ABSTRACT
Orderly division of radial glial progenitors (RGPs) in the developing mammalian cerebral cortex generates deep and superficial layer neurons progressively. However, the mechanisms that control RGP behavior and precise neuronal output remain elusive. Here, we show that the oxidative stress level progressively increases in the developing mouse cortex and regulates RGP behavior and neurogenesis. As development proceeds, numerous gene pathways linked to reactive oxygen species (ROS) and oxidative stress exhibit drastic changes in RGPs. Selective removal of PRDM16, a transcriptional regulator highly expressed in RGPs, elevates ROS level and induces expression of oxidative stress-responsive genes. Coinciding with an enhanced level of oxidative stress, RGP behavior was altered, leading to abnormal deep and superficial layer neuron generation. Simultaneous expression of mitochondrially targeted catalase to reduce cellular ROS levels significantly suppresses cortical defects caused by PRDM16 removal. Together, these findings suggest that oxidative stress actively regulates RGP behavior to ensure proper neurogenesis in the mammalian cortex.
KEY WORDS: Cortical development, Neocortical progenitor cells, Neurogenesis transition, Oxidative stress
Summary: Reactive oxygen species and oxidative stress regulated by the transcriptional regulator PRDM16 actively influence radial glial progenitor mitotic behavior and precise neuronal output in the developing mouse cortex.
INTRODUCTION
The cerebral cortex is a complex yet highly organized brain structure that plays a fundamental role in processing sensory information as well as supporting motor and cognitive functions. It consists of a network of excitatory and inhibitory neurons that are distributed throughout the six distinct cortical layers. Distinct cell types and neuronal connections further define the importance of preserving the precise formation of cortical laminae (Greig et al., 2013; Kwan et al., 2012). The principal neural progenitor cell population, radial glial progenitors (RGPs), supports the intricate production of diverse neurons to assemble the cortex. Derived from neuroepithelial cells, RGPs are located in the ventricular zone (VZ) of the developing cortex (Anthony et al., 2004; Florio and Huttner, 2014; Gao et al., 2014; Homem et al., 2015; Malatesta et al., 2000; Miyata et al., 2001; Noctor et al., 2001; Noctor et al., 2004; Tamamaki et al., 2001). They exhibit a unique and highly recognizable bipolar morphology with a long basal radial glial fiber that extends to the pia surface and a short apical endfoot that forms junctions with neighboring apical endfeet at the luminal surface of the VZ (Bultje et al., 2009; Chenn et al., 1998; Rakic, 2003).
At the early stage of cortical development (e.g. prior to embryonic day 12, E12, in mice), RGPs mainly undergo symmetric proliferative division to amplify themselves (Florio and Huttner, 2014; Homem et al., 2015; Kriegstein and Alvarez-Buylla, 2009). As development proceeds, RGPs transition from symmetric proliferative to asymmetric neurogenic division to produce diverse cortical neurons either directly or indirectly via transit amplifying progenitors such as intermediate progenitor cells (IPs) (Englund et al., 2005; Haubensak et al., 2004; Miyata et al., 2004). While a majority of RGPs exit the cell cycle as they complete the neurogenesis process, a defined fraction of RGPs proceeds to gliogenesis to produce glial cells, including astrocytes and oligodendrocytes (Anthony et al., 2004; Gao et al., 2014; Kessaris et al., 2006; Noctor et al., 2004). Newborn neurons migrate radially along the mother radial glial fibers to assemble the cortical layers in a birth date-dependent inside-out fashion (Angevine and Sidman, 1961; Hatten, 1999; Marin and Rubenstein, 2003; Noctor et al., 2001; Rakic, 1988). Therefore, the precise behavior and division dynamics of RGPs determine the number and type of neurons and orchestrate the formation of the intricate cerebral cortex.
Although there is a comprehensive understanding on the overall process of RGP division and cortical neurogenesis, the key mechanisms that regulate RGP division behavior and the precise neuronal output remain not fully understood. Previous studies have suggested that cellular reactive oxygen species (ROS) and oxidative stress may play a pivotal role in regulating neural stem cell self-renewal and maturation (Hernandez-Garcia et al., 2010; Kwon et al., 2004; Liu et al., 2009; Sundaresan et al., 1995). Moreover, cellular ROS level varies between stem cells and differentiated cells, and has a contrasting effect on their transition and cell fate specification (Ito et al., 2004; Ito et al., 2006; Jang and Sharkis, 2007; Lee et al., 2018b; McGraw and Mittal, 2010; Owusu-Ansah and Banerjee, 2009; Sauer et al., 2001; Singh et al., 2013). Embryonic neural stem cells possess relatively high cellular ROS levels that promote their proliferation and survival (Inoue et al., 2017; Le Belle et al., 2011). ROS levels further increase as stem cells transition from proliferation to differentiation (Bigarella et al., 2014). Unregulated high concentrations of cellular ROS can lead to premature senescence of cells or even cell death (Bae et al., 2011; Bigarella et al., 2014; Blanchetot and Boonstra, 2008; Droge, 2002; Guo et al., 2010; Zhang et al., 2011).
Endogenous ROS are largely produced as a natural product of the normal metabolism by NADPH oxidase complexes in mitochondria, cell membranes, peroxisomes and endoplasmic reticulum (Phaniendra et al., 2015). Emerging data continue to support the correlation between ROS levels and their roles in cell proliferation, differentiation and death (Kwon et al., 2004; Le Belle et al., 2011; Paik et al., 2009; Renault et al., 2009; Sundaresan et al., 1995; Tsatmali et al., 2005; Tsatmali et al., 2006). However, our understanding of the precise regulation of ROS levels and oxidative stress in the developing cortex, and its link to RGP behavior and cortical neurogenesis is limited. In this study, we found that ROS levels and oxidative stress regulated by PR domain-containing 16 (PRDM16), a transcriptional regulator preferentially expressed in RGPs, actively influence RGP mitotic behavior and precise neuronal output in the developing cortex.
RESULTS
Progressive increase in oxidative stress in the developing mouse cortex
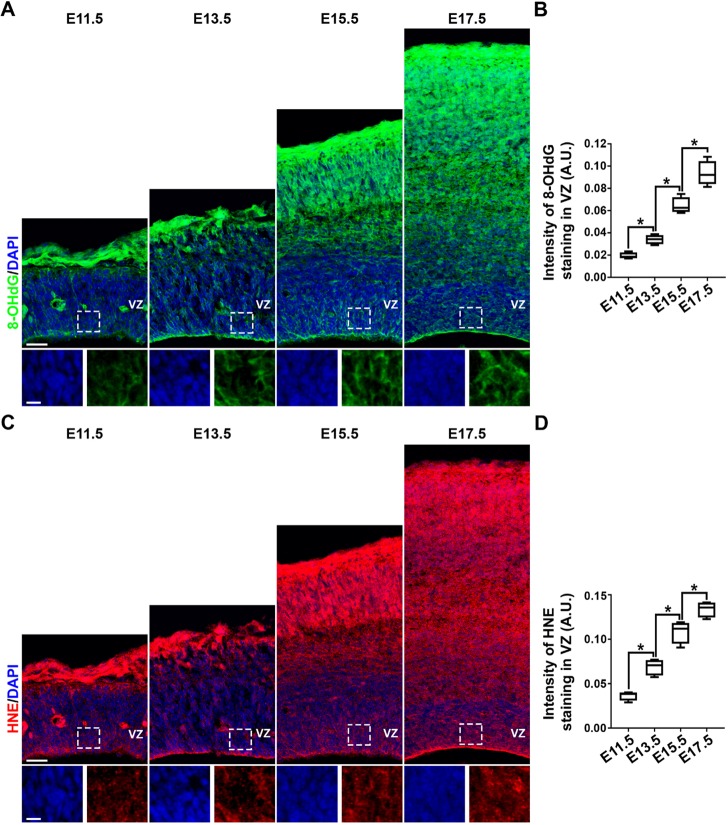
Two critical biomarkers have been well established to measure oxidative stress and visually examine the effect of oxidative damage to DNA and lipids; 8-hydroxy-2′-deoxyguanosine (8-OHdG), an oxidized DNA damage marker (Valavanidis et al., 2009); and 4-hydroxynonenal (HNE), an oxidized lipid damage marker (Zhong and Yin, 2015). To assess oxidative stress during mouse cortical development, we stained embryonic brain sections at E11.5, E13.5, E15.5 and E17.5 with antibodies against 8-OHdG (Fig. 1A,B) or HNE (Fig. 1C,D). Interestingly, as development proceeded, the levels of both 8-OHdG and HNE progressively increased in the developing cortex, including in RGPs in the VZ. To further measure the ROS level in live RGPs during mouse cortical development, we collected the VZ tissues at E11.5, E13.5, E15.5 and E17.5, and prepared neurospheres in culture. We stained the neurospheres with CellROX Green, a cell-permeant dye that measures ROS levels and oxidative stress in live cells (Vlaski-Lafarge and Ivanovic, 2015), and found that ROS levels and oxidative stress progressively increased in the neurospheres prepared at different developmental stages (Fig. S1). These results suggest that there is a steady increase in ROS levels and oxidative stress in RGPs during cortical development.
Fig. 1.
Progressive increase in oxidative stress in the developing mouse cortex. (A,C) Representative images of E11.5, E13.5, E15.5 and E17.5 cortical sections stained using an antibody against the oxidative DNA-damage marker 8-OHdG (green, A) or lipid-damage marker HNE (red, C), and counterstained with DAPI (blue). High-magnification images of the VZ (outlined) are shown at the bottom. Scale bars: 50 µm (top row) and 20 µm (bottom row). (B,D) Quantification of the staining intensity of 8-OHdG (B) and HNE (D) in the VZ (in arbitrary units, A.U.). n=3 brains. *P<0.05. For box and whisker plots: center line, median; box, interquartile range; whiskers, minimum and maximum. Statistical analysis was performed using an unpaired Student's t-test.
Robust and persistent changes in ROS-related gene expression in RGPs
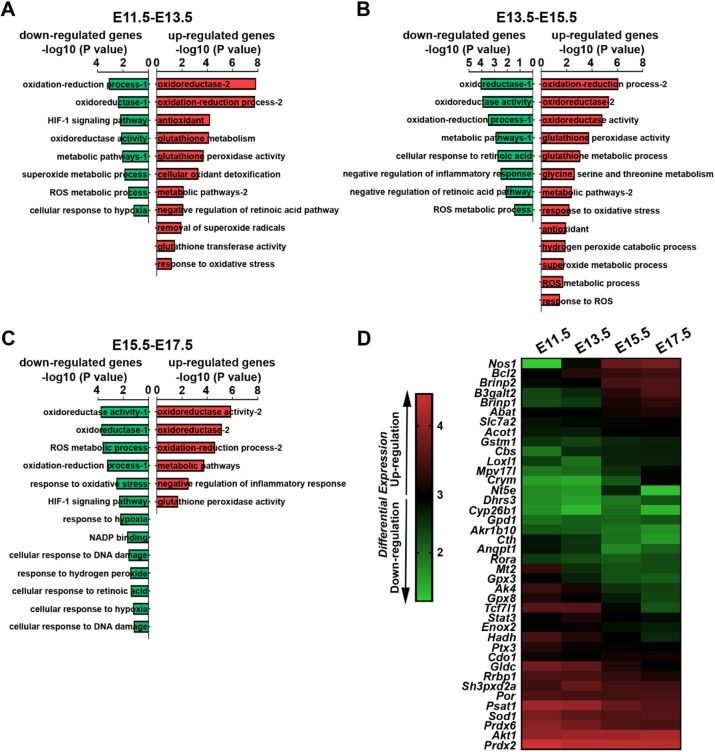
To understand the mechanisms linked to the progressive increase in ROS level and oxidative stress in RGPs, we manually isolated the cortical VZ tissues at E11.5, E13.5, E15.5 and E17.5, and carried out RNA-sequencing (RNA-seq) (Fig. 2). We validated the identity of cells in the VZ tissues to be predominantly in RGPs by staining the dissociated cultures of the VZ tissues with an antibody against PAX6, a transcription factor highly expressed in cortical RGPs (Englund et al., 2005; Gotz et al., 1998) (Fig. S2). Interestingly, through gene ontology analysis and literature search for established regulators of ROS (Bigarella et al., 2014) (Table S1), we found that various upstream and downstream pathways associated with cellular ROS regulation exhibited drastic changes in expression throughout cortical development. Gene Set Enrichment Analysis (GSEA) showed that multiple ROS-related pathways were upregulated or downregulated (Fig. 2A-C), suggesting that ROS levels are highly controlled in RGPs. Notably, numerous ROS-related genes became progressively upregulated or downregulated throughout cortical development (Fig. 2D). Among them, Prdx2 and Prdx6, members of the PRDX antioxidant enzyme family that has been suggested to suppress the production of ROS (Kwon et al., 2016), exhibited a steady decrease in expression. Sod1, an isoenzyme responsible for destroying free superoxide radicals (Wang et al., 2018), also exhibited a strong downregulation. Gpx3 and Gpx8, two ROS scavengers and part of the glutathione peroxidase family that are crucial to maintain ROS levels at a certain range (Herault et al., 2012; Ramming et al., 2014), were progressively downregulated. On the other hand, Nos1, a member of the nitric oxide synthase family that synthesizes the reactive free radical nitric oxide (Liu et al., 2002; Monteiro et al., 2019), displayed a drastic increase in expression. Together, these results suggest that multiple gene pathways are in action to regulate ROS levels in RGPs during cortical development. Moreover, these results indicate that ROS levels are robustly regulated in RGPs, which is likely crucial to ensure proper RGP behavior and neurogenesis in the developing cortex.
Fig. 2.
Robust changes in numerous oxidative stress-related genes in cortical VZ RGPs. (A-C) GSEA of the RNA-seq data from E11.5 to E13.5 (A), E13.5 to E15.5 (B) and E15.5 to E17.5 (C) VZ tissues. Downregulated gene sets are shown in green and upregulated gene sets in red. Forty well-established genes known to regulate ROS with the cutoff P<0.05 (see Table S1) were used to perform GSEA. (D) Heat map showing the robust changes in the expression level of ROS and oxidative stress-related genes from E11.5 to E17.5 in the cortical VZ RGPs.
PRDM16 removal elevates ROS level and drives oxidative stress responsive gene expression
We next wanted to examine whether alteration in ROS levels and oxidative stress would disrupt RGP behavior and cortical neurogenesis. Previous studies have suggested that PRDM16 influences ROS levels in hematopoietic and nervous systems (Chuikov et al., 2010; Inoue et al., 2017; Shimada et al., 2017). As shown previously (Baizabal et al., 2018), PRDM16 was enriched in mouse cortical VZ RGPs, with a lateral high to medial low gradient, as well as in the choroid plexus (Fig. S3A, left). Moreover, its expression in RGPs progressively increased as development proceeded, coinciding with the progressive change in oxidative stress and ROS-related gene expression.
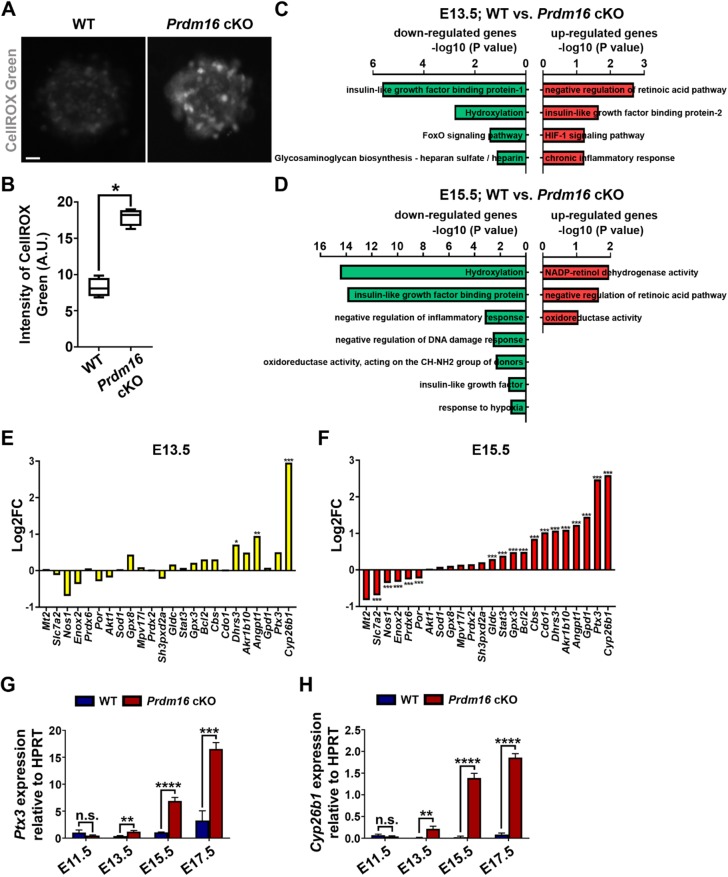
To test whether PRDM16 regulates ROS levels and oxidative stress in cortical RGPs, we took advantage of the floxed Prdm16 mutant mice possessing two loxP sites flanking exon 9, Prdm16fl/fl (Cohen et al., 2014), and crossed them with the Emx1-Cre mice (Gorski et al., 2002), in which Cre recombinase is selectively expressed in cortical RGPs starting around ∼E9-10. Based on immunohistochemistry and western blot analyses, PRDM16 was largely eliminated from cortical RGPs by E13.5 (Fig. S3A,B). To test whether PRDM16 influences ROS production and oxidative stress in live RGPs, we collected the VZ tissues from Prdm16 conditional knockout (cKO) and wild-type littermate control cortices at E15.5, and prepared neurospheres in culture. We stained the neurospheres with CellROX Green and found that ROS level and oxidative stress were substantially increased in the Prdm16 cKO neurosphere compared with the wild-type neurosphere (Fig. 3A,B). These results suggest that PRDM16 removal increases ROS level and oxidative stress in cortical RGPs.
Fig. 3.
PRDM16 removal causes drastic changes in genes associated with oxidoreductase activity. (A) Representative images of E15.5 wild-type and Prdm16 cKO neurospheres stained for CellROX Green (gray) (in arbitrary units, A.U.). Scale bar: 10 µm. (B) Quantification of ROS levels in individual neurospheres. Wild type, n=4 brains; Prdm16 cKO, n=4 brains. *P<0.05. (C,D) GSEA of the RNA-seq data from E13.5 (C) and E15.5 (D) wild-type and Prdm16 cKO VZ tissues showing the drastic changes in ROS pathway gene sets. Downregulated gene sets in Prdm16 cKO versus wild type are in green and upregulated gene sets in red. Cutoff: P<0.05. (E,F) Quantification of the changes in the expression level of ROS-related genes in E13.5 (E) and E15.5 (F) Prdm16 cKO VZ compared with wild-type VZ. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. ***P<0.005, **P<0.01, *P<0.05. (G,H) Quantification of Ptx3 (G) and Cyp26b1 (H) expression in the wild-type and Prdm16 cKO cortices relative to the housekeeping gene hypoxanthine guanine phosphoribosyltransferase (Hprt) at different embryonic stages based on qRT-PCR analysis. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. ****P<0.0001; ***P<0.005; **P<0.01; n.s., not significant. For bar charts, data are mean±s.e.m. For box and whisker plots: center line, median; box, interquartile range; whiskers, minimum and maximum. Statistical analysis was performed using an unpaired Student's t-test.
To explore the mechanisms by which PRDM16 regulates ROS levels and oxidative stress in RGPs, we collected the VZ tissues from the wild-type and Prdm16 cKO cortices at E13.5, E15.5 and E17.5, and carried out RNA-seq analysis (Fig. 3C,D and Fig. S4). Using unbiased transcriptome and GSEA on the top 500 differentially expressed genes (Table S2), we found that multiple ROS-related pathways involved in suppressing ROS levels were downregulated, whereas those associated with increasing ROS levels were upregulated in the Prdm16 cKO VZ compared with the wild-type VZ at E13.5, E15.5 and E17.5 (Fig. 3C,D and Fig. S4A), indicating an upregulation of ROS levels as well as oxidative stress responsive genes in the absence of PRDM16.
Interestingly, we found that cytochrome P450 family 26 subfamily B member 1 (Cyp26b1) and pentraxin 3 (Ptx3), two oxidative stress inducible or responsive genes (Dell'Oglio et al., 2017; Lee et al., 2018a; Zangar et al., 2004), were among the highest upregulated genes in the Prdm16 cKO VZ RGPs compared with the wild-type VZ RGPs at E13.5, E15.5 and E17.5 (Fig. 3E,F and Fig. S4B), consistent with the notion that PRDM16 removal leads to elevated ROS level and induction of oxidative stress responsive gene expression in RGPs. Additionally, through gene ontology analysis and literature searching, we identified additional key ROS pathway-related genes exhibiting significant changes in expression in the Prdm16 cKO RGPs compared with the wild-type RGPs (Fig. 3E,F and Fig. S4B). Among them, Enox2, a member of the NOX family of NADPH oxidases that has been suggested to balance ROS level in cancer cells (Chen et al., 2018), became consistently downregulated. Notably, these identified key ROS pathway-related genes largely overlapped with the genes reflecting the progressive increase in ROS level and oxidative stress in RGPs during normal cortical development (Fig. 2).
We confirmed the upregulation of Ptx3 and Cyp26b1 expression by performing quantitative real-time PCR (qRT-PCR) analysis and found that, compared with the wild-type VZ RGPs, Ptx3 and Cyp26b1 transcripts progressively increased in the Prdm16 cKO VZ RGPs between E13.5 and E17.5 (Fig. 3G,H). Moreover, we also found that PTX3 protein expression was progressively upregulated in the Prdm16 cKO cortex (Fig. S5). Collectively, these results suggest that PRDM16 removal leads to elevated ROS levels and oxidative stress in RGPs, which drives the expression of oxidative stress responsive genes.
PRDM16 removal alters cortical neuronal composition and positioning
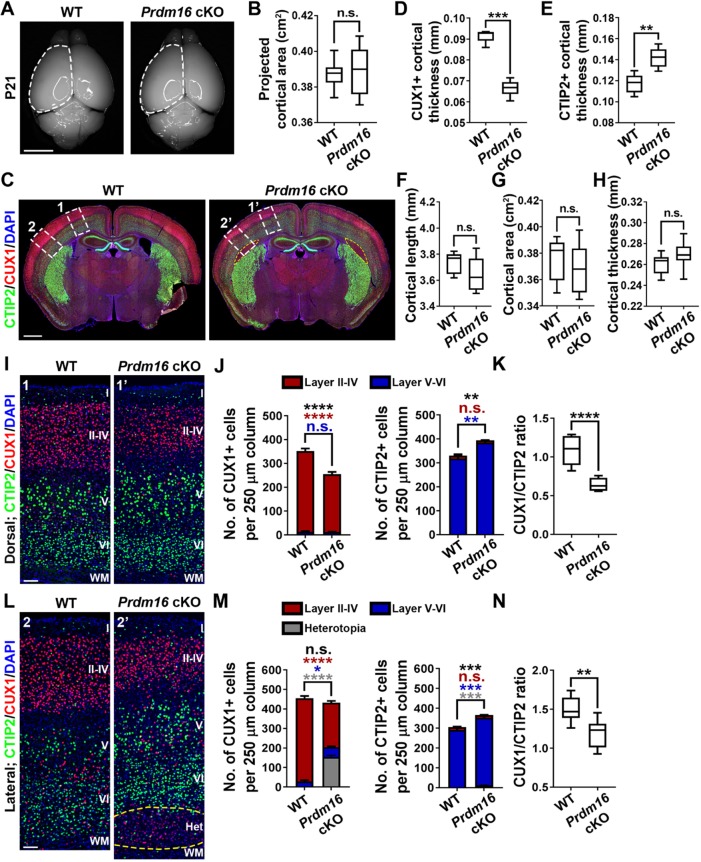
To examine the effect of elevated oxidative stress upon PRDM16 removal on RGPs and cortical development, we examined the wild-type and Prdm16 cKO cortices at postnatal day (P) 21. Prdm16 cKO mice were born at the expected frequency and survived to adulthood. We observed no obvious change in the overall projected cortical area in the Prdm16 cKO mice compared with the wild-type mice (Fig. 4A,B). To further assess whether Prdm16 deletion alters neuronal production and organization in the cortex, we stained P21 brain sections with antibodies against CTIP2 (green), a deep layer V-VI neuronal marker (Greig et al., 2013), and CUX1 (red), a superficial layer II-IV neuronal marker (Greig et al., 2013) (Fig. 4C). Interestingly, we observed a substantial decrease in the thickness of CUX1+ superficial layers (II-IV) but a significant increase in the thickness of CTIP2+ deep layers (V-VI) in the Prdm16 cKO cortex compared with the wild-type control cortex (Fig. 4D,E). Moreover, a prominent subcortical heterotopia was consistently found in the lateral region of the Prdm16 cKO cortex (Fig. 4C, yellow broken lines), as shown previously (Baizabal et al., 2018). The overall cortical length, area or thickness was comparable between the wild-type and Prdm16 cKO brains (Fig. 4F-H). Together, these results suggest that PRDM16 removal leads to altered neuronal composition as well as positioning in the cortex.
Fig. 4.
PRDM16 removal in RGPs alters cortical neuronal composition and organization. (A) Whole-mount images of P21 wild-type and Prdm16 cKO brains. Broken lines indicate the cerebral hemisphere. Scale bar: 0.5 cm. (B) Quantification of the average projected cortical area. Wild type, n=12 brains; Prdm16 cKO, n=13 brains. n.s., not significant. (C) Representative images of P21 wild-type and Prdm16 cKO brain cortices stained for CUX1 (red) and CTIP2 (green), and with DAPI (blue). Yellow broken line delineates the heterotopia. White broken rectangles indicate the dorsal (1 and 1′) and lateral (2 and 2′) regions of the cortex shown in I and L, respectively. Scale bar: 1 mm. (D,E) Quantification of the thickness of CUX1+ (D) and CTIP2+ (E) labeled layers in P21 wild-type and Prdm16 cKO cortices. Wild type, n=5 brains; Prdm16 cKO, n=8 brains. ***P<0.001; **P<0.01. (F-H) Quantification of P21 wild-type and Prdm16 cKO cortical length (F), area (G) and thickness (H). Wild type, n=7 brains; Prdm16 cKO, n=13 brains. n.s., not significant. (I) Representative images of the dorsal region of P21 wild-type and Prdm16 cKO cortices stained for CUX1 (red) and CTIP2 (green), and with DAPI (blue). Scale bar: 200 µm. (J) Quantification of the number of CUX1+ (top) and CTIP2+ (bottom) neurons per 250 µm column. Asterisks indicate the statistical significance of the differences in the number of total CUX1+ or CTIP2+ cells (black), superficial layer CUX1+ or CTIP2+ cells (red), or deep layer CUX1+ or CTIP2+ cells (blue). Wild type, n=5 brains; Prdm16 cKO, n=5 brains. ****P<0.0001; **P<0.01; n.s., not significant. (K) Quantification of the ratio of CUX1+/CTIP2+ neurons per 250 µm column. Wild type, n=5 brains; Prdm16 cKO, n=5 brains. ****P<0.0001. (L) Representative images of the lateral region of P21 wild-type and Prdm16 cKO cortices stained for CUX1 (red) and CTIP2 (green), and with DAPI (blue). Yellow broken line delineates the heterotopia. Scale bar: 200 µm. (M) Quantification of the number of CUX1+ (top) and CTIP2+ (bottom) neurons per 250 µm column. Wild type, n=5 brains; Prdm16 cKO, n=5 brains. ****P<0.0001; ***P<0.001; *P<0.05; n.s., not significant. (N) Quantification of the ratio of CUX1+/CTIP2+ neurons per 250 µm column. Wild type, n=5 brains; Prdm16 cKO, n=5 brains. **P<0.01. For box and whisker plots: center line, median; box, interquartile range; whiskers, minimum and maximum. For bar charts, data are mean±s.e.m. Statistical analysis was performed using an unpaired Student's t-test.
To further characterize the phenotype, we quantitatively analyzed the densities of neurons in deep and superficial layers of the cortex in two separate regions: the dorsal region without heterotopia (Fig. 4C, areas 1 and 1′); and the lateral region harboring the heterotopia (Fig. 4C, areas 2 and 2′). In both the dorsal and lateral regions, the density of CUX1+ superficial layer neurons was substantially decreased, while the density of CTIP2+ deep layer neurons was significantly increased in the Prdm16 cKO cortex compared with the wild-type control cortex (Fig. 4I,J,L,M). Consequently, the ratio of CUX1+ superficial layer neurons to CTIP2+ deep layer neurons was significantly decreased (Fig. 4K,N). To determine whether PRDM16 removal alters the total number of neurons in the dorsal or lateral regions, we stained P21 brain sections with an antibody against NEUN (green), a differentiation neuronal marker (Gusel'nikova and Korzhevskiy, 2015) (Fig. S6A,C). While the density of NEUN+ superficial layer neurons was substantially decreased and the density of NEUN+ deep layer neurons was significantly increased in the Prdm16 cKO cortex compared with the wild-type control cortex, we observed no change in the total density of neurons in the dorsal or lateral region (Fig. S6B,D). The heterotopia formed in the lateral region of the Prdm16 cKO cortex consisted mainly of CUX1+ neurons (Fig. 4L, yellow broken lines). However, the expression level of CUX1 in the heterotopia neurons was clearly weaker than that in the normal superficial layers in either the wild-type or Prdm16 cKO cortex (Fig. S7), indicating that neurons in the heterotopia may not be the typical superficial layer neurons.
The formation of the heterotopia raises the possibility that PRDM16 may function in postmitotic neurons to control neuronal migration and positioning. To directly test this, we crossed the Prdm16fl/fl mouse with the Nex-Cre mouse line (Goebbels et al., 2006), in which Cre recombinase is selectively expressed in postmitotic cortical neurons. Compared with the wild-type littermate control, we observed no obvious changes in the number or distribution of CUX1+ superficial and CTIP2+ deep layer neurons in the Nex-Cre;Prdm16fl/fl cKO cortex at P21 (Fig. S8), suggesting that the defects observed in the Emx1-Cre;Prdm16fl/fl cKO cortex are unlikely due to PRDM16 function in postmitotic neurons. Taken together, these results suggest that PRDM16 removal in RGPs leads to an increase in deep layer neuron production but a concomitant decrease in superficial layer neuron production, as well as abnormal neuronal positioning that may be related to identity specification.
PRDM16 removal promotes excessive RGP proliferation at E13.5
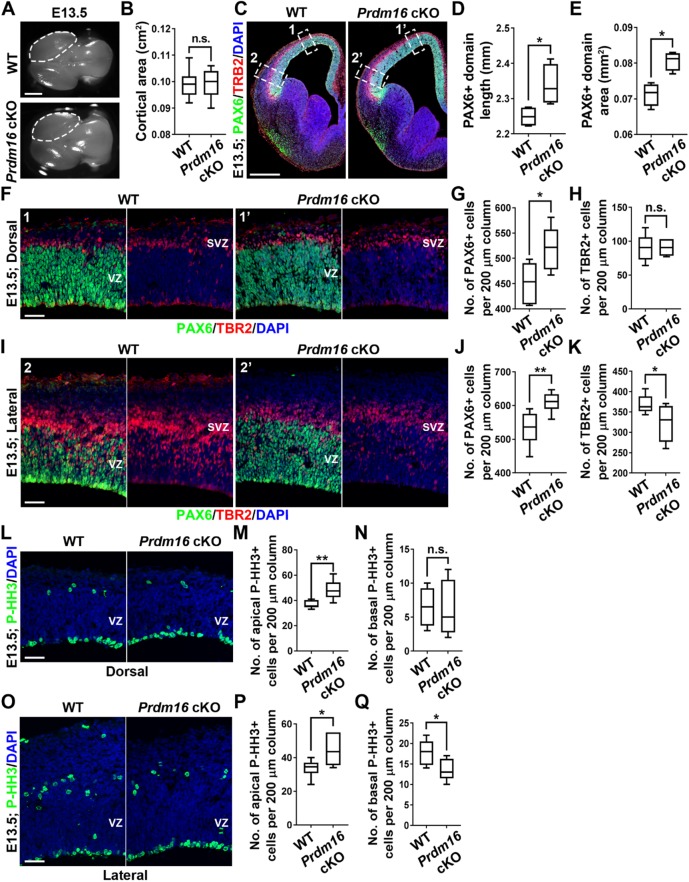
To understand the origins of altered neurogenesis and cortical malformation in the Prdm16 cKO cortex, we next examined RGP behavior and organization at the embryonic stage (Fig. 5). At E13.5, we observed no obvious change in the overall projected cortical area in the Prdm16 cKO brain compared with the wild-type brain (Fig. 5A,B). Interestingly, when we stained the brain sections with an antibody against PAX6, we observed a significant increase in the length and area of PAX6+ domain in the Prdm16 cKO cortex compared with the wild-type cortex (Fig. 5C-E), indicating an increase in RGPs in the Prdm16 cKO cortex at E13.5. We further quantified the density of PAX6+ RGPs in the VZ of the Prdm16 cKO and wild-type cortices in both the dorsal (areas 1 and 1′) and lateral (areas 2 and 2′) regions. Notably, the density of PAX6+ RGPs was significantly higher in the Prdm16 cKO cortex than that in the wild-type cortex (Fig. 5F,G,I,J). Together, these results strongly suggest that removal of PRDM16 in RGPs leads to a drastic increase in the total number of RGPs at E13.5, when deep layer neurons are generated.
Fig. 5.
PRDM16 removal causes excessive RGP proliferation and generation at E13.5. (A) Whole-mount images of E13.5 wild-type and Prdm16 cKO brains. Broken lines indicate the cerebral hemispheres. Scale bar: 1 mm. (B) Quantification of the average projected cortical area. Wild type, n=17 brains; Prdm16 cKO, n=7 brains. n.s., not significant. (C) Representative images of E13.5 wild-type and Prdm16 cKO cortices stained for PAX6 (green), TBR2 (red) and with DAPI (blue). White broken rectangles indicate dorsal (1 and 1′) and lateral (2 and 2′) regions of the cortex shown in F and I, respectively. Scale bar: 0.5 mm. (D,E) Quantification of PAX6+ domain length (D) and area (E). Wild type, n=4 brains; Prdm16 cKO, n=4 brains. *P<0.05. (F) Representative images of the dorsal region of E13.5 wild-type and Prdm16 cKO cortices stained for PAX6 (green) and TBR2 (red), and with DAPI (blue). Scale bar: 50 µm. (G,H) Quantification of the number of PAX6+ (G) and TBR2+ (H) cells per 200 µm column in F. Wild type, n=4 brains; Prdm16 cKO, n=4 brains. *P<0.05; n.s., not significant. (I) Representative images of the lateral region of E13.5 wild-type and Prdm16 cKO cortices stained for PAX6 (green), TBR2 (red) and DAPI (blue). Scale bar: 50 µm. (J,K) Quantification of the number of PAX6+ (J) and TBR2+ (K) cells per 200 µm column in I. Wild type, n=4 brains; Prdm16 cKO, n=4 brains. **P<0.01; *P<0.05. (L) Representative images of the dorsal region of E13.5 wild-type and Prdm16 cKO cortices stained for P-HH3 (green) and DAPI (blue). Scale bar: 50 µm. (M,N) Quantification of the number of apical (M) and basal (N) P-HH3+ cells per 200 µm column as shown in L. Wild type, n=4 brains; Prdm16 cKO, n=4 brains. **P<0.01; n.s., not significant. (O) Representative images of the lateral region of E13.5 wild-type and Prdm16 cKO cortices stained for P-HH3 (green) and with DAPI (blue). Scale bar: 50 µm. (P,Q) Quantification of the number of apical (P) and basal (Q) P-HH3+ cells per 200 µm column as shown in O. Wild type, n=4 brains, Prdm16 cKO, n=4 brains. *P<0.05. For box and whisker plots: center line, median; box, interquartile range; whiskers, minimum and maximum. Statistical analysis was performed using an unpaired Student's t-test.
RGPs divide at the VZ surface to produce neurons as well as IPs that continue to divide in the subventricular zone (SVZ) (Englund et al., 2005; Haubensak et al., 2004; Noctor et al., 2004). We therefore stained the brain sections with an antibody against TBR2, a T-box transcription factor highly expressed in IPs (Englund et al., 2005), and found that the density of TBR2+ IPs in the Prdm16 cKO cortex was comparable with that in the wild-type cortex in the dorsal region (Fig. 5F,H). However, in the lateral region, the density of TBR2+ IPs in the Prdm16 cKO cortex was significantly decreased compared with the wild-type cortex (Fig. 5I,K). Consistent with the changes in the densities of PAX6+ RGPs in the VZ and TBR2+ IPs in the SVZ, we observed a substantial increase in the densities of mitotic cells labeled by phosphorylated histone H3 (P-HH3) at the VZ surface where RGPs divide in both the dorsal and lateral regions, and a decrease in the density of P-HH3+ mitotic cells in the SVZ of the lateral region in the Prdm16 cKO cortex compared with the wild-type cortex (Fig. 5L-Q). Together, these results suggest that PRDM16 removal results in an increase in RGP number and division, as well as a decrease in IP number and division at the lateral region, at E13.5.
