Abstract
Background
Lifestyle changes and cardiovascular prevention measures are a primary treatment for intermittent claudication (IC). Symptomatic treatment with vasoactive agents (Anatomic Therapeutic Chemical Classification (ATC) for medicines from the World Health Organisation class CO4A) is controversial.
Objectives
To evaluate evidence on the efficacy and safety of oral naftidrofuryl (ATC CO4 21) versus placebo on the pain‐free walking distance (PFWD) of people with IC by using a meta‐analysis based on individual patient data (IPD).
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched October 2012) and CENTRAL (2012, Issue 9).
For the original review the authors handsearched the European Journal of Vascular and Endovascular Surgery (1984 to 1994) and checked relevant bibliographies. They contacted the registration holder of naftidrofuryl and the authors of identified trials for any unpublished data.
Selection criteria
We included only randomized controlled trials (RCTs) with low or moderate risk of bias for which the IPD were available.
Data collection and analysis
We collected data from the electronic data file or from the case report form and checked the data by a statistical quality control procedure. All randomized patients were analyzed following the intention‐to‐treat (ITT) principle. The geometric mean of the relative improvement in PFWD was calculated for both treatment groups in all identified studies.
The effect of the drug was assessed compared with placebo on final walking distance (WDf) using multilevel and random‐effect models and adjusting for baseline walking distance (WD0). For the responder analysis, therapeutic success was defined as an improvement of walking distance of at least 50%.
Main results
We included seven studies in the IPD (n = 1266 patients). One of these studies (n = 183) was only used in the sensitivity analysis so that the main analysis included 1083 patients. The ratio of the relative improvement in PFWD (naftidrofuryl compared with placebo) was 1.37 (95% confidence interval (CI) 1.27 to 1.49, P < 0.001). The absolute difference in responder rate, or proportion successfully treated, was 22.3% (95% CI 17.1% to 27.6%). The calculated number needed to treat was 4.5 (95% CI 3.6 to 5.8).
Authors' conclusions
Oral naftidrofuryl has a statistically significant and clinically meaningful, although moderate, effect of improving walking distance in the six months after initiation of therapy for people with intermittent claudication. Access by researchers to data from RCTs that are suitable for IPD analysis should be possible through repositories of data from pharmacological trials. Regular formal appraisal of the balance of risk and benefit is needed for older pharmaceutical products.
Keywords: Humans; Administration, Oral; Intermittent Claudication; Intermittent Claudication/drug therapy; Nafronyl; Nafronyl/therapeutic use; Randomized Controlled Trials as Topic; Vasodilator Agents; Vasodilator Agents/therapeutic use; Walking; Walking/physiology
Plain language summary
Naftidrofuryl for intermittent claudication
Patients with narrowed arteries of the lower limbs may be hampered by pain in their calves after relatively short walks. This limits the distance they can walk, and hence their quality of life. This is a sure sign of atherosclerosis. These patients are at greater risk of cardiovascular death and should take preventive measures. The symptoms of the disease can be alleviated by smoking cessation and exercise. The question is whether specific drugs such as naftidrofuryl also reduce symptoms, more than placebo. To answer the question, we collected all published reports of randomized trials where the drug was compared with placebo. In addition, we went back to the original data of individual patients and made one big database with all data from all patients from all trials. We included seven studies with a total of 1266 patients. The improvement of pain‐free walking distance was 37% larger in the naftidrofuryl group than the improvement observed in the placebo group. In the naftidrofuryl group 55% of the patients improved by more than 50%, compared with 30% of patients on placebo. Naftidrofuryl 200 mg (taken three times a day by mouth) improved walking distance in the six months after the start of therapy.
Background
Atherosclerosis is a disease in which arteries become narrowed and hardened. Deposits of yellowish plaques (atheromas) containing cholesterol, lipoid material and lipophages are formed within the inner layer of blood vessels (the intima) and the inner media of large and medium‐sized arteries causing arterial narrowing and reduced blood flow to the lower limbs, at rest or during exercise. Atherosclerosis of the arteries of the lower extremities is commonly labeled as peripheral arterial occlusive disease (PAOD) and more recently as peripheral arterial disease (PAD).
A widely accepted international classification of PAD is Fontaine's classification (Fontaine 1954): asymptomatic patients (stage I), intermittent claudication (stages IIa and IIb), rest pain (stage III) and trophic lesions (stage IV) that include lower extremity ulcers, critical leg ischemia and ultimately gangrene of the affected extremity.
It is estimated that PAD, either symptomatic or asymptomatic, occurs in approximately 12% of the adult population in the Western world. The prevalence of PAD increases with advancing age such that almost 20% of people over the age of 70 years have the disease (Hiatt 1995). In the United Kingdom, one in five of the late middle‐aged (65 to 75 years) population have evidence of PAD on clinical examination, although only a quarter of them have symptoms (Fowkes 1991). In a German cross‐sectional study of a population of 6880 unselected patients who were aged 65 years or older the prevalence of PAD, as indicated by an ankle brachial index (ABI) < 0.9, was 19.8% and 16.8% for men and women, respectively. Patients with PAD were slightly older than patients without PAD and suffered more frequently from diabetes (36.6% compared with 22.6%, adjusted odds ratio (OR) 1.8), hypertension (78.8% compared with 61.6%, OR 2.2), lipid disorders (57.2% compared with 50.7%, OR 1.3) and other co‐existing atherothrombotic diseases (any cerebrovascular event: 15.0% compared with 7.6%, OR 1.8; any cardiovascular event: 28.9% compared with 17.0%, OR 1.5) (Diehm 2004). PAOD is also prevalent in developing countries (16% prevalence in patients with a mean age of 68 years) (Li 2003).
In a recent epidemiological study in the Netherlands of 3649 participants (40 to 78 years of age) over a mean follow‐up time of 7.2 years, asymptomatic PAD was significantly associated with a cardiovascular morbidity hazard ratio (HR) of 1.6 (95% confidence interval (CI) 1.3 to 2.1), a total mortality HR of 1.4 (95% CI 1.1 to 1.8) and cardiovascular mortality HR of 1.5 (95% CI 1.1 to 2.1) (Hooi 2004). The natural history of patients with PAD is influenced primarily by the extent of co‐existent coronary artery and cerebrovascular disease (Coffman 1979). The majority of deaths are either sudden or secondary to myocardial infarction. The evolution from asymptomatic to symptomatic disease, the severity of symptoms in intermittent claudication and the transition to stages III and IV are influenced by the extent of the anatomical narrowing of the affected vessel(s) and any existence of a collateral circulation.
The symptoms of intermittent claudication (IC) are pain, ache, cramp or sense of fatigue in the muscles of the affected limb(s); which occurs during exercise and is relieved with rest. The occurrence of IC increases with age with a prevalence of < 1% among people younger than 49 years and as high as 24% among those aged 85 years and over; the prevalence of IC in people aged over 70 years approaches 18% (Carman 2000). Prevalence of IC is higher in males than females (two to three men for every woman) and there is no change in this ratio with increasing age (Dormandy 2000). According to the Edinburgh Artery Study the prevalence in men aged 50 to 59 years is 2.2% and rises to 7.7% for men aged 70 to 74 years (Fowkes 1991). Patients with IC have a three times higher incidence of cardiovascular mortality compared with patients without IC due to the presence of systemic atherosclerosis (Criqui 1985; Hiatt 1995). Smoking cessation plays a vital role in the management (Verhaege 1998). Regular exercise and smoking cessation can improve the symptoms of IC and may be beneficial for any associated coronary artery or cerebrovascular disease (Leng 2000). Conservative treatment should achieve improvement of functional capacity, that is an increase in walking distances, a reduction in symptoms, enhanced quality of life, inhibition of progression of atherosclerotic lesions and reduction of cardiovascular and cerebrovascular morbidity and mortality. To conform with the latest European and American regulatory guidelines (TASC II 2007), improvement of functional capacity for a patient is assessed by measuring the improvement of walking distance as the main study parameter.
The pharmacological management of IC remains to be defined precisely. To date, drugs with proven efficacy in the prevention of major cardiovascular and cerebrovascular events in PAD patients include antiplatelet agents (CLIPS 2007) and lipid‐lowering drugs (statins) (Burns 2003; Mohler 2003). Agents such as 'vasodilators' (naftidrofuryl, pentoxifylline, buflomedil), cilostazol and others (levocarnitine, ginkgo biloba and L‐carnitine) have been used although the availability of robust data to support their role remains lacking (Moher 2000). With regard to the so‐called vasoactive agents (the C04A class in the Anatomic Therapeutic Chemical Classification for medicines from the World Health Organisation) (WHO), controversy has reigned because evidence of clinical efficacy in improving functional capacity is not convincing. In an attempt to resolve this issue, several meta‐analyses of these drugs for intermittent claudication have been undertaken (De Backer 2000; Girolami 1999; Hood 1996; Lehert 1990; Walker 1995). Overall the conclusions of these reviews are that the effects of the C04A drugs are modest. However, since this class consists of such a heterogenous group of products, in terms of their mechanism of action, it is possible that there are differences between them in clinical efficacy. Hence, new reviews should focus on placebo‐controlled trials of the clinical efficacy of single drugs. In the most recent systematic review dedicated specifically to the class of vasoactive drugs, naftidrofuryl was singled out as a potential candidate for further research (De Backer 2000). The most recent narrative review, specifically on the use of naftidrofuryl in the treatment of intermittent claudication (Goldsmith 2005), also supports a clinical relevant effect of naftidrofuryl in patients with intermittent claudication.
All the previous reviews of class C04A drugs which engaged in meta‐analyses were based on published aggregate data, where the average results of several studies are pooled into one meta‐analytic result. Although this is the dominant method, and a valuable approach for synthesizing efficacy data on medicinal products, it may have several drawbacks. In meta‐analyses based on published aggregate data there may be insufficient information available on the variability of the results. Confidence intervals may be provided for baseline and final values but not for the mean change in the primary measure. It is possible to provide approximate estimates in such a situation by calculating the standard deviation (SD) of a difference from the SD of the initial and final visits but these estimates are inaccurate. In older papers the change in walking distance was recorded as an arithmetic mean. In more recent papers it is the geometric mean of the ratio between the walking distance at baseline and the walking distance at final assessment that is recorded. The difference between these two statistics could be important, depending on the skewness of the distribution of the results.
To overcome the limitations of a meta‐analysis based on published aggregate data methodology we undertook a meta‐analysis based on individual patient data (IPD). This allowed us to overcome differences in populations in the different studies over the last 20 years due to changes in study selection guidelines issued by regulatory authorities. In addition, it was possible to correct the usual approach of older studies, to report on a per‐protocol (PP) population, by performing an intention‐to‐treat (ITT) analysis of all randomized participants as insisted upon in recent guidelines for randomized controlled trials. The inherent drawback of an IPD meta‐analysis is the considerable work involved in finding and reorganizing raw data. In this respect carrying out an IPD analysis for the C04A drugs combined would be a large and probably unfeasible task. Hence, we focused on naftidrofuryl as this is one of the only drugs in the class where a minimum of studies of acceptable methodological quality are available (De Backer 2000). In addition, preliminary contacts with the manufacturer (Merck laboratories) and with the study investigators led us to believe that it would be possible to get access to the individual patient data for most of the trials.
Naftidrofuryl is a vasoactive drug that has been marketed since 1968 for the treatment of PAD. The putative mechanisms of action are selective blockade of vascular and platelet 5‐hydroxytryptamine (5‐HT2) receptors and stimulation of the intracellular tricarboxylic acid cycle with increased adenosine triphosphate (ATP) production (Lehert 1990). Although not a conventional vasodilator, naftidrofuryl is included in the ATC therapeutic class of peripheral vasodilators (C04A). Successive small trials with this drug have shown moderate but significant effects. However, the clinical relevance of a classical meta‐analysis based on the literature was questioned because of the heterogeneity among the trials (De Backer 2000). Naftidrofuryl is an old drug with an acceptable safety record that has been widely used. The recommended oral dosage for the indication of intermittent claudication has remained unchanged at 200 mg three times per day. Its patent has expired and there is a wide choice of generics in most countries. Consequently daily treatment costs are rather low (around one euro per day).
Objectives
To determine the efficacy of oral naftidrofuryl (600 mg daily) versus placebo in improving functional capacity in patients with intermittent claudication (IC) by conducting a meta‐analysis of individual patient data (IPD).
Methods
Criteria for considering studies for this review
Types of studies
Randomized placebo‐controlled clinical trials of oral naftidrofuryl.
Types of participants
Patients with intermittent claudication (Fontaine stage II) (Fontaine 1954).
Types of interventions
Oral naftidrofuryl (200 mg three times a day) compared with placebo.
Types of outcome measures
Primary outcome
Pain‐free walking distance (PFWD), defined as the distance walked (in meters) during a standardized exercise test before the onset of calf pain.
Secondary outcomes
Maximum walking distance (MWD), defined as the maximum distance walked (in meters) during a standardized exercise test.
Safety issues, defined as adverse effects directly or possibly related to the use of naftidrofuryl.
Search methods for identification of studies
There was no restriction on language.
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched October 2012) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2012, Issue 9, part of The Cochrane Library, www.thecochranelibrary.com. See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
Searching other resources
For the original review the authors handsearched the European Journal of Vascular and Endovascular Surgery (1984 to 1994), checked relevant bibliographies and contacted the registration holder of naftidrofuryl and the authors of identified trials for any unpublished data.
Data collection and analysis
Study selection
From the results of the above search strategy, TDB and RVS selected the relevant RCTs based on the abstracts of the identified articles or, if necessary, the original publication.
Quality assessment
Assessment of the quality of trials based on the published reports
We assessed the methodological quality of the selected publications of trials and unpublished reports, for both the quality of the trial and the reporting, using the approach of a previous systematic review of vasodilators (De Backer 2000). The original report of all selected trials was transformed into a structured abstract (prepared by TDB and RVS).
We assessed internal validity using the validated scale developed by Jadad et al (Jadad 1996) which includes appropriateness of randomization, double blinding and a description of dropouts and withdrawals by intervention group.
As in the previous systematic review based on the literature (De Backer 2000b), we set three additional quality criteria: sufficient reporting of variability of results, minimum sample size of the study (30 participants) and minimum duration of the study (three months), in accordance with generally accepted methodological standards (Cameron 1988).
Two authors (TDB and RVS) assessed for quality the full publications and the structured abstracts of the clinical trials not identified in the previous systematic review (De Backer 2000b). A third author (LVB) arbitrated if there was disagreement. We only used data for the final analysis from unpublished reports or publications of trials with an acceptable quality. If trials were eliminated by quality criteria we performed a sensitivity analysis to assess the stability of the results.
Additional elements used to appraise the quality of the studies were:
obsolete dosage (< 600 mg daily);
a target population not corresponding to PAD stage II;
study conducted before 1980 (quality control considered too poor);
cross‐over design (because of important carry‐over effects).
Assessment of the quality of trials based on the individual patient data (IPD)
We checked the availability of IPD data for the selected trials by sending a request to the registration holder for unconditional delivery of blinded data.
Reasons to exclude studies were:
too many missing variables or an important overall rate of missing data (> 10% after case report form (CRF) consultation);
the following variables missing in > 5% of patients: randomization treatment code, walking distance (WD) at baseline, WD at final stage of analysis;
the following variables missing in > 20% of patients: age, gender, end‐of‐file status;
doubt about reliability of the computer data compared with CRF data;
questionable data (published results different from database). In our checks for data reliability we were able to isolate, in our database of all randomized patients, only the patients for which data were used in each of the historical publications (some data were per protocol and some by an intention to treat, see Methods section). Hence we were able to check whether the aggregate data from these patients, as presented in our IPD database, fully matched the results of the patients used in the historical publication; only a very small margin of error was allowed (less than 1% deviation).
This quality procedure resulted in the exclusion and inclusion of studies through a consensus discussion process involving all the authors of the original protocol. Studies were excluded because of serious quality flaws or unavailability of IPD data, or both. The included studies were divided into a group of main studies (acceptable quality and IPD available) and a second group of supportive studies (not compliant with all of the above quality criteria but IPD data available). Data from the main and supportive studies constituted the IPD database.
Data collection for the IPD meta‐analysis
Search for the original patient data
After identification of the relevant trials, the data collection in the IPD meta‐analysis constituted a long and complex process involving the different research teams of the included trials, and possibly the manufacturing company, with a request to access either the original data on printed CRFs or the databases into which the raw data were entered. Computerized data entry files are available in various mainframe formats whereas for other older studies only the CRFs are likely to have been retained. All available data were entered in a common pooled database.
Data integrity checks
For data collected from secondary databases, we performed a statistical quality control procedure against the original CRFs by using a military control process, 105D (Mil‐STD 105D), based on random sampling of cases to be checked. At database completion we sent the registration holder (keeper of the CRFs) a list of values randomly distributed among patients and variables, with patient and variable identification but without the value, with the aim of filling in each value at the CRF level. The returned values were compared with our database and a failure rate was calculated. A random subset of 150 values were sent, with a failure rate cut‐off of k = 0. The following key variables were retained: study, centre, patient number; gender; walking distance at baseline (WD0), last carried forward claudication distance (WDf); weight (kg) and age (years).
Checks for publication bias
We intended to assess possible publication bias through funnel plots and the method of Egger that is based on visual representation of the correlation between effect size and sample size (Egger 1997).
Final decision to proceed with the IPD analysis
Two authors (TDB and PL) supervised this procedure. As soon as the information on quality and availability of the individual patient data from the selected trials was finalized, a decision was made as to the continuation of this research. This decision was made by the authors, with consensus.
Data extraction
Construction of the pooled database
Any randomized patient for whom a CRF or database record was available was entered in the pooled database (using the intention‐to‐treat (ITT) principle or full analysis set (FAS) procedure). In particular, data from patients lost to follow up and excluded in per‐protocol analyses were retrieved. In addition, in some older studies it was possible that analysts retrospectively excluded patients from the final analyses based on post‐hoc criteria although fully completed CRFs or database records of these patients (excluded from the analysis but randomized) were available. The data on these patients were also retrieved.
Main variables
We considered the following variables to be important. If these variables were not documented in the secondary databases we then re‐examined the CRFs.
(1) Dates of visits, centre number and patient number.
(2) Termination status (or end‐of‐trial status), including detailed definition of the reason for early termination: 0 = normal termination; 1 = lost to follow up with no apparent reason; 2 = adverse drug‐related reaction; 3 = intercurrent disease, not drug related; 4 = interruption due to lack of compliance; 5 = refusal to participate any longer; 6 = interruption due to protocol violation; 7 = death or serious cardiovascular event; 8 = interruption for surgery; 9 = discontinuation due to local deterioration or progression to PAD stage III.
(3) Follow‐up variables. The ability to evaluate were described as: patient was excluded based on post‐hoc criteria; patient not evaluated on a per‐protocol analysis; patient included in the original analysis (ITT, restricted ITT or per protocol).
(4) Demographic variables: gender, age, height, weight, body mass index (BMI), illness duration, month of randomization, brachial systolic blood pressure and ankle systolic blood pressure at baseline.
(5) Various risk factors: history of smoking, hypertension, obesity, diabetes mellitus, hyperlipidemia, cerebrovascular or cardiovascular events.
(6) Walking distance measurement: pain‐free walking distance (PFWD) measured at selection, baseline, day 90, day 180 and last observation carried forward; maximum walking distance (MWD), if available.
Statistical analysis
Patient selection
The main analysis was based on intention to treat, where all the randomized patients were considered. Two other selections were used for support, essentially for checking consistency with historical results: per‐protocol patients (study completers and fully compliant to the regimen) and restricted intention‐to‐treat (rIT) patients (randomized patients with at least one post‐baseline value).
Analysis organization
During the data collection a statistical analysis plan (SAP) was agreed by the team. This analysis conformed with the European Agency for the Evaluation of Medicinal Products (EMEA) regulatory guidelines for PAD (CPMP Working Party; EMEA). All the analyses were carried out with SAS (version 9.1). After the data integrity check the database was locked. The treatment codes were unknown at the time of the data collection and only provided at the end of the analysis process.
Missing data imputation
For missing main endpoint data, the worst case value was attributed to patients who interrupted the trial early for a reason related to PAD (progression to stage III and IV, aggravation, hospitalization or surgery). For all the other randomized patients who stopped for a reason unrelated to PAD, the last observation carried forward (LOCF) was used as occurs in most PAD trials. However, for sensitivity assessment purposes we carried out an alternative analysis by using summary statistics (mean of intermediate, non‐missing post‐baseline values) when at least two intermediate observations were available. For covariables, missing data were checked for at random and imputed by a systematic full information maximum likelihood (FIML) technique (Anderson 1957).
Main endpoint
Our primary measurement was the pain‐free walking distance (PFWD) measured at baseline and on final assessment, in both naftidrofuryl and placebo treatment groups. We used as the main endpoint the relative improvement RI = WDf/WD0, where WDf and WD0 designated final and baseline PFWD values respectively. The geometric mean with 95% confidence intervals (CI) was used for normality purposes. Treatment effect was measured by the ratio RInaftidrofuryl /RIplacebo. In addition, we calculated the traditional Cohen's effect size (Cohen 1988).
In our main analysis, the PFWD at the end of the study was taken as the final (WDf) if available, and the LOCF was used if the WDf was not available. Four studies had their final WD at six months, one at 12 months (Boccalon 2001) and two at three months (Kriessman 1988; Maass 1984). The LOCF is an approximation justified by: a) its recommendation in PAD guidelines; b) the short expected follow‐up time (six months in general); and c) for a majority of patients the final values were available. In addition, we did summary statistics analysis where we included all intermediate values of the PFWD (often measured at one, three and six months).
Main meta‐analysis statistical test based on the geometric means of WDf and WD0
We conducted the analysis in two independent ways: a one‐stage approach in which the database was considered as one data set and a two‐stage approach in which the aggregated results of each study were pooled and then analyzed with traditional peripheral artery disease meta‐analysis (MA‐PAD) techniques.
For the ratio RInaftidrofuryl / RIplacebo and for effect size we used, in the one‐stage approach, the multilevel (patient and trial) general linear mixed model (GLMM) (Higgins 2001) by considering random treatment effects, fixed‐study effects and adjusting for WD0. We conducted a supportive two‐stage analysis by calculating aggregate RI estimates for each treatment group within each study followed by a conventional random‐effects model (DerSimonian 1986) that is more familiar to clinicians. Our postulated model is based on WD0 covariates, study and treatment factors. Exploratory stepwise research was carried out on all the available predictors (risk factors in particular) to detect the possible existence of additional determinant predictors.
Threshold for significance
The alpha level of error for a significant treatment effect was set at 0.01. We also calculated 95% CIs.
Responder analysis
We also analyzed the data as binary variables. A patient was considered as a responder to therapy when the PFWD improved by at least 50%, from baseline. We estimated the difference in success rates between naftidrofuryl and placebo, the derived number needed to treat (NNT), the relative benefit and the odds ratio (OR). These statistics were calculated with a similar mixed model as above for the one‐stage approach, adapted for binary data (Turner 2000). For the two‐step approach, again we used the random‐effects model of DerSimonian and Laird (DerSimonian 1986).
Secondary outcome measure
For analysis of the maximal walking distance (MWD) data in the trials for which data were available we used the same technique as for PFWD .
Sensitivity analysis
We tested the stability of the results by:
using all the available trials;
excluding or including trials considered as debatable;
comparing the results on the full intention‐to‐treat (ITT) sample with the other a priori selected subgroups, namely restricted intention to treat (rIT) and per‐protocol;
comparing LOCF and use of summary statistics allocation of missing data.
Safety
1. From the publications of the randomized controlled trials
A short description was given of reports on safety issues in the publications.
2. From the IPD
From the IPD, the safety of oral naftidrofuryl was assessed mainly from the mean of the reported adverse events in the naftidrofuryl‐treated patients compared with the mean in the placebo group. We extracted data from the source documents, clinical trials or the publications.
We carried out safety analysis from RCTs by estimating the proportion of participants experiencing at least one:
adverse event (AE) at a moderate level;
serious cardiovascular AE (including death);
serious non‐cardiovascular AE;
gastric AE.
For each of these events we calculated the relative risk in the naftidrofuryl and placebo groups, calculating the difference for each study and using the DerSimonian and Laird model.
3. From all trials and from post‐marketing surveillance (PMS) and periodic safety update (PSU) reports
From all the studies of naftidrofuryl (22,187 patients), two safety studies were carried out and the upper limit 95% CI of the serious adverse‐event incidence was calculated. From the PMS and PSU reports provided by the local authorities, the number of treatment years, during the period 1995 to 1998 and 1998 to 2000, and the number of declared serious adverse events, labeled and suspected drug‐related reports were calculated.
Results
Description of studies
Results of the search
See Appendix 1.
No additional studies were identified for inclusion or exclusion in this update.
We were able to retrieve from the international library system the original publication of all the trials. Subsequent confirmatory searches, based on this set of 11 trials and 20 reviews, using the "related articles" algorithm in PubMED and a search in the Science Citation Index did not reveal additional references; nor did a request for information to the company and to the authors of the retrieved studies. Our request for IPD data to the authors and to the company resulted in the information, provided by the company, that IPD data for nine of the 11 studies were available.
Of the 11 trials, three were published before 1984, six between 1984 to 2000 and two after 2000. Three trials used obsolete doses of naftidrofuryl, either 100 mg four times daily or 100 mg three times daily, and eight used either 200 mg three times daily or 300 two times daily. One study (Karnik 1988) was a cross‐over study. IPD were available from nine studies and covariates from seven studies.
Characteristics of excluded studies
(see also table 'Characteristics of excluded studies' and Table 1; Table 2; Table 3)
