Abstract
The use of lidocaine in spinal anesthesia may increase the risk of transient neurological symptoms (TNS) according to previous meta-analyses. However, the previous meta-analyses lacked data on some other local anesthetics and thus, more evaluations are still needed to compare the effect of lidocaine on the development of TNS. The objective of this study was to compare the risk of TNS according to lidocaine versus other local anesthetics in patients undergoing spinal anesthesia. A total of 39 randomized controlled trials with 4733 patients were analyzed. The incidence of TNS was 10.8% in the lidocaine group and was 2.2% in the control groups (risk ratio (RR) 4.12, 95% confidence interval (CI) 3.13 to 5.43, p < 0.001). In subgroup analysis, lidocaine increased the incidence of TNS compared with other local anesthetics except mepivacaine, ropivacaine or sameridine. The risk of TNS was higher in the hyperbaric (p < 0.001) or isobaric lidocaine (p < 0.001) group compared with the control group, but there were no differences found between the two groups when hypobaric lidocaine was administered (p = 1.00). This study confirmed that lidocaine for spinal anesthesia still causes TNS more frequently than most other local anesthetics, especially when hyperbaric or isobaric lidocaine was used.
Keywords: Lidocaine, Anesthesia, spinal, Postoperative Complications
1. Introduction
Lidocaine is an attractive regional anesthetic for ambulatory surgery. It offers a rapid onset and fast recovery of both motor and sensory block [1]. However, when compared with other local anesthetics, the use of lidocaine in spinal anesthesia has been known to be associated with increased risk of transient neurological symptoms (TNS) [2,3], hindering its application in ambulatory spinal anesthesia. Other local anesthetics including mepivacaine, low-dose bupivacaine, procaine, articaine, levobupivacaine, ropivacaine and 2-chloroprocaine have been suggested as replacement drugs.
TNS generally occurs in patients with single injection spinal anesthesia within the first 24 hours [2]. TNS consists of pain in the lower extremities without abnormalities in neurologic and radiologic examination [2]. In previous systematic reviews and meta-analyses [2,3], lidocaine has a significantly higher relative risk of developing TNS compared with most other local anesthetics, but the previous meta-analyses lacked data on ropivacaine, levobupivacaine, and chloropocaine; thus, more evaluations are still needed to confirm favorable results for these aforementioned local anesthetics. In the last decade, many randomized controlled trials (RCTs) comparing between the incidence of TNS after lidocaine and other local anesthetics have been conducted. More subsequent studies evaluating the effect of lidocaine on the risk of TNS have been published [2,3]. Furthermore, among them, many studies reported no patients suffering from TNS after spinal anesthesia with lidocaine [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Therefore, it is still controversial on the safety of lidocaine for spinal anesthesia during ambulatory surgery in terms of TNS. The objective of this systematic review and meta-analysis is to compare the incidence of TNS between lidocaine and other local anesthetics and to evaluate the frequency of TNS with various types of local anesthetics in adult surgical patients after spinal anesthesia.
2. Materials and Methods
2.1. Literature Search
This systematic review and meta-analysis was conducted according to the Preferred Items for Systematic Reviews and Meta Analyses (PRISMA) statements guideline [19]. A predefined protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42019137819). RCTs comparing lidocaine versus other local anesthetics during spinal anesthesia were searched on the following databases: PubMed, EMBASE, CENTRAL, CINAHL, Scopus, Web of Science and KoreaMed. The final search was performed on March 31st, 2019. Search strategies were established with MeSH terms and keywords, including “spinal anesthesia”, “lidocaine”, or “lignocaine”. Each finding was combined with the Boolean operator, such as “AND”, “OR”. Detailed search strategies for each database were described in Table S1. The title, abstract, and authors of all retrieved articles were extracted and collected, regardless of the publication year, language or region.
2.2. Study Selection
C.-H.K. and H.-J.S. independently accessed the titles and abstracts of the articles to screen for relevant studies. Subsequently, full-texts of relevant articles were obtained via hand-search, library service or contacting the authors. C.-H.K. and J.-H.R. read the full text to select studies that were appropriate for this meta-analysis. The inclusion criteria were (1) randomized controlled trials, (2) surgical patients under spinal anesthesia, (3) lidocaine use for spinal anesthesia in at least in one group, and (4) use of other local anesthetic for spinal anesthesia in the control group. The exclusion criteria were: (1) abstract, protocol, conference poster or review; and (2) The study which did not report the incidence of TNS. S.-H.H. participated on selection if any disagreement existed.
2.3. Data Extraction
C.-H.K. and H.-J.S. independently investigated and collected the following data from final full-texts: author, publication year, language, sample size, type of surgery, type of anesthesia, patient’s position during surgery, needle type, characteristics of lidocaine (concentration, baricity, dose, and adjuvants), characteristics of local anesthetics used in the control group (type, concentration, baricity, dose, and adjuvants) and the incidence of TNS.
2.4. Risk of Bias Assessment
C.-H.K. and J.-H.R. independently assessed the risk of bias of the included studies using the Cochran Risk of Bias tool [20]. It consists of seven items: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases. Each item was graded as low, unclear or high. S.-H.H. settled any disagreements between the aforementioned assessors.
2.5. Data Synthesis and Statistical Analysis
Data synthesis and meta-analysis were performed using Revman 5.3 software (Cochrane Collaboration, Oxford, UK) and R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Since the incidence of TNS was dichotomous variable, the authors calculated the risk ratio (RR) as a pooled estimate. The inverse variance method and random effect models were employed. A continuity correction of 0.5 was applied to zero total events trials [21]. The findings were presented as a forest plot with 95% confidence intervals. A subgroup analysis was conducted according to the local anesthetics which were used in the control group. Additional subgroup analyses were carried out to investigate any relationship between the incidence of TNS and baricity/concentration. The heterogeneity among the studies was evaluated by I2 statistic. I2 could be interpreted in the following manner: 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% considerable heterogeneity [22]. A publication bias was assessed by construction of a funnel plot and the linear regression test. Sensitivity analysis (leave one study out) was conducted to confirm the robustness of the results.
3. Results
3.1. Descriptions of Trials
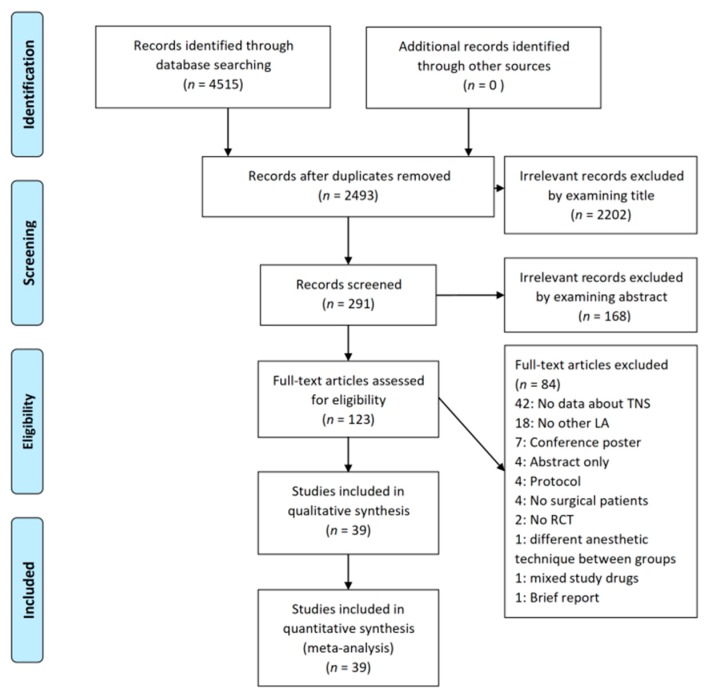
A total of 4515 articles were found on the initial database search. Among them, 2493 articles were removed due to duplication. Subsequently, 2202 articles and 168 articles were considered as irrelevant based on their title and abstract, respectively. The full-text of 123 articles were evaluated, and then, 84 articles were excluded due to the following reasons: no results about the incidence of TNS (n = 42); no other local anesthetics were used (n = 18); conference posters (n = 7); abstracts only (n = 4); protocols (n = 4); healthy subjects (n = 4); non-randomized studies of intervention (n = 2); different anesthetic techniques between groups (n = 1); mixed spinal anesthetics (n = 1); and a brief report (n = 1). Therefore, a total of 39 RCTs were included in the final analysis (Figure 1) [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46].
