Abstract
Study Objectives:
The chronic pain disorder, fibromyalgia, is associated with sleep disturbance, typically sleep maintenance. No studies have evaluated the effect of sleep medication on pain sensitivity in this population. Suvorexant, an orexin antagonist approved for treatment of insomnia, was evaluated for effects on both sleep and the pain of fibromyalgia.
Methods:
Women age 21 to 65 years with fibromyalgia and comorbid insomnia (n = 10) were treated, double-blind, for 9 nights each with suvorexant, 20 mg and placebo in counterbalanced order. All were in good psychiatric and stable physical health and met American College of Rheumatology 2010 criteria for fibromyalgia and Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition criteria for insomnia. Screening 8-hour polysomnography (PSG) was used to rule out other sleep disorders. On nights 8 and 9 of each treatment 8-hour PSG were collected and on days 1 and 8 pain sensitivity was assessed at 1100 and 1500 hours by measuring finger withdrawal latency (FWL) to a radiant heat stimulus at 5 randomly presented intensity levels.
Results:
Suvorexant versus placebo increased total sleep time (7.2 versus 6.7 hours, P < .05) and reduced wake after sleep onset (37 versus 67 minutes, P < .04) with no night effects or interaction. Latency to persistent sleep and sleep stage measures were not altered. FWL on both am and pm tests varied as a function of intensity (P < .001). Average FWL (over 5 intensities and both days) was increased relative to placebo on both the am (13.9 versus 13.1 seconds) and pm tests (15.8 versus 14.1 seconds, P < .03) following suvorexant the previous night.
Conclusions:
Suvorexant 20 mg in patients with fibromyalgia, improved sleep time and reduced next-day pain sensitivity on assessments of FWL to a radiant heat stimulus.
Clinical Trial Registry:
Registry: ClinicalTrials.gov; Name: A double-blind cross-over, study to compare the hypnotic, daytime sleepiness/fatigue, and pain effects of nighttime administration of suvorexant 20 mg versus placebo in patients with fibromyalgia and comorbid insomnia; Identifier: NCT02684136; URL: https://clinicaltrials.gov/ct2/show/NCT02684136
Citation:
Roehrs T, Withrow D, Koshorek G, Verkler J, Bazan L, Roth T. Sleep and pain in humans with fibromyalgia and comorbid insomnia: double-blind, crossover study of suvorexant 20 mg versus placebo. J Clin Sleep Med. 2020;16(3):415–421.
Keywords: comorbid insomnia, fibromyalgia, pain sensitivity, suvorexant
INTRODUCTION
Typically, insomnia is comorbid with various medical and psychiatric disorders and among the disorders frequently comorbid with insomnia are the various chronic pain disorders. A prevalent musculoskeletal chronic pain disorder is fibromyalgia, in which pain is widespread and not specifically localized.1 Its etiology is unknown, although a prominent hypothesis suggests the pain is associated with enhanced central nervous system sensitization shown over all sense modalities.2 Between 60% to 80% of patients with fibromyalgia complain of sleep disturbance, which most typically manifests as a sleep maintenance problem, a complaint that has been confirmed with polysomnography (PSG). Another important persisting symptom in fibromyalgia is daytime sleepiness and fatigue, which may relate to the disturbed sleep of fibromyalgia or the underlying pathophysiology of the disorder, putative proinflammatory cytokine activation.
The PSG studies in fibromyalgia, dating back as early as 1975, have consistently reported reduced total sleep time (TST) relative to age-matched control patients, primarily due to increased wake after sleep onset (WASO).3 However, despite having reduced nocturnal sleep compared to age-matched healthy control patients and reporting elevated levels of daytime sleepiness and fatigue as seen in patients with rheumatoid arthritis, patients with fibromyalgia show elevated daytime arousal on an objective measure of daytime sleepiness/alertness, the Multiple Sleep Latency Test (MSLT).4 Elevated MSLT latencies, despite shorter total nocturnal sleep times, have also been seen in some people with primary insomnia, which is consistent with the sensory hypersensitization model of fibromyalgia.2
It has now become clear that the relation of sleep and pain is bidirectional; acute and chronic pain are associated with disturbed sleep and shortened and disturbed sleep enhances pain. Experimental studies have shown that reduced and fragmented sleep in pain-free healthy patients increases their pain sensitivity, and daily self-report studies in patients with chronic pain have shown a poor night of sleep is followed by enhanced next-day pain.3 In mediation analyses of large clinical datasets it is found that the sleep-pain side of the bidirectional relation, as opposed to the pain-sleep side, accounts for the greater variance.5 These data then would suggest that improving sleep in chronic pain disorders should attenuate daytime pain.
Drugs from a number of different drug classes have been assessed as treatments in chronic pain disorders including analgesics, antidepressants, antiepileptic drugs, and hypnotic agents. In many of these studies pain is the major focus and if sleep is measured, it is a secondary outcome and typically only measured by self-report. Furthermore, rarely are patients in these studies specifically included for the presence of comorbid insomnia and degree of sleep disturbance.
Although analgesics, antidepressants, and antiepileptic drugs are typically used and studied in chronic pain disorders, only a few studies have assessed hypnotic agents and with quite mixed results. In patients with fibromyalgia, zolpidem,6 but not zopiclone,7 improved some of the sleep measures, but neither drug improved pain. Triazolam improved sleep and pain in patients with rheumatoid arthritis,8 whereas zopiclone only improved pain.9 A large (n = 153) study of eszopiclone, the S isomer of zopiclone, assessed its sleep and pain effects in patients with insomnia comorbid with rheumatoid arthritis.10 Eszopiclone compared to placebo improved self-reported measures of sleep and almost all of the self-report pain measures. Hospitalized patients with mucositis associated with chemotherapy for hematologic malignancies were randomized to 2 nights of eszopiclone or placebo.11 Pain scores throughout the day were improved with eszopiclone and patients reported increased sleep time and fewer awakenings.
Most of the drugs used to treat chronic pain facilitate inhibitory central nervous system mechanisms as their primary mechanism of action. Suvorexant, recently approved by the US Food and Drug Administration for the treatment of insomnia has a unique mechanism of action. Suvorexant is a selective antagonist for orexin receptors (OX1R and OX2R).12 Orexin is considered to be involved in arousal and maintenance of the waking state. In support of this view, findings have shown that orexin neurons send dense projections to brain arousal areas including the locus coeruleus, tuberomammillary nucleus, and the basal forebrain cholinergic system.13 In PSG studies in healthy men, suvorexant 10 mg increased sleep efficiency and reduced WASO14 and in patients with primary insomnia it increased sleep efficiency by reducing latency to persistent sleep (10 min of continuous sleep) and WASO.15
As such, suvorexant may provide unique clinical benefit as a treatment in chronic pain conditions with comorbid insomnia, and specifically for fibromyalgia with its putative central hyperarousal and hypersensitization. This study assessed objective and self-report measures of sleep, pain, and daytime sleepiness and fatigue in patients with fibromyalgia and comorbid insomnia while treated short-term with suvorexant 20 mg versus placebo.
Relative to placebo, we hypothesized the following in persons with fibromyalgia and comorbid insomnia: (1) suvorexant 20 mg would increase PSG TST and reduce sleep latency and wake after sleep onset; (2) suvorexant 20 mg would reduce self-reported next-day pain and nociceptive sensitivity as measured by finger withdrawal testing and (3) suvorexant 20 mg would reduce self-reported next-day fatigue and normalize MSLT scores.
METHODS
Participants
Ten women, aged 20 to 50 years, completed the study (Table 1). Each patient signed an institutional review board-approved informed consent, underwent a brief physical examination, a physician interview to confirm the fibromyalgia diagnosis using American College of Rheumatology 2010 criteria,16 and a sleep specialist interview to confirm the comorbid insomnia diagnosis using Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition criteria.17 All were in stable physical health based on the physician examination and standard laboratory test results. All were in good psychiatric health based on history and Hamilton Depression and Anxiety Inventories with those scores being in the mild to moderate range. Patients reported long sleep latencies, short TST, and increased WASO (Table 1). Severity of sleep disturbance and absence of other sleep disorders was confirmed by the screening PSG. Concomitant medications included over-the-counter and prescribed analgesics and nonsedating antidepressants (Table 2). Patients were instructed to report any change in their concomitant medication use. Social drug use included infrequent weekly alcohol use and one to two cups of coffee a day (Table 1). No patient reported or tested positive for marijuana use.
Table 1.
Participant demographics.
| Sex, female (n) | 10 |
| Age, years | 50.0 (9.1) |
| Race (n) | |
| African American | 1 |
| Caucasian | 9 |
| Widespread pain indexa | 10.2 (14.6) |
| Somatic symptom severityb | 8.2 (2.1) |
| Fibromyalgia impact scorec | 50.3 (12.6) |
| Self-reported sleep latency (minutes) | 47.0 (32.3) |
| Self-reported total sleep time (hours) | 5.2 (1.2) |
| Self-reported wake after sleep onset (minutes) | 70.0 (49.9) |
| Hamilton Depression Inventoryd | 11.7 (6.4) |
| Hamilton Anxiety Inventorye | 14.2 (7.7) |
| No. alcoholic beverages per week | 1.7 (1.9) |
| No. caffeinated beverages per week | 11.9 (9.7) |
| Marijuana use | None |
Data presented as mean (standard deviation) unless otherwise indicated. aScore range from 1 to 19 reflects bodily regions with pain. bScore range from 1 to 12 reflects level of problem with pain. cScore range from 1 to 100 reflects effect on daily function. dScore range 0 to 7 = normal, 8 to 13 = mild, 14 to 18 = moderate, 19 to 22 = severe, ≥ 23 = very severe depression. eScore range < 17 = mild, 18 to 24 = mild to moderate, 25 to 30 = moderate to severe anxiety.
Table 2.
Concomitant medications.
| ID | Medications |
|---|---|
| 1 | Acetaminophen PM |
| 2 | Dicyclomine, Loratidine, Azelastine, Liraglutide, Cyclobenzaprine, Excedrin Migraine, Eletriptan, Spironolactone, Montelukast, Metformin, Pancrelipase, Metoclopramide, Ondansetron, Pantoprazole, Ipratropium, Metoprolol Succinate |
| 3 | Metformin, CalMag, Naproxen, Acetaminophen, Vitamin C, Aspirin |
| 5 | Duloxetine, Nyquil, Dayquil, Hydrocodone |
| 8 | Tramadol, Armorthyroid, Duloxetine, Vitamin D |
| 9 | Levothyroxine, Atorvastatin, Cetirizine, Belvig |
| 10 | Naproxen, Multivitamin, Potassium |
| 12 | Duloxetine, Pantoprazole, Tramadol, Premarin, Magnesium, Prasterone, Cortisol Manager Supplement |
| 13 | Metoprolol tartrate, Magnesium, Aspirin, Vitamin B12, Albuterol, Acetaminophen |
| 14 | Biotin, Aspirin, Acyclovir, Cyclobenzaprine |
Design
The study was conducted as a double-blind, placebo-controlled trial using a repeated-measures design. Patients with fibromyalgia and comorbid insomnia were treated for 9 nights each with suvorexant 20 mg and placebo with the order of treatments counterbalanced and 7 nights of washout between the 9-night treatments. PSG tests were collected on the screening night and on nights 8 and 9 of each condition. During the day following night 8 an MSLT (1000, 1200, 1400, and 1600 hours) was done and on days 1 (after night 1) and 8 (after night 8) nociceptive sensitivity [finger withdrawal latency (FWL)] testing to a radiant heat stimulus (1100 and 1500 hours) was conducted. On days 1 and 8 self-reported mood and pain indices were also completed prior to each nociceptive sensitivity test. On the nonlaboratory days and nights patients slept at home and engaged in their normal daytime routines.
Procedures
Nocturnal polysomnography
For each PSG night, participants arrived 2 hours prior to their reported bedtime to complete check-in procedures and undergo electrode placement for standard 8-hour PSG.18 Bedtimes were determined using the participant’s self-reported normal sleep schedule with time in bed fixed to 8 hours. For the screening PSG, central and occipital electroencephalogram (EEG) leads (C3-A2 and O2-A1) were placed to measure EEG activity and left and right horizontal electrooculograms were used to measure eye movements. A V5 electrocardiogram lead and a submental electromyogram lead were placed. Airflow (by pressure transducer), body position via video recording, thoracic and abdominal excursion (inductance plethysmography), oxygen saturation (finger pulse oximetry), leg movement (with electrode over left anterior tibialis muscle), and sound were recorded. For the study nights 8 and 9, the 8-hour PSGs consisted of continuous monitoring of two channels of EEG (C3-A2 and O2-A1), left and right electrooculograms, submental electromyogram, and V5 electrocardiogram.
All recordings were scored in 30-second epochs for standard sleep stages according to the standards of Rechtschaffen and Kales.18 Scoring was done by technicians with established intralaboratory scoring concordance and blind as to treatment on a given night. Respiratory events and periodic leg movement events were scored according to established American Academy of Sleep Medicine published criteria.19 Based on the screening PSG, patients with respiratory or periodic leg movement event indices > 10 were excluded.
Multiple Sleep Latency Testing
Each test of the MSLT was conducted according to the standard MSLT protocol.20 Participants were placed in bed in quiet, darkened rooms and instructed to close their eyes, relax, and fall asleep at 1000, 1200, 1400 and 1600 hours. Each test was concluded after 20 minutes of continuous wake or 15 minutes after one 30-second epoch of any sleep stage. Latency to sleep onset was scored as minutes to the first epoch of sleep or 20 minutes if sleep did not occur. The latencies for the four tests were averaged to generate a single latency value.
