Abstract
Previous studies have shown that the aging kidney has a marked loss of α(E)-catenin in proximal tubular epithelium. α-Catenin, a key regulator of the actin cytoskeleton, interacts with a variety of actin-binding proteins. Cisplatin-induced loss of fascin2, an actin bundling protein, was observed in cells with a stable knockdown of α(E)-catenin (C2 cells), as well as in aging (24 mon), but not young (4 mon), kidney. Fascin2 co-localized with α-catenin and the actin cytoskeleton in NRK-52E cells. Knockdown of fascin2 increased the susceptibility of tubular epithelial cells to cisplatin-induced injury. Overexpression of fascin2 in C2 cells restored actin stress fibers and attenuated the increased sensitivity of C2 cells to cisplatin-induced apoptosis. Interestingly, fascin2 overexpression attenuated cisplatin-induced mitochondrial dysfunction and oxidative stress in C2 cells. These data demonstrate that fascin2, a putative target of α(E)-catenin, may play important role in preventing cisplatin-induced acute kidney injury.
Keywords: α-Catenin, Actin, Aging, Apoptosis, Fascin2, Mitochondria
1. Introduction
During the last century, human lifespan has increased dramatically, which will contribute to a substantial increase in the geriatric population (Bolignano et al., 2014). It is well established that aging is associated with structural and functional renal changes that increase susceptibility to acute kidney injury (AKI) (Wang et al., 2014a; Hain and Paixao, 2015). Elderly patients (≥65 years) have ten times the incidence rate of AKI compared with those less than 65 years of age in Italy (Baraldi et al., 1998). Xue et al. also established age as a risk factor for AKI; the incidence of AKI was 1.9% in patients younger than 65 which rose to 2.9% in those older than 85 (Xue et al., 2006). Moreover, AKI in the elderly is more severe and less reversible due to delayed, or decreased, repair. Recovery from AKI was 3-times longer (32 days versus 11.4 days) in elderly (mean 67.1 years) versus young (32.2 years) individuals (Arora et al., 1993). In a meta-analysis of 17 studies, it was found that a higher percentage of elderly patients did not recover renal function as compared to younger patients (Schmitt et al., 2008).
α-Catenin, which connects the cadherin-β-catenin complex to F-actin, is important in the relationship between the adherens junction (AJ) and cytoskeleton that is essential for cell adhesion (Desai et al., 2013). Besides linking the cadherin/catenin complex to the cytoskeleton, α-catenin also interacts with a variety of actin-binding proteins, including α-actinin and vinculin, as well as actin itself (Knudsen et al., 1995; Rimm et al., 1995). There are three forms of α-catenin: neural (N), epithelial (E) and testis/heart (T) (Kobielak and Fuchs, 2004). Recent studies indicate that in addition to the well-established role in cell adhesion, α-catenin is also involved in multiple pathways controlling membrane and actin dynamics, cell proliferation, migration and apoptosis (Benjamin and Nelson, 2008). Our laboratory has reported a dramatic decrease of α(E)-catenin expression in proximal tubular epithelium in aged male Fisher 344 rats (Jung et al., 2004). Our laboratory has shown that C2 cells, an NRK-52E cell line with a stable knockdown of α(E)-catenin, are characterized by increased monolayer permeability, increased cisplatin-induced apoptosis, decreased cell-cell aggregation, and decreased repair in a wound healing assay due to migration deficits (Nichols et al., 2014a,b; Wang et al., 2014b).
Fascin is a highly conserved actin-binding and bundling protein that plays an important role in maintenance and stability of parallel filamentous actin bundles, regulating cell proliferation, adhesion, migration and apoptosis (Jayo and Parsons, 2010; Kim et al., 2015). There are 3 isoforms of fascin: fascin 1, encoded by fscn1, is mainly expressed in mesenchymal and nervous tissues; fascin 2, encoded by fscn2, is most prevalent in retinal cells; fscn3, encoding for fascin 3, is restricted to the testes (Adams, 2004). While fascin has been shown to regulate actin bundle assembly, more specific roles for fascin have been demonstrated recently in the formation and turnover of cell adhesive structures which suggests fascin may be a potential mediator between α(E)-catenin and F-actin (Adams, 2004). In terms of cell death, it has been shown that increased fascin1 prevents apoptosis and is important for tumor cell survival (Lai et al., 2015). Furthermore, increased fascin levels enhance cholangiocarcinoma rat brain endothelial cell proliferation, migration and invasion (Zhao et al., 2015). The current studies were designed to test the hypothesis that fascin2 is a mediator of the cellular response to injury and repair.
2. Methods
2.1. Animals
Male Fisher 344 (F344) rats were obtained from the National Institutes of Aging (NIA) colony housed at Charles River Laboratory. On the day of the experiment, rats were anesthetized by intraperitoneal injection of ketamine (80–120 mg/kg)/xylazine (5–10 mg/kg). Kidneys were collected and 1 mm cross sections were snap frozen in liquid nitrogen. In certain experiments, tissue from young (4 mon) and aged (24 mon) animals challenged with 2.75 mg/kg cisplatin for 72 h, a protocol that induces injury in aged, but not young rats, were analyzed (Wang et al., 2014b). All experimental procedures were approved by the Animal Care and Use Committee of the University of Missouri in accordance with the Guide for the Care and Use of Laboratory Animals.
2.2. Cell culture
Cells were plated at a density of 5 × 104 cells/cm2 and cultured in DMEM/F12 (1:1), containing L-Glutamine and HEPES (Gibco), supplemented with 5–10% fetal bovine serum (FBS) (Altanta Biologicals) plus penicillin-streptomycin (50 I.U./ml and 50 μg/ml, respectively) and incubated at 37 °C with 5% CO2. Cells were harvested with TrypLE Express (Gibco) and pelleted at 1500 rpm for 5 min at room temperature. C2 cells have stable knockdown of α(E)-catenin; they are derived from NRK-52E cells as previously described by our laboratory and NT3 cells are the non-targeted control cell line (Nichols et al., 2014a,b). Cells were grown in the presence of 5 μg/ml puromycin (Sigma-Aldrich) for maintenance of lentiviral shRNA construct.
