Abstract
Background.
The emergence of callous unemotional (CU) traits, and associated externalizing behaviors, is believed to reflect underlying dysfunction in the amygdala. Studies of adults with CU traits or psychopathy have linked characteristic patterns of amygdala dysfunction to reduced amygdala volume, but studies in youths have not thus far found evidence of similar amygdala volume reductions. The current study examined the association between CU traits and amygdala volume by modeling CU traits and externalizing behavior as independent continuous variables, and explored the relative contributions of callous, uncaring, and unemotional traits.
Methods.
CU traits and externalizing behavior problems were assessed in 148 youths using the Inventory of Callous Unemotional Traits (ICU) and the Child Behavior Checklist (CBCL). For a subset of participants (n = 93), high-resolution T1-weighted images were collected and volume estimates for the amygdala were extracted.
Results.
Analyses revealed that CU traits were associated with increased externalizing behaviors and decreased bilateral amygdala volume. These results were driven by the callous and uncaring sub-factors of CU traits, with unemotional traits unrelated to either externalizing behaviors or amygdala volume. Results persisted after accounting for covariation between CU traits and externalizing behaviors. Bootstrap mediation analyses indicated that CU traits mediated the relationship between reduced amygdala volume and externalizing severity.
Conclusions.
These findings provide evidence that callous-uncaring traits account for reduced amygdala volume among youths with conduct problems. These findings provide a framework for further investigation of abnormal amygdala development as a key causal pathway for the development of callous-uncaring traits and conduct problems.
Keywords: Amygdala, callous unemotional traits, externalizing behaviors
Callous unemotional (CU) traits in childhood and adolescence characterize a relatively homogenous subgroup of youths with conduct problems who engage in severe and persistent antisocial and aggressive behaviors (Frick et al., 2005; Rowe et al., 2010; Kahn et al., 2013) and who are at particularly high risk of developing psychopathic traits in adulthood (Vasey et al., 2005; Salekin, 2006; Burke et al., 2007). CU traits include limited empathy and remorse and reduced displays of emotion, and are most commonly assessed using the Inventory of Callous Unemotional Traits (ICU), which is comprised of three subfactors: callousness, uncaring, and unemotionality (Frick and Ray, 2015). The emergence of these traits and subsequent behavior problems has been consistently linked to dysfunction in the amygdala (Marsh et al., 2008, 2011a; Finger et al., 2012; Viding et al., 2012; Herpers et al., 2014; Lozier et al., 2014; Breeden et al., 2015). Although it has been posited that amygdala dysfunction in CU youths may stem from major anatomical aberrations, no evidence of atypical amygdala volume in children with CU traits has yet emerged. This may be considered surprising in light of consistent findings of reduced amygdala volume in adults with psychopathy or CU traits (Yang et al., 2009; Pardini et al., 2014; Vieira et al., 2015). In the present study, we explored whether a relationship between CU traits and amygdala volume would emerge in a moderately large sample of children and adolescents in which CU traits and externalizing behaviors were simultaneously modeled as continuous variables. We also considered the relationship between the three independent CU subfactors and amygdala volume.
Theories of the development of CU traits frequently focus on deficits in affective and reinforcement learning processes that rely on the amygdala (Blair, 2013). Impaired amygdala functioning in CU youths is believed to impede their ability to learn to avoid behaviors like aggression and making threats, in part because the amygdala is important for recognizing and responding appropriately to signs of others’ distress (Marsh, 2016). Without appropriate signaling in the amygdala, CU youths fail to develop appropriate guilt and empathy in response to others’ distress, and so persist in behaviors like aggression and violence that would normally be inhibited by these emotional responses (Kochanska, 1993; Frick and Morris, 2004; Blair, 2005, 2013; Marsh, 2016; Seara-Cardoso et al., 2016). These theories are reinforced by consistent findings of reduced amygdala responsivity to fearful facial expressions in high CU youths (Marsh et al., 2008; Jones et al., 2009; Viding et al., 2012; White et al., 2012; Lozier et al., 2014) as well as aberrant functional connectivity between the amygdala and other regions implicated in emotion processing (Marsh et al., 2011a; Finger et al., 2012; Aghajani et al., 2016).
Despite a robust literature examining amygdala activity in CU youths, relatively few structural magnetic resonance imaging (MRI) studies have examined the association between CU traits and brain structure. Reduced amygdala volume has repeatedly been found in both studies of youths with conduct disorder (Sterzer et al., 2007; Huebner et al., 2008; Fairchild et al., 2013; Wallace et al., 2014; Rogers and De Brito, 2016) and studies of adults with psychopathic traits, who are distinguished from other antisocial populations primarily by their elevated CU traits (Yang et al., 2009; Cope et al., 2014; Pardini et al., 2014). These findings strongly implicate reduced amygdala volume in the development of antisociality and CU traits, but four studies of children and adolescents have not yet found an association between amygdala volume and CU traits (De Brito et al., 2009; Wallace et al., 2014; Cohn et al., 2016; Sebastian et al., 2016) (although Cohn et al. found that increased CU traits were associated with reduced amygdala gray matter concentration). This could be interpreted to mean that CU traits are associated with amygdala volume only in adulthood, but not childhood or adolescence. Alternately, methodological considerations may have concealed a relationship between amygdala volume and CU traits in youths. For example, although CU traits are continuously distributed and more accurately assessed using continuous analyses (Guay et al., 2007; Moffitt et al., 2008; Lozier et al., 2014), three of the four studies of volumetric differences in CU youths employed primarily group-based approaches (De Brito et al., 2009; Wallace et al., 2014; Sebastian et al., 2016). In addition, Wallace et al. and De Brito et al. compared youths with both CU traits and conduct problems to healthy control youths, an approach that hinders the dissociation of correlates of CU traits and conduct problems more generally (De Brito et al., 2009; Wallace et al., 2014). Sebastian et al. compared groups of youths with conduct problems and both low and high levels of CU traits to healthy controls (Sebastian et al., 2016). However, similar to the prior two studies, their use of a primarily group-based approach lacked the power of a continuous analysis, and may have been affected by suppressor effects (Sebastian et al., 2012).
Moreover, these studies all examined CU traits as a unitary construct, although, increasingly, assessments of CU traits have focused on the three subfactors comprising CU traits (callous, uncaring, and unemotional traits) (Kimonis et al., 2008a, b). Examining the independent associations between callous, uncaring, and unemotional traits with aberrant amygdala volume may clarify the relevance of amygdala development to the emergence of CU traits. Given the key role of the amygdala in emotional processing, it may be primarily the unemotional component of CU traits that is associated with reduced amygdala volume, a finding that would implicate the amygdala in global affective deficits in CU youths. By contrast, associations with the callous and/or uncaring components of CU traits would suggest that the amygdala may play a more complex role in interpersonal empathy and caring. Examination of associations between unemotional traits and amygdala volume may also provide important insight into the validity of assessments of unemotional traits, the nature of which have been subject to recent concerns (Henry et al., 2016; Cardinale and Marsh, 2017).
The current study therefore examined associations between amygdala gray matter volume and externalizing behaviors as well as callous, uncaring, and unemotional traits in a sample of youths with varying levels of conduct problems and CU traits. Through a series of multiple linear regression analyses, we investigated how total ICU scores and the three subfactor scores correspond to both aberrant structural development of the amygdala and the emergence of externalizing behaviors, and whether CU traits mediate the relationship between reduced amygdala volume and externalizing behavior severity. We hypothesized that CU traits would be associated with increased externalizing behavior problems and decreased bilateral amygdala volume. Furthermore, we predicted that these relationships would be driven by the callous and uncaring subscales of the ICU.
