Abstract
Currently, five classes of drug are approved for the treatment of pulmonary arterial hypertension (PAH): phosphodiesterase 5 inhibitors (PDE5i); endothelin receptor antagonists; prostacyclin analogs; the IP receptor agonist selexipag; and the soluble guanylate cyclase (sGC) stimulator riociguat. For patients with inoperable or persistent/recurrent chronic thromboembolic pulmonary hypertension (CTEPH), riociguat is currently the only approved pharmacotherapy. Despite the development of evidence-based guidelines on appropriate use of specific drugs, in clinical practice patients are often prescribed PAH-targeted therapies off label or at inadequate doses.
PDE5i are the most often prescribed class of drugs as initial therapy, either alone or in combination with other drug classes. However, a proportion of patients receiving PAH therapies do not reach or maintain treatment goals. As PDE5i and riociguat target different molecules in the nitric oxide-sGC-cyclic guanosine monophosphate (NO-sGC-cGMP) signaling pathway, for patients with PAH without an initial or sustained response to PDE5i, there is a biological rationale for switching to riociguat. However, robust data from randomized controlled trials on the safety and efficacy of switching are lacking, as is formal guidance for clinicians. Here we review studies of sequential combination therapy, and trial data and case studies that have investigated switching between PAH-approved therapies, particularly from PDE5i to riociguat in patients with PAH with an insufficient response to PDE5i, and in patients with CTEPH who were receiving off-label treatment. These studies summarize the current evidence and practical real-life experience on the concept of switching treatments.
Keywords: pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, sGC stimulator, transition, phosphodiesterase 5 inhibitor
Introduction
Pulmonary hypertension (PH) is a progressive disease that leads to pulmonary arteriolar vasoconstriction, right-ventricular overload, and ultimately death. Pulmonary arterial hypertension (PAH) is characterized by reduced levels of the vasodilators nitric oxide (NO), cyclic guanosine monophosphate (cGMP), and prostacyclin, and overexpression of the vasoconstrictor endothelin. This results in increased vascular tone, and promotes vascular remodeling and the progressive destruction of the pulmonary vascular bed. Chronic thromboembolic pulmonary hypertension (CTEPH) is caused by obstruction of the pulmonary vasculature by residual organized thrombi, leading to increased pulmonary vascular resistance (PVR), progressive PH, and ultimately right ventricular failure.1–3
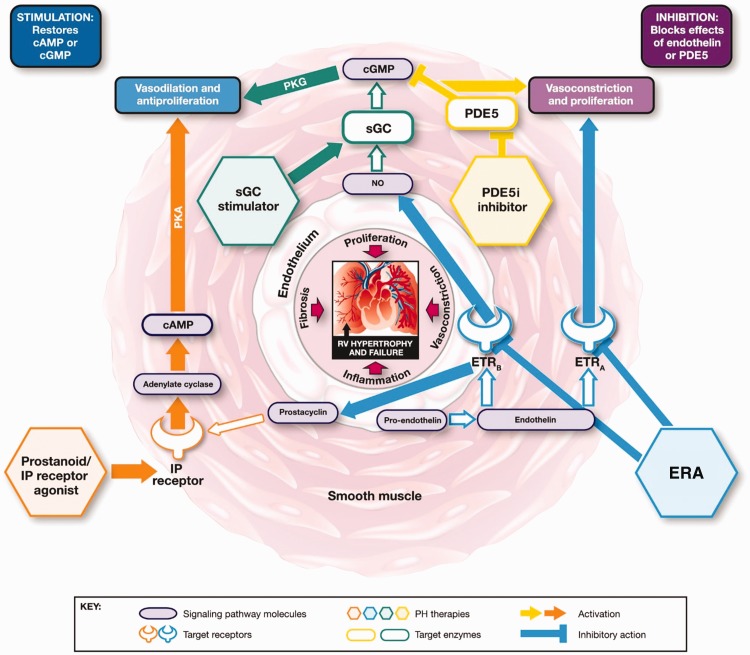
Five classes of drug are approved for the treatment of PAH, targeting three signaling pathways (Fig. 1):4–7 prostacyclin analogs (PCAs) epoprostenol, iloprost, treprostinil, and beraprost; the IP receptor agonist selexipag; the endothelin receptor antagonists (ERAs) bosentan, ambrisentan, and macitentan; the phosphodiesterase (PDE5) inhibitors (PDE5i) sildenafil and tadalafil; and the soluble guanylate cyclase (sGC) stimulator riociguat, also approved for patients with inoperable CTEPH or persistent/recurrent CTEPH after surgery.
Fig. 1.
Mechanisms of action of approved classes of PAH-targeted therapies.6 cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; endothelin receptor antagonists (ERA), endothelin receptor A; ETRB, endothelin receptor B; IP, prostacyclin; NO, nitric oxide; PDE5, phosphodiesterase type 5; PH phosphodiesterase 5 inhibitors (PDE5i); pulmonary hypertension; PKA, phosphate kinase A; PKG, cGMP-dependent protein kinase; RV, right ventricular; sGC, soluble guanylate cyclase.
Impairment of the NO sGC-cGMP signaling pathway (Fig. 2) is central to the pathogenesis of PAH and CTEPH,8–10 where endothelial dysfunction leads to impaired NO synthesis.10–14 The progression of PAH and CTEPH also correlates with low NO production.
Fig. 2.
The role of the NO signaling pathway in PAH and CTEPH pathogenesis16 (amended from Buglioni and Burnett. Annu Rev Med 2016; 67: 229–243). cGMP, cyclic guanosine monophosphate; GC-A, particulate guanylate cyclase A; GTP, guanosine triphosphate; NO, nitric oxide; NOS, nitric oxide synthase; PDE, phosphodiesterase; PKG, cGMP-dependent protein kinase; sGC, soluble guanylate cyclase.
The purpose of this review is to provide a summary of published experience of trials and case studies that have investigated switching between approved PAH therapies, particularly switching within the NO pathway in patients with PAH and switching from off-label therapies to riociguat in patients with CTEPH, and an overview of the options for sequential combination therapy. Given that switching patients from PDE5i to riociguat is already taking place in clinical practice despite a lack of guideline recommendations, we also provide some cautionary notes on best practice.
Methods
To summarize a broad review of trials and case studies, a PubMed literature search was performed using the following search terms: “pulmonary arterial hypertension,” “pulmonary arterial hypertension” AND “transition,” and “pulmonary arterial hypertension” AND “switch.” To identify studies of combination therapy, we searched for the following drug names: riociguat, sildenafil, tadalafil, bosentan, ambrisentan, macitentan, selexipag, epoprostenol, treprostinil, iloprost, and beraprost.
