Abstract
Objective: To determine if a 12-week yoga intervention (YI) was associated with increased gamma aminobutyric acid (GABA) levels and decreased depressive symptoms in participants with major depressive disorder (MDD).
Methods: Subjects were randomized to a high-dose group (HDG) of three YIs a week and a low-dose group (LDG) of two YIs a week. Thalamic GABA levels were obtained using magnetic resonance spectroscopy at Scan-1 before randomization. After the assigned 12-week intervention, Scan-2 was obtained, immediately followed by a YI and Scan-3. Beck Depression Inventory II (BDI-II) scores were obtained before Scan-1 and Scan-3.
Settings/Location: Screenings and interventions occurred at the Boston University Medical Center. Imaging occurred at McLean Hospital.
Subjects: Subjects met criteria for MDD.
Intervention: Ninety minutes of Iyengar yoga and coherent breathing at five breaths per minute plus homework.
Outcome measures: GABA levels and the BDI-II.
Results: BDI-II scores improved significantly in both groups. GABA levels from Scan-1 to Scan-3 and from Scan-2 to Scan-3 were significantly increased in the LDG (n = 15) and showed a trend in the total cohort. Post hoc, participants were divided into two groups based on having an increase in GABA levels at Scan-2. Increases in Scan-2 GABA levels were observed in participants whose mean time between their last YI and Scan-2 was 3.93 ± 2.92 standard deviation (SD) days, but not in those whose mean time between their last YI and Scan-2 was 7.83 ± 6.88 SD.
Conclusions: This study tentatively supports the hypothesis that one of the mechanisms through which yoga improves mood is by increasing the activity of the GABA system. The observed increase in GABA levels following a YI that was no longer observed 8 days after a YI suggests that the associated increase in GABA after a YI is time limited such that at least one YI a week may be necessary to maintain the elevated GABA levels.
Keywords: yoga, depression, anxiety, breathing, GABA, MRS, progesterone
Introduction
The World Health Organization ranked depression as the single largest contributor to global disability in 2015.1 Although pharmacologic treatments targeting monoamine systems are effective in reducing symptoms of major depressive disorder (MDD), 40% to 50% of individuals with MDD do not achieve remission with medication(s) alone.2,3 Individuals with complete remission (asymptomatic recovery) from an MDD episode have a 61% lower risk of recurrence, and remain free of relapse or recurrence 4.2 times longer than those who continue to exhibit subsyndromal depressive symptoms.2,4 New treatments based on other mechanisms of action are needed. The monoamine deficit hypothesis has led to the development of antidepressant medications; however, it does not explain the delay in symptom reduction or failure to remit among depressed patients treated with antidepressants that target monoamine systems.5
Gamma aminobutyric acid (GABA), an amino acid neurotransmitter, has emerged as a contributing factor in mood disorders.5 The GABA deficit hypothesis of MDD points to an association between depressive symptoms and deficits in the GABA system and the GABAA receptor.6,7 Using magnetic resonance spectroscopy (MRS), GABA levels have been shown to be low in MDD subjects.8,9 This article tests the hypothesis that a yoga intervention (YI), by addressing a deficit in the GABA system, would improve depressive symptoms in MDD.
The connection between the GABA system and depressive symptoms is supported by the relationship between decreasing progesterone levels and increased depressive symptoms. Progesterone is metabolized to allopregnanolone, which by directly binding at GABAA receptors modulates the function of the GABA system.10 Allopregnanolone has anxiolytic and antidepressant effects.11 Serum allopregnanolone concentrations temporarily follow changes in progesterone levels during the menstrual cycle, but with a 1- to 2-day delay.12 Progesterone is low during the follicular stage, rises in the midluteal phase, and falls in the late luteal phase before the onset of menstruation.13 The failure of the GABAA receptor to adapt to abrupt decreases in progesterone and allopregnanolone levels may contribute to postpartum depression (PPD).14 Brexanolone, a synthetic form of allopregnanolone and allosteric modulator of the GABAA receptor, has been approved for the treatment of PPD.15 Premenstrual dysphoric disorder (PMDD) is characterized by recurrent irritability, depression, anxiety, and emotional lability during the luteal phase.16 Serotonin selective reuptake inhibitors (SSRIs) are used to treat PMDD and MDD.17 Subjects with MDD treated for 8 weeks with SSRIs, fluoxetine, or citalopram, had increases in occipital cortical GABA levels and decreases in depressive symptoms.18 The use of SSRIs to treat disorders related to low progesterone, allopregnanolone, and GABA levels combined with a delay of clinical improvement for weeks is consistent with the theory that monoamine antidepressants eventually exert their effects by correcting deficits in the GABA system.7
There is increasing evidence from randomized controlled trials that YIs are associated with decreased depressive symptoms.19,20 The mechanisms through which yoga exerts its effects are relatively unexplored. YIs have been associated with increased GABA levels in experienced practitioners after a 60-min hatha yoga session, yoga-naive healthy controls after a 12-week Iyengar YI, and individuals with MDD after a 12-week Iyengar YI.9,21,22 This study reports changes in thalamic GABA levels measured by MRS in participants with MDD randomized to either a high- or low-dose YI. While this article focuses on changes in GABA levels, it is part of a larger/parent study that tested the Vagal-GABA theory.9,21,22 The overall hypothesis is that an Iyengar YI, including coherent breathing at five breaths per minute, increases parasympathetic input into the brain, which is associated with increased GABA levels and decreased depressive symptoms.
