to 5.48, participants = 851) and OR 2.01 (95% CI 0.18 to 22.24, participants = 851), respectively (Analysis 10.2).
10.2. Analysis.

Comparison 10: Clopidogrel and ASA versus ASA alone, all grafts, Outcome 2: Side effects
When we evaluated graft subgroups, there was still less mild bleeding in the ASA alone group for venous grafts: OR 2.46 (95% CI 1.33 to 4.53, participants = 598), but the occurrence of mild bleeding events was more similar between the treatment groups for prosthetic grafts: OR 1.86 (95% CI 0.67 to 5.21, participants = 253). The same trend was seen for moderate bleeding: venous grafts: OR 5.75 (95% CI 1.26 to 26.17, participants = 598) and prosthetic grafts: OR 2.50 (95% CI 0.48 to 13.13, participants = 253). Severe bleeding was similar between treatment groups for both venous and prosthetic grafts: OR 2.75 (95% CI 0.72 to 10.47, participants = 598) and OR 0.48 (95% CI 0.04 to 5.41, participants = 253), respectively (Analysis 11.3).
11.3. Analysis.

Comparison 11: Clopidogrel and ASA versus ASA alone, subgroups, Outcome 3: Side effects
Limb amputation
For all grafts, amputations were similar between groups, with slightly higher occurrence in the ASA alone group: OR 0.67 (95% CI 0.41 to 1.08, participants = 851) (Analysis 10.3). Amputation occurrence was also similar between treatment groups for venous grafts: OR 0.91 (95% CI 0.48 to 1.73, participants = 598), but more occurred in the ASA alone group for prosthetic grafts: OR 0.44 (95% CI 0.21 to 0.91, participants = 253) (Analysis 11.4).
10.3. Analysis.

Comparison 10: Clopidogrel and ASA versus ASA alone, all grafts, Outcome 3: Limb amputation
11.4. Analysis.

Comparison 11: Clopidogrel and ASA versus ASA alone, subgroups, Outcome 4: Limb amputation
Cardiovascular events and mortality
For all graft types, death was similar between both treatment groups: OR 1.44 (95% CI 0.76 to 2.72, participants = 851), which was also the case for both venous and prosthetic grafts: OR 1.43 (95% CI 0.69 to 2.97, participants = 598), and OR 1.49 (95% CI 0.41 to 5.40, participants = 253), respectively (Analysis 10.4; Analysis 11.5).
10.4. Analysis.

Comparison 10: Clopidogrel and ASA versus ASA alone, all grafts, Outcome 4: Mortality
11.5. Analysis.

Comparison 11: Clopidogrel and ASA versus ASA alone, subgroups, Outcome 5: Mortality
Discussion
Summary of main results
Antiplatelet therapy effect on bypass patency
In the present meta‐analysis we evaluated the effect of postoperatively administered antiplatelet treatment in people with peripheral arterial disease (PAD) receiving infrainguinal bypasses. It was shown that antiplatelet treatment with aspirin (ASA) or a combination of ASA and dipyridamole (ASA/DIP) has an overall positive effect on primary patency 12 months after the procedure. Interestingly, the size of the effect differed between participants receiving prosthetic grafts and those receiving venous grafts. When we limited the analysis to the subgroups receiving prosthetic (polytetrafluoroethylene (PTFE) or Dacron) grafts, the effect was statistically significant at all time points. In contrast, there was no evidence to support an effect of ASA or ASA/DIP in participants receiving venous graft bypasses. The evidence for secondary patency is too limited to draw any conclusions at this time. It should be noted that we did not perform a formal test for interaction to statistically demonstrate a differential effect of antiplatelet agents by graft type.
There was no difference in primary patency for the comparison between ASA/DIP versus pentoxifylline (PTX) at one, three, six and 12 months. However, for most of the time points we evaluated only a single study. For the comparisons between ASA and DIP versus indobufen (prosthetic grafts only), ASA or ASA/DIP versus vitamin K antagonists (VKA), as well as ASA/DIP versus low molecular weight heparin (LMWH), there were no differences between the treatment groups in primary patency at any time point. The analyses of indobufen and LMWH only had single study reporting and VKA included only two studies.
A single study evaluated ticlopidine versus placebo in participants receiving venous graphs only. Primary patency was significantly improved at six, 12 and 24 months after the procedure, but not in the first month post‐procedure. Thus, ticlopidine seems to be the only antiplatelet agent achieving a favourable effect on venous graft patency. ASA versus prostaglandin E1, also only with venous grafts, was reported in a single study, with few outcome events and no difference between the treatment groups for early occlusions. Comparing ASA with naftidrofuryl in a single study, there was no difference in primary patency at 12 months.
Clopidogrel with ASA versus ASA alone was reported in a single study, with no difference in primary patency at 24 months for all participants, as well as no difference for venous grafts. Prosthetic grafts showed improvement in patency at 24 months for the clopidogrel with ASA group.
Side effects and limb amputations
For the comparison of ASA or ASA/DIP versus placebo or nothing, there were no differences between the treatment groups for any of the evaluated side effects, complications or amputations. Comparing ASA or ASA/DIP to PTX there was a possible increase in gastric intolerance, but no differences for gastric haemorrhage, vertigo or amputations. There were also no differences in amputation comparing ASA or ASA/DIP to VKA. Both general and gastrointestinal side effects were increased in the ASA group, compared with naftidrofuryl, but vertigo and stomach bleeding were similar between the two groups. Comparing clopidogrel/ASA with ASA alone, there were increased total, mild and moderate bleeding events in the one study that evaluated this treatment comparison, in all participants, but no difference for severe or fatal bleeding events. In venous grafts within this study, there was an increase in mild and moderate events, but no difference in severe bleeding events, and there was no difference in any of the bleeding categories for prosthetic grafts. Within this comparison, amputations were similar between treatment groups for all participants, including venous grafts, but there was a slight decrease in amputations in the clopidogrel/ASA group.
Cardiovascular events and mortality
For the comparisons of ASA or ASA/DIP versus placebo or nothing and ASA or ASA/DIP versus VKA there were no differences between the treatment groups for cardiovascular events or mortality. There was also no difference in mortality when comparing ASA or ASA/DIP versus PTX, ASA versus naftidrofuryl, or clopidogrel/ASA versus ASA alone; cardiovascular events were not evaluated within these comparisons.
Although very few events were reported in the single study, there appeared to be decreased mortality in the ASA/DIP treatment group, compared with LMWH.
In conclusion, the results of our meta‐analysis suggest that the administration of platelet inhibitors such as ASA or ASA/DIP could result in improved venous and prosthetic graft patency compared to no treatment. However, subgroup analysis by graft type, i.e. venous versus PTFE or Dacron, shows that participants receiving a prosthetic graft are likely to benefit more from ASA or ASA and DIP administration than those treated with a venous graft. For most of the comparisons included in this review, there is currently not enough data to draw any conclusions.
Overall completeness and applicability of evidence
With 16 studies included in the review and meta‐analysis, there was much relevant data available to provide evidence for the review question. However, we separately evaluated a total of nine different comparisons, with only one comparison having more than two studies providing data for meta‐analysis. Therefore, while this review does bring many trials together touching on many antiplatelet treatments, the review does not reach a meaningful level of evidence for all of the predefined comparisons. Also, none of the included studies addressed the outcomes of assisted primary patency, objective assessment of blood flow or quality of life, and many studies did not report on any of the other outcomes. Assisted primary patency as an outcome is of specific importance, as even a highly stenotic graft may remain patent, and a graft at a highly stenotic stage would be more easily treated, both technically and for the patient, than a fully occluded graft. Therefore, knowing whether antiplatelet treatments help reduce full occlusion and allow treatment at a stenotic but not occluded stage would be of clinical importance.
Data presentation did not distinguish by graft type for the site of the distal anastomosis. Furthermore, the number of grafts frequently differed from the number of participants, without appropriate explanation. Insufficient information was available in the included studies to assess the effects of antiplatelet agents in different clinical settings such as diabetes and other co‐morbidities. Another concern with the applicability of evidence is the fact that different dosages of the various treatments were used between the included studies. Further information on the dosages can be found in Characteristics of included studies tables.
Quality of the evidence
This review included 16 studies with a total of 5683 randomised participants. Currently we can only draw a robust conclusion regarding antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery for the comparison between ASA or ASA/DIP versus placebo or nothing, showing a positive reduction in occlusion at 12 months in participants who received ASA or ASA/DIP. The comparisons of ASA or ASA/DIP versus vitamin K antagonists and of clopidogrel and ASA versus ASA alone only included one or two studies, but they encompassed a large number of participants. Neither comparison found any difference in primary graft patency at any of the time points. All other comparisons only included one or two smaller studies. Also limiting the quality of the evidence is the fact that several included studies provided their results by the number of bypasses and not by the number of participants, indicating bilaterally‐treated patients. This could alter results, although currently only a few studies provided results in this manner, with few participants in their study population receiving multiple bypasses. Also of concern is the reporting of outcomes at varying time points, which vary between studies. This reduces our ability to combine outcome data in the most robust way possible.
Overall, study quality was moderate, with the largest problem being that the majority of studies did not adequately describe their methods of randomisation or outcome assessor blinding. The other main issue with study quality was not blinding participants or personnel to the treatment received. These concerns, along with the previously reported issues with paucity of data for many of the comparisons, unit of analysis issues, variation of treatment dosages and lack of data on our review's prespecified outcomes, leaves the quality of the evidence of the review moderate to low. We expect that with future updates and more high‐quality studies, the quality of evidence will improve.
Potential biases in the review process
In order to reduce potential bias two review authors assessed studies for inclusion and performed data extraction and quality assessment. We made every attempt to include all relevant studies, but it is still possible that we did not identify all studies.
The majority of included studies randomised individuals as the unit for analysis, but four studies included participants with multiple or bilateral reconstructions or re‐randomised participants, and used grafts as the unit of analysis. In order to include these data we performed analyses with the four studies with unit of analyses issues, but also performed sensitivity analyses by excluding them to see if the results were affected. Only the comparison between ASA or ASA/DIP versus nothing or placebo contained enough studies to evaluate the effects of the unit of analysis issues by sensitivity analysis, and within this comparison we could not evaluate all outcomes. Overall there was very little change in the results when the studies were removed, but when the odds ratios (ORs) did change, it was an expected attenuation and decreasing of power due to a reduction in the overall number of participants included in the calculation. Readers should keep in mind our methods of addressing these unit of analysis issues when evaluating the results of this review, as other reviews may take a different approach.
For the outcome 'primary patency', the numbers recorded for analyses were actually the number of grafts that occluded during the specified time period as reported by the trialists. Both patency and occlusion are used to describe the treatment outcomes within the included studies and within this review, which should be kept in mind when interpreting the presented data. As assisted primary patency was not reported by the trialists of the included studies, it is unclear if the reported occlusion is actual occlusion i.e. thrombosis of the graft, or if it also includes participants whose grafts were revised prior to occlusion/thrombosis.
We calculated data for graft failure from the survival curves if raw data were not reported or were unavailable after contacting the authors of the trials.
