Abstract
The delivery of ophthalmic drugs is challenging despite easy accessibility via the ocular surface. Topical instillation of eye drops is a relatively easy and most commonly used as a conduit for drug delivery for treating a myriad of ocular morbidities, particularly involving the anterior segment, and has an additional benefit of avoiding the first-pass metabolism while passing through the systemic circulation. The primary challenges of drug administration through traditional methods include—inadequate patient education for proper drug instillation technique, compliance, adherence, and persistence. Various dynamic (choroidal and conjunctival blood flow, lymphatic clearance, and tear dilution) and static (namely, different layers of cornea, sclera, and retina including blood aqueous and blood–retinal barriers) ocular barriers limit drug delivery to the target ocular tissues. The maintenance of the therapeutic drug levels on the ocular surface for a prolonged duration is an added challenge, thus preventing persistent delivery for longer durations. These factors result in inadequate management, leading to poor prognosis in vision loss in as many as 27% of the patients diagnosed with glaucoma. We have reviewed the research and advancements in the development of novel and well-tolerated drug delivery systems with the common goal of overcoming the factors limiting adequate drug delivery to the target tissues in glaucomatous patients with traditional techniques. In the recent past, multiple research groups have successfully designed noninvasive, sustained drug delivery systems, promoting the efficacy as well as the feasibility of delivering topical drugs to the anterior segment.
Keywords: compliance, drug delivery, glaucoma, nanoparticles, neuroprotection, ocular drugs, ocular inserts, therapeutic contact lenses
Introduction
Glaucoma is the second most common cause of blindness in the world. Patients with glaucoma clinically present with raised ocular pressures which cause optic neuropathy precipitating in the corresponding visual field loss.1 Currently, about 60 million people are estimated to have optic neuropathy secondary to glaucoma globally.2 The studies have shown that primary open angle glaucoma (POAG) leads to bilateral blindness in 9% patients and unilateral blindness in 27% patients, within 20 years of the first glaucomatous changes.3 In 2010, an estimated 4.5 and 3.9 million people were diagnosed with bilateral blindness due to open angle glaucoma (OAG) and angle closure glaucoma (ACG), respectively. The numbers of bilaterally blind people due to OAG and ACG are predicted to increase to 5.9 and 5.3 million in 2020, respectively.2 The visual field loss due to glaucoma is progressive and irreversible; however, attaining the target intraocular pressure (IOP) within the normal range can alleviate the progression of visual field loss. Lowering the IOP is the initial management strategy for ‘high-risk’ glaucoma suspect or a patient diagnosed with OAG using topical antiglaucoma drugs. However, despite the timely diagnosis and easy availability of pharmacotherapy, clinicians continue to face the uphill task of successfully slowing down the progression of vision and visual field loss. Various studies have highlighted the shortcomings of current treatment regimens other than drug efficacy factors causing short precorneal residence time and reduced absorption, that is, rapid turnover of lacrimal fluid, extensive nasolacrimal drainage, and rapid blinking reflex, as well as human factors, that is, poor compliance and persistence.4–7
An ideal drug delivery system in theory should deliver the prescribed amount of drug to the ocular tissue with comfort, should be easy to administer, and should not interfere with vision or normal functioning of the eye. The development of novel drug delivery vehicles has been an ongoing quest to improve the compliance among the patients and subsequently prevent blindness with the aim of managing ocular hypertension and promoting neuroprotection.
In this review, we discuss the different novel drug delivery techniques that are currently available or are under different stages of development across the world.
Ocular inserts
Ocular inserts are sterile drug-impregnated microdevices, placed in or around the eye for the release of therapeutic drugs over a prolonged duration. On the basis of their physical and chemical properties, the inserts are classified into insoluble, soluble, or bioerodible.8 The inserts increase the ocular surface contact time of the drug to a few days, thereby increasing the bioavailability by manifold, due to reduced washout by tears.
Pilocarpine ocular inserts
In 1976, Bensinger and colleagues demonstrated the use of a synthetic biosoluble matrix in the conjunctival cul-de-sac to increase the contact time of pilocarpine with the corneal tear film for IOP control. A significant reduction in the IOP was recorded for different doses ranging from 0.5 to 2 mg, 32 h post-insertion.9 The IOP had the maximum lowering of 6.25 ± 2.48 mmHg on placement of 0.5 mg pilocarpine inset, whereas a higher dose of 1.5 mg pilocarpine reduced the IOP by 8.14 ± 0.96, 5 h post placement. Only the pilocarpine inserts loaded with 1 mg pilocarpine significantly lowered the IOP at 32 h after placement.
Soluble ophthalmic drug inserts
Soluble ocular drug insert (SODI) is an oval-shaped ocular insert made up of a copolymer of polyacrylamide, ethyl acrylate, and vinyl pyrrolidone. Maichuk10 first reported its use to administer drugs including pilocarpine through the inferior cul-de-sac. The drug insert softens and converts into a viscous polymer solution after 10- to 15-s contact with the tear film, followed by conversion to a polymer solution within 60–90 min of administration. There is a change in the consistency of the SODI immediately after insertion in the conjunctival sac and acquires a globular, followed by steady dissolution to release the drug, completely dissolving within a few hours. A single application of SODI loaded with 2.7 mg pilocarpine was as efficacious as 4–12 eye drop instillations or 3–6 ointment applications and could be efficaciously used for the once-a-day treatment of glaucoma as per the author of the report.
Ocusert
Ocusert was one of the earliest models of ocular inserts developed by Armaly and Rao,11 made commercially available by Alza Corporation Inc. Ocusert releases the drug at a constant rate of 20 or 40 μg/h for an extended period of 7 days. Pilocarpine was loaded in a polymer membrane system consisting of an inner layer of pilocarpine in alginate gel within di-(ethylhexyl)phthalate for a release enhancer, sandwiched between two outer layers of ethylene-vinyl acetate (EVA) layers designed to release the drug at a predetermined constant rate. It is inserted in the upper or lower cul-de-sac and requires weekly replacement. Pilocarpine released from Ocusert acts on target organs in the iris, ciliary body, and trabecular meshwork. The authors reported a 45.4% ± 3.86% and a 48.0% ± 3.18% reduction with pilocarpine-loaded Ocusert 50 and 80 inserts, respectively, as compared with a 28.4% ± 3.63% reduction with conventional eye drops of pilocarpine.
Zimmerman and colleagues used Ocusert for Pilocarpine delivery in 40 patients with a target release rate of 20 µg/h. At the initiation of the study, the mean IOP was recorded to be 25.6 ± 5.6 mmHg. Using pilocarpine-loaded Ocusert, IOP was reduced to 19.9 ± 3.9 mmHg. The group also reported that the patients preferred the Ocusert system over the pilocarpine drops. No side effects from the Ocusert were noted.12 A study by Pavan-Langston and colleagues with 29 patients showed a satisfactory control of the IOP by pilocarpine-loaded Ocuserts releasing either 20 or 40 μg/h of pilocarpine. The side effects of the Ocuserts were minimal or absent.13
Although the clinical studies showed positive outcomes, Ocusert did not become a widely accepted method of drug delivery because of failure to satisfactorily control IOP in many patients, the difficulty of device insertion, irritation during insertion, and ejection of the device from the eye.14
Poly(vinyl methyl ether-maleic anhydride)anhydride ocular inserts
Poly(vinyl methyl ether-maleic anhydride)anhydride (PVMMA) and its alkyl monoesters have been used as bioerodible polymers that can be used for controlled timolol release in animals. The polymer-assisted drug release was found to reduce the systemic effects of timolol. Finne and colleagues15 found a peak concentration of timolol in tear fluid (64 ± 9 μg/ml) and a lower steady-state concentration (1.0 ± 0.1 ng/ml) in plasma 3 h after administration. They also reported a 1.6-fold increase in timolol concentration in tears (104 ± 8 μg/ml) on addition of disodium phosphate as a buffer. The IOP-lowering effect, as well as the pharmacokinetics of silicone tubing-based timolol inserts, was as effective as 0.5% timolol eye drops in glaucoma patients. They also found a decrease in side effects due to decreased systemic absorption.
