Abstract
Background:
Sonic hedgehog (Shh) and Nrf2 play a critical role in chemotherapeutic resistance. These two genes have been found to be dysregulated in head and neck squamous cell carcinomas (HNSCC). The purpose of this study was to analyze the expression, function and clinical prognostic relationship of Shh and Nrf2 in HNSCC in the context of therapeutic resistance and cancer stem cells (CSCs).
Methods:
We analyzed a cohort of patients with HNSCC to identify potential therapeutic biomarkers correlating with overall survival (OS) as well as disease-free survival (DFS) from our own data and validated these results using The Cancer Genome Atlas dataset. Expression of Shh and Nrf2 was knocked down by siRNA and cell growth, sphere growth and chemotherapeutic resistance were evaluated.
Results:
Widespread abundant expression of Shh and Nrf2 proteins were associated with shorter OS and DFS. The combination of Shh and Nrf2 expression levels was found to be a significant predictor of patient DFS. The tumor stromal index was correlated with Shh expression and inversely associated with shorter OS and DFS. Inhibition of Shh by siRNA or cyclopamine resulted in the attenuation of resistant CSC self-renewal, invasion, clonogenic growth and re-sensitization to the chemotherapeutic agents. Concomitant upregulation of Shh and Nrf2 proved to be an independent predictor of poor OS and DFS in patients with HNSCC.
Conclusions:
These findings suggest that Shh and Nrf2 could serve as therapeutic targets as well as promising dual prognostic therapeutic biomarkers for HNSCC.
Keywords: cisplatin, disease-free survival, HNSCC, Nrf2, overall survival, Shh
Introduction
Squamous cell cancer of the head and neck (HNSCC) continues to be a major health risk worldwide. The incidence of HNSCC in Bangladesh has shown a sharp increase in recent years due to socioeconomic conditions and a diverse population. Platinum-based chemotherapy and chemoradiotherapy (CRT) are the standards of care for many patients with locally advanced inoperable HNSCC.1 Furthermore, CRT has emerged as an adjuvant therapeutic option for operable high-risk patients.1
Despite the improvements mediated by radiation treatment followed by administration of adjuvant chemotherapy for HNSCC, the 5-year overall survival (OS) and disease-free survival (DFS) rates have not been improved.2 Although therapeutic and curative successes have been shown in a subgroup of patients, approximately 50% of patients with stage III and stage IV experience locoregional recurrence and metastatic spread within 2 years.3 Moreover, a group of patients develop resistance due to an increase in tumor cell proliferation rate.4,5 Therefore, clinical markers are essential to identify therapeutic resistance and efficiently gauge prognosis and recurrence after chemotherapy.
Several studies have evaluated a number of molecular markers including epidermal growth factor receptor (EGFR), RB1, and cyclin D1 as predictive and prognostic indicators in HNSCC.6 Many gene expression signatures with potential promise as biomarkers may originate from organ- and tissue-specific stem or progenitor cells that have not been explored extensively. For example, sonic hedgehog (Shh) is a critical morphogen for motor neuron differentiation both spatially and temporally.7 In cancer, Shh overexpression has been identified as a factor in the initiation of tumor growth and metastasis due to its stem cell-modulating properties.8 Evidence suggests that Shh is also critical for cancer stem cell (CSC) maintenance and functions.9 Activation of Shh signaling is associated with both chemotherapy and radiotherapy resistance in those patients who have received adjuvant chemotherapy.10 A recent study reported the significance of Shh pathway expression on outcome of human papilloma virus (HPV)-negative patients with head and neck carcinoma after surgery and adjuvant radiotherapy.4 All this evidence suggests that Shh could have potential as a therapeutic biomarker. On the other hand, nuclear factor erythroid 2 p45-related factor 2 (Nrf2), is a master transcription factor that regulates genes and is involved in antioxidant and detoxification pathways in normal cells.11 Nrf2 is overexpressed in 90% of HNSCC tumors12 and genomic alterations in the Nrf2 pathway are correlated with worse OS.13 Studies have shown that elevated levels of Nrf2 lead to chemoresistance in many cancer types.14 Inhibition of Nrf2 leading to reversal of the resistance in cisplatin-resistant head and neck cancer cells has been described recently.15 This evidence highlights the individual potential of Shh and Nrf2 as biomarkers in many cancers; however, their combined clinical significance in HNSCC has not yet been studied.
Cisplatin (cis-diaminedichloroplatinum) remains as the preferred anticancer drug for HNSCC; however, some patients frequently develop cisplatin resistance, posing a major therapeutic challenge. Therefore, it would be clinically beneficial if tumor sensitivity to cisplatin could be predicted, which would enable the design of combination therapeutic interventions to efficiently reduce cisplatin-mediated resistance. In this study, we examined the expressions of Shh and Nrf2 in tumor samples from patients with HNSCC and correlated expression with post-chemotherapy tumor resistance as well as clinical prognosis. We further explored the role of Shh and Nrf2 in cisplatin resistance, CSC maintenance, cell survival and invasive behavior of HNSCC cells.
Materials and methods
Study population-patients and samples
A retrospective part of the study included 183 patients [Chittagong Medical College and Hospital (CMCH) discovery cohort] with confirmed stage 1 to 4 HNSCC, who received treatment at the CMCH affiliated with the University of Chittagong, Bangladesh between September 2014 and October 2016. This study was approved by the central Bangladesh Medical Research Council (BMRC) ethics committee of Bangladesh (approval ID 052(l) 04 06 2014), and was performed in accordance with the 1964 Declaration of Helsinki and all subsequent revisions. Written informed consent was obtained from all patients used in this study concerning patient inclusion, data collection and analysis, patient’s biological data collection, patient’s privacy, risk and facility classification and storage and assessment. This study was conducted in strict compliance with current REMARK guidelines, in that it describes relevant information concerning the design, hypothesis, patient’s clinical characteristics and samples, experimental methods and detailed statistical analysis. The grade and stage of tumors were determined by an independent pathologist on the basis of the histologic findings and classified accordingly. To be eligible for the study, only patients with confirmed stage I, II, III and IV (oral cavity, oropharyngeal, laryngeal) HNSCC, or adjuvant chemotherapy, with complete information on T stage and metastatic spread to regional lymph nodes (N1 and N2) were included. The TNM staging was conducted following the American Joint Committee on Cancer, 8th edition.
Cell isolation and culture
After obtaining patient’s informed consent and following local and international regulations, healthy and HNSCC tumor tissues were obtained at the time of surgery. Collected tumors were first minced and enzymatically dissociated with 2 mg/ml of dispase (Roche, USA), and then incubated with 0.25% trypsin–EDTA, passed through a 21-gauge syringe and filtered through a 23-µm cell filter (Merck Millipore). Cells were either directly cultured in supplemented CSC medium or cryopreserved in freezing medium, consisting of 80% fetal bovine serum (FBS) and 20% dimethyl sulfoxide (DMSO) until further use. The FaDu and SCC61 cell lines were originally obtained from the American Type Cell Culture and cultured in Dulbecco’s modified Eagle’s medium, supplemented with 10% FBS with 1% penicillin–streptomycin antibiotics in a 37°C humidified incubator.
Invasion assay
Invasion assay was performed as described previously.16 Briefly, monolayer-grown cells were first treated either with cyclopamine (10 µM) or with vehicle (DMSO) for 48 h. Then, cells were harvested and seeded in low serum (0.1%) FBS on Matrigel-coated inserts at 50,000 cells/well. After 24 h of incubation, cells were stained with 1% crystal violet for 30 min and washed with phosphate-buffered saline (PBS). Invaded cells were counted under phase contrast microscope ×4 magnification) and quantified using ImageJ software. The experiments were performed in triplicate.
Colony-forming assay
Colony-forming assay was performed as described previously.17 Briefly, monolayer-grown cells were treated either with cyclopamine (10 µM) or with vehicle (DMSO) for 48 h. Cells were trypsinized and re-suspended (2 × 104 cells/ml) in 40% methyl cellulose in culture medium and plated in 35-mm culture plates and incubated for 2 weeks. After 2 weeks, the number of colonies were counted under a phase-contrast microscope (×10 magnification). Clonogenic efficiency was determined as the average number of colonies per dish, comparing the treatment group with the untreated group.
Western blotting
Cell lysates were prepared directly in sample buffer and analyzed as previously described.18 The primary antibodies used included Shh, Gli1, Nrf2, HO-1 and GAPDH. An enhanced chemiluminescence detection kit (Thermo Fisher) was used for signal detection.
Immunofluorescence
Spheres were grown on Matrigel-coated cover slips for 2 weeks in supplemented CSC medium, fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton-X for 15 min followed by 30 min blocking with 4% bovine serum albumin [BSA, (Sigma)]. Cells were incubated with Shh and Nrf2 primary antibodies overnight at 4°C, washed in PBS and incubated with Alexa fluorophore-conjugated secondary antibodies. Slides were mounted in Vectashield with DAPI (Vector Laboratories) and images were captured using an Optima fluorescence microscope. Digital images were processed using Adobe Photoshop CS2.
Real-time PCR
RNA was isolated using the Qiagen RNA isolation kit (Qiagen). Real-time PCR was performed using the QuantStudio 5 system. Primers used are listed below (Table 1).
Table 1.
