Abstract
Inflammatory cells contribute to irreversible damage in pulmonary arterial hypertension (PAH). We hypothesized that in PAH, dysfunctional BMPR2 signaling in macrophages contributes to pulmonary vascular injury and phenotypic changes via proinflammatory cytokine production. Studies were conducted in: (1) Rosa26-rtTA2 3 X TetO7-Bmpr2delx4 FVB/N mice (mutant Bmpr2 is universally expressed, BMPR2delx4 mice) given a weekly intra-tracheal liposomal clodronate injections for four weeks; and (2) LysM-Cre X floxed BMPR2 X floxed eGFP monocyte lineage-specific BMPR2 knockout (KO) mouse model (Bmpr2 gene expression knockdown in monocytic lineage cells) (BMPR2KO) following three weeks of sugen/hypoxia treatment. In the BMPR2delx4 mice, increased right ventricular systolic pressure (RVSP; P < 0.05) was normalized by clodronate, and in monocyte lineage-specific BMPR2KO mice sugen hypoxia treatment increased (P < 0.05) RVSP compared to control littermates, suggesting that suppressed BMPR2 in macrophages modulate RVSP in animal models of PH. In addition, in these mouse models, muscularized pulmonary vessels were increased (P < 0.05) and surrounded by an increased number of macrophages. Elimination of macrophages in BMPR2delx4 mice reduced the number of muscularized pulmonary vessels and macrophages surrounding these vessels. Further, in monocyte lineage-specific BMPR2KO mice, there was significant increase in proinflammatory cytokines, including C-X-C Motif Chemokine Ligand 12 (CXCL12), complement component 5 a (C5a), Interleukin-16 (IL-16), and secretory ICAM. C5a positive inflammatory cells present in and around the pulmonary vessels in the PAH lung could potentially be involved in pulmonary vessel remodeling. In summary, our data indicate that, in BMPR2-related PAH, macrophages with dysfunctional BMPR2 influence pulmonary vascular remodeling and phenotypic outcomes via proinflammatory cytokine production.
Keywords: pulmonary arterial hypertension, BMPR2 mutation, macrophages, inflammatory cytokines, animal models of pulmonary hypertension
Introduction
Pulmonary arterial hypertension (PAH) is a disease involving progressive occlusion of small pulmonary arteries. This leads to an increase in pulmonary vascular resistance and eventually death due to right heart failure.1–6 The role of altered immunity and inflammation has been increasingly recognized and shown to contribute to irreversible pulmonary vascular damage in many sub groups of human PAH including idiopathic and heritable forms of PAH. Animal models of PAH also show that progressive remodeling of pulmonary arteries is dependent on a pro-inflammatory signaling environment.3,7–13 However, in almost all classical animal models, and in humans with some secondary forms of PH, when the damaging stimulus is removed, inflammation spontaneously resolves with time.14–16 This is not true in human PAH, as inflammation is an integral part of PAH vascular lesions, thus, becoming an important contributing factor to the disease manifestation rather than only being a consequence of the disease.17
Macrophages can function as agents of defense or destruction18 to orchestrate pathophysiology of a disease.19–22 Adaptation of macrophages in pathological conditions23 depends on their response to specific stimuli.18 BMP proteins via the BMPR2 receptor recruit activated macrophages to the site of injury to promote disease progression.24–29 BMPR2 suppression modulates disease pathogenesis by failure to resolve inflammation and alter immune processes and cytokine production.10,30–36. We and others have shown that mutation or reduced expression of BMPR2 results in increase in pulmonary vascular inflammation with inflammatory cell recruitment—especially macrophages—and constitutive activation of tissue macrophages.10,37
Genetic linkage studies show heterozygous germline mutations in the BMPR-II gene (BMPR2) are associated with 80% of the heritable form of PAH (HPAH). We have shown that a mouse model universally expressing a Bmpr2delx4+ mutation phenotype (a truncated receptor just after the transmembrane domain which acts as a dominant negative for SMAD signaling) is dominated by inflammatory phenotype in pulmonary vasculature and airways, which appeared to be driven by a primary defect in tissue macrophages in the absence of stimulus.35 Follow-up studies in bone marrow-derived macrophages (BMDM) demonstrated that Bmpr2 mutant macrophages had mild constitutive, possibly classical, activation, and that activated wild-type macrophages secreted BMP inhibitors to an extent sufficient to alter BMP reporter and BMP-dependent behavior in smooth muscle cells with co-culture or conditioned media.10 Similarly, Song et al. have also shown that heterozygous BMPR2 mutant mice develop PAH under inflammatory stress.39 Moreover, conditional ablation of BMPR2 in PA endothelial cells of transgenic mice with perivascular infiltration of CD68-positive cells resulted in some of the animals developing PAH.40 In addition, loss of function of BMPR2 in smooth muscle cells increased the proinflammatory cytokine IL-6 via phospho-p38-dependent signaling mechanism.41 Similarly, in human studies, HPAH patients with BMPRII mutations have shown increased pulmonary artery pressures than those without the mutations42 with pronounced remodeling of the lung intima associated with perivascular and interstitial inflammatory infiltrates.43 We, therefore, sought to test the hypothesis that suppression of BMPR2 signaling in macrophages fail to resolve pulmonary vascular injury and contribute to phenotypic changes via proinflammatory cytokine production.
Methods
Rosa26-rtTA2 X TetO7-Bmpr2delx4+ mice
For animal studies, we did the following: (1) we bred Rosa26-rtTA2 mice44 (Rosa26 or control mice) with Rosa 26 Bmpr2delx4+ mice45 (BMPR2delx4 mice) to create mice heterozygous in both genes and expression of the mutations inducible with doxycycline, in chow for eight weeks. Both strains were on a FVB/N background and mice were used in early adulthood (aged 12–14 weeks at sacrifice). Clodronate (dichloromethylene diphosphonic acid; Sigma-Aldrich) or sterile phosphate buffered saline (PBS)-containing liposomes were prepared as previously described46 and mice were injected intra-tracheally weekly for four weeks to eliminated monocytes/macrophages.47,48 (2) We created a LysM-Cre X floxed BMPR2 X floxed eGFP monocyte lineage-specific BMPR2 knockout (KO) mouse model of pulmonary hypertension (PH) (LysM Cre BMPR2KO or BMPR2KO mice), in which Bmpr2 gene expression was completely knockdown in monocytic lineage cells. LysM Cre mice (Strain name: B6.129P2-Lyz2tm1(cre)Ifo/J, Stock number: 004781) and Cre GFP mice (Strain name: B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, Stock number: 007676) were obtained from Jackson Laboratories; BMPR2-KO mice were obtained from MMRRC UNC (Strain name: B6.129S4(Cg)-Bmpr2tm1.1Enl, Strain ID: 29883). LysM Cre mice without BMPR2 floxed alleles were used as controls. Studies were conducted (in mice aged 12–14 weeks at sacrifice) at baseline or after three weeks of sugen/hypoxia treatment.49 All the animals underwent echocardiography38 and, at the time of sacrifice, invasive hemodynamics was performed through a closed chested technique for measurement of right ventricular systolic pressure (RVSP).50 Blood was drawn for insulin and glucose measurements and assayed by the Mouse Metabolic Phenotyping Core at Vanderbilt University Medical Center. Homeostatic model assessment insulin resistance (HOMA-IR) was calculated as previously described.51 All animal procedures were approved by the Vanderbilt Institutional Animal Care and Use Committee (IACUC).
