Abstract
Background:
We constructed a high-volume registry to identify whether risk factors of intracranial atherosclerotic plaque (ICAP) features differ in the posterior and anterior circulation in patients with symptomatic intracranial atherosclerotic stenosis (ICAS) investigated by high-resolution magnetic resonance imaging (HRMRI).
Methods:
The registry was constructed for patients with symptomatic ICAS who underwent HRMRI for culprit plaques. ICAP-vulnerable features included positive remodelling, diffuse distribution, intraplaque haemorrhage and strong enhancement.
Results:
We analysed risk factors for the same ICAP features between the posterior and anterior circulation in data of 97 patients in the posterior circulation and 105 patients in the anterior circulation ICAPs. In patients with diffuse distribution, the probability of being female were lower [odds ratio (OR):0.08; 95% confidence interval (CI):0.02–0.34; p = 0.001] and having diabetes mellitus was higher (OR: 7.75; 95% CI:1.75–34.39; p = 0.007) in posterior circulation patients. In patients with strong enhancement, the probability of having diabetes was higher in posterior circulation patients (OR:6.71; 95% CI:1.37–32.81; p = 0.019).
Conclusions:
Our results demonstrate more risk factors in the posterior than in the anterior circulation in patients with the same ICAP-vulnerable features, highlighting the need for stratification of risk factors in symptomatic ICAPs.
Trial Registration:
URL: http://www.clinicaltrials.gov. Unique identifier: NCT02705599.
Keywords: risk factors, intracranial atherosclerosis plaque, high resolution MRI, location
Introduction
Intracranial atherosclerotic stenosis (ICAS) is a major cause of ischemic stroke, and is highly prevalent in East Asia.1,2 Epidemiological reports have identified gender, age, hypertension, diabetes mellitus, smoking and dyslipidaemia as risk factors for intracranial atherosclerotic disease.3–5 Differences in risk factors (including age, gender, hypertension and diabetes mellitus) have been identified between the posterior and anterior circulation in ICAS.6,7
The Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis (SAMMPRIS)8 and Warfarin–Aspirin for Symptomatic Intracranial Disease (WASID)9 trials reported that modifying risk factors reduced stroke recurrence in patients with ICAS. However, despite aggressive medical treatment, around 15% of severe ICAS patients still experience recurrent stroke.10 In addition, differences in recurrent stroke have been identified between patients with posterior and anterior circulation ICAS.11
However, it remains unclear whether the incidence of risk factors and intracranial atherosclerotic plaque (ICAP)-vulnerable features in the posterior circulation are comparable to those in the anterior circulation. Identifying ICAP-vulnerable features, and understanding differences in risk factors and plaque features between the posterior and anterior circulation, may, therefore, have utility for risk stratification for recurrent stroke.
High-resolution magnetic resonance imaging (HRMRI) can subtract the signal from blood flow in the vessel lumen and allow direct visualization of ICAP in vivo.12,13 In previous studies, ICAP features based on HRMRI including positive remodelling,14 diffuse distribution,15 intraplaque haemorrhage and strong enhancement have been identified as markers for future stroke risk.16,17 We therefore designed a prospective and observational registry focusing on patients with symptomatic ICAS who underwent HRMRI to evaluate differences in risk factors of ICAP-vulnerable features between the posterior and anterior circulation.
Methods
This was a observational study conducted at two high-volume stroke centres. All study protocols were approved by the ethics committees of Beijing Tiantan hospital and Chinese PLA General Hospital. Written informed consent was obtained from the patients or their legal guardians, and all research was performed in accordance with the relevant guidelines and regulations.
