Abstract
Bovine mastitis is the most important infectious disease, causing significant losses in the dairy industry, in which Streptococcus agalactiae is a major pathogen. In this study, lysin CHAPk, derived from bacteriophage K, was expressed heterogeneously, and its antimicrobial and anti-biofilm effects against S. agalactiae isolated from bovine mastitis were further analyzed. CHAPk was expressed in Escherichia coli BL21 (DE3), in which the purified yield of CHAPk was up to 14.6 mg/L with the purity of 95%. Time-killing kinetic curves showed that CHAPk fastly killed S. agalactiae in TSB medium and in milk within 25 min (by 3.3 log10 CFU/mL and 2.4 log10 CFU/mL, respectively). Observation of scanning electron microscope (SEM) showed cells wrinkled and ruptured after the treatment of CHAPk. CHAPk effectively inhibited early biofilms by 95% in 8 × MIC, and eradicated mature biofilms by 89.4% in 16 × MIC. Moreover, CHAPk killed 99% bacteria in mature biofilms. Confocal laser scanning microscopy (CLSM) also demonstrated the potent antimicrobial and anti-biofilm action of CHAPk. It was firstly demonstrated CHAPk had the characters of inhibition/elimination of S. agalactiae biofilms and killing the bacteria in biofilms. CHAPk has the potential to develop a new antibacterial agent for mastitis treatment of S. agalactiae infections.
Keywords: Lysin, CHAPk, expression, anti-biofilm ability, Streptococcus agalactiae
1. Introduction
Streptococcus agalactiae of group B (GBS) is one of the important pathogens which is associated with clinical/subclinical mastitis in bovine and neonatal sepsis and meningitis in humans. Bovine mastitis is the primary health hazard, leading to severe reduction of milk production and effect of milk quality. It is responsible for significant economic losses in the dairy industry worldwide [1,2]. Infection control measures of S. agalactiae were performed during the 1960s to reduce the occurrence of S. agalactiae mastitis in several European countries. However, the prevalence of S. agalactiae mastitis in bovine has re-emergence in Norway, Denmark, and other countries [3,4,5]. In China, S. agalactiae is also a prevalent problem in bovine diagnosed with subclinical mastitis [6]. The sequence types (STs) of S. agalactiae in bovine mainly belong to ST67, ST103, and ST568, which harbor the virulence characteristics of biofilms formation ability, growth ability in milk and adhesion ability, and can persist indefinitely within the mammary gland [1,7]. β-lactam antibiotics (BLAs) (penicillin, ampicillin, cefalexin, and ceftiofur sodium) are officially approved and extensively used to treat S. agalactiae mastitis. However, clinical isolated S. agalactiae from cows with mastitis showed unpredicted mutations in the penicillin-binding proteins encoding (PBP) genes which lead to the high BLAs-resistant rate [8]. Eight S. agalactiae strains isolated from cow mastitis showed 37.5% resistant to tetracycline and were highly resistant to trimethoprim [9]. Therefore, there is an urgent need to search for novel agents against S. agalactiae with low possibility to develop resistance.
Lysins are peptidoglycan hydrolase produced by bacteriophages. Among the different sources of bacteriophages, lysins show specificity on the pathogen. Compared with phages, non-biological lysins are safer and have no effect on commensal microflora [10,11]. Previous studies have demonstrated that lysins were active against streptococcus and staphylococcus pathogens, causing mucosal and systemic infections [12,13,14,15]. Lysins rapidly induce bacterial lysis and death by hydrolysing covalent bonds, which are essential for cell wall integrity and viability, and consequently develop little resistance compared with antibiotics [16,17,18]. CHAPk (18.6 kDa), comprised solely of the lytic domain, is a truncated derivative of native lysin (LysK, 54 kDa) of Staphylococcus aureus, which belongs to cysteine-and histidine-dependent amidohydrolase/peptidase and specifically cleaves staphylococcal cell wall on the peptide bond between D-alanine and the first glycine in the pentaglycine cross bridge [19,20,21]. Previous studies have reported that CHAPk exhibited strong activity against S. aureus and effected on the S. aureus biofilms [22,23,24,25]. Interestingly, researchers discovered that CHAPk also had antibacterial activity against streptococcus, which may be related to the similar peptidoglycan cross-bridge of staphylococcal and streptococcal pathogens [19,26]. In this study, CHAPk gene was synthesized and expressed in E. coli BL21 (DE3), and the efficacy of recombinant CHAPk against S. agalactiae biofilms was evaluated.
2. Materials and Methods
2.1. Strains, Plasmid, and Reagents
The tested strains of Streptococcus agalactiae CAU-FRI 1, S. agalactiae CAU-FRI 2, and S. agalactiae CAU-FRI 3 isolated from bovine mastitis were obtained from China Agricultural University, S. agalactiae CAU-FRI 4 isolated from tilapia was stored at our laboratory, Streptococcus uberis CAU-FRI 1, S. uberis CAU-FRI 2, S. uberis CAU-FRI 3, Streptococcus dysgalactiae CAU-FRI 1, S. dysgalactiae CAU-FRI 2, and S. dysgalactiae CAU-FRI 3 isolated from bovine mastitis were obtained from China Agricultural University, S. agalactiae ATCC 13813, S. aureus ATCC 43300, Streptococcus epidermidis ATCC 12228, S. epidermidis ATCC 35984, and Escherichia coli ATCC 25922 were purchased from the American Type Culture Collection; S. aureus CVCC 546, Streptococcus pneumoniae CVCC 3309, S. pneumoniae CVCC 2350, Streptococcus suis CVCC 606, S. suis CVCC 3928, and Salmonella enteritidis CVCC 3377 were purchased from the China Veterinary Culture Collection Center (Beijing, China); S. aureus KY, S. aureus KR and S. aureus FJ isolated from bovine with endometritis were stored at our laboratory; Staphylococcus hyicus NCTC 10350 was purchased from National Collection of Type Cultures; S. pneumoniae CGMCC 1.8747 and Candida albicans CGMCC 2.2411 were purchased from China General Microbiological Culture Collection Center. pET28a vector and E. coli BL21 (DE3) (Novagen, Beijing, China) were used for cloning and expression. DNA restriction enzymes and T4 DNA ligase were purchased from NEB (USA). The kits for plasmid extraction and DNA purification were purchased from TIANGEN (China). Other chemical reagents were analytical grade.
2.2. Expression and Purification of CHAPk
The synthesized CHAPk (The sequence was acquired from NCBI PDB:4CT3_D and synthesized by Sangon Biotech (Shanghai) Co., Ltd.) nucleotide sequence was optimized by reverse translate tool (http://bioinformatics.org//sms2/rev_trans.html) based on the preferential codon usage of E. coli (http://www.kazusa.or.jp/codon/) and amplified with primers of CHAPk-F: 5′-CATGCCATGGCGAAAACCCAGGCGGAAA-3′, CHAPk-R: 5′-CCGCTCGAGCTATTAGTGGTGGTGGTG-3′. The DNA fragment contained the XhoI and NcoI restriction site, and 8 × His-tag was added to the C-terminus. After confirmed by sequencing the polymerase chain reaction (PCR) fragment, the purified CHAPk fragment digested with XhoI and NcoI and ligated into pET28a vectors which digested with the same restriction enzymes to generate the pET28a-CHAPk plasmid, and the plasmid was transformed into E. coli BL21 (DE3). The positive transforms were screened on luria broth (LB, 1% NaCl, 0.5% Yeast extract, 1% Tryptone) plates containing 50 μg/mL kanamycin and confirmed by PCR and DNA sequencing.
