Abstract
Microorganisms related to plant roots are vital for plant growth and health and considered to be the second genome of the plant. When the plant is attacked by plant pathogens, the diversity and community structure of plant-associated microbes might be changed. The goal of this study is to characterize differences in root-associated endophytic actinobacterial community composition and antifungal activity between Fusarium wilt diseased and healthy cucumber and screen actinobacteria for potential biological control of Fusarium wilt of cucumber. In the present research, three healthy plants (also termed “islands”) and three obviously diseased plants (naturally infected by F. oxysporum f. sp. cucumerinum) nearby the islands collected from the cucumber continuous cropping greenhouse were chosen as samples. Results of culture-independent and culture-dependent analysis demonstrated that actinomycetes in the healthy roots were significantly more abundant than those of diseased roots. Moreover, there were seven strains with antifungal activity against F. oxysporum f. sp. cucumerinum in healthy cucumber roots, but only one strain in diseased cucumber roots. Out of these eight strains, the isolate HAAG3-15 was found to be best as it had the strongest antifungal activity against F. oxysporum f. sp. cucumerinum, and also exhibited broad-spectrum antifungal activity. Thus, strain HAAG3-15 was selected for studying its biocontrol efficacy under greenhouse conditions. The results suggested that the disease incidence and disease severity indices of cucumber Fusarium wilt greatly decreased (p < 0.05) while the height and shoot fresh weight of cucumber significantly increased (p < 0.05) after inoculating strain HAAG3-15. On the basis of morphological characteristics, physiological and biochemical properties and 100% 16S ribosomal RNA (rRNA) gene sequence similarity with Streptomyces sporoclivatus NBRC 100767T, the isolate was assigned to the genus Streptomyces. Moreover, azalomycin B was isolated and identified as the bioactive compound of strain HAAG3-15 based on analysis of spectra using a bioactivity-guided method. The stronger antifungal activity against F. oxysporum f. sp. cucumerinum, the obvious effect on disease prevention and growth promotion on cucumber seedlings in the greenhouse assay, and the excellent broad-spectrum antifungal activities suggest that strain HAAG3-15 could be developed as a potential biocontrol agent against F. oxysporum f. sp. cucumerinum used in organic agriculture. These results suggested that the healthy root nearby the infected plant is a good source for isolating biocontrol and plant growth-promoting endophytes.
Keywords: Fusarium oxysporum f. sp. cucumerinum, endophytic actinomycetes, HAAG3-15, biocontrol agent
1. Introduction
Cucumber (Cucumis sativus L.), belonging to the family Cucurbitaceae [1], is a very important vegetable which possesses remarkable economic and dietary value. It has been around for over three thousand years as a monoecious annual cultivable plant [2,3]. In addition, cucumber is well known for its softness and succulence and contains a variety of nutrients, such as potassium, copper, manganese, phosphorus, pantothenic acid, dietary fibers, and vitamins (A, C, K, and B6) [4].
However, cucumber is susceptible to many pathogens and pests [5]. Cucumber Fusarium wilt, induced by the pathogen Fusarium oxysporum f. sp. cucumerinum, is a typical soil-borne fungal disease and also one of the most important cucumber diseases in worldwide [6,7]. The disease could reduce ~10% to 30% of cucumber production and cause quality degradation, which results in serious economic losses [8,9]. Fusarium wilt of cucumber disease may appear throughout the whole growth period of cucumber plant, and the disease incidence at early stages is more serious [10,11]. The symptoms of the disease are vascular and root wilt which eventually cause plant death [11,12]. Chemical control agents are implicated in ecological, environmental, and human health problems, and pathogens can develop resistance to them [13]. Traditional ways of crop rotation and seeding grafting could be applicable for controlling Fusarium wilt; however, these methods are high-cost and laborious [14]. Moreover, soil fumigation is also an efficient approach to control the extension of soil-borne disease, but this strategy is labor-consuming and inconvenient, which limits its application [9].
Up to now, agricultural scientists paid much attention to an efficient, environmentally friendly, and sustainable method, biocontrol, which is used for protecting plants against soil-borne diseases [15]. A number of antagonistic microbes were investigated and studied to control various plant pathogens, such as Pseudomonas spp., Bacillus spp., Streptomyces spp., Trichoderma spp., and Paenibacillus spp [13,16,17,18,19,20,21,22]. Kareem et al. reported that Trichoderma longibrachiatum NGJ167 could be used to control Fusarium wilt of cucumber [13], Bacillus subtilis 9407 was recorded as a biocontrol agent against bacterial fruit blotch of melon [20], and Pseudomonas aeruginosa BRp3 could reportedly be applied in controlling bacterial leaf blight of rice [22]. Streptomyces genus is one of the most efficient groups, with the capacity of preventing plant fungal diseases [17,19,21,23,24]. S. exfoliates FT05W and S. cyaneus ZEA17 were documented as biocontrol agents against lettuce drop caused by Sclerotinia sclerotiorum [19], S. griseochromogenes and S. lydicus WYEC108 were reported as biocontrol agents against the rice blast fungus, Magnaporthe oryzae (Pyricularia oryzae) [21], and S. albospinus CT205 was found to have biocontrol potential against cucumber Fusarium wilt [24]. Streptomyces with efficient rhizosphere and/or the inner regions of plant tissue colonization could prevent fungal pathogens and promote plant growth by inoculating spore suspensions on seeds or seedlings [19,25,26,27]. The genus Streptomyes is known for its capacity of producing abundant secondary metabolites with bioactivities against plant pathogens [28]. Thus, the isolation of Streptomyces with antibacterial activity is recognized as a crucial strategy in the prevention and control of plant diseases and development of agriculture, as well as ecosystem safety.
Endophytes which can reside within the plants through parasitic, symbiotic, or mutualistic modes without inducing apparent infections or symptoms of disease for the whole or part of their life history [29,30] represent a portion of the microbes associated with plant. The phylum Actinobacteria was reported as the major portion of endophytic microbes, while Streptomyces was reported as the main content of endophytic actinomycetes in most plants [31,32,33,34,35,36,37,38,39,40,41,42]. It was also reported that many endophytic actinobacteria could control plant pathogens, improve plant stress resistance, and promote plant growth [43,44,45,46]. Therefore, the application of endophytic biotcontrol agents is the current research hotspot [47] and could provide biocontrol strategies in future.
Microorganisms related to plant roots are vital for plant growth and health and considered to be the second genome of the plant. When the plant is attacked by plant-pathogenic microorganisms, the diversity and community structure of plant-associated microbes may be changed [48,49]. The goal of this study is to characterize differences in root-associated endophytic actinobacterial community composition and antifungal activity between Fusarium wilt diseased and healthy cucumber, as well as to screen actinobacteria for potential biological control of Fusarium wilt of cucumber. In our present research, three healthy plants (also termed “islands”) [50] and three obviously diseased plants nearby the islands collected from a cucumber continuous cropping greenhouse were chosen as samples for culture-independent and culture-dependent analysis. A preliminary study of antifungal activities of these strains against F. oxysporum f. sp. cucumerinum was performed and compared. Strain HAAG3-15 with the strongest antifungal activity was selected for investigating its biocontrol effect on potted plants. Furthermore, the bioactive constituent with antifungal activity of strain HAAG3-15 was isolated and the chemical identity was determined. This would be of high importance for the source of antagonistic strains and biocontrol of cucumber Fusarium wilt, as well as other plant fungal diseases.
2. Materials and Methods
2.1. Sampling of Healthy and Diseased Plants
In June 2017, three healthy cucumber plants (also termed “islands”) and three obviously diseased cucumber plants (infected by F. oxysporum f. sp. cucumerinum) nearby the islands collected from the cucumber continuous cropping greenhouse (320 m2, plastic film) of Northeast Agricultural University, Heilongjiang province, northeast China (45°41′ north (N), 126°37′ east (E)) were chosen as samples. Each group included one healthy plant and one obviously diseased plant nearby the island. These three groups were named H1-D1, H2-D2, and H3-D3. All cucumber plants surveyed in the current study were in the initial flowering stage of cucumber.
2.2. DNA Extraction, Sequencing, and Data Analysis
The loose soil attached to the cucumber roots was firstly removed by gentle shaking. Then, the roots were washed in water with an ultrasonic step (160 W, 15 min) to thoroughly clean off surface soils and adherent epiphytes. Then, the total DNA was isolated from the roots using the Cetyltrimethylammonium Ammonium Bromide (CTAB) method. The purity of DNA was checked using 1% agarose gels, and DNA concentration was determined with a NanoPhotometer spectrophotometer (Implen, München, Germany). The V3–V4 regions of 16S ribosomal RNA (rRNA) genes of bacterial DNA were amplified using the primer pair 341F (forward; 5′–CCTAYGGGRBGCASCAG–3′) and 806R (reverse; 5′–GGACTACNNGGGTATCTAAT–3′) with the barcode and sequenced on an Ion S5TM XL platform at Beijing Novogene Technology Co. Ltd. (Beijing, China), generating 600 bp single-end reads. The raw data were filtered (removing low-quality reads less than 17) according to the Cutadapt (V1.9.1, http://cutadapt.readthedocs.io/en/stable/) [51] quality control process. The reads were compared with Silva database (https://www.arb-silva.de/) [52] using the UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html) [53], and then the chimera sequences [54] were removed to obtain clean data. The Uparse software (v7.0.1001, http://drive5.com/uparse/) [55] was used for sequences analysis. All sequences were clustered into operational taxonomic units (OTUs) on the basis of a sequence similarity of ≥97%, and the representative sequence was annotated with taxonomic information based on the Silva Database (https://www.arb-silva.de/) [52] using the Mothur algorithm. Determination of the difference in dominant species among different samples (groups) and multiple sequence alignment were carried out using the MUSCLE software (Version 3.8.31, http://www.drive5.com/muscle/) [56]. Observed species and Chao 1 were calculated with QIIME (Version1.9.1, http://qiime.org), and principal component analysis (PCA) was used for analyzing the ordinations of community patterns.
2.3. Isolation and Maintenance of Endophytic Actinomycetes
The cucumber root samples were air dried for 24 h at room temperature and weighed. The roots were cut into pieces of 5–10 mm in length and then subjected to a seven-step surface sterilization procedure [57]. The samples were then ground with a sterile mortar and pestle, employing 1 mL of 0.5 M phosphate buffer saline (pH 7.0) per 100 mg tissue. Tissue particles were allowed to settle down at 4 °C for 20–30 min. The suspensions of each sample were all spread on plates of humic acid–vitamin agar [58], Gause’s synthetic agar No. 1 [59], dulcitol–proline agar [57], cellulose–proline agar [57], and arginine–alanine–granulose agar [57], supplemented with cycloheximide (50 mg∙L−1) and nalidixic acid (20 mg∙L−1). Endophytic strains were incubated at 28 °C until single colonies were observed. Single actinomycete colonies growing on the plates were isolated and purified on oatmeal agar (International Streptomyces Project medium 3, ISP 3) [60]. The isolates were prepared on ISP 3 medium and kept at −80 °C (under 30% glycerol) for long-term storage and at 4 °C as source cultures.
2.4. Screening the Isolates with Antifungal Activity
To screen antagonistic actinomycetes, the antifungal activity of these isolates was determined against the pathogen F. oxysporum f. sp. cucumerinum. These isolates were streaked on ISP 3 medium and cultivated for seven days (five repetitions). The pathogenic fungus (F. oxysporum f. sp. cucumerinum) was cultured on potato dextrose agar (PDA) for one week [61]. Mycelia discs (6 mm diameter) of each pathogen were picked up and put in the center of different plates which contained freshly prepared PDA medium, and the strains were point-inoculated at the margin areas which were 3 cm away from the central pathogen colony using an inoculating needle, and then cultured in an incubator at 28 °C for seven days. All the experiments above were repeated three times. The inhibition of fungal growth on each plate was calculated as described below [62]. The antifungal activity of antagonistic strains against other nine pathogenic fungi (Corynespora cassiicola, Setosphaeriaturcica turcicaf, Colletotrichum orbiculare, Alternaria solani, Helminthosporium maydis, Sphacelotheca reiliana, Sclerotinia sclerotiorum, Phytophthora sojae, Rhizoctonia solani) was also determined as described in Equation (1).
| (1) |
where A is the mycelial growth of fungal pathogen in the absence of antagonists, and B is the mycelial growth of fungal pathogen in the presence of antagonists.
2.5. Morphological and Physiological Characterization
Gram staining was performed using a standard method. Morphological characteristics of strain HAAG3-15 were observed by light microscopy (Nikon ECLIPSE E200, Nikon Corporation, Tokyo, Japan) and scanning electron microscopy (Hitachi SU8010, Hitachi Co., Tokyo, Japan) using cultures grown on ISP 3 medium at 28 °C for three weeks; samples for scanning electron microscopy were prepared as described by Jin et al. [63].
2.6. Genomic and Phylogenetic Analysis
For DNA extraction, strain HAAG3-15 was cultured in ISP 2 medium at 28 °C for four days, and then the cultures were centrifuged to harvest the cells. Genomic DNA extraction was carried out using a TIANamp Bacteria DNA Kit (TIANGEN Biotech, Co., Ltd., Beijing, China). PCR amplification of the 16S rRNA gene was performed using a standard procedure [64]. PCR products were purified and cloned into the vector pMD19-T (Takara Bio Inc., Dalian, China) and sequenced using an Applied Biosystems DNA sequencer (model 3730XL, Applied Biosystems Inc., Foster City, CA, USA). The almost full-length 16S rRNA gene sequence of strain HAAG3-15 (1519 bp) was obtained and submitted to the EzBioCloud server (https://www.ezbiocloud.net/) for comparison with type strains [65], retrieved using NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi;), and then submitted to the GenBank database. The phylogenetic tree was built based on the 16S rRNA gene sequences of strain HAAG3-15 and related reference species. Sequences were multiply aligned in Molecular Evolutionary Genetics Analysis (MEGA) software version MEGA7.0 using Clustal W algorithm and manually modified if necessary. The phylogenetic tree was constructed using the neighbor-joining [66] algorithm with MEGA [67]. A bootstrap method with 1000 replicates was used to evaluate the stability of the topology of the phylogenetic tree [68]. Kimura’s two-parameter model was used for generating a distance matrix [69]. All positions in the dataset containing gaps and missing data were deleted (complete deletion option).
2.7. Greenhouse Biocontrol Assay Using HAAG3-15
The capacity of strain HAAG3-15 to control cucumber Fusarium wilt and promote the growth of cucumbers (Jinyan four varieties) was evaluated using a pot experiment with four treatments (F, F + S, N, and S) under greenhouse conditions. The soil used in the present study was steam-sterilized three times (121 °C, 30 min). In the treatment F, the spore suspension of F. oxysporum f. sp. cucumerinum (2 mL, 4–5 × 104 colony-forming units (CFU)∙mL−1) was irrigated in the soil while cucumber was transplanted. In the treatment F + S, the spore suspension of isolate HAAG3-15 (2 mL, 4–5 × 106 CFU∙mL−1), together with that of F. oxysporum (2 mL, 4–5 × 104 CFU∙mL−1), was irrigated in the soil while the cucumber was transplanted. In the treatment N, no microbial suspension was added to the soil. For the treatment S, the spore suspension of isolate HAAG 3-15 (2 mL, 4–5 × 106 CFU∙mL−1) was irrigated in the soil when the cucumber was transplanted. For each treatment, 30 two-week old cucumber seedlings were used and cultivated in plastic pots (15 cm diameter, one cucumber seedling per pot). This study was performed under greenhouse conditions with average temperature of 25 °C, relative humidity of about 60%, and 12 h of illumination (11.8 W/m2) per day. The cucumber seedlings were watered every two days and no fertilizers were used. Fifteen cucumber seedlings randomly harvested from the pots of each treatment were used to measure their shoot fresh weights and heights after cultivating after four weeks. The disease symptoms of all cucumber seedlings per treatment were investigated in this study. Severity of disease symptoms was recorded using an index ranging from 0 (healthy plant) to 4 (dead plant). The plant disease index (DI) was calculated according to the following formula: DI = [∑ (Ni × i)/ (N × 4)] × 100, where i means a 0–4 disease level, and Ni means the plant number of reaction i [70].
2.8. Isolation and Characterization of the Antifungal Compound
The antifungal compound was separated based on the antifungal (F. oxysporum f. sp. cucumerinum) activity-guided method from the extraction. Strain HAAG3-15 was inoculated into 250-mL Erlenmeyer flasks filled with 50 mL of sterile tryptic soy broth (seed medium, TSB, Beijing AOBOXING BIO-TECH CO., LTD., Beijing, China) and cultured for two days at 28 °C. Then, the seed culture (12.5 mL) was transferred into 1000-mL Erlenmeyer flasks containing 250 mL of production medium (soybean flour 20 g, peptone 2 g, glucose 20 g, soluble starch 5 g, yeast extract 2 g, NaCl 4 g, K2HPO4 3H2O 0.5 g, MgSO4 7H2O 0.5 g, CaCO3 2 g, and distilled water 1 L; pH 7.2–7.4) and incubated at 28 °C for seven days on a rotary shaker (250 rpm). Next, 50-L cultures were obtained and filtered, and 30 L of mycelial cake was harvested. Then, the mycelial cake was washed with 3 L of distilled water and extracted with 3 L of methyl alcohol. The supernatant and wash water were subjected to a Diaion HP-20 resin column (500 mm × 100 mm inner diameter (i.d.)) eluting with 95% EtOH (5 L). The MeOH extract and the EtOH eluents were evaporated under reduced pressure to 1 L at 50 °C, and the resulting concentrate was extracted three times using EtOAc (5 L) and then concentrated to yield a residue (22 g) in the same conditions. The crude extract was resolved by a silica gel (100–200 mesh) column eluted with a stepwise gradient of CHCl3/MeOH mixtures with growing polarity (100:0–50:50, v/v) to obtain three fractions (Fr. 1–3) based on the Thin-Layer Chromatography (TLC) profiles, performed with a solvent system of CHCl3/MeOH (9:1). Fr. 2 showed antifungal activity and was further purified by a Sephadex LH-20 gel column eluted with CHCl3/MeOH (1:1, v/v), giving two fractions Fr. 2-1 and Fr. 2-2, referring to the TLC profiles. Fr. 2-1 showed antifungal activity and was further separated by semi-preparative HPLC eluting with a CH3CN–H2O mixture (48:52, v/v) using a reversed-phase column (Zorbax SB-C18, 5 mm, 250 × 9.4 mm inner diameter) to obtain compound 1 (tR 27.0 min, 12 mg). The eluates were detected by a photodiode array detector at 254 nm with a flow rate of 1.5 mL/min at 25 °C.
