Abstract
One-bead-one-compound (OBOC) libraries constructed by solid-phase split-and-pool synthesis are a valuable source of protein ligands. Most OBOC libraries are comprised of oligoamides, particularly peptides, peptoids, and peptoid-inspired molecules. Further diversification of the chemical space covered by OBOC libraries is desirable. Toward this end, we report here the efficient proline-catalyzed asymmetric Mannich reaction between immobilized aldehydes and soluble ketones and anilines. The reaction conditions do not compromise the amplification of DNA by the PCR. Thus, this chemistry will likely be useful for the construction of novel DNA-encoded libraries by solid-phase synthesis.
Keywords: Mannich reaction, solid-phase synthesis, organocatalysis, one-bead-one-compound libraries, DNA-encoded libraries
Graphical Abstract
INTRODUCTION
An important first step in many probe/drug discovery campaigns is a high throughput screen against a target of interest. Traditional high-throughput screening (HTS) is expensive and requires elaborate infrastructure. Thus, there is continuing interest in the development of faster and cheaper ways to screen large numbers of compounds. For example, Houghten and colleagues have demonstrated the power of positional scanning libraries1–3 for this purpose. Another popular format has been one bead one compound (OBOC) libraries created by solid-phase split and pool synthesis.4 These libraries can be mined for protein ligands using binding assays with labeled proteins or even cells.5–16 While the identification of protein ligands in OBOC libraries was slowed by nagging technical problems, most of these issues have now been solved.17–20
In most OBOC screening efforts, the structures of the molecules on beads scored as hits had to be characterized de novo, usually by tandem mass spectrometry or Edman sequencing5,21,22 after cleavage of the compound from the bead. This limited OBOC libraries to mostly oligoamides, such as peptides,4 peptoids,5,23 and peptoid-like conformationally constrained species.24–29 This limitation can be circumvented by encoding strategies,30 such as Lam’s one bead two compound strategy, in which the synthetic history of a molecule displayed on the bead surface is encoded by a peptide or peptoid created in the protein-inaccessible interior of the bead.31 More recently, DNA-encoding technology32 has been applied to OBOC libraries,33 which has the advantage of allowing much smaller beads, such as 10 μm TentaGel microspheres, to be employed for library construction. This is because the encoding tags on the beads scored as hits are amplified by PCR prior to sequencing. Thus, very little material is needed. Ten μm beads pass readily through a flow cytometer,34 vastly increasing the number of beads that can be analyzed and thus the size of the libraries that can be employed. This advance has highlighted the need to expand the chemical diversity of DNA-encoded OBOC libraries beyond oligoamides and related molecules. We are particularly interested in exploring solid-phase reactions that produce conformationally restricted chiral centers, since there is general agreement that molecules with more “three-dimensionality” will be more selective ligands than the highly aromatic, hydrophobic species that dominate many typical screening collections.35–38
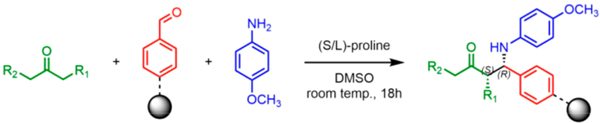
Of course, a central issue in the development of new reaction schemes in this format is compatibility with the encoding DNA. Enough DNA on each bead must remain amplifiable at the end of the library synthesis to allow facile identification of hits. Therefore, we were attracted to organocatalytic methods,39–41 which generally proceed under mild conditions. In this Research Article, we focus on the proline-catalyzed Mannich reaction, pioneered by List and co-workers.42–44 While Mannich reactions have been used for solid-phase synthesis previously,45,46 these efforts did not employ organocatalysis or produce optically enriched products. We demonstrate that addition of a variety of soluble ketones and anilines with a resin-bound aldehyde proceeds with good yield and stereo-selectivity in many cases. The process is shown to have little effect on the amplifiability of DNA using a quantitative PCR assay47 and thus will likely be of utility for the construction of DNA-encoded OBOC libraries.