The recombinant CHAPk protein was expressed in E. coli BL21 (DE3) by IPTG induction [27]. In brief, 1% of the positive transformant cultured overnight was inoculated into LB medium containing 50 μg/mL kanamycin and incubated to OD600 nm of 0.4–0.6. CHAPk expression was induced by IPTG at 1.0 mM, 37 °C for 6 h. The pellets were harvested by centrifugation at 4722 g for 5 min and analyzed by 12% SDS-PAGE. Cells were lysed by sonicated for 15 min at 0 °C, then collected precipitation and dissolved it in the binding buffer (20 mM sodium phosphate, 500 mM NaCl, 5 mM imidazole, 8 M Urea), samples were loading into Ni2+–Nitriloacetate (NTA) super flow resin column at a rate of 4 mL/min with elution buffer (20 mM sodium phosphate, 500 mM NaCl, 175 mM imidazole, 8 M Urea, pH 7.4). Renaturation of the CHAPk by TGE buffer (50 mM Tris, 50 mM NaCl, 0.5 mM Ethylenediaminetetraacetic acid disodium salt (EDTA-Na2), 0.1% L-Arginine, 10% glycerol, 6 M–4 M–2 M–1 M Urea gradient dialysis until changed to distilled water) at 4 °C. To confirm the purified protein, the mixture of purified protein and matrix SA was detected by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) (New UltrafleXtreme, Bruker, Germany) in linear mode (at the Laboratory of Proteomics, Institute of Biophysics, Chinese Academy of Sciences). Purified protein was freeze-dried and stored in –20 °C [28].
2.3. Antibacterial Activity Assay
The minimal inhibitory concentrations (MICs) of CHAPk were measured by the broth microdilution method. Overnight cultures were inoculated to the tryptic soy broth medium (TSB, Qingdao Hope Bio-Technology Co., Ltd.) containing 3% (w/v) BSA, cultured to the mid-log phage. The bacterial suspension was then diluted to 1 × 105 CFU/mL. CHAPk was two-fold diluted to final concentration with 0.625–1280 μg/mL. Then CHAPk (10 μL) and bacterial suspension (90 μL) were added into 96-well plates (polystyrene, Beijing Huamei World Trade Technology Development Co., Ltd.). The plates were incubated at 37 °C for 16–18 h [29]. All assays were performed in triplicate. The MIC was defined as the lowest protein concentration at which there was no visible bacterial growth.
2.4. Time-Killing Kinetic Curves in TSB and Milk
After S. agalactiae ATCC 13813 inoculated into TSB containing 3% BSA at 37 °C and 250 rpm for 3 h, the mid-log phase bacteria were diluted to 5 × 108 CFU/mL. CHAPk was mixed with bacteria suspension to the final concentration of 16 × MIC (1.72 μM) in TSB, 32 × MIC (3.44 μM) in pasteurized whole-fat milk (6% fat, 2% carbohydrate, Beijing Sanyuan food Co., Ltd.). Meanwhile, the bacteria suspension without CHAPk as the blank control, and 16 × MIC and 32 × MIC of vancomycin as the positive control, respectively. Sample (100 μL) was taken at 1, 3, 5, 10, 15, 20, and 25 min for colony counting on the Tryptic Soy Agar (TSA, Qingdao Hope Bio-Technology Co., Ltd.).
2.5. Scanning Electron Microscope (SEM) Observation
The mid-log phase S. agalactiae cells were incubated with 8 × MIC CHAPk for 2 h at 37 °C. After being washed with PBS for three times, S. agalactiae cells treated with CHAPk were fixed with 2.5% glutaric dialdehyde (0.01 M PBS) at 4 °C overnight. Fixation fluid was discarded, and cells were washed with deionized water three times to removed fixation fluid for 4, 5, and 6 min, respectively. Then the samples were covered on the coverslips and various concentrations of ethanol (50%, 70%, 85%, 95%, and 100% × 3 times) were dehydrated for 15 min. Finally, the samples were dried by CO2, coated with platinum, and observed on a QUANTA200 SEM [30,31].
2.6. Ability of CHAPk against Biofilms and Bacteria of S. agalactiae
2.6.1. Biofilms Formation Assay
The ability of biofilms formation was evaluated by crystal violet staining, which was referenced as described by Tremblay et al. [32]. Briefly, S. agalactiae ATCC 13813 cells were inoculated to TSB medium containing 3% BSA, incubated until mid-log phase, and diluted to 1 × 108 CFU/mL by TSB medium. A 200 μL bacterial suspension was inoculated into 96-well plates. After incubation for 24 h at 37 °C, the medium was gently rinsed by PBS for three times, excess liquid was dried in room temperature with an inverted position, and the wells were fixed with 2.5% glutaric dialdehyde for 1.5 h at 4 °C. After being washed with PBS for three times, 100 μL 0.1% crystal violet were filled into wells for dyeing and incubated for 15 min. Following removal of the crystal violet solution, the plates were washed with PBS and dried. The crystal violet was dissolved in 200 μL 95% ethanol for 30 min. The value of OD570 nm was measured, and the blank control was TSB medium with the same operation [33]. Identification by crystal violet staining: the mean OD570 of the blank control was ODc, OD was the mean value of tested strain, and the biofilm formation ability of the strain was divided into four categories: negative (-): OD < ODc; A small amount (1 +): ODc < OD ≤ 2 ODc; Medium (2 +): 2 ODc < OD ≤ 4 ODc; A large amount (3 +): OD > 4 ODc.
2.6.2. Effect on Early Biofilms Formation
Mid-log phase S. agalactiae ATCC 13813 cells were diluted to 1 × 108 CFU/mL by TSB medium. A 180 μL bacterial suspension and a range of concentrations (0.25–8 × MIC) of CHAPk (20 μL) were tested and the most effective concentration was chosen for the subsequent biofilm assays. The plates were cultured for early biofilms formation for 24 h at 37 °C, then the effect of CHAPk on biofilms formation was evaluated by crystal violet staining, as described above [33]. The untreated bacteria were used as control (A). The inhibition effect of CHAPk on biofilms was determined by the following equation: Biofilms (%) = [(A-ACHAPk)/A] × 100.
2.6.3. Effect on Mature Biofilms
S. agalactiae ATCC 13813 cells (1 × 108 CFU/mL) were cultured in TSB medium in 96-well plates at 37 °C for 24 h. Following the final concentration of 0.25–8 × MIC CHAPk were mixed into the plates and cultured for another 24 h, the plates were cultured for mature biofilms formation and dyed by crystal violet, as described above [33].
