Abstract
Context
Women are at increased risk for depressive symptoms during the menopause transition. Changes in estradiol secretion and presence of vasomotor symptoms (VMS) contribute to perimenopausal depressive symptoms, but links with progesterone have not been investigated.
Objective
To determine whether estradiol variability, ovulatory levels of progesterone, and VMS burden are independently associated with perimenopausal depressive symptomatology.
Design and Intervention
Depressive symptoms, serum levels of estradiol and progesterone, and VMS frequency were assessed weekly in an 8-week observational study. Association of mood with estradiol variability, ovulatory levels of progesterone, and VMS frequency were estimated using generalized estimating equation models.
Setting
Academic medical center.
Patients
Fifty unmedicated perimenopausal women with mild-to-moderate depressive symptoms (mean Montgomery-Åsberg Depression Rating Scale [MADRS] score 15.5 ± 5.3).
Main Outcome Measure
Depressive symptoms (MADRS score).
Results
During the study, 90.0% of participants had varying estradiol levels, 51.1% had ovulatory progesterone levels, and 90% had VMS. Greater estradiol variability and absence of progesterone levels consistent with ovulation, but not VMS frequency, are associated with higher levels of depressive symptoms (β = 0.11 [95% confidence interval (95% CI), 0.04 to 0.18; P = 0.001]; β = −2.62 [95% CI, −4.52 to −0.71; P = 0.007], respectively), after accounting for higher body mass index, lifetime history of depression, and stressful life events.
Conclusions
Increasing dysregulation of ovarian hormones, but not VMS, associates with more depressive symptom burden during perimenopause. These results suggest that perimenopausal mood instability is driven by the underlying hormonal dysregulation of the menopause transition involving changes in both estradiol and progesterone.
Keywords: perimenopause, depression, estradiol, progesterone, ovulation, mood
Women are at increased risk for mood disturbance across the perimenopause, with 45% to 68% developing depressive symptoms during this reproductive transition (1). While mood disturbances may be severe and constitute a full-blown major depressive episode, subsyndromal depressive symptoms are more common and remain important as they cause significant distress, impair quality of life, and increase health care utilization (2, 3). Identifying factors that drive vulnerability for depressive symptoms in the perimenopause helps women and their clinicians understand the potential course of depressive symptoms across the menopause transition and optimize therapeutic approaches.
Menopause-specific risk factors for the development of perimenopausal depressive symptoms include greater variability in serum levels of estradiol, higher and more variable levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), as well as the presence of vasomotor symptoms (VMS) (4–10). These associations highlight the potential interactions of changing gonadal steroids dynamics with neural mechanisms regulating affect (11–13) and the impact of VMS, particularly nocturnal VMS and associated sleep disturbance (8), in the emergence of depressive symptoms in the perimenopause. Gonadal steroid changes during the menopause transition involve wide excursions in estradiol and less frequent production of progesterone with the emergence of anovulation. However, the potential independent impact of progesterone on mood, possibly mediated through its neurosteroid metabolite allopregnanolone, has received little attention (13).
In the current study, we investigated whether depressive symptom severity in perimenopausal women is linked independently with variability of estradiol, production of postovulatory levels of progesterone, as well as VMS frequency, after accounting for a priori potential confounders such as depression history and stressful life events. To achieve this goal, we conducted an 8-week clinical observational study of 50 perimenopausal women with untreated depressive symptoms who completed weekly assessments of mood, serum gonadal steroid levels, and VMS. We hypothesized that estradiol variability, progesterone levels consistent with ovulation, and VMS frequency would independently predict higher levels of depressive symptoms in perimenopausal women.
Materials and Methods
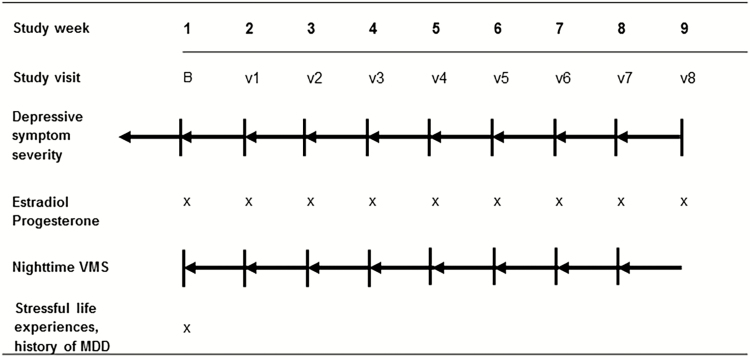
Women with mild-to-moderate depressive symptoms who were not using psychotropic or systemic hormonal medications were enrolled in an 8-week observational study (see Fig. 1). Throughout the study, participants completed assessments of depressive symptom severity, weekly gonadal steroid and gonadotropin assays, and a daily VMS diary. All subjects provided written informed consent for study procedures, which were approved by the Partners HealthCare Institutional Review Board and conducted at Massachusetts General Hospital and Brigham and Women’s Hospital.