List of primers.
| Gene names | Sense primers | Antisense primers |
|---|---|---|
| Shh | AGGACCCGGTTTGATCTTCT | GCCATGTGACACAGACAACC |
| Gli1 | GTGCAAGTCAAGCCAGAACA | ATAGGGGCCTGACTGGAGAT |
| Ptch1 | ACAAACTCCTGGTGCAAACC | CTTTGTCGTGGACCCATTCT |
| Smo | GGGAGGCTACTTCCTCATCC | GGCAGCTGAAGGTAATGAGC |
| NFE2L2/Nrf2 | GCGACGGAAAGAGTATGAGC | GTTGGCAGATCCACTGGTTT |
| HMOX1/HO-1 | TCCGATGGGTCCTTACACTC | TAAGGAAGCCAGCCAAGAGA |
| CD44 | AGCAACCAAGAGGCAAGAAA | GTGTGGTTGAAATGGTGCTG |
| Nanog | TTCCTTCCTCCATGGATCTG | TCTGCTGGAGGCTGAGGTAT |
| Oct3/4 | GTACTCCTCGGTCCCTTTCC | CAAAAACCCTGGCACAAACT |
Silencing Shh and Nrf2
To knockdown Shh and Nrf2 gene expression, FaDu cells were seeded and transfected 2 days later with Shh and Nrf2 small interfering RNA (siRNA) or nontargeting scrambled siRNA (Origene, MD, USA) for 24 h. The transfection and silencing efficiency were confirmed by Western blotting for each sample.
Tumor stromal index/density analysis
Tumor sections were stained with hematoxylin and eosin for microscopic analysis. A representative slide from each tumor section (n = 53) was evaluated by two independent pathologists and the tumor–stromal ratio (TSR) was visually estimated and scored as 10%, 20%, 30%, 40% and with a cut-off value as 50%. If the TSR value was less than 50% it was defined as a poor stromal index (SI), whereas 50% or more was defined as a high SI.
Cell proliferation assay
Treatment naïve HNSCC tumor cells and FaDu and HCC61 cells were cultured in 96-well plates and treated with increasing concentrations of cyclopamine for 5 days. AlamarBlue reagent was added to the cells and incubated for 4 h. Plates were read in a BioRad spectrophotometer and survival of cells was calculated.
Shh and Nrf2 immunohistochemistry
Normal and tumor tissues were formalin-fixed, paraffin-embedded, cut into 4-µm sections, deparaffinized with xylene and rehydrated through 100%, 90%, 70% and 50% ethanol to water. After rehydration, sections were heated in citrate buffer (pH 6.0) for antigen retrieval. Sections were stained with 2 µg/ml of goat polyclonal anti-Shh (Santa Cruz, cat. no. sc1194), monoclonal mouse anti-Shh (dilution 1:100 Sigma, cat. no. S8321) and polyclonal rabbit anti-Nrf2 (dilution 1:100, Abcam, cat. no. ab137550) followed by incubation in horseradish peroxidase-conjugated (Invitrogen) secondary antibody incubation for 1 h at room temperature. Antibody-stained sections were analyzed by ImageJ software.
Analysis of tissue sections
Complete clinicopathological information was obtained for all patients from independent as well as primary care hospital sources. A detailed description of evaluation and selection of the patient cohort is provided in Figure 1(a). A blindfolded scoring system was used to analyze Shh and Nrf2 expression and every attempt was taken to avoid double scoring from the same sample. All tumor sections showing widespread nuclear or cytoplasmic expression of Shh and Nrf2 in either all or the majority of cancer cells were scored as Shhhigh/Nrf2high. On the other hand, tumors were scored as Shhlow/Nrf2low when the nuclear compartment lacked Shh/Nrf2 expression or showed very weak nuclear or cytoplasmic expression in a small number of cancer cells. The agreement between the scoring results obtained from two independent observers was evaluated with the use of contingency tables and calculated with Cohen’s kappa index.
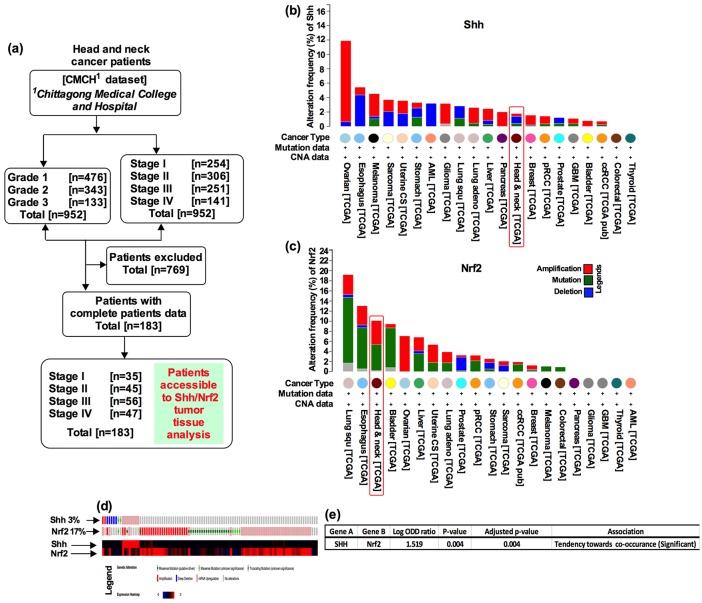
Figure 1.
Patient selection criteria, cohort characteristics and identification of biomarkers in HNSCC. (a) The CMCH datasets containing 952 tumor samples from patients with HNSCC. Based on the patient’s complete medical information and serum sample availability, 183 patients out of 952 were selected for further analysis. Distribution and frequency of genetic alterations for (b) Shh and (c) Nrf2, in major cancer types. A vertical red color box delineates the TCGA results for head and neck cancer. (d) Alteration in the Shh and Nrf2 genes (columns represent individual samples). Heat map representing the intensity of the alterations shown below. (e) Significant co-occurrence association of Shh-Nrf2 genes. TCGA datasets were used to generate graphs and to calculate the significance of co-occurrence.
CMCH, Chittagong Medical College and Hospital; HNSCC, head and neck squamous cell carcinoma; TCGA, The Cancer Genome Atlas.
Measurement of serum Shh and Nrf2 transcriptional activity
After obtaining patients’ written consent, blood samples were collected and centrifuged to separate serum and plasma and stored at −80°C until use. The Shh concentration and transcriptional activity of Nrf2 in blood serum were measured using human Shh enzyme-linked immunosorbent assay (cat. no. ELH-ShhN, RayBioTech) and human Nrf2 transcription factor assay kit (cat. no. ab207223, Abcam) following manufacturer’s procedures. We used 50 pg/l for Shh and 400 pg/l for Nrf2 as the serum cut-off levels to discriminate normal concentrations from those of patients with HNSCC.
TCGA datasets (validation datasets)
The validation datasets used in this study were from TCGA (http://cancergenome.nih.gov/). TCGA datasets were downloaded from cBioPortal. We analyzed the OS and DFS using the R Statistical Language software (Foundation for Statistical Computing, Vienna, Austria, version 3.4.3) package ‘survival’ and ‘survminer’ (www.R-project.org/).
Statistical analysis
We used R version 3.4.3 software for all statistical analysis and graphs. Patients’ subgroup and clinicopathological variables were compared with respect to survival outcomes with use of Kaplan–Meier curves, log-rank tests, and univariate and multivariate analysis based on a Cox proportional hazard ratio (HR) method. The relationship and comparisons between the Shh/Nrf2 status (negative versus positive) and adjuvant chemotherapy (chemo-yes versus chemo-no) were analyzed using the Cox proportional HR method. A Student’s t test was used to compare the serum concentration and relative gene expression in each group.
Results
Patient selection criteria, cohort characteristics and identification of biomarkers in HNSCC
A total of 952 patients with HNSCC were screened for eligibility, and 183 patients who were HPV-negative fulfilled the study criteria for this translational study [Figure 1(a)]. A large number of the patients had received cisplatin-based adjuvant chemotherapy. The majority of specimens originated from oropharyngeal (n = 85; 46.45%), oral cavity (n = 56; 30.61%) and laryngeal (n = 42; 22.95%) sites (Table S1; S2). In this cohort, undifferentiated (grade 3) and moderately differentiated (grade 2) patients significantly correlated with OS [Figure S1(a); grade 3 median OS 32 months, grade 2 median OS 42 months, log-rank p < 0.0001] as well as stage 3 and stage 4 patients [Figure S1(b); stage 3 median OS 39 months, stage 4 median OS 29 months, log-rank p < 0.0001] significantly correlated with OS. HRs for death were 1.65 for tumor grades 1–3 [95% confidence interval (CI), 1.021–2.587, p < 0.05], 1.97 for tumor stage 1–4 (95% CI, 1.125–2.754, p < 0.001), 1.89 for tumor T stage (T1–T4, 95% CI, 1.105–2.229, p < 0.01) and 1.24 for tumor N stage [N0–N3, 95% CI, 1.02–2.054, p = 0.234; Figure S1(c)].
To determine whether Shh and Nrf2 could serve as possible dual biomarkers in a tumor-specific context, we first surveyed the genetic alterations of Shh and Nrf2 genes in 21 major cancers in the TCGA dataset. As a comparison, Shh was altered 12% in ovarian, 3% in melanoma and glioma, and 2% in uterine, pancreas and lung adenocarcinoma respectively. However, Shh was altered only 1% in tumors of patients with HNSCC [Figure 1(b)]. On the other hand, Nrf2 locus was altered 4% in HNSCC, compared with 2% in lung, and 8% in ovarian cancer patient tumors [Figure 1(c)]. To further explore the relationship between Shh and Nrf2, we analyzed the mRNA expression data from the TCGA head and neck cancer cohort (n = 537). It revealed that Shh and Nrf2 were individually amplified by 3% and 17%, respectively [Figure 1(d)], and showed a significant tendency towards co-occurrence in the TCGA dataset [Figure 1(e), p < 0.001)]. Thus, this analysis highlighted a potential close association between Shh and Nrf2 in HNSCC.