Figure 1.
Flow diagram of the included and excluded studies. A total of 4515 articles were found during the literature search. Among them, 2493 articles were duplicated retrievals. A total of 2202 articles and 168 articles were obviously irrelevant studies. We excluded 84 articles due to various reasons. Finally, 39 articles were included in the final analysis. Abbreviations: TNS = transient neurological symptoms, LA = local anesthetics, RCT = randomized controlled trial
All these RCTs compared lidocaine with other local anesthetics (bupivacaine, prilocaine, mepivacaine, levobupivacaine, chloroprocaine, ropivacaine, procaine, articaine, or sameridine) and reported the incidence of TNS. We found that 28 RCTs had two groups [4,5,6,7,8,9,10,11,12,14,15,18,23,26,27,29,30,34,35,36,37,38,39,40,41,42,44,46] of lidocaine more than other local anesthetics, while 11 RCTs had multiple groups [13,16,17,24,25,28,31,32,33,43,45]. Five out of 11 RCTs have more than two lidocaine groups [16,24,28,33,43]. According to the Cochrane guidelines [22], all lidocaine groups were pooled into a single group. In another five RCTs, more than two other local anesthetics were used for spinal anesthesia [17,25,31,32,45]. In the remaining RCT [13], three doses of sameridine were used for spinal anesthesia and the results of three sameridine groups were combined. Patients who received general anesthesia due to insufficient spinal block were excluded from the final analysis because it was unclear whether local anesthetics were administered in the cerebrospinal fluid. Details of each trial are summarized in Table 1. The 5% hyperbaric lidocaine was most used, followed by 2% isobaric. The dose of lidocaine used in each trial varied from 10 to 100mg. Specific details, including concentration, baricity, doses and adjuvants of study drugs, are summarized in Table 2.
Table 1.
Baseline characteristics of the included randomized trials (n = 39).
| Author | Year | Language | Anesthesia | Type of Operation | Number of Groups | Local Anesthetics (Control Group) |
Needle | Position during Surgery | Follow-up Periods |
|---|---|---|---|---|---|---|---|---|---|
| Ali | 2015 | English | SA a | Knee arthroscopy | 2 | Bupivacaine | 25G Quincke | Supine | 24,72,168 h |
| Aouad | 2001 | English | SA a | Cesarean section | 2 | Bupivacaine | 25G Whitacre | Supine | 24,48,72 h |
| Beilin | 2003 | English | CSE b | Cervical cerclage | 2 | Bupivacaine | 25G Sprotte | Lithotomy | 24 h |
| Breebaart | 2003 | English | SA a | Knee arthroscopy | 3 | G1: Levobupivacaine G2: Ropivacaine |
27G Whitacre | Supine | 48 h |
| Breebaart | 2014 | English | SA a | Knee arthroscopy | 4 | Chloroprocaine | 27G Whitacre | Supine | 168 h |
| Buckenmaier | 2003 | English | SA a | Anorectal surgery | 2 | Ropivacaine | 25G Pencan | Jackknife | 24,48,72,168 h |
| Casati | 2007 | English | SA a | Knee arthroscopy | 2 | Chloroprocaine | 25G Whitacre | Not described | 24, 168 h |
| de Santiago | 2009 | English | SA a | Tubal sterilization | 2 | Levobupivacaine | 27G Whitacre | Trendelenburg | 168 h |
| de Santiago | 2010 | Spanish | SA a | Anorectal surgery | 2 | Levobupivacaine | 27G Whitacre | Jackknife | 72, 168 h |
| de Weert | 2000 | English | SA a | Short surgery of the lower body | 2 | Prilocaine | 25G pencil-point | Supine | 24 h |
| Etezadi | 2013 | English | SA a | Varicocele, surgical fixation of lower extremities, transurethral resection of prostate, transurethral lithotripsy, herniorrhaphy | 4 | Bupivacaine | 25G Sprotte or Quincke | Supine or lithotomy | 8, 16, 24, 32, 40, 48, 72 h |
| Fanelli | 2009 | English | SA a | Knee arthroscopy | 2 | Ropivacaine | 25G Whitacre | Supine | 24, 168 h |
| Gozdemir | 2010 | English | SA a | Minor orthopedic, varicose vein, inguinal hernia, appendectomy | 2 | Levobupivacaine | 25G Quincke | Supine | 48, 168 h |
| Gozdemir | 2016 | English | SA a | Minor orthopedic, cesarean section, varicose vein, inguinal hernia, appendectomy | 4 | G1: Levobupivacaine G2: Bupivacaine G3: Articaine |
27G pencil-point | Not described | 24,48,72 h |
| Hampl | 1995 | English | SA a | Short gynecological procedure | 3 | Bupivacaine | 25G pencil-point | Lithotomy | 24 h |
| Hampl | 1998 | English | SA a | Short gynecological procedure | 3 | G1: Prilocaine G2: Bupivacaine |
25G pencil-point | Lithotomy | 24 h |
| Hodgson | 2000 | English | SA a | Knee arthroscopy | 2 | Procaine | 24 or 25G pencil-point | Supine | 72 h |
| Imbelloni | 2010 | English | SA a | Anorectal surgery | 2 | Bupivacaine | 27G Quincke | Jackknife | Until 30th day |
| Keld | 2000 | English | SA a | Inguinal hernia, femoral hernia, knee arthroscopy, removal of osteosynthetic material, fractures in the lower extremities, incision of infraumbilical abscess | 2 | Bupivacaine | 25G pencil-point | Supine | 24, 72 h |
| Khant | 2017 | English | SA a | Urologic surgery | 2 | Bupivacaine | 26G Quincke | Supine or lithotomy | Not described |
| Kyokong | 2001 | English | SA a | Cesarean section | 2 | Bupivacaine | 27G Quincke | Supine | 24 h |
| Le Truong | 2001 | English | SA a | General, gynecological, or other surgery | 2 | Procaine | 27G Whitacre | Supine or lithotomy | 48 h |
| Liguori | 1998 | English | SA a | Knee arthroscopy | 2 | Mepivacaine | 27G Whitacre | Supine | 48 h |
| Maliachi | 1999 | Portuguese | SA a | Femur surgery | 2 | Bupivacaine | 22G, not described | Supine | 24, 48, 72 h |
| Martin | 2005 | English | SA a | Knee arthroscopy | 2 | Prilocaine | 25G Whitacre | Not described | 48h |
| Martinez | 1998 | English | SA a | Orthopedic, urologic, gynecologic, vascular, general surgery | 2 | Prilocaine | 25G pencil-point | Not described | 72–120 h |
| Mulroy | 1999 | English | SA a | Inguinal hernia | 4 | Sameridine | 25G Whitacre | Supine | 24 h |
| Orozco | 2006 | Spanish | SA a | Surgery below the umbilicus | 2 | Bupivacaine | Not described | Not described | Not described |
| Ostgaard | 2000 | English | SA a | Urology surgery | 2 | Prilocaine | 25,26,27,29G Quincke | Supine or lithotomy | 24 h |
| Pawlowski | 2012 | English | CSE b | Anterior cruciate ligament repair | 2 | Mepivacaine | 27G Pencan | Supine | 24,48,72 h |
| Philip | 2001 | English | SA a | Postpartum tubal ligation | 2 | Bupivacaine | 25G Whitacre | Supine | 24,48 h |
| Pollock | 1996 | English | SA a | Knee arthroscopy or inguinal hernia | 3 | Bupivacaine | 22 or 25G Quincke or Whitacre | Supine | 72 h |
| Pradhan | 2010 | English | SA a | Cesarean section | 2 | Bupivacaine | 26G Quincke | Supine | Not described |
| Punj | 2013 | English | SA a | pelvic surgery | 4 | Bupivacaine | 24G Quincke | Supine | 120 h |
| Salazar | 2001 | English | SA a | Minor surgery of lower extremities | 2 | Mepivacaine | 26 or 27G Quincke | Supine | 24 h |
| Salmela | 1998 | English | SA a | Urologic surgery, varicose vein, hemorrhoidectomy, hernia | 3 | G1: Mepivacaine G2: Bupivacaine |
27G Quincke or Whitacre | Supine or lithotomy | 24 h |
| Teunkens | 2016 | English | SA a | Knee arthroscopy | 3 | G1: Chloroprocaine G2: Bupivacaine |
27G Whitacre | Supine | 24 h |
| Vaghadia | 2012 | English | SA a | Transurethral resection of prostate | 2 | Chloroprocaine | 25 or 27G Whitacre | Lithotomy | 96–168 h |
| Yea | 1998 | Korean | SA a | Surgery of lower body | 2 | Mepivacaine | 25G Quincke | Supine | 24 h |
Abbreviations: a SA = spinal anesthesia; b CSE = combined spinal-epidural anesthesia.