Open reading frame clones of mouse fscn1 and rat fscn2 in the pCMV6 Entry vector (Origene) were used to generate stable overexpressing cell lines in C2 cells (C2\Fscn1, C2\Fscn2). Non-targeted vector controls were generated in both NT3 and C2 cells (NT3\V, C2\V). Cells were grown in 5 μg/ml puromycin and 200 μg/ml geneticin (Gibco) for vector maintenance.
For the knockdown studies, 4 unique 29mer shRNA constructs in a retroviral vector (pGFP-V-RS) were purchased from Origene. The constructs were transfected into NRK-52E cells, 5 ug/ml puromycin was used for selection and subsequent maintenance of cell lines. The targeting sequences were A1 - tactgcctcaagtcttatgacagccgcta; B1-gtgtgccaccgccgaggctccaaccagct; C1-cgtgactgtcgcttcttggtcttgccgca; D1-ccttgtgaacgatgccgaccgctacctca. This strategy generated 5 stable cell lines; Fscn2A1, Fscn2B1, Fscn2C1, Fscn2D1 and the vector control (NRK\V1).
2.3. Western blot
Confluent cells were washed twice with ice-cold DPBS (Gibco) and lysed with lysis buffer (10 mM Tris-HCl, 1% SDS) containing HaltTM Protease/Phosphatase inhibitors (Thermo Scientific). Cells were scraped and incubated on a rocker for 15 min at 4 °C. Cells were further disrupted by pipetting 15 times and spun at 12,000 g for 15 min at 4 °C. Protein concentration was determined by NanoDrop 2000c Spectrophotometer at 280 nm. The following antibodies were used: fascin1 (GeneTex, GTX63842; 1:1000), fascin2 (NOVUS; Ab78599, 1:1000), BH3 interacting-domain death agonist (BID; NOVUS, NB100–56106, 1:1000), B-cell lymphoma 2 (bcl-2; Cell Signaling, 2876, 1:1000), cleaved poly (ADP-ribose) polymerase (PARP; Sigma, SAB4500487, 1:1000), α(E)-catenin (GeneTex, GTX 61621, 1:1000) and anti-β-actin (Sigma, A2228, 1:2500). Goat-anti-mouse HRP conjugate and goat-anti-rabbit HRP conjugate (Jackson ImmunoResearch Laboratories) were used at 1:20,000 dilutions. Blots were developed using SuperSignal West Femto Chemiluminescent Substrate (Pierce), imaged using the ChemiDocTM imaging system (Bio-Rad), and quantitation performed using the ImageLab 3.0 software (Bio-Rad); single bands were seen with the western blots.
2.4. Immunofluorescence and phalloidin staining
Cells were grown on 2-well glass chamber slides (Ibidi) overnight. Cells were washed with PBS, fixed in 2% paraformaldehyde for 10 min, permeabilized with 1% TritonX-100 for 10 min and blocked with 1% bovine serum albumin (BSA) for 1 h. Cells were incubated in primary antibody or stained with 200 ng/ml FITC phalloidin (Sigma) in blocking buffer overnight on rocker at 4 °C. Cells were subsequently washed 2x in PBS, incubated in conjugated secondary antibody for 2 h on the rocking plate at RT (this step was omitted during phalloidin staining), shaken dry, and counterstained with Fluoroshield with DAPI (Sigma). Cells were imaged on an Olympus IX51 microscope (Olympus) with a 40 x or 60 x oil immersion lens with a UC50 digital camera using cellSense software (Olympus) at identical exposure times. The following antibodies were used: fascin 2 (NOVUS) and α(E)-catenin (GeneTex), Goat-anti-mouse FITC conjugate (Sigma), Goat-anti-rabbit TRITC conjugate (Sigma), Goat-anti-mouse TRITC conjugate (Sigma).
2.5. Atomic force microscopy (AFM)
A MFP-3D System (Asylum Research Inc., Santa Barbara, CA) AFM mounted on an Olympus 81X inverted microscope (Olympus) was used in constant force, contact mode operation to acquire topographic images of cultured renal cells. The AFM probes used were silicon nitride probes (model MLCT, Bruker-Nano Inc., Goleta, CA) with tip diameter of 20 nm and spring constant ranging from 10 to 14 pN/nm. Renal epithelial cells were plated on 60 mm tissue culture dishes overnight at ~60% confluency, and were imaged in cell culture medium at room temperature. The AFM probe was scanned across cell surface at a speed of 20 μm/s, with a tracking force of ~400 pN. The tracking force ensures AFM probes maintain contact with the cell surface to obtain high-resolution surface topographical features of the cells and does not damage the cells. AFM Images were acquired at a resolution of 380 × 380 pixels in an area of 72 μm × 72 μm.
2.6. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Assay
Cells were seeded in a 96 well flat bottom tissue culture plate (MidSci) at a density of 5 × 104 cells/cm2. After 24 h, the culture media was replaced by serum free (SF) media supplemented with cisplatin. Three hr before harvest, 10 μl of 5 mg/ml MTT (Sigma), dissolved in DPBS, was added to each well. Upon harvesting, cells were washed with cold DPBS and dissolved by adding 50 μl solubilization solution (10% Triton X-100, 0.1N HCl in isopropanol). The plates were read at 570/690 nm on the Synergy HT Multi-Detection Microplate Reader (BioTek). The results are expressed as percent viability [Abs570–690 treated/Abs570–690 control × 100].
2.7. Caspase activity assay
Confluent cultures of NT3 and C2 cells in 96 well plates were challenged with cisplatin. Caspase activity was determined by Caspase-Glo® 3/7 Assay (Promega) according to the manufacturer’s instructions.
2.8. ATP detection assay
Confluent cultures of cells in 96 well plates were challenged with cisplatin. Cellular ATP was determined by Mitochondrial ToxGloTM Assay (Promega), according to the manufacturer’s instructions.