Materials and Methods
Instruments and data management
Study methods and the specifics of the YI, previously published in detail, are summarized below.23 The Boston University Medical Center (BUMC) Institutional Review Board approved this study. Written informed consent was obtained at the screening interview. The Structured Clinician Interview for DSM-IV Axis I Disorders was used to diagnose Axis I Disorders.24 The Beck Depression Inventory II (BDI-II), a self-report instrument, was used to assess depressive symptoms.25 The Columbia-Suicide Severity Rating Scale (C-SSRS) was used to assess suicide risk.26
Study design
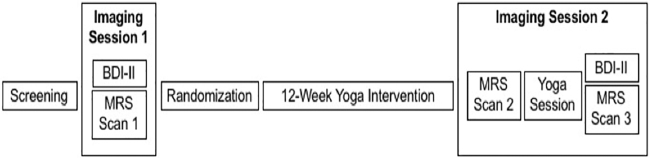
The screening and interventions occurred at BUMC. The imaging occurred at McLean Hospital, Belmont, MA, from October 2013 to October 2015. Admissions were rolling with recruitment from the Internet and advertisements. Phone screens assessed eligibility. In-person screening interviews were conducted by either a clinical psychologist or psychiatrist who medically cleared participants. Participants had Scan-1 before being randomized to either a high-dose group (HDG) or low-dose group (LDG) for the 12-week YI. The HDG intervention consisted of three 90-min yoga sessions and four 30-min homework sessions per week. The LDG intervention consisted of two 90-min yoga sessions and three 30-min homework sessions per week. After the 12-week YI, participants had Scan-2 that was immediately followed by a 90-min yoga session and then Scan-3 (Fig. 1).
FIG. 1.
Study design. BDI-II, Beck Depression Inventory II; MRS, magnetic resonance spectroscopy.
Inclusion and exclusion criteria
At screening, the following inclusion criteria were initially used: 18 to 55 years old; current diagnosis of MDD, BDI-II score of ≥14 (mild depression) to <28 (severe depression). Comorbid anxiety disorder(s) were allowable if they would not interfere with participation. The following were exclusionary: treatment with antidepressants, benzodiazepines, mood stabilizers, or psychotherapy in the 3 months before screening; >6 one-hour mind–body practices in the last 6 months; current prayer practice >2 h per week; bipolar illness; history of psychosis; suicide attempt and/or suicidal ideation within the last year using the C-SSRS criteria; current alcohol or substance abuse or dependence; and inability to complete the study protocol.
The eligibility criteria were found to exclude subjects who would have been appropriate for the study. To increase recruitment, inclusion criteria were expanded approximately half-way through the study to remove the BDI-II upper limit of 28; increase the upper age limit from 55 to 65 years; allow suicidal ideation without intent but continue to exclude for suicidal ideation with intent in the prior year (using C-SSRS criteria); exclude for suicide attempts within the prior year instead of within the lifetime; and allow subjects on a stable dose of antidepressant(s) for at least three months at screening with no anticipated dose changes during the study.
To avoid changes in GABA levels due to menstrual cycle stage, all females were required to have a serum progesterone of ≤0.3 ng/mL, consistent with the follicular stage before Scan-1. The same level of ≤0.3 ng/mL was sought before Scan-2 and 3, but if the scan data would have otherwise been lost, Scan-2 and 3 were allowed with a serum progesterone of >0.3 ng/mL if pregnancy tests were negative.
Allocation, randomization, masking, and dosing
The allocation sequence was generated using a permuted block design (n = 4) by the study statistician and placed in sealed sequentially numbered envelopes that were opened by staff at randomization. Subjects could not be blinded to group assignment. McLean Hospital staff who collected and analyzed spectral data for GABA levels were blind to group assignment. BUMC study staff were not blinded to group assignment. To reduce the risk of outcome assessor bias, the BDI-II was completed by the participants. Subject compliance was monitored by sign-in sheets at each YI and weekly self-report forms assessing compliance with homework.
Yoga plus coherent breathing intervention
The manualized 90-min yoga protocol included ∼60 min of Iyengar yoga, 10 min of relaxation, and 20 min of a coherent breathing exercise at five breaths per minute with equal inhalation and exhalation paced by an audio file.22 Each homework assignment consisted of 15 min of postures and 15 min of audio-paced coherent breathing.27 The complete manual has been previously published.23 Completers were defined as having completed at least three evaluations, at baseline, at least one of the week 4 or 8 evaluations, and the week 12 evaluation.
Magnetic resonance imaging/MRS
Magnetic resonance imaging (MRI) and proton (1H) MRS scans were acquired on a 4.0-Tesla Varian Unity/INOVA whole-body MRI/MRS scanner (Varian, Inc., Palo Alto, CA) using a volumetric head coil (XLR Imaging, London, Canada). Head placement was confirmed before high-contrast 3D fast low-angle shot T1-weighted images for voxel placement, followed by global shimming, and placement of a 20 (left to right) × 20 (inferior to superior) × 30 (anterior to posterior)-mm voxel in the left thalamus, whose inferior aspect was aligned with the anterior commissure–posterior commissure line on sagittal view, and the medial aspect of the central sulcus and anterior aspect of the corpus callosum on axial view. The left thalamus was the region of interest because it is functionally connected to brain regions associated with mood regulation and provides high-quality spectral data from deep gray nuclei with high concentrations of GABA and thus a high GABA signal to detect changes.28 Due to time constrains, only spectral data from the left thalamus were obtained.
Difference-edited GABA-optimized spectra were obtained using MEGAPRESS.29–31 The 3.00-ppm GABA doublet resonance and coedited resonance structures of glutamate (Glu), glutamine (Gln), N-acetylaspartate (NAA), and the .93-ppm macromolecule (MM) resonance from MEGAPRESS 68 msec and difference-edited spectra were fitted using LCModel.32 GABA metabolite ratios (GABA levels) were determined using GABA from MEGAPRESS difference-edited spectra relative to creatine levels derived from the MEGAPRESS 68 msec spectrum (GABA/Cr ratio). T1-weighted axial image sets were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid binary-tissue maps (FSL, Oxford, United Kingdom), to extract tissue percentages for each voxel.33–36 Tissue percentages also were compared to confirm reliability of voxel placement across scans.
Statistics
Study data were analyzed on an intention-to-treat basis using SPSS v 24 (IBM SPSS Statistics; IBM corp., worldwide). Between-group comparisons of participant characteristics were made using Fisher's exact test for categorical data and two-sample t-tests for continuous data. Independent samples t-tests were used to compare GABA levels between the two groups. One-sample t-tests were used to evaluate changes in GABA levels between Scans-1 and 2, Scans-2 and 3, and Scans-1 and 3. Effect-size was measured using Cohen's d, or Hedge's-g when group sizes were unequal. Logistic regression and independent t-tests were used to evaluate potential factors besides yoga dose-group assignment that may effect changes in GABA levels. P-values for all statistical comparisons were two-tailed, using an alpha of 0.05 for statistical significance.
Results
Recruitment and demographics
Recruitment resulted in 265 volunteers who were screened by telephone; of these, 86 participated in an in-person screening visit, 32 were randomized (16 HDG and 16 LDG), 30 completed the 12-week intervention (15 HDG and 15 LDG), and 28 completed the second scanning session (15 LDG and 13 HDG) that included Scans-2 and 3. A flow chart of subject recruitment through intervention completion has already been published.23 The only anxiety disorder that resulted in exclusion was claustrophobia that prevented scanning. As reported previously, both groups were compliant with the assigned dosage, such that the HDG received significantly more yoga minutes than the LDG (p = 0.001).23
One subject in each group was lost to follow-up before the first assessment (reasons for dropouts unknown). A comparison of the participant characteristics between the LDG and HDG is shown in Table 1. Both groups had similar demographic and health status characteristics, except that the HDG had a significantly higher percentage of postmenopausal women (p = 0.04) and a trend toward lower basal metabolic index (p = 0.06). At Scan-1, 23 females and at Scan-2, 22 females had serum progesterone levels of ≤0.3 ng/mL. One LDG female had a serum progesterone level of 11.45 ng/mL (luteal phase) at Scan-2. A sensitivity analysis omitting that participant was performed, but did not alter the interpretation of the results reported below (data not shown).