Table 2.
Concentration, baricity, doses and adjuvants of study drugs (n = 39).
| Study | Sample Size | LDC a | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LDC | Control | Concentration | Baricity | Dose | Added | Type | Concentration | Baricity | Dose | Added | |
| Ali 2015 | 25 | 25 | 0.6% | Hypobaric | 20 mg | FTN b 25 µg | Bupivacaine | 0.375% | Hyperbaric | 3 mg | FTN b 10 µg |
| Aouad 2001 | 100 | 100 | 5% | Hyperbaric | 75 mg | - | Bupivacaine | 0.75% | Hyperbaric | 12 mg | - |
| Beilin 2003 | 29 | 30 | 1% | Isobaric | 30 mg | FTN b 20 µg | Bupivacaine | 0.175% | Hyperbaric | 5.25 mg | FTN b 20 µg |
| Breebaart 2003 | 30 | G1: 30 G2: 30 |
2% | Isobaric | 60 mg | - | G1: Levobupivacaine G2: Ropivacaine |
G1: 0.33% G2: 0.5% |
G1:Isobaric G2:Isobaric |
G1: 10 mg G2: 15 mg |
- |
| Breebaart 2014 | 50 | 50 | 1.5% | Isobaric | 60 mg | - | Chloroprocaine | 1% | Isobaric | 40 mg | - |
| Buckenmaier 2003 | 37 | 35 | 2.5% | Hyperbaric | 25 mg | FTN b 20 µg | Ropivacaine | 0.5% | Hyperbaric | 4 mg | FTN b 20 µg |
| Casati 2007 | 15 | 15 | 1% | Isobaric | 50 mg | - | Chloroprocaine | 1% | Isobaric | 50 mg | |
| de Santiago 2009 | 26 | 26 | 0.3% | Hypobaric | 10 mg | FTN b 10 µg | Levobupivacaine | 0.1% | Hypobaric | 3 mg | FTN b 10 µg |
| de Santiago 2010 | 30 | 30 | 0.6% | Hypobaric | 18 mg | FTN b 10 µg | Levobupivacaine | 0.5% | Hypobaric | 3 mg | FTN b 10 µg |
| de Weert 2000 | 35 | 34 | 2% | Isobaric | 80 mg | - | Prilocaine | 2% | Isobaric | 80 mg | - |
| Etezadi 2013 | 125 | 125 | 5% | Hyperbaric | 75–100 mg | - | Bupivacaine | 0.5% | Isobaric | 12.5–15 mg | - |
| Fanelli 2009 | 15 | 15 | 1% | Isobaric | 50 mg | - | Ropivacaine | 0.5% | Isobaric | 10 mg | - |
| Gozdemir 2010 | 30 | 30 | 2% | Isobaric | 80 mg | - | Levobupivacaine | 0.5% | Isobaric | 20 mg | - |
| Gozdemir 2016 | 100 | G1: 100 G2: 100 G3: 100 |
2% | Isobaric | 60 mg | - | G1: Levobupivacaine G2: Bupivacaine G3: Articaine |
G1: 0.5% G2: 0.5% G3: 2% |
G1: Isobaric G2: Isobaric G3: Isobaric |
G1: 15 mg G2: 15 mg G3: 60 mg |
- |
| Hampl 1995 | G1: 15 G2: 13 |
16 |
G1:5% (7.5% dextrose) G2:5% (2.7% dextrose) |
G1:Hyperbaric G2: Hyperbaric |
G1:75 mg G2: 75 mg |
- | Bupivacaine | 0.5% | Hyperbaric | 7.5 mg | - |
| Hampl 1998 | 30 | G1: 30 G2: 30 |
2% | Hyperbaric | 50 mg | - | G1: Prilocaine G2: Bupivacaine |
G1: 2% G2: 0.5% |
G1: Hyperbaric G2: Hyperbaric |
G1: 50 mg G2:12.5 mg |
- |
| Hodgson 2000 | 35 | 35 | 2.5% | Hyperbaric | 50 mg | - | Procaine | 5% | Hyperbaric | 100 mg | |
| Imbelloni 2010 | 75 | 75 | 0.6% | Hypobaric | 18 mg | - | Bupivacaine | 0.15% | Hypobaric | 4.5 mg | - |
| Keld 2000 | 35 | 34 | 5% | Hyperbaric | 100 mg | - | Bupivacaine | 0.5% | Hyperbaric | 12.5 mg | - |
| Khant 2017 | 498 | 492 | 3.125% | Not described | 25 mg | Butorphanol 0.3 mg |
Bupivacaine | 0.5% | Not described | 5 mg | - |
| Kyokong 2001 | 71 | 71 | 5% | Hyperbaric | 60 mg | MPc 0.2 mg EPId 0.1 mg |
Bupivacaine | 0.5% | Hyperbaric | 11 mg | MPc 0.2 mg |
| Le Truong 2001 | 29 | 25 | 5% | Hyperbaric | 100 mg | - | Procaine | 5% | Isobaric | 100 mg | - |
| Liguori 1998 | 27 | 30 | 2% | Not described | 60 mg | - | Mepivacaine | 1.5% | Not described | 45 mg | - |
| Maliachi 1999 | 20 | 20 | 5% | Not described | 1 mg/kg | - | Bupivacaine | 0.5% | Not described | 7–15 mg | - |
| Martin 2005 | 40 | 40 | 1.5% | Not described | 45 mg | - | Prilocaine | 1.5% | Not described | 45 mg | - |
| Martinez 1998 | 98 | 100 | 5% | Hyperbaric | 67.7 ± 8.7 mg | - | Prilocaine | 5% | Hyperbaric | 68.6±9.7 mg | - |
| Mulroy 1999 | 32 | G1: 23 G2: 43 G3: 42 |
2.5% | Hyperbaric | 100 mg | - | Sameridine | Not described | Isobaric | G1: 15 mg G2: 20 mg G3: 23 mg |
- |
| Orozco 2006 | 109 | 97 | 5% | Not described | Not described | - | Bupivacaine | 0.5% | Not described | Not described | - |
