Abstract
Background:
Circulating tumor cells (CTCs) have recently emerged as a new dynamic soluble marker for several malignancies including cutaneous melanoma (CM) and are suitable for prognostic evaluations and treatment monitoring. However, to date many limitations still hamper the wide-scale application of CTCs in CM setting, including the lack of standardized methods as well as both low levels and heterogeneity of these cells.
Methods:
We developed a protocol for CTC detection in CM based on immune-magnetic sorting to deplete CD45-, CD31- or CD34-positive cells, followed by dielectrophoretic DEPArray separation according to cell morphology and immunophenotype. To this end, we explored the expression of melanoma stem cell antigens (CD271, ABCB5, and RANK) and the epithelial-to-mesenchymal transition markers (N-Cad, -CD44, and -MCAM/CD146) on CTCs from 17 stage IV CM patients, and investigated their BRAF mutational status by droplet digital PCR.
Results:
The number of CTCs isolated from CM patients ranged from 2 to 91 cells (38 ± 6.4) with respect to healthy donors (p < 0.0002). To confirm the melanoma origin of isolated cells, we observed an 80% agreement between their BRAFV600 mutational status and matched primary tumors. The characterization of the immune phenotype of isolated cells revealed high interindividual and intraindividual heterogeneity that was found to correlate with the clinical outcome.
Conclusions:
The dual-step protocol of immune-magnetic sorting and subsequent dielectrophoretic DEPArray separation, turned out to be a suitable method to isolate viable CTCs from stage IV melanoma patients and enabled quantitative and qualitative analyses on these cells, which may deserve prospective evaluation for potential use in the clinical practice.
Keywords: Circulating tumor cells, EMT, liquid biopsy, melanoma, melanoma stem cells
Introduction
The incidence of cutaneous melanoma (CM) has increased worldwide and a defined improvement of survival has been recently achieved by new treatments primarily including targeted agents and immunotherapy.1,2 The prognosis of CM mostly depends on the primary cancer histopathology features while circulating tumor cells (CTCs) are the underpinning resemblance for metastatic dissemination.3,4 On the other hand, based on the tumor spreading risk in performing biopsies of metastatic sites, CTCs are currently considered a real surrogate of tumors. Nevertheless, molecular data from the excised primary CM are perhaps inadequate to reflect the evolution of the genomic landscape particularly in the subclinical progressing disease that evolves with high intratumor heterogeneity.5 To this regard, CTCs appear highly feasible for genomic and transcriptomic profiles of tumors as CM thus providing insights to the metastatic machinery as well as for monitoring the disease progression in relation to the amounts of CTCs, or for revealing the minimal residual disease by the MCAM/CD146 levels expression.6 Furthermore, CTCs have been proposed for the early identification of acquired mutations of resistance to new therapies.7,8 Other than CTCs, tumor cell-free (ct)-DNA, microRNAs, and exosomes are actually in intensive investigation in CM for prognostic use and selection of responders to antiprogrammed death (PD)-1 monoclonal antibody (MoAb) treatments.4,9,10
Previous work in breast, colon, prostate and lung cancers has recently proved that CTC levels correlate with treatment outcomes, and their sorting by CellSearch has been previously standardized and translated to clinical use as CTC counts for prognostic purposes.11 Similarly, the number of CTCs in melanoma patients has been correlated with prognosis, and suggests the complete lymph node dissection (CLND) in those with positive sentinel lymph nodes.3,7 However, melanoma cells express specific melanoma-associated antigens (MAAs) and are extremely heterogeneous in their genomic and transcriptome aberrations. Thus, it is fundamental to detect multi-MAAs on CTCs to improve their sensitivity and specificity in clinical use though the efficiency of their characterization is frequently denied by both the low number of recoverable cells, and their extended genomic abnormalities.
To optimize the recruitment of CM CTCs, original attempts were based on their capture by detecting specific MAAs such as Melan-A, MART-1, or MAGE-3, while subsequent work focused on the expression of MCAM/CD146 that is up-regulated during the epithelial-to-mesenchymal transition (EMT) process in the metastatic phase of melanoma. Recent reports, however, showed that other techniques including micro manufactured filters,12,13 CTC-iChip,14 Cluster-Chip,15 and the slanted spiral microfluidic device16 have been fruitfully adopted to isolate CTCs in CM. In addition, several CTC subsets displaying tumor-initiating or stemness properties are marked by Nestin, ABCB5 (ATP-binding cassette sub-family B member 5), CD133, CD271, and RANK,17–21 and their circulating levels have been variably correlated with the disease progression.
Here, we describe a novel method to isolate in a highly specific fashion CTCs from patients with metastatic CM. In addition, by detecting typical melanoma markers, we adopted the DEPArray technology and recorded an apparent linearity of CTC molecular features with the primary tumors as well as a correlation of peripheral counts with clinical outcome.
Methods
Study population
Peripheral blood from 17 hospitalized or out-clinic patients in treatment at the Medical Oncology Unit of the University of Bari Aldo Moro with stage IV CM, and 5 healthy donors were collected after written informed consent (Table 1). The study was carried out in accordance with the Declaration of Helsinki and the protocol was approved by the Independent Ethics Committee (Identification code ID: 44100). Histopathological and clinical data were collected and reported in Supplementary Table 1 (S1). The eligibility criteria for patient recruitment were (a) age ⩾18 years and (b) diagnosis of metastatic CM and known BRAFV600 mutational status of the primary tumor. Enrolled patients were either systemic-treatment naïve or with radiological evidence of disease progression during systemic therapy for metastatic disease; the latter patients were enrolled at least 21 days after the last cycle of treatment. Exclusion criteria included a personal history of other malignancies and patients bearing NRAS or rare BRAFV600 (K, N, R) mutations.
Table 1.
Demographics and major clinical features of enrolled patients.
| Patient ID | Sex | Age at recruitment | BRAF status | Sites of distant metastases | Number of metastatic sites (<3; ⩾3) |
Previous systemic treatment | Number of total CTCs | Number of PE+ CTCs | PFS (months) |
OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 231 | M | 43 | V600E | bone, lung, liver, lymph nodes, subcutaneous tissue | ⩾3 | dabrafenib + trametinib | 74 | 56 | 0.3 | 0.3 |
| 292 | F | 55 | wild type | brain, lung, liver, lymph nodes, subcutaneous tissue | ⩾3 | none | 74 | 27 | 0.4 | 0.4 |
| 297 | F | 60 | V600E | bone, lung, lymph nodes, subcutaneous tissue | ⩾3 | none | 3 | 2 | 9.8 | 16.3 |
| 315 | M | 48 | wild type | bone, lung, lymph nodes, muscle | ⩾3 | none | 91 | 3 | 15.7 | 15.7 |
| 349 | M | 53 | wild type | brain, lung, lymph nodes, muscle | ⩾3 | nivolumab | 72 | 64 | 3.3 | 4.1 |
| 352 | M | 58 | V600E | brain, liver, bone, lymph nodes, parotid gland | ⩾3 | dabrafenib + trametinib | 75 | 59 | 7 | 8.2 |
| 369 | M | 77 | V600K | brain, liver, bone, lung | ⩾3 | dabrafenib + trametinib | 45 | 41 | 1.1 | 1.1 |
| 373 | F | 69 | V600E | liver, lymph nodes | <3 | none | 10 | 5 | 10.9 | 10.9 |
| 376 | F | 68 | wild type | brain, liver, bone, lung | ⩾3 | none | 47 | 42 | 0.5 | 0.5 |
| 390 | M | 64 | V600K | lung, lymph nodes | <3 | none | 13 | 4 | 11.4 | 11.4 |
| 393 | F | 80 | V600E | brain | <3 | none | 3 | 2 | 1 | 2.6 |
| 416 | M | 63 | V600E | subcutaneous tissue | <3 | none | 60 | 34 | 1.1 | 4.2 |
| 417 | M | 50 | wild type | brain, bone, lung, lymph nodes, subcutaneous tissue | ⩾3 | none | 50 | 32 | 9.5 | 9.5 |
| 419 | M | 60 | wild type | bone, liver, lymph nodes, adrenal glands |
⩾3 | none | 18 | 13 | 2.3 | 14.9 |
| 484 | M | 76 | wild type | subcutaneous tissue | <3 | none | 11 | 10 | 1.7 | 1.7 |
| 521 | F | 38 | V600E | brain, bone, lung, subcutaneous tissue, muscle, ovary | ⩾3 | none | 2 | 2 | 2.6 | 2.6 |
| 526 | F | 62 | V600E | lymph nodes, subcutaneous tissue | <3 | none | 13 | 13 | 2.6 | 2.6 |
CTC, circulating tumor cells; OS, overall survival; PFS, progression free survival.
Melanoma cell phenotyping
The specific melanoma antigenic profile was evaluated by flow cytometry in several melanoma cell lines, namely LCP, LCM, M-397, and WM115,6 and SKMEL2 (American Type Culture Collection, USA). The cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin-glutamine (Thermo Fisher Scientific). After gentle cell detachment from culture flasks, the expression of membrane antigens was explored by incubating 1 × 105 cells with 5 μl fluorescein isothiocyanate (FITC)-conjugated anti-CD271 (Bio-Legend, CA), anti-ABCB5 (Novus Biologicals, MN), and anti-RANK (Novus Biological) MoAbs, as well with phycoerythrin (PE)-conjugated anti-MCAM/CD146 (Becton-Dickinson), anti-N-cadherin (N-Cad; Invitrogen, CA), and anti-CD44 (Bio-Legend) MoAbs. After 30 min of incubation at 4°C, cells were analyzed for their antigenic profile by BD Accuri™ C6-plus (Becton Dickinson, CA). Each experiment was performed in triplicate by determining the positivity with respect to the relative isotype controls.
Melanoma cell line spiking and CTC isolation
A preliminary phase required the instrument set-up that was completed using in vitro established cell lines. For this purpose, both LCM and M-397 melanoma cells were used in relation to both expression of MAAs and assessment of specificity and efficiency of the DEPArray-based isolation.22 Briefly, 10, 50, and 100 viable melanoma cells were spiked into 10 ml of peripheral blood from healthy donors and separated by Ficoll density gradient. Then, blood mononuclear cells were depleted by negative immune-magnetic sorting23,24 with microbeads conjugated with both anti-CD45 and antiglycophorin (AutoMACS®, Miltenyi, Germany). The next set of experiments was dedicated to select melanoma cells by DEPArray technology (Menarini Silicon Biosystem, Italy) after their incubation with fluorochrome-conjugated MoAbs. For this purpose, two cocktails of MoAbs were used, respectively FITC- (anti-CD271, -ABCB5, and -RANK) and PE-conjugated (anti-N-Cad, -CD44, and -MCAM/CD146) while both contaminating mononuclear cells and endothelial cells were excluded by a cocktail of APC-conjugated MoAbs (anti-CD45, -CD3,1 and -CD34; Invitrogen). In particular, 5 µl of each fluorescent antibody were added to the enriched samples, followed by 30 minute-incubation at 4°C.
Nuclei were visualized by 20 µg/ml Hoechst 33342 as a cell-permeable DNA dye that served also to exclude apoptotic and dead cells. Then, specimens were analyzed by dielectrophoretic DEPArray system after sample loading into dedicated cartridges according to the manufacturer’s instructions. The cartridge loaded with cells was scanned under an automated fluorescence microscope to generate an image gallery by the Cell Browser Software favoring the selection of cells in relation to specific fluorescence pattern. Selected cells were then transferred throughout dielectrophoretic cages, recovered and collected as pool of cells. For the CTC isolation, we used 10 ml of blood from patients and healthy donors while the recovered CTCs were numbered and stored at −80°C for molecular analysis.
