Abstract
Purpose:
To establish an efficient new risk index for screening patients with endometrial cancer from patients with abnormal vaginal bleeding or discharge.
Method:
A total of 254 patients with abnormal vaginal bleeding or discharge were included in this study. Several candidate markers, including HE4, CA125, CA199, CA153, AFP, CEA, d-dimer, and fibrinogen, were employed. A new risk index for endometrial cancer screening was established by binary logistic regression. The diagnostic value of the candidate markers and the new risk index were assessed by a receiver operating characteristic curve, sensitivity, and specificity.
Results:
The most valuable diagnostic indicator for endometrial cancer was HE4, followed by d-dimer and then fibrinogen (area under the receiver operating characteristic curve: HE4 = 0.794, d-dimer = 0.717, fibrinogen = 0.690). The new risk index was superior to a single application of markers and a widely used combination (HE4 and CA125). At the ideal cutoff level, the sensitivity and specificity were 91.34% and 70.08%, respectively. In addition, only patients without organic disease served as controls, which further increase its performance (area under the receiver operating characteristic curve = 0.932, sensitivity = 94.49%, and specificity = 77.42%).
Conclusions:
The new risk index combining HE4, d-dimer, fibrinogen, and CA199 was the ideal combination for the screening of endometrial cancer. As a simple, rapid, nondestructive detection method, the new risk index is worth promotion in clinical practice, especially in primary medical institutions.
Keywords: CA199, d-dimer, HE4, fibrinogen, diagnosis, endometrial cancer
Introduction
Endometrial cancer (EC), the primary symptom of which is abnormal vaginal bleeding or discharge, is the most common gynecological malignancy.1,2 Since 1987, its incidence and mortality have increased continuously, especially in Asia.3 According to a study conducted by the China Cancer Society in 2015, 63.4 out of 1000 women will be diagnosed with EC and 21.8 per 1000 women will die per year.4 With disease progression, the prognosis becomes worse. The 5-year survival rates for localized, regional, and distant disease were 91%, 57%, and 17%, respectively.3 Therefore, early diagnosis and management are key factors to improve the outcomes of EC. Currently, the gold standard way to diagnose EC is pathological examination.5,6 However, it is not a routine examination, especially in some primary medical institutions. Therefore, under the system of the hierarchical medical system, screening for suspected patients with EC and referring them to the Regional Medical Center for early diagnosis and selection of a treatment strategy is very important.
Serum markers are widely used in the diagnosis of malignant tumors. With in-depth study, researchers have found that their standalone application does not work well and have tried to improve the use of serum markers in other ways. Combined detection is a promising method. Many studies have been carried out and have achieved some results. Dong et al and Saarelainen et al suggested that the combined detection of HE4 and CA125 could achieve a higher detection rate and was superior to the single application of HE4 or CA125.7,8 There were also some studies that combined multiple serum markers.9,10 However, their results were not very satisfactory. For example, the combined detection of HE4, CA125, CA724, and CA199 was not very greatly superior to the combined detection of any 3 of them alone.11 Considering the literature review of common serum markers (CA125, HE4, CA199, CA153, AFP, and CEA) in gynecological malignancies, we surmised that the detection rate of suspected patients with EC does not increase with the number of markers in the combined detection. Therefore, the introduction of new serum markers to distinguish suspected patients with EC is needed.
Serum d-dimer and fibrinogen are used as coagulation parameters and change with changes in coagulation function. Emerging data suggest that they are correlated with tumor stage and prognosis in several cancer types.12-16 The hypercoagulable microenvironment is very closely related to carcinoma tumorigenesis and progression.17-19 The procoagulants, tissue factors, or inflammatory cytokines expressed by tumor cells cause blood hypercoagulability, even in the early stages.20 Therefore, tumors could cause an increase in the levels of serum d-dimer and fibrinogen. Some studies have confirmed that their levels are associated with the prognosis of patients with malignant tumors.14,21-23 However, few studies have concentrated on their application in the diagnosis of cancer, especially EC. Therefore, the intensive study of their diagnostic value in EC is the focus of this study.
The primary aim of this study was to detect the diagnostic value of d-dimer and fibrinogen in EC. Next, we developed a new risk forecast model based on candidate markers for different diseases to improve the detection rate of suspected patients with EC.