1. Patient Baseline Characteristics.
| Characteristic | Placebo n=626 | Naftidrofuryl n=640 | All n= 1266 | ||||
| mean | sd | mean | sd | mean | sd | p | |
| Age (y) | <0.001 | ||||||
| Maass 1984 | 57.06 | 8.67 | 57.04 | 8.32 | 57.05 | 8.46 | |
| Adhoute 1986 | 59.34 | 8.85 | 59.43 | 8.68 | 59.39 | 8.73 | |
| Kriessman 1988 | 61.04 | 8.51 | 61.97 | 8.72 | 61.48 | 8.61 | |
| Adhoute 1990 | 61.87 | 8.29 | 60.37 | 8.3 | 61.12 | 8.3 | |
| Moody 1994 | 63.31 | 8.93 | 63.01 | 7.9 | 63.16 | 8.43 | |
| Boccalon 2001 | 65.62 | 8.9 | 66.07 | 9.33 | 65.86 | 9.11 | |
| Kieffer 2001 | 66.43 | 11.43 | 67.43 | 10.07 | 66.93 | 10.76 | |
| All studies | 62.36 | 9.58 | 62.45 | 9.39 | 62.41 | 9.48 | |
| Gender | <0.001 | ||||||
| Maass 1984 | 0.09 | 0.04 | 0.06 | ||||
| Adhoute 1986 | 0.06 | 0.17 | 0.12 | ||||
| Kriessman 1988 | 0.16 | 0.21 | 0.18 | ||||
| Adhoute 1990 | 0.11 | 0.07 | 0.09 | ||||
| Moody 1994 | 0.23 | 0.28 | 0.26 | ||||
| Boccalon 2001 | 0.21 | 0.29 | 0.25 | ||||
| Kieffer 2001 | 0.21 | 0.22 | 0.22 | ||||
| All studies | 0.16 | 0.19 | 0.18 | ||||
| Height (m) | 0.012 | ||||||
| Maass 1984 | 1.71 | 0.08 | 1.72 | 0.06 | 1.72 | 0.07 | |
| Adhoute 1986 | 1.7 | 0.05 | 1.68 | 0.05 | 1.69 | 0.05 | |
| Kriessman 1988 | 1.7 | 0.07 | 1.7 | 0.08 | 1.7 | 0.07 | |
| Adhoute 1990 | 1.69 | 0.04 | 1.7 | 0.05 | 1.69 | 0.05 | |
| Moody 1994 | 1.7 | 0.08 | 1.68 | 0.09 | 1.69 | 0.08 | |
| Boccalon 2001 | 1.68 | 0.06 | 1.69 | 0.07 | 1.69 | 0.07 | |
| Kieffer 2001 | 1.68 | 0.08 | 1.67 | 0.08 | 1.67 | 0.08 | |
| All studies | 1.69 | 0.07 | 1.69 | 0.07 | 1.69 | 0.07 | |
| Weight (kg) | <0.001 | ||||||
| Maass 1984 | 76.35 | 9.35 | 75.51 | 10.23 | 75.92 | 9.79 | |
| Adhoute 1986 | 70.94 | 8.88 | 68 | 8.68 | 69.31 | 8.86 | |
| Kriessman 1988 | 74.34 | 11.13 | 74.03 | 11.87 | 74.19 | 11.46 | |
| Adhoute 1990 | 69.24 | 8.47 | 69.97 | 9.33 | 69.6 | 8.9 | |
| Moody 1994 | 70.55 | 10.05 | 70.03 | 11.25 | 70.03 | 10.62 | |
| Boccalon 2001 | 72.3 | 10.94 | 72.03 | 12.99 | 72.15 | 12.04 | |
| Kieffer 2001 | 69.32 | 12.34 | 72.34 | 15.57 | 70.83 | 14.09 | |
| All | 71.82 | 10.62 | 71.75 | 11.94 | 71.79 | 11.3 | |
| BMI(kg/m2) | <0.001 | ||||||
| Maass 1984 | 26.01 | 2.4 | 25.36 | 2.97 | 25.67 | 2.72 | |
| Adhoute 1986 | 24.61 | 2.17 | 24.09 | 2.21 | 24.32 | 2.2 | |
| Kriessman 1988 | 25.66 | 3.2 | 25.63 | 3.17 | 25.65 | 3.18 | |
| Adhoute 1990 | 24.19 | 2.15 | 24.22 | 2.52 | 24.2 | 2.34 | |
| Moody 1994 | 24.35 | 3.07 | 24.68 | 3.14 | 24.51 | 3.1 | |
| Boccalon 2001 | 24.14 | 7.05 | 22.78 | 8.26 | 23.41 | 7.73 | |
| Kieffer 2001 | 24.53 | 3.41 | 25.81 | 4.24 | 25.17 | 3.89 | |
| All | 24.79 | 3.75 | 24.67 | 4.42 | 24.73 | 4.1 | |
| SBP (mmHg) | <0.001 | ||||||
| Maass 1984 | 152.13 | 20.9 | 154.42 | 22.91 | 153.32 | 21.93 | |
| Adhoute 1986 | 145.62 | 14.99 | 148.7 | 12.89 | 147.33 | 13.9 | |
| Kriessman 1988 | 151.3 | 19.34 | 151.93 | 19.23 | 151.6 | 19.25 | |
| Adhoute 1990 | 149.98 | 23.1 | 145.55 | 19.04 | 147.76 | 21.22 | |
| Moody 1994 | 162.12 | 28.05 | 158.69 | 26.47 | 160.47 | 27.29 | |
| Boccalon 2001 | 144.19 | 17.64 | 143.3 | 21.83 | 143.72 | 19.91 | |
| Kieffer 2001 | 137.4 | 12.34 | 136.73 | 14 | 137.07 | 13.16 | |
| All | 149.14 | 21.42 | 148.24 | 20.94 | 148.69 | 21.17 | |
| ABI | <0.001 | ||||||
| Maass 1984 | 0.61 | 0.13 | 0.61 | 0.14 | 0.61 | 0.14 | |
| Adhoute 1986 | 0.66 | 0.1 | 0.66 | 0.12 | 0.66 | 0.11 | |
| Kriessman 1988 | 0.58 | 0.12 | 0.58 | 0.13 | 0.58 | 0.12 | |
| Adhoute 1990 | 0.65 | 0.19 | 0.67 | 0.16 | 0.66 | 0.18 | |
| Moody 1994 | 0.69 | 0.17 | 0.68 | 0.15 | 0.69 | 0.16 | |
| Boccalon 2001 | 0.73 | 0.08 | 0.74 | 0.17 | 0.74 | 0.14 | |
| Kieffer 2001 | 0.65 | 0.26 | 0.63 | 0.23 | 0.64 | 0.24 | |
| All | 0.65 | 0.17 | 0.65 | 0.17 | 0.65 | 0.17 | |
| Duration of PAD (y) | <0.001 | ||||||
| Maass 1984 | 2.77 | 3.51 | 2.74 | 3.05 | 2.75 | 3.27 | |
| Adhoute 1986 | 2.65 | 3.19 | 3.85 | 5.68 | 3.32 | 4.46 | |
| Kriessman 1988 | 2.47 | 2.52 | 2.15 | 2.54 | 2.32 | 2.53 | |
| Adhoute 1990 | 2.89 | 3.55 | 3.41 | 4 | 3.15 | 3.78 | |
| Moody 1994 | 3.48 | 3.17 | 3.8 | 3.82 | 3.63 | 3.49 | |
| Boccalon 2001 | 3.65 | 0.61 | 3.69 | 0.64 | 3.67 | 0.62 | |
| Kieffer 2001 | 5.38 | 5.15 | 5 | 4.36 | 5.19 | 4.77 | |
| All | 3.35 | 3.47 | 3.51 | 3.76 | 3.43 | 3.62 |
2. Patient Baseline Characteristics.
| Characteristic | Placebo n=626 | Naftidrofuryl n=640 | All n=1266 | ||||
| n | % | n | % | n | % | p | |
| Obesity (n, %) | <0.001 | ||||||
| Maass 1984 | 17 | 25 | 18 | 24.32 | 35 | 24.65 | |
| Adhoute 1986 | 12 | 18.46 | 11 | 13.58 | 23 | 15.75 | |
| Kriessman 1988 | 40 | 32.26 | 39 | 35.14 | 79 | 33.62 | |
| Adhoute 1990 | 12 | 13.19 | 12 | 13.19 | 24 | 13.19 | |
| Moody 1994 | 19 | 20 | 21 | 23.86 | 40 | 21.86 | |
| Boccalon 2001 | 18 | 22.22 | 18 | 19.78 | 36 | 20.93 | |
| Kieffer 2001 | 21 | 21.43 | 36 | 36.73 | 57 | 29.08 | |
| All studies | 139 | 22.35 | 155 | 24.45 | 294 | 23.41 | |
| Smoking (n, %) | <0.001 | ||||||
| Maass 1984 | 38 | 55.88 | 49 | 66.22 | 87 | 61.27 | |
| Adhoute 1986 | 42 | 64.62 | 46 | 56.79 | 88 | 60.27 | |
| Kriessman 1988 | 44 | 35.48 | 47 | 42.34 | 91 | 38.72 | |
| Adhoute 1990 | 49 | 53.85 | 50 | 54.95 | 99 | 54.4 | |
| Moody 1994 | 41 | 43.16 | 50 | 56.82 | 91 | 49.73 | |
| Boccalon 2001 | 41 | 50.62 | 51 | 56.04 | 92 | 53.49 | |
| Kieffer 2001 | 29 | 29.59 | 27 | 27.55 | 56 | 28.57 | |
| All studies | 284 | 45.66 | 320 | 50.47 | 604 | 48.09 | |
| AHT (n,%) | 0.103 | ||||||
| Maass 1984 | 25 | 36.76 | 29 | 39.19 | 54 | 38.03 | |
| Adhoute 1986 | 14 | 21.54 | 26 | 32.1 | 40 | 27.4 | |
| Kriessman 1988 | 39 | 31.45 | 41 | 36.94 | 80 | 34.04 | |
| Adhoute 1990 | 25 | 27.47 | 22 | 24.18 | 47 | 25.82 | |
| Moody 1994 | 33 | 34.74 | 26 | 29.55 | 59 | 32.24 | |
| Boccalon 2001 | 22 | 27.16 | 36 | 39.56 | 58 | 33.72 | |
| Kieffer 2001 | 36 | 36.73 | 37 | 37.76 | 73 | 37.24 | |
| All | 194 | 31.19 | 217 | 34.23 | 411 | 32.72 | |
| Angina (n, %) | <0.001 | ||||||
| Maass 1984 | 3 | 4.41 | 7 | 9.46 | 10 | 7.04 | |
| Adhoute 1986 | 11 | 16.92 | 12 | 14.81 | 23 | 15.75 | |
| Kriessman 1988 | 11 | 8.87 | 13 | 11.71 | 24 | 10.21 | |
| Adhoute 1990 | 8 | 8.79 | 13 | 14.29 | 21 | 11.54 | |
| Moody 1994 | 14 | 14.74 | 17 | 19.32 | 31 | 16.94 | |
| Boccalon 2001 | 9 | 10.59 | 17 | 17.53 | 26 | 14.29 | |
| Kieffer 2001 | 0 | 0 | 0 | ||||
| All | 56 | 10.61 | 79 | 14.58 | 135 | 12.62 | |
| Diabetes (n, %) | <0.001 | ||||||
| Maass 1984 | 7 | 10.29 | 15 | 20.27 | 22 | 15.49 | |
| Adhoute 1986 | 10 | 15.38 | 10 | 12.35 | 20 | 13.7 | |
| Kriessman 1988 | 6 | 4.84 | 7 | 6.31 | 13 | 5.53 | |
| Adhoute 1990 | 11 | 12.09 | 15 | 16.48 | 26 | 14.29 | |
| Moody 1994 | 12 | 12.63 | 9 | 10.23 | 21 | 11.48 | |
| Boccalon 2001 | 5 | 6.17 | 17 | 19.68 | 22 | 12.79 | |
| Kieffer 2001 | 23 | 23.47 | 22 | 22.45 | 45 | 22.96 | |
| All | 74 | 11.9 | 95 | 14.98 | 169 | 13.46 | |
| Hyperlipaemia (n, %) | 0.015 | ||||||
| Maass 1984 | 27 | 39.71 | 33 | 44.59 | 60 | 42.25 | |
| Adhoute 1986 | 2 | 33.85 | 23 | 28.4 | 45 | 30.82 | |
| Kriessman 1988 | 55 | 44.35 | 53 | 47.75 | 108 | 45.96 | |
| Adhoute 1990 | 30 | 32.97 | 29 | 31.87 | 59 | 32.42 | |
| Moody 1994 | 36 | 37.89 | 31 | 35.23 | 67 | 36.61 | |
| Boccalon 2001 | 38 | 46.91 | 39 | 42.86 | 77 | 44.77 | |
| Kieffer 2001 | 36 | 36.73 | 34 | 34.69 | 70 | 35.71 | |
| All | 244 | 39.23 | 242 | 38.17 | 486 | 38.69 | |
| Sedentarism (n, %) | 0.05 | ||||||
| Maass 1984 | 23 | 33.82 | 32 | 43.24 | 55 | 38.73 | |
| Adhoute 1986 | 35 | 53.85 | 35 | 43.21 | 70 | 47.95 | |
| Kriessman 1988 | 58 | 46.77 | 60 | 54.05 | 118 | 50.21 | |
| Adhoute 1990 | 43 | 47.25 | 39 | 42.86 | 82 | 45.05 | |
| Moody 1994 | 40 | 42.11 | 36 | 40.91 | 76 | 41.53 | |
| Boccalon 2001 | 29 | 35.8 | 31 | 34.07 | 60 | 34.88 | |
| Kieffer 2001 | 39 | 39.8 | 47 | 47.96 | 86 | 43.88 | |
| All | 267 | 42.93 | 280 | 44.16 | 547 | 43.55 |
3. Dates of accrual and of publication of the studies.
| Studies | Date of accrual | Date of publication |
| EXCLUDED | ||
| Ruckley | 1975‐1976 | 1978 |
| Pohle | 1976‐1978 | 1979 |
| Clyne | 1977‐1979 | 1980 |
| Karnik | 1985‐1987 | 1987 |
| INCLUDED | ||
| Maass | 1980‐1983 | 1984 |
| Adhoute | 1982‐1984 | 1986 |
| Kriessmann | 1983‐1985 | 1988 |
| Adhoute | 1985‐1989 | 1990 |
| Moody | 1987‐1989 | 1994 |
| Kieffer | 1996‐1999 | 2001 |
| Boccalon | 1996‐1999 | 2001 |
Ruckley 1978 This was a study of small but acceptable size (50 patients) that was of short duration (three months) with an obsolete dosage of 100 mg three times daily and with a flawed internal validity. Individual patient data were partially available but not the data on the covariables. An effect size of 1.25 was calculated.
Pohle 1979 This was a study of small but acceptable size (51 patients), a short study (12 weeks) with an obsolete dosage of 100 mg once daily and with an acceptable internal validity. Individual patient data were partially available but not the data on the covariables. An effect size of 0.63 was calculated.
Clyne 1980 This was a study of acceptable size (128 patients of whom 30 dropped out), duration (six months) and internal validity with an obsolete dosage of 100 mg once daily. There were no IPD and no covariables data available. An effect size of 0.45 was calculated.
Karnik 1988 This was a small trial (40 patients) using the usual dosage of 600 mg daily but of short duration in a cross‐over design (2 x 8 weeks). There were no IPD data available and no data on covariables. An effect size of 1.11 was calculated.
Characteristics of included studies (see also table 'Characteristics of included studies')
Maass 1984 This was a double‐blind, randomized, placebo‐controlled multicenter (seven) study performed in hospital and ambulatory care settings in Germany. One hundred and thirty‐three patients with PAOD stage II over at least six months and aged between 40 and 70 years were included. Twenty‐nine patients dropped out. After a four‐week wash‐out period the patients were randomly divided into two groups: 54 in the treatment group (51 men and three women) and 50 in the placebo group (46 men and four women) with a mean age of 57 years + 8.2 years and 57 years + 8.6 years, respectively. The patients were assigned to 12 weeks of oral treatment with either 200 mg naftidrofuryl three times daily or placebo. Measurements were taken in the wash‐out period and at one, two and three months. Pain‐free walking distance and MWD were measured on a treadmill (5 km/h at 10% elevation). Ankle brachial index was measured by Doppler and using a cuff. IPD sample size: 142.
Adhoute 1986 This was a double‐blind, placebo‐controlled, randomized multicenter clinical trial performed in hospital and ambulatory care in France. One hundred and eighty‐six patients of both sexes, aged between 40 and 70 years with Fontaine stage II PAOD were selected out of 222 recruited patients following a wash‐out period of one month. Thirty‐six patients were excluded because they did not correspond to the protocol of the trial; 32 patients dropped out of the study because of a variation of PFWD > 20%. After the four‐week run‐in period patients were divided randomly in two groups: 64 in the treatment group (55 men and nine women) and 54 in the placebo group (50 men and four women). During the four‐week run‐in period patients received placebo tablets three times daily. Afterwards, patients received naftidrofuryl 200 mg three times daily or an identical placebo tablet for six months. Measurements were taken at days ‐30, 0, 90 and 180. Pain‐free walking distance was measured on a treadmill (3 km/h at 10% elevation). Ankle brachial index was measured by Doppler. Clinical compliance and tolerance were measured at each visit. IPD sample size: 146.
Kriessman 1988 This was a double‐blind, placebo‐controlled, randomized multicenter trial performed in hospital and ambulatory care settings in Germany. Two hundred and seventy‐four patients aged between 40 and 70 years with Fontaine stage II PAOD were recruited. Fifty‐four patients were excluded from the study because they did not correspond to the study protocol; 27 stopped the therapy; 39 were not included in the study because their center did not reach the minimal level of recruitment and 18 were lost to follow up. One hundred and thirty‐six patients were included in the trial. After a four‐week run‐in period the patients were divided randomly into two groups: 71 in the treatment group (57 men and 14 women) with a mean age of 63+8 years and 65 in the placebo group (52 men and 13 women) with a mean age of 60 + 8 years. After a two‐week run‐in period where all patients received placebo, the patients received 12 weeks of oral treatment with either 600 mg naftidrofuryl (300 mg two times daily) or placebo. Measurements were taken at days ‐14, 0, 40 and 82. Pain‐free walking distance was measured on a treadmill (5 km/h at 7% elevation). Ankle brachial index was measured by Doppler. IPD sample size: 235.
Adhoute 1990 This was a double‐blind, placebo‐controlled, randomized multicenter study performed in hospital and ambulatory care in France. One hundred and eighty‐three patients of both sexes, aged between 40 and 70 years with Fontaine stage II PAOD were recruited. Seventy‐one patients were excluded as they did not correspond with the protocol; 15 were lost to follow up and three discontinued treatment. After the four‐week run‐in period, 94 patients were divided randomly in two groups: 52 in the treatment group (48 men and four women) and 42 in the placebo group (36 men and six women). During the four‐week run‐in period all patients received placebo. During the trial the patients received either 316.5 mg naftidrofuryl or identical placebo tablets two times daily for six months. Measurements were taken at days ‐30, 0, 90 and 180. Pain‐free walking distance and MWD were measured on a treadmill (3 km/h at 10% elevation). Ankle brachial index was measured by Doppler. IPD sample size: 182.