Current treatment strategies for PAH
PDE5i and riociguat both target the NO-sGC-cGMP signaling pathway to promote vasodilation with different mechanisms of action (MoAs) (Fig. 1).6 PDE5 deactivates and degrades cGMP, is abundantly expressed in pulmonary vasculature, and is upregulated in PAH. PDE5i occupy the catalytic site on PDE5, blocking degradation of cGMP (Fig. 1).6,15 However, the MoA of PDE5i is dependent on endogenous NO bioavailability, and evidence suggests that NO and intracellular levels of cGMP are depleted during the progression of PAH, which could render PDE5i less effective. This may explain why some patients do not have a sufficient sustained response to PDE5i. Riociguat has a dual MoA; it sensitizes sGC to endogenous NO and directly stimulates sGC via a second binding site, independent of NO, and has been shown to increase sGC activity regardless of NO and cGMP levels, resulting in increased cGMP. ERAs, PCAs, and selexipag target different pathways. ERAs prevent endothelin-1 (ET-1)- mediated vasoconstriction by blocking the binding of ET-1 to ET-1 receptors (Fig. 1), which are upregulated in PAH. PCAs are synthetic analogs of the pulmonary vasodilator prostacyclin (also known as prostaglandin I2) and selexipag is a high-affinity agonist of the human IP receptor. In PAH, prostacyclin synthase is downregulated and thus, prostacyclin levels are decreased (Fig. 1).
Medical therapy may be prescribed as monotherapy or, alternatively, as initial or sequential combination therapy. With combination therapy, multiple signaling pathways involved in the pathogenesis of the disease may be targeted. Initial combined therapy with ambrisentan and tadalafil is recommended in the 2015 European Society of Cardiology/European Respiratory Society (ESC/ERS) guidelines, following the results of the AMBITION study. However, several other studies of sequential combination therapy with bosentan and a PDE5i did not show a significant effect, possibly due to pharmacokinetic interaction (Table 1).17–20 Macitentan added to PAH background therapy (mostly sildenafil) was shown to be effective in a subgroup analysis of the SERAPHIN study,21 as was selexipag added to background ERA or PDE5i in the GRIPHON study.22 Similarly, riociguat added to existing ERA treatment was shown to be effective in the PATENT-1 and -2 studies, and is recommended in the 2015 ESC/ERS treatment guidelines.4,23,24 An overview of studies of sequential combination therapy is given in Table 1.
Table 1.
Sequential combination therapy strategies tested in clinical randomized controlled trials.
| Study name | Combination | Significant benefit | Reference |
|---|---|---|---|
| PACES | Epoprostenol + sildenafil | Yes | Simonneau et al., 200841 |
| TRIUMPH | Bosentan + inhaled treprostinil | Yes | McLaughlin et al., 201042 |
| Sildenafil + inhaled treprostinil | No | McLaughlin et al., 201042 | |
| PATENT | ERAs/non-intravenous prostanoids + riociguat | Yes | Ghofrani et al., 201323 |
| SERAPHIN | PDE5i/non-intravenous prostanoids + macitentan | Yes | Pulido et al., 201321 |
| AMBITION | First-line ambrisentan + tadalafil | Yes | Galiè et al., 201543 |
| STEP | Bosentan + inhaled iloprost | Yes | McLaughlin et al., 200644 |
| GRIPHON | PDE5i/ERAs + selexipag | Yes | McLaughlin et al., 201845 |
| MERIT-1 | PDE5i + macitentan | Yes | Ghofrani et al., 201734 |
| FREEDOM-C | ERAs/non-intravenous prostanoids + oral treprostinil | No | Tapson et al., 201246 |
| PHIRST | Bosentan + tadalafil | No | Galiè et al., 200919 |
| COMPASS-2 | Sildenafil + bosentan | No | McLaughlin et al., 201447 |
| NCT00323297 | Bosentan + sildenafil | No | Vizza et al., 201720 |
| BREATHE-2 | Epoprostenol + bosentan | No | Humbert et al., 200448 |
| PATENT-PLUS | Sildenafil + riociguat | No | Galiè et al., 201549 |
ERA, endothelin receptor analog; PDE5i, phosphodiesterase 5 inhibitor.
The concomitant administration of PDE5i and riociguat is contraindicated due to the risk of systemic hypotension. For patients already receiving an ERA and either a PDE5i or riociguat, a PCA or IP receptor agonist may be administered. Although triple combination therapy has the advantage of acting on three different molecular targets, it also increases pharmacologic complexity and the potential risk of adverse events (AEs), and may lead to increased drug-related healthcare costs. The addition of PCAs or selexipag to an existing treatment regimen may cause additional side effects such as headache, flushing, and jaw pain, and the intravenous administration of PCAs carries the risk of sepsis and embolic phenomena.
PDE5i are the most frequently prescribed treatment for PAH, either as monotherapy or combination therapy. However, clinical data indicate that 20–60% of patients receiving PDE5i do not reach or maintain treatment goals as set out in the 2015 ESC/ERS guidelines. Furthermore, in AMBITION, 73% of patients with PAH receiving tadalafil as monotherapy, and 61% of patients receiving combined ambrisentan and tadalafil, did not achieve the secondary composite endpoint of satisfactory clinical response at week 24. In the SERAPHIN study of macitentan in PAH, the morbidity/mortality event-free survival of patients receiving PDE5i monotherapy was approximately 50% at three years.
The 2015 ESC/ERS treatment guidelines advise that the treatment objective for patients with PAH is to achieve a low-risk status, indicated by stable, non-progressive disease, an approach supported by analyses of registry data. Patients who do not reach or maintain a low-risk status are described as having an insufficient response to treatment; the current treatment guidelines recommend sequential addition of oral therapies and then the addition of a prostanoid. However, as a direct stimulator of sGC, riociguat targets a different molecule in the same signaling pathway as PDE5i. For patients without a sustained response to PDE5i, there is therefore a biological rationale for switching to riociguat in this setting to augment pharmacological effects and lower risk. However, data are scarce on switching between drug classes in PAH, including riociguat data, and the majority of studies have focused on switching within a drug class, e.g. from parenteral to inhaled prostanoids. The concept of switching to riociguat from a PDE5i is an attractive approach, particularly in patients at low/intermediate risk and not rapidly deteriorating, to avoid therapy complexity, mitigate overlapping side effects, and minimize costs. Furthermore, in adults, switching to riociguat does not preclude subsequent escalation to triple therapy. However, further evidence to support this concept is still required and it is important to remember that switching between classes is not included in the 2015 ESC/ERS guidelines.