Agreements and disagreements with other studies or reviews
A systematic review and meta‐analysis that was published in 1999 also set out to evaluate antiplatelet therapy (specifically aspirin) in the prevention of graft occlusion after infrainguinal bypass surgery (Tangelder 1999). This review differs from our own, as it was interested in oral anticoagulants, as well as antiplatelet agents, and they used risk ratios (RRs) and not odds ratios for their dichotomous outcomes. The Tangelder 1999 review included five studies that we also included in our own review, and an additional two studies, one of which was specific to oral anticoagulants, while the other compared an anticoagulant with aspirin to aspirin alone. The findings in Tangelder 1999 were similar to our own meta‐analyses findings for the comparison between ASA or ASA/DIP versus nothing or placebo. They found a decrease in bypass occlusions in the aspirin group (RR 0.78, 95% CI 0.64 to 0.95), and no difference between the treatment groups for total mortality (RR 0.92, 95% CI 0.64 to 1.32).
Authors' conclusions
Implications for practice.
There is evidence to suggest that the administration of platelet inhibitors such as ASA or ASA/DIP will result in improved venous and prosthetic graft patency compared to no treatment. However, patients with a prosthetic graft may benefit more from ASA or ASA/DIP administration than those with a venous graft, but this needs further investigation. The results of this review should be interpreted with caution, due to the unit of analysis issues described within the review, as well as the fact that many of the comparisons were only reported in a single study, and that the dosages often differed between studies.
Implications for research.
Further high‐quality randomised clinical trials, with adequate sample sizes, are required to evaluate the efficacy of antiplatelet medications for graft patency in infrainguinal venous and prosthetic grafts for all treatment comparisons. Presentation of data should be detailed and not only show survival curves for overall patency. Tables showing the raw data would improve the transparency of the trial performance and allow comparison of endpoints at consecutive time points. The reader would be able to identify the number of occlusions, or other endpoints, at different time points in each comparison group as well as in subgroups defined by bypass material, above‐ and below‐knee anastomosis, and inflow and outflow conditions. Other concerns for research within this area would be determining the optimal dosage of antiplatelet medications and also outcome stratification by co‐morbidities, such as diabetes. A more complete outcome profile should be evaluated, such as assisted primary patency. Uniformity of unit of analysis issues should be addressed in studies of peripheral vascular disease, as there needs to be clarity around single and multiple grafts and the time points at which these occurred.
Feedback
Anticoagulant feedback, 14 February 2011
Summary
Feedback received on this review, and other reviews and protocols on anticoagulants, is available on the Cochrane Editorial Unit website at http://www.editorial-unit.cochrane.org/anticoagulants-feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 23 January 2023 | Amended | This review has been superseded by Cochrane Reviews: 'Antithrombotics after infra‐inguinal bypass grafting' (https://doi.org/10.1002/14651858.CD015141) and 'Antithrombotics after infra‐inguinal peripheral endovascular treatment' (https://doi.org/10.1002/14651858.CD015142) and will no longer be updated. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 2 September 2014 | New search has been performed | Searches rerun, one new study included, three new studies excluded |
| 2 September 2014 | New citation required but conclusions have not changed | Searches rerun, one new study included, three new studies excluded, risk of bias tables completed, two new authors joined review team. Conclusions not changed |
| 14 February 2011 | Amended | Link to anticoagulant feedback added |
| 23 April 2008 | Amended | Converted to new review format. |
| 23 April 2008 | New citation required and conclusions have changed | This review was updated in 2008. New studies were identified and added to the included and excluded tables. |
| 26 May 2004 | Amended | Graph created and text amended |
| 18 August 2003 | Amended | Published note added to effect that Donald Adam wrote protocol but did not have any involvement with the review |
Notes
Acknowledgements
A special thank you to Karen Welch for assistance with German translation for several of the included studies.
We would like to thank the authors of the previous versions of the this review: Julie Brown, Andrew Wawryzniak, Janine Dörffler Melly, Marianne Koopmann, Harry Büller and Donald Adam.
The authors would like to thank the Peripheral Vascular Diseases Review group for their support and guidance.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Arteriosclerosis] this term only | 895 |
| #2 | MeSH descriptor: [Arteriolosclerosis] this term only | 0 |
| #3 | MeSH descriptor: [Arteriosclerosis Obliterans] this term only | 73 |
| #4 | MeSH descriptor: [Atherosclerosis] this term only | 513 |
| #5 | MeSH descriptor: [Arterial Occlusive Diseases] this term only | 810 |
| #6 | MeSH descriptor: [Intermittent Claudication] this term only | 768 |
| #7 | MeSH descriptor: [Ischemia] this term only | 814 |
| #8 | MeSH descriptor: [Peripheral Vascular Diseases] explode all trees | 2293 |
| #9 | MeSH descriptor: [Vascular Diseases] this term only | 424 |
| #10 | MeSH descriptor: [Leg] explode all trees and with qualifier(s): [Blood supply ‐ BS] | 1145 |
| #11 | MeSH descriptor: [Femoral Artery] explode all trees | 796 |
| #12 | MeSH descriptor: [Popliteal Artery] explode all trees | 289 |
| #13 | MeSH descriptor: [Iliac Artery] explode all trees | 159 |
| #14 | MeSH descriptor: [Tibial Arteries] explode all trees | 32 |
| #15 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD) | 19399 |
| #16 | (arter*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 6127 |
| #17 | (vascular) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1656 |
| #18 | (vein*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 951 |
| #19 | (veno*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1174 |
| #20 | (peripher*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1631 |
| #21 | peripheral near/3 dis* | 3705 |
| #22 | arteriopathic | 25 |
| #23 | (claudic* or hinken*) | 1600 |
| #24 | (isch* or CLI) | 20013 |
| #25 | dysvascular* | 35 |
| #26 | leg near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 201 |
| #27 | limb near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 261 |
| #28 | (lower near/3 extrem*) near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 158 |
| #29 | (aort* or iliac or femoral or popliteal or femoro* or fempop* or crural or *inguinal) near/3 (obstruct* or occlus*) | 402 |
| #30 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 | 46395 |
| #31 | bypass*:ti,ab,kw (Word variations have been searched) | 11328 |
| #32 | MeSH descriptor: [Vascular Grafting] explode all trees | 6012 |
| #33 | MeSH descriptor: [Blood Vessel Prosthesis] explode all trees | 472 |
| #34 | MeSH descriptor: [Blood Vessel Prosthesis Implantation] explode all trees | 536 |
| #35 | revascul*:ti,ab,kw (Word variations have been searched) | 4850 |
| #36 | graft*:ti,ab,kw (Word variations have been searched) | 13673 |
| #37 | reconstruct*:ti,ab,kw (Word variations have been searched) | 9 |
| #38 | #31 or #32 or #33 or #34 or #35 or #36 or #37 | 23761 |
| #39 | #30 and #38 in Trials | 5278 |
| #40 | MeSH descriptor: [Platelet Aggregation Inhibitors] explode all trees | 3038 |
| #41 | MeSH descriptor: [Phosphodiesterase Inhibitors] explode all trees | 970 |
| #42 | MeSH descriptor: [Tetrazoles] this term only | 1681 |
| #43 | antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg*:ti,ab,kw (Word variations have been searched) | 2314 |
| #44 | ((platelet or thromboxane or thrombocyte or cyclooxygenase or cyclo‐oxygenase or phosphodiesterase or fibrinogen or PAR‐1) near/3 (antagonist or inhibitor)):ti,ab,kw (Word variations have been searched) | 6624 |
| #45 | (gp* or glycoprotein* or protease or P2Y12 or TXA2) near/3 inhibit*:ti,ab,kw (Word variations have been searched) | 2401 |
| #46 | thienopyridine:ti,ab,kw (Word variations have been searched) | 144 |
| #47 | ticlopidine or Ticlid:ti,ab,kw (Word variations have been searched) | 1583 |
| #48 | clopidogrel or Plavix:ti,ab,kw (Word variations have been searched) | 1849 |
| #49 | Prasugrel or Effient or Efient or Prasita:ti,ab,kw (Word variations have been searched) | 182 |
| #50 | ticagrelor or AZD6140 or Brilinta:ti,ab,kw (Word variations have been searched) | 126 |
| #51 | elinogrel or PRT060128 or PRT‐060128:ti,ab,kw (Word variations have been searched) | 7 |
| #52 | cangrelor or AR‐C6993* or ARC6993*:ti,ab,kw (Word variations have been searched) | 25 |
| #53 | SCH530348 or SCH‐530348:ti,ab,kw (Word variations have been searched) | 11 |
| #54 | E5555:ti,ab,kw (Word variations have been searched) | 3 |
| #55 | terutroban or Triplion:ti,ab,kw (Word variations have been searched) | 11 |
| #56 | (aspirin* or nitroaspirin or ASA or "acetyl salicylic acid*" or "acetylsalicylic acid" or "acetyl‐salicylic acid"):ti,ab,kw (Word variations have been searched) | 15346 |
| #57 | triflusal or disgren:ti,ab,kw (Word variations have been searched) | 88 |
| #58 | Cilostazol or Pletal or Pletaal:ti,ab,kw (Word variations have been searched) | 356 |
| #59 | dipyridamol* or Persantine:ti,ab,kw (Word variations have been searched) | 1086 |
| #60 | OPC‐13013 or OPC13013:ti,ab,kw (Word variations have been searched) | 5 |
| #61 | picotamide or picotinamide:ti,ab,kw (Word variations have been searched) | 43 |
| #62 | satigrel:ti,ab,kw (Word variations have been searched) | 4 |
| #63 | vorapaxar:ti,ab,kw (Word variations have been searched) | 22 |
| #64 | indobufen | 102 |
| #65 | #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 | 25325 |
| #66 | 39 and #65 in Trials | 1259 |
Data and analyses
Comparison 1. ASA or ASA/DIP vs placebo or nothing, all grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Primary graft patency at 12 months | 6 | 952 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.22, 0.83] |
| 1.2 Side effects and complications | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Side effects‐ general | 5 | 913 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.00, 2.41] |
| 1.2.2 Gastrointestinal side effects | 6 | 952 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.92, 2.24] |
| 1.2.3 Major bleeding | 2 | 598 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.85, 4.16] |
| 1.2.4 Minor bleeding | 1 | 148 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.31, 1.70] |
| 1.2.5 Wound or graft infection | 1 | 549 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.71] |
| 1.3 Limb amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Cardiovascular events | 4 | 811 | Odds Ratio (M‐H, Random, 95% CI) | 1.27 [0.43, 3.80] |
| 1.5 Mortality | 4 | 799 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.56, 1.26] |
Comparison 2. ASA or ASA/DIP vs placebo or nothing, subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Primary graft patency, venous grafts, 1 month | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.26, 2.25] |
| 2.2 Primary graft patency, venous grafts, 3 months | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.54, 1.35] |
| 2.3 Primary graft patency, venous grafts, 6 months | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 2.4 Primary graft patency, venous grafts, 12 months | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.48, 0.99] |
| 2.5 Primary graft patency, venous grafts, 24 months | 2 | 620 | Odds Ratio (M‐H, Random, 95% CI) | 1.03 [0.32, 3.28] |
| 2.6 Primary graft patency, prosthetic grafts, 1 month | 3 | 157 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.04, 0.51] |
| 2.7 Primary graft patency, prosthetic grafts, 3 months | 4 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.14, 0.66] |
| 2.8 Primary graft patency, prosthetic grafts, 6 months | 4 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.41] |
| 2.9 Primary graft patency, prosthetic grafts, 9 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.10 Primary graft patency, prosthetic grafts, 12 months | 4 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.10, 0.36] |
Comparison 3. ASA or ASA/DIP versus pentoxifylline (PTX), all grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Primary graft patency, 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Primary graft patency, 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Primary graft patency, 6 months | 2 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.56, 3.11] |
| 3.4 Primary graft patency, 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5 Side effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5.1 Gastric intolerance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5.2 Gastric haemorrhage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.5.3 Vertigo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6 Limb amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.7 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. ASA/DIP versus indobufen, prosthetic grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Primary graft patency, 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.2 Primary graft patency, 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.3 Primary graft patency, 9 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.4 Primary graft patency, 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. ASA or ASA/DIP versus vitamin K antagonists, all grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Primary graft patency, 3 months | 2 | 2781 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.14] |
| 5.2 Primary graft patency, 6 months | 2 | 2781 | Odds Ratio (M‐H, Random, 95% CI) | 0.72 [0.27, 1.96] |
| 5.3 Primary graft patency, 12 months | 2 | 2781 | Odds Ratio (M‐H, Random, 95% CI) | 0.68 [0.27, 1.69] |
| 5.4 Primary graft patency, 24 months | 2 | 2781 | Odds Ratio (M‐H, Random, 95% CI) | 0.64 [0.25, 1.63] |
| 5.5 Limb amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.6 Cardiovascular events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.6.1 Myocardial infarction | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.6.2 Stroke | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.7 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. ASA/DIP versus LMWH, all grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Primary graft patency, 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 Primary graft patency, 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.3 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Ticlopidine versus placebo, venous grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Primary graft patency, 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.2 Primary graft patency, 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.3 Primary graft patency, 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.4 Primary graft patency, 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 8. ASA versus prostaglandin (PGE1), venous grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Early occlusion | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. ASA versus naftidrofuryl.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Primary graft patency at 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2 Side effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2.1 Side effects‐ general | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2.2 Gastrointestinal side effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2.3 Vertigo | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.2.4 Stomach bleeding | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.3 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Clopidogrel and ASA versus ASA alone, all grafts.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Primary graft patency at 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2 Side effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2.1 Bleeding‐ total | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2.2 Bleeding‐ mild | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2.3 Bleeding‐ moderate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2.4 Bleeding‐ severe | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2.5 Bleeding‐ fatal | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3 Limb amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.4 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Clopidogrel and ASA versus ASA alone, subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Primary graft patency, venous grafts, 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.2 Primary graft patency, prosthetic grafts, 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3 Side effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.1 Bleeding‐ mild, venous grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.2 Bleeding‐ mild, prosthetic grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.3 Bleeding‐ moderate, venous grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.4 Bleeding‐ moderate, prosthetic grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.5 Bleeding‐ severe, venous grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3.6 Bleeding‐ severe, prosthetic grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.4 Limb amputation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.4.1 Amputation, venous grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.4.2 Amputation, prosthetic grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5 Mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5.1 Mortality, venous grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.5.2 Mortality, prosthetic grafts | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Becquemin 1997.
| Study characteristics | ||
| Methods |
Study type: Multicentre, double‐blind, randomised controlled trial Study aim: To determine whether ticlopidine (TIC) could reduce the rate of late occlusion of saphenous‐vein grafts below the knee Country: France |
|
| Participants |
Number randomised: Total n = 243 (TIC n = 122; placebo n = 121) Age‐ mean years: TIC 67.1; placebo 67.7 Gender n (M/F):TIC 96/26; placebo 92/29 Inclusion criteria: All patients 18 to 80 years old who required femoropopliteal or femorotibial bypass graft for atheromatous occlusive disease; had a saphenous vein suitable for grafting Exclusion criteria: Acute ischaemia or aneurysm; marked stenosis in the ipsilateral iliac artery; previous arterial surgery on the same limb; reduced life expectancy; pregnancy; inability to comply with the protocol; associated conditions requiring treatment with platelet‐inhibiting drugs or anticoagulants; abnormalities of haemostasis Co‐morbidity: current angina or previous MI (TIC 20.5%; placebo 24.8%), impaired left ventricular function (TIC 10.7%; placebo 7.4%), arrhythmia (TIC 9.0%; placebo 15.7%), carotid stenosis (TIC 22.1%; placebo 21.5%), hypertension (TIC 48.4%; placebo 53.7%), current smoker (TIC 25.4%; placebo 19.0%), diabetes (TIC 27.0%; placebo 21.5%), hyperlipidaemia (TIC 23.8%; placebo 25.6%), previous vascular surgery (TIC 32.8%; placebo 30.6%) Severity of occlusive disease:Leriche‐Fontaine stage of disease‐ stage IIb (TIC 27.0; placebo 22.3), stage III (TIC 30.3; placebo 41.3), stage IV (TIC 42.6; placebo 36.4) Site of distal anastomosis:popliteal (TIC n = 66; placebo n = 82), tibial (TIC n = 56; placebo n = 39) Type of graft:autologous saphenous‐vein grafts More than 70% of the participants in both groups suffered from critical limb ischaemia |
|
| Interventions |
Treatment:250 mg ticlopidine in bottles with a 1‐month supply; given in boxes containing 6‐month supply dispensed on day 0 and at 6, 12 and 18 months; participants instructed to take 1 tablet twice a day for the 24 months of the study Control: placebo identically matched to treatment in bottles with a 1‐month supply; given in boxes containing 6‐month supply dispensed on day 0 and at 6, 12 and 18 months; participants instructed to take 1 tablet twice a day for the 24 months of the study Duration: 24 months |
|
| Outcomes | Primary endpoint was primary patency at 2 years postoperatively; Secondary endpoints were secondary graft patency, death from any cause, nonfatal MI, nonfatal stroke, limb ischaemia, mesenteric infarct, amputation of leg or thigh Evaluated at 1, 3, 6, 12, 18 and 24 months |
|
| Notes | Randomisation took place between the 3rd and 14th postoperative day and follow‐up continued for 2 years | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…according to a predetermined randomization list of numbers balanced in blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Treatment bottles containing a one‐month supply of ticlopidine (250‐mg tablets) or matching placebo were prepared by Sanofi and labelled according to a predetermined randomization list of number balanced in block of four" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All records concerning graft patency were reviewed by a validation committee that was unaware of the patients' treatment assignments. The clinical events were reviewed and adjudicated blindly by an independent panel…" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data; used intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
BOA 2000.
| Study characteristics | ||
| Methods |
Study type:An open, randomised, multicentre clinical trial Study aim:To compared the effectiveness of oral anticoagulants with that of aspirin in the prevention of infrainguinal bypass‐graft occlusion and other clinical events Country: the Netherlands |
|
| Participants |
Number randomised: Total n = 2690 (ASA n = 1351; coumarin n = 1339) Age‐ mean years (SD): ASA 69 (10); coumarin 69 (10) Gender‐ M%: ASA 63%; coumarin 65% Inclusion criteria: All patients who required an infrainguinal bypass graft for obstructive arterial disease Exclusion criteria:Contraindication or absolute indication for one of the trial medications; substantially shortened life expectancy; MI or stroke within 1 month before surgery; abnormalities of blood platelets or erythrocytes; anaemia; inability to adhere to the protocol; inability to give informed consent Co‐morbidity: All participants: 54% smokers, 39% with hypertension, 26% with diabetes mellitus, and 16% with hyperlipidaemia Severity of occlusive disease: Claudicants (ASA 52%; coumarin 50%), Pain at rest (ASA 21%; coumarin 21%), Ischaemic ulceration (ASA 25%; coumarin 27%), gangrene (ASA 2%; coumarin 1%) Site of distal anastomosis: popliteal above knee (ASA 48%; coumarin 45%), popliteal below knee (ASA 32%; coumarin 36%), crural (ASA 19%; coumarin 18%), pedal (ASA 1%; coumarin 2%) Type of graft: vein (ASA 58%; coumarin 59%), biograft (ASA 6%; coumarin 6%), prosthetic (ASA 34%; coumarin 31%), composite (ASA 2%; coumarin 3%) |
|
| Interventions |
Treatment: Aspirin, 100 mg daily pulverised carbasalate calcium (equivalent to 80 mg daily), started within 5 days after surgery Control:coumarin derivatives (phenprocoumon or acenocoumarol) with intended International Normalised Ratio (INR) range was 3.0 ‐ 4.5 (achieved in 50% of the observed patient years), started within 5 days after surgery Duration:unclear, but mean observation time was 21 months |
|
| Outcomes | Primary endpoint was graft patency assessed clinically by Doppler flow measurement or duplex sonography, or by arteriography if indicated. Secondary endpoints were vascular death, MI, stroke, amputation, vascular intervention, and major haemorrhage. Follow‐up was at 3, 6, 12, 18, and 24 months, with 6 monthly visits thereafter. Mean observation time was 21 months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by telephone through a central trial office, using computer‐generated sequences of numbers |
| Allocation concealment (selection bias) | Low risk | Performed by telephone through central trial office |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for drop‐outs, and final numbers for each treatment group are similar |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
CASPAR 2010.
| Study characteristics | ||
| Methods |
Study type: Multicentre, prospective, randomised, placebo‐controlled trial Study aim: To determine whether clopidogrel plus ASA had better limb outcomes compared to ASA alone, in patients undergoing below‐knee bypass grafting Country:UK |
|
| Participants |
Number randomised:Total n = 851 (Clopidogrel + ASA n = 425; ASA + placebo n = 426) Age‐ mean years (SD):Clopidogrel + ASA 66.5 (8.7); ASA + placebo 65.6 (8.5) Gender‐ M%: Clopidogrel + ASA 75.5%; ASA + placebo 75.8% Inclusion criteria: ≥ 40 and ≤ 80 years; informed consent obtained before conducting any study‐related procedure; chronic background treatment with daily ASA of any done, started at least 4 weeks before surgery; a post‐randomisation dose of ASA between 75 and 100 mg/day; unilateral below‐knee bypass graft for atherosclerotic PAD; patent index graft demonstrated during bypass surgery or between surgery and time of randomisation; no clinical evidence of graft occlusion at randomisation Exclusion criteria: Onset of PAD symptoms before age of 40; nonatherosclerotic vascular disease; patients receiving aortobifemoral, iliac‐femoral or cross‐over (femoral‐femoral) grafts or undergoing peripheral transcutaneous angioplasty during the same surgery; significant bleeding risk such as current active bleeding at the surgical site; withdrawal of an epidural catheter less than 12 hours before randomisation; peptic ulceration within 12 months of randomisation; previous or current intracranial haemorrhage or haemorrhagic stroke; any history of severe spontaneous bleeding; current warfarin therapy or anticipated need for warfarin; concomitant additional antiplatelet agents or thrombolytic agents Co‐morbidity: Hypertension (Clopidogrel + ASA 70.1%, ASA + placebo 70.0%); Hyperlipidemia (Clopidogrel + ASA 50.4%, ASA + placebo 48.8%); CAD and/or CRVD (Clopidogrel + ASA 38.4%, ASA + placebo 31.0%); Diabetes (Clopidogrel + ASA 37.4%, ASA + placebo 38.0%), claudication only (Clopidogrel + ASA 34.1%, ASA + placebo 32.6%), rest pain (Clopidogrel + ASA 26.1%, ASA + placebo 26.5%), ulcers/gangrene (Clopidogrel + ASA 39.3%, ASA + placebo 39.9%) Severity of occlusive disease (determined by ABPI):ABPI (SD) ‐ Clopidogrel + ASA 0.44 (0.25), ASA + placebo 0.46 (0.26) Site of distal anastomosis:Below‐knee popliteal (Clopidogrel + ASA 75.5%, ASA + placebo 74.8%); below‐knee popliteal crural (Clopidogrel + ASA 20.7%, ASA + placebo 22.1%); beyond popliteal pedal (Clopidogrel + ASA 3.8%, ASA + placebo 3.1%) Type of graft: venous and prosthetic grafts (Clopidogrel + ASA venous = 297 prosthetic = 128; ASA + placebo venous = 301 prosthetic = 125) |
|
| Interventions |
Treatment:Clopidogrel + ASA: ASA 75 ‐ 100 mg/day plus clopidogrel 75 mg/day Control:ASA + placebo: ASA 75 ‐ 100 mg/day plus placebo 1 tablet/day Duration:Beginning 2‐ 4 days after bypass surgery; follow‐up from 6 to 24 months after surgery; Follow‐up evaluations at 1 month and then every 6 months thereafter |
|
| Outcomes | Primary efficacy: composite of index‐graft occlusion or revascularisation, above‐ankle amputation of affect limb or death Primary safety: severe bleeding (GUSTO classification), moderate and mild bleeding ABPI |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used an interactive voice‐response system with a pre‐established randomisation scheme, stratified by graft type (venous or prosthetic) |
| Allocation concealment (selection bias) | Low risk | Interactive voice‐response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double‐blind" and used placebo tablets in ASA‐only group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All occurrences of the primary endpoint were adjudicated on a blinded basis by the Clinical Enpoints Committee" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs and losses to follow up were clearly described and similar between groups; efficacy analysis performed on an intention‐to‐treat basis, with all randomised participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Clyne 1987.
| Study characteristics | ||
| Methods |
Study type: Prospective randomised single‐centre trial Study aim:To discover whether improved patency could be achieved by a short course of maximum thrombogenicity Country:UK |
|
| Participants |
Number randomised:Total n = 140 (148 bypasses) (ASA/DIP = 78 bypasses; control = 70 bypasses) Age: not indicated Gender n (M/F): Total 107/41 Inclusion criteria: patients undergoing femorodistal bypasses for rest pain or very short distance claudication Exclusion criteria:not indicated Co‐morbidity: smoker (ASA/DIP 62%; control 61%), diabetes (ASA/DIP 13%; control 13%), duration of claudication‐ months (SD) (ASA/DIP 15.6 (24.9); control 24.7 (35)), rest pain (ASA/DIP = 42; control = 46) Severity of occlusive disease (determined by ABPI): ABPI (SD) ‐ ASA/DIP 0.39 (0.25); control 0.35 (0.27) Site of distal anastomosis: below knee 119 (ASA/DIP = 64; control = 55) Type of graft: autogenous vein bypasses ‐ 93 (63 %) (ASA/DIP = 49; control = 44), prosthetic including PTFE, Dacron and umbilical vein ‐ 55 (ASA/DIP = 29; control = 26), 28 participants received secondary reconstructions |
|
| Interventions |
Treatment: ASA/DIP ‐ 200 mg twice daily DIP started 48 hours before surgery, continued on day of surgery and postoperatively 10 mg iv DIP; followed by 300 mg ASA and 200 mg DIP twice daily for 6 weeks after surgery. All participants received heparin perioperatively Control: no specific anti‐thrombotic treatment or placebo Duration: 6 weeks |
|
| Outcomes | Primary endpoints were graft failure, limb loss, death at 12 months follow‐up. Graft patency was assessed clinically and by Doppler ankle blood pressure before discharge, at 1, 3, 6, and 12 months postoperatively. Arteriography was performed when further operative treatment was considered | |
| Notes | All data were related to the numbers of bypasses instead of numbers of participants (8 of 140 participants received 2 grafts) 98 participants underwent operation for rest pain, 42 for claudication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open trial |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No evidence of incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | All data were related to the numbers of bypasses instead of numbers of participants (8 of 140 participants received bilateral grafts) |
D'Addato 1992.
| Study characteristics | ||
| Methods |
Study type:Multicentre, double‐blind, randomised clinical trial Study aim:To compare the efficacy of indobufen (IND) and the combination of ASA/DIP in improving long‐term patency of the femoropopliteal prosthetic bypass Country:Italy |
|
| Participants |
Number randomised:Total n = 113 (ASA/DIP n = 57; IND n = 56) Age‐ mean years (SD):ASA/DIP 65.6 (8.4); IND 66.6 (8.4) Gender n (M/F):ASA/DIP 47/10; IND 49/7 Inclusion criteria:Patients with vascular disease complaining of rest pain or trophic lesions or both; occlusion of the femoropoplitea‐tibial tract; candidates for revascularising with PTFE bypass graft Exclusion criteria:Patients with severe obesity; severe or unstable diabetes; life expectancy < 12 months; hypertensive patients with mean arterial pressure > 115 mmHg not controllable by suitable therapy; patients with severe hepatic or renal insufficiency; chronic anaemia; haemorrhagic diathesis; present or previous peptic ulcer; need for treatment with drugs that could affect platelet aggregation or clotting or both Co‐morbidity:Diabetes mellitus (ASA/DIP 15.8%; IND 14.3%), hypercholesterolaemia (ASA/DIP 14.0%; IND 7.2%), smoking (ASA/DIP 68.4%; IND 71.4%), hypertension (ASA/DIP 22.8%; IND 19.6%), critical ischaemia (ASA/DIP 75.4%; IND 66.1%) Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:Above‐knee anastomosis (ASA/DIP 29%; IND 35%), below‐knee anastomosis (ASA/DIP 28%; IND 21%) Type of graft:PTFE |
|
| Interventions |
Treatment:900 mg ASA combined with 225 mg DIP, started 2 days before surgery Control:400 mg IND daily, started 2 days before surgery 7‐day washout period during which no anticoagulants or inhibitors of prostaglandin synthesis were used Duration:12 months |
|
| Outcomes | Graft patency was assessed by angiography 6 days and 12 months postoperatively, and clinically every 3 months by physical examination, Doppler ultrasound measurements on the graft, and by angiography if indicated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "...blindly assigned to treatment"; insufficient description of blinding methods |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for drop‐outs, and final numbers for each treatment group are similar |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Donaldson 1985.
| Study characteristics | ||
| Methods |
Study type:A randomised, double‐blind clinical trial performed between 1979 and 1981 Study aim:To evaluate the effect of a combination of ASA and DIP on the early patency of femoro‐popliteal Dacron bypass grafts implanted to relieve disabling claudication Country:UK |
|
| Participants |
Number randomised:Total n = 65 (73 grafts) (ASA/DIP n = 33 (35 grafts); placebo n = 32 (38 grafts)) Age‐ mean years: ASA/DIP = 59.3; placebo 60.0 Gender n (M/F):ASA/DIP 27/6; placebo 25/7 Inclusion criteria:Patients with disabling intermittent claudication suitable for femoro‐popliteal bypass Exclusion criteria:a history of dyspepsia, peptic ulceration or hypersensitivity to ASA or DIP Co‐morbidity:diabetic (ASA/DIP n = 3; placebo n = 2), mean MWD‐ m (ASA/DIP 61.2; placebo 54.7) Severity of occlusive disease (determined by ABPI):ASA/DIP 0.63; placebo 0.61 Site of distal anastomosis:below the knee (ASA/DIP 11; placebo 10) Type of graft:Dacron bypass grafts |
|
| Interventions |
Treatment:3 times daily 330 mg ASA and 75 mg DIP Control:placebo Duration:Therapy was started preoperatively. 1 trial capsule given on the evening prior to surgery then resumed as soon as oral intake was permitted postoperatively) and continued for 12 months postoperatively All participants received heparin intraoperatively |
|
| Outcomes | Assessment of graft patency was performed by a standardised treadmill test, ankle/brachial systolic pressure index (ABPI), Doppler ultrasound and by palpation at 3, 6, 9, and 12 months postoperatively. Plasma drug levels were measured for ASA and DIP. Graft patency by clinical examination, ABPI, Doppler on graft, maximum walking distance, platelet adhesion/aggregation, bleeding time | |
| Notes | 2 bilateral participants in the ASA/DIP group and 6 in the placebo group All concomitant antithrombotic therapy had been stopped at least 3 weeks prior to surgery |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patient sample was divided into two groups depending on whether the distal anastomosis was to be above or below the knee. Each group was then randomised to receive either placebo or active capsules…". Insufficient information to determine adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "… randomised to receive either placebo or active capsules…" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although drop‐out reasons given, withdrawals were much greater in the intervention treatment group |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on; it should be noted that adverse events were only reported for those that withdrew from treatment and not the whole randomised group, but as adverse events were not a specified outcome, this would not increase reporting bias |
| Other bias | Unclear risk | All data were related to the numbers of bypasses instead of numbers of participants (2 participants received bilateral grafts) |
Edmondson 1994.
| Study characteristics | ||
| Methods |
Study type:An open, multicentre, randomised controlled trial Study aim:To determine if low molecular weight heparin (LMWH) would be more effective than ASA and DIP in maintaining graft patency in patients undergoing femoropopliteal bypass grafting Country:UK |
|
| Participants |
Number randomised:Total n = 200 (ASA/DIP n = 106; LMWH n = 94) Age‐ mean years (SD):ASA/DIP 68.4 (9.6); LMWH 66.8 (9.2) Gender n (M/F):ASA/DIP 74/32; LMWH 61/33 Inclusion criteria:> 40 years old; angiographically‐proven arterial occlusive disease; scheduled to undergo femoropopliteal bypass surgery Exclusion criteria:currently taking oral anticoagulants; there was a known allergy to heparin, ASA or DIP; limb for operation had undergone previous femoropopliteal bypass grafting; the indication for bypass surgery was aneurysmal disease or trauma; there was a serious concomitant disease Co‐morbidity:smoking (ASA/DIP 31.1%; LMWH 28.7%), diabetes (ASA/DIP 19.8%; LMWH 14.9%), hyperlipidaemia (ASA/DIP 6.6%; LMWH 5.3%) Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:above‐knee (ASA/DIP 69.8%; LMWH 68.1%), below‐knee (ASA/DIP 27.4%; LMWH 27.7%), tibial vessels (ASA/DIP 2.8%; LMWH 4.3%) Type of graft:autologous vein (ASA/DIP 24.5%; LMWH 28.7%), prosthetic (PTFE and Dacron (ASA/DIP 46.2%; LMWH 30.9%)) |
|
| Interventions |
Treatment:ASA/DIP‐ 300 mg ASA and 100 mg DIP, both 3 times per day Control:2500 IU LMWH (Fragmin) subcutaneously once daily Duration: 3 months All the participants received LMWH subcutaneously 2 hours preoperatively, continuing for 1 week postoperatively, and at 1 week participants were randomised to continue LMWH or receive ASA/DIP |
|
| Outcomes | On the 7th postoperative day participants were assessed for primary graft occlusion, wound infection, thrombectomy, bleeding, and death. Total follow‐up was 1 year with visits at 1, 3, 6, and 12 months. Graft patency was evaluated clinically and by ABPI, and, if indicated, supplemented by arteriography or duplex sonography | |
| Notes | Indication for surgery was disabling claudication in 107 participants and salvage in 93 participants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation using computer‐generated random numbers was used 1 week after operation. |
| Allocation concealment (selection bias) | Low risk | Used central telephone service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as open |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Goldman 1984.
