Abstract
BACKGROUND:
Depressive disorders are considered to be one of the leading causes of morbidity and mortality accounting for 4.3% of total disability-adjusted life years. Selective serotonin reuptake inhibitors and serotonin–norepinephrine reuptake inhibitors have greater efficacy and lesser side effects; at the same time, these drugs cause sexual dysfunction and weight gain. Vilazodone was supposed to have better efficacy and less sexual dysfunction and weight gain.
AIM OF THE STUDY:
The aim is to compare efficacy (in terms of Hamilton Depression Rating Scale [HDRS]), sexual dysfunction (in terms of Arizona Sexual Experience Scale [ASEX]), and weight gain caused due to vilazodone and sertraline.
MATERIALS AND METHODS:
This is a randomized controlled study; 60 patients diagnosed with depressive episode were divided into two groups of 30 each; using block randomization technique, one group was prescribed vilazodone and another sertraline. The groups were compared on the basis of efficacy, weight gain, and sexual dysfunction using HDRS and ASEX at baseline, 4-week, and 12-week intervals. Statistical analysis was done by applying Chi-square test, Fisher's exact test, t-test, and repeated measures ANOVA using Wilks' lambda test.
RESULTS:
On comparing both vilazodone and sertraline, it was observed that both molecules have equal efficacy in terms of HDRS, but vilazodone does not cause weight gain and sexual dysfunction in terms of ASEX, and these findings are statistically very highly significant.
CONCLUSIONS:
Our study shows that vilazodone has similar efficacy but can be a better antidepressant due to lesser weight gain and sexual dysfunction compared to sertraline.
Keywords: Sertraline, sexual dysfunction, vilazodone, weight gain
Introduction
The WHO estimates that 300 million people all around the world are suffering from depression.[1] This disorder on the top of disability-adjusted life years (4.3%). It has been estimated that if such trends continue, it will be the most leading cause of morbidity throughout the world by 2030.[2,3] The prevalence of depression in India is reported to be 15.9%.[4]
Initially, the goal of antidepressants was up to 50% reduction in depressive symptoms (response on a standard scale). However, it was possible with selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs) to achieve more than 50% reduction in symptoms. Although there was improved efficacy of SSRIs and SNRIs, high incidence of SSRI-induced sexual dysfunction (24%–73%)[5] and weight gain (4%–25%) of original body weight[6] leads to premature discontinuation of treatment.
Sertraline (SSRI)[7] has excellent tolerability, but sexual dysfunction (3.5%–67%)[8] and weight gain (4.2%) of original body weight[6] lead to discontinuation of treatment, thus causing a relapse.
Purpose of the study
There remains a need for a newer antidepressant which has a better side effect profile in terms of sexual dysfunction and weight gain with more or at least equal efficacy as that of SSRIs. Vilazodone is found to have no or less sexual dysfunction and no weight gain with efficacy equivalent to as that of SSRIs. It is approved recently in 2011 by the Food and Drug Administration for depressive disorders. It is also known as serotonin partial agonist–reuptake inhibitor due to its dual action on serotonin reuptake inhibition and partial agonism on 5-HT1A which increases its antidepressant effect and its tolerability.
Most of the studies and trials on vilazodone till date have been conducted in animals and a very few in humans; there is no study comparing specifically sertraline and vilazodone. Hence, this particular study was planned to compare both the drugs in terms of efficacy, sexual dysfunction, and weight gain, especially so when vilazodone is being marketed as the antidepressant that does not cause weight gain and sexual dysfunction.
Materials and Methods
A longitudinal study (12 weeks for each patient) was conducted at the Department of Psychiatry, Maharishi Markandeshwar Institute of Medical Sciences and Research, Mullana. The Institutional Ethics Committee approval was obtained before the study with reference number IEC/MMIMSR/812.
The total duration of study was 6 months.
Enrollment of the patients
After assessing the patients' eligibility for the study, the patients were asked to participate in the study. After this, written informed consent was obtained. The patient was referred to the junior consultant, who prescribed the treatment as per the envelope in the sequence (based on sealed envelope technique). Block randomization (2 × 2) was used; the sequence was enclosed and sealed in an opaque envelope which was sequentially numbered. Care was taken not to manipulate the series. A total of 71 patients were recruited for the study, but 11 dropped out from the study, as shown in Figure 1. As a result of the process, we had two groups of 30 patients; each prescribed vilazodone or sertraline (30 each). This activity was completed within 3 months; thereafter, the patients were followed up for another 12 weeks (3 months). Hence, the whole process of data acquisition and the assessment was completed in a span of 6 months.
Figure 1.
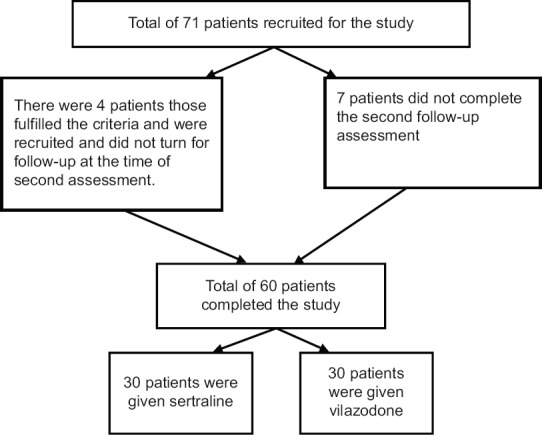
Recruitment of patients for the study
Design of the study
This was a randomized controlled study conducted in an outpatient setting. The principal investigator (MB) recruited the patient according to the inclusion criteria; after that, co-investigator (SA) carried out the block randomization as per the protocol and the treatment prescribed accordingly; finally, the rating was done by co-investigator (SA). The assessment of every patient recruited into the study was done at baseline/first visit, 4-week, and 12-week intervals by co-investigator (SA) on follow-up visits. The same co-investigator SA did all the ratings.
Inclusion criteria
Married patients (males/females) of age 21 years and above were included given the marriageable age)
All the patients who were drug naïve (patients who were not taking any psychotropic medication for the past 3 months at least)
Patients with ICD-10 criteria for depressive episode or recurrent depressive disorder
Patients who give written informed consent.
Exclusion criteria
Patients with comorbid psychiatric disorders (except depression or recurrent depression)
Depression with psychotic symptoms
Patients with mental retardation
Patients who are having a comorbid substance dependence
Patients with any other medical disorder which is confounding our inclusion criteria
Patients requiring the use of other psychotropic medication use of any concomitant medications (except zolpidem 5 mg once daily).
Dosing
Sertraline was started in a dose of 50 mg once daily and gradually increased to a maximum of 200 mg/day in divided doses if required by assessing the severity on the Hamilton Rating Scale for depression. Vilazodone started in a dose of 10 mg once daily and slowly increased to a maximum of 40 mg/day if needed. The dosage of both the drugs was increased only if tolerated well. (The medications were purchased by the patients from the hospital pharmacy; it was ensured that the medications used were of the same batch and brands).
Compliance
All patients and family members were psycho-educated regarding the course and prognosis of the illness and also the need for the treatment. Every patient was prescribed medications for 10 days at one time. The quantity of medication dispensed was recorded on the outpatient department record file by co-investigator (SA), and the patient was asked to bring the empty wrappers on the next follow-up and was matched from the previously recorded number of medications. Moreover, it was confirmed from the patient's primary caregiver.
All patients and family members were psycho-educated regarding the course and prognosis of the illness and also the need for the treatment.
Tools used
Sociodemographic data specially designed for the study
Hamilton Depression Rating Scale (HDRS)[9] – To grade the severity of depression. It is a 17-item scale; 8 items are scored on a 5-point scale ranging from 0 to 4, and 9 items are scored from 0 to 2; it is a clinician-administered scale
Arizona Sexual Experience Scale (ASEX)[10] – To assess sexual functioning. It is a five-item rating scale that quantifies sex drive, arousal, vaginal lubrication/penile erection, ability to reach orgasm, and satisfaction from orgasm. Possible total scores range from 5 to 30, with the higher scores indicating more sexual dysfunction. It is a clinician-administered scale.
Statistical analysis
The statistical analysis was done using independent t-test, Chi-square test, Fisher's exact test, and repeated measures ANOVA (MANOVA) using Wilks' lambda test and descriptive analysis in SPSS Statistics for Windows, Version 17.0. (SPSS Inc., Chicago, IL, USA).
Results
The mean age of patients in the vilazodone group was 40 ± 8.137 years, and it was 37 ± 4.250 years in the sertraline group [Table 1].
Table 1.
Mean±standard deviation of the age of the patients on vilazodone and sertraline
| Mean±SD | t | P | 95% CI of the difference | |||
|---|---|---|---|---|---|---|
| Vilazodone | Sertraline | Lower | Upper | |||
| Age | 40.00±8.137 | 37.27±4.250 | 1.631 | 0.108 | −0.622 | 6.088 |
SD=Standard deviation, CI=Confidence interval
Most of the patients were graduate (n = 27) which was a statistically highly significant finding (P = 0.00 with χ2= 21.6), and the rest were below the 12th standard. In our sample, the most common diagnosis was depressive disorder with somatic syndrome in both the groups (n = 36); of which, 21 patients were prescribed vilazodone and 15 were prescribed sertraline randomly which was statistically significant (with P = 0.017 and χ2= 12.07) [Table 2]. There was a mean ± standard deviation score of participants according to HDRS, ASEX score, and weight of the patients at 0 (baseline), 4, and 12 weeks after treatment with vilazodone and sertraline, which shows that at all the visits, both the molecules had equal efficacy (on HDRS), but there were no weight gain (P = 0.010)and no sexual dysfunction (on ASEX with P = 0.000) which were statistically significant findings [Table 3].
Table 2.
Association of sociodemographic factors with the two treatment groups
| Sociodemographic variables | Group | Total | χ2 | P | |
|---|---|---|---|---|---|
| Vilazodone | Sertraline | ||||
| Sex | |||||
| Female | 10 | 15 | 25 | 1.714 | 0.190 |
| Male | 20 | 15 | 35 | ||
| Locality | |||||
| Rural | 13 | 15 | 28 | 0.268 | 0.605 |
| Urban | 17 | 15 | 32 | ||
| Occupation | |||||
| Unskilled | 8 | 9 | 17 | 3.182 | 0.204 |
| Semi-skilled | 4 | 9 | 13 | ||
| Skilled | 18 | 12 | 30 | ||
| Education | |||||
| Uneducated | 0 | 6 | 6 | 21.600 | 0.000* |
| Up to Class V | 5 | 0 | 5 | ||
| Up to Class X | 7 | 0 | 7 | ||
| Up to Class XII | 9 | 6 | 15 | ||
| Graduate | 9 | 18 | 27 | ||
| Marital status | |||||
| Married | 30 | 30 | 60 | ||
| Religion | |||||
| Hindu | 26 | 27 | 53 | 4.019 | 0.134 |
| Muslim | 3 | 0 | 3 | ||
| Sikh | 1 | 3 | 4 | ||
| ICD-10 | |||||
| F 32.00 | 0 | 3 | 3 | 12.077 | 0.017* |
| F 32.01 | 0 | 6 | 6 | ||
| F 32.11 | 21 | 15 | 36 | ||
| F 32.2 | 7 | 6 | 13 | ||
| F 33.11 | 2 | 0 | 2 | ||
*Statistically significant. F 32.00: Mild depressive episode without somatic syndrome, F 32.01: Mild depressive episode with somatic syndrome, F 32.11: Moderate depressive episode with somatic syndrome, F 32.2: Severe depressive episode without psychotic symptoms, F 33.11: Recurrent depressive disorder, current episode moderate, with somatic syndrome. ICD - 10=International Classification of Disease - 10
Table 3.