2.6.4. Effect on Bacteria in Early Biofilms
The mid-log phase S. agalactiae ATCC 13813 cells (1 × 108 CFU/mL) were inoculated into in 96-well plates and cultured for 24 h at 37 °C. Plates were washed twice by PBS. Subsequently, the final concentration of 0.5–64 × MIC CHAPk or vancomycin were added into plates for 2 h at 37 °C, with PBS added wells as negative control. The plates were treated with ultrasound for 5 min, and the viable bacteria were counted on the TSA plates [33].
2.6.5. Effect on Bacteria in Mature Biofilms
Bacteria in mature biofilms were obtained by the following operation: the S. agalactiae ATCC 13813 cells (1 × 108 CFU/mL) were incubated in 96-well plates (200 μL/well) for 24 h, and the planktonic bacteria were rinsed by PBS. Biofilms were incubated in 200 μL TSB containing 25 × MIC vancomycin for another 24 h at 37 °C, then the biofilm-encased bacteria were counted after the planktonic bacteria were gently removed by PBS. Subsequently, biofilms were treated with 8 × MIC of CHAPk and vancomycin for 24 h at 37 °C, and the same volume of PBS was treated as control (CK), and viable bacteria in biofilms were counted by colonies counting on TSA plates [33].
2.6.6. Observation of Biofilms by Confocal Laser Scanning Microscopy (CLSM)
S. agalactiae ATCC 13813 cells (1 × 108 CFU/mL) were seeded in a confocal dish, CHAPk or vancomycin were added into the dish at the final concentration of 8 × MIC and incubated for 24 h, the bacteria treated without CHAPk or vancomycin were used as control. Planktonic bacteria were gently rinsed twice, and the biofilms were dyed with SYTO9 and propidium iodide (PI) (LIVE/DEAD BacLight Bacterial Viability Kit) for 15 min. After being washed with PBS, the biofilms were observed by Zeiss LSM880 confocal microscope [33].
2.7. Statistical Analysis
All data were analyzed by GraphPad Prism 6 and the results are presented as means ± standard deviation (SD). A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Expression and Purification of CHAPk
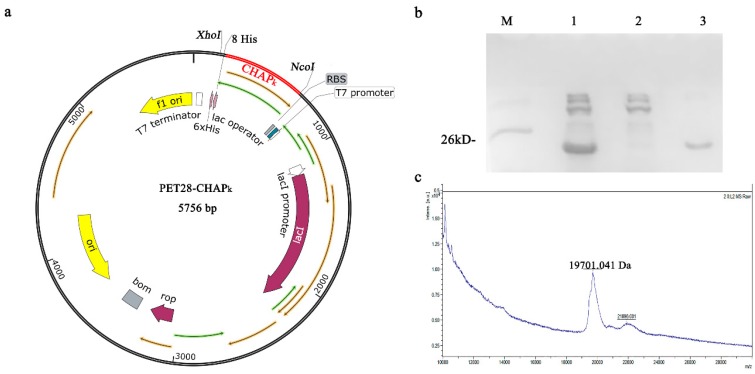
CHAPk nucleotide sequence was successfully inserted into the XhoI and NcoI sites of pET28a to construct the pET28a-CHAPk plasmid after being confirmed by DNA sequencing (Figure 1a). The results of SDS-PAGE analysis showed that the CHAPk with C-terminal 8 × His-tag was purified by a one-step affinity chromatography, and the target peak band was about 19 kDa (Figure 1b). The MALDI-TOF MS analysis showed that the molecular mass of CHAPk is 19.701 kDa, which is consistent with its theoretical value of 19.683 kDa. The yield and purity of the CHAPk were 14.6 mg/L and 95%, respectively. These results indicated that CHAPk is successfully expressed in E. coli.
Figure 1.
Expression and purification of CHAPk in E. coli. (a) The schematic diagram of the pET28a-CHAPk expression vector. (b) SDS-PAGE analysis of the purified CHAPk. M: Protein ladder, lane 1: total protein of E.coli BL21-pET28a-CHAPk, lane 2: the peak of penetration, lane 3: the peak of elution. (c) MALDI-TOF MS analysis of the purified CHAPk.
3.2. Antibacterial Activity Assay
The antimicrobial activity of CHAPk was determined by MICs assay (Table 1). The MIC values of CHAPk against S. agalactiae were 0.05–0.11 μM, which were better than those of vancomycin (0.67 μM). CHAPk also had the potent antimicrobial activity against other streptococcus, with MICs of 0.03–0.11 μM against S. uberis, 0.11–0.22 μM against S. dysgalactiae, 0.05–0.86 μM against S. epidermidis, 0.11 μM against S. suis, which were superior to those of vancomycin (streptococcus: 0.34–2.69 μM). For the S. aureus strains, the MIC values of CHAPk were 0.43–3.44 μM, that of S. hyicus were 1.72 μM, those of S. epidermidis were 0.86–1.72 μM. There was almost no antimicrobial activity against Gram-negative and C. albicans (>6.88 μM). These results suggested that CHAPk displays potent antimicrobial activity for staphylococcus and streptococcus, especially for streptococcus.
Table 1.
The MIC values of CHAPk and vancomycin.
| Strain | MIC | |||
|---|---|---|---|---|
| CHAPk | Vancomycin | |||
| μg/mL | μM | μg/mL | μM | |
| Gram-positive bacteria | ||||
| Streptococcus agalactiae ATCC 13813 | 2 | 0.11 | 1 | 0.67 |
| S. agalactiae CAU-FRI 1 | 1 | 0.05 | 1 | 0.67 |
| S. agalactiae CAU-FRI 2 | 1 | 0.05 | 1 | 0.67 |
| S. agalactiae CAU-FRI 3 | 1 | 0.05 | 1 | 0.67 |
| S. agalactiae CAU-FRI 4 | 2 | 0.11 | 1 | 0.67 |
| Streptococcus uberis CAU-FRI 1 | 2 | 0.11 | 0.5 | 0.34 |
| S. uberis CAU-FRI 2 | 0.5 | 0.03 | 0.5 | 0.34 |
| S. uberis CAU-FRI 3 | 1 | 0.05 | 0.5 | 3.34 |
| Streptococcus dysgalactiae CAU-FRI 1 | 4 | 0.22 | 0.5 | 3.34 |
