Abstract
Background:
Joint bleeds are the hallmark of haemophilia, and can lead to disabling haemophilic arthropathy. Consequently, the movement behaviour of adults with haemophilia differs from that of healthy adults. It seems unlikely that a single outcome is able to reflect all relevant information regarding movement behaviour. The aim of the current study was to identify patterns of movement behaviour within persons with haemophilia (PWH) and compare clinical characteristics between patterns of movement behaviour.
Methods:
A total of 105 PWH [70% severe haemophilia; median age 43 years (30.0–54.0)] were included in the study. Hierarchical cluster analysis was used to identify patterns of movement behaviour. Clustering variables included seven parameters of movement behaviour: sitting, standing, walking, biking, running, frequency of active bouts and length of active bouts. Clinical characteristics included age, severity of haemophilia, joint health, physical functioning and pain. Clinical characteristics were compared between identified clusters by Kruskall–Wallis test. Movement behaviour was assessed with the Activ8 accelerometer, joint health was assessed on the Haemophilia Joint Health Score, physical functioning on the Haemophilia Activity List and the 40 m self-paced walk test and pain on the Numerical Pain Rating Score.
Results:
Cluster analysis identified three clusters, which were defined as: ‘sedentary’ (57%), ‘bikers and runners’ (22%) and ‘walkers’ (20%). The ‘bikers and runners’ showed better joint health and experienced fewer limitations in activities than the ‘walkers’ and the ‘sedentary’. The ‘walkers’ perceived fewer limitations in activities than the ‘sedentary’, with comparable joint health. We did not identify differences in pain, walking speed and age between the clusters.
Conclusions:
We identified three patterns of movement behaviour. The majority of PWH was identified as sedentary, whereas less sitting and regular walking during the day seemed to be more beneficial.
Keywords: accelerometer, arthropathy, haemophilia, movement behaviour, physical activity
Introduction
Haemophilia is an inherited genetic disorder that impairs the body’s ability to make blood clots. This results in longer bleeding time after injury, easy bruising and an increased risk of bleeding inside joints and muscles. Joint bleeds cause haemophilic arthropathy (HA) through synovial inflammation and direct blood-related osteochondral changes.1,2 HA is characterized by joint pain, limitations in range of motion and muscle atrophy, resulting in limitations to activities and participation. Consequently, adults with severe haemophilia are less physically active than healthy adults.3
In patients with osteoarthritis (OA) avoidance of activities and more sedentary time induce a negative vicious circle of disuse, loss of muscle mass, reduced joint stability, proprioception and postural control and increased limitations in activities.4 Given the similarities in pathogenesis of HA and OA, similar effects in persons with haemophilia (PWH) can be postulated.5 Increasing daily physical activity and limiting sedentary behaviour may improve physical functioning.6,7 Although several exercise interventions have been developed directed at improving physical functioning in PWH, none are aimed at changing movement behaviour in daily life.8
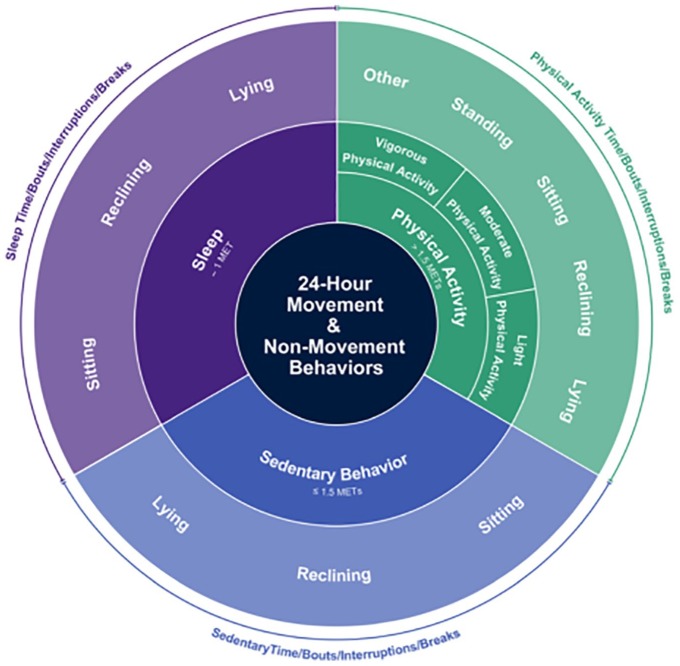
A person’s daily movement behaviour is considered as any combination of daytime sedentary behaviour, physical activity (PA) and sleep.9 In turn, this can be divided in activities and postures (e.g. sitting, walking and standing) and PA can be divided in PA of different intensities (light PA, moderate PA, etc.), as presented in Figure 1. In previous studies, movement behaviour in PWH was investigated using only a single parameter (e.g. average amount of moderate and vigorous PA), or several parameters were analysed separately.10,11 However, individuals who appear similar in one parameter (total amount of moderate and vigorous PA) may score very differently for other metrics (total amount of sedentary behaviour).12 