Mean±standard deviation score of participants according to Hamilton Depression Rating Scale score, Arizona Sexual Experience Scale score, and weight of the patients at 0, 4, and 12 weeks of treatment with vilazodone and sertraline
| Mean±SD | t | P | 95% CI of the difference | |||
|---|---|---|---|---|---|---|
| Vilazodone | Sertraline | Lower | Upper | |||
| HDRS | ||||||
| 0 week | 20.03±4.013 | 19.53±3.540 | 0.512 | 0.611 | −1.456 | 2.456 |
| 4 weeks | 15.57±3.530 | 14.60±2.660 | 1.198 | 0.236 | −0.649 | 2.582 |
| 12 weeks | 10.57±3.559 | 9.10±2.657 | 1.809 | 0.076 | −0.157 | 3.090 |
| ASEX | ||||||
| 0 week | 17.13±2.763 | 16.50±3.298 | 0.806 | 0.423 | −0.939 | 2.206 |
| 4 weeks | 15.30±2.984 | 17.20±3.388 | −2.305 | 0.025 | −3.550 | −0.250 |
| 12 weeks | 11.77±3.598 | 19.37±3.917 | −7.827 | 0.000 | −9.544 | −5.656 |
| Weight (kg) | ||||||
| 0 week | 69.90±7.526 | 72.53±7.943 | −1.318 | 0.193 | −6.632 | 1.366 |
| 4 weeks | 69.93±7.697 | 72.90±7.980 | −1.466 | 0.148 | −7.018 | 1.085 |
| 12 weeks | 69.83±7.530 | 75.33±8.483 | −2.656 | 0.010 | −9.645 | −1.355 |
HDRS=Hamilton Depression Rating Scale, ASEX=Arizona Sexual Experience Scale, CI=Confidence interval, SD=Standard deviation
On performing repeated measures ANOVA test, it has seen that with vilazodone the sexual score on ASEX remains the same over time till 12 weeks with Wilks' lambda test value of 0.310, F (2,28) = 31.127, and P = 0.000 which is very highly significant [Table 4]. While the patients on sertraline have an increasing score on ASEX over time till 12 weeks with Wilks' lambda test value of 0.488, F (2,28) = 14.686, and P = 0.000 which is also very highly significant [Table 5]. On performing repeated measures ANOVA test, it has seen that vilazodone and sertraline both are very highly significant in terms of efficacious for depression when given for 12 weeks with Wilks' lambda test value [Table 6].
Table 4.
Repeated measures ANOVA test for vilazodone for various parameters of the study
| Mean±SD | |||
|---|---|---|---|
| HDRS scores | ASEX scores | Weight (kg) | |
| Timeline | |||
| At 0 weeks | 20.03±4.013 | 17.13±2.763 | 69.9±7.52 |
| At 4 weeks | 15.57±3.530 | 15.30±2.984 | 69.93±7.69 |
| At 12 weeks | 10.57±3.559 | 11.77±3.598 | 69.83±7.53 |
| Wilks’ lambda test | |||
| Value | 0.125 | 0.310 | 0.996 |
| F | 2, 28=97.867 | 2, 28=31.127 | 2, 28=0.055 |
| P | 0.000 | 0.000 | 0.946 |
HDRS=Hamilton Depression Rating Scale, ASEX=Arizona Sexual Experience Scale, SD=Standard deviation
Table 5.
Repeated measures ANOVA test for sertraline for various parameters of the study
| Mean±SD | |||
|---|---|---|---|
| HDRS scores | ASEX scores | Weight (kg) | |
| Timeline | |||
| At 0 weeks | 19.53±3.540 | 16.50±3.298 | 72.53±7.94 |
| At 4 weeks | 14.60±2.660 | 17.20±3.388 | 72.90±7.98 |
| At 12 weeks | 9.10±2.657 | 19.37±3.917 | 75.33±8.48 |
| Wilks’ lambda test | |||
| Value | 0.056 | 0.488 | 0.304 |
| F | 2, 28=237.325 | 2, 28=14.686 | 2, 28=32.077 |
| P | 0.000 | 0.000 | 0.000 |
HDRS=Hamilton Depression Rating Scale, ASEX=Arizona Sexual Experience Scale, SD=Standard deviation
Table 6.
Repeated measures ANOVA for difference of scores between two groups
| Wilks’ lambda | Value | f | P |
|---|---|---|---|
| For HDRS | 0.977 | 0.686 (2, 57) | 0.508 |
| For ASEX | 0.382 | 46.135 (2, 57) | 0.000 |
| For weight | 0.602 | 18.826 (2, 57) | 0.000 |
f=Degree of freedom, HDRS=Hamilton Depression Rating Scale, ASEX=Arizona Sexual Experience Scale, SD=Standard deviation
On performing repeated measures ANOVA test, it has seen that with vilazodone, there are no weight changes over time till 12 weeks with Wilks' lambda test value of 0.996, F (2,28) = 0.055, and P = 0.946 which is statistically insignificant Table 4. While the patients on sertraline have weight gain over time till 12 weeks with Wilks' lambda test value of 0.304, F (2,28) = 32.077, and P = 0.000 which is very highly significant Table 5. Repeated measures ANOVA for difference of scores between two groups. When scores of the two groups were compared, it was found that change in depression scores was not significant with Wilks' lambda value of 0.977, F = 0.686, and P = 0.508 in terms of HDRS, whereas ASEX scores and weight gain between the two groups were significantly different from Wilks' lambda value of 0.382, F = 46.135, and P = 0.000 for ASEX and Wilks' lambda value of 0.602, F = 18.826, and P = 0.000 for weight [Table 6].
Discussion
In our study, the mean age of patients was 40 ± 8.13 and 37 ± 4.2 years in the vilazodone and sertraline groups, respectively. It was a male predominant study, most of whom were from the urban locality. Majority of the patients were graduate, married, and belonged to the Hindu religion. A latest national mental health survey conducted in India 2015–2016 showed consistent findings regarding the demographic profile.[11]
Efficacy
In our study, we have seen that both the groups that are vilazodone as well as sertraline groups had an improvement in depressive symptoms on the HDRS scale showing that both the drugs are equally efficacious. There are studies which have shown similar kind of results with vilazodone[12] as well as with sertraline[13] in the context of improvement of depressive symptoms. A meta-analysis done in 2018 showed the results similar to our finding in terms of efficacy.[1] Furthermore, the study done by Bathla et al.[3] showed a similar kind of result for vilazodone. As we reviewed the past literature, we could not find any study against our finding which might be explained by the fact that data are sparse for vilazodone as it is a newer antidepressant and needs to be studied further individually.
Sexual side effects
As normal sexual functioning is one of the significant factors for maintaining self-esteem and natural well-being in humans. Thus, it is one of the priorities of the psychiatric patient to get it corrected and so it remains a significant concern for the psychiatrist as well. Sexual dysfunction in depressive disorder can be due to disease per se, and it can be due to antidepressant-induced sexual side effects, which is one of the primary reasons for premature discontinuation of antidepressant therapy. In our study, we have observed that sexual dysfunction occurred with sertraline but not with vilazodone; instead, there was an improvement on the ASEX scale of sexual function, and consistent finding was seen in a meta-analysis[14] showing consistent result that second-generation antidepressants including sertraline lead to sexual dysfunction while vilazodone does not; instead, it improves sexual functioning on the given scale (sexual functioning questionnaire score).[14]
Another meta-analysis[15] showed that up to 80% of patients had sexual dysfunction with sertraline. A study was done by Bathla et al.,[3] and Robinson et al.[16] also showed a similar finding regarding vilazodone.
There was a randomized controlled trial[12,14] with inconsistent findings showing that the patients on vilazodone were having higher sexual dysfunction than the placebo. This difference in outcome can be explained by the fact that in our study, vilazodone was compared to the older SSRI which has an established side effect of sexual dysfunction, whereas in that randomized controlled trial, the comparison was in between vilazodone and placebo which is not known to us that which drug or substance was given in the form of placebo; hence, we cannot confirm whether this placebo has sexual side effects or not.
Weight gain
Modernization is bringing a new trend in people becoming slim both for cosmetic as well as for fitness purpose because of which everyone remains fervent for staying slim. Depressive illness is an entity which, despite bringing mental disability, also brings physical disability in the form of weight gain. Studies have shown that depressive patients are at double the risk of gaining weight than nondepressive people (50% vs. 24%).[17] The weight gain in depressive patients can be due to the pathogenesis of depression, easy fatigability, and avolition leading to sedentary routine, overeating/munching as a stress buster to relieve depression (especially in atypical depression) and of course the use of antidepressants which brings happiness at the cost of weight gain. After the initiation of treatment with antidepressant the improvement in depressive symptoms is seen later however, the weight gain as a side effects of the treatment is observed earlier ultimately leading to discontinuation of the treatment.[18] The weight gain and depression flourishes each other by a common neurobiological pathway and thus increases the risk of obesity, hypertension, cardiac problems, diabetes mellitus, and other metabolic problems. Thus, weight gain directly or indirectly adds to the burden on the health system as well as on our economy by absenteeism. Thus, it has become a priority for a psychiatrist to consider weight gain issues in depressive patients and to choose an antidepressant which is either weight neutral or increases the weight to a minimal level.
Thus, our study considered this factor by comparing weight gain in patients taking vilazodone and sertraline. In our study, there was weight gain in patients who were taking sertraline, whereas there was no weight gain in the patients who were prescribed vilazodone. Studies are showing consistent results showing that weight gain remained persistent in patients on SSRI[6] (including sertraline) leading to treatment nonadherence.[6] Consistent results were seen by Bathla et al.,[3] Mathew et al.,[14] and Robinson et al.,[16] all of them showing that vilazodone is a safe drug in terms of weight gain. We could not find any study that shows weight gain with vilazodone though that may be explained by the fact that vilazodone is a newer drug; thus, literature regarding this molecule is very scanty and needs to be studied further.
Strengths of the study
Vilazodone is a novel SSRI, so very sparse literature exists, and our study may help the readers to enhance their knowledge regarding vilazodone. This is one of the very few studies study done in India regarding sexual dysfunction and weight gain as a result of vilazodone treatment and only study which compares vilazodone and sertraline in PubMed search till the time of our submission. Furthermore, this study could manage to get the report of sexual dysfunction which is always considered a stigmatic issue for the patients in the Indian scenario.
Limitations
There may be the chance of biasing as scales used were clinician rated. The dietary habits of the patients were not taken into consideration and hence would have confounded the results. Furthermore, only weight gain and sexual side effects were screened out, and no other side effect was assessed. It is a short-term study, and results may vary if treatment with both the drugs is prolonged. Furthermore, the sample size was very, and these findings could not be generalized and need further research.
Directions for future research
A large sample size, multicenter, long duration follow-up study, assessing the complete side effect profile (not only the weight gain and sexual dysfunction), the use of patient-rated scales and lastly the comparison to the other antidepressants and not only one single molecule.
Conclusions
In our study, we have reached a conclusion that vilazodone and sertraline have similar efficacy, but vilazodone has no weight gain and lesser sexual dysfunction as compared to sertraline, and thus, vilazodone can be considered a better antidepressant.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Nil.
References
- 1.Wagner G, Schultes MT, Titscher V, Teufer B, Klerings I, Gartlehner G. Efficacy and safety of levomilnacipran, vilazodone and vortioxetine compared with other second-generation antidepressants for major depressive disorder in adults: A systematic review and network meta-analysis. J Affect Disord. 2018;228:1–2. doi: 10.1016/j.jad.2017.11.056. [DOI] [PubMed] [Google Scholar]
- 2.Pattanayak RD, Sagar R. Depressive disorders in Indian context: A review and clinical update for physicians. J Assoc Physicians India. 2014;62:827–32. [PubMed] [Google Scholar]
- 3.Bathla M, Anjum S, Singh M, Panchal S, Singh GP. A 12-week comparative prospective open-label randomized controlled study in depression patients treated with vilazodone and escitalopram in a tertiary care hospital in North India. Indian J Psychol Med. 2018;40:80–5. doi: 10.4103/IJPSYM.IJPSYM_368_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poongothai S, Pradeepa R, Ganesan A, Mohan V. Prevalence of depression in a large urban South Indian population – The Chennai Urban Rural Epidemiology Study (CURES-70) PLoS One. 2009;4:e7185. doi: 10.1371/journal.pone.0007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy SH, Eisfeld BS, Dickens SE, Bacchiochi JR, Bagby RM. Antidepressant-induced sexual dysfunction during treatment with moclobemide, paroxetine, sertraline, and venlafaxine. J Clin Psychiatry. 2000;61:276–81. doi: 10.4088/jcp.v61n0406. [DOI] [PubMed] [Google Scholar]
- 6.Deshmukh R, Franco K. Managing weight gain as a side effect of antidepressant therapy. Cleve Clin J Med. 2003;70:614–23. doi: 10.3949/ccjm.70.7.614. [DOI] [PubMed] [Google Scholar]
- 7.Carrasco JL, Díaz-Marsá M, Sáiz-Ruiz J. Sertraline in the treatment of mixed anxiety and depression disorder. J Affect Disord. 2000;59:67–9. doi: 10.1016/s0165-0327(99)00138-x. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin DS. Depression and sexual dysfunction. Br Med Bull. 2001;57:81–99. doi: 10.1093/bmb/57.1.81. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGahuey CA, Gelenberg AJ, Laukes CA, Moreno FA, Delgado PL, McKnight KM, et al. The Arizona Sexual Experience Scale (ASEX): Reliability and validity. J Sex Marital Ther. 2000;26:25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]
- 11.Murthy RS. National mental health survey of India 2015-2016. Indian J Psychiatry. 2017;59:21–6. doi: 10.4103/psychiatry.IndianJPsychiatry_102_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz MP. Vilazodone HCl (Viibryd): A serotonin partial agonist and reuptake inhibitor for the treatment of major depressive disorder. PT. 2012;37:28–31. [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor CM, Jiang W, Kuchibhatla M, Silva SG, Cuffe MS, Callwood DD, et al. Safety and efficacy of sertraline for depression in patients with heart failure: Results of the SADHART-CHF (Sertraline Against Depression and Heart Disease in Chronic Heart Failure) trial. J Am Coll Cardiol. 2010;56:692–9. doi: 10.1016/j.jacc.2010.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathews M, Gommoll C, Chen D, Nunez R, Khan A. Efficacy and safety of vilazodone 20 and 40 mg in major depressive disorder: A randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 2015;30:67–74. doi: 10.1097/YIC.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: A meta-analysis. J Clin Psychopharmacol. 2009;29:259–66. doi: 10.1097/JCP.0b013e3181a5233f. [DOI] [PubMed] [Google Scholar]
- 16.Robinson DS, Kajdasz DK, Gallipoli S, Whalen H, Wamil A, Reed CR. A 1-year, open-label study assessing the safety and tolerability of vilazodone in patients with major depressive disorder. J Clin Psychopharmacol. 2011;31:643–6. doi: 10.1097/JCP.0b013e31822c6741. [DOI] [PubMed] [Google Scholar]
- 17.Angst J. Sexual problems in healthy and depressed persons. Int Clin Psychopharmacol. 1998;13(Suppl 6):S1–4. doi: 10.1097/00004850-199807006-00001. [DOI] [PubMed] [Google Scholar]
- 18.Fava M, Dunner DL, Greist JH, Preskorn SH, Trivedi MH, Zajecka J, et al. Efficacy and safety of mirtazapine in major depressive disorder patients after SSRI treatment failure: An open-label trial. J Clin Psychiatry. 2001;62:413–20. doi: 10.4088/jcp.v62n0603. [DOI] [PubMed] [Google Scholar]


