Abstract
Although the effect of beetroot supplementation on exercise performance has been widely demonstrated to improve the performance of cyclists, runners, and swimmers, its effect on combat sports remains inconclusive. The present study assessed the effect of beetroot-based gel (BG) supplementation on maximal voluntary contraction (MVC), exercise time until fatigue (ETF), muscle O2 saturation (SmO2), and blood volume (tHb) in response to handgrip isotonic exercise (HIE) in recreational combat sport athletes. In a randomized, crossover, double-blind study, 14 combat sports athletes performed three sets of HIE (at 40% MVC) until fatigue after BG or nitrate-depleted gel (PLA) supplementation, in which forearm SmO2 and tHb were continuously monitored using near-infrared spectroscopy. MVC was evaluated at baseline and 20 min after HIE. MVC values were analysed as the change from baseline values (ΔMVC). There was a significant increase accompanied by a large effect size in ΔMVC (p = 0.036, d = 0.94) after HIE in the BG condition compared to PLA. However, there were no changes in SmO2 parameters (p> 0.05), tHb (p> 0.05) or ETF (p = 0.161) throughout the three sets of HIE. Additionally, a trivial to small effect size was observed in near-infrared spectroscopy (NIRS) parameters and ETF (d = ≤ 0.2 to 0.5). Therefore, a single dose of beetroot gel supplementation may be considered as a good nutritional strategy to improve strength recovery in combat sports athletes.
Keywords: Muscle isometric strength, Sport nutrition, Combat sport, Muscle oxygenation, Handgrip exercise
INTRODUCTION
Combat sports such as jiu-jitsu, judo, and wrestling require high production of forearm muscle strength, since the main technique used for adversary immobilization during combat is grappling [1, 2]. Previous studies have assessed the isometric maximal handgrip strength before and after bouts of combat sports, in which a significant decline in forearm muscle strength in judo and jiu-jitsu athletes was found [1, 3]. Since the reduced ability to produce force in response to exercise may be defined as fatigue [4], the decline in handgrip strength during these sports activities suggests the high prevalence of fatigue in the forearm muscle of the athletes [3].
Increased blood lactate concentration after handgrip exercise has been linked to reduced grip strength in combat sport athletes [1]. Increased blood lactate and consequent muscle acidosis in exercising muscles impair metabolic pathways (adenosine triphosphate (re)synthesis rate), leading to fatigue [5]. Nitric oxide (NO) is a well-known molecule responsible for playing a crucial role in modulating vascular tonus and metabolic control of skeletal muscle during exercise, contributing to attenuation of muscle fatigue [6, 7]. Beetroot-based supplements have been widely studied due to their high nitrate content that can be converted to nitrite by nitrate-reducing bacteria present in the oral cavity [8]. Reduction of nitrite into NO can occur spontaneously in favourable conditions (low pH and hypoxia), such as during muscle contraction in exercise [9]. Therefore, previous studies have investigated the effect of dietary nitrate supplementation on exercise performance due to its potential effect on increasing NO bioavailability [10–15].
Larsen et al. [10] reported a significant reduction of O2 cost in healthy male subjects during submaximal exercise after 3 days of dietary nitrate supplementation. This effect was not associated with increases in blood lactate concentration, thereby indicating that the energy production had become more efficient. The authors suggested that these effects might have been attributed to the improvement in mitochondrial function (reduced proton leakage over the inner mitochondrial membrane) after dietary nitrate ingestion. Furthermore, several studies have reported improvements in muscle force [11, 12], muscle energy economy during exercise [10, 13] and exercise tolerance [14, 15] in non-athlete subjects (lower physical levels). Although the ergogenic effect of beetroot consumption on exercise performance has been confirmed in non-athlete subjects [16], the current pool of data reporting the effect of beetroot supplementation on exercise performance in athletes (high physical levels) is not enough to delineate the optimum nutritional strategies for this population.
While previous studies have failed to demonstrate a positive effect of dietary nitrate in trained athletes at different sport modalities such as cycling, kayaking, and swimming [17–21], we have recently shown that Jiu-jitsu trained individuals improved grip isometric strength after 8 days of beetroot-based supplementation [22]. Given the importance of improving grip strength for combat sports athletes and the divergent findings for beetroot supplementation in trained individuals, the present study investigates whether a single dose of beetroot-based gel ingestion improves muscle strength and muscle O2 saturation in combat sport trained athletes. Instead of multi-day, beetroot-based supplementation, a single dose may be a more practical and convenient nutritional strategy for combat sport athletes, since such individuals already ingest a high amount of food daily. Thus, it was hypothesized that a single dose of beetroot-based gel supplement would improve muscle strength and muscle O2 saturation in combat sport trained athletes.
MATERIALS AND METHODS
Participants
Fourteen adult male Brazilian and recreational combat sports athletes (age: 29.92 ± 8.51 years; height: 1.74 ± 0.05 cm; body weight: 79.72 ± 10.09 kg; body mass index: 26.39 ± 2.70) were recruited through lecture and by flyers at the fighters’ gyms. Although only recreationally trained, all combat athletes included in the present study had at least 5 years of frequent combat sports training experience (at least five times per week), totalling ~15 hours or more of training per week. Of the fourteen combat sport athletes, one was graded as brown belt, three were purple belts and ten were black belts. Athletes were excluded if they had any disease, such as diabetes mellitus, kidney and lung failure, cardiovascular disease and/or skeletal muscle, tendon and bone injuries which compromised handgrip exercise, and usage of antioxidants, amino acids, pre-working supplementation, or androgenic anabolic steroids (≤ 6 months prior to analyses). All participants were fully informed of the nature and purpose of the investigation and provided written consent to participate. All experimental procedures were performed in accordance with the ethical standards of the Declaration of Helsinki and approved by the institutional ethics committee.
Experimental design
The study was conducted in a crossover, randomized, placebo-controlled, and double-blind way. All individuals came to the laboratory on three occasions. The first visit was used to explain the experimental procedures, to take anthropometric measurements, to familiarize subjects with rhythmic handgrip exercise, and to randomize subjects to either 100 g of beetroot-based nutritional gel (BG) or a control nitrate-depleted gel (PLA). The BG (12.2 ± 0.2 mmol of nitrate) and PLA (0.2 ± 0.02 mmol of nitrate) were prepared according to Oliveira et al. [23] and Morgado et al. [24]. On the second and third visit, maximal forearm muscle isometric strength was measured (T0) followed by a single dose of BG or PLA supplementation. Approximately 120 minutes (T120) after the nutritional supplementation, three sets of rhythmic handgrip exercise were performed. Based on previous studies that have shown bioconversion of dietary nitrate into nitrite at approximately 120–150 min after beetroot supplementation, exercise performance was assessed at 120 minutes after beetroot gel ingestion [11, 22, 25, 26]. The near-infrared spectroscopy (NIRS) device was used to assess the forearm muscle O2 saturation (SmO2) during exercise and exercise recovery. Maximal voluntary contraction (MVC) of the forearm muscles was assessed before (baseline) and at 20 min after handgrip exercise in order to measure the muscle strength recovery. The three visits were held between 07:00 and 12:00 a.m. For each participant, one week elapsed between the nutritional interventions (visits). Subjects were instructed to fast for at least 8 hours before each visit and not use any antibacterial mouthwash for the duration of the experimental period. On the day before the study, participants were instructed to restrict nitrate- and nitrite-rich foods. A list containing the food groups and foods to be avoided was distributed.
Forearm muscle isometric strength
The maximal voluntary contraction was performed as described in the previous study of our laboratory [22]. Briefly, MVC of the forearm muscles was assessed through three maximal repetitions interposed by 30 s of recovery between each MVC and the highest value was considered. When the highest MVC value was not reached by the third attempt, an additional MVC was performed until isometric strength reached a plateau or decreased. MVC was adjusted according to each subject’s body weight and the MVC values were expressed by the change from baseline values (ΔMVC).
Exercise protocol
The handgrip exercise protocol was performed according to previous studies of our laboratory [22]. Briefly, all participants were in a supine position and three sets of handgrip exercise were performed at 40% of the MVC interposed by 60 s of exercise recovery. The concentric and eccentric muscle contraction phases were controlled throughout the handgrip exercise by using a metronome sound at 0.5 s for each muscle contraction phase (60 contractions per minute). The exercise began 120 min after the BG or PLA supplementation and all participants performed the handgrip exercise until fatigue, defined as the participant’s inability to maintain the exercise rate (as contraction rhythms mismatch with metronome sound) and/or range of motion was reduced for more than 5 consecutive contractions. The exercise time until fatigue (ETF) was recorded in order to assess exercise tolerance.
Muscle oxygenation and blood volume changes
Muscle O2 saturation was assessed with a NIRS device (PortaMon, Artinis Medical Systems, Elst, Netherlands) placed on the anteromedial aspect of the dominant forearm, exactly 2 cm distal of the medial epicondyle of the humerus. The NIRS probe was wrapped around the forearm muscle (flexor muscles) by using an elastic bandage in order to minimize movement during handgrip exercise. The NIRS system was connected to a personal computer via Bluetooth for data acquisition at a frequency of 10 Hz, analogue-to-digital conversion. Subsequent analysis of the raw data (i.e. no filter was used) was conducted using native software (Oxysoft, version 2.1.6; Artinis Medical Systems, Elst, Netherlands). The following NIRS parameters were taken into account: Baseline SmO2 (SmO2base) was calculated as the average of SmO2 values during 30 s immediately before the onset of the exercise. Minimum SmO2 (SmO2min) was determined as the minimum SmO2 values reached during exercise (Ex) following 5, 10, 20, 30, and 60 s of exercise recovery. SmO2 desaturation rate (SmO2slope_1) was calculated as the downslope of SmO2 during the exercise. SmO2 resaturation rate of the SmO2 (SmO2slope_2) was calculated as the upslope of SmO2 during exercise recovery. Averages for SmO2 during exercise (SmO2Ex) and exercise recovery (SmO2Rec) were recorded. Averages for total haemoglobin (tHb) during exercise (tHbEx) and the exercise recovery period (tHbRec) were recorded.
Statistical analysis
The normality, homogeneity of variances and sphericity of the data were examined with the Shapiro-Wilk, Levene and Mauchly tests, respectively. Paired t-test was performed to identify differences in ΔMVC between BG and PLA supplementation. A two-way ANOVA with repeated measurements was performed to identify differences in MVC, SmO2 parameters, and ETF between BG and PLA supplementation. The magnitude (effect size) of the nutritional intervention was calculated by Cohen’s d, with values < 0.2 considered to indicate a trivial effect, 0.2 – < 0.5 a small effect, 0.5– < 0.8 a moderate effect, and ≥ 0.8 a large effect. Statistical significance was set at the 0.05 level of confidence. All analyses were performed using a commercially available statistical package (IBM SPSS Statistics version 22 for Mac, Armonk, N.Y., USA), and the results were expressed as means ± SD.
RESULTS
At the end of the study, the participants were asked if they were able to recognize the beetroot-based gel. Most of the participants were unsure about which supplement they had been taking (i.e. nitrate-rich beetroot-based gel or placebo intervention). Although some of the participants expressed certainty that they ingested the nitrate-rich beetroot-based gel, it had in fact been the placebo.
Forearm muscle performance
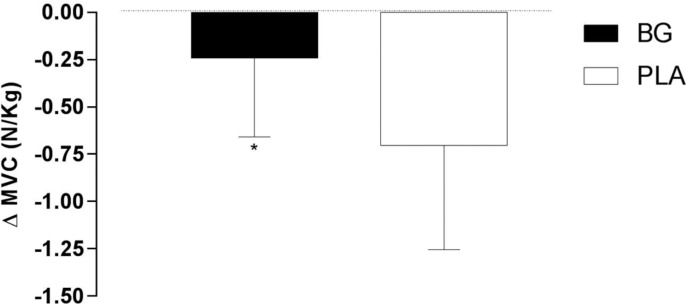
Values of maximal voluntary contraction (ΔMVC) of the forearm muscle after 20 min of the handgrip exercise are presented in Figure 1. Paired t-test revealed a significant difference in ΔMVC after BG compared to PLA supplementation (p = 0.036) and a large effect size was observed (d = 0.94). Additionally, two-way ANOVA test revealed no significant difference in MVC at baseline (PLA: 6.67± 0.82 vs. BG: 6.64 ± 0.50 N/kg, p = 0.904) and 20 min after 3 sets of handgrip exercise (PLA: 5.97 ± 0.93 vs. BG: 6.40 ± 0.60 N/kg, p = 0.161). However, a moderate effect size (d = 0.54) at 20 min was observed. Values of ETF are shown in Table 1. There was no significant difference between nutritional supplementation in ETF (interaction effect for set of exercise by treatment, p= 0.161). Additionally, a small effect size after BG ingestion in ETF in set 1 (d = 0.40), set 2 (d = 0.30), and set 3 (d = 0.13) was observed.
Fig. 1.
Changes from baseline in maximal voluntary contraction (ΔMVC, N/kg) of the forearm muscle at 20 min of exercise recovery of beetroot-based nutritional gel (BG) and nitrate-depleted gel (PLA) supplementation.
* significant difference between BG and PLA inervention (p < 0.05).
TABLE 1.
NIRS-derived forearm muscle oxygenation saturation parameters, blood volume, and exercise time until fatigue during exercise after nutritional supplementation.
| Set 1 |
Set 2 |
Set 3 |
||||
|---|---|---|---|---|---|---|
| BG | PLA | BG | PLA | BG | PLA | |
| ETF (sec) | 51.98 ± 14.11 | 46.79 ± 11.45 | 31.64 ± 12.08 | 35.15 ± 10.81 | 30.65 ± 8.66 | 29.45 ± 9.26 |
| SmO2Ex (%) | 65.32 ± 4.70 | 64.55 ± 6.27 | 63.26 ± 8.61 | 60.65 ± 6.86 | 60.10 ± 8.53 | 59.82 ± 7.20 |
| SmO2Slope_1 (% · s-1) | -0.33 ± 0.26 | -0.37 ± 0.28 | -0.41 ± 0.39 | -0.52 ± 0.29 | -0.41 ± 0.39 | -0.62 ± 0.50 |
| SmO2Rec (%) | 63.88 ± 8.14 | 65.04 ± 5.58 | 66.50 ± 8.07 | 64.70 ± 5.74 | 65.34 ± 7.62 | 64.77 ± 5.80 |
| SmO2Slope_2 (% · s-1) | 0.12 ± 0.19 | 0.17 ± 0.13 | 0.14 ± 0.12 | 0.16 ± 0.11 | 0.15 ± 0.11 | 0.16 ± 0.12 |
| SmO2min (%) | 52.80 ± 9.22 | 51.39 ± 9.95 | 53.41 ± 10.55 | 48.46 ± 8.62 | 49.46 ± 8.46 | 48.31 ± 8.71 |
| tHbEx (μM) | 63.28 ± 10.41 | 61.99 ± 6.68 | 62.65 ± 7.35 | 62.85 ± 6.00 | 61.40 ± 7.78 | 61.58 ± 6.83 |
| tHbRec (μM) | 73.00 ± 11.65 | 69.03 ± 9.30 | 69.84 ± 9.98 | 68.59 ± 7.67 | 68.96 ± 9.91 | 70.81 ± 9.86 |
Values are expressed as means ± SD. BG, beetroot-based nutritional gel; PLA, control nitrate depleted gel; ETF, exercise time until fatigue; SmO2slope_1, SmO2 desaturation rate; SmO2Ex, SmO2 during exercise; SmO2slope_2, resaturation rate; SmO2Rec, SmO2 during exercise recovery; SmO2min, minimum SmO2; tHbEx, tHb during exercise; tHbRec, tHb during exercise recovery.
Muscle oxygenation and blood volume changes
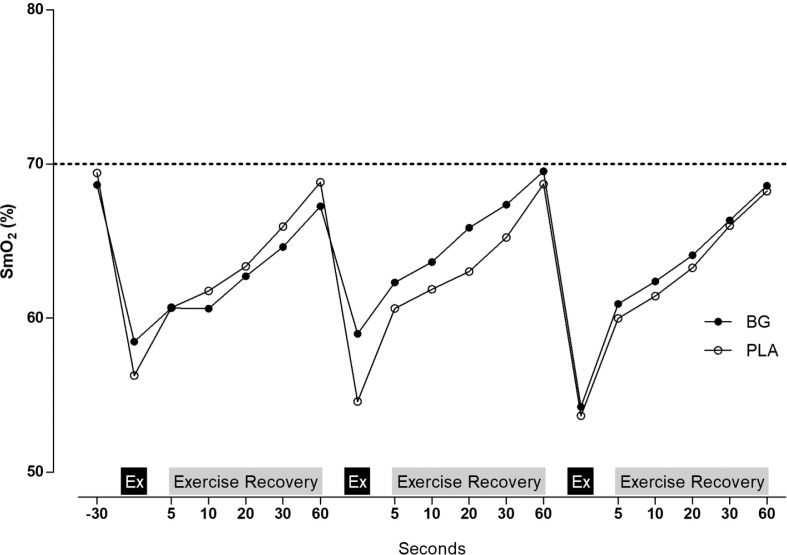
Values of forearