Introduction
In Wuhan, China, a novel and alarmingly contagious primary atypical (viral) pneumonia broke out in December 2019. It has since been identified as a zoonotic coronavirus, similar to SARS coronavirus and MERS coronavirus and named COVID-19. As of 8 February 2020, 33 738 confirmed cases and 811 deaths have been reported in China.
Here we review the basic reproduction number (R0) of the COVID-19 virus. R0 is an indication of the transmissibility of a virus, representing the average number of new infections generated by an infectious person in a totally naïve population. For R0 > 1, the number infected is likely to increase, and for R0 < 1, transmission is likely to die out. The basic reproduction number is a central concept in infectious disease epidemiology, indicating the risk of an infectious agent with respect to epidemic spread.
Methods and Results
PubMed, bioRxiv and Google Scholar were accessed to search for eligible studies. The term ‘coronavirus & basic reproduction number’ was used. The time period covered was from 1 January 2020 to 7 February 2020. For this time period, we identified 12 studies which estimated the basic reproductive number for COVID-19 from China and overseas. Table 1 shows that the estimates ranged from 1.4 to 6.49, with a mean of 3.28, a median of 2.79 and interquartile range (IQR) of 1.16.
Table 1.
Published estimates of R0 for 2019-nCoV
| Study (study year) | Location | Study date | Methods | Approaches | R 0 estimates (average) | 95% CI |
| Joseph et al.1 | Wuhan | 31 December 2019–28 January 2020 | Stochastic Markov Chain Monte Carlo methods (MCMC) | MCMC methods with Gibbs sampling and non-informative flat prior, using posterior distribution | 2.68 | 2.47–2.86 |
| Shen et al.2 | Hubei province | 12–22 January 2020 | Mathematical model, dynamic compartmental model with population divided into five compartments: susceptible individuals, asymptomatic individuals during the incubation period, infectious individuals with symptoms, isolated individuals with treatment and recovered individuals |
R
0 = 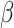 / / 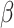 = mean person-to-person transmission rate/day in the absence of control interventions, using nonlinear least squares method to get its point estimate = mean person-to-person transmission rate/day in the absence of control interventions, using nonlinear least squares method to get its point estimate = isolation rate = 6 = isolation rate = 6 |
6.49 | 6.31–6.66 |
| Liu et al.3 | China and overseas | 23 January 2020 | Statistical exponential Growth, using SARS generation time = 8.4 days, SD = 3.8 days | Applies Poisson regression to fit the exponential growth rateR0 = 1/M(−𝑟)M = moment generating function of the generation time distributionr = fitted exponential growth rate | 2.90 | 2.32–3.63 |
| Liu et al.3 | China and overseas | 23 January 2020 | Statistical maximum likelihood estimation, using SARS generation time = 8.4 days, SD = 3.8 days | Maximize log-likelihood to estimate R0 by using surveillance data during a disease epidemic, and assuming the secondary case is Poisson distribution with expected value R0 | 2.92 | 2.28–3.67 |
| Read et al.4 | China | 1–22 January 2020 | Mathematical transmission model assuming latent period = 4 days and near to the incubation period | Assumes daily time increments with Poisson-distribution and apply a deterministic SEIR metapopulation transmission model, transmission rate = 1.94, infectious period =1.61 days | 3.11 | 2.39–4.13 |
| Majumder et al.5 | Wuhan | 8 December 2019 and 26 January 2020 | Mathematical Incidence Decay and Exponential Adjustment (IDEA) model | Adopted mean serial interval lengths from SARS and MERS ranging from 6 to 10 days to fit the IDEA model, | 2.0–3.1 (2.55) | / |
| WHO | China | 18 January 2020 | / | / | 1.4–2.5 (1.95) | / |
| Cao et al.6 | China | 23 January 2020 | Mathematical model including compartments Susceptible-Exposed-Infectious-Recovered-Death-Cumulative (SEIRDC) | R = K 2 (L × D) + K(L + D) + 1L = average latent period = 7,D = average latent infectious period = 9,K = logarithmic growth rate of the case counts | 4.08 | / |
| Zhao et al.7 | China | 10–24 January 2020 | Statistical exponential growth model method adopting serial interval from SARS (mean = 8.4 days, SD = 3.8 days) and MERS (mean = 7.6 days, SD = 3.4 days) | Corresponding to 8-fold increase in the reporting rateR0 = 1/M(−𝑟)𝑟 =intrinsic growth rateM = moment generating function | 2.24 | 1.96–2.55 |
| Zhao et al.7 | China | 10–24 January 2020 | Statistical exponential growth model method adopting serial interval from SARS (mean = 8.4 days, SD = 3.8 days) and MERS (mean = 7.6 days, SD = 3.4 days) | Corresponding to 2-fold increase in the reporting rateR0 = 1/M(−𝑟)𝑟 =intrinsic growth rateM = moment generating function | 3.58 | 2.89–4.39 |
| Imai (2020)8 | Wuhan | January 18, 2020 | Mathematical model, computational modelling of potential epidemic trajectories | Assume SARS-like levels of case-to-case variability in the numbers of secondary cases and a SARS-like generation time with 8.4 days, and set number of cases caused by zoonotic exposure and assumed total number of cases to estimate R0 values for best-case, median and worst-case | 1.5–3.5 (2.5) | / |
| Julien and Althaus9 | China and overseas | 18 January 2020 | Stochastic simulations of early outbreak trajectories | Stochastic simulations of early outbreak trajectories were performed that are consistent with the epidemiological findings to date | 2.2 | |
| Tang et al.10 | China | 22 January 2020 | Mathematical SEIR-type epidemiological model incorporates appropriate compartments corresponding to interventions | Method-based method and Likelihood-based method | 6.47 | 5.71–7.23 |
| Qun Li et al.11 | China | 22 January 2020 | Statistical exponential growth model | Mean incubation period = 5.2 days, mean serial interval = 7.5 days | 2.2 | 1.4–3.9 |
| Averaged | 3.28 | |||||
CI, Confidence interval.