Moreover, sedentary behaviour and PA are independent predictors of health outcomes, and the distribution of activities during the day predicts health outcomes, independently of absolute time spent in these activities.13 Using a single parameter, or analysing several parameters separately, ignores the co-occurrence of activities and postures and would result in an incomplete or inaccurate picture of a person’s overall movement behaviour. It seems unlikely that a single outcome is able to reflect all relevant information of movement behaviour. We therefore propose to identify patterns of movement behaviour, including all relevant metrics.
Figure 1.
The conceptual model of movement behaviour.9
Previous studies in PWH did not find a relationship between activity level and the degree of HA,11 although adult PWH were found to be less physically active than healthy adults. It is hypothesized that clinical characteristics (e.g. joint health, pain and physical functioning) are related to patterns of movement behaviour rather than total amount of PA. Insight into patterns of movement behaviour, and the relationship of these patterns with clinical characteristics, could enable us to identify patients at risk of deterioration. Furthermore, insights into patterns of movement behaviour are essential in developing interventions directed at changing movement behaviour.
The aim of this study was to identify patterns of movement behaviour in adults with haemophilia and to compare clinical outcomes between the identified patterns.
Methods
Design
This study was a cross-sectional study based on medical health record data.
Participants
Data from PWH were extracted from medical health records of regular check-up visits of all adult patients (⩾18 years) with haemophilia A or B, who visited their physiotherapist between 2014 and 2016 for a regular check up at the van Creveldkliniek, University Medical Centre Utrecht. Within our clinic, we aim to schedule check-up visits with the physiotherapist every 3 years (scheduled subsequently to a visit with the physician). Joint health, physical functioning and pain were evaluated during regular physiotherapy check ups. Movement behaviour was assessed in the week prior to the clinical visit. The current study excluded patients who had no data available on movement behaviour, were wheelchair dependent, experienced a joint or muscle bleed <6 weeks before the check up, underwent total knee or total hip replacement <24 months before the check up or underwent an ankle arthrodesis <12 months before the check up. The research protocol was approved by the Institutional Review Board of the University Medical Centre Utrecht, the Netherlands.
Outcome measures
Movement behaviour
Movement behaviour was measured with the Activ8 activity monitor, carried in trouser pockets.14 Activ8 has been validated and distinguishes between six postures and activities: lying down, sitting, standing, walking, running and cycling,15 enabling accurate measurement of a person’s movement behaviour.16,17 Postures and activities are determined every 5 s. Subsequently, the relative period spent per body position or activity is stored every 5 min. For example, between 10.00 and 10.05 a.m., a person might spend 3.0 min sitting and 2.0 min standing. The sequence of postures and activities within these 5 min can therefore not be distinguished. Nonwear is reported as lying down, and therefore it is not possible to distinguish between lying down and nonwear. PWH were instructed to wear the Activ8 for 7 consecutive days, except during swimming, bathing, showering and sleep. The minimum requirement for data inclusion was 10 h of wear time (corresponding to <14 h lying/nonwear) on at least 4 days.18 Time spent on activities was reported in hours per day (lying/nonwear, sitting, standing and walking) or minutes per day (running and biking). Frequency and length of active bouts were calculated to gain insight in distribution of PA over the day. An active bout is defined as consecutive periods of 5 min consisting of a combination of walking, running or biking with allowance of standing <5min.
Clinical characteristics
Joint health was measured using the Haemophilia Joint Health Score (HJHS) version 2.1. The HJHS evaluates swelling, muscle atrophy, crepitus, range of motion, joint pain and strength of the knees, the ankles and the elbows. Additionally, gait is assessed with a single global gait score. HJHS scores consist of 20 points per joint and 4 points for global gait, adding up to a maximum of 124 points. A higher score indicates worse joint health. The HJHS is validated for children and young adults with haemophilia19,20 ; however, it is the most commonly used instrument in adult PWH.
Physical functioning was measured using a self-assessment tool and a performance based tool; the Haemophilia Activity List (HAL) and the 40-m self-paced walk test (40-m SPWT), respectively. The HAL is a haemophilia-specific questionnaire assessing self-perceived limitations in activities. The HAL requires patients to consider difficulties they experienced in activities during the previous month due to haemophilia-related complaints. Internal consistency and validity of the HAL are considered good.21 A summary score, as well as component scores involving upper extremity, basic lower extremity and complex lower extremity activities, can be calculated. Normalized scores ranging from 0 to 100, with higher scores representing better functional status, can be obtained for the summary score and the component scores. Walking speed is measured with the 40-m SPWT; time used to walk 40 m was recorded.22 Patients are instructed to walk 40 m straight on in a hallway, at a self-chosen (habitual) pace; the use of a walking aid is allowed. Walking speed is considered an important predictor for health outcome in well-functioning older adults, and is complementary to self-perceived limitations in activities. The 40-m SPWT is a reliable tool to assess walking speed.
Pain was assessed using the numerical pain rating score (NRS).23 The NRS is an 11-point rating scale ranging from 0 to 10 points. A score of 0 represents ‘no pain’ and a score of 10 represents ‘worst pain possible’. Patients indicated the number that corresponds to their maximum pain level in the past week. The NRS is considered a valid and reliable instrument to measure pain. Furthermore, age and severity of haemophilia were extracted from medical health records.
Data analysis
Statistical analyses were performed with R version 0.99.903.24 Descriptive results were presented as means [standard deviation (SD)] for normally distributed data or medians [interquartile range (IQR)] for not normally distributed data. Normality of the data was checked by visual inspection of histograms and Q-Qplots and additional Shapiro–Wilk tests. In order to identify patterns of movement behaviour, agglomerative hierarchical cluster analysis was performed, using package ‘stats’ version 3.3.2.25 Ward’s linkage method with Euclidean distance was used to merge clusters. Clustering variables included absolute time spent in postures or activities (sitting, standing, walking, biking, running) and distribution of postures and activities during the day (frequency and length of active bouts). Given the different constructs of the clustering variables, the clustering variables were standardised. Hierarchical clustering does not require predetermining the number of clusters. The number of clusters was determined based on visual inspection of the dendrogram and interpretability of the clusters. To correct for differences in wear time, sensitivity analysis was performed with activities as percentage of wear time. Differences in patient characteristics between the identified patterns were evaluated with the Kruskall–Wallis test. Complete case analysis was performed for missing data on clinical characteristics.
Results
A total of 105 PWH were included in the study; 70% suffered from severe haemophilia. Median age of the participants was 43 years (30.0–54.0). Clinical characteristics of included patients are shown in Table 1. Descriptive statistics of the clustering variables are presented in Table 2. Data were missing on 6.3% of clinical outcome values. Missing values were due to logistic reasons and were therefore considered ‘Missing Completely at Random’. Participants with missing data did not differ from participants with complete data based on clustering variables or clinical characteristics.
Table 1.
Clinical characteristics.
| n (%)/median (IQR) | n | |
|---|---|---|
| Age | 43.0 (30.0–54.0) | 105 |
| Severe | 73 (70) | 105 |
| Prophylaxis | 71 (68) | 105 |
| HJHS total | 15.0 (3.0–31.0) | 100 |
| HAL total | 83.4 (66.2–94.3) | 96 |
| 40-m SPWT | 27.5 (25.0–30.5) | 96 |
| NRS max | 3.0 (0.0–7.0) | 88 |
HAL, haemophilia activity list; HJHS, haemophilia joint health score; IQR, interquartile range; NRS, numerical pain rating score; SPWT, self-paced walk test.