RESULTS AND DISCUSSION
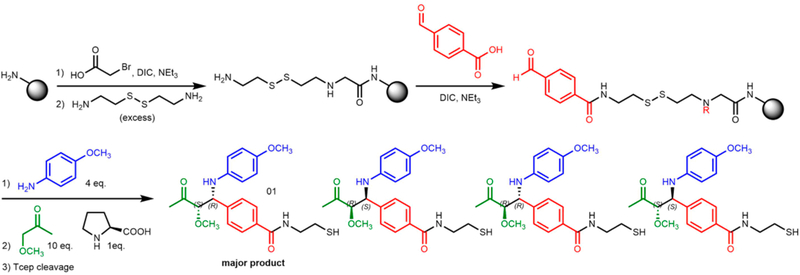
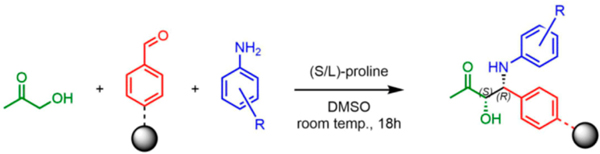
TentaGel microspheres (90 μm; 0.29 mmol loading per gram) were modified with a disulfide-containing linker (Figure 1) to allow the release of compounds from the solid support under reductive conditions (treatment with TCEP)48 to avoid potential racemization of the Mannich product under the usual acidic cleavage conditions. The amino groups in the linker were acylated with 4-formylbenzoic acid using Oxyma coupling conditions49 (Figure 1). The aldehyde-displaying resin (50 mg) was incubated for 5 min at room temperature with solution of 4-methoxyaniline (7 mg; 4 equiv) in 145 μL of DMSO, then a presonicated solution of (S)-proline catalyst (1.67 mg, 1 equiv) and 1-methoxypropan-2-one (13.5 L; 10 equiv) in 145 μL of DMSO was added to reaction vessel without removal of the excess aniline. After it was incubated for 18 h at room temperature with gentle shaking, the beads were washed thoroughly to remove excess reactants. The disulfide linkage was reduced using TCEP (16.63 mg, 4 equiv) and NaHCO3 (19.49 mg; 16 equiv) in 290 μL H2O). The organic products were extracted with dichloromethane, dried with MgSO4, and analyzed by liquid chromatography—mass spectrometry (LC-MS). Two peaks having the mass of the expected Mannich products were observed (Supporting Figure 4a), suggesting that they are either diastereomers or regioisomers. To distinguish between these possibilities, each compound was purified by HPLC and analyzed by 1H-NMR spectroscopy (Supporting Figure 4). These spectra indicated that the only regioisomer produced is the one in which the methoxy-substituted carbon acts as the nucleophile. Furthermore, the NMR spectra argued that the syn isomer is the major product, having the smaller 3.0 Hz vicinal coupling constant at chiral centers, instead of 5.6 Hz for the anti isomer. This result is analogous to that obtained in the solution-phase Mannich reaction using similar sub-strates.42,43 The order of elution of the anti and syn isomers was also consistent with the order of elution of the known anti and syn isomers produced in the solution phase reaction, (Supporting Figures 4b and 3b). Assuming the diastereomers have similar molar extinction coefficients at 254 nm, the diastereomeric ratio (d.r.) is 79:21 (syn:anti). Chiral LC analysis of the cleaved mixtures from Mannich reactions catalyzed by (R)-proline and (S)-proline indicated that the enantiomer excess (e.e.) for the syn diastereomer is 81%, while the value for the antidiastereomer is 56% (Supporting Figure 4b).
Figure 1.
Mannich reaction on TentaGel microsphere, with disulfide-containing linker for cleavage using TCEP. Note that the addition of 4-formylbenzoic acid and subsequent Mannich couplings likely occurs on the secondary amine in the linker as well.48 Since this material is never released from the bead and analyzed, it is abbreviated as “R”.
The absolute configuration of the two enantiomers comprising the syn diastereomer was assigned by similar order of elution in Chiral LC, in comparison to the analogous solution phase reaction (Supporting Figure 3b) where the product has been characterized by X-ray crystallography:43 (S, R) for the major enantiomer, (R, S) for the minor enantiomer. As the reaction was performed on solid phase in small scale, a metric was devised to relatively quantify reaction yield: conversion—the ratio of products’ peak area over total peak area of product and starting material at 254 nm. The conversion for the reaction is 83%.
