ABSTRACT
Membrane mucins cover most mucosal surfaces throughout the human body. The intestine harbors complex population of microorganisms (the microbiota) and numerous exogenous molecules that can harm the epithelium. In the colon, where the microbial burden is high, a mucus barrier forms the first line of defense by keeping bacteria away from the epithelial cells. In the small intestine where the mucus layer is less organized, microbes are kept at bay by peristalsis and antimicrobial peptides. Additionally, a dense glycocalyx consisting of extended and heavily glycosylated membrane mucins covers the surface of enterocytes. Whereas many aspects of mucosal barriers are being discovered, the function of membrane mucins remains a largely overlooked topic, mainly because we lack the necessary reagents and experimental animal models to investigate these large glycoproteins. In this Cell Science at a Glance article and accompanying poster, we highlight central concepts of membrane mucin biology and the role of membrane mucins as integral components of intestinal mucosal barriers. We also present the current consensus concerning the role of membrane mucins in host–microbe interactions. Moreover, we discuss how regulatory circuits that govern membrane mucins in the healthy gut display strong overlap with pathways that are perturbed during chronic inflammation. Finally, we review how dysregulation of intestinal membrane mucins may contribute to human diseases, such as inflammation and cancer.
KEY WORDS: Barrier, Glycocalyx, Intestine, Microvilli, Mucus, Mucin
Summary: Transmembrane mucins cover the surface of many cells, especially those in the intestine. They form a dense glycan coat (glycocalyx) that protects the apical surface of enterocytes.
Introduction
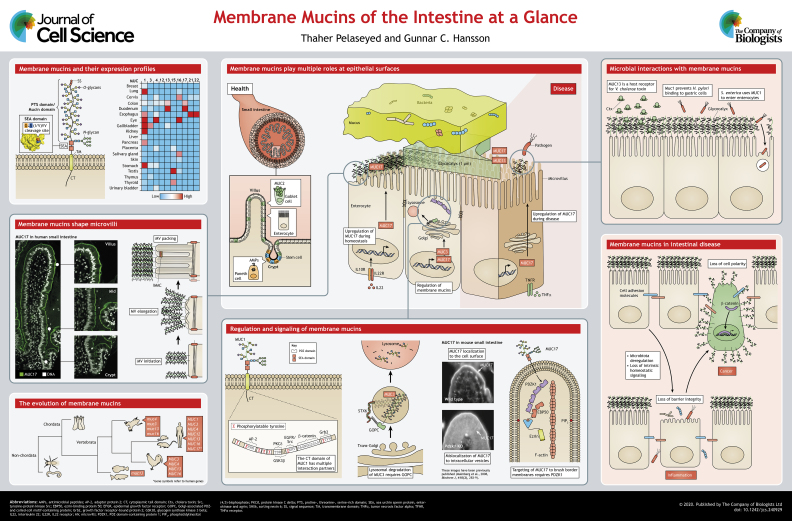
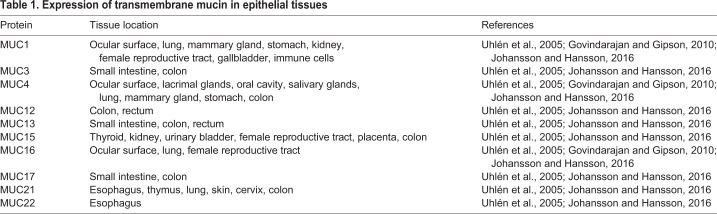
Membrane mucins are large and extended glycoproteins that are attached to the cell membrane through a single-pass transmembrane domain. The family of membrane mucins constitutes MUC1, MUC3, MUC4, MUC12, MUC13, MUC15, MUC16, MUC17, MUC21 and MUC22 (see poster and Table 1). Membrane mucins are expressed at all mucosal surfaces, such as the eye, lungs and the gastrointestinal tract and in reproductive organs like the cervix but, here, we will mostly focus on those expressed in the intestine (see poster).
Table 1.
Expression of transmembrane mucin in epithelial tissues
General features of membrane mucins
Membrane mucins are characterized by a specific domain with multiple and repetitive amino acid sequences rich in Pro, Thr and Ser residues, i.e. the PTS domain. Within this domain amino acids Thr and Ser are extensively O-glycosylated as the membrane mucin travels through the secretory pathway (Lang et al., 2007). The O-glycosylated proline-, threonine- and serine-rich (PTS) domain forms the typical mucin domain that is also a characteristic feature of secreted mucins, such as MUC2, MUC5AC, MUC5B and MUC6. All membrane mucins, except MUC4, MUC21 and MUC22, hold a sea urchin sperm protein, enterokinase and agrin (SEA) domain in their extracellular region (see poster). MUC4 is an interesting exception to the other membrane mucins because it has an extracellular domain assembly comprising three unique domains. These are the (i) nidogen (NIDO) domain, the (ii) adhesion-associated domain in MUC4 and other proteins (AMOP) domain, and the (iii) von Willebrand factor type D (vWD) domain. This configuration is only found in the sushi domain-containing 2 (Susd2) protein. Susd2 lacks a PTS domain (Duraisamy et al., 2006) and has homologs in frog as well as invertebrates, such as C. elegans and D. melanogaster. See text Box 1 for the evolutionary origins of membrane mucins.
Box 1. The evolution of membrane mucins.
The evolutionary origins of mucins cannot be traced by merely exploring genomes of organisms for the characteristic PTS domain, as amino acid sequences of PTS domains are poorly conserved between species. The characteristic trademark of mucins lies, instead, in the frequency of Pro, Thr and Ser residues within long, often recurring sequences or tandem repeats (Lang et al., 2004). Additional domains, such as AMOP, SEA and vWD, have also been looked at when investigating exon sequences in different organisms for the existence of membrane mucins (Lang et al., 2007). Whereas SEA domains are found in Drosophila melanogaster and Caenorhabditis elegans, SEA domain-containing proteins with a PTS domain emerged first in vertebrates, such as frog (Xenopus tropicalis) and zebrafish (Danio rerio) (Lang et al., 2007). Orthologues of the human MUC1 gene are found among mammals, but MUC1 is not present in other vertebrates, such as chick, frog or fish (see poster) (Spicer et al., 1995). Human MUC3, MUC12 and MUC17 are clustered in tandem on human chromosome locus 7q22. Most likely, this locus, with its three membrane mucin genes and their corresponding exon-intron organizations, is conserved from human down to frog (Lang et al., 2007). In analogy with the human 7q22 locus, the locus comprising murine Muc17 is flanked by those of the acetylcholinesterase Ache and the E3 ubiquitin-protein ligase Trim56. This also supports the existence of Muc3 and Muc12 in mouse, in which they have not yet been fully sequenced (Lang et al., 2007). MUC17 paralogs have been identified in opossum and MUC17-like proteins are expressed in zebrafish (Lang et al., 2007). The NIDO–vWD–AMOP domains of MUC4 appeared first in Xenopus through fusion with a characteristic N-terminal PTS domain.