Studies of switching between PAH-approved therapies
Multiple studies have evaluated switching to PAH therapies other than riociguat, switching within a drug class, and from one drug class to another (Table 2). However, switching between drug classes is not recommended by the guidelines due to the lack of randomized controlled trial (RCT)-based evidence.4 PCAs are the longest-established therapy for PAH, and switching between PCAs has been shown to be well tolerated, prevent clinical deterioration, and maintain functional status.25–27 In a retrospective assessment of switching from intravenous epoprostenol to iloprost, after one year 78% of patients remained on iloprost and 81% were free from clinical worsening. Patients in these studies were switched either because of drug-related AEs, because clinical improvement during PCA therapy allowed a switch to a non-intravenous formulation, or to achieve a more convenient administration schedule when switching between inhaled PCAs.25–27 Other studies have assessed switching from a prostanoid to an ERA such as bosentan28 or a PDE5i such as sildenafil.29 These studies demonstrated a prolonged and stable functional class, and improved 6-minute walking distance (6MWD) and quality of life.28 After switching to sildenafil, approximately 70% of patients maintained improved quality of life and improved 6MWD for up to 3 months.29 Reasons for switching from a PCA to an ERA included having met specific disease stability criteria while receiving a PCA,28 and for those patients who switched to a PDE5i, concerns over the availability of PCA therapy in the study region.29 Switching within other drug classes such as ERAs30 and PDE5i31–33 has also been evaluated and, although data are limited, exercise capacity and World Health Organization functional class (WHO FC) were sustained. The patients who switched from sitaxsentan to ambrisentan were US patients enroled in an open-label study of sitaxsentan in PAH and switched at study closure because sitaxsentan did not receive Food and Drug Administration (FDA) approval.30 Patients in studies of switching between PDE5i did so due to drug-related AEs,31 the desire for greater adherence and convenience,32,33 as well as potentially lower costs and higher quality of life.33 The SITAR study assessed safety and patient satisfaction after switching from sildenafil to tadalafil, and demonstrated that tadalafil was well tolerated with improved convenience for patients.32 Other studies have also supported these findings demonstrating tolerance and reduced deterioration.31,33
Table 2.
Studies of BPA combined with background medical therapy.
| Study type | Background therapy | Patients (n) | Medical treatment before BPA (%) | Significant benefit | Reference |
|---|---|---|---|---|---|
| Retrospective | PEA | 136 | 85 | Yes | Inami et al., 201450 |
| PEA | 29 | 100 | Yes | Taniguchi et al., 201451 | |
| PEA | 20 | 75 | Yes | Fukui et al., 201452 | |
| PEA | 170 | 91 | Yes | Inami et al., 201653 | |
| PEA | 80 | 61 | Yes | Ogo et al., 201754 | |
| Observational | Epoprostenol | 68 | 100 | Yes | Mizoguchi et al., 201255 |
| PEA | 20 | 10 | Yes | Andreassen et al., 201356 | |
| PEA | 56 | 80 | Yes | Kurzyna et al., 201757 | |
| Prospective | PEA | 12 | 100 | Yes | Sugimura et al., 201258 |
| PDE5i/ERA | 29 | 100 | Yes | Kataoka et al., 201259 | |
| PDE5i/prostaglandin | 24 | 92 | Yes | Aoki et al., 201660 | |
| PDE5i | 11 | 66 | Yes | Roik et al., 201661 | |
| PDE5i | 56 | Almost all | Yes | Olsson et al., 201762 | |
| Riociguat | 123 | 100 | Yes | Wiedenroth et al., 201863 | |
| PEA | 10 | 100 | Yes | Yanaka et al., 201864 |
BPA, balloon pulmonary angioplasty; ERA, endothelin receptor analog; PDE5i, phosphodiesterase 5 inhibitor; PEA, pulmonary endarterectomy.
Current treatment strategies in CTEPH
Pulmonary endarterectomy (PEA) is the recommended treatment for CTEPH as it is potentially curative and can result in the near normalization of pulmonary hemodynamics. However, up to 40% of patients are ineligible for surgery, and although balloon pulmonary angioplasty (BPA) may be an option for some of these patients, the remainder will require pharmacologic therapy, as will the 51% of patients undergoing PEA who experience persistent/recurrent PH after surgery. Moreover, patients or physicians may be reluctant to consider surgery, despite the recommendations of treatment guidelines. Before the approval of riociguat for inoperable and persistent/recurrent CTEPH, patients with CTEPH were often prescribed drugs approved for PAH off label.
At the time of writing, riociguat is the only approved drug for the treatment of inoperable or persistent/recurrent CTEPH, based on the phase 3 CHEST-1 study and its open-label, long-term extension CHEST-2. However, the recent phase 2 MERIT study of macitentan in patients with inoperable CTEPH demonstrated encouraging results, including in patients who were receiving background PDE5i therapy (46%).34 In patients who are inoperable or have persistent/recurrent CTEPH after PEA, and are not reaching treatment goals on off-label medical therapy, switching to riociguat is a logical treatment choice.35 WHO FC III and 6MWD of 165–440 m were part of the definition of unsatisfactory response to previous therapy in the RESPITE and REPLACE clinical studies, and may also be useful as criteria for patients with CTEPH who are not reaching treatment goals and could benefit from switching to riociguat.
BPA
An emerging treatment option for these patients may be BPA, in which the pulmonary arteries are dilated using a balloon inserted via a catheter. Although this can open obstructed vessels and widen stenotic lesions without the need for invasive surgery, it has no impact on the small-vessel disease component of CTEPH or the underlying pathophysiology.36 The ESC/ERS treatment guidelines currently give a lower class of recommendation for BPA (IIb) than riociguat (I), and state that BPA should be performed only in expert centers. BPA is usually performed in parallel with underlying medical therapy, and the combination of pharmacologic treatment with BPA has been investigated in numerous smaller studies (Table 2); however, large-scale randomized controlled trials (RTCs) are still required to determine the benefit of BPA.4
Table 3.