Table 1.
Participant Characteristics
| LDG |
HDG |
DF | T-stat chi-sq | p-Value | |
|---|---|---|---|---|---|
| n = 15 | n = 13 | ||||
| Age (years, mean ± SD) | 34.67 ± 10.38 | 39.62 ± 15.61 | 20.39 | 0.972 | 0.343 |
| Education (years, mean ± SD) | 16.67 ± 2.16 | 16.46 ± 1.90 | 26 | 0.265 | 0.793 |
| Female (%) | 80.0 | 84.6 | 1 | 0.101 | 0.750 |
| Menopausal (%) | 6.7 | 38.3 | 1 | 4.182 | 0.041* |
| Caucasian (%) | 86.7 | 69.2 | 2 | 2.598 | 0.273 |
| BMI (mean ± SD) | 28.08 ± 5.63 | 24.69 ± 3.26 | 26 | 1.910 | 0.067** |
| Baseline BDI score | 27.73 ± 8.00 | 24.38 ± 7.21 | 26 | 1.157 | 0.258 |
| Days (last class to Scan-2) | 7.07 ± 7.06 | 4.54 ± 2.79 | 18.89 | 1.278 | 0.217 |
| Antidepressant medication | n = 1*** | n = 1**** |
Significant p < 0.05, **p < 0.10, ***venlafaxine, and ****bupropion.
BDI, Beck Depression Inventory; BMI, body mass index; Chi-Sq, chi-squared; DF, degree of freedom; HDG, high-dose group; LDG, low-dose group; SD, standard deviation; T-stat, T-statistic.
MRI/MRS metabolite data
Manual voxel shimming of the thalamic voxel yielded an average water line width = 9.0 ± 2.7 Hz, which did not differ significantly between groups or across scan sessions. Within-subject coefficients of variation using this MEGAPRESS sequence for GABA acquisition from the thalamus, according to Licata et al., were 8.6% ± 3.3%.34 Average GABA Cramer Rao lower bounds observed for GABA in the present study were 8.6% ± 3.3%, which did not differ significantly between groups or scan-time (F2,52 = 2.24, p = 0.12). GABA was the a priori metabolite of interest. Other 68 msec “OFF” subspectra metabolites were examined to verify MRS reliability, and to rule out systematic metabolite differences. No significant differences appeared for either group in tissue segmentation data across Scans-1, 2, or 3, for GM content (55.1% ± 16.1%, F2,52 = 0.15, p = .86), confirming reproducibility of the voxel placement algorithm across MRS sessions.
Changes in thalamic GABA
Mean GABA concentrations for the total cohort, and for LDG and HDG, are presented in Table 2. Independent samples t-tests revealed no statistically significant differences between the LDG and HDG for GABA concentrations for any of the three scans, although there was a trend at Scan-3 for higher GABA concentrations in the LDG (p = 0.087). The percentage change in GABA concentrations between the three scans is shown in Table 3.
Table 2.
Gamma Aminobutyric Acid Level Summary Statistics
| Whole (mean ± SD) | Low-dose group (mean ± SD) | High-dose group (mean ± SD) | Hedges-g (effect size) | t | df | p-Value | |
|---|---|---|---|---|---|---|---|
| GABA | |||||||
| Scan-1 | 0.267 ± 0.055 | 0.255 ± 0.063 | 0.282 ± 0.043 | 0.494 | −1.32 | 26 | 0.200 |
| Scan-2 | 0.280 ± 0.063 | 0.278 ± 0.061 | 0.282 ± 0.067 | 0.063 | −0.203 | 26 | 0.841 |
| Scan-3 | 0.297 ± 0.068 | 0.317 ± 0.073 | 0.273 ± 0.055 | 0.677 | 1.780 | 26 | 0.087** |
p < 0.10.
GABA, gamma aminobutyric acid; SD, standard deviation.
Table 3.