The SEA domain of membrane mucins is autocatalytically cleaved at a G/S[I/V]VV consensus sequence during protein folding in the endoplasmic reticulum (ER) (Ligtenberg et al., 1992). The cleaved SEA domain is folded into a globular structure formed by four α-helices that cradle four parallel β-sheets, held together by strong non-covalent forces that are resistant to thermal and chemical denaturation (Macao et al., 2006; Pelaseyed et al., 2013b). When a SEA domain-containing membrane mucin finally reaches the plasma membrane, it is a heteromeric glycoprotein comprising a long, heavily O-glycosylated extracellular fragment that is non-covalently linked through the SEA domain to a shorter fragment containing a transmembrane domain and a cytoplasmic tail domain.
The function of the conserved SEA domain is currently unknown. Notch receptors that mediate intercellular signaling harbor an extracellular domain called the negative regulatory region (NRR), which shares high structural homology with canonical SEA domains found in membrane mucins. However, the NRR domain lacks the characteristic autoproteolytic G/S[V/I]VV cleavage site (Gordon et al., 2009; Pei and Grishin, 2017). Instead, NRR adopts an autoinhibited conformation that conceals a proteolytic cleavage site that is revealed once Notch binds its ligand on an opposing cell, resulting in cleavage of Notch through a disintegrin and metalloprotease (ADAM) proteases and in subsequent signaling (Gordon et al., 2015). Mechanical forces can induce cleavage through ADAM proteases in the NRR, which is in line with findings showing that the SEA domain of MUC1 unfolds in response to mechanical forces (Pelaseyed et al., 2013b). In mouse, the endogenous ADAM17 protease can also act directly on membrane mucins. MUC1, for example, is cleaved by ADAM17 on the surface of uterine epithelial cells to allow embryo implantation (Thathiah et al., 2003).
MUC4 has a cleavage site located between Asp and Pro in the Gly–Asp–Pro–His (GDPH) sequence within the vWD domain that is typical for von Willebrand factor-derived proteins (Lidell et al., 2003). The cleavage of GDPH takes place in the ER but is probably autocatalytic and protease independent (Soto et al., 2006). Interestingly, the vWD domain is also found in secreted mucins, such as MUC2, MUC5AC and MUC5B, with MUC2 and MUC5AC having an autocatalytically cleaved GDPH motif (Ambort et al., 2012; Ridley et al., 2014; Trillo-Muyo et al., 2018). One of the vWD domains in these mucins mediates their oligomerization, suggesting that membrane mucins with a vWD domain participate in interactions with secreted mucins.
The SEA domain-containing membrane mucins MUC1, MUC3, MUC12, MUC13, MUC16 and MUC17 display a common domain organization, i.e. an extracellular fragment starting with an N-terminal signal sequence (SS) is followed by a PTS and SEA domain. A transmembrane domain is then followed by a cytoplasmic tail (CT) domain (see poster). MUC1, MUC3, MUC13 and MUC17 also contain EGF-like domains that flank the SEA domain on the extracellular fragment (Parry et al., 2001; Williams et al., 2001; Gum et al., 2002; Duraisamy et al., 2006). It has been suggested that EGF-like domains of membrane mucins function as ligands that can activate EGF receptor signaling (Carraway et al., 1999).
The fact that all surface membrane mucins are cleaved, non-covalently attached heteromers is intriguing and not yet fully understood. Membrane mucins cover epithelial surfaces that are subjected to environmental insults, suggesting that membrane mucins can simply shed their mucin domains to protect against biochemical and mechanical factors that, otherwise, might disrupt the epithelial monolayer. After shedding, the remaining membrane-attached mucin fragment could participate in intracellular signaling through specific intracellular motifs and sequences.
Glycosylation of membrane mucins
One of the hallmark features of mucins is glycosylation. Membrane mucins comprise PTS domains, in which >80% of all Ser and Thr residues carry O-linked glycans (see poster). Here, MUC17 serves as an example with its 4073 amino acid-long PTS domain with >2000 Thr and Ser residues, resulting in >1600 O-glycosylation sites. O-glycosylation is initiated by addition of N-acetylgalactosamine (GalNAc) to Ser or Thr residues, followed by stepwise extension of this first epitope into more-complex and branched glycan chains that forces the long membrane mucin protein to adopt the extended and linear conformation that is often evident in electron micrographs (Ito, 1965). The dense glycan chains also protect the protein backbone from digestive enzymes and microbial proteases (van der Post et al., 2013; Bergstrom et al., 2017). In addition, O-glycosylation has been reported to regulate apical targeting of membrane mucins (Kinlough et al., 2011).
Most membrane mucins also carry several N-linked glycans on Asn residues. MUC17 carries nine Asn residues flanking the extracellular SEA domain, which potentially undergo N-glycosylation. N-glycosylation occurs in the lumen of the ER, and is required for correct folding and export of membrane mucins through interactions with mannose-binding lectin chaperones in the ER. Impaired N-glycosylation in the ER results in protein misfolding followed by degradation (Lamriben et al., 2016).
There are regional differences in glycosylation caused by selective and differential expression of glycosyltransferases along the digestive tract, and differences between healthy or diseased states (Robbe et al., 2003; Holmen-Larsson et al., 2013; Johansson et al., 2015). Microbiota also induce expression of glycosyltransferases, such as sialyltransferases, as shown when comparing glycosyltransferase expression and O-glycosylation of mucins between germ-free and conventional microbiota-harboring mice (Johansson et al., 2015; Arike et al., 2017). Microbial regulation of host protein glycosylation is crucial for the etiology of intestinal disease, such as inflammatory bowel disease (IBD), the collective term for Crohn's disease and ulcerative colitis. There, altered bacterial communities affect MUC2 glycosylation and may decimate its capacity to act as a barrier protecting the epithelium (Larsson et al., 2011). These external and intrinsic regulatory processes can also affect glycosylation of membrane mucins expressed along the length of the intestine.