Studies evaluating switching between other PAH-approved therapies.
| Transition | Drug class | Reference |
|---|---|---|
| Sildenafil to tadalafil | PDE5i | Lichtblau et al., 2015;31 Frantz et al., 2014;32 Shapiro et al., 201365 |
| Prostacyclin to iloprost | Prostaglandin | Channick et al., 201325 |
| Iloprost to treprostinil | Prostaglandin | Bourge et al., 201326 |
| Sitaxsentan to ambrisentan | ERA | Safdar, 201130 |
| Prostacyclin to bosentan | Prostaglandin to ERA | Safdar, 200928 |
| Treprostinil to sildenafil | Prostaglandin to PDE5i | Keogh et al., 200729 |
| Epoprostenol to treprostinil | Prostaglandin | Rubenfire et al., 200727 |
ERA, endothelin receptor analog; PAH, Pulmonary arterial hypertension.
Clinical trials of switching to riociguat
RESPITE
Riociguat clinical Effects Studied in Patients with Insufficient Treatment response to PDE5 inhibitors (RESPITE) investigated whether it is feasible, safe, and beneficial to replace PDE5i with riociguat. RESPITE was a 24-week, prospective, open-label, multi-center, uncontrolled, phase 3b pilot study. Patients had symptomatic PAH, and an insufficient response to stable treatment with tadalafil or sildenafil. Additional inclusion criteria were: WHO FC III, 6MWD 165–440 m, cardiac index < 3.0 L/min/m2, mean pulmonary arterial pressure (mPAP) > 30 mmHg, pulmonary capillary wedge pressure ≤ 15 mmHg, and PVR > 400 dynċsċcm−5.
PDE5i were stopped 1–3 days before the first dose of riociguat to allow a PDE5i treatment-free period of at least 24 h for sildenafil and at least 72 h for tadalafil. The exploratory endpoints included 6MWD, N-terminal prohormone of brain natriuretic peptide (NT-proBNP), WHO FC, pulmonary hemodynamics, EuroQol 5 Dimensions (EQ-5D) quality of life score, clinical worsening, and safety.
Of the 61 patients enrolled in RESPITE, 51 completed 24 weeks of treatment; at baseline, 50 patients (82%) were receiving combination therapy with ERAs. The majority of patients in RESPITE reached the maximum dose of riociguat after switching from PDE5i. In those patients completing the study, mean ± standard deviation (SD) 6MWD increased by +31 ± 63 m (95% confidence interval [CI] = 13–49 m; P = 0.0010), mean ± SD NT-proBNP decreased by −347 ± 1235 pg/mL, a relative reduction of 22% (95% CI = 5–37; P = 0.0170), and 28 patients (54%) had improved WHO FC (52% to WHO FC II and 2% to WHO FC I; P < 0.001). Hemodynamic data, available for 49 patients at baseline and week 24, showed that mean ± SD PVR was reduced by −103 ± 296 dyn·s·cm–5 (95% CI = −188–−18; P = 0.0184), mean ± SD mPAP was reduced by −2.8 ± 8.8 mm Hg, and right atrial pressure was reduced by −0.8 ± 4.2 mmHg. Mean ± SD cardiac index and mixed venous oxygen saturation increased by +0.3 ± 0.5 L/min/m2 (95% CI = 0.2–0.5 L/min/m2; P = 0.0001) and +1.0 ± 6.3%, respectively. A post hoc analysis indicated that at week 24, 41% of the overall population achieved a low-risk status according to the ESC/ERS treatment guidelines risk assessment (where an overall low-risk status was assumed when > 50% of the available variables met the low-risk thresholds).37
The switch to riociguat was tolerated by the majority of patients. Fifty-eight patients (95%) experienced an AE, the most common of which were dyspepsia (n = 14 [23%]), headache (n = 12 [20%]), and diarrhea (n = 11 [18%]). No serious adverse events (SAEs) or clinical worsening events were reported during the PDE5i treatment-free period; two patients (3%) experienced SAEs during the first two weeks of riociguat treatment. Two patients (3%) died (one subdural hematoma and one pneumonia, both not related to riociguat) during the study. Overall, the safety profile of riociguat was consistent with the results of the PATENT-1 and PATENT-2 studies.
The RESPITE study was limited by the lack of a control group, lack of blinding, and the drop-out rate (n = 10 [16%]), which may have biased the analysis. To address this potential bias, a sensitivity analysis was performed using worst-possible values for 6MWD, WHO FC, and EQ-5D, and the last observation carried forward for hemodynamics to provide the most conservative view of the data. Improvement was still in line with the overall results of the study, but no longer statistically significant for 6MWD and NT-proBNP; the change for WHO FC remained significant.37
RESPITE was the first study to investigate the clinical safety and efficacy outcomes of switching from PDE5i to riociguat in PAH, and has provided preliminary evidence that switching may be safe, tolerable, and beneficial in patients with PAH with an insufficient response to PDE5i. The findings also offer preliminary support to the hypothesis that the NO-sGC-cGMP pathway might be optimized by riociguat. However, given the limitations of RESPITE noted above, larger controlled studies are needed to confirm this.
REPLACE
Following the preliminary signals of improvement observed in RESPITE, the phase 4, prospective, randomized, controlled, open-label study REPLACE (Riociguat rEplacing PDE5i Therapy evaLuated Against Continued PDE5i thErapy; NCT02891850) was initiated and will investigate the efficacy of riociguat as a replacement for PDE5i in patients with PAH. Patients will be randomized to switch to riociguat or remain on PDE5i therapy (with or without concomitant ERAs) for 24 weeks. The primary endpoint of the study will be satisfactory clinical response, defined as free from clinical worsening and fulfilling at least two of the following criteria: an increase in 6MWD ≥ 10% or ≥ 30 m from baseline to week 24; WHO FC I/II at week 24; or a reduction in NT-proBNP ≥ 30% from baseline to week 24. Secondary outcomes include 6MWD, NT-proBNP, WHO FC, REVEAL risk score, and clinical worsening. REPLACE commenced in December 2016 and is estimated to finish in August 2019.
CTEPH Early Access Study
The CTEPH Early Access Study assessed the safety and tolerability of riociguat in 300 patients in real-world clinical practice. This open-label, uncontrolled, phase 3b, long-term surveillance study included patients with inoperable or persistent/recurrent CTEPH after PEA, who were not satisfactorily treated, or treatment-naïve and could not participate in another CTEPH trial. The study provided early access to riociguat before market launch and consisted of three phases: an eight-week dose-adjustment phase; a maintenance phase until riociguat was available in a patient’s respective country; and a safety follow-up phase. All patients underwent a PDE5i/ERA/PCA treatment-free period of at least 72 h before initiating riociguat. Concomitant treatment with an ERA or PCA was permitted after the dose-adjustment phase.