Materials and Methods
Patients
A total of 254 patients with abnormal vaginal bleeding or discharge who were treated at the Affiliated Obstetrics and Gynecology Hospital of Nanjing Medical University between June 2017 and February 2019 were included in this retrospective study. A total of 127 cases of EC confirmed histologically were classified as the experimental group (EG), and 127 other cases (including 96 patients with atypical hyperplasia of the endometrium [AHE] and 31 patients without organic disease) were classified as the control group (CG). All patients in this study had no other complications (including malignant tumors of other systems, poor blood glucose or blood pressure control, thrombotic diseases, coagulation dysfunction, endocrine system diseases, liver and kidney dysfunction, etc). This study was approved by the Medical Ethics Committee of Nanjing Maternal and Child Health Hospital (No. NFKSL-003) and was registered at the Chinese Clinical Trial Registry (ChiCTR1900023149). Because of the retrospective nature of the analysis, the ethics committee waived the requirement for written informed consent from the participants.
Candidate Marker Serum Concentration Measurements
Preoperative CA125, HE4, CA199, CA153, AFP, and CEA concentrations were measured using a COBAS 6000 analyzer (Roche, Switzerland) with the chemiluminescent reagent kit supplied by Roche. Preoperative d-dimer and fibrinogen concentrations were measured using a CA7000 automated coagulation analyzer (Sysmex, Japan) with particular reagents. All measurements were performed at room temperature according to the manufacturers’ instructions blinded to the results of the histopathologic report.
Statistical Analysis
Statistical analysis was performed with SPSS version 23 (Chicago, Illinois) and MedCalc Statistical Software version 15.6.1 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015). Normally distributed data are presented as the mean and standard deviation (SD). Nonnormally distributed data are presented as the median (M) and interquartile range (Q). The Mann-Whitney U test was implemented to assess the differences between groups. Logistic regression model analysis was employed to test the relationship between candidate markers and EC and construct a new risk forecast model. A receiver operating characteristic curve (ROC) was employed to assess the diagnostic efficacy of the candidate tumor markers.24-26 The estimation of sensitivity and specificity was based on the Youden index.27 The DeLong method was used to compare the area under the receiver operating characteristic curve (AUROC).28 Value of P < .05 was considered to be statistically significant.
Results
Table 1 briefly shows the basic clinical data of all included patients. By comparison with EG, we noted whether patients with atypical hyperplasia of the endometrium or without organic disease had markedly lower levels of all candidate markers except AFP.
Table 1.
The Characteristics of the Patient Population.
| Numerical Display | EC | Control Group | P Value | ||
|---|---|---|---|---|---|
| AHE | No Organic Lesions | ||||
| Number | N | 127 | 96 | 31 | |
| Age, year | mean (SD) | 54.45 (8.48) | 45.39 (12.820) | 44.0 (13.90) | .000 |
| BMI, kg/m2 | mean (SD) | 25.131 (4.035) | 25.269 (3.729) | 23.217 (3.268) | .02 |
| Fibrinogen, g/L | M (Q) | 2.602 (2.303-2.987) | 2.235 (1.95-2.59) | 2.36 (1.73-2.73) | .000 |
| d-dimer, mg/L | M (Q) | 0.28 (0.2-0.4) | 0.18 (0.11-0.27) | 0.18 (0.12-0.28) | .000 |
| CA125, U/mL | M (Q) | 20.4 (15.29-31.49) | 18.2 (12.673-33.033) | 16.45 (11.15-26.02) | .041 |
| HE4, pmol/L | M (Q) | 74.52 (58.43-114.0) | 54.37 (45.57-62.298) | 44.45 (36.78-56.32) | .000 |
| CA199, U/mL | M (Q) | 15.86 (9.08-31.39) | 9.56 (6.89-15.686) | 9.28 (6.89-12.68) | .000 |
| CA153, U/mL | M (Q) | 9.18 (6.39-12.49) | 8.785 (6.238-11.635) | 6.12 (2.12-9.72) | .002 |
| AFP, ng/mL | M (Q) | 2.79 (2.08-3.73) | 2.505 (1.72-3.805) | 2.36 (1.68-3.22) | .115 |
| CEA, ng/mL | M (Q) | 1.55 (1.06-2.28) | 1.215 (0.748-1.87) | 1.2 (0.84-1.83) | .002 |
Abbreviations: AHE, atypical hyperplasia of endometrium; BMI, body mass index; EC, endometrial cancer; M, median; Q, interquartile range; SD, standard deviation.
Univariate analysis demonstrated that the d-dimer, fibrinogen, HE4, CA199, and CA153 levels were correlated with EC. Multivariate analysis was used to verify the results and confirmed that the relationship remained significant except for CA153 (Table 2).