Table 2.
Clustering variables.
| Activ8 | Median (IQR) | n |
|---|---|---|
| Sitting (hours/day) | 9.2 (7.4–10.6) | 105 |
| Standing (hours/day) | 2.8 (2.0–3.6) | 105 |
| Walking (hours/day) | 1.9 (1.4–2.5) | 105 |
| Biking (min/day) | 14.2 (5.8–28.7) | 105 |
| Running (min/day) | 0.6 (0.2–1.9) | 105 |
| Frequency of active bouts (per day) | 10.0 (7.1–12.7) | 105 |
| Length active bout (min) | 11.8 (10.6–14.3) | 105 |
IQR, interquartile range.
Identification of patterns
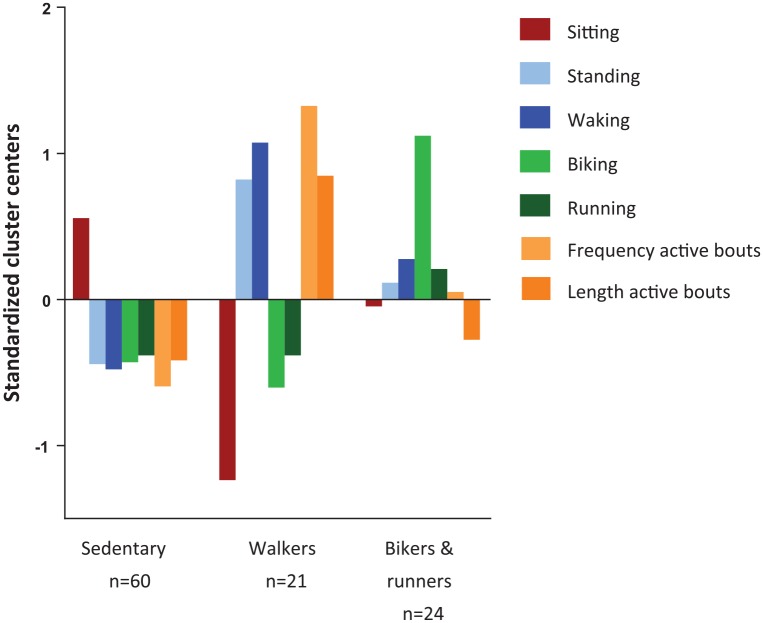
Hierarchical cluster analysis identified three clusters. The dendrogram of the cluster analysis is shown in Figure 2. One cluster included the majority of patients (57%), the other two were of comparable size (23% and 20%). The largest cluster was characterised by extensive sitting and little standing, walking, running and biking. The length of active bouts was short and the frequency low. This cluster was described as the ‘sedentary’ cluster. Persons in the second cluster performed extensive biking and reasonable amounts of running, standing and walking; the cluster is therefore named ‘bikers and runners’ cluster. Frequency and length of active bouts approached the average of the total included sample. The smallest cluster was characterised by little sitting, biking and running, extensive walking and standing and a high frequency and longer duration of active bouts. This cluster is therefore described as ‘walkers’. Standardised cluster centres are shown in Figure 3. Comparison of the medians of the clustering variables confirmed differences in the clustering variables between the clusters, as presented in Table 3. Although persons in the ‘bikers and runners’ cluster did more running than in the other clusters, even in the ‘bikers and runners’ cluster half of the people did less than 20 min running per week [median 2.4 (IQR 0.6–7.5)]. Sensitivity analysis with activities as percentage of wear time did not change the allocation to a cluster of any of the participants.
Figure 2.
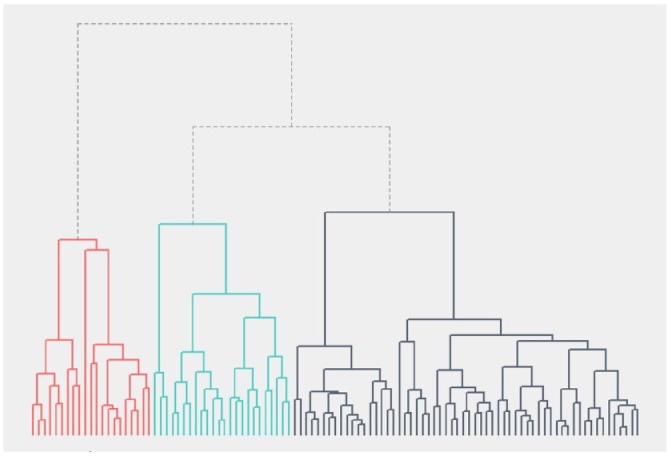
Dendrogram of hierarchical clustering.