Percentage Change in Gamma Aminobutyric Acid Levels
| GABA | % Δ ± SD | Scan-3 − Scan-1 |
|
Scan-3 − Scan-2 |
|
Scan-2 − Scan-1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t | df | Cohen's d | p | % Δ ± SD | T | df | Cohen's d | p | % Δ ± SD | t | df | Cohen's d | p | ||
| Total | 17.24 ± 46.03 | 1.98 | 27 | 0.375 | 0.058** | 8.80 ± 27.28 | 1.71 | 27 | 0.323 | 0.099 | 11.54 ± 49.59 | 1.23 | 27 | 0.233 | 0.229 |
| LDG | 34.03 ± 55.39 | 2.38 | 14 | 0.614 | 0.032* | 17.05 ± 28.43 | 2.32 | 14 | 0.600 | 0.036* | 20.32 ± 63.66 | 1.24 | 14 | 0.319 | 0.237 |
| HDG | −2.14 ± 20.70 | −0.37 | 12 | 0.103 | 0.716 | −0.71 ± 23.41 | −0.11 | 12 | 0.030 | 0.915 | 1.40 ± 24.44 | 0.21 | 12 | 0.057 | 0.840 |
Significant p < 0.05, **p < 0.10.
GABA, gamma aminobutyric acid; HDG, high-dose group; LDG, low-dose group; SD, standard deviation; t, one-sample-t-test.
Scan-3 − Scan-1
A one-sample t-test comparing the relative percentage change in GABA from Scan-1 to Scan-3 [(Scan-3 − Scan-1)/Scan-1] for the total cohort showed a trend for increased thalamic GABA levels at Scan-3 (t = 1.98, df = 27, p = 0.058). When separated into LDG and HDG, the same comparison showed a statistically significant increase in GABA for the LDG (t = 2.38, df = 14, p = 0.032), but not for the HDG (t = −0.372, df = 12, p = 0.716).
Scan-3 − Scan-2
A one-sample t-test comparing the relative percentage change in GABA from Scan-2 to Scan-3 [(Scan-3 − Scan-2)/Scan-2] for the total cohort showed a trend for increased GABA concentrations at Scan-3 (t = 1.71, df = 27, p = 0.099). When separated into LDG and HDG, the same comparison showed a statistically significant increase in GABA for the LDG (t = 2.32, df = 14, p = 0.036), but not for the HDG (t = −0.109, df = 12, p = 0.915). The lack of increase in GABA levels in the HDG group was not explained by participant characteristics.
Scan-2 − Scan-1
A one-sample t-test comparing the relative percentage change in GABA from Scan-1 to Scan-2 [(Scan-2 − Scan-1)/Scan-1] for the total cohort was not statistically significant (t = 1.231, df = 27, p = 0.229). When separated into LDG and HDG, the same comparison did not show significant changes in GABA for either group (t = 1.236, df = 14, p = 0.237, and t = 0.206, df = 12, p = 0.840, respectively).
Potential factors affecting GABA levels
Of the 28 participants who completed Scan-2, 14 had an increase in thalamic GABA levels from Scan-1 to Scan-2 (HDG = 7 and LDG = 7) and 14 had a decrease in thalamic GABA levels from Scan-1 to Scan-2 (HDG = 6 and LDG = 8). Since these findings were unexpected, a logistic regression was performed to determine whether there were participant characteristics that predicted whether an individual was a GABA “responder” (i.e., their GABA levels increased over the course of the study). Table 4 shows the results of this analysis. Of the characteristics evaluated, the number of days between the last YI and Scan-2 was significantly associated with increases in GABA levels from Scan-1 to Scan-2 (β = 0.392, standard error of the mean [SEM] ±0.171, p = 0.022). Using the independent t-test, those participants whose GABA levels were higher at Scan-2 than Scan-1 had an average of 3.93 ± 2.92 days between the last YI and Scan-2. The average time between the last YI and Scan-2 for those participants whose GABA levels did not increase was 7.83 ± 6.88. This difference in days between last YI and Scan-2 approached significance (t = −1.97, df = 17.54, p = 0.065). There was also a trend in that participants with higher BDI-II scores at baseline were more likely to have increased GABA levels at Scan-2 (β = 0.137, SEM ±0.077, p = 0.077).
Table 4.
Participant Characteristics and Increases in Gamma Aminobutyric Acid Levels Between Scans-1 and 2
| β | SEM | p-Value | |
|---|---|---|---|
| Last class—Scan-2 (days) | −0.392 | 0.171 | 0.022* |
| Menopausal (yes/no) | −2.588 | 2.407 | 0.282 |
| Age | −0.098 | 0.076 | 0.198 |
| Male (yes/no) | −1.906 | 1.701 | 0.263 |
| Education (years) | 0.055 | 0.237 | 0.815 |
| Caucasian (yes/no) | 0.214 | 1.217 | 0.860 |
| BMI | −0.154 | 0.115 | 0.179 |
| BDI-II baseline score | 0.137 | 0.077 | 0.077** |
| Cumulative yoga minutes | −0.001 | 0.000 | 0.149 |
Significant p < 0.05, **p < 0.10.