Table 2.
Univariate and Multivariate Analysis of Candidate Marker Expression in EC.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Relative Risk | 95% CI | P Value | Relative Risk | 95% CI | P Value | |
| Fibrinogen | 3.237 | 1.958-5.353 | .000 | 3.229 | 1.760-5.922 | .000 |
| d-dimer | 32.483 | 6.133-172.048 | .000 | 30.372 | 4.233-217.906 | .001 |
| CA125 | 1.002 | 0.997-1.007 | .518 | - | ||
| HE4 | 1.046 | 1.030-1.061 | .000 | 1.044 | 1.027-1.061 | .000 |
| CA199 | 1.044 | 1.021-1.069 | .000 | 1.033 | 1.008-1.058 | .01 |
| CA153 | 1.052 | 1.008-1.098 | .019 | 0.982 | 0.924-1.042 | .541 |
| AFP | 1.089 | 0.931-1.275 | .285 | - | ||
| CEA | 1.042 | 0.961-1.130 | .321 | - | ||
Abbreviation: CI, confidence interval.
The risk index of endometrial cancer (RIEC) screening was established based on combining the serum levels of d-dimer, fibrinogen, HE4, and CA199. The P value of the Hosmer-Lemeshow test of the prediction equation was greater than .05, indicating an adequate model formulation.29
The coefficients in the prediction equation were as follows:
The predicted probability (PP) was:
The areas under the ROC curve were used to evaluate the efficiency of d-dimer, fibrinogen, HE4, CA199, HE4+CA125, and RIEC in differentiating EC (Table 3). The results showed that the performance of HE4 was greater when it was used as a single marker in EC screening, and RIEC showed the best efficiency of diagnosis compared with the single markers and the most common combination (P value < .001; Table 3).
Table 3.
Area Under the Receiver Operating Characteristic Curve and Sensitivity for Individual and Combined Tumor Marker Assays for Endometrial Cancer.
| AUROC | 95% CI | P Value | Cutoff | Specificity (Sensitivity = 94.5%) | P Valuea | |
|---|---|---|---|---|---|---|
| Fibrinogen | 0.690 | 0.625-0.754 | .000 | 2.25 | 24.4% | .000 |
| CA199 | 0.681 | 0.615-0.746 | .000 | 21.45 | 4.7% | .000 |
| HE4 | 0.794 | 0.740-0.849 | .000 | 59.07 | 31.5% | .000 |
| d-dimer | 0.717 | 0.654-0.779 | .000 | 0.17 | 34.6% | .000 |
| CA125 + HE4 | 0.797 | 0.743-0.844 | .000 | 0.46 | 33.1% | .000 |
| RIEC | 0.884 | 0.838-0.920 | .000 | 0.36 | 66.1% | - |
Abbreviations: CI, confidence interval; AUROC, area under the receiver operating characteristic curve; RIEC, risk index of endometrial cancer.
a Value of P compared with the AUROC of RIEC.
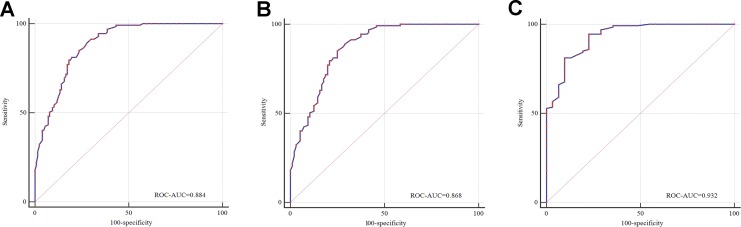
According to the pathological type, International Federation of Gynecology and Obstetrics (FIGO) stage and grade, subgroup analysis was performed. When only patients without organic disease served as a control, the AUROC was high and reached 0.932, and the sensitivity and specificity were 94.49% and 77.42%, respectively (Table 4 and Figure 1). In addition, the value of RIEC in screening patients with stage II to IV or middle to low differentiation was higher (Table 4).
Table 4.