The interactome of membrane mucins
The CT domains of membrane mucins hold sequences and motifs involved in protein interaction, targeting and signaling through phosphorylation. The CT domain of MUC1 contains several conserved Ser, Thr and Tyr phosphorylation sites that modulate interactions with various binding partners (Spicer et al., 1995; Schroeder et al., 2001; Wang et al., 2003; Singh et al., 2007). For example, the proto-oncogene tyrosine-protein kinase Src, the members of the epidermal growth factor receptor (EGFR) ErbB2 and ErbB3, glycogen synthase kinase 3 beta (GSK3β) and protein kinase Cδ (PKCδ), all interact with MUC1, together with other partners that lack kinase activity, such as adaptor protein complex 2 (AP-2), β-catenin and growth factor receptor-bound protein 2 (Grb2) (Kinlough et al., 2004; Funes et al., 2006; Singh and Hollingsworth, 2006). MUC3, MUC12 and MUC17 contain class I PDZ binding motifs, i.e. x[S/T]ɸ (where x represents any amino acid and ɸ indicates any hydrophobic amino acid) in their cytoplasmic tail domains, making them ligands for PDZ domain-containing proteins involved in assembly of protein and signaling complexes (Malmberg et al., 2008). MUC3 interacts with the trans-Golgi-resident PDZ protein Golgi-associated PDZ and coiled-coil motif-containing protein (GOPC, also known as CAL) and functions as a part of a larger protein complex that consists of the Q-SNARE protein syntaxin 6 (STX6) and the small GTPase Rho-related GTP-binding protein RhoQ (RHOQ, also known as TC10). Together, they mediate cargo trafficking from the trans-Golgi to the lysosome for degradation (Cheng et al., 2010) (see poster). Another target for GOPC-facilitated degradation is the cystic fibrosis transmembrane conductance regulator (CFTR) anion channel that transports bicarbonate and fluids into the gut lumen to unfold mucus (Gustafsson et al., 2012). Overexpression of either MUC3 or CFTR rescues either of these GOPC-binding partners from lysosomal degradation, demonstrating a direct link between membrane mucins and CFTR function in epithelial cells with yet unknown consequences (Pelaseyed and Hansson, 2011). MUC17 binds to three out of four PDZ domains in PDZ domain-containing protein 1 (PDZK1) that organizes protein complexes, as exemplified by PDZK1 augmenting CFTR channel activity by assembling dimers of the anion channel (Wang et al., 2000). Mouse MUC17, which normally resides at the apical membrane of small intestinal enterocytes, localizes to intracellular vesicles in Pdzk1 knockout mice (Pdzk1 KO), suggesting that PDZK1 regulates apical targeting or retention of MUC17 at the plasma membrane (Malmberg et al., 2008). Intriguingly, cholinergic stimulation of cultured epithelial cells or mouse duodenal cells results in activation of the CFTR and its relocalization to the apical membrane, alongside removal of MUC17 from apical surfaces followed by colocalization with PDZK1 (Pelaseyed et al., 2013a). The CT domain of human MUC17 contains two phosphorylation sites that are conserved in the mouse MUC17; however, the consequence of MUC17 phosphorylation is not yet understood (Schneider et al., 2019). Interaction between membrane mucins and multivalent PDZ proteins supports the idea that membrane mucins are components of regulated protein complexes, including ion channels, cytoskeletal proteins and regulatory kinases, which act in concert in response to stimuli. The diversity of membrane mucin interaction partners testifies to the fact that membrane mucins have important biological functions at mucosal surfaces, as discussed below.
Membrane mucins of the intestines
In humans, the intestinal tract constitutes the largest surface area that is in contact with the harsh environment of the outside world. Our intestines are lined with a rapidly renewing monolayer of epithelial cells, which is turned over every three to five days (Cheng and Leblond, 1974). Valuable intestinal stem cells at the base of intestinal crypts differentiate into specialized epithelial lineages that, collectively, contribute to mucosal barriers against chemical and microbial challenges. Therein, intestinal stem cells (ISCs) differentiate into specialized epithelial lineages that, collectively, contribute to mucosal barriers against chemical and microbial challenges. Paneth cells within the small intestine secrete antimicrobial peptides that safeguard the neighboring stem cells. Goblet cells produce, store and secrete mucus that protects epithelial surfaces (Johansson et al., 2008; Vaishnava et al., 2008). Transporting epithelial cells – termed enterocytes in the small intestinal and colonocytes in the colon – account for 70–80% of all intestinal epithelial cells, and participate in nutrient uptake and ion exchange. Enterocytes are characterized by their apical brush border membrane, shaped by ∼1000 microvilli that cover the surface of each cell. Each microvillus is 1–2 µm long, has a diameter of 100–150 nm and is capped with extended membrane mucins that are likely to be the main glycoprotein component of the glycocalyx (see poster). This morphological term was first used and described in the late 1950s for the surface of red blood cells (Bartlett, 1958). The glycocalyx that decorates the surface of intestinal epithelial cells was first observed in bat and later in cat intestines (Ito and Winchester, 1963; Ito, 1965).
The role of membrane mucins in shaping apical membrane domains
Membrane mucin MUC17 is highly expressed in the small intestine, whereas expression levels are lower in the colon (Uhlén et al., 2015). Recent advances in single-cell RNA sequencing of mouse small intestine show that MUC17 is almost exclusively expressed in enterocytes, with lowest levels in progenitor enterocytes and highest in mature differentiated enterocytes (Haber et al., 2017). The distinct spatiotemporal expression pattern of MUC17 is shared with a cluster of proteins, such as Cdhr2, Cdhr5, Ebp50, Ezrin and Ush1c (see poster). These proteins are responsible for assembly, elongation and formation of stable bundles of packed microvilli that are, in turn, decorated with membrane mucins. Microvilli are evolutionarily conserved actin-based membrane protrusions that shape the membrane of enterocytes by generating a brush border membrane (see poster on how membrane mucins shape microvilli) (Pelaseyed and Bretscher, 2018). The cellular cues that initiate microvilli formation have not yet been defined but it is known that microvilli assembly requires: (i) actin cytoskeleton treadmilling towards the tip of the microvillus (Meenderink and Tyska, 2019), (ii) continuous cycles of phosphorylation and dephosphorylation of ezrin – a crosslinker between the membrane and F-actin cytoskeleton; a process that, in turn, requires phosphatidylinositol (4,5)-bisphosphate (PIP2) and an ezrin-specific kinase to assemble microvilli (Viswanatha et al., 2012; Pelaseyed et al., 2017) (see poster). Assembled microvilli are then tightly packed via an inter-microvillar adhesion complex (IMAC) that comprises Cdhr2, Cdhr5, Myo7b and Ush1c, an ensemble of proteins that form stable crosslinks between microvilli (Crawley et al., 2014). Once stable packing of microvilli through the adhesion complex occurs, membrane mucins are likely to be locked in place at the tip of microvilli until the cell is shed. The stable retention of the glycocalyx has been shown by using in vivo labeling of surface glycoproteins covering enterocytes (Schneider et al., 2018).
The correlation between MUC17 and microvillar components, and the fact that each microvillus is decorated with a certain number of membrane mucins, suggest a functional association between membrane mucins and microvilli that is not yet fully understood. A recent publication suggests that the densely O-glycosylated domains of membrane mucins at the plasma membrane generate physical forces that bend the membranes to form microvillus protrusions (Shurer et al., 2019).
The brush border membrane of enterocytes constitutes a crucial interface with the gut lumen. The fact that this surface is covered with membrane mucins alludes to their function in host–microbe interactions. To determine how membrane mucin expression and microvillar architecture are interconnected will be crucial in order to understand how epithelial barriers contribute to intestinal homeostasis.
Microbial interactions with membrane mucins
The gut lumen harbors trillions of microorganisms that contribute to our well-being by priming our immune system, promoting intestinal maturation, extracting nutrients from ingested food and generating metabolites that are important for intestinal homeostasis (Ley et al., 2005). Membrane mucins present dense arrays of glycans to the luminal content of the gut. On one hand, the numerous multivalent glycan moieties in the mucin domain of membrane mucins offer excellent stoichiometric power to allow specific interactions with glycan-binding proteins of gut microbes. On the other hand, membrane mucins decorate the tip of microvilli and form a thick carbohydrate-rich coat that may act as a highly specific barrier based on charge, chain length and branching of mucin glycans. Another possibility is that membrane mucins act as binding and attachment sites for bacteria (see poster). Thus, in a combined binding and barrier function, membrane mucins can act as decoys that limit microbial binding to cells surfaces. This has been shown for murine MUC1 that is highly expressed in the gastric epithelium; there, it limits acute and chronic Helicobacter pylori colonization by preventing H. pylori from binding directly to enterocytes (McGuckin et al., 2007). In another study, oral infection of mice with Campylobacter jejuni upregulated protein levels of MUC1 along the gastrointestinal tract and, in turn, suppressed the pro-apoptotic action of the C. jejuni toxin, explaining why the bacterium induces epithelial damage and translocates systemically in Muc1−/− mice (McAuley et al., 2007). Studies of in vitro cancer cell cultures have also suggested that Salmonella enterica strains invade epithelial cells by binding to sialyated MUC1 through their giant adhesin SiiE (Li et al., 2019). Human MUC13 has been identified as a host receptor for the pentameric B-subunit of Vibrio cholerae toxin (Ctx) in human T84 cells; however, whether MUC13 is an entry route or a protective decoy for Ctx has not yet been explored (Wands et al., 2015). Recently, we showed that overexpression of MUC17 in a 2D human epithelial Caco-2 cell culture with low endogenous MUC17 expression reduced binding of enteropathogenic Escherichia coli (EPEC) to cell surfaces in a TNF-dependent manner (Schneider et al., 2019), supporting the concept that membrane mucins function as cell-autonomous barriers against bacteria (see poster).
Regulation of membrane mucins in health and disease
Several membrane mucins show aberrant expression in cancers. MUC1 overexpression in colon, gall bladder and pancreas cancers often correlates with metastasis and poor prognosis (Nakamori et al., 1994; Hiraga et al., 1998; Kashiwagi et al., 2000; Lüttges et al., 2002). Moreover, epigenetic changes, such as DNA methylation and histone acetylation, have been shown to upregulate MUC4 and MUC17 in pancreatic cancer (Vincent et al., 2008; Kitamoto et al., 2011). Although the role of membrane mucins in cancer is not fully understood, several mechanisms have been suggested. Loss of regulated cell polarity and dissociation of cancer cells from neighboring cells has been attributed to the heavily glycosylated mucin domain (Maher et al., 2011) (see poster). Overexpression of MUC1 has also been shown to both mediate and block cell–cell adhesion due to interactions between its mucin domain and proteins, such as selectins and intracellular adhesion molecule 1 (ICAM1) (McDermott et al., 2001). Moreover, the CT domain of MUC1 engages several binding partners, such as β-catenin, that are known to be involved in carcinogenesis (see poster).
How external signals and intrinsic programs regulate membrane mucins in health and disease is only beginning to be understood. Insight into the signaling pathways that govern membrane mucins can be gained by investigating human diseases, such as inflammatory bowel disease (IBD), and mouse models of acute enteropathogenic infections. In the mouse colon, in vivo interleukin 22 (IL22) upregulates gene expression of Muc1, Muc13 and Muc17 (Sugimoto et al., 2008). IL22 and interferon gamma (IFNγ) also upregulates Muc17 in organoids derived from mouse small intestine but not in those from colon (Price et al., 2018). IL22 is a homeostatic interleukin involved in epithelial cell regeneration and barrier reinforcement (Sanos et al., 2009). Together with its downstream signaling pathway components, such as the transcription factor signal transducer and activator of transcription 3 (STAT3), IL22 has been identified as a susceptibility gene in IBD (Glocker et al., 2009; Silverberg et al., 2009; Khor et al., 2011; Chi et al., 2014). Indeed, an earlier study has reported that the membrane mucin genes MUC1, MUC4, MUC12, MUC13 and MUC17 are downregulated in humans suffering from Crohn's disease (CD) or ulcerative colitis (Moehle et al., 2006). A more-recent study has reported abnormal microvillar morphology in CD patients, and correlated this observation to the significant downregulation of genes involved in microvillus assembly and maintenance in CD patients as compared to the control group (VanDussen et al., 2018). An intriguing explanation for the role of membrane mucins in IBD is that perturbations in membrane mucin expression and microvillar assembly can result in defective glycocalyx, leading to loss of epithelial barrier integrity.