Spectroscopic analysis was used to determine the structure of the antifungal compound. 1H and 13C NMR spectra were measured with a Bruker DRX-600 (600 MHz for 1H and 150 MHz for 13C) spectrometer (Bruker, Rheinstetten, Germany). Electrospray ionization (ESI) MS data were obtained using an Agilent G6230 Q-TOF mass instrument (Agilent Corp., Santa Clara, CA, USA).
2.9. Statistical Analysis
The data were analyzed using analysis of variance (ANOVA) followed by Duncan’s multiple-range test (p ≤ 0.05) using statistical software SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). The results were expressed as means ± SD.
3. Results
3.1. Culture-Independent Communities
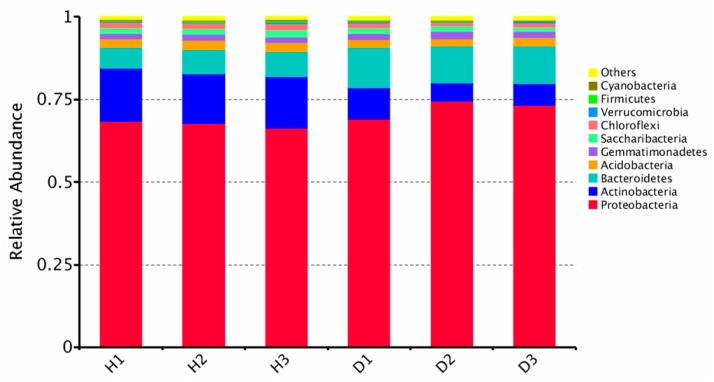
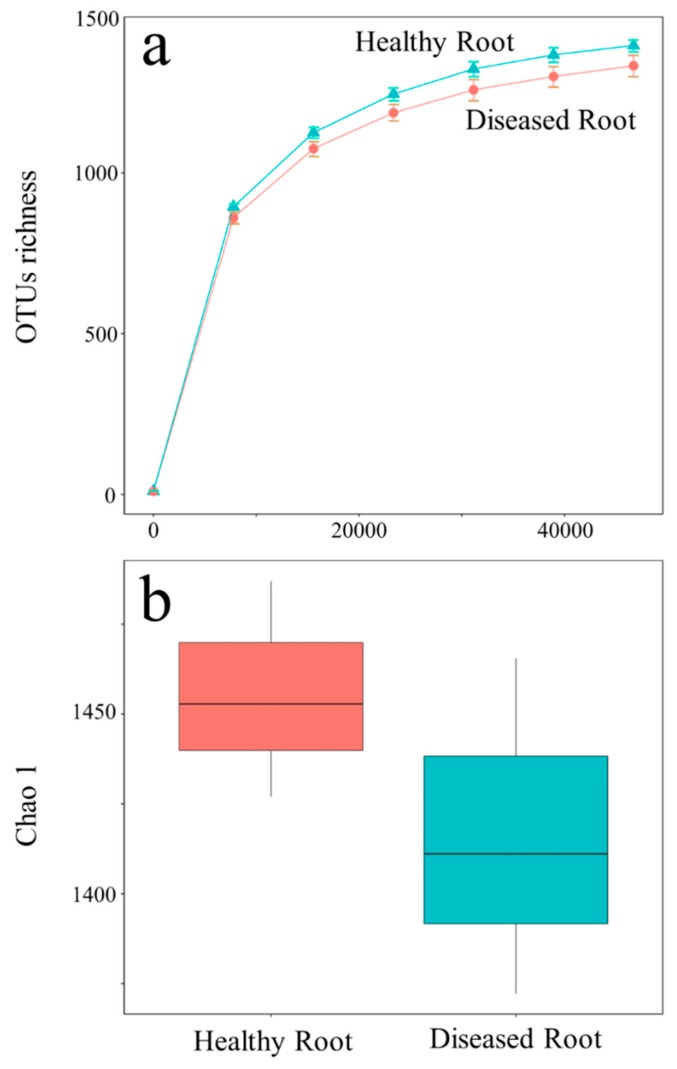
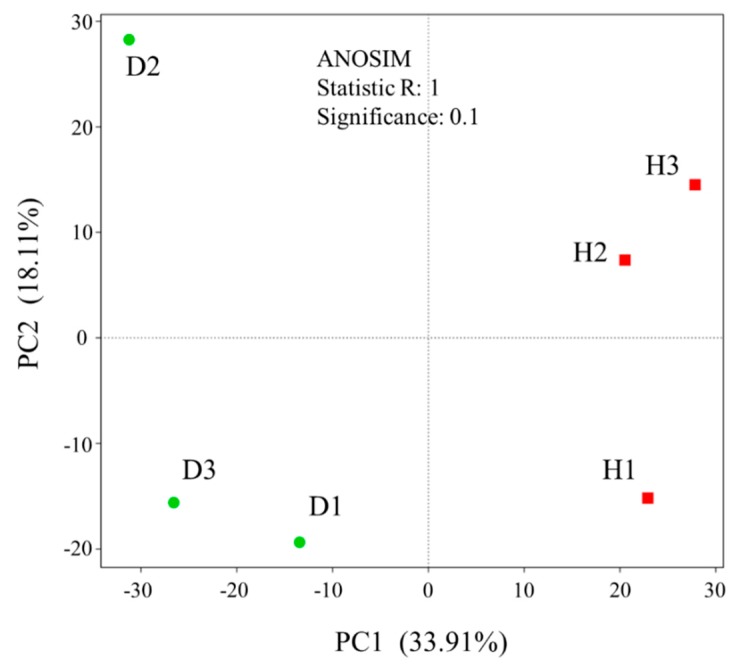
In the current study, a total of 300,923 high-quality reads classified as 8708 OTUs from the microbiome in cucumber root were determined. The raw sequencing reads were deposited to NCBI SRA (National Center for Biotechnology Information Short Read Archive) for this project under accession numbers SRR10589211–SRR10589216. The predominant bacterial phyla of healthy and diseased cucumber roots were all Proteobacteria, Actinobacteria, and Bacteroidetes, but the relative abundance of phylum Actinobacteria in the healthy samples was more significant (p < 0.05) than in diseased samples (Figure 1). Including Streptomyces, the relative abundance of genera in Actinobacteria (top 30) in the healthy samples was greater than in diseased samples (Figure S1, Supplementary Materials), except for Sporichthya (Figure S1, Supplementary Materials). The bacteria α-diversity Chao 1 index of the healthy root was significantly higher than that of the diseased root (Figure 2). Principal component analysis (PCA) suggested a clear difference between the bacterial community (Analysis of similarities (ANOSIM) for bacteria, p = 0.1) of healthy and diseased cucumber roots (Figure 3). The principal component analysis (PCA) explained 34% and 18% of the variation in the bacterial communities.
Figure 1.
Analysis of culture-independent endophytic communities at phylum level in the cucumber roots. H1–3, healthy samples; D1–3, diseased samples.
Figure 2.
The rarefaction curve (a) and Chao 1 (b) α-diversity of the healthy and diseased cucumber roots.
Figure 3.
Principal component analysis (PCA) of bacterial community beta-diversity based on Bray–Curtis dissimilarity among all samples of the healthy and diseased roots.
3.2. Isolation of Endophytic Actinomycetes
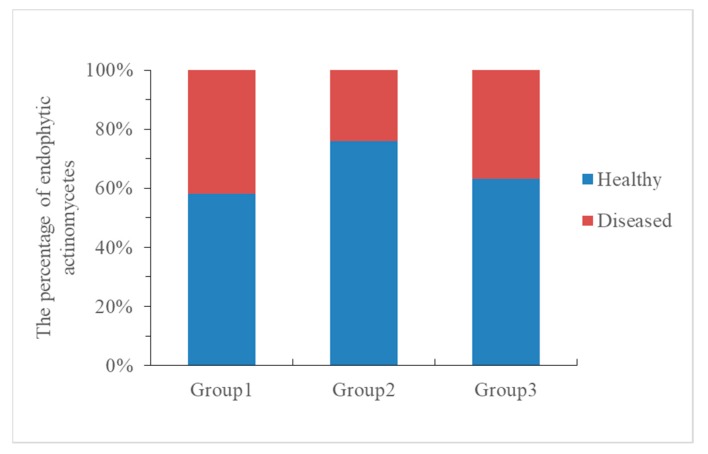
A total of 263 endophytic actinomycetes colonies were isolated from healthy and diseased cucumber roots. Out of this number, 50 (58.1%), 66 (75.8%), and 57 (63.3%) isolates originated from the roots of healthy cucumber in Group 1 (H1-D1), Group 2 (H2-D2), and Group 3 (H3-D3), respectively, whereas 36 (41.9%), 21 (24.2%), and 33 (36.7%) isolates originated from the roots of diseased cucumber in all three groups. The colony-forming units (CFU) per gram of root varied widely among healthy and diseased plants, while also indicating that endophytic actinomycetes in healthy cucumber roots were more significantly (p < 0.05) abundant than in diseased cucumber roots (Figure 4).
Figure 4.
The percentage of endophytic actinomycetes in Group1 (H1-D1), Group2 (H2-D2), and Group3 (H3-D3).