Measurement for pulmonary vessels
After euthanizing the mice, the left atrium was removed and the pulmonary circulation was perfused with PBS using a syringe-generated flow. The left lung was inflated with 0.8% low melt agarose at constant inflation pressure and embedded in paraffin. Samples were transversely sectioned at 6-µm thickness and immunostained with antibody against smooth muscle alpha actin (αSMA, Sigma-Aldrich, St. Louis, MO, USA) with a fluorescent (GFP) secondary and counterstained with DAPI. Random fields were selected with DAPI, avoiding fields with large airways or bronchi, and then vessel size and muscularization measured for each vessel identified by αSMA staining. Ten fields at 100× magnification were selected per mouse lung for analysis.
Flow cytometric evaluation of cells from mouse bone marrow and peripheral blood
Flow cytometric assay was done as previously described.52,53 Briefly, bone marrow cells isolated by flushing the tibias and femurs of the euthanized mice, resuspended in a single-cell suspension and undiluted whole blood cells were used for flow cytometric analysis. Following the removal of red blood cells, the remaining cells were stained with a cocktail of antibodies (CD11b (Mac-1)-PE, Gr-1-PECy7, CD68 APC, CD4-eFluoro450 (PB), B220APC, and CD8-APCCy7 from eBiosciences) to identify the population of myeloid, B cells, and T cells. Flow cytometry analysis was performed on a Becton Dickinson 5-laser LSRII instrument.
Cytokine array panel
Plasma, lung, and BMDM samples collected from control and BMPR2KO mice were used to conduct cytokine arrays. Pooled plasma and lung samples from three animals per group were used for the assay. BMDM were enriched from mouse bone marrow as described previously.10 2.5 × 105 BMDM from each mouse were plated in each well of a six-well plate and incubated overnight in DMEM, 10% FBS, and 1% pen/strep+1% l-glutamine and 300 ng/mL doxycycline. The next day, cells were treated with IL-6 or vehicle for 4 h, resulting in three wells per condition per mouse and five mice per genotype. Cells were washed with media; cell supernatants were harvested after 24 h. Supernatants from each of the four conditions (+/− monocytic lineage specific BMPR2KO, +/− IL6) were pooled before application to the arrays, to reduce variability. R&D systems mouse cytokine antibody array kit (Catalog no. ARY006) was used according to the manufacturer’s instructions.
Immunohistochemical analysis for C5a in human right ventricle
Immunolocalization of C5a was performed on archival paraffin-embedded human lung tissue obtained from controls and HPAH and IPAH patients. The study protocol was approved by the institutional research and ethics committees of Vanderbilt University Medical Center. Briefly, lung sections were deparaffinized, rehydrated, and blocked with 5% normal goat serum, followed by overnight incubation with primary antibody (C5a, Thermo Fisher Scientific, Rockford, IL) at 4℃. The sections were then incubated with AlexFluor595-labeled secondary antibody (Zymed Laboratories Invitrogen, Carlsbad, CA, USA) for 1 h. The slides were mounted using Vectashield mounting media containing DAPI (Vector Laboratories, Burlingame, CA, USA) for confocal microscopy.
Statistical analysis
Data expressed as means ± SE. Statistical analysis was carried out using a two-way ANOVA with the Bonferroni post-test or TTEST (GraphPad Prism Software, La Jolla CA, USA). A P value < 0.05 was considered significant.
Results
Elimination of macrophages by clodronate normalizes RVSP in BMPR2delx4+ mice
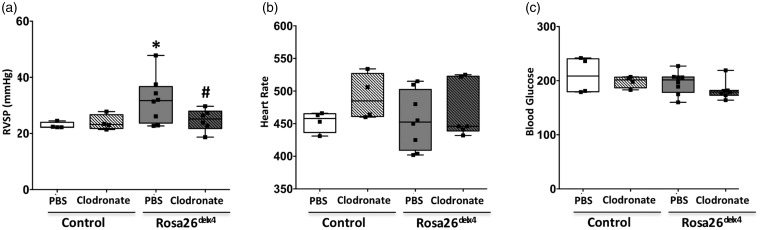
We determined the influence of macrophages on phenotypic outcome in Bmpr2delx4+ mice. The four experimental groups were: (1) Rosa26; (2) Rosa26 + clodronate; (3) Rosa26-Bmpr2delx4+; and (4) Rosa26-Bmpr2delx4+ + clodronate. There was no difference in systemic systolic pressures: controls averaged 95 mmHg and Bmpr2delx4+ averaged 96 mmHg (data not shown). In Bmpr2delx4+ mice, RVSP was significantly higher (P < 0.01) (average RVSP =31.8 mmHg, median = 31.7 mmHg) compared to control mice (average RVSP = 22.8 mmHg, median = 22.3 mmHg). Following clodronate injections, the RVSP remained unchanged (average = 23.9 mmHg, median = 22.2 mmHg) in control mice but significantly decreased (P < 0.01) in Bmpr2delx4+ mice (average = 24.8 mmHg, median = 25.1 mmHg) (Fig. 1a). In all animals, a heart rate >400 bpm indicated that anesthesia provided during RVSP measurement was appropriate and similar levels of the blood glucose across groups indicated a no-stress response (Fig. 1b and 1c). Thus, our results indicate that suppression of BMPR2 in monocytes/macrophages can normalize RVSP in a Bmpr2delx4+ mouse model of PH.
Fig. 1.
Elimination of macrophages by clodronate normalizes RVSP in BMPR2delx4+ mutant mice. (a) Right ventricular systolic pressure (RVSP); (b) heart rate; and (c) blood glucose. *P < 0.05 control (Rosa 26-rtTA2 mice) and Rosa26delx4, #P < 0.05 PBS vs. clodronate treated. Black squares represent individual animals.