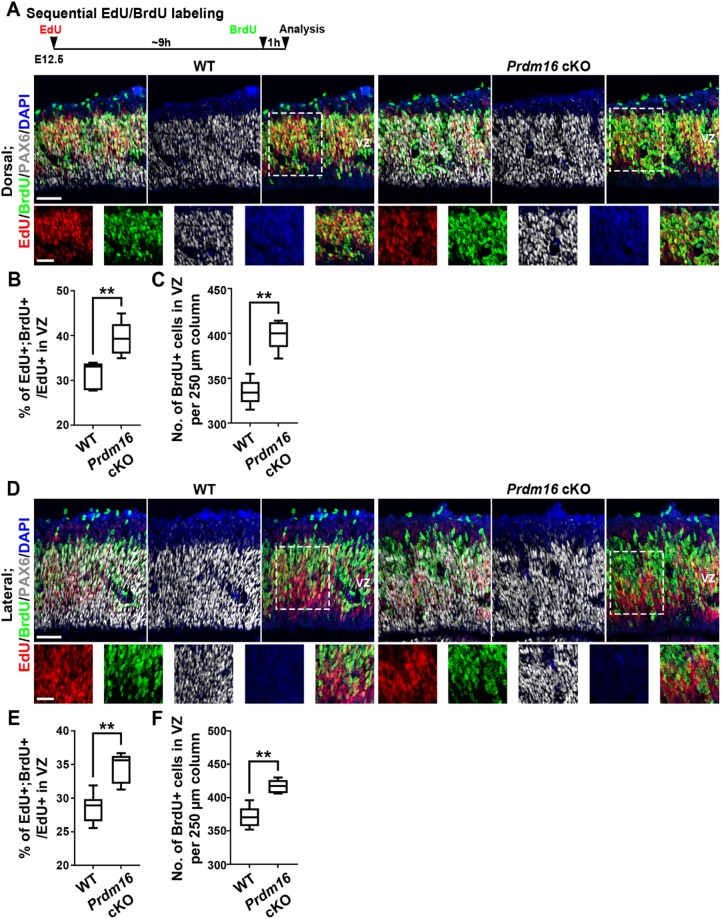
The drastic increase in RGPs upon PRDM16 removal at E13.5 likely arises from enhanced RGP proliferation. To test this, we performed sequential pulse-chase experiments to assess the cell cycle dynamics of RGPs in the Prdm16 cKO and wild-type cortices prior to E13.5 (Fig. 6). We administered a single dose of 5-ethynyl-2′-deoxyuridine (EdU), a modified nucleoside, at E12.5 followed by a single dose of 5-bromo-2′-deoxyuridine (BrdU), a thymidine analog, ∼9 h apart and collected the brains 1 h later for analysis (Fig. 6A, top). A previous study has shown that cell cycle duration at E12.5 is ∼9-10 h (Caviness et al., 1995). We found that the percentage of EdU+ RGPs in the VZ that were BrdU+ was substantially increased in the dorsal and lateral regions of the Prdm16 cKO cortex compared with that in the wild-type cortex (Fig. 6A,B,D,E), suggesting that dividing RGPs in the Prdm16 cKO cortex re-enter the cell cycle faster than those in the wild-type cortex. The enhanced RGP cell cycle progression in the Prdm16 cKO cortex was corroborated by an increased density of BrdU+ RGPs in the VZ of the dorsal and lateral regions (Fig. 6A,C,D,F). Collectively, these results suggest that at the early phase of cortical neurogenesis, PRDM16 removal promotes RGP cell cycle progression, resulting in excessive proliferation and generation of RGPs for deep layer neuron production.
Fig. 6.
PRDM16 removal accelerates RGP cell cycle progression at E12.5. (A,D) Representative images of the dorsal and lateral regions of E12.5 wild-type and Prdm16 cKO cortices subjected to EdU (red) and BrdU (green) sequential pulse-chase labeling, and stained for PAX6 (white) and with DAPI (blue), respectively. Schematic paradigm is shown at the top. High-magnification images (broken lines) are shown at the bottom. Scale bars: 50 µm (top) and 20 µm (bottom). (B,C) Quantification of the percentage of EdU+;BrdU+ cells out of the total EdU+ cells in the VZ (B) and the number of BrdU+ cells in the VZ per 250 µm column (C) in A. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. **P<0.01. (E,F) Quantification of the percentage of EdU+;BrdU+ cells out of the total EdU+ cells in the VZ (E) and the number of BrdU+ cells in the VZ per 250 µm column (F) in D. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. **P<0.01. For box and whisker plots: center line, median; box, interquartile range; whiskers, minimum and maximum. Statistical analysis was performed using an unpaired Student's t-test.
PRDM16 removal accelerates RGP differentiation and depletion at E15.5
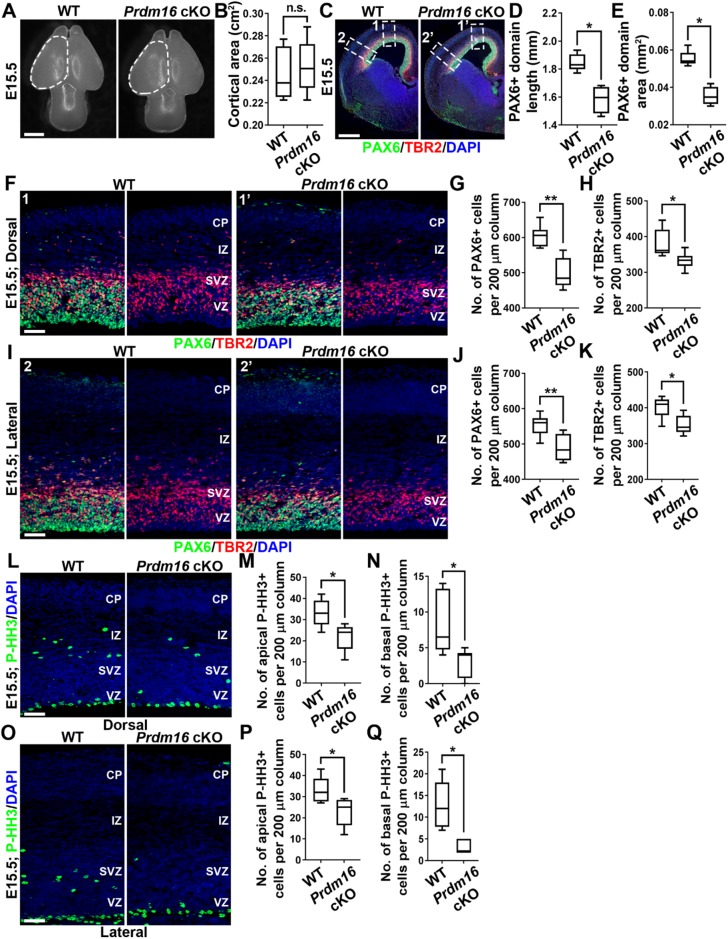
To further dissect the basis of the superficial layer neuron reduction in the Prdm16 cKO cortex, we examined the number and behavior of cortical progenitors at E15.5 when superficial layer neurons are generated (Fig. 7). The overall projected cortical area of the Prdm16 cKO brain was similar to that of the wild-type control (Fig. 7A,B). In contrast to that at E13.5, the length and area of PAX6+ RGP domain were significantly decreased in the Prdm16 cKO cortex compared with the control (Fig. 7C-E), indicating a loss of RGPs at E15.5. We further analyzed the density of PAX6+ RGPs in the VZ and the density of TBR2+ IPs in the SVZ, and found that both were significantly reduced in the dorsal and lateral regions of the Prdm16 cKO cortex (Fig. 7F-K). Consistent with the loss of RGPs and IPs, we observed a substantial decrease in P-HH3-labeled mitotic cells at the VZ surface and in the SVZ of the dorsal and lateral regions of the Prdm16 cKO cortex (Fig. 7L-Q). Together, these results suggest that removal of PRDM16 leads to a premature loss of RGPs, as well as IPs, at E15.5 when superficial layer neurons are generated.
Fig. 7.
PRDM16 removal results in a loss of RGPs and IPs at E15.5. (A) Whole-mount images of E15.5 wild-type and Prdm16 cKO brains. Broken lines indicate the cerebral hemispheres. Scale bar: 1 mm. (B) Quantification of the average projected cortical area. Wild type, n=6 brains; Prdm16 cKO, n=6 brains. n.s., not significant. (C) Representative images of E15.5 wild-type and Prdm16 cKO cortices stained for PAX6 (green) and TBR2 (red), and with DAPI (blue). White broken rectangles indicate dorsal (1 and 1′) and lateral (2 and 2′) regions of the cortex shown in F and I, respectively. Scale bar: 0.5 mm. (D,E) Quantification of PAX6+ domain length (D) and area (E). Wild type, n=5 brains; Prdm16 cKO, n=4 brains. *P<0.05. (F) Representative images of the dorsal region of E15.5 wild-type and Prdm16 cKO cortices stained for PAX6 (green) and TBR2 (red), and with DAPI (blue). Scale bar: 50 µm. (G,H) Quantification of the number of PAX6+ (G) and TBR2+ (H) cells per 200 µm column. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. **P<0.01; *P<0.05. (I) Representative images of the lateral region of E15.5 wild-type and Prdm16 cKO cortices stained for PAX6 (green) and TBR2 (red), and with DAPI (blue). Scale bar: 50 µm. (J,K) Quantification of the number of PAX6+ (J) and TBR2+ (K) cells per 200 µm column. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. **P<0.01; *P<0.05. (L) Representative images of the dorsal region of E15.5 wild-type and Prdm16 cKO cortices stained for P-HH3 (green) and DAPI (blue). Scale bar: 50 µm. (M,N) Quantification of the number of apical (M) and basal (N) P-HH3+ cells per 200 µm column. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. *P<0.05. (O) Representative images of the lateral region of E15.5 wild-type and Prdm16 cKO cortices stained for P-HH3 (green) and DAPI (blue). Scale bar: 50 µm. (P,Q) Quantification of the number of apical (P) and basal (Q) P-HH3+ cells per 200 µm column. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. *P<0.05. For box and whisker plots: center line, median; box, interquartile range; whiskers, minimum and maximum. Statistical analysis was performed using an unpaired Student's t-test.
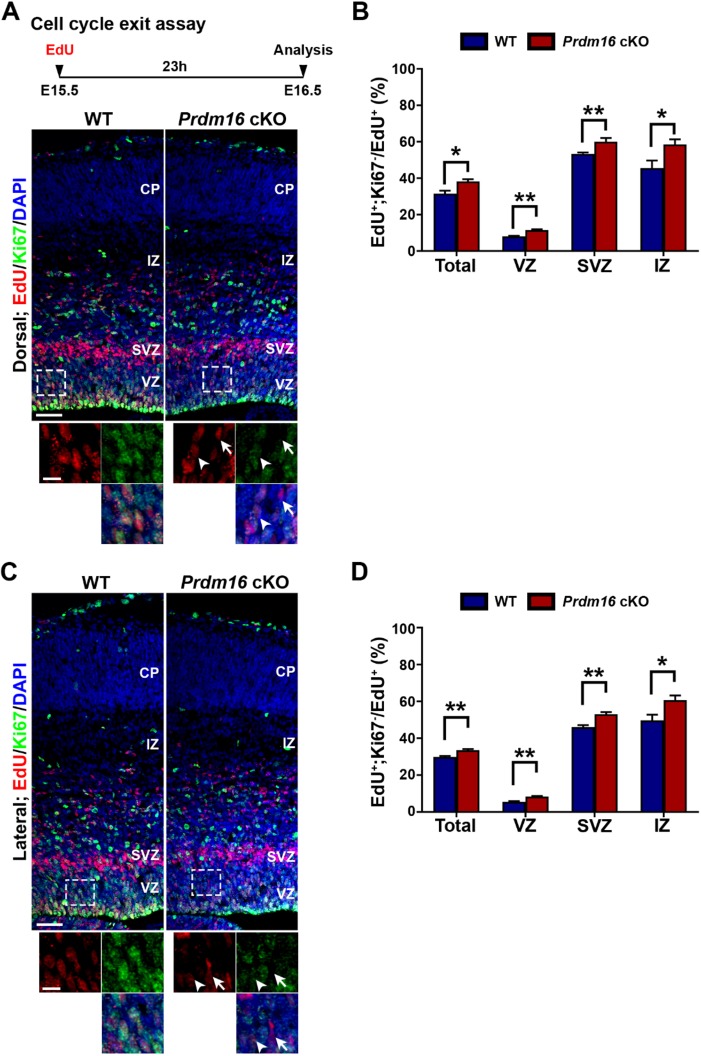
The loss of RGPs upon PRDM16 removal at E15.5 likely arises from premature cell cycle exit of RGPs. To test this, we examined the cell cycle exit index of cortical progenitors at E15.5 (Fig. 8). A single pulse of EdU was administered at E15.5 and brains were extracted at E16.5 and stained for the proliferative marker Ki67 (Fig. 8A, top). We analyzed the fraction of EdU+ cells that were Ki67− (i.e. exited the cell cycle) and found that there was a significant increase in cell cycle exit in the dorsal and lateral regions of the Prdm16 cKO cortex compared with that in the wild-type cortex (Fig. 8A-D). The enhanced rate of cell cycle exit is consistent with a decrease in RGPs (Fig. 7F,G,I,J). Together, these results suggest that PRDM16 removal leads to premature cell cycle exit and depletion of RGPs at late stage of cortical neurogenesis when superficial layer neurons are generated. Notably, the biphasic change in RGP behavior upon PRDM16 removal is well in line with the bidirectional regulation of cell proliferation and differentiation by ROS level, indicating that PRDM16 functions via controlling ROS levels to regulate RGP behavior and proper neurogenesis in the developing cortex.
Fig. 8.