BDI-II, Beck Depression Inventory II; BMI, basal metabolic index; SEM, standard error of the mean.
BDI-II scores and relationship with GABA levels
The results of the BDI-II have been previously published but are provided as context for the changes in GABA levels in Table 5.37 Pearson correlation analysis was used to evaluate the relationship between BD-II scores and GABA levels at Scan-1, Scan-3, and percentage change between Scans-1 and 3. At Scan-1 and Scan-3 time points, there were no statistically significant relationships between BDI scores and GABA levels for the total group, HDG, or LDG. When the relationship between changes in BDI-II scores at the Scan-1 and Scan-3 time points was compared with changes in GABA levels between the two scans, results showed no significant relationship for the total group or the LDG. There was, however, a statistically significant inverse relationship between changes in BDI scores and GABA levels for the HDG (Pearson corr. = −0.562, p = 0.045). This means that as GABA levels increased, depressive symptoms decreased.
Table 5.
Beck Depression Inventory Summary Statistics for Participants Who Completed Scans-1–3
| Pre-Scan-1 | Pre-Scan-3 | t | df | p-Value | |
|---|---|---|---|---|---|
| Whole (mean ± SD) | 26.18 ± 7.69 | 7.75 ± 8.39 | 10.08 | 27 | 0.000* |
| Low-dose group (mean ± SD) | 27.73 ± 8.00 | 10.20 ± 10.21 | 6.15 | 14 | 0.000* |
| High-dose group (mean ± SD) | 24.38 ± 7.21 | 4.92 ± 4.54 | 8.63 | 12 | 0.000* |
Significant p < 0.05.
SD, standard deviation.
Two participants were on antidepressants at randomization and study conclusion. Participant-1 was taking venlafaxine. Participant-2 was taking bupropion. Both had decreases in their BDI-II scores from 40 to 14 and from 16 to 9, respectively.
Discussion
In summary, the LDG exhibited a significant increase in GABA levels from Scan-1 to Scan-3 and from Scan-2 to Scan-3. There were also trends for the total cohort to have an increase in GABA levels from Scan-1 to Scan-3 and from Scan-2 to Scan-3. The lack of statistically significant within-group difference in GABA levels between Scan-1 and Scan-2 may be explained by the differences in length of time between the last yoga session to Scan-2. The difference between Scan-1 and Scan-3 reflects both the 12-week YI and the immediate effect of a yoga class. Table 1 shows that the days from the last yoga class to Scan-2 were not statistically significant between groups. However, on average, it was the participants with the shorter time interval between the last yoga class and Scan-2 who were statistically more likely to show increases in GABA levels at Scan-2, with the number of subjects who had increases in GABA levels at Scan-2 evenly distributed between the HDG (n = 7) and the LDG (n = 7). This suggests that the time from the last yoga class to scanning may have a greater association with increased GABA levels than assignment to the HDG or LDG. This would be consistent with the observed increase from Scan-2 to Scan-3. It is probable that the effects of yoga sessions, like pharmacologic treatments, are time limited. The yoga tradition advocates daily practice. The increase in GABA levels seen after a YI was observed after an average of 4 days, but no longer observed after an average of 8 days. These findings suggest that at least one yoga session a week may be necessary to maintain elevated GABA levels.
No significant correlations were observed between BDI-II scores and GABA levels for the total group. However, there was a significant association between changes in BDI-II scores and changes in GABA scores in the HDG, consistent with the GABA deficit hypothesis of depression.6,7 In previous studies, GABA levels increased and depressive symptoms decreased after SSRI treatment of participants with MDD, but no correlation between GABA levels and depressive symptoms was found.18 The lack of correlation between BDI-II scores and GABA levels could be due to the discrepancy in the time periods represented, with the BDI-II covering a 2-week period and GABA levels reflecting the current state. The two participants who did not achieve full remission despite treatment with antidepressants had clinically significant decreases in their depressive symptoms when the YI was added to their treatment.
Study strengths include a randomized controlled trial in which the primary dependent variable (thalamic GABA levels) was obtained and analyzed by staff who were blind to group assignment. All females, except one, were scanned in the follicular stage for all three scans, such that changing progesterone levels were not a confounding variable with regard to GABA levels.