Enrolment of patients
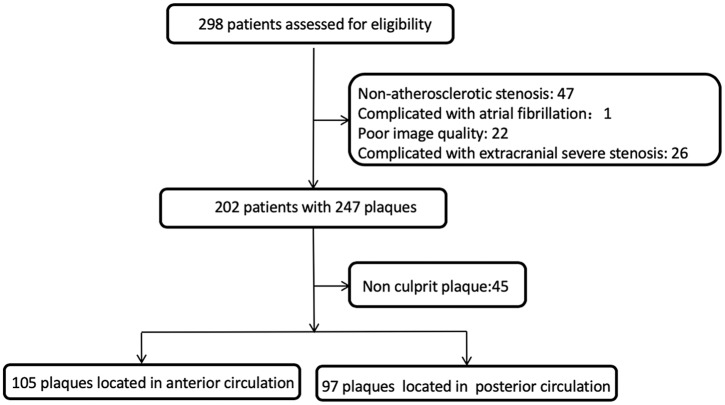
Patients admitted with suspected symptomatic intracranial arterial disease between September 2014 and January 2017 were enrolled. Patients received one or more types of examination to determine the cause of ischemic events, including transient ischemic attack or ischemic stroke, using carotid duplex ultrasound, transcranial Doppler, echocardiography, electrocardiography, computer tomography (CT), CT angiography (CTA), magnetic resonance imaging (MRI), magnetic resonance angiography (MRA) and (or) digital subtraction angiography (DSA). If these examinations indicated that ischemic events were considered to be due to ICAS, patients were referred to the MRI centre for scanning of the culprit lesions by HRMRI. Patients were enrolled in this study according to the following criteria: (1) ischemic events in the target regions of intracranial anterior or posterior circulation within 90 days of presentation, (2) lack of coexistent ipsilateral extracranial carotid artery or vertebral artery stenosis of ⩾50%, (3) no potential sources of cardioaortic embolism based on the modified Trial of ORG 10 172 in Acute Stroke Treatment (TOAST) classification,18 (4) age ⩾18 years and (5) one or more atherosclerotic risk factors. The study flow chart is shown in Figure 1.
Figure 1.
Flow chart of study.
Patients with the following conditions were excluded: (1) nonatherosclerotic vasculopathy, such as vasculitis and arterial dissection, diagnosis through comprehensive laboratory work (such as erythrocyte sedimentation rate or C-reactive protein elevation, antinuclear antibody or antiphospholipid antibody positivity), vascular imaging or clinical evaluation; or (2) contraindication to MR examination or medical instability precluding MR examination.
Definitions of risk factors
Hypertension was defined as systolic blood pressure of ⩾140 mmHg, diastolic blood pressure ⩾90 mmHg or current antihypertensive drug use. Hypercholesterolemia was defined as a total cholesterol level ⩾6.22 mmol/l, low-density lipoprotein cholesterol level ⩾4.14 mmol/l or current use of cholesterol-lowering medication. Patients who used antidiabetic medications (insulin or oral hypoglycaemics) were considered to have diabetes mellitus. Patients who smoked in the past or currently were considered to have a history of smoking cigarettes. Obesity was defined as a body mass index greater than 30 kg/m2.
HRMRI acquisition
All HRMRI studies were performed using a 3T GE DISCOVERY MR 750 (GE Healthcare, Waukesha, WI, USA) or a 3T Siemens Trio MR scanner (Siemens Healthcare, Ehrlangen, Germany). The multiple pulse sequences included three-dimensional time of flight MR angiography (3D TOF MRA), 3D T1-weighted imaging, proton attenuation weighted imaging, magnetization-prepared rapid acquisition with gradient-echo sequence (MPRAGE), and contrast enhanced T1-weighted imaging. Details of the sequence parameters are presented in the supplemental eTable 1 online. Images were reconstructed using the Reformate tool in the AW 4.5 workstation (GE Healthcare) or the D multiple planer reconstruction tool in the Siemens workstation.
Imaging analysis and measurements
All HRMR images were analysed by two neuroradiologists (L.X. and L.J.H.). All readers were blinded to the patients’ clinical data. An image-quality rating (1 = poor, 2 = adequate and 3 = good) was given to each image by the two neuroradiologists. Patients with poor-quality images due to severe motion artefacts or low signal-to-noise ratio were excluded. In the initial group of 10 patients evaluated using both scanners, these images were also reviewed by two neuroradiologists (S.B.B. and S.M.) for the inter-observer and intra-observer variability of the two scanners. The present study did not display intra- and inter-observer variabilities with the same or different scanners in light of previous research showing that these variabilities are small.19
Remodelling index was calculated as the ratio of the vessel area at the maximal lumen narrowing site to that at the reference site. The reference site was selected based on the WASID method.20 A remodelling index ⩾1.05 was defined as positive remodelling, 0.95–1.05 as intermediate remodelling, ⩽0.95 as negative remodelling (eFigure S1 in the online-only Data Supplement).21
Distribution patterns were identified at the narrowest slices. Plaque distribution was recorded as one of four quadrants of the vessel wall on cross-sectional images (eFigure 2 in the online-only Data Supplement). Plaques that were distributed across at least three quadrants of the lumen perimeter were defined as diffuse, and those across at least two were defined as nondiffuse. Intraplaque haemorrhage was defined as a signal intensity of >150% of that of the adjacent grey matter on all pulse sequences (eFigure 3 in the online-only Data Supplement).22
Contrast enhancement was performed on black blood T1WI (three-dimensional T1 CUBE and T1 SPACE) 5 min after Gadolinium administration (0.1 mmol/kg gadopentetate dimeglumine, Magnevist; Bayer Schering Pharma, Berlin, Germany) using the same parameters as with the precontrast black blood T1WI.23 The enhancement was classified into one of three grades: nonenhancement, moderate enhancement or strong enhancement. Moderate enhancement was defined as less than that of the pituitary infundibulum. Strong enhancement was equal to or stronger than that of the pituitary infundibulum (eFigure 4 in the online-only Data Supplement).19
In this study, positive remodelling, diffuse distribution, intraplaque haemorrhage and strong enhancement were recognized as ICAP-vulnerable features as previous studies have showed their close relationships with future ischemic events.14–17
Image reconstruction was performed at the workstation. MR images were subsequently processed using freely available software ImageJ (Rasband, National Institute of Mental Health, Bethesda, USA).24
ICAP location
The culprit plaques located in the intracranial internal carotid artery, middle cerebral artery, intracranial vertebral artery or basilar artery were recorded. Culprit plaques in the intracranial internal carotid and middle cerebral arteries were categorized as anterior circulation ICAPs, while those in the intracranial vertebral and basilar arteries were categorized as posterior circulation ICAPs. Culprit plaques were determined by two experienced neurologists through clinical symptoms and vascular imaging including DWI and MRA, CTA or DSA.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD) and categorical variables as percentages. The differences in risk factors of patients with the same ICAP features between posterior and anterior circulation were analysed. Chi-square tests were used to analyse the differences in risk factors and ICAP-vulnerable features between posterior and anterior circulation. Chi-square tests and multiple variable logistic regression analysis were used to evaluate differences in risk factors of patients with same ICAP-vulnerable features between posterior and anterior circulation. All statistical tests were two-sided, and p < 0.05 was considered statistically significant.
Results
General subject characteristics
A study flow chart is presented in Figure 1. In total, 202 patients were enrolled, 247 plaques were found and 202 culprit plaques were available for analysis (Table 1). We analysed data from 97 patients with posterior circulation plaques and 105 patients with anterior circulation plaques. The mean age of the patients was 52.96 ± 11.71 years (range: 26–82) and the median age was 54 years. Of the subjects, 158 (78.2%) were male, 23 (11.5%) were obese, 135 (66.8%) had hypertension, 65 (32.2%) had diabetes mellitus, 96 (47.5%) had hyperlipidaemia and 124 (61.4%) were cigarette smokers. The mean low-density lipoprotein cholesterol (LDL-C) level was 2.10 ± 0.78 mmol/l and the mean body mass index was 26.14 ± 3.21 kg/m2.
Table 1.
General subject characteristics.
| Variable | Patients (n = 202) |
|---|---|
| Risk factors | |
| Age, yearsa | 53.77 (45–62) |
| Male | 158 (78.2) |
| Obesity | 23 (11.5) |
| Hypertension | 135 (66.8) |
| Diabetes mellitus | 65 (32.2) |
| Hyperlipidemia | 96 (47.5) |
| Smoking | 124 (61.4) |
| LDL-C, mmola | 1.96 (1.51–2.65) |
| Body mass index, kg/m2 a | 25.92 (23.75–27.90) |
| Clinical events | |
| TIA | 38 (18.8) |
| Cerebral infarction | 164 (81.2) |
| Plaque location | |
| Anterior circulation | 105 (52.0) |
| Posterior circulation | 97 (48.0) |
Data are presented as median (interquartile range).