BMPR2 knockdown in monocytic lineage cells shows worsened phenotype following sugen/hypoxia injury in a mouse model of PH
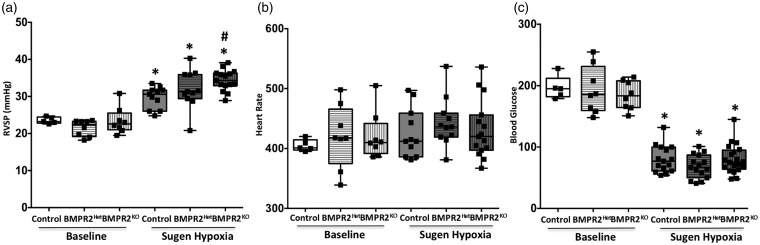
We used newly generated a LysM-Cre × floxed gene BMPR2 × floxed eGFP monocytic lineage-specific BMPR2KO mouse model of PH (BMPR2KO mice). At baseline, RVSP in these mice was similar to control mice (LysM Cre mice). Following treatment with VEGF inhibitor (sugen) and 10% oxygen, as expected,49 the control mice showed a significant (P < 0.01) increase in RVSP, which was further exacerbated (P < 0.01) in BMPR2KO mice (P < 0.01) indicating a worsened phenotype (Fig. 2a) compared to controls. The heart rate in the BMPR2KO mice was similar to controls with and without sugen hypoxia treatment (Fig. 2b); however, the blood glucose was significantly decreased in all the groups following sugen hypoxia treated (Fig. 2c). Hypoxic conditions have shown to have detrimental effects on glucose homeostasis.54,55 Thus, our results indicate that loss of BMPR2 expression in macrophages leads to increased susceptibility to worsened phenotypic outcome following injury.
Fig. 2.
BMPR2 knockdown in monocytic lineage cells show worsened phenotype following sugen/hypoxia injury in mouse model of PH: (a) RVSP; (b) heart rate; and (c) blood glucose. *P < 0.01 baseline vs. sugen/hypoxia treatment, #P < 0.05 control (LysM Cre mice) vs. BMPR2KO (LysM Cre BMPR2KO mice). Black squares represent individual animals.
Suppression of BMPR2 expression in macrophages contribute to pulmonary vascular remodeling in a mouse model of PH
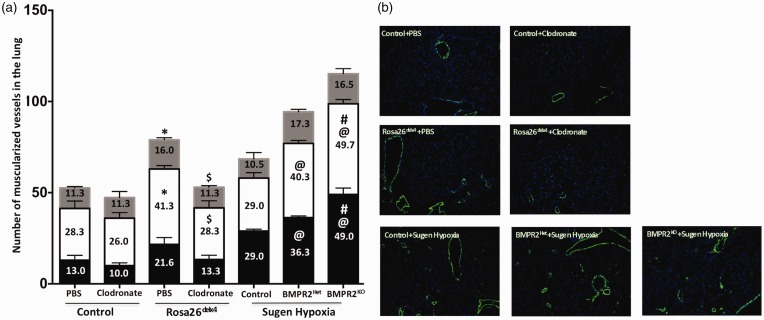
Pulmonary vascular remodeling in an integral part of PAH. We determined whether altered BMPR2 expression or elimination of monocytic lineage cells with BMPR2 knockdown influences pulmonary vessel remodeling by measuring the muscularization of 0–25 uM, 25–50 uM, and 50–100 uM size pulmonary vessels. In the Bmpr2delx4+ mice, the total number of muscularized pulmonary vessels was increased, with a significant increase (P < 0.05) in 25–50 uM and 50–100 uM size pulmonary vessels compared to control mice (Fig. 3a and 3b). Treatment with clodronate reduced total number of muscularized pulmonary vessels in Bmpr2delx4+ mice and, in particular, significantly (P < 0.05) reduced 25–50 uM and 50–100 uM size pulmonary vessels (Fig. 3a and 3b). In sugen/hypoxia-treated control mice (LysM Cre mice), the total number of muscularized pulmonary vessels were as expected,49 and in BMPR2KO mice, we observed a further increase (P < 0.05) in the muscularization of small and mid-size pulmonary vessels (0–25 uM and 25–50 uM) in BMPR2Het and BMPR2KO mice compared to sugen/hypoxia-treated controls (Fig. 3a and 3b). Thus, altered BMPR2 expression as a result of mutation or knockdown of gene expression specifically in monocytic lineage cells may further accelerate muscularization of pulmonary vessels; elimination of these cells can reverse this pulmonary vessel remodeling.
Fig. 3.
Suppression of BMPR2 expression in macrophages contributes to pulmonary vascular remodeling in mouse model of PH: (a) Number of muscularized vessels in lung. Black bar = 0–25 uM, white bar = 25–50 uM, and gray bar = 50–100 uM size vessels. *P < 0.05 control (PBS) vs. Rosa26delx4 (PBS), $P < 0.05 Rosa26delx4 (PBS) vs. Rosa26delx4 (clodronate), @P < 0.05 control (sugen/hypoxia) vs. BMPR2Het and BMPR2KO, #P < 0.05 BMPR2Het (sugen/hypoxia) vs. and BMPR2KO (sugen/hypoxia); and (b) Immunofluorescence staining for smooth muscle actin in green (magnification 100×).