Collagen shield
Dr Svyatoslav Fyodorov first developed the collagen shields as postoperative corneal bandages.16 Bloomfield and colleagues developed the drug delivery model for the collagen shields. They demonstrated a higher level of gentamicin in the tear film, and tissues in rabbit eyes using wafer-shaped collagen inserts impregnated with gentamicin as compared with eye drops, ointment, or subconjunctival injection.17 In collagen shields, hydrophilic drugs are loaded in the collagen matrix by soaking a dry shield in the aqueous solution of the drug. The water-insoluble drugs are directly added to the shield during the manufacturing process. Agban and colleagues18 developed cross-linked collagen shields consisting of nanoparticles consisting of titanium dioxide (TiO2), zinc oxide (ZnO), and polyvinylpyrrolidone (PVP) capped zinc oxide (ZnO/PVP), for controlled delivery of pilocarpine hydrochloride in glaucoma patients over a prolonged duration, which is currently undergoing animal trials. The results from the group show a sustained release of pilocarpine hydrochloride when cross-linked with ZnO/PVP nanoparticles over a period of 14 days.
Ocufit SR
Ocufit SR developed by Escalon Ophthalmics Inc. is a flexible rod-shaped silicon elastomer device designed for retention in the conjunctival fornix for controlled release of drugs over long periods. The different models are a maximum of 1.9 mm in diameter and range between 25 and 30 mm in length. Katz and Blackman19 later reported that expulsion of rod-shaped devices was significantly less frequent than that of oval, flat inserts. The insoluble Ocufit had favorable properties of both long retention and sustained drug release. When placed in the upper fornix, the placebo devices were retained for 2 weeks or more in 70% of the cases.
Minidisc
Bawa and colleagues20 developed the Minidisc or Ocular Therapeutic System (OTS), a miniature contact lens, with a diameter of 4–5 mm with a convex and a concave face, the latter conforming substantially to the sclera of the eye. The minidisc is a polymer of hydroxyethyl methacrylate and ethylene glycol methacrylate. The size and shape of the OTS allow an easy placement of the device under either upper or lower lid without any foreign body sensation, distortion in vision, or decreased oxygen permeability.
New Ophthalmic Delivery System
New Ophthalmic Delivery System (NODS) is a system used for delivering drugs in precise amounts to the eye, through the lower conjunctival sac using a water-soluble film loaded with the drug.21 The device consists of 20-µm-thick, 4 × 6 mm2 medicated flag, attached to a 0.7-mm-long paper-covered handle and 3- to 4-µm-thick membrane. NODS is manufactured using water-soluble polyvinyl alcohol (PVA). Greaves and colleagues22 used radiolabeled NODS loaded with pilocarpine nitrate, to evaluate the pharmacokinetics and bioavailability in human subjects. They found an eightfold precorneal bioavailability and higher compliance among the test subjects pilocarpine NODS as compared with a 2% w/v pilocarpine nitrate solution. Results obtained following administration of the NODS were compared with those obtained after administration of a 25-µl drop of a 2% w/v pilocarpine nitrate solution. Dissolution of the radiolabel from the NODS in vivo showed considerable variation in the subjects, with the time taken for the dissolution of 50% of the drugs ranging from 46 to 833 s. The half-time of clearance of the radiolabel from the NODS and corneal region of interest was 406 ± 214 s, whereas the radiolabeled solution had a mean ocular surface residence time of 2.9 ± 1.5 s. The IOP 5 h after the application was significantly reduced in the NODS group (–11.90 ± 2.61 mmHg) as compared with the control eye (–5.20 ± 1.86 mmHg).
Topical bimatoprost ocular insert
A bimatoprost-loaded insert consisting of a silicone matrix with a polypropylene backbone for sustained delivery to treat glaucoma underwent randomized phase II clinical trial.23 The diameter of the insert ranges from 24 to 29 mm and was placed between the upper and lower fornices. The bimatoprost ocular insert elutes the drug at a variable rate over a period of 6 months, depending on the polymer–drug matrix properties. On the onset, the elution rate was 35 mg/day which reduces to 6 mg/day after 6 months. The results from the trial showed a mean reduction from baseline IOP of −3.2 to −6.4 mmHg for the bimatoprost insert group compared with −4.2 to −6.4 mmHg for the eye drop group over 6 months, without any reported complications. The recorded adverse events were consistent with bimatoprost, and no unexpected ocular adverse events were recorded or observed.
In 2016, De Souza and colleagues developed an ocular insert with mucoadhesive properties, developed from a polymer of chitosan. The data from the in vitro studies showed sustained release of brimonidine tartrate. Moreover, the authors highlighted the adherent properties of the chitosan-based polymer on the conjunctiva. They also confirmed the rate of constant release for a prolonged period of 30 days without initial burst. The insert had biocompatibility with the surrounding ocular tissues.24
Patient education continues to be a significant challenge when it comes to successful use of the inserts as it requires a fine manual technique to manipulate and place the insert. It has been seen that the level of education and age continue to be the factors that govern the success of these devices when used for glaucoma.25
Hitoshi and colleagues studied the efficacy of ophthalmic inserts of timolol based on poly(2-hydroxypropyl methacrylate) and poly(2-hydroxyethyl methacrylate) polymers. The results from the study indicated that the prepared inserts resulted in a controlled release and an improved ocular bioavailability of timolol.26
Therapeutic contact lenses
The ease of availability and application of contact lenses make them an ideal drug delivery system. The therapeutic contact lenses help in sustained and regulated ocular drug delivery, due to their unique properties like extended wear and more than 50% bioavailability in comparison with eye drop formulations.27,28 Soft contact lenses are water-soluble polymeric hydrogels, cross-linked to form networks. These hydrogel lenses are widely used for drug delivery, even though the delivery of water-soluble drugs, such as timolol and dorzolamide, elutes from the highly hydrated polymer networks rapidly.29 In comparison, the soft contact lenses manufactured by polymerization N,N-diethyl acrylamide and methacrylic acid deliver timolol over a prolonged period.30
Soak and release
Waltman and Kaufman31 first demonstrated the potential use of hydrophilic polymers of 2-hydroxyethyl methacrylate (HEMA) for drug delivery using fluorescein stain. In 1974, Hillman demonstrated the delivery of antiglaucoma drugs through soft contact lenses. He used polymers of vinyl pyrrollidone soaked in 1% pilocarpine for drug delivery. He reported the system to be as efficacious as 4% pilocarpine topical eye drops.32
Microemulsion-loaded lenses
The microemulsions for drug dispersal are favored due to their high drug-loading capacity, thermodynamic stability, ease of preparation, increased wettability, and easy tailoring of drug release pattern. Multiple groups have developed drug-loaded microemulsion-incorporating contact lenses. Gulsen and Chauhan encapsulated timolol in microemulsion stabilized within a silica shell using octadecyltrimethoxysilane (OTMS), followed by dispersion in hydrogel lens. This model has shown sustained release of up to 8 days without affecting the transparency of the lens.33 Li and colleagues34 developed contact lenses loaded with timolol, with oil-in-water-type microemulsions using a combination of ethyl butyrate and Pluronic F127. The group fabricated the microemulsion-laden gels, ethyl butyrate/water microemulsions stabilized by Pluronic F127 surfactant, and subsequently polymerized after the addition of HEMA. The timolol microemulsion–loaded gels exhibited a slow and extended drug release in DI water. The delivery rates showed a sigmoidal shape due to long-term degradation of the gel. These systems also have a very high timolol loading and can be used as a vehicle for hydrophobic drug delivery to the ocular surface.