Conclusions and future perspectives
Membrane mucins are a neglected family of membrane-bound glycoproteins that cover many epithelial surfaces of the human body. In this Cell Science at a Glance article and poster, we have discussed structure, function, glycosylation and microbial interactions of membrane mucins, particularly, in the intestinal tract. However, many crucial gaps of knowledge remain to be addressed. What is the fundamental role of membrane mucins in epithelial cells? Do membrane mucins act as receptors for specific bacteria or as specialized barriers against pathogens? How are these large glycoproteins trafficked to the cell surface and how are the spatiotemporal dynamics of membrane mucins coordinated with cell differentiation in tissue with a high cell turnover? At various sites through the human body, two or more membrane mucins are expressed in the same cell type. How are these membrane mucins connected in terms of localization and function? Finally, the comprehensive scrutiny of regulatory pathways that dictate gene expression of membrane mucins will add another layer of understanding regarding infectious and inflammatory diseases in the intestine.
Acknowledgements
The content of this Review is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Funding
This work was supported by the European Research Council (ERC, grant no. 694181), National Institute of Allergy and Infectious Diseases (grant no. U01AI095473), the Knut and Alice Wallenberg Foundation (grant no. 2017.0028), Vetenskapsrådet (grant no. 2017-00958), Cancerfonden, IngaBritt and Arne Lundberg Foundation, Sahlgrenska Universitetssjukhuset (The ALF agreement 236501), Bill and Melinda Gates Foundation, Wilhelm and Martina Lundgren Foundation, Svenska Sällskapet för Medicinsk Forskning (SSMF), the Sahlgrenska Akademin and the Wenner-Gren Foundation. Deposited in PMC for release after 12 months.
Cell science at a glance
A high-resolution version of the poster and individual poster panels are available for downloading at http://jcs.biologists.org/lookup/doi/10.1242/jcs.240929.supplemental
References
- Ambort D., Johansson M. E. V., Gustafsson J. K., Nilsson H. E., Ermund A., Johansson B. R., Koeck P. J. B., Hebert H. and Hansson G. C. (2012). Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc. Natl Acad. Sci. USA 109, 5645-5650. 10.1073/pnas.1120269109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arike L., Holmén-Larsson J. and Hansson G. C. (2017). Intestinal Muc2 mucin O-glycosylation is affected by microbiota and regulated by differential expression of glycosyltranferases. Glycobiology 27, 318-328. 10.1093/glycob/cww134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett G. R. (1958). Organization of red cell glycolytic enzymes: cell coat phosphorus transfer. Ann. N. Y. Acad. Sci. 75, 110-114. 10.1111/j.1749-6632.1958.tb36855.x [DOI] [PubMed] [Google Scholar]
- Bennett E. P., Mandel U., Clausen H., Gerken T. A., Fritz T. A. and Tabak L. A. (2012). Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736-756. 10.1093/glycob/cwr182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom K., Fu J., Johansson M. E. V., Liu X., Gao N., Wu Q., Song J., McDaniel J. M., McGee S., Chen W. et al. (2017). Core 1- and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol. 10, 91-103. 10.1038/mi.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L. III, Rossi E. A., Komatsu M., Price-Schiavi S. A., Huang D., Guy P. M., Carvajal M. E., Fregien N., Carraway C. A. C. and Carraway K. L. (1999). An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J. Biol. Chem. 274, 5263-5266. 10.1074/jbc.274.9.5263 [DOI] [PubMed] [Google Scholar]
- Cheng H. and Leblond C. P. (1974). Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine I. Columnar cell. Am. J. Anat. 141, 461-479. 10.1002/aja.1001410403 [DOI] [PubMed] [Google Scholar]
- Cheng J., Cebotaru V., Cebotaru L. and Guggino W. B. (2010). Syntaxin 6 and CAL Mediate the Degradation of the Cystic Fibrosis Transmembrane Conductance Regulator. Mol. Biol. Cell 21, 1153-1314. 10.1091/mbc.e09-03-0229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. G., Zheng X. B., Wu Z. G., Dai S. X., Wan Z. and Zou Y. (2014). Association of the interleukin-22 genetic polymorphisms with ulcerative colitis. Diagn. Pathol. 9, 183 10.1186/s13000-014-0183-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley S. W., Shifrin D. A. Jr, Grega-Larson N. E., Mcconnell R. E., Benesh A. E., Mao S., Zheng Y., Zheng Q. Y., Nam K. T., Millis B. A. et al. (2014). Intestinal brush border assembly driven by protocadherin-based intermicrovillar adhesion. Cell 157, 433-446. 10.1016/j.cell.2014.01.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisamy S., Ramasamy S., Kharbanda S. and Kufe D. (2006). Distinct evolution of the human carcinoma-associated transmembrane mucins, MUC1, MUC4 AND MUC16. Gene 373, 28-34. 10.1016/j.gene.2005.12.021 [DOI] [PubMed] [Google Scholar]
- Funes M., Miller J. K., Lai C., Carraway K. L. and Sweeney C. (2006). The Mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J. Biol. Chem. 281, 19310-19319. 10.1074/jbc.M603225200 [DOI] [PubMed] [Google Scholar]
- Glocker E.-O., Kotlarz D., Boztug K., Gertz E. M., Schäffer A. A., Noyan F., Perro M., Diestelhorst J., Allroth A., Murugan D. et al. (2009). Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N. Engl. J. Med. 361, 2033-2045. 10.1056/NEJMoa0907206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon W. R., Roy M., Vardar-Ulu D., Garfinkel M., Mansour M. R., Aster J. C. and Blacklow S. C. (2009). Structure of the Notch1-negative regulatory region: implications for normal activation and pathogenic signaling in T-ALL. Blood 113, 4381-4390. 10.1182/blood-2008-08-174748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon W. R., Zimmerman B., He L., Miles L. J., Huang J., Tiyanont K., McArthur D. G., Aster J. C., Perrimon N., Loparo J. J. et al. (2015). Mechanical allostery: evidence for a force requirement in the proteolytic activation of notch. Dev. Cell 33, 729-736. 10.1016/j.devcel.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan B. and Gipson I. K. (2010). Membrane-tethered mucins have multiple functions on the ocular surface. Exp. Eye Res. 90, 655-663. 10.1016/j.exer.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gum J. R., Crawley S. C., Hicks J. W., Szymkowski D. E. and Kim Y. S. (2002). MUC17, a novel membrane-tethered mucin. Biochem. Biophys. Res. Commun. 291, 466-475. 10.1006/bbrc.2002.6475 [DOI] [PubMed] [Google Scholar]