3.3. In Vitro Antagonistic Activity Assays
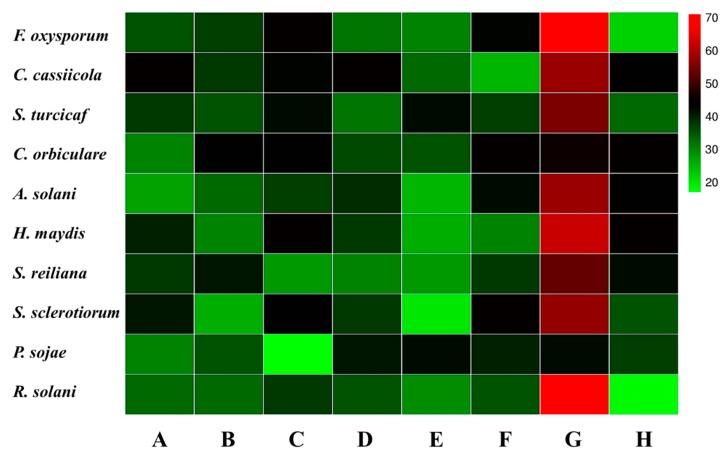
There were eight strains showing antagonism to F. oxysporum f. sp. cucumerinum. Among these eight antagonistic strains, seven strains (HGS1-1, HGS2-18, HGS3-17, HAAG3-4, HAAG3-8, HCPA2-26, and HAAG3-15) were isolated from healthy cucumber roots, while only one strain (DCPA1-15) was isolated from diseased cucumber roots. Strain HAAG3-15 isolated from the root of healthy cucumber showed 71% inhibition of mycelial growth (Figure S2, Supplementary Materials), whereas the other seven strains only exhibited 17% to 45% inhibition. In addition, strain HAAG3-15 also exhibited stronger antifungal activities against other nine pathogenic fungi (Corynespora cassiicola, Setosphaeriaturcica turcicaf, Colletotrichum orbiculare, Alternaria solani, Helminthosporium maydis, Sphacelotheca reiliana, Sclerotinia sclerotiorum, Phytophthora sojae, and Rhizoctonia solani) than other antagonistic strains (Figure 5).
Figure 5.
Eight isolates showed antagonism to Fusarium oxysporum f. sp. cucumerinum and nine other pathogenic fungi. A, HGS1-1; B, HGS2-18; C, HGS3-17; D, HAAG3-4; E, HAAG3-8; F, HCPA2-26; G, HAAG3-15; H, DCPA1-15. The color of the checkerboard represents the inhibition rate of fungal mycelial growth (%) of A–H. The legend on the right side represents the color corresponding to the different inhibition rates.
3.4. Characterization and Identification of the Isolate HAAG3-15
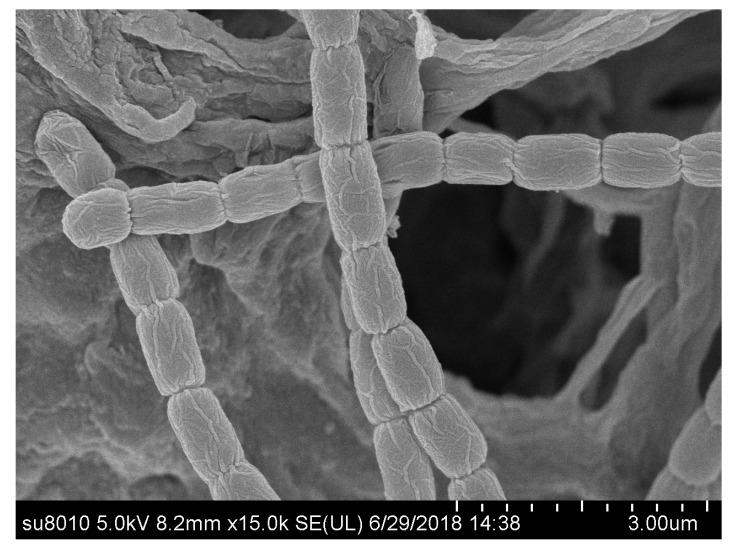
The morphological characteristics of strain HAAG3-15 showed that it belonged to the genus Streptomyces [71]. The strain formed well-developed, branched substrate hyphae and aerial mycelium that differentiated into straight or flexuous spore chains consisting of cylindrical spores (0.55–0.81 μm × 0.75–1.22 μm), and the spore surface was rough (Figure 6) after cultivation for three weeks. The strain exhibited good growth on all tested media. Diffusible pigments were not observed on any of the media used in this study for strain HAAG3-15. The isolate was observed to grow in a pH range of 6.0–8.0 (optimum pH 7.0) and 10–40 °C (optimum 28 °C), as well as in the presence of 0%–2% NaCl (w/v, optimally 0%).
Figure 6.
Scanning electron micrograph of strain HAAG3-15 grown on International Streptomyces Project (ISP) 3 medium for three weeks at 28 °C.
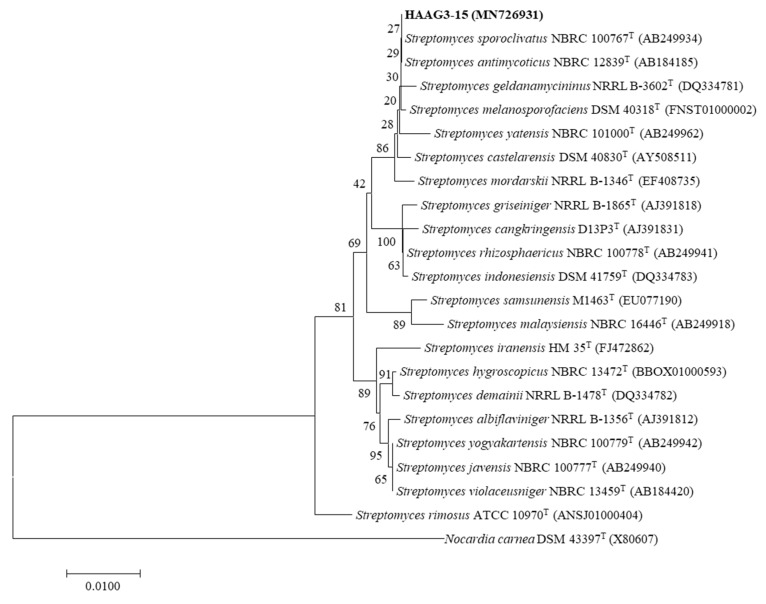
The almost-full length 16S rRNA gene sequence (1519 bp) of strain HAAG3-15 was deposited as MN726931 in the GenBank/EMBL/DDBJ (European Molecular Biology Laboratory/DNA Data Bank of Japan) databases. Based on EzBioCloud analysis, strain HAAG3-15 belongs to the genus Streptomyces and is most closely related to Streptomyces sporoclivatus NBRC 100767T (100% identity) and S. antimycoticus NBRC 12839T (100%). Phylogenetic analysis based on 16S rRNA gene sequences with the neighbor-joining tree suggested that the strain clustered within the genus Streptomyces and formed a stable subclade with S. sporoclivatus NBRC 100767T and S. antimycoticus NBRC 12839T (Figure 7).
Figure 7.
Neighbor-joining phylogenetic tree, based on almost-complete 16S ribosomal RNA (rRNA) gene sequences, showing the phylogenetic relationships of strain HAAG3-15 and the closest strains within the genus Streptomyces. Nocardia carnea DSM43397T was used as the outgroup. Bar, 0.0100 substitutions per nucleotide position.
3.5. Greenhouse Biocontrol Assay of Strain HAAG3-15
In the experiment under greenhouse conditions, the disease index and incidence, shoot fresh weight, and height of cucumber seedlings were measured upon transplanting after four weeks; the average values were calculated, and the results are presenting in Table 1. After two weeks of the inoculation of F. oxysporum f. sp. cucumerinum, some visual external wilt symptoms (yellowing of leaves and stems) of cucumber seedlings were exhibited in both F (inoculated only F. oxysporum f. sp. cucumerinum) and F + S (inoculated both HAAG3-15 and F. oxysporum f. sp. cucumerinum), and the typical symptom of chlorosis emerged and spread from older leaves to younger leaves. With the application of strain HAAG3-15, the treatment F + S could significantly reduce the disease severity of F. oxysporum f. sp. cucumerinum on cucumber seedlings after transplanting at four weeks (Figure 8). For F + S treatment, only 10 (30%) infected plantlets showed typical symptoms of the disease with a disease index of just 12 because of the inoculation of strain HAAG3-15, while 27 (90%) infected plantlets had a disease index of 45 in the F treatment. In addition, the other two treatments N (inoculated no microorganism) and S (inoculated only HAAG3-15) exhibited no symptoms of disease and still stayed healthy (Figure not shown). Moreover, the application of strain HAAG3-15 could also significantly increase the shoot fresh weight and height of cucumber (p < 0.05); the average shoot fresh weight and height of cucumber (4.62 g, 12.55 cm) in treatment S (inoculated only HAAG3-15) were greater than those in treatment N (no microorganism; 4.06 g, 11.76 cm), which clearly indicated that strain HAAG3-15 could promote the growth of cucumber seedlings. The employment of strain HAAG3-15 could also markedly reduce the impact of F. oxysporum f. sp. cucumerinum on cucumber shoot fresh weight and height (p < 0.05). For treatment F (inoculated only F. oxysporum f. sp. cucumerinum), the average shoot fresh weight and height were 3.16 g and 10.32 cm, respectively, which were lower than those for treatment F + S (inoculated both HAAG3-15 and F. oxysporum f. sp. cucumerinum; 3.95 g, 11.58 cm).