Evaluating Shh and Nrf2 protein expression in tumor tissues by immunostaining
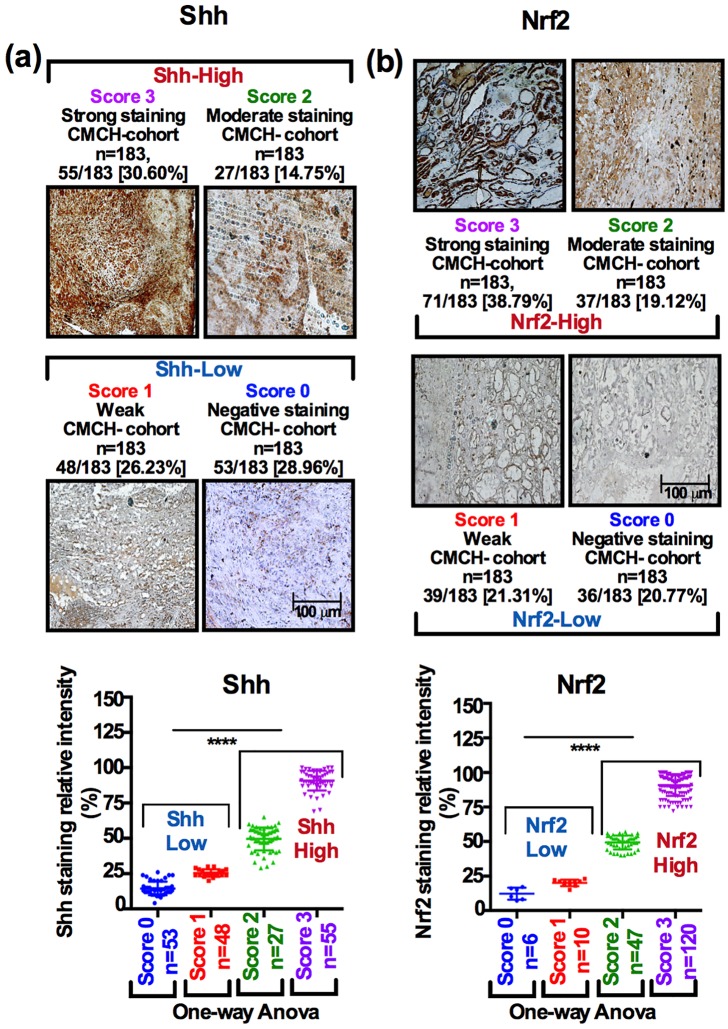
Immunohistochemical results were assessed in all tumors and a good inter-rater agreement was achieved on the basis of unweighted Cohen’s kappa index values. A full description of scoring method, performance analysis and two independent rater agreements are presented in the Figure S2(a–f). Of the available patient tumor samples (n = 183), 82 of 183 tumors (44.81%) exhibited strong to moderate nuclear Shh immunostaining and 101 of 183 tumors (55.19%) exhibited weak to negative Shh immunostaining [Figure 2(a); bar graph, one-way analysis of variance (ANOVA)]. On the other hand, 108 of 183 tumors (59.01%) exhibited strong to moderate Nrf2 staining and 75 of 183 tumors (42.08%) exhibited weak to negative immunostaining [Figure 2(b); bar graph, one-way ANOVA].
Figure 2.
Evaluating Shh and Nrf2 protein expression in tumor tissues by immunostaining. (a, b) We classified the HNSCC tumors as Shhhigh/Shhlow and Nrf2high/Nrf2low. Shhhigh and Nrf2high samples were scored as Score2 and Score3 if the staining intensity of Shh and Nrf2 showed dense nuclear expression in the entire section of tumors. Similarly, Shhlow and Nrf2low were scored as Score0 and Score1, if the staining intensity was completely lacking or the minority of cells were positive for either Shh or Nrf2. The samples were subclassified as Score0 if Shh expression was completely lacking (28.96%, 53 of 183); Score1 if staining was weak or sporadic (26.23%, 48 of 183); Score2 if staining intensity was moderate (14.75%, 27 of 183); and Score3 if staining intensity was strong (30.60%, 55 of 183). For Nrf2, samples were subclassified as Score0 if Nrf2 expression was completely lacking (20.77%, 38 of 183); Score1 if staining intensity was weak and sporadic (21.30%, 39 of 183); Score2 if the staining intensity was moderate (19.20%, 37 of 183); and Score3 if staining was strong and widespread (38.79%, 71 of 183). For staining intensity comparison one-way ANOVA was used, p < 0.0001).
ANOVA, analysis of variance; HNSCC, head and neck squamous cell carcinoma.
The immunostaining scores for Shh ranged from 55 of 183 (30.05%, Score3), 27 of 183 (14.75%, Score2), 48 of 183 (26.23%, Score1) and 53 of 183 [28.96%, Score0; Figure 2(a)]. In a similar fashion, the staining scores for Nrf2 ranged from 71 of 183 (38.79%, Score3), 37 of 183 (19.12%, Score2), 39 of 183 (21.31%, Score1) and 36 of 183 [19.67%, Score0; Figure 2(b)]. Based on the scoring method, we defined Score2 as medium expression (cells expressing Shh/Nrf2 between 25–50%) and Score3 as strong expression (cells expressing Shh/Nrf2 over 50%) corresponding to the Shhhigh /Nrf2high group, and Score0 as negative expression (cells expressing Shh/Nrf2 0–10%) and Score1 as weak expression (cells expressing Shh/Nrf2 10–25%), which corresponds to the Shhlow /Nrf2low group [Figures 2(a,b); graphs, p < 0.0001 for Shh and p < 0.0001 for Nrf2].
Shh and Nrf2 expression predicts overall survival in HNSCC
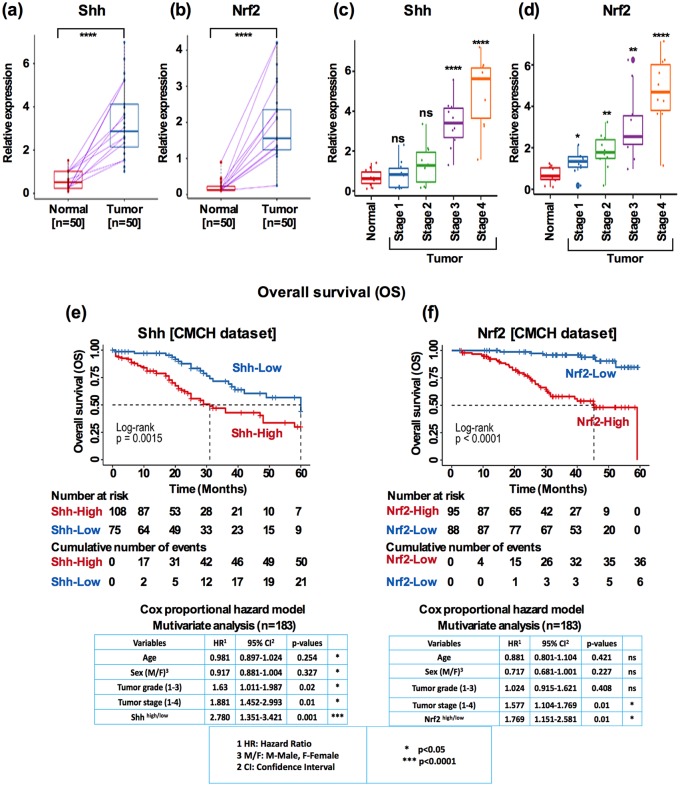
In order to evaluate the clinical significance of Shh and Nrf2 in HNSCC, we first analyzed whether the individual expressions of Shh and Nrf2 were associated with clinical prognosis. Using a small cohort (n = 50) of matched adjacent normal and tumor tissues, we found that both Shh and Nrf2 expressions were frequently increased in the tumor tissues [Figure 3(a), Shh: p < 0.0001, Figure 3(b), Nrf2: p < 0.0001]. In HNSCC, tumor stage was the best predictor for OS. Given the clinical importance of tumor stage, we analyzed the expression of Shh and Nrf2 in early- (stage 1 and 2) and late-stage (stage 3 and 4) tumors. Both Shh and Nrf2 were significantly higher in late-stage tumors [Figure 3(c); Fisher’s exact test, Shh: p < 0.0001, Figure 3(d), Nrf2: p < 0.01; p < 0.0001]. High Shh and high Nrf2 expression were clearly independent predictors of poorer OS. The median OS was worse for patients with high expression of Shh [Figure 3(e), median OS: 31.0 versus 60.0 months, log-rank p < 0.001] and Nrf2 [Figure 3(f), median OS: 45.5 versus over 60 months, log-rank p < 0.0001]. The association remained significant after a multivariate Cox proportional HR analysis when adjusting for age, sex, tumor grade, and tumor stage. The HR was 2.780 for Shh [Figure 3(e), bottom panel; 95% CI, 1.351–3.421, p < 0.001] and 1.769 for Nrf2 [Figure 3(f), bottom panel; 95% CI, 1.151–2.581; p < 0.01].
Figure 3.
Shh and Nrf2 expression predicts OS in HNSCC. (a) Shh and Nrf2 expression in matched normal and tumor samples (Mann–Whitney U test, p < 0.0001) (b) Shh and Nrf2 expression stratified by tumor stage (one-way ANOVA, p < 0.0001, p < 0.05, p < 0.01). (c) Kaplan–Meier survival curves comparing the OS for patients with HNSCC grouped as Shhhigh and Shhlow (log-rank p < 0.001), and Nrf2high and Nrf2low expression (log-rank p < 0.0001; high: Score3/Score2; low: Score1/Score0 expression groups). Comparison was made between high and low expression groups. Below each graph showed the Cox proportional hazard ratio in a multivariate analysis (p < 0.01 and p < 0.001).
