Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia, and it is associated with an increased risk of heart failure, stroke, dementia, and death. Recently, titin-truncating variants (TTNtv), which are predominantly associated with dilated cardiomyopathy (DCM), were associated with early-onset AF. Furthermore, genome-wide association studies (GWAS) associated AF with other structural genes. In this study, we investigated whether early-onset AF was associated with loss-of-function variants in DCM-associated genes encoding cytoskeletal proteins. Using targeted sequencing, we examined a cohort of 527 Scandinavian individuals with early-onset AF and a control group of individuals free of AF (n = 383). The patients had onset of AF before 50 years of age, normal echocardiogram, and no other cardiovascular disease at onset of AF. We identified six individuals with rare loss-of-function variants in three different genes (dystrophin (DMD), actin-associated LIM protein (PDLIM3), and fukutin (FKTN)), of which two variants were novel. Loss-of-function variants in cytoskeletal genes were significantly associated with early-onset AF when patients were compared with controls (p = 0.044). Using publicly available GWAS data, we performed genetic correlation analyses between AF and 13 other traits, e.g., showing genetic correlation between AF and non-ischemic cardiomyopathy (p = 0.0003). Our data suggest that rare loss-of-function variants in cytoskeletal genes previously associated with DCM may have a role in early-onset AF, perhaps through the development of an atrial cardiomyopathy.
Keywords: atrial fibrillation, genetics, arrhythmia, cardiology, next-generation sequencing, cardiomyopathy
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and affects more than 30 million individuals worldwide [1]. AF is associated with an increased risk of serious complications such as stroke, heart failure, dementia, and death [2]. At present, the disease represents a significant healthcare burden [3], and the prevalence is expected to increase more than two-fold in the coming decades [4]. The complex pathophysiological mechanisms behind AF initiation and maintenance are not yet completely understood. Current pharmacological treatments are limited in efficacy or associated with significant adverse effects [5]. AF also has a genetic component, which currently includes 166 common genetic variants [6] and rare variants in a variety of genes [7]. Whereas AF is traditionally regarded as an electrical disease, two recent studies identified loss-of-function (LOF) variants in the structural sarcomere gene titin (TTN) to be significantly enriched in early-onset AF patients [8,9]. In addition, several large genome-wide association studies (GWAS) associated structural genes with the disease [6,10], and an expert consensus document from several scientific societies suggested AF to overlap with an atrial cardiomyopathy [11].
There is substantial evidence that genes encoding cytoskeletal proteins are involved in skeletal muscle myopathies, many of which also have cardiac involvement [12]. Interestingly, the cytoskeletal gene SGCG, encoding the gamma-sarcoglycan protein, was recently associated with AF in a large GWAS [6]. SGCG was previously associated with skeletal muscle myopathies, and it is thought to be linked with dilated cardiomyopathy (DCM) [13]. The newly identified association between SGCG and AF suggests that cytoskeletal genes might play a role in AF, supporting the hypothesis of an atrial cardiomyopathy.
Here, we aim to assess the role of cytoskeletal genes in AF, by focusing on rare LOF variants in a highly selected cohort of AF patients with early onset of disease.
2. Materials and Methods
2.1. Study Populations
Our case population included 527 Danish and Norwegian patients with onset of AF at age < 50 and presenting with no other cardiovascular disease. The diagnosis of AF was defined by ICD-10 code I48. Patients with diabetes, hypertension, hyperthyroidism, congenital heart disease, valvular heart disease, or congestive heart failure were excluded. We also excluded patients with a left ventricular ejection fraction <55%, as determined by echocardiographic examination. Danish patients were identified through the National Danish Patient Registry, while Norwegian patients were identified and recruited through clinical practice and the Norwegian Atrial Fibrillation Registry. The study was approved by the Scientific Ethics Committee of Copenhagen and Frederiksberg (Protocol reference number: H-KF-01313322) and the Regional Ethics Committee (REK) in Norway (Protocol reference number: 2009/2224-5).
The control population consisted of 383 Danish individuals from the Copenhagen Holter Study. These individuals were monitored for arrhythmia with Holter monitors for 48 h and were known to be free of AF and cardiovascular disease. The cohort was previously described in detail elsewhere [14]. Characteristics of the study populations are summarized in Table 1. The control group was approved by the Scientific Ethics Committee of Copenhagen and Frederiksberg (Protocol reference number: KF 01 25304).
Table 1.
Clinical characteristics of populations.
| Early-Onset AF Cohort (n = 527) | Control Cohort (n = 383) | |
|---|---|---|
| Sex, male, n (%) | 441 (83.6) | 257 (67) |
| Age, years, median (IQR) | 30 (24–36) a | 71 (66–76) b |
| Height, cm, mean (SD) | 183 (±9) | 172 (±9) |
| Weight, kg, mean (SD) | 89 (±17) | 77 (±15) |
| BMI, kg/m2, mean (SD) | 26 (±5) | 26 (±5) |
| Comorbidities: | ||
| Hypertension, n (%) | 0 (0) | 246 (64.2) |
| Diabetes, n (%) | 0 (0) | 39 (10.2) |
| Heart failure, n (%) | 0 (0) | 0 (0) |
| Ischemic heart disease, n (%) | 0 (0) | 0 (0) |
| Valvular heart disease, n (%) | 0 (0) | 0 (0) |
AF, atrial fibrillation; BMI, body mass index; IQR, interquartile range; SD, standard deviation. a Age of AF onset; b age at enrolment in cohort.
2.2. Genetic Sequencing
We extracted genomic DNA (gDNA) from leukocytes in peripheral blood samples from study participants in both case and control populations. The gDNA was fragmented with endonucleases and hybridized with gene-specific probes from the Illumina TruSight Cardio Sequencing Kit. The hybridized fragments were captured with magnetic beads, PCR-amplified, and sequenced using Illumina HiSeq 2500 and NextSeq technology. Reads were aligned to the Human Reference Genome (NCBI Genome Build 37) using the Burrow Wheelers Aligner algorithm [15], and post-processed in accordance with Genome Analysis Toolkit version 3.4 (GATK) guidelines [16].
2.3. Selection of Candidate Genes
We focused on genes encoding cytoskeletal proteins in cardiomyocytes, which were reported to be linked with DCM in previous literature [17]. Our candidate genes consisted of the following genes: DMD (dystrophin), CRYAB (crystallin alpha B), DES (desmin), PDLIM3 (actin-associated LIM protein), FKTN (fukutin), FKRP (fukutin-related protein), LAMA4 (laminin subunit alpha 4), and SGCD (delta-sarcoglycan).
2.4. Evaluation of Identified Variants
Annotation of variant coding DNA and consequence of variants were performed using the tool TransVar [18]. LOF variants were defined as variants leading to a premature stop codon in coding DNA, or variants affecting splice donor or splice acceptor sites. In genes producing multiple protein isoforms, we focused on isoforms expressed in atrial tissue. Isoform expression analysis was conducted using the online database The Genotype Tissue Expression Database version 8 (GTEx v8) [19].
2.5. Pathway Analysis
We performed a pathway analysis of protein-protein interactions, using tool STRING [20]. Pathway analysis was performed on the human protein products of all genes in which LOF variants were identified. The results were filtered, and interactions were assigned confidence scores. The methods for calculating these confidence scores were described in detail elsewhere [21]. For each gene, we focused on the ten protein-protein interactions with the highest confidence scores, and we excluded interactions with a confidence score <0.700.
2.6. Statistical Analyses
Due to the small sample size, we used Fisher’s exact test for comparisons of variants in patients versus controls. Using an LD regression score [19], we also examined the genetic correlation between AF and 13 other traits: alcohol dependence, angina, body mass index (BMI), coronary heart disease, depression, diabetes type 2, smoking, hand grip strength, heart failure, height, hypertension, non-ischemic cardiomyopathy, and overall health rating. These analyses were based on a similar analysis conducted in a previous study [22], modified to also include non-ischemic cardiomyopathy. The GWAS data were derived from publicly available summary statistics, summarized in Table S1 (Supplementary Materials). We applied Bonferroni correction to account for multiple testing, and p < 0.05/13 was considered significant. Statistical analyses were performed using the software R, version 3.6.0.
3. Results
3.1. Clinical Characteristics
We identified six patients with LOF variants in genes encoding cytoskeletal proteins. The clinical characteristics of the individuals with LOF variants are summarized in Table 2. Four Danish patients (I through IV) harbored LOF variants in DMD, whereas two Norwegian patients carried LOF variants in FKTN (patient V) and PDLIM3 (patient VI), respectively.
Table 2.
Clinical characteristics of variant carriers.
| Patient | Gene | Variant | RefSNP | Gender | Genotype | Onset of AF (age in years) |
AF Type | LVEF (%) |
Family History of AF (self-reported) |
|---|---|---|---|---|---|---|---|---|---|
| I | DMD | p.D615Efs*6 | rs752332058 | Male | Hemizygote | 28 | Persistent | >55 | No |
| II | DMD | p.D615Efs*6 | rs752332058 | Male | Hemizygote | 25 | Persistent | >55 | Yes |
| III | DMD | c.10262+1G > A | rs145603325 | Male | Hemizygote | 21 | Persistent | >55 | Yes |
| IV | DMD | c.10262+1G > A | rs145603325 | Male | Hemizygote | 28 | Persistent | >55 | No |
| V | FKTN | Chr9:108358933C > T | NA | Male | Heterozygote | 31 | Paroxysmal | NA | No |
| VI | PDLIM3 | Chr4:186425651_186425652del | NA | Female | Heterozygote | 40 | Paroxysmal | >55 | Yes |
AF, atrial fibrillation; LVEF, left-ventricular ejection fraction; NA, not available; RefSNP, reference single-nucleotide polymorphism.