Vitamin E–loaded lenses
Chauhan and colleagues developed the technique using Vitamin E as an in situ transport barrier for timolol. The drug release was significantly increased, by elevating the loading concentration of Vitamin E from 10% to 40% in contact lenses.28 The group demonstrated a quadratic increase in drug release duration in Vitamin E loading. Loadings of 10% and 40% Vitamin E increased the release time of timolol by a factor of about 5 and 400, respectively. However, Vitamin E loading in the lens led to an increase in lens sizes, a reduction in oxygen diffusion, and a significant reduction in ion permeability.
Film impregnation in contact lens
Ciolino and colleagues35 designed a latanoprost-eluting contact lens for the treatment of glaucoma, manufactured by encapsulating the drug film enclosed in methafilcon lenses. These lenses have shown sustained release for up to 1 month in glaucomatous monkeys. The amount of drug delivered to the eye exceeded or was comparable to that delivered by topical drops. Contact lenses with polymer drug films (40–45 mm in thickness) demonstrated an initial burst of latanoprost in the aqueous humor and subsequently a steady concentration that was similar to the average hourly concentration delivered from a drop of commercially available latanoprost solution. The contact lenses were produced in high (149 g latanoprost; CLHI) and low (97 g latanoprost; CLLO) dose variations. The IOP-lowering efficacy of the high-dose (149 g) and low-dose (97 g) latanoprost-eluting contact lenses was compared with latanoprost eye drops in adult monkeys with glaucoma with a 3-week washout period between the two treatments. The authors reported a mean IOP reduction in the ranges of 2.9 ± 1.0 to 6.6 ± 1.3 mmHg with topical latanoprost, 4.0 ± 1.1 to 7.8 ± 3.8 mmHg in 97-g contact lenses, and 6.0 ± 4.4 to 10.2 ± 2.5 mmHg with 149-g contact lenses. The results from the study showed that IOP lowering with the latanoprost-eluting contact lens was as more persistent and equally efficacious as topical latanoprost eye drops.
Enzyme-triggered timolol release
Kim and colleagues36 embedded nanodiamonds (NDs) loaded with timolol in contact lenses. The ND–nanogel embedded contact lens acts as an enzyme trigger for the delivery of timolol. The nanogels sequester timolol prior activation of the lysozyme that causes chitosan degradation and subsequently allow sustained drug release. After 24-h treatment with lysozyme, the total steady drug release from the lens was 9.41 μg. However, some variability was reported in the drug release after 48 h of lysozyme treatment. Optical clarity, water content, and oxygen permeability are maintained at practical levels.
The decreased ion and oxygen permeability, protein adherence, alterations in mechanical properties, and lens-related infections due to continuous wear significantly limit the application of contact lenses for drug delivery.
Intraocular implants
Intravitreal implants
Intravitreal implants are capable of delivering drugs for prolonged duration in the eye. Although surgical implants present a viable option for long-term drug delivery, the invasive nature of the initial and subsequent surgical procedures to remove the implants does not make them a favorable choice for drug delivery. OZURDEX is a degradable dexamethasone intravitreal implant produced by Allergan, which has been used to treat macular edema and noninfectious uveitis. The device slowly degrades after implantation in the vitreous while delivering dexamethasone. Currently, the manufacturer is conducting clinical trials of the implants loaded with brimonidine tartrate in the proprietary NOVADUR poly(lactic-co-glycolic acid) (PLGA) intravitreal polymer matrix platform for the management of geographic atrophy due to age-related macular degeneration. Currently, topical daily ophthalmic brimonidine tartrate drops are prescribed for IOP reduction and neuroprotective effect; if the NOVADUR PLGA platform implants with brimonidine tartrate are approved, they can also be adapted for use in glaucoma patients.37
Subconjunctival inserts
The subconjunctival inserts are used as implants as a replacement for viscoelastic depot delivery injections. The VS101 ocular insert is one such insert developed by ViSci Inc. in 2014 and is currently undergoing a phase I/II multicentric randomized control study to evaluate the safety and effectiveness of subconjunctival latanoprost insert in subjects with ocular hypertension or OAG.38
PCL-PEG inserts
Ng and colleagues used a biodegradable microfilm synthesized by a combination of poly(lactide)/poly(ε-caprolactone) (PLC) and poly(ε-caprolactone)/poly(ethylene glycol) (PLC/PCL-PEG). The polymer was loaded with timolol maleate and inserted by conjunctival dissection.39 The authors reported a reduction of 50.1% ± 8.5% in IOP from baseline in primates with ocular hypertension, which was sustained for 140 days.
AP-PCL inserts
Alkoxylphenacyl-based polycarbonate copolymers in combination with polycaprolactone (AP-PCL) were used by Manickavasagam and colleagues40 for sustained delivery of brimonidine tartrate for up to 3 months. The major drawback attributed to the subconjunctival inserts is the requirement of a surgically invasive procedure, which creates a small opening in the conjunctiva with a possibility of subconjunctival migration, infection, and need of an Operating Room procedure for insertion and removal of the device.
Microelectromechanical system
The microelectromechanical system (MEMS) works on the principle of bubble generation by electrolysis to mechanically push the loaded drug out of the reservoir. The device, currently in a preclinical development phase, also allows loading the drug multiple times.41 Saati and colleagues demonstrated that the use of the MEMS pumping mechanism is based on electrolysis connected to a drug refill port as well as a check valve to control delivery. The procedure is similar to implantation of a glaucoma aqueous drainage device. MEMS enables ophthalmologists to instill drug in the outpatient setting without any invasive procedure. The single dosage loading of drug usually lasts for a very long duration of 3–4 months and the rate of electrolysis can be used to regulate the rate of drug release. It can be used for intravitreal delivery of multiple drugs with minor modifications.
The only disadvantage is that it requires a minimally invasive procedure for implantation in the eye with associated short- and long-term risks.
Liposomes
The liposome-encapsulated drug is delivered as a solution as an eye drop. Natarajan and colleagues used latanoprost-loaded egg-phosphatidylcholine liposomes for delivery. The liposomes remained stable for at least 6 months on storage at 4°C and at least 1 month at 25°C. A slow and sustained release of 60% of latanoprost was achieved by 2 weeks in vitro. A greater sustained IOP-lowering effect was recorded in liposome-treated animals (4.8 ± 1.5 mmHg) compared with daily administration of topical latanoprost (2.5 ± 0.9 mmHg) beyond 90 days.42 Monem and colleagues used multilamellar vesicles (MLVs) as a vehicle for delivering pilocarpine. They reported neutral MLVs encapsulating pilocarpine HCl exhibited the most prolonged efficacy in the reduction of IOP. They also reported that negatively charged MLVs encapsulating pilocarpine HCl and free pilocarpine HCl exhibited a significantly shorter period of drug action.43 The group speculated that the frequency of administration of drug administration in humans would be reduced to half with the usage of MLV vehicle, thus promoting better compliance.