ANOVA, analysis of variance; HNSCC, head and neck squamous cell carcinoma; OS, overall survival.
OS and DFS from TCGA validation dataset
To evaluate the strength of the Shh and Nrf2 analysis in our own cohort, we used an independent validation cohort to test the reproducibility for Shh and Nrf2 based on mRNA-sequence expression data for HNSCC tumors from cBioportal for Cancer Genomics (www.cbioportal.org/data_sets.jsp). These datasets contain harmonized information of patient’s tumor recurrence status and OS status as well as long-term follow-up records.
To explore the relationship between Shh and Nrf2 expression and OS and DFS, we stratified the TCGA validation dataset (n = 324) into two groups as Shhhigh and Shhlow based on log2 normalized expression values. In this validation cohort, Shhhigh displayed a significant association with 5-year OS [Figure S3(a); Shhhigh versus Shhlow: 60 months versus over 100 months, log-rank p < 0.001]; DFS was relatively lower in magnitude [Figure S3(b); Shhhigh versus Shhlow: 24 versus 80 months, log-rank p < 0.0001]. A similar grouping method was applied for Nrf2 expression status (n = 224); however, here the association did not reach statistical significance for OS [Figure S3(c); Nrf2high versus Nrf2low, 40 months versus 90 months, log-rank p = 0.24] and DFS [Figure S3(d); Nrf2high versus Nrf2low; 64 months versus 100 months, log-rank p = 0.12].
Shh and Nrf2 expression are correlated with tumor prognostic factors
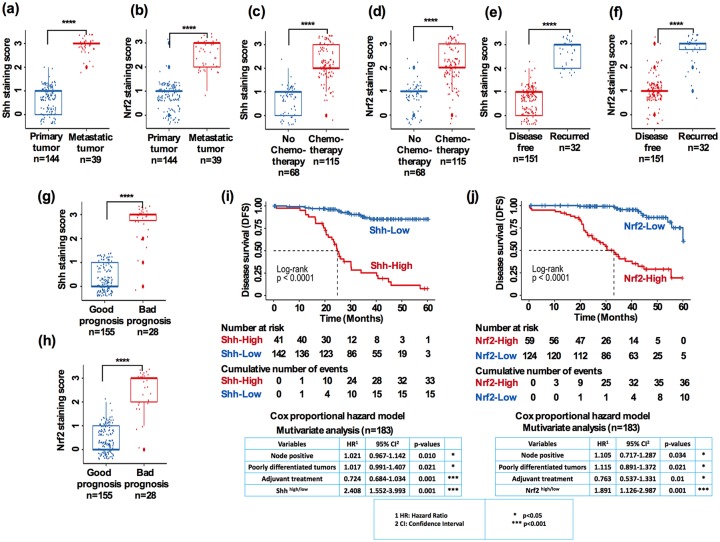
We then analyzed the correlation and the distribution of several tumor prognostic features with the expressions of Shh and Nrf2 individually and determined DFS. There was a significant difference in Shh and Nrf2 expression between primary and metastatic tumors [Figure 4(a); Shh: p < 0.0001; Figure 4(b); Nrf2: p < 0.0001]. When comparing the expression between no chemotherapy and chemotherapy-treated tumors, we found significant differences between the patients who received chemotherapy and those who did not [Figure 4(c); Shh: p < 0.0001, Figure 4(d); Nrf2: p < 0.0001]. We further analyzed the patients’ follow-up data and their expressions of Shh and Nrf2 in the recurred and disease-free patient tumors. Both Shh and Nrf2 expression were significantly higher in patients with tumor recurrence than patients who were disease free [Figure 4(e), Shh: p < 0.0001, Figure 4(f); Nrf2: p < 0.0001]. Furthermore, there was a significant difference between the patients who had good prognosis and those with worse prognosis [Figure 4(g); Shh: p < 0.001, Figure 4(h); Nrf2: p < 0.0001]. Next, we evaluated the relationship between Shh and Nrf2 expression and patient DFS. The Kaplan–Meier analysis revealed a worse DFS in patients with high Shh and high Nrf2 expression. The median DFS was 25.5 months versus >60 months for high versus low Shh [Figure 4(i); log-rank p < 0.0001], and 32.5 versus 60 months for high versus low Nrf2 [Figure 4(j); log-rank p < 0.0001]. This suggests that high Shh and high Nrf2 expressions were independent worse DFS predictors among other variables [Figure 4(i), bottom panel; Shh: HR-2.408; p < 0.0001; and Figure 4(j); bottom panel; Nrf2: 1.891, p < 0.0001].
Figure 4.
Shh and Nrf2 expression are correlated with tumor prognostic factors. (a, b) Correlation between primary versus metastatic tumors on the basis of staining score for Shh and Nrf2. (c, d) Correlation between no chemotherapy versus chemotherapy tumors on the basis of staining score for Shh and Nrf2. (e, f) Correlation between disease free versus recurred tumors on the basis of staining score for Shh and Nrf2. (g, h) Correlation between good prognosis versus bad prognosis for patients on the basis of staining score for Shh and Nrf2. (i) Kaplan–Meier survival curves comparing DFS for patients with HNSCC, grouped as Shhhigh and Shhlow (log-rank p < 0.0001; bottom panel: Cox proportional hazard ratio in a multivariate analysis, p < 0.01, p < 0.001, p < 0.001). (j) Nrf2high and Nrf2low expression groups (log-rank p < 0.0001; bottom panel: Cox proportional hazard ratio in a multivariate analysis, p < 0.05, p < 0.05, p < 0.01, p < 0.001).
DFS, disease-free survival; HNSCC, head and neck squamous cell carcinoma.
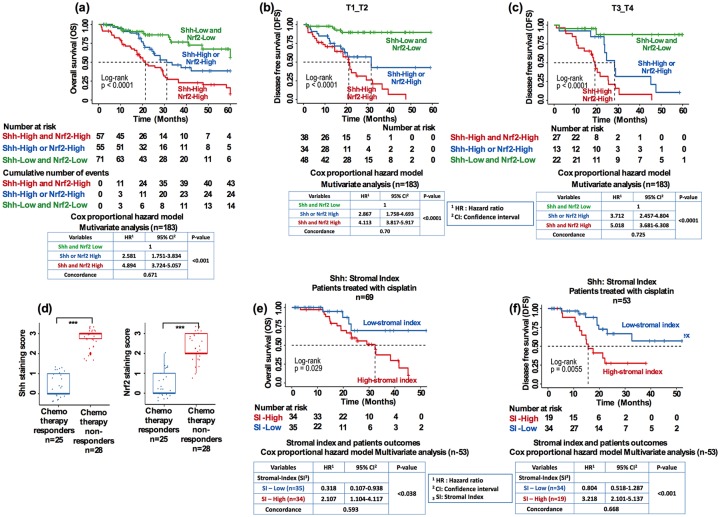
Dual prognostic values based on stratification of patients with HNSCC with respect to invasion and lymph node involvement, chemotherapy response, and stromal index
Shh signaling is linked to the epithelial-to-mesenchymal (EMT) transition phenotype following chemotherapy.19 On the other hand, cancer cells frequently showing Nrf2 overexpression are linked to increased resistance to chemotherapy.15 Therefore, we felt it would be interesting to evaluate the combined outcome of Shh and Nrf2 in respect to chemotherapeutic resistance. First, we analyzed the favorable and unfavorable outcomes based on combined Shh and Nrf2 expression levels. Patients with low Shh and low Nrf2 expression levels showed a very favorable OS outcome [Figure 5(a), median OS: over 60 months, HR: set at 1 constant]. Patients with either high Shh or high Nrf2 levels showed an intermediate outcome [Figure 5(a), median OS: 31 months, HR = 2.581], whereas the most unfavorable outcome was seen in both the high Shh and high Nrf2 patient group [Figure 5(a), median OS: 21.5 months, HR = 4.894; Figure 5(a), bottom panel; Cox proportional HR in a multivariate analysis]. These results identified that low expression of both Shh and Nrf2 predicts the most favorable outcome and less aggressive tumors.
Figure 5.
Dual prognostic values based on stratification of patients with HNSCC with respect to invasion and lymph node involvement, chemotherapy response, and stromal index. (a) Kaplan–Meier cumulative OS of all patients (n = 183; bottom panel: Cox proportional hazard ratio in a multivariate analysis, p < 0.001). (b, c) Cumulative DFS of all patients (n = 183) patients stratified based on depth of invasion (T1 versus T2, bottom panel: Cox proportional hazard ratio in a multivariate analysis, p < 0.0001) and T3 versus T4 (bottom panel: Cox proportional hazard ratio in a multivariate analysis, p < 0.0001). (d) Comparison of Shh (p < 0.001) and Nrf2 (p < 0.001) expression between chemo-responders and nonresponders (Student’s t test). (e) Kaplan–Meier cumulative DFS of patients with HNSCC (bottom panel: Cox proportional hazard ratio in a multivariate analysis, p < 0.001; independent two-tailed Student’s t test).
DFS, disease-free survival; HNSCC, head and neck squamous cell carcinoma; OS, overall survival.
Secondly, to analyze which subgroup of patients with HNSCC predicts a worse DFS outcome, we stratified patients with known risk factors, such as the status of regional lymph nodes (N0 versus N1 and N2 versus N3) in patients with HNSCC. The dual biomarkers Shhhigh/Nrf2high group preferentially discriminated the DFS status in patients with lymph nodes stages N0–N1 versus N2–N3 in the CMCH cohort [Figure S4(a,b); log-rank p < 0.0001 and p < 0.0001, respectively]. These results remained significant in a multivariate analysis [Figure S4(a,b), Cox proportional HR model in multivariate analysis]. Furthermore, when patients were stratified by the depth of invasion, combined biomarkers discriminated the differences in DFS in patients in the T1–T2 versus T3–T4 groups [Figure 5(b,c); log-rank p < 0.0001; p < 0.0001, respectively]. The multivariate analysis remained significant for combined Shhhigh/Nrf2high groups [Figure 5(b,c) bottom panel; Cox proportional HR model in multivariate analysis].