2.9. Oxygen consumption assay
Oxygen consumption was measured polarographically at 25 °C using a Clark-type electrode in the medium used for swelling measurements supplemented with 1 mM MgCl2 and either 5 mM glutamate/5 mM malate or 10 mM sussinate. State 3 was initiated by adding 2 μM ADP to the reaction mixture. State 4 was started by adding 2 μM oligomycin to the reaction mixture. Respiratory ratio = state 3/state 4.
2.10. Oxidative stress assay
Confluent cultures of cells in 96 well plates were challenged with cisplatin. Oxidative stress was determined by HNE Adduct Competitive ELISA kit (Cell Biolabs), according to the manufacturer’s instructions.
2.11. Statistical analysis
All experiments were independently performed in triplicate at a minimum. All data were expressed as mean ± S.E. Statistical analysis was performed using Analysis of Variance (ANOVA, Bonferoni post hoc) with the statistical software GraphPad Prism 6 (GraphPad Software). The differences were considered statistically significant when p < 0.05.
3. Results
3.1. Decreased expression of Fscn2 in aged kidney
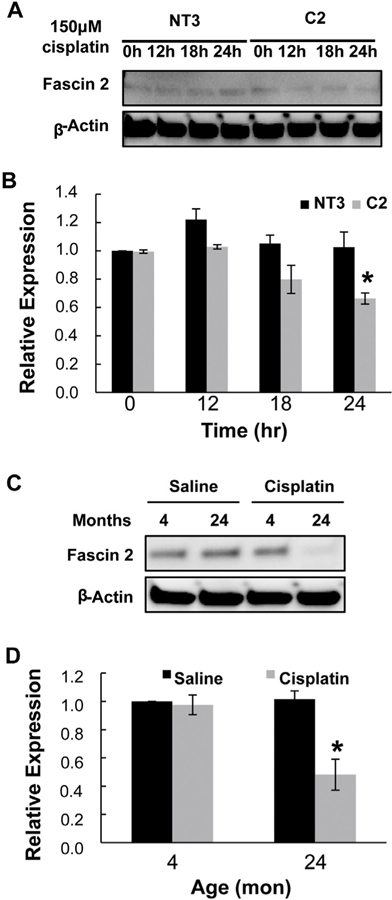
Fascin2 was identified as a downstream target of α(E)-catenin by a plate-based array approach to identify actin cytoskeleton regulators altered in C2 cells migrating in a wound healing assay. Fascin2 gene expression is decreased in migrating C2 cells relative to NT3 cells using methods previously described by our laboratory (Nichols et al., 2014a) (Supplemental Fig. 1). Interestingly, fascin2 protein expression is similar in basal C2 and NT3 cells; however, challenging cells with cisplatin induced a decrease of fascin2 protein in C2, but not NT3, cells (Fig. 1A and B). While the basal protein level expression of fascin2 was not altered in the aging kidney, protein levels of fascin2 were significantly decreased (approximately 50%) in aged kidney after cisplatin challenge, without a corresponding loss of expression in the young kidney (Fig. 1C and D).
Fig. 1.
Fascin2 Expression is Decreased During Acute Injury. A. A loss of fascin2 protein expression was seen in C2, but not NT3, cells following challenge with 150 μM cisplatin for 12–24 h. B. Densitometric analysis of three replicate experiments; protein expression is shown as the fold decrease from control (C2, 0 h). Each data point represents the mean + SE of three samples, * indicates a significant difference from control. C. Fascin2 expression is decreased in aged (24 mon), but not young (4 mon) kidney 72 h (peak of injury) after cisplatin challenge (2.75 mg/kg). Each lane is a sample from an individual rat. D. Densitometric analysis of four replicate experiments; protein expression is shown as the fold decrease from control (4 mon control). Each data point represents the mean + SE of four samples, * indicates a significant difference from control.
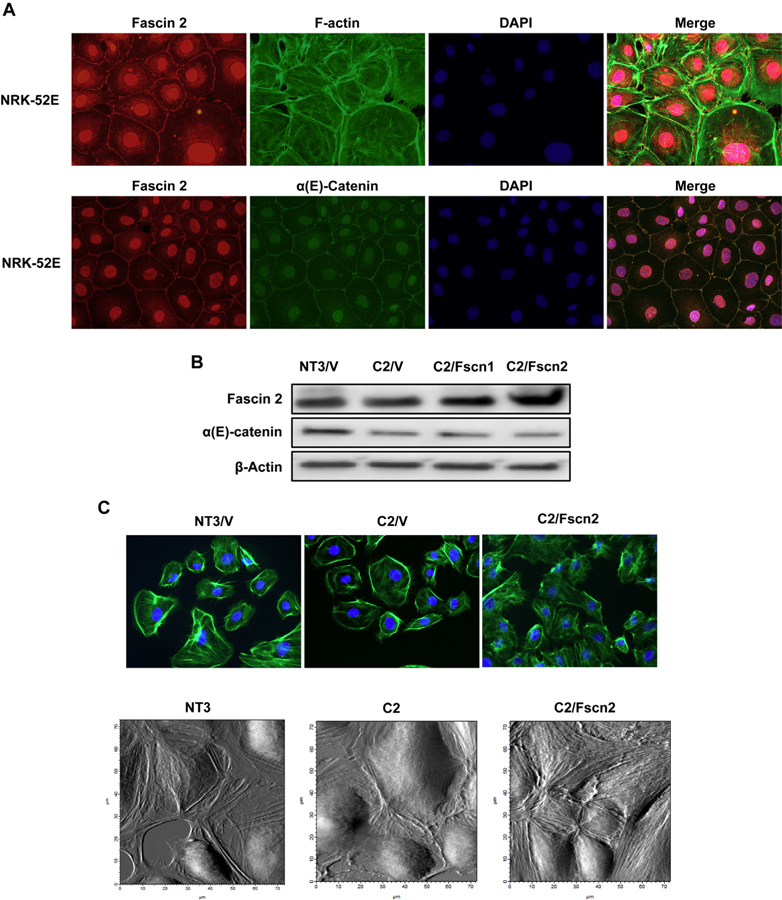
3.2. Co-localization of fascin2 and α(E)-catenin
Fascin2 is expressed and localized within the cytosol and along the cell membrane in NRK-52E cells; there is also perinuclear and strong nuclear staining with this antibody (Fig. 2A). Co-staining with FITC-phalloidin to visualize the F-actin cytoskeleton indicated that fascin2 accumulated around actin stress fibers, actin bundles that line the cell periphery and filopodia; this is consistent with the role of fascin2 in stress fiber formation (Yamashiro-Matsumura and Matsumura, 1986) (Fig. 2A). Interestingly, a co-localization of fascin2 and α(E)-catenin was also observed (Fig. 2A). Although there is no direct evidence showing fascin2 localizes at adherens junctions (AJs), it may play a role in cell adhesion (Kuo et al., 2011; Schiller et al., 2011). However, fascin2 was not detectable in anti-α(E)-catenin immunoprecipitates (data not shown), which indicates fascin2, and α(E)-catenin do not interact directly.