- Gustafsson J. K., Ermund A., Ambort D., Johansson M. E. V., Nilsson H. E., Thorell K., Hebert H., Sjövall H. and Hansson G. C. (2012). Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J. Exp. Med. 209, 1263-1272. 10.1084/jem.20120562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber A. L., Biton M., Rogel N., Herbst R. H., Shekhar K., Smillie C., Burgin G., Delorey T. M., Howitt M. R., Katz Y. et al. (2017). A single-cell survey of the small intestinal epithelium. Nature 551, 333-339. 10.1038/nature24489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga Y., Tanaka S., Haruma K., Yoshihara M., Sumii K., Kajiyama G., Shimamoto F. and Kohno N. (1998). Immunoreactive MUC1 expression at the deepest invasive portion correlates with prognosis of colorectal cancer. Oncology 55, 307-319. 10.1159/000011868 [DOI] [PubMed] [Google Scholar]
- Holmen-Larsson J. M, Thomsson K.A., Rodríguez-Piñeiro A.M., Karlsson H. and Hansson G.C. (2013). Gastrointestinal Muc5ac and Muc2 mucin O-glycan pattern reveal a regio-specific distribution - 3. Studies of mucus in mouse stomach, small intestine, and colon. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G357-G363. 10.1152/ajpgi.00048.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. (1965). The enteric surface coat on cat intestinal microvilli. J. Cell Biol. 27, 475-491. 10.1083/jcb.27.3.475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S. and Winchester R. J. (1963). The fine structure of the gastric mucosa in the bat. J. Cell Biol. 16, 541-577. 10.1083/jcb.16.3.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E. V. and Hansson G. C. (2016). The Mucins. Encyclopedia of Immunobiol. 2, 381-388. 10.1016/B978-0-12-374279-7.02019-1 [DOI] [Google Scholar]
- Johansson M. E. V., Phillipson M., Petersson J., Velcich A., Holm L. and Hansson G. C. (2008). The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 105, 15064-15069. 10.1073/pnas.0803124105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M. E. V., Jakobsson H. E., Holmén-Larsson J., Schütte A., Ermund A., Rodríguez-Piñeiro A. M., Arike L., Wising C., Svensson F., Bäckhed F. et al. (2015). Normalization of host intestinal mucus layers requires long-term microbial colonization. Cell Host Microbe. 18, 582-592. 10.1016/j.chom.2015.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi H., Kijima H., Dowaki S., Ohtani Y., Tobita K., Tsukui M., Tanaka Y., Matsubayasi H., Tsuchida T., Yamazaki H. et al. (2000). DF3 expression in human gallbladder carcinoma: significance for lymphatic invasion. Int. J. Oncol. 16, 455-464. 10.3892/ijo.16.3.455 [DOI] [PubMed] [Google Scholar]
- Khor B., Gardet A. and Xavier R. J. (2011). Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307-317. 10.1038/nature10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinlough C. L., Poland P. A., Bruns J. B., Harkleroad K. L. and Hughey R. P. (2004). MUC1 membrane trafficking is modulated by multiple interactions. J. Biol. Chem. 279, 53071-53077. 10.1074/jbc.M409360200 [DOI] [PubMed] [Google Scholar]
- Kinlough C. L., Poland P. A., Gendler S. J., Mattila P. E., Mo D., Weisz O. A. and Hughey R. P. (2011). Core-glycosylated mucin-like repeats from MUC1 are an apical targeting signal. J. Biol. Chem. 286, 39072-39081. 10.1074/jbc.M111.289504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto S., Yamada N., Yokoyama S., Houjou I., Higashi M., Goto M., Batra S. K. and Yonezawa S. (2011). DNA methylation and histone H3-K9 modifications contribute to MUC17 expression. Glycobiology 21, 247-256. 10.1093/glycob/cwq155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamriben L., Graham J. B., Adams B. M. and Hebert D. N. (2016). N-glycan-based ER molecular chaperone and protein quality control system: the Calnexin binding cycle. Traffic 17, 308-326. 10.1111/tra.12358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T. Alexandersson M., Hansson G. C. and Samuelsson T. (2004). Bioinformatic identification of polymerizing and transmembrane mucins in the puffer fish Fugu rubripes. Glycobiology 14, 521-527. 10.1093/glycob/cwh066 [DOI] [PubMed] [Google Scholar]
- Lang T., Hansson G. C. and Samuelsson T. (2007). Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA 104, 16209-16214. 10.1073/pnas.0705984104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J. M. H., Karlsson H., Crespo J. G., Johansson M. E. V., Eklund L., Sjövall H. and Hansson G. C. (2011). Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 17, 2299-2307. 10.1002/ibd.21625 [DOI] [PubMed] [Google Scholar]
- Ley R. E., Bäckhed F., Turnbaugh P., Lozupone C. A., Knight R. D. and Gordon J. I. (2005). Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. USA 102, 11070-11075. 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Bleumink-Pluym N. M. C., Luijkx Y. M. C. A., Wubbolts R. W., van Putten J. P. M. and Strijbis K. (2019). MUC1 is a receptor for the Salmonella SiiE adhesin that enables apical invasion into enterocytes. PLoS Pathog. 15, e1007566 10.1371/journal.ppat.1007566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell M. E., Johansson M. E. V. and Hansson G. C. (2003). An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J. Biol. Chem. 278, 13944-13951. 10.1074/jbc.M210069200 [DOI] [PubMed] [Google Scholar]
- Ligtenberg M. J., Kruijshaar L., Buijs F., van Meijer M., Litvinov S. V. and Hilkens J. (1992). Cell-associated episialin is a complex containing two proteins derived from a common precursor. J. Biol. Chem. 267, 6171-6177. [PubMed] [Google Scholar]