Of the 300 patients enrolled in the study, 84 (28%) switched to riociguat from other single or combination PAH-targeted therapies, with a median treatment-free period of four days (range = 3–74 days). Fifty-eight patients (19%) previously received PDE5i (most frequently sildenafil [14%]), 44 patients (15%) ERAs (most commonly bosentan [12%]), and seven patients PCAs (most commonly iloprost [2%]). Twenty-four patients (8%) received dual (n = 23 [8%]) or triple (n = 1 [<1%]) combination therapy before switching.
The safety and tolerability of riociguat was similar between patients who switched from other PAH-targeted therapies and those who were treatment-naïve, and was comparable with that of the CHEST-1 and CHEST-2 studies. During the PDE5i/ERA/PCA treatment-free period, AEs were experienced by 11 patients (13%), eight of which were mild in severity. Two patients experienced SAEs during the treatment-free period (syncope and hospitalization due to septicemia), both of which resolved.
Overall, other PAH-targeted therapies were initiated or restarted by 42 patients (14%), the majority due to worsening CTEPH. In patients who switched from prior PAH-targeted therapy, 17 patients (20%) started an ERA and one patient (1%) started a PCA during the maintenance phase. Two patients (2%) restarted PDE5i treatment on the same day as discontinuing riociguat. Overall, 38 patients (13%) discontinued riociguat during the study, with AEs being the most frequent reason (n = 14), and four patients discontinued riociguat treatment during the safety follow-up phase.
Assessment of 6MWD was optional during the study; therefore, data were not available for all patients. In switched patients, mean ± SD baseline 6MWD was 389 ± 87 m. At week 12, mean ± SD change from baseline was + 28 ± 39 m (n = 32). The percentage of switched patients in WHO FC I/II/III/IV at baseline was 2% (n = 84). After 12 weeks of treatment, WHO FC had improved/stabilized/worsened in 21/76/3% (n = 70).
Case studies of switching to riociguat
In addition to preliminary clinical trial data, some case study reports and retrospective analyses have also provided initial data around switching to riociguat in clinical settings (Table 4). For example, a retrospective analysis by Davey et al. of 12 patients with PH showed that switching to riociguat led to an increase in cardiac index by the Fick method of 0.41 ± 0.16 L/min/m2 (P = 0.026), a concomitant decrease in PVR from 649 ± 103 to 524 ± 102 dyn.s.cm−5 (P = 0.037), and an improvement in WHO FC from 2.8 ± 0.01 to 2.5 ± 0.2 (P = 0.018) after 12 weeks of therapy. Headache and lowering of blood pressure were commonly reported following the switch, but patients experiencing these side effects did not discontinue riociguat. Weir et al. performed an independent retrospective analysis of 31 patients with PAH who had switched from PDE5i to riociguat. Twenty-two patients (71%) successfully switched; in this group, mPAP decreased from 43.7 to 38.3 mmHg (P = 0.0285), PVR from 7.93 to 4.6 WU (P = 0.0036), cardiac index increased from 2.9 to 3.13 L/min/m2 (P = 0.53), and 6MWD by 21 m (P = 0.19). Nine patients (29%) did not tolerate the switch due to worsening symptoms (78%) or side effects (22%). Raina et al. described three patients with PAH associated with connective tissue disease (PAH-CTD), where the switch to riociguat from PDE5i was associated with clinical and hemodynamic improvements, similar to those observed in patients with PAH-CTD in the PATENT-1 and PATENT-2 studies. Yamamoto et al. described eight patients with CTEPH who switched from PDE5i treatment to riociguat and experienced a significant reduction of −116.5 pg/mL ± SD (P = 0.0156) in BNP, and improvements in mPAP, PVR, cardiac index, and partial pressure of oxygen in arterial blood. Finally, Darocha et al. performed a retrospective analysis of 28 patients with inoperable CTEPH who switched from sildenafil to riociguat. Patients demonstrated reductions in PVR, mPAP, and increased cardiac output.38
Table 4.
Case studies of patients with PAH and CTEPH switching from PDE5i therapy to riociguat.
| Patient presentation | Prior PAH-targeted therapy | Reason for switching | Treatment-free period | Outcome of switching | Reference |
|---|---|---|---|---|---|
| 59-year-old patient with PAH, a history of anorexigen use, and NYHA FC II | Sildenafil + bosentan | No insurance cover for high-dose sildenafil in addition to ERA | Overnight Continued ERA | Improved 6MWD (from 547 to 606 m) Improved general exercise capacity Improved symptoms | Poch, 201666 |
| 44-year-old patient with Sjögren’s syndrome and associated PAH, WHO FC II | Tadalafil + macitentan + IV epoprostenol | Decrease in exercise tolerance | 48 h Continued ERA and PCA | Improved 6MWD (from 430 to 480 m) Stabilized WHO FC Normalized hemodynamics | Sulica et al., 201567 |
| Patient with idiopathic PAH, WHO FC III | Sildenafil + ambrisentan | Deterioration in hemodynamics | 8 h Continued ambrisentan | Patient discontinued due to lack of efficacy | Andersen et al.68 |
| Patient with persistent/ recurrent CTEPH following PEA, WHO FC II | Sildenafil + bosentan + inhaled iloprost | An attempt to improve clinical condition | 6 h Continued ERA and PCA | Improved 6MWD (from 421 to 437 m) Improved WHO FC Improved symptoms Concomitant iloprost discontinued, bosentan dose halved | |
| Retrospective analysis of 12 patients with PH | PDE5i (n = 10) PDE5i + ERA (n = 2) | Clinical worsening (n = 9) PDE5i side effects (n = 1) Approval of riociguat for CTEPH (n = 2) | Not specified | Significant increase in cardiac index Fall in PVR, Improvement in WHO FC | Davey et al., 201769 |
| Three patients with PAH-CTD | Tadalafil + bosentan + imatinib (n = 1) Sildenafil (n = 1) Inhaled treprostinil + macitentan + tadalafil | Worsening PAH | Not specified | Improvements in PVR, cardiac output and cardiac index | Raina et al., 201770 |
| Eight patients with CTEPH | PDE5i | Inadequate clinical response to PDE5i | Not specified | BNP significantly decreased Improved mPAP, PVR, cardiac index, and PaO2 | Yamamoto et al., 201771 |
| Retrospective analysis of 31 patients with PAH | Sildenafil (n = 16) Tadalafil (n = 16) Of these, 17 patients were receiving prostanoids, 9 were receiving dual therapy, and 10 were receiving triple therapy | Suboptimal therapeutic response to PDE5i | Not specified | 22 patients successfully switched to riociguat Improvement in mPAP, PVR, and cardiac index, with no change in RAP and PCWP Improved 6MWD (+21 m) | Weir et al., 201772 |
| Retrospective analysis of 107 patients with non-operable CTEPH or PH persistent after PEA | Sildenafil | Unsatisfactory clinical response (WHO FC II–IV) | 24-h wash-out | Reduced PVR and PAP Improved pulmonary hemodynamics and WHO FC | Darocha et al., 201838 |
6MWD, 6-min walking distance; BNP, brain natriuretic peptide; CTEPH, chronic thromboembolic pulmonary hypertension; ERA, endothelin receptor antagonist; IV, intravenous; mPAP, mean pulmonary arterial pressure; NYHA FC, New York Heart Association functional class; PAH, pulmonary arterial hypertension; PAH-CTD, PAH associated with connective tissue disease; PaO2, the partial pressure of O2 in arterial blood; PAP, pulmonary arterial pressure; PCA, prostacyclin analog; PDE5i, phosphodiesterase 5 inhibitor; PEA, pulmonary endarterectomy; PCWP; pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RAP, right arterial pressure; WHO FC, World Health Organization functional class.