PRDM16 removal accelerates RGP cell cycle exit at E15.5. (A,C) Representative images of the dorsal and lateral regions of E15.5 wild-type and Prdm16 cKO cortices stained for EdU (red) and proliferating marker Ki67 (green), and with DAPI (blue). Schematic paradigm of the cell cycle exit assay is shown at the top. High-magnification images (broken lines) are shown at the bottom. Arrows indicate EdU+/Ki67− cells that exit the cell cycle and arrowheads indicate EdU+/Ki67+ cells that remain in the cell cycle. Scale bars: 50 µm (top) and 20 µm (bottom). (B) Quantification of the percentage of EdU+;Ki67− cells out of the total EdU+ cells in A. (D) Quantification of the percentage of EdU+;Ki67− cells out of the total EdU+ cells in C. Wild type, n=3 brains; Prdm16 cKO, n=3 brains. **P<0.01; *P<0.05. Statistical analysis was performed using unpaired Student's t-test. For bar charts, data are mean±s.e.m.
mCAT expression suppresses cortical defects caused by PRDM16 removal
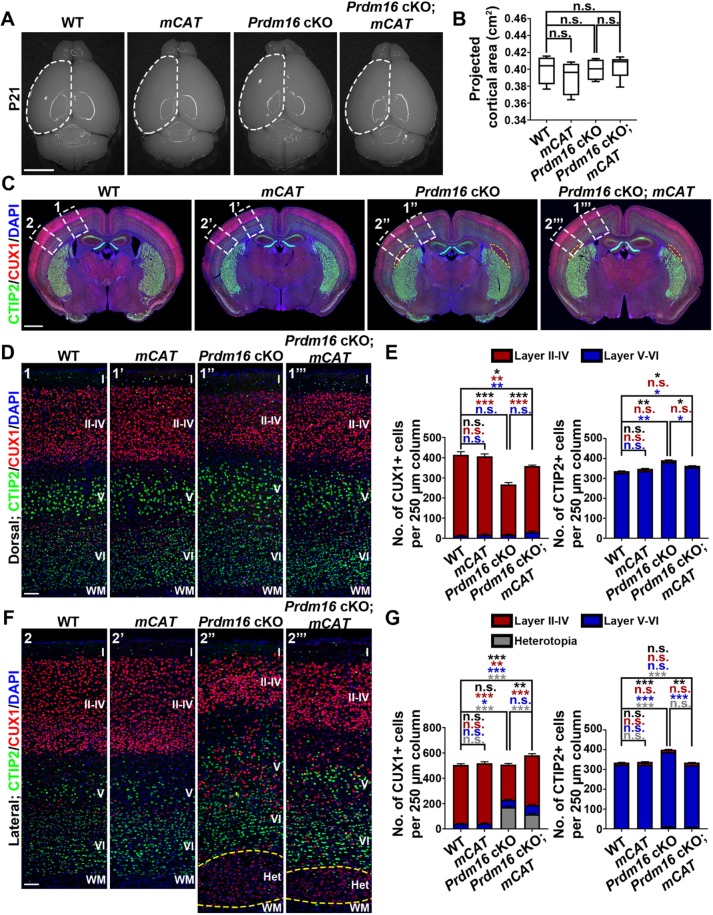
To directly test whether PRDM16 functions via regulating ROS levels to control RGP behavior and cortical neurogenesis, we took advantage of the transgenic mouse line mitochondrially targeted catalase (mCAT), which over-expresses the catalase gene in mitochondria and thereby reduces mitochondria-generated ROS (Schriner et al., 2005). Catalase catalyzes the decomposition of ROS such as hydrogen peroxide to water and oxygen (Dai et al., 2017). We integrated the mCAT transgene with the Prdm16 cKO mice and generated the wild-type, mCAT, Prdm16 cKO and Prdm16 cKO; mCAT mice, which were born at the expected frequency (Fig. 9). There was no obvious change in the overall projected cortical area among the four different genotypes at P21 (Fig. 9A,B).
Fig. 9.
Simultaneous mCAT expression significantly rescues cortical defects caused by PRDM16 removal. (A) Whole-mount images of P21 wild-type, mCAT, Prdm16 cKO and Prdm16 cKO; mCAT brains. Broken lines indicate the cerebral hemisphere. Scale bar: 0.5 cm. (B) Quantification of the average projected cortical area. Wild type, n=4 brains; mCAT, n=4 brains; Prdm16 cKO, n=4 brains; Prdm16 cKO; mCAT, n=4 brains. n.s., not significant. (C) Representative images of P21 wild-type, mCAT, Prdm16 cKO and Prdm16 cKO; mCAT cortices stained for CUX1 (red) and CTIP2 (green), and with DAPI (blue). Yellow broken outline delineates the heterotopia. White broken rectangles indicate dorsal (1, 1′, 1″ and 1‴) and lateral (2, 2′, 2″ and 2‴) regions of the cortex shown in D and F, respectively. Scale bar: 1 mm. (D) Representative images of the dorsal region of P21 wild-type, mCAT, Prdm16 cKO and Prdm16 cKO; mCAT cortices stained for CUX1 (red) and CTIP2 (green), and with DAPI (blue). Scale bar: 200 µm. (E) Quantification of the number of CUX1+ (top) and CTIP2+ (bottom) neurons per 250 µm column. Wild type, n=4 brains; mCAT, n=4 brains; Prdm16 cKO, n=4 brains; Prdm16 cKO; mCAT, n=4 brains. ***P<0.001; **P<0.01; *P<0.05; n.s., not significant. (F) Representative images of the lateral region of P21 wild-type, mCAT, Prdm16 cKO and Prdm16 cKO; mCAT cortices stained for CUX1 (red) and CTIP2 (green), and with DAPI (blue). Yellow broken line delineates the heterotopia. Scale bar: 200 µm. (G) Quantification of the number of CUX1+ (top) and CTIP2+ (bottom) neurons per 250 µm column. Wild type, n=4 brains; mCAT, n=4 brains; Prdm16 cKO, n=4 brains; Prdm16 cKO; mCAT, n=4 brains. ***P<0.001; **P<0.01; *P<0.05; n.s., not significant. For box and whisker plot: center line, median; box, interquartile range; whiskers, minimum and maximum. For bar charts, data are mean±s.e.m. Statistical analysis was performed using an unpaired Student's t-test.
To assess neuronal production and cortical development, we stained the brain sections with antibodies against CTIP2 (green) and CUX1 (red) (Fig. 9C-G). Compared with the wild-type control, we observed no obvious change in the overall number or distribution of CUX1+ superficial and CTIP2+ deep layer neurons in the mCAT cortex, indicating that mCAT expression alone does not affect cortical neurogenesis and development. As shown above, in addition to the heterotopia formation in the lateral region, the number of CUX1+ superficial layer neurons was drastically reduced and the number of CTIP2+ deep layer neurons was significantly increased in the Prdm16 cKO cortex. Interestingly, compared with the Prdm16 cKO cortex, we observed a significant increase in CUX1+ superficial layer neurons and a significant decrease in CTIP2+ deep layer neurons in the Prdm16 cKO; mCAT cortex. Moreover, the size of the heterotopia in the Prdm16 cKO; mCAT cortex also became significantly smaller (Fig. 9C,F, yellow broken lines). Together, these results clearly suggest that mCAT expression significantly rescues cortical neurogenesis and neuronal organization defects caused by PRDM16 removal. Notably, compared with the wild-type cortex, the number of CUX1+ superficial layer neurons remained decreased and the number of CTIP2+ deep layer neurons remain increased in the Prdm16 cKO; mCAT cortex, indicating that mCAT expression does not fully rescue the cortical defects caused by PRDM16 removal.
mCAT expression suppresses elevated oxidative stress caused by PRDM16 removal
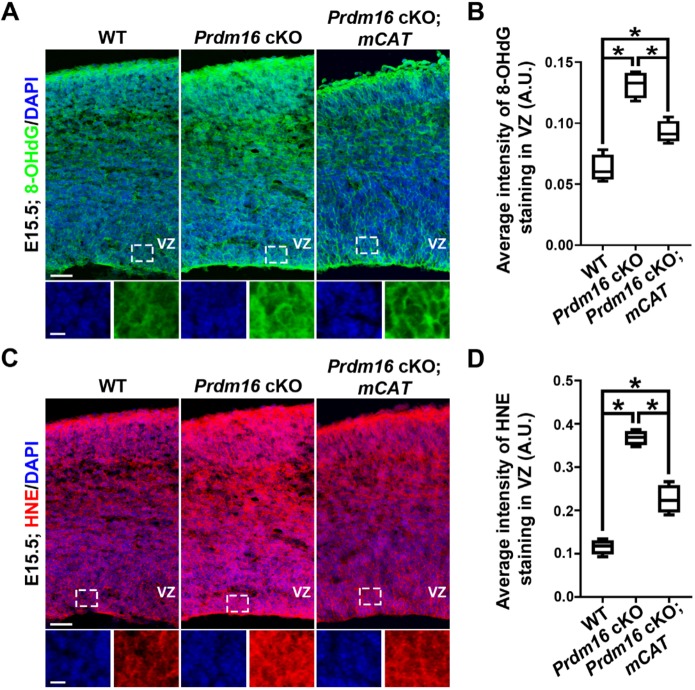
To further assess whether PRDM16 regulates ROS level and oxidative stress in RGPs in the developing cortex in vivo, we stained the wild-type, Prdm16 cKO and Prdm16 cKO; mCAT brain sections with antibodies against 8-OHdG and HNE at E13.5 (Fig. S9A,C) and E15.5 (Fig. 10A,C). As expected, we observed a significant increase in both 8-OHdG and HNE staining in the VZ of the Prdm16 cKO cortex compared with that of the wild-type cortex (Fig. S9 and Fig. 10), suggesting that PRDM16 removal enhances ROS level and oxidative stress in RGPs of the developing cortex. The increase was more drastic at E15.5 than at E13.5. Notably, compared with the Prdm16 cKO cortex, we also observed a significant decrease in both 8-OHdG and HNE staining in the VZ of the Prdm16 cKO; mCAT cortex (Fig. S9 and Fig. 10). Together, these results suggest that mCAT expression suppresses the enhanced ROS level and oxidative stress caused by PRDM16 removal. Expression of mCAT did not fully suppress the elevated oxidative stress caused by PRDM16 removal (Fig. S9 and Fig. 10), consistent with our observation that mCAT expression does not fully rescue the cortical defects observed in the Prdm16 cKO cortex (Fig. 9). The increase in ROS level and oxidative stress upon PRDM16 removal raises the possibility that it may cause cell death. To test this, we stained the wild-type and Prdm16 cKO brain sections with an antibody against cleaved-caspase 3 (CASP3), an apoptotic cell death marker, and observed no obvious increase in cell death in the Prdm16 cKO cortex compared with the wild-type cortex (Fig. S10). Together, these results further support the possibility that PRDM16 regulates ROS level and oxidative stress in RGPs, and thereby controls cortical neurogenesis and development.
Fig. 10.
PRDM16 removal leads to a drastic increase in oxidative stress at E15.5. (A) Representative images of the dorsal region of E15.5 wild-type, Prdm16 cKO and Prdm16 cKO; mCAT cortices stained for 8-OHdG (green) and counterstained with DAPI (blue). High-magnification images of the VZ (outlined) are shown at the bottom. Scale bars: 50 µm (top) and 20 µm (bottom). (B) Quantification of the 8-OHdG expression level in the VZ (A.U.). Wild type, n=4 brains; Prdm16 cKO, n=4 brains; Prdm16 cKO; mCAT, n=4. *P<0.05. (C) Representative images of the dorsal region of E15.5 wild-type, Prdm16 cKO and Prdm16 cKO; mCAT cortices stained for HNE (red) and with DAPI (blue). High magnification images of the VZ (outlined) are shown at the bottom. Scale bars: 50 µm (top) and 20 µm (bottom). (D) Quantification of the HNE expression level in the VZ (A.U.). Wild type, n=4 brains; Prdm16 cKO, n=4 brains; Prdm16 cKO; mCAT, n=4. *P<0.05. For box and whisker plots: center line, median; box, interquartile range; whiskers, minimum and maximum. Statistical analysis was performed using an unpaired Student's t-test.
DISCUSSION
In this study, we uncover the critical regulation of ROS levels and oxidative stress in controlling proper RGP behavior, cortical neurogenesis and neuronal organization. Moreover, through the selective removal of PRDM16 in RGPs, our data demonstrate that an increase in ROS level and oxidative stress impairs proper RGP behavior and the precise generation of deep versus superficial layer neurons in the mammalian cortex. At the early stage of cortical neurogenesis, an increase in oxidative stress causes RGP over-proliferation and excessive production of deep layer neurons. On the other hand, at the late stage of cortical neurogenesis, more elevated oxidative stress accelerates RGP differentiation and depletion, resulting in a loss of superficial layer neurons.
