Abstract
BACKGROUND:
Dexmedetomidine is a clinically useful drug for providing sedation, but concern regarding its cardiovascular side effect profile can limit its widespread use during routine diagnostic flexible bronchoscopy (FB).
MATERIALS AND METHODS:
Patients between 18 and 65 years of age, who required diagnostic FB, were screened. Eligible patients were randomized to either receive 0.5 μg/kg intravenous dexmedetomidine over 10 min or intravenous midazolam 0.035 mg/kg over 1 min. If required, rescue medication (intravenous midazolam 0.5 mg bolus) was administered. The primary outcome measure was the composite score. Other parameters observed were numerical rating scale, hemodynamic variables, oxygen saturation, number of doses of rescue medication, visual analog scale score for cough, ease of bronchoscopy, Ramsay Sedation Score, and postprocedure patient response after 24 h of bronchoscopy.
RESULTS:
A total of 54 patients were enrolled, 27 in each group. Total composite score (mean ± standard deviation) in dexmedetomidine and midazolam group at nasopharynx was 7.04 ± 2.19 and 6.59 ± 1.55 (P = 0.387), respectively. The corresponding values at the level of trachea were 9.22 ± 3.69 and 8.63 ± 2.13 (P = 0.475). In the dexmedetomidine group, patient response after 24 h of bronchoscopy showed the quality of sedation to be excellent in three patients, good in 10, fair in 11, and poor in 3 and discomfort to be nil in 14, mild 7, moderate in 3, and severe in 3. The corresponding values in the midazolam group for the quality of sedation were 0, 9, 18, 0 and for discomfort 10, 16, 1, 0. Other parameters did not reveal any statistically significant difference.
CONCLUSION:
Dexmedetomidine at a dose of 0.5 μg/kg may provide clinically useful conscious sedation, comparable to midazolam.
Keywords: Dexmedetomidine, flexible bronchoscopy, midazolam, sedation
Introduction
Flexible bronchoscopy (FB) is a procedure to visualize the lumen and mucosa of the airways. It is routinely used for the diagnostic purpose to obtain specimens for evaluating pulmonary pathologies, to assess centrally located tumors, and to identify and localize the cause of hemoptysis, etc., It also has therapeutic indications such as removal of mucus impaction and foreign bodies, tumor debulking, airway stenting, and others.
FB is a safe procedure but can be noxious, for some patients, if the procedure is done without using sedation. Hence, over time the practice of offering conscious sedation to the patients has become the standard of care.[1] Various sedatives, such as midazolam, fentanyl, and propofol, have been used; however, as with most scientific endeavors, the search for the ideal option is still on.[2,3,4] Efforts in this direction have led to a number of studies on identifying and establishing the safety and efficacy of dexmedetomidine (an α2 agonist) use during FB.[5,6,7] On being administered at a dose of 1 μg/kg (over 10 min), it has provided reliable and satisfactory sedation with the added advantage of the lack of respiratory depression.[7,8] However, the downside of this drug is its side effect profile on the cardiovascular system – bradycardia and hypotension. These cardiovascular side effects appear to decrease when the dose is reduced to 0.5 μg/kg.[9,10] We hypothesized that if the dose of dexmedetomidine is reduced to 0.5 μg/kg, the efficacy could be retained to a great extent. Thus, the present study was conducted to assess the efficacy of reduced dose (0.5 μg/kg) of dexmedetomidine in comparison with the standard of care, i.e., midazolam 0.035 mg/kg, when used during FB.
Materials and Methods
This prospective, randomized, and double-blinded study was conducted in the Department of Respiratory Medicine of a tertiary care teaching hospital in southern India. After clearance from the institutional ethics committee (ECR/146/Inst/KA/2013) was obtained, a written informed consent (in the native language of the patients) was obtained from willing participants. The study was conducted between May 2016 and March 2018. Patients between 18 and 65 years of age, who requiring diagnostic FB, were screened. Patients with renal or hepatic insufficiency, seizure disorder, hemodynamic instability including cardiac failure, hypovolemia, heart rate <50 beats/min or second- or third-degree heart block, chronic obstructive pulmonary disease having forced expiratory volume in one second <50%, psychiatric disorder, bodyweight >70 kg, pregnant and lactating women, known or suspected allergy to midazolam or dexmedetomidine were excluded from the study. The enrolled patients were randomized into two groups – dexmedetomidine group and the midazolam group. Randomization was done by block randomization (chit method).
In this study, double blinding was ensured by labeling two syringes as 1 and 2, before the procedure while study drugs were being prepared. Syringe 1 contained either dexmedetomidine 0.5 μg/kg (rounded off to the nearest decimal) diluted in normal saline to make up to 10 mL or 10 mL of normal saline, which was infused over 10 min starting from time 0 to 10th min. Syringe 2 had either the drug (midazolam 0.035 mg/kg) diluted with normal saline to make it to 4 mL or had 4 ml of normal saline, which was given at the beginning of 9th min. Three observers were involved in the study. The first observer (Observer A) prepared the study drug after picking randomization slip and labeled the syringes 1 and 2 to ensure blinding. This observer was not involved in the study after this point. Second observer (Observer B) performed the bronchoscopy, was not aware of the contents of syringe 1 and 2. Third observer (Observer C) after taking consent and explaining the study methodology, injected the study drugs, assessed the patients, and made a recording of the various variables. This person was also not aware of the contents of syringes 1 and 2. Both observers B and C were blinded to the study drug. If any of the study participants developed persistent adverse events, they would be observed and managed as long as necessary till they were safe in the poststudy period.
At baseline, patients were monitored for respiratory rate, oxygen saturation (SpO2), pulse rate, noninvasive blood pressure, and Ramsay Sedation Score (RSS) for sedation status. Bronchoscopy was started at the end of the 10th min.
Before beginning the FB procedure, 2 mL of 2% lignocaine jelly was administered into one of the nostrils of the patient. After starting the bronchoscopy, lignocaine (2 mL of 2%) was sprayed at the oropharynx and then again at vocal cords. After waiting for a minute for the local anesthetic to act, the bronchoscope was localized between the vocal cords and 1 mL of 2% lignocaine was sprayed over tracheal mucosa. On reaching carina, lignocaine (1 mL of 2%) was sprayed. Subsequently, having passed the carina, lignocaine was sprayed (1 ml of 2%) into each main bronchus. Cough having subsided, the bronchoscope was introduced into the main bronchi to examine the bronchial tree and the planned sampling procedure was completed. Additional 1 mL of 2% lignocaine was sprayed if the patient developed a recurrent cough. If required, rescue medication in the form of intravenous midazolam 0.5 mg bolus (kept in an open labeled syringe) was administered with a gap of at least 2 min between the doses. RSS was used to assess the sedation status of the patient – before, during, and after 10 min of competition of bronchoscopy – and was maintained between a score of 2–3. Supplemental oxygen was given to all the patients during the procedure at 4 L/min through nasal cannula.
The primary outcome measure was composite score [Appendix Table 1] during the FB assessed by observer C, at least for 1 min when the scope was at the level of the nasopharynx and at the time of passage of the scope through the trachea. The composite score included sedation, respiratory response, calmness, facial tension, and physical movement; and higher the score worse the performance of the drug.[7]
On a scale of 0–10 (0 equal to no pain/discomfort and 10 is unbearable pain/discomfort) pain intensity and distress were assessed by the Observer C using numerical rating scale (NRS) before, during, and 10 min after the bronchoscopy.[11] SpO2 (<93%), heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), number of doses of rescue medication (intravenous midazolam 0.5 mg bolus) required during the procedure; duration of bronchoscopy procedure and lignocaine dose used were noted. The observer C used the visual analog scale (VAS) to assess cough during the bronchoscopy, and the patient also used the VAS, but 24 h after the procedure.[12,13,14] Observer C and the patient marked on a 10-cm VAS (0 denoting no cough and 10 representing worst cough ever). Ease of bronchoscopy was assessed by the observer B. Postprocedure patient response, after 24 h of bronchoscopy, was assessed by a questionnaire which contained six questions.[7]
Sample size
Sample size was based on comparison of mean composite scores across two groups. From the pilot study, we had observed the standard deviation of 5 in the composite score. Assuming a minimum clinically significant difference of 4 between two groups, the sample size required was 27 in each group at 80% power and 5% type 1 error.
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). For continuous variables such as composite score, age, height, weight, body mass index, and duration of bronchoscopy, the independent t-test was used. For categorical variables such as gender, numerical rating scale, ease of bronchoscopy, RSS, requirement of rescue medication, and postprocedure patient assessment Chi-square test and Fischer's exact test was used and for VAS for cough, Mann–Whitney U-test. Values of P < 0.05 were taken as statistically significant.
Results
Of a total of 82 patients who were screened for eligibility, 54 patients who satisfied the study criteria and consented for the study, were enrolled (27 in each arm). The study was completed by all the enrolled patients [Figure 1]. Table 1 shows the baseline characteristics of the patients. At the level of the nasopharynx, the mean composite scores in the dexmedetomidine group and midazolam group were 7.04 ± 2.19 and 6.59 ± 1.55 (P = 0.387), respectively. At the level of trachea, the mean composite scores in the dexmedetomidine group and midazolam group were 9.22 ± 3.69 and 8.63 ± 2.13 (P = 0.475), respectively. Comparison of total composite score and components of the composite score at the nasopharynx and trachea are shown in Tables 2 and 3, respectively. Comparison of NRS for pain and distress is shown in Table 4. Other study parameters are shown in Table 5.