Moody 1994 This was a double‐blind, placebo‐controlled, randomized parallel group trial performed in a hospital setting. One hundred and eighty‐eight patients with intermittent claudication and aged between 40 and 80 years old were recruited from two centers with inclusion based on self‐estimated walking distance. Five patients did not enter the study and three were lost to follow up, leaving 180 patients. In the course of the study five patients in the active group and 10 patients in the control group withdrew from the study. After a four‐week run in period, the active treatment group (n = 85) received 316.5 mg naftidrofuryl fumarate two times daily for 24 weeks while the placebo group (n = 95) received placebo two times daily. Measurements were taken at weeks 0, 8, 16, and 24. Pain‐free walking distance and MWD were measured on a treadmill (slope of 100 at 3 km/h). Ankle brachial index was also measured. IPD sample size: 183. Boccalon 2001 This was a double‐blind, placebo‐controlled randomized trial performed in an ambulatory care setting in France. The outpatients selected were of either sex, aged 40 to 80 years with chronic stable intermittent claudication (< 500 m) and an ankle brachial index (ABI) between 0.60 and 0.90. The patients received naftidrofuryl 200 mg three times daily or placebo for 12 months. They were seen by their general practitioner every three months: M0, M3, M6, M9 and M12; when ABI, tolerance and treatment effects were evaluated. Four visits were to the angiologists' office: M0, M3, M6 and M12; where walking distance was evaluated. The measurement of walking distance was undertaken using a device called the PADHOC (peripheral arterial disease holter control) worn by the patient. This was a registering box attached to a belt and linked to two ultrasonic patches and a command box held in the hand. The principle of the device is the measurement of walking distance and the speed profile in an ambulatory person. One hundred and eighty‐two patients were randomized and 168 entered the intention‐to‐treat analysis. The two groups were well matched for demographic variables, risk factors and history of vascular disease. IPD sample size: 182.
Kieffer 2001 This was a double‐blind, placebo‐controlled randomized study performed in outpatients recruited from five hospitals in Paris and assessed in one single centre. Outpatients of either sex (80% male, mean age 66.9 years) aged between 35 and 85 years with moderately severe chronic, stable intermittent claudication of at least six months duration and who had been clinically stable during the last three months, with the diagnosis of PAOD confirmed by arteriography or duplex scan, were recruited. All patients had already undergone a course of exercise therapy. Only patients whose PFWD and MWD on the treadmill were between 100 and 300 meters were included in the study. It was also a requirement that the walking distance of the patients did not vary by more than 25% between recruitment and randomization. Of the 221 selected patients, 25 did not satisfy the entry criteria. One hundred and ninety‐six patients were randomized following a four‐week placebo run‐in period to: naftidrofuryl (n = 98) or placebo (n = 98) for six months. The patients were followed up for a further six months after discontinuation of treatment. The two groups were well matched for demographic variables (with the exception of BMI, which was significantly higher in the naftidrofuryl group), risk factors and history of vascular disease. Fifteen patients did not supply any further information after baseline, nine in the naftidrofuryl group and six in the placebo group, leaving 181 who entered the intention‐to‐treat analysis. A further 29 patients (13 naftidrofuryl, 16 placebo) did not complete the study according to the protocol, which left 152 patients available for the on‐treatment analysis. Following the end of the six months treatment phase, a further 18 patients dropped out before the final treadmill test at eight months, leaving 134 patients eligible for the final treadmill test at eight months. The primary outcome measures were PFWD and MWD measured on the treadmill using a constant workload and a specific device which recorded both time and gait (results of the latter measurements are not included in this review). The secondary measures of efficacy were the ABI. These measures were performed at all assessment points before, during and at the end of the active treatment phase and at eight months. Clinical tolerance was assessed at all timepoints. Biological tolerance, in particular measurement of liver and renal function, was also evaluated. IPD sample size: 196.
Risk of bias in included studies
(see also Table 4)
4. Quality evaluation of the excluded and included trials.
| Study | Sample size | Duration | Variability | Internal Validity | IPD available | Covariables availabl | Grade |
| EXCLUDED studies | |||||||
| Ruckley 1978 | 0 | 1 | 2 | 2 | incomplete | no | C |
| Pohle 1979 | 1 | 1 | 0 | 1 | incomplete | no | C |
| Clyne 1980 | 0 | 0 | 0 | 1 | no | no | C |
| Karnik 1988 | 1 | 1 | 2 | 2 | no | no | C |
| INCLUDED studies | |||||||
| Maass 1984 | 0 | 1 | 0 | 1 | yes | yes | B |
| Adhoute 1986 | 0 | 0 | 0 | 1 | yes | yes | B |
| Kriessman 1988 | 0 | 1 | 0 | 1 | yes | yes | B |
| Adhoute 1990 | 0 | 0 | 0 | 1 | yes | yes | B |
| Moody 1994 | 0 | 0 | 2 | 2 | yes | yes | B |
| Boccalon 2001 | 0 | 0 | 1 | 0 | yes | yes | B |
| Kieffer 2001 | 0 | 0 | 1 | 0 | yes | yes | B |
Assessment of methodological quality
Excluded studies
We graded the trials of Ruckley 1978 and Karnik 1988 as C (high risk of bias) because of problems of internal validity. This evaluation was in line with our previous review (De Backer 2000). Two trials (Clyne 1980; Pohle 1979) were excluded, in contrast with evaluation of published results in our previous review. Pohle 1979 was excluded because of: incomplete IPD data, bad quality, no availability of covariables and the use of an obsolete drug dosage. The effect size was calculated as 0.63. Clyne 1980 was excluded because IPD were only available for 60% of the patients, covariates were not available and an obsolete dosage was used. The effect size was 0.45.
In conclusion, four trials (Clyne 1980; Karnik 1988; Pohle 1979; Ruckley 1978) were excluded and the reasons for exclusion are listed in the 'Characteristics of excluded studies' table.
Included studies
We included seven trials, which we graded B (moderate risk of bias). All trials were conducted with a common dosage of 600 mg oral naftidrofuryl daily and were from the period between 1984 and 2001. Individual patient data and covariables were available for all studies (see table 'Characteristics of included studies' and Table 3).
We did not exclude the trial by Moody et al (Moody 1994) as we did in our previous review, because no data on variability were given in the published report. In this review IPD data were available and hence variability could be directly assessed.
Checks for publication bias
The sample size of the included studies ranged from 142 to 235 so no study could be considered as small or large; in statistical terms this is interpreted by the square root of the sample size (11.9 to 15.3). This range is very small, thus funnel plots based on detection of a positive monotonic relationship between relative efficacy and sample size were of limited relevance here.
We did, however, apply a range of techniques to find unpublished trials: searches of trials registers, contacts with other researchers and contact with the manufacturing company. None of these approaches revealed evidence of unpublished studies. In addition, we meticulously reviewed the reference lists of relevant articles to find 'hidden' studies (a process called snowballing). In our literature search, undertaken over 10 years, we did not find any evidence of unpublished studies for naftidrofuryl. This is in contrast with documented publication bias for other products in this field where we did find references in the bibliography to studies which, judging by the abstract, were negative but never published (De Backer 2000).
Data extraction for the IPD meta‐analysis
Access to the original patient data
At our request, the company agreed to provide the IPD data unconditionally. Data were stored in CRFs and in individual databases per study, each with a different computer format. The data were extracted from these study databases and transferred to one common database with a common structure.
Data integrity checks
For data collected from secondary databases, we performed a check against the original CRFs using a military control process 105D (Mil‐STD 105D). We checked a random subset of 150 values (study, centre, patient number; gender; WD0, WDf; weight (kg) and age (years). We were able to retrieve all data except for the weight values in the Kriessman study and, instead of 150 values, 146 values were available. After comparison of the computer output with the results manually reported by Merck the number of mistakes was 0/146. This test provided some basic evidence of data reliability of at least 99%.
A total of 7.8% of secondary covariables were missing. After analysis these missing data were considered as missing at random and allocated by a full information maximum likelihood procedure (FIML).
Comparison with published results
For each trial and each treatment group we calculated the mean WD0, WDf and difference (WDf‐WD0); this was the calculation in all published results. We were able to determine which patients in our pooled database contributed to the results as published, from a selection by study design of the published study (per protocol or rIT). The analyses were originally carried out on a restricted intention‐to‐treat basis (Boccalon 2001; Kieffer 2001; Moody 1994) and per protocol for all other studies. This enabled us to check whether the mean values documented in the primary analyses matched with our own results in the pooled IPD database and this check was successful (margin of error less than 1%).
Final decision to proceed with the IPD analysis
Given the limited number of excluded trials, the successful data integrity check and match with the historical data as published and the size of the available pooled database, the authors decided to proceed with the IPD analysis.
There was some debate about two studies (Boccalon 2001; Moody 1994). The latter study (Moody 1994) that had been excluded in the previous review was now included because IPD were available. However, we considered the study to be of rather poor quality with regard to internal validity and more than 70% of the patients had a baseline PFWD of < 100 m. Therefore, this study was classified as a supportive study; it was not included in the main analyses and only analyzed in the sensitivity analysis.
Boccalon 2001 was considered to be of good quality, complying with the guidelines and internal validity criteria. The variability of the results was not well reported but this could be corrected by the IPD data. The trialists used the non‐conventional PADHOC method for measuring walking distance, claimed to represent a higher but more physiological WD0 and WDf. The inclusion of this trial had two consequences. Firstly, it was decided that a sensitivity analysis without the results of this study would be conducted. Secondly, in the main analysis where the Boccalon study was included, results could not be expressed in absolute terms (for example the number of meters gained in WDf ‐ WD0) and were expressed as relative improvement (WDf/WD0).
Effects of interventions
Sample description
(see Additional tables Table 1; Table 2)
One thousand, two hundred and sixty‐six patients (ITT) constituted the whole database of this IPD meta‐analysis (626 placebo, 640 naftidrofuryl); seven studies contributed to the database: Adhoute 1986 (146); Adhoute 1990 (182); Boccalon 2001 (182); Kieffer 2001 (196); Kriessman 1988 (235); Maass 1984 (142); and Moody 1994 (183).
The sample was characterized by a mean age of 62.8 years, 18% women, mean body mass index (BMI) 24.78 ± 4.29 kg/m2, mean systolic blood pressure (SBP) 148.86 ± 21.95 mm Hg, mean ankle brachial index (ABI) 0.65 ± 0.17, mean duration of illness 3.45 ± 3.44 years, 23.4% obese patients, 48% currently smoking, 32.7% hypertensives, 12.6% with angina pectoris, 13.4% type 2 diabetes patients, 38.7% of patients with hyperlipidemia and 43.6% sedentary patients (not performing any physical exercise). As expected, there were important differences among the studies for all these variables (inter‐study heterogeneity) but the two treatment groups matched well for each study as well as for the full set.
Final assessment data were available (per protocol) for 896 patients (71%). For 305 patients (24%) at least one assessment after baseline (not final assessment) was available (rIT). For 65 patients (5%) a randomization code was available but no outcome assessment data (ITT).
Details on the reasons for termination of data are given for 370 patients in Additional Table 5 and Table 6.
5. Termination Status by Study and by Treatment.
| Study | Treatment | Normal | Lost to FU | Adverse Drug R | Intercurr dis |
| Maass 1984 | Placebo | 59 | 3 | 1 | |
| Naftidrofuryl | 69 | 3 | |||
| Adhoute 1986 | Placebo | 46 | 3 | ||
| Naftidrofuryl | 58 | 7 | 4 | 1 | |
| Kriessman 1988 | Placebo | 102 | 6 | 3 | |
| Naftidrofuryl | 97 | 5 | 3 | ||
| Adhoute 1990 | Placebo | 60 | 9 | ||
| Naftidrofuryl | 75 | 7 | |||
| Moody 1994 | Placebo | 82 | 1 | 1 | |
| Naftidrofuryl | 80 | 4 | 1 | ||
| Boccalon 2001 | Placebo | 55 | 8 | 1 | 2 |
| Naftidrofuryl | 67 | 8 | 6 | ||
| Kieffer 2001 | Placebo | 69 | 10 | 3 | |
| Naftidrofuryl | 66 | 9 | 1 |
6. Termination status by Study and by Treatment.
| Study | Treatment | Non Compliance | Refusal further part | Protocol violation | Dead+CVE | Surgery | Local deterioration |
| Maass 1984 | Placebo | 1 | 2 | 2 | |||
| Naftidrofuryl | 1 | 1 | |||||
| Adhoute1986 | Placebo | 1 | 3 | 10 | 2 | ||
| Naftidrofuryl | 2 | 7 | 2 | ||||
| Kriessman 1988 | Placebo | 2 | 6 | 5 | |||
| Naftidrofuryl | 1 | 1 | 4 | ||||
| Adhoute 1990 | Placebo | 1 | 2 | 16 | 3 | ||
| Naftidrofuryl | 1 | 7 | 1 | ||||
| Moody 1994 | Placebo | 1 | 4 | 6 | |||
| Naftidrofuryl | 2 | 1 | |||||
| Boccalon 2001 | Placebo | 9 | 1 | 2 | 2 | 5 | |
| Naftidrofuryl | 9 | 3 | 1 | 3 | |||
| Kieffer 2001 | Placebo | 1 | 13 | 3 | |||
| Naftidrofuryl | 22 | 2 |
Outcome evaluation
Goodness of fit of the proposed model
We carried out a stepwise linear regression analysis of the WDf as a dependent variable, potential predictors being WD0, risk factors and other covariables (age, sex, duration of illness, obesity, diabetes, sedentary life, hypertension, angina pectoris, smoking, hyperlipemia). The analysis revealed that WD0 was the key predictor (R2 = 0.479), accounting for almost all the variability of the other baseline predictors.
Main analysis
Details of the relative improvement of PFWD by study and by treatment are given in Table 7.
7. Relative Improvement of Pain Free Walking Distance (PFWD) by study and by treatment.
| WDf/WD0 | Placebo | CI | Naftidrofuryl | CI |
| Maass 1984 | 1.167 | 0.944‐1.465 | 1.302 | 1.062‐1.656 |
| Adhoute 1986 | 1.316 | 1 ‐1.739 | 1.6 | 1.036‐2.069 |
| Kriessman 1988 | 1.293 | 0.933‐2.04 | 1.486 | 1.217‐2.111 |
| Adhoute 1990 | 1.176 | 1‐1.429 | 1.5 | 1.167‐2 |
| Moody 1994 | 1.16 | 0.838‐1.5 | 1.316 | 1‐1.841 |
| Boccalon 2001 | 1 | 0.713‐1.665 | 1.639 | 1.042‐2.688 |
| Kieffer 2001 | 1.135 | 0.946‐1.419 | 1.801 | 1.414‐2.248 |
| All | 1.176 | 0.926‐1.645 | 1.53 | 1.124‐2.149 |
The main analysis was carried out on a full ITT basis for all participants of six trials (excluding Moody 1994). As explained above, Moody 1994 was not considered in the main analysis, leaving a total of 1083 patients (531 placebo, 552 naftidrofuryl).