The data from these case studies suggest that switching to riociguat has been considered in the clinical setting, with the suggestion of therapeutic efficacy, although the current role of this therapeutic modality in the treatment algorithm is not yet defined.
Non-interventional studies
The EXPERT registry (NCT02092818) has collected evidence from real-world clinical practice on patients with PAH or CTEPH receiving riociguat. Safety data from EXPERT on patients with PAH or CTEPH who switched from other PAH-targeted therapies indicated that tolerability was similar between switched and treatment-naïve patients, and that AEs and SAEs in switched patients were consistent with the known safety profile of riociguat.39 The retrospective chart review study CAPTURE enrolled 125 patients with PAH, or inoperable or persistent/recurrent CTEPH, who switched to riociguat from another PH-targeted medical therapy. The aim of the study was to understand real-world procedures for switching to riociguat, and the results suggest that switching to riociguat from other PH-targeted therapies appears to be feasible in the context of current label recommendations.
How to switch within the NO pathway
Given the limited existing evidence and the lack of a recommendation for switching in published guidelines, we would not encourage switching until randomized data supporting this strategy are available. As noted above, the rationale for switching from PDE5i to riociguat is to optimize the NO-sGC-cGMP signaling pathway. Concomitant administration of riociguat and PDE5i exerts an additive effect on systemic blood pressure, and clinically significant hypotension can occur. Concomitant use of all PDE5i, or non-specific PDE5i and riociguat, is contraindicated and a PDE5i treatment-free period must be initiated before switching to riociguat. The recommended PDE5i treatment-free periods, as used in the RESPITE and REPLACE studies, are based on the pharmacokinetics of sildenafil and tadalafil, and data from real-world clinical practice. Sildenafil should be discontinued for at least 24 h and tadalafil should be discontinued for at least 48 h before administering riociguat. In addition, it is recommended to monitor for signs and symptoms of hypotension on initiation. Should a patient need to be switched from riociguat to a PDE5i, riociguat must be discontinued at least 24 h before initiation of the PDE5i, and the patient should be monitored for signs and symptoms of hypotension upon initiation of the new therapy.40
If individual clinicians wish to attempt a switch to riociguat based on their medical judgment, patient enrolment in the ongoing REPLACE study should be considered. We suggest that possible candidates for a switch include: patients who have not reached a low-risk profile (e.g. one or two intermediate-risk characteristics) but who do not have any high-risk characteristics by ESC/ERS criteria, or patients with a REVEAL risk score of 6–8 who are stable/not declining rapidly for several months. Indeed, preliminary data from RESPITE suggest that patients with lower PVR and right arterial pressure, and higher 6MWD at baseline, were more likely to respond to a switch. Any decision regarding a change in therapy should be based on individual clinical status and/or the AEs of any previously administered therapy. If switching to riociguat from other PAH-specific drugs is considered appropriate or necessary, the decision regarding the execution of such a switch can only be made at the discretion of the experienced treating PH physician after discussion with the patient. REPLACE has the potential to provide further data on when switching might be considered over sequential combination therapy in patients not meeting treatment goals.
Expert opinion/clinical perspectives
Early, limited clinical evidence from RESPITE and case series data indicate that switching to riociguat may be a viable treatment option in carefully selected patients with PH who are not achieving treatment goals with their current regimen. There are, however, no RCTs comparing switching with either continued therapy or sequential combination therapy. Further trials are taking place that may determine whether switching will become a goal-oriented treatment strategy for minimizing risk in patients with PAH, and this may identify where switching could be placed in the PAH treatment algorithm. Although switching to riociguat may be of benefit to some patients, it is important to note that it is not possible to identify responders upfront. Previous data indicated that switching would be less appropriate in patients with deteriorating and advanced symptoms, and these patients are candidates for treatment with sequential combination therapy and/or parenteral prostanoids, as recommended in the ERS/ESC guidelines. We recommend that a patient’s risk is assessed by either REVEAL score or ERS/ESC risk criteria when making treatment decisions.
Conclusion
Data on switching between PAH-approved therapies in patients with PAH or CTEPH are still limited. The studies reviewed here summarize the available evidence and practical experience in a situation where there is no formal protocol or recommendation available for switching. In line with the recommendation of goal-oriented therapy to keep patients at a low-risk status, switching may present a potential additional strategy to sequential combination therapy in low- to intermediate-risk patients, keeping the option of adding a third drug class for a later stage in the disease course. There are no data to support switching between therapies for patients undergoing triple drug therapy.
The studies discussed here suggest that switching to riociguat may be safe, tolerable, and beneficial in patients with PAH who have an insufficient response to PDE5i, and in patients with CTEPH who are receiving off-label treatment. However, additional data from RCTs are needed to determine if this strategy is an effective approach to the management of patients with PAH and CTEPH.
Acknowledgments
Editorial assistance was provided by Adelphi Communications Ltd (Bollington, UK), supported by Bayer AG (Berlin, Germany).