| Study characteristics | ||
| Methods |
Study type:Double‐blind, randomised controlled trial Study aim:To assess the effect of ASA and DIP on the patency of prosthetic femoropopliteal bypass grafts Country:UK |
|
| Participants |
Number randomised:Total n = 53 (ASA/DIP n = 22; placebo n = 31) Age‐ mean years:ASA/DIP 64.3; placebo 63.2 Gender n (M/F):ASA/DIP 16/6; placebo 27/4 Inclusion criteria:patients awaiting femoropopliteal bypass for occlusive disease Exclusion criteria:patients already receiving platelet inhibitory drugs; with a significant history of dyspepsia Co‐morbidity:Claudicants (ASA/DIP 3; placebo 8), rest pain/gangrene (ASA/DIP 19; placebo 23), smokers (ASA/DIP 19; placebo 24), diabetes mellitus (ASA/DIP 2; placebo 3) hypertension (ASA/DIP 4; placebo 6), ischaemic heart disease (ASA/DIP 7; placebo 11) Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:above‐knee anastomoses (ASA/DIP 10; placebo 19), below‐knee anastomoses (ASA/DIP 12; placebo 12) Type of graft:prosthetic (Dacron or PTFE) |
|
| Interventions |
Treatment:ASA/DIP‐ combination of 300 mg ASA and 75 mg DIP 3 times daily Control:placebo‐ identical to treatment drug Duration:started 48 h before surgery and continued for 12 months |
|
| Outcomes | Graft patency was assessed in monthly follow‐up visits up to 1 year based on reported symptoms, palpation, directional Doppler, and isotope angiography | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "… double blind to receive identical capsules containing either placebo or a combination of aspirin 300mg plus dipyridamole 75mg…" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for drop‐outs and all participants accounted for |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Groups did not appear comparable at baseline (differences in gender and above‐/below‐knee proportions) |
Green 1982.
| Study characteristics | ||
| Methods |
Study type:Double‐blind, randomised controlled trial Study aim:To asses the effect of aspirin alone or in combination with DIP on the patency rates of expanded PTFE grafts placed in the infrainguinal position Country:US |
|
| Participants |
Number randomised:Total n = 49 (ASA alone n = 16; ASA/DIP n = 16; placebo n = 17) Age:not indicated Gender:not indicated Inclusion criteria:All private patients of study authors RMG and JAD seen in the Peripheral Vaular Surgical Service of the University of Rochester Medical Center, Strong Memorial Hospital, beginning May 1979 2 required an infrainguinal bypass Exclusion criteria:Patients with known sensitivity to ASA or DIP; platelet or clotting abnormalities; gastrointestinal disturbances with a tendency to bleeding; require ASA for other medical conditions; infrainguinal autogenous saphenous vein bypass available; concomitant inflow disease Co‐morbidity:smoking (ASA 56%; ASA/DIP 56%; placebo 53%), diabetes (ASA 37%; ASA/DIP 50%; placebo 48%), previous femoropopliteal bypass (ASA 6%; ASA/DIP 19%; placebo 41%), Severity of occlusive disease:procedure performed for limb salvage (ASA 75%; ASA/DIP 87%; placebo 88%) Site of distal anastomosis:below‐knee (ASA 38%; ASA/DIP 56%; placebo 47%) Type of graft:prosthetic PTFE grafts |
|
| Interventions |
Treatment: 1) ASA alone‐ 325 mg ASA plus a red placebo tablet 3 times daily 2) ASA/DIP‐ 325 mg ASA and 75 mg DIP 3 times daily Control:Placebo‐ received red and white placebo tablets 3 times daily Duration:Started preoperatively: DIP or matching placebo started 48 hours prior to operation and ASA or matching placebo started 24 hours prior to surgery, and then for 1 year Patients were randomised to either treatment with 325 mg ASA once daily plus a placebo 3 times daily or 325 mg ASA once daily combined with 75 mg DIP 3 times per day for 1 year. Medication was started 48 hours preoperatively and continued for 1 year |
|
| Outcomes | Primary graft occlusion, withdrawal, and death were considered as treatment failures. Graft patency was assessed by clinical examination and ABPI change of 0.4 to 1.0. Follow‐up visits were every 3 months, or when problems arose, until death or withdrawal or for at least 1 year | |
| Notes | PTFE grafts when prosthetic graft was obligatory; all patients with available autologous saphenous veins for grafting were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although authors state "key to the randomization was kept in a sealed envelope and was opened only on emergency basis by an independent observer", the sequence generation was not adequately described |
| Allocation concealment (selection bias) | Low risk | "The key to the randomization was kept in a sealed envelope and was opened only on emergency basis by an independent observer" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The placebo group received red and white placebo tablets three times daily, the ASA group received 325 mg ASA and a red placebo tablet three times daily and the ASA/DIP group received 325 mg ASA and 75 mg DIP three times daily"; Use of coded bottles |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for drop‐outs and all participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | Adverse events not clearly reported |
| Other bias | Low risk | No indication of other bias |
Gruss 1991.
| Study characteristics | ||
| Methods |
Study type:Prospective, randomised controlled trial Study aim:Can a permanent intra‐arterial PGE1 therapy reduce the number of postoperative immediate and early closures in femoro‐crural great saphenous vein in situ bypasses Country:Germany |
|
| Participants |
Number randomised:Total n = 100 (ASA n = 50; PGE1 n = 50) Age:not indicated Gender (M/F):not indicated Inclusion criteria: patients with femorotibial greater saphenous vein in situ bypasses Exclusion criteria:not indicated Co‐morbidity:not indicated Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:not indicated Type of graft:autologous saphenous vein grafts |
|
| Interventions |
Treatment:ASA, 0.5 g 3 times daily and 15,000 IU heparin continuously Control:Intra‐arterial PGE1, 0.2 ng per kg body weight/min and 15,000 IU heparin continuously Duration:24 hours for the first 10 postoperative days |
|
| Outcomes | Early graft occlusion was the primary outcome of this study | |
| Notes | Study information came from a single abstract, with the majority of information in German | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No indication of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no discussion of drop‐outs or withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Not enough information in text to determine |
| Other bias | Low risk | No indication of other bias |
Kohler 1984.
| Study characteristics | ||
| Methods |
Study type:Double‐blind, randomised controlled trial performed from 1978 to 1982 Study aim:To determine whether ASA and DIP administered postoperatively to patients with PTFE or autologous vein infrainguinal bypasses would improve graft patency during the first 24 months after operation Country:US |
|
| Participants |
Number randomised:Total n = 100 (grafts = 102) (ASA/DIP n = 44 (grafts = 51); placebo n = 44 (grafts = 51) 12 patients were not included in the study post‐randomisation Age‐ mean years (SD):ASA/DIP 66 (12); placebo 66 (10) Gender n (M/F):ASA/DIP 34/10; placebo 34/10 Inclusion criteria:Private patients at the Beth Israel or Brigham and Women's Hospital who were scheduled for infrainguinal bypass procedures with either autologous saphenous vein or expanded PTFE grafts Exclusion criteria:Concomitant inflow disease; hypersensitivity to ASA or DIP; gastrointestinal bleeding; recently taken platelet‐activating drugs or anticoagulants for any reason; or if geographical inaccessibility prevented follow‐up Co‐morbidity:Diabetic (ASA/DIP n = 15; placebo n = 15), history of smoking (ASA/DIP n = 32; placebo n = 33), claudication indication for operation (ASA/DIP n = 16; placebo n = 19), threatened limb indication for operation (ASA/DIP n = 35; placebo n = 32) Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:Tibial (ASA/DIP n = 8; placebo n = 10), proximal popliteal (ASA/DIP n = 21; placebo n = 22), distal popliteal (ASA/DIP n = 22; placebo n = 24) Type of graft:vein grafts (ASA/DIP n = 36; placebo n = 35), PTFE grafts (ASA/DIP n = 15; placebo n = 16) |
|
| Interventions |
Treatment:ASA/DIP‐ ASA 325 mg and DIP 75 mg 3 times daily, oral route Control:placebo‐ identical placebo tablets 3 time daily, oral route Duration:Starting on first day postoperatively, continuing 24 months |
|
| Outcomes | Graft patency, the primary endpoint, was assessed at 3 and 6 weeks, further at 3, 6, 12, 18, 24 months postoperatively by evaluation of clinical symptoms, palpation of the graft, ABPI assessment, and if indicated by angiography | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…the research pharmacist randomized the patients to receive treatment or placebo according to a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used identical placebo with same schedule as active treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Discussed drop‐outs, but not consistently by which treatment group, drop‐outs > 10% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Lucas 1984.
| Study characteristics | ||
| Methods |
Study type:Randomised controlled trial Study aim:To compare post‐operative course over 6 months of 2 parallel cohorts of peripheral arteriopathy patients, 1 treated with pentoxifylline (PTX) and the other with conventional anti‐aggregant medication, ASA and DIP, with regard to the incidence of rethrombosis Country:Argentina |
|
| Participants |
Number randomised:Total n = 100 (ASA/DIP n = 49; PTX n = 48), three patients were excluded post‐randomisation Age‐ mean years (SD):ASA/DIP 66.1 (9.9); PTX 68.5 (8.2) Gender:ASA/DIP 71.4% males; PTX 80% males Inclusion criteria:not indicated Exclusion criteria:not indicated Co‐morbidity: Claudicants (ASA/DIP 28.6%; PTX 50%), ischaemia (ASA/DIP 71.4%; PTX 50%) Severity of occlusive disease (determined by ABPI):ASA/DIP 0.33 ± 0.33; PTX 0.46 ± 0.27 Site of distal anastomosis:not indicated Type of graft:autologous vein (ASA/DIP 12; PTX 12), synthetic prosthetic‐ Dacron and Foretex (ASA/DIP 34; PTX 30) |
|
| Interventions |
Treatment:ASA/DIP‐ 1050 mg/day ASA (divided into 3 doses) plus 150 mg/day DIP Control:PTX‐ 1200 mg/day (divided into 3 doses of 400 mg) Duration:6 months |
|
| Outcomes | Graft occlusion within 6 months was the primary endpoint, evaluated by clinical examination, ABPI, treadmill assessment of walking distance, and angiography. Follow‐up controls were at 1, 3, 4, and 6 months | |
| Notes | The study included patients with aortoiliac or femoropopliteal bypass or both; for the purposes of this review, only participants receiving femoropopliteal bypass were included and analysed; 14 participants were included in our analysis randomised to ASA/DIP and 19 to PTX | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned to one of the treatments using a random number table" |
| Allocation concealment (selection bias) | Low risk | "Once patients had been assigned, the individual envelope containing the treatment to be followed in each case was opened" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No indication of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis is performed with all randomised participants |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
McCollum 1991.