Fig. 2.
Fascin2 Colocalizes with α-Catenin and the Actin Cytoskeleton; Overexpression of Fascin2 Rescues Stress Fibers in C2Cells. A. Immunofluorescence images (60x) of NRK-52E cells treated with rabbit anti-fascin2 (red), FITC-phalloidin (green) or anti-α-catenin (green) and DAPI (blue) demonstrates that fascin2 colocalizes with both actin and α-catenin. B. Overexpression of fascin1 (C2/Fscn1) or fascin2 (C2/Fscn2) in C2 cells. C. Phalloidin staining demonstrates increased actin stress fibers in C2/Fscn2 cells, while the bottom panel shows stress fibers using cell surface scanning with AFM. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We next overexpressed full-length fscn1 and fscn2 in C2 cells (C2/Fscn1; C2/Fscn2); neither construct affected α(E)-catenin expression (Fig. 2B). Overexpression of fascin2 rescues stress fiber formation in C2/V cells as evidenced by both phalloidin staining and topographic imaging with atomic force microscopy (Fig. 2C).
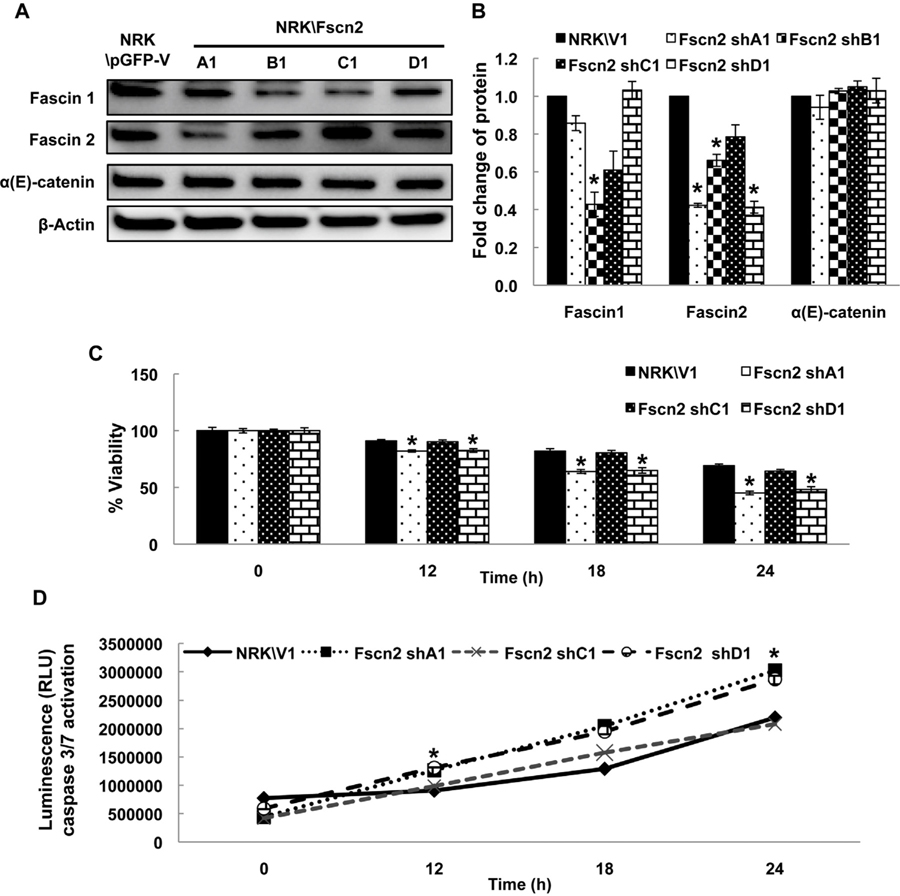
3.3. Fascin2 knockdown increases the susceptibility of tubular cells to cisplatin-induced apoptosis
shRNA vectors designed to knock down fascin2 were expressed in NRK-52E cells; these cell lines have varied expression of fascin2 (Fig. 3A and B). None of the constructs affected α(E)-catenin expression. shC1 did not elicit substantial knockdown of fascin2 (or fascin1) and, as expected, viability following cisplatin challenge was not affected with this construct (Fig. 3C). The shA1 and shD1 constructs knocked down fascin2, with slight change in fascin1 expression and showed increased susceptibility to cisplatin as compared with NRK/V1 control cells (Fig. 3C). Of note, shB1 cells, which significantly reduced both fascin2 and fascin1 did not survive following cisplatin challenge. Consistent with these observations, higher caspase 3/7 activities were observed in shA1 and shD1 cells than other cell lines following the cisplatin treatment (Fig. 3D). Taken together, these data suggest that the loss of fascin2 increases the susceptibility of tubular epithelial cells to cisplatin injury.
Fig. 3.