All six patients had very early onset of disease; the maximum age at onset of disease was 40 years. All four patients with DMD variants had onset of AF before age 30 and were all diagnosed with persistent AF. Interestingly, five of the six patients were male, including all of the four patients with DMD variants, making them hemizygous for the variants. The individuals with LOF variants in FKTN and PDLIM3 were both heterozygous for the variants.
Three of the six patients self-reported family history of AF (Table 2). These family members were unfortunately not available for genetic sequencing.
3.2. Genetic Variation
We identified four different LOF variants in three cytoskeletal genes. Two of the LOF variants were identified in DMD, one in FKTN, and one in PDLIM3.
Two Danish AF patients carried a variant in DMD, which affected the splice donor sites in multiple DMD isoforms expressed in cardiac tissue, notably isoform Dp427m (ENST00000357033) and isoform Dp71b (ENST00000378723). In Dp427m, it affected the donor splice site between exon 71 and exon 72, while affecting the donor splice site between exon 10 and 11 in Dp71b. Another variant in DMD was present in two other Danish patients. This variant resulted in a frameshift in several DMD isoforms, including the isoforms Dp71b (ENST00000378723) and Dp260 (ENST00000358062). We identified the variant FKTN p.Q54X that caused a stop codon in exon 3 in one Norwegian AF patient. Finally, one Norwegian individual harbored a frameshift variant in PDLIM3 (p.C246*fs*1), resulting in a premature stop-codon in exon 6. The characteristics of all variants are summarized in Table 3. Variants affecting multiple protein isoforms are summarized in Table S2 (Supplementary Materials).
Table 3.
Loss-of-function variants identified in cytoskeletal genes.
| Gene | Genomic Position | RefSNP | Transcript | Consequence | Effect | GnomAD MAF (%) |
|---|---|---|---|---|---|---|
| DMD | ChrX:31140001_31140013del | rs752332058 | ENST00000378723 | p.D615Efs*6 | Frameshift variant | 0.02491 |
| DMD | ChrX:31196048C>T | rs145603325 | ENST00000357033 | c.10262+1G>A | Splice donor | 0.02689 |
| FKTN | Chr9:108358933C>T | NA | ENST00000223528 | p.Q54* | Nonsense variant | NA |
| PDLIM3 | Chr4:186425651_186425652del | NA | ENST00000284771 | p.C246*fs*1 | Frameshift variant | NA |
GnomAD, Genome Aggregation Database; MAF, minor allele frequency; NA, not available; RefSNP, reference single-nucleotide polymorphism.
The association test showed a significantly larger proportion of LOF variants in cytoskeletal genes in early-onset AF patients compared with the controls free of AF (p = 0.044).
3.3. Genetic Correlation
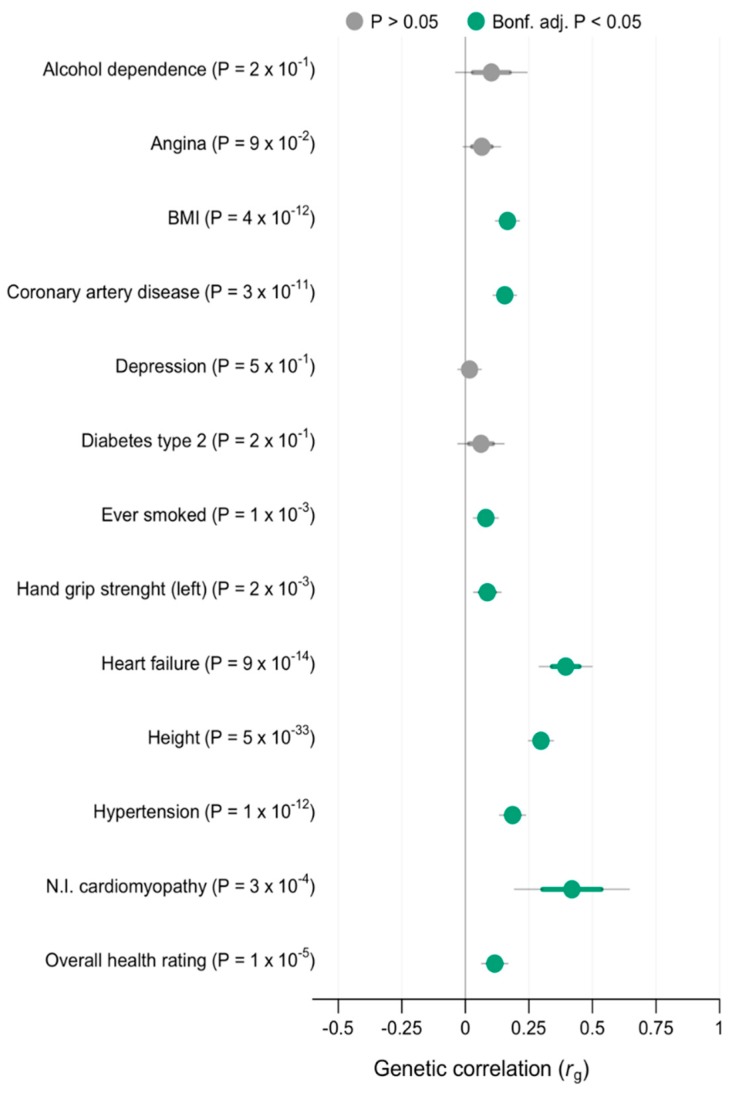