Conflict of interest
The authors declare the following conflicts of interest: RLB reports grants from Bayer AG, Actelion, EIGER, United Therapeutics, and Gilead paid to his institution, and honoraria from Gilead, Bayer, and Actelion; PAC reports grant support from Bayer (University Research Fund), and fees for advisory boards and talks from Bayer, Actelion, and GSK; H-AG reports grants from DFG (German Research Foundation), honoraria from Actelion, Bayer, Ergonex, Gilead, GSK, Novartis, and Pfizer, consultancy fees from AbbVie, Actelion, Bayer, Bellerophon Pulse Technologies, Ergonex, Gilead, GSK, Medscape, MSD Sharpe & Dohme, Novartis, OMT, Pfizer, and Web MD Global, and speaker’s bureau fees from Actelion, Bayer, Ergonex, Gilead, GSK, Novartis, and Pfizer; GS reports grants, personal fees, and non-financial support from Bayer, Actelion, GSK, Lilly, Pfizer, and Novartis; VVM reports grants, personal fees, and non-financial support from Actelion, Bayer, Gilead, United Therapeutics, and Ikaria, and grants from Novartis, EIGER and SoniVie; AR reports honoraria from Bayer, research support from United Therapeutic, and consulting fees from St. Jude. MK has no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Lang IM, Klepetko W. Chronic thromboembolic pulmonary hypertension: an updated review. Curr Opin Cardiol 2008; 23: 555–559. [DOI] [PubMed] [Google Scholar]
- 2.Peacock A, Simonneau G, Rubin L. Controversies, uncertainties and future research on the treatment of chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc 2006; 3: 608–614. [DOI] [PubMed] [Google Scholar]
- 3.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009; 54: S43–S54. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 5.Hoeper MM, McLaughlin VV, Dalaan AM, et al. Treatment of pulmonary hypertension. Lancet Respir Med 2016; 4: 323–336. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, Ghofrani HA. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2016; 71: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humbert M, Lau EM, Montani D, et al. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation 2014; 130: 2189–2208. [DOI] [PubMed] [Google Scholar]
- 8.Ghofrani HA, Humbert M, Langleben D, et al. Riociguat: mode of action and clinical development in pulmonary hypertension. Chest 2017; 151: 468–480. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt HH, Walter U. NO at work. Cell 1994; 78: 919–925. [DOI] [PubMed] [Google Scholar]
- 10.Stasch JP, Evgenov OV. Soluble guanylate cyclase stimulators in pulmonary hypertension. Handb Exp Pharmacol 2013; 218: 279–313. [DOI] [PubMed] [Google Scholar]
- 11.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004; 109: 159–165. [DOI] [PubMed] [Google Scholar]
- 12.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995; 333: 214–221. [DOI] [PubMed] [Google Scholar]
- 13.Schermuly RT, Ghofrani HA, Wilkins MR, et al. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011; 8: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011; 123: 2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corbin JD, Francis SH. Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction. J Androl 2003; 24: S38–S41. [DOI] [PubMed] [Google Scholar]
- 16.Buglioni and Burnett. Annu Rev Med 2016; 67: 229–243. [DOI] [PubMed]
- 17.McLaughlin V, Channick RN, Ghofrani HA, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015; 46: 405–413. [DOI] [PubMed] [Google Scholar]
- 18.Paul GA, Gibbs JS, Boobis AR, et al. Bosentan decreases the plasma concentration of sildenafil when coprescribed in pulmonary hypertension. Br J Clin Pharmacol 2005; 60: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 20.Vizza CD, Jansa P, Teal S, et al. Sildenafil dosed concomitantly with bosentan for adult pulmonary arterial hypertension in a randomized controlled trial. BMC Cardiovasc Disord 2017; 17: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 22.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 23.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. [DOI] [PubMed] [Google Scholar]
- 24.Rubin LJ, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension: a long-term extension study (PATENT-2). Eur Respir J 2015; 45: 1303–1313. [DOI] [PubMed] [Google Scholar]
- 25.Channick RN, Frantz RP, Kawut SM, et al. A multicenter, retrospective study of patients with pulmonary arterial hypertension transitioned from parenteral prostacyclin therapy to inhaled iloprost. Pulm Circ 2013; 3: 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourge RC, Tapson VF, Safdar Z, et al. Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. Cardiovasc Ther 2013; 31: 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rubenfire M, McLaughlin VV, Allen RP, et al. Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. Chest 2007; 132: 757–763. [DOI] [PubMed] [Google Scholar]
- 28.Safdar Z. Outcome of pulmonary hypertension subjects transitioned from intravenous prostacyclin to oral bosentan. Respir Med 2009; 103: 1688–1692. [DOI] [PubMed] [Google Scholar]
- 29.Keogh AM, Jabbour A, Weintraub R, et al. Safety and efficacy of transition from subcutaneous treprostinil to oral sildenafil in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2007; 26: 1079–1083. [DOI] [PubMed] [Google Scholar]
- 30.Safdar Z. Effect of transition from sitaxsentan to ambrisentan in pulmonary arterial hypertension. Vasc Health Risk Manag 2011; 7: 119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lichtblau M, Harzheim D, Ehlken N, et al. Safety and long-term efficacy of transition from sildenafil to tadalafil due to side effects in patients with pulmonary arterial hypertension. Lung 2015; 193: 105–112. [DOI] [PubMed] [Google Scholar]
- 32.Frantz RP, Durst L, Burger CD, et al. Conversion from sildenafil to tadalafil: results from the sildenafil to tadalafil in pulmonary arterial hypertension (SITAR) study. J Cardiovasc Pharmacol Ther 2014; 19: 550–557. [DOI] [PubMed] [Google Scholar]
- 33.Shapiro S, Traiger G, Hill W, et al. Safety, tolerability, and efficacy of overnight switching from sildenafil to tadalafil in patients with pulmonary arterial hypertension. Cardiovasc Ther 2013; 31: 274–279. [DOI] [PubMed] [Google Scholar]
- 34.Ghofrani HA, Simonneau G, D’Armini AM, et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med 2017; 5: 785–794. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin V, Jansa P, Nielsen-Kudsk JE, et al. Riociguat in patients with chronic thromboembolic pulmonary hypertension: results from an early access study. BMC Pulm Med 2017; 17: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simonneau G, Torbicki A, Kim N. The pathophysiology and pathobiology of CTEPH. Eur Respir Rev 2017; 26: 160112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoeper MM, Simonneau G, Corris PA, et al. RESPITE: Switching to riociguat in PAH patients with inadequate response to PDE5i. Eur Respir J 2017; 50: 1602425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Darocha S, Banaszkiewicz M, Pietrasik A, et al. Sequential treatment with sildenafil and riociguat in patients with persistent or inoperable chronic thromboembolic pulmonary hypertension improves functional class and pulmonary hemodynamics. Int J Cardiol 2018; 269: 283–288. [DOI] [PubMed] [Google Scholar]
- 39.Grünig E, Gall H, Ghofrani HA, et al. Safety of switching to riociguat for the treatment of pulmonary hypertension: data from the EXPERT registry. World Symposium on Pulmonary Hypertension. Nice 2018.