Cellular ROS levels have been demonstrated to regulate the property and behavior of different cell types, especially stem cells (Bigarella et al., 2014; Ito et al., 2004; Liu et al., 2009; Madhavan et al., 2006; Yoneyama et al., 2010). It has been suggested that neural stem cells require relatively high endogenous ROS levels to propel them to a high proliferative state, while low endogenous ROS levels keep them in a quiescent state (Le Belle et al., 2011). Moreover, an excessive amount of ROS can cause cell differentiation, senescence or even death (Bae et al., 2011; Bigarella et al., 2014; Blanchetot and Boonstra, 2008; Droge, 2002; Guo et al., 2010; Zhang et al., 2011). Our findings are consistent with previous studies suggesting that a low-level increase in ROS status promotes cell proliferation and survival, whereas a continuous buildup of ROS levels drives premature cell differentiation and senescence (Bigarella et al., 2014; Guo et al., 2010; Inoue et al., 2017; Le Belle et al., 2011). In the developing cortex, RGPs progress through a program of symmetric proliferation, asymmetric neurogenesis and cell cycle exit/depletion. ROS are produced as a product of normal cellular metabolism and may therefore act as a crucial regulator of proper RGP behavior and lineage progression.
PRDM16 was originally identified as a proto-oncogene in leukemias (Kinameri et al., 2008). It is a member of the PRDM transcription factor family that functions as transcriptional regulators as well as methyltransferases (Hohenauer and Moore, 2012). In a previous suppressor screen of BMI-1 polycomb epigenetic regulator, PRDM16 was identified to promote stem cell maintenance in the fetal hematopoietic and nervous systems (Chuikov et al., 2010). However, the precise function of PRDM16 in regulating RGP division and cortical neurogenesis is not fully understood, as the systematic Prdm16 deletion mice die soon after birth (Chuikov et al., 2010). On the other hand, conditional deletion of Prdm16 using Nestin-Cre results in a loss of ependymal cells, severe hydrocephalus and adult neural stem cell abnormalities in the SVZ, with no obvious defect in embryonic cortical neurogenesis (Shimada et al., 2017). We did not observe any obvious hydrocephalus in the Prdm16 cKO brain using Emx1-Cre. A recent study showed that conditional deletion of Prdm16 using Emx1-Cre leads to a loss and defective migration (i.e. heterotopia) of superficial layer neurons, while the number of deep layer neurons is not affected (Baizabal et al., 2018). Moreover, this study suggested that PRDM16 regulates the epigenetic state of transcriptional enhancers to activate genes involved in IP generation and to repress genes involved in cell migration, such as the E3 ubiquitin ligase PDZRN3 (Baizabal et al., 2018). In comparison, we found that in the Emx1-Cre;Prdm16fl/fl cKO cortex, in addition to a loss of superficial layer neurons and a heterotopia in the lateral region, the number of deep layer neurons is significantly increased, suggesting that PRDM16 regulates the production of both deep and superficial layer neurons. Moreover, we found that the opposite change in the generation of deep and superficial layer neurons in the absence of PRDM16 is due to an initial over-proliferation of RGPs at E13.5, resulting in more deep layer neuron production, followed by an accelerated cell cycle exit of RGPs at E15.5 leading to fewer superficial layer neuron generation. These results suggest that PRDM16 actively regulates the precise behavior of RGPs throughout cortical neurogenesis.
Importantly, the defects in the production and positioning of deep and superficial layer neurons in the Prdm16 cKO cortex were significantly rescued by the expression of catalase targeted to mitochondria, which reduces ROS levels and ameliorates oxidative stress. These results strongly suggest that PRDM16 regulates RGP behavior and cortical neurogenesis, as well as neuronal position via modulating ROS levels. Consistent with this, the expression of oxidative stress responsive genes was robustly and progressively induced in the Prdm16 cKO RGPs, indicating that PRDM16 removal results in elevated ROS levels and oxidative stress. Interestingly, upon PRDM16 removal at E15.5 and E17.5, there is an upregulation of genes negatively regulating the retinoic acid pathway, which regulates early neurogenesis by RGPs, suppresses ROS levels and can induce apoptosis (Haushalter et al., 2017; Jeong and Joo, 2016). One of these crucial regulators is Cyp26b1, which controls the level of retinoic acid by catalyzing a multistep reaction to oxidize retinoic acid and thereby preventing abnormal cell signaling via this pathway (Rhinn and Dolle, 2012). Future efforts to assess the precise function of CYP26B1 in cortical RGPs will provide new insight into the regulation of the retinoic acid pathway and its influence on ROS level and RGP behavior. Notably, Hamlet, the PRDM16 homolog in Drosophila, has been identified as a key regulator of Drosophila neuroblast lineage progression (Eroglu et al., 2014; Moore et al., 2002). In this context, Hamlet deletion led to increased ROS levels and oxidative stress. Consistent with this, we found that PRDM16 removal in cortical RGPs drives the expression of oxidative stress responsive genes and affects their division and output, indicating that a conserved function of PRDM16 is to regulate neural progenitor behavior by modulating ROS levels and oxidative stress.
PRDM16 has also been shown to regulate mitochondrial ROS levels, and neuronal morphological changes and migration in the developing mouse cortex (Baizabal et al., 2018; Chuikov et al., 2010; Inoue et al., 2017). We also found a few similar cell migration genes within the top 500 differentially expressed genes in our RNA-seq analysis of the wild-type and Emx1-Cre;Prdm16fl/fl cKO cortices (Table S3). Compared with the wild-type cortex, most of the identified cell migration genes were increased in the Prdm16 cKO cortex. We did not observe any obvious migration defect in the Nex-Cre;Prdm16 cKO cortex, indicating that the migration defect is unlikely due to PRDM16 function in postmitotic neurons.
It is important to point out that mCAT expression does not fully rescue cortical defects caused by PRDM16 removal. This may reflect at least two possible scenarios. First, additional mechanisms, such as epigenetic state regulation, are involved in mediating the function of PRDM16 in regulating RGP behavior and cortical development (Baizabal et al., 2018). Second, mCAT expression does not fully revert ROS level elevation caused by PRDM16 removal. Indeed, we found that mCAT expression could not fully suppress the elevated oxidative stress caused by PRDM16 removal. ROS are generated mainly by NADPH oxidase complexes in cell membranes, mitochondria, peroxisomes and endoplasmic reticulum (Phaniendra et al., 2015). Reducing mitochondria-generated ROS may not be sufficient to fully suppress ROS level elevation and, consequently, cortical defects caused by PRDM16 removal. It will be interesting to examine whether further decrease in ROS generation may fully rescue the defects observed in the Prdm16 cKO cortex.
While the overall cortex size, area, length and thickness in the Prdm16 cKO brain were not significantly changed compared with the wild-type brain, the composition and positioning of neurons were altered. Notably, the changes in the number of deep and superficial layer neurons are largely consistent in the dorsal and lateral regions of the Prdm16 cKO cortex. However, the formation of the heterotopia consisting of predominantly neurons expressing relatively weak superficial layer neuron marker occurs only in the lateral region. While the exact basis of this region-specific heterotopia formation remains to be determined, it indicates that PRDM16 may have a stronger influence on the cortical lamination in the lateral region. This may be related to its high lateral to low medial expression gradient at the early developmental stages. Furthermore, it would be interesting to assess whether the generation of astrocytes and/or oligodendrocytes by RGPs was altered upon PRDM16 removal and ROS level change.
ROS are naturally generated in cells during metabolic processes, and are tightly regulated by antioxidant enzymes to maintain a safe and proper ROS level. ROS are crucial factors for multiple biological processes such as gene expression, protein translation, cellular signaling and metabolism, cell proliferation and differentiation, and chromatin remodeling (Bigarella et al., 2014; Kanda et al., 2011; Ray et al., 2012). However, an excess accumulation of ROS may lead to cytotoxic levels of oxidative stress and cause damage to cellular components. Future efforts towards unraveling additional key regulators of ROS will be essential for understanding oxidative stress and mammalian cortical development under normal and disease conditions.
MATERIALS AND METHODS
Animals
Prdm16fl/fl (fl, floxed allele, stock#024992) and Emx1-Cre (stock#005628) mice were obtained from The Jackson Laboratory. Genotyping was carried out using standard PCR protocols. Prdm16fl/fl mice were crossed to Emx1-Cre mice to subsequently generate Prdm16−/− conditional knockout mice. Emx1-Cre and Nex-Cre (Goebbels et al., 2006) mice were used to conditionally delete Prdm16. mCAT (stock number 016197) mice were obtained from The Jackson Laboratory. The mice were maintained at the facilities of Memorial Sloan Kettering Cancer Center (MSKCC) and all animal procedures were approved by the MSKCC Institutional Animal Care and Use Committee (IACUC). For timed pregnancies, the plug date was designated as E0.5 and the date of birth was defined as P0.
Acutely dissociated cell culture and immunostaining
Acutely dissociated VZ cell culture was performed as described previously (Gotz et al., 1998) with modifications. Timed pregnant females were anesthetized, embryos were removed, live brains were extracted and sectioned, and cortical VZ tissues were selectively removed, digested with Accutase (Innovative Cell Technologies) at 37°C for 5 min and washed with DMEM. Cortical VZ tissues were then dissociated, passed through a 70 µm cell strainer (BD Biosciences), plated on Poly-L-Ornithine/Fibronectin-coated glass coverslips in a 12-well plate, and cultured in NpGrow medium (BrainBits) for 3 h. Cells were fixed with 4% paraformaldehyde (PFA) for 20 min and immunostained as previously described (Cai et al., 2013).
Western blot assay
Timed pregnant females that carried conditional mutant alleles were anesthetized, embryos were removed, live brain tissues were sectioned and VZ tissue were selectively removed and directly frozen with liquid nitrogen. VZ tissues were homogenized in RIPA buffer containing protease inhibitor (Roche). Protein levels were quantified using the Pierce BCA Protein Assay kit (ThermoFisher). Protein samples were separated via 10% SDS-PAGE gel and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% non-fat milk and blotted with primary antibody against Prdm16 (1:200, Millipore). Loading control membranes were blotted with a primary antibody against β-Actin (1:5000, Cell Signaling Technology). After treatment with secondary antibodies (anti-rabbit, 1:5000; anti-mouse, 1:7500; Santa Cruz), blots were incubated with ECL western blotting detection reagent (Bio-Rad). The radiographic film images were scanned and analyzed with ImageJ.
Tissue preparation, immunohistochemistry, confocal imaging and quantification
Timed pregnant females that carried conditional mutant alleles were anesthetized, and embryos were removed and perfused with ice-cold phosphate buffered saline (PBS, pH 7.4), followed by 4% PFA. Brains were post-fixed with 4% PFA for 6 h, cryo-protected and sectioned at 20 μm for immunohistochemistry. Postnatal animals were similarly processed and cryo-sectioned at 40 μm. Sections were blocked in 10% serum and 0.1% Triton-X in PBS, and incubated with the primary antibody at 4°C overnight. The following primary antibodies were used: mouse anti-PAX6 (1:100, Developmental Hybridoma Bank), rabbit anti-CUX1 (1:200, Santa Cruz, sc-13024), rabbit anti-PAX6 (1:500, BioLegend, 901301), mouse anti-Ki67 (1:200, eBioscience, 14-5698-82), rabbit anti-PTX3 (1:100, Abcam, ab190838), rabbit anti-P-HH3 (1:1000, Abcam, ab47297), rat anti-BrdU (1:1000, Abcam, ab6326), rat anti-CTIP2 (1:1000, Abcam, ab18465), rat anti-TBR2 (1:100, eBioscience, 14-4875-82), mouse anti-NEUN (1:300, Millipore, MAB377), rabbit anti-CASP3 (1:300, Cell Signaling Technology, 9661), goat anti-8-OHdG (1:1000, Millipore, AB5830) and rabbit anti-HNE (1:1000, Alpha Diagnostic, HNE11-S). Rabbit anti-PRDM16 (1:100) was kindly provided by Dr. Patrick Seale (University of Pennsylvania, Philadelphia, USA). Nuclei were stained with DAPI (1:500, Sigma). Alexa fluor 488-, 546- or 647-conjugated secondary antibodies (Life Technologies) were used. For EdU and BrdU double pulse-chase analysis, animals were weighed and injected with EdU (10 µg per gram weight) and BrdU (50 µg per gram weight) sequentially. EdU staining was performed using Click-IT EdU Alexa Fluor 647 Imaging Kit (ThermoFisher). Before proceeding with BrdU staining, tissue sections were blocked with azidomethyl phenyl sulfide to minimize cross-reactivity of anti-BrdU antibody against EdU (Liboska et al., 2012). BrdU staining was performed as described previously (Insolera et al., 2014). Coronal sections were mounted on glass slides, imaged using a FV1200 confocal microscope (Olympus) and NanoZoomer 2.0 HT slide scanner (Hamamatsu Photonics), and analyzed with Volocity (ImproVision), Photoshop (Adobe Systems) and ImageJ.