Molecular characterization of CTCs
The mutational status of putative CTCs was investigated by Droplet Digital polymerase chain reaction (ddPCR) by using the ddPCR Supermix for probes (no dUTP, Bio-Rad) as well as ddPCR Mutant Assay BRAFV600Eand BRAFwild type (Bio-Rad). The amplification was completed in the T100 thermal cycler (Bio-Rad) while the fluorescence intensity of individual droplets was investigated by the QX200 droplet and data analyzed by the QuantaSoft software. DNA was purified by Single Shot Cell Lysis Kit (Bio-Rad) according to the manufacturer’s instructions. Detection of BRAF V600E mutation by ddPCR was carried out by using ddPCR Supermix for Probes (no dUTP, Bio-Rad) and validated Prime PCR ddPCR Mutation Assays including BRAF MUT V600E-FAM probe (Id: dHsaCP2000027, Bio-Rad) and BRAF WT V600E-HEX probe (Id: dHsaCP2000028, Bio-Rad). ddPCR reaction consisted of 10 µl 2X ddPCR Master Mix (Bio-Rad), 1 µl lBRAF MUT V600E-FAM probe, 1 µl BRAF WT V600E-HEX probe, 5 µl sample DNA and 3 µl deionized water. This solution was converted to droplets with the QX200 droplet generator (Bio-Rad). Amplifications were performed in T100 thermal cycler (Bio-Rad) with the following cycling conditions: 1 × (95°C for 10 min), 40 × (94°C for 30 s, 55°C for 1 min), 1 ×(98°C for 10 min) with 2°C/s ramp rate. After PCR, the fluorescence intensity of individual droplets was analyzed with the QX200 droplet (Bio-Rad) and the data analysis was performed with Quanta Soft analysis software (Bio-Rad).
Statistical analysis
The Kruskal–Wallis (nonparametric) test explored the difference of total CTC levels between groups of patients stratified for clinical parameters or BRAFV600 status. The Kaplan–Meier analysis of survival was based on the number of CTCs per 10 ml of blood at baseline. For all survival analyses, the progression-free survival (PFS) was calculated as intervals between the first dose of targeted or immunotherapy after the enrolment and the date of disease progression, death, or the last follow-up visit, while overall survival (OS) was the interval between the treatment and death or the last follow-up visit. Survival curves were compared with the use of log-rank testing. To select a cut-off of CTCs that most clearly distinguished patients with different prognosis, thresholds of 2–91 total baseline CTC levels were systematically correlated with OS for the 17 patients enrolled in the study. Then, median OS among patients with levels above each threshold was calculated, while hazard ratio served to select the appropriate cut-off able to stratify patients with different prognosis, as already described.11,25 The statistical analyses were completed with the Medcalc software (version 12.7.0.0) accepting as statistically significant p < 0.05.
Results
Patients
Overall, patients were followed up for a median period of 4.8 months. The male-to-female ratio was 1.42, while median age at the time of CTC collection was 60 years. At the time of enrollment all patients had a stage IV CM, while at the time of melanoma diagnosis their clinical stage ranged from IB to IV. Most patients (8/17) had a nodular melanoma that also exhibited polypoid features in 2 out of 8 cases, whereas a superficial spreading melanoma occurred in seven patients. The BRAFV600E mutation was detected in 10 out of 17 patients. The most frequent primary tumor sites were trunk and extremities, while a single patient had an unknown primary melanoma. At least three different anatomical sites of distant metastases were described in 63% of patients. A total of 13 out of 17 patients were naïve from systemic therapies, three patients had received anti-BRAF and anti-MEK agents whereas one patient progressed from anti-PD1 immunotherapy (Tables 1 and S1).
Melanoma cell phenotype and spiking experiments
Preliminary experiments were completed in melanoma cell lines to define the expression levels of melanoma-related molecules as well as to select those useful for the CTC isolation. As shown in Figure 1, not metastatic (WM115 and LCP) and metastatic (M-397 and LCM) melanoma cells showed different levels of those antigens. Both CD44 and MCAM/CD146 were highly expressed by all populations (96.1 ± 2.3%) while the expression of the stem cell marker CD271 was high in M-397 (92% on average) and similarly lower in the other cells (21.3 ± 18.5%). Moreover, RANK was mostly detected in metastatic melanoma cells as compared with the others, and ABCB5 was undetectable in WM115 cells and expressed in 25%, 27%, and 31% of LCP, M-397, and LCM cells, respectively. Finally, melanoma cells exhibited a mesenchymal phenotype with N-Cad expression detected in more than 90% of LCM and M-397 cells, whereas lower levels were demonstrated in WM115 and LCP (59% and 63%). Based on the prevalent expression of these markers by LCP and M-397 cells, they were selected for next experiments.
Figure 1.

Phenotypic characterization of melanoma cell lines. Metastatic (M-397 and LCM) and not-metastatic (WM115 and LCP) cell lines were analyzed for the expression of melanoma associated antigens. The panel shows the high expression of both MCAM/CD146 and CD44 (>95%) by most cells. The marker of stemness CD271 was over-expressed in M-398 (on average 92%) and moderately present in the other cell lines, whereas highest levels of RANK occurred in metastatic cells (>95%) in a fashion almost similar to N-Cad. In addition, the ABCB5 antigen had low expression except for WM115, which was negative. Histograms and standard deviations were calculated from biological triplicates.
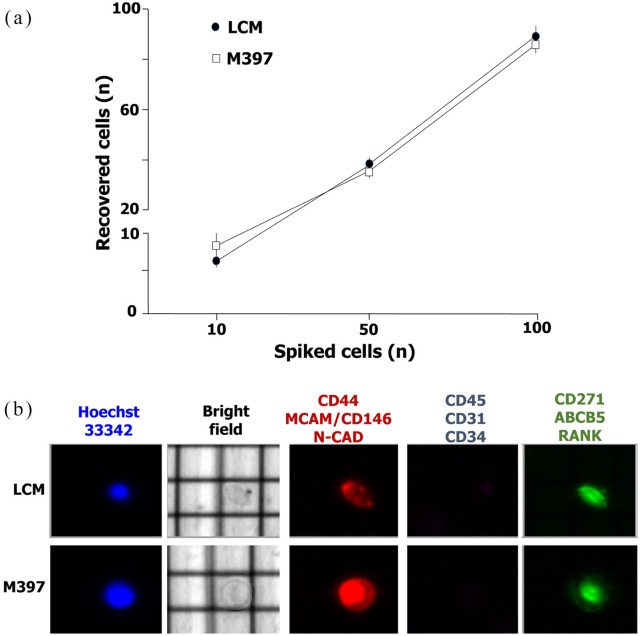
The evaluation of the DEPArray performance in capturing rare CTCs permitted to detect at least 79 ± 7% of spiked M-397 and 81 ± 5% of LCM (Figure 2) and the recovery rate was independent of the number of spiked cells. The viability of spiked melanoma cells from lines was evaluated by nuclear staining with Hoechst in parallel with the previously mentioned surface antigens (Figure 2b), whereas Hoechst+ cells expressing at least one of them among CD45, CD34 and CD31, were defined as peripheral mononuclear cells. As shown, the phenotypic features of melanoma cells allowed their efficient capture by combining MoAbs specific26 for mesenchymal (CD44, MCAM/CD146 and N-Cad) or melanoma stem cell antigens (RANK, ABCB5 and CD271) that resulted in them being over-expressed.
Figure 2.
Detection of melanoma cell lines by DEPArray technology. Based on the melanoma associated antigen profile, both LCM and M-397 were selected for the spiking experiments required for the evaluation of performance in circulating tumor cell detection. As shown in (a), we detected 79 ± 7% of spiked (n = 10, 50,100) M-397 (empty box) and 81 ± 5% of LCM (black box). After capturing by DEPArray, melanoma cells were characterized (b) in relation to their size as well as nucleus/cytoplasm ratio and nuclear integrity by Hoechst-33342 staining. Representative cell lines were negative for CD45, CD31 and CD34 expression. By contrast, both cell lines were stained by a cocktail of PE-conjugated (anti-CD44, -MCAM/CD146 and -N-CAD) or FITC-conjugated (anti-CD271, -ABCB5 and -RANK) monoclonal antibodies.
Melanoma CTC recovery by DEPArray technology
After capturing by DEPArray, melanoma cells were further identified in relation to their morphological features such as size, high nucleus/cytoplasm ratio, nuclear integrity, and negative expression of CD45, CD34 and CD31. Most isolated CTCs showed a variable size (diameter range: 8.6–13.3 µm) that was, however, smaller with respect to spiked melanoma cell lines (diameter range: 11.1–17.4 µm). As shown in Figure 3, the number of CTCs purified from melanoma patients ranged from 2 to 91 cells (mean 38 ± 6.4), thus being significantly higher as compared with healthy donors whose circulating cells were isolated with the same panel of antigens. In fact, the range was up to one single cell (mean 0.62 ± 0.32; p < 0.0002.
Figure 3.
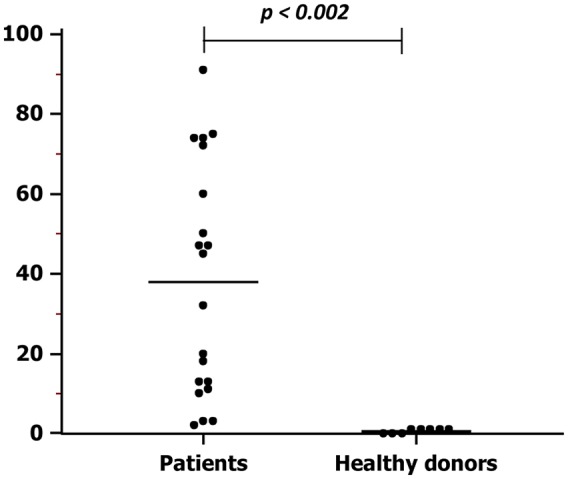
Numbers of circulating tumor cells in melanoma patients and healthy donors. The average number of CTCs isolated from patients with metastatic melanoma was higher (mean: 38.0 ± 6.4 cells; range 2–91 cells) with respect to that obtained from healthy subjects (mean: 0.62 ± 0.52 cells; range 0–1 cells) by using the same cocktail of monoclonal antibodies (p < 0.002).
Furthermore, the phenotypic characterization of CTCs showed high heterogeneity of melanoma CTCs (Figure 4a and b). In all patients we identified CTCs expressing at least one marker belonging to the CD44/MCAM-CD146/N-cad cocktail, in the absence of RANK, ABCB5 and CD271 (PE+ CTCs; mean number/ml: 2.4; range/10 ml: 1–64). In a single patient (#349) we found FITC+ CTCs with the RANK/ABCB5/CD271 positive phenotype. Moreover, in 10 out of 17 patients we identified ‘double positive’ (FITC+/PE+) CTCs, namely cells expressing at least one marker from both antibody mixtures (mean number of CTCs/ml: 1.3; range/10 ml: 1–88), whereas 11 patients harbored ‘double negative’ (FITC−/PE−) CTCs (mean number of CTCs/ml: 1; range/10 ml: 1–26).
Figure 4.
Detection of circulating tumor cells (CTCs) in melanoma patients. (a) shows representative patterns of CTCs isolated from a patient with metastatic melanoma (#349). As shown, nuclei were not damaged (Hoechst-33342 positive cells) while all isolated populations were negative for the expression of CD45, CD31 and CD34. Line 1 reveals PE+ CTCs (CD44+ or CD146+ or NCAD+), line 2 the positivity for the FITC-conjugated monoclonal antibodies (CD271 or ABCB5 or RANK), line 3 the double positivity (PE+/FITC+) and line 4 the CTCs negative for all the mentioned antigens. The bottom panel in (a) shows the negative control represented by lymphocytes detected by the expression of CD45 or CD31 or CD34. (b) shows the phenotypic profile of CTCs isolated from the 17 patients with metastatic melanoma. (c) is representative of the mutational assessment by Droplet Digital polymerase chain reaction (ddPCR) of CTCs from two patients previously investigated for the presence of BRAFV600E mutation in primary tumor. The ddPCR analysis produced the same results obtained in primary melanoma specimens.