| S. dysgalactiae CAU-FRI 2 | 2 | 0.11 | 1 | 0.67 |
| S. dysgalactiae CAU-FRI 3 | 4 | 0.22 | 0.5 | 0.34 |
| Streptococcus pneumoniae CVCC 3309 | 1 | 0.05 | 1 | 0.67 |
| S. pneumoniae CVCC 2350 | 8 | 0.43 | 4 | 2.69 |
| S. pneumoniae CGMCC 1.8747 | 16 | 0.86 | 4 | 2.69 |
| Streptococcus suis CVCC 606 | 2 | 0.11 | 1 | 0.67 |
| S. suis CVCC 3928 | 2 | 0.11 | 1 | 0.67 |
| Staphylococcus aureus KY | 8 | 0.43 | 4 | 2.69 |
| S. aureus KR | 8 | 0.43 | 4 | 2.69 |
| S. aureus FJ | 32 | 1.72 | 8 | 5.38 |
| MRSA S. aureus ATCC 43300 | 64 | 3.44 | 8 | 5.38 |
| S. aureus CVCC 546 | 64 | 3.44 | 8 | 5.38 |
| Staphylococcus hyicus NCTC 10350 | 32 | 1.72 | 4 | 2.69 |
| Staphylococcus epidermidis ATCC 12228 | 32 | 1.72 | 4 | 2.69 |
| S. epidermidis ATCC 35984 | 16 | 0.86 | 4 | 2.69 |
| Gram-negtive bacteria | ||||
| Escherichia coli ATCC 25922 | >128 | >6.88 | >128 | 86.14 |
| Salmonella enteritidis CVCC 3377 | >128 | >6.88 | >128 | 86.14 |
| Fungi | ||||
| Candida albicans CGMCC 2.2411 | >128 | >6.88 | >128 | 86.14 |
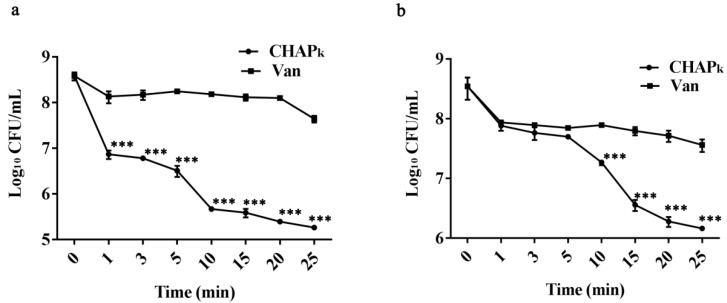
3.3. Time-Killing Kinetic Curves in TSB and Milk
Assessment of bactericidal activity of CHAPk was performed by time-killing kinetic curves. This is an important indicator of efficacy evaluation, indicating the bactericidal rate of CHAPk. The results showed that CHAPk displayed rapid bactericidal activity. With 16 × MIC CHAPk incubation, the bacteria number were reduced by 1.7 log10 CFU/mL in 1 min, which was four times higher than that of vancomycin (0.4 log10 CFU/mL). CHAPk reduced bacteria by 3.3 log10 CFU/mL within 25 min, whereas that of vancomycin was 0.9 log10 CFU/mL, which showed significant differences in bacteria kiling activity between CHAPk and vancomycin groups (p < 0.001) (Figure 2a,b). Considering the potential use of CHAPk as mastitis therapeutics, the antimicrobial activity of CHAPk in milk was evaluated. CHAPk also retained high activity in fresh milk, which caused 2.4 log10 CFU/mL reduction of S. agalactiae within 25 min, however, vancomycin showed only 0.9 log10 CFU/mL reduction.
Figure 2.
Time-killing kinetic curves in TSB and milk (a) Time-killing kinetic curves in TSB medium. (b) Time-killing kinetic curves in milk. All assays were performed in triplicate. The analyses were measured by one-way ANOVA, with Duncan’s multiple comparisons test. A probability value of < 0.05 was considered significant. (*) Indicates the significance between control and treatment groups. ***p < 0.001. The results are given as the mean ± SD (n = 3).
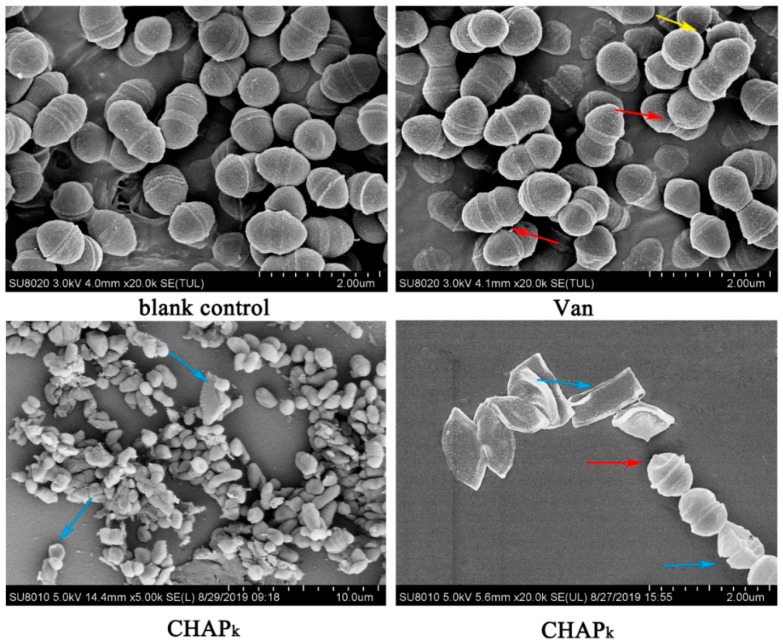
3.4. SEM Observation
The changes in cell morphology, integrity, and cellular structure of S. agalactiae ATCC 13813 were directly observed by SEM after treatment with CHAPk or vancomycin. The untreated bacteria were ovoid and clustered together, displaying morphologic integrality, and the cell surface was smooth (Figure 3). After treatment with vancomycin, wrinkles on the cell surface were observed, some cells were slightly deformed, but most of the cells remained intact. However, after treatment with CHAPk, the number of bacteria was significantly reduced, cells wrinkled seriously and even ruptured, implying that CHAPk and vancomycin exhibit different bactericidal mechanisms.
Figure 3.
Scanning electron microscope observation. The magnification of the image is 20,000 (blank control), 20,000 (Van), 5000 (CHAPk-left bottom), and 20,000 (CHAPk-right bottom), respectively. Red arrows: Cell shrinkage; Yellow arrows: Vesicular bulge; Blue arrows: Cell rupture; Van: vancomycin.
3.5. Ability of CHAPk against Biofilms and Bacteria of S. agalactiae
3.5.1. Biofilms Formation Capacity of S. agalactiae ATCC 13813
According to the evaluation criteria of biofilms formation capacity based on crystal violet staining method, the absorbance value of S. agalactiae ATCC 13813 (0.361 ± 0.04) was four times higher than that of blank control (0.089 ± 0.01) at OD570 nm, which indicated that S. agalactiae ATCC 13813 is a strong biofilm-forming strain.
3.5.2. Effect of CHAPk on S. agalactiae ATCC 13813 Early and Mature Biofilms
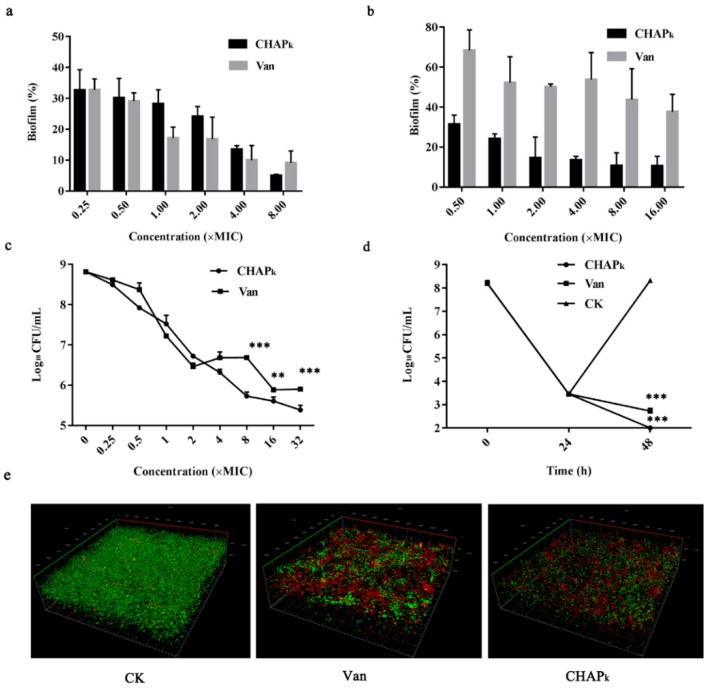
Early biofilm showed obvious signs of cell-cell aggregation. To investigate the inhibitory effect of CHAPk on early biofilms, different concentrations of CHAPk were exposed to S. agalactiae. After treatment with 0.25–8 × MIC CHAPk or vancomycin, the biofilms were inhibited by 67.3–95% and 67.3–90.9%, respectively, whose inhibitory effect was displayed in a concentration-dependent manner (Figure 4a). These data suggested that CHAPk has a potent inhibition ability to early biofilms, which is similar to vancomycin.
Figure 4.
Effects of CHAPk on S. agalactiae biofilms and bacteria in biofilms. (a) Inhibition of biofilms formation by CHAPk. (b) Eradication of mature biofilms by CHAPk. (c) Bactericidal activity against the early biofilms of S. agalactiae. (d) Bactericidal activity of CHAPk against the mature biofilms of S. agalactiae. (e) Observation of S. agalactiae biofilms by CLSM. S. agalactiae was incubated with 8 × MIC CHAPk or vancomycin for 24 h; after removing planktonic bacteria, biofilms were stained with dyes and visualized by CLSM. Live cells are stained in green by SYTO9 and dead cells are stained in red by PI, CK: the untreated S. aureus biofilms. Van: vancomycin. All assays were performed in triplicate. The analyses were measured by one-way ANOVA, with Duncan’s multiple comparisons test. A probability value of < 0.05 was considered significant. (*) Indicates the significance between control and treatment groups. ** p < 0.01; *** p < 0.001. The results are given as the mean ± SD (n = 3).
Peptidoglycan further bound and fully deployed to form mature biofilms. Mature biofilms are more difficult to remove than early biofilms. The eradication rate of mature biofilms was only 31% after exposed to 0.5 × MIC vancomycin, which was much lower than that of early biofilms. After treatment with 16 × MIC vancomycin, 62.3% mature biofilms were eradicated (Figure 4b). At the concentration of 0.5–16 × MIC CHAPk, the eradication rate of mature biofilms reached 68.4–89.4%, which was superior to that of vancomycin. CHAPk eradicated the mature biofilms in a concentration-dependent manner. It demonstrated that CHAPk has a stronger ability to eradicate the mature biofilms than vancomycin.
3.5.3. Effect on Bacteria in Early and Mature Biofilms
The growth of bacteria in early biofilms was investigated by plate counting. S. agalactiae ATCC 13813 cells in early biofilms significantly declined after exposure to CHAPk (Figure 4c). At the concentrations of 0.25–4 × MIC, there were no significant differences in bacterial reduction between CHAPk and vancomycin, but at the concentrations of 8–32 × MIC, the bacterial reduction caused by CHAPk was superior to that of vancomycin, and there were significant differences between CHAPk and vancomycin groups (p < 0.01). The results indicated that CHAPk can effectively kill bacteria in early biofilms of S. agalactiae in a concentration-dependent manner, which was in accordance with results of its inhibitory effect on biofilms in early stages.
To further explore the activity of CHAPk against bacteria in mature biofilms which were resistant to vancomycin, the mature biofilms of S. agalactiae ATCC 13813 were exposed to 25 × MIC vancomycin for 24 h, the bacteria encapsulated in biofilms reduced from 108 CFU/mL to 103 CFU/mL and no colonies were regrown. The bacteria in mature biofilms were significantly killed by 99% with 4 × MIC CHAPk in 24 h, which was superior to vancomycin (Figure 4d). It indicated that CHAPk has the potential activity to kill the vancomycin-resistant bacteria.
3.5.4. Observation of Biofilms by CLSM
To further confirm the inhibition and eradication effects of biofilms and internal bacteria, the S. agalactiae ATCC 13813 cells were treated with SYTO9 and PI, and observed by CLSM (Figure 4e), the thickness of biofilms formed by untreated S. agalactiae in confocal dish reached 20.72 μm. Compared with untreated group, the biofilms significantly became thinner (thickness of 11.28 μm) and dead bacteria increased in CHAPk treatment group, which was superior to vancomycin treatment group, implying the strong activity of CHAPk against S. agalactiae and the biofilms.
4. Discussion
S. agalactiae is a contagious pathogen which mainly causes bovine intramammary infections and spreads to the herd [34,35,36,37,38]. With the increase of antibiotics resistance, especially for β-lactam antibiotics [8], novel antimicrobial agents are urgently needed. More recently, the original application of phage as therapeutics to treat human and animal infections has been rekindled, lysins have attracted attention again due to their specific antimicrobial activity after being discovered for a century [12,39]. Deeper research is now involved in lysins, especially for their effect on Gram-positive bacteria. CHAPk is reported truncated phage lysin and it presents a broader antimicrobial spectrum as same as the original LysK enzyme. Compared with native enzyme LysK, the truncated single-domain lysin, CHAPk, showed the same lytic activity [22]. CHAPk displays strong inhibition activity against S. aureus, including methicillin resistant S. aureus (MRSA) and vancomycin-intermediate S. aureus (VISA) strains [19,22,23,24,25,26]. CHAPk also has some antibacterial effects against streptococcus [21]. In this study, it was firstly demonstrated that CHAPk had the anti-biofilm and inhibition of bacteria in biofilms effects on S. agalactiae. In this study, CHAPk has potent antibacterial activity against S. aureus, the MIC values were 0.43–3.44 μM (Table 1), this result is consistent with the conclusions of former research in this field, which demonstrated the antimicrobial activity against S. aureus in vitro [19,21,22,25,40]. Meanwhile, CHAPk also displayed antimicrobial activity for S. agalactiae isolated from bovine mastitis. Previous studies have demonstrated that CHAPk can rapidly lyse S. aureus, for Streptococcus (S. mutans DSM 6178 and S. pneumoniae DSM 11865), small decrease in turbidity was observed after treatment with CHAPk, which means less cell death [26], but little research in this field about S. agalactiae. In this study, the antimicrobial activity of CHAPk has been investigated against S. agalactiae. CHAPk exhibited high antibacterial activity against S. agalactiae (MIC: 0.05–0.11 μM), and also displayed a potent effect against the other streptococcus, including S. uberis and S. dysgalactiae, which were also the pathogens causing bovine mastitis. These results suggest that CHAPk may work at a common part in the peptidoglycan cross-bridge of staphylococcal and streptococcal pathogens. CHAPk cleaves the staphylococcal cell wall on the peptide bond between D-alanine and the first glycine in the pentaglycine cross bridge [19,26], whereas the streptococcal peptidoglycan cross-bridge contains a D-alanine-L-alanine bond and no glycine residues [41]. It was reported that the Cpl-7 cell wall binding domains of the streptococcal phage lysin λSa2 were replaced by staphylococcal SH3b domains from phage lysin LysK, which resulted in increased staphylolytic activity by five times based on the maintained streptolytic activity, suggesting that the staphylococcal domains have certain broad-spectrum antibacterial properties that are not always staphylococcal-specific [42]. The activity of CHAPk against S. agalactiae is worth further investigation. In this study, time-killing kinetic curves showed that CHAPk had a rapid effect on bacteria both in TSB medium and in milk within 25 min, which was superior to vancomycin (Figure 2), and the result was accordance with the former research that CHAPk retained antimicrobial activity in raw bovine milk [40]. However, the effect of CHAPk was weaker in milk than that in TSB medium, this might include reduced affinity to the S. agalactiae cell envelope which was changed when grown in milk or CHAPk bound to milk components [43]. Because of the special mode of inhibition, lysins have no effect on commensal microflora, which is also a reason for the safety of using CHAPk [44,45,46]. In addition, the destructed cell wall and leaked contents indicated that CHAPk kills S. agalactiae by causing cells to rupture (Figure 3). The activity of CHAPk truncated cell-wall binding domain (CBD) has been suggested to be connected with the overall charge of the enzymatically active domain (EAD) alone, seemingly because EAD with positive charge can independently bind the bacterial cell wall of the CBD [44].