| Study characteristics | ||
| Methods |
Study type:Multicentre, double‐blind, randomised controlled trial, performed between 1984 and 1989 Study aim:To investigate whether ASA/DIP therapy improved graft patency in autologous saphenous vein femoropopliteal bypasses Country:UK |
|
| Participants |
Number randomised:Total n = 549 (ASA/DIP n = 286; placebo n = 263 Age‐ mean years (SEM):ASA/DIP 66.8 (0.5); placebo 66.6 (0.6) Gender:ASA/DIP 75.5% men; placebo 74.9% men Inclusion criteria:All patients undergoing femoropopliteal bypass; informed consent given Exclusion criteria:Contraindication to ASA or DIP; patient taking drugs with platelet‐inhibitory or anticoagulant effects that could not be stopped; adequate follow‐up could not be achieved Co‐morbidity:smoking (current) (ASA/DIP 42.2%; placebo 40.5%), hypertension (ASA/DIP 36.0%; placebo 35.0%), diabetes (ASA/DIP 18.2%; placebo 18.3%), IHD (ASA/DIP 26.2%; placebo 22.4%), claudication only (ASA/DIP 40.6%; placebo 39.6%), rest pain/gangrene (ASA/DIP 59.4%; placebo 60.4%) Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:Above‐knee (ASA/DIP 36%; placebo 46%) Type of graft:autologous saphenous vein |
|
| Interventions |
Treatment:ASA/DIP‐ 300 mg ASA plus 150 mg DIP, twice daily orally Control: Identical placebo, twice daily orally Duration:Starting 48 h before surgery, taken indefinitely (mean follow‐up was 35 months) |
|
| Outcomes | Primary endpoints were graft occlusion, MI and stroke. Patency assessment was performed by pulse palpation, Doppler signal or duplex sonography, DSA, isotope angiography at 3‐monthly intervals of follow‐ups during the first year, and 6‐monthly for 5 years | |
| Notes | Participants that required a prosthetic implant were given ASA/DIP and randomised to HUV or PTFE grafts; results of these participants are not currently reported Also known as the Femoropopliteal Bypass Trial |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was in batches of 10 to ensure that each hospital recruited similar numbers of patients on ASA + DPM or placebo"; Insufficient description of sequence generation |
| Allocation concealment (selection bias) | Low risk | Allocation centrally controlled by pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Used identical placebo with same schedule as active treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons given for drop‐outs and all participants accounted for. Analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Power calculation reported a study population of 800 participants to detect a 10% reduction in occlusion rate, but only 549 patients were randomised; groups not comparable at baseline (proportions with above‐knee grafts, which is a prognostic factor that can influence the outcomes) |
Noppeney 1988.
| Study characteristics | ||
| Methods |
Study type:Randomised controlled trial Study aim:To evaluate the antithrombotic efficacy of naftidrofuryl in comparison to ASA Country:Germany |
|
| Participants |
Number randomised:Total n = 99 (ASA n = 49; naftidrofuryl n = 50) Age‐ years:0 ‐ 39 (ASA 2%; naftidrofuryl 0%), 40 ‐ 49 (ASA 2%; naftidrofuryl 0%), 50 ‐ 59 (ASA 27%; naftidrofuryl 12%), 60 ‐ 69 (ASA 33%; naftidrofuryl 38%), 70 ‐ 79 (ASA 33%; naftidrofuryl 48%), 80 ‐ 89 (ASA 4%; naftidrofuryl 2%) Gender (M/F):not indicated Inclusion criteria:not indicated Exclusion criteria:not indicated Co‐morbidity:Hypertension (ASA/DIP n = 25; naftidrofuryl n = 23), hyperlipidaemia (ASA/DIP n = 36; naftidrofuryl n = 34), hyperuricaemia (ASA/DIP n = 14; naftidrofuryl n = 12), diabetes mellitus (ASA/DIP n = 32; naftidrofuryl n = 28), obesity (ASA/DIP n = 10; naftidrofuryl n = 11), smoker (ASA/DIP n = 34; naftidrofuryl n = 38) Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:not indicated Type of graft:PTFE grafts |
|
| Interventions |
Treatment:ASA 1500mg daily orally Control:naftidrofuryl 600 mg daily orally Duration:commenced on day 1 postoperatively for 12 months |
|
| Outcomes | Graft occlusion within 12 months was the primary endpoint, also reported side effects and treatment withdrawal | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No indication of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for; analysis on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Low risk | No indication of other bias |
Raithel 1987.
| Study characteristics | ||
| Methods |
Study type:Open, randomised controlled trial Study aim:To examine whether a protective effect associated with a low risk of side effects could be achieved with pentoxifylline (PTX), compared with ASA Country:Germany |
|
| Participants |
Number randomised:Total n = 118 (ASA n = 59; PTX n = 59) Age‐ mean years (range):Total study population 67.2 (42 to 87) Gender n (M/F):Total study population 88/30 Inclusion criteria:Patients who had undergone a prosthetic bypass operation in the femoropopliteal region Exclusion criteria:not indicated Co‐morbidity:Angina pectoris (ASA 15; PTX 21), myocardial infarction (ASA 39; PTX 42), coronary insufficiency (ASA 11; PTX 14), arrhythmias (ASA 5; PTX 8), hypertension (ASA 31; PTX 22), hyperlipidaemia (ASA 38; PTX 41), hyperuraemia (ASA 20; PTX 17), obesity (ASA 3; PTX 6), diabetes mellitus (ASA 45; PTX 32), smoking (ASA 42; PTX 46), operated on for critical ischemias (ASA 94.9 %; PTX 96.6%) Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:not indicated Type of graft:prosthetic grafts |
|
| Interventions |
Treatment:ASA‐ 500 mg 3 times daily Control:PTX ‐ 400 mg daily Duration:Starting 2 days before operation and continuing until 12 months postoperatively. |
|
| Outcomes | Graft patency was assessed by clinical examination, pulses, Doppler pressure, and arteriography when indicated, before and 3, 6, 9, 12 months postoperatively. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as an open study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Authors state: "One hundred eighteen patients… were available for the final assessment"; unclear how many were initially randomised, and whether the data are incomplete |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Groups do not appear to be comparable at baseline with respect to risk factors |
Schneider 1979.
| Study characteristics | ||
| Methods |
Study type:Single‐centre, double‐blind, randomised controlled trial, performed between 1974 and 1978 Study aim:To evaluate the prophylactic value of long‐term oral anticoagulation and inhibition of platelet aggregation Country:Switzerland |
|
| Participants |
Number randomised:Total n = 213 randomised to femoropopliteal bypass or thromboendarterectomy (TEA); n = 91 randomised to femoropopliteal bypass (ASA or ASA/DIP n = 61; coumarin n = 30) Age‐ mean years (SD):All participants receiving femoropopliteal bypass 63.9 (8.8) Gender (M:F ratio):All participants receiving femoropopliteal bypass 3.3:1 Inclusion criteria:Patients with atherosclerosis obliterans undergoing femoropopliteal reconstruction Exclusion criteria:not indicated Co‐morbidity:not indicated Severity of occlusive disease (determined by ABPI):not indicated Site of distal anastomosis:not indicated Type of graft:not indicated |
|
| Interventions |
Treatment:ASA or ASA/DIP‐ 1 g/day ASA or 1 g ASA and 225 mg DIP per day Control:coumarin ‐ (Quick, aim 25 ‐ 30%) Duration:2 years All participants were treated with therapeutic doses of heparin and coumarins in the first 1 to 2 postoperative weeks and were then randomly allocated to either coumarins (Quick, aim 25 ‐ 30%) or aspirin, or aspirin/dipyridamole |
|
| Outcomes | Graft patency was assessed angiographically before dismissal, further by evaluation of segmental oscillography and a fall of the systolic pressure of at least 20 mm Hg; Signs of occlusions were measured angiographically; Follow‐up period was 2 years with visits every 3 months during the first year and every 6 months in the second year; Drug assessment was performed by measurement of prothrombin time for coumarins and ASA levels in urine; Death and bleeding complications were recorded; Side effects like gastrointestinal bleeding or ulceration or pain or other bleeding complications could not be disclosed, as results were only presented for all participants, including those undergoing TEA | |
| Notes | This study randomised patients to receive either femoropopliteal bypass or TEA; only patients receiving femoropopliteal bypass are considered in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient description of randomisation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods not adequately described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors mention double‐blinding but the methods are unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not enough descriptive information |
| Selective reporting (reporting bias) | Unclear risk | Not enough information in text to determine |
| Other bias | Low risk | No indication of other bias |
ABPI: ankle brachial pressure index ASA: aspirin CAD: coronary artery disease CRVD: cerebrovascular disease DIP: dipyridamole DPM: dipyridamole (equivalent to DIP) DSA: digital subtraction angiography h: hour HUV: human umbilical vein IND: indobufen IU: international units iv: intravenous kg: kilograms LMWH: low molecular weight heparin m: metre mg: milligram MI: myocardial infarction min: minute MWD: maximum walking distance ng: nanograms PAD: peripheral arterial disease PGE1: prostaglandin E1 PTFE: polytetrafluoroethylene PTX: pentoxifylline SD: standard deviation TEA: thromboendarterectomy TIC: ticlopidine
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Böhmig 1995 | Not a controlled trial. 20‐year follow‐up study of infrainguinal venous bypass grafts. All participants received 100 IU/kg heparin iv intraoperatively, followed by 5000 IU 4 times daily sc in the first postoperative days, then dicoumarol lifelong. No control group |
| Burdess 2010 | Heterogeneous study population: bypass, femoral endartectomy and lower limb amputation without stratification |
| DeWeese 1971 | Non‐randomised, non‐controlled study on femoropopliteal venous bypass patency |
| Ehersmann 1977 | Heterogeneous cohort of patients. Results not separated for infrainguinal bypass surgery |
| EUCTR2007 2008 | Intervention is anticoagulant (fondaparinux) versus anticoagulant (enoxaparin) with no antiplatelet treatment |
| Gorter 2001 | Comparison of 2 studies, one of which is the BOA 2000 trial |
| Harjola 1981 | No clear distinction between bypass operations and other reconstructive therapies. No definition of 'major' and 'minor' injury. No details on claudicants, ischaemic participants, on distal insertion of the grafts, other risk factors like diabetes No description of randomisation procedure |
| Jivegard 2005 | Intervention is anticoagulant (LMWH) versus placebo. All participants received 75 mg of ASA daily |
| Johnson 2002 | Intervention is anticoagulant (warfarin) + ASA versus ASA alone i.e. both groups received ASA |
| Johnson 2004 | Intervention is anticoagulant (warfarin) + ASA versus ASA alone i.e. both groups received ASA |
| Kibbe 2002 | Intervention is anticoagulant (warfarin) + ASA versus ASA alone i.e. both groups received ASA |
| Lassila 1991 | 144 participants, randomised controlled trial. Mainly endarterectomy involved, ASA vs nothing. Results not presented separately |
| Monaco 2012 | Intervention is anticoagulant + clopidogrel versus clopidogrel + ASA. Only 1 treatment group received the anticoagulant, so there could be a drug interaction between the clopidogrel and anticoagulant that would make it impossible to directly compare with ASA |
| Nevelsteen 1991 | No relevant outcomes |
| Raithel 1987b | 99 participants assigned to treatment by ASA or naftidrofuryl in a randomised trial. Time point and method of randomisation are unclear. Data are only presented in an abstract mentioning cumulative patency rate 12 months postoperatively, which was 80% in both groups without any significant differences. The authors state that side effects occurred in 23 participants of the ASA group versus 3 participants in the naftidrofuryl group, without describing the type of side effect. Too little information is available for inclusion in the present meta‐analysis |
| Reichle 1979 | Retrospective non‐controlled, non‐randomised study |
| Rosenthal 1987 | Retrospective study |
| Satiani 1985 | Excluded because of pseudo‐randomisation (odd ages receiving ASA) |
| Shionoya 1990 | Intention‐to‐treat analysis is not possible as the results are only analysed for operated arterial segments and not for number of participants. In addition, bias has been introduced by exclusion of prosthetic grafts when final data were analysed |
| Smout 2004 | All patients received aspirin |
ASA: aspirin iv: intravenous LMWH: low molecular weight heparin sc: subcutaneous
Differences between protocol and review
We amended the outcome 'Limb salvage rate: survival rates with limb intact' to included 'or limb amputation', as most studies reported amputations, but not necessarily limb salvage rate.