In order to confirm the melanoma derivation of isolated CTCs, we searched the BRAFV600E mutational status that showed an 80% of concordance with respect to the primary tumors. The BRAF mutational status was confirmed in all subgroups of collected CTCs in 4 randomly selected BRAFV600E mutated patients. Moreover, in all CTC-enriched samples from BRAFwild type patients, no mutant copies of the gene were detected by ddPCR. Figure 4c shows representative panels of BRAFV600wt (left) and BRAFV600E (right) CTCs.
Correlation between CTC features and clinical-pathological parameters
We next sought to determine any potential correlation among CTC characteristics, clinical and pathological features as well as the patients’ outcome. By arbitrarily defining 60 years as the cut-off for age, we found a statistically significant association of higher numbers of CTCs in younger than in over-sixty patients (p < 0.05), whereas no correlations were detected with either the BRAFV600E mutation or peculiar histopathology aspects of the primary tumor. Similarly, we did not find any association between CTC counts and serum melanoma biomarkers, as lactate dehydrogenase and S100B (data not shown).
With respect to the prognostic role in melanoma, the number of metastatic sites was investigated for the potential correlation with CTC number, and more viable CTCs were observed in patients suffering of at least three different metastatic sites, although no statistically significant values were detected (data not shown). Moreover, the potential influence of systemic treatments on both CTC count and phenotype distribution was also evaluated. As shown in Figure 5 (a), the total CTC number was higher in pretreated patients (p = 0.06) who also harbored significantly higher numbers of PE+ CTCs (Figure 5b) than treatment-naïve ones (p = 0.005). The latter patients were then defined as responders or nonresponders (NR) to subsequent first line therapy with the purpose to reveal potential differences between these groups in terms of CTC counts and phenotype distribution. The preliminary analysis showed a trend to higher amounts of both total and PE+ CTCs in NR patients (Figure 5c), which might deserve further investigation for predictive purposes.
Figure 5.
Correlation of circulating tumor cell (CTC) number with clinical and laboratory parameters. As shown in (a), the total number of CTCs was higher in pretreated patients with respect to the naïve ones (p < 0.05) and showed higher numbers of PE+ CTCs (p < 0.05; b). By dividing patients into responders (R) and nonresponders (NR) following the first line therapy (c), a trend to the increase of PE+ CTCs was demonstrated.
Finally, we investigated the association between CTC number and clinical outcome in terms of both PFS and OS, respectively (Figure 6).The median OS among patients with levels above or below each threshold differed for each cut-off of CTCs and reached a plateau at approximately 20 CTCs per 10 ml of blood. Thus, a cut-off of 20 CTCs was arbitrarily used to separate patients with unfavorable from those with favorable prognosis. We observed that the total CTC count was not prognostic for PFS, while the presence of at least 20 PE+ CTCs was associated with worse outcome (median PFS 1.1 months versus not reached, HR: 3.05, 95% CI: 0.82–11.38; p < 0.05) (Figure 5, panel a). Moreover, the presence of ⩾20 cells significantly correlated with shorter median OS, as compared with smaller amounts (4.1 versus 14.9 months, HR: 4.07, 95% CI: 1.10–15.11; p < 0.05). However, by limiting the analysis on PE+ CTC subset (Figure 5, panel b), we estimated a median OS of 2.6 months in patients with at least 20 cells/10 ml as compared with those with fewer PE+ CTCs, for whom the median OS was not reached (HR 6.64, 95% CI: 1.68–26.28, p < 0.005).
Figure 6.
Correlation of circulating tumor cell (CTC) number with outcome. To verify the impact of CTC release on both PFS and overall survival (OS), a cut-off of 20 CTCs per 10 ml of blood was arbitrarily applied. As shown in (a), the CTC count was not prognostic for the calculation of the PFS (right), whereas the presence of a number of PE+ CTC > 20 was associated with a worse outcome (p < 0.05; left). Moreover, a number of CTC higher than 20 was correlated with shorter OS (b), which was further worsened in patients whose CTCs expressed a PE phenotype (p < 0.05).
CI, confidence interval; HR, hazard ratio.
Discussion
Metastasis onset is a hallmark of cancer and liquid biopsy became an innovative tool for the characterization of genetic signature of CTCs, thus providing predictive and prognostic information as well as options for tailored therapeutic strategies. We standardized a new method for the isolation of CTCs in metastatic melanoma based on dual immune-magnetic selection and DEPArray separation and propose peculiar CTC phenotypes for potentially prognostic purposes.