Biofilms provide a habitat or reservoir for bacteria, which make it difficult for antibiotics to work, leading to resistances and reinfections [47,48]. A previous study has demonstrated that CHAPk has the anti-biofilm properties for S. aureus [41]. In this study, CHAPk has the effect on early biofilms (24 h growth) and mature biofilms (48 h growth) in a concentration-dependent manner (Figure 4a,b) [49]. For the early biofilms, CHAPk had potent inhibited ability which is similar to vancomycin, the reason may be that in the early stage of biofilms production, low yield of biofilms is insufficient to provide protection for bacteria, antibiotics can inhibit the formation of biofilms by killing the S. agalactiae. However, for mature biofilms, CHAPk displayed higher eradication rate than that of vancomycin, and previous research indicated there was no changes in other streptococcus (Streptococcus anginosus) biofilms by vancomycin exposure [50]. The results demonstrated that CHAPk had a more potent anti-biofilm ability than vancomycin, which is consistent with the observation of biofilms by CLSM (Figure 4e). CHAPk also successfully disrupted the S. aureus biofilms at a concentration as low as 3.91 μg/mL, there was little or no visible biofilms detected at a concentration of 62.5 μg/mL [51]. The antibiofilm activity of chimeric phage endolysin Ply187 on biofilms of MRSA strains were measured by live/dead staining assay, which indicated effective antimicrobial activity, whereas gentamycin had a poor effect on biofilms viability [48], which indicated that the lysins have better anti-biofilm activity than antibiotics. The bacteria in biofilms is a major reason that causes reinfections. CHAPk can effectively reduce the number of S. agalactiae ATCC 13813 in biofilms, which showed a significant difference with vancomycin. Earlier studies have shown that vancomycin that inhibit cell wall synthesis reduce bacterial adherence, thus reducing bacteria and biofilm formation [52]. Recent research showed that the resistance of Streptococcus anginosus to vancomycin in a multispecies biofilm is due to increased thickness of the cell wall [50]. However, the elimination of biofilms by lysin is in connection with lysin inhibiting poly-intercellular adhesion (PIA) binding to peptidoglycan by rapidly degrading the cell wall and significantly decreasing the eDNA content. This may be the reason why CHAPk is more potent in killing S. agalactiae and inhibiting biofilms formation than vancomycin [53]. The bacteria in biofilms show reduced susceptibility to antibiotics among several important pathogens [54], in contrast, lysins have high efficiency activity against bacteria in biofilms. CHAPk killed the bacteria in biofilms, which were resistant to 25 × MIC vancomycin. Survival of bacteria in S. aureus biofilms were formed by treatment with 2 mg/mL of rifampicin or 3 mg/mL of ciprofloxacin for 4 h, which could be eliminated with 0.5 mM endolysin LysH5 [55]. After treatment with 1 × MIC of P128, the survival of S. epidermidis in 50 × MIC daptomycin or 100 × MIC vancomycin could be drastically reduced [56].
In summary, recombinant CHAPk was successfully expressed in E. coli via pET28a, CHAPk exhibited potent antimicrobial activity against Gram-positive, especially for S. agalactiae which were isolated from bovine mastitis. CHAPk could rapidly lyse S. agalactiae cells in a short time, and it also significantly inhibited its biofilms formation at an early stage and eliminated its mature biofilms. For the bacteria in biofilms, CHAPk showed efficient antimicrobial ability, which was superior to vancomycin. The potent antimicrobial activity of CHAPk against the S. agalactiae isolated from bovine mastitis is the basis for its clinical application. It is suggested that CHAPk may be a candidate for novel antimicrobial agents against streptococcal and even staphylococcal infections in mastitis treatment.
Acknowledgments
We acknowledge Chunli Li from the Core Facility at the Institute of Microbiology at the Chinese Academy of Sciences (CAS) for his technical support with SEM analysis, and Dan Zhang from the Core Facility at the Center of Biomedical Analysis at Tsinghua University for her Confocal Microscopy analysis.
Author Contributions
J.W., D.T. and H.F. conceived and designed experiments. Y.S. and N.Y. carried out all experiments. Y.S., N.Y., X.W., R.M., Y.H. and X.M. conducted the data analysis and created the methodology. Y.S. wrote the original draft of the manuscript. J.W., D.T. and N.Y. contributed to the writing, review, and editing of the manuscript. J.W. and H.F. contributed in funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Innovation Program of Agricultural Science and Technology in CAAS (CAAS-ZDXT2018008 and CAAS-ASTIP-2013-FRI-02) and Tianjin Science and Technology Planning Project (18YFZCNC01130).
Conflicts of Interest
All the authors declare no competing financial interest.
References
- 1.Keefe G.P. Streptococcus agalactiae mastitis: A review. Can. Vet. J. 1997;38:429–437. [PMC free article] [PubMed] [Google Scholar]
- 2.Holmoy I.H., Toftaker I., Kirkeby C., Osteras O., Jorgensen H.J., Nodtvedt A. A cohort study of the effect of Streptococcus agalactiae on milk yield and somatic cell count in Norwegian dairy cows. J. Dairy Sci. 2019;102:8385–8399. doi: 10.3168/jds.2018-16155. [DOI] [PubMed] [Google Scholar]
- 3.Østerås O., Sølverød L. Streptococcus agalactiae-an increasing problem in big herds which must be taken seriously (in Norwegian) Nor. Vet. Tidsskr. 2011;123:519–520. [Google Scholar]
- 4.Zadoks R.N., Middleton J.R., McDougall S., Katholm J., Schukken Y.H. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J. Mammary Gland Biol. Neoplasia. 2011;16:357–372. doi: 10.1007/s10911-011-9236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jørgensen H.J., Nordstoga A.B., Sviland S., Zadoks R.N., Sølverød L., Kvitle B., Mørk T. Streptococcus agalactiae in the environment of bovine dairy herds–rewriting the textbooks? Vet. Microbiol. 2016;184:64–72. doi: 10.1016/j.vetmic.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y., Liu Y., Ding Y., Yi L., Ma Z., Fan H., Lu C., Zhou D. Molecular characterization of Streptococcus agalactiae isolated from bovine mastitis in eastern China. PLoS ONE. 2013;8:e67755. doi: 10.1371/journal.pone.0067755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ebrahimi A., Moatamedi A., Lotfalian S., Mirshokraei P. Biofilm formation, hemolysin production and antimicrobial susceptibilities of Streptococcus agalactiae isolated from the mastitis milk of dairy cows in Shahrekord district, Iran. Vet. Res. Forum. 2014;4:269–272. [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Y., Kan Y., Zhang Z., Lu Z., Li Y., Leng C., Ji J., Song S., Shi H. New mutations of penicillin-binding proteins in Streptococcus agalactiae isolates from cattle with decreased susceptibility to penicillin. Microb. Drug Resist. 2018;24:1236. doi: 10.1089/mdr.2017.0223. [DOI] [PubMed] [Google Scholar]
- 9.Guérin-Faublée V., Tardy F., Bouveron C., Carret G. Antimicrobial susceptibility of Streptococcus species isolated from clinical mastitis in dairy cows. Int. J. Antimicrob. Agents. 2002;19:219–226. doi: 10.1016/S0924-8579(01)00485-X. [DOI] [PubMed] [Google Scholar]
- 10.Schuch R., Nelson D., Fischetti V.A. A bacteriolytic agent that detects and kills Bacillus anthracis. Nature. 2002;418:884–889. doi: 10.1038/nature01026. [DOI] [PubMed] [Google Scholar]
- 11.Schmelcher M., Loessner M.J. Bacteriophage endolysins: Applications for food safety Curr. Opin. Biotech. 2016;37:76–87. doi: 10.1016/j.copbio.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Nelson D., Loomis L., Fischetti V.A. Prevention and elimination of upper respiratory colonization of mice by group a streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA. 2001;98:4107–4112. doi: 10.1073/pnas.061038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniel A., Euler C., Collin M., Chahales P., Gorelick K.J., Fischetti V.A. Synergism between a novel chimeric lysin and oxacillin protects against infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2010;54:1603. doi: 10.1128/AAC.01625-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenton M., Casey P.G., Hill C., Gahan C.G., Ross R.P., McAuliffe O., O’Mahony J., Maher F., Coffey A. The truncated phage lysin CHAP k eliminates Staphylococcus aureus in the nares of mice. Bioeng Bugs. 2010;1:404–407. doi: 10.4161/bbug.1.6.13422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mccullers J.A., Asa K.M., Iverson A.R., Loeffler J.M., Fischetti V.A. Novel strategy to prevent otitis media caused by colonizing Streptococcus pneumoniae. PLoS Pathog. 2007;3:e28. doi: 10.1371/journal.ppat.0030028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loeffler M.J., Nelson D., Fischetti V.A. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science. 2001;294:2170–2172. doi: 10.1126/science.1066869. [DOI] [PubMed] [Google Scholar]
- 17.Loessner M.J. Bacteriophage endolysins-Current state of research and applications. Curr. Opin. Microbiol. 2005;8:480–487. doi: 10.1016/j.mib.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Borysowski J., Weber-Dabrowska B., Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006;231:366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- 19.Becker S.C., Dong S., Baker J., Foster-Frey J., Pritchard D., Donovan D. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. Fems Microbiol. Lett. 2010;294:52–60. doi: 10.1111/j.1574-6968.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- 20.Sanz-Gaitero M., Keary R., Garcia-Doval C., Coffey A., van Raaij M.J. Crystallization of the CHAP domain of the endolysin from Staphylococcus aureus bacteriophage K. Acta Crystallogr. 2013;69:1393–1396. doi: 10.1107/S1744309113030133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keary R., Sanz-Gaitero M., van Raaij M.J., O’Mahony J., Fenton M., McAuliffe O., Hill C., Ross R.P., Coffey A. Characterization of a bacteriophage-derived murein peptidase for elimination of antibiotic-resistant Staphylococcus aureus. Curr. Protein Pept. Sci. 2016;17:183–190. doi: 10.2174/1389203716666151102105515. [DOI] [PubMed] [Google Scholar]
- 22.Horgan M., O’Flynn G., Garry J., Cooney J., Coffey A., Fitzgerald G.F., Ross R.P., McAuliffe O. Phage lysin LysK can be truncated to its CHAP domain and retain lytic activity against live antibiotic-resistant staphylococci. Appl. Environ. Microb. 2009;75:872–874. doi: 10.1128/AEM.01831-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanzgaitero M., Keary R., Garciadoval C., Coffey A., Raaij M.J.V. Crystal structure of the lytic CHAP K domain of the endolysin LysK from Staphylococcus aureus bacteriophage K. Virol. J. 2014;11:1–11. doi: 10.1186/1743-422X-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini E.S., Moniri R., Goli Y.D., Kashani H.H. Purification of antibacterial CHAPK protein using a self-cleaving fusion tag and its activity against methicillin-resistant Staphylococcus aureus. Probiotics Antimicro. 2016;8:1–9. doi: 10.1007/s12602-016-9236-8. [DOI] [PubMed] [Google Scholar]
- 25.Hathaway H., Ajuebor J., Stephens L., Coffey A., Potter U., Sutton J.M., Jenkins A.T.A. Thermally triggered release of the bacteriophage endolysin CHAPK and the bacteriocin lysostaphin for the control of methicillin resistant Staphylococcus aureus (MRSA) J. Control. Release. 2017;245:108–115. doi: 10.1016/j.jconrel.2016.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fenton M., Ross R.P., Mcauliffe O., O’Mahony J., Coffey A. Characterization of the staphylococcal bacteriophage lysin CHAP(K) J. Appl. Microbiol. 2011;111:1025–1035. doi: 10.1111/j.1365-2672.2011.05119.x. [DOI] [PubMed] [Google Scholar]