We amended the outcome 'Side effects of treatment' to include 'and complications', as in several studies these two outcome types were grouped together.
Contributions of authors
RB: performed study selection, data extraction and quality assessment and wrote the review update AL: performed study selection, data extraction and quality assessment HM: provided support in updating the manuscript SA: provided support in updating the manuscript MP: provided support in updating the manuscript
Sources of support
Internal sources
No sources of support provided
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
RB: none known AL: none known HM: none known SA: none known MP: Prof Prins' institution has received a grant from ZON‐MW (not‐for‐profit organisation) for demonstrating cost‐effectiveness on the topic of this review. Prof Prins' institution has received funding for his board membership of clinical studies and consultancy from Bayer, Boeringer Ingelheim, Daiichi Sankyo, Pfizer and ISIS Pharmaceuticals. These were not related to this review.
Edited (no change to conclusions)
References
References to studies included in this review
Becquemin 1997 {published data only}
- Becquemin JP. Effect of ticlopidine on the long-term patency of saphenous-vein bypass grafts in the legs. New England Journal of Medicine 1997;337(24):1726-31. [DOI] [PubMed] [Google Scholar]
BOA 2000 {published data only}
- Algra A. A randomised trial of oral anticoagulants versus aspirin after infrainguinal bypass surgery. Tijdschrift voor Sociale Gezondheidszorg 1999;77(4):11.
- Ariesen MJ, Tangelder MJD, Lawson JA, Eikelboom BC, Grobbee DE, Algra A. Risk of major haemorrhage in patients after infrainguinal venous bypass surgery: Therapeutic consequences? The Dutch BOA (Bypass Oral Anticoagulants or Aspirin) study. European Journal of Vascular and Endovascular Surgery 2005;30(2):154-9. [DOI] [PubMed] [Google Scholar]
- Dutch Bypass Oral anticoagulants of Aspirin (BOA) Study Group. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral anticoagulants or Aspirin study): a randomised trial. Lancet 2000;355(9201):346-51. [PubMed] [Google Scholar]
- Eikelboom BC. Prevention of occlusions after infrainguinal bypass surgery with oral anticoagulants or acetylsalicylic acid: A randomised trial. Nederlands Tijdschrift voor Geneeskunde 2001;145(22):1060-7. [PubMed] [Google Scholar]
- Oostenbrink JB, Tangelder MJ, Busschbach JJ, Van Hout BA, Buskens E, Algra A, et al. Cost effectiveness of oral anticoagulants versus aspirin in patients after infrainguinal bypass grafting surgery. Journal of Vascular Surgery 2001;34(2):254-62. [DOI] [PubMed] [Google Scholar]
- Smeets L, Ho GH, Tangelder MJ, Algra A, Lawson JA, Eikelboom BC, et al, Dutch BOA Study Group. Outcome after occlusion of infrainguinal bypasses in the Dutch BOA Study: comparison of amputation rate in venous and prosthetic grafts. European Journal of Vascular and Endovascular Surgery 2005;30(6):604-9. [DOI] [PubMed] [Google Scholar]
- Tangelder MJ, Algra A, Lawson JA, Eikelboom BC. Risk factors for occlusion of infrainguinal bypass grafts. European Journal of Vascular and Endovascular Surgery 2000;20(2):118-24. [DOI] [PubMed] [Google Scholar]
- Tangelder MJ, Algra A, Lawson JA, Hennekes S, Eikelboom BC. Optimal oral anticoagulant intensity to prevent secondary ischemic and hemorrhagic events in patients after infrainguinal bypass graft surgery. Dutch BOA Study Group. Journal of Vascular Surgery 2001;33(3):522-7. [DOI] [PubMed] [Google Scholar]
- Tangelder MJ, Eikelboom BC, Lawson JA, Algra A. Prevention of occlusion following peripheral bypass surgery using oral anticoagulants or acetylsalicylic acid: a randomized comparison within the Dutch BOA study [Preventi van occlusies na perifere bypass-chirurgie met orale anticoagulantia of acetylsalicylzuur: een gerandomiseerde vergelijking in het Nederlands BOA-onderzoek]. Nederlands Tijdschrift voor Geneeskunde 1995;139(29):1504-6. [PubMed] [Google Scholar]
- Tangelder MJ, McDonnel J, Van Busschbach JJ, Buskens E, Algra A, Lawson JA, Eikelboom BC. Quality of life after infrainguinal bypass grafting surgery. Dutch Bypass Oral Anticoagulants or Aspirin (BOA) Study Group. Journal of Vascular Surgery 1999;29(5):913-9. [DOI] [PubMed] [Google Scholar]
- Van Hattum ES, Algra A, Lawson JA, Eikelboom BC, Moll FL, Tangelder MJD. Bleeding increases the risk of ischemic events in patients with peripheral arterial disease. Circulation 2009;120(16):1569-76. [DOI] [PubMed] [Google Scholar]
- Van Hattum ES, Tangelder MJD, Lawson JA, Moll FL, Algra A. The quality of life in patients after peripheral bypass surgery deteriorates at long-term follow-up. Journal of Vascular Surgery 2011;53(3):643-50. [DOI] [PubMed] [Google Scholar]
CASPAR 2010 {published data only}
- Anonymous. Clopidogrel and acetyl salicylic acid in bypass surgery for peripheral arterial disease. clinicaltrials.gov/ct2/show/NCT00174759?term=CASPAR&rank=2 (accessed 5 May 2014).
- Anonymous. Dual antiplatelet therapy benefits prosthetic but not venous grafts. Vascular News 2009;Feb:17. [Google Scholar]
- Belch JJ, Dormandy J. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. Journal of Vascular Surgery 2010;52(4):825-33. [DOI] [PubMed] [Google Scholar]
- The Caspar Investigators, Belch J. Effect of adding clopidogrel to aspirin on the success of below knee arterial bypass grafts. A randomised placebo controlled study. In: Vascular Annual Meeting; 2007 Jun; Baltimore, USA. 2007.
Clyne 1987 {published data only}
- Clyne CA, Archer TJ, Atuhaire LK, Chant AD, Webster JH. Random control trial of a short course of aspirin and dipyridamole (Persantin) for femorodistal grafts. British Journal of Surgery 1987;74(4):246-8. [DOI] [PubMed] [Google Scholar]
D'Addato 1992 {published data only}
- D'Addato M, Curti T, Bertini D, Donini I, Ferrero R, Fiorani P, et al. Indobufen vs acetylsalicylic acid plus dipyridamole in long-term patency after femoropopliteal bypass. International Angiology 1992;11(2):106-12. [PubMed] [Google Scholar]
Donaldson 1985 {published data only}
- Donaldson DR, Salter MC, Kester RC, Rajah SM, Hall TJ, Sreeharan N, et al. The influence of platelet inhibition on the patency of femoro-popliteal Dacron bypass grafts. Vascular Surgery 1985;19(4):224-30. [Google Scholar]
Edmondson 1994 {published data only}
- Edmondson RA, Cohen AT, Das SK, Wagner BM, Kakkar VV. Low-molecular weight heparin versus aspirin and dipyridamole after femoropopliteal bypass grafting. Lancet 1994;344(8927):914-8. [DOI] [PubMed] [Google Scholar]
Goldman 1984 {published data only}
- Goldman M, McCollum C. A prospective randomized study to examine the effect of aspirin plus dipyridamole on the patency of prosthetic femoro-popliteal grafts. Vascular Surgery 1984;18(4):217-21. [Google Scholar]
Green 1982 {published data only}
- Green RM, Roedersheimer RL, DeWeese JA. Effects of aspirin and dipyridamole on expanded polytetrafluoethylene graft patency. Surgery 1982;92(6):1016-26. [PubMed] [Google Scholar]
Gruss 1991 {published data only}
- Gruss JD, Hiemer W, Kroiss A, Geissler C. Experiences with adjuvant prostaglandin therapy in vascular surgery interventions. Vasa-Supplementum 1991;33:353-4. [PubMed] [Google Scholar]
Kohler 1984 {published data only}
- Kohler TR, Kaufman JL, Kacoyanis G, Clowes A, Donaldson MC, Kelly E, et al. Effect of aspirin and dipyridamole on the patency of lower extremity bypass grafts. Surgery 1984;96(3):462-6. [PubMed] [Google Scholar]
Lucas 1984 {published data only}
- Lucas MA. Prevention of post-operative thrombosis in peripheral arteriopathies. Pentoxifylline vs conventional antiaggregants: a six-month randomized follow-up study. Angiology 1984;35(7):443-50. [DOI] [PubMed] [Google Scholar]
McCollum 1991 {published data only}
- Franks PJ, Sian M, Kenchington GF, Alexander CE, Powell JT. Aspirin usage and its influence on femoro-popliteal vein graft patency. European Journal of Vascular Surgery 1992;6(2):185-8. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Sian M, Kenchington GF, Alexander CE, Powell JT. Aspirin usage and its influence on femoropopliteal vein graft patency. In: European Society for Vascular Surgery 5th Annual Meeting, 25-27 September 1991; Warsaw, Poland. 1991.