Figure 1.
Study overview. Figure shows data sampling of 50 perimenopausal women during the 8-week study period. At every visit the severity of depressive symptoms (Montgomery-Asberg Depression Rating Scale [MADRS]) was assessed for the preceding 7 days and blood was drawn in order to determine estradiol and progesterone levels. Additionally, every participant reported the number of vasomotor symptoms (VMS) on a daily diary during the week preceding each visit.
Participants
Fifty-eight women from the ages of 35 to 56 years who were perimenopausal, defined by the Stages of Reproductive Aging Workshop (STRAW) criteria as being in the early menopause transition (menstrual cycle length varying > 7 days in consecutive cycles) or late menopause transition (> 60 days without menses but amenorrhea < 12 months in the prior 12 months), participated in the study (14). Participants were recruited from the community through local advertising and enrolled if they met eligibility criteria during the period of study accrual. They were required to have current mild-to-moderate depressive symptoms, defined as a score from 10 to 25 on the clinician-rated Montgomery-Åsberg Depression Rating Scale (MADRS) (15), and/or a score > 7.7 on the self-rated Kellner Symptom Questionnaire Anger-Hostility subscale (16). Exclusion criteria included menstrual irregularities attributable to other conditions (pregnancy, lactation, hyperprolactinemia, thyroid dysfunction, polycystic ovarian syndrome, hypothalamic amenorrhea), suicidal ideation, a MADRS score > 25 indicating severe depression, history of severe psychiatric illness (bipolar disorder, psychosis, psychiatric hospitalization or suicide attempts within the past 5 years), substance use disorder in the past year, sleep disorder diagnosis, and use of antidepressants, systemic hormones, or other centrally active medications known to alter mood or VMS. Sleep was monitored at home once using the ApneaLink sleep monitor (ResMed Corp., Poway, CA) to exclude women with obstructive sleep apnea.
Study overview
After obtaining written informed consent and confirming eligibility, including assessment of depressive symptom severity on the MADRS, blood was drawn to measure serum estradiol, progesterone, and FSH at baseline (see Fig. 1). Participants also completed a daily VMS diary to determine a weekly average of VMS frequency. These assessments were repeated thereafter at the weekly visits and during the week between visits for the remainder of the 8-week study duration. At baseline, current major and minor depression was assessed using the Patient Health Questionnaire-9 (PHQ9) (17) and lifetime history of depression was assessed by study psychiatrists using a clinical interview. In addition, recent stressful life events were self-reported using Life Experience Survey (LES) (18).
Severity of depressive symptoms
Severity of depressive symptoms was measured using the clinician-rated 10-item MADRS (range 0–60) as described previously (15). The MADRS has good internal consistency (Cronbach’s α = 0.82), test-retest reliability (correlation = 0.90), and validity, and is highly sensitive to changing symptom levels (19, 20). Scores of 7–19 and 20–30 (out of 60) are commonly used for identifying mild and moderate depression, respectively (21).
Hormonal assays
Serum levels of estradiol were measured by liquid chromatography-mass spectrometry (LC/MS) (Mayo Clinic, Rochester, NY), with 10 pg/ml as the lower limit of quantitation (22, 23). The inter-assay coefficient of variation (CV) for estradiol in this range is 8.6% (22). Serum progesterone was measured by chemiluminescence immunoassays (Abbott Architect ci8200 and Beckman Coulter, Fullerton, CA). The lower analytical sensitivities of these assays were 0.1 ng/ml and 0.08 ng/ml, respectively and both inter-assay CV were ≤10%. Serum FSH was measured by a chemiluminescence immunoassay (Abbott Architect ci8200). The analytical sensitivity was 0.05 IU/L and the inter-assay CV was estimated to be ≤10%.
Vasomotor symptoms
Subjective daily VMS were assessed using a self-report vasomotor symptoms diary, as previously described (24). VMS frequency was then categorized as daytime or nighttime events. These data were then averaged separately for the week between visits to obtain weekly averages of daytime, nighttime, and total VMS frequency, consistent with established procedures (24).
Statistical analyses
This analysis was restricted to the 50 (86.2%) of 58 eligible subjects who completed a minimum of 4 consecutive weekly visits and provided complete information on the key predictors and outcome. The enrolled sample of 50 did not differ from the 8 ineligible women on key demographic and clinical characteristics.
As the data involved multiple within-person observations, we used generalized estimating equation (GEE) models to examine the association of depressive symptom severity with estradiol variability, progesterone peaks, and VMS frequency, accounting for within-person correlation (25). Visits with estradiol levels below the detection limit (N = 83) were given values of 9 pg/ml. Estradiol was log transformed (ln-estradiol) due to its right-skewed distribution. The CV of ln-estradiol was calculated for each subject to integrate the extent to which serum estradiol levels varied within each subject over the 8-week period. A progesterone level > 6 ng/mL in weekly samples was used to define presumed ovulation or its absence in the analysis (26). VMS were analyzed as average daily frequency over the previous week. Therefore, analyses linked the time-invariant predictors CV of ln-estradiol and presence of ovulation and the time-variant predictor VMS with weekly depressive symptom severity (MADRS) scores describing the same week.