Fascin2 Knockdown Increases Susceptibility to Cisplatin-Induced Nephrotoxicity. A. shRNA knockdown of fascin2 expression by four different targeting constructs; significant knockdown was seen with A1, B1, and D1 constructs. The impact on fascin1 and fascin2 expression is shown in B. C. Cell viability was determined by the MTT assay in cells treated 150 μM cisplatin for the indicated time periods. The results are presented as the percent viability of untreated NRK/V1 cells in SF media. D. The activities of caspase 3/7 in cells treated with 150 μM cisplatin for the indicated time periods were determined by luminescence. Data points represent the mean ± SE of four samples; *indicates a significant difference from control; similar results were seen in replicate experiments.
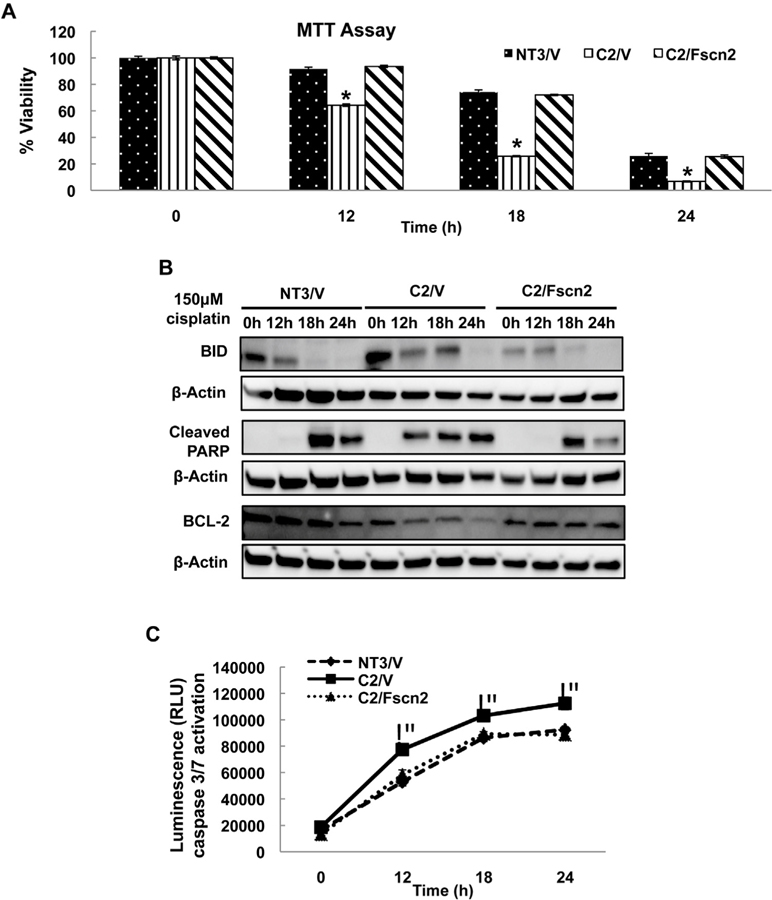
3.4. Overexpression of fascin2 decreases the susceptibility of C2 cells to cisplatin-induced apoptosis
C2 cells are characterized by increased sensitivity to cisplatin-induced toxicity. The increased susceptibility is due to increased apoptosis, but not necrosis (Wang et al., 2014b; Wang and Parrish, 2015); a finding that has been confirmed in the aging kidney in vivo (Wang et al., 2014b). Consistent these previous studies, C2/V cells exhibited a significant loss of viability after cisplatin injury (Fig. 4A). In addition, there was increased apoptosis as indicated by increased BID and PARP cleavage, decreased Bcl-2 levels and higher caspase 3/7 activity after cisplatin challenge as compared with NT3/V cells (Fig. 4B and C). Overexpression of fascin2 completely attenuated the increased susceptibility of C2 cells to cisplatin injury (Fig. 4A), as well as decreasing cisplatin-induced BID and cleaved PARP, and attenuating loss of bcl-2 (Fig. 4B). The increased caspase 3/7 activation following cisplatin challenge was also prevented by fascin2 (Fig. 4C). Interestingly, similar protective effects were seen with overexpression of fascin1 (Supplemental Fig. 2). C2 cells are also characterized by a deficiency in repair, as assessed by the failure to migrate in a wound healing assay (Nichols et al., 2014a, 2014b). Interestingly, Fascin2, but not fascin1, overexpression was also able to increased wound healing in C2 cells (Supplemental Fig. 3).
Fig. 4.
Fascin2 Rescues the Increased Susceptibility to Cisplatin-Induced Nephrotoxicity. A. Overexpression of fascin2 attenuates loss of viability in C2 cells relative to NT3. B. Overexpression of fascin2 attenuates markers of apoptosis in C2 cells, including BID, PARP cleavage and Bcl-2 expression. C. Activation of caspase3/7 is decreased in C2 cells by fascin2 overexpression. Data points represent the mean ± SE of four samples; *indicates a significant difference from control; similar results were seen in replicate experiments.
The role of the actin cytoskeleton in regulating susceptibility to injury is supported by the finding that latrunculin A, a compound that inhibits actin polymerization (Coue et al., 1987) decreased the viability of C2/Fscn1, C2/Fscn2 and NT3/V to the same extent as C2/V in response to cisplatin (data not shown). Correspondingly, stabilization of actin polymerization with jasplakinolide rescued C2/V to control (NT3) values (Bubb et al., 1994). Collectively, these findings suggested that fascin2 stabilization of actin stress fibers increases resistance to cisplatin-induced apoptosis.