- 27.Haghighat S., Siadat S.D., Sorkhabadi S.M.R., Sepahi A.A., Mahdavi M. Cloning, expression and purification of autolysin from methicillin-resistant Staphylococcus aureus: Potency and challenge study in Balb/c mice. Mol. Immunol. 2017;82:10–18. doi: 10.1016/j.molimm.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Yang N., Liu X., Teng D., Li Z., Wang X., Mao R., Wang X., Hao Y., Wang J. Antibacterial and detoxifying activity of NZ17074 analogues with multi-layers of selective antimicrobial actions against Escherichia coli and Salmonella enteritidis. Sci. Rep. 2017;7:3392. doi: 10.1038/s41598-017-03664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X., Wang X., Teng D., Mao R., Hao Y., Yang N., Zong L., Wang J. Mode of action of plectasin-derived peptides against gas gangrene-associated Clostridium perfringens type A. PLoS ONE. 2017;12:e0185215. doi: 10.1371/journal.pone.0185215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao Y., Yang N., Wang X., Teng D., Mao R., Wang X., Li Z., Wang J. Killing of Staphylococcus aureus and Salmonella enteritidis and neutralization of lipopolysaccharide by 17-residue bovine lactoferricins: Improved activity of Trp/Ala-containing molecules. Sci. Rep. 2017;7:44278. doi: 10.1038/srep44278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zong L., Teng D., Wang X., Mao R., Yang N., Hao Y., Wang J. Mechanism of action of a novel recombinant peptide, MP1102, against Clostridium perfringens type C. Appl. Microbiol. Biotechnol. 2016;100:5045–5057. doi: 10.1007/s00253-016-7387-x. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay Y.D., Deslandes V., Jacques M. Actinobacillus pleuropneumoniae genes expression in biofilms cultured under static conditions and in a drip-flow apparatus. BMC Genom. 2013;14:364. doi: 10.1186/1471-2164-14-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang N., Teng D., Mao R., Hao Y., Wang X., Wang Z., Wang X., Wang J. A recombinant fungal defensin-like peptide-P2 combats multidrug-resistant Staphylococcus aureus and biofilms. Appl. Microbiol. Biot. 2019;103:5193–5213. doi: 10.1007/s00253-019-09785-0. [DOI] [PubMed] [Google Scholar]
- 34.Bi Y., Wang Y.J., Qin Y., Guix V.R., Maldonado G.J., Sun W., Li S., Cao Z. Prevalence of bovine mastitis pathogens in bulk tank milk in China. PLoS ONE. 2016;11:e0155621. doi: 10.1371/journal.pone.0155621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira J.C., Gomes M.S., Ecr B., Canisso I.F., Garrett E.F., Stewart J.L., Zhou Z., Lima F.S. Comparative analysis of four commercial on-farm culture methods to identify bacteria associated with clinical mastitis in dairy cattle. PLoS ONE. 2018;13:e0194211. doi: 10.1371/journal.pone.0194211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forsman P., Tilsala-Timisjärvi A., Alatossava T. Identification of staphylococcal and streptococcal causes of bovine mastitis using 16S-23S rRNA spacer regions. Microbiology. 1997;143:3491–3500. doi: 10.1099/00221287-143-11-3491. [DOI] [PubMed] [Google Scholar]
- 37.Levison L.J., Miller-Cushon E.K., Tucker A.L., Bergeron R., Leslie K.E., Barkema H.W., Devries T.J. Incidence rate of pathogen-specific clinical mastitis on conventional and organic Canadian dairy farms. J. Dairy Sci. 2016;99:1341–1350. doi: 10.3168/jds.2015-9809. [DOI] [PubMed] [Google Scholar]
- 38.Suleiman T.S., Karimuribo E.D., Mdegela R.H. Prevalence of bovine subclinical mastitis and antibiotic susceptibility patterns of major mastitis pathogens isolated in Unguja island of Zanzibar, Tanzania. Trop. Anim. Health Pro. 2017;50:1–8. doi: 10.1007/s11250-017-1424-3. [DOI] [PubMed] [Google Scholar]
- 39.Twort F.W. An investigation on the nature of ultramicroscopic viruses. Bacteriophage. 1915;2:1241–1243. [Google Scholar]
- 40.Verbree C.T., Twyler S.M., Meile S., Eichenseher F., Donovan D.M., Loessner M.J., Schmelcher M., Schaffner D.W. Identification of peptidoglycan hydrolase constructs with synergistic staphylolytic activity in cow’s milk. Appl. Environ. Microb. 2017;83 doi: 10.1128/AEM.03445-16. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Pritchard D.G., Dong S., Baker J.R., Engler J.A. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology. 2004;150:2079–2087. doi: 10.1099/mic.0.27063-0. [DOI] [PubMed] [Google Scholar]
- 42.Becker S.C., Foster-Frey J., Stodola A.J., Anacker D., Donovan D.M. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene. 2009;443:32–41. doi: 10.1016/j.gene.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 43.Schmelcher M., Powell A.M., Becker S.C., Camp M.G., Donovan D.M. Chimeric phage lysins act synergistically with lysostaphin to kill mastitis-causing Staphylococcus aureus in murine mammary glands. Appl. Environ. Microb. 2012;78:2297–2305. doi: 10.1128/AEM.07050-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Low L.Y., Yang C., Perego M., Osterman A., Liddington R. Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J. Biol. Chem. 2011;286:34391–34403. doi: 10.1074/jbc.M111.244160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoong P., Schuch R., Nelson D., Fischetti V.A. Identification of a broadly active phage lytic enzyme with lethal activity against antibiotic-resistant Enterococcus faecalis and Enterococcus faecium. J. Bacteriol. 2004;186:4808–4812. doi: 10.1128/JB.186.14.4808-4812.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischetti V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010;300:357–362. doi: 10.1016/j.ijmm.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.David D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- 48.Singh P.K., Donovan D.M., Kumar A. Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob. Agents Chemother. 2014;58:4621–4629. doi: 10.1128/AAC.00126-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S.J., Chang J., Rimal B., Yang H., Schaefer J. Surface proteins and the formation of biofilms by Staphylococcus aureus. BBA Biomembr. 2017;1860:749–756. doi: 10.1016/j.bbamem.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tavernier S., Sass A., De Bruyne M., Baeke F., De Rycke R., Crabbé A., Vandecandelaere I., Van Nieuwerburgh F., Coenye T. Decreased susceptibility of Streptococcus anginosus to vancomycin in a multispecies biofilm is due to increased thickness of the cell wall. J. Antimicrob. Chemoth. 2018;73:2323–2330. doi: 10.1093/jac/dky216. [DOI] [PubMed] [Google Scholar]
- 51.Fenton M., Keary R., Mcauliffe O., Ross R.P., O’Mahony J., Coffey A. Bacteriophage-derived peptidase CHAP(K) eliminates and prevents staphylococcal biofilms. Int. J. Microbiol. 2013;2013:625341. doi: 10.1155/2013/625341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernard J.P., Francioli P., Glauser M.P. Vancomycin prophylaxis of experimental Streptococcus sanguis. Inhibition of bacterial adherence rather than bacterial killing. J. Clin. Invest. 1981;68:1113–1116. doi: 10.1172/JCI110337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chopra S., Harjai K., Chhibber S. Potential of sequential treatment with minocycline and S. aureus specific phage lysin in eradication of MRSA biofilms: An in vitro study. Appl. Microbiol. Biot. 2015;99:3201–3210. doi: 10.1007/s00253-015-6460-1. [DOI] [PubMed] [Google Scholar]
- 54.Proctor R.A., von Eiff C., Kahl B.C., Becker K., McNamara P., Herrmann M., Peters G. Small colony variants: A pathogenic form of bacteria that facilitates persistent and recurrent infections. Nat. Rev. Microbiol. 2009;4:295–305. doi: 10.1038/nrmicro1384. [DOI] [PubMed] [Google Scholar]
- 55.Gutiérrez D., Ruas-Madiedo P., Martínez B., Rodríguez A., García P. Effective removal of staphylococcal biofilms by the endolysin LysH5. PLoS ONE. 2014;9:e107307. doi: 10.1371/journal.pone.0107307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poonacha N., Nair S., Desai S., Tuppad D., Hiremath D., Mohan T., Vipra A., Sharma U. Efficient killing of planktonic and biofilm embedded coagulase-negative staphylococci by bactericidal protein P128. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]