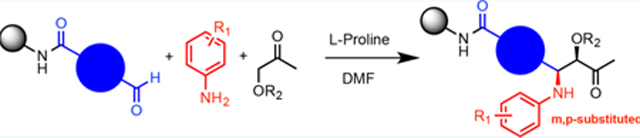
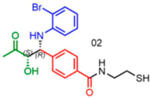
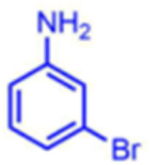
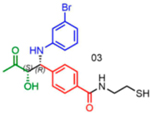
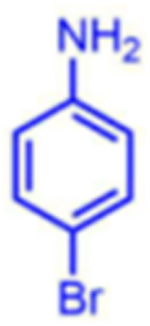
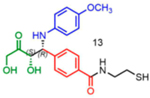
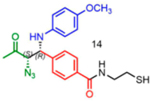
Attempts to improve conversion and stereoselectivity of the reaction by modifying the solvent and temperature were unsuccessful. Hence, the conditions described above were used to explore the scope of the reaction with respect to the aniline and ketone components. We focused on anilines that provide an additional handle for further elaboration of the products since we are interested in the solid-phase synthesis of novel, conformationally constrained oligomers.29 Using 1-hydroxy-propan-2-one as the nucleophile, we found that meta- and para-halogenated anilines work well, proceeding with high conversion and good stereoselectivity (Table 1). Excellent conversion was also observed with 4-Fmoc-protected amino-and aminomethyl-substituted anilines. Unfortunately, the latter reaction provided only a 54% e.e. The e.e. of the product produced from 4-Fmoc-protected amino-aniline could not be determined because the enantiomers failed to separate on the chiral column. Ortho-substituted anilines did not yield the desired Mannich products in high yield, perhaps because of steric hindrance. Benzylamine was also investigated as a possible substitute for aniline. The conversion was an acceptable 66%, but the diastereomeric ratio was poor, being close to 1:1 (Table 1). This may be acceptable for library preparation, where mixtures of stereoisomers can be tolerated, but it would be important to have a more selective method for hit resynthesis to validate which of the stereoisomers is the active compound in a screening experiment.
Table 1.
Proline-Catalyzed Mannich Reaction of Various Anilines with 1-Hydroxypropan-2-one and Immobilized Aldehydea
 | ||||
|---|---|---|---|---|
| Aniline | Product | Conversion (%) | dr | ee (%) |
 |
 |
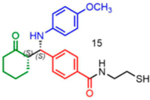
11 | 23:77 | 51 |
 |
 |
84 | 05:95 | 96 |
 |
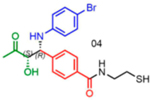 |
76 | 21:79 | 86 |
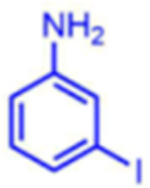 |
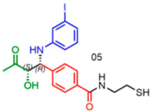 |
86 | 23:77 | 84 |
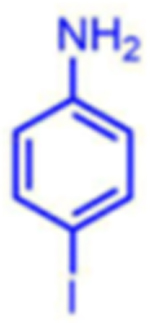 |
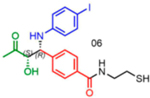 |
92 | 08:92 | 92 |
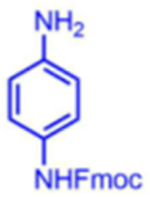 |
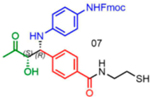 |
99 | 28:72 | nd1 |
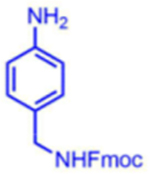 |
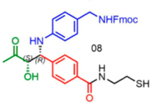 |
96 | 07:93 | 54* |
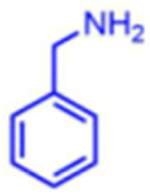 |
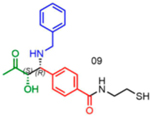 |
66 | 56:44 | nd1 |
nd: Not determined. Superscript 1: The enantiomers were not resolvable by chiral LC. Asterisk (*): Determined from analysis of derived molecules, after Fmoc deprotection and acylation with Fmoc-Phe-OH (Supporting Figure 21a).