We found a significant, moderate genetic correlation (rg = 0.40–0.59) between AF and non-ischemic cardiomyopathy (rg = 0.42 (SE = 0.12); p = 3 × 10−4). We also found a significant, small correlation between AF and heart failure (rg = 0.39 (SE = 0.05); p = 9 × 10−14), height (rg = 0.30 (SE = 0.02); p = 5 × 10−33), hypertension (rg = 0.19 (SE = 0.02); p = 1 × 10−12) BMI (rg = 0.17 (SE = 0.02); p = 4 × 10−12), coronary heart disease (rg = 0.16 (SE = 0.02); p = 3 × 10−11), overall health rating (rg = 0.12 (SE = 0.03); p = 1 × 10−5), smoking (rg = 0.08 (SE = 0.03); p = 1 × 10−3), and hand grip strength (left hand) (rg = 0.08 (SE = 0.03); p = 2 × 10−3). We found no significant genetic correlation between AF and alcohol dependence, angina, depression, and diabetes type 2. Genetic correlation results are illustrated in Figure 1.
Figure 1.
Genetic correlation between AF and 13 other traits with 95% and 99% confidence intervals. Correlations that were significant when accounting for multiple testing are marked in green. Bonf. adj., Bonferroni adjusted; BMI, body mass index; N.I. cardiomyopathy, non-ischemic cardiomyopathy.
3.4. Pathway Analysis
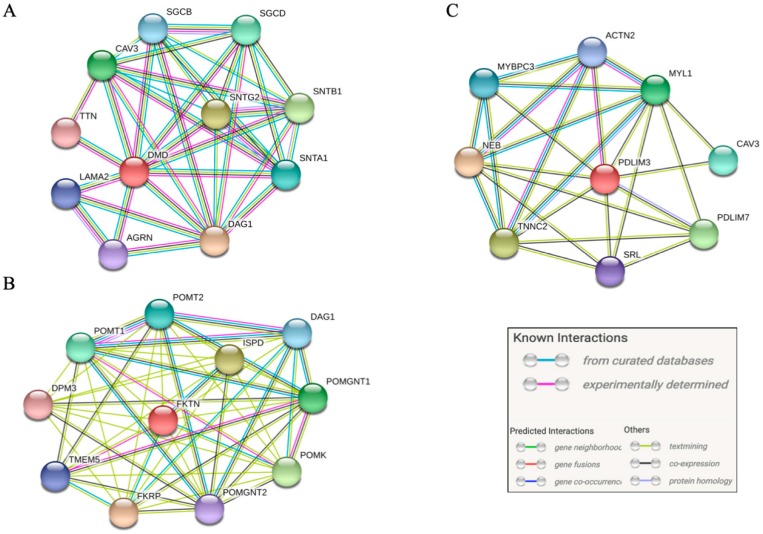
We found interactions between the protein products of DMD, FKTN, and PDLIM3 and numerous other genes. DMD had protein-protein interactions with more than 10 other genes, with the TTN gene among top ten interactions with highest confidence score. FKTN also interacted with more than 10 other genes, while PDLIM3 had protein-protein interactions with eight other genes. The top ten protein-protein interactions with highest confidence scores for all three genes are summarized in Table S3 (Supplementary Materials). Interaction networks are illustrated in Figure 2.
Figure 2.
Protein-protein interactions with high confidence score (confidence score >0.7). (A) interactions of the product of DMD, (B) interactions of the product of FKTN, and (C) interactions of the product of PDLIM3.
4. Discussion
In this study, we identified four different LOF variants in three cytoskeletal genes (DMD, FKTN, and PDLIM3) carried by six individuals with early-onset AF. There was a significant enrichment of LOF variants in cytoskeletal genes in the case population compared to an arrhythmia-free control population, in which no individuals carried LOF variants.
4.1. Variants in DMD
Four Danish patients with early-onset AF carried two different LOF-variants in DMD. The variant-carriers had a considerable burden of disease; all four presented with very early onset of disease (age <30 years) and developed persistent AF (Table 3). Deleterious variants in DMD are associated with the serious muscular dystrophies Duchenne and Becker, in which skeletal muscle function is severely impaired along with serious cardiomyopathies [23]. However, none of the participants harboring variants in DMD were diagnosed with skeletal muscle dysfunction. DMD produces numerous isoforms [24], and predicting the effect of variants in the different isoforms on cardiac function is challenging. Several cases of DMD-associated, X-linked cardiomyopathy without a skeletal muscle phenotype were reported [25], which emphasizes the complex pathophysiology of DMD variants. Notably, all four individuals with DMD variants were male and, therefore, hemizygous for the variants. It is possible that these variants, while having arrhythmogenic effects in males, are less pathogenic in females who have a second copy of the DMD gene.
Of the two DMD LOF variants, the frameshift variant (p.D615Efs*6) did not affect the primary isoform expressed in skeletal and cardiac muscle, Dp427m. The variant did, however, affect several other isoforms expressed in cardiac tissue, including Dp71b (ENST00000378723), a product of alternative splicing of the ubiquitously expressed Dp71(24), and numerous other isoforms such as Dp260 (see Table S2, Supplementary Materials). Although the exact function of these isoforms in the heart is not yet known, the Dp71b isoform with the transcript ENST00000378723 is highly expressed in the atria (Figure S1, Supplementary Materials), and previous studies showed the Dp260 isoform to be expressed in cardiac tissue [26].
The DMD splice donor variant (c.10262+1G > A) affected numerous isoforms, including Dp427m, an isoform important for the function of skeletal and cardiac muscle function. Interestingly, this variant was previously reported in a patient who suffered from sudden cardiac death [27], which supports possible proarrhythmic effects of the variant. Studies of lethal cardiac diseases in recent years emphasizes that the pathogenicity of genetic variants in conserved genes may not be as malignant as previously thought [28,29]. It is acknowledged that rare variants have different disease-causing effects and that disease burden and severity are affected by a combination of rare and common variants, in addition to other non-genetic factors [30]. Therefore, the DMD splice donor variant may predispose to lethal arrhythmia in one individual while causing less lethal disease in another. The variant c.10262+1G > A had a minor allele frequency (MAF) of 0.027% in gnomAD, indicating that it is not a major contributor of highly malignant disease.
Additionally, pathway analysis (see Figure 2A) suggests protein-protein interactions between DMD and several other genes expressed in the heart. Notably, DMD interacts with TTN, a gene which was previously linked with AF [6,8].
4.2. Variants in FKTN and PDLIM3
We identified LOF variants in the cytoskeletal genes FKTN and PDLIM3 in two Norwegian patients with early-onset AF. Both variants identified were novel and, to the best of our knowledge, not reported elsewhere.
FKTN is associated with the rare muscular dystrophy, Fukuyama-type muscular dystrophy [31]. However, FKTN variants were in some cases also linked with DCM with minimal skeletal muscle involvement [17,32], and our pathway analysis (Figure 2B) predicts protein-protein interactions between the product of FKTN and the product of FKRP (fukutin-related protein), among others. FKRP was previously associated with DCM [33].
Similarly, PDLIM3 was also associated with both DCM [17,34] and muscular dystrophy, in the form of myotonic dystrophy [35]. The protein product of PDLIM3 is predicted to interact with the protein products of several other genes that are linked with heart disease, including MYBPC3, ACTN2, and CAV3 [13] (Figure 2C).
4.3. Atrial Cardiomyopathy
Our understanding of the pathophysiological disease mechanisms of disease-causing genetic variants changed in recent years. Evidence from large-scale GWAS and high-throughput sequencing projects [6,10] suggests that diseases, which previously were thought to have completely different underlying mechanisms, may be more closely related, and even part of a continuum. For instance, recent reports of an enrichment of titin-truncating variants (TTNtv) in familial and early-onset AF [8], and the association of exonic SNPs in TTN with AF in a large GWAS [6] support the hypothesis of an atrial cardiomyopathy [11].
Moreover, our analysis of genetic correlation between AF and other traits (Figure 1) shows a significant genetic correlation between AF and non-ischemic cardiomyopathy (rg = 0.42; p = 3 × 10−4), further supporting the hypothesis of atrial cardiomyopathy playing a role in AF development.
4.4. Treatment Consequences
Catheter ablation of AF is a class I recommendation when antiarrhythmic treatment fails [2]. Several studies showed that AF patients with a higher degree of fibrosis in the atria have higher recurrence rates after catheter ablation [36]. It was, therefore, discussed whether ablative therapy is beneficial when AF occurs as a result of atrial cardiomyopathy [37]. Ongoing studies are evaluating whether detection of fibrosis before ablation, using late gadolinium enhancement magnetic resonance, may improve the ablation of AF in fibrotic hearts [38]. Several of the genes examined in this study are linked with cardiac fibrosis. Cardiac fibrosis is a well-known clinical feature of DMD-associated DCM [39], and PDLIM3 was shown to be involved in cardiac collagen deposition [40].
Finally, these patients might be at higher risk of tachycardia-induced cardiomyopathy in the ventricles, as they are already predisposed for DCM by carrying variants in genes associated with DCM. Understanding the pathogenesis of disease in patients carrying variants in these genes could yield valuable knowledge about the treatment of AF and atrial cardiomyopathy.