Colours represent the three-cluster solution.
Figure 3.
Clusters centres of identified clusters.
Table 3.
Differences in clustering variables between clusters.
| Sedentary (n = 60) | Walkers (n = 21) | Bikers and runners (n = 24) | p value | |
|---|---|---|---|---|
| Sitting (hours/day) | 10.0 (8.7–11.2) | 6.5 (5.607.6) | 9.0 (8.2–9.7) | <0.01 |
| Standing (hours/day) | 2.4 (1.9–3.0) | 4.0 (3.3–5.8) | 3.1 (2.1–3.5) | <0.01 |
| Walking (hours/day) | 1.6 (1.1–2.0) | 2.9 (2.4–3.8) | 2.2 (1.6–2.6) | <0.01 |
| Biking (min/day) | 12.6 (4.8–18.6) | 10.5 (3.5–19.8) | 39.6 (35.6–52.8) | <0.01 |
| Running (min/day) | 0.6 (0.0–1.2) | 0.6 (0.0–1.2) | 2.4 (0.6–7.5) | <0.01 |
| Frequency of active bouts (per day) | 7.7 (6.3–10.2) | 15.7 (13.5–17.0) | 10.4 (8.7–13.1) | <0.01 |
| Length of active bout min) | 11.3 (10.5–13.3) | 15.9 (12.3–17.9) | 11.8 (10.6–13.2) | <0.01 |
Median (IQR).
IQR, interquartile range.
Differences in clinical characteristics between clusters
Self-perceived limitations in activities differed between the clusters. PWH in the ‘sedentary’ cluster perceived the most limitation in activities, PWH in the ‘walkers’ cluster perceived fewer limitations and persons in the ‘bikers and runners’ cluster perceived the least limitations in activities [74.5 (61.0–92.5), 85.6 (76.7–93.9) and 90.3 (69.1–100) respectively, p = 0.04]. A trend was found towards better joint health in PWH in the ‘bikers and runners’ cluster compared with PWH in the ‘sedentary’ cluster or the ‘walkers’ cluster [4.0 (1.0–29.0), 15.5 (5.8–25.8) and 16.0 (7.5–34.0) respectively, p = 0.07]. No differences were found between the clusters based on age, pain and walking speed. Differences in clinical characteristics between clusters are presented in Table 4.
Table 4.
Differences in clinical characteristics between clusters.
| Sedentary (n = 60) | Walkers (n = 21) | Bikers and runners (n = 24) | p value | |
|---|---|---|---|---|
| Severe | 78.3% (66.4–86.9) | 52.4% (32.4–71.7) | 62.5% (42.7–78.8) | 0.28 |
| Age | 43.0 (31.0–53.0) | 44.0 (32.0–59.0) | 34.5 (24.5–55.3) | 0.40 |
| HJHS total | 16.0 (7.5–34.0) | 15.5 (5.8–25.8) | 4.0 (1.0–29.0) | 0.07 |
| HAL total | 74.5 (61.0–92.5) | 85.6 (76.7–93.9) | 90.3 (69.1–100) | 0.04 |
| 40m SPWT | 27.6 (25.1–31.0) | 26.5 (24.5–29.3) | 27.3 (25.8–28.9) | 0.52 |
| VAS max | 4.5 (0.0–7.0) | 3.0 (2.0–7.0) | 1.0 (0.0–6.5) | 0.24 |
Median (IQR) and % (95% confidence interval).
Data were missing on 6.3% of clinical outcome values. Participants with missing data did not differ from participants with complete data based on clustering variables or clinical characteristics.
HAL, haemophilia activity list; HJHS, haemophilia joint health score; IQR, interquartile range; SPWT, self-paced walk test; VAS, visual analogue scale.