Based on ITT samples of six trials, the main analysis based on RI = WDf/WD0 and secondary analyses on responder rate were carried out in a one‐stage multilevel linear mixed model. The treatment effect estimate was RInaftidrofuryl / RIplacebo 1.37 (95% CI 1.27 to 1.49, P < .001). The unadjusted geometric mean RIs were 1.21 and 1.60 for placebo and naftidrofuryl, respectively.
The treatment effect estimates of the two meta‐analytic techniques (one‐stage and two‐stage conventional random approach) were very similar, with somewhat wider estimates of CI in the two‐stage approach (Table 8).
8. Comparison of efficacy between placebo and naftidrofuryl for 1‐step and 2‐steps.
| Analysis | 1‐Step Estimates | 2‐Step Estimates |
| Ratio Relative Improvement PFWD: WDf/WD0 Naf/Plac | 1.37; 95% CI 1.27 to 1.49 | 1.38; 95%;CI 1.24 to1.56 |
| Effect Size ES WDf/WD0 | 0.69; 95% CI 0.55 to 0.84 | 0.57; 95%;CI 0.31 to 0.82 |
| Ratio Relative Improvement MWD: MWDf/MWD0 Naf/Plac | 1.40; 95% CI 1.19 to 1.63 | 1.38; 95%;CI 1.18 to 1.61 |
| Absolute difference succes proportion | 22.3; 95% CI 17.1 to 27.6 | 24.8; 95%;CI 12.2 to 37.4 |
| NNT | 4.48; 95% CI 3.62 to 5.85 | 4.03; 95%;CI 2.51 to 8.19 |
| Relative Benefit | 1.75; 95% CI 1.50 to 2.03 | 1.84; 95%;CI 1.36 to 2.45 |
| Odds Ratio | 2.65; 95% CI 2.10 to 3.37 | 2.90; 95%;CI 1.70 to 4.94 |
Our responder analysis identified 30.2% and 54.7% responders for placebo and naftidrofuryl, respectively. The absolute difference in responder rate, or success proportion, was 22.3% (95% CI 17.1 to 27.6). The number needed to treat was 4.48 (95% CI 3.62 to 5.85). Forest plots based on the Peto OR are shown with responder rates for naftidrofuryl over placebo. Additional tables (Table 9; Table 10; Table 11) show the responder analysis.
9. Responder rate (>50%). Response by study by treatment.
| Study ID | Rp | Np | Rn | Nn |
| Maass 1984 | 15 | 68 | 29 | 74 |
| Adhoute 1986 | 25 | 65 | 47 | 81 |
| Kriessmann 1988 | 51 | 124 | 54 | 111 |
| Adhoute 1990 | 21 | 91 | 44 | 91 |
| Moody 1994 | 24 | 95 | 32 | 88 |
| Boccalon 2001 | 26 | 85 | 57 | 97 |
| Kieffer 2001 | 22 | 98 | 71 | 98 |
| All | 184 | 626 | 334 | 640 |
10. Responder analysis. Difference in success proportion Rn‐Rp.
| Study | Responder rate Rp | Responder rate Rn | Difference Rn‐Rp | CI 1 | CI2 |
| Maass 1984 | 22.06 | 39.19 | 17.13 | ‐13.56 | 47.82 |
| Adhoute 1986 | 38.46 | 58.02 | 19.56 | ‐11.68 | 50.81 |
| Kriessman 1988 | 41.13 | 48.65 | 7.52 | ‐22.18 | 37.22 |
| Adhoute 1990 | 23.08 | 48.35 | 25.27 | ‐4.74 | 55.29 |
| Moody 1994 | 25.26 | 36.36 | 11.1 | ‐18.87 | 41.07 |
| Boccalon 2001 | 30.59 | 58.76 | 28.17 | ‐2.04 | 58.39 |
| Kieffer 2001 | 22.45 | 72.45 | 50 | 20.55 | 79.45 |
| All | 22.84 | 11.44 | 34.24 |
11. Responder analysis. Odds Ratio (Rn/1‐Rn)/(Rp/1‐Rp).
| Study | Rp/1‐Rp | Rn/1‐Rn | (Rn/1‐Rn)/(Rp/1‐Rp) | CI 1 | CI 2 |
| Maass 1984 | 28.3 | 64.44 | 227.7 | 97.75 | 357.66 |
| Adhoute 1986 | 62.5 | 138.24 | 221.18 | 95.21 | 347.14 |
| Kriessman 1988 | 69.86 | 94.74 | 135.6 | 16.91 | 254.29 |
| Adhoute 1990 | 30 | 93.62 | 312.06 | 187.59 | 436.52 |
| Moody 1994 | 33.8 | 57.14 | 169.05 | 44.74 | 293.35 |
| Boccalon 2001 | 44.07 | 142.5 | 323.37 | 200.13 | 446.6 |
| Kieffer 2001 | 28.95 | 262.96 | 908.42 | 783.36 | 1033.47 |
| All | 268.19 | 167.58 | 429.19 |
Heterogeneity
In the two‐stage approach, the test for heterogeneity using Peto OR was significant (P < 0.001). Hence we studied the influence of baseline handicap on treatment effect (presented in Additional Figure 1). The baseline handicap did not significantly affect the relative improvement (mixed model, P > 0.05), which remained constant for the naftidrofuryl group and the placebo group except for a larger walking distance at baseline where the placebo effect seemed to decrease. The heterogeneity was taken into account in the one‐stage approach by using multilevel techniques and in the two‐stage approach by using the random‐effects model of DerSimonian and Laird (DerSimonian 1986).
1.
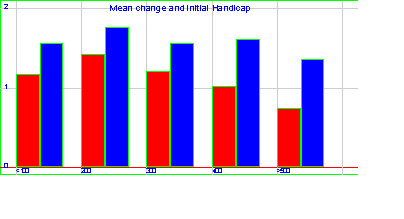
Mean change and baseline handicap
Sensitivity analysis
We carried out various alternative analyses (Table 12); in particular, adding the excluded study (Moody 1994) resulted in a treatment effect estimate with a relative improvement ratio RInaftidrofuryl / RIplacebo of 1.37 (95% CI 1.28 to 1.48, P <0.001, n = 1266). Eliminating Boccalon 2001 (based on physiological walking distance), the ratio was 1.31 (95% CI 1.24 to 1.39, P < 0.001). In comparing our full intention‐to‐treat analysis with the per‐protocol sample (n = 726), we found a higher value of 1.42 (95% CI 1.22 to 1.65). The LOCF versus summary statistics showed a ratio of 1.37 (95% CI 1.28 to 1.48) versus 1.52 (95% CI 1.44 to 1.62).
12. Summary of efficacy in the different analyses. One stage analysis.
| Analysis | Sample size | Treatment estimate | RInaf/RIplac | ||
| n | Treatment | LogWD0 | Study | ||
| Main Analysis (All‐Moody) | 1083 | <0.001 | <0.001 | 0.115 | 1.37; CI 95% 1.27 to 1.48 |
| All studies | 1266 | <0.001 | <0.001 | <0.01 | 1.37; CI 95% 1.28 to 1.48 |
| All studies ‐Boccalon | 1084 | <0.001 | <0.001 | 0.052 | 1.31; CI 95% 1.24 to 1.39 |
| All studies ‐Moody‐Boccalon | 901 | <0.001 | <0.001 | 0.01 | 1.32; CI 95% 1.24 to 1.40 |
| Per Protocol | 726 | <0.001 | <0.001 | <0.01 | 1.42; CI 95% 1.22 to 1.65 |
Analysis of the secondary outcome
The maximum walking distance (MWD) was determined with the same selection and statistical techniques. This endpoint was not measured in all studies and for some studies only on a subset of patients (n = 968, six studies). The relative improvement was 1.40 (95% CI 1.19 to 1.63) and the responder rate absolute risk difference was 23.9% (95% CI 15.7 to 32.1).
Details of the relative improvement of MWD (WDf/WD0) by study and by treatment are given in Table 13.
13. Relative Improvement of Maximal Walking Distance (MWD) by study and by treatment.
| WDf/WD0 | Placebo | CI | Naftidrofuryl | CI |
| Maass 1984 | 1.25 | 1.09‐1.43 | 1.41 | 1.28‐1.54 |
| Kriessman 1988 | 1.44 | 1.27‐1.63 | 1.74 | 1.59‐1.89 |
| Adhoute 1990 | 1.18 | 1.05‐1.32 | 1.60 | 1.48‐1.73 |
| Moody 1994 | 1.13 | 1.02‐1.25 | 1.21 | 1.08‐1.35 |
| Boccalon 2001 | 1.01 | 0.86‐1.119 | 1.74 | 1.48‐2.05 |
| Kieffer 2001 | 1.14 | 1.07‐1.21 | 1.83 | 1.72‐1.94 |
| All | 1.17 | 1.12‐1.23 | 1.57 | 1.50‐1.64 |
Safety analysis
1. As reported in the publications of the randomized controlled trials
1. Maass 1984 reported as serious adverse events (SAE): in the naftidrofuryl group one requiring vascular surgery and one stroke during the wash‐out period; in the placebo group one embolectomy, one ischialgia (pain in the hip) and one ulcus cruris (foot ulcer). Reported non‐serious adverse events were: in the naftidrofuryl group one with nausea plus gastric pain, one urticaria, one dry mouth, one nausea and one loss of body weight; in the placebo group one with urticaria, one nausea and gastric pain. No relevant variations in biological data were detected.
2. Adhoute 1986 reported seven adverse events in the naftidrofuryl group and three in the placebo group. These were mainly gastrointestinal problems. In one patient receiving placebo the gastrointestinal problems were severe enough to discontinue treatment. In both groups laboratory data remained unchanged during the study period.
3. Kriessman 1988 reported serious adverse events for which treatment was discontinued: in the naftidrofuryl group four experienced deterioration of PAOD, two gastric pain and one death with reason unknown; in the placebo group five had deterioration of PAOD, two gastric pain, one fever and one pulmonary disorder. Other adverse events were: in the naftidrofuryl group 14 with gastric disorders, one dry mouth, one skin erythema (reddening of the skin), one articular stiffness, one pressure in the eyes and two with no details given; in the placebo group three experienced gastric disorders, one memory disorder, one sweating, one sleep disturbance, two headaches and three with no details given.
4. Adhoute 1990 reported 27 patients (30%) in the naftidrofuryl group with at least one adverse event. Of these, 20 reported at least one important adverse event and eight reported at least one non‐serious adverse event. In the placebo group 31 (34%) patients reported at least one adverse event of which 10 were important, five patients reported at least one non‐serious adverse event.
5. Moody 1994 stated that in the naftidrofuryl group there was one with angina pectoris and one suspected myocardial infarction. In the placebo group one participant experienced acute ischemia followed by bypass surgery, one myocardial infarction and one vascular occlusion followed by angioplasty. In the naftidrofuryl group a higher incidence of minor gastrointestinal symptoms was recorded.
6. Boccalon 2001 found that in the naftidrofuryl group 28 patients (29%) had at least one adverse event versus 15 (17%) in the placebo group during the double‐blind treatment period. In the naftidrofuryl group 17 patients (29%) had at least one serious adverse event versus eight (17%) in the placebo group, although this was not statistically significant. This difference is due to hospitalization for various pathologies (eight in the naftidrofuryl group versus three in the placebo group), among other factors. Five deaths occurred during the study (three in the naftidrofuryl group versus two in the placebo group). In the naftidrofuryl group eight non‐serious adverse events were reported, mainly gastro‐intestinal disorders (four); compared with five in the placebo group.
7. Kieffer 2001 reported that in the naftidrofuryl group 18 patients (18%) experienced one or more adverse events compared with 21 patients (21%) in the placebo group. In the naftidrofuryl group 11%, 12% in the placebo group, reported at least one serious adverse event. No serious adverse event was directly related to the study during the double‐blind treatment phase. One patient died in the naftidrofuryl group due to lung cancer. In the naftidrofuryl group nine non‐serious adverse events were reported versus 12 in the placebo group. The non‐serious adverse event for which a possible relationship with naftidrofuryl existed was one case of digestive disorder.
In summary, there were no significant differences for serious adverse events between naftidrofuryl and placebo. For the non‐serious adverse events there was a significantly higher incidence of gastro‐intestinal disorders.
2. From the IPD of randomized controlled trials
Table 14 shows the number of patients experiencing at least one adverse event at a moderate level for naftidrofuryl and for placebo. Table 15 shows the number of patients experiencing at least one gastric adverse event for naftidrofuryl and for placebo. Table 16 shows the number of patients experiencing at least one serious non‐cardiovascular adverse event for naftidrofuryl and for placebo. Table 17 shows the number of patients experiencing at least one serious cardiovascular event for naftidrofuryl and for placebo. Overall, there was no significant difference between both groups for moderately severe adverse events; the proportion of non‐cardiovascular adverse events was not significantly different and the proportion of cardiovascular adverse events was slightly higher in the placebo group. Only the proportion of gastric disorders was higher in the naftidrofuryl with a risk difference of 2.85% (95% CI 0.78 to 4.91%) compared with placebo. Table 5 and Table 6 show the reasons for prematurely leaving the study, including adverse drug reactions and cardiovascular events.
14. Safety calculation of Placebo versus Naftidrofuryl: Moderate AE.
| Study | Placebo | N Placebo | Naftidrofuryl | N Naftidrofuryl |
| Maass 1984 | 0 | 68 | 0 | 74 |
| Adhoute 1986 | 19 | 65 | 17 | 81 |
| Kriessman 1988 | 20 | 124 | 27 | 111 |
| Adhoute 1990 | 0 | 91 | 0 | 91 |
| Moody 1994 | 0 | 95 | 0 | 88 |
| Boccalon 2001 | 15 | 85 | 28 | 97 |
| Kieffer 2001 | 21 | 98 | 18 | 98 |
| All | 75 | 626 | 90 | 640 |
15. Safety calculation of Placebo versus Naftidrofuryl: Gastric AE.
| Study | Placebo | N Placebo | Naftidrofuryl | N Naftidrofuryl |
| Maass 1984 | 2 | 68 | 4 | 74 |
| Adhoute 1986 | 3 | 65 | 7 | 81 |
| Kriessman 1988 | 8 | 124 | 18 | 111 |
| Adhoute 1990 | 10 | 91 | 18 | 91 |
| Moody 1994 | 5 | 95 | 7 | 88 |
| Boccalon 2001 | 3 | 85 | 4 | 97 |
| Kieffer 2001 | 2 | 98 | 1 | 98 |
| All | 33 | 626 | 59 | 640 |
16. Safety calculation of Placebo versus Naftidrofuryl: Non CV AE.
| Study | Placebo | N Placebo | Naftidrofuryl | N Naftidrofuryl |
| Maass 1984 | 1 | 68 | 0 | 74 |
| Adhoute 1986 | 0 | 65 | 5 | 81 |
| Kriessman 1988 | 3 | 124 | 3 | 111 |
| Adhoute 1990 | 0 | 91 | 0 | 91 |
| Moody 1994 | 1 | 95 | 1 | 88 |
| Boccalon 2001 | 3 | 85 | 6 | 97 |
| Kieffer 2001 | 0 | 98 | 0 | 98 |
| All | 8 | 626 | 15 | 640 |
17. Safety calculation Placebo versus naftidrofuryl: CV AE.
| Study | Placebo | N Placebo | Naftidrofuryl | N Naftidrofuryl |
| Maass 1984 | 5 | 68 | 1 | 74 |
| Adhoute 1986 | 15 | 65 | 11 | 81 |
| Kriessman 1988 | 11 | 124 | 5 | 111 |
| Adhoute 1990 | 21 | 91 | 9 | 91 |
| Moody 1994 | 10 | 95 | 3 | 88 |
| Boccalon 2001 | 10 | 85 | 20 | 97 |
| Kieffer 2001 | 13 | 98 | 12 | 98 |
| All | 85 | 626 | 61 | 640 |
3. From periodic safety update reports (PSUR)
From all the studies of naftidrofuryl (n = 22,187 patients) two safety studies were carried out. The upper limit of serious adverse‐event incidence was 1.35 x 10‐4 (1/10,000).
During the period from 1995 to1998 there were 1,224,908 treatment years with 19 labeled adverse events. During the period 1998 to 2000 there were 2,823,546 treatment years with 18 labeled adverse events. These events were mainly gastric disorders. The observed incidence rate was 0.305 x 10‐5 and the upper 95% CI was 0.89 x 10‐5, so that the rate of drug‐related serious adverse events was likely to be less than 10‐5 (1/100,000).
In summary, the few observed lethal or serious adverse events were not considered to be attributable to the treatments either by the investigator or by the sponsor; they either had another cause or were a complication of an underlying disease. Except for gastric disorders, no disorder of other specific organ systems was shown to have a clinically relevant incidence. The analysis of gastric disorders provided a relative risk of 1.75. The incidence of gastric disorders was variable across studies. The maximum incidence, at a 95% CI, of drug‐related serious adverse events was 0.89 x 10‐5 and essentially consisted of gastric disorders. There were a few reports of neurological or skin adverse reactions from the spontaneous reporting system but the incidence was similar on placebo.
Discussion
The evidence base of symptomatic treatment of peripheral arterial occlusive disease (PAD)
In the management of PAD caused by atherosclerosis, risk factor modification plays a crucial role as cardiovascular morbidity and mortality is very high in these patients. All patients with PAD should receive all measures recommended in the secondary prevention of cardiovascular disease. Exercise therapy and smoking cessation are essential elements of the treatment of intermittent claudication from the beginning, both for cardiovascular protection and for the symptomatic treatment of PAD itself.
Although for symptomatic treatment the non‐pharmacological measures of "stop smoking and keep walking" are important and efficacious, pharmacological treatment of disabling symptoms could be justified in the management of PAD. If proven efficacious and safe, pharmacological treatment could have a clinically relevant impact on the quality of life and a facilitating effect on exercise therapy. However, the available evidence on the efficacy of pharmaceuticals is limited in this field. There is evidence that statins have a positive effect on symptoms of PAD besides their cardiovascular protective effects (Aung 2007).
A series of drugs labeled as vasoactive agents with differing presumed modes of action have been promoted for symptomatic treatment of peripheral arterial disease. However, no single pharmacological agent has been accepted and used worldwide. These drugs do not replace risk factor modification, lifestyle changes and exercise training programs but they can have a place as adjunctive treatment for those who do not perform well or benefit from exercise therapy or for those who have a contra‐indication or no indication (yet) for invasive therapy. Drugs for which no evidence for improving claudication is available are cinnarizine, cyclandelate, nicotinic acid, isoxsuprine (De Backer 2000) and omega‐3 fatty acids (Sommerfield 2007). Drugs with a limited number of studies, providing insufficient evidence, are vitamin E (Kleijnen 1998 1998) and prostanoids (Reiter 2003). Drugs for which several studies exist but they are of doubtful quality and insufficiently support claims of efficacy are buflomedil and pentoxifylline (De Backer 2000). The use of two pharmacological agents is supported by an evidence base; they have limited efficacy and are mentioned in the European and Transatlantic guidelines. These are cilostazol and naftidrofuryl (Robless 2007; SIGN 2006; TASC II 2007).
For cilostazol, a Cochrane review exists based on six RCTs, comparing cilostazol 100 mg twice daily versus placebo. The weighted mean difference (WMD) for the PFWD was 31.1 m (95% C) 21.4 to 40.9). The side effects included headache, diarrhea, peripheral edema and nausea. Cilostazol is contra‐indicated in heart failure. There are no data on the reduction of cardiovascular events (Robless 2007). For naftidrofuryl, about 20 narrative reviews and one systematic review have been published. However, in the systematic review the authors made the decision not to undertake a meta‐analysis, because of the methodological limitations of pooling heterogenous aggregated data. To perform a final decisive evaluation of the efficacy of naftidrofuryl we decided to repeat the analysis on individual patient data within the framework of The Cochrane Collaboration. Health technology assessment in pharmaceuticals is usually limited to new products. An IPD meta‐analysis is rarely attempted in clinical pharmacotherapy for new products as the number of pivotal trials is limited in the first phases of the life cycle of a product. With older products it is often too difficult to collect IPD data from trials, especially when the earliest studies were conducted decades ago before the era of Good Clinical Practice (GCP).
Regulatory control of the quality of drugs on the market should not only be limited to new agents and safety issues. When initial claims of efficacy and efficiency of older products are not corroborated as experience accumulates and when research fails to deliver confirmation of effect, marketing should be put into question, even in the absence of safety crises. Authorities should not just wait for the slow relinquishment process of obsolete products or the withdrawal by the manufacturer because the product is no longer economically viable.
Strengths of the IPD
For naftidrofuryl we managed to conduct an IPD meta‐analysis based on seven RCTs from a period spanning from 1978 to 2001. The 1266 patients in the IPD database provide a solid basis for analysis. A full intention‐to‐treat analysis (ITT) was performed where all randomized patients were included irrespective of their outcome. Robust methods for missing endpoint allocation were applied and a common robust measure of central tendency (the geometric mean) was used. As the EMEA guidelines recommend, we chose PFWD as the primary endpoint and MWD as the secondary endpoint (EMEA). As individual patient data (IPD) were available, we were able to use both the one‐stage and two‐stage approach in analyzing results, expressed as continuous and binary data, allowing us to assess the impact of different meta‐analytical statistical techniques. Our sensitivity analysis was based on various study exclusions and inclusions and the techniques used pointed out the stability of the results.
Another potential strength of IPD is the ability to perform analyses within different patient subgroups. In this analysis we did a first assessment on the possible influence of covariates by carrying out a stepwise exploratory linear regression in which we entered all covariates (age, gender, duration of illness, risk factors, ABI) and we found that only baseline walking distance (WD0) and treatment effects were useful predictors of the final walking distance, WDf. However, we intend to analyze the data on the influence of covariates (including patient subgroups) on baseline walking distance in another context.
Limitations of this IPD
The outcome in these studies was measured with a standardized treadmill test (in one study with a physiological method, the PADHOC). In some studies graded‐load treadmill tests and in other studies constant‐load treadmill tests were used. The studies were conducted in a limited number of countries in Europe (UK, France and Germany). The maximal walking distance was not recorded in all studies. For four of the 11 identified trials no or only incomplete IPD were available (two of which had quality flaws serious enough to be excluded). The studies dated from different time periods with intervening major events such as the generalized introduction of Good Clinical Practice (GCP) and changing international guidelines to conduct trials in PAD.
Can these results be trusted ?
The authors were dependent on the goodwill of the marketing authorization holder for access to the data. We found references and full text of all identified studies in the medical literature but for the individual patient data we had to rely on the permission of the company, as the data were not available from the principal investigators. The company, who funded most of the studies in this review, provided the data without preliminary conditions. The meta‐analysis in itself was not funded by the company, as directed by The Cochrane Collaboration. Despite precise data integrity checks some uneasiness with regard to the authenticity of the original data remains as some studies date from before the period of the implementation of rigorous GCP checks. Our successful comparison of the per protocol and restricted ITT data in our database with the published results provided reassurance in this regard.
Despite searching since 1997 for unpublished trials carried out at any time in the past, uncertainty about publication bias remains. It is still possible that trials were executed, analyzed and hidden because of negative results. Unfortunately, classical numerical tests for publication bias can hardly be applied in this meta‐analysis because the number of trials is small and with little range in the sample size. Funnel plots based on detection of a positive monotonic relationship between relative efficacy and sample size are of limited relevance here. The only possible check in this context would be to examine the relationship of relative efficacy across the time when these trials were conducted, with the underlying assumption that early trials might show a better efficacy than more recent trials since the historical early studies could be expected to have less scientific rigor or due to the phenomenon of 'fading of reported effectiveness' (Gehr 2006). In our analysis the findings were that the expected efficacy does not manifestly decrease from older to more recent historical studies.
Our results could be affected by choices made in the inclusion and exclusion of trials and the subsequent quality evaluation of included trials. We excluded four studies. Two had serious flaws in quality, three had an obsolete dosage of 100 mg once daily and IPD data were not available or incomplete for all of these four studies. The excluded trials had an effect size of 0.45 (Clyne 1980), 0.63 (Pohle 1979), 1.11 (Karnik 1988) and 1.25 (Ruckley 1978), while the overall effect size of the included studies was 0.54 (Table 18). Hence, it is unlikely that the exclusion of these trials have inflated the results.
18. Hedges and Olkin table on Effect Size.
| Study ID | Effect size | CI1 | CI2 |
| Maass 1984 | 0.47 | ‐0.12 | 1.06 |
| Adhoute 1986 | 0.39 | ‐0.2 | 0.98 |
| Kriessmann 1988 | 0.33 | ‐0.22 | 0.88 |
| Adhoute 1990 | 0.46 | ‐0.11 | 1.03 |
| Moody 1994 | 0.39 | ‐0.17 | 0.96 |
| Boccalon 2001 | 0.6 | 0.03 | 1.17 |
| Kieffer 2001 | 1.17 | 0.6 | 1.75 |
| All | 0.54 | 0.33 | 0.76 |
We conducted sensitivity analyses which showed that the inclusion or exclusion of the studies by Moody 1994 (with a majority of patients with very high disease severity at baseline) and Boccalon (using a physiological instead of standardized outcome measure) did not affect the conclusions in a meaningful way.
Clinical relevance
The question is whether our statistically significant results are clinically relevant. The geometric mean of the relative improvement was 1.60 for naftidrofuryl and 1.21 for placebo. There is an absence of interaction of baseline severity with treatment, which means that the efficacy of the treatment remains constant irrespective of the magnitude of the baseline handicap. We set the threshold for response at 50% improvement. One could argue that a 100% improvement is needed. Although somewhat arbitrary, the value of 50% improvement is based on the rationale that above a baseline walking distance of 200 meters (the average of our database) an improvement of 50% is at least 100 meters, a value considered as clinically meaningful to help maintain essential activities of daily living (D'Hooge 2001).
In this analysis little attention was paid to baseline risk factors. It is known that PAD is strongly affected by smoking and sedentary lifestyle. The effect of physical exercise on walking distance improvement has been widely demonstrated but one key question remains, does a particular drug have a positive or synergistic interaction with physical exercise? Today the regulatory authorities require not only that the physicians motivate their patients to improve lifestyle conditions but also constrains the drug manufacturer to show that the effect of the studied drug is not simply suppressed when a patient modifies his or her lifestyle. This is the key question in PAD therapy and is apparently without an answer today. The question is difficult as it requires longitudinal recording of smoking habits and exercise and a methodological solution to disentangle the pharmacological effects and the lifestyle change effects. Based on the response analysis, a number needed to treat of 4.48 (95% CI 3.62 to 5.85) was calculated, which may be considered as an acceptable result for symptomatic treatment. Our results on maximal walking distance (MWD) corroborated the results with pain‐free walking distance (PFWD) as an outcome. In the results of previously published individual trials often a significant difference was found for PFWD but not for MWD. In fact, there are few scientific arguments to favor one measurement over the other. However, historically PFWD data are more readily available. Walking distance improvement consists of the relief of a symptom considered as an important impairment because it has a causal effect on disability, reduces the activities of daily living that are linked with a person's autonomy and influences social life, role achievements, mood and quality of life (D'Hooge 2001). In a previous consensus conference on oral vasoactive medication for intermittent claudication that was organized by our group, we and a panel of general practitioners, medical specialists, pharmacists, nurses, health insurers and patients concluded that priority should be given to financing health education and patient education programs (prevention, rehabilitation, physical exercise) rather than spending resources on vasoactive medications. A cost‐benefit study should now be performed for naftidrofuryl (currently sold at a cost of approximately one Euro a day).
Safety
Based on the published reports and the IPD of adverse events in the included RCTs, and on the periodic safety update reports from post‐marketing surveillance, naftidrofuryl seems to be a well‐tolerated oral treatment with side effects limited to minor gastric symptoms (for example esophagitis, diarrhea).
Was it worth the effort?
IPD meta‐analyses generally necessitate a huge effort for collecting data, and this analysis was no exception. In the future when company data from pivotal trials are archived in a standardized way and made available for independent researchers, it will be easier to conduct IPD analyses.
We were able to model the PFWD final value by adjusting for baseline PFWD because we included a covariance analysis, not simply a mean change, and assessed treatment effect under an appropriate multi‐level mixed model that accounted for study effect. We were also able to include data from two trials with poorly published results on variability. The IPD allowed us to provide a uniform calculation of endpoint measurement (relative improvement) for all randomized patients and to apply statistical techniques which take into account inter‐study heterogeneity. Hence, the analysis led to a clear conclusion, permitting us to resolve continuing uncertainty about the efficacy of the pharmacological approach to symptomatic treatment in PAD with naftidrofuryl. It kept us from throwing out the baby with the bath water, by discarding the group of vasoactive drugs as a whole.
We enlarged the results through a systematic intention‐to‐treat approach; the results of our main analysis using a full intention to treat (RI 1.37, 95% CI 1.27 to 1.49) differ from the per‐protocol analysis (RI 1.42, 95% CI 1.22 to 1.65; n = 726), which is a higher value (Table 12). Hence, the IPD meta‐analysis resulted in a more conservative but more robust estimate of the effect. Now that this database is constructed, additional interesting research questions could be explored, such as unravelling the effects of covariates on disease severity, subgroup analyses and time‐to‐event analyses. From a methodological point of view, a more thorough comparison of the results of the one‐stage and two‐stage approaches for the different expressions of outcome could be interesting.
Authors' conclusions
Implications for practice.
Naftidrofuryl has a statistically significant and clinically meaningful, although moderate effect compared with placebo for improving walking distance in intermittent claudication in the first six months after initiation of therapy. Lifestyle changes such as stopping smoking, physical exercise and use of antiplatelet drugs remain cornerstones in the prevention and treatment of PAD and should accompany symptomatic drug prescription.
Implications for research.