Future studies would benefit from a larger sample size, a control group, the administration of a clinician-administered depression scale by a blinded rater. The use of a reliable and valid self-assessment questionnaire, the BDI-II, mitigated the lack of a blinded rater. MRS is limited by only measuring the presence of GABA, not cellular location or receptor activity. The lack of bilateral thalamic data prevented the examination of lateralized changes. This article only addresses changes in GABA levels; other factors that could be related to changes in depressive symptoms in this study have been previously reported.9,22,28,37,38
Conclusions
Given the small sample size, tentative support is provided for the hypothesis that one of the mechanisms through which yoga plus coherent breathing affect mood is through increasing GABA levels. For the post hoc analysis, the responders, defined as an increase in GABA from Scan-1 to Scan-2, had on average a 4-day interval between the last class and Scan-2; the nonresponders had on average an 8-day interval. However, 4 versus 8 days should not be used as hard cutoffs due to the overlap in those ranges. It must be considered that the time from the last yoga class to scanning had a greater association with increased GABA levels than group assignment. This observation provides tentative support that a yoga practice of at least once a week would be needed to sustain elevated GABA levels.
Acknowledgments
Dr. Brown and Dr. Gerbarg teach pro bono and for-profit Breath-Body-Mind™, a multicomponent program that includes coherent breathing and other mind–body practices coordinated with breathing. Several of their books include information about breathing and other mind–body practices. Dr. Streeter is certified to teach Breath-Body-Mind.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
R21AT004014 and R01AT007483 (C.C.S.), M01RR00533 (Boston University Clinical and Translational Science Institute (CTSI), U11RR025771 (General Clinical Research Unit at Boston University Medical Center), and K23AT008043 (M.B.N.).
References
- 1. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva, Switzerland: World Health Organization, 2017 [Google Scholar]
- 2. Rush AJ, Fava M, Wisniewski SR, et al. Sequenced treatment alternatives to relieve depression (STAR*D): Rationale and design. Control Clin Trials 2004;25:119–142 [DOI] [PubMed] [Google Scholar]
- 3. Judd LL, Schettler PJ, Rush AJ, et al. A new empirical definition of major depressive episode recovery and its positive impact on future course of illness. J Clin Psychiatry 2016;77:1065–1073 [DOI] [PubMed] [Google Scholar]
- 4. Fava M, Rush AJ, Wisniewski SR, et al. A comparison of mirtazapine and nortriptyline following two consecutive failed medication treatments for depressed outpatients: A STAR*D report. Am J Psychiatry 2006;163:1161–1172 [DOI] [PubMed] [Google Scholar]
- 5. Wilkinson ST, Sanacora G. A new generation of antidepressants: An update on the pharmaceutical pipeline for novel and rapid-acting therapeutics in mood disorders based on glutamate/GABA neurotransmitter systems. Drug Discov Today 2019;24:606–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharmacol 2015;73:97–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luscher B, Shen Q, Sahir N. The GABAergic deficit hypothesis of major depressive disorder. Mol Psychiatry 2011;16:383–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanacora G, Mason GF, Rothman DL, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry 1999;56:1043–1047 [DOI] [PubMed] [Google Scholar]
- 9. Streeter C, Gerbarg P, Nielsen G, et al. Effects of yoga on thalamic gamma-aminobutyric acid, mood and depression: Analysis of two randomized controlled trials. Neuropsychiatry (London) 2018;8:1923–1939 [Google Scholar]
- 10. Locci A, Pinna G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: A biomarker axis in stress-induced cognitive and emotional impairment. Br J Pharmacol 2017;174:3226–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pineles SL, Nillni YI, Pinna G, et al. PTSD in women is associated with a block in conversion of progesterone to the GABAergic neurosteroids allopregnanolone and pregnanolone measured in plasma. Psychoneuroendocrinology 2018;93:133–141 [DOI] [PubMed] [Google Scholar]
- 12. Ottander U, Poromaa IS, Bjurulf E, et al. Allopregnanolone and pregnanolone are produced by the human corpus luteum. Mol Cell Endocrinol 2005;239:37–44 [DOI] [PubMed] [Google Scholar]
- 13. Reed B, Carr B. The Normal Menstrual Cycle and the Control of Ovulation. In: Feingold K, Anawalt B, Boyce A, et al., eds. Endotext. South Dartmouth, MA: MDText.com, Inc., 2018 [Google Scholar]