Nociceptive sensitivity (finger withdrawal latency)
A radiant heat method was used to assess nociceptive sensitivity.21 Patients were seated in a comfortable chair at a desk with their hand resting on top of a metal box housing the heat source. The pad of the index finger (fingerprint whorl) was centered over a 3-mm hole through which the heat radiated. The heat source was a 100-W projection bulb, located 10 mm from the finger. A potentiometer controlled the amount of current delivered to the light bulb, thereby varying heat intensity. On each trial, patients were instructed to place their index finger on the hole through which the heat source radiated and to withdraw their index finger when they first began to feel pain. After finger withdrawal occurred, a photocell (mounted on a post located above the finger) detected the light (from the bulb underneath) and stopped a digital timer connected to the circuit. Both index fingers were tested and the heat intensities were adjusted on each trial such that five different heat intensities (ranging from 83.4 to 101.6°F, measured at steady-state after 10-second duration) were presented in a randomized order. The FWL from left and right index fingers were averaged. The primary dependent measure was mean FWL (to a resolution of 0.01 seconds) for each of the 5 heat intensities.
Patients initially underwent a training session to familiarize themselves with the nociceptive testing equipment and procedures. The threshold at which a finger withdrawal response was elicited for each participant was established. The threshold radiant heat intensity was defined as the intensity that produces FWL < 21 seconds, which was then used as the baseline lowest stimulus intensity for that patient in all subsequent testing sessions. In each test session the low intensity and four additional incrementing intensities were randomly presented to both right and left index fingers and the average of both fingers served as the withdrawal latency for a given intensity. Testing was conducted blind as to treatment the previous night.
Regarding validity of the radiant heat method, our studies have shown FWL is systematically related to stimulus intensity (greater intensity = shorter FWL), basal level of sleepiness/alertness (greater sleepiness = shorter FWL), and codeine versus placebo (codeine = longer FWL).21 Importantly, in healthy patients without sleep disturbance, reducing sleep time by as little as 2 hours reduced FWL and increasing sleep time by as little as 1 hour increased FWL.22,23 Further, in patients with moderate to severe obstructive sleep apnea treated with continuous positive airway pressure (CPAP), thereby eliminating obstructive sleep apnea and consolidating sleep, FWL was increased and when the CPAP was discontinued for 2 nights FWL was again reduced.24
Morning self-reported sleep and previous-day pain assessment
Each morning after arising participants completed a brief questionnaire regarding the previous night’s sleep and the previous day’s pain. The Red Cap questionnaires were accessed over the Internet by participants using their own password-protected login identification credential with a daily time-limited completion interval (before 12:00 noon each day) imposed. The questionnaire queried regarding latency to fall asleep, wake time after falling asleep, TST, pain disruption of sleep, and alertness after awakening in the morning. Daytime pain the previous day was assessed using the Short Form McGill Pain Questionnaire.25
Safety monitoring
Each participant completed the Columbia Suicidality Scale at screening and on study day 1 and 8. Additionally the Hamilton Depression Rating Scale and Hamilton Anxiety Rating Scale also were completed on those days.26,27 The rating scales were administered between the first and second nociceptive sensitivity testing (at approximately 1130 hours) on each day. Changes on any of these scales from the scores established at the baseline were reported as adverse events. On day three of each of the treatment periods the patients were contacted by phone to determine whether they were experiencing AEs.
Analyses
At first pass, we conducted two-factor (nights [PSG] or days [FWL] and drug) repeated-measures analyses of variance of the objective sleep parameters and the finger withdrawal latency measure of pain sensitivity. In both sets of analyses the results were borderline (P = .10) showing improvement associated with suvorexant versus placebo. Not surprisingly, given the small sample size, variability was high in the PSG sleep and FWL pain datasets and compared to the baseline there was a significant (P < .05) placebo effect on both the primary sleep (TST) and pain (FWL) outcome data. Given the placebo effect, we tested for a treatment order effect (placebo first [n = 5] versus suvorexant first [n = 5]) and found no significant order effect. We used each patient’s baseline values as covariates and found significant suvorexant effects on both the PSG sleep and FWL pain data with improved sleep and reduced pain. In secondary analyses, standard sleep stages and two derived sleep stage measures (ie, combined minutes of wake and stage 1, the ratio of minutes of stage 1 to stages 3 to 4) were also compared. Having found no night/day main effects on PSG or FWL analyses or interactions, data tabled are means of nights 8 and 9 or days 1 and 8.