Area Under the Receiver Operating Characteristic Curve, Sensitivity, and Specificity of RIEC in the Diagnosis of Patients With Endometrial Cancer With Different Clinical Characteristics.
| AUROC | 95% CI | P Value | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| EG vs CG | 0.884 | 0.838-0.920 | .000 | 91.34 | 70.08 |
| Histological type | |||||
| EC vs AHE | 0.868 | 0.817-0.910 | .000 | 85.04 | 75.0 |
| EC vs POD | 0.932 | 0.881-0.966 | .000 | 94.49 | 77.42 |
| FIGO | |||||
| I vs CG | 0.869 | 0.817-0.910 | .000 | 89.80 | 70.08 |
| II-IV vs CG | 0.935 | 0.884-0.968 | .000 | 93.10 | 82.68 |
| Grade | |||||
| G1 vs CG | 0.857 | 0.796-0.905 | .000 | 91.84 | 70.08 |
| G2-3 vs CG | 0.906 | 0.856-0.944 | .000 | 86.15 | 81.89 |
Abbreviations: AHE, atypical hyperplasia of endometrium; AUROC, area under the receiver operating characteristic curve; CG, control group; CI, confidence interval; EC, endometrial cancer; EG, experimental group; FIGO, International Federation of Gynecology and Obstetrics; POD, patient without organic disease; RIEC, risk index of endometrial cancer.
Figure 1.
Receiver operating characteristic curves for RIEC. A, Patients with endometrial atypical hyperplasia and patients without organic diseases served as the control group. B, Only patients with endometrial atypical hyperplasia served as the control group. C, Only patients without organic diseases served as the control group.
Discussion
Endometrial cancer is one of the most common malignant tumors in females, and the primary symptom is abnormal vaginal bleeding or discharge. However, this primary symptom is not specific for EC.30-32 Pathological examination, the diagnostical gold standard for EC, is not a routine inspection due to the shortage of pathologists, especially in areas underdeveloped at the medical level. Thus, the timely screening of suspected patients with EC from patients with abnormal vaginal bleeding or discharge to achieve a definite diagnosis and select a treatment strategy as early as possible are very important. The detection of serum markers is simple, quick, accurate, stable, and inexpensive, so it is a very promising approach, especially the combined detection of markers.
At present, the most widely used serum markers in screening EC are CA125 and HE4.33 However, their diagnostic value is not very satisfactory.34 Accordingly, clinicians have been working toward establishing new screening methods and have achieved some results. The combined detection of HE4 and CA125 was a useful attempt and could improve the accuracy of screening. Unfortunately, the effect was not very ideal.7,8 In this study, the performance of HE4 combined with CA125 was also presented, and the result, which was consistent with that of previous studies, showed that the accuracy of screening was increased.35 Furthermore, some researchers have attempted to increase the number of markers in the combined detection to improve the accuracy of screening. Bian et al tried to develop a new risk index based on the combined detection of HE4, CA125, CA724, and CA199. However, their method did not work well. The AUROC of the new risk index was only increased by 0.05 compared with that of the single marker HE4. The sensitivity only increased 1.1%.11 Therefore, we suspected that simply increasing the number of markers in the combined detection could not significantly improve the screening efficiency.
This study attempted to develop an innovative coagulation index (d-dimer and fibrinogen) for the screening of EC. Surprisingly, the application value of d-dimer and fibrinogen, only next to HE4, was higher than that of the other markers. Based on this finding, a new risk index (RIEC) was developed. The results showed that with the application of RIEC, the accuracy of EC screening increased significantly. The AUROC was 0.844, which was considerably greater than that of the combined detection of HE4 and CA125. In the subgroup analysis, when only patients without organic lesions served as controls, the AUROC was high and reached 0.932, and the sensitivity and specificity were 94.49% and 77.42%, respectively. The study also found that RIEC had more diagnostic value in patients with advanced disease and patients with middle to low differentiation.
Another characteristic of this study was the CG, which consisted of patients with AHE or without organic lesions. The primary symptoms were abnormal vaginal bleeding or discharge. The members of the CG were more in accordance with clinical practice. This study indicated that the performance of RIEC was still satisfactory (AUROC = 0.868) when only patients with AHE were regarded as controls.
This study demonstrated that RIEC is a useful tool in screening for suspected patients with EC, especially applicable in areas underdeveloped at the medical level. Limited by the single-center retrospective nature of the study, the conclusions still need to be confirmed by a prospective multicenter study.
Abbreviations
- AHE
atypical hyperplasia of endometrium
- AUROC
area under the receiver operating characteristic curve
- BMI
body mass index
- CG
control group
- EC
endometrial cancer
- EG
experimental group
- FIGO
International Federation of Gynecology and Obstetrics
- M
median
- Q
interquartile range
- PP
predicted probability
- RIEC
the new risk index of endometrial cancer
- ROC
receiver operating characteristic curve
- SD
standard deviation.