The scope of the reaction with respect to the ketone component is described in Table 2. Ketones with a hydroxyl or alkoxy substituent on the nucleophilic carbon were the best substrates. Simple ketones coupled more sluggishly. Only about 30% of the starting aldehyde was converted to products when acetone was employed in the reaction, though the enantiose-lectivity was good. Chloroacetone gave a better conversion (53%), with good stereoselectivity.
Table 2.
Proline-Catalyzed Mannich Reaction of Various Ketones with 4-Methoxyaniline and Immobilized Aldehydea
 | ||||
|---|---|---|---|---|
| Aniline | Product | Conversion (%) | dr | ee (%) |
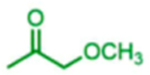 |
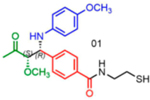 |
83 | 21:79 | 81 |
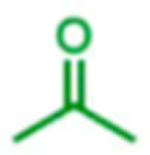 |
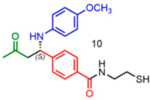 |
31 | Na | 99 |
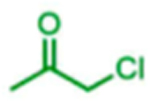 |
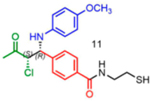 |
53 | 32:68 | nd1 |
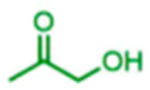 |
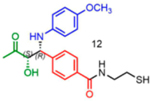 |
62 | 19:81 | 65 |
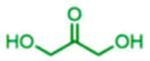 |
 |
19 | 29:71 | nd2 |
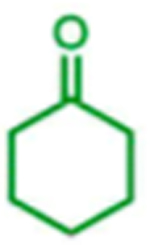
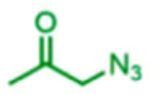 |
 |
trace | nd2 | nd2 |
 |
 |
trace | nd2 | nd2 |
na: Not applicable. nd: not determined. Superscript 1: The enantiomers were not resolvable by chiral LC. Superscript 2: Low conversion.
Given the mild conditions of the Mannich reaction, we anticipated it would be compatible with DNA encoding tags. To test this, we used an assay described by Malone and Paegel, in which bead-displayed duplex DNA is subjected to the reaction conditions and the amount of DNA that remains amplifiable is determined by quantitative PCR.47 We found that the in the presence of the reactants used in Figure 1, 87% of DNA remained amplifiable relative to a control sample not subjected to any chemistry (Supporting Figure 23 and Table 6). This chemistry will therefore be of utility in the synthesis of DNA-encoded OBOC libraries.
Finally, while we employed a disulfide linker in these studies to eliminate any chance of racemization of the chiral center upon release of the product from the bead, subsequent investigations of the stereochemical integrity of the Mannich products demonstrated that this concern was unfounded. Exposure of the Mannich products to the conditions used to free compounds from RAM resins (dilute trifluoroacetic acid for 15 min) did not result in racemization of the product. Similarly, the standard conditions used to remove the Fmoc group also did not racemize the stereocenters (data not shown).
In conclusion, the proline-catalyzed asymmetric Mannich reaction42,43 has been adapted to solid-phase synthesis. A variety of meta- and para-substituted anilines work well in a reaction employing an immobilized benzaldehyde and soluble hydroxyacetone (Table 1). The syn product is favored with the diastereoisomeric ratio ranging from approximately 20:1 in the best case to 3:1 in the worst. The enantiomeric excess of the syn product varies from 96% to 54%. The scope of the reaction with respect to the ketone is somewhat limited. Only 1-hydroxypropan-2-one and 1-methoxypropan-2-one provided a high yield of Mannich products under these conditions, suggesting that a hydroxy or alkoxy substituent is required at the nucleophilic carbon. Importantly, the reaction conditions are tolerant of DNA, so this chemistry should be useful in the preparation of novel DNA-encoded OBOC libraries.33
EXPERIMENTAL PROCEDURES
Linker Synthesis and General Solid-Phase Mannich Reaction Protocol.