RESULTS
A total of 199 people expressed interest regarding the study and were sent information about the study via email. Of those 199, 79 had continued interest and were phone screened with 17 people being eligible after the phone screen. The major phone screen exclusions included multiple comorbid physical and mental health problems and medication use. After consenting and explanation of the study requirements 24% of the 17 eligible people declined due to time constraints. The study design, a within-subject crossover design with 9 nights of placebo and 9 nights of suvorexant with a 7-day washout between conditions required almost 1 month to complete. Three of the 17 people failed the PSG screening and 10 completed the study.
For PSG-defined sleep (Table 3, Table 4, and Table 5), on nights 8 and 9 suvorexant versus placebo increased TST (mean night 8 and 9: 7.2 versus 6.7 hours, P < .05) and reduced WASO (mean night 8 and 9: 37 versus 67 minutes, P < .04) with no night effects or interaction. Duration of awakenings over the entire night were reduced (1.2 minutes versus 2.3 minutes, P < .03), but number of awakenings was not (29.5 versus 30.3). Suvorexant also reduced wake during the last half (20 versus 41 minutes, P < .03) and quarter (13 versus 20 minutes, P < .03) of the night. Latency to persistent sleep, sleep stage, and derived measures were not altered by suvorexant. Two of the 10 participants showed alpha-delta sleep, which was not altered on either night by suvorexant.
Table 3.
Polysomnography sleep efficiency measures (mean nights 8 and 9).
| TST | LPS | WASO | No. of Wakes | Dur Wake | |
|---|---|---|---|---|---|
| Base | 346.90 (52.32) | 62.25 (42.30) | 110.43 (50.45) | 37.6 (9.0) | 3.01 (1.35) |
| Placebo | 400.54 (57.34) | 28.65 (23.04) | 66.79 (55.75) | 30.3 (9.75) | 2.33 (2.03) |
| Suvorexant | 429.31 (29.39)a | 24.02 (25.75) | 37.67 (22.57)b | 29.5 (10.94) | 1.21 (0.45)c |
Data are mean (standard deviation) of minutes or number. aP < .05, bP < .04, cP < .03 versus placebo. Dur Wake = duration of awakenings, LPS = latency to persistent sleep (10 minutes of continuous sleep), No. of Wakes = number of 1 minute or greater awakenings, TST = total sleep time, WASO = wake after sleep onset.
Table 4.
Polysomnography sleep staging measures (mean nights 8 and 9).
| % Stage 1 | % Stage 3-4 | % REM | Min 1+W | R 1/3-4 | |
|---|---|---|---|---|---|
| Base | 11.26 (4.31) | 8.94 (4.17) | 15.42 (7.19) | 171.34 (53.47) | 1.85 (1.72) |
| Placebo | 10.12 (5.06) | 12.38 (6.35) | 20.8 (5.66) | 118.04 (62.87) | 1.75 (1.93) |
| Suvorexant | 9.02 (4.37) | 11.79 (5.18) | 21.57 (4.36) | 88.58 (39.20) | 1.28 (1.41) |
Data are mean (standard deviation) of percentages or minutes. Min 1+W = min of stage 1 plus wake; R 1/3-4 = ratio of minutes stage 1 to minutes stages 3 to 4; % REM = percent stage rapid eye movement; % Stage 1 = percent stage 1 sleep; % Stage 3-4 = percent stage 3 plus stage 4.
Table 5.
Analyses of wake time by halves and quarters of the night (mean night 8 and 9).
| Half 2 | Quarter 3 | Quarter 4 | |
|---|---|---|---|
| Base | 52.35 (25.41) | 24.60 (17.88) | 27.75 (25.27) |
| Placebo | 40.92 (32.65) | 19.67 (23.60) | 20.48 (15.88) |
| Suvorexant | 20.53 (16.33)a | 6.88 (6.29) | 13.65 (10.81)a |
Data are mean (standard deviation) in minutes. aP < .03 versus placebo. Half 2 = last 4 hours of the 8-hour time in bed; Quarter 3 = third 2 hours of the 8-hour time in bed; Quarter 4 = last 2 hours of the 8-hour time in bed.
FWL on both am and pm tests varied as a function of stimulus intensity (P < .001) with no time-of-day effects or interaction (Figure 1; low intensity = long latency, high = short latency). On days 1 and 8 (Table 6) after suvorexant versus placebo pain sensitivity was reduced (ie, latency increased). Average FWL (over 5 intensities and both days) was increased (treatment condition main effect, P < .03) on both the am test (13.9 versus 13.1 seconds) and pm tests (15.8 versus 14.1 seconds) following suvorexant the previous night.
Figure 1. Finger withdrawal latency as a function of stimulus intensity.