We used GEE models to determine the association of severity of depressive symptoms on the MADRS with each of the 3 main predictors (CV ln-estradiol, occurrence of ovulatory progesterone levels, VMS frequency) and then each of the following a priori covariates: age, race, body mass index (BMI), marital status, education, lifetime history of depression, and number of stressful life events. For each of the univariate models for which the predictor variable was statistically significant, multivariate models were developed by adjusting first for the other primary predictors and covariates examined that achieved the same significance threshold. Sensitivity analyses divided VMS according to daytime and nighttime frequency. In order to examine whether the associations were stronger for women who had experienced recent stressful life events or who had suffered from a depression previously, the interaction terms stressful life events × estradiol variability and stressful life events × presence of presumed ovulation, and lifetime depression × estradiol variability and lifetime depression × presence of presumed ovulation were added to the multivariate model.
All analyses were adjusted for the number of days between visits (average 1 week). Data were analyzed using STATA 15 (College Station, Texas), using a P value of 0.05 as the threshold for statistical significance.
Results
Participant characteristics at baseline
Baseline characteristics of the 50 subjects in this analysis are reported in Table 1. Briefly, the mean age of study participants was 48.4 ± 3.9 years, mean BMI was 27.2 ± 6.7 kg/m2, and the majority were either Caucasian (56%) or African American (36%). Menopause status was evenly divided between those in the early and late menopause transition. The median FSH and estradiol at baseline were 24.8 (interquartile range [IQR], 6.9–54.4) IU/L and 83.0 (IQR, 41.0–148.0) pg/ml, respectively. VMS were reported by 87.5% of participants. The mean baseline MADRS score was 15.5 ± 5.3, reflecting mild-to-moderate depressive symptom burden. Depressive symptoms were subthreshold for a current major depressive episode in the vast majority of participants (87.3%). One-third of participants reported previously experiencing an episode of depression. The current episode of mood symptoms developed concurrently or after onset of perimenopause in 70% of participants and concurrent with or after VMS in 56% of participants.
Table 1.
Baseline Characteristics of Perimenopausal Women With Mild-to-Moderate Depressive Symptoms (n = 50)
| Baseline | Follow-up Visits (including baseline) | |
|---|---|---|
| Mean ± SD, median (25th–75th centile), or N (%) | Mean ± SD, median (25th–75th centile), or N (%) | |
| Demographics | ||
| Age, years | 48.4 ± 3.9 | - |
| Race, n (%) | ||
| Caucasian | 28 (56.0) | - |
| African American | 18 (36.0) | - |
| Other | 4 (8.0) | - |
| Married, n (%) | 25 (50.0) | - |
| College graduate, n (%) | 28 (56.0) | - |
| Body-mass index, kg/m2 | 27.2 ± 6.7 | - |
| Menstrual Cycle Characteristics | ||
| Menopause status, n (%) | ||
| Early menopause transition | 25 (50.0) | - |
| Late menopause transition | 25 (50.0) | - |
| Duration of irregular menses, months | 21.2 ± 22.5 | - |
| Number of menstrual bleeds in past year | 8.2 ± 4.0 | - |
| Days since last menstrual bleed | 28.5 (11–73) | - |
| No. participants with menstrual bleeding or spotting, n (%) | - | 36 (72.0) |
| Gonadal Hormone Profile | ||
| FSH, IU/L | 24.8 (6.9–54.4)a | 22.4 (6.9–52.5) |
| Estradiol, pg/ml | 83.0 (41.0–148.0)b | 77.0 (30.0–144.0)c |
| No. participants with an ovulatory progesterone level (>6 ng/dL) on at least 1 occasion | - | 23 (51.1)c |
| Vasomotor Symptom Characteristics | ||
| No. participants reporting VMS, n (%) | 42 (87.5)d | 44 (89.8)e |
| VMS Frequency | ||
| 24 hours | 2.5 (1.4–4.7)d | 2.1 (0.9–4.6)e |
| Night | 1.3 (0.7–2.1)f | 1.2 (0.6–2.1)e |
| Day | 1.5 (1.0–3.0)g | 1.4 (0.6–3.1)e |
| Depressive Symptoms | ||
| MADRS scores | 15.5 ± 5.3 | 10.8 ± 6.5 |
| No. participants with current MDE, n (%) | 6 (12.7)h | - |
| No. participants with lifetime history of MDD, n (%) | 16 (32.0) | - |
| No. stressful life events in past 6 months, n (%) | 44 (88.0) | - |
Continuous measures are reported as mean ± standard deviation or in case of nonnormal distribution as median and interquartile range (IQR). Categorical are presented as number of events with percent. Early and late menopause transition were defined based on menstrual markers consistent with the STRAW+10 criteria.