Besides registries of randomized controlled trials, we should have repositories of data from pharmacological trials that are suitable for individual patient data (IPD) analysis. Raw data should be available to the principal investigators, regulatory authorities and researchers, with due consideration of patient privacy. In addition, regular formal appraisal of the balance of risk and benefit is needed for older products.
What's new
| Date | Event | Description |
|---|---|---|
| 15 October 2012 | New citation required but conclusions have not changed | The searches were re‐run. No new studies were identified. Minor copy edits made. |
| 15 October 2012 | New search has been performed | The searches were re‐run. No new studies were identified. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 4 May 2011 | Amended | Maximal Walking Distance table added, table label and error in abstract corrected. |
| 5 May 2008 | Amended | Converted to new review format. |
| 14 December 2007 | New citation required and conclusions have changed | Substantive amendment. Submitted for publication in Issue 2, 2008. |
| 11 November 2004 | New search has been performed | Protocol revised and updated as the review will now include individual patient data. Submitted for publication in Issue 1, 2005. Changes to team of authors led by Dr Tine de Backer. |
| 23 May 2003 | Amended | Revised protocol submitted for publication in Issue 3, 2003. New team of authors (Mr Ous Alozairi, Mr Paul Bachoo and Miss Julie Brittenden). |
| 18 November 1998 | New citation required and conclusions have changed | Protocol by a team led by Professor David Moher submitted for publication in Issue 1,1999 |
Acknowledgements
The original protocol for this review was published by a team led by Professor David Moher, Mr Paul Bachoo and Dr Julie Brittenden, Aberdeen Royal Infirmary, UK. They assisted in the revised protocol and in the quality evaluation of the trials.
We thank Mrs Heather Maxwell for coordinating the editorial process and for her help in the search for studies. We thank the Cochrane Peripheral Vascular Diseases Group and Professor Mike Clarke for their feedback and advice in the peer review process of this IPD systematic meta‐analysis.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Arteriosclerosis] this term only 893 #2 MeSH descriptor: [Arteriolosclerosis] this term only 0 #3 MeSH descriptor: [Arteriosclerosis Obliterans] this term only 71 #4 MeSH descriptor: [Atherosclerosis] this term only 377 #5 MeSH descriptor: [Arterial Occlusive Diseases] this term only 753 #6 MeSH descriptor: [Intermittent Claudication] this term only 708 #7 MeSH descriptor: [Ischemia] this term only 746 #8 MeSH descriptor: [Peripheral Vascular Diseases] explode all trees 2137 #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 5042 #10 atherosclero* or arteriosclero* or PVD or PAOD or PAD 16413 #11(arter* or vascular or vein* or veno* or peripher*) near (occlus* or steno* or obstuct* or lesio* or block*)7222 #12 peripheral near/3 dis* 3147 #13 claudic* or IC 3311 #14 isch* or CLI 16581 #15 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 39447 #16 MeSH descriptor: [Nafronyl] explode all trees 99 #17 naftidrofuryl* 226 #18 praxilene* 27 #19 dusodril* 22 #20 nafronyl 102 #21 #16 or #17 or #18 or #19 or #20 251 #22 #15 and #21 108 in Trials
Data and analyses
Comparison 1. Naftidrofuryl (NFD) versus placebo: responder rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Responder rate naftidrofuryl versus placebo | 7 | 1266 | Peto Odds Ratio (95% CI) | 2.59 [2.06, 3.24] |
1.1. Analysis.
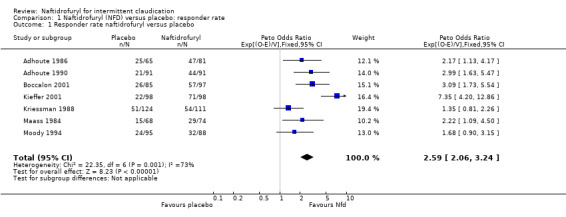
Comparison 1 Naftidrofuryl (NFD) versus placebo: responder rate, Outcome 1 Responder rate naftidrofuryl versus placebo.
Comparison 2. Naftidrofuryl (Nfd) versus placebo: WDf‐WD0. Mean gain naftidrofuryl over placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean gain in meters PFWD of naftidrofuryl over placebo | 6 | 1084 | Mean Difference (IV, Fixed, 95% CI) | 48.44 [35.94, 60.95] |
2.1. Analysis.
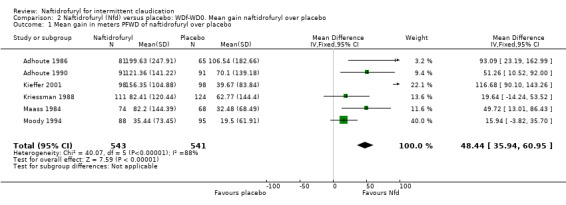
Comparison 2 Naftidrofuryl (Nfd) versus placebo: WDf‐WD0. Mean gain naftidrofuryl over placebo, Outcome 1 Mean gain in meters PFWD of naftidrofuryl over placebo.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adhoute 1986.
| Methods | Study design: double‐blind placebo‐controlled, randomized multicenter trial in hospitalized and ambulatory care settings in France. Measurements were done at days ‐30, 0, 90, and 180. PFWD was measured by treadmill (3 km/h at 10% elevation). ABI was measured by Doppler. Clinical compliance and tolerance were measured at each visit. | |
| Participants | Publication: 186 patients of both sexes between 40 and 70 years with Fontaine stage II PAOD were selected out of 222 recruited patients during a wash‐out period of 1 month. 36 patients were excluded because they did not correspond to the protocol of the trial, 32 patients dropped out of the study (variation PFWD > 20%). After a 4‐week run‐in period patients were divided randomly in 2 groups: 64 in treatment group (55 men and 9 women) and 54 in placebo group (50 men and 4 women). IPD: 146 patients. | |
| Interventions | During the 4‐week run‐in period, patients received placebo tablets TID followed by either naftidrofuryl 200 mg TID or an identical placebo tablet for 6 months. | |
| Outcomes | Publication: A statistically significant increase at days 90 and 180 in PFWD was observed in both groups. The comparison between the two groups at day 90 and 180 showed a significant difference in favor of the naftidrofuryl group (P < 0.02): 103 m (CI 67 to 139 m). The variations in the pressure index between the two groups showed no significant difference. IPD: The mean change (geometric mean) over baseline was 163% for naftidrofuryl versus 122% for placebo. Effect size = 0.39. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Adhoute 1990.
| Methods | Study design: double‐blind randomized placebo‐controlled multicenter study in hospitalized and ambulatory care settings in France. Measurements were done at days ‐30, 0, 90 and 180. PFWD and MWD were measured by treadmill (3 km/h at 10% elevation). ABI was measured by Doppler. | |
| Participants | Publication: 183 patients of both sexes between 40 and 70 years with Fontaine stage II PAOD were recruited. 71 patients were not included for not keeping to the protocol, 15 were lost to follow up and 3 discontinued treatment. After a 4‐week run‐in period, 94 patients were divided randomly in 2 groups: 52 in the treatment group (48 men and 4 women) and 42 in placebo group (36 men and 6 women). IPD: 182 patients. | |
| Interventions | During the 4‐week run‐in period all patients received placebo. During the trial the patients received either 316.5 mg naftidrofuryl or identical placebo tablets BID for 6 months. | |
| Outcomes | Publication: A statistically significant increase (P < 0.05) at day 180 in PFWD (from 227.21 m ± 54.31 m to 350.92 m ± 155.10 m) and MWD (from 292.61 m ± 92.50 m to 469.39 m ± 181.85 m) in the naftidrofuryl group was measured. Between day 0 and 180, the residual pressure index increased significantly (P < 0.02) in the naftidrofuryl group and remained stable in the placebo group. The difference in pressure index between the 2 groups did not reach significance at day 180. There was a significant difference in treatment benefit observed by the patient or physician at the end of the study in favor of naftidrofuryl. IPD: The mean change over baseline was 154% for naftidrofuryl versus 124% for placebo. Effect size = 0.46. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Boccalon 2001.
| Methods | Study design: double‐blind, placebo‐controlled, randomized trial in an ambulatory care in France. Measurements were done at 0, 3, 6 and 12 months of treatment. The primary variables were PFWD and MWD measured with the Peripheral Arterial Disease Holter Control device (PADHOC), measuring the intermalleolar distance using ultrasound telemetry. Patients were seen by the generalist every 3 months: M0, M3, M6, M9 and M12. Then ABI, tolerance and therapeutic observation were measured. Four visits were done at the angiologist's office: M0, M3, M6 and M12, where the walking distance test was performed. | |
| Participants | Publication: The outpatients were of both sexes, aged 40 to 80 years, with a chronic stable intermittent claudication (PFWD <500 m) and an ABI between 0.60 and 0.90. Exclusion criteria were PAOD stage I, II, IV, claudication of other origin than arteriosclerosis, antecedents of myocardial infarction, angina or stroke in the last 2 months, surgical intervention in the last 2 months or surgery planned, isolated stenosis at the aorto‐iliac level, insulin dependent diabetes, neuropathy, uncontrolled arterial hypertension 9 (S > 180 mmHg, D > 115 mmHg), renal or hepatic failure. 182 patients were randomized and 168 entered the intention‐to‐treat analysis (88 = naftidrofuryl and 80 = placebo). 14 patients did not come back after the initial visit (9 = naftidrofuryl and 5 = placebo). The 2 groups were well matched for demographic variables, risk factors and history of vascular disease. The per‐protocol population consisted of 122 patients: 67 who received naftidrofuryl and 55 who received placebo. All patients had an optimal medical treatment including antiplatelets, advice of stop smoking and physical exercise. IPD: 182 patients. | |
| Interventions | Patients received naftidrofuryl or placebo during 12 months. The dosage of naftidrofuryl was 600 mg/d given as 200 mg tid. | |
| Outcomes | Publication: After a 12‐month treatment patients who received naftidrofuryl had a 107% improvement of geometric physiological PFWD versus 12% in the placebo group (P < 0.001) and 74% improvement of geometric maximal walking distance versus 1% in the placebo group. IPD: The mean change over baseline was 183% for naftidrofuryl versus 97% for placebo. Effect size = 0.6. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kieffer 2001.
| Methods | Study design: double‐blind, placebo‐controlled, randomized trial in 5 hospitals in Paris but assessed at a single centre. The primary outcome measures of efficacy were PFWD distance and MWD measured on the treadmill using a constant workload and using a specific device which recorded both time and gait (results of the latter measurements are not included in this paper). The secondary measure of efficacy were ankle‐brachial indices. All these measures were performed at all assessments points before, during and at the end of active treatment phase, as well as 8 months. Clinical tolerance was assessed at all time points. Biological tolerance, in particular measurements of renal and liver function, was also evaluated. | |
| Participants | Outpatients of both sexes (80% male with a mean age of 66.9 y), aged 35 to 85 years, with moderately severe chronic, stable intermittent claudication of at least 6 months and which had been clinically stable during the last 3 months and the diagnosis of which was confirmed by arteriography or duplex scan. All patients had already undergone a course of exercise therapy. Only the patients whose PFWD and MWD on the treadmill were between 100 and 300 m were included in the study. It was also a requirement that the WD of patients did not vary by more than 25% between recruitment and randomization. Of the 221 selected patients ‐ 25 did not satisfy the entry criteria: 196 were randomized following a 4‐week placebo run‐in period: naftidrofuryl (n= 98) or placebo (n= 98). 15 patients did not supply any further information after baseline, 9 in the naftidrofuryl group and 6 in the placebo group, leaving 181 who entered the intention‐to‐treat analysis. A further 29 patients (13 naftidrofuryl, 16 placebo) did not complete the study according to the protocol, which left 152 patients available for the on‐treatment analysis. The patients were followed up for a further 6 months after treatment discontinuation. Following the end of the 6 months treatment phase a further 18 patients dropped out before the final treadmill test at 8 months, leaving 134 patients eligible for the final treadmill test at 8 months. IPD: 196 patients. | |
| Interventions | Naftidrofuryl 200 mg TID or placebo for six months. | |
| Outcomes | Publication: In the naftidrofuryl group there was a 91.8% increase in geometric PFWD and a 82% increase in geometric MWD compared to 16.8% and 13.9% in the placebo group (P < 0.001). The change in ABI was not significantly different between the two groups. There were no differences between the two groups in terms of number of serious or non‐serious adverse events. There were no clinically significant changes in any of the biological parameters between baseline and the end of the treatment period. IPD: The mean change over baseline was 181% for naftidrofuryl versus 116% for placebo. Effect size = 1.17. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Kriessman 1988.
| Methods | Study design: double‐blind placebo‐controlled, randomized multicenter trial in hospitalized and ambulatory care settings in Germany. Measurements were done at days ‐14, 0, 40 and 82. PFWD was measured by treadmill (5km/h at 7% elevation). ABI was measured by Doppler. | |
| Participants | Publication: 274 patients between 40 and 70 years with Fontaine stage II PAOD for at least 6 months. 54 were excluded from the study because they did not correspond to the study protocol, 27 stopped the therapy, 39 were not included because their center did not reach the minimal level of recruitment and 18 were lost to follow up. 136 patients were included in the trial. After a 4‐week run‐in period the patients were randomly divided in 2 groups: 71 in treatment group (57 men and 14 women) with a mean age of 63 ± 8 years, 65 in the placebo group (52 men and 13 women) with a mean age of 60± 8 years. IPD: 235 patients. | |
| Interventions | After a 2‐week run‐in period where all patients received placebo, the patients received 12 weeks of oral treatment with either 600 mg naftidrofuryl (delayed action, 300 mg BID) daily or placebo. | |
| Outcomes | Publication: PFWD increased in both groups between the beginning of the trial and the end: 91.85 m for the naftidrofuryl group and 42.38 m for the placebo group. The difference in increase was significant (P < 0.05) in favor of the treatment group: 49 m (CI 16 to 82 m). IPD: The mean change over baseline was 157% for naftidrofuryl versus 121% for placebo. Effect size = 0.33. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Maass 1984.
| Methods | Study design: double‐blind placebo‐controlled randomized multicenter (7) study in a hospitalized and ambulatory care in Germany. Measurements were done in the wash‐out period and at months 1, 2 and 3. PFWD and MWD were measured by treadmill (5 km/h at 10% elevation). ABI was measured by Doppler and cuff. | |
| Participants | Publication: 133 patients between 40 and 70 years with Fontaine stage II PAOD for at least 6 months. 29 patients dropped out. After a 4‐week wash‐out period the patients were divided randomly in 2 groups: 54 (51 men and 3 women, mean age 57 ± 8.2 years) in treatment group and 50 (46 men and 4 women, mean age 57± 8.6 years) in placebo group. IPD: 142 patients. | |
| Interventions | 12 weeks of oral treatment with either 200 mg naftidrofuryl TID or placebo. | |
| Outcomes | Publication: The WDs increased in both groups. The difference in increase in PFWD between placebo and treatment group was significant (P < 0.02): 59 m (CI 8 to 110 m). For MWD the difference was not significant. IPD: The mean change over baseline was 144% for naftidrofuryl versus 115% for placebo. Effect size = 0.47. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Moody 1994.
| Methods | Study design: double‐blind placebo controlled randomized controlled trial in a hospital setting. PFWD, MWD measured by treadmill (slope 10% at 3 km/h) and ABI at weeks 0, 8, 16 and 24. | |
| Participants | Publication: 180 patients with IC between 40 and 80 years recruited in 2 hospitals. In the course of the study 5 patients in active group and 10 patients in control group withdrew from the study. IPD: 183 patients. | |
| Interventions | After a 4‐week run‐in period the active treatment group (85) received 316.5 mg naftidrofuryl fumarate bid for 24 weeks while the placebo group (95) received placebo BID. | |
| Outcomes | Publication: The results for PFWD and MWD did not reach statistical significance. An ad hoc constructed combined index of PFWD, MWD and ABI revealed a positive effect in 44% of patients (P < 0.047). IPD: the mean change over baseline was 138 % for naftidrofuryl versus 113% for placebo. Effect size = 0.39. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ABI: ankle brachial index BID: twice daily CI: confidence interval IC: intermittent claudication IPD: individual patient data MWD: maximum walking distance PAOD: peripheral arterial occlusive disease PFWD: pain‐free walking distance TID: three times daily WD: walking distance
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Clyne 1980 | ‐ dosage is obsolete (100 mg QD) ‐ IPD data available for only 60% of patients ‐ covariates not available |
| Karnik 1988 | ‐ high risk of bias (small short study with internal validity problems) ‐ is a cross‐over study ‐ IPD data incomplete and of bad quality ‐ no covariates available |
| Pohle 1979 | ‐ IPD data incomplete and of bad quality ‐ dosage is obsolete (100 mg QD) ‐ covariates not available |
| Ruckley 1978 | ‐ high risk of bias (short trial with internal validity problems) ‐ use of obsolete dosage (100 mg TID) ‐ IPD data not available ‐ covariates not available |
IPD: individual patient data QD: once daily TID: three times daily
Contributions of authors
TDB co‐ordinated the project and searched for trials with the Cochrane Peripheral Vascular Diseases Group. TDB selected and assessed the quality of trials, extracted data, interpreted the results and assisted in writing the text of the review.