Polymeric nanoparticles
Due to their molecular scale size, nanoparticles efficaciously deliver drugs in the anterior chamber as well as in the posterior compartment via the blood–aqueous and the blood–retina barrier, respectively.44 Different types of nanoparticles are classified on the basis of the origin of the constituent monomers. The emphasis is also being laid on effective drug loading on the nanoparticles through process such as electrospraying and electrospinning. Mehta and colleagues demonstrated a single-needle electrohydrodynamic process for adding a stable nanocoating to the contact lenses with timolol maleate. The in vitro studies showed biphasic release of the drug, with an initial burst release followed by sustained release.45
Chitosan-based polymeric nanoparticles
Chitosan, a 2-amino-2-doexy-beta-D-glucan, is being widely tested for synthesizing nanoparticles for drug delivery.46 The biodegradable, biocompatible, and mucoadhesive properties of chitosan make it highly suitable for delivering antiglaucoma drugs. Li and colleagues47 developed chitosan nanoparticles loaded with beta-adrenergic agent betaxolol, prescribed for lower IOP. The ex vivo data published by the authors show a 1.75 times higher value compared with the topical eye drops. Zhao and colleagues48 have developed timolol maleate–loaded nanoparticles from glycosylated polymers of chitosan for ocular delivery. The authors reported an augmented transcorneal penetration due to high lipid solubility. The authors reported that the data from the in vivo experiments showed a sustained release over a significantly longer duration of time. Mehta and colleagues used electrohydrodynamic atomization of timolol maleate–loaded PVP and poly(N-isopropylacrylamide). The authors used the formulation approach for sustained timolol maleate release using the chitosan and borneol combination. The authors reported biphasic and triphasic release, depending on composition.49
Poly(lactic-co-glycolic acid) nanoparticles
PLGA is a copolymer of cyclic dimers (1,4-dioxane-2,5-diones) of glycolic acid and lactic acid.50 Salama and colleagues51 used PLGA nanoparticles for the delivery of brinzolamide subconjunctivally. The authors reported the release of drug prolonged for a period of up to 10 days after a single dosage. Khan and colleagues used PLGA-based nanoparticles for the delivery of forskolin, a natural extract with potent noradrenergic stimulatory action on adenylate cyclase.52,53 The authors reported a steady release of the drug from the PLGA polymer, with 90% release over 72 h compared with eye drops (96.6% release in 12 h).
Gelatin nanoparticles
The ease of availability and high biocompatibility make gelatin a favorable polymeric vehicle for delivery of antiglaucoma drugs to the eye. Recently, Shokry and colleagues reported the use of gelatin nanoparticles for delivering timolol maleate. The authors reported increased mucoadhesion and transcorneal permeability due to its positive charge attracted to negatively charged lipid layers in the cornea.54 The in vitro release studies showed a burst effect of timolol release followed by a sustained profile over a prolonged duration. The in vivo studies in the albino rabbits showed a sustained and higher efficacy in IOP lowering. In another study, Liao and colleagues55 used silica-based mesoporous nanoparticles for pilocarpine with gelatin coating. The in vitro data showed a 36-day release profile of the gelatin-coated mesoporous nanoparticles with an efficacious in vivo IOP-lowering effect for 21 days.
Propoxylated glyceryl triacylate nanoparticles
Jung and colleagues56 developed a contact lens based on the principle of dispersing timolol-loaded propoxylated glyceryl triacylate (PGT) nanoparticles within the lens. Timolol–PGT particles release drug for an extended period (>30 days at room temperature) by steady hydrolysis of the ester bond. The bioavailability of timolol delivered through the contact lens showed 50% bioavailability as compared with only 1–2% through eye drops. A significant decrease in the IOP was also recorded albeit over a very short term (<5 days). A major disadvantage of the model was the reduction in both ion and oxygen permeability and an increase in storage modulus.
PGT–ethylene glycol dimethacrylate nanoparticles
Jung and Chauhan57 also developed a lens with highly cross-linked particles consisting of monomeric units with multivinyl functionalities such as PGT and ethylene glycol dimethacrylate (EGDMA). The 3.5-nm nanoparticles encapsulated 48–66% of the drugs. The rate constant of ester hydrolysis was significantly less than those of the previous models developed by the same group, possibly due to steric effects and the low water content of the highly cross-linked hydrophobic particles. The nanoparticles dispersing timolol were encapsulated in highly linked nanoparticles enclosed within contact lenses, which increases the duration of drug release from 1 to 2 h to about 4 weeks. The drug-dispensing particles were dispersed in hydroxymethyl methacrylate (HEMA) gels, which are commonly used for manufacturing contact lenses.
Xu and colleagues58 developed micelles that could be loaded on the contact lenses for sustained release of timolol and latanoprost simultaneously for management of glaucoma. The micelles were synthesized by free radical polymerization of HEMA monomer with timolol and latanoprost. The lenses released timolol and latanoprost in tear fluid for 144 and 120 h, respectively. The in vivo data showed sustained timolol and latanoprost release for 120 and 96 h in tear fluid, respectively. The mean residence time of the drugs was 79.6- and 122.2-fold, and bioavailability was 2.2- and 7.3-fold for timolol and latanoprost release from the micelles loaded in the lenses, respectively, compared with the topical eye drop. The studies performed on rabbits showed a sustained reduction in the IOP for over 168 h and almost a 10-fold increase in pharmacological availability compared with the eye drops, with minimal side effects.
Nanospheres/microspheres
The penetration of drug loaded on the nanosphere depends on the size, charge, architecture, and surface chemistry of the carrier nanoparticle systems.59,60 The architecture of the nanospheres consists of a diblock copolymer, that is, a hydrophobic block [polycaprolactone (PCL)] and a hydrophilic component [polyethylene glycol (PEG)]. The unique structure of nanospheres allows a longer residence time on the surface of the cornea to provide the drug with a carrier followed by fusion with the corneal epithelial membrane, hence reducing the dosing frequency.61 Chiang and colleagues62 reported a reduction in IOP for 1 month in normotensive rabbit eyes, initially by 6 mmHg with brimonidine polylactic acid (PLA) microspheres. The in vitro analysis of the brimonidine microspheres showed a sustained release of drug for 35 days.
OHR1031
OHR1031 is a micromolecular drug for glaucoma management, which is incorporated into microparticles using a dissolvable hydrogel template technology.63 The drug is dissolved into a PLGA polymer–solvent mixture and the microparticles formed using the dissolvable template technology. The median size of drug-loaded particles is 60 μm. The authors reported that the OHR1031 content in the microparticles was 57% with almost 100% incorporation efficiency. The drug release rate was of nearly zero order for over 3 months with virtually no initial burst.
SoliDrop
SoliDrop by Otero Therapeutics consists of a thermoresponsive hydrogel carrier and drug-loaded polymer microspheres. On administering a single brimonidine-loaded gel/microsphere drop, the IOP-lowering efficacy (reduced by 30% of baseline IOP) was comparable to that of rabbits receiving twice-daily, standard brimonidine drops for a period of 28 days. The gel/microsphere drop was retained in the fornix during the entire period of the study.64
Injectable systems
Injectable systems are passive delivery systems, capable of delivering medications to the target tissues for an extended period. The injectable systems are typically implanted at the site of drug release through a minimally invasive procedure, usually in an outpatient setting. The injectable systems use a polymer delivery vehicle to prolong delivery up to a few months in the surrounding tissue. Recently, both degradable as well as nondegradable polymers have been developed for use as injectable systems for drug delivery in the eye.65 Degradable PLGAs are materials of choice for developing such a system. The nondegradable alternative such as the polymer of ethylene-co-vinyl acetate may lead to an immune response due to the prolonged presence of a foreign body.66 The rate of dispersion of drugs from these systems is variable, with initial more massive quantity release. The water solubility of the drug affects the efficacy because hydrophilic drugs interact poorly with degradable polymers as they are hydrophobic.67
ENV 515/travoprost XR
ENV515 PGA/travoprost XR therapy is a particle replication in nonwetting template–based biodegradable polymer drug delivery system. The implant is rod shaped and fits the anatomy of the iridocorneal angle in the anterior chamber and allows its administration via an acceptably sized needle. The results from the phase II clinical trial report that a single low dose of ENV515 decreased the mean IOP by 6.7 ± 3.7 mmHg over 11 months. The mean IOP after a single low dose of ENV515 was 19.5 mmHg over the 11-month period.68
Bimatoprost SR
Bimatoprost SR is a biodegradable implant for the reduction of IOP with a 4-month sustained release period.69 In the first phase III clinical study, Bimatoprost SR reduced IOP by 30% over the 12-week primary efficacy period. The results showed no requirement of supplementary treatment for IOP lowering for 1 year after the last implant insertion. The magnitude of IOP-lowering efficacy with Bimatoprost SR observed in this study is similar to that observed with daily topical prostaglandin analogues. Bimatoprost SR was well tolerated in the majority of patients.