- McCollum C, Alexander C, Kenchington G, Franks PJ, Greenhalgh R. Antiplatelet drugs in femoropopliteal vein bypasses: A multicenter trial. Journal of Vascular Surgery 1991;13(1):150-62. [PubMed] [Google Scholar]
- Powell JT, Greenhalgh RM. Smoking and factors influencing the outcome of arterial reconstruction. Annales Chirurgiae et Gynaecologiae 1992;81(2):236-41. [PubMed] [Google Scholar]
Noppeney 1988 {published data only}
- Noppeney T, Kasprzak P, Raithel D, Hirche H. Naftidrofuryl in postoperative treatment after femoropopliteal bypass. Die Medizinische Welt 1988;39:1545-50. [Google Scholar]
Raithel 1987 {published data only}
- Raithel D, Kasprzak P, Noppeney Th. Relapse prophylaxis after femoropopliteal reconstruction with PTFE-prostheses. Die Medizinische Welt 1986;37:664-7. [Google Scholar]
- Raithel D. Prevention of reocclusion after prosthetic bypass operations in the femoro-popliteal region: a comparative study of pentoxifylline versus acetylsalicylic acid. Vascular Surgery 1987;21(3):208-14. [Google Scholar]
Schneider 1979 {published data only}
- Schneider E, Brunner U, Bollinger A. No English title available [Medikamentose Rezidivprophylaxe nach femoro-poplitealer Arterienrekonstruktion]. Angio 1979;2(1):73-7. [Google Scholar]
References to studies excluded from this review
Böhmig 1995 {published data only}
- Böhmig HJ, Zeidler G, Schwierz TH, Loy E. Orthograde vein bypass procedure for inguinal arterial reconstructions - 20 years experience [20 Jahre orthograder venen-bypass für infrainguinale arterielle rekonstruktion]. Chirurg 1995;66(2):120-6. [PubMed] [Google Scholar]
Burdess 2010 {published data only}
- Burdess A, Nimmo A, Garden OJ, Murie JA, Dawson AR, Fox KA, et al. Dual antiplatelet therapy in surgery for critical limb ischaemia. The Vascular Society of Great Britain & Ireland Yearbook 2008 2008:47.
- Burdess A, Nimmo AF, Garden OJ, Murie JA, Dawson AR, Fox KA, et al. Randomized controlled trial of dual antiplatelet therapy in patients undergoing surgery for critical limb ischemia. Annals of Surgery 2010;252(1):37-42. [DOI] [PubMed] [Google Scholar]
DeWeese 1971 {published data only}
- DeWeese JA, Rob CG. Autogenous venous bypass grafts five years later. Annals of Surgery 1971;174(3):346-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehersmann 1977 {published data only}
- Ehresmann U, Alemany J, Loew D. Use of acetylsalicylic acid in the prevention of re-occlusion following revascularization interventions. Results of a double blind long term study [Prophylaxe von rezidivverschlüssen nach revaskularisationseingriffen mit acetylsalicylsäure]. Medizinische Welt 1977;28(26):1157-62. [PubMed] [Google Scholar]
EUCTR2007 2008 {published data only}
- EUCTR2007-003746-15-DE. Prospective randomized open study on the comparison of fondaparinux with the low-molecular-weight heparin enoxaparin in patients undergoing femoro-distal venous bypass operation. www.clinicaltrialsregister.eu/ctr-search/trial/2007-003746-15/DE 2008.
Gorter 2001 {published data only}
- Gorter JW, Oostenbrink JB, Tangelder MJ, Stroke Pi, Reversible Ischemia Trial (SPIRIT) Study Group, Dutch Bypass O, Anticoagulants or Aspirin Study (BOA). Costs of outpatient anticoagulant treatment in patients with cerebral and peripheral arterial occlusive disease. Thrombosis and Haemostasis 2001;85(1):52-6. [PubMed] [Google Scholar]
Harjola 1981 {published data only}
- Harjola PT, Meurala H, Frick MH. Prevention of early reocclusion by dipyridamole and ASA in arterial reconstructive surgery. Journal of Cardiovascular Surgery 1981;22(2):141-4. [PubMed] [Google Scholar]
Jivegard 2005 {published data only}
- Jivegard L, Drott C, Gelin J, Groth O, Hensater M, Jensen N, et al. Effects of three months of low molecular weight heparin (Dalteparin) treatment after bypass surgery for lower limb ischemia - A randomised placebo-controlled double blind multicentre trial. European Journal of Vascular and Endovascular Surgery 2005;29(2):190-8. [DOI] [PubMed] [Google Scholar]
Johnson 2002 {published data only}
- Johnson WC, Williford WO, Department of Veterans Affairs Cooperative Study # 362. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: a prospective randomized study. Journal of Vascular Surgery 2002;35(3):413-21. [DOI] [PubMed] [Google Scholar]
Johnson 2004 {published data only}
- Johnson WC, Williford WO, Corson JD, Padberg FT Jr. Hemorrhagic complications during long-term postoperative warfarin administration in patients undergoing lower extremity arterial bypass surgery. Vascular 2004;12(6):362-8. [DOI] [PubMed] [Google Scholar]
Kibbe 2002 {published data only}
- Kibbe MR, Hassett AL, McSherry F, Conner P, Bontempo FA, Williford W, et al. Can screening for genetic markers improve peripheral artery bypass patency? Journal of Vascular Surgery 2002;36(6):1198-206. [DOI] [PubMed] [Google Scholar]
Lassila 1991 {published data only}
- Lassila R, Lepäntalo M, Lindfors O. The effect of acetylicsalicylic acid on the outcome after lower limb arterial surgery with special reference to cigarette smoking. World Journal of Surgery 1991;15(3):378-82. [DOI] [PubMed] [Google Scholar]
Monaco 2012 {published data only}
- Monaco M, Di Tommaso L, Pinna GB, Lillo S, Schiavone V, Stassano P. Combination therapy with warfarin plus clopidogrel improves outcomes in femoropopliteal bypass surgery patients. Journal of Vascular Surgery 2012;56(1):96-105. [DOI] [PubMed] [Google Scholar]
Nevelsteen 1991 {published data only}
- Nevelsteen A, Mortelmans L, Van de Cruys A, Merckx E, Verhaeghe R. Effect of ticlopidine on blood loss, platelet turnover and platelet deposition on prosthetic surfaces in patients undergoing aorto-femoral bypass grafting. Thrombosis Research 1991;64(3):363-9. [DOI] [PubMed] [Google Scholar]
Raithel 1987b {published data only}
- Raithel D, Noppeney T, Kasprzak P. Medikamentöse Therapie nach femoro.poplitealem Kunststoffbypass - naftidrofuryl versus ASS. In: Vasa-Supplementum. Vol. 20. 1987:223-Abstract No 78.
Reichle 1979 {published data only}
- Reichle FA, Rankin KP, Tyson RR, Finestone AJ, Shuman C. Long-term results of 474 arterial reconstructions for severely ischemic limbs: A fourteen year follow-up. Surgery 1979;85(1):93-100. [PubMed] [Google Scholar]
Rosenthal 1987 {published data only}
- Rosenthal D, Mittenthal MH, Ruben DM, Jones DH, Estes JW, Stanton PE, et al. The effects of aspirin, dipyridamole and warfarin in femorodistal reconstruction: long-term results. American Surgeon 1987;53(9):477-81. [PubMed] [Google Scholar]
Satiani 1985 {published data only}
- Satiani B. A prospective randomized trial of aspirin in femoral popliteal and tibial bypass grafts. Angiology 1985;36(9):608-16. [DOI] [PubMed] [Google Scholar]
Shionoya 1990 {published data only}
- Shionoya S, Sakurai T, Ueyama T, Kusakawa M, Sakaguchi S, Tsuchioka H, et al. Effect of ticlopidine on graft patency following arterial reconstructive surgery in the lower extremity: a multicenter three-year prospective study. Vascular Surgery 1990;24(8):541-7. [Google Scholar]
Smout 2004 {published data only}
- Smout JD, Mikhailidis DP, Shenton BK, Stansby G. Combination antiplatelet therapy in patients with peripheral vascular bypass grafts. Clinical and Applied Thrombosis/Hemostasis 2004;10(1):9-18. [DOI] [PubMed] [Google Scholar]
Additional references
Abbott 1997
- Abbott WM, Green RM, Matsumoto T, Wheeler JR, Miller N, Veith FJ, et al. Prosthetic above-knee femoropopliteal bypass grafting: results of a multicenter randomized prospective trial. Above-Knee Femoropopliteal Study Group. Journal of Vascular Surgery 1997;25(1):19-28. [DOI] [PubMed] [Google Scholar]
Baril 2013
- Baril D, Patel V, Judelson D, Goodney P, McPhee J, Hevelone N, et al. Outcomes of lower extremity bypass performed for acute limb ischemia. Journal of Vascular Surgery 2013;58(4):949-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2011
- Becker F, Robert-Ebadi H, Ricco JB, Setacci C, Cao P, De Donato G. Guidelines for critical limb ischaemia and diabetic foot, Chapter I: Definitions, Epidemiology, Clinical presentation and Prognosis. European Journal of Vascular and Endovascular Surgery 2011;42:4-12. [DOI] [PubMed] [Google Scholar]
Consensus 1991
- European Working Group on Critical Limb Ischemia Second European Consensus Document on Chronic Critical Leg Ischemia. Second European Consensus Document on Chronic Critical Leg Ischemia. Circulation 1991;84 Suppl 4:1-26. [Google Scholar]
Cooper 1990
- Cooper GG, Austin C, Fitzsimmons E, Brannigan PD, Hood JM, D'Sa AA. Outflow resistance and early occlusion of infrainguinal bypass grafts. European Journal of Vascular Surgery 1990;4(3):279-83. [DOI] [PubMed] [Google Scholar]
Fujitani 1988
- Fujitani RM, Nordestgaard AG, Marcus CS, Wilson SE. Perioperative suppression of platelet adherence to small-diameter polytetrafluoroethylene (PTFE) grafts. Journal of Surgical Research 1988;44(4):455-60. [DOI] [PubMed] [Google Scholar]
Geraghty 2011
- Geraghty AJ, Welch K. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No: CD000536. [DOI: 10.1002/14651858.CD000536.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
NICE 2013
- Anon. Antiplatelet treatment- summary. National Institue for Health and Care Excellence (NICE) 2013;cks.nice.org.uk/antiplatelet-treatment#!topicsummary((Accessed July 1 2014)).
Rutherford 1988
- Rutherford RB, Jones DN, Bergentz SE, Bergqvist D, Comerota AJ, Dardik H, et al. Factors affecting the patency of infrainguinal bypass. Journal of Vascular Surgery 1988;8(3):236-46. [PubMed] [Google Scholar]
Tangelder 1999
- Tangelder MJ, Lawson JA, Algra A, Eikelboom BC. Systematic review of randomized controlled trials of aspiring and oral anticoagulants in the prevention of graft occlusion and ischemic events after infrainguinal bypass surgery. Journal of Vascular Surgery 1999;30(4):701-9. [DOI] [PubMed] [Google Scholar]
Tangelder 2000
- Tangelder MJ, Algra A, Lawson JA, Eikelboom, BC. Risk factors for occlusion of infrainguinal bypass grafts. European Journal of Vascular and Endovascular Surgery 2000;20(2):118-24. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Adams 1997
- Adams D, Stonebridge PA. Antiplatelet therapy after peripheral arterial bypass surgery. Cochrane Database of Systematic Reviews 1997, Issue 4. Art. No: CD000535. [DOI: 10.1002/14651858.CD000535] [DOI] [Google Scholar]
Brown 2008
- Brown J, Lethaby A, Maxwell H, Wawrzyniak AJ, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD000535. [DOI: 10.1002/14651858.CD000535.pub2] [DOI] [PubMed] [Google Scholar]
Dörffler‐Melly 2003
- Dörffler-Melly J, Büller HR, Koopman MM, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD000535. [DOI: 10.1002/14651858.CD000535] [DOI] [PubMed] [Google Scholar]