TentaGel S NH2 resin (0.29 mmol per g loading) was used as the solid support. To 50 mg of DMF-equilibrated resin was added 217 μL of 2 M bromoacetic acid (BAA) (60 mg, 30 equiv) in DMF and 217 μL of 2 M DIC (68 μL, 30 equiv) in DMF (149.4 μL). After vortexing briefly (∼2 s), the mixture was shaken gently for 5 min at 37 °C. The supernatant was separated from resin by applying vacuum. The resin was then washed three times with DMF, three times with MeOH, and re-equilibrated in MeOH. 435 μL of 1 M cystamine (97.96 mg, 30 equiv) in 2/1 v/v MeOH/DIEPA was then added to the resin, which was then incubated for 1 h at 37 °C with gentle shaking. The resin was pelleted by applying vacuum to remove supernatant, then washed three times with MeOH, three times with DMF, and re-equilibrated in DMF.
To immobilize the aldehyde, 290 μL of 0.2 M 4-formylbenzoic acid (8.71 mg), 0.2 M Oxyma (8.24 mg), 0.2 M DIC (9.1 μL) in DMF (280 L) (4 eq. each compared to resin loading) were preincubated for 5 min at room temperature. The solution was then added to the resin and the beads were incubated for 1 h at room temperature with gentle shaking. After pelleting the beads, the supernatant was removed and the beads were washed three times with DMF, three times with DMSO and then equilibrated in DMSO.
The Mannich reaction was conducted by first adding 145 μL of 0.4 M aniline (4 equiv) in DMSO to the beads and incubating at room temperature for 5 min with gentle shaking. During this time, a solution was prepared containing 1 M ketone (10 equiv) and 0.1 M D- or L-proline (1.7 mg, 1 equiv) in a total volume of 145 μL. This solution was sonicated for 5 min, then added to the beads without draining the excess aniline. The mixture was then shaken gently for 18 h at room temperature. The beads were then pelleted and washed three times with DMSO.
To release the products from the beads, the beads were first equilibrated in water. Then 290 μL of an aqueous solution containing 0.2 M tris(2-carboxyethyl)phosphine hydrochloride (TCEP-HCl) (16.63 mg, 4 equiv) and 0.8 M NaHCO3 (19.49 mg, 16 equiv) was added to the beads, which were shaken gently for 1.5 h at room temperature. The beads were pelleted and both the bead and the supernatant was extracted with three times 500 μL of CH2Cl2. The organic layer was separated and dried with MgSO4. Finally, the liquid was filtered into a vial, the solution was flushed with argon and sealed until analysis.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Ted Kamenecka (TSRI) for his help with chiral chromatography; Dr. Xiaohai Li and Dr. Michael Cameron (TSRI) (NIH grant 1S10OD010603) for their help with HRMS; Dr. Jeremy Mason for useful discussions concerning interpretation of NMR spectra; Vuong Dang, Kevin Pels, Paige Dickson, and Scott Simanski (TSRI) for their help with the DNA damage assay and for technical advice.
Funding
This work was supported by a grant from the National Institutes of Health (AG052813).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acscombs-ci.7b00151.