Graybug
Graybug is a drug-encapsulated microparticle formulation to provide continuous IOP lowering that is administered by the treating physician every 3–6 months using a subconjunctival injection. GB-203 is a preclinical stage dual model of action, single molecular entity agent that can hydrolyze into an active agent that has the potential to lower IOP and a second active agent that can provide long-term neuroprotection.70 Another pilot polymer depot formulation of GB-6249-103 developed on the Graybug platform safely has been shown to deliver its payload in a sustained manner both in vitro and in vivo.71 A significant reduction in IOP was observed within the first week following injection of the formulation in rabbits. The results recorded a sustained maximum IOP lowering of ~20% for over a period of 2 months.
Punctal plugs
Punctal occlusion with the plugs blocks the tear drainage system and prevents the runoff of natural tears on the ocular surface. Over the past decade, multiple solid or semisolid models have been developed with an added drug-eluting functionality to the punctal plugs for use in glaucoma for sustained release of drug for 3–4 months.
The ease of insertion of the plugs in an outpatient setting makes it a widely accepted drug delivery system. However, foreign body sensation post-placement may be a significant deterrent in some patients.
OTX-TP
The OTX-TP (Ocular Therapeutix) delivers travoprost to the ocular surface via an intracanalicular punctal plug for up to 3 months, as well as resorbs and drains through the nasolacrimal system.72 It consists of PEG-based hydrogel with embedded travoprost–loaded PLA microspheres. These microspheres slowly degrade and show a sustained drug release over a period of 30 days. A study by Perera and colleagues73 reported 100% retention rate of the plugs, 10 days post-implantation, and a reduction in IOP by 5.4–7.5 mmHg. It is minimally invasive, contains fluorescein to monitor any retention, and clears from the body through absorption. The studies have shown an enhanced therapeutic benefit for 90 days with a consistent 90% retention rate. The phase II trial did not find any serious adverse effects and showed only a slightly less hypotensive effect as compared with timolol.
Latanoprost punctal plug delivery system
Goldberg and Williams74 used the Latanoprost Punctal Plug Delivery System (L-PPDS) for lowering IOP in OAG patients. The data reported by the authors showed a reduction in mean IOP by 5.7 mmHg. They also reported that 60% of subjects in the study showed at least 5 mmHg or greater IOP reduction and 47% of the subjects showed a reduction of at least 6 mmHg. A statistically significant 22.3% mean change in IOP was recorded in the subjects with L-PPDS when compared with controls.
Evolute
Evolute, a punctal plug delivery system developed by Mati Therapeutics has been tested with latanoprost in patients with OAG or ocular hypertension.75 The plug consists of a drug core, which allows unidirectional sustained drug elution into the tear film. In the phase II clinical trial, an overall punctal plug retention rate of 96% was reported at 12 weeks. In the second phase, the retention rate of plugs was 92% at the 12th week. The punctal plugs loaded with travoprost reduced the pressure by 7 mm compared with a 5 mmHg decrease in pressure with latanoprost.
Pentablock copolymer gels
The pentablock copolymer gels are used as a vehicle for topical and intraocular delivery of glaucoma drugs like bimatoprost. Currently, the Food and Drug Administration (FDA) has approved five different pentablock copolymers for use in the eye. These include polyglycolic acid (PGA), PCL, PEG, PLA, and PLGA.76
The drug is introduced as an eye drop, which then undergoes a change in physical characteristics on the basis of body temperature at contact. The change in viscosity gives the vehicle copolymer gel-like characteristics, which gets accumulated under the lower palpebra, releasing the drug over a longer period. Depending on the dose, the time course can be 2, 4, or even 6 months. DuraSite ISV-215 (InSite Vision, Inc.) uses pentablock copolymers to form a stable mucoadhesive matrix of bimatoprost 0.03% that provides sustained release of the drug over a more extended period.77 ISV-215 had a 2.2-fold higher bimatoprost concentration than the control in the aqueous humor and a 6.1-fold increase in the iris–ciliary body.
Microneedles
Microneedles are drug delivery devices manufactured using metals or polymers with dimensions between 10 and 200 μm. The ultradimensions of these devices make the drug delivery less invasive and more targeted to the sites of drug action. Jiang and colleagues used 500- to 750-μm-long coated stainless steel microneedles delivering pilocarpine into the anterior chamber via the intrascleral route. The authors reported a 45-fold increase in drug absorption compared with conventional eye drops.78
Discussion
Multiple studies have laid emphasis on the importance of adherence, compliance, and persistence for appropriate management of glaucoma. Although the available drugs are efficacious in lowering IOP as well as neuroprotection, the traditional methods of topical drug delivery have been deemed as inefficient due to poor target bioavailability, increased systemic absorption, and poor patient compliance.
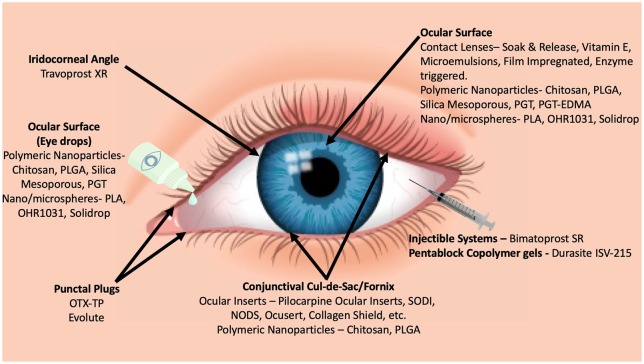
This review attempts to highlight the direction of the ongoing innovation and research to address the challenge of safer and more effective drug delivery. A majority of the devices reviewed in this article are currently in various stages of development, and not commercially available. The impact of these devices on the patients can only be gauged once they are available for clinical use and extensive clinical data are available for scrutiny. Notwithstanding the lack of data, the critical role of these devices in glaucoma management in the near future needs to be emphasized. The potential of increasing patient compliance and persistence for optimum outcomes with the help of these devices is unprecedented. Eye drop instillation has always been a challenge, especially in the geriatric and pediatric age group patients. Effective localized delivery will prevent drug loss due to systemic absorption and first-pass metabolism, thereby minimizing drug wastage. The high prevalence of ocular surface disease in patients instilling antiglaucoma drugs with added preservatives may be overcome with the newer devices. The most significant advantage of these devices will be the improvement in the quality of life of the patients who currently have to adhere to a strict regime of repeatedly putting eye drops throughout the day (Figure 1 and Table 1).
Figure 1.
Different sustained release drug delivery systems in various stages of development.
Table 1.
Summary of the antiglaucoma drug delivery systems in different stages of development.
| Drug delivery system | Drug | Material | Placement location | Current stage of development | References |
|---|---|---|---|---|---|
| Ocular inserts | |||||
| Pilocarpine ocular inserts | Pilocarpine | Soluble collagen biomatrix | Conjunctival cul-de-sac | Clinical | Bensinger and colleagues9 |
| Soluble ocular drug insert | Pilocarpine | Polyacrylamide, ethyl acrylate, and vinyl pyrrolidone | Inferior cul-de-sac | Clinical | Maichuk10 |
| Ocusert | Pilocarpine | Di-(ethylhexyl)phthalate, ethylene-vinyl acetate | Conjunctival cul-de-sac | Clinical | Armaly and Rao11 |
| Poly(vinyl methyl ether-maleic anhydride)anhydride | Timolol | Poly (vinyl methyl ether-maleic anhydride) anhydride (PVMMA) and its alkyl monoesters | Conjunctival cul-de-sac | Clinical | Finne and colleagues15 |
| Collagen shield | Pilocarpine | Collagen matrix with titanium dioxide (TiO2), zinc oxide (ZnO), and polyvinylpyrrolidone (PVP) | Ocular surface | Preclinical | Agban and colleagues 18 |
| Ocufit SR | Various | Silicon elastomer | Conjunctival cul-de-sac/fornix | Clinical | Katz and Blackman19 |
| Minidisc | Various | Hydroxyethyl methacrylate, ethylene glycol methacrylate | Under the eyelid | Phase I clinical trial | Kumari and colleagues8 |
| New ophthalmic delivery system | Pilocarpine | Polyvinyl alcohol | Lower conjunctival sac | Phase I clinical trial | Greaves and colleagues21 |
| Topical bimatoprost ocular insert | Bimatoprost | Silicone matrix, polypropylene | Conjunctival cul-de-sac/fornix | Phase II clinical trial | De Souza and colleagues23 |
| Therapeutic contact lens | |||||
| Soak and release | Pilocarpine | Vinyl pyrrollidone | Ocular surface | Clinical | Hillman31 |
| Microemulsions | Timolol | Octadecyltrimethoxysilane (OTMS)/ethyl butyrate and Pluronic F127 | Ocular surface | Preclinical | Gulsen and Chauhan32 and Li and colleagues33 |
| Vitamin E | Timolol | Vitamin E | Ocular surface | Preclinical | Peng and colleagues27 |
| Film impregnation in contact lens | Latanoprost | Methafilcon | Ocular surface | Preclinical | Ciolino and colleagues34 |
| Enzyme triggered | Timolol | Nanodiamond–nanogel | Ocular surface | Preclinical | Kim and colleagues35 |
| Intraocular implants | |||||
| Intravitreal | Brimonidine | NOVADUR-poly(D, L) sustained lactide-co-glycolic acid (PLGA) | Vitreous humor | Phase III Clinical Trial | ClinicalTrials.gov36 |
| Subconjunctival inserts | Latanoprost | – | Subconjunctival space | Phase IIa clinical trial | ClinicalTrials.gov37 |
| PCL-PEG inserts | Timolol | Poly(lactide)/poly(ε-caprolactone) (PLC) and poly(ε-caprolactone)/poly(ethylene glycol) (PLC/PCL-PEG) | Subconjunctival space | Preclinical | Ng and colleagues38 |
| AP-PCL inserts | Brimonidine | Alkoxylphenacyl-based polycarbonates, polycaprolactone | Subconjunctival space | Preclinical | Manickavasagam and colleagues39 |
| Microelectromechanical system (MEMS) | Various | Subconjunctival space | Preclinical | Saati and colleagues40 | |
| Liposomes | Latanoprost | Egg-phosphatidylcholine | Ocular surface | Preclinical | Venkatraman and colleagues41 |
| Polymeric nanoparticles | |||||
| Chitosan | Betaxolol | 2-Amino-2-doexy-beta-D-glucan | Ocular surface | Preclinical | Li and colleagues46 |
| Chitosan | Timolol | 2-Amino-2-doexy-beta-D-glucan | Ocular surface | Preclinical | Zhao and colleagues47 |
| Chitosan | Timolol | 2-Amino-2-doexy-beta-D-glucan, borneol, polyvinylpyrrolidone, poly(N-isopropylacrylamide) | Ocular surface—contact lens | Preclinical | Mehta and colleagues48 |
| Polylactic-co-glycolic acid (PLGA) | Brinzolamide | 1,4-Dioxane-2,5-diones of glycolic acid and lactic acid | Subconjunctival space | Preclinical | Salama and colleagues50 |
| Polylactic-co-glycolic acid (PLGA) | Forskolin | 1,4-Dioxane-2,5-diones of glycolic acid and lactic acid | Subconjunctival space | Preclinical | Khan and colleagues51 and Caprioli and Sears52 |
| Gelatin | Timolol | Gelatin | Ocular surface | Preclinical | Shokry and colleagues53 |
| Silica mesoporous | Pilocarpine | Silica mesoporous polymer with gelatin coating | Ocular surface | Preclinical | Liao and colleagues54 |
| PGT nanoparticles | Timolol | Propoxylated glyceryl triacylate | Ocular surface—contact lens | Preclinical | Jung and colleagues55 |
| PGT-EGDMA | Timolol | propoxylated glyceryl triacylate (PGT) and ethylene glycol dimethacrylate (EGDMA) | Ocular surface | Preclinical | Jung and Chauhan56 |
| Nano/microspheres | |||||
| Polylactic acid (PLA) microspheres | Brimonidine | PLA | Ocular surface | Preclinical | Uchida58 |
| OHR1031 | OHR1031 | Poly(lactic-co-glycolic acid) polymer solvent | Ocular surface | Preclinical | Chiang and colleagues59 |
| SoliDrop | Brimonidine | – | Ocular surface | Preclinical | Malavia and colleagues60 |
| Injectable systems | |||||
| ENV515/PGA or travoprost XR | Travoprost | – | Iridocorneal angle | Phase II clinical trial | Bao and colleagues64 |
| Bimatoprost SR | Bimatoprost | – | Ocular surface | Phase III clinical trial | ClinicalTrials.gov65 |
| Graybug | Various | – | Subconjunctival space | Preclinical | Allergan66 |
| Punctal plugs | |||||
| OTX-TP | Travoprost | Polyethylene glycol–based hydrogel with loaded PLA | Puncta | Phase II clinical trial | Hoang and colleagues68 |
| Evolute | Latanoprost | – | Puncta | Phase II clinical trial | Perera and colleagues70 |
| Latanoprost Punctal Plug Delivery System (L-PPDS) | Latanoprost | – | Puncta | Phase II clinical trial | Goldberg and Williams71 |
| Pentablock copolymer gels | |||||
| DuraSite ISV-215 | Bimatoprost | – | Ocular surface | Preclinical | Utkhede and William72 |
| Microneedles | |||||
| Clearside microneedles | Various | Metal/polymer | Supraciliary space | Preclinical | Shafiee and colleagues74 |
AP-PCL, alkoxylphenacyl-based polycarbonate copolymers in combination with polycaprolactone; PGA, polyglycolic acid.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors. The manuscript is limited to a review of literature about the drug delivery systems that are currently available or are under development. Therefore, no approval from ethical review board is required.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Our manuscript does not include any patient data or results, and thus patient consent is not applicable for this manuscript.
ORCID iD: Rohan B. Singh  https://orcid.org/0000-0002-2426-3900
https://orcid.org/0000-0002-2426-3900
Contributor Information
Rohan B. Singh, Massachusetts Eye and Ear, Department of Ophthalmology, Harvard Medical School, Boston, MA 02114, USA.
Parul Ichhpujani, Department of Ophthalmology, Government Medical College & Hospital, Chandigarh, Chandigarh, India.
Sahil Thakur, Department of Ophthalmology, Government Medical College & Hospital, Chandigarh, Chandigarh, India; Singapore Eye Research Institute, Singapore.
Sumeet Jindal, Department of Ophthalmology Virginia Commonwealth University School of Medicine Richmond, VA, USA.