Combination chemotherapy such as cisplatin with 5-fluorouracil (5-FU) treatment have shown good response rates (60–90%) in patients with locoregionally advanced head and neck cancer, with half of the patients achieving complete response.19 However, cisplatin-mediated drug resistance has been a major obstacle due to the activation of multiple resistance pathways. We therefore evaluated whether Shh and Nrf2 expression could be associated with administration of chemotherapy and resulting chemoresistance. Among 183 patients with HNSCC, 53 patient samples were available after cisplatin-based chemotherapy. Overall, 25 patients (47.12%) showed complete response to cisplatin (good responders) while 28 patients (52.83%) showed resistance or failed cisplatin treatment (nonresponders). Interestingly, resistant and nonresponder patients showed significantly higher Shh and higher Nrf2 expression compared with good responders [Figure 5(d); p < 0.001, independent two-tailed Student’s t test].
A recent study demonstrated that hedgehog (Hh) pathway genes play a crucial role at the tumor–stromal intersection in patients with HNSCC treated with radiotherapy and contribute to stromal-mediated resistance.20 This prompted us to evaluate stromal abundance and Shh expression in patients treated with cisplatin. The SI correlated with Shh abundance and poor OS and DFS [Figure 5(e,f); log-rank: p < 0.05 (OS) and p < 0.001 (DFS); Figure 5(e,f) bottom panel; Cox proportional HR model].
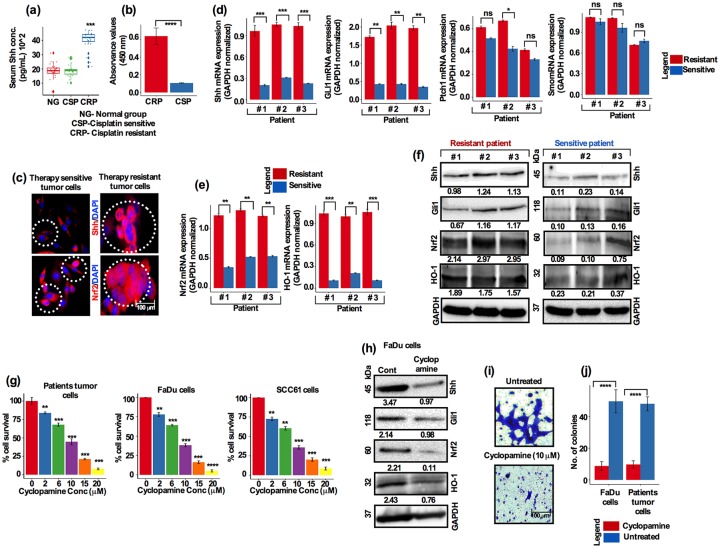
Shh and Nrf2 are highly expressed in treatment-resistant HNSCC tumors
Tissue immunohistochemistry and fluorescence in situ hybridization remain the preferred methods for prognostic biomarker diagnosis; however, these require high quality tissue samples. Therefore, serum or plasma concentrations of a particular marker could be used as an alternative to these methods for prognostication. We therefore evaluated Shh and Nrf2 in blood serum from a group of patients who were either sensitive or resistant to chemotherapy. Analysis of serum Shh showed that, cisplatin-resistant patient serum showed high Shh abundance compared with sensitive and normal patient serum [Figure 6(a), p < 0.001]. In addition, analysis of Nrf2 transcriptional activity in blood serum was found to be higher in cisplatin-resistant patients and significantly lower in sensitive patients [Figure 6(b), p < 0.0001].
Figure 6.
Shh and Nrf2 are highly expressed in treatment-resistant HNSCC patient tumors. (a) Serum Shh concentrations differ from healthy (n = 30), cisplatin-sensitive and cisplatin-resistant patients with HNSCC. Serum Shh concentrations measured (pg/ml, p = 0.0014, Fisher’s exact test). (b) Nrf2 transcriptional activity from healthy, cisplatin-resistant and cisplatin-sensitive patient samples (p = 0.0001, Student’s t test). Inhibition of Shh by cyclopamine decreased cell survival, invasion and cisplatin-induced resistance in HNSCC cells. (c) Immunofluorescence staining showing the localization of Shh and Nrf2 in therapy-sensitive versus therapy-resistant patients with HNSCC tumors (red: Shh/Nrf2 and blue: DAPI; scale bar, 100 µm) mRNA expression of (d) Shh, Gli1, Ptch1, Smo, (e) Nrf2 and HO-1 in patients with HNSCC tumors assessed by quantitative reverse transcriptase PCR. (f) Western blots showing the expression of Shh, Gli1, Nrf2 and HO-1 from three independent patients’ tumor samples. Serum Shh and Nrf2 transcriptional activity analysis. (g) Freshly isolated HNSCC tumor cells, FaDu and HCC61 cells treated with increasing concentrations of cyclopamine for 3 days. Cell viability was assessed by alamarBlue assay. (h) FaDu cells were treated with cyclopamine (10 µM) and subjected to Western blot for the expression of the indicated proteins. (i) FaDu cells were treated as described in (h) and cell invasion assay was performed in Matrigel-coated Boyden chambers for 24 h (scale bar, 100 µm). (j) Colony-forming efficiency assay after cyclopamine treatment.
HNSCC, head and neck squamous cell carcinoma; PCR, polymerase chain reaction.
A recent study reported that tumors with an upregulated Gli1 and EMT pathway have been found to be more chemo- and radio-resistant in locally invasive and metastatic cancers.21 To determine the possible role of Shh and the Ptch1–Smo–Gli1 pathway in therapeutic resistance, we examined the cellular localization of Shh and Nrf2 from a pool of therapy-resistant and therapy-sensitive HNSCC patient tumors by immunostaining. Shh was localized in the cytoplasm and nucleus in both therapy sensitive and resistant cells [Figure 6(c); white circle]; however, Nrf2 was mainly localized in the cytoplasm in therapy-sensitive cells [Figure 6(a); white circle], whereas strong nuclear (white big circle) as well as sporadic cytoplasmic (white small circle) expression was observed in therapy-resistant cells [Figure 6(c)]. Real-time PCR results showed the relative expression of Shh, Gli1, Ptch1, Smo, Nrf2 and HO-1. Shh and Gli1 were significantly upregulated in therapy-resistant tumors [Figure 6(d)]. In contrast, Ptch1 and Smo expression showed no detectable significance between resistant and sensitive tumors [Figure 6(d)]. Nrf2 inhibition that reverses the resistance of cisplatin-resistant head and neck cancer cells has recently been described.15 Therefore, it was essential to determine whether the administration of chemotherapy increases Nrf2 expression and its target gene HO-1 via an antioxidant response element in patients with HNSCC. Similarly to Shh expression, Nrf2 and HO-1 were highly expressed in resistant tumors compare with sensitive counterparts [Figure 6(e)]. Furthermore, Western blot results from treatment-resistant and treatment-sensitive tumors from patients further confirmed that Shh, Gli1, Nrf2 and HO-1 were all elevated in the cisplatin-resistant tumors compared with cisplatin-sensitive counterparts [Figure 6(f)].
Regrowth of treatment-resistant tumor cells compromises the effectiveness of treatment, suggesting that resistant cells repopulate and tumors recur over time. Previous studies have reported the contribution of the Hh signaling pathway in the repopulation of these progenitor cells.22,23 Targeting Hh signaling by cyclopamine both suppresses HNSCC and enhances chemotherapeutic effects;24 however, it was not shown whether targeting Shh signaling by cyclopamine might have any suppressive effects on Nrf2 and its target genes. We therefore assessed the effects of cyclopamine on cell survival in treatment-naïve patient tumor cells. We observed a significant dose-dependent cell death effect on HNSCC cells [Figure 6(g)]. We then tested the dose response on FaDu and HCC61 cells. FaDu and HCC61cells showed a strong dose-dependent suppressive growth response to cyclopamine [Figure 6(g)]. These results were confirmed by Western blot analysis and it was found that treatment of FaDu cells with cyclopamine inhibited the expression of Shh, Gli1, Nrf2 and HO-1 [Figure 6(h)]. Next, we wanted to ascertain whether blocking of the Shh pathway by cyclopamine had any effects on cell invasiveness and colony-forming capacity. Cells were treated as described in Figure 6(h); trypsinized and cultured in Matrigel-coated Boyden chambers for 24 h. Cyclopamine (10 µM) treatment significantly inhibited the invasion of cells [Figure 6(i)]. In addition, cyclopamine significantly inhibited the colony-forming efficiency of patient tumor cells and FaDu cells [Figure 6(j); p < 0.001 for patient cells and FaDu cells]. These observations indicated that the Shh pathway was prominently involved in the invasive and aggressive behaviors of HNSCC tumor cells.