3.5. Fscn2 rescues mitochondrial dysfunction in C2 cells
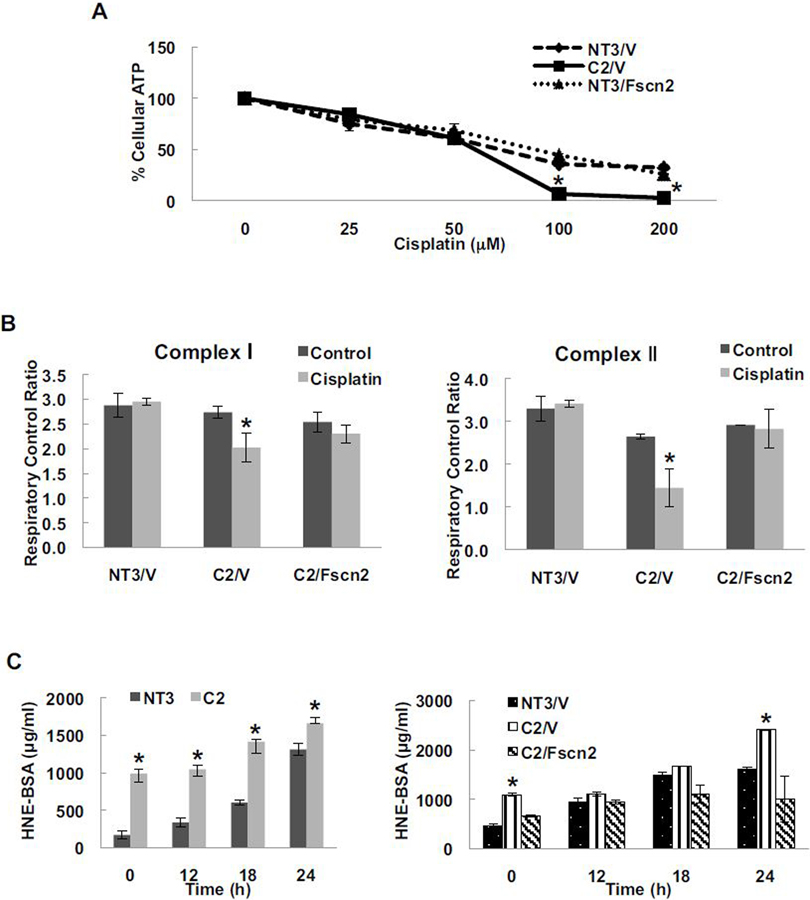
Abnormal mitochondria DNA and increased levels of reactive oxygen species (ROS) have been observed in aged kidneys (Shigenaga et al., 1994). Several studies suggest that cisplatin accumulates in renal mitochondria, hampering the respiratory chain and increasing ROS production that leads to apoptosis of renal tubular epithelial cells (Santos et al., 2008). Consistent with these observations, our study showed that cisplatin impaired ATP production in a concentration-dependent manner (Fig. 5A). C2/V exhibited lower ATP levels in response to cisplatin as compared with NT3/V cells and overexpression of fascin2 was able to completely abolish these differences (Fig. 5A). Cisplatin induces the loss of mitochondrial coupling in response to both complex-1 and complex-II substrates in C2/V cells, but not in NT3/V cells. Fscn2 overexpression rescued these deficits in C2/V cells in response to cisplatin (Fig. 5B). Consistent with mitochondrial dysfunction was our finding that more oxidative stress, as measured by the 4-hydroxynonenal (HNE) assay, was induced by cisplatin in C2 cells than in NT3 cells (Fig. 5C). Overexpression of Fscn2 in C2 cells significantly reduced the HNE level in response to cisplatin treatment (Fig. 5C). Therefore, these data demonstrated that fascin2 reduces cisplatin-induced mitochondrial dysfunction.
Fig. 5.
Fascin2 Rescues Mitochondrial Dysfunction in Cisplatin-Induced Nephrotoxicity. A. Fascin2 attenuates loss of ATP following cisplatin challenge. B. Oxygen consumption of complex I and II in C2 cells is corrected by fascin2 overexpression. C. Cisplatin induces increased oxidative stress in C2 cells; an effect attenuated by fascin2. Data points represent the mean ± SE of 12 replicates; *indicates a significant difference from control (NT3/V).
4. Discussion
A relationship between AKI and aging has long been recognized. AKI in the elderly is more severe and patients are less likely to recover, presumably due to impaired proliferation and migration of renal tubular epithelial cells (Haagsma and Pound, 1980; Toback, 1992). Previous studies from our laboratory showed marked loss of α(E)-catenin in proximal tubular epithelium in aged kidney (Jung et al., 2004; Wang et al., 2014b). α-Catenin, a tension-sensing, key regulator of the actin cytoskeleton, interacts with a variety of actin-binding proteins (Knudsen et al., 1995; Rimm et al., 1995). Fascin2 is an actin bundling protein that interacts with adhesion molecules and F-actin (Hwang et al., 2008; Jayo and Parsons, 2010). While many previous studies have focused on the function of fascin2 in retina (Horák et al., 2006), its role in renal tubular epithelium is not yet known. In this study, a stress-induced loss of fascin2 was observed in aged kidney and C2 cells. Overexpression of fascin2 abolished the increased cisplatin-induced apoptosis, mitochondrial dysfunction and oxidative stress in C2 cells compared with NT3 cells. Moreover, there was an inverse correlation between fascin2 levels and the susceptibility of tubular epithelial cells to cisplatin injury. These data suggest that fascin2 regulates cisplatin-induced apoptosis.
It has been shown that disruption of the actin cytoskeleton is also associated with apoptosis in kidney injury. Disruption of the actin cytoskeleton precedes apoptosis in cisplatin-induced nephrotoxicity (Kruidering et al., 1998). Furthermore, a large amount of evidence indicates changes to the dynamics of actin cytoskeleton cause release of reactive oxygen species from mitochondria and subsequent cell apoptosis (Gourlay and Ayscough, 2005). Overexpression of gelsolin, an actin binding protein that regulates actin filament assembly and disassembly, maintains the mitochondrial membrane potential and reduces cytochrome c release by closing the voltage-dependent anion channel in the mitochondrial outer membrane (Harms et al., 2004). Staurosporine has been shown to phosphorylate cofilin, an actin binding protein that disassembles actin filaments. The activated cofilin then relocates into mitochondria, triggers the release of cytochrome c and initiates apoptosis. Importantly, the actin-binding domain of cofilin is essential for this pro-apoptosis function (Chua et al., 2003). Although the specific mechanisms still remain to be studied, these studies suggested a link between the actin cytoskeleton, mitochondria and apoptosis.