Full experimental details and characterization of the products (PDF)
REFERENCES
- (1).Houghten RA; Pinilla C; Blondelle SE; Appel JR; Dooley CT; Cuervo JH Generation and use of synthetic peptide combinatorial libraries for basic research and drug discovery. Nature 1991, 354 (6348), 84–6. [DOI] [PubMed] [Google Scholar]
- (2).Hoesl CE; Nefzi A; Ostresh JM; Yu Y; Houghten RA Mixture-based combinatorial libraries: from peptides and peptidomi-metics to small molecule acyclic and heterocyclic compounds. Methods Enzymol. 2003, 369, 496–517. [DOI] [PubMed] [Google Scholar]
- (3).Fleeman R; LaVoi TM; Santos RG; Morales A; Nefzi A; Welmaker GS; Medina-Franco JL; Giulianotti MA; Houghten RA; Shaw LN Combinatorial Libraries As a Tool for the Discovery of Novel, Broad-Spectrum Antibacterial Agents Targeting the ESKAPE Pathogens. J. Med. Chem 2015, 58 (8), 3340–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Lam KS; Salmon SE; Hersh EM; Hruby VJ; Kazmierski WM; Knapp RJ A new type of synthetic peptide library for identifying ligand-binding activity. Nature 1991, 354, 82–84. [DOI] [PubMed] [Google Scholar]
- (5).Alluri PG; Reddy MM; Bachhawat-Sikder K; Olivos HJ; Kodadek T. Isolation of protein ligands from large peptoid libraries. J. Am. Chem. Soc 2003, 125 (46), 13995–4004. [DOI] [PubMed] [Google Scholar]
- (6).Lam KS; Lake D; Salmon SE; Smith JD; Chen M-L; Wade S; Abdul-Latif F; Knapp RJ; Leblova Z; Ferguson RD; Krchnak V; Sepetov NF; Lebl M. A one-bead one-peptide combinatorial library method for B-cell epitope mapping. Methods 1996, 9, 482–493. [DOI] [PubMed] [Google Scholar]
- (7).Wu CY; Wang DH; Wang X; Dixon SM; Meng L; Ahadi S; Enter DH; Chen CY; Kato J; Leon LJ; Ramirez LM; Maeda Y; Reis CF; Ribeiro B; Weems B; Kung HJ; Lam KS Rapid Discovery of Functional Small Molecule Ligands against Proteomic Targets through Library-Against-Library Screening. ACS Comb. Sci 2016, 18 (6), 320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Liu T; Liu Y; Kao HY; Pei D. Membrane Permeable Cyclic Peptidyl Inhibitors against Human Peptidylprolyl Isomerase Pin1. J. Med. Chem 2010, 53, 2494–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Liu T; Qian Z; Xiao Q; Pei D. High-throughput screening of one-bead-one-compound libraries: identification of cyclic peptidyl inhibitors against calcineurin/NFAT interaction. ACS Comb. Sci 2011, 13 (5), 537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Lim HS; Archer CT; Kodadek T. Identification of a peptoid inhibitor of the proteasome 19S regulatory particle. J. Am. Chem. Soc 2007, 129 (25), 7750–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Moon H; Lim HS Synthesis and screening of small-molecule alpha-helix mimetic libraries targeting protein-protein interactions. Curr. Opin. Chem. Biol 2015, 24, 38–47. [DOI] [PubMed] [Google Scholar]
- (12).Lau DH; Guo L; Liu R; Song A; Shao C; Lam KS Identifying peptide ligands for cell surface receptors using cell-growth-on-bead assay and one-bead one-compound combinatorial library. Biotechnol Lett. 2002, 24, 497–500. [Google Scholar]
- (13).Kumaresan PR; Wang Y; Saunders M; Maeda Y; Liu R; Wang X; Lam KS Rapid discovery of death ligands with one-bead-two-compound combinatorial library methods. ACS Comb. Sci 2011, 13 (3), 259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hintersteiner M; Buehler C; Uhl V; Schmied M; Muller J; Kottig K; Auer M. Confocal nanoscanning, bead picking (CONA): PickoScreen microscopes for automated and quantitative screening of one-bead one-compound libraries. J. Comb. Chem 2009, 11 (5), 886–94. [DOI] [PubMed] [Google Scholar]
- (15).Hintersteiner M; Kimmerlin T; Garavel G; Schindler T; Bauer R; Meisner NC; Seifert JM; Uhl V; Auer M. A highly potent and cellularly active beta-peptidic inhibitor of the p53/hDM2 interaction. ChemBioChem 2009, 10 (6), 994–8. [DOI] [PubMed] [Google Scholar]