4.5. Limitations
While the study included a relatively large number of participants, we only identified a total of six patients with LOF variants. Four of these were in the DMD gene, likely because of its large size (79 exons) and numerous isoforms, while only two of the variants were in other cytoskeletal genes. Furthermore, most of the individuals with LOF variants were male, including the four carriers of DMD variants, who were hemizygous for the variants. It cannot be excluded that these variants, or similar LOF variants in cytoskeletal genes could have other effects in other population groups.
Several of the participants reported family members with AF. However, these individuals were not genetically tested and, therefore, it was not possible to perform co-segregation analyses. The lack of genetic testing of family members is a significant limitation of the study.
Furthermore, our genetic analyses were focused on genes encoding cytoskeletal proteins of cardiomyocytes. These genes only represent a subgroup of structural genes associated with DCM, and the results of this study cannot be extrapolated to all DCM-associated genes.
Therefore, while our data suggest a possible link between LOF variants in structural, cytoskeletal genes and AF, our findings should be regarded as hypothesis-generating and be interpreted with caution.
5. Conclusions
We identified six individuals with LOF variants in three cytoskeletal genes in a cohort of 527 early-onset AF patients. We also found significant genetic correlation between AF and non-ischemic cardiomyopathy using publicly available summary statistics from recent genome-wide association studies. Our data suggest that cytoskeletal genes previously associated with ventricular cardiomyopathy may also play a role in AF. Combined with numerous recent studies on other structural genes, these data add to the hypothesis that structural genes could possibly predispose to development of atrial cardiomyopathy.
Acknowledgments
This work was supported by the John and Birthe Meyer foundation, the Villadsen family foundation, the Arvid Nilsson Foundation, and Fondsbørsvekselerer Henry Hansen og Hustru Karla Hansen, født Westergaards Legat.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/2/372/s1: Figure S1: DMD isoform expression; Figure S2: FKTN isoform expression; Figure S3: PDLIM3 isoform expression; Table S1: Descriptive information on studies used for genetic correlation analysis of AF and other traits; Table S2: All affected isoforms of DMD, FKTN, and PDLIM3; Table S3: Summary of protein-protein interactions of DMD, FKTN, and PDLIM3.
Author Contributions
Conceptualization, J.H.S., A.T., and M.S.O.; methodology, O.B.V., M.S.O., G.A., and J.G.; software, G.A.; validation, O.B.V., C.P.-M., and M.S.O.; formal analysis, O.B.V., G.A., and J.G.; investigation, L.A. and I.E.C.; resources, A.S., I.E.C., and L.A.; data curation, G.A.; writing—original draft preparation, O.B.V., C.P.-M., and M.S.O.; writing—review and editing, G.A., S.M.K., J.G., L.A., S.H., A.T., A.S., I.E.C., and J.H.S.; visualization, O.B.V. and G.A.; supervision, M.S.O. and J.H.S.; project administration, M.S.O., J.H.S., A.T., and S.H.; funding acquisition, M.S.O., J.H.S., S.H., and A.T. All authors have read and agreed to the published version of the manuscript.
Funding
O.B.V. is funded by a Novo Nordisk Foundation Pregraduate Scholarship (NNF18OC0053094). M.S.O. holds a Novo Nordisk Foundation Hallas-Møller Emerging Investigator grant (NNF17OC0031204). I.E.C. is funded by a mobility grant from the Research Council of Norway. S.M.K. is funded by a three-year PhD-grant from the Southeastern Norway Health Authority.
Conflicts of Interest
I.E.C. previously gave a presentation at a symposium for general practitioners arranged by MSD Norway. The authors declare no conflict of interest.