LDL-C, low-density lipoprotein cholesterol; TIA, transient ischaemic attack.
Differences in risk factors between posterior and anterior circulation
In patients with posterior circulation ICAP, there were higher incidences of age ⩾54 years (61.9% versus 34.3%, p = 0.001), hypertension (79.4% versus 55.2%, p = 0.001) and diabetes mellitus (42.3% versus 22.9%, p = 0.003) in patients with posterior circulation ICAP compared with patients with anterior circulation ICAP except females (28.57% versus 14.43%, p = 0.002) (Table 2).
Table 2.
The association risk factors with location of intracranial atherosclerosis plaques, using multiple logistic regression model.
| Variable | Anterior (n = 105) | Posterior (n = 97) | OR (95%CI) | p |
|---|---|---|---|---|
| Female | 30 (28.57) | 14 (14.43) | 0.21 (0.08–0.56) | 0.002 |
| Age, ⩾54 years | 36 (34.29) | 60 (61.86) | 3.66 (1.87–7.14) | 0.001 |
| Obese | 10 (9.52)a | 13 (13.40) | 1.89 (0.70–5.12) | 0.209 |
| Hypertension | 58(55.24) | 77 (79.38) | 2.26 (1.08–4.71) | 0.029 |
| Diabetes | 24 (22.86) | 41 (42.27) | 2.77 (1.34–5.72) | 0.006 |
| Hyperlipidemia | 53 (50.48) | 43 (44.33) | 0.57 (0.30–1.10) | 0.093 |
| Smoking | 63 (60.00) | 61 (62.89) | 0.92 (0.43–1.97) | 0.832 |
Data are presented from 103 patients.
OR, odds ratio.
Differences in ICAP-vulnerable features between posterior and anterior circulation
There were no differences in ICAP-vulnerable features between posterior and anterior circulation, including positive remodelling (31.9% versus 39.2%, p = 0.511), intraplaque haemorrhage (19.6% versus 18.1%, p = 0.786) and strong enhancement (37.9% versus 54.0%, p = 0.094) between posterior and anterior circulation, respectively. However, diffuse distribution was significantly different between posterior and anterior circulation (73.6% versus 31.6%, respectively, p = 0.001).
Differences in risk factors in patients with the same ICAP-vulnerable features between posterior and anterior circulation
Multivariate logistic regression analysis showed that, in patients with diffuse distribution, there were higher odds of diabetes [odds ratio (OR): 7.75; 95% confidence interval (CI): 1.75–34.39; p = 0.07] in the posterior than the anterior circulation, and higher odds of being female (OR: 0.08; 95% CI: 0.02–0.34; p = 0.001) in the anterior than in the posterior circulation. In patients with strong enhancement, the odds of diabetes (OR: 6.71; 95% CI: 1.37–38.81; p = 0.019) were higher in the posterior than in the anterior circulation (Table 3).
Table 3.
The association of related variables with position of intracranial atherosclerosis plaques, using multiple logistic regression model by each characteristics of plaques.
| Variable | Diffuse distribution (n = 98) |
|
|---|---|---|
| OR (95%CI) | p | |
| Female | 0.08 (0.02–0.34) | 0.001 |
| Age, ⩾54 years | 2.47 (0.85–7.21) | 0.098 |
| Obese | 2.67 (0.49–14.65) | 0.259 |
| Hypertension | 1.34 (0.41–4.46) | 0.628 |
| Diabetes | 7.75 (1.75–34.39) | 0.007 |
| Intraplaque haemorrhage (n = 38) |
||
| OR (95%CI) | p | |
| Female | 0.48 (0.08–3.00) | 0.436 |
| Age, ⩾54 years | 0.97 (0.17–5.46) | 0.969 |
| Obese | 0.39 (0.02–8.49) | 0.550 |
| Hypertension | 8.03 (0.71–90.98) | 0.093 |
| Diabetes | 3.16 (0.6–16.57) | 0.174 |
| Positive remodelling (n = 67) |
||
| OR (95%CI) | p | |
| Female | 0.3 0.07–1.27) | 0.103 |
| Age, ⩾54 years | 2.04 (0.63–6.65) | 0.236 |
| Obese | 1.64 (0.17–15.77) | 0.667 |
| Hypertension | 2.59 (0.71–9.4) | 0.149 |
| Diabetes | 1.62 (0.5 -5.23) | 0.421 |
| Strong enhancement (n = 49) |
||
| OR (95%CI) | p | |
| Female | 0.25 (0.05–1.34) | 0.106 |
| Age, ⩾54 years | 2.85 (0.66–12.42) | 0.162 |
| Obese | 4.86 (0.71–33.37) | 0.107 |
| Hypertension | 1.67 (0.33–8.5) | 0.538 |
| Diabetes | 6.71 (1.37–32.81) | 0.019 |
OR, odds ratio.
Discussion
In this study, we found that risk factors were different between the posterior circulation and the anterior circulation in patients with the same ICAP-vulnerable features.
In patients with diffuse distribution, we found that there were higher odds of having diabetes mellitus and lower odds of being female in the posterior than in the anterior circulation. Use of HRMRI enables clear visualization of plaque distribution.25,26 Changes in plaque distribution from focal to diffuse indicate increasing plaque progression. Our data indicated that female patients may be more susceptible to diffuse plaque distribution in the anterior circulation, and that this may be related to metabolic factors secondary to diabetes mellitus.
The patients with strong enhancement of plaques had higher odds of diabetes in the posterior circulation than in the anterior circulation. Plaque enhancement on HRMRI is a marker of plaque vulnerability and progression is strongly associated with stroke.27 Plaque enhancement is also associated with endothelial dysfunction and neovascularization of the artery wall.17 Several studies have found that arterial wall enhancement is closely related to age and the degree of intracranial artery stenosis.28,29 Our data indicated that strong enhancement in the posterior circulation may be more related to metabolic factors caused by dyslipidaemia.
In patients with positive remodelling and intraplaque haemorrhage, risk factors did not differ between the posterior and the anterior circulation. Artery remodelling is a compensatory response to stenosis that may involve haemodynamic changes and inflammatory mechanisms.28 A previous study showed that there is a strong correlation between positive remodelling and ischemic events.14 Positive remodelling in the coronary artery is associated with several risk factors including hypertension, diabetes and dyslipidaemia.30 The lack of difference in risk factors in patients with positive remodelling between the posterior and the anterior circulation may be due to the small sample size and should be studied further in the future.
Although there were no risk factor differences between the posterior and anterior circulation in patients with intraplaque haemorrhage, there were higher odds of hypertension in the posterior than in the anterior circulation (p = 0.093). The small sample may be the major reason that the tendency did not reach significance. Intraplaque haemorrhage is a well-recognized marker of plaque destabilization and is also associated strongly with plaque progression, thin or ruptured fibrous caps, active inflammation within plaques and ischemic events.31 Intraplaque haemorrhage may arise because the plaque cannot obtain sufficient nutrients from the vasa vasorum during progression, causing extravasation of red blood cells.32 A study focusing on basilar artery plaques found that intraplaque haemorrhage was closely related to the degree of stenosis.16
This study showed that there were higher incidences in older, hypertensive and diabetes mellitus patients with posterior circulation ICAPs compared with patients with anterior circulation ICAPs. These findings were similar to those reported in previous studies showing that age and diabetes mellitus were significantly related to basilar artery plaques, while being male and hypertensive were associated with intracranial vertebral plaques.29 A previous study found that hypertension and diabetes mellitus were related to posterior circulation disease.7 The results of subgroup analysis from the WASID trial showed that basilar stenosis was associated with age and hyperlipidaemia, that intracranial vertebral artery stenosis was associated with coronary artery disease and that intracranial carotid artery stenosis was associated with diabetes mellitus and middle cerebral artery stenosis in females.6
In addition, our study showed no significant differences in ICAP features, including positive remodelling, intraplaque haemorrhage and strong enhancement between the posterior and anterior circulation; however, diffuse distribution showed a significant difference between the two groups. Larger ICAPs with diffuse distribution appeared to be a specific feature of posterior circulation lesions over those in the anterior circulation (74.3% in posterior, 31.8% in anterior circulation), which is consistent with the results of a previous study.33
There are several limitations of the current study. First, all enrolled patients are Chinese, and therefore the results may not be applicable to other ethnicities. Second, the sample size was relatively small. Third, ICAP features based on HRMRI may not reflect the true intracranial plaque features in vivo because of its limitations in terms of resolution. In patients with the same ICAP-vulnerable features, there are more risk factors in the posterior circulation than in the anterior circulation, suggesting that stratification may be required for risk factor management in patients with symptomatic intracranial ICAP. These findings should be confirmed or refuted with future studies.