The prognostic role of CTCs has been well established in several malignancies since the CTC enumeration is being used for monitoring the outcome in advanced, refractory, and early solid cancers as well as for identifying indolent or aggressive tumor genotype. CTCs act as biomarker for the disease progression in breast and metastatic castration-resistant prostate cancer and have been suggested as mirror of the tumor heterogeneity in lung, prostate, and colon cancer.27–30 A similar contribution emerged in other neoplasms including noncutaneous and CM although the results have not been translated into the clinical practice. A modest predictive role has been demonstrated in CM and few studies suggest a possible application of CTC enumeration in patients bearing stage III of the disease. By contrast, a prognostic role in the metastatic setting emerged in the majority of studies, with a variability that regards the optimal cut-off. Furthermore, a peculiar association between CTC enrichment in peripheral blood and liver metastasis has been revealed in uveal melanoma.8,31–35
The lack of consensus on the best method to be employed for CTC detection in melanoma still hampers their routine application in the clinical setting. To this regard, many technologies have been attempted, including filtration-based, microfluidic, and dielectric procedures to obtain CTCs according to their physical properties, while immune phenotyping of these cells has also been proposed for their detection and enrichment.36,37 In this context, Hong and colleagues have recently developed a 19-gene RNA signature applied to microfluidically-enriched CTCs, for the longitudinal monitoring of stage IIIC/IV melanoma patients receiving immunotherapy and described a significant correlation between variations of the ‘CTC score’ and clinical outcome.34 In addition, the detection by CellSearch Circulating Melanoma assay of at least 1 CTC per 7.5 ml of blood has been significantly correlated with poor PFS in metastatic melanoma.33 However, a disagreement on the cut-off to be applied for prognostic purposes is today persistently considerable.3,31 Further challenges to be faced include the rarity of CTCs in the bloodstream and their heterogeneity, which may provide incomplete or inaccurate information.26,36–38
In the last decade, EMT has emerged as one of the key mechanisms involved in cancer progression and metastasis39–43 and several researchers investigated its activation state by monitoring CTC phenotype, based on the expression of epithelial or mesenchymal markers, and their variations over time.38,44–46 Furthermore, recent evidence suggests that the acquisition of a mesenchymal phenotype by CTCs is often associated with stemness properties, which might result in both tumor-initiating capability and resistance to anticancer drugs.47–49
Melanoma CTCs with stemness features have been described in both early stage and metastatic melanoma patients.26 The latter apparently exfoliate more circulating melanoma cells with heterogeneous phenotypic profile, thus underlying the importance of multiple antigen detection for their characterization in the clinical setting. In this context, a phase III multicenter trial demonstrated the prognostic meaning of multiple MAA expression in CTCs from stage III melanoma patients after CLND.8
In our work, following the depletion of blood and endothelial cells by immune-magnetic sorting, the dielectrophoretic DEPArray system was employed for CTC detection mostly based on morphological and immunophenotypical features. In particular, we mutually detected CD271, ABCB5 and RANK stem cell antigens, while mesenchymal markers including N-cad, CD44 and MCAM/CD146 were investigated through a separate antibody mixture. In agreement with previous reports in melanoma and other malignancies,26,50–52 the majority of patients spread CTCs with mesenchymal features either alone or in combination with stemness properties, that might account for their enhanced invasiveness and self-renewal potential.
In order to confirm the presence of melanoma cells in our CTC-enriched fractions, we tested them for BRAFV600E mutation by ddPCR, due to the high sensitivity of this method.53,54 Hence, we verified whether or not both CTC detection and enumeration by DEPArray could be appropriate to establish any potential correlation between clinical-pathological parameters and CTC count, to be applied in subsequent studies on a wider patient series. Interestingly, we observed a significant association between CTC number and patient age, whose prognostic role in CM is still debated40,55,56 while, in accordance with previous reports,3,33 no correlation was found between the number of viable CTCs and primary tumor features, including BRAFV600E status. By contrast, exploring a potential relationship with other well-known prognostic factors in CM as the number of metastatic sites we observed that CTC counts reflected the extent of metastatic disease.31,57–59 However, our small sample size did not reach statistical significance.
To the best of our knowledge, this is the first report in which immune-magnetic sorting and DEPArray technology were combined to detect and isolate viable CTCs in stage IV melanoma patients with the purpose to simultaneously investigate amounts, morphological patterns (e.g. nucleus/cytoplasm ratio, cell size, and nuclear integrity), phenotype, and molecular properties in melanoma. Our highly sensitive method also enabled the prognostic stratification of patients by using the cut-off of 20 CTCs/10 ml, and suggested that, alongside the total CTC count, phenotypical features of these cells should be carefully evaluated, since the occurrence of at least 20 PE+CTCs with mesenchymal properties was associated, in our cohort, with a worse outcome in terms of PFS and OS and may deserve further investigation on a wider population. This is in line with current studies that support the role of EMT in melanoma metastasis and progression41 since subjects enrolled in our study were characterized by high disease burden in relation to their status of treatment naïve or relapsed metastatic patients.
In conclusion, here we described a dual-step procedure for the CTC recruitment in CM that may also validate this modality of liquid biopsy for the molecular characterization of this tumor. We proved that the phenotype pattern of the CTCs is apparently correlated with prognosis in line with the evidence that their number reflects both PFS and OS in metastatic patients. These data are, however, preliminary and mostly based on a small sample size without longitudinal sampling in the same patients but in line with the most innovative research in melanoma. However, a larger study could be of great benefit for a definite validation of CTC enumeration by this new method with the final aim of monitoring the CM evolution including the development of drug resistance, thus permitting timely variations of the therapeutic strategies.
Supplemental Material
Supplemental material, Tucci_M_et_al_CTC_Table_S1_R1 for Dual-procedural separation of CTCs in cutaneous melanoma provides useful information for both molecular diagnosis and prognosis by Marco Tucci, Stella D’Oronzo, Francesco Mannavola, Claudia Felici, Domenica Lovero, Paola Cafforio, Raffaele Palmirotta and Franco Silvestris in Therapeutic Advances in Medical Oncology
Acknowledgments
Marco Tucci and Stella D’Oronzo contributed equally to this work.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the Italian Association for Cancer Research (grant number 173536) and the Apulia Region (‘Oncogenomic’, ‘Jonico-Salentino’, and ‘Precision Medicine’ projects).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Marco Tucci  https://orcid.org/0000-0003-4008-4897
https://orcid.org/0000-0003-4008-4897
Raffaele Palmirotta  https://orcid.org/0000-0002-9401-7377
https://orcid.org/0000-0002-9401-7377
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marco Tucci, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’, P.za Giulio Cesare, 11, Bari, 70124, Italy; I.R.C.C.S - Giovanni Paolo II Cancer Institute, Bari, Italy.
Stella D’Oronzo, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’ Italy; I.R.C.C.S - Giovanni Paolo II Cancer Institute, Bari, Italy.
Francesco Mannavola, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’ Italy.
Claudia Felici, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’ Italy.
Domenica Lovero, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’ Italy.
Paola Cafforio, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’ Italy.
Raffaele Palmirotta, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’ Italy.