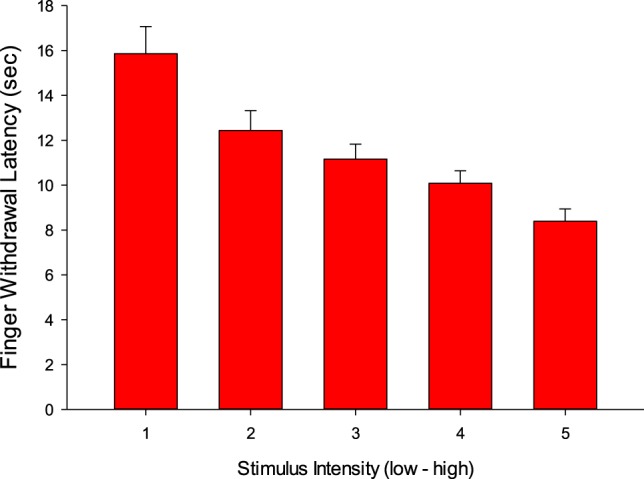
Table 6.
Daytime pain and sleepiness measures.
| AM FWL | PM FWL | MSLT | |
|---|---|---|---|
| Placebo | 13.34 (4.11) | 14.17 (4.94) | 6.95 (4.19) |
| Suvorexanta | 13.90 (3.23) | 15.83 (5.39) | 5.37 (4.00) |
Data are mean (standard deviation) in seconds or minutes. aP < .05 versus placebo. AM FWL = finger withdrawal latency (seconds) on am test; MSLT = Multiple Sleep Latency Test (mean of four tests in minutes); PM FWL = finger withdrawal latency (seconds) on pm test.
Regarding daytime sleepiness as measured by the MSLT (Table 6), there were no significant changes on average daily sleep latency. No significant drug effects on self-reported ratings of sleep, pain, or fatigue were observed.
Adverse events are reported on Table 7. No serious adverse events were reported. Adverse events were more frequently reported with suvorexant than placebo, with four individuals reporting residual sedation and three nausea.
Table 7.
Adverse events.
| ID | Placebo | Suvorexant |
|---|---|---|
| 1 | Stomach pain | Nausea, restlessness |
| 2 | None | None |
| 3 | None | Dizziness, residual sedation |
| 5 | None | Upper respiratory infection |
| 8 | None | Euphoria, stomachache, nausea |
| 9 | None | Daytime sleepiness |
| 10 | None | Residual sedation |
| 12 | Vivid nightmares | None |
| 13 | Hallucination | Decreased appetite, irregular heartbeat |
| 14 | None | Headaches, muscle tremors, nausea, tinnitus |
DISCUSSION
To summarize our findings, suvorexant produced borderline improvements in PSG TST and WASO in 10 people with fibromyalgia and comorbid insomnia. Given the variability in this population, when the sleep data were covaried with the baseline PSG data the suvorexant associated improvements in TST and WASO achieved statistical significance. Suvorexant also reduced duration of nocturnal awakenings, but not the frequency of those awakenings. Finally, suvorexant produced a borderline reduction in pain sensitivity (FWL), which achieved statistical significance when covaried by baseline measures. No changes in daytime sleepiness (MSLT) or self-reported assessments of sleep, pain, or fatigue were observed.
The absence of self-report effects was expected given the sample size. This study was powered for n = 30 based on the two objective sleep (PSG) and pain (FWL) measures as our primary outcomes. Given the between-subject variability, as well as the difficulty in recruiting patients with fibromyalgia and comorbid insomnia the study was designed as a crossover study. We exercised scientific rigor, employing standard diagnostic criteria (American College of Rheumatology 2010 criteria for fibromyalgia and Diagnostic and Statistical Manual for Mental Disorders, Fifth Edition for insomnia) and excluded multiple comorbid physical and mental health problems, which also contributed to the small study sample size. Given our crossover design we allowed various concomitant pain medications and we requested patients report any change in their pain medication usage; none was reported (Table 2).
Consistent with studies of suvorexant in patients with insomnia disorder, in patients with fibromyalgia and comorbid insomnia suvorexant also maintained sleep through the last 2 hours of the night. In hour-by-hour analyses of 493 patients receiving suvorexant 15/20 mg relative to 767 patients receiving placebo, suvorexant continued to reduce wakefulness through hour 8 of the night.28 In this current study suvorexant reduced the duration of awakenings, but not the frequency of awakenings. This is consistent with a large “N” pooled analysis of wake bout frequency and duration done in patients with insomnia disorder receiving suvorexant.29 It is remarkable, then, that in the current study of patients with comorbid insomnia and the small sample size we replicated the findings of these large clinical trials.