Methods
Participants
One hundred forty-eight children, aged 9–18 (M = 13.96, s.d. = 2.44, % male = 59.46), were recruited from Washington, DC and surrounding regions through referrals, advertisements, and fliers seeking both healthy children and children with conduct problems. All participants and their parents first completed an initial visit during which demographic and clinical measures were completed along with IQ testing using the Kauffman Brief Intelligence Test (Kaufman and Kaufman, 2004). Participants reported a wide range of scores on our clinical measures, confirming that our sample included both healthy youths and youths with elevated conduct problems and varying CU traits, as well as psychiatric symptoms including externalizing behaviors, internalizing behaviors, and attentional difficulties (Table 1). Consistent with our recruitment effort to specifically target both healthy children and children with elevated conduct problems, 77 participants reported clinical levels of externalizing behavior as assessed by an age and gender standardized externalizing symptomology score on the Child Behavior Checklist (CBCL) that placed them above the 98th percentile (Achenbach, 1991).
Table 1.
Descriptive statistics
| Total sample (n = 148) | Scanned participants (n = 84) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Range | Skew | Kurt | Mean | s.d. | Min | Skew | Kurt | p | |
| Demographic variables | |||||||||||
| Age | 13.96 | 2.44 | 9.56–18.07 | 0.01 | 1.75 | 14.60 | 2.31 | 10.46–18.45 | −0.07 | 1.75 | 0.05* |
| IQ | 101.59 | 15.84 | 60–136 | 0.05 | 2.64 | 104.92 | 14.10 | 80–136 | 0.30 | 2.34 | 0.11 |
| Gender, % male | 58.78% | 55.95% | 0.60 | ||||||||
| Race/ethnicity, n | 0.69 | ||||||||||
| White, non-Hispanic | 40 | 29 | |||||||||
| African-American, non-Hispanic | 81 | 41 | |||||||||
| Hispanic | 14 | 7 | |||||||||
| Other | 13 | 7 | |||||||||
| Clinical measures | |||||||||||
| CU | 36.64 | 12.11 | 13–63 | 0.05 | 2.19 | 35.89 | 13.15 | 13–62 | 0.24 | 1.86 | 0.66 |
| Callous | 11.33 | 6.55 | 0–29 | 0.57 | 2.67 | 10.94 | 6.77 | 0–29 | 0.47 | 2.49 | 0.67 |
| Uncaring | 14.83 | 5.25 | 2–24 | −0.30 | 2.14 | 14.58 | 5.61 | 5–23 | −0.17 | 1.75 | 0.73 |
| Unemotional | 8.98 | 2.54 | 3–15 | 0.01 | 2.69 | 8.79 | 2.55 | 3–15 | 0.10 | 2.75 | 0.59 |
| Externalizing | 17.99 | 15.64 | 0–57 | 0.50 | 2.07 | 18.79 | 16.73 | 0–52 | 0.34 | 1.69 | 0.72 |
| Internalizing | 10.88 | 10.82 | 0–48 | 1.43 | 4.69 | 11.43 | 11.71 | 0–48 | 1.41 | 4.44 | 0.72 |
| Attentional difficulties | 7.05 | 5.67 | 0–19 | 0.33 | 1.89 | 6.45 | 5.63 | 0–18 | 0.45 | 1.92 | 0.44 |
| Brain volume estimates (mm3) | |||||||||||
| Left amygdala | – | – | – | – | – | 1686 | 221 | 1194–2163 | 0.21 | 2.53 | – |
| Right amygdala | – | – | – | – | – | 1704 | 233 | 1171–2273 | 0.11 | 2.81 | – |
| Intracranial volume | – | – | – | – | – | 1 499 457 | 159 859 | 1 193 760–1 864 635 | 0.19 | 2.29 | – |
p Values are reported for comparisons of total sample v. scanned participants.
Of participants who completed the initial visit, 93 were eligible for and consented to participate in an MRI scan. Participants were excluded from MRI scanning for: history of head trauma or neurological disorder, symptoms of pervasive developmental disorder, IQ <80, or MRI contraindications such as claustrophobia or metallic implants including braces or permanent retainers. The MRI sample consisted of children aged 10–17 (M = 13.98, s.d. = 2.36, % male = 59.14) and varied widely in externalizing behavior, including 46 participants with clinically significant externalizing scores. The MRI sample did not differ from the full sample in terms of externalizing and CU scores or any other clinical or demographic measures, with the exception of a trend-level difference in age between the full sample and the scanned sample (Table 1). All participants were native English speakers. Written informed assent and consent were obtained from children and parents before testing. Approval for all procedures was obtained from the Georgetown University Institutional Review Board. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Clinical measures
Inventory of Callous Unemotional Traits
The ICU was used to assess CU traits (Kimonis et al., 2008a, b). The ICU was completed separately by parents and participants. Scores on the ICU were calculated by summing the highest item response from either the child or parent version (Jones et al., 2009; Sebastian et al., 2012; Viding et al., 2012; Lozier et al., 2014; Breeden et al., 2015). This scoring approach follows the recommended scoring practices for the parent scale of the ICU (Frick and Hare, 2001), and has been shown to reduce susceptibility to social desirability biases and optimize accuracy across multiple contexts (Piacentini et al., 1992; Frick et al., 2003).
Child Behavior Checklist
The CBCL is a parent report-based assessment of behavioral and emotional problems in children and adolescents (Achenbach, 1991). Externalizing and internalizing syndrome scales were calculated for each participant. Attentional difficulties were also measured using the attention difficulties syndrome scale. The use of the CBCL to assess the severity of various clinical symptoms in community samples has been demonstrated to be reliable and valid (Biederman et al., 1993; Warnick et al., 2008).
Image acquisition and analysis
Three-dimensional anatomical images were acquired using a 3.0 Tesla Siemens (Erlangen, Germany) TIM Trio. High-resolution T1-weighted images were collected for each participant (TR = 1900 ms, TE = 2.52 ms, TI = 900.0 ms, 1.0 mm3 voxels, 176 slices, matrix = 246 × 256, field of view = 250 mm2). Prior to analyses, all images were visually inspected for motion artifacts. Any potential motion artifacts were examined by three independent evaluators and only scans for which all three evaluators reached agreement were included in the dataset. Thirty-nine participants completed more than one anatomical scan; the scan with the fewest motion artifacts and clearest contrast was selected for these participants. Data from nine participants could not be analyzed due to excessive motion artifacts in all completed anatomical scans, resulting in a final sample size of 84 participants. Images were collected using an eight-channel phased-array head coil for 16 participants and using a 12-channel phased-array head coil for the remaining 68 participants. For all analyses investigating neural volume, a dummy coded variable for use of the 8 v. 12-channel was included as a covariate (Breeden et al., 2015). Because participants completed the scan during a separate visit, we also included age at the time of the scan in addition to age at the time of the initial visit for all analyses investigating neural volume.
Anatomical images were analyzed using FreeSurfer version 5.3.0. Automated segmentation of subcortical regions occurred during the first stage of the FreeSurfer cortical reconstruction process (Fischl et al., 2002, 2004; Fischl, 2012). During this stage, neuroanatomical labels are automatically assigned to each voxel based on probabilistic information acquired through an a priori knowledge of spatial relationships acquired through a manually labeled training set. This classification technique is robust to anatomical variation typical in pediatric populations through the use of a non-linear registration procedure. Segmentation occurs following three automated strategies to disambiguate voxel labels, which assess the prior probability of the tissue class occurring at an atlas location, and given the tissue class, the likelihood of the image and the probability of the local spatial configuration. The resulting subcortical segmentation, has been shown to be reliable (Morey et al., 2010) and comparable to manual segmentation (Fischl et al., 2002; Morey et al., 2009; Grimm et al., 2015; Schoemaker et al., 2016). Following segmentation, 42 subcortical regions were identified and labeled using both subject-independent probabilistic atlases and subject-specific measured variables for each subject. All images were visually inspected following segmentation. Volume estimates for all subcortical regions, including the left amygdala and right amygdala, as well as total intracranial volume were extracted and exported for analysis in STATA (Table 1; online Supplementary Table S2).