- (16).Liu R; Shih TC; Deng X; Anwar L; Ahadi S; Kumaresan P; Lam KS Design, synthesis, and application of OB2C combinatorial peptide and peptidomimetic libraries. Methods Mol. Biol 2015, 1248, 3–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Chen X; Tan PH; Zhang Y; Pei D. On-bead screening of combinatorial libraries: reduction of nonspecific binding by decreasing surface ligand density. J. Comb. Chem 2009, 11 (4), 604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Doran TM; Gao Y; Mendes K; Dean S; Simanski S; Kodadek T. The utility of redundant combinatorial libraries in distinguishing high and low quality screening hits. ACS Comb. Sci 2014, 16, 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Olivos HJ; Bachhawat-Sikder K; Kodadek T. Quantum dots as a visual aid for screening bead-bound combinatorial libraries. ChemBioChem 2003, 4, 1242–1245. [DOI] [PubMed] [Google Scholar]
- (20).Hintersteiner M; Kimmerlin T; Kalthoff F; Stoeckli M; Garavel G; Seifert JM; Meisner NC; Uhl V; Buehler C; Weidemann T; Auer M. Single bead labeling method for combining confocal fluorescence on-bead screening and solution validation of tagged one-bead one-compound libraries. Chem. Biol 2009, 16 (7), 724–35. [DOI] [PubMed] [Google Scholar]
- (21).Paulick MG; Hart KM; Brinner KM; Tjandra M; Charych DH; Zuckermann RN Cleavable hydrophilic linker for one-bead-one-compound sequencing of oligomer libraries by tandem mass spectrometry. J. Comb. Chem 2006, 8 (3), 417–426. [DOI] [PubMed] [Google Scholar]
- (22).Thakkar A; Cohen AS; Connolly MD; Zuckermann RN; Pei D. High-Throughput Sequencing of Peptoids and Peptide-Peptoid Hybrids by Partial Edman Degradation and Mass Spectrometry. J. Comb. Chem 2009, 11, 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Figliozzi GM; Goldsmith R; Ng SC; Banville SC; Zuckermann RN Synthesis of N-substituted glycine peptoid libraries. Methods Enzymol. 1996, 267, 437–447. [DOI] [PubMed] [Google Scholar]
- (24).Aditya A; Kodadek T. Incorporation of heterocycles into the backbone of peptoids to generate diverse peptoid-inspired one bead one compound libraries. ACS Comb. Sci 2012, 14 (3), 164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Gao Y; Kodadek T. Synthesis and screening of stereochemically diverse combinatorial libraries of peptide tertiary amides. Chem. Biol 2013, 20, 360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Suwal S; Kodadek T. Synthesis of libraries of peptidomimetic compounds containing a 2-oxopiperazine unit in the main chain. Org. Biomol. Chem 2013, 11, 2088–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Suwal S; Kodadek T. Solid-phase synthesis of peptoid-like oligomers containing diverse diketopiperazine units. Org. Biomol. Chem 2014, 12 (31), 5831–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Aquino C; Sarkar M; CHalmers MJ; Mendes K; Kodadek T; Micalizio G. A biomimetic polyketide-inspired approach to small molecule ligand discovery. Nat. Chem 2012, 4, 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kodadek T; McEnaney PJ Towards vast libraries of scaffold-diverse, conformationally constrained oligomers. Chem. Commun 2016, 52 (36), 6038–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Ohlmeyer MH; Swanson RN; Dillard LW; Reader JC; Asouline G; Kobayashi R; Wigler M; Still WC Complex synthetic chemical libraries indexed with molecular tags. Proc. Natl. Acad. Sci. U. S. A 1993, 90, 10922–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Liu R; Marik J; Lam KS A novel peptide-based encoding system for “one-bead one-compound” peptidomimetic and small molecule combinatorial libraries. J. Am. Chem. Soc 2002, 124, 7678–7680. [DOI] [PubMed] [Google Scholar]
- (32).Clark MA; Acharya RA; Arico-Muendel CC; Belyanskaya SL; Benjamin DR; Carlson NR; Centrella PA; Chiu CH; Creaser SP; Cuozzo JW; Davie CP; Ding Y; Franklin GJ; Franzen KD; Gefter ML; Hale SP; Hansen NJ; Israel DI; Jiang J; Kavarana MJ; Kelley MS; Kollmann CS; Li F; Lind K; Mataruse S; Medeiros PF; Messer JA; Myers P; O’Keefe H; Oliff MC; Rise CE; Satz AL; Skinner SR; Svendsen JL; Tang L; van Vloten K; Wagner RW; Yao G; Zhao B; Morgan BA Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol 2009, 5 (9), 647–54. [DOI] [PubMed] [Google Scholar]