Table 1.
Height, shoot fresh weight, disease index (DI), and number of infected plantlets with four treatments in greenhouse biocontrol assay.
| Treatments | Height (cm) | Shoot Fresh Weight (g) | Disease Index | Infected Plantlets |
|---|---|---|---|---|
| F | 10.32 ± 0.52 c | 3.16 ± 0.36 c | 45 ± 3.8 a | 27(90%) |
| F + S | 11.58 ± 0.63 b | 3.95 ± 0.18 b | 12 ± 2.2 b | 10(30%) |
| N | 11.76 ± 0.46 b | 4.06 ± 0.27 b | 0 | 0 |
| S | 12.55 ± 0.32 a | 4.62 ± 0.15 a | 0 | 0 |
Average shoot fresh weight and height of 15 plantlets for each treatment (mean ± SD). Different letters in the same column indicate significant differences (p < 0.05). Cucumber plants grown in soil containing F, the spore suspension of F. oxysporum f. sp. cucumerinum (2 mL of 4–5 × 104 CFU/mL); F + S, the spore suspension of F. oxysporum f. sp. cucumerinum (2 mL of 4–5 × 104 CFU/mL) and the spore suspension of strain HAAG3-15 (2 mL of 4–5 × 106 CFU/mL); N, no microorganism (2 mL of sterile tap water); and S, the spore suspension of HAAG3-15 (2 mL of 4–5 × 106 CFU/mL).
Figure 8.
Biocontrol assay of inoculation with F. oxysporum f. sp. cucumerinum and strain HAAG3-15 on cucumber seedlings in greenhouse. Inoculation with F. oxysporum f. sp. cucumerinum and HAAG3-15 (left, F + S), and only inoculated F. oxysporum f. sp. cucumerinum (right, F).
3.6. Structure Elucidation of the Antifungal Compound
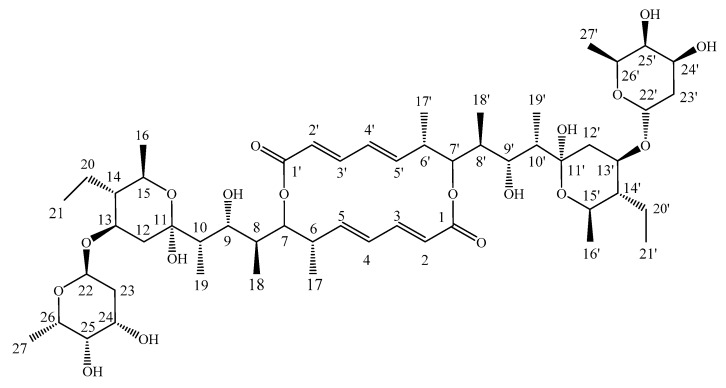
The antifungal compound was separated based on the antifungal activity-guided method from the extraction of fermentation broth of strain HAAG3-15, and compound 1 was obtained as its active constituent. Then, compound 1 was identified as Azalomycin B (Figure 9) based on the spectral data (Figures S3–S5, Supplementary Materials) and literature values [72].
Figure 9.
Structure of Azalomycin B. Structural elucidation of compound 1: Azalomycin B, C54H88O18, white microcrystals. UV (MeOH) λmax 252nm; electrospray ionization (ESI) MS m/z: 1047 [M + Na]+; 1H NMR (600 MHz, MeOD) δH 6.93 (1H, dd, J = 15.2, 11.2 Hz, H-3), 6.17 (1H, dd, J = 15.0, 11.2 Hz, H-4), 5.74 (1H, d, J = 15.4 Hz, H-2), 5.67 (1H, dd, J = 15.1, 9.9 Hz, H-5), 5.04 (2H, m, H-7, H-22), 4.02 (1H, d, J = 9.7 Hz, H-13), 3.94 (2H, m, H-24, H-26), 3.90 (2H, m, H-9, H-15), 3.53 (1H, m, H-25), 2.58 (1H, m, H-6), 2.34 (1H, dd, J = 12.1, 4.5 Hz, H-12), 1.95 (1H, m, H-8), 1.94 (1H, dd, J = 12.3, 3.9 Hz, H-23), 1.72 (1H, m, H-10), 1.66 (1H, m, H-20), 1.62 (1H, dd, J = 12.6, 4.7 Hz, H-23), 1.47 (1H, m, H-20), 1.20 (3H, d, J = 6.6 Hz, H-27), 1.16 (1H, m, H-16), 1.13 (3H, d, J = 6.0 Hz, H-16), 1.12 (1H, m, H-12), 1.05 (3H, d, J = 6.6 Hz, H-17), 0.97 (3H, d, J = 7.1 Hz, H-19), 0.87 (6H, t, J = 6.2 Hz, H-21, H-18). 13C NMR (150 MHz, MeOD) δC 170.4 (C-1), 146.9 (C-3), 146.1 (C-5), 132.7 (C-4), 122.6 (C-2), 100.9 (C-11), 94.8 (C-22), 78.2 (C-7), 72.4 (C-25), 71.8 (C-9), 70.9 (C-13), 68.2 (C-15), 68.1 (C-26), 67.0 (C-24), 49.8 (C-14), 44.0 (C-10), 42.7 (C-6), 38.9 (C-12), 37.8 (C-8), 33.7 (C-23), 20.3 (C-20), 19.5 (C-16), 17.3 (C-27), 15.8 (C-17), 9.6 (C-18), 9.5 (C-21), 7.1 (C-19).
The antifungal activities of compound 1 against F. oxysporum f. sp. Cucumerinum, Corynespora cassiicola, Setosphaeriaturcica turcicaf, Colletotrichum orbiculare, Alternaria solani, Helminthosporium maydis, Sphacelotheca reiliana, Sclerotinia sclerotiorum, Phytophthora sojae, and Rhizoctonia solani were determined in vitro. The compound showed significant antifungal activity against F. oxysporum f. sp. cucumerinum and also exhibited certain antifungal activities against the nine other fungi. Therefore, Azalomycin B was identified as the main antifungal component produced by strain HAAG3-15.
4. Discussion
Microorganisms related to plant roots are vital for plant growth and health, and they are considered to be the second genome of the plant. When plants are attacked by pathogens, the diversity and community structure of plant-associated microbes may be changed [48,49,73]. Here we studied the differences in root-associated endophytic actinobacterial community composition and antifungal activity between Fusarium wilt diseased and healthy cucumber under natural greenhouse field conditions in China using culture-independent and culture-dependent analysis, and we screened actinomycetes for potential biological control of Fusarium wilt of cucumber. Microbiome biodiversity is known as a driver of plant health. Abundant bacterial flora predetermines the future plant health [49,74]. In this study, culture-independent analysis suggested that the healthy cucumber roots had higher actinobacteria richness and abundance than the diseased plants. This result was similar to the previous study showing that tomato plant resistance to infection with Ralstonia solanacearum depended on more abundance and diversity of rhizospheric bacteria than diseased plants [49]. Moreover, the relative abundance of phylum Actinobacteria in the healthy samples was more significant than in diseased samples. It is known that Actinobacteria can produce various metabolites with important potential application in the agriculture, food, and pharmaceutical industries [75,76], such as antibiotics, enzymes, enzyme inhibitors, vitamins, and so on. The reduction of the relative abundance of Actinobacteria may have had a positive effect on the Fusarium oxysporum f. sp. cucumerinum growth as a result of weakened pathogen suppression via antibiosis.
In addition, culture-dependent analysis was also performed using five isolation media to isolate strains from the cucumber roots. In total, 173 endophytic actinomycetes colonies were isolated from the healthy cucumber roots, and 90 endophytic actinomycetes colonies originated from the diseased cucumber roots (nearby the healthy cucumber collected from the cucumber continuous cropping greenhouse), which indicated that the culturable endophytic actinomycetes in the healthy cucumber roots were more abundant than in the diseased cucumber roots (Figure 4). The result was in agreement with the culture-independent analysis. To obtain actinobacteria with potential biological control of Fusarium wilt of cucumber, all strains were selected for testing its antifungal activity against F. oxysporum f. sp. cucumerinum. Results showed that there were seven strains with antifungal activity against F. oxysporum f. sp. cucumerinum in healthy cucumber roots, but only one strain in diseased cucumber roots with weak antifungal activity. In previous studies, several biocontrol and plant growth-promoting endophytes were isolated from infected plants [57,77,78] or healthy plants [79,80,81]. Our results seem to be different from previous observations, and the healthy cucumber root nearby the diseased plant contained more abundant microbes, as well as more actinomycetes with antifungal activities. Among the eight antagonistic strains, isolate HAAG3-15 in healthy cucumber root was found to be best as it had the strongest antifungal activity against F. oxysporum f. sp. cucumerinum and also exhibited broad-spectrum antifungal activity. Moreover, biocontrol of cucumber Fusarium wilt showed that strain HAAG3-15 had an obvious effect in terms of disease prevention and growth promotion on cucumber seedlings in greenhouse assay.