Discussion
In this study we identified three patterns of movement behaviour in PWH: the ‘sedentary’, the ‘walkers’ and the ‘bikers and runners’. The majority of the PWH showed a ‘sedentary’ movement pattern. PWH showing a ‘bikers and runners’ pattern had better joint health and perceived fewer limitations in activities than PWH with a ‘walkers’ or a ‘sedentary’ movement pattern. PWH showing a ‘walkers’ movement pattern perceived fewer limitations in activities than those showing a ‘sedentary’ pattern, but had comparable joint health. We did not identify differences in pain, walking speed, severity and age between the subgroups.
The use of patterns of movement behaviour has been proposed in previous studies investigating outcome measures of movement behaviour.12 However, studies investigating patterns of movement behaviour in different populations are still scarce. One study aimed to identify patterns of day-to-day moderate-to-vigorous PA (MVPA) in the general population.26 Patterns of persons who show more MVPA on weekdays, or who show more MVPA on weekend days, were identified. This is a different approach than that used in the current study, in which persons are categorised based on type of activity. In accordance with the current study, the majority of participants had low levels of MVPA. Previous research already showed that PWH are less physically active than healthy adults, but show similar sedentary behaviour.3 Furthermore, previous studies indicated that PA level is explained only in small part by joint health.11 This is supported by the findings in the current study, which show that only persons with a ‘bikers and runners’ movement pattern have a better joint health, but that joint health of PWH with a ‘walkers’ or ‘sedentary’ movement pattern is comparable. We found no evidence of pain as an explaining factor. Explanation for the difference in movement behaviour between ‘walkers’ and ‘sedentary’ could be both haemophilia specific (fear of bleeding, restrictions in PA in childhood) and of a general nature (work-related activity, social influences, financial influences, comorbidities).
The main limitation of the current study was the small sample size of the subgroups with a ‘walkers’ and ‘bikers and runners’ movement pattern. Give the small sample sizes of these subgroups, we were not able to perform post hoc analysis. This could also have impaired the ability to identify differences in clinical characteristics between the subgroups. With a greater sample size, borderline differences between groups in the proportion of persons with severe haemophilia might have been more evident. Furthermore, large variation and small sample size may have impaired the ability to identify differences in pain between the subgroups. The overall sample size was sufficient to perform cluster analysis with the included seven clustering variables. Furthermore, movement behaviour might have been influenced by the recommendations of clinical staff, since this was a single-centre study; the view of the comprehensive care team within the included centre is to stimulate PA. It can be hypothesised that the proportion of persons allocated to the sedentary cluster would be higher in centres that discourage persons with haemophilia to be physically active. A last limitation of this study is that the HJHS is not validated in adults with haemophilia, only in children and adolescents with haemophilia and adults with von Willebrand disease.19,20,27 The main strength of the current study was that we included a large spectrum of movement behaviour, instead of a single measure.12 We defined two measures to describe the distribution of PA during the day (frequency and length of active bouts). Currently, there is no consensus on an optimal definition to determine active bouts.28 Using a different definition for an active bout may have resulted in a cluster with a distinct distribution of PA (e.g. walkers with bouts of high frequency and short length and walkers with bouts of low frequency and extended length). Frequency and duration of sedentary bouts were not included since the number of clustering variables was limited by our sample size.
Similar joint health in PWH with a ‘walkers’ and a ‘sedentary’ movement pattern implies that movement behaviour is dependent on more factors than joint and muscle function. Future research is needed to identify barriers and facilitators of the identified patterns of movement behaviour. Although causality cannot be determined given the design of the current study, fewer limitations in activities in PWH showing a ‘walkers’ movement pattern compared with PWH showing a ‘sedentary’ movement pattern suggests that this movement behaviour could be beneficial for self-perceived limitations in activities. Given the influence of PA and sedentary behaviour on physical functioning in persons with OA, longitudinal research in PWH is needed to identify whether a ‘walkers’ movement pattern is beneficial for perceived limitations in activities compared with a ‘sedentary’ movement pattern. Furthermore, the current study indicates that interventions directed at changing movement behaviour should include the whole spectrum of movement behaviour instead of a single measure. Using the whole spectrum of movement behaviour allows for interventions adapted to a person’s possibilities and preferences. We recommend exploring movement behaviour of other disease groups with a similar approach. Investigating patterns of movement behaviour consisting of a wide range of relevant parameters will enable a more complete picture of movement behaviour to emerge. Further research is needed to determine which parameters need to be included. We expect that the results of the current study can be generalised to patients in countries with similar treatment possibilities. However, cultural differences may influence movement behaviour.