- Lüttges J., Feyerabend B., Buchelt T., Pacena M. and Klöppel G. (2002). The mucin profile of noninvasive and invasive mucinous cystic neoplasms of the pancreas. Am. J. Surg. Pathol. 26, 466-471. 10.1097/00000478-200204000-00008 [DOI] [PubMed] [Google Scholar]
- Macao B., Johansson D. G. A., Hansson G. C. and Härd T. (2006). Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 13, 71-76. 10.1038/nsmb1035 [DOI] [PubMed] [Google Scholar]
- Maher D. M., Gupta B. K., Nagata S., Jaggi M. and Chauhan S. C. (2011). Mucin 13: structure, function, and potential roles in cancer pathogenesis. Mol. Cancer Res. 9, 531-537. 10.1158/1541-7786.MCR-10-0443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg E. K., Pelaseyed T., Petersson Å. C., Seidler U. E., De Jonge H., Riordan J. R. and Hansson G. C. (2008). The C-terminus of the transmembrane mucin MUC17 binds to the scaffold protein PDZK1 that stably localizes it to the enterocyte apical membrane in the small intestine. Biochem. J. 410, 283-289. 10.1042/BJ20071068 [DOI] [PubMed] [Google Scholar]
- McAuley J. L., Linden S. K., Png C. W., King R. M., Pennington H. L., Gendler S. J., Florin T. H., Hill G. R., Korolik V. and Mcguckin M. A. (2007). MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313-2324. 10.1172/JCI26705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott K. M., Crocker P. R., Harris A., Burdick M. D., Hinoda Y., Hayashi T., Imai K. and Hollingsworth M. A. (2001). Overexpression of MUC1 reconfigures the binding properties of tumor cells. Int. J. Cancer 94, 783-791. 10.1002/ijc.1554 [DOI] [PubMed] [Google Scholar]
- McGuckin M. A., Every A. L., Skene C. D., Linden S. K., Chionh Y. T., Swierczak A., Mcauley J., Harbour S., Kaparakis M., Ferrero R. et al. (2007). Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133, 1210-1218. 10.1053/j.gastro.2007.07.003 [DOI] [PubMed] [Google Scholar]
- Meenderink L. M. and Tyska M. J. (2019). Actin dynamics drive microvillar motility and clustering during brush border assembly. Dev. Cell 50, 545-556. 10.1101/432294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehle C., Ackermann N., Langmann T., Aslanidis C., Kel A., Kel-Margoulis O., Schmitz-Madry A., Zahn A., Stremmel W. and Schmitz G. (2006). Aberrant intestinal expression and allelic variants of mucin genes associated with inflammatory bowel disease. J. Mol. Med. 84, 1055-1066. 10.1007/s00109-006-0100-2 [DOI] [PubMed] [Google Scholar]
- Nakamori S., Ota D. M., Cleary K. R., Shirotani K. and Irimura T. (1994). MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 106, 353-361. 10.1016/0016-5085(94)90592-4 [DOI] [PubMed] [Google Scholar]
- Parry S., Silverman H. S., Mcdermott K., Willis A., Hollingsworth M. A. and Harris A. (2001). Identification of MUC1 proteolytic cleavage sites in vivo. Biochem. Biophys. Res. Commun. 283, 715-720. 10.1006/bbrc.2001.4775 [DOI] [PubMed] [Google Scholar]
- Pei J. and Grishin N. V. (2017). Expansion of divergent SEA domains in cell surface proteins and nucleoporin 54. Protein Sci. 26, 617-630. 10.1002/pro.3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed T. and Bretscher A. (2018). Regulation of actin-based apical structures on epithelial cells. J. Cell Sci. 131, jcs221853 10.1242/jcs.221853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed T. and Hansson G. C. (2011). CFTR anion channel modulates expression of human transmembrane mucin MUC3 through the PDZ protein GOPC. J. Cell Sci. 124, 3074-3083. 10.1242/jcs.076943 [DOI] [PubMed] [Google Scholar]
- Pelaseyed T., Gustafsson J. K., Gustafsson I. J., Ermund A. and Hansson G. C. (2013a). Carbachol-induced MUC17 endocytosis is concomitant with NHE3 internalization and CFTR membrane recruitment in enterocytes. Am. J. Physiol. Cell Physiol. 305, C457-C467. 10.1152/ajpcell.00141.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed T., Zäch M., Petersson Å. C., Svensson F., Johansson D. G. A. and Hansson G. C. (2013b). Unfolding dynamics of the mucin SEA domain probed by force spectroscopy suggest that it acts as a cell-protective device. FEBS J. 280, 1491-1501. 10.1111/febs.12144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaseyed T., Viswanatha R., Sauvanet C., Filter J. J., Goldberg M. L. and Bretscher A. (2017). Ezrin activation by LOK phosphorylation involves a PIP2-dependent wedge mechanism. eLife 6, e22759 10.7554/eLife.22759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. E., Shamardani K., Lugo K. A., Deguine J., Roberts A. W., Lee B. L. and Barton G. M. (2018). A map of toll-like receptor expression in the intestinal epithelium reveals distinct spatial, cell type-specific, and temporal patterns. Immunity 49, 560-575.e6. 10.1016/j.immuni.2018.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley C., Kouvatsos N., Raynal B. D., Howard M., Collins R. F., Desseyn J.-L., Jowitt T. A., Baldock C., Davis C. W., Hardingham T. E. et al. (2014). Assembly of the respiratory Mucin MUC5B: a new model for a gel-forming Mucin. J. Biol. Chem. 289, 16409-16420. 10.1074/jbc.M114.566679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe C., Capon C., Maes E., Rousset M., Zweibaum A., Zanetta J.-P. and Michalski J.-C. (2003). Evidence of regio-specific glycosylation in human intestinal mucins: presence of an acidic gradient along the intestinal tract. J. Biol. Chem. 278, 46337-46348. 10.1074/jbc.M302529200 [DOI] [PubMed] [Google Scholar]
- Sanos S. L., Bui V. L., Mortha A., Oberle K., Heners C., Johner C. and Diefenbach A. (2009). RORγt and commensal microflora are required for the differentiation of mucosal interleukin 22–producing NKp46+ cells. Nat. Immunol. 10, 83-91. 10.1038/ni.1684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Pelaseyed T., Svensson F. and Johansson M. E. V. (2018). Study of mucin turnover in the small intestine by in vivo labeling. Sci. Rep. 8, 5760 10.1038/s41598-018-24148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Berger E., Dolan B., Martinez-Abad B., Arike L., Pelaseyed T. and Hansson G. C. (2019). The human transmembrane mucin MUC17 responds to TNFα by increased presentation at the plasma membrane. Biochem. J. 476, 2281-2295. 10.1042/BCJ20190180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. A., Thompson M. C., Gardner M. M. and Gendler S. J. (2001). Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J. Biol. Chem. 276, 13057-13064. 10.1074/jbc.M011248200 [DOI] [PubMed] [Google Scholar]
- Shurer C. R., Kuo J. C., Roberts L. M., Gandhi J. G., Colville M. J., Enoik T. A., Pan H., Su J., Noble J. M., Hollander M. J. et al. (2019). Physical principles of membrane shape regulation by the Glycocalyx. Cell 177, 1757-1770.e21. 10.1016/j.cell.2019.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg M. S., Cho J. H., Rioux J. D., Mcgovern D. P. B., Wu J., Annese V., Achkar J.-P., Goyette P., Scott R., Xu W. et al. (2009). Ulcerative colitis–risk loci on chromosomes 1p36 and 12q15 found by genome-wide association study. Nat. Genet. 41, 216-220. 10.1038/ng.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P. K. and Hollingsworth M. A. (2006). Cell surface-associated mucins in signal transduction. Trends Cell Biol. 16, 467-476. 10.1016/j.tcb.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Singh P. K., Wen Y., Swanson B. J., Shanmugam K., Kazlauskas A., Cerny R. L., Gendler S. J. and Hollingsworth M. A. (2007). Platelet-derived growth factor receptor β-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res. 67, 5201-5210. 10.1158/0008-5472.CAN-06-4647 [DOI] [PubMed] [Google Scholar]
- Soto P., Zhang J. and Carraway K. L. (2006). Enzymatic cleavage as a processing step in the maturation of Muc4/sialomucin complex. J. Cell. Biochem. 97, 1267-1274. 10.1002/jcb.20718 [DOI] [PubMed] [Google Scholar]
- Spicer A. P., Duhig T., Chilton B. S. and Gendler S. J. (1995). Analysis of mammalian MUC1 genes reveals potential functionally important domains. Mamm. Genome 6, 885-888. 10.1007/BF00292441 [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A. K., Blumberg R. S., Xavier R. J. and Mizoguchi A. (2008). IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Investig. 118, 534-544. 10.1172/JCI33194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathiah A., Blobel C. P. and Carson D. D. (2003). Tumor necrosis factor-alpha converting enzyme/ADAM 17 mediates MUC1 shedding. J. Biol. Chem. 278, 3386-3394. 10.1074/jbc.M208326200 [DOI] [PubMed] [Google Scholar]
- Trillo-Muyo S., Nilsson H. E., Recktenwald C. V., Ermund A., Ridley C., Meiss L. N., Bähr A., Klymiuk N., Wine J. J., Koeck P. J. B. et al. (2018). Granule-stored MUC5B mucins are packed by the noncovalent formation of N-terminal head-to-head tetramers. J. Biol. Chem. 293, 5746-5754. 10.1074/jbc.RA117.001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlén M., Björling E., Agaton C., Szigyarto C. A.-K., Amini B., Andersen E., Andersson A.-C., Angelidou P., Asplund A., Asplund C. et al. (2005). A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteomics 4, 1920-1932. 10.1074/mcp.M500279-MCP200 [DOI] [PubMed] [Google Scholar]
- Uhlén M., Fagerberg L., Hallstrom B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A. et al. (2015). Tissue-based map of the human proteome. Science 347, 1260419 10.1126/science.1260419 [DOI] [PubMed] [Google Scholar]
- Vaishnava S., Behrendt C. L., Ismail A. S., Eckmann L. and Hooper L. V. (2008). Paneth cells directly sense gut commensals and maintain homeostasis at the intestinal host-microbial interface. Proc. Natl. Acad. Sci. USA 105, 20858-20863. 10.1073/pnas.0808723105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Post S., Subramani D. B., Bäckström M., Johansson M. E. V., Vester-Christensen M. B., Mandel U., Bennett E. P., Clausen H., Dahlén G., Sroka A. et al. (2013). Site-specific O-glycosylation on the MUC2 mucin protein inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB). J. Biol. Chem. 288, 14636-14646. 10.1074/jbc.M113.459479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandussen K. L., Stojmirović A., Li K., Liu T.-C., Kimes P. K., Muegge B. D., Simpson K. F., Ciorba M. A., Perrigoue J. G., Friedman J. R. et al. (2018). Abnormal Small Intestinal Epithelial Microvilli in Patients With Crohn's Disease. Gastroenterology 155, 815-828. 10.1053/j.gastro.2018.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Ducourouble M.-P. and Van Seuningen I. (2008). Epigenetic regulation of the human mucin gene MUC4 in epithelial cancer cell lines involves both DNA methylation and histone modifications mediated by DNA methyltransferases and histone deacetylases. FASEB J. 22, 3035-3045. 10.1096/fj.07-103390 [DOI] [PubMed] [Google Scholar]
- Viswanatha R., Ohouo P. Y., Smolka M. B. and Bretscher A. (2012). Local phosphocycling mediated by LOK/SLK restricts ezrin function to the apical aspect of epithelial cells. J. Cell Biol. 199, 969-984. 10.1083/jcb.201207047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wands A. M., Fujita A., McCombs J. E., Cervin J., Dedic B., Rodriguez A. C., Nischan N., Bond M. R., Mettlen M., Trudgian D. C. et al. (2015). Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife 4, e09545 10.7554/eLife.09545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yue H., Derin R. B., Guggino W. B. and Li M. (2000). Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell 103, 169-179. 10.1016/S0092-8674(00)00096-9 [DOI] [PubMed] [Google Scholar]
- Wang H., Lillehoj E. P. and Kim K. C. (2003). Identification of four sites of stimulated tyrosine phosphorylation in the MUC1 cytoplasmic tail. Biochem. Biophys. Res. Commun. 310, 341-346. 10.1016/j.bbrc.2003.09.030 [DOI] [PubMed] [Google Scholar]
- Williams S. J., Wreschner D. H., Tran M., Eyre H. J., Sutherland G. R. and Mcguckin M. A. (2001). MUC13, a Novel Human Cell Surface Mucin Expressed by Epithelial and Hemopoietic Cells. J. Biol. Chem. 276, 18327-18336. 10.1074/jbc.M008850200 [DOI] [PubMed] [Google Scholar]