It was reported that biocontrol of the Fusarium wilt pathogens illustrated the use of suppressive soils and antagonistic bacteria to inhibit the propagation of germination and penetration growth by the pathogen [82]. However, a rapid decline in the size of populations of active cells to ineffective levels was achieved following introduction into soil, due to the hostility of the soil environment to incoming microbes [83]. However, endophytes are not subject to competition from soil microbes, and they colonize in the plant tissue. They have the ability to penetrate plant cells, stimulating the plant defense response and producing antifungal metabolites in situ. Strain HAAG3-15 was screened from the cucumber root, belonging to the group of endophytic actinomycetes, which would not affect the structure of the actinomycetes in the root of cucumber. If the endophytic actinomycetes were introduced into the cucumber seedlings at the breeding stage, they would become the principal parts of the microbial flora in the cucumber plant at the time of transplanting and could protect their host plant from F. oxysporum f. sp. cucumerinum. This is a promising prospect for biological control of Fusarium wilt of cucumber. It provides a new method for the prevention and cure of F. oxysporum f. sp. cucumerinum in agricultural production. Therefore, the endophytic strain HAAG3-15 as a biological control agent against Fusarium wilt of cucumber has great potential application in organic agriculture.
In addition, we also screened and identified antifungal components from strain HAAG3-15 and a bioactive compound, Azalomycin B, was obtained. Azalomycin, as a broad-spectrum antibiotic, is widely applicable [84,85,86,87,88]. Previous studies showed that Azalomycin has a good inhibitory effect on Gram-positive bacteria, parasites, and fungi, and the application prospect of Azalomycin in agricultural production was also presented [35,89,90,91]. Azalomycin B isolated in the current study also showed significant antifungal activity against F. oxysporum f. sp. cucumerinum, as also previously reported. Further research is needed to confirm the efficacy of the active compound against cucumber Fusarium wilt under greenhouse conditions.
Above all, the result of the present study indicated that a pathogen-prevalent environment, such as for healthy roots nearby infected plants, is also a good source for isolating biocontrol and plant growth-promoting endophytes, and the endophytic actinomycete strain HAAG3-15 has potential as a biocontrol agent against F. oxysporum f. sp. cucumerinum.
5. Conclusions
In conclusion, healthy cucumber roots had higher actinobacteria richness and abundance than diseased cucumber roots based on culture-independent and culture-dependent analysis. This suggested that the healthy root nearby the infected plant is a good source for isolating biocontrol and plant growth-promoting actinobacteria endophytes. In addition, strain HAAG3-15 showed stronger antifungal activity against F. oxysporum f. sp. cucumerinum than seven other strains, with an obvious effect in terms of disease prevention and growth promotion on cucumber seedlings in the greenhouse assay. Its excellent broad-spectrum antifungal activities suggest that it could be a potential candidate for the development of a potential biocontrol agent against F. oxysporum f. sp. cucumerinum used in organic agriculture.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-2607/8/2/236/s1: Figure S1: Analysis of culture-independent endophytic actinobacteria communities at genus level top 30 in the cucumber roots; Figure S2: Strain HAAG3-15 showed antagonism to F. oxysporum f. sp. Cucumerinum; Figure S3: 1H NMR (600 MHz) spectrum of compound 1 in MeOD; Figure S4: 13C NMR (150 MHz) spectrum of compound 1 in MeOD; Figure S5: ESI-MS spectrum of compound 1.
Author Contributions
P.C. and C.L. performed the culture-dependent and culture-independent community analysis, isolation, characterization, and greenhouse assay of strain HAAG3-15. X.X. performed the antifungal test. H.W. and Z.Y. carried out the structure elucidation of the antifungal compound. X.W. prepared the figures and tables. J.Z. and W.X. designed the experiments and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part by grants from the National Natural Youth Science Foundation of China (No. 31701858), the China Postdoctoral Science Foundation (2018M631907), the Heilongjiang Postdoctoral Fund (LBH-Z17015), the University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2017017), and the “Young Talents” Project of Northeast Agricultural University (17QC14).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Fareed G., Atiq M., Abbas M., Usman M., Abbas G., Hayat K. In Vitro and in vivo Management of Fusarium Wilt of Cucumber (FWC) Through Various Chemicals. Adv. Zool. Bot. 2015;3:169–174. [Google Scholar]
- 2.Whitaker T.W., Davis G.N. Cucurbits: Botany, Cultivation and Utilization. Leonard Hill; London, UK: Leonard Hill; New York, NY, USA: 1962. [Google Scholar]
- 3.Adetula O., Denton L. Performance of vegetative and yield accessions of cucumber (Cucumis sativa L.) Horticultural Society of Nigeria (HORTSON); Proceedings of the 21st Annual Conference; San Francisco, CA, USA. 12–15 October 2003. [Google Scholar]
- 4.Vimala P., Ting C.C., Salbiah H., Ibrahim B., Ismail L. Biomass production and nutrient yields of four green manures and their effects on the yield of cucumber. J. Trop. Agric. Food Sci. 1999;27:47–55. [Google Scholar]
- 5.Lebeda A., Ryder E.J., Grube R., DoleŽalovÁ I., KŘÍstkovÁ E. Lettuce (Asteraceae; Lactuca spp.) In: Singh R.J., editor. Genetic Resources, Chromosome Engineering, and Crop Improvement, Vegetable Crops. Volume 3. CRC Press; Boca Raton, FL, USA: 2007. pp. 377–472. [Google Scholar]
- 6.El-Sharkawy E.E.S., Abdalla M.Y., El-Shemy A.O. Biological Control and Induction of Systemic Resistance against Cucumber Fusarium Wilt by Plant Growth Promoting Rhizo-organisms. Egypt. J. Biol. Pest Control. 2015;25:407–413. [Google Scholar]
- 7.Shi L., Du N., Shu S., Sun J., Li S., Guo S. Paenibacillus polymyxa nsy50 suppresses Fusarium wilt in cucumbers by regulating the rhizospheric microbial community. Sci. Rep. 2017;7:41234. doi: 10.1038/srep41234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J., Yang Q., Zhang S.M., Wang Y.X., Zhao X.Y. Evaluation of biocontrol efficiency and security of a Bacillus subtilis strain B29 against cucumber Fusarium wilt in field. China Veg. 2009;2:30–33. [Google Scholar]
- 9.Shi L., Du N., Yuan Y., Shu S., Sun J., Guo S. Vinegar residue compost as a growth substrate enhances cucumber resistance against the Fusarium wilt pathogen Fusarium oxysporum by regulating physiological and biochemical responses. Environ. Sci. Pollut. Res. Int. 2016;23:1–11. doi: 10.1007/s11356-016-6798-7. [DOI] [PubMed] [Google Scholar]
- 10.Ahn P., Chung H.S., Lee Y.H. Vegetative compatibility groups and pathogenicity among isolates of Fusarium oxysporum f. sp. cucumerinum. Plant Dis. 1998;82:244–246. doi: 10.1094/PDIS.1998.82.2.244. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J., Mei Z., Zhang X., Xue C., Zhang C., Ma T., Zhang S. Suppression of Fusarium wilt of cucumber by ammonia gas fumigation via reduction of Fusarium population in the field. Sci. Rep. 2017;7:43103. doi: 10.1038/srep43103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vakalounakis D.J., Wang Z., Fragkiadakis G.A., Skaracis G.N., Li D.B. Characterization of Fusarium oxysporum isolates obtained from cucumber in China by pathogenicity, VCG, and RAPD. Plant Dis. 2004;88:645–649. doi: 10.1094/PDIS.2004.88.6.645. [DOI] [PubMed] [Google Scholar]
- 13.Kareem T.K., Ugoji O.E., Aboaba O.O. Biocontrol of Fusarium wilt of cucumber with Trichoderma longibrachiatum NGJ167 (Rifai) Br. Microbiol. Res. J. 2016;16:1–11. doi: 10.9734/BMRJ/2016/28208. [DOI] [Google Scholar]
- 14.King S.R., Davis A.R., Liu W., Levi A. Grafting for disease resistance. HortScience. 2008;43:1673–1676. doi: 10.21273/HORTSCI.43.6.1673. [DOI] [Google Scholar]
- 15.Koch E., Becker J.O., Berg G., Hauschild R., Jehle J., Köhl J., Smalla K. Biocontrol of plant diseases is not an unsafe technology. J. Plant Dis. Prot. 2018;125:121–125. doi: 10.1007/s41348-018-0158-4. [DOI] [Google Scholar]
- 16.El-Tarabily K.A., Hardy G.E.S.J., Sivasithamparam K. Performance of three endophytic actinomycetes in relation to plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber under commercial field production conditions in the United Arab Emirates. Eur. J. Plant Pathol. 2010;128:527–539. doi: 10.1007/s10658-010-9689-7. [DOI] [Google Scholar]