Abbreviations: FSH, follicle stimulating hormone; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder. MDE, major depressive episode; VMS, vasomotor symptoms;
a n = 49 women with baseline serum
b n = 39 women with detectable estradiol levels
c n = 45 women who had at least one detectable estradiol level
d n = 48 women who completed baseline VMS diary
e n = 49 women who completed at least one VMS diary
f n = 39 women whose VMS were distinguishable as occurring during the night
g n = 37 women whose VMS were distinguishable as occurring during the day
h n = 47 women with available Patient Health Questionnaire (PHQ-9) data
Course of depressive symptoms during the weekly study visits
Across the study, MADRS scores remained in the mild-to-moderate depressive range at most assessments (10.8 ± 6.5), but there was variability in depressive symptom course between and within subjects. The majority of women showed some degree of variability in depressive symptom levels during their study participation. MADRS scores varied across the study by 12.7 ± 4.3 (range, 3–26), reflecting a wide range of within-person variability in depressive symptom levels during follow-up.
Changes in reproductive hormones, menstrual patterns, and vasomotor symptoms during follow-up
As expected for women in the menopause transition, there was substantial between-subject variability in menstrual patterns, estradiol, and progesterone profiles during follow-up. The majority (72.0%) had some menstrual bleeding or spotting, while a quarter (28.0%) were amenorrheic. The CV for estradiol ranged from 0% to 36%. Five (10%) women had estradiol levels that were consistently nondetectable throughout the study (< 10 pg/ml). Of the 45 (90%) women whose estradiol exceeded the detectable threshold at least once, estradiol levels were detectable at the majority (93%) of their study visits. Among those with detectable estradiol levels, 51.1% had at least one progesterone level consistent with ovulation. VMS were near universal, with a median of 2.1 (IQR, 0.9–4.6) VMS/24 hours and only 10.2% of women reporting no VMS during follow-up.
Predictors of depressive symptom severity during follow-up
Associations of depressive symptom severity with estradiol variability, presence of presumed ovulation, and VMS frequency are shown in Table 2. Univariate models revealed that greater variability in serum estradiol levels was associated with higher levels of depressive symptoms (β = 0.11; 95% confidence interval [CI], [0.02-0.21]; P = 0.02). Univariate models also revealed that presence of at least one progesterone level > 6 ng/dL was associated with lower depressive symptom levels (β = −2.62 [−4.52 to −0.71]; P = 0.007). VMS frequency was not associated with depression symptom severity (β = 0.05 [−0.24 to 0.34]; P = 0.75). Results were unchanged when VMS were analyzed separately by nighttime and daytime (data not shown). Other predictors of worse depressive symptoms in univariate models were higher BMI (β = 0.23 [0.08-0.37]; P = 0.002), history of depression (β = 3.77 [1.97-5.57]; P < 0.001), and more stressful life events (β = 0.31 [0.01-0.62]; P = 0.04), but not the subset of stressful life events that were severe, nor age, race, marital status, or education (all P > 0.10).
Table 2.
Associations of Estradiol Variability, Ovulatory Progesterone, and Vasomotor Symptoms With Severity of Depressive Symptoms in 50 Perimenopausal Women With Mild-to-Moderate Depressive Symptoms
| Univariate Models | Multivariate Model | |||
|---|---|---|---|---|
| B (95% CI) | P value | B (95% CI) | P value | |
| Coefficient of variability of ln-E2 | 0.11 (0.02, 0.21) | 0.02 | 0.11 (0.04, 0.18) | 0.002 |
| Presence of ovulatory progesterone | −2.62 (−4.52, −0.71) | 0.007 | −2.68 (−4.10, −1.27) | <0.001 |
| VMS frequency | 0.05 (−0.24, 0.33) | 0.75 | ||
| Demographic covariates | ||||
| Age | 0.18 (−0.08, 0.43) | 0.17 | ||
| Race (Caucasian yes/no) | 0.25 (−1.74, 2.24) | 0.81 | ||
| Married (yes/no) | −0.46 (−2.44, 1.52) | 0.65 | ||
| College graduate (yes/no) | −1.57 (−3.54, 0.40) | 0.12 | ||
| BMI, kg/m2 | 0.23 (0.08, 0.37) | 0.002 | 0.22 (0.12, 0.32) | <0.001 |
| Lifecourse experience | ||||
| No. recent stressful life events | 0.31 (0.01, 0.62) | 0.04 | 0.30 (0.08, 0.51) | 0.007 |
| Lifetime history of depression | 3.77 (1.97, 5.57) | <0.001 | 3.38 (1.93, 4.84) | <0.001 |
Estimates are B-coefficients determined by generalized estimating equation analyses. All models were adjusted for the number of days between study visits. Severity of depressive symptoms was assessed by the Montgomery-Asberg Depression Rating Scale (MADRS).