Cell cycle exit analysis
To label proliferating cells, pregnant females were intraperitoneally injected with EdU (10 µg per gram weight). At 23 h after the injection, embryonic brains were collected, sectioned and stained with the antibodies to EdU and Ki67. The cell cycle exit index was analyzed as the fraction of EdU+/Ki67− cells among all EdU+ cells.
Neurosphere culture
Neural stem cell medium (NSC) was prepared by mixing Neurobasal media (Gibco, Life Technologies), supplemented with N2 (Gibco, Life Technologies), B27 (without vitamin A) (Gibco, Life Technologies), 20 ng/ml of human recombinant EGF (Gibco, Life Technologies) and 20 ng/ml FGF2 (Gibco, Life Technologies) together and filtering the entire medium. Timed pregnant females were anesthetized, embryos were removed, live brains were extracted and sectioned, and VZ tissues were selectively removed, digested with Accutase (Innovative Cell Technologies) at 37°C for 5 min and washed with DMEM. Tissues were then dissociated, passed through a 70 µm cell strainer (BD Biosciences), plated at a suitable density in complete NSC medium in a non-treated six-well plate, placed in a 37°C humidified incubator with 5% CO2 and allowed neurospheres to form. After 2 days, the neurospheres were plated onto a matrigel coated plate for 3-4 h. The cells were stained with 5 µM CellROX Green Reagent from Life Technologies at 37°C for 30 min and then washed three times with DMEM before being imaged and analyzed.
RNA-seq differential expression analysis
VZ tissue were manually isolated from three pairs of wild-type and Prdm16 cKO mutant E13.5, E15.5 and E17.5 embryos. RNA was isolated using Qiagen RNeasy Micro Kit (QIAGEN). After RiboGreen quantification and quality control using an Agilent BioAnalyzer, 125-150 ng of total RNA underwent polyA selection and TruSeq library preparation according to instructions provided by Illumina (TruSeq Stranded mRNA LT Kit, RS-122-2102). Samples were barcoded and ran on the HiSeq 4000 platform, using the HiSeq 3000/4000 SBS Kit (Illumina). The output FASTQ files were mapped to the UCSC Mus musculus (mm10) genome assembly using the RNA-STAR aligner. Sample groups were normalized and differential expression analysis between samples groups was conducted using the Bioconductor package DESeq (Maza, 2016). Gene annotation was based on the iGenomes UCSC Mus musculus gene annotation. Gene Ontology was generated using DAVID Bioinformatics Resources 6.8. The genes with P<0.05 were considered to be differentially expressed. Similar methods and materials were used to conduct RNA-seq differential expression analysis on wild-type VZ tissue at E11.5, E13.5, E15.5 and E17.5 to further understand ROS-related pathways during cortical development. Forty well-established genes known to regulate ROS level with P<0.05 were used to perform GSEA.
qRT-PCR expression analysis
Cortices were isolated from three pairs of wild-type and Prdm16 cKO mutant E11.5, E13.5, E15.5 and E17.5 embryos. RNA was isolated using Qiagen RNeasy Micro Kit (QIAGEN). cDNA was prepared using QuantiTect Reverse Transcription Kit (QIAGEN). Quantitative real-time PCR (qRT-PCR) was performed using TaqMan Fast Advanced Master Mix (Applied Biosystems). qRT-PCR was performed using inventoried TaqMan Gene Expression Assays for Ptx3 (Mm00477268_m1), Cyp26b1 (Mm00558507_m1) and Hprt (Mm03024075_m1). Fold changes in expression were calculated using the ΔCt method. The HPRT gene was used to normalize the results. Statistical differences were determined using Student's two-tailed t-test. Statistical significance was set as P<0.05.
Quantification and statistical analysis
Age-matched wild-type littermates were used as the controls in all experiments. For cell number quantification, all cells positive for the corresponding markers were counted in a 200 μm width (embryonic) or 250 μm width (postnatal) columnar area from the lateral ventricle to the pial surface in similar regions of the cortex (embryonic) or the primary somatosensory (includes heterotopia)/motor cortex (postnatal). At least three animals (six hemispheres) in each group were analyzed in all experiments. Both male and female mice were used in experiments. The intensity of 8-OHdG and HNE staining, ROS levels by CellROX Green and CUX1 staining were calculated using the measurement corrected total cell fluorescence (CTCF), as described previously (McCloy et al., 2014). All statistical tests were performed with Prism (GraphPad). Data are presented as mean+s.e.m. and statistical differences were determined using Student's two-tailed t-test or a nonparametric Mann–Whitney test. Statistical significance was set at P<0.05.
Supplementary Material
Acknowledgements
We thank members of the Shi laboratory for valuable discussion and input. Core Facilities at the Memorial Sloan Kettering Cancer Center are funded by the National Institutes of Health, P30CA008748.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.C., S.-H.S.; Methodology: A.C., Q.Z., Q.D.; Validation: A.C., Q.Z.; Formal analysis: A.C.; Investigation: A.C., Q.Z.; Resources: S.-H.S.; Data curation: A.C., S.S.; Writing - original draft: A.C.; Writing - review & editing: A.C., Q.Z., S.-H.S.; Visualization: A.C., S.-H.S.; Supervision: S.-H.S.; Project administration: S.-H.S.; Funding acquisition: S.-H.S.
Funding
This work was supported by grants from the National Institutes of Health (R01DA024681, R01NS102904 and R01NS085004 to S.-H.S.), the Simons Foundation (GC232866 to S.-H.S.) and the Howard Hughes Medical Institute (S.-H.S.). Deposited in PMC for release after 12 months.
Data availability
RNA-seq data have been deposited in Gene Expression Omnibus under accession number GSE142684.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.184150.supplemental
Peer review history
The peer review history is available online at https://dev.biologists.org/lookup/doi/10.1242/dev.184150.reviewer-comments.pdf
References
- Angevine J. B. Jr. and Sidman R. L. (1961). Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766-768. 10.1038/192766b0 [DOI] [PubMed] [Google Scholar]
- Anthony T. E., Klein C., Fishell G. and Heintz N. (2004). Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41, 881-890. 10.1016/S0896-6273(04)00140-0 [DOI] [PubMed] [Google Scholar]
- Bae Y. S., Oh H., Rhee S. G. and Yoo Y. D. (2011). Regulation of reactive oxygen species generation in cell signaling. Mol. Cells 32, 491-509. 10.1007/s10059-011-0276-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baizabal J. M., Mistry M., Garcia M. T., Gómez N., Olukoya O., Tran D., Johnson M. B., Walsh C. A. and Harwell C. C. (2018). The epigenetic statE of PRDM16-Regulated enhancers in radial glia controls cortical neuron position. Neuron 98, 945-962.e8. 10.1016/j.neuron.2018.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigarella C. L., Liang R. and Ghaffari S. (2014). Stem cells and the impact of ROS signaling. Development 141, 4206-4218. 10.1242/dev.107086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchetot C. and Boonstra J. (2008). The ROS-NOX connection in cancer and angiogenesis. Crit. Rev. Eukaryot. Gene Expr. 18, 35-45. 10.1615/CritRevEukarGeneExpr.v18.i1.30 [DOI] [PubMed] [Google Scholar]
- Bultje R. S., Castaneda-Castellanos D. R., Jan L. Y., Jan Y. N., Kriegstein A. R. and Shi S. H. (2009). Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron 63, 189-202. 10.1016/j.neuron.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Zhang Y., Shen Q., Rubenstein J. L. and Yang Z. (2013). A subpopulation of individual neural progenitors in the mammalian dorsal pallium generates both projection neurons and interneurons in vitro. Stem Cells 31, 1193-1201. 10.1002/stem.1363 [DOI] [PubMed] [Google Scholar]
- Caviness V. S. Jr., Takahashi T. and Nowakowski R. S. (1995). Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 18, 379-383. 10.1016/0166-2236(95)93933-O [DOI] [PubMed] [Google Scholar]
- Chen H.-Y., Islam A., Yuan T.-M., Chen S.-W., Liu P.-F. and Chueh P. J. (2018). Regulation of tNOX expression through the ROS-p53-POU3F2 axis contributes to cellular responses against oxaliplatin in human colon cancer cells. J. Exp. Clin. Cancer Res. 37, 161 10.1186/s13046-018-0837-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenn A., Zhang Y. A., Chang B. T. and McConnell S. K. (1998). Intrinsic polarity of mammalian neuroepithelial cells. Mol. Cell Neurosci. 11, 183-193. 10.1006/mcne.1998.0680 [DOI] [PubMed] [Google Scholar]
- Chuikov S., Levi B. P., Smith M. L. and Morrison S. J. (2010). Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat. Cell Biol. 12, 999-1006. 10.1038/ncb2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Levy J. D., Zhang Y., Frontini A., Kolodin D. P., Svensson K. J., Lo J. C., Zeng X., Ye L., Khandekar M. J. et al. (2014). Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156, 304-316. 10.1016/j.cell.2013.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D. F., Chiao Y. A., Martin G. M., Marcinek D. J., Basisty N., Quarles E. K. and Rabinovitch P. S. (2017). Mitochondrial-Targeted catalase: extended longevity and the roles in various disease models. Prog. Mol. Biol. Transl. Sci. 146, 203-241. 10.1016/bs.pmbts.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Dell'Oglio M. P., Simone S., Ciccone M., Corciulo R., Gesualdo M., Zito A., Cortese F., Castellano G., Gigante M., Gesualdo L. et al. (2017). Neutrophil-dependent pentraxin-3 and reactive oxygen species production modulate endothelial dysfunction in haemodialysis patients. Nephrol. Dial. Transplant. 32, 1540-1549. 10.1093/ndt/gfw363 [DOI] [PubMed] [Google Scholar]
- Dröge W. (2002). Free radicals in the physiological control of cell function. Physiol. Rev. 82, 47-95. 10.1152/physrev.00018.2001 [DOI] [PubMed] [Google Scholar]
- Englund C., Fink A., Lau C., Pham D., Daza R. A., Bulfone A., Kowalczyk T. and Hevner R. F. (2005). Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 25, 247-251. 10.1523/JNEUROSCI.2899-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu E., Burkard T. R., Jiang Y., Saini N., Homem C. C. F., Reichert H. and Knoblich J. A. (2014). SWI/SNF complex prevents lineage reversion and induces temporal patterning in neural stem cells. Cell 156, 1259-1273. 10.1016/j.cell.2014.01.053 [DOI] [PubMed] [Google Scholar]
- Florio M. and Huttner W. B. (2014). Neural progenitors, neurogenesis and the evolution of the neocortex. Development 141, 2182-2194. 10.1242/dev.090571 [DOI] [PubMed] [Google Scholar]
- Gao P., Postiglione M. P., Krieger T. G., Hernandez L., Wang C., Han Z., Streicher C., Papusheva E., Insolera R., Chugh K. et al. (2014). Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 159, 775-788. 10.1016/j.cell.2014.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebbels S., Bormuth I., Bode U., Hermanson O., Schwab M. H. and Nave K. A. (2006). Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44, 611-621. 10.1002/dvg.20256 [DOI] [PubMed] [Google Scholar]
- Gorski J. A., Talley T., Qiu M., Puelles L., Rubenstein J. L. and Jones K. R. (2002). Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J. Neurosci. 22, 6309-6314. 10.1523/JNEUROSCI.22-15-06309.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M., Stoykova A. and Gruss P. (1998). Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21, 1031-1044. 10.1016/S0896-6273(00)80621-2 [DOI] [PubMed] [Google Scholar]
- Greig L. C., Woodworth M. B., Galazo M. J., Padmanabhan H. and Macklis J. D. (2013). Molecular logic of neocortical projection neuron specification, development and diversity. Nat. Rev. Neurosci. 14, 755-769. 10.1038/nrn3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y. L., Chakraborty S., Rajan S. S., Wang R. and Huang F. (2010). Effects of oxidative stress on mouse embryonic stem cell proliferation, apoptosis, senescence, and self-renewal. Stem Cells Dev. 19, 1321-1331. 10.1089/scd.2009.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusel'nikova V. V. and Korzhevskiy D. E. (2015). NeuN as a neuronal nuclear antigen and neuron differentiation marker. Acta Naturae 7, 42-47. 10.32607/20758251-2015-7-2-42-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M. E. (1999). Central nervous system neuronal migration. Annu. Rev. Neurosci. 22, 511-539. 10.1146/annurev.neuro.22.1.511 [DOI] [PubMed] [Google Scholar]
- Haubensak W., Attardo A., Denk W. and Huttner W. B. (2004). Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA 101, 3196-3201. 10.1073/pnas.0308600100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haushalter C., Asselin L., Fraulob V., Dollé P. and Rhinn M. (2017). Retinoic acid controls early neurogenesis in the developing mouse cerebral cortex. Dev. Biol. 430, 129-141. 10.1016/j.ydbio.2017.08.006 [DOI] [PubMed] [Google Scholar]
- Herault O., Hope K. J., Deneault E., Mayotte N., Chagraoui J., Wilhelm B. T., Cellot S., Sauvageau M., Andrade-Navarro M. A., Hébert J. et al. (2012). A role for GPx3 in activity of normal and leukemia stem cells. J. Exp. Med. 209, 895-901. 10.1084/jem.20102386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Garcia D., Wood C. D., Castro-Obregón S. and Covarrubias L. (2010). Reactive oxygen species: A radical role in development? Free Radic. Biol. Med. 49, 130-143. 10.1016/j.freeradbiomed.2010.03.020 [DOI] [PubMed] [Google Scholar]