- 17.Wang H., Han L., Feng J., Zhang X. Evaluation of two Streptomyces spp. and compost for growth promotion and biocontrol potential against Rhizoctonia solani on pepper. Biocontrol Sci. Technol. 2015;25:852–857. doi: 10.1080/09583157.2015.1015485. [DOI] [Google Scholar]
- 18.Abdallah R.A.B., Jabnoun-Khiareddine H., Nefzi A., Mokni-Tlili S., Daami-Remadi M. Biocontrol of Fusarium Wilt and Growth Promotion of Tomato Plants Using Endophytic Bacteria Isolated from Solanum elaeagnifolium Stems. Biol. Control. 2016;97:80–88. doi: 10.1016/j.biocontrol.2016.03.005. [DOI] [Google Scholar]
- 19.Chen X., Pizzatti C., Bonaldi M., Saracchi M., Erlacher A., Kunova A., Berg G., Cortesi P. Biological control of lettuce drop and host plant colonization by rhizospheric and endophytic Streptomycetes. Front. Microbiol. 2016;7:714. doi: 10.3389/fmicb.2016.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan H., Zhang Z., Li Y., Zhang X., Duan Y., Wang Q. Biocontrol of bacterial fruit blotch by Bacillus subtilis 9407 via surfactin-mediated antibacterial activity and colonization. Front. Microbiol. 2017;8:1973. doi: 10.3389/fmicb.2017.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law W.F., Ser H.L., Khan T.M., Chuah L.H., Pusparajah P., Chan K.G., Goh B.H., Lee L.H. The Potential of Streptomyces as Biocontrol Agents against the Rice Blast Fungus, Magnaporthe oryzae (Pyricularia oryzae) Front. Microbiol. 2017;8:1398. doi: 10.3389/fmicb.2017.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yasmin S., Hafeez F.Y., Mirza M.S., Rasul M., Arshad H.M.I., Zubair M., Iqbal M. Biocontrol of bacterial leaf blight of rice and profiling of secondary metabolites produced by rhizospheric Pseudomonas aeruginosa BRp3. Front. Microbiol. 2017;8:1895. doi: 10.3389/fmicb.2017.01895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ara I., Rizwana H., Al-Othman M.R., Bakir M.A. Studies of actinomycetes for biological control of Colletotrichum musae pathogen during post-harvest anthracnose of banana. Afr. J. Microbiol Res. 2012;6:3879–3886. [Google Scholar]
- 24.Wang S., Liang Y., Shen T., Yang H., Shen B. Biological characteristics of ct205 and its biocontrol potential against cucumber Fusarium wilt. Biocontrol Sci. Technol. 2016;26:1–23. doi: 10.1080/09583157.2016.1172203. [DOI] [Google Scholar]
- 25.Tahvonen R., Avikainen H. The biological control of seed-borne Alternaria brassicicola of cruciferous plants with a powdery preparation of Streptomyces sp. Agric. Food Sci. 1987;59:199–208. doi: 10.23986/afsci.72264. [DOI] [Google Scholar]
- 26.Nur Azura A.B., Yusoff M., Tan G.Y.A., Jegadeesh R., Appleton D.R., Vikineswary S. Streptomyces sanglieri which colonised and enhanced the growth of Elaeis guineensis jacq. seedlings was antagonistic to Ganoderma boninense in in vitro studies. J. Ind. Microbiol. Biotechnol. 2016;43:485–493. doi: 10.1007/s10295-015-1724-4. [DOI] [PubMed] [Google Scholar]
- 27.Jog R., Pandya M., Nareshkumar G., Rajkumar S. Mechanism of phosphate solubilization and antifungal activity of Streptomyces spp. isolated from wheat roots and rhizosphere and their application in improving plant growth. Microbiology. 2014;160:778–788. doi: 10.1099/mic.0.074146-0. [DOI] [PubMed] [Google Scholar]
- 28.Ueno M., Quyet N.T., Shinzato N., Matsui T. Antifungal activity of collected in subtropical region, Okinawa, against Magnaporthe oryzae. Trop. Agric. Dev. 2016;60:48–52. [Google Scholar]
- 29.Hallmann J., Quadt-Hallmann A., Mahaffee W.F., Kloepper J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997;43:895–914. doi: 10.1139/m97-131. [DOI] [Google Scholar]
- 30.Eljounaidi K., Lee S.K., Bae H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases-review and future prospects. Biol. Control. 2016;103:62–68. doi: 10.1016/j.biocontrol.2016.07.013. [DOI] [Google Scholar]
- 31.Park M.S., Jung S.R., Lee M.S., Kim K.O., Do J.O., Lee K.H., Kim S.B., Bae K.S. Isolation and characterization of bacteria associated with two sand dune plant species, Calystegia soldanella and Elymus mollis. J. Microbiol. 2005;43:219–227. [PubMed] [Google Scholar]
- 32.Rosenblueth M., Martínez-Romero E. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 2006;19:827–837. doi: 10.1094/MPMI-19-0827. [DOI] [PubMed] [Google Scholar]
- 33.Tian X., Cao L., Tan H., Han W., Chen M., Liu Y., Zhou S. Diversity of cultivated and uncultivated actinobacterial endophytes in the stems and roots of rice. Microb. Ecol. 2007;53:700–707. doi: 10.1007/s00248-006-9163-4. [DOI] [PubMed] [Google Scholar]
- 34.Lee S.O., Choi G.J., Choi Y.H., Jang K.S., Park D.J., Kim C.J., Kim J.C. Isolation and characterization of endophytic actinomycetes from Chinese cabbage roots as antagonists to Plasmodiophora brassicae. J. Microbiol. Biotechnol. 2008;18:1741–1746. doi: 10.4014/jmb.0800.108. [DOI] [PubMed] [Google Scholar]
- 35.Yuan G., Lin H., Wang C., Hong K., Liu Y., Li J. 1H and 13C assignments of two new macrocyclic lactones isolated from Streptomyces sp. 211726 and revised assignments of Azalomycins F3a, F4a and F5a. Magn. Reson. Chem. 2011;49:30–37. doi: 10.1002/mrc.2697. [DOI] [PubMed] [Google Scholar]
- 36.Verma V.C., Gond S.K., Kumar A., Mishra A., Kharwar R.N., Gange A.C. Endophytic actinomycetes from Azadirachta indica A. Juss.: Isolation, diversity, and anti-microbial activity. Microb. Ecol. 2009;57:749–756. doi: 10.1007/s00248-008-9450-3. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y., Lu C., Qian X., Huang Y., Shen Y. Diversities within genotypes, bioactivity and biosynthetic genes of endophytic actinomycetes isolated from three pharmaceutical plants. Curr. Microbiol. 2009;59:475–482. doi: 10.1007/s00284-009-9463-2. [DOI] [PubMed] [Google Scholar]
- 38.Marquez-Santacruz H.A., Hernandez-Leon R., Orozco-Mosqueda M.C., Velazquez-Sepulveda I., Santoyo G. Diversity of bacterial endophytes in roots of Mexican husk tomato plants (Physalis ixocarpa) and their detection in the rhizosphere. Genet. Mol. Res. 2010;9:2372–2380. doi: 10.4238/vol9-4gmr921. [DOI] [PubMed] [Google Scholar]
- 39.Zhao K., Penttinen P., Guan T., Xiao J., Chen Q., Xu J., Lindstrom K., Zhang L., Zhang X., Strobel G.A. The diversity and anti-microbial activity of endophytic actinomycetes isolated from medicinal plants in Panxi plateau, China. Curr. Microbiol. 2011;62:182–190. doi: 10.1007/s00284-010-9685-3. [DOI] [PubMed] [Google Scholar]
- 40.Golinska P., Wypij M., Agarkar G., Rathod D., Dahm H., Rai M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie Leeuwenhoek. 2015;108:267–289. doi: 10.1007/s10482-015-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruasuwan W., Thamchaipenet A. Diversity of culturable plant growth-promoting bacterial endophytes associated with sugarcane roots and their effect of growth by co-inoculation of diazotrophs and actinomycetes. J. Plant Growth Regul. 2016;35:1–14. doi: 10.1007/s00344-016-9604-3. [DOI] [Google Scholar]
- 42.Matsumoto A., Takahashi Y. Endophytic actinomycetes: Promising source of novel bioactive compounds. J. Antibiot. 2017;70:514–519. doi: 10.1038/ja.2017.20. [DOI] [PubMed] [Google Scholar]
- 43.Cao L.X., Qiu Z.Q., You J.L., Tan H.M., Zhou S. Isolation and characterization of endophytic Streptomycete antagonists of Fusarium wilt pathogen from surface-sterilized banana roots. FEMS Microbiol. Lett. 2005;247:147–152. doi: 10.1016/j.femsle.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Misk A., Franco C.M.M. Biocontrol of chickpea root rot using endophytic actinobacteria. BioControl. 2011;56:811–822. doi: 10.1007/s10526-011-9352-z. [DOI] [Google Scholar]
- 45.Sziderics A., Rasche F., Trognitz F., Sessitsch A., Wilhelm E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.) Can. J. Microbiol. 2007;53:1195–1202. doi: 10.1139/W07-082. [DOI] [PubMed] [Google Scholar]
- 46.Qin S., Zhang Y.J., Yuan B., Xu P.Y., Xing K., Wang J., Jiang J.H. Isolation of ACC deaminase-producing habitat-adapted symbiotic bacteria associated with halophyte Limonium sinense (Girard) Kuntze and evaluating their plant growth-promoting activity under salt stress. Plant Soil. 2014;374:753–766. doi: 10.1007/s11104-013-1918-3. [DOI] [Google Scholar]
- 47.Card S., Johnson L., Teasdale S., Caradus J. Deciphering endophyte behaviour: The link between endophyte biology and efficacious biological control agents. FEMS Microbiol. Ecol. 2016;92:114. doi: 10.1093/femsec/fiw114. [DOI] [PubMed] [Google Scholar]