Abbreviations: ln-E2, natural log of estradiol; BMI, body mass index; VMS, vasomotor symptoms.
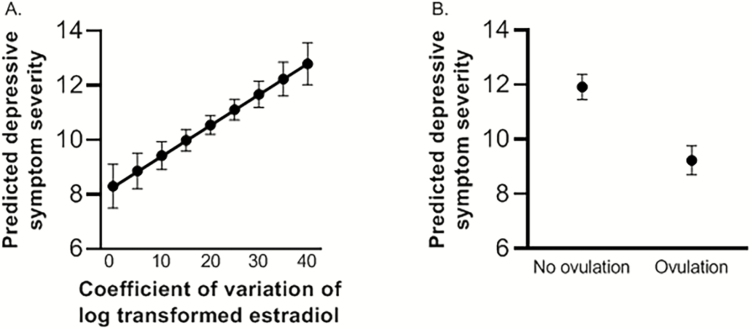
A final multivariate model was built adjusting for BMI, history of depression, and stressful life events, covariates that were significantly associated with MADRS scores in univariate models. The association of the severity of depressive symptoms with the primary predictors of interest, estradiol variability and ovulatory progesterone levels, remained significant (adjusted β = 0.11 [0.04-0.18]; P = 0.002, and adjusted β = −2.68 [−4.10 to −1.27]; P < 0.001, respectively, Table 2). This translates to an increase of 1.1 on the MADRS depression score for each 10% increase in the relative variability of estradiol and a 2.7-point decrease of the MADRS depression score for those who had at least one progesterone level consistent with ovulation relative to those who did not. In the same model, all covariates (higher BMI, history of depression, stressful life events) remained independent predictors of more severe depressive symptoms (P < 0.01). Fig. 2A and 2B present the marginal means of depressive symptom severity with estradiol variability and by the presence of progesterone levels consistent with ovulation, respectively. When the adjusted analysis was re-run with the number of presumed ovulatory cycles as a 3-category variable (1 or 2+ vs 0), the difference in depressive symptom severity compared with the reference group was significant for both the group that had only 1 (P < 0.001) and for those who had 2 or more ovulatory cycles (P = 0.013). However, there was no “dose response” relationship; those with multiple ovulatory cycles did not have lower levels of depressive symptoms than those with 1 cycle (P = 0.36).
Figure 2.
Predicted depressive symptom severity by A) variability in serum levels of estradiol and by B) detection or not of serum progesterone level indicating recent ovulation during the 8-week study period
The association of estradiol variability and ovulatory progesterone levels with depressive symptom severity did not vary by the presence of recent stressful life events nor by a lifetime diagnosis of depression (P > 0.10 for all interaction terms).
Discussion
Results of this study show that depression symptom severity during the perimenopause is linked to a greater degree of concurrent estradiol variability and the absence of progesterone at levels suggesting recent ovulation, but not with the presence of VMS. These findings in women with mild-to-moderate depressive symptoms remain after accounting for other factors known to be associated with depression symptom severity during the perimenopause, including a prior history of major depression, the presence of recent stressful life events, and a higher BMI. Our results therefore suggest that perimenopausal mood instability is driven in part by the underlying dysregulation of both estradiol and progesterone that is characteristic of the menopause transition.
Our analysis focused uniquely on concurrent changes in hormonal profiles in women actively experiencing depressive symptoms. To achieve this, we used a repeated-measures approach to capture weekly measures of mood, serum gonadal steroid levels, and VMS over an 8-week time period. This is in contrast to epidemiologic approaches that have examined reproductive hormone levels in women as predictors of subsequent depressive symptoms and mood disorders that emerged months or years later (4, 7, 10, 28). Such approaches contrast with our underlying hypothesis that depressive symptoms are caused by concurrent exposure of neural circuity linked with mood to highly variable perimenopausal hormone dynamics. During the menopause transition, excursions in estradiol and the occurrence of ovulation vary widely both within women over time and between women, even among those with relatively comparable menstrual patterns (29, 30). This marked variability may explain why depressive symptoms can ebb and flow over time during the perimenopause and why some women are susceptible to mood disturbance at some, but not all, times over the duration of this transition.
Results of our study highlight the importance and independent roles of both estradiol variability and ovulatory levels of progesterone in perimenopausal mood regulation. Numerous studies have focused on serum estradiol levels in relation to menopause-related depression (4, 10, 27, 28, 31, 32), but associations with serum progesterone have not been explored. Thus, we observed for the first time that lower levels of depressive symptoms occur surrounding an ovulatory cycle (33). It is notable that women are at increased risk for depressive symptoms during both the early menopause transition—when ovulation is more common, as well as during the late menopause transition—when prolonged amenorrhea and anovulation occur more commonly (1). Therefore, while estradiol variability is more common in the early menopause transition and anovulatory cycles are more common in the late menopause transition, both occur in either phase of the transition, as does the increased prevalence of depressive symptoms. Our population was evenly divided between early and late menopause, which gave us the opportunity to examine associations across the menopause transition and a wide range of hormone profiles. Of the 90% of women who had at least one detectable estradiol level, half had at least one progesterone level consistent with ovulation. Therefore, regardless of whether women were in the early or late menopause transition or whether estradiol levels fluctuated more or less markedly, depressive symptoms were less severe in our population across the 8-week period of observation when progesterone levels indicated recent ovulation.