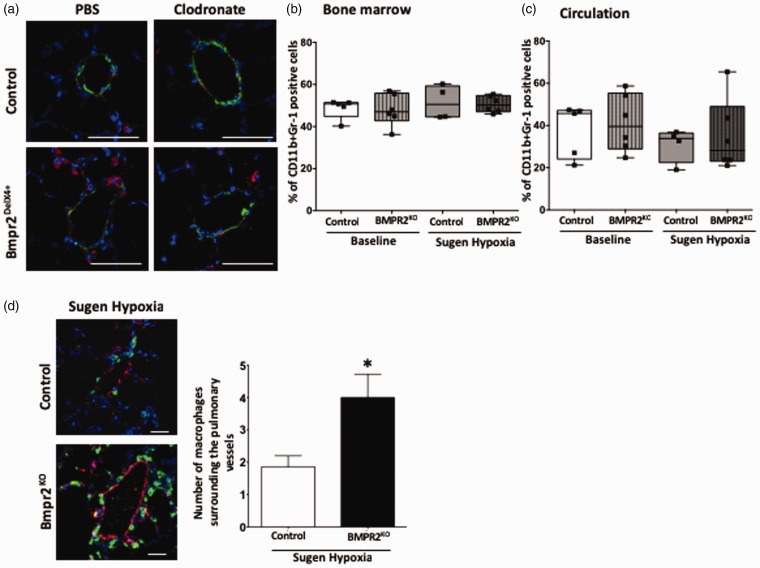
Suppressed BMPR2 expression in macrophages drives macrophage localization in pulmonary vessels in a mouse model of PH
We have shown an increase in pulmonary inflammation in the mouse model of PAH with BMPR2 mutation;10; therefore, we investigated whether suppression of BMPR2 in macrophages can influence localization of these cells in pulmonary vessels. In the Bmpr2delx4+ mouse model, using a classic macrophage marker, CD68,56 we first confirmed whether the number of CD68 positive lung macrophages (red color) is reduced by clodronate. We observed marked reduction number of macrophages surrounding the small pulmonary vessels (green color: alpha smooth muscle actin positive cells) in a Bmpr2delx4+ mouse lung (Fig. 4a) following clodronate injections, suggesting that elimination of macrophages in pulmonary vascular is associated with a reduction in RVSP. Next, we determined the population of CD11b+Gr-1-positive monocytic lineage cells in bone marrow and circulation. Myeloid-derived suppressor cells (MDSCs) express CD11b, a marker for myeloid cells of the macrophage lineage, and a marker for granulocytes, Gr-1; thus, these cells are referred to as Gr-1+CD11b+ cells.57 At baseline and after sugen/hypoxia treatment, the population of CD11b+Gr-1-positive cells in the bone marrow and circulation remained relatively unchanged in BMPR2KO mice compared to controls (Fig. 4b and 4c). We then investigated whether monocytic lineage cells reside in the lung following sugen/hypoxia treatment. In the BMPR2KO mouse lung, we observed a twofold increase in the number of GFP-positive cells surrounding the pulmonary vessels (red color: von Willebrand factor [vWF] positive cells) compared to controls (Fig. 4d). These results indicate that BMPR2 knockdown and hypoxic vascular injury did not alter the population of CD11b+Gr-1 macrophages in bone marrow and in circulation but led to the increased localization of monocytic lineage-specific cells surrounding the pulmonary vessels.
Fig. 4.
Suppressed BMPR2 expression in macrophages drives macrophage localization in pulmonary vessels in mouse model of PH: (a) Immunofluorescence staining for CD68-positive macrophages (in red) and smooth muscle actin (in green). Nucleus is stained blue (magnification 900×, scale bar 50 uM). (b, c) Number of CD11b+Gr-1-positive cells in bone marrow and in circulation (plasma). (d) Immunofluorescence staining for vWF (in red) and macrophages are green in color (by lineage tracing). Nucleus is stained blue (magnification 600×, scale bar 50 uM). *P < 0.01 control (LysM Cre mice) (sugen/hypoxia) vs. BMPR2Het (LysM Cre BMPR2-KO mice). Bar graph represents number of macrophages surrounding the pulmonary vessels in control and BMPR2KO mice following sugen/hypoxia treatment.
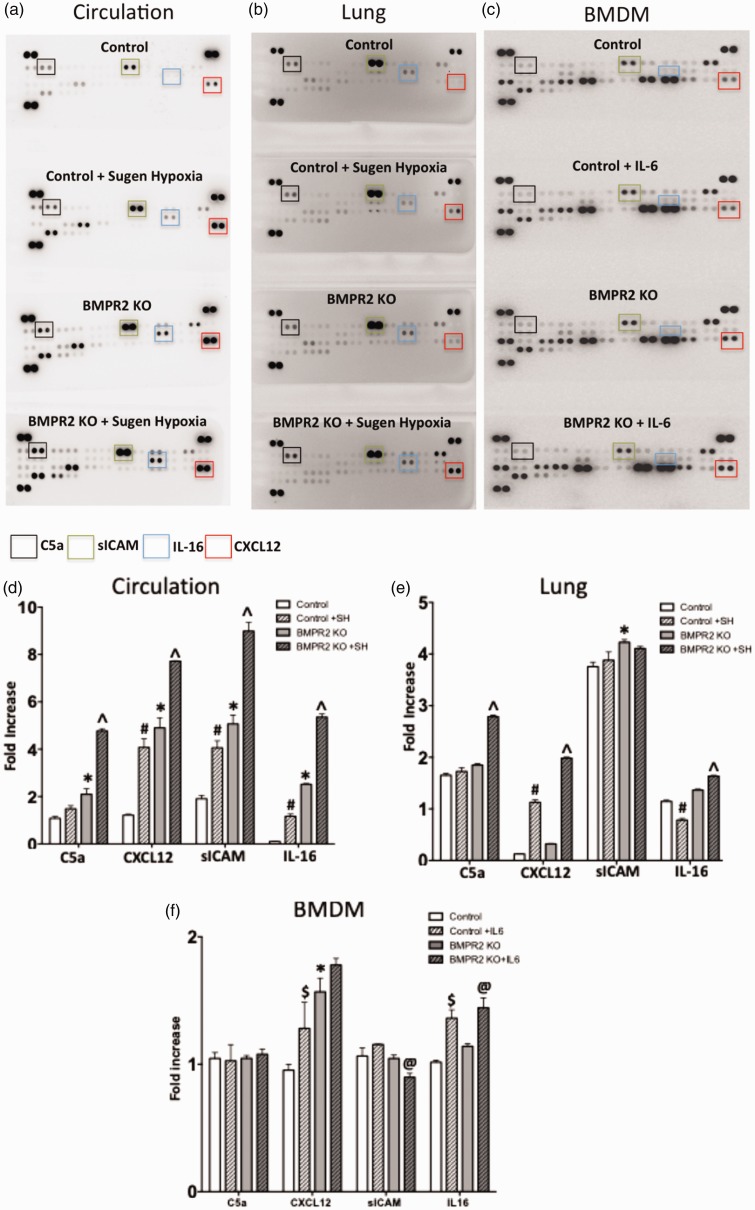
Vascular and hypoxic injury increases proinflammatory cytokines in circulation and lung in monocytic lineage-specific BMPR2KO mice
Proinflammatory cytokines are increased in lung and remodeled vessels in PAH patients.31,33,58 We investigated whether BMPR2 knockdown in monocytic lineage cells increases proinflammatory cytokines by performing cytokine assay using plasma and lung tissue obtained from BMPR2KO and controls with and without sugen/hypoxia treatment. There was a striking increase in number of circulating cytokines in BMPR2KO mice with sugen/hypoxia treatment compared to BMPR2KO mice at baseline or control animals with or without sugen/hypoxia treatment. The circulating cytokines C5a, CXCL12, sICAM, and IL-16 appeared to have a gradient increase as a result of BMPR2KO and sugen/hypoxia treatment (Fig. 5a and 5d). At baseline, a BMPR2KO mouse lung demonstrated a modest increase for most of the cytokines compared to controls (with or without sugen/hypoxia treatment) and a striking increase in C5a and CXC12 following sugen/hypoxia treatment (Fig. 5b and 5e). Using BMDM, we performed cytokine arrays (with and without IL6 treatment) to investigate whether increased cytokines in circulation in BMPR2KO mice (especially C5a, CXCL12, sICAM, and IL-16) is a result of increased secretion by monocytic lineage cells in BMPR2KO mice. At baseline, the cytokine profile for BMDM from BMPR2KO mice was similar to control BMDM except for CXCL12, which was increased in BMDM from BMPR2KO mice. IL-6 stimulation increased CXCL12 in control BMDM and IL-16 (Fig. 5c and 5f) in control and BMPR2KO BMDM. Thus, macrophages in circulation, and not derived from bone marrow, are associated with an increase in proinflammatory cytokines. This suggests that loss of BMPR2 expression may directly cause an increase in cytokine expression in macrophages in circulation or it may increase susceptibility to macrophage activation. Moreover, even with no additional stimulus, BMPR2KO in macrophages leads to an inflammatory profile in BMPR2KO animals comparable or a little worse than the profile in control mice that have received sugen/hypoxia. Thus, even unstimulated, interaction with some other cell type is sufficient to drive an inflammatory phenotype in BMPR2KO macrophages, but not in control macrophages.