Results
For all analyses, variables were mean centered, and known correlates of amygdala volume and/or externalizing behaviors were entered as covariates. For analyses of clinical symptomology, gender, IQ, and age at initial visit were included as covariates. For analyses of brain volume, we included total intracranial volume, age at time of scan, and headcoil as additional covariates. Robust standard errors were used to account for heteroscedasticity of the experimental variables and to control for sibling effects. All analyses were repeated with dummy variables coding for the 18 sibling groups present in these data (n = 41). Findings were not affected by the inclusion of these covariates. Therefore, for ease of interpretation of results, we report the findings from analyses excluding sibling status as a covariate.
ICU scores and clinical symptomology
The internal consistency of total ICU, a = 0.90, callous subscale, a = 0.84, and uncaring subscale, a = 0.87 were acceptable, whereas the unemotional subscale showed relatively low internal consistency, a = 0.58. Intercorrelations among scores on the ICU were all large (online Supplementary Table S1).
Results of a multiple linear regression analysis across all participants (n = 148) predicting externalizing behaviors from ICU scores confirmed that as total ICU scores increased, externalizing behaviors increased, β = 0.73, t(143) = 11.73, p<0.001 (Fig. 1). This association remained significant after controlling for attentional difficulty and internalizing behavior scores, β = 0.31, t(141) = 4.55, p < 0.001. Next, a multiple regression with all three ICU subscales predicting externalizing behavior problems found that scores on the callous, β = 0.53, t(141) = 4.56, p <0.001, and uncaring, β = 0.34, t(141) = 3.30, p = 0.001, subscales were independently associated with increased externalizing, whereas unemotional subscale scores were not, β = −0.07, t(141) = −1.13, p = 0.26 (Fig. 1). Associations between externalizing and the callous, β = 0.21, t(139) = 3.03, p = 0.003, and uncaring, β = 0.20, t(139) = 3.19, p = 0.002, subscales persisted when controlling for attentional difficulties and internalizing behaviors, whereas the unemotional subscale scores remained non-significant, β = −0.08, t(139) = −1.37, p = 0.17.
Fig. 1.
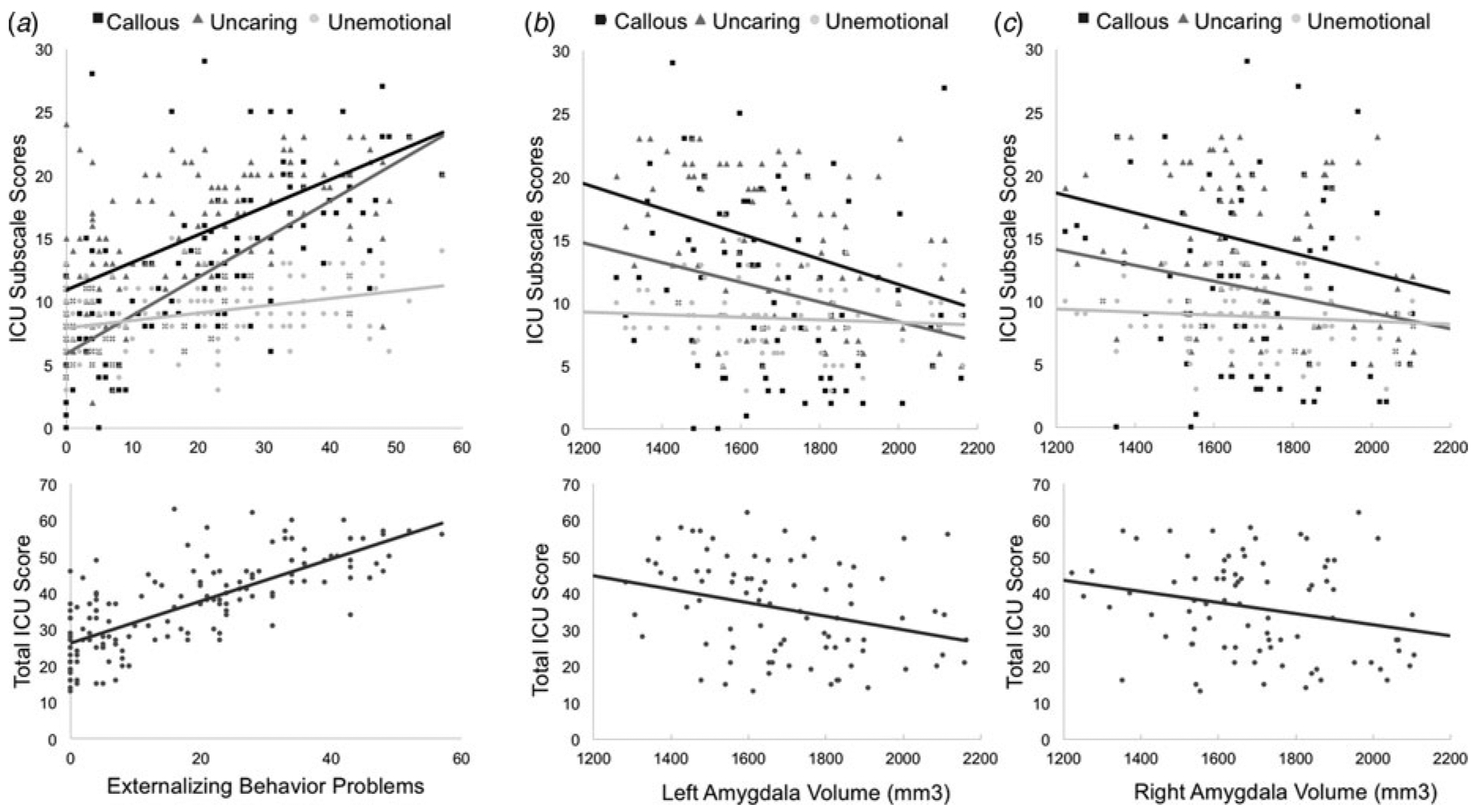
Scatter plots for the associations between total scores, callous, uncaring, and unemotional subscale scores on the ICU with (a) externalizing behaviors and (b) left and (c) right amygdala volumes.
We repeated all of the above analyses examining the relationship between ICU scores and clinical symptomologies restricted to only those participants who qualified for inclusion in MRI scanning (n = 84). All patterns of significant findings persisted when analyses were limited to this sample (online Supplementary Text S1), supporting the reliability of the identified patterns.
Amygdala volume
We next investigated associations between amygdala volume, scores on the ICU, and externalizing symptoms using a region of interest (ROI) approach, consistent with the approaches used in previous studies of neural correlates of CU traits and psychopathy (Sebastian et al., 2012; Lozier et al., 2014; Pardini et al., 2014; Wallace et al., 2014; Breeden et al., 2015; Vieira et al., 2015; Sebastian et al., 2016). Again, analyses included age at scanning, headcoil type, and total intracranial volume as covariates in addition to age at time of initial visit, gender, and IQ. Results of separate multiple linear regression analyses revealed that total ICU scores were associated with decreased left, β = −0.36, t(76) = −3.21, p = 0.002, and right, β = −0.27, t(76) = −2.64, p = 0.01, amygdala volume (Fig. 2).
Fig. 2.