Acknowledgments
Authors Ziqi Xu, Mingyao Li, Benyan Luo and Ning Ma contributed equally to the manuscript. We thank Jingyi Liu from the Neurology Department of Beijing Tiantan Hospital for polishing the article.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (Contract grant numbers: 81671126 and 81730048 to X.L., 81471390 to N.M., 81371290 to Z.R.M., and 81361120402 to B.B.S and M.S.), Beijing High-level Personnel Funds (Contract grant number: 2013-2-19 to Z.R.M.), and National Key R&D Program of China (Contract grant number: 2016YFC0100100 to X.L.),Medical and Health Science and Technology of Zhejiang Province (Contract grant number: 2015KYA080 to Z.Q.X).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Ziqi Xu  https://orcid.org/0000-0002-5516-4817
https://orcid.org/0000-0002-5516-4817
Benyan Luo  https://orcid.org/0000-0002-9892-5778
https://orcid.org/0000-0002-9892-5778
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ziqi Xu, Department of Neurology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Mingyao Li, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Jinhao Lyu, Department of Radiology, Chinese PLA General Hospital, Beijing, China.
Zhikai Hou, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Jianfeng He, Department of Radiology, Chinese PLA General Hospital, Beijing, China.
Dapeng Mo, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Feng Gao, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Xin Liu, Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China.
Binbin Sui, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Mi Shen, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Yuesong Pan, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Yongjun Wang, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Xin Lou, Department of Radiology, Chinese PLA General Hospital, Beijing, China.
Zhongrong Miao, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, Center of Stroke, Beijing Institute for Brain Disorders, Beijing, China.
Benyan Luo, Department of Neurology, The First Affiliated Hospital, College of Medicine, Zhejiang University, No.79 Qingchun Road, Hangzhou, 310003, China.
Ning Ma, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, China National Clinical Research Center for Neurological Diseases, No.119 Nansihuanxilu, Fengtai District, Beijing 100070, China.
References
- 1. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013; 12: 1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Y, Zhao X, Liu L, et al. ; CICAS Study Group. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke 2014; 45: 663–669. [DOI] [PubMed] [Google Scholar]
- 3. Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet 2014; 383: 984–998. [DOI] [PubMed] [Google Scholar]
- 4. Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation 2014; 130: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 5. Kim JS, Caplan LR, Wong KS. Intracranial atherosclerosis: pathophysiology, diagnosis and treatment. Front Neurol Neurosci 2016; 40: 47–57.27960158 [Google Scholar]
- 6. Turan TN, Makki AA, Tsappidi S, et al. ; WASID Investigators. Risk factors associated with severity and location of intracranial arterial stenosis. Stroke 2010; 4: 1636–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim JS, Nah HW, Park SM, et al. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke 2012; 43: 3313–3318. [DOI] [PubMed] [Google Scholar]
- 8. Chimowitz MI, Lynn MJ, Derdeyn CP, et al. ; SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011; 365: 993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. ; Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005; 352: 1305–1316. [DOI] [PubMed] [Google Scholar]