- 14. Maguire J, Mody I. GABA(A)R plasticity during pregnancy: Relevance to postpartum depression. Neuron 2008;59:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanes S, Colquhoun H, Gunduz-Bruce H, et al. Brexanolone (SAGE-547 injection) in post-partum depression: A randomised controlled trial. Lancet (London, England) 2017;390:480–489 [DOI] [PubMed] [Google Scholar]
- 16. Bixo M, Johansson M, Timby E, et al. Effects of GABA active steroids in the female brain with a focus on the premenstrual dysphoric disorder. J Neuroendocrinol 2018;30 DOI: 10.1111/jne.12553 [DOI] [PubMed] [Google Scholar]
- 17. Marjoribanks J, Brown J, O'Brien PM, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev 2013;6:CD001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanacora G, Mason GF, Rothman DL, et al. Increased occipital cortex GABA concentrations in depressed patients after therapy with selective serotonin reuptake inhibitors. Am J Psychiatry 2002;159:663–665 [DOI] [PubMed] [Google Scholar]
- 19. Cramer H, Anheyer D, Lauche R, et al. A systematic review of yoga for major depressive disorder. J Affect Disord 2017;213:70–77 [DOI] [PubMed] [Google Scholar]
- 20. Uebelacker LA, Epstein-Lubow G, Gaudiano BA, et al. Hatha yoga for depression: Critical review of the evidence for efficacy, plausible mechanisms of action, and directions for future research. J Psychiatr Pract 2010;16:22–33 [DOI] [PubMed] [Google Scholar]
- 21. Streeter CC, Jensen JE, Perlmutter RM, et al. Yoga Asana sessions increase brain GABA levels: A pilot study. J Altern Complement Med 2007;13:419–426 [DOI] [PubMed] [Google Scholar]
- 22. Streeter CC, Whitfield TH, Owen L, et al. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: A randomized controlled MRS study. J Altern Complement Med 2010;16:1145–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Streeter CC, Gerbarg PL, Whitfield TH, et al. Treatment of major depressive disorder with Iyengar yoga and coherent breathing: A randomized controlled dosing study. J Altern Complement Med 2017;23:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. First MB, Gibbon M, Spitzer R, et al. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP). New York: New York State Psychiatric Institute, 1997. [Google Scholar]
- 25. Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual, 2nd ed. San Antonio, TX: Psychological Corporation, 1996 [Google Scholar]
- 26. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brown RP, Gerbarg PL. The Healing Power of the Breath: Simple Techniques to Reduce Stress and Anxiety, Enhance Concentration, and Balance Your Emotions. Boston, MA: Shambhala Publications, Inc., 2012 [Google Scholar]
- 28. Streeter CC, Gerbarg PL, Saper RB, et al. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses 2012;78:571–579 [DOI] [PubMed] [Google Scholar]
- 29. Rothman DL, Petroff OA, Behar KL, et al. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci U S A 1993;90:5662–5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mescher M, Merkle H, Kirsch J, et al. Simultaneous in vivo spectral editing and water suppression. NMR Biomed 1998;11:266–272 [DOI] [PubMed] [Google Scholar]
- 31. Silveri MM, Sneider JT, Crowley DJ, et al. Frontal lobe gamma-aminobutyric acid levels during adolescence: Associations with impulsivity and response inhibition. Biol Psychiatry 2013;74:296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–679 [DOI] [PubMed] [Google Scholar]
- 33. Jensen JE, Licata SC, Ongur D, et al. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR Biomed 2009;22:762–769 [DOI] [PubMed] [Google Scholar]
- 34. Licata SC, Jensen JE, Penetar DM, et al. A therapeutic dose of zolpidem reduces thalamic GABA in healthy volunteers: A proton MRS study at 4 T. Psychopharmacology (Berl) 2009;203:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi IY, Lee SP, Merkle H, et al. In vivo detection of gray and white matter differences in GABA concentration in the human brain. Neuroimage 2006;33:85–93 [DOI] [PubMed] [Google Scholar]
- 36. Jensen J, Frederick B, Renshaw P. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed 2005;18:570–576 [DOI] [PubMed] [Google Scholar]
- 37. Streeter C, Gerbarg P, Whitfield T, et al. Treatment of major depressive disorder with Iyengar yoga and coherent breathing: A randomized controlled dosing study. J Altern Complement Med 2017;23:201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nyer M, Gerbarg P, Silveri M, et al. A randomized controlled dosing study of Iyengar Yoga and coherent breathing for the treatment of major depressive disorder: Impact on suicidal ideation and safety findings. Complement Ther Med 2018;37:136–142 [DOI] [PubMed] [Google Scholar]