RVS assisted in the search, selection and quality assessment of the trials, in the interpretation of the results, and in writing the text of the review.
PL assisted in data extraction and analysis, and in the interpretation of the results.
LVB assisted in the selection and quality assessment of the trials.
Sources of support
Internal sources
Allocation of working time by the Ghent University, Belgium.
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
The PVD Group editorial base is supported by a programme grant from the NIHR
Declarations of interest
Tine de Backer, Robert Vander Stichele and Luc Van Bortel have no conflict of interests. Philippe Lehert has performed statistical consultancy for a number of pharmaceutical companies, including Merck which holds the marketing authorization for naftidrofuryl.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adhoute 1986 {published and unpublished data}
- Adhoute G, Bacourt F, Barral M, Cardon JM, Chevalier JM, Cuny A, et al. Naftidrofuryl in chronic arterial disease. Results of a six month controlled multicenter study using naftidrofuryl tablets 200 mg. Angiology 1986;37:160‐7. [DOI] [PubMed] [Google Scholar]
Adhoute 1990 {published and unpublished data}
- Adhoute G, Andreassian B, Boccalon H, Cloarec M, Maria G, Lefebvre O, et al. Treatment of stage II chronic arterial disease of the lower limbs with the serotonergic antagonist naftidrofuryl: Results after 6 months of a controlled, multicenter study. Journal of Cardiovascular Pharmacology 1990;16(Suppl):75‐80. [PubMed] [Google Scholar]
Boccalon 2001 {published and unpublished data}
- Boccalon H, Lehert P, Mosnier M. Effect of naftidrofuryl on physiological walking distance in patients with intermittent claudication. Annales de Cardiologie et d Angeiologie 2001;50(3):175‐82. [DOI] [PubMed] [Google Scholar]
Kieffer 2001 {published and unpublished data}
- Kieffer E, Bahnini A, Mouren X, Gamand S. A new study demonstrates the efficacy of naftidrofuryl in the treatment of intermittent claudication International. Angiology 2001;20(1):58‐65. [PubMed] [Google Scholar]
Kriessman 1988 {published and unpublished data}
- Kriessman A, Neiss A. Clinical effectiveness of naftidrofuryl in intermittent claudication [Naftidrofuryl bei der peripher arteriellen verschlusskrankheit]. VASA 1988;17(Suppl 24):27‐32. [PubMed] [Google Scholar]
Maass 1984 {published and unpublished data}
- Maass U, Amberger H‐G, Bohme H, Diehm C, Dimroth H, Heidrich H, et al. Naftidrofuryl in arterial occlusive disease [Naftidrofuryl bei arterieller Verschlusskrankheit. Kontrollierte multizentrische Doppelblindstudie mit oraler Applikation]. Deutsche Medizinische Wochenschrift 1984;109:745‐50. [DOI] [PubMed] [Google Scholar]
Moody 1994 {published and unpublished data}
- Moody AP, Al‐Khaffaf HS, Lehert P, Harris PL, Charlesworth D. An evaluation of patients with severe intermittent claudication and the effect of treatment with naftidrofuryl. Journal of Cardiovascular Pharmacology 1994;23(Suppl 3):44‐7. [PubMed] [Google Scholar]
References to studies excluded from this review
Clyne 1980 {published and unpublished data}
- Clyne CAC, Galland RB, Fox MJ, Gustave R, Jantet GH, Jamieson CW. A controlled trial of naftidrofuryl (Praxilene) in the treatment of intermittent claudication. British Journal of Surgery 1980;67:347‐8. [DOI] [PubMed] [Google Scholar]
Karnik 1988 {published and unpublished data}
- Karnik R, Valentin A, Stollberger C, Slany J. Effects of naftidrofuryl in patients with intermittent claudication. Angiology 1988;39(3):234‐40. [DOI] [PubMed] [Google Scholar]
Pohle 1979 {published data only}
- Pohle W, Hirche H, Barmeyer J, Schumichen C, Hoffman G. A double‐blind trial of naftidrofuryl hydrogen oxalate in patients suffering from occlusive peripheral arterial disease. Medizinische Welt 1979;30(7):269‐72. [PubMed] [Google Scholar]
Ruckley 1978 {published and unpublished data}
- Ruckley CV, Callam MJ, Ferrington CM, Prescott RJ. Naftidrofuryl for intermittent claudication: a double‐blind controlled trial. British Medical Journal 1978;1:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Anderson 1957
- Anderson TW. Maximum likelihood estimates for a multivariate normal distribution when some observations are missing. Journal of the American Statistical Association 1957;52:200‐3. [Google Scholar]
Aung 2007
- Aung PP, Maxwell HG, Jepson RG, Price JF, Leng GC. Lipid‐lowering for peripheral arterial disease of the lower limb. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000123.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burns 2003
- Burns P, Gough S, Bradbury AW. Management of peripheral arterial disease in primary care. BMJ 2003;326(7389):584‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cameron 1988
- Cameron HA, Waller PC, Ramsay LE. Drug treatment of intermittent claudication: a critical analysis of the methods and findings of published clinical trials, 1965‐1985. British Journal of Clinical Pharmacology 1988;26(5):569‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carman 2000
- Carman TL, Fernandez BB Jr. A primary care approach to the patient with claudication. American Family Physician 2000;61(4):1027‐32,1034. [PubMed] [Google Scholar]
CLIPS 2007
- Critical Leg Ischaemia Prevention Study (CLIPS) Group, Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double‐blind trial. Journal of Internal Medicine 2007;261(3):276‐84. [DOI] [PubMed] [Google Scholar]
Coffman 1979
- Coffman JD. Intermittent claudication and rest pain: physiologic concepts and therapeutic approaches. Progress in Cardiovascular Diseases 1979;22(1):53‐72. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. New Jersey: Lawrence Erlbaum, 1988. [Google Scholar]
CPMP Working Party
- CPMP Working Party. Clinical investigations of medicinal products in the treatment of chronic peripheral arterial disease. Note for guidance EMEA Status as of October 2002. http://www.emea.europa.eu/pdfs/human/ewp/071498en.pdf (accessed 23 May 2007).
Criqui 1985
- Criqui MH, Fronek A, Barrett‐Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation 1985;71(3):510‐5. [DOI] [PubMed] [Google Scholar]
D'Hooge 2001
- D'Hooge D, Lehert P, Clement DL. Naftidrofuryl in quality of life (NIQOL). A Belgian study. International Angiology 2001;20(4):288‐94. [PubMed] [Google Scholar]
De Backer 2000
- Backer TL, Vander Stichele RH, Bogaert MG. Buflomedil for intermittent claudication. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000988.pub2] [DOI] [PubMed] [Google Scholar]
De Backer 2000b
- Backer TL, Vander Stichele RH, Warie HH, Bogaert MG. Oral vasoactive medication in intermittent claudication: utile or futile?. European Journal of Clinical Pharmacology 2000;56(3):199‐206. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Diehm 2004
- Diehm C, Schuster A, Allenberg JR, Darius H, Haberl R, Lange S, et al. High prevalence of peripheral arterial disease and co‐morbidity in 6880 primary care patients: cross‐sectional study. Atherosclerosis 2004;172(1):95‐105. [DOI] [PubMed] [Google Scholar]
Dormandy 2000
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAltlantic Inter‐Society Consensus (TASC). Journal of Vascular Surgery 2000;31(Pt 2):1‐296. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EMEA
- The European Agency for the Evaluation of Medicinal Products (EMEA). ICH Topic E 6. Guideline for Good Clinical Practice. http://www.emea.eu.int/pdfs/human/ich/013595en.pdf (accessed 28 August 2004).
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;21:499‐533. [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FG, Housley E, Cawood EH, MacIntyre CA, Ruckley CV, Prescott RJ. Edinburgh artery study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. International Journal of Epidemiology 1991;20(2):384‐92. [DOI] [PubMed] [Google Scholar]
Gehr 2006
- Gehr BT, Weiss C, Porzsolt F. The fading of reported effectiveness. A meta‐analysis of randomized controlled trials. BMC Medical Research Methodology 2006;6(25):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Girolami 1999
- Girolami B, Bernardi E, Prins MH, Cate JW, Hettiarachchi R, Prandoni P, et al. Treatment of intermittent claudication with physical training, smoking cessation, pentoxifylline, or nafronyl: a meta‐analysis. Archives of Internal Medicine 1999;159(4):337‐45. [DOI] [PubMed] [Google Scholar]
Goldsmith 2005
- Goldsmith DR, Wellington K. Naftidrofuryl: a review of its use in the treatment of intermittent claudication. Drugs and Aging 2005;22(11):967‐77. [DOI] [PubMed] [Google Scholar]
Hiatt 1995
- Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease. The San Luis Valley Diabetes Study. Circulation 1995;91(5):1472‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2001
- Higgins JP, Whitehead A, Turner RM, Omar RZ, Thompson SG. Meta‐analysis of continuous outcome data from individual patients. Statistics in Medicine 2001;20:2219‐41. [DOI] [PubMed] [Google Scholar]
Hood 1996
- Hood SC, Moher D, Barber GG. Management of intermittent claudication with pentoxifylline: meta‐analysis of randomized controlled trials. Canadian Medical Association Journal 1996;155(8):1053‐9. [PMC free article] [PubMed] [Google Scholar]
Hooi 2004
- Hooi JD, Kester AD, Stoffers HE, Rinkens PE, Knottnerus JA, Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7‐year follow‐up study. Journal of Clinical Epidemiology 2004;57(3):294‐300. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Kleijnen 1998
- Kleijnen J, Mackerras D. Vitamin E for intermittent claudication. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000987] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehert 1990
- Lehert P, Riphagen FE, Gamand S. The effect of naftidrofuryl on intermittent claudication: a meta‐analysis. Journal of Cardiovascular Pharmacology 1990;16(Suppl 3):81‐6. [PubMed] [Google Scholar]
Leng 2000
- Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD000990] [DOI] [PubMed] [Google Scholar]
Li 2003
- Li XY, Wang J, He Y, Fan L. The relation between peripheral arterial occlusive disease and cardiovascular diseases in elderly population: a cross‐section study in Wanshoulu area,Beijing. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2003;83(21):1847‐51. [PubMed] [Google Scholar]
Mil‐STD 105D
- Defense Standardization Program. Military Standards 105D ‐ Quality on Data Entry process. http://www.dsp.dla.mil (accessed 27 August 2004).
Moher 2000
- Moher D, Pham B, Ausejo M, Saenz A, Hood S, Barber GG. Pharmacological management of intermittent claudication: a meta‐analysis of randomised trials. Drugs 2000;59(5):1057‐70. [DOI] [PubMed] [Google Scholar]
Mohler 2003
- Mohler ER 3rd. Peripheral arterial disease: identification and implications. Archives of Internal Medicine 2003;163(19):2306‐14. [DOI] [PubMed] [Google Scholar]
Reiter 2003
- Reiter M, Bucek RA, Stümpflen A, Minar E. Prostanoids for intermittent claudication. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD000986.pub2] [DOI] [PubMed] [Google Scholar]
Robless 2007
- Robless P, Mikhailidis DP, Stansby GP. Cilostazol for peripheral arterial disease. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003748.pub2] [DOI] [PubMed] [Google Scholar]
SIGN 2006
- Scottish Intercollegiate Guidelines Network. Diagnosis and management of peripheral arterial disease. A national clinical guideline. Scottish Intercollegiate Guidelines Network (SIGN) 2006;89:1‐41. [Google Scholar]
Sommerfield 2007
- Sommerfield T, Price J, Hiatt WR. Omega‐3 fatty acids for intermittent claudication. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003833.pub3] [DOI] [PubMed] [Google Scholar]
TASC II 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, TASCII Working Group. Inter‐society consensus for the management of peripheral arterial disease (TASC II). European Journal of Vascular and Endovascular Surgery 2007;33 Suppl 1:1‐75. [DOI] [PubMed] [Google Scholar]
Turner 2000
- Turner RM, Omar RZ, Yang M, Goldstein H, Thompson SG. A multilevel model framework for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2000;19(24):3417‐32. [DOI] [PubMed] [Google Scholar]
Verhaege 1998
- Verhaeghe R. Epidemiology and prognosis of peripheral obliterative arteriopathy. Drugs 1998;56(Suppl 3):1‐10. [DOI] [PubMed] [Google Scholar]
Walker 1995
- Walker GA, Mac Hannaford JC. A meta‐analysis of randomized, double‐blind, placebo‐controlled studies of the effect of buflomedil on intermittent claudication. Fundamental and Clinical Pharmacology 1995;9(4):387‐94. [DOI] [PubMed] [Google Scholar]
WHO
- World Health Organization. The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). http://www.who.int/classifications/atcddd/en/ (accessed July 2007).
References to other published versions of this review
de Backer 2008
- Backer TLM, Vander Stichele R, Lehert P, Bortel L. Naftidrofuryl for intermittent claudication. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD001368.pub3] [DOI] [PubMed] [Google Scholar]