- Hohenauer T. and Moore A. W. (2012). The prdm family: expanding roles in stem cells and development. Development 139, 2267-2282. 10.1242/dev.070110 [DOI] [PubMed] [Google Scholar]
- Homem C. C., Repic M. and Knoblich J. A. (2015). Proliferation control in neural stem and progenitor cells. Nat. Rev. Neurosci. 16, 647-659. 10.1038/nrn4021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Iwai R., Tabata H., Konno D., Komabayashi-Suzuki M., Watanabe C., Iwanari H., Mochizuki Y., Hamakubo T., Matsuzaki F. et al. (2017). Prdm16 is crucial for progression of the multipolar phase during neural differentiation of the developing neocortex. Development 144, 385-399. 10.1242/dev.136382 [DOI] [PubMed] [Google Scholar]
- Insolera R., Bazzi H., Shao W., Anderson K. V. and Shi S. H. (2014). Cortical neurogenesis in the absence of centrioles. Nat. Neurosci. 17, 1528-1535. 10.1038/nn.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Matsuoka S., Takubo K., Hamaguchi I., Nomiyama K., Hosokawa K., Sakurada K., Nakagata N. et al. (2004). Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431, 997-1002. 10.1038/nature02989 [DOI] [PubMed] [Google Scholar]
- Ito K., Hirao A., Arai F., Takubo K., Matsuoka S., Miyamoto K., Ohmura M., Naka K., Hosokawa K., Ikeda Y. et al. (2006). Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat. Med. 12, 446-451. 10.1038/nm1388 [DOI] [PubMed] [Google Scholar]
- Jang Y. Y. and Sharkis S. J. (2007). A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110, 3056-3063. 10.1182/blood-2007-05-087759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong C. H. and Joo S. H. (2016). Downregulation of reactive oxygen species in apoptosis. J. Cancer Prev. 21, 13-20. 10.15430/JCP.2016.21.1.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda Y., Hinata T., Kang S. W. and Watanabe Y. (2011). Reactive oxygen species mediate adipocyte differentiation in mesenchymal stem cells. Life Sci. 89, 250-258. 10.1016/j.lfs.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Kessaris N., Fogarty M., Iannarelli P., Grist M., Wegner M. and Richardson W. D. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173-179. 10.1038/nn1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinameri E., Inoue T., Aruga J., Imayoshi I., Kageyama R., Shimogori T. and Moore A. W. (2008). Prdm proto-oncogene transcription factor family expression and interaction with the notch-Hes pathway in mouse neurogenesis. PLoS One 3, e3859 10.1371/journal.pone.0003859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A. and Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149-184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K. Y., Sestan N. and Anton E. S. (2012). Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex. Development 139, 1535-1546. 10.1242/dev.069963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J., Lee S. R., Yang K. S., Ahn Y., Kim Y. J., Stadtman E. R. and Rhee S. G. (2004). Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA 101, 16419-16424. 10.1073/pnas.0407396101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J., Wang A., Burke D. J., Boudreau H. E., Lekstrom K. J., Korzeniowska A., Sugamata R., Kim Y. S., Yi L., Ersoy I. et al. (2016). Peroxiredoxin 6 (Prdx6) supports NADPH oxidase1 (Nox1)-based superoxide generation and cell migration. Free Radic. Biol. Med. 96, 99-115. 10.1016/j.freeradbiomed.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle J. E., Orozco N. M., Paucar A. A., Saxe J. P., Mottahedeh J., Pyle A. D., Wu H. and Kornblum H. I. (2011). Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8, 59-71. 10.1016/j.stem.2010.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. H., Kim S. Y., Na J. C., Yoon Y. E. and Han W. K. (2018a). Exogenous pentraxin-3 inhibits the reactive oxygen species-mitochondrial and apoptosis pathway in acute kidney injury. PLoS ONE 13, e0195758 10.1371/journal.pone.0195758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Cho Y. S., Jung H. and Choi I. (2018b). Pharmacological regulation of oxidative stress in stem cells. Oxid. Med. Cell Longev. 2018, 4081890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liboska R., Ligasova A., Strunin D., Rosenberg I. and Koberna K. (2012). Most anti-BrdU antibodies react with 2′-deoxy-5-ethynyluridine – the method for the effective suppression of this cross-reactivity. PLoS One 7, e51679 10.1371/journal.pone.0051679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. K., Robertson C. S. and Valadka A. (2002). The association between neuronal nitric oxide synthase and neuronal sensitivity in the brain after brain injury. Ann. N Y Acad. Sci. 962, 226-241. 10.1111/j.1749-6632.2002.tb04071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao L., Chen J., Song S., Lee I. H., Quijano C., Liu H., Keyvanfar K., Chen H., Cao L. Y. et al. (2009). Bmi1 regulates mitochondrial function and the DNA damage response pathway. Nature 459, 387-392. 10.1038/nature08040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan L., Ourednik V. and Ourednik J. (2006). Increased “vigilance” of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells 24, 2110-2119. 10.1634/stemcells.2006-0018 [DOI] [PubMed] [Google Scholar]
- Malatesta P., Hartfuss E. and Gotz M. (2000). Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127, 5253-5263. [DOI] [PubMed] [Google Scholar]
- Marín O. and Rubenstein J. L. (2003). Cell migration in the forebrain. Annu. Rev. Neurosci. 26, 441-483. 10.1146/annurev.neuro.26.041002.131058 [DOI] [PubMed] [Google Scholar]
- Maza E. (2016). In papyro comparison of TMM (edgeR), RLE (DESeq2), and MRN normalization methods for a simple two-Conditions-Without-Replicates RNA-Seq experimental design. Front. Genet. 7, 164 10.3389/fgene.2016.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloy R. A., Rogers S., Caldon C. E., Lorca T., Castro A. and Burgess A. (2014). Partial inhibition of Cdk1 in G2 phase overrides the SAC and decouples mitotic events. Cell Cycle 13, 1400-1412. 10.4161/cc.28401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw T. E. and Mittal V. (2010). Stem cells: metabolism regulates differentiation. Nat. Chem. Biol. 6, 176-177. 10.1038/nchembio.324 [DOI] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi A., Okano H. and Ogawa M. (2001). Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727-741. 10.1016/S0896-6273(01)00420-2 [DOI] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi A., Saito K., Kawano M., Muto T. and Ogawa M. (2004). Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 131, 3133-3145. 10.1242/dev.01173 [DOI] [PubMed] [Google Scholar]
- Monteiro H. P., Rodrigues E. G., Amorim Reis A. K. C., Longo L. S. Jr., Ogata F. T., Moretti A. I. S., da Costa P. E., Teodoro A. C. S., Toledo M. S. and Stern A. (2019). Nitric oxide and interactions with reactive oxygen species in the development of melanoma, breast, and colon cancer: a redox signaling perspective. Nitric Oxide 89, 1-13. 10.1016/j.niox.2019.04.009 [DOI] [PubMed] [Google Scholar]
- Moore A. W., Jan L. Y. and Jan Y. N. (2002). hamlet, a binary genetic switch between single- and multiple-dendrite neuron morphology. Science 297, 1355-1358. 10.1126/science.1072387 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Flint A. C., Weissman T. A., Dammerman R. S. and Kriegstein A. R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714-720. 10.1038/35055553 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Martínez-Cerdeno V., Ivic L. and Kriegstein A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136-144. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E. and Banerjee U. (2009). Reactive oxygen species prime drosophila haematopoietic progenitors for differentiation. Nature 461, 537-541. 10.1038/nature08313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J. H., Ding Z., Narurkar R., Ramkissoon S., Muller F., Kamoun W. S., Chae S. S., Zheng H., Ying H., Mahoney J. et al. (2009). FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5, 540-553. 10.1016/j.stem.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phaniendra A., Jestadi D. B. and Periyasamy L. (2015). Free radicals: properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 30, 11-26. 10.1007/s12291-014-0446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. (1988). Specification of cerebral cortical areas. Science 241, 170-176. 10.1126/science.3291116 [DOI] [PubMed] [Google Scholar]
- Rakic P. (2003). Developmental and evolutionary adaptations of cortical radial glia. Cereb. Cortex 13, 541-549. 10.1093/cercor/13.6.541 [DOI] [PubMed] [Google Scholar]
- Ramming T., Hansen H. G., Nagata K., Ellgaard L. and Appenzeller-Herzog C. (2014). GPx8 peroxidase prevents leakage of H2O2 from the endoplasmic reticulum. Free Radic. Biol. Med. 70, 106-116. 10.1016/j.freeradbiomed.2014.01.018 [DOI] [PubMed] [Google Scholar]
- Ray P. D., Huang B. W. and Tsuji Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981-990. 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault V. M., Rafalski V. A., Morgan A. A., Salih D. A., Brett J. O., Webb A. E., Villeda S. A., Thekkat P. U., Guillerey C., Denko N. C. et al. (2009). FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5, 527-539. 10.1016/j.stem.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M. and Dolle P. (2012). Retinoic acid signalling during development. Development 139, 843-858. 10.1242/dev.065938 [DOI] [PubMed] [Google Scholar]
- Sauer H., Wartenberg M. and Hescheler J. (2001). Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol. Biochem. 11, 173-186. 10.1159/000047804 [DOI] [PubMed] [Google Scholar]
- Schriner S. E., Linford N. J., Martin G. M., Treuting P., Ogburn C. E., Emond M., Coskun P. E., Ladiges W., Wolf N., Van Remmen H. et al. (2005). Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308, 1909-1911. 10.1126/science.1106653 [DOI] [PubMed] [Google Scholar]
- Shimada I. S., Acar M., Burgess R. J., Zhao Z. and Morrison S. J. (2017). Prdm16 is required for the maintenance of neural stem cells in the postnatal forebrain and their differentiation into ependymal cells. Genes Dev. 31, 1134-1146. 10.1101/gad.291773.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. K., Williams C. A., Klarmann K., Burkett S. S., Keller J. R. and Oberdoerffer P. (2013). Sirt1 ablation promotes stress-induced loss of epigenetic and genomic hematopoietic stem and progenitor cell maintenance. J. Exp. Med. 210, 987-1001. 10.1084/jem.20121608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan M., Yu Z. X., Ferrans V. J., Irani K. and Finkel T. (1995). Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296-299. 10.1126/science.270.5234.296 [DOI] [PubMed] [Google Scholar]
- Tamamaki N., Nakamura K., Okamoto K. and Kaneko T. (2001). Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci. Res. 41, 51-60. 10.1016/S0168-0102(01)00259-0 [DOI] [PubMed] [Google Scholar]
- Tsatmali M., Walcott E. C. and Crossin K. L. (2005). Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Res. 1040, 137-150. 10.1016/j.brainres.2005.01.087 [DOI] [PubMed] [Google Scholar]
- Tsatmali M., Walcott E. C., Makarenkova H. and Crossin K. L. (2006). Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol. Cell Neurosci. 33, 345-357. 10.1016/j.mcn.2006.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A., Vlachogianni T. and Fiotakis C. (2009). 8-hydroxy-2′-deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 27, 120-139. 10.1080/10590500902885684 [DOI] [PubMed] [Google Scholar]
- Vlaski-Lafarge M. and Ivanovic Z. (2015). Reliability of ROS and RNS detection in hematopoietic stem cells--potential issues with probes and target cell population. J. Cell Sci. 128, 3849-3860. 10.1242/jcs.171496 [DOI] [PubMed] [Google Scholar]
- Wang Y., Branicky R., Noë A. and Hekimi S. (2018). Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 217, 1915-1928. 10.1083/jcb.201708007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Kawada K., Gotoh Y., Shiba T. and Ogita K. (2010). Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem. Int. 56, 740-746. 10.1016/j.neuint.2009.11.018 [DOI] [PubMed] [Google Scholar]
- Zangar R. C., Davydov D. R. and Verma S. (2004). Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol. Appl. Pharmacol. 199, 316-331. 10.1016/j.taap.2004.01.018 [DOI] [PubMed] [Google Scholar]
- Zhang J., Khvorostov I., Hong J. S., Oktay Y., Vergnes L., Nuebel E., Wahjudi P. N., Setoguchi K., Wang G., Do A. et al. (2011). UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 30, 4860-4873. 10.1038/emboj.2011.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H. and Yin H. (2015). Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: focusing on mitochondria. Redox Biol. 4, 193-199. 10.1016/j.redox.2014.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.