- 48.Berendsen R.L., Pieterse C.M., Bakker P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Wei Z., Hu J., Gu Y.A., Yin S.X., Xu Y.C., Jousset A., Shen Q.R., Friman V.P. Ralstonia solanacearum pathogen disrupts bacterial rhizosphere microbiome during an invasion. Soil Biol. Biochem. 2018;118:8–17. doi: 10.1016/j.soilbio.2017.11.012. [DOI] [Google Scholar]
- 50.Schneider R. Effects of nonpathogenic strains of Fusarium oxysporum on celery root infection by F. oxysporum f. sp. apii and a novel use of the Lineweaver-Burk double reciprocal plot technique. Phytopathology. 1984;74:646–653. doi: 10.1094/Phyto-74-646. [DOI] [Google Scholar]
- 51.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011;17:10–12. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 52.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucl. Acids Res. 2013;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edgar R.C., Haas B.J., Clemente J.C., Quince C., Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haas B.J., Gevers D., Earl A.M., Feldgarden M., Ward D.V., Giannoukos G., Ciulla D., Tabbaa D., Highlander S.K., Sodergren E., et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edgar R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 56.Edgar R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao P., Liu C.X., Sun P.Y., Fu X.P., Wang S.X., Wu F.Z., Wang X.J. An endophytic Streptomyces, sp. strain DHV3-2 from diseased root as a potential biocontrol agent against Verticillium dahliae, and growth elicitor in tomato (Solanum lycopersicum) Antonie Leeuwenhoek. 2016;109:1573–1582. doi: 10.1007/s10482-016-0758-6. [DOI] [PubMed] [Google Scholar]
- 58.Hayakawa M., Nonomura H. Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J. Ferment. Technol. 1987;65:501–509. doi: 10.1016/0385-6380(87)90108-7. [DOI] [Google Scholar]
- 59.Atlas R.M. Handbook of microbiological media. In: Parks L.C., editor. Microbiology. CRC Press; Boca Raton, FL, USA: 1993. [Google Scholar]
- 60.Shirling E.T., Gottlieb D. Methods for characterization of Streptomyces species. Int. J. Syst. Evol. Microbiol. 1966;16:313–340. doi: 10.1099/00207713-16-3-313. [DOI] [Google Scholar]
- 61.Shomura T., Yoshida J., Amano S., Kojima M., Inouye S., Niida T. Studies on Actinomycetales producing antibiotics only on agar culture. I. Screening, taxonomy and morphology-productivity relationship of Streptomyces halstedii, strain SF-1993. J. Antibiot. 1979;32:427–435. doi: 10.7164/antibiotics.32.427. [DOI] [PubMed] [Google Scholar]
- 62.Riungu G.M., Muthomi J.W., Narla R.D., Wagacha J.M., Gathumbi J.K. Management of Fusarium head blight of wheat and deoxynivalenol accumulation using antagonistic microorganisms. Plant Pathol. J. 2008;7:13–19. [Google Scholar]
- 63.Jin L.Y., Zhao Y., Song W., Duan L.P., Jiang S.W., Wang X.J., Zhao J.W., Xiang W.S. Streptomyces inhibens sp. nov., a novel actinomycete isolated from rhizosphere soil of wheat (Triticum aestivum L.) Int. J. Syst. Evol. Microbiol. 2019;69:688–695. doi: 10.1099/ijsem.0.003204. [DOI] [PubMed] [Google Scholar]
- 64.Kim S.B., Brown R., Oldfield C., Gilbert S.C., Goodfellow M. Gordonia amicalis sp. nov., a novel benzothiophene-desulphurizing actinomycete. Int. J. Syst. Bacteriol. 1999;49:1845–1851. doi: 10.1099/00207713-49-4-1845. [DOI] [PubMed] [Google Scholar]
- 65.Yoon S.H., Ha S.M., Kwon S., Lim J., Kim Y. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Boil. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 67.Kumar S., Stecher G., Tamura K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 69.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 70.Fang Z.D. Research Methodology for Plant Diseases. 3rd ed. Chinese Agriculture Press; Beijing, China: 1998. [Google Scholar]
- 71.Waksman S.A., Henrici A.T. The nomenclature and classification of the actinomycetes. J. Bacteriol. 1943;46:337–341. doi: 10.1128/JB.46.4.337-341.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakakoshi M., Kimura K.I., Nakajima N., Yoshihama M., Uramoto M. SNA-4606-1, a new member of elaiophylins with enzyme inhibition activity against testosterone 5 α-reductase. J. Antibiot. 2010;30:175–177. doi: 10.7164/antibiotics.52.175. [DOI] [PubMed] [Google Scholar]
- 73.Bulgarelli D., Schlaeppi K., Spaepen S., Themaat E.V.L.V., Schulze-Lefert P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013;64:807. doi: 10.1146/annurev-arplant-050312-120106. [DOI] [PubMed] [Google Scholar]
- 74.Muhammad S., Hu J., Alexandre J. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019;50:145–168. [Google Scholar]
- 75.Labeda D.P., Goodfellow M., Brown R., Ward A.C., Lanoot B. Phylogenetic study of the species within the family Streptomycetaceae. Antonie Leeuwenhoek. 2012;101:73–104. doi: 10.1007/s10482-011-9656-0. [DOI] [PubMed] [Google Scholar]
- 76.Bérdy J. Bioactive microbial metabolites, a personal view. J. Antibiot. 2005;58:1–26. doi: 10.1038/ja.2005.1. [DOI] [PubMed] [Google Scholar]
- 77.Passari A.K., Mishra V.K., Saikia R., Gupta V.K., Singh B.P. Isolation, abundance and phylogenetic affiliation of endophytic actinomycetes associated with medicinal plants and screening for their in vitro antimicrobial biosynthetic potential. Front. Microbiol. 2015;6:273. doi: 10.3389/fmicb.2015.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faheem M., Raza W., Zhong W., Nan Z., Shen Q., Xu Y. Evaluation of the biocontrol potential of Streptomyces goshikiensis YCXU against Fusarium oxysporum, f. sp. niveum. Biol. Control. 2015;81:101–110. doi: 10.1016/j.biocontrol.2014.11.012. [DOI] [Google Scholar]
- 79.Rybakova D., Cernava T., Köberl M., Liebminger S., Etemadi M., Berg G. Endophytes-assisted biocontrol: Novel insights in ecology and the mode of action of Paenibacillus. Plant Soil. 2016;405:125–140. doi: 10.1007/s11104-015-2526-1. [DOI] [Google Scholar]
- 80.Yang W., Xu Q., Liu H.X., Wang Y.P., Wang Y.M., Yang H.T., Guo J.H. Evaluation of biological control agents against Ralstonia wilt on ginger. Biol. Control. 2012;62:144–151. doi: 10.1016/j.biocontrol.2012.05.001. [DOI] [Google Scholar]
- 81.Maldonado-Gonza’lez M.M., Bakker P.A., Prieto P., Mercado-Blanco J. Arabidopsis thaliana as a tool to identify traits involved in Verticillium dahliae biocontrol by the olive root endophyte Pseudomonas fluorescens PICF7. Front. Microbiol. 2015;6:266. doi: 10.3389/fmicb.2015.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cook R.J., Baker K.F. The Nature and Practice of Biological Control of Plant Pathogens. American Phytopathological Society; Austin, TX, USA: 1983. pp. 233–282. [Google Scholar]
- 83.Veen J.A., Overbeek L.S., Elsas J.D. Fate and activity of microorganisms introduced into soil. Microbiol. Mol. Biol. Rev. 1997;61:121–135. doi: 10.1128/.61.2.121-135.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arcamone F., PEREGO M. Isolation and characteristics of a new antibiotic: Etruscomycin. Ann. Chim. 1959;49:345–351. [Google Scholar]
- 85.Arai M. Azalomycins B and F, two new antibiotics. II. Properties of azalomycins B and F. J. Antibiot. 1960;13:51. [PubMed] [Google Scholar]
- 86.Kiriakov A., Khlebarova M., Staneva-Stoicheva D., Panova I. The effect of prolonged treatment hypertensive rats with antihypertensive agents with different mechanisms of action on blood pressure and noradrenaline concentration in the myocardium, brain and aorta. Eksp. Med. Morfol. 1972;12:135–141. [PubMed] [Google Scholar]
- 87.Chandra A. Azalomycin F complex from Streptomyces hygroscopicus, MSU/MN-4-75B. J. Antibiot. 1995;48:896–898. doi: 10.7164/antibiotics.48.896. [DOI] [PubMed] [Google Scholar]
- 88.Xu D.B., Ye W.W., Han Y., Deng Z.X., Hong K. Natural products from mangrove actinomycetes. Mar. Drugs. 2014;12:2590–2613. doi: 10.3390/md12052590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sugawara S. Effect of Azalomycin F on Bacteria. J. Antibiot. 1968;21:83–84. doi: 10.7164/antibiotics.21.83. [DOI] [PubMed] [Google Scholar]
- 90.Lee S.Y., MI-Soon K.I.M., Hang-Sub K.I.M., Young-Ho K.I.M., Soon-Duck H.O.N.G., Jung-Joon L.E.E. Structure determination and biological activities of elaiophylin produced by Streptomyces sp. MCY-846. J. Microbiol. Biotechnol. 1996;6:245–249. [Google Scholar]
- 91.Subramanian K.S., Muniraj I., Uthandi S. Plant Growth Promoting Actinobacteria. Springer; Singapore: 2016. Role of Actinomycete-Mediated Nanosystem in Agriculture; pp. 233–247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.