- 10. Derdeyn CP, Chimowitz MI, Lynn MJ, et al. ; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis Trial Investigators. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet 2014; 383: 333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waters MF, Hoh BL, Lynn MJ, et al. ; Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial Investigators. Factors Associated With Recurrent Ischemic Stroke in the Medical Group of the SAMMPRIS Trial. JAMA Neurol 2016; 73: 308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turan TN, Rumboldt Z, Granholm AC, et al. Intracranial atherosclerosis: correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis 2014; 237: 460–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang WJ, Chen XY, Zhao HL, et al. In Vitro assessment of histology verified intracranial atherosclerotic disease by 1.5T magnetic resonance imaging: concentric or eccentric? Stroke 2016; 47: 527–530. [DOI] [PubMed] [Google Scholar]
- 14. Zhang DF, Chen YC, Chen H, et al. A high-resolution MRI study of relationship between remodeling patterns and ischemic stroke in patients with atherosclerotic middle cerebral artery stenosis. Front Aging Neurosci 2017; 9: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dieleman N, Yang W, Abrigo JM, et al. Magnetic resonance imaging of plaque morphology, burden, and distribution in patients with symptomatic middle cerebral artery stenosis. Stroke 2016; 47: 1797–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu JH, Kwak HS, Chung GH, et al. Association of intraplaque hemorrhage and acute infarction in patients with basilar artery plaque. Stroke 2015; 46: 2768–2772. [DOI] [PubMed] [Google Scholar]
- 17. Skarpathiotakis M, Mandell DM, Swartz RH, et al. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol 2013; 34: 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ay H, Furie KL, Singhal A, et al. An evidence-based causative classification system for acute ischemic stroke. Ann Neurol 2005; 58: 688–697. [DOI] [PubMed] [Google Scholar]
- 19. Ma N, Xu Z, Lyu J, et al. Association of perforator stroke after basilar artery stenting with negative remodeling. Stroke 2019; 50: 745–749. [DOI] [PubMed] [Google Scholar]
- 20. Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol 2000; 21: 643–646. [PMC free article] [PubMed] [Google Scholar]
- 21. Ma N, Jiang WJ, Lou X, et al. Arterial remodeling of advanced basilar atherosclerosis: a 3-tesla MRI study. Neurology 2010; 75: 253–258. [DOI] [PubMed] [Google Scholar]
- 22. Turan TN, LeMatty T, Martin R, et al. Characterization of intracranial atherosclerotic stenosis using high-resolution MRI study-rationale and design. Brain Behav 2015; 5: e00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lou X, Ma N, Ma L, et al. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR Am J Neuroradiol 2013; 34: 513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012; 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013; 44: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang WJ, Yu W, Ma N, et al. High resolution MRI guided endovascular intervention of basilar artery disease. J Neurointerv Surg 2011; 3: 375–378. [DOI] [PubMed] [Google Scholar]
- 27. Millon A, Boussel L, Brevet M, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012; 43: 3023–3028. [DOI] [PubMed] [Google Scholar]
- 28. Ward MR, Pasterkamp G, Yeung AC, et al. Arterial remodeling. Mechanisms and clinical implications. Circulation 2000; 102: 1186–1191. [DOI] [PubMed] [Google Scholar]
- 29. Subramanian G, Silva J, Silver FL, et al. ; Investigators of the Registry of the Canadian Stroke Network. Risk factors for posterior compared to anterior ischemic stroke: an observational study of the Registry of the Canadian Stroke Network. Neuroepidemiology 2009; 33: 12–16. [DOI] [PubMed] [Google Scholar]
- 30. Pant R, Marok R, Klein LW. Pathophysiology of coronary vascular remodeling: relationship with traditional risk factors for coronary artery disease. Cardiol Rev 2014; 22: 13–16. [DOI] [PubMed] [Google Scholar]
- 31. Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012; 71: 195–198. [DOI] [PubMed] [Google Scholar]
- 32. Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 2005; 25: 2054–2061. [DOI] [PubMed] [Google Scholar]
- 33. Chen Z, Liu AF, Chen H, et al. Evaluation of basilar artery atherosclerotic plaque distribution by 3D MR vessel wall imaging. J Magn Reson Imaging 2016; 44: 1592–1599. [DOI] [PubMed] [Google Scholar]