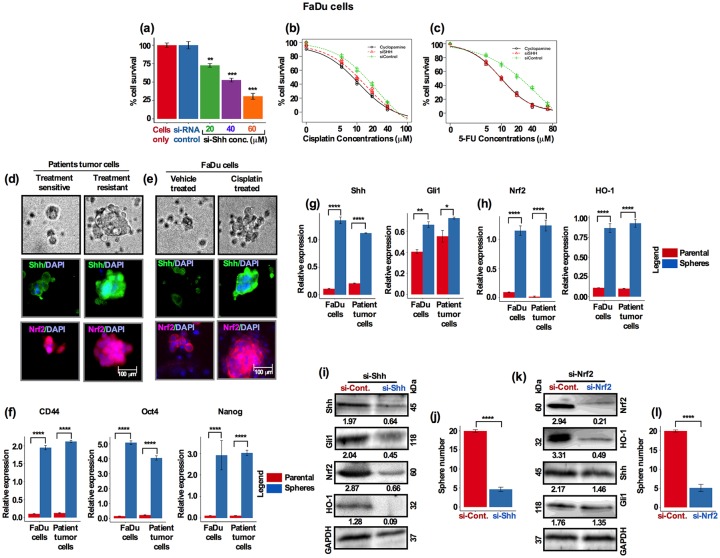
High expression of Shh and Nrf2 correlates with induced stem cell-like characteristics and contributes to chemoresistance of HNSCC cells
In order to validate the role of Shh in HNSCC tumor cell growth and resistance, we knocked down Shh with Shh specific siRNA and assessed cell survival. Transfected cells were incubated for 72 h and assessed for cell survival by alamarBlue assay. Similarly to cyclopamine alone [shown in Figure 6(g)], there was a concentration-dependent suppressive behavior of siRNA-Shh on cell survival. No changes in cell growth inhibition were observed in the untreated and control siRNA-treated cells [Figure 7(a); Figure S5(a)]. To further determine the role of Shh in chemoresistance, we assessed the effects of Shh inhibition by sequentially treating cells with cisplatin, cyclopamine and siRNA-Shh. First, cells were treated with siRNA-Shh for 24 h, then washed and further treated with cyclopamine (10 µM) for 24 h, trypsinized and re-cultured followed by a series of cisplatin doses for 48 h. Low-dose cisplatin (2 µM) alone had small effect (18–20% inhibition) on cell survival 72 h after treatment; however, the combination treatment either with cyclopamine (10 µM) or siRNA-Shh reduced cell survival by 47% (siRNA-Shh) and 60% [cyclopamine 10 µM; Figure 7(b) and Figure S5(b)]. We then tested chemoresistance of cells by a second anticancer agent, 5-FU. Cells were treated with siRNA-Shh and cyclopamine as described above followed by a series of 5-FU doses for 48 h. Resistance to 5-FU was identical to that seen for cisplatin and cell survival was greatly reduced when cyclopamine (10 µM) and siRNA-Shh were applied in combination [Figure 7(c) and Figure S5(c)].
Figure 7.
High expression of Shh and Nrf2 correlates with induced stem cell-like characteristics and contributes to chemoresistance of HNSCC cells. (a) FaDu cells were treated with three different concentrations of ShhsiRNA for 24 h and cell survival was analyzed. (b) FaDu cells were treated with Shh-siRNA and cyclopamine (10 µM) for 2 days, washed and treated with increasing concentrations of cisplatin and cell viability was assessed by alamarBlue assay. (c) FaDu cells were treated with Shh-siRNA and cyclopamine (10 µM) for 2 days, washed and treated with increasing concentrations of 5-fluorouracil (5-FU) and cell viability was assessed by alamarBlue assay. Dose–response data were analyzed by the R statistical software ‘DRC’ package. (d) Freshly isolated treatment-sensitive and treatment-resistant HNSCC cells and (e) FaDu cells were cultured in cancer stem cell (CSC) supplemented medium for 10 days. Resulting spheres were immunofluorescently stained for Shh and Nrf2 (green: Shh; red: Nrf2, blue: DAPI; scale bar, 100 µm). Patients’ tumor cells and FaDu cells were cultured in the supplemented sphere growth medium and analyzed for expression of (f) CD44, Oct4 and Nanog. (g, h) Shh, Gli1, Nrf2 and HO-1 by Real-time PCR and compared for relative expression. (i) FaDu cells were transfected with Shh-siRNA and analyzed by Western blots for expression of Shh, Gli1, Nrf2 and HO-1. (j) FaDu cells were cultured and transfected as in (i) and sphere growth and numbers were analyzed (p = 6.3×10−10, Student’s t test). (k) FaDu cells were transfected with Nrf2-siRNA and analyzed by Western blots for expression of Shh, Gli1, Nrf2 and HO-1. (l) FaDu cells were cultured and transfected as in (i) and sphere growth and numbers were analyzed (p < 0.0001, Student’s t test).
HNSCC, head and neck squamous cell carcinoma; PCR, polymerase chain reaction.
To elucidate the relationship between Shh and CSC-like characteristics and enhanced resistance to chemotherapy, freshly dissociated treatment-sensitive and treatment-resistant HNSCC tumor cells were cultured in a serum-free supplemented growth medium (as for CSC), and assessed for sphere formation efficiency. The resistant cells formed comparatively larger spheres than the sensitive cells and showed enhanced tolerance to cisplatin treatment [Figure 7(d)]. Immunostaining of spheres showed that treatment-resistant spheres had intense Shh and Nrf2 expression compared with spheres from sensitive cells [Figure 7(d)]. Similar results were obtained for the FaDu cells. Cells were treated with 5 µM cisplatin or vehicle, and analyzed for sphere formation efficiency. Cisplatin-treated cells generated significantly larger sized spheres as well as showing stronger Shh and Nrf2 expression, suggesting that Shh and Nrf2 are probably involved in enhanced chemoresistance [Figure 7(e)].
Recent evidence suggests that, resistance to cisplatin is closely linked to the existence of CSCs, primarily CD44+, in HNSCC.25 Because CD44 was recently reported as a CSC marker in HNSCC, we examined the expression of CD44, Oct4 and Nanog in HNSCC tumor cells. Patient-derived treatment-naïve and FaDu cells were treated with cisplatin (5 µM) for 7 days, trypsinized and cultured in growth factor-supplemented CSC medium. Cells were allowed to form spheres to enrich for higher CSC and stemness activity, and gene expression was subsequently analyzed by Real-time PCR. The CSC marker CD44 and stemness markers Nanog and Oct4 were significantly elevated in spheres compared with parental cells [Figure 7(f)]. Furthermore, Shh and Gli1 expression was also elevated in spheres [Figure 7(g]). In addition, Nrf2 and HO-1 expression was also significantly upregulated in spheres [Figure 7(h)]. Taken together these results suggest that cisplatin treatment induces more CSC activity and an increase in the CSC-like cell population, thus acquiring HNSCC with an enhanced resistance to chemotherapy.
To further investigate the functional involvement of Shh and Nrf2, we silenced both Shh and Nrf2 independently. Cells were treated with siRNA-Shh and siRNA-Nrf2 for 48 h. Silencing of the Shh gene showed markedly lower expression levels of Shh, Gli1, Nrf2 and HO-1 [Figure 7(i) and Figure S5(d)]. These results suggest that chemotherapy-mediated Shh activation contributed to CSC-like characteristics and thereby enhanced resistance to chemotherapy. In addition, silencing of Shh significantly attenuated the growth and the number of spheres [Figure 7(j) and Figure S5(e)]. Recent studies have reported that Nrf2 signaling is also involved in supporting CSC-like characteristics in many cancers.26 Knockdown of Nrf2 inhibited the self-renewal efficiency of glioma stem cells.26,27 Nrf2 knockdown showed lower expression of Nrf2 and HO-1 than in the control/scramble siRNA-treated cells [Figure 7(k)]; however, no visible changes in Shh and Gli1 protein expression was observed after Nrf2 knockdown [Figure 7(k) and Figure S5(f)]. Furthermore, sphere-forming efficiency was significantly decreased after Nrf2 knockdown [Figure 7(l) and Figure S5(g)]. These results suggest that Shh potentially regulates Nrf2 pathway-dependent CSC regulation and activation upon chemotherapy.
Discussion
Although cisplatin is widely used for the treatment of patients with locally advanced HNSCC, unfortunately, a group of patients develop cisplatin resistance after an initial good response. This suggests that the efficacy of cisplatin diminishes over time; however, the adverse effects after administration remain detrimental. Currently, cisplatin-based chemotherapy is administered without knowledge of any biomarkers to predict the response. The goal of the study reported here was to determine whether Shh and Nrf2, two candidate molecular biomarkers, could be used to predict response to cisplatin-based chemotherapy. The patient data we used in this study were obtained from a uniformly selected and treated cohort of patients. We conducted a series of experiments to demonstrate that Shh and Nrf2 confer resistance to cisplatin-based chemotherapy in HNSCC, and found that the resistance was in part attributed to emergence of CSC-like characteristics. Our data suggest that combined higher expression of Shh and Nrf2 had a worse OS and DFS than that of low expression of Shh and Nrf2. Additionally, our data indicated that the expressions of Shh and Nrf2 could be important therapeutic biomarkers for patients with locally advanced HNSCC who undergo cisplatin treatment and thus there is the potential for therapeutic benefit.
Prognostic biomarkers are primarily used for risk stratification of patients and to predict chemotherapeutic response. Current available prognostic factors for HNSCC largely rely on the size of tumor (T stage), presence and extent of cervical node metastasis (N stage), and presence of distant metastasis (M stage). In addition to TNM staging, HPV-negative and HPV-positive HNSCC cancers have received much attention from the use of potential prognostic markers.28,29 However, a recent study by Psyrii and colleagues30 reported in a large European Organisation for Research and Treatment of Cancer study that the treatment effects were insignificant between HPV(+) or HPV(−) patients. In addition, several molecular and clinical risk factors have been investigated with limited clinical use.7 For example, EGFR overexpression was shown to be a negative prognostic factor after radiotherapy31 and a potential predictive biomarker for cisplatin-based chemotherapy in patients with HNSCC.21,32 To our knowledge, no studies have reported the predictive usefulness of two key signaling and regulatory pathway factors, Shh and Nrf2, as dual therapeutic biomarkers in the adjuvant chemotherapeutic setting. In the present study, we used patient tumor samples and compared Shh and Nrf2 expression taking several factors into consideration. Most importantly, our comparisons between cisplatin-sensitive and cisplatin-resistant, and no chemotherapy versus patients who underwent chemotherapy, helped us to identify true predictive biomarkers in patients with HNSCC, where high expression of Shh and Nrf2 clearly correlated with shorter OS and DFS in these patients. In addition to the use of tumor tissues, a serum-based prognostic assay might provide the potential to improve the accuracy of prognostic estimation. To this end, we further demonstrated the use of serum Shh and Nrf2 transcriptional activity as potential prognostic and therapeutic biomarkers in patients with HNSCC. Taken all together, these results suggest that dual high expression of Shh and Nrf2 could be a potential therapeutic biomarker for patients with HNSCC.