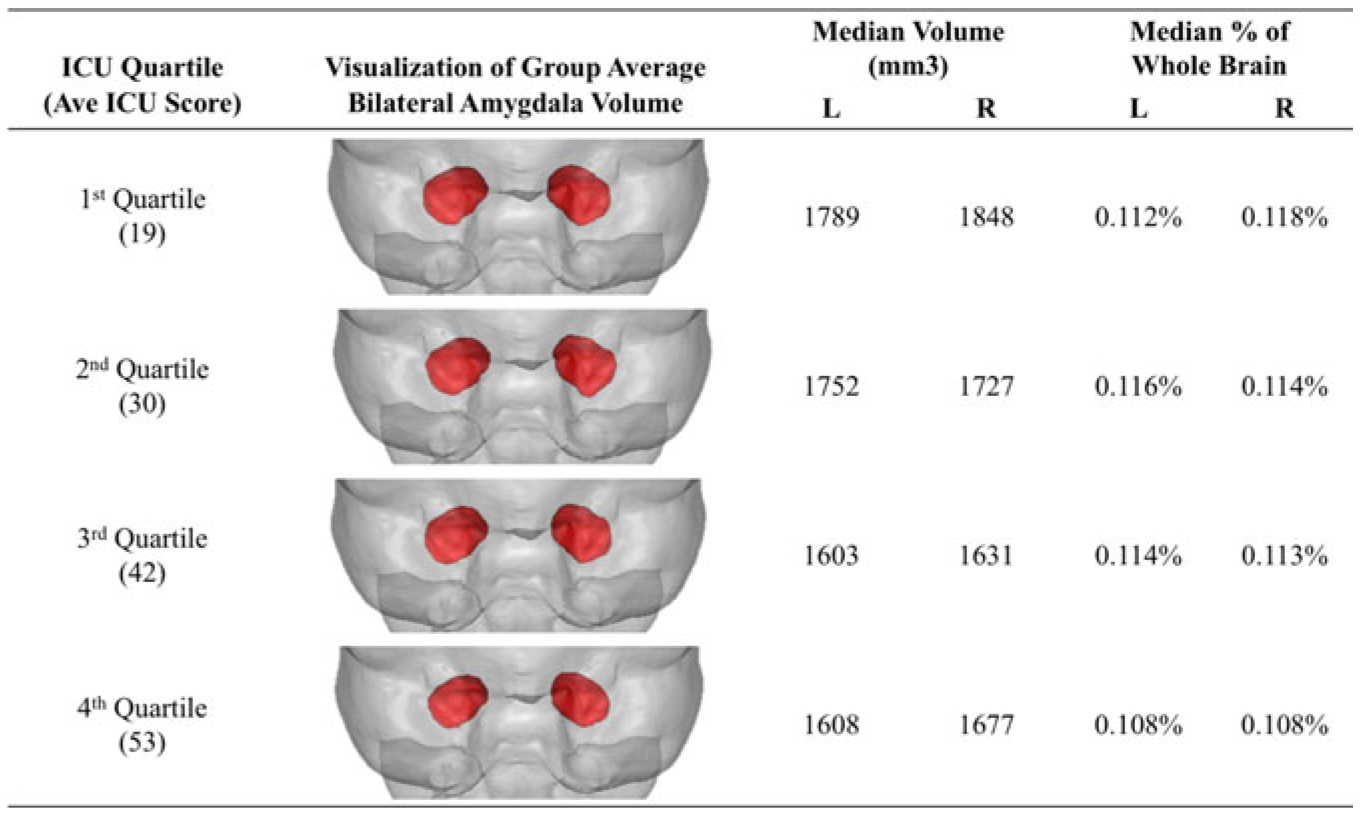
Visualizations of average amygdala volumes for each ICU total score quartile rendered within the mean brain volume of all subjects. Shown in anterior view with the right hemisphere displayed on the right.
Similar results were obtained for callous [left: β = −0.32, t(76) = −3.00, p = 0.004; right: β = −0.24, t(76) = −2.40, p = 0.02] and uncaring [left: β = −0.35, t(76) = −3.07, p = 0.003; right: β = −0.24, t(76) = −2.34, p = 0.02] subscale scores. Unemotional subscale scores were associated with right amygdala volume at a trend level, β = −0.17, t(76) = −1.97, p = 0.05, but not left amygdala volume, β = −0.18, t(76) = −1.64, p = 0.11 (Fig. 1).
To assess the specificity of these findings, we conducted parallel analyses examining associations between CU traits and subcortical volume estimates for the nucleus accumbens, caudate, hippocampus, pallidum, putamen, thalamus, and ventral diencephalon (DC). Across all of these subcortical regions, no significant associations (all p >0.10) were found with ICU total (online Supplementary Table S3) or subscale scores (online Supplementary Table S4), or with externalizing behaviors (online Supplementary Table S5), with one exception: decreased right ventral DC volume was associated with increased externalizing behaviors, β = −0.18, t(76) = −2.18, p = 0.03, and uncaring traits, β = −0.17, t(76) = −2.18, p = 0.03, but no other measure of CU traits. Of note, neither ICU scores nor externalizing behaviors were associated with total intracranial volume, all p >0.10.
Multiple linear regression analyses in which externalizing behavior problems were entered as predictors of left and right amygdala volumes found that externalizing problems were associated with decreased left, β = −0.27, t(76) = −2.54, p = 0.01, but not right, β = −0.18, t(76) = −1.88, p = 0.06, amygdala volume. When both externalizing behaviors and total ICU scores were included simultaneously in the model, CU traits remained predictors of both left, β = −0.44, t(75) = −2.42, p = 0.02, and right, β = −0.40, t(76) = −2.21, p = 0.03, amygdala volume, but the relationship between externalizing behaviors and amygdala volume was rendered non-significant. Consistent with this pattern of findings, mediation analyses across all participants using the SPSS PROCESS macro revealed a significant indirect effect of amygdala volumes on externalizing behaviors through CU traits (online Supplementary Fig. S1), such that the observed direct statistical relationship between decreased left and right amygdala volumes with increased externalizing behaviors was explained by the statistical relationship between each of these variables with CU traits (left: Sobel Z = −3.50, p = .001; right: Sobel Z = −2.60, p = 0.01).
Following persistent findings that the unemotional subscale was not closely associated with either externalizing behaviors or amygdala volumes, we created a composite callous-uncaring score by summing responses to items comprising only the callous and uncaring subscales (scale reliability was acceptable, α = 0.90). Callous-uncaring scores predicted right and left amygdala volumes, even after accounting for externalizing scores (Table 2). Mediation analyses that included callous-uncaring composite scores revealed that callous-uncaring traits, specifically, mediated the statistical relationship between decreased amygdala volume and increased externalizing behaviors (left: Sobel Z = −3.56, p < 0.001; right: Sobel Z = −2.55, p = 0.01).
Table 2.