Fig. 5.
Vasular and hypoxic injury increases proinflammatory cytokines in circulation and lung in monocytic lineage-specific BMPR2 KO mice: (a–c) Dot blot depicting cytokine profile in circulation, lung and bone marrow-derived macrophages (BMDM). (d–f) Bar graph representing fold increase in cytokines C5a, CXCL12, sICAM, and IL-16 in circulation, lung, and BMDM. Control mice are LysM Cre mice and BMPR2KO mice are LysM Cre BMPR2-KO mice. *P < 0.01 BMPR2KO vs. controls, #P < 0.01 controls at baseline vs. sugen/hypoxia, ^P < 0.01 BMPR2KO at baseline vs. sugen/hypoxia, $P < 0.01 control at baseline vs. with IL6 treatment, and @P < 0.01 BMPR2KO at baseline vs. with IL6 treatment.
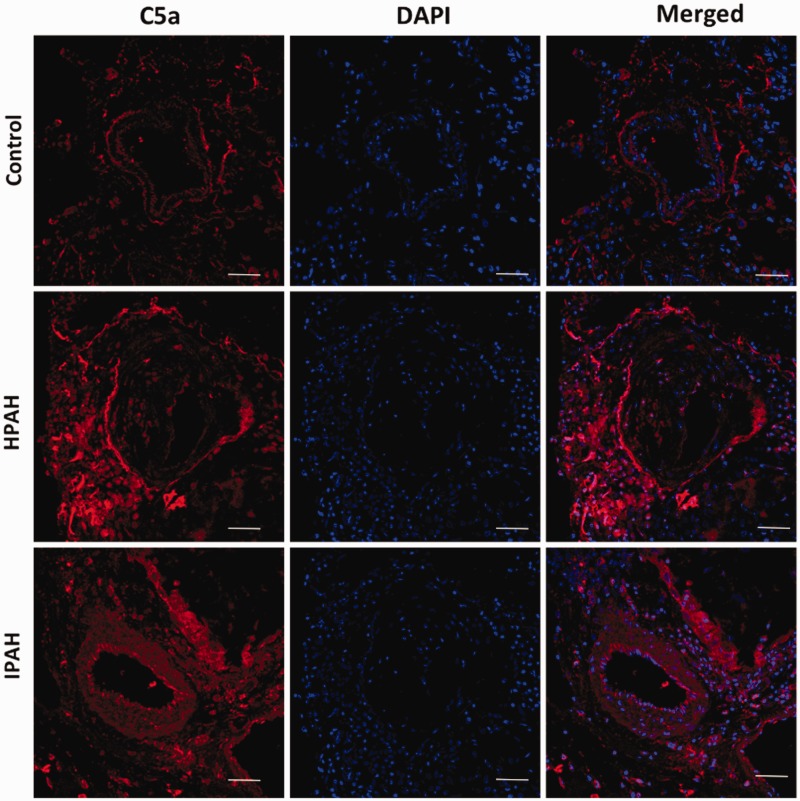
In PAH, C5a is increased in cells within pulmonary vessels and inflammatory cells surrounding these vessels
C5a, complement component 5a, is an anaphylatoxin that can recruit inflammatory cells, increase vascular permeability,59–63 and aggravate microvascular injury.64 Genetic mutations can also lead to excessive complement activation and tissue injury involving vascular cells.65–68 Complement components have been identified in PAH patients.69,70 In our previous publication, we have shown spontaneous doubling of lung interstitial macrophages10 in mutant Bmpr2delx4 mice suggesting increased permeability of the endothelium to circulating cells; potentially, it could be a functional consequence of C5a. We determined the localization of C5a in the lung vasculature of PAH patients. In the HPAH and idiopathic PAH lung, unlike the control lung, C5a immunolocalization was seen in inflammatory cells surrounding the remodeled pulmonary vessels as well as cells within these vessels (Fig. 6). Thus, an increase in C5a protein in the lung may be associated with increase production and secretion of this anaphylatoxin in the lung and in circulation, which may have a role to play in pulmonary vessel remodeling.
Fig. 6.
In PAH, C5a is increased in cells within pulmonary vessels and inflammatory cells surrounding these vessels: immunofluorescence staining for C5a (red color) is seen in inflammatory cells surrounding the pulmonary vessels and in cells of pulmonary vessels. Nucleus is stained blue (magnification 400×, scale bar 50 uM).
Discussion
In the present study, we demonstrate that, in mice universally overexpressing patient-derived BMPR2 mutation,10 elimination of macrophages by clodronate (Fig. 4) normalizes RVSP (Fig. 1) and reduces muscularized pulmonary vessels (Fig. 3) in these animals. In contrast, in our newly created monocytic lineage-specific BMPR2 knockout mouse model of PH, we demonstrate a significant increase in RVSP with sugen/hypoxia treatment (Fig. 2) and increased muscularization of pulmonary vessels (Fig. 3) associated with a significant increase in the number of macrophages surrounding these pulmonary vessels (Fig. 4). Further, BMPR2 knockdown in monocytic lineage cells increased proinflammatory molecules in circulation and, to a lesser extent, in the lung but did not directly lead to a striking increase in expression of these molecules in macrophages (Fig. 5), suggesting that BMPR2 suppression in macrophages may precondition these cells to modulate the pulmonary vasculature (Fig. 6) and influence the release and accumulation of proinflammatory molecules in the development of BMPR2-related PAH. Thus, our findings indicate that macrophages with dysfunctional BMPR2 are important mediators in the clinical manifestation of PAH.