Consistent with our observation of an inverse relationship between depressive symptoms and concurrent exposure to progesterone is the recent finding of an antidepressive effect of the progesterone-derived neurosteroid allopregnanolone for treatment of postpartum depression (34), leading to the approval of allopregnanolone by the FDA for postpartum depression. As a treatment for another reproductive hormone-associated mood disturbance (35), allopregnanolone’s efficacy for postpartum depression challenges earlier presumptions that progestins adversely affect mood. Allopregnanolone may act as a neurosteroid to mediate the protective effect of peripheral progesterone on mood through direct inhibition of γ-aminobutyric acid (GABA) receptors. However, the relationship of ovarian production of progesterone to centrally active allopregnanolone and GABA inhibition remains to be determined in women with hormonally sensitive mood disturbance. Collectively, these data illustrate a beneficial effect of progesterone and its metabolites on mood in women with hormonally linked mood disturbance.
We observed higher levels of depressive symptoms in association with more marked concurrent variability in serum estradiol. Levels of estradiol are known to be more variable during the early menopause transition than during the follicular phase of premenopausal menstrual cycles (13, 36, 37). Variability in estradiol levels during the perimenopause may adversely impact mood through mediation of serotonergic and dopaminergic systems within the central nervous system since estradiol receptors are widely dispersed across these neuronal networks involved in mood-regulating neurotransmission (38). Alternatively, because estradiol increases allopregnanolone (13), marked variability of estradiol during perimenopause may cause fluctuations in allopregnanolone, resulting in failure of the GABAA receptor to regulate overall GABA-ergic tone. Dysregulation in GABA-ergic tone may also lead to perturbation of the hypothalamic-pituitary-adrenal axis, potentially conferring vulnerability to depression (13).
Our finding that variability in estradiol is associated with depressed mood is consistent with those of some (4, 10, 28), but not all (27, 31, 32), studies examining associations of serum estradiol with depressive symptoms. In contrast to our study, others did not examine estradiol changes in relation to concurrent mood, but in relation to subsequent emergence of depression (4, 10, 27, 28). Differences between our and other studies also include the frequency of estradiol sampling. Our findings using weekly assessments are generally consistent with others in which estradiol was measured more frequently (weekly (28), monthly (4, 39), or every 6 months (10)) rather than less (every 8–12 months (27, 31, 32)) frequently. Given the changes in estradiol across the menopause transition, comparison between studies examining associations between estradiol variability with adverse mood that utilize different estradiol sampling frequencies must be interpreted cautiously. Our study population also differs from previous studies that were restricted to women with clinically relevant mood disorders (4, 27, 28, 39), as the vast majority of participants in the current study had less severe depressive symptoms that did not constitute a major depressive episode. This is important because factors associated with mood disturbance during the perimenopause differ between those who have major depressive episodes and those with less severe depressive symptoms (5).
In contrast with previous studies, we did not find stronger associations of estradiol variability (or postovulatory levels of progesterone) with depressive symptoms in women who recently experienced stressful life events (10) nor in those who had a history of major depression (28). Results of our analysis therefore suggest that previous exposure to these independent risk factors for depression does not sensitize women to greater depressive symptom burden when estradiol varies nor when progesterone levels are low.
VMS, especially when nocturnal, are associated with risk for subthreshold depressive symptoms (1, 8, 9), but not with clinically relevant depressive disorders in perimenopausal women (1, 9, 39). We did not observe an association between VMS and depressive symptom severity. Our findings are consistent with previous studies in perimenopausal women who had variable estradiol levels (39), but not with other studies in which hypo-estrogenism was persistent (8). It is possible that our sample might be too homogeneous to detect an association with VMS as almost 90% of participants reported VMS and only 5% had sustained hypoestrogenism. Alternatively, the impact of VMS on mood may only be evident in perimenopausal women during a period of hypo-estrogenism, further contributing to the complex interrelationships among gonadal steroids, VMS and mood across the perimenopause.
Our study has important strengths, particularly the weekly, concurrent assessment of depressive symptoms, gonadal steroids, and VMS in a population whose mental health and menopause profile was well-characterized. This approach informs how co-varying menopause-specific factors contribute to concurrent depressive symptom burden. To date, the majority of studies examining hormonal predictors of depressive symptoms have assessed mood, reproductive hormones, and VMS as infrequently as every 8–12 months (27, 31, 32). While these studies powerfully characterize the course and predictors of depressive symptoms over a prolonged period of time, they do not provide critical insight into the concurrent relationship between real-time reproductive hormone dynamics and depressive symptom levels during this dynamic phase of a woman’s life, as our design achieves. Another strength is that estradiol was assayed using the gold-standard LC/MS, which has improved reliability over immunoassays in the low estradiol range (40).