References
- 1. Kingman S. Glaucoma is second leading cause of blindness globally. Bull World Health Organ 2004; 82: 887–888. [PMC free article] [PubMed] [Google Scholar]
- 2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006; 90: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology 1998; 105: 2099–2104. [DOI] [PubMed] [Google Scholar]
- 4. Mohindroo C, Ichhpujani P, Kumar S. How ‘drug aware’ are our glaucoma patients. J Curr Glaucoma Pract 2015; 9: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wilson CG, Zhu YP, Frier M, et al. Ocular contact time of a carbomer gel (GelTears) in humans. Br J Ophthalmol 1998; 82: 1131–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol 1986; 2: 67–108. [DOI] [PubMed] [Google Scholar]
- 7. Wei G, Xu H, Ding PT, et al. Thermosetting gels with modulated gelation temperature for ophthalmic use: the rheological and gamma scintigraphic studies. J Control Release 2002; 83: 65–74. [DOI] [PubMed] [Google Scholar]
- 8. Kumari A, Sharma PK, Garg VK, et al. Ocular inserts—advancement in therapy of eye diseases. J Adv Pharm Technol Res 2010; 1: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bensinger R, Shin DH, Kass MA, et al. Pilocarpine ocular inserts. Invest Ophthalmol 1976; 15: 1008–1010. [PubMed] [Google Scholar]
- 10. Maichuk YF. Soluble ophthalmic drug inserts. Lancet 1975; 305: P173. [DOI] [PubMed] [Google Scholar]
- 11. Armaly MF, Rao KR. The effect of pilocarpine Ocusert with different release rates on ocular pressure. Invest Ophthalmol 1973; 12: 491–496. [PubMed] [Google Scholar]
- 12. Worthen DM, Zimmerman TJ, Wind CA. An evaluation of the pilocarpine Ocusert. Invest Ophthalmol 1974; 13: 296–299. [PubMed] [Google Scholar]
- 13. Macoul KL, Pavan-Langston D. Pilocarpine Ocusert system for sustained control of ocular hypertension. Arch Ophthalmol 1975; 93: 587–590. [DOI] [PubMed] [Google Scholar]
- 14. Sihvola P, Puustjarvi T. Practical problems in the use of Ocusert-pilocarpine delivery system. Acta Ophthalmol (Copenh) 1980; 58: 933–937. [DOI] [PubMed] [Google Scholar]
- 15. Finne U, Väisänen V, Urtti A. Modification of ocular and systemic absorption of timolol from ocular inserts by a buffering agent and a vasoconstrictor. Int J Pharm 1990; 65: 19–27. [Google Scholar]
- 16. Tannebaum S., Svyatoslav Fyodorov M.D: innovative eye surgeon. J Am Optom Assoc 1995; 66: 652–654. [PubMed] [Google Scholar]
- 17. Bloomfield SE, Miyata T, Dunn MW, et al. Soluble gentamicin ophthalmic inserts as a drug delivery system. Arch Ophthalmol 1978; 96: 885–887. [DOI] [PubMed] [Google Scholar]
- 18. Agban Y, Lian J, Prabakar S, et al. Nanoparticle cross-linked collagen shields for sustained delivery of pilocarpine hydrochloride. Int J Pharm 2016; 501: 96–101. [DOI] [PubMed] [Google Scholar]
- 19. Katz IM, Blackman WM. A soluble sustained-release ophthalmic delivery unit. Am J Ophthalmol 1977; 83: 728–734. [DOI] [PubMed] [Google Scholar]
- 20. Bawa R, Nandu M. Physico-chemical considerations in the development of an ocular polymeric drug delivery system. Biomaterials 1990; 11: 724–728. doi:10.1016/0142-9612(90)90035-O [DOI] [PubMed] [Google Scholar]
- 21. Diestelhorst M, Krieglstein GK. The ocular tolerability of a new ophthalmic drug delivery system (NODS). Int Ophthalmol 1994; 18: 1–4. [DOI] [PubMed] [Google Scholar]
- 22. Greaves J, Wilson C, Birmingham A, et al. Scintigraphic studies on the corneal residence of a New Ophthalmic Delivery System (NODS): rate of clearance of a soluble marker in relation to duration of pharmacological action of pilocarpine. Br J Clin Pharmacol 1992; 33: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brandt JD, Sall K, DuBiner H, et al. Six-month intraocular pressure reduction with a topical bimatoprost ocular insert: results of a phase II randomized controlled study. Ophthalmology 2016; 123: 1685–1694. [DOI] [PubMed] [Google Scholar]
- 24. De Souza JF, Maia KN, De Oliveira Patrício PS, et al. Ocular inserts based on chitosan and brimonidine tartrate: development, characterization and biocompatibility. J Drug Deliv Sci Technol 2016; 32: 21–30. [Google Scholar]
- 25. Stewart RH, Novak S. Introduction of the Ocusert ocular system to an ophthalmic practice. Ann Ophthalmol 1978; 10: 325–330. [PubMed] [Google Scholar]
- 26. Hitoshi S, Choyu T, Koyo N, et al. Drug release from an ophthalmic insert of a beta-blocker as an ocular drug delivery system. J Control Release 1993; 27: 127–137. [Google Scholar]
- 27. Li CC, Chauhan A. Modeling ophthalmic drug delivery by soaked contact lenses. Ind Eng Chem Res 2006; 45: 3718–3734. [Google Scholar]
- 28. Peng C-C, Kim J, Chauhan A. Extended delivery of hydrophilic drugs from silicone-hydrogel contact lenses containing Vitamin E diffusion barriers. Biomaterials 2010; 31: 4032–4047. [DOI] [PubMed] [Google Scholar]
- 29. Peppas NA, Bures P, Leobandung W, et al. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm 2000; 50: 27–46. [DOI] [PubMed] [Google Scholar]
- 30. Hiratani H, Alvarez-Lorenzo C. Timolol uptake and release by imprinted soft contact lenses made of N, N-diethylacrylamide and methacrylic acid. J Control Release 2002; 83: 223–230. [DOI] [PubMed] [Google Scholar]
- 31. Waltman SR, Kaufman HE. Use of hydrophilic contact lenses to increase ocular penetration of topical drugs. Invest Ophthalmol 1970; 9: 250–255. [PubMed] [Google Scholar]
- 32. Hillman JS. Management of acute glaucoma with Pilocarpine soaked hydrophilic lens. Br J Ophthalmol 1974; 58: 674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gulsen D, Chauhan A. Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. Int J Pharm 2005; 292: 95–117. [DOI] [PubMed] [Google Scholar]
- 34. Li C-C, Abrahamson M, Kapoor Y, et al. Timolol transport from microemulsions trapped in HEMA gels. J Colloid Interface Sci 2007; 315: 297–306. [DOI] [PubMed] [Google Scholar]
- 35. Ciolino JB, Stefanescu CF, Ross AE, et al. In vivo performance of a drug-eluting contact lens to treat glaucoma for a month. Biomaterials 2014; 35: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim H-J, Zhang K, Moore L, et al. Diamond nanogel-embedded contact lenses mediate lysozyme-dependent therapeutic release. ACS Nano 2014; 8: 2998–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ClinicalTrials.gov. A safety and efficacy study of brimonidine intravitreal implant in geographic atrophy secondary to age-related macular degeneration (BEACON). https://clinicaltrials.gov/ct2/show/NCT02087085.
- 38. ClinicalTrials.gov. A phase 1/2 multicenter, randomized, study to evaluate the safety and efficacy of VS101 subconjunctival latanoprost insert in subjects with open-angle glaucoma or ocular hypertension. https://clinicaltrials.gov/ct2/show/NCT02129673 (accessed 25 August 2017).