| Ostgaard 2000 | 49 | 50 | 2% | Isobaric | 80 mg | - | Prilocaine | 2% | Isobaric | 80 mg | - |
| Pawlowski 2012 | 41 | 38 | 2% | Isobaric | 80 mg | - | Mepivacaine | 2% | Isobaric | 80 mg | - |
| Philip 2001 | 29 | 28 | 5% | Hyperbaric | 60–80 mg | - | Bupivacaine | 0.75% | Hyperbaric | 10.5–12 mg | - |
| Pollock 1996 | G1:51 G2:51 |
50 |
G1: 5% G2: 2% |
Hyperbaric or isobaric |
60 or 75 mg | EPI d or none | Bupivacaine | 0.75% | Hyperbaric | 7.5 or 9 mg | - |
| Pradhan 2010 | 26 | 26 | 5% | Hyperbaric | 75 mg | - | Bupivacaine | 0.5% | Hyperbaric | 12.5 mg | - |
| Punj 2013 | G1:20 G2:20 |
G1: 20 G2: 20 |
G1: 5% G2: 2.5% |
Hyperbaric | Not described | - | Bupivacaine | G1: 0.5% G2: 0.25% |
Hyperbaric | G1: 10 mg G2: 5 mg |
- |
| Salazar 2001 | 40 | 40 | 2% | Isobaric | 40–60 mg | - | Mepivacaine | 2% | Isobaric | 40–60 mg | - |
| Salmela 1998 | 30 | G1:30 G2: 30 |
2.5% | Hyperbaric | 60–100 mg | - | G1: Mepivacaine G2: Bupivacaine |
G1: 4% G2: 0.5% |
G1: Hyperbaric G2: Hyperbaric |
G1:40–80 mg G2:7.5–17 mg |
- |
| Teunkens 2016 | 28 | G1: 30 G2: 34 |
1% | Isobaric | 40 mg | - | G1: Chloroprocaine G2: Bupivacaine |
G1: 1% G2: 0.5% |
G1: Isobaric G2: Isobaric |
G1: 40 mg G2: 7.5 mg |
|
| Vaghadia 2012 | 20 | 20 | 1.75% | Not described | 35 mg | FTN b 12.5 µg | Chloroprocaine | 1.77% | Isobaric | 40 mg | FTN b 12.5 µg |
| Yea 1998 | 30 | 30 | 1.5% | Hyperbaric | 75 mg | - | Mepivacaine | 2% | Hyperbaric | - | |
Abbreviations: a LDC = Lidocaine; b FTN = Fentanyl; c MP = Morphine; d EPI = Epinephrine.
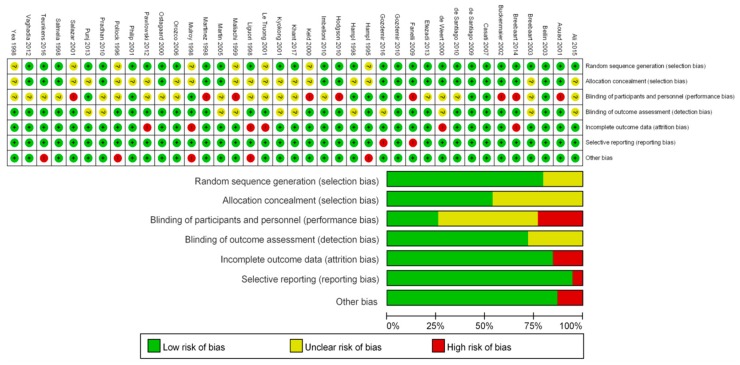
3.2. Methodology Quality and Risk of Bias
The methodology quality and risk of bias are summarized in Figure 2. In each study, all patients were randomized to receive intrathecal lidocaine or other local anesthetics; however, the randomization method was unclear in eight studies. Twenty one studies maintained allocation concealment, but the other studies failed to describe it clearly. The risk of performance bias was high in 9 studies and unclear in 20 studies. It might be important for anesthesiologists to be aware of drugs for patient safety and sufficient block when performing spinal anesthesia. Unlike performance bias, the risk of detection bias was low overall. The risk of attrition bias, reporting bias, and other biases were low in more than 75% of the studies evaluated. Reasons for each risk of bias are shown in Table S2.
Figure 2.
Risk of bias summary and graph using the Cochrane Risk of Bias tool [20]. The randomization and allocation concealment were well performed in most studies, but the method was not described in several studies. The performance bias was unclear or high in most studies, whereas the detection bias was low overall. The risk of attrition bias, reporting bias and other biases were low in most studies. Abbreviations: + = low risk of bias, ? = unclear risk of bias, - = high risk of bias.
3.3. Outcome Synthesis
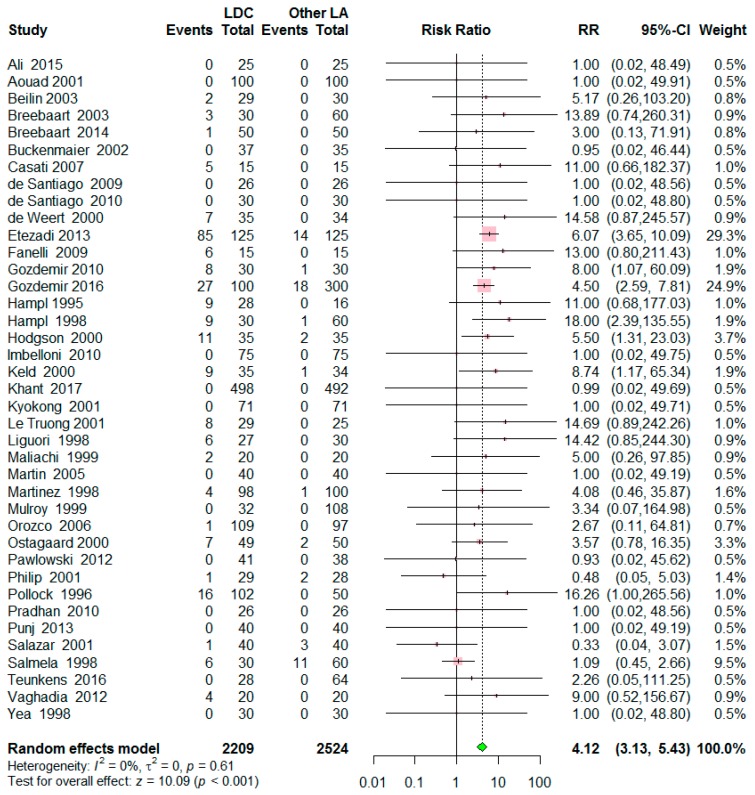
A total of 39 studies included 4733 patients; 2209 patients were allocated to the lidocaine group and 2524 patients to the control group. The incidence of TNS was 10.8% (238/2209) in the lidocaine group and was 2.2% (56/2524) in the control group. The risk of TNS after spinal anesthesia was significantly higher in the lidocaine group than in the control group (Risk ratio (RR) = 4.12, 95% confidence interval (CI) = 3.13 to 5.43, p < 0.001), with a low level of heterogeneity (I2 = 0%, p = 0.61) (Figure 3). A symmetrical funnel plot and linear regression test showed insignificant results for publication bias (p = 0.206) (Figure S1). Sensitivity analysis revealed the robustness of the results (Table S3). Omitting one study [28] decreased the RR to 3.51, but still maintained the significance.
Figure 3.
Forest plot for the risk of transient neurologic symptoms (TNS) after spinal anesthesia with lidocaine versus other local anesthetics. The incidence of TNS 10.8 % in the lidocaine group while 2.2% in the control group. The risk of TNS was significantly higher in the lidocaine group than in the control group (p < 0.001). Abbreviations: LDC = lidocaine, LA = local anesthetics, RR = risk ratio, CI = confidence interval.
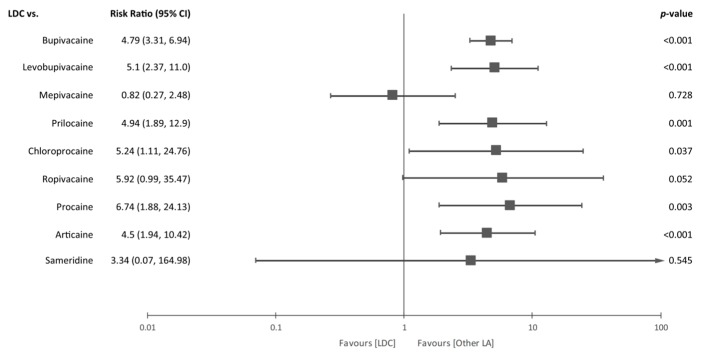
In subgroup analysis as shown in Figure 4, lidocaine increased the incidence of TNS compared with most other local anesthetics, such as bupivacaine (RR = 4.79, 95% CI = 3.31 to 6.94, p < 0.001), levobupivacaine (RR = 5.1, 95% CI = 2.37 to 11.0, p < 0.001), prilocaine (RR = 4.94, 95% CI = 1.89 to 12.9, p = 0.001), chloroprocaine (RR = 5.24, 95% CI = 1.11 to 24.76, p = 0.037), procaine (RR = 6.74, 95% CI = 1.88 to 24.13, p = 0.003), and articaine (RR = 4.5, 95% CI = 1.94 to 10.42, p < 0.001). However, no significant difference was observed between the lidocaine group and the mepivacaine (RR = 0.82, 95% CI = 0.27 to 2.48, p = 0.728), ropivacaine (RR = 5.92, 95% CI = 0.99 to 35.47, p = 0.052) or sameridine group (RR = 3.34, 95% CI = 0.07 to 164.98, p = 0.545). A low level of heterogeneity was found in each subgroup analysis.
Figure 4.
Forest plot for subgroup analysis. A subgroup analysis was conducted according to the local anesthetics which were used in the control group. The incidence of TNS was significantly higher in the lidocaine group than in the bupivacaine (p < 0.001), levobupivacaine (p < 0.001), prilocaine (p = 0.001), chloroprocaine (p = 0.037), procaine (p = 0.003) and articaine group (p < 0.001). However, there were no differences in the risk of TNS between the lidocaine group and mepivacaine (p =0.728), ropivacaine (p = 0.052) and sameridine group (p = 0.545). Abbreviations: LDC = lidocaine, LA = local anesthetics, CI = confidence interval.
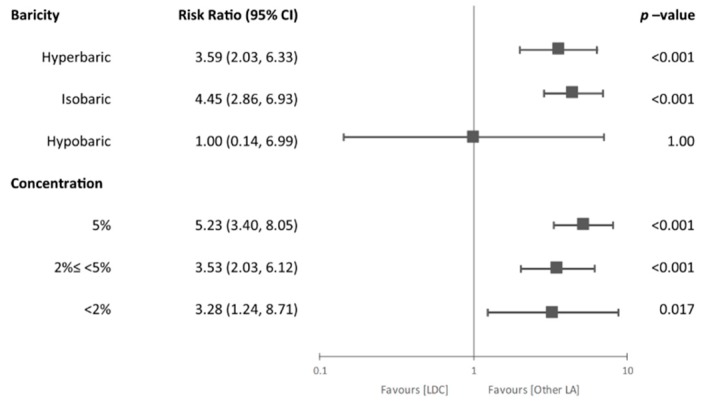
The relationship between the incidence of TNS and baricity or concentration is shown in Figure 5. Sixteen studies used hyperbaric lidocaine [5,6,11,13,15,16,18,28,32,33,34,35,36,39,42,45], 12 studies used isobaric [14,17,23,24,25,26,27,29,30,31,41,44], and 4 studies used hypobaric lidocaine for spinal anesthesia [4,7,26,33]. One study had two treatment groups that administered hyperbaric or isobaric lidocaine [43]. Since 6 studies failed to report the baricity of lidocaine [10,12,37,38,40,46], those RCTs were excluded from subgroup analysis. The risk of TNS was higher in the lidocaine group compared with the control group when hyperbaric (RR = 3.59, 95% CI = 2.03 to 6.33, p < 0.001) or isobaric lidocaine (RR = 4.45, 95% CI = 2.86 to 6.93, p < 0.001) was used. However, there were no differences between the two groups with respect to the risk of TNS when hypobaric lidocaine was administered (RR = 1.00, 95% CI = 0.14 to 6.99, p = 1.00). As shown in Table 2, the concentration used in each study varied from 0.3% to 5%. Two studies have multiple groups with different concentration [16,43]. Most studies used 2% or 5% lidocaine and subgroups were categorized into 3 groups: (1) 5%, (2) 2%≤ <5%, and (3) <2%. The incidence of TNS was significantly higher in the lidocaine group compared with the control group in all categories (5%: RR = 5.23, 95% CI = 3.40 to 8.05, p < 0.001; 2%≤ <5%: RR = 3.53, 95% CI = 2.03 to 6.12, p < 0.001; <2%: RR = 3.28, 95% CI = 1.24 to 8.71, p = 0.017).
Figure 5.
Forest plot for subgroup analysis. Additional subgroup analyses were conducted according to the baricity or concentration of the lidocaine. The risk of TNS was significantly higher in hyperbaric (p < 0.001), isobaric lidocaine group (p < 0.001) compared to the control group. In terms of concentration, lidocaine showed a higher incidence of TNS regardless of concentrations compared to the control group (p < 0.05). Abbreviations: LDC = lidocaine, LA = local anesthetics, CI = confidence interval.
4. Discussion
The present meta-analysis confirmed that lidocaine used for spinal anesthesia still causes TNS more frequently than most other local anesthetics (bupivacaine, levobupivacaine, prilocaine, chloroprocaine, procaine, and articaine) except for mepivacaine, ropivacaine or sameridine. This is the first study that analyzed the role of baricity and concentration of lidocaine as potential risk factors for TNS, and the subgroup analysis showed that hyperbaric and isobaric lidocaine showed higher TNS rates than the others, and higher rates of TNS have been observed in all concentration categories of lidocaine.