Macrophages are a prominent component of the inflammatory infiltrates in experimental and clinical PAH9,35,71–74 and are thought to play a very important role in modulating the disease.9,74 Macrophage depletion or inactivation is shown to prevent experimentally induced hypoxic PH and portopulmonary hypertension.14,73 Our mouse model of mild PH with universal Bmpr2delx4+ mutation, dominated by inflammatory phenotype in pulmonary vasculature and appeared to be driven by a primary defect in tissue macrophages,10 indicates normalization of RVSP as a result of depletion of macrophages. In contrast, in our newly created mouse model of PAH, deletion of BMPR2 gene in monocytic lineage cells results in worsening of the phenotypic outcome with a second hit (hypoxic and vascular injury). These two experimental mouse models of PH provide direct evidence indicating that macrophages can play a very important role in modulating the progression of disease in PAH.
We and others have shown that in PAH, inflammatory cells play a pathological role in pulmonary vascular remodeling, as indicated by increased muscularization of pulmonary vessels, which leads to progression of the disease.9,13,14,75,76 Both the mouse models used in our studies corroborate with these previous findings and suggest an important role of macrophages in pulmonary vessel remodeling. In PAH, monocytes/macrophages present in pulmonary vascular lesions and surrounding the remodeled pulmonary vessels are significantly increased.74,77 Our lineage-tracing studies in the monocyte-specific BMPR2-KO mouse indicate that dysfunctional BMPR2 in macrophages accelerate the migration of these cells to pulmonary vessels following injury, which may influence pulmonary vessel remodeling; elimination of these cells by clodronate is shown to reverse this pulmonary vessel remodeling. Further, circulating and bone marrow-derived myeloid cells do not appear to directly influence macrophage infiltration and pulmonary vessel remodeling. Thus, taken together, these results indicate that in animal models of PH, suppression of BMPR2 in macrophages may be involved in infiltration of macrophages in pulmonary vasculature, which can potentially facilitate pulmonary vessel remodeling.
In human and animal models of PAH, inflammatory cytokines released by various pulmonary cell types (endothelial, smooth muscle, fibroblast, and inflammatory cells) can influence the migration of inflammatory cells including macrophages to the lung.9,13 In monocytic lineage-specific BMPR2KO mice, C5a, sICAM, IL-16, and CXCL12 (proinflammatory molecules) released in circulation and the lung, can therefore potentially affect macrophage infiltration in pulmonary vasculature. C5a is shown to increase vascular leak,59 smooth muscle proliferation,78 and induce autocrine signaling79 by binding to the C5aR on VSMCs80 and thus can potentially influence pulmonary vessels remodeling. C5a levels are significantly upregulated in COPD individuals81 and C3 and C4a, other components of the complementary cascade, have been implicated as biomarkers in patients with suppressed BMPR2.69,70 Similarly, CXCL12 is increased in PAH patients82 and proposed as a biomarker of heart failure.83 It is highly expressed at sites where macrophages are present in either physiologic or pathologic situations84 and plays a critical role in monocyte extravasation84 and chemotaxis.85 Serum sICAM is elevated in children with congenital heart disease86 and in PH associated with sickle cell anemia;87 it is shown to activate alveolar macrophages causing intensified lung injury.88 However, the role of IL-16 in PAH is conflicting.89,90 Our results suggest that, in PAH, proinflammatory molecules, C5a and CXCL12, hold a promising role in monocyte/macrophage infiltration and pulmonary vessel remodeling, which needs to be explored further.
Our study does have some limitations. First, in PAH, it is well described that the perivascular inflammatory cell infiltrates in the lung are composed of various cell types, which include, but are not limited to, macrophages, T cells, B cells, dendritic cells, and mast cells.9,13 This study does not demonstrate the crosstalk between macrophages and other cells types in pulmonary vascular bed, an essential piece of the aberrant pro-inflammatory signaling which can be driven by lost BMPR2 signaling in macrophages. It is quite possible that these cells may potentially play a role in macrophage recruitment and remodeling as a result of increased proinflammatory cytokine production in monocytic lineage specific BMPR2KO mice, which is an important area for future studies. Second, this study is limited to demonstrating the increase in proinflammatory cytokine production but is unable to demonstrate to the cell type involved in this increase. Finally, an in-depth cell culture studies using specific cell types to understand the functional role of macrophages with dysfunctional BMPR2 and proinflammatory cytokines in pulmonary vessel remodeling is warranted.
In conclusion, in animal models of PH, suppression of BMPR2 in macrophages can exacerbate phenotypic outcome, potentially by influencing pulmonary vessel remodeling via proinflammatory cytokine production as a result of injury. A deeper understanding of the complex mechanisms of pulmonary vasculature remodeling by macrophages with dysfunctional BMPR2 will aid in the development of targeted treatment in PAH.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research was funded by P01 HL 108800-01A1, R01 HL095797; 1R01 HL136748.