The patients with fibromyalgia and comorbid insomnia complained of daytime sleepiness and fatigue, and on the MSLT they were moderately sleepy (Table 6). The sleepiness in this sample directly contrasts with results from an earlier study comparing patients with fibromyalgia, rheumatoid arthritis, and healthy control patients.4 In that study the patients with fibromyalgia had unusually high MSLT scores compared to both the rheumatoid and control groups. Rather than being hyperaroused, the current patients were moderately sleepy. The current study specifically selected patients with both insomnia and fibromyalgia, whereas the earlier study selected patients for fibromyalgia only. It has been documented in a large study of people with insomnia that one-fourth are “sleepy,”30 such as observed in this study.
It is helpful to understand the effect that the suvorexant-associated improvement in sleep in these patients with fibromyalgia and insomnia had on next-day pain sensitivity, as measured with the FWL methodology, by comparing the current results to previous FWL studies. This will help to put into context the clinical significance of the 1-second increase in FWL observed in this study. A study in excessively sleepy versus nonsleepy, otherwise healthy patients, administered codeine 30 mg twice a day versus placebo.31 In the nonsleepy group codeine increased FWL by 14%, while having no effect in the sleepy group. By comparison, the FWL improvement in the afternoon testing of the present study was 11% and 5% in the morning testing. In a study of mildly sleepy patients because of chronic sleep loss, half were randomized to 4 nights of an extended bedtime, whereas the others remained on their habitual schedule.23 Nightly sleep time on the 4 nights averaged 8.9 hours versus 7.1 hours and the MSLT increased from 5 minutes to 9 minutes in the extended group, but not the habitual group; FWL increased by 20%. Finally, FWL was tested in patients with severe sleep apnea syndrome before and after treatment with CPAP.24 Before treatment, sleep was fragmented by 51 arousals (apnea events) per hour on average, which CPAP reduced to one arousal per hour and FWL was improved by 28% in association with the improvement of sleep continuity.
In this study suvorexant increased sleep time by approximately 30 minutes relative to placebo and by 80 minutes relative to baseline. Pain sensitivity was improved by approximately 8%. We correlated FWL scores with sleep times and sleep stages 1, 3 through 4, and rapid eye movement the previous night, but the correlation coefficients were low and nonsignificant. Overall then, for these patients with fibromyalgia with comorbid insomnia suvorexant improved sleep which was associated with a modest reduction of pain sensitivity.
ABBREVIATIONS
- CPAP
continuous positive airway pressure
- EEG
electroencephalogram
- FWL
finger withdrawal latency
- MSLT
Multiple Sleep Latency Testing
- OSA
obstructive sleep apnea
- PSG
polysomnography
- TST
total sleep time
- WASO
wake after sleep onset
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Work for this study was performed at Henry Ford Hospital. Financial support for all authors provided by Henry Ford Hospital. Off-label or investigational use: Suvorexant broadly approved for use in insomnia, but not specifically in individuals with fibromyalgia. T Roehrs reports research funding from NIDA and Merck (Merck MISP grant #53918) and consulting for Cadent. T Roth reports consulting for Flamel, Novion, Merck, Jazz, Eisai, SEQ, and Idorsia. The other authors report no conflicts of interest.
REFERENCES
- 1.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arth Care Res. 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 2.Yunus MB. Fibromyalgia and overlapping disorders: The unifying concept of central sensitivity syndromes. Semin Arthritis Rheum. 2007;36(6):339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Roehrs T, Roth T. Pain and sleep. In: Babson KA, Feldner MT, eds. Sleep and Affect. New York, NY: Elsevier; 2015:377-397. [Google Scholar]
- 4.Roehrs T, Diederichs C, Gillis M, et al. Nocturnal sleep, daytime sleepiness and fatigue in fibromyalgia patients compared to rheumatoid arthritis patients and healthy controls: a preliminary study. Sleep Med. 2013;14(1):109–115. doi: 10.1016/j.sleep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Russell IJ, Crofford LJ, Leon T, et al. The effects of pregabalin on sleep disturbance symptoms among individuals with fibromyalgia syndrome. Sleep Med. 2009;10(6):604–610. doi: 10.1016/j.sleep.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Moldofsky H, Lue FA, Mously C, Roth-Schechter B, Reynolds WJ. The effect of zolpidem in patients with fibromyalgia: a dose ranging, double-blind, placebo controlled, modified crossover study. J Rheumatol. 1996;23:529–533. [PubMed] [Google Scholar]
- 7.Grönbald M, Nykänen J, Konttinen Y, Järvinen E, Helve T. Effect of zolpiclone on sleep quality, morning stiffness, widespread tenderness and pain and general discomfort in primary fibromyalgia patients. A double-blind randomized trial. Clin Rheumatol. 1993;12(2):186–191. doi: 10.1007/BF02231524. [DOI] [PubMed] [Google Scholar]
- 8.Walsh JK, Muehlbach MJ, Lauter SA, Hilliker A, Schweitzer PK. Effects of triazolam on sleep, daytime sleepiness, and morning stiffness in patients with rheumatoid arthritis. J Rheumatol. 1996;23:245–252. [PubMed] [Google Scholar]
- 9.Drewes AM, Bjerregard K, Taaghold SJ, Svendsen L, Nielsen KD. Zopiclone as night medication in rheumatoid arthritis. Scand J Rheumatol. 1998;27(3):180–187. doi: 10.1080/030097498440787. [DOI] [PubMed] [Google Scholar]
- 10.Roth T, Lankford A, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res (Hoboken) 2012;64(4):597–606. doi: 10.1002/acr.21595. [DOI] [PubMed] [Google Scholar]
- 11.Dimsdale JE, Ball ED, Carrier E, et al. Effect of eszopiclone on sleep, fatigue, and pain in patients with musocitis associated with hematologic malignancies. Support Care Cancer. 2011;19(12):2015–2020. doi: 10.1007/s00520-010-1052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoyer D, Jacobson LH. Orexin in sleep, addiction and more: Is the perfect insomnia drug at hand. Neuropeptides. 2013;47(6):477–488. doi: 10.1016/j.npep.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 13.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. TINS. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 14.Sun H, Kennedy WP, Wilbraham D, et al. Effects of suvorexant, an orexin receptor antagonist, on sleep parameters as measured by polysomnography in healthy men. Sleep. 2013;36:259–267. doi: 10.5665/sleep.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herring WJ, Snyder E, Budd K, et al. Orexin receptor antagonism for treatment of insomnia: a randomized clinical trial of suvorexant. Neurology. 2012;79(23):2265–2274. doi: 10.1212/WNL.0b013e31827688ee. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe F. Fibromyalgia. Rheum Dis Clin North Am. 1990;16(3):681–698. [PubMed] [Google Scholar]
- 17.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 18.Rechtschaffen A, Kales A, editors. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 19.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.1. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 20.Carskadon MA, Dement WC, Mitler MM, Roth T, Westbrook PR, Keenan S. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519–524. doi: 10.1093/sleep/9.4.519. [DOI] [PubMed] [Google Scholar]
- 21.Chhangani BS, Roehrs TA, Harris EJ, et al. Pain sensitivity in sleepy pain-free normals. Sleep. 2009;32(8):1011–1017. [PMC free article] [PubMed] [Google Scholar]
- 22.Roehrs TA, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29(2):145–151. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 23.Roehrs TA, Harris E, Randall S, Roth T. Pain sensitivity and recovery from mild chronic sleep loss. Sleep. 2012;35:1602–1608. doi: 10.5665/sleep.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalid I, Roehrs TA, Hudgel DW, Roth T. Continuous positive airway pressure in severe obstructive sleep apnea reduced pain sensitivity. Sleep. 2011;34(12):1687–1691. doi: 10.5665/sleep.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawker GA, Mian S, Kendzerske T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63 Suppl 11:S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. The Hamilton Rating Scale for Depression. In: Sartorius N, Ban TA, eds. Assessment of Depression. Berlin, Heidelberg, Germany: Springer; 1986. [Google Scholar]
- 27.Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61–68. doi: 10.1016/0165-0327(88)90072-9. [DOI] [PubMed] [Google Scholar]
- 28.Herring WJ, Connor KM, Snyder E, et al. Suvorexant in patients with insomnia: Pooled analyses of three-month day from phase-3 randomized controlled clinical trials. J Clin Sleep Med. 2016;12(9):1215–1225. doi: 10.5664/jcsm.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svetnik V, Snyder ES, Tao P, et al. Insight into reduction of wakefulness by suvorexant in patients with insomnia: analysis of wake bouts. Sleep. 2018;41:1–9. doi: 10.1093/sleep/zsx178. [DOI] [PubMed] [Google Scholar]
- 30.Roehrs TA, Randall S, Harris E, Maan R, Roth T. MSLT in primary insomnia: stability and relation to nocturnal sleep. Sleep. 2011;34(12):1647–1652. doi: 10.5665/sleep.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinmiller CL, Roehrs TA, Harris E, Hyde M, Greenwald MK. Differential effect of codeine on thermal nociceptive sensitivity in sleepy versus non-sleepy healthy subjects. Exp Clin Psychopharmacol. 2010;18(3):277–283. doi: 10.1037/a0018899. [DOI] [PubMed] [Google Scholar]