- 39. Ng XW, Liu KL, Veluchamy AB, et al. A biodegradable ocular implant for long-term suppression of intraocular pressure. Drug Deliv Transl Res 2015; 5: 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manickavasagam D, Wehrung D, Chamsaz EA, et al. Assessment of alkoxylphenacyl-based polycarbonates as a potential platform for controlled delivery of a model anti-glaucoma drug. Eur J Pharm Biopharm 2016; 107: 56–66. [DOI] [PubMed] [Google Scholar]
- 41. Saati S, Lo R, Li P-Y, et al. Mini drug pump for ophthalmic use. Trans Am Ophthalmol Soc 2009; 107: 60–70. [PMC free article] [PubMed] [Google Scholar]
- 42. Natarajan JV, Ang M, Darwitan A, et al. Nanomedicine for glaucoma: liposomes provide sustained release of latanoprost in the eye. Int J Nanomedicine 2012; 7: 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monem AS, Ali FM, Ismail MW. Prolonged effect of liposomes encapsulating pilocarpine HCl in normal and glaucomatous rabbits. Int J Pharm 2000; 198: 29–38. [DOI] [PubMed] [Google Scholar]
- 44. Zarbin MA, Montemagno C, Leary JF, et al. Nanotechnology in ophthalmology. Can J Ophthalmol 2010; 45: 457–476. [DOI] [PubMed] [Google Scholar]
- 45. Mehta P, Al-Kinani AA, Haj-Ahmad R, et al. Electrically atomised formulations of timolol maleate for direct and on-demand ocular lens coatings. Eur J Pharm Biopharm 2017; 119: 170–184. [DOI] [PubMed] [Google Scholar]
- 46. Ibrahim KA, El-Eswed BI, Abu-Sbeih KA, et al. Preparation of chito-oligomers by hydrolysis of chitosan in the presence of zeolite as adsorbent. Mar Drugs 2016; 14: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li J, Tian S, Tao Q, et al. Montmorillonite/chitosan nanoparticles as a novel controlled-release topical ophthalmic delivery system for the treatment of glaucoma. Int J Nanomedicine 2018; 13: 3975–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao R, Li J, Wang J, et al. Development of timolol-loaded galactosylated chitosan nanoparticles and evaluation of their potential for ocular drug delivery. AAPS Pharmscitech 2017; 18: 997–1008. [DOI] [PubMed] [Google Scholar]
- 49. Mehta P, Al-Kinani AA, Arshad MS, et al. Engineering and development of chitosan-based nanocoatings for ocular contact lenses. J Pharm Sci 2019; 108: 1540–1551. [DOI] [PubMed] [Google Scholar]
- 50. Samadi N, Abbadessa A, Di Stefano A, et al. The effect of lauryl capping group on protein release and degradation of poly(d, l-lactic-co-glycolic acid) particles. J Control Release 2013; 172: 436–443. [DOI] [PubMed] [Google Scholar]
- 51. Salama HA, Ghorab M, Mahmoud AA, et al. PLGA nanoparticles as subconjunctival injection for management of glaucoma. AAPS Pharmscitech 2017; 18: 2517–2528. [DOI] [PubMed] [Google Scholar]
- 52. Khan N, Ameeduzzafar, Khanna K, et al. Chitosan coated PLGA nanoparticles amplify the ocular hypotensive effect of forskolin: statistical design, characterization and in vivo studies. Int J Biol Macromol 2018; 116: 648–663. [DOI] [PubMed] [Google Scholar]
- 53. Caprioli J, Sears M. Forskolin lowers intraocular pressure in rabbits, monkeys, and man. Lancet 1983; 1: 958–960. [DOI] [PubMed] [Google Scholar]
- 54. Shokry M, Hathout RM, Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for timolol maleate: augmented in-vivo efficacy and safe histological profile. Int J Pharm 2018; 545: 229–239. [DOI] [PubMed] [Google Scholar]
- 55. Liao Y, Te Lee CH, Chen ST, et al. Gelatin-functionalized mesoporous silica nanoparticles with sustained release properties for intracameral pharmacotherapy of glaucoma. J Mater Chem B 2017; 5: 7008–7013. [DOI] [PubMed] [Google Scholar]
- 56. Jung HJ, Abou-Jaoude M, Carbia BE, et al. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J Control Release 2013; 165: 82–89. [DOI] [PubMed] [Google Scholar]
- 57. Jung HJ, Chauhan A. Temperature sensitive contact lenses for triggered ophthalmic drug delivery. Biomaterials 2012; 33: 2289–2300. [DOI] [PubMed] [Google Scholar]
- 58. Xu J, Ge Y, Bu R, et al. Co-delivery of latanoprost and timolol from micelles-laden contact lenses for the treatment of glaucoma. J Control Release 2019; 305: 18–28. [DOI] [PubMed] [Google Scholar]
- 59. Cai X, Conley S, Naash M. Nanoparticle applications in ocular gene therapy. Vision research 2008; 48: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cellular and Molecular Life Sciences 2009; 66: 2873–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Uchida T. Introduction of macromolecules into mammalian cells by cell fusion. Exp Cell Res 1988; 178: 1–17. [DOI] [PubMed] [Google Scholar]
- 62. Chiang B, Kim YC, Doty AC, et al. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J Control Release 2016; 228: 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Malavia N, Reddy L, Szinai I, et al. Biodegradable sustained-release drug delivery systems fabricated using a dissolvable hydrogel template technology for the treatment of ocular indications. Invest Ophthalmol Vis Sci 2015; 56: 1296–1296. [Google Scholar]
- 64. Fedorchak MV, Conner IP, Schuman JS, et al. Long term glaucoma drug delivery using a topically retained gel/microsphere eye drop. Sci Rep 2017; 7: 8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Mansoor S, Kuppermann BD, Kenney MC. Intraocular sustained-release delivery systems for triamcinolone acetonide. Pharm Res 2009; 26: 770–784. [DOI] [PubMed] [Google Scholar]
- 66. Okabe K, Kimura H, Okabe J, et al. Intraocular tissue distribution of betamethasone after intrascleral administration using a non-biodegradable sustained drug delivery device. Invest Ophthalmol Vis Sci 2003; 44: 2702–2707. [DOI] [PubMed] [Google Scholar]
- 67. Bao W, Zhou J, Luo J, et al. PLGA microspheres with high drug loading and high encapsulation efficiency prepared by a novel solvent evaporation technique. J Microencapsul 2006; 23: 471–479. [DOI] [PubMed] [Google Scholar]
- 68. ClinicalTrials.gov. Safety and efficacy of ENV515 travoprost extended release (XR) in patients with bilateral ocular hypertension or primary open angle glaucoma, 2019, https://clinicaltrials.gov/ct2/show/NCT02371746
- 69. Allergan. Allergan announces positive topline phase 3 clinical data for bimatoprost SR (sustained-release) implant for IOP lowering in patients with open-angle glaucoma or ocular hypertension. https://www.allergan.com/news/news/thomson-reuters/allergan-announces-positive-topline-phase-3-clinic (accessed 18 March 2019).
- 70. GB—201, GB—202 and GB—203—glaucoma products. Graybug Vision. https://graybug.com/pipeline/gb-201-202-203-glaucoma/ (accessed 18 March 2019).
- 71. Hoang BP, Crean CS, Yang ML, et al. An injectable depot formulation of an outflow prodrug for sustained reduction of intraocular pressure. Invest Ophthalmol Vis Sci 2018; 59: 5710. [Google Scholar]
- 72. Gebhart F. Drug-delivery platforms offer more options and promise for clinicians. Ophthalmology Times, 2016. https://www.ophthalmologytimes.com/article/sustained-drug-delivery-moving-toward-clinical-use (accessed 27 August 2017).
- 73. Perera SA, Ting DSW, Nongpiur ME, et al. Feasibility study of sustained-release travoprost punctum plug for intraocular pressure reduction in an Asian population. Clin Ophthalmol 2016; 10: 757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Goldberg DF, Williams R. A phase 2 study evaluating safety and efficacy of the latanoprost punctal plug delivery system (L-PPDS) in subjects with ocular hypertension (OH) or open-angle glaucoma (OAG). IOVS ARVO Journals. https://iovs.arvojournals.org/article.aspx?articleid=2358808 (accessed 22 November 2019).
- 75. Utkhede D, William R. Improving retention rates for sustained therapeutic delivery through punctal plugs. Invest Ophthalmol Vis Sci 2018; 59: 5675. [Google Scholar]
- 76. Patel SP, Vaishya R, Pal D, et al. Novel pentablock copolymer-based nanoparticulate systems for sustained protein delivery. AAPS PharmSciTech 2015; 16: 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Shafiee A, Bowman LM, Hou E, et al. Ocular pharmacokinetics of bimatoprost formulated in DuraSite compared to bimatoprost 0.03% ophthalmic solution in pigmented rabbit eyes. Clin Ophthalmol 2013; 7: 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jiang J, Gill HS, Ghate D, et al. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci 2007; 48: 4038–4043. [DOI] [PubMed] [Google Scholar]