In conclusion, within adult PWH, three different patterns of movement behaviour can be distinguished: ‘sedentary’, ‘walkers’ and ‘bikers and runners’. The majority show a sedentary pattern of movement behaviour. A ‘bikers and runners’ movement pattern is associated with better joint health and fewer perceived limitations in activities. A ‘walkers’ movement pattern is associated with comparable joint health and fewer perceived limitations in activities compared with a ‘sedentary’ pattern.
Footnotes
Author contributions: MT and MP developed the study. MT and MP performed statistical analysis. All authors contributed to data interpretation. All authors contributed to critical reviewing and editing of the manuscript. All authors approved the final manuscript.
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
Ethics approval: The research protocol was approved by the Institutional Review Board of the University Medical Centre Utrecht, the Netherlands.
Consent for publication: Not applicable.
ORCID iD: Merel A. Timmer  https://orcid.org/0000-0003-1910-999X
https://orcid.org/0000-0003-1910-999X
Availability of data and material: The datasets used and analysed during the current study are available from the DANS website on reasonable request.
Contributor Information
Merel A. Timmer, Van Creveldkliniek, University Medical Centre Utrecht, Heidelberglaan 100, Utrecht, the Netherlands; Physical Therapy Research, Department of Rehabilitation, Physical Therapy Science and Sport, Brain Centre Rudolf Magnus, University Medical Centre Utrecht, Utrecht University, Utrecht, the Netherlands.
Cindy Veenhof, Physical Therapy Research, Department of Rehabilitation, Physical Therapy Science and Sport, Brain Centre Rudolf Magnus, University Medical Centre Utrecht, Utrecht University, Utrecht, the Netherlands; Research Group Innovation of Human Movement Care, University of Applied Sciences Utrecht, the Netherlands.
Piet de Kleijn, Van Creveldkliniek, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands.
Rob A. de Bie, Department of Epidemiology, CAPHRI School for Public Health and Primary Care, Maastricht University, Maastricht, Utrecht, the Netherlands
Roger E. G. Schutgens, Van Creveldkliniek, University Medical Centre Utrecht, Utrecht University, Utrecht, The Netherlands
Martijn F. Pisters, Centre for Physical Therapy Research and Innovation in Primary Care, Julius Health Care Centres, Utrecht University, the Netherlands
References
- 1. Jansen NW, Roosendaal G, Lafeber FP. Understanding haemophilic arthropathy: an exploration of current open issues. Br J Haematol 2008; 143: 632–640. [DOI] [PubMed] [Google Scholar]
- 2. Roosendaal G, Lafeber FP. Blood-induced joint damage in hemophilia. Semin Thromb Hemost 2003; 29: 37–42. [DOI] [PubMed] [Google Scholar]
- 3. Timmer MA, Pisters MF, de Kleijn P, et al. Movement behaviour in adults with haemophilia compared to healthy adults. Haemophilia 2018; 24: 445–451. [DOI] [PubMed] [Google Scholar]
- 4. Pisters MF, Veenhof C, van Dijk GM, et al. Avoidance of activity and limitations in activities in patients with osteoarthritis of the hip or knee: a 5 year follow-up study on the mediating role of reduced muscle strength. Osteoarthritis Cartilage 2014; 22: 171–177. [DOI] [PubMed] [Google Scholar]
- 5. Roosendaal G, van Rinsum AC, Vianen ME, et al. Haemophilic arthropathy resembles degenerative rather than inflammatory joint disease. Histopathology 1999; 34: 144–153. [DOI] [PubMed] [Google Scholar]