References
- 1.Chugh S.S., Havmoeller R., Narayanan K., Singh D., Rienstra M., Benjamin E.J., Gillum R.F., Kim Y.H., McAnulty J.H., Zheng Z.J., et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirchhof P., Benussi S., Kotecha D., Ahlsson A., Atar D., Casadei B., Castella M., Diener H.C., Heidbuchel H., Hendriks J., et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016;37:2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S., Murphy N.F, Murphy N., Walker A., McGuire A., McMurray J.J.V. Cost of an emerging epidemic: An economic analysis of atrial fibrillation in the UK. Heart. 2004;90:286–292. doi: 10.1136/hrt.2002.008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go A.S., Hylek E.M., Phillips K.A., Chang Y., Henault L.E., Selby J.V., Singer D.E. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention: The AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 5.Dobrev D., Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212–1223. doi: 10.1016/S0140-6736(10)60096-7. [DOI] [PubMed] [Google Scholar]
- 6.Nielsen J.B., Thorolfsdottir R.B., Fritsche L.G., Zhou W., Skov M.W., Graham S.E., Herron T.J., McCarthy S., Schmidt E.M., Sveinbjornsson G., et al. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat. Genet. 2018;50:1234–1239. doi: 10.1038/s41588-018-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olesen M.S., Nielsen M.W., Haunsø S., Svendsen J.H. Atrial fibrillation: The role of common and rare genetic variants. Eur. J. Hum. Genet. 2014;22:297–306. doi: 10.1038/ejhg.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahlberg G., Refsgaard L., Lundegaard P.R., Andreasen L., Ranthe M.F., Linscheid N., Nielsen J.B., Melbye M., Haunsø S., Sajadieh A., et al. Rare truncating variants in the sarcomeric protein titin associate with familial and early-onset atrial fibrillation. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-06618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi S.H., Weng L.-C., Roselli C., Lin H., Haggerty C.M., Shoemaker M.B., Barnard J., Arking D.E., Chasman D.I., Albert C.M., et al. Association Between Titin Loss-of-Function Variants and Early-Onset Atrial Fibrillation. JAMA. 2018;320:2354–2364. doi: 10.1001/jama.2018.18179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roselli C., Chaffin M.D., Weng L.-C., Aeschbacher S., Ahlberg G., Albert C.M., Almgren P., Alonso A., Anderson C.D., Aragam K.G., et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat. Genet. 2018;50:1225–1233. doi: 10.1038/s41588-018-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goette A., Kalman J.M., Aguinaga L., Akar J., Cabrera J.A., Chen S.A., Chugh S.S., Corradi D., D’Avila A., Dobrev D., et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. doi: 10.1093/europace/euw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arbustini E., Di Toro A., Giuliani L., Favalli V., Narula N., Grasso M. Cardiac Phenotypes in Hereditary Muscle Disorders: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018;72:2485–2506. doi: 10.1016/j.jacc.2018.08.2182. [DOI] [PubMed] [Google Scholar]
- 13.McNally Elizabeth M., Mestroni L. Dilated Cardiomyopathy. Circ. Res. 2017;121:731–748. doi: 10.1161/CIRCRESAHA.116.309396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sajadieh A., Nielsen O.W., Rasmussen V., Hein H.O., Hansen J.F. Prevalence and prognostic significance of daily-life silent myocardial ischaemia in middle-aged and elderly subjects with no apparent heart disease. Eur. Heart J. 2005;26:1402–1409. doi: 10.1093/eurheartj/ehi169. [DOI] [PubMed] [Google Scholar]
- 15.Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van der Auwera G.A., Carneiro M.O., Hartl C., Poplin R., Del Angel G., Levy-Moonshine A., Jordan T., Shakir K., Roazen D., Thibault J., et al. From FastQ data to high confidence variant calls: The Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinform. 2013;43:11.10.1–11.10.33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilde A.A.M., Behr E.R. Genetic testing for inherited cardiac disease. Nat. Rev. Cardiol. 2013;10:571–583. doi: 10.1038/nrcardio.2013.108. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W., Chen T., Chong Z., Rohrdanz M.A., Melott J.M., Wakefield C., Zeng J., Weinstein J.M., Meric-Bernstam F., Mills G.B., et al. TransVar: A multilevel variant annotator for precision genomics. Nat. Methods. 2015;12:1002. doi: 10.1038/nmeth.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GTEx Portal [Internet] [(accessed on 28 November 2019)]; Available online: https://www.gtexportal.org/home/
- 20.Szklarczyk D., Gable A.L., Lyon D., Junge A., Wyder S., Huerta-Cepas J., Simonovic M., Doncheva N.T., Morris J.H., Bork P., et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic. Acids. Res. 2019;47:D607–D613. doi: 10.1093/nar/gky1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Mering C., Jensen L.J., Snel B., Hooper S.D., Krupp M., Foglierini M., Jouffre N., Huynen M.A., Bork P. STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005;33:D433–D437. doi: 10.1093/nar/gki005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadji-Turdeghal K., Andreasen L., Hagen C.M., Ahlberg G., Ghouse J., Bækvad-Hansen M., Bybjerg-Grauholm J., Hougaard D.M., Hedley P., Haunsø S., et al. Genome-wide association study identifies locus at chromosome 2q32.1 associated with syncope and collapse. Cardiovasc. Res. 2020;116:138–148. doi: 10.1093/cvr/cvz106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flanigan K.M. Duchenne and Becker muscular dystrophies. Neurol. Clin. 2014;32:671–688. doi: 10.1016/j.ncl.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Dystrophin Isoforms and Their Expression [Internet] [(accessed on 28 November 2019)]; Available online: https://www.dmd.nl/isoforms.html.