References
- 1.Austin ED, Loyd JE. The genetics of pulmonary arterial hypertension. Circ Res 2014; 115: 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin ED, West J, Loyd JE, et al. Molecular medicine of pulmonary arterial hypertension: from population genetics to precision medicine and gene editing. Am J Respir Crit Care Med 2017; 195: 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Jesus Perez VA. Molecular pathogenesis and current pathology of pulmonary hypertension. Heart Fail Rev 2016; 21: 239–257. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation 2010; 122: 156–163. [DOI] [PubMed] [Google Scholar]
- 5.Rubin LJ. Pulmonary arterial hypertension. Proc Am Thorac Soc 2006; 3: 111–115. [DOI] [PubMed] [Google Scholar]
- 6.Runo JR, Loyd JE. Primary pulmonary hypertension. Lancet 2003; 361: 1533–1544. [DOI] [PubMed] [Google Scholar]
- 7.Dorfmuller P, Perros F, Balabanian K, et al. Inflammation in pulmonary arterial hypertension. Eur Respir J 2003; 22: 358–363. [DOI] [PubMed] [Google Scholar]
- 8.Nicolls MR, Taraseviciene-Stewart L, Rai PR, et al. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J 2005; 26: 1110–1118. [DOI] [PubMed] [Google Scholar]
- 9.Savai R, Pullamsetti SS, Kolbe J, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 897–908. [DOI] [PubMed] [Google Scholar]
- 10.Talati M, West J, Zaynagetdinov R, et al. BMP pathway regulation of and by macrophages. PLoS One 2014; 9: e94119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voelkel NF, Tamosiuniene R, Nicolls MR. Challenges and opportunities in treating inflammation associated with pulmonary hypertension. Expert Rev Cardiovasc Ther 2016; 14: 939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schafer M, Myers C, Brown RD, et al. Pulmonary arterial stiffness: toward a new paradigm in pulmonary arterial hypertension pathophysiology and assessment. Curr Hypertens Rep 2016; 18: 4. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian W, Jiang X, Tamosiuniene R, et al. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med 2013; 5: 200ra117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueira-Ferreira R, Vitorino R, Ferreira R, et al. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach. Pulm Pharmacol Ther 2015; 35: 8–16. [DOI] [PubMed] [Google Scholar]
- 16.de Raaf MA, Schalij I, Gomez-Arroyo J, et al. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respir J 2014; 44: 160–168. [DOI] [PubMed] [Google Scholar]
- 17.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 2011; 109: 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laskin DL, Sunil VR, Gardner CR, et al. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol 2011; 51: 267–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016; 138: 16–27. [DOI] [PubMed] [Google Scholar]
- 20.Cao Z, Lis R, Ginsberg M, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med 2016; 22: 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gill SE, Yamashita CM, Veldhuizen RA. Lung remodeling associated with recovery from acute lung injury. Cell Tissue Res 2017; 367: 495–509. [DOI] [PubMed] [Google Scholar]
- 22.Han X, Wang Y, Chen H, et al. Enhancement of ICAM-1 via the JAK2/STAT3 signaling pathway in a rat model of severe acute pancreatitis-associated lung injury. Exp Ther Med 2016; 11: 788–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11: 889–896. [DOI] [PubMed] [Google Scholar]
- 24.Chen NY, S DC, Luo F, et al. Macrophage bone morphogenic protein receptor 2 depletion in idiopathic pulmonary fibrosis and Group III pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2016; 311: L238–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee GT, Kwon SJ, Lee JH, et al. Induction of interleukin-6 expression by bone morphogenetic protein-6 in macrophages requires both SMAD and p38 signaling pathways. J Biol Chem 2010; 285: 39401–39408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moral-Sanz J, Lopez-Lopez JG, Menendez C, et al. Different patterns of pulmonary vascular disease induced by type 1 diabetes and moderate hypoxia in rats. Exp Physiol 2012; 97: 676–686. [DOI] [PubMed] [Google Scholar]
- 27.Rocher C, Singla DK. SMAD-PI3K-Akt-mTOR pathway mediates BMP-7 polarization of monocytes into M2 macrophages. PLoS One 2013; 8: e84009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simoes Sato AY, Bub GL, Campos AH. BMP-2 and -4 produced by vascular smooth muscle cells from atherosclerotic lesions induce monocyte chemotaxis through direct BMPRII activation. Atherosclerosis 2014; 235: 45–55. [DOI] [PubMed] [Google Scholar]
- 29.Singla DK, Singla R, Wang J. BMP-7 Treatment increases M2 macrophage differentiation and reduces inflammation and plaque formation in Apo E-/- mice. PLoS One 2016; 11: e0147897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dorfmuller P, Zarka V, Durand-Gasselin I, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 534–539. [DOI] [PubMed] [Google Scholar]
- 31.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 32.Itoh T, Nagaya N, Ishibashi-Ueda H, et al. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology 2006; 11: 158–163. [DOI] [PubMed] [Google Scholar]
- 33.Price LC, Wort SJ, Perros F, et al. Inflammation in pulmonary arterial hypertension. Chest 2012; 141: 210–221. [DOI] [PubMed] [Google Scholar]
- 34.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 35.Talati M, Mutlak H, Lane KB, et al. NF-kB activation exacerbates, but is not required for murine Bmpr2-related pulmonary hypertension. Diseases 2014; 2: 148–167. [Google Scholar]
- 36.Vergadi E, Chang MS, Lee C, et al. Early macrophage recruitment and alternative activation are critical for the later development of hypoxia-induced pulmonary hypertension. Circulation 2011; 123: 1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawada H, Saito T, Nickel NP, et al. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J Exp Med 2014; 211: 263–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song Y, Jones JE, Beppu H, et al. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation 2005; 112: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong KH, Lee YJ, Lee E, et al. Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 2008; 118: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagen M, Fagan K, Steudel W, et al. Interaction of interleukin-6 and the BMP pathway in pulmonary smooth muscle. Am J Physiol Lung Cell Mol Physiol 2007; 292: L1473–1479. [DOI] [PubMed] [Google Scholar]
- 41.Girerd B, Montani D, Eyries M, et al. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res 2010; 11: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stacher E, Graham BB, Hunt JM, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson JA, Hemnes AR, Perrien DS, et al. Cytoskeletal defects in Bmpr2-associated pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2012; 302: L474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.West J, Fagan K, Steudel W, et al. Pulmonary hypertension in transgenic mice expressing a dominant-negative BMPRII gene in smooth muscle. Circ Res 2004; 94: 1109–1114. [DOI] [PubMed] [Google Scholar]
- 45.Everhart MB, Han W, Parman KS, et al. Intratracheal administration of liposomal clodronate accelerates alveolar macrophage reconstitution following fetal liver transplantation. J Leukoc Biol 2005; 77: 173–180. [DOI] [PubMed] [Google Scholar]
- 46.Zaynagetdinov R, Sherrill TP, Polosukhin VV, et al. A critical role for macrophages in promotion of urethane-induced lung carcinogenesis. J Immunol 2011; 187: 5703–5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferenbach DA, Sheldrake TA, Dhaliwal K, et al. Macrophage/monocyte depletion by clodronate, but not diphtheria toxin, improves renal ischemia/reperfusion injury in mice. Kidney Int 2012; 82: 928–933. [DOI] [PubMed] [Google Scholar]
- 48.Vitali SH, Hansmann G, Rose C, et al. The Sugen 5416/hypoxia mouse model of pulmonary hypertension revisited: long-term follow-up. Pulm Circ 2014; 4: 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West J, Harral J, Lane K, et al. Mice expressing BMPR2R899X transgene in smooth muscle develop pulmonary vascular lesions. Am J Physiol Lung Cell Mol Physiol 2008; 295: L744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mather K. Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab 2009; 296: E398–399. [DOI] [PubMed] [Google Scholar]
- 51.Yan L, Chen X, Talati M, et al. Bone marrow-derived cells contribute to the pathogenesis of pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 193: 898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan L, Womack B, Wotton D, et al. Tgif1 regulates quiescence and self-renewal of hematopoietic stem cells. Mol Cell Biol 2013; 33: 4824–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woolcott OO, Ader M, Bergman RN. Glucose homeostasis during short-term and prolonged exposure to high altitudes. Endocr Rev 2015; 36: 149–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen XQ, Dong J, Niu CY, et al. Effects of hypoxia on glucose, insulin, glucagon, and modulation by corticotropin-releasing factor receptor type 1 in the rat. Endocrinology 2007; 148: 3271–3278. [DOI] [PubMed] [Google Scholar]
- 55.Zaynagetdinov R, Sherrill TP, Kendall PL, et al. Identification of myeloid cell subsets in murine lungs using flow cytometry. Am J Respir Cell Mol Biol 2013; 49: 180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010; 40: 2969–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balabanian K, Foussat A, Dorfmuller P, et al. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med 2002; 165: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 58.Bjork J, Hugli TE, Smedegard G. Microvascular effects of anaphylatoxins C3a and C5a. J Immunol 1985; 134: 1115–1119. [PubMed] [Google Scholar]
- 59.Morel DR, Zapol WM, Thomas SJ, et al. C5a and thromboxane generation associated with pulmonary vaso- and broncho-constriction during protamine reversal of heparin. Anesthesiology 1987; 66: 597–604. [DOI] [PubMed] [Google Scholar]
- 60.Morganroth ML, Schoeneich SO, Till GO, et al. C3a57-77, a C-terminal peptide, causes thromboxane-dependent pulmonary vascular constriction in isolated perfused rat lungs. Am Rev Respir Dis 1990; 141: 296–300. [DOI] [PubMed] [Google Scholar]
- 61.Bauer EM, Zheng H, Comhair S, et al. Complement C3 deficiency attenuates chronic hypoxia-induced pulmonary hypertension in mice. PLoS One 2011; 6: e28578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller-Redetzky HC, Suttorp N, Witzenrath M. Dynamics of pulmonary endothelial barrier function in acute inflammation: mechanisms and therapeutic perspectives. Cell Tissue Res 2014; 355: 657–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan MA, Maasch C, Vater A, et al. Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc Natl Acad Sci U S A 2013; 110: 6061–6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walport MJ. Complement. Second of two parts. N Engl J Med 2001; 344: 1140–1144. [DOI] [PubMed] [Google Scholar]
- 65.Walport MJ. Complement. First of two parts. N Engl J Med 2001; 344: 1058–1066. [DOI] [PubMed] [Google Scholar]
- 66.Engstrom G, Hedblad B, Berglund G, et al. Plasma levels of complement C3 is associated with development of hypertension: a longitudinal cohort study. J Hum Hypertens 2007; 21: 276–282. [DOI] [PubMed] [Google Scholar]
- 67.Gan W, Wu J, Lu L, et al. Associations of CFH polymorphisms and CFHR1-CFHR3 deletion with blood pressure and hypertension in Chinese population. PLoS One 2012; 7: e42010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdul-Salam VB, Paul GA, Ali JO, et al. Identification of plasma protein biomarkers associated with idiopathic pulmonary arterial hypertension. Proteomics 2006; 6: 2286–2294. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, Zhang Y, Li N, et al. Potential diagnostic biomarkers in serum of idiopathic pulmonary arterial hypertension. Respir Med 2009; 103: 1801–1806. [DOI] [PubMed] [Google Scholar]
- 70.Frid MG, Brunetti JA, Burke DL, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol 2006; 168: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Overbeek MJ, Mouchaers KT, Niessen HM, et al. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. Int J Rheumatol 2010; 2010: 604615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thenappan T, Goel A, Marsboom G, et al. A central role for CD68(+) macrophages in hepatopulmonary syndrome. Reversal by macrophage depletion. Am J Respir Crit Care Med 2011; 183: 1080–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuder RM, Groves B, Badesch DB, et al. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994; 144: 275–285. [PMC free article] [PubMed] [Google Scholar]
- 74.Otsuki S, Sawada H, Yodoya N, et al. Potential contribution of phenotypically modulated smooth muscle cells and related inflammation in the development of experimental obstructive pulmonary vasculopathy in rats. PLoS One 2015; 10: e0118655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Majka S, Hagen M, Blackwell T, et al. Physiologic and molecular consequences of endothelial Bmpr2 mutation. Respir Res 2011; 12: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perros F, Dorfmuller P, Souza R, et al. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J 2007; 29: 462–468. [DOI] [PubMed] [Google Scholar]
- 77.Zhao Y, Xu H, Yu W, et al. Complement anaphylatoxin C4a inhibits C5a-induced neointima formation following arterial injury. Mol Med Rep 2014; 10: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shagdarsuren E, Bidzhekov K, Mause SF, et al. C5a receptor targeting in neointima formation after arterial injury in atherosclerosis-prone mice. Circulation 2010; 122: 1026–1036. [DOI] [PubMed] [Google Scholar]
- 79.Weaver DJ, Jr, Reis ES, Pandey MK, et al. C5a receptor-deficient dendritic cells promote induction of Treg and Th17 cells. Eur J Immunol 2010; 40: 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marc MM, Korosec P, Kosnik M, et al. Complement factors c3a, c4a, and c5a in chronic obstructive pulmonary disease and asthma. Am J Respir Cell Mol Biol 2004; 31: 216–219. [DOI] [PubMed] [Google Scholar]
- 81.Costello CM, McCullagh B, Howell K, et al. A role for the CXCL12 receptor, CXCR7, in the pathogenesis of human pulmonary vascular disease. Eur Respir J 2012; 39: 1415–1424. [DOI] [PubMed] [Google Scholar]
- 82.Subramanian S, Liu C, Aviv A, et al. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arterioscler Thromb Vasc Biol 2014; 34: 2100–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petit I, Jin D, Rafii S. The SDF-1-CXCR4 signaling pathway: a molecular hub modulating neo-angiogenesis. Trends Immunol 2007; 28: 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanchez-Martin L, Estecha A, Samaniego R, et al. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood 2011; 117: 88–97. [DOI] [PubMed] [Google Scholar]
- 85.Sungprem K, Khongphatthanayothin A, Kiettisanpipop P, et al. Serum level of soluble intercellular adhesion molecule-1 correlates with pulmonary arterial pressure in children with congenital heart disease. Pediatr Cardiol 2009; 30: 472–476. [DOI] [PubMed] [Google Scholar]
- 86.Kato GJ, Martyr S, Blackwelder WC, et al. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol 2005; 130: 943–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmal H, Czermak BJ, Lentsch AB, et al. Soluble ICAM-1 activates lung macrophages and enhances lung injury. J Immunol 1998; 161: 3685–3693. [PubMed] [Google Scholar]
- 88.Bull TM, Coldren CD, Moore M, et al. Gene microarray analysis of peripheral blood cells in pulmonary arterial hypertension. Am J Respir Crit Care Med 2004; 170: 911–919. [DOI] [PubMed] [Google Scholar]