Gan and colleagues20 previously reported that radiotherapy elevated the expression of Shh and Gli1 in HNSCC tumors as well as in the tumor–stromal intersection, which contributes to stromal-mediated resistance and that these effects were inhibited by the Hh inhibitor cyclopamine. This suggests that the tumor stroma plays a critical role in therapeutic resistance. Taking this lead, using a clinical HNSCC patient cohort, we demonstrated that in patients treated with cisplatin the SI was significantly higher in the Shhhigh patient group than in the Shhlow patient group, and that a high SI was a predictive factor for DFS in patients. Furthermore, patients responding to chemotherapy had lower Shh expression than those who did not respond to therapy. These data suggest that Shh alone can serve as a predictive therapeutic biomarker for patients with HNSCC. At the molecular level, the relationship between Shh and Nrf2, and their combined role in tumor–stromal interaction as regards treatment resistance is largely unknown. Although, as our study shed some light on tumor–stromal interaction and the relationship of Shh and Nrf2, future studies should focus more on deciphering the interplay between Shh and Nrf2 signaling.
Until recently, overexpression of Shh and Nrf2 individually have been reported in a number of cancer types and linked to increased resistance to chemotherapy.19,28,33–35 However, their combined effect on therapeutic outcome in patients with HNSCC had not yet been reported. To date, reliable predictive biomarkers have not been established for cisplatin-based chemotherapy at least for patients with HNSCC. Therefore, there is a precedent as Shh and Nrf2 have distinct roles in therapeutic resistance and the availability of inhibitors that target both pathways can be exploited. Our results show that both Shh and Nrf2 are abundantly expressed in tumors treated with cisplatin. The frequent overexpression of these two biomarkers accompanied with the increase in growth and proliferation of cells suggests critical roles in growth and proliferation particularly in post-therapy resistant cells. The Gli1 transcription factor studied in a retrospective analysis (RTOG 90-03) found that high expression of Gli1 levels before treatment of patients with HNSCC correlated with poor local control rate, aggressive distant metastases and poor OS.36 On the other hand, HO-1 (heme oxygenase 1) a downstream effector of Nrf2-dependent cell response,37 was shown to play an important role in malignant transformation of cancer cells. A high level of HO-1 has been reported to correlate with poor OS in many cancers38,39 and correlated with resistance to chemotherapy.40 To verify the biological functions and role of therapeutic resistance of Shh and Nrf2 in HNSCC, we conducted knockdown experiments for both Shh and Nrf2 individually and assessed effect on cell growth and survival. Our results clearly demonstrate a reduction in HNSCC cell growth. Furthermore, the Shh inhibitor cyclopamine dose-dependently decreased the growth of cells and reduced the invasive potential of HNSCC cells. These findings suggest that Shh and Nrf2 may play crucial roles in the survival and proliferation of resistant tumor cells after chemotherapy.
One of the limitations of our present work was the lack of validation using an animal model, particularly for the CSC population and resulting resistance. As a well-recognized alternative, we employed three-dimensional sphere cultures generated from patient tumor cells and FaDu, an HNSCC cell line. The three-dimensional cell models have been authenticated to be more realistic for translating in vitro study results for in vivo application.41 We have confirmed that cells isolated from cisplatin-resistant tumor cells produced greater sphere size and increased numbers of spheres than cells that were cisplatin sensitive, concomitantly with increased Shh and Nrf2 expression in the resistant tumor sphere cells. Furthermore, we found that inhibition of either Shh or Nrf2 inhibited the growth and size of spheres compared with untreated cells. One interesting finding of this study was that the inhibition of Nrf2 by siRNA-Nrf2 did not alter Shh and Gli1 expressions, suggesting that Shh possibly regulates Nrf2 and its target genes. However, more experimental evidence is required to prove the interrelation of Shh and Nrf2 in the regulation of chemotherapeutic resistance. Nevertheless, in this study we have provided a convincing body of data that lays out the foundation about the combined function of Shh and Nrf2 and patient outcome in HNSCC. Together, these results suggest that Shh and Nrf2 play major roles in the chemotherapeutic resistance process and the growth and proliferation of tumor cells after chemotherapy.
The data presented here lead us to propose that combined Shh and Nrf2 overexpression in resected tumors is significantly associated with OS and DFS in patients with HNSCC and chemoresistance. The data further support the notion that Shh and Nrf2 are probably important regulators of cancer stemness and chemotherapeutic resistance. Thus, both Shh and Nrf2 overexpression are probably associated with disease recurrence and prognosis. These findings overall suggest that Shh and Nrf2 may be potential therapeutic biomarkers and may offer potential new therapeutic targets in combination with chemotherapy.
Supplemental Material
Supplemental material, Supplementary_Figure_S1 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S2 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S3 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S4 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S5 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Table_S2 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Table__S1 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Acknowledgments
We sincerely thank all patients and the core pathology department who assisted us to prepare and process the tumor samples and preparation of slides. We also thank the independent pathologist who rated and scored the expression of relevant proteins.
Footnotes
Author contributions: Conception and design was provided by S.S.I. and A.S.M.N. Experimental design and execution was conducted by S.S.I., A.S.M.N., J.C.S., S.A., N.M.J., A.S., S.R.K., S.A., P.R.R., R.M.I. and R.M.L. Administrative support was provided by A.S.M.N., R.M.I., S.A., U.S.B., S.A. and S.S.I. Study materials and patient support was provided by A.S.M.N., M.Z.R., C.A.A., B.T. and S.S.I. Clinical identification and therapeutic management support was provided by C.A.A. Liaison with pathologists was provided by A.S.M.N., J.C.S., S.A., N.M.J., A.S., S.R.K., S.A., P.R.R., R.M.I. and R.M.L. Liaison with oncologists and radiologists was provided by N.A.S.M., R.M.Z., R.M.M., S. A., M.R., A. S., S.R.K., P.R.R., R.M.I. and R.M.L. Analysis of RNA-seq data from TCGA was provided by S.S.I. Contribution to pathological studies was provided by R.M.Z. Collection of patient’s clinical records was performed by J.C.S., S.A., N.M.J., S.A., M.R., R.M.L., P.R.R., C.A., B.S.B., H.A., B.S., H.S.M.I. and A.S. Patient interviews and collection of patient samples were conducted by J.C.S., S.A., N.M.J., R.M.L., S.A., M.R., P.R.R., C.A., B.S.B., H.A., B.S., H.S.M.I. and A.S. Hospital and clinic visits were conducted by J.C.S., S.A., S.A, N.M.J, M.R, A.S., S.R.K. and A.J. Data collection and assembly was conducted by P.R.R., R.M.I., J.C.S., S.A., N.M.J., S.A., R.M.L., A.S., S.R.K., M.R., A.S., C.A., B.S.B., H.A., B.S., H.S.M.I., R.M.M., A.J. and S.A. Data analysis and interpretation was conducted by S.S.I. Manuscript writing and revision was conducted by S.S.I., A.S.M.N., B.K., W.A.F. and H.Y. Financial support was provided by A.S.M.N. and S.S.I. Overall project supervision was conducted by S.S.I. and A.S.M.N. Final approval of the manuscript was conducted by all authors.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was partially supported by the Grant-in-Aid for Scientific Research from BMRC and from University Grant Commission, Ministry of Education with the assistance of the World Bank under Higher Education Quality Enhancement Project (HEQEP)-Window-4 (grant ID CP-4023).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Chandsultana Jerin  https://orcid.org/0000-0002-0040-6196
https://orcid.org/0000-0002-0040-6196
Syed S. Islam  https://orcid.org/0000-0003-2201-8642
https://orcid.org/0000-0003-2201-8642
Supplementary material: Supplementary material for this article is available online.
Contributor Information
Abu Shadat M. Noman, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh Department of Pathology, McGill University, Montreal, Quebec, Canada.
Rashed R. Parag, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh
Muhammad I. Rashid, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh
Mohammad Z. Rahman, Department of Pathology, Chittagong Medical College and Hospital, Chittagong, Bangladesh
Ali A. Chowdhury, Department of Radiotherapy, Chittagong Medical College and Hospital, Chittagong, Bangladesh
Afrin Sultana, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Chandsultana Jerin, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Ayesha Siddiqua, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Lutfur Rahman, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Afsana Shirin, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Junayed Nayeem, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Reaz Mahmud, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Sonam Akther, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Rajib K. Shil, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh
Ikram Hossain, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Sharmin Alam, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Arfina Chowdhury, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Shabnam B. Basher, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh
Abul Hasan, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Shammy Bithy, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Jannatul Aklima, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Mizanur Rahman, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh; Rangamati Medical College, Rangamati, Bangladesh.
Nabila Chowdhury, Department of Biochemistry and Molecular Biology, The University of Chittagong, Chittagong, Bangladesh.
Tahmina Banu, Chittagong Research Institute of Children Surgery, Chittagong, Bangladesh.
Bedri Karakas, Department of Molecular Oncology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia.