- 6. Song J, Gilbert AL, Chang RW, et al. Do inactive older adults who increase physical activity experience less disability: evidence from the osteoarthritis initiative. J Clin Rheumatol 2017; 23: 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song J, Lindquist LA, Chang RW, et al. Sedentary behavior as a risk factor for physical frailty independent of moderate activity: results from the osteoarthritis initiative. Am J Public Health 2015; 105: 1439–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strike K, Mulder K, Michael R. Exercise for haemophilia. Cochrane Database Syst Rev 2016; 12: CD011180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tremblay MS, Aubert S, Barnes JD, et al. Sedentary behavior research network (SBRN) - terminology consensus project process and outcome. Int J Behav Nutr Phys Act 2017; 14: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez LM, Peiro-Velert C, Devis-Devis J, et al. Comparison of physical activity and sedentary behaviours between young haemophilia A patients and healthy adolescents. Haemophilia 2011; 17: 676–682. [DOI] [PubMed] [Google Scholar]
- 11. Baumgardner J, Elon L, Antun A, et al. Physical activity and functional abilities in adult males with haemophilia: a cross-sectional survey from a single US haemophilia treatment centre. Haemophilia 2013; 19: 551–557. [DOI] [PubMed] [Google Scholar]
- 12. Thompson D, Batterham AM. Towards integrated physical activity profiling. PLoS One 2013; 8: e56427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biswas A, Oh PI, Faulkner GE, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015; 162: 123–132. [DOI] [PubMed] [Google Scholar]
- 14. Activ8. VitaMove, https://www.activ8all.com/product/activ8-professional-activity-monitor/
- 15. University Medical Center Rotterdam. Validation of the Active8 Activity Monitor: detection of body postures and movements, https://www.activ8all.com/front15/wp-content/uploads/2014/08/ReportActiv8_EMC.pdf (2013).
- 16. Fanchamps MHJ, Horemans HLD, Ribbers GM, et al. The accuracy of the detection of body postures and movements using a physical activity monitor in people after a stroke. Sensors (Basel) 2018; 18: pii: E2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lankhorst K, van den Berg-Emons RJ, Bussmann JBJ, et al. A novel tool for quantifying and promoting physical activity in youths with typical development and youths who are ambulatory and have motor disability. Phys Ther 2019; 99: 354–363. [DOI] [PubMed] [Google Scholar]
- 18. Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc 2005; 37(Suppl. 11): S531–S543. [DOI] [PubMed] [Google Scholar]
- 19. Fischer K, De Kleijn P. Using the hemophilia joint health score (HJHS) in adult patients: Testing inter-rater reliability. Haemophilia 2012; 18: 116. [Google Scholar]
- 20. Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia 2006; 12: 518–525. [DOI] [PubMed] [Google Scholar]
- 21. van Genderen FR, Fischer K, Heijnen L, et al. Pain and functional limitations in patients with severe haemophilia. Haemophilia 2006; 12: 147–153. [DOI] [PubMed] [Google Scholar]
- 22. Bennell K, Dobson F, Hinman R. Measures of physical performance assessments: self-paced walk test (SPWT), stair climb test (SCT), six-minute walk test (6MWT), chair stand test (CST), timed up and go (TUG), sock test, lift and carry test (LCT), and car task. Arthritis Care Res (Hoboken) 2011; 63(Suppl. 11): S350–S370. [DOI] [PubMed] [Google Scholar]
- 23. Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011; 152: 2399–2404. [DOI] [PubMed] [Google Scholar]
- 24. R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2016. [Google Scholar]
- 25. Mächler M, Rousseeuw P, Struyf S, et al. Cluster: cluster analysis basics and extensions. R Package Version 2.0.1, 2015. [Google Scholar]
- 26. Metzger JS, Catellier DJ, Evenson KR, et al. Patterns of objectively measured physical activity in the United States. Med Sci Sports Exerc 2008; 40: 630–638. [DOI] [PubMed] [Google Scholar]
- 27. van Galen KPM, Timmer MA, de Kleijn P, et al. Joint assessment in von Willebrand disease. Validation of the haemophilia joint health score and haemophilia activities list. Thromb Haemost 2017; 117: 1465–1470. [DOI] [PubMed] [Google Scholar]
- 28. Ayabe M, Kumahara H, Morimura K, et al. Epoch length and the physical activity bout analysis: an accelerometry research issue. BMC Res Notes 2013; 6: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]