Fascin is a small globular protein which selectively cross-links actin filaments that have been arranged in a parallel orientation into tightly packed actin bundles (Courson and Rock, 2010). These bundles play a critical role in the formation and organization of a variety of highly contractile and dynamic subcellular structures, including lamellipodia, filopodia and stress fibers (Yamashiro-Matsumura and Matsumura, 1986). The formation of structures such as filopodia and stress fibers play key roles in cell survival (Mattila and Lappalainen, 2008; Kuo et al., 2003). Lai and colleagues demonstrated that microRNA-133a levels are inversely related to fascin1, i.e., when miR-133a is up-regulated, proliferation and migration are inhibited, whereas apoptosis of gastric cancer cells is promoted and this process can be reversed by up-regulation of fascin1 (Lai et al., 2015). Moreover, fascin is a suppressor of caspase-associated anoikis in colon adenocarcinoma cells (Kanda et al., 2014). Importantly, there is evidence that filopodia actin bundles can be recycled into stress fibers for use in cytoskeletal tension and retraction. Fascin-containing filopodia are tethered by adhesion complexes that initiate stress fiber assembly in motile fish keratocytes. In addition, untethered filopodia actin bundles incorporate actin stress fiber along with myosin II and integrate in lamellipodia (Nemethova et al., 2008). Furthermore, the association of fascin with tropomyosin and the competition between fascin, caldesmon and tropomyosin for F-actin may also play an important role in the interconversion of filopodial bundles and stress fibers (Ishikawa et al., 1998; Creed et al., 2011).
In summary, we have shown that fascin2 ameliorates cisplatin-induced injury in renal cells. This newly identified role of fascin2 may lay the groundwork for new therapeutic approaches to AKI, including the potential to enhance cisplatin-based chemotherapy by alleviating nephrotoxicity. Furthermore, there are many different actin cross-linking proteins that also interact with α-catenin. Understanding the properties and functions of these cross-linking proteins will lead to a more complete understanding of the role of actin cytoskeleton in regulating the response of tubular epithelial cells to injury, as well as repair.
Supplementary Material
HIGHLIGHTS.
Cisplatin induces loss of fascin2 protein expression in vitro and in vivo.
Fascin2 is regulated, in part, by α(E)-catenin.
Knockdown of fascin2 increases susceptibility to cisplatin nephrotoxicity in vitro.
Overexpression of fascin2 is renoprotective against cisplatin injury in vitro.
Acknowledgements
Research reported in this publication was supported by the National Institutes of Health under award numbers RO1AG034154 (ARP), HL094404 (CPB) and PO1HL095468 (GAM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxlet.2016.11.021.
References
- Adams JC, 2004. Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol 16, 590–596. [DOI] [PubMed] [Google Scholar]
- Arora P, Kher V, Kohli HS, Sharma RK, Gupta A, Jha R,1993. Acute renal failure in the elderly: experience from a single centre in India. Nephrol. Dial. Transplant 8, 827–830. [PubMed] [Google Scholar]
- Baraldi A, Ballestri M, Rapana R, Lucchi L, Borella P, Leonelli M, Furci L, Lusvarghi E, 1998. Acute renal failure of medical type in an elderly population. Nephrol. Dial. Transplant 13 (S7), 25–29. [DOI] [PubMed] [Google Scholar]
- Benjamin JM, Nelson WJ, 2008. Bench to bedside and back again: molecular mechanisms of alpha-catenin function and roles in tumorigenesis. Semin. Cancer Biol 18, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolignano D, Mattace-Raso F, Sijbrands EJ, Zoccali C, 2014. The aging kidney revisited: a systematic review. Ageing Res. Rev 14, 65–80. [DOI] [PubMed] [Google Scholar]
- Bubb MR, Senderowicz AM, Sausville EA, Duncan KL, Korn ED, 1994. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem 269, 14869–14871. [PubMed] [Google Scholar]
- Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P, 2003. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat. Cell Biol 5, 1083–1089. [DOI] [PubMed] [Google Scholar]
- Coue M, Brenner SL, Spector I, Korn ED, 1987. Inhibition of actin polymerization by latrunculin A. FEBS Lett 213, 316–318. [DOI] [PubMed] [Google Scholar]
- Courson DS, Rock RS, 2010. Actin cross-link assembly and disassembly mechanics for α-actinin and fascin. J. Biol. Chem 285, 26350–26357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed SJ, Desouza M, Bamburg JR, Gunning P, Stehn J, 2011. Tropomyosin isoform 3 promotes the formation of filopodia by regulating the recruitment of actin-binding proteins to actin filaments. Exp. Cell Res 317, 249–261. [DOI] [PubMed] [Google Scholar]
- Desai R, Sarpal R, Ishiyama N, Pellikka M, Ikura M, Tepass U, 2013. Monomeric α-catenin links cadherin to the actin cytoskeleton. Nat. Cell Biol 15, 261–273. [DOI] [PubMed] [Google Scholar]
- Gourlay CW, Ayscough KR, 2005. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat. Rev. Mol. Cell Biol 6, 583–589. [DOI] [PubMed] [Google Scholar]
- Haagsma BH, Pound AW, 1980. Mercuric chloride-induced tubulonecrosis in the rat kidney: the recovery phase. Br. J. Exp. Pathol 61, 229–241. [PMC free article] [PubMed] [Google Scholar]
- Hain D, Paixao R, 2015. The perfect storm: older adults and acute kidney injury. Crit. Care Nurs 3, 271–279. [DOI] [PubMed] [Google Scholar]
- Harms C, Bösel J, Lautenschlager M, Harms U, Braun JS, Hörtnagl H, Dimagl U, Kwiatkowski DJ, Fink K, Endres M, 2004. Neuronal gelsolin prevents apoptosis by enhancing actin depolymerization. Mol. Cell. Neurosci 25, 69–82. [DOI] [PubMed] [Google Scholar]