Figure 1.
CONSORT flow diagram
Table 1.
Baseline characteristics of the patients
| Patient characteristics | Dexmedetomidine group (n=27) | Midazolam group (n=27) | P |
|---|---|---|---|
| Age (years), mean±SD | 47.07±13.40 | 45.00±14.26 | 0.584# |
| Male:female | 19:8 | 14:13 | 0.163* |
| Height (cm), mean±SD | 161.02±9.38 | 158.82±8.88 | 0.379# |
| Weight (kg), mean±SD | 53.23±8.69 | 50.37±9.84 | 0.263# |
| BMI (kg/m2), mean±SD | 20.61±3.27 | 19.98±3.51 | 0.503# |
| Indications for bronchoscopy | |||
| Malignancy | 2 | 5 | 0.224* |
| Infection | 25 | 22 | |
| Others | 0 | 0 | |
| Procedures performed | |||
| BAL/bronchial washing | 27 | 27 | 1 |
| Bronchial brushing | 9 | 15 | 0.100* |
| Endo bronchial biopsy | 2 | 3 | 0.639* |
| Trans bronchial needle aspiration | 5 | 4 | 0.715* |
| Trans bronchial lung biopsy | 1 | 1 | 1 |
| Route of bronchoscopy | |||
| Trans nasal | 25 | 27 | 0.150* |
| Trans oral | 2 | 0 | |
| Duration of bronchoscopy (min) | 13.30±6.83 | 14.67±7.17 | 0.476# |
*χ2, #Independent t-test. SD=Standard deviation, BMI=Body mass index, BAL=Bronchoalveolar lavage
Table 2.
Comparison of composite score (total score)
| Total score | At the level of nasopharynx | At the level of trachea | ||
|---|---|---|---|---|
| Group dexmedetomidine (n=27) | Group midazolam (n=27) | Group dexmedetomidine (n=27) | Group midazolam (n=27) | |
| 5 | 9 | 9 | 2 | 1 |
| 6-10 | 15 | 18 | 17 | 21 |
| 11-15 | 3 | 0 | 5 | 5 |
| 16-20 | 0 | 0 | 3 | 0 |
| Mean±SD | 7.04±2.19 | 6.59±1.55 | 9.22±3.69 | 8.63±2.13 |
| P | 0.387# | 0.475# | ||
#Independent t-test. SD=Standard deviation
Table 3.
Components of the composite score at the nasopharynx and trachea (n=27)
| Components of the composite score | At nasopharynx | At trachea | ||
|---|---|---|---|---|
| Dexmedetomidine group, n (%) | Midazolam group, n (%) | Dexmedetomidine group, n (%) | Midazolam group, n (%) | |
| Sedation | ||||
| 1 | 26 (96.3) | 22 (81.5) | 19 (70.4) | 22 (81.5) |
| 2 | 1 (3.7) | 5 (18.5) | 8 (29.6) | 5 (18.5) |
| 3 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 |
| Calmness | ||||
| 1 | 21 (77.8) | 24 (88.9) | 14 (51.9) | 17 (63) |
| 2 | 6 (22.2) | 3 (11.1) | 9 (33.3) | 10 (37) |
| 3 | 0 | 0 | 1 (3.7) | 0 |
| 4 | 0 | 0 | 3 (11.1) | 0 |
| 5 | 0 | 0 | 0 | 0 |
| Respiratory response | ||||
| 1 | 17 (63) | 18 (66.7) | 11 (40.7) | 5 (18.5) |
| 2 | 6 (22.2) | 5 (18.5) | 8 (29.6) | 10 (37) |
| 3 | 4 (14.8) | 4 (14.8) | 6 (22.2) | 12 (44.4) |
| 4 | 0 | 0 | 2 (7.4) | 0 |
| 5 | 0 | 0 | 0 | 0 |
| Physical movement | ||||
| 1 | 16 (59.3) | 19 (70.4) | 10 (37) | 12 (44.4) |
| 2 | 8 (29.6) | 8 (29.6) | 9 (33.3) | 9 (33.3) |
| 3 | 3 (11.1) | 0 | 5 (18.5) | 6 (22.2) |
| 4 | 0 | 0 | 3 (11.1) | 0 |
| 5 | 0 | 0 | 0 | 0 |
| Facial tension | ||||
| 1 | 12 (44.4) | 15 (55.6) | 9 (33.3) | 6 (22.2) |
| 2 | 10 (37) | 10 (37) | 9 (33.3) | 14 (51.9) |
| 3 | 5 (18.5) | 2 (7.4) | 6 (22.2) | 7 (25.9) |
| 4 | 0 | 0 | 1 (3.7) | 0 |
| 5 | 0 | 0 | 2 (7.4) | 0 |
Table 4.
Comparison of numerical rating scale for pain and distress
| Prior to FB | During FB | 10 min after FB | |||||
|---|---|---|---|---|---|---|---|
| 0-3 | 4-7 | 8-10 | 0-3 | 4-7 | 8-10 | ||
| NRS pain | |||||||
| Group dexmedetomidine | 0 | 24 | 3 | 0 | 23 | 4 | 0 |
| Group midazolam | 0 | 24 | 3 | 0 | 25 | 2 | 0 |
| P | 1.00* | 0.669* | |||||
| NRS distress | |||||||
| Group dexmedetomidine | 0 | 18 | 9 | 0 | 22 | 4 | 1 |
| Group midazolam | 0 | 20 | 7 | 0 | 23 | 4 | 0 |
| P | 0.766* | 1* | |||||
*Fischer’s exact test. NRS=Numerical rating scale, FB=Flexible bronchoscopy
Table 5.
Other observations during the study
| Time in relation to bronchoscopy | Ramsay Sedation Score | Dexmedetomidine group | Midazolam group |
|---|---|---|---|
| During bronchoscopy | 1 | 4 | 2 |
| 2 | 18 | 23 | |
| 3 | 5 | 2 | |
| P | 0.337 (Fisher’s exact test) | ||
| 10 min after bronchoscopy | 1 | 20 | 21 |
| 2 | 7 | 6 | |
| P | 1 (Fisher’s exact test) | ||
| Ease of bronchoscopy | |||
| Easy | 18 | 15 | |
| Slightly difficult | 6 | 12 | |
| Very difficult | 3 | 0 | |
| P | 0.087 (Fisher’s exact test) | ||
| VAS for cough | |||
| During bronchoscopy (median) | 2 | 2.5 | |
| P | 0.205 (Mann-Whitney U-test) | ||
| As perceived by patient, 24 h after bronchoscopy (median) | 2.5 | 3 | |
| P | 0.467 (Mann-Whitney U test) | ||
| Patient’s opinion 24 h after the bronchoscopy | |||
| Quality of sedation | |||
| Excellent | 3 | 0 | |
| Good | 10 | 9 | |
| Fair | 11 | 18 | |
| Poor | 3 | 0 | |
| P | 0.033 (Fisher’s exact test) | ||
| Discomfort | |||
| Nil | 14 | 10 | |
| Mild | 7 | 16 | |
| Moderate | 3 | 1 | |
| Severe | 3 | 0 | |
| P | 0.030 (Fisher’s exact test) | ||
| Number of rescue doses of midazolam given | |||
| 0 | 24 | 25 | |
| 1 | 1 | 2 | |
| 2 | 2 | 0 | |
| P | 0.610 (Fisher’s exact test) | ||
VAS=Visual analog scale
The mean HR (beats per minute) at different time points in dexmedetomidine group and midazolam group, respectively, was as follows: baseline (87.85 ± 16.19, 87.41 ± 16.31; P = 0.920), start of bronchoscopy (84.56 ± 17.65, 92.00 ± 19.25; P = 0.145), at 5 min (96.74 ± 19.02, 106.22 ± 18.33; P = 0.068), at 10 min (91.89 ± 22.03, 105.09 ± 19.99; P = 0.054), at the end of bronchoscopy (102.37 ± 21.34, 105.04 ± 18.33; P = 0.624), and at 10 min postbronchoscopy (89.56 ± 18.70, 97.04 ± 18.50; P = 0.146).
The mean SBP (mm Hg) at different time points in dexmedetomidine group and midazolam group, respectively, was as follows: baseline (131.63 ± 22.56, 126.67 ± 18.92; P = 0.385), start of bronchoscopy (125.11 ± 20.31, 129.19 ± 18.76; P = 0.447), at 5 min (139.78 ± 26.51, 135.41 ± 20.71; P = 0.503), at 10 min (134.78 ± 24.94, 133.91 ± 24.68; P = 0.913), at the end of bronchoscopy (136.22 ± 30.87, 136.96 ± 19.05; P = 0.916), and at 10 min postbronchoscopy (120.96 ± 19.91, 118.15 ± 16.25; P = 0.572) [Figure 2].
Figure 2.
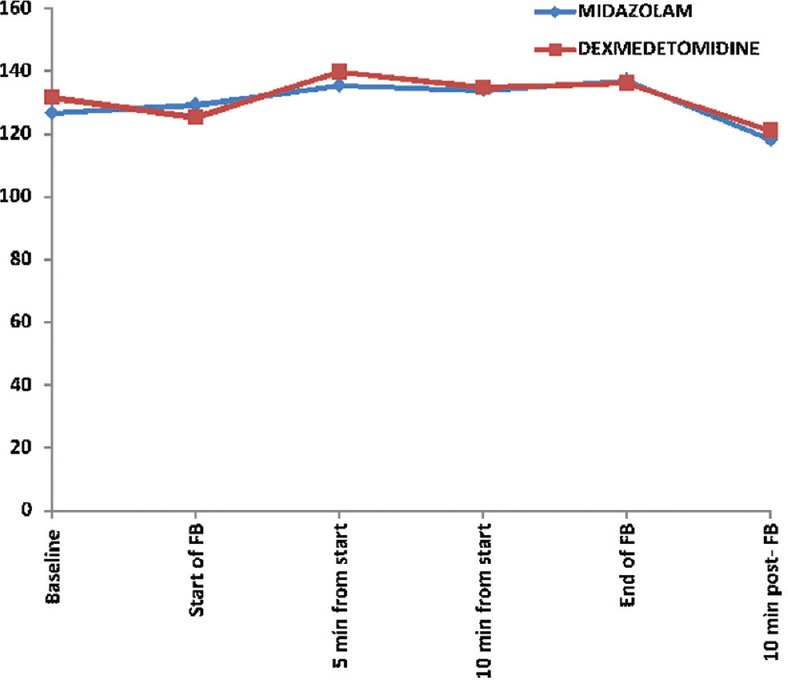
Comparison of systolic blood pressure at different time points
The mean DBP (mm Hg) at different time points in dexmedetomidine group and midazolam group, respectively, was as follows: baseline (77.85 ± 9.53, 77.19 ± 11.49; P = 0.817), start of bronchoscopy (74.07 ± 9.85, 78.37 ± 12.23; P = 0.161), at 5 min (85.26 ± 15.88, 86.07 ± 19.06; P = 0.865), at 10 min (81.44 ± 15.83, 85.27 ± 16.22; P = 0.458), at the end of bronchoscopy (85.63 ± 18.32, 81.63 ± 12.73; P = 0.356), and at 10 min postbronchoscopy (75.26 ± 14.32, 77.56 ± 13.53; P = 0.547).
The mean SpO2 (%) at different time points in dexmedetomidine group and midazolam group, respectively, was as follows: baseline (100.00 ± 0, 99.85 ± 0.53; P = 0.155), start of bronchoscopy (100.00 ± 0, 99.78 ± 0.69; P = 0.104), at 5 min (99.70 ± 1.20, 99.63 ± 0.97; P = 0.804), at 10 min (99.67 ± 1.19, 98.32 ± 3.75; P = 0.151), at the end of bronchoscopy (99.33 ± 1.92, 99.15 ± 1.97; P = 0.728), and at 10 min postbronchoscopy (99.74 ± 0.76, 99.33 ± 1.77; P = 0.279).
No patient developed hypotension or bradycardia in either group. Only two patients developed complications (oxygen desaturation) during the study and both belonged to the midazolam group. However, the frequency of occurrence of complications showed no statistically significant difference between the two groups (P = 0.49, Fisher's exact test).
The lignocaine dose used was similar between the dexmedetomidine group and midazolam group (209.63 ± 12.86, 210.37 ± 15.06; P = 0.847, independent t-test).
Discussion
Dexmedetomidine is an α-adrenergic agonist and acts on α-2 receptors in the brain and spinal cord and thereby decreases the plasma concentration of catecholamines, leading to sedation, analgesia, and loss of anxiety.[15]
By inhibiting sympathetic nerve excitement, increasing vagal activity, it induces bradycardia and decreases blood pressure.[16]
A study done to compare the safety and efficacy of dexmedetomidine 1 μg/kg versus midazolam among patients put on mechanical ventilation in ICU revealed that in dexmedetomidine group there was noticed a decrease in the total duration of mechanical ventilation, and this led to quicker extubation.[17] Authors comparing dexmedetomidine 1 μg/kg with fentanyl 12 μg/kg–midazolam 0.02 mg/kg combination on sedation and safety during awake fiberoptic intubation observed that dexmedetomidine provided better and safe intubating condition and better hemodynamic stability.[18] Endoscopic retrograde cholangiography is an uncomfortable procedure where conscious sedation is required. In this procedure also, dexmedetomidine 1 μg/kg has shown better sedation, stable hemodynamics, and quick recovery as compared to midazolam at a dose of 0.05 mg/kg intravenous bolus.[19] Researchers comparing the addition of low dose of ketamine to dexmedetomidine against propofol in awake fiberoptic intubation observed that the former was better with regard to patient tolerance, better analgesia, and less effect on hemodynamic variables and thus making it an appropriate drug for conscious sedation.[20]
Notwithstanding the clinical efficacy of dexmedetomidine as a sedative and analgesic, the bronchoscopists may be averse to its use during routine FB because of its side effect profile – hypotension, bradycardia, etc. These side effects though manageable at routinely used therapeutic doses may put extra burden on the resources and the time schedule of a busy bronchoscopy unit. Goneppanavar et al. compared the dexmedetomidine group (loading dose of 1 μg/kg, with no maintenance dose, unlike in most other studies) with the midazolam group (0.02 mg/kg) during diagnostic FB, and found that dexmedetomidine provided very good patient comfort. The results of this study led us to consider the possibility of further reducing the dose of dexmedetomidine and retaining meaningful clinical efficacy.[7]
The fact that in our study the composite score between the two study groups showed no significant difference suggests that a reduced dose of dexmedetomidine (0.5 μg/kg) may produce an acceptable level of conscious sedation. However, contrary to our results, a pilot study from Australia, using dexmedetomidine at 0.5 μg/kg, has shown that more than half of the study subjects required rescue doses and hence, the investigators concluded that this dose of dexmedetomidine does not produce effective sedation during FB.[21]
In this study, the dexmedetomidine group had a higher number of patients requiring rescue doses of midazolam, but the requirement for the rescue doses did not show any significant difference between the study groups. A similar trend being also observed in the ease of doing bronchoscopy, as judged by the bronchoscopist, but again no significant difference between the groups was noticed. However, in the dexmedetomidine group, there was a higher number of cases in a very difficult category. The NRS for pain and distress showed similar values between the two groups in our study prior, during, and 10 min after bronchoscopy. Among the patients receiving dexmedetomidine, the distribution of patients as per NRS (for distress and pain) during bronchoscopy was similar to that in another study where 1 μg/kg of dexmedetomidine was used.[7] Hence, it seems that the reduced dose of dexmedetomidine we had used may be maintaining clinical effectiveness during diagnostic FB. However, when comparing the NRS for pain and distress 10 min after bronchoscopy the results, though grossly similar, revealed that the dexmedetomidine group in the study by Goneppanavar et al. had lesser number of patients with higher score compared to the present study. This may indicate that 0.5 μg/kg dexmedetomidine without a maintenance dose may not be maintaining patient comfort after the procedure. This, obviously, can become a problem even during FB, if the procedure time is longer. In our study, patients' responses after 24 h of bronchoscopy reveal a mixed picture, with patients perceiving excellent to good quality of sedation being more in dexmedetomidine group but those having nil to mild discomfort being less.
In a previous study (total dose dexmedetomidine of 1 μg/kg), only one patient had developed hypotension from among 27 patients recruited in the dexmedetomidine arm of the study. Although it was not statistically significant, such an event happening in a clinical setting, even occasionally, can put unnecessary burden on the set-up, as mentioned above, and on the patient as well.[7] Our line of thinking of using lower dose of 0.5 μg/kg dexmedetomidine has been further supported by a recent study, using a reduced dose of dexmedetomidine (total dose of 0.5 μg/kg), which compared its efficacy with alfentanil, and found no difference between the two groups during FB. HR changes, desaturations, and hypoxia were most prevalent in the alfentanil group. Hypotension being observed mostly in the dexmedetomidine group; however, there was no significant difference in the need for circulatory support between the groups.[9] Quite a few other studies – in clinical settings other than FB – have observed that lower doses of dexmedetomidine compared with higher doses had, interestingly, similar clinical effectiveness but produced lesser cardiovascular side effects. In a study comparing a dose of 1 μg/kg dexmedetomidine with 2 μg/kg dexmedetomidine, in patients undergoing upper limb surgeries, it was found that by increasing the dose the duration of analgesia was not prolonged, but the higher dose resulted in lower HR and blood pressure.[22] Similarly, in a study done during laryngoscopy and tracheal intubation, the investigators reported that 0.5 μg/kg and 1.0 μg/kg dexmedetomidine were equally efficacious. Moreover, the incidence of cardiovascular adverse effects such as bradycardia and hypotension was lesser.[10]
In a study comparing dexmedetomidine group (at a loading dose up to 1 μg/kg given over 10 min and then followed up with an infusion of 0.5 μg/kg/h, as maintenance dose) with midazolam group (2 mg intravenous followed by 1 mg midazolam boluses as directed by the bronchoscopist) significantly higher number of bradycardia events were noted (13.1% vs. 4% P = 0.04) in the former group. In addition, hypotension was observed in 6.1% of patients in the dexmedetomidine group, though not statistically significant.[23] In the present study, we did not encounter bradycardia or hypotension in any of the patients – thus, further strengthening the opinion that at the lower dose, the cardiovascular adverse effects of dexmedetomidine are reduced. Moreover, two events of desaturation were observed in the midazolam group and none in dexmedetomidine, though no significant difference between the groups was noticed. This observation points to the fact that dexmedetomidine has an advantage over midazolam when it comes to the absence of ventilatory depression – thus, potentially making it an option in patients who are likely to be sensitive to the respiratory depressive effects of drugs like benzodiazepines.[24,25]
Limitations of the study
Comparison of dexmedetomidine with midazolam-fentanyl combination would have shed more light on the comparative sedative and analgesic effects of these regimens. However, since midazolam is the most common sedative used in our country during FB practice,[26] we planned to study it. Dexmedetomidine is routinely used as a loading dose followed by a maintenance dose, but in our study, we not only reduced the dose but also skipped the maintenance dose, and this could compromise its effectiveness. However, since we were studying the drug in the setting of routine diagnostic FB the average duration of the procedures was relatively short and we expected the drug to stay effective in that time frame.
Conclusion
The present study put forth that 0.5 μg/kg dexmedetomidine may provide clinically useful conscious sedation during FB. However, the trends in the data may point to the fact that in some cases, the patient's and/or bronchoscopist's comfort levels may not be optimal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix
Appendix Table 1.
Composite score during bronchoscopy (5-25)[7]
| Parameter | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Sedation | Awake to voice >10 s | Light sedation, briefly awakens to voice | Moderate sedation, no eye contact | Deep sedation, response only for physical stimulation | Unarousable, no response to physical stimulation |
| Calmness | Alert and calm | Anxious Not aggressive | Frequent nonpurposeful movement | Pulls or removes bronchoscope; aggressive | Combative, violent |
| Respiratory response | Spontaneous respiration No coughing | Occasional cough | Coughing regularly | Frequent coughing or choking | Vigorous cough preventing bronchoscopy |
| Physical movement | No movement | Occasional slight movement | Frequent slight movements | Vigorous movement limited to the extremities | Vigorous movements including torso and head |
| Facial tension | Facial muscle tone normal | Mild muscle tension evident | Tension evident in all facial muscles | Grimacing | Grimacing and crying |
References
- 1.Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: Accredited by NICE. Thorax. 2013;68(Suppl 1):i1–44. doi: 10.1136/thoraxjnl-2013-203618. [DOI] [PubMed] [Google Scholar]
- 2.Barnett AM, Jones R, Simpson G. A survey of bronchoscopy practice in Australia and New Zealand. J Bronchology Interv Pulmonol. 2016;23:22–8. doi: 10.1097/LBR.0000000000000251. [DOI] [PubMed] [Google Scholar]
- 3.Hwang J, Jeon Y, Park HP, Lim YJ, Oh YS. Comparison of alfetanil and ketamine in combination with propofol for patient-controlled sedation during fiberoptic bronchoscopy. Acta Anaesthesiol Scand. 2005;49:1334–8. doi: 10.1111/j.1399-6576.2005.00842.x. [DOI] [PubMed] [Google Scholar]
- 4.Prabhudev AM, Chogtu B, Magazine R. Comparison of midazolam with fentanyl-midazolam combination during flexible bronchoscopy: A randomized, double-blind, placebo-controlled study. Indian J Pharmacol. 2017;49:304–11. doi: 10.4103/ijp.IJP_683_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan F, Fu H, Yang P, Sun K, Wu S, Lv M, et al. Dexmedetomidine-fentanyl versus propofol-fentanyl in flexible bronchoscopy: A randomized study. Exp Ther Med. 2016;12:506–12. doi: 10.3892/etm.2016.3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryu JH, Lee SW, Lee JH, Lee EH, Do SH, Kim CS. Randomized double-blind study of remifentanil and dexmedetomidine for flexible bronchoscopy. Br J Anaesth. 2012;108:503–11. doi: 10.1093/bja/aer400. [DOI] [PubMed] [Google Scholar]
- 7.Goneppanavar U, Magazine R, Periyadka Janardhana B, Krishna Achar S. Intravenous dexmedetomidine provides superior patient comfort and tolerance compared to intravenous midazolam in patients undergoing flexible bronchoscopy. Pulm Med. 2015;2015:727530. doi: 10.1155/2015/727530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Riachy M, Khayat G, Ibrahim I, Aoun Z, Dabar G, Bazarbachi T, et al. A randomized double-blind controlled trial comparing three sedation regimens during flexible bronchoscopy: Dexmedetomidine, alfentanil and lidocaine. Clin Respir J. 2018;12:1407–15. doi: 10.1111/crj.12669. [DOI] [PubMed] [Google Scholar]
- 10.Sharma N, Mehta N. Therapeutic efficacy of two different doses of dexmedetomidine on the hemodynamic response to intubation, the intubating conditions, and the effect on the induction dose of propofol: A randomized, double-blind, placebo-controlled study. Anesth Essays Res. 2018;12:566–71. doi: 10.4103/aer.AER_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, et al. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 12.Stolz D, Chhajed PN, Leuppi JD, Brutsche M, Pflimlin E, Tamm M. Cough suppression during flexible bronchoscopy using combined sedation with midazolam and hydrocodone: A randomised, double blind, placebo controlled trial. Thorax. 2004;59:773–6. doi: 10.1136/thx.2003.019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spinou A, Birring SS. An update on measurement and monitoring of cough: What are the important study endpoints? J Thorac Dis. 2014;6:S728–34. doi: 10.3978/j.issn.2072-1439.2014.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morice AH, Fontana GA, Belvisi MG, Birring SS, Chung KF, Dicpinigaitis PV, et al. ERS guidelines on the assessment of cough. Eur Respir J. 2007;29:1256–76. doi: 10.1183/09031936.00101006. [DOI] [PubMed] [Google Scholar]
- 15.Ihmsen H, Saari TI. Dexmedetomidine. Pharmacokinetics and pharmacodynamics. Anaesthesist. 2012;61:1059–66. doi: 10.1007/s00101-012-2114-1. [DOI] [PubMed] [Google Scholar]
- 16.Constantin JM, Momon A, Mantz J, Payen JF, De Jonghe B, Perbet S, et al. Efficacy and safety of sedation with dexmedetomidine in critical care patients: A meta-analysis of randomized controlled trials. Anaesth Crit Care Pain Med. 2016;35:7–15. doi: 10.1016/j.accpm.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Tripathi M, Kumar V, Kalashetty MB, Malviya D, Bais PS, Sanjeev OP. Comparison of dexmedetomidine and midazolam for sedation in mechanically ventilated patients guided by bispectral index and sedation – Agitation scale. Anesth Essays Res. 2017;11:828–33. doi: 10.4103/aer.AER_48_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yousuf A, Ahad B, Mir AH, Mir AW, Wani JG, Hussain SQ. Evaluation of effectiveness of dexmedetomidine and fentanyl-midazolam combination on sedation and safety during awake fiberoptic intubation: A randomized comparative study. Anesth Essays Res. 2017;11:998–1003. doi: 10.4103/aer.AER_150_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pushkarna G, Sarangal P, Pushkarna V, Gupta R. Comparative evaluation of dexmedetomidine versus midazolam as premedication to propofol anesthesia in endoscopic retrograde cholangiopancreatography. Anesth Essays Res. 2019;13:297–302. doi: 10.4103/aer.AER_62_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Sharkawy RA. Efficacy of adding low-dose ketamine to dexmedetomidine versus low-dose ketamine and propofol for conscious sedation in patients undergoing awake fiber-optic intubation. Anesth Essays Res. 2019;13:73–8. doi: 10.4103/aer.AER_181_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee K, Orme R, Williams D, Segal R. Prospective pilot trial of dexmedetomidine sedation for awake diagnostic flexible bronchoscopy. J Bronchology Interv Pulmonol. 2010;17:323–8. doi: 10.1097/LBR.0b013e3181f2a002. [DOI] [PubMed] [Google Scholar]
- 22.Sinha C, Kumar A, Kumari P, Singh AK, Sharma S, Kumar A, et al. Comparison of two doses of dexmedetomidine for supraclavicular brachial plexus block: A randomized controlled trial. Anesth Essays Res. 2018;12:470–4. doi: 10.4103/aer.AER_33_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao W, Ma G, Su QG, Fang Y, Gu BC, Zou XM. Dexmedetomidine versus midazolam for conscious sedation in postoperative patients undergoing flexible bronchoscopy: A randomized study. J Int Med Res. 2012;40:1371–80. doi: 10.1177/147323001204000415. [DOI] [PubMed] [Google Scholar]
- 24.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afonso J, Reis F. Dexmedetomidine: Current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:118–33. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 26.Madan K, Mohan A, Agarwal R, Hadda V, Khilnani GC, Guleria R. A survey of flexible bronchoscopy practices in India: The Indian bronchoscopy survey (2017) Lung India. 2018;35:98–107. doi: 10.4103/lungindia.lungindia_417_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