Footnotes
Authors’ Note: Guangquan Liu, Kai Hu, and Ke Huang contributed equally to this work. This study was approved by the Medical Ethics Committee of Nanjing Maternal and Child Health Hospital (No. NFKSL-003) and had been registered at Chinese Clinical Trial Registry (ChiCTR1900023149). A new risk index combining d-dimer, fibrinogen, HE4, and CA199 differentiates suspected patients with endometrial cancer from those with abnormal vaginal bleeding or discharge.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was financially supported by the National Natural Science Foundation of China (No. 81572556) and Science and Technology Development Fund of Nanjing Medical University (No. NMUB2018126).
ORCID iD: Xuemei Jia  https://orcid.org/0000-0002-9323-4425
https://orcid.org/0000-0002-9323-4425
References
- 1. Pakish JB, Lu KH, Sun CC, et al. Endometrial cancer associated symptoms: a case-control study. J Womens Health (Larchmt). 2016;25(11):1187–1192. doi:10.1089/jwh.2015.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sorosky JI. Endometrial cancer. Obstet Gynecol. 2012;120(2 pt 1):383–397. doi:10.1097/AOG.0b013e3182605bf1. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi:10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 4. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5. Prat J, Gallardo A, Cuatrecasas M, Catasus L. Endometrial carcinoma: pathology and genetics. Pathology. 2007;39(1):72–87. doi:10.1080/00313020601136153. [DOI] [PubMed] [Google Scholar]
- 6. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi:10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 7. Dong C, Liu P, Li C. Value of HE4 combined with cancer antigen 125 in the diagnosis of endometrial cancer. Pak J Med Sci. 2017;33(4):1013–1017. doi:10.12669/pjms.334.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saarelainen SK, Peltonen N, Lehtimaki T, Perheentupa A, Vuento MH, Maenpaa JU. Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrial carcinoma. Am J Obstet Gynecol. 2013;209(2):142.e1-e6. doi:10.1016/j.ajog.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 9. Kemik P, Saatli B, Yildirim N, et al. Diagnostic and prognostic values of preoperative serum levels of YKL-40, HE-4 and DKK-3 in endometrial cancer. Gynecol Oncol. 2016;140(1):64–69. doi:10.1016/j.ygyno.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 10. Li M, Zhao L, Shen D, Li X, Wang J, Wei L. Clinical implications and prognostic value of single and combined biomarkers in endometrial carcinoma. Chin Med J (Engl). 2014;127(8):1459–1463. [PubMed] [Google Scholar]
- 11. Bian J, Sun X, Li B, Ming L. Clinical significance of serum HE4, CA125, CA724, and CA19-9 in patients with endometrial cancer. Technol Cancer Res Treat. 2017;16(4):435–439. doi:10.1177/1533034616666644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ge LP, Li J, Bao QL, Chen P, Jiang Q, Zhu LR. Prognostic and predictive value of plasma D-dimer in advanced non-small cell lung cancer patients undergoing first-line chemotherapy. Clin Transl Oncol. 2015;17(1):57–64. doi:10.1007/s12094-014-1198-2. [DOI] [PubMed] [Google Scholar]
- 13. Krenn-Pilko S, Langsenlehner U, Stojakovic T, et al. An elevated preoperative plasma fibrinogen level is associated with poor disease-specific and overall survival in breast cancer patients. Breast. 2015;24(5):667–672. doi:10.1016/j.breast.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 14. Zhang C, Jia Y, Jia Y, Zhang X, Li K. Prognostic and predictive value of plasma D-dimer levels in patients with small-cell lung cancer. Int J Clin Oncol. 2018;23(6):1070–1075. doi:10.1007/s10147-018-1320-5. [DOI] [PubMed] [Google Scholar]
- 15. Zhao K, Deng H, Qin Y, Liao W, Liang W. Prognostic significance of pretreatment plasma fibrinogen and platelet levels in patients with early-stage cervical cancer. Gynecol Obstet Invest. 2015;79(1):25–33. doi:10.1159/000365477. [DOI] [PubMed] [Google Scholar]
- 16. Xu L, He F, Wang H, Gao B, Wu H, Zhao S. A high plasma D-dimer level predicts poor prognosis in gynecological tumors in East Asia area: a systematic review and meta-analysis. Oncotarget. 2017;8(31):51551–51558. doi:10.18632/oncotarget.17936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boccaccio C, Medico E. Cancer and blood coagulation. Cell Mol Life Sci. 2006;63(9):1024–1027. doi:10.1007/s00018-005-5570-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lima LG, Monteiro RQ. Activation of blood coagulation in cancer: implications for tumour progression. Biosci Rep. 2013;33(5):e00064 doi:10.1042/BSR20130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wojtukiewicz MZ, Hempel D, Sierko E, Tucker SC, Honn KV. Protease-activated receptors (PARs)—biology and role in cancer invasion and metastasis. Cancer Metastasis Rev. 2015;34(4):775–796. doi:10.1007/s10555-015-9599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diao D, Wang Z, Cheng Y, et al. D-dimer: not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS One. 2014;9(7):e10112 5. doi:10.1371/journal.pone.0101125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ge LP, Li J, Bao QL, Chen P, Jiang Q, Zhu LR. Prognostic and predictive value of plasma D-dimer in advanced non-small cell lung cancer patients undergoing first-line chemotherapy. Clin Transl Oncol. 2015;17(1):57–64. doi:10.1007/s12094-014-1198-2. [DOI] [PubMed] [Google Scholar]
- 22. Yang L, Dong H, Li Z, Pan Y, Qu Tan Z. Correlation between circulating tumor cells and D-D and platelet in patients with pulmonary malignancies. Oncology Lett. 2018;15(2):2169–2172. doi:10.3892/ol.2017.7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu J, Fu Z, Liu G, Xu P, Xu J, Jia X. Clinical significance of plasma D-dimer in ovarian cancer: a meta-analysis. Medicine (Baltimore). 2017;96(25):e7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chatziioannou SN, Georgakopoulos AT, Pianou NK, Kafiri GT, Pavlou SN, Kallergi M. Recurrent thyroid cancer diagnosis: ROC study of the effect of a high-resolution head and neck 18F-FDG PET/CT scan. Acad Radiol. 2014;21(1):58–63. doi:10.1016/j.acra.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 25. Kottas M, Kuss O, Zapf A. A modified Wald interval for the area under the ROC curve (AUC) in diagnostic case-control studies. BMC Med Res Methodol. 2014;14:26 doi:10.1186/1471-2288-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zivanovic O, Abramian A, Kullmann M, et al. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer. 2015;136(3):699–708. doi:10.1002/ijc.29011. [DOI] [PubMed] [Google Scholar]
- 27. Martinez-Camblor P, Pardo-Fernandez JC. The Youden index in the generalized receiver operating characteristic curve context. Int J Biostat. 2019;15(1):/j/ijb.2019. doi:10.1515/ijb-2018-0060. [DOI] [PubMed] [Google Scholar]
- 28. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 29. Bascom KE, Dziodzio J, Vasaiwala S, et al. Derivation and validation of the CREST model for very early prediction of circulatory etiology death in patients without ST-segment-elevation myocardial infarction after cardiac arrest. Circulation. 2018;137(3):273–282. doi:10.1161/CIRCULATIONAHA.116.024332. [DOI] [PubMed] [Google Scholar]
- 30. Cheong Y, Cameron IT, Critchley HOD. Abnormal uterine bleeding. Br Med Bull. 2017;123(1):103–114. doi:10.1093/bmb/ldx027. [DOI] [PubMed] [Google Scholar]
- 31. Marnach ML, Laughlin-Tommaso SK. Evaluation and management of abnormal uterine bleeding. Mayo Clin Proc. 2019;94(2):326–335. doi:10.1016/j.mayocp.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 32. Mullins TL, Miller RJ, Mullins ES. Evaluation and management of adolescents with abnormal uterine bleeding. Pediatr Ann. 2015;44(9):e218–222. doi:10.3928/00904481-20150910-09. [DOI] [PubMed] [Google Scholar]
- 33. Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem. 2011;57(11):1534–1544. doi:10.1373/clinchem.2010.157073. [DOI] [PubMed] [Google Scholar]
- 34. Hsieh CH, ChangChien CC, Lin H, et al. Can a preoperative CA 125 level be a criterion for full pelvic lymphadenectomy in surgical staging of endometrial cancer? Gynecol Oncol. 2002;86(1):28–33. doi:10.1006/gyno.2002.6664. [DOI] [PubMed] [Google Scholar]
- 35. Moore RG, Brown AK, Miller MC, et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol. 2008;110(2):196–201. doi:10.1016/j.ygyno.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]