- Horák P, Knoll A, Dvorák J, 2006. The retinal fascin gene 2 (FSCN2)—partial structural analysis and polymorphism detection in dogs with progressive retinal atrophy (PRA). J. Appl. Genet 47, 361–364. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Smith CA, Salhia B, Rutka JT, 2008. The role of fascin in the migration and invasiveness of malignant glioma cells. Neoplasia 10, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R, Yamashiro S, Kohama K, Matsumura F, 1998. Regulation of actin binding and actin bundling activities of fascin by caldesmon coupled with tropomyosin. J. Biol. Chem 273, 26991–26997. [DOI] [PubMed] [Google Scholar]
- Jayo A, Parsons M, 2010. Fascin: a key regulator of cytoskeletal dynamics. Int. J. Biochem. Cell Biol 42, 1614–1617. [DOI] [PubMed] [Google Scholar]
- Jung K-Y, Dean D, Jiang J, Gaylor S, Griffith WH, Burghardt RC, Parrish AR, 2004. Loss of N-cadherin and alpha-catenin in the proximal tubules of aging male Fischer 344 rats. Mech. Ageing Dev 125, 445–453. [DOI] [PubMed] [Google Scholar]
- Kanda Y, Kawaguchi T, Kuramitsu Y, Kitagawa T, Kobayashi T, Takahashi N, Tazawa H, Habelhah H, Hamada J, Kobayashi M, Hirahata M, Onuma M, Nakamura K, Kitagawa T, Hosokawa M, Okada F, 2014. Fascin regulates chronic inflammation-related human colon carcinogenesis by inhibiting cell anoikis. Proteomics 14, 1031–1041. [DOI] [PubMed] [Google Scholar]
- Kim J-K, Jang S-W, Suk K, Lee W-H, 2015. Fascin regulates TLR4/PKC-mediated translational activation through miR-155 and miR-125b, which targets the 30 untranslated region of TNF-α mRNA. Immunol. Invest 44, 309–320. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ, 1995. Interaction of alpha-actinin with the cadherin/catenin cell-cell adhesion complex via alpha-catenin. J. Cell Biol 130, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E, 2004. Alpha-catenin: at the junction of intercellular adhesion and actin dynamics. Nat. Rev. Mol. Cell Biol 5, 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruidering M, van de Water B, Zhan Y, Baelde JJ, Heer E, Mulder GJ, Stevens JL, Nagelkerke JF, 1998. Cisplatin effects on F-actin and matrix proteins precede renal tubular cell detachment and apoptosis in vitro. Cell Death Differ 5, 601– 614. [DOI] [PubMed] [Google Scholar]
- Kuo J-C, Lin J-R, Staddon JM, Hosoya H, Chen R-H, 2003. Uncoordinated regulation of stress fibers and focal adhesions by DAP kinase. J. Cell Sci 116, 4777–4790. [DOI] [PubMed] [Google Scholar]
- Kuo J-C, Han X, Hsiao C-T, Yates JR, Waterman CM, 2011. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat. Cell Biol 13, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C, Chen Z, Li R, 2015. MicroRNA-133a inhibits proliferation and invasion, and induces apoptosis in gastric carcinoma cells via targeting fascin actin-bundling protein 1. Mol. Med. Rep 12, 1473–1478. [DOI] [PubMed] [Google Scholar]
- Mattila PK, Lappalainen P, 2008. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol 9, 446–454. [DOI] [PubMed] [Google Scholar]
- Nemethova M, Auinger S, Small JV, 2008. Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella. J. Cell Biol 180, 1233–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols LA, Grunz-Borgmann EA, Wang X, Parrish AR, 2014a. A role for the age-dependent loss of α(E)-catenin in regulation of N-cadherin expression and cell migration. Physiol. Rep 2, e12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols LA, Slusarz A, Grunz-Borgmann EA, Parrish AR, 2014b. α(E)-Catenin regulates BMP-7 expression and migration in renal epithelial cells. Am. J. Nephrol 39, 409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS, 1995. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. U. S. A 92, 8813–8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos NAG, Bezerra CSC, Martins NM, Curti C, Bianchi MLP, Santos AC, 2008. Hydroxyl radical scavenger ameliorates cisplatin-induced nephrotoxicity by preventing oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Cancer Chemother. Pharmacol 61, 145–155. [DOI] [PubMed] [Google Scholar]
- Schiller HB, Friedel CC, Boulegue C, Fässler R, 2011. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep 12, 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt R, Coca S, Kanbay M, Tinetti ME, Cantley LG, Parikh CR, 2008. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. Am. J. Kidney Dis 52, 262–271. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN, 1994. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. U. S. A 91, 10771–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toback FG, 1992. Regeneration after acute tubular necrosis. Kidney Int 41, 226–246. [DOI] [PubMed] [Google Scholar]
- Wang X, Parrish AR, 2015. Loss of α(E)-catenin promotes Fas mediated apoptosis in tubular epithelial cells. Apoptosis 20, 921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bonventre JV, Parrish AR, 2014a. The aging kidney: increased susceptibility to nephrotoxicity. Int. J. Mol. Sci 15, 15358–15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Grunz-Borgmann EA, Parrish AR, 2014b. Loss of α(E)-catenin potentiates cisplatin-induced nephrotoxicity via increasing apoptosis in renal tubular epithelial cells. Toxicol. Sci 141, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ, 2006. Incidence and mortality of acute renal failure in medicare beneficiaries, 1992 to 2001. J. Am. Soc. Nephrol 17, 1135–1142. [DOI] [PubMed] [Google Scholar]
- Yamashiro-Matsumura S, Matsumura F, 1986. Intracellular localization of the 55-kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actinin, tropomyosin, and fimbrin. J. Cell Biol 103, 631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Yang F, Zhao W, Zhang C, Liu J, 2015. Fascin overexpression promotes cholangiocarcinoma RBE cell proliferation, migration, and nvasion. Technol. Cancer Res. Treat 15, 322–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.