Multiple regression models predicting left and right amygdala volumes
| Left amygdala | Right amygdala | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (1) | (2) | (3) | (4) | (5) | |
| Total ICU score | −0.36 (0.11)** | −0.44 (0.18)* | −0.27 (0.10)* | −0.40 (0.18)* | ||||||
| Callous uncaring traits | −0.36 (0.11)** | −0.47 (0.19)* | −0.26 (0.10)* | −0.39 (0.20)* | ||||||
| Externalizing behaviors | −0.27 (0.11)* | 0.10 (0.18) | 0.13 (0.19) | −0.18 (0.09) | 0.16 (0.17) | 0.16 (0.18) | ||||
| Covariates | ||||||||||
| Age at time of screen | 0.04 (0.38) | −0.12 (0.33) | −0.17 (0.31) | −0.13 (0.34) | −0.19 (0.32) | 0.45 (0.25) | 0.34 (0.23) | 0.27 (0.24) | 0.34 (0.24) | 0.27 (0.24) |
| Age at time of scan | 0.15 (0.34) | 0.27 (0.30) | 0.31 (0.29) | 0.27 (0.31) | 0.30 (0.30) | −0.43 (0.23) | −0.34 (0.22) | −0.30 (0.22) | −0.35 (0.22) | −0.31 (0.22) |
| Gender | 0.17 (0.09) | 0.22 (0.09)* | 0.24 (0.10)* | 0.21 (0.09)* | 0.22 (0.10)* | 0.12 (0.10) | 0.16 (0.09) | 0.18 (0.10) | 0.15 (0.10) | 0.16 (0.10) |
| IQ | −0.01 (0.13) | −0.06 (0.13) | −0.05 (0.13) | −0.06 (0.13) | −0.06 (0.13) | −0.03 (0.10) | −0.07 (0.10) | −0.06 (0.10) | −0.07 (0.10) | −0.07 (0.10) |
| Headcoil | −0.02 (0.07) | −0.004 (0.07) | −0.003 (0.07) | 0.003 (0.07) | 0.01 (0.07) | −0.03 (0.10) | −0.01 (0.10) | −0.01 (0.10) | −0.01 (0.10) | −0.004 (0.10) |
| Total intracranial volume | 0.49 (0.09)*** | 0.49 (0.08)*** | 0.50 (0.08)*** | 0.49 (0.09)*** | 0.50 (0.08)*** | 0.61 (0.08)*** | 0.61 (0.08)*** | 0.62 (0.07)*** | 0.61 (0.08)*** | 0.61 (0.08)*** |
p < 0.05,
p < 0.01,
p < 0.001.
We report five independent regression models for the prediction of left and right amygdala volume separately. Each regression model is reported in its own column as indicated by the numbers 1–5. Standardized betas with standard error in parentheses. Significant effects in bold.
Age and gender as moderators
We next examined whether age or gender moderated the relationship between CU traits and amygdala volume. Moderation analyses were conducted using the SPSS PROCESS macro. We selected a model such that both age and gender were entered as moderators of the relationship between CU traits and amygdala volume. Results revealed that both age, β = 0.19, t(73) = 2.10, p = 0.04, and gender, β = −0.31, t(73) = −2.86, p = 0.01, were moderators, such that the conditional effect of CU traits on predicting amygdala volume is greater at younger ages and in male participants (Fig. 3). Whereas in male participants, the conditional effect of CU traits on amygdala volume is significant across all age ranges but greatest at younger ages; within female participants, the conditional effect of CU traits on right amygdala volume is non-significant at all ages and only in younger females is the conditional effect of CU traits on left amygdala volume significant. Of note, IQ, age, externalizing behaviors, and CU traits were not significantly different across male and female participants in our sample. Whereas age was unrelated to IQ, gender, or CU traits, we observed a significant bivariate correlation between age and externalizing behaviors, r(84) = 0.26, p = 0.02.
Fig. 3.
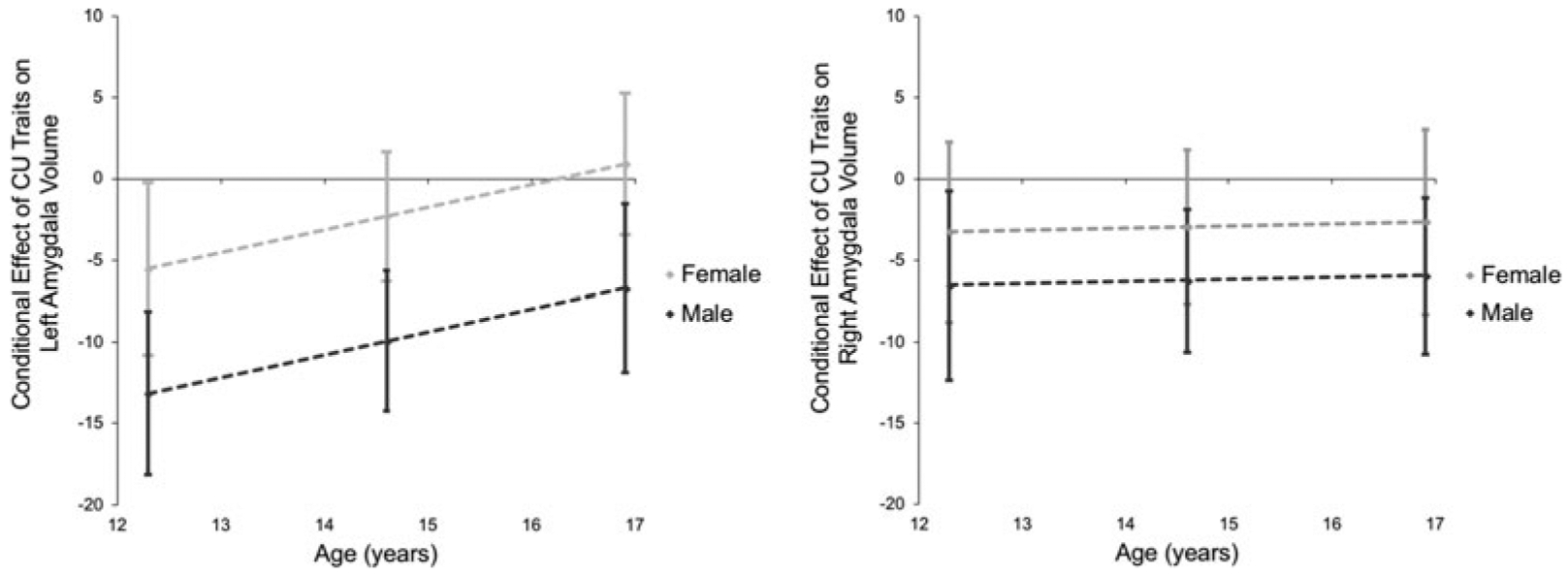
Age and gender moderate the relationship between CU traits and amygdala volume. The conditional effect of CU traits on amygdala volume plotted as a function of age separately for male and female participants. Note: 95% CI depicted as error bars at the mean age and ±1s.d.
Discussion
These findings provide the first evidence linking CU traits to reduced amygdala gray matter volume in youths. Across a mixed-gender sample of children with varying levels of externalizing behavior and CU traits, we found that variation in amygdala volume is associated with levels of both callous-uncaring traits and antisocial and externalizing behaviors, even after accounting for variation in children’s age, sex, cognitive abilities, and total intracranial volume. In our sample, the volume of left amygdala was associated with engagement in externalizing behaviors (e.g. aggression, theft, rule-breaking) and the volume of both left and right amygdala was associated with CU traits including limited empathy, remorse, and guilt. Multiple regression analyses revealed, however, that when externalizing behaviors and CU traits were modeled simultaneously, only CU traits remained associated with amygdala volume. Moreover, the relationship between amygdala volume and CU traits primarily reflected callous and uncaring subscale scores (both of which were also robustly associated with externalizing behavior), supporting a role for the amygdala in interpersonal empathy and caring. Alone, unemotional traits as measured by the ICU were unrelated to externalizing behaviors or amygdala volume. Comparable patterns were not observed in parallel analyses of subcortical volume, suggesting that findings were specific to the amygdala rather than limbic structures generally.
Together, these findings suggest that aberrant development of the amygdala, particularly relative reductions in bilateral amygdala volume as a proportion of total intracranial volume, may play a role in the emergence of CU traits and subsequent externalizing behavior. Structural amygdala abnormalities in high CU youths may lead to functional impairments in stimulus reinforcement learning and empathy, two processes that typically promote the avoidance of externalizing behaviors that cause distress in others (Marsh, 2016; Seara-Cardoso et al., 2016). Among callous and uncaring youths, developmental deficits in the amygdala may stunt the development of empathy and guilt, leading to engagement in increased externalizing behaviors such as aggression (Kochanska, 1993; Frick and Morris, 2004; White et al., 2009). The results of our moderator analyses suggest a developmental trajectory for the association between amygdala volume and CU traits such that the association between reduced amygdala volume with elevated CU traits was greatest at younger ages within our sample. This is consistent with the hypothesis that the amygdala plays a key role in moral development in childhood and early adolescence, such that anatomical abnormalities during this period are particularly important. Gender also emerged as a significant moderator. While we observe similar patterns in both genders, the association between CU traits and amygdala volume was stronger and more consistent in males. Future studies should investigate younger developmental periods, as the associations between amygdala volume and CU traits in female children may be stronger at even younger ages given evidence that brain maturation occurs earlier in females in comparison to males (Giedd et al., 1999; Lenroot et al., 2007).
Our findings contrast with those of four previous investigations that have observed no correspondence between amygdala gray matter volume and CU traits (De Brito et al., 2009; Wallace et al., 2014; Cohn et al., 2016; Sebastian et al., 2016). However, the present investigation benefited from several alternate analytical approaches that may explain this disparity. We employed continuous analyses of CU traits rather than group-based analyses, in keeping with emerging trends in evaluating CU traits (and psychopathology more generally) (Guay et al., 2007; Moffitt et al., 2008; Lozier et al., 2014). Given that CU traits are highly correlated with externalizing behaviors, groups defined by CU traits may also be characterized by high levels of externalizing behaviors, making it more difficult to isolate variables that are specifically associated with CU traits. Furthermore, lack of group differences in amygdala volume could result from suppressor effects arising from the strong positive correlation between externalizing behaviors and CU traits but inverse associations of CU traits and externalizing behaviors with various aspects of neural development (Sebastian et al., 2012; Viding et al., 2012; Lozier et al., 2014). Our findings are consistent with the existence of suppressor effects for the associations between CU traits, externalizing behaviors, and amygdala volume. Examined separately, both externalizing behaviors and CU traits were associated with decreased amygdala volume. But when entered together in a multiple regression, the suppressor effect became evident through the emergence of CU traits as associated with decreased amygdala volume while externalizing behaviors were (non-significantly) associated with increased amygdala volume.
In addition, the current study employed FreeSurfer to extract measures of subcortical volume, whereas three of the four previous studies used voxel-based morphometry (VBM) in Statistical Parametric Mapping (SPM) (De Brito et al., 2009; Cohn et al., 2016; Sebastian et al., 2016). There is some evidence that the use of different analytic techniques can produce different results for the examination of subcortical gray matter structures (Heinen et al., 2016; Katuwal et al., 2016; Popescu et al., 2016), which could be due to fundamental methodological differences or their varied statistical requirements. The main aim of VBM is to characterize differences in the local composition of brain tissues (at the voxel level) while discounting gross anatomical and positional differences (Mechelli et al., 2005) accomplished through spatial normalization to a template space. Each voxel is then assigned a value indicating the concentration of a given tissue class (i.e. gray matter), which is then statistically analyzed using mass-univariate testing. By contrast, FreeSurfer’s subcortical segmentation pipeline labels each voxel as being part of a particular brain region based on anatomical priors. The resulting metrics are not at the voxel level but rather the volume of the segmented subcortical structure. While VBM is highly applicable for data-driven analyses, variations in local gray matter (i.e. within the medial temporal lobe) may be anatomically imprecise and difficult to interpret. FreeSurfer was chosen for the current study given our anatomically specific hypotheses, desire for strong interpretability and detailed statistical modeling procedure. One previous study employed similar analyses in FreeSurfer as the current study (Wallace et al., 2014). However, the group-level statistical models primarily employed a group-based approach and did not account for covariation between CU traits and conduct problems.
Our findings are also consistent with recent concerns about the validity of the unemotional subscale of the ICU (Roose et al., 2010; Byrd et al., 2013; Kimonis et al., 2013; Hawes et al., 2014; Waller et al., 2015; Henry et al., 2016). Among the studies that have investigated associations between externalizing behaviors and subfactors of CU traits, callous and uncaring traits generally demonstrate stronger associations with externalizing behaviors than do unemotional traits (Essau et al., 2006; Kimonis et al., 2008b; Ciucci et al., 2014; Gluckman et al., 2016) and may emerge from distinct etiologies (Henry et al., 2016), which, as our findings suggest, may influence the growth of the amygdala during adolescence. We found no association between the unemotional subscale and either externalizing behaviors or amygdala volume, suggesting that the unemotional subscale of the ICU may fail to capture the affective deficits underlying CU traits. This could be due to poor psychometric properties of the scale, such as poor internal reliability (α = 0.58 in our sample) and small correlations with total ICU scores. Alternatively, unemotionality as a construct may fail to capture the nature of affective deficits underlying CU traits, which are not uniformly associated with deficits in all aspects of emotion (Cardinale et al., 2018). Whereas high CU youths frequently report and exhibit decreased experience of fear (Kimonis et al., 2008a; Muñoz et al., 2008; Jones et al., 2010; Marsh et al., 2011b), reports and experiences of, for example, disgust and happiness may be relatively unaffected (Marsh and Blair, 2008; Marsh et al., 2011b; Dawel et al., 2012).
The current study is limited in its ability to draw causal conclusions regarding reduced amygdala volume and the emergence of CU traits and externalizing behavior problems due to the cross-sectional design of this study. Future longitudinal work assessing amygdala volume at various stages in childhood, as well as the trajectory of CU traits and externalizing behavior problems across childhood, adolescence, and into adulthood, would better allow for a more direct investigation of the causal role of the amygdala in the development of CU traits and externalizing behavior. In addition, the current study is limited in that it only investigated associations with subcortical structure. Previous work has linked CU traits to structural abnormalities in cortical regions such as the ventromedial prefrontal cortex, insula, and anterior cingulate cortex (Wallace et al., 2014; Sebastian et al., 2016). As such, future investigation of the association between dimensionally assessed CU traits and subsequent externalizing behaviors with measures of cortical thickness, surface area, and curvature using surface-based methods is necessary to acquire a full understanding of neuroanatomical deficits underlying the development of CU traits.
Despite this limitation, these findings provide the first evidence for volumetric abnormalities in the amygdala associated with CU traits in childhood and adolescence. Our study, along with findings linking psychopathy and decreased amygdala volume in adulthood (Pardini et al., 2014), supports theories that CU traits reflect underlying neuroanatomical deficits during development (Blair et al., 2006; Blair, 2013) and provides the framework for further investigation of abnormal amygdala growth as a key causal pathway for the development of CU traits and conduct problems. This has implications for the potential identification of biomarkers for CU traits early in development and suggests that early interventions aimed at fostering healthy amygdala development may reduce the emergence of CU traits and conduct problems in youths.
Supplementary Material
Financial support.
This research was supported by NIH/NICHD (A.A., R03 HD 064906-01); The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) (J.V.M., NIH/NCATS 1KL2RR031974-01); Intellectual and Developmental Disabilities Research Center (IDDRC) at Children’s National Medical Center (J.V.M., NIH/NICHD 2P30HD040677-11).
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001927.