Franco Silvestris, DIMO, Department of Biomedical Sciences and Clinical Oncology, University of Bari ‘Aldo Moro’ Italy.
References
- 1. Passarelli A, Mannavola F, Stucci LS, et al. Immune system and melanoma biology: a balance between immunosurveillance and immune escape. Oncotarget 2017; 8: 106132–106142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Khoja L, Lorigan P, Zhou C, et al. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J Invest Dermatol 2012; 133: 1582–1590. [DOI] [PubMed] [Google Scholar]
- 4. Palmirotta R, Lovero D, Cafforio P, et al. Liquid biopsy of cancer: a multimodal diagnostic tool in clinical oncology. Ther Adv Med Oncol 2018; 10: 1758835918794630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gerami P, Cook RW, Wilkinson J, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res 2015; 21: 175–183. [DOI] [PubMed] [Google Scholar]
- 6. Rapanotti MC, Campione E, Spallone G, et al. Minimal residual disease in melanoma: circulating melanoma cells and predictive role of MCAM/MUC18/MelCAM/CD146. Cell Death Discov 2017; 3: 17005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoshimoto S, Faries MB, Morton DL, et al. Assessment of prognostic circulating tumor cells in a phase III trial of adjuvant immunotherapy after complete resection of stage IV melanoma. Ann Surg 2011; 255: 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoshimoto S, Shingai T, Morton DL, et al. Association between circulating tumor cells and prognosis in patients with stage III melanoma with sentinel lymph node metastasis in a phase III international multicenter trial. J Clin Oncol 2012; 30: 3819–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mannavola F, Tucci M, Felici C, et al. miRNAs in melanoma: a defined role in tumor progression and metastasis. Expert Rev Clin Immunol 2015; 12: 79–89. [DOI] [PubMed] [Google Scholar]
- 10. Mannavola F, Tucci M, Felici C, et al. Tumor-derived exosomes promote the in vitro osteotropism of melanoma cells by activating the SDF-1/CXCR4/CXCR7 axis. J Transl Med 2019; 17: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351: 781–791. [DOI] [PubMed] [Google Scholar]
- 12. Zheng S, Lin HK, Lu B, et al. 3D microfilter device for viable circulating tumor cell (CTC) enrichment from blood. Biomed Microdevices 2010; 13: 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tang Y, Shi J, Li S, et al. Microfluidic device with integrated microfilter of conical-shaped holes for high efficiency and high purity capture of circulating tumor cells. Sci Rep 2014; 4: 6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ozkumur E, Shah AM, Ciciliano JC, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med 2013; 5: 179ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sarioglu AF, Aceto N, Kojic N, et al. A microfluidic device for label-free, physical capture of circulating tumor cell clusters. Nat Methods 2015; 12: 685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aya-Bonilla CA, Marsavela G, Freeman JB, et al. Isolation and detection of circulating tumour cells from metastatic melanoma patients using a slanted spiral microfluidic device. Oncotarget 2017; 8: 67355–67368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature 2008; 451: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kupas V, Weishaupt C, Siepmann D, et al. RANK is expressed in metastatic melanoma and highly upregulated on melanoma-initiating cells. J Invest Dermatol 2011; 131: 944–955. [DOI] [PubMed] [Google Scholar]
- 19. Grichnik JM, Burch JA, Schulteis RD, et al. Melanoma, a tumor based on a mutant stem cell? J Invest Dermatol 2006; 126: 142–153. [DOI] [PubMed] [Google Scholar]
- 20. Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res 2008; 21: 39–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boiko AD, Razorenova OV, van de Rijn M, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010; 466: 133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. D’Oronzo S, Lovero D, Palmirotta R, et al. Dissection of major cancer gene variants in subsets of circulating tumor cells in advanced breast cancer. Sci Rep 2019; 9: 17276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cafforio P, Savonarola A, Stucci S, et al. PTHrP produced by myeloma plasma cells regulates their survival and pro-osteoclast activity for bone disease progression. J Bone Miner Res 2013; 29: 55–66. [DOI] [PubMed] [Google Scholar]
- 24. Tucci M, Ciavarella S, Strippoli S, et al. Immature dendritic cells from patients with multiple myeloma are prone to osteoclast differentiation in vitro. Exp Hematol 2011; 39: 773–783.e1. [DOI] [PubMed] [Google Scholar]
- 25. Moreno JG, Miller MC, Gross S, et al. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology 2005; 65: 713–718. [DOI] [PubMed] [Google Scholar]
- 26. Gray ES, Reid AL, Bowyer S, et al. Circulating melanoma cell subpopulations: their heterogeneity and differential responses to treatment. J Invest Dermatol 2015; 135: 2040–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kapeleris J, Kulasinghe A, Warkiani ME, et al. The prognostic role of circulating tumor cells (CTCs) in lung cancer. Front Oncol 2018; 8: 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thery L, Meddis A, Cabel L, et al. Circulating tumor cells in early breast cancer. JNCI Cancer Spectr 2019; 3: pkz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lorente D, Olmos D, Mateo J, et al. Circulating tumour cell increase as a biomarker of disease progression in metastatic castration-resistant prostate cancer patients with low baseline CTC counts. Ann Oncol 2018; 29: 1554–1560. [DOI] [PubMed] [Google Scholar]
- 30. Cristofanilli M, Pierga JY, Reuben J, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol 2018; 134: 39–45. [DOI] [PubMed] [Google Scholar]
- 31. Bidard FC, Madic J, Mariani P, et al. Detection rate and prognostic value of circulating tumor cells and circulating tumor DNA in metastatic uveal melanoma. Int J Cancer 2013; 134: 1207– [DOI] [PubMed] [Google Scholar]
- 32. Rao C, Bui T, Connelly M, et al. Circulating melanoma cells and survival in metastatic melanoma. Int J Oncol 2011; 38: 755–760. [DOI] [PubMed] [Google Scholar]
- 33. Hall CS, Ross M, Bauldry JBB, et al. Circulating tumor cells in stage IV melanoma patients. J Am Coll Surg 2018; 227: 116–124. [DOI] [PubMed] [Google Scholar]
- 34. Hong X, Sullivan RJ, Kalinich M, et al. Molecular signatures of circulating melanoma cells for monitoring early response to immune checkpoint therapy. Proc Natl Acad Sci USA 2018; 115: 2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anand K, Roszik J, Gombos D, et al. Pilot study of circulating tumor cells in early-stage and metastatic uveal melanoma. Cancers (Basel) 2019; 11: pii: E856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marsavela G, Aya-Bonilla CA, Warkiani ME, et al. Melanoma circulating tumor cells: benefits and challenges required for clinical application. Cancer Lett 2018; 424: 1–8. [DOI] [PubMed] [Google Scholar]
- 37. De Souza LM, Robertson BM, Robertson GP. Future of circulating tumor cells in the melanoma clinical and research laboratory settings. Cancer Lett 2017; 392: 60–70. [DOI] [PubMed] [Google Scholar]
- 38. Aya-Bonilla C, Gray ES, Manikandan J, et al. Immunomagnetic-enriched subpopulations of melanoma circulating tumour cells (CTCs) exhibit distinct transcriptome profiles. Cancers (Basel) 2019; 11: pii: E157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tucci M, Stucci S, Strippoli S, et al. Dendritic cells and malignant plasma cells: an alliance in multiple myeloma tumor progression? Oncologist 2011; 16: 1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ribero S, Stucci LS, Marra E, et al. Effect of age on melanoma risk, prognosis and treatment response. Acta Derm Venereol 2018; 98: 624–629. [DOI] [PubMed] [Google Scholar]
- 41. Meng F, Wu G. The rejuvenated scenario of epithelial-mesenchymal transition (EMT) and cancer metastasis. Cancer Metastasis Rev 2012; 31: 455–467. [DOI] [PubMed] [Google Scholar]
- 42. Nieto MA, Huang RY, Jackson RA, et al. EMT: 2016. Cancer Lett 2016; 166: 21–45. [DOI] [PubMed] [Google Scholar]
- 43. Silvestris F, Ciavarella S, De Matteo M, et al. Bone-resorbing cells in multiple myeloma: osteoclasts, myeloma cell polykaryons, or both? Oncologist 2009; 14: 264–275. [DOI] [PubMed] [Google Scholar]
- 44. McInnes LM, Jacobson N, Redfern A, et al. Clinical implications of circulating tumor cells of breast cancer patients: role of epithelial-mesenchymal plasticity. Front Oncol 2015; 5: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen Y, Li S, Li W, et al. Circulating tumor cells undergoing EMT are poorly correlated with clinical stages or predictive of recurrence in hepatocellular carcinoma. Sci Rep 2019; 9: 7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wei T, Zhu D, Yang Y, et al. The application of nano-enrichment in CTC detection and the clinical significance of CTCs in non-small cell lung cancer (NSCLC) treatment. PLoS One 2019; 14: e0219129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kasimir-Bauer S, Hoffmann O, Wallwiener D, et al. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res 2012; 14: R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chiotaki R, Polioudaki H, Theodoropoulos PA. Stem cell technology in breast cancer: current status and potential applications. Stem Cells Cloning 2016; 9: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jia D, Li X, Bocci F, et al. Quantifying cancer epithelial-mesenchymal plasticity and its association with stemness and immune response. J Clin Med 2019; 8: pii: E725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chang K, Kong YY, Dai B, et al. Combination of circulating tumor cell enumeration and tumor marker detection in predicting prognosis and treatment effect in metastatic castration-resistant prostate cancer. Oncotarget 2015; 6: 41825–41836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Papadaki MA, Stoupis G, Theodoropoulos PA, et al. Circulating tumor cells with stemness and epithelial-to-mesenchymal transition features are chemoresistant and predictive of poor outcome in metastatic breast cancer. Mol Cancer Ther 2018; 18: 437–447. [DOI] [PubMed] [Google Scholar]
- 52. Ning Y, Zhang W, Hanna DL, et al. Clinical relevance of EMT and stem-like gene expression in circulating tumor cells of metastatic colorectal cancer patients. Pharmacogenomics J 2016; 18: 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hindson BJ, Ness KD, Masquelier DA, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem 2011; 83: 8604–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Reid AL, Freeman JB, Millward M, et al. Detection of BRAF-V600E and V600K in melanoma circulating tumour cells by droplet digital PCR. Cancer Lett 2014; 48: 999–1002. [DOI] [PubMed] [Google Scholar]
- 55. Gassenmaier M, Keim U, Leiter U, et al. Age as key factor for pattern, timing, and extent of distant metastasis in patients with cutaneous melanoma: a study of the German central malignant melanoma registry. J Am Acad Dermatol 2019; 80: 1299–1307.e7. [DOI] [PubMed] [Google Scholar]
- 56. Balch CM, Soong SJ, Gershenwald JE, et al. Age as a prognostic factor in patients with localized melanoma and regional metastases. Ann Surg Oncol 2013; 20: 3961–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol 2016; 17: 1743–1754. [DOI] [PubMed] [Google Scholar]
- 58. Wei T, Zhang X, Zhang Q, et al. Vimentin-positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patients with pancreatic cancer. Cancer Lett 2019; 452: 237–243. [DOI] [PubMed] [Google Scholar]
- 59. Bidard FC, Hajage D, Bachelot T, et al. Assessment of circulating tumor cells and serum markers for progression-free survival prediction in metastatic breast cancer: a prospective observational study. Breast Cancer Res 2012; 14: R29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Tucci_M_et_al_CTC_Table_S1_R1 for Dual-procedural separation of CTCs in cutaneous melanoma provides useful information for both molecular diagnosis and prognosis by Marco Tucci, Stella D’Oronzo, Francesco Mannavola, Claudia Felici, Domenica Lovero, Paola Cafforio, Raffaele Palmirotta and Franco Silvestris in Therapeutic Advances in Medical Oncology