Limitations in our study include inherent limitations of any observational study; namely, data interpretation is confined to associations rather than causal relationships. While frequent, our assessments were weekly, reducing our ability to capture all occurring ovulatory cycles based on progesterone levels. However this would have biased any association toward the null, thereby limiting our ability to detect an association. Because all analyses were adjusted for time between assessments, any between- and within-participant variation due to variable time intervals was accounted for in the analysis.
In summary, we observed that a more optimal concurrent mood state is present when cycles are ovulatory and have less variability in estradiol; conversely, greater depressive symptom burden is present during anovulatory periods when progesterone is low and estradiol is especially variable. Therefore, regardless of whether women are in the early or late menopause transition, the highly variable and unpredictable reproductive hormone dynamics during the perimenopause explain, at least in part, the variability in depressive symptoms even within an individual.
Acknowledgments
Financial Support: Supported by National Institute of Mental Health R01MH082922 (HJ).
Glossary
Abbreviations
- BMI
body mass index
- CV
coefficient of variation
- FSH
follicle-stimulating hormone
- GABA
γ-aminobutyric acid
- IQR
interquartile range
- LH
luteinizing hormone
- MADRS
Montgomery-Åsberg Depression Rating Scale
- VMS
vasomotor symptoms
Additional Information
Disclosure Summary: AW, JC, SC, AW, GA, KS, and JH have nothing to declare. HJ serves as a consultant/advisory board member for NeRRe/KaNDy, Sojournix, and Eisai; receives research support from NIH, Merck, NeRRe/KaNDy, Pfizer, and QUE Oncology; and her spouse is an employee of Merck Research Lab, consults for and holds equity in Arsenal Biosciences, and holds equity in Tango. SK is employed by TYME, Inc. LC has received research support from Alkermes Biopharmaceuticals, Forest/Actavis Pharmaceuticals, Otsuka Pharmaceuticals, Sunovion Pharmaceuticals, Inc., Teva Pharmaceuticals, Brain & Behavior Research Foundation, JayMac Pharmaceuticals, NIH, and SAGE Therapeutics. MF works with the Massachusetts General Hospital National Pregnancy Registry which is currently sponsored by Teva, Alkermes, Inc., Otsuka America Pharmaceutical, Inc., Forest/Actavis, and Sunovion Pharmaceuticals, Inc.; works with the Massachusetts General Hospital Clinical Trials Network and Institute which has received research funding from multiple pharmaceutical companies and NIMH; has spoken to the US Psychiatric Congress and Medscape; serves on the Janssen Independent Data Safety and Monitoring Committee and on Otsuka, Alkermes, Janssen, Sage, and Sunovion advisory boards; and is involved in investigator-initiated trials/research for Takeda, JayMac, and SAGE.
Data Availability: The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Maki PM, Kornstein SG, Joffe H, et al. Guidelines for the evaluation and treatment of perimenopausal depression: summary and recommendations. J Womens Health (Larchmt). 2019;28(2):117–134. [DOI] [PubMed] [Google Scholar]
- 2. Rodríguez MR, Nuevo R, Chatterji S, Ayuso-Mateos JL. Definitions and factors associated with subthreshold depressive conditions: a systematic review. BMC Psychiatry. 2012;12(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cuijpers P, van Straten A, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta-analytic review of psychological interventions. Am J Psychiatry. 2008;165(10):1272–1280. [DOI] [PubMed] [Google Scholar]
- 4. Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. [DOI] [PubMed] [Google Scholar]
- 5. Bromberger JT, Kravitz HM, Youk A, Schott LL, Joffe H. Patterns of depressive disorders across 13 years and their determinants among midlife women: SWAN mental health study. J Affect Disord. 2016;206:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4(3):214–220. [DOI] [PubMed] [Google Scholar]
- 7. Joffe H, Hall JE, Soares CN, Hennen J, Reilly CJ, Carlson K, Cohen LS. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9(6):392–398. [DOI] [PubMed] [Google Scholar]
- 8. Joffe H, Crawford SL, Freeman MP, et al. Independent contributions of nocturnal hot flashes and sleep disturbance to depression in estrogen-deprived women. J Clin Endocrinol Metab. 2016;101(10):3847–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Worsley R, Bell R, Kulkarni J, Davis SR. The association between vasomotor symptoms and depression during perimenopause: a systematic review. Maturitas. 2014;77(2):111–117. [DOI] [PubMed] [Google Scholar]
- 10. Gordon JL, Rubinow DR, Thurston RC, Paulson J, Schmidt PJ, Girdler SS. Cardiovascular, hemodynamic, neuroendocrine, and inflammatory markers in women with and without vasomotor symptoms. Menopause. 2016;23(11):1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28(2):139–168. [DOI] [PubMed] [Google Scholar]
- 12. Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44(9):798–811. [DOI] [PubMed] [Google Scholar]
- 13. Gordon JL, Girdler SS, Meltzer-Brody SE, et al. Response to Eskola et al. Am J Psychiatry. 2015;172(8):797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harlow SD, Gass M, Hall JE, et al. ; STRAW + 10 Collaborative Group Executive summary of the stages of reproductive aging workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab. 2012;97(4):1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 16. Kellner R. A symptom questionnaire. J Clin Psychiatry. 1987;48(7):268. [PubMed] [Google Scholar]
- 17. Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007;22(11):1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46(5):932–946. [DOI] [PubMed] [Google Scholar]
- 19. Mundt JC, Katzelnick DJ, Kennedy SH, Eisfeld BS, Bouffard BB, Greist JH. Validation of an IVRS version of the MADRS. J Psychiatr Res. 2006;40(3):243–246. [DOI] [PubMed] [Google Scholar]
- 20. Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The montgomery-asberg depression scale: reliability and validity. Acta Psychiatr Scand. 1986;73(5):544–548. [DOI] [PubMed] [Google Scholar]
- 21. Herrmann N, Black SE, Lawrence J, Szekely C, Szalai JP. The sunnybrook stroke study: a prospective study of depressive symptoms and functional outcome. Stroke. 1998;29(3):618–624. [DOI] [PubMed] [Google Scholar]
- 22. Nelson RE, Grebe SK, OKane DJ, Singh RJ. Liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of estradiol and estrone in human plasma. Clin Chem. 2004;50(2):373–384. [DOI] [PubMed] [Google Scholar]
- 23. Siekmann L. Determination of oestradiol-17 beta in human serum by isotope dilution-mass spectrometry. Definitive methods in clinical chemistry, II. J Clin Chem Clin Biochem. 1984;22(8):551–557. [DOI] [PubMed] [Google Scholar]
- 24. Newton KM, Carpenter JS, Guthrie KA, et al. Methods for the design of vasomotor symptom trials: the menopausal strategies: finding lasting answers to symptoms and health network. Menopause (New York, NY). 2014;21(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzmaurice GM, Laird NM, Ware JH.. Applied Longitudinal Analysis. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2011. [Google Scholar]
- 26. Leiva R, Bouchard T, Boehringer H, Abulla S, Ecochard R. Random serum progesterone threshold to confirm ovulation. Steroids. 2015;101:125–129. [DOI] [PubMed] [Google Scholar]
- 27. Bromberger JT, Kravitz HM, Chang YF, Cyranowski JM, Brown C, Matthews KA. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gordon JL, Eisenlohr-Moul TA, Rubinow DR, Schrubbe L, Girdler SS. Naturally occurring changes in estradiol concentrations in the menopause transition predict morning cortisol and negative mood in perimenopausal depression. Clinical Psychological Science. 2016;4(5):919–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Santoro N, Crawford SL, El Khoudary SR, et al. Menstrual cycle hormone changes in women traversing menopause: Study of Women’s Health Across the Nation. J Clin Endocrinol Metab. 2017;102(7):2218–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O’Connor KA, Ferrell R, Brindle E, et al. Progesterone and ovulation across stages of the transition to menopause. Menopause. 2009;16(6):1178–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Avis NE, Crawford S, Stellato R, Longcope C. Longitudinal study of hormone levels and depression among women transitioning through menopause. Climacteric. 2001;4(3):243–249. [PubMed] [Google Scholar]
- 32. Woods FN, Smith-Dijulio BK, Percival YD, Tao SE, Mariella SA, Mitchell SE. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15(2):223–232. [DOI] [PubMed] [Google Scholar]
- 33. Yen & Jaffe’s Reproductive Endocrinology : Physiology, Pathophysiology, and Clinical Management. Eighth edition Philadelphia, PA: Elsevier; 2018. [Google Scholar]
- 34. Meltzer-Brody S, Colquhoun H, Riesenberg R, et al. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392(10152):1058–1070. [DOI] [PubMed] [Google Scholar]
- 35. Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: conceptualizing models and moving toward etiology. Harv Rev Psychiatry. 2009;17(2):72–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hale GE, Robertson DM, Burger HG. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol. 2014;142:121–131. [DOI] [PubMed] [Google Scholar]
- 37. Butler L, Santoro N. The reproductive endocrinology of the menopausal transition. Steroids. 2011;76(7):627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barth C, Villringer A, Sacher J. Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front Neurosci. 2015;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Joffe H, Petrillo LF, Koukopoulos A, et al. Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab. 2011;96(7):E1044–E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Demers LM. Testosterone and estradiol assays: current and future trends. Steroids. 2008;73(13):1333–1338. [DOI] [PubMed] [Google Scholar]