The incidence of TNS of lidocaine was about 4 times higher than that of other local anesthetics in this study, which supported the result of the previous meta-analysis by Zaric et al. [2] which included 16 RCTs with a total of 1467 patients. Since then, many RCTs have been still conducted to compare the incidence of TNS between lidocaine and other local anesthetics. A recent meta-analysis including 24 studies with 2226 patients compared the risk of TNS by using direct and indirect comparison [3]. However, in this meta-analysis, more RCTs, including 39 studies with 4733 patients (more than twice) were analyzed and lidocaine was compared with more various local anesthetics. However, the risk ratio and prevalence of developing TNS of the current study was slightly lower than those of the previous studies; Zaric et al. (risk ratio 4.62, prevalence 14.2%) [2], Forget et al. (prevalence 18%) [3]. These difference can be explained in part by that many RCTs of the present meta-analysis reported no case of TNS in the lidocaine group [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18], and a continuity correction of 0.5 was applied to these zero total events trials to prevent the overestimation of the risk of TNS [21]. Among other local anesthetics, chloroprocaine and mepivacaine have similar characteristics with lidocaine in terms of rapid onset time and short duration [47,48]. Subgroup analysis suggested that the incidence of TNS of chloroprocaine was lower than that of lidocaine. This finding significantly differs from the previous results [2,3]. However, the previous meta-analyses included only one or two RCTs comparing the effect of lidocaine and chloroprocaine. This study included four RCTs and no case of TNS in the chloroprocaine group was reported [17,24,26,46]. This would appear to indicate that chloroprocaine may be an attractive alternative to lidocaine for the short ambulatory surgery with fast onset and quick recovery time [48]. On the other hand, there was no difference in the incidence of TNS between lidocaine and mepivacaine, which was consistent with the result of the previous studies [2,3]. The idea that ropivacaine could decrease the development of TNS is still controversial. Although Zaric et al. [2] found that there was no difference in the risk of TNS between lidocaine and ropivacaine, Forget et al. [3] found that ropivacaine could decrease the risk of TNS than lidocaine. However, as expected, two studies included smaller number of studies and the latter study estimated pooled effect size by mostly indirect comparison. Surprisingly, the present study found more studies comparing the effect of lidocaine and ropivacaine, and estimated the pooled effect size by using a direct comparison.
Subgroup analysis suggested that no cases of TNS were found in the hypobaric lidocaine group whereas previous studies showed that the TNS of the lidocaine group occurred regardless of the baricity and concentration [49,50,51]. This result can be explained by low doses (10–20 mg) of hypobaric lidocaine group administered. Ben-David et al. [52] also reported that small doses of hypobaric lidocaine reduced the risk of TNS more than large doses of hypobaric lidocaine. However, this needs to be interpreted with caution since low doses of local anesthetics may be insufficient for adequate regional block [53]. Regarding the concentration of lidocaine, higher rates of TNS have been observed in all categories of concentration, which confirms the previous finding that altering the lidocaine concentration had no influence on the prevention of TNS [54].
This meta-analysis has a few limitations. First, various definitions of TNS were used in each study. Generally, TNS is defined as pain originating in the gluteal region and radiating to both lower extremities [2]. Some studies included considered TNS as only pain [4], while several other studies regarded TNS as pain and abnormal sensation (hypoesthesia or dysesthesia) [26,30]. Moreover, the anatomical regions (back, thigh, buttock or lower extremity), involving TNS, varied in each study. Furthermore, some studies did not specify details and/or a definition of TNS [35,47]. This variance with respect to TNS may have created bias, influencing the exact frequency of TNS. Second, specific types of surgery and position may be considered as risk factors of TNS, such as knee arthroscopy and lithotomy position. In the present study, various types of surgery and surgical position were included, and this may induce a bias in the results. Subgroup analysis according to the surgical position may provide a better overview. However, surgical position is heterogeneous and is not defined in some trials and the subgroup analysis according to the position, which may induce inaccurate results with bias. Third, only one study compared lidocaine to articaine with 134 patients, which may not be enough to conclude that the frequency of TNS with articaine is less than with lidocaine. Similarly, one RCT compared sameridine with lidocaine with 140 patients and there were no cases of TNS in the sameridine group.
5. Conclusions
In conclusion, the risk of developing TNS after spinal anesthesia with lidocaine was significantly higher than with bupivacaine, levobupivacaine, prilocaine, chloroprocaine, procaine or articaine. In addition, hyperbaric and isobaric lidocaine showed higher TNS rates than other lidocaines.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/2/493/s1, Figure S1: Funnel plot of comparison, lidocaine vs. other local anesthetics, Table S1: Search strategy for each database, Table S2: Details for judgement for each risk of bias, Table S3: Sensitivity analysis of comparison.
Author Contributions
Conceptualization, H.-J.S., S.-H.H. and J.-H.R.; methodology, C.-H.K. and H.-J.S..; software, C.-H.K.; validation, C.H.-K., H.-J.S., S.-H.H. and J.-H.R.; formal analysis, C.-H.K. and H.-J.S.; investigation, C.-H.K., H.-J.S., S.-H.H., and J.-H.R.; resources, C.-H.K. and J.-H.R.; data curation, C.-H.K. and H.-J.S..; writing—original draft preparation, C.-H.K. and H.-J.S.; writing—review and editing, S.-H.H. and J.-H.R..; visualization, C.-H.K.; supervision, J.-H.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Liam B.L., Yim C.F., Chong J.L. Dose response study of lidocaine 1% for spinal anaesthesia for lower limb and perineal surgery. Can. J. Anaesth. 1998;45:645–650. doi: 10.1007/BF03012094. [DOI] [PubMed] [Google Scholar]
- 2.Zaric D., Pace N.L. Transient neurologic symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics. Cochrane Database Syst. Rev. 2009;15:CD003006. doi: 10.1002/14651858.CD003006.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Forget P., Borovac J.A., Thackeray E.M., Pace N.L. Transient neurological symptoms (TNS) following spinal anaesthesia with lidocaine versus other local anaesthetics in adult surgical patients: A network meta-analysis. Cochrane Database Syst. Rev. 2019;12:CD003006. doi: 10.1002/14651858.CD003006.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali Hassan H.I. Comparison between two different selective spinal anesthesia techniques in ambulatory knee arthroscopy as fast-track anesthesia. Anesth. Essays Res. 2015;9:21–27. doi: 10.4103/0259-1162.150157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aouad M.T., Siddik S.S., Jalbout M.I., Baraka A.S. Does pregnancy protect against intrathecal lidocaine-induced transient neurologic symptoms? Anesth. Analg. 2001;92:401–404. doi: 10.1213/00000539-200102000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Buckenmaier C.C., 3rd, Nielsen K.C., Pietrobon R., Klein S.M., Martin A.H., Greengrass R.A., Steele S.M. Small-dose intrathecal lidocaine versus ropivacaine for anorectal surgery in an ambulatory setting. Anesth. Analg. 2002;95:1253–1257. doi: 10.1097/00000539-200211000-00028. [DOI] [PubMed] [Google Scholar]
- 7.de Santiago J., Santos-Yglesias J., Giron J., de Oca F.M., Jimenez A., Diaz P. Low-Dose 3 mg Levobupivacaine Plus 10 microg Fentanyl Selective Spinal Anesthesia for Gynecological Outpatient Laparoscopy. Anesth. Analg. 2009;109:1456–1461. doi: 10.1213/ANE.0b013e3181ba792e. [DOI] [PubMed] [Google Scholar]
- 8.de Santiago J., Santos-Yglesias J., Giron J., Jiménez A., Errando C.L. Low-dose hypobaric spinal anesthesia for anorectal surgery in jackknife position: Levobupivacaine-fentanyl compared to lidocaine-fentanyl. Rev. Espaola Anestesiol. Reanim. 2010;57:565–570. doi: 10.1016/S0034-9356(10)70283-8. [DOI] [PubMed] [Google Scholar]
- 9.Imbelloni L.E., Gouveia M.A., Cordeiro J.A. Hypobaric 0.15% bupivacaine versus hypobaric 0.6% lidocaine for posterior spinal anesthesia in outpatient anorectal surgery. Braz. J. Anestesiol. 2010;60:113–120. doi: 10.1016/S0034-7094(10)70015-5. [DOI] [PubMed] [Google Scholar]
- 10.Khant S.R., Chaudhari R., Kore R.A., Bhagwat S., Jakhalekar R.P. Low dose lignocaine + butorphanol vs. low dose bupivacaine for spinal anaesthesia in day care urological surgeries: A prospective randomized control trial. Turk. J. Urol. 2017;43:189–195. doi: 10.5152/tud.2017.14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyokong O., Charuluxananan S., Pothimamaka S., Leerapun R. Hypotension in spinal anesthesia for cesarean section: A comparison of 0.5% hyperbaric bupivacaine and 5% hyperbaric lidocaine. J. Med. Assoc. Thai. 2001;84(Suppl. 1):S256–S262. [PubMed] [Google Scholar]
- 12.Martin M.A., Olle G., Oferil F., Opisso L.l., Serra-Prat M., Hidalgo L. Recovery time and patient satisfaction in ambulatory knee arthroscopy: Prospective study comparing three anaesthetic methods. Ambul. Surg. 2005;12:75–79. doi: 10.1016/j.ambsur.2005.06.005. [DOI] [Google Scholar]
- 13.Mulroy M.F., Greengrass R., Ganapathy S., Chan V., Heierson A. Sameridine is safe and effective for spinal anesthesia: A comparative dose-ranging study with lidocaine for inguinal hernia repair. Anesth. Analg. 1999;88:815–821. doi: 10.1213/00000539-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Pawlowski J., Orr K., Kim K.M., Pappas A.L., Sukhani R., Jellish W.S. Anesthetic and recovery profiles of lidocaine versus mepivacaine for spinal anesthesia in patients undergoing outpatient orthopedic arthroscopic procedures. J. Clin. Anesth. 2012;24:109–115. doi: 10.1016/j.jclinane.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Pradhan B. Spinal anesthesia for cesarean section: Comparison of 5.0% lignocaine and 0.5% bupivacaine. Nepal Med. Coll. J. 2010;12:30–33. [PubMed] [Google Scholar]
- 16.Punj J., Khan R.M. Spinal anaesthesia for pelvic surgery: Low concentrations of lignocaine and bupivacaine are effective with less adverse events. Middle E. J. Anaesthesiol. 2013;22:71–77. [PubMed] [Google Scholar]
- 17.Teunkens A., Vermeulen K., Van Gerven E., Fieuws S., Van de Velde M., Rex S. Comparison of 2-Chloroprocaine, Bupivacaine, and Lidocaine for Spinal Anesthesia in Patients Undergoing Knee Arthroscopy in an Outpatient Setting: A Double-Blind Randomized Controlled Trial. Reg. Anesth. Pain Med. 2016;41:576–583. doi: 10.1097/AAP.0000000000000420. [DOI] [PubMed] [Google Scholar]
- 18.Yea K.H., Lee S.C., Kim J.S., Chung C.J. Spinal Anesthesia with Hyperbaric 1.5% Lidocaine and 1.5% Mepivacaine. Korean J. Anesthesiol. 1998;35:1095–1099. doi: 10.4097/kjae.1998.35.6.1095. [DOI] [Google Scholar]
- 19.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrich J.O., Adhikari N.K., Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med. Res. Methodol. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Intervetions Version 5.1.0. The Cochrane Collaboration; Chichester, UK: 2011. [Google Scholar]
- 23.Beilin Y., Zahn J., Abramovitz S., Bernstein H.H., Hossain S., Bodian C. Subarachnoid small-dose bupivacaine versus lidocaine for cervical cerclage. Anesth. Analg. 2003;97:56–61. doi: 10.1213/01.ANE.0000068940.36040.54. [DOI] [PubMed] [Google Scholar]
- 24.Breebaart M.B., Teune A., Sermeus L.A., Vercauteren M.P. Intrathecal chloroprocaine vs. lidocaine in day-case surgery: Recovery, discharge and effect of pre-hydration on micturition. Acta Anaesthesiol. Scand. 2014;58:206–213. doi: 10.1111/aas.12247. [DOI] [PubMed] [Google Scholar]
- 25.Breebaart M.B., Vercauteren M.P., Hoffmann V.L., Adriaensen H.A. Urinary bladder scanning after day-case arthroscopy under spinal anaesthesia: Comparison between lidocaine, ropivacaine, and levobupivacaine. Br. J. Anaesth. 2003;90:309–313. doi: 10.1093/bja/aeg078. [DOI] [PubMed] [Google Scholar]
- 26.Casati A., Fanelli G., Danelli G., Berti M., Ghisi D., Brivio M., Putzu M., Barbagallo A. Spinal anesthesia with lidocaine or preservative-free 2-chlorprocaine for outpatient knee arthroscopy: A prospective, randomized, double-blind comparison. Anesth. Analg. 2007;104:959–964. doi: 10.1213/01.ane.0000258766.73612.d8. [DOI] [PubMed] [Google Scholar]
- 27.de Weert K., Traksel M., Gielen M., Slappendel R., Weber E., Dirksen R. The incidence of transient neurological symptoms after spinal anaesthesia with lidocaine compared to prilocaine. Anaesthesia. 2000;55:1020–1024. doi: 10.1046/j.1365-2044.2000.01618-4.x. [DOI] [PubMed] [Google Scholar]
- 28.Etezadi F., Karimi Yarandi K., Ahangary A., Shokri H., Imani F., Safari S., Khajavi M.R. The effect of needle type, duration of surgery and position of the patient on the risk of transient neurologic symptoms. Anesth. Pain Med. 2013;2:154–158. doi: 10.5812/aapm.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fanelli G., Danelli G., Zasa M., Baciarello M., Di Cianni S., Leone S. Intrathecal ropivacaine 5 mg/ml for outpatient knee arthroscopy: A comparison with lidocaine 10 mg/ml. Acta Anaesthesiol. Scand. 2009;53:109–115. doi: 10.1111/j.1399-6576.2008.01815.x. [DOI] [PubMed] [Google Scholar]
- 30.Gozdemir M., Muslu B., Sert H., Usta B., Demircioglu R.I., Karatas O.F., Surgit O. Transient neurological symptoms after spinal anaesthesia with levobupivacaine 5 mg/ml or lidocaine 20 mg/ml. Acta Anaesthesiol. Scand. 2010;54:59–64. doi: 10.1111/j.1399-6576.2009.02141.x. [DOI] [PubMed] [Google Scholar]
- 31.Gozdemir M., Muslu B., Sert H., Usta B., Demircioglu R.I., Kasikara H. Transient neurological symptoms after spinal anesthesia. Clin. Invest. Med. 2016;39:27512. doi: 10.25011/cim.v39i6.27512. [DOI] [PubMed] [Google Scholar]
- 32.Hampl K.F., Heinzmann-Wiedmer S., Luginbuehl I., Harms C., Seeberger M., Schneider M.C., Drasner K. Transient neurologic symptoms after spinal anesthesia: A lower incidence with prilocaine and bupivacaine than with lidocaine. Anesthesiology. 1998;88:629–633. doi: 10.1097/00000542-199803000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Hampl K.F., Schneider M.C., Thorin D., Ummenhofer W., Drewe J. Hyperosmolarity does not contribute to transient radicular irritation after spinal anesthesia with hyperbaric 5% lidocaine. Reg. Anesth. 1995;20:363–368. [PubMed] [Google Scholar]
- 34.Hodgson P.S., Liu S.S., Batra M.S., Gras T.W., Pollock J.E., Neal J.M. Procaine compared with lidocaine for incidence of transient neurologic symptoms. Reg. Anesth. Pain Med. 2000;25:218–222. doi: 10.1016/s1098-7339(00)90001-4. [DOI] [PubMed] [Google Scholar]
- 35.Keld D.B., Hein L., Dalgaard M., Krogh L., Rodt S.A. The incidence of transient neurologic symptoms (TNS) after spinal anaesthesia in patients undergoing surgery in the supine position. Hyperbaric lidocaine 5% versus hyperbaric bupivacaine 0.5% Acta Anaesthesiol. Scand. 2000;44:285–290. doi: 10.1034/j.1399-6576.2000.440311.x. [DOI] [PubMed] [Google Scholar]
- 36.Le Truong H.H., Girard M., Drolet P., Grenier Y., Boucher C., Bergeron L. Spinal anesthesia: A comparison of procaine and lidocaine. Can. J. Anaesth. 2001;48:470–473. doi: 10.1007/BF03028311. [DOI] [PubMed] [Google Scholar]
- 37.Liguori G.A., Zayas V.M., Chisholm M.F. Transient neurologic symptoms after spinal anesthesia with mepivacaine and lidocaine. Anesthesiology. 1998;88:619–623. doi: 10.1097/00000542-199803000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Maliachi Revuelta R., Calzada Grijalva J.F., Gonzalez A., Dosta Herrera J.J., Flores Lopez D. Syndrome of transient radicular irritation secondary to spinal blockade in pelvis traumatologic surgery. Anest. Mex. 1999;11:109–114. [Google Scholar]
- 39.Martínez-Bourio R., Arzuaga M., Quintana J.M., Aguilera L., Aguirre J., Saez-Eguilaz J.L., Arizaga A. Incidence of transient neurologic symptoms after hyperbaric subarachnoid anesthesia with 5% lidocaine and 5% prilocaine. Anesthesiology. 1998;88:624–628. doi: 10.1097/00000542-199803000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Orozco E.G., Arguelles R.A.F., Ortega A.D., Bastanzuri M.C.L. Cost-effectiveness evaluation of 5% lidocaine and 0,5% bupivacaine in spinal anesthesia. Rev. Cubana Farm. 2006;40 [Google Scholar]
- 41.Østgaard G., Hallaråker O., Ulveseth O.K., Flaatten H. A randomised study of lidocaine and prilocaine for spinal anaesthesia. Acta Anaesthesiol. Scand. 2000;44:436–440. doi: 10.1034/j.1399-6576.2000.440413.x. [DOI] [PubMed] [Google Scholar]
- 42.Philip J., Sharma S.K., Gottumukkala V.N., Perez B.J., Slaymaker E.A., Wiley J. Transient neurologic symptoms after spinal anesthesia with lidocaine in obstetric patients. Anesth. Analg. 2001;92:405–409. doi: 10.1213/00000539-200102000-00024. [DOI] [PubMed] [Google Scholar]
- 43.Pollock J.E., Neal J.M., Stephenson C.A., Wiley C.E. Prospective study of the incidence of transient radicular irritation in patients undergoing spinal anesthesia. Anesthesiology. 1996;84:1361–1367. doi: 10.1097/00000542-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Salazar F., Bogdanovich A., Adalia R., Chabás E., Gomar C. Transient neurologic symptoms after spinal anaesthesia using isobaric 2% mepivacaine and isobaric 2% lidocaine. Acta Anaesthesiol. Scand. 2001;45:240–245. doi: 10.1034/j.1399-6576.2001.450216.x. [DOI] [PubMed] [Google Scholar]
- 45.Salmela L., Aromaa U. Transient radicular irritation after spinal anesthesia induced with hyperbaric solutions of cerebrospinal fluid-diluted lidocaine 50 mg/ml or mepivacaine 40 mg/ml or bupivacaine 5 mg/ml. Acta Anaesthesiol. Scand. 1998;42:765–769. doi: 10.1111/j.1399-6576.1998.tb05319.x. [DOI] [PubMed] [Google Scholar]
- 46.Vaghadia H., Neilson G., Lennox P.H. Selective spinal anesthesia for outpatient transurethral prostatectomy (TURP): Randomized controlled comparison of chloroprocaine with lidocaine. Acta Anaesthesiol. Scand. 2012;56:217–223. doi: 10.1111/j.1399-6576.2011.02599.x. [DOI] [PubMed] [Google Scholar]
- 47.Liu S.S., McDonald S.B. Current issues in spinal anesthesia. Anesthesiology. 2001;94:888–906. doi: 10.1097/00000542-200105000-00030. [DOI] [PubMed] [Google Scholar]
- 48.Goldblum E., Atchabahian A. The use of 2-chloroprocaine for spinal anaesthesia. Acta Anaesthesiol. Scand. 2013;57:545–552. doi: 10.1111/aas.12071. [DOI] [PubMed] [Google Scholar]
- 49.Hampl K.F., Schneider M.C., Pargger H., Gut J., Drewe J., Drasner K. A similar incidence of transient neurologic symptoms after spinal anesthesia with 2% and 5% lidocaine. Anesth. Analg. 1996;83:1051–1054. doi: 10.1213/00000539-199611000-00026. [DOI] [PubMed] [Google Scholar]
- 50.Pollock J.E., Liu S.S., Neal J.M., Stephenson C.A. Dilution of spinal lidocaine does not alter the incidence of transient neurologic symptoms. Anesthesiology. 1999;90:445–450. doi: 10.1097/00000542-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Tong D., Wong J., Chung F., Friedlander M., Bremang J., Mezei G., Streiner D. Prospective study on incidence and functional impact of transient neurologic symptoms associated with 1% versus 5% hyperbaric lidocaine in short urologic procedures. Anesthesiology. 2003;98:485–494. doi: 10.1097/00000542-200302000-00030. [DOI] [PubMed] [Google Scholar]
- 52.Ben-David B., Maryanovsky M., Gurevitch A., Lucyk C., Solosko D., Frankel R., Volpin G., DeMeo P.J. A comparison of minidose lidocaine-fentanyl and conventional-dose lidocaine spinal anesthesia. Anesth. Analg. 2000;91:865–870. doi: 10.1097/00000539-200010000-00018. [DOI] [PubMed] [Google Scholar]
- 53.Nair G.S., Abrishami A., Lermitte J., Chung F. Systematic review of spinal anaesthesia using bupivacaine for ambulatory knee arthroscopy. Br. J. Anaesth. 2009;102:307–315. doi: 10.1093/bja/aen389. [DOI] [PubMed] [Google Scholar]
- 54.Freedman J.M., Li D.K., Drasner K., Jaskela M.C., Larsen B., Wi S. Transient neurologic symptoms symptoms after spinal anesthesia: An epidemiologic study of 1,863 patients. Anesthesiology. 1998;89:633–641. doi: 10.1097/00000542-199809000-00012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.