- (33).MacConnell AB; McEnaney PJ; Cavett VJ; Paegel BM DNA-Encoded Solid-Phase Synthesis: Encoding Language Design and Complex Oligomer Library Synthesis. ACS Comb. Sci 2015, 17, 518–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Mendes K; Malone ML; Ndungu JM; Suponitsky-Kroyter I; Cavett VJ; McEnaney PJ; MacConnell AB; Doran TM; Ronacher K; Stanley K; Utset O; Walzl G; Paegel BM; Kodadek T. High-throughput identification of DNA-encoded IgG ligands that distinguish active and latent mycobacterium tuberculosis infections. ACS Chem. Biol 2017, 12, 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lovering F; Bikker J; Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem 2009, 52 (21), 6752–6. [DOI] [PubMed] [Google Scholar]
- (36).Ritchie TJ; Macdonald SJ The impact of aromatic ring count on compound developability-are too many aromatic rings a liability in drug design? Drug Discovery Today 2009, 14 (21–22), 1011–20. [DOI] [PubMed] [Google Scholar]
- (37).Ritchie TJ; Macdonald SJ; Young RJ; Pickett SD The impact of aromatic ring count on compound developability: further insights by examining carbo- and hetero-aromatic and -aliphatic ring types. Drug Discovery Today 2011, 16 (3–4), 164–71. [DOI] [PubMed] [Google Scholar]
- (38).Bauer RA; Wurst JM; Tan DS Expanding the range of “druggable” targets with natural product-based libraries: an academic perspective. Curr. Opin. Chem. Biol 2010, 14, 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Notz W; Tanaka F; Barbas CF 3rd Enamine-based organocatalysis with proline and diamines: the development of direct catalytic asymmetric Aldol, Mannich, Michael, and Diels-alder reactions. Acc. Chem. Res 2004, 37 (8), 580–91. [DOI] [PubMed] [Google Scholar]
- (40).Jacobsen EN; MacMillan DW Organocatalysis. Proc. Natl. Acad. Sci. U. S. A 2010, 107 (48), 20618–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Pan SC; List B. New concepts for organocatalysis. Ernst Schering Res. Found. Workshop 2008, 2, 259–300. [PubMed] [Google Scholar]
- (42).List B. The direct catalytic asymmetric three-component Mannich reaction. J. Am. Chem. Soc 2000, 122, 9336–9337. [Google Scholar]
- (43).List B; Pojarliev P; Biller WT; Martin HJ The proline-catalyzed direct asymmetric three-component Mannich reaction: scope, optimization, and application to the highly enantioselective synthesis of 1,2-amino alcohols. J. Am. Chem. Soc 2002, 124 (5), 827–33. [DOI] [PubMed] [Google Scholar]
- (44).Notz W; Tanaka F; Watanabe S; Chowdari NS; Turner JM; Thayumanavan R; Barbas CF 3rd The direct organocatalytic asymmetric Mannich reaction: unmodified aldehydes as nucleophiles. J. Org. Chem 2003, 68 (25), 9624–34. [DOI] [PubMed] [Google Scholar]
- (45).Youngman MA; Dax SL Solid-phase Mannich condensation of amines, aldehydes, and alkynes: investigation of diverse aldehyde inputs. J. Comb. Chem 2001, 3 (5), 469–72. [DOI] [PubMed] [Google Scholar]
- (46).Schlienger N; Bryce MR; Hansen TK The boronic Mannich reaction on a solid-phase approach. Tetrahedron 2000, 56, 10023–10030. [Google Scholar]
- (47).Malone ML; Paegel BM What is a “DNA-Compatible” Reaction? ACS Comb. Sci 2016, 18, 182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Fisher KJ; Turkett JA; Corson AE; Bicker KL Peptoid Library Agar Diffusion (PLAD) Assay for the High-Throughput Identification of Antimicrobial Peptoids. ACS Comb. Sci 2016, 18, 287–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Subiros-Funosas R; Prohens R; Barbas R; El-Faham A; Albericio F. Oxyma: an efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. - Eur. J 2009, 15 (37), 9394–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.