Figure 1.

Timeline of the R0 estimates for the 2019-nCoV virus in China
The first studies initially reported estimates of R0 with lower values. Estimations subsequently increased and then again returned in the most recent estimates to the levels initially reported (Figure 1). A closer look reveals that the estimation method used played a role.
The two studies using stochastic methods to estimate R0, reported a range of 2.2–2.68 with an average of 2.44.1,9 The six studies using mathematical methods to estimate R0 produced a range from 1.5 to 6.49, with an average of 4.2.2,4–6,8,10 The three studies using statistical methods such as exponential growth estimated an R0 ranging from 2.2 to 3.58, with an average of 2.67.3,7,11
Discussion
Our review found the average R0 to be 3.28 and median to be 2.79, which exceed WHO estimates from 1.4 to 2.5. The studies using stochastic and statistical methods for deriving R0 provide estimates that are reasonably comparable. However, the studies using mathematical methods produce estimates that are, on average, higher. Some of the mathematically derived estimates fall within the range produced the statistical and stochastic estimates. It is important to further assess the reason for the higher R0 values estimated by some the mathematical studies. For example, modelling assumptions may have played a role. In more recent studies, R0 seems to have stabilized at around 2–3. R0 estimations produced at later stages can be expected to be more reliable, as they build upon more case data and include the effect of awareness and intervention. It is worthy to note that the WHO point estimates are consistently below all published estimates, although the higher end of the WHO range includes the lower end of the estimates reviewed here.
R 0 estimates for SARS have been reported to range between 2 and 5, which is within the range of the mean R0 for COVID-19 found in this review. Due to similarities of both pathogen and region of exposure, this is expected. On the other hand, despite the heightened public awareness and impressively strong interventional response, the COVID-19 is already more widespread than SARS, indicating it may be more transmissible.
Conclusions
This review found that the estimated mean R0 for COVID-19 is around 3.28, with a median of 2.79 and IQR of 1.16, which is considerably higher than the WHO estimate at 1.95. These estimates of R0 depend on the estimation method used as well as the validity of the underlying assumptions. Due to insufficient data and short onset time, current estimates of R0 for COVID-19 are possibly biased. However, as more data are accumulated, estimation error can be expected to decrease and a clearer picture should form. Based on these considerations, R0 for COVID-19 is expected to be around 2–3, which is broadly consistent with the WHO estimate.
Author contributions
J.R. and A.W.S. had the idea, and Y.L. did the literature search and created the table and figure. Y.L. and A.W.S. wrote the first draft; A.A.G. drafted the final manuscript. All authors contributed to the final manuscript.
Conflict of interest
None declared.
Teaser: Our review found the average R0 for COVID-19 to be 3.28, which exceeds WHO estimates from 1.4 to 2.5.
References
- 1. Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study, The Lancet, 2020.
- 2. Shen M, Peng Z, Xiao Y, Zhang L. Modelling the epidemic trend of the 2019 novel coronavirus outbreak in China. bioRxiv 2020. doi: https://doi.org/10.1101/2020.01.23.916726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu T, Hu J, Kang M et al. Transmission dynamics of 2019 novel coronavirus (2019-nCoV). bioRxiv 2020. doi: https://doi.org/10.1101/2020.01.25.919787. [Google Scholar]
- 4. Read JM, Bridgen JRE, Cummings DAT, Ho A, Jewell CP. Novel coronavirus 2019-nCoV: early estimation of epidemiological parameters and epidemic predictions. medRxiv 2020. doi: https://doi.org/10.1101/2020.01.23.20018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Majumder M, Mandl KD. (2020) Early transmissibility assessment of a novel coronavirus in Wuhan, China. https://papers.ssrn.com/abstract=3524675(27 January 2020, date last accessed).
- 6. Cao Z Zhang Q, Lu X et al. Estimating the effective reproduction number of the 2019-nCoV in China. medRxiv 2020. doi: https://doi.org/10.1101/2020.01.27.20018952.
- 7. Zhao S, Ran J, Musa SS et al. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a datadriven analysis in the early phase of the outbreak. bioRxiv 2020. doi: https://doi.org/10.1101/2020.01.23.916395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Imai N, Cori A, Dorigatti I et al. Report 3: transmissibility of 2019-nCoV. 2020. WHO Collaborating Centre for Infectious Disease Modelling, MRC Centre for Global Infectious Disease Analysis, J-IDEA, Imperial College London, UK. [Google Scholar]
- 9. Riou J, Althaus CL. Pattern of early human-to-human transmission of Wuhan 2019-nCoV. bioRxiv 2020. https://www.biorxiv.org/content/10.1101/2020.01.23.917351v1.full.pdf(27 January 2020, date last accessed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tang B, Wang X, Li Q et al. Estimation of the transmission risk of 2019-nCov and its implication for public health interventions (January 24, 2020). https://ssrn.com/abstract=3525558 or https://doi.org/10.2139/ssrn.3525558 (9 February 2020, date last accessed). [DOI] [PMC free article] [PubMed]
- 11. Qun L, et al. 2020. Early transmission dynamics in wuhan, china, of novel coronavirus-infected pneumonia. New England Journal of Medicine. 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed]