References
- Achenbach TM (1991) Manual for the Child Behavior Checklist: 4–18. Burlington: University of Vermont. [Google Scholar]
- Aghajani M, Klapwijk ET, van der Wee NJ, Veer IM, Rombouts SA, Boon AE, van Beelen P, Popma A, Vermeiren R, and Colins OF (2016) Disorganized amygdala networks in conduct-disordered juvenile offenders With callous-unemotional traits. Biological Psychiatry 82, 283–293. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Doyle A, Lehman BK, Kraus I, Perrin J and Tsuang MT (1993) Convergence of the Child Behavior Checklist with structured interview-based psychiatric diagnoses of ADHD children with and without comorbidity. Journal of Child Psychology and Psychiatry 34, 1241–1251. [DOI] [PubMed] [Google Scholar]
- Blair RJ (2013) The neurobiology of psychopathic traits in youths. Nature Reviews Neuroscience 14, 786–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DGV and Pine DS (2006) The development of psychopathy. Journal of Child Psychology and Psychiatry 47, 262–276. [DOI] [PubMed] [Google Scholar]
- Blair RJR (2005) Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology 17, 865–891. [DOI] [PubMed] [Google Scholar]
- Breeden AL, Cardinale EM, Lozier LM, VanMeter JW and Marsh AA (2015) Callous-unemotional traits drive reduced white-matter integrity in youths with conduct problems. Psychological Medicine 45, 3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JD, Loeber R and Lahey BB (2007) Adolescent conduct disorder and interpersonal callousness as predictors of psychopathy in young adults. Journal of Clinical Child and Adolescent Psychology 35, 334–346. [DOI] [PubMed] [Google Scholar]
- Byrd AL, Kahn RE and Pardini DA (2013) A validation of the Inventory of Callous-Unemotional Traits in a community sample of young adult males. Journal of Psychopathology Behavioral Assessment 35, 20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale EM, Breeden AL, Robertson EL, Lozier LM, VanMeter JW and Marsh AA (2018) Externalizing behavior severity in youths with callous-unemotional traits corresponds to patterns of amygdala activity and connectivity during judgments of causing fear. Development and Psychopathology 30, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale EM and Marsh AA (2017) The Reliability and Validity of the Inventory of Callous Unemotional Traits. A Meta-Analytic Review. Assessment, 1073191117747392. [DOI] [PubMed] [Google Scholar]
- Ciucci E, Baroncelli A, Franchi M, Golmaryami FN and Frick PJ (2014) The association between callous-unemotional traits and behavioral and academic adjustment in children: further validation of the Inventory of Callous-Unemotional Traits. Journal of Psychopathology and Behavioral Assessment 36, 189–200. [Google Scholar]
- Cohn MD, Viding E, McCrory E, Pape L, van den Brink W, Doreleijers TAH, Veltman DJ and Popma A (2016) Regional grey matter volume and concentration in at-risk adolescents: untangling associations with callous-unemotional traits and conduct disorder symptoms. Psychiatry Research 254, 180–187. [DOI] [PubMed] [Google Scholar]
- Cope LM, Ermer E, Nyalakanti PK, Calhoun VD and Kiehl KA (2014) Paralimbic gray matter reductions in incarcerated adolescent females with psychopathic traits. Journal of Abnormal Child Psychology 42, 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawel A, O’Kearney R, McKone E and Palermo R (2012) Not just fear and sadness: meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neuroscience & Biobehavioral Review 36, 2288–2304. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, Hodgins S and Viding E (2009) Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain 132, 843–852. [DOI] [PubMed] [Google Scholar]
- Essau CA, Sasagawa S and Frick PJ (2006) Callous-unemotional traits in a community sample of adolescents. Assessment 13, 454–469. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Hagan CC, Walsh ND, Passamonti L, Calder AJ and Goodyer IM (2013) Brain structure abnormalities in adolescent girls with conduct disorder. Journal of Child Psychology and Psychiatry 54, 86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012) Freesurfer. Neuroimage 62, 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B and Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT and Dale AM (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23, S69–S84. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh AM, Blair KS, Majestic C, Evangelou I, Gupta K, Schneider MR, Sims C, Pope K, Fowler K, Sinclair S, Tovar-Moll F, Pine D and Blair RJ (2012) Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research 202, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT and Loney BR (2003) Callous-unemotional traits and developmental pathways to severe conduct problems. Developmental Psychology 39, 246–260. [DOI] [PubMed] [Google Scholar]
- Frick PJ and Hare RD (2001) Antisocial Process Screening Device: APSD. Toronto: Multi-Health Systems. [Google Scholar]
- Frick PJ and Morris AS (2004) Temperament and developmental pathways to conduct problems. Journal of Clinical Child and Adolescent Psychology 33, 54–68. [DOI] [PubMed] [Google Scholar]
- Frick PJ and Ray JV (2015) Evaluating callous-unemotional traits as a personality construct. Journal of personality 83, 710–722. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Stickle TR, Dandreaux DM, Farrell JM and Kimonis ER (2005) Callous–unemotional traits in predicting the severity and stability of conduct problems and delinquency. Journal of Abnormal Child Psychology 33, 471–487. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC and Rapoport JL (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience 2, 861–863. [DOI] [PubMed] [Google Scholar]
- Gluckman NS, Hawes DJ and Russell AM (2016) Are callous-unemotional traits associated with conflict adaptation in childhood. Child Psychiatry & Human Development 47, 583–592. [DOI] [PubMed] [Google Scholar]
- Grimm O, Pohlack S, Cacciaglia R, Winkelmann T, Plichta MM, Demirakca T and Flor H (2015) Amygdalar and hippocampal volume: a comparison between manual segmentation, FreeSurfer and VBM. Journal of Neuroscience Methods 253, 254–261. [DOI] [PubMed] [Google Scholar]
- Guay JP, Ruscio J, Knight RA and Hare RD (2007) A taxometric analysis of the latent structure of psychopathy: evidence for dimensionality. Journal of Abnormal Psychology 116, 701–716. [DOI] [PubMed] [Google Scholar]
- Hawes SW, Byrd AL, Henderson CE, Gazda RL, Burke JD, Loeber R and Pardini DA (2014) Refining the parent-reported Inventory of Callous-Unemotional Traits in boys with conduct problems. Psychological Assessment 26, 256–266. [DOI] [PubMed] [Google Scholar]
- Heinen R, Bouvy WH, Mendrik AM, Viergever MA, Biessels GJ and de Bresser J (2016) Robustness of automated methods for brain volume measurements across different MRI field strengths. PLoS ONE 11, e0165719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J, Pingault JB, Boivin M, Rijsdijk F and Viding E (2016) Genetic and environmental aetiology of the dimensions of callous-unemotional traits. Psychological Medicine 46, 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpers PC, Scheepers FE, Bons DMA, Buitelaar JK and Rommelse NNJ (2014) The cognitive and neural correlates of psychopathy and especially callous-unemotional traits in youths: a systematic review of the evidence. Development and Psychopathology 26, 245–273. [DOI] [PubMed] [Google Scholar]
- Huebner T, Vloet TD, Marx I, Konrad K, Fink GR, Herpertz SC and Herpertz-Dahlmann B (2008) Morphometric brain abnormalities in boys with conduct disorder. Journal of the American Academy of Child Adolescent Psychiatry 47, 540–547. [DOI] [PubMed] [Google Scholar]
- Jones AP, Happe FG, Gilbert F, Burnett S and Viding E (2010) Feeling, caring, knowing: different types of empathy deficit in boys with psychopathic tendencies and autism spectrum disorder. Journal of Child Psychology and Psychiatry 51, 1188–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ and Viding E (2009) Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry 166, 95–102. [DOI] [PubMed] [Google Scholar]
- Kahn RE, Byrd AL and Pardini DA (2013) Callous-unemotional traits robustly predict future criminal offending in young men. Law and Human Behavior 37, 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katuwal GJ, Baum SA, Cahill ND, Dougherty CC, Evans E, Evans DW, Moore GJ and Michael AM (2016) Inter-method discrepancies in brain volume estimation may drive inconsistent findings in autism. Frontiers in Neuroscience 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS and Kaufman NL (2004) Kaufman brief intelligence test (2nd ed.). In Encyclopedia of Special Education. Hoboken, NJ: John Wiley & Sons, Inc. [Google Scholar]
- Kimonis ER, Branch J, Hagman B, Graham N and Miller C (2013) The psychometric properties of the Inventory of Callous-Unemotional Traits in an undergraduate sample. Psychological Assessment 25, 84–93. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Munoz LC and Aucoin KJ (2008a) Callous·-unemotional traits and the emotional processing of distress cues in detained boys: testing the moderating role of aggression, exposure to community violence, and histories of abuse. Development and Psychopathology 20, 569–589. [DOI] [PubMed] [Google Scholar]
- Kimonis ER, Frick PJ, Skeem JL, Marsee MA, Cruise K, Munoz LC, Aucoin KJ and Morris AS (2008b) Assessing callous-unemotional traits in adolescent offenders: validation of the Inventory of Callous-Unemotional Traits. International Journal of Law and Psychiatry 31, 241–252. [DOI] [PubMed] [Google Scholar]
- Kochanska G (1993) Toward a synthesis of parental socialization and child temperament in early development of conscience. Child Development 64, 325–347. [Google Scholar]
- Lenroot RK, Gogtay N, Greenstein DK, Wells EM, Wallace GL, Clasen LS, Blumenthal JD, Lerch J, Zijdenbos AP, Evans AC and Thompson PM (2007) Sexual dimorphism of brain developmental trajectories during childhood and adolescence. NeuroImage 36, 1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW and Marsh AA (2014) Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry 71, 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA (2016) Understanding amygdala responsiveness to fearful expressions through the lens of psychopathy and altruism. Journal of Neuroscience Research 94, 513–525. [DOI] [PubMed] [Google Scholar]
- Marsh AA and Blair RJ (2008) Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neuroscience & Biobehavioral Review 32, 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Fowler KA, Jurkowitz IT, Schechter JC, Yu HH, Pine DS and Blair RJ (2011a) Reduced amygdala-orbitofrontal connectivity during moral judgments in youths with disruptive behavior disorders and psychopathic traits. Psychiatry Research 194, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS and Blair RJR (2008) Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry 165, 712–720. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Shechter JC, Jurkowitz IT, Reid ME and Blair RJ (2011b) Adolescents with psychopathic traits report reductions in physiological responses to fear. Journal of Child Psychology and Psychiatry 52, 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Price CJ, Friston KJ and Ashburner J (2005) Voxel-based morphometry of the human brain: methods and applications. Current Medical Imaging Reviews 1, 105–113. [Google Scholar]
- Moffitt TE, Arseneault L, Jaffee SR, Kim-Cohen J, Koenen KC, Odgers CL, Slutske WS and Viding E (2008) Research review: DSM-V conduct disorder: research needs for an evidence base. Journal of Child Psychology and Psychiatry 49, 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner II HR, Lewis DV, LaBar KS, Styner M and McCarthy G (2009) A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage 45, 855–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RA, Selgrade ES, Wagner HR, Huettel SA, Wang L and McCarthy G (2010) Scan-rescan reliability of subcortical brain volumes derived from automated segmentation. Human Brain Mapping 31, 1751–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz LC, Frick PJ, Kimonis ER and Aucoin KJ (2008) Types of aggression, responsiveness to provocation, and callous-unemotional traits in detained adolescents. Journal of Abnormal Child Psychology 36, 15–28. [DOI] [PubMed] [Google Scholar]
- Pardini DA, Raine A, Erickson K and Loeber R (2014) Lower amygdala volume in men is associated with childhood aggression, early psychopathic traits, and future violence. Biological Psychiatry 75, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini JC, Cohen P and Cohen J (1992) Combining discrepant diagnostic information from multiple sources: are complex algorithms better than simple ones. Journal of Abnormal Child Psychology 20, 51–63. [DOI] [PubMed] [Google Scholar]
- Popescu V, Schoonheim MM, Versteeg A, Chaturvedi N, Jonker M, Xavier de Menezes R, Garre FG, Uitdehaag BM, Barkhof F and Vrenken H (2016) Grey matter atrophy in multiple sclerosis: clinical interpretation depends on choice of analysis method. PLoS ONE 11, e0143942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JC and De Brito SA (2016) Cortical and subcortical gray matter volume in youths with conduct problems: a meta-analysis. JAMA Psychiatry 73, 64–72. [DOI] [PubMed] [Google Scholar]
- Roose A, Bijttebier P, Decoene S, Claes L and Frick PJ (2010) Assessing the affective features of psychopathy in adolescence: a further validation of the inventory of callous and unemotional traits. Assessment 17, 44–57. [DOI] [PubMed] [Google Scholar]
- Rowe R, Maughan B, Moran P, Ford T, Briskman J and Goodman R (2010) The role of callous and unemotional traits in the diagnosis of conduct disorder. Journal of Child Psychology and Psychiatry 51, 688–695. [DOI] [PubMed] [Google Scholar]
- Salekin RT (2006) Factor structure of psychopathy in youth: testing the applicability of the new four-factor model. Criminal Justice and Behavior 33, 135–157. [Google Scholar]
- Schoemaker D, Buss C, Head K, Sandman CA, Davis EP, Chakravarty MM, Gauthier S and Pruessner JC (2016) Hippocampus and amygdala volumes from magnetic resonance images in children: assessing accuracy of FreeSurfer and FSL against manual segmentation. NeuroImage 129, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seara-Cardoso A, Sebastian CL, Viding E and Roiser JP (2016) Affective resonance in response to others’ emotional faces varies with affective ratings and psychopathic traits in amygdala and anterior insula. Social Neuroscience 11, 140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, De Brito SA, McCrory EJ, Hyde ZH, Lockwood PL, Cecil CA and Viding E (2016) Grey matter volumes in children with conduct problems and varying levels of callous-unemotional traits. Journal of Abnormal Child Psychology 44, 639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJP, Cecil CAM, Lockwood PL, De Brito SA, Fontaine NMG and Viding E (2012) Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Archives of General Psychiatry 69, 814–822. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Poustka F and Kleinschmidt A (2007) A structural neural deficit in adolescents with conduct disorder and its association with lack of empathy. Neuroimage 37, 335–342. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Kotov R, Frick PJ and Loney BR (2005) The latent structure of psychopathy in youth: a taxometric investigation. Journal of Abnormal Child Psychology 33, 411–429. [DOI] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CAM, De Brito SA and McCrory EJ (2012) Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. American Journal of Psychiatry 169, 1109–1116. [DOI] [PubMed] [Google Scholar]
- Vieira JB, Ferreira-Santos F, Almeida PR, Barbosa F, Marques-Teixeira J and Marsh AA (2015) Psychopathic traits are associated with cortical and subcortical volume alterations in healthy individuals. Social Cognitive and Affective Neuroscience 10, 1693–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace GL, White SF, Robustelli B, Sinclair S, Hwang S, Martin A and Blair RJ (2014) Cortical and subcortical abnormalities in youths with conduct disorder and elevated callous-unemotional traits. Journal of the American Academy of Child and Adolescent Psychiatry 53, 456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Wright AG, Shaw DS, Gardner F, Dishion TJ, Wilson MN and Hyde LW (2015) Factor structure and construct validity of the parent-reported inventory of callous-unemotional traits among high-risk 9-year-olds. Assessment 22, 561–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnick EM, Bracken MB and Kasl S (2008) Screening efficiency of the Child Behavior Checklist and strengths and difficulties questionnaire: a systematic review. Child and Adolescent Mental Health 13, 140–147. [DOI] [PubMed] [Google Scholar]
- White SF, Cruise KR and Frick PJ (2009) Differential correlates to self-report and parent-report of callous-unemotional traits in a sample of juvenile sexual offenders. Behavioral Sciences and the Law 27, 910–928. [DOI] [PubMed] [Google Scholar]
- White SF, Marsh AA, Fowler KA, Schechter JC, Adalio CJ, Pope K, Sinclair S, Pine DS and Blair RJR (2012) Reduced amygdala response in youths with disruptive behavior disorders and psychopathic traits: decreased emotional response versus increased top-down attention to none-motional features. American Journal of Psychiatry 169, 750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletti P and Toga AW (2009) Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry 66, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