- 40.Bayer AG. Adempas-US prescribing information. 2017. Available at: http://labelingbayerhealthcarecom/html/products/pi/Adempas_PIpdf.
- 41.Simonneau G, Rubin LJ, Galiè N, et al. Addition of sildenafil to long-term intravenous epoprostenol therapy in patients with pulmonary arterial hypertension: a randomized trial. Ann Intern Med 2008; 149: 521–530. [DOI] [PubMed] [Google Scholar]
- 42.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 43.Galiè N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 44.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174: 1257–1263. [DOI] [PubMed] [Google Scholar]
- 45.McLaughlin VV, Hoeper MM, Channick RN, et al. Pulmonary arterial hypertension-related morbidity is prognostic for mortality. J Am Coll Cardiol 2018; 71: 752–763. [DOI] [PubMed] [Google Scholar]
- 46.Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin V, Channick RN, Ghofrani HA, et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. Eur Respir J 2015; 46: 405–413. [DOI] [PubMed] [Google Scholar]
- 48.Humbert M, Barst RJ, Robbins IM, et al. Combination of bosentan with epoprostenol in pulmonary arterial hypertension: BREATHE-2. Eur Respir J 2004; 24: 353–359. [DOI] [PubMed] [Google Scholar]
- 49.Galiè N, Muller K, Scalise AV, et al. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in PAH. Eur Respir J 2015; 45: 1314–1322. [DOI] [PubMed] [Google Scholar]
- 50.Inami T, Kataoka M, Shimura N, et al. Pressure-wire-guided percutaneous transluminal pulmonary angioplasty: a breakthrough in catheter-interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv 2014; 7: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 51.Taniguchi Y, Miyagawa K, Nakayama K, et al. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. Euro Intervention 2014; 10: 518–525. [DOI] [PubMed] [Google Scholar]
- 52.Fukui S, Ogo T, Tsuji A, et al. Short-term impact of balloon pulmonary angioplasty on exercise capacity and ventilatory inefficiency in patients with inoperable chronic thromboembolic pulmonary hypertension. Eur Heart J 2014; 35(Suppl.): 916. [Google Scholar]
- 53.Inami T, Kataoka M, Yanagisawa R, et al. Long-term outcomes after percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Circulation 2016; 134: 2030–2032. [DOI] [PubMed] [Google Scholar]
- 54.Ogo T, Fukuda T, Tsuji A, et al. Efficacy and safety of balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension guided by cone-beam computed tomography and electrocardiogram-gated area detector computed tomography. Eur J Radiol 2017; 89: 270–276. [DOI] [PubMed] [Google Scholar]
- 55.Mizoguchi H, Ogawa A, Munemasa M, et al. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755. [DOI] [PubMed] [Google Scholar]
- 56.Andreassen AK, Ragnarsson A, Gude E, et al. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 57.Kurzyna M, Darocha S, Pietura R, et al. Changing the strategy of balloon pulmonary angioplasty resulted in a reduced complication rate in patients with chronic thromboembolic pulmonary hypertension. A single-centre European experience. Kardiol Pol 2017; 75: 645–654. [DOI] [PubMed] [Google Scholar]
- 58.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488. [DOI] [PubMed] [Google Scholar]
- 59.Kataoka M, Inami T, Hayashida K, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762. [DOI] [PubMed] [Google Scholar]
- 60.Aoki T, Sugimura K, Nochioka K, et al. Effects of balloon pulmonary angioplasty on oxygenation in patients with chronic thromboembolic pulmonary hypertension. Importance of intrapulmonary shunt. Circ J 2016; 80: 2227–2234. [DOI] [PubMed] [Google Scholar]
- 61.Roik M, Wretowski D, Labyk A, et al. Refined balloon pulmonary angioplasty driven by combined assessment of intra-arterial anatomy and physiology — Multimodal approach to treated lesions in patients with non-operable distal chronic thromboembolic pulmonary hypertension–Technique, safety and efficacy of 50 consecutive angioplasties. Int J Cardiol 2016; 203: 228–235. [DOI] [PubMed] [Google Scholar]
- 62.Olsson KM, Wiedenroth CB, Kamp JC, et al. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J 2017; 49: 1602409. [DOI] [PubMed] [Google Scholar]
- 63.Wiedenroth CB, Ghofrani HA, Adameit MSD, et al. Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm Circ 2018; 8: 2045894018783996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yanaka K, Nakayama K, Shinke T, et al. Sequential hybrid therapy with pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Am Heart Assoc 2018; 7: e008838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shapiro S, Traiger G, Hill W, et al. Safety, tolerability, and efficacy of overnight switching from sildenafil to tadalafil in patients with pulmonary arterial hypertension. Cardiovasc Ther 2013; 31: 274–279. [DOI] [PubMed] [Google Scholar]
- 66.Poch DS. Case report: a patient with pulmonary arterial hypertension transitioning from a PDE-5 inhibitor to Riociguat. BMC Pulm Med 2016; 16: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sulica R, Fenton R, Cefali F. Early observations on the use of riociguat in a large, metropolitan pulmonary arterial hypertension/chronic thromboembolic pulmonary hypertension treatment center. Cardiol Ther 2015; 4: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersen A, Korsholm K, Mellemkjaer S, et al. Switching from sildenafil to riociguat for the treatment of PAH and inoperable CTEPH: real-life experiences. Respir Med Case Rep 2017; 22: 39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davey RA, Benza RL, Murali S, et al. Phosphodiesterase type 5 inhibitor to riociguat transition is associated with hemodynamic and symptomatic improvement in pulmonary hypertension. Pulm Circ 2017; 7: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raina A, Benza RL, Farber HW. Replacing a phosphodiesterase-5 inhibitor with riociguat in patients with connective tissue disease-associated pulmonary arterial hypertension: a case series. Pulm Circ 2017; 7: 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamamoto K, Tanabe N, Suda R, et al. Riociguat for patients with chronic thromboembolic pulmonary hypertension: Usefulness of transitioning from phosphodiesterase type 5 inhibitor. Respir Investig 2017; 55: 270–275. [DOI] [PubMed] [Google Scholar]
- 72.Weir N, Brown AW, DelaSantina J, et al. Transition from PDE-5 inhibitors to riociguat in pulmonary hypertension. Am J Crit Care Med 2017; 195: A2282. [Google Scholar]