- 25.Towbin J.A., Hejtmancik J.F., Brink P., Gelb B., Zhu X.M., Chamberlain J.S., McCabe E.R., Swift M. X-linked dilated cardiomyopathy. Molecular genetic evidence of linkage to the Duchenne muscular dystrophy (dystrophin) gene at the Xp21 locus. Circulation. 1993;87:1854–1865. doi: 10.1161/01.CIR.87.6.1854. [DOI] [PubMed] [Google Scholar]
- 26.Tokarz S.A., Duncan N.M., Rash S.M., Sadeghi A., Dewan A.K., Pillers D.A. Redefinition of dystrophin isoform distribution in mouse tissue by RT-PCR implies role in nonmuscle manifestations of duchenne muscular dystrophy. Mol. Genet. Metab. 1998;65:272–281. doi: 10.1006/mgme.1998.2763. [DOI] [PubMed] [Google Scholar]
- 27.Scheiper S., Ramos-Luis E., Blanco-Verea A., Niess C., Beckmann B.-M., Schmidt U., Kettner M., Geisen C., Verhoff M.A., Brion M., et al. Sudden unexpected death in the young—Value of massive parallel sequencing in postmortem genetic analyses. Forensic Sci. Int. 2018;293:70–76. doi: 10.1016/j.forsciint.2018.09.034. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen C., Nielsen J.B., Refsgaard L., Holst A.G., Christensen A.H., Andreasen L., Sajadieh A., Haunsø S., Svendsen J.H., Olesen M.S. New population-based exome data are questioning the pathogenicity of previously cardiomyopathy-associated genetic variants. Eur. J. Hum. Genet. 2013;21:918–928. doi: 10.1038/ejhg.2012.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paludan-Müller C., Ghouse J., Vad O.B., Herfelt C.B., Lundegaard P., Ahlberg G., Schmitt N., Svendsen J.H., Haunsø S., Bundgaard H., et al. Reappraisal of variants previously linked with sudden infant death syndrome: Results from three population-based cohorts. Eur. J. Hum. Genet. 2019;27:1427–1435. doi: 10.1038/s41431-019-0416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreasen L., Nielsen J.B., Olesen M.S. Genetic aspects of lone atrial fibrillation: What do we know? Curr. Pharm. Des. 2015;21:667–678. doi: 10.2174/1381612820666140825143610. [DOI] [PubMed] [Google Scholar]
- 31.Saito K. In: Fukuyama Congenital Muscular Dystrophy. Adam M.P., Ardinger H.H., Pagon R.A., editors. University of Washington; Seattle, WA, USA: [(accessed on 2 January 2020)]. 1993–2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1206/ [PubMed] [Google Scholar]
- 32.Murakami T., Hayashi Y.K., Noguchi S., Ogawa M., Nonaka I., Tanabe Y., Ogino M., Takada F., Eriguchi M., Kotooka N., et al. Fukutin gene mutations cause dilated cardiomyopathy with minimal muscle weakness. Ann. Neurol. 2006;60:597–602. doi: 10.1002/ana.20973. [DOI] [PubMed] [Google Scholar]
- 33.Mercuri E., Brockington M., Straub V., Quijano-Roy S., Yuva Y., Herrmann R., Brown S.C., Torelli S., Dubowitz V., Blake D.J., et al. Phenotypic spectrum associated with mutations in the fukutin-related protein gene. Ann. Neurol. 2003;53:537–542. doi: 10.1002/ana.10559. [DOI] [PubMed] [Google Scholar]
- 34.Wang D., Fang J., Lv J., Pan Z., Yin X., Cheng H., Gou X. Novel polymorphisms in PDLIM3 and PDLIM5 gene encoding Z-line proteins increase risk of idiopathic dilated cardiomyopathy. J. Cell Mol. Med. 2019;23:7054–7062. doi: 10.1111/jcmm.14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohsawa N., Koebis M., Suo S., Nishino I., Ishiura S. Alternative splicing of PDLIM3/ALP, for α-actinin-associated LIM protein 3, is aberrant in persons with myotonic dystrophy. Biochem. Biophys. Res. Commun. 2011;409:64–69. doi: 10.1016/j.bbrc.2011.04.106. [DOI] [PubMed] [Google Scholar]
- 36.Marrouche N.F., Wilber D., Hindricks G., Jais P., Akoum N., Marchlinski F., Kholmovski E., Burgon N., Hu N., Mont L., et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation: The DECAAF Study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 37.Nattel S., Dobrev D. Controversies About Atrial Fibrillation Mechanisms. Circ. Res. 2017;120:1396–1398. doi: 10.1161/CIRCRESAHA.116.310489. [DOI] [PubMed] [Google Scholar]
- 38.Siebermair J., Kholmovski E.G., Marrouche N. Assessment of Left Atrial Fibrosis by Late Gadolinium Enhancement Magnetic Resonance Imaging: Methodology and Clinical Implications. JACC Clin. Electrophysiol. 2017;3:791–802. doi: 10.1016/j.jacep.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 39.Meyers T.A., Townsend D. Cardiac Pathophysiology and the Future of Cardiac Therapies in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2019;20:4098. doi: 10.3390/ijms20174098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lodder E.M., Scicluna B.P., Beekman L., Arends D., Moerland P.D., Tanck M.W.T., Adriaens M.E., Bezzina C.R. Integrative genomic approach identifies multiple genes involved in cardiac collagen deposition. Circ. Cardiovasc. Genet. 2014;7:790–798. doi: 10.1161/CIRCGENETICS.114.000537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.