Citation:
Garcia GJM, Woodson BT. The collapsing anatomical structure is not always the primary site of flow limitation in obstructive sleep apnea. J Clin Sleep Med. 2020;16(3):345–346.
Optimal surgical outcomes for obstructive sleep apnea (OSA) require accurate identification of the primary site of flow limitation. However, identifying the primary site of flow limitation is challenging. The upper airway has multiple collapsible structures (soft palate, lateral walls, tongue, and epiglottis) that interact directly (via soft tissue forces) and indirectly (by modulating the pressure and flow experienced by other structures). Furthermore, muscle tone is sharply reduced during sleep, thus the narrowest site observed endoscopically in the awake patient does not necessarily correspond to the site of obstruction when the patient is asleep. To evaluate the upper airway, surgeons often perform drug induced sedated endoscopy (DISE), which allows visualization of airway motion in a physiological state of reduced muscle tone and the selection of anatomical structures that may be targeted with surgery.
In this issue of the Journal of Clinical Sleep Medicine, Yanagisawa-Minami and colleagues1 quantified the pressure-flow relationship in the upper airway of 20 children aged 4 to 8 years using computational fluid dynamics (CFD). A statistically significant correlation was found between CFD-derived airway resistance and OSA severity measured by the apnea-hypopnea index, which is consistent with previous publications.2 Importantly, the authors suggest that the primary site of flow limitation does not always correspond to the site of airway collapse. The CFD simulations predicted a more pronounced drop in luminal pressure downstream of constrictions in which air velocity (V) exceeded 12 m/s. This led the authors to conclude that airway collapse would occur downstream of such constrictions and to propose that the primary site of OSA may be identified as the most anterior location where V > 12 m/s. For a 20-kg child with an inhalation rate (Q) of 240 mL/s, this criterion is equivalent to airspace cross-sectional area falling under 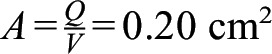 .
.
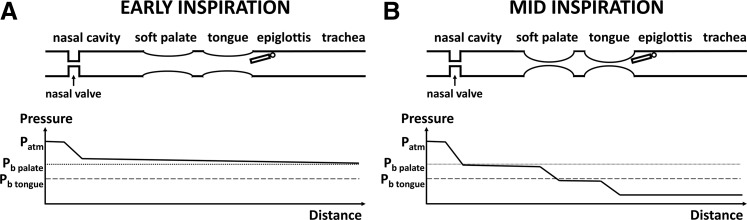
The concept that the primary site of flow limitation does not necessarily correspond to the site of airway collapse can be understood with the following hypothetical scenario. In a patient with a severe constriction at the nasal valve, assuming no mouth breathing, most of the pressure loss occurs at the nasal cavity during early inspiration (Figure 1A). As luminal pressure continues to decrease during inspiration, the highly negative luminal pressure in the pharynx causes the collapse of the soft palate and tongue, which further increases pressure loss in the upper airway (Figure 1B). In this example, the nasal valve is the primary site of flow limitation, but collapse occurs at the soft palate and tongue. Enlarging the constriction at the nasal valve would lessen the pressure loss in the nasal cavity, leading to less negative luminal pressure in the pharynx. This may be enough to prevent airway collapse if luminal pressure does not fall below the critical threshold (ie, the buckling pressure) of each collapsible structure. A similar example would be a constriction at the nasopharynx causing a pressure reduction in the oropharynx, in which case the flow-limiting segment would be the nasopharynx, but only tongue collapse would be observed. This thought experiment implies that precise quantification of each anatomical structure’s contribution to flow limitation will likely require measuring the pressure profile along the entire airway, which can be performed using pressure catheters during DISE or estimated from computer simulations.
Figure 1. Diagram illustrating the concept that the site of flow limitation may be different from the site of airway collapse.
In a hypothetical patient, the nasal valve is the site of flow limitation, but the airway collapses at the soft palate and tongue. (A) In early inspiration most of the pressure drop occurs in the rigid nasal cavity due to a constriction at the nasal valve. (B) Once the local pressure falls below the buckling pressure of each anatomical structure, the airway collapses at that location, which further increases the pressure loss along the upper airway. Symbols: Patm = atmospheric pressure; Pb palate = buckling pressure of the soft palate; Pb tongue = buckling pressure of the tongue.
One shortcoming of the CFD simulations reported by Yanagisawa-Minami and colleagues is that tissue compliance was not incorporated in the CFD model, but rather rigid walls were assumed. Therefore, the authors did not simulate airway collapse. An emerging area of research is the application of fluid-structure interaction (FSI) simulations to investigate upper airway biomechanics.3–6 Future studies should explore the interplay between flow limitation at one site and airway collapse at another site. CFD-FSI technology may one day become the foundation for patient-specific virtual surgery planning.7
DISE is routinely used as part of the evaluation for cranial nerve stimulation8 but its use for clinically selecting patients for other airway surgeries remains uncertain. In a multicentre cohort study using the VOTE classification scheme, Green and coauthors9 found that oropharyngeal lateral wall-related obstruction and complete tongue-related obstruction were associated with poorer surgical outcomes, but DISE findings concerning the velum or epiglottis were not clearly associated with surgical outcomes. The study by Yanagisawa-Minami and colleagues1 may provide some insight into these results. Airway collapse in OSA is a complex process that involves not only sites of collapse but more fundamentally areas of flow limitation. The pharyngeal conduit has complex structural shapes, flow patterns, and pressure-flow profiles that ultimately cause collapse. This passive biomechanical behavior, in turn, is affected by each individual patient’s means of physiologic compensation.10 Current methods of DISE interpretation do not measure or account for these factors and this may partly explain why they are not more predictive of surgical outcomes. In summary, more research on the interplay between fluid dynamic forces, upper airway biomechanics, and physiologic compensation may lead to novel methods to evaluate the upper airway and ultimately lead to more predictive surgical outcomes.
DISCLOSURE STATEMENT
All authors confirm that they have seen and approved the manuscript. The authors acknowledge the financial support from the Advancing a Healthier Wisconsin Endowment (AHW REP project 5520494). The authors report no conflicts of interest.
REFERENCES
- 1.Yanagisawa-Minami A, Sugiyama T, Iwasaki T, Yamasaki Y. Primary site identification in children with obstructive sleep apnea by computational fluid dynamics analysis of the upper airway. J Clin Sleep Med. 2020;16(3):431–439. doi: 10.5664/jcsm.8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wootton DM, Luo H, Persak SC, et al. Computational fluid dynamics endpoints to characterize obstructive sleep apnea syndrome in children. J Appl Physiol. 2014;116(1):104–112. doi: 10.1152/japplphysiol.00746.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pirnar J, Dolenc-Grošelj L, Fajdiga I, Žun I. Computational fluid-structure interaction simulation of airflow in the human upper airway. J Biomech. 2015;48(13):3685–3691. doi: 10.1016/j.jbiomech.2015.08.017. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Mitchell J, Chen Y, Yim W, Chu W, Wang RC. Study of the upper airway of obstructive sleep apnea patient using fluid structure interaction. Respir Physiol Neurobiol. 2018;249:54–61. doi: 10.1016/j.resp.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Subramaniam DR, Arens R, Wagshul ME, Sin S, Wootton DM, Gutmark EJ. Biomechanics of the soft-palate in sleep apnea patients with polycystic ovarian syndrome. J Biomech. 2018;76:8–15. doi: 10.1016/j.jbiomech.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le TB, Moghaddam MG, Woodson BT, Garcia GJM. Airflow limitation in a collapsible model of the human pharynx: Physical mechanisms studied with fluid-structure interaction simulations and experiments. Physiol Rep. 2019;7(10):e14099. doi: 10.14814/phy2.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanhille DL, Garcia GJM, Asan O, et al. Virtual surgery for the nasal airway: A preliminary report on decision support and technology acceptance. JAMA Facial Plast Surg. 2018;20(1):63–69. doi: 10.1001/jamafacial.2017.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strollo PJ, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 9.Green KK, Kent DT, D’Agostino MA, et al. Drug-induced sleep endoscopy and surgical outcomes: a multicenter cohort study. Laryngoscope. 2019;129(3):761–770. doi: 10.1002/lary.27655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes M. Role of respiratory control mechanisms in the pathogenesis of obstructive sleep disorders. J Appl Physiol. 2008;105(5):1389–1405. doi: 10.1152/japplphysiol.90408.2008. [DOI] [PubMed] [Google Scholar]