Herman Yeger, Developmental and Stem Cell Biology, Research Institute, The Hospital for Sick Children, Toronto, ON, Canada.
Walid A. Farhat, Department of Urology, University of Wisconsin School of Medicine and Public Health, Madison, WI, USA
Syed S. Islam, Department of Molecular Oncology, Cancer Biology and Experimental Therapeutics, King Faisal Specialist Hospital and Research Centre, School of Medicine, Alfaisal University, Thakassussi Street, Riyadh, 11211, Saudi Arabia.
References
- 1. Cullen KJ, Schumaker L, Nikitakis N, et al. beta-Tubulin-II expression strongly predicts outcome in patients receiving induction chemotherapy for locally advanced squamous carcinoma of the head and neck: a companion analysis of the TAX 324 trial. J Clin Oncol 2009; 27: 6222–6228. [DOI] [PubMed] [Google Scholar]
- 2. Huang SH, Xu W, Waldron J, et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J Clin Oncol 2015; 33: 836–845. [DOI] [PubMed] [Google Scholar]
- 3. Alterio D, Marvaso G, Maffini F, et al. Role of EGFR as prognostic factor in head and neck cancer patients treated with surgery and postoperative radiotherapy: proposal of a new approach behind the EGFR overexpression. Med Oncol 2017; 34: 107. [DOI] [PubMed] [Google Scholar]
- 4. Enzenhofer E, Parzefall T, Haymerle G, et al. Impact of Sonic hedgehog pathway expression on outcome in HPV negative head and neck carcinoma patients after surgery and adjuvant radiotherapy. PLoS One 2016; 11: e0167665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu Z, Weinberger PM, Haffty BG, et al. Cyclin D1 is a valuable prognostic marker in oropharyngeal squamous cell carcinoma. Clin Cancer Res 2005; 11: 1160–1166. [PubMed] [Google Scholar]
- 6. Beck TN, Georgopoulos R, Shagisultanova EI, et al. EGFR and RB1 as dual biomarkers in HPV-negative head and neck cancer. Mol Cancer Ther 2016; 15: 2486–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015; 517: 576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dimitrova K, Stoehr M, Dehghani F, et al. Overexpression of the Hedgehog signalling pathway in head and neck squamous cell carcinoma. Onkologie 2013; 36: 279–286. [DOI] [PubMed] [Google Scholar]
- 9. Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature 2009; 458: 776–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yoshikawa R, Nakano Y, Tao L, et al. Hedgehog signal activation in oesophageal cancer patients undergoing neoadjuvant chemoradiotherapy. Br J Cancer 2008; 98: 1670–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Namani A, Matiur Rahaman M, Chen M, et al. Gene-expression signature regulated by the KEAP1-NRF2-CUL3 axis is associated with a poor prognosis in head and neck squamous cell cancer. BMC Cancer 2018; 18: 46–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinez VD, Vucic EA, Thu KL, et al. Disruption of KEAP1/CUL3/RBX1 E3-ubiquitin ligase complex components by multiple genetic mechanisms: association with poor prognosis in head and neck cancer. Head Neck 2015; 37: 727–734. [DOI] [PubMed] [Google Scholar]
- 14. Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev 2013; 27: 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roh J-L, Kim EH, Jang H, et al. Nrf2 inhibition reverses the resistance of cisplatin-resistant head and neck cancer cells to artesunate-induced ferroptosis. Redox Biol 2017; 11: 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Islam SS, Mokhtari RB, Noman AS, et al. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol Carcinog 2016; 55: 537–551. [DOI] [PubMed] [Google Scholar]
- 17. Islam SS, Mokhtari RB, Akbari P, et al. Simultaneous targeting of bladder tumor growth, survival, and epithelial-to-mesenchymal transition with a novel therapeutic combination of acetazolamide (AZ) and sulforaphane (SFN). Target Oncol 2016; 11: 209–227. [DOI] [PubMed] [Google Scholar]
- 18. Islam SS, Uddin M, Noman ASM, et al. Antibody-drug conjugate T-DM1 treatment for HER2+ breast cancer induces ROR1 and confers resistance through activation of Hippo transcriptional coactivator YAP1. EBioMedicine 2019; 43: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forastiere AA, Adelstein DJ, Manola J. Induction chemotherapy meta-analysis in head and neck cancer: right answer, wrong question. J Clin Oncol 2013; 31: 2844–2846. [DOI] [PubMed] [Google Scholar]
- 20. Gan GN, Eagles J, Keysar SB, et al. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res 2014; 74: 7024–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma J, Tian L, Cheng J, et al. Sonic hedgehog signaling pathway supports cancer cell growth during cancer radiotherapy. PLoS One 2013; 8: e65032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for hedgehog ligand stimulation in growth of digestive tract tumours. Nature 2003; 425: 846–851. [DOI] [PubMed] [Google Scholar]
- 23. Sims-Mourtada J, Izzo JG, Apisarnthanarax S, et al. Hedgehog: an attribute to tumor regrowth after chemoradiotherapy and a target to improve radiation response. Clin Cancer Res 2006; 12: 6565–6572. [DOI] [PubMed] [Google Scholar]
- 24. Mozet C, Stoehr M, Dimitrova K, et al. Hedgehog targeting by cyclopamine suppresses head and neck squamous cell carcinoma and enhances chemotherapeutic effects. Anticancer Res 2013; 33: 2415–2424. [PubMed] [Google Scholar]
- 25. Miyazaki H, Takahashi R-U, Prieto-Vila M, et al. CD44 exerts a functional role during EMT induction in cisplatin-resistant head and neck cancer cells. Oncotarget 2018; 9: 10029–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim D, Choi B-H, Ryoo I-G, et al. High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis 2018; 9: 896–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu J, Wang H, Sun Q, et al. Nrf2 is required to maintain the self-renewal of glioma stem cells. BMC Cancer 2013; 13: 380–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheraghlou S, Torabi SJ, Husain ZA, et al. HPV status in unknown primary head and neck cancer: prognosis and treatment outcomes. Laryngoscope 2019; 129: 684–691. [DOI] [PubMed] [Google Scholar]
- 29. Albergotti WG, Schwarzbach HL, Abberbock S, et al. Defining the prevalence and prognostic value of perineural invasion and angiolymphatic invasion in human papillomavirus-positive oropharyngeal carcinoma. JAMA Otolaryngol Head Neck Surg 2017; 143: 1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res 2002; 62: 7350–7356. [PubMed] [Google Scholar]
- 31. Burtness B, Bauman JE, Galloway T. Novel targets in HPV-negative head and neck cancer: overcoming resistance to EGFR inhibition. Lancet Oncol 2013; 14: e302–e309. [DOI] [PubMed] [Google Scholar]
- 32. Burtness B, Goldwasser MA, Flood W, et al. ; Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 2005; 23: 8646–8654. [DOI] [PubMed] [Google Scholar]
- 33. Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 2013; 12: 931–947. [DOI] [PubMed] [Google Scholar]
- 34. Ren D, Villeneuve NF, Jiang T, et al. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA 2011; 108: 1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chung CH, Dignam JJ, Hammond ME, et al. Glioma-associated oncogene family zinc finger 1 expression and metastasis in patients with head and neck squamous cell carcinoma treated with radiation therapy (RTOG 9003). J Clin Oncol 2011; 29: 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Na H-K, Surh Y-J. Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic Biol Med 2014; 67: 353–365. [DOI] [PubMed] [Google Scholar]
- 37. Tsai J-R, Wang H-M, Liu P-L, et al. High expression of heme oxygenase-1 is associated with tumor invasiveness and poor clinical outcome in non-small cell lung cancer patients. Cell Oncol (Dordr) 2012; 35: 461–471. [DOI] [PubMed] [Google Scholar]
- 38. Miyake M, Fujimoto K, Anai S, et al. Clinical significance of heme oxygenase-1 expression in non-muscle-invasive bladder cancer. Urol Int 2010; 85: 355–363. [DOI] [PubMed] [Google Scholar]
- 39. Ma D, Fang Q, Wang P, et al. Induction of heme oxygenase-1 by Na+-H+ exchanger 1 protein plays a crucial role in imatinib-resistant chronic myeloid leukemia cells. J Biol Chem 2015; 290: 12558–12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ravi M, Paramesh V, Kaviya SR, et al. 3D cell culture systems: advantages and applications. J Cell Physiol 2015; 230: 16–26. [DOI] [PubMed] [Google Scholar]
- 41. Ma H, Seebacher NA, Hornicek FJ, et al. Cyclin-dependent kinase 9 (CDK9) is a novel prognostic marker and therapeutic target in osteosarcoma. EBioMedicine 2019; 39: 182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Figure_S1 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S2 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S3 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S4 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Figure_S5 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Table_S2 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology
Supplemental material, Supplementary_Table__S1 for Widespread expression of Sonic hedgehog (Shh) and Nrf2 in patients treated with cisplatin predicts outcome in resected tumors and are potential therapeutic targets for HPV-negative head and neck cancer by Abu Shadat M. Noman, Rashed R. Parag, Muhammad I. Rashid, Mohammad Z. Rahman, Ali A. Chowdhury, Afrin Sultana, Chandsultana Jerin, Ayesha Siddiqua, Lutfur Rahman, Afsana Shirin, Junayed Nayeem, Reaz Mahmud, Sonam Akther, Rajib K. Shil, Ikram Hossain, Sharmin Alam, Arfina Chowdhury, Shabnam B. Basher, Abul Hasan, Shammy Bithy, Jannatul Aklima, Mizanur Rahman, Nabila Chowdhury, Tahmina Banu, Bedri Karakas, Herman Yeger, Walid A. Farhat and Syed S. Islam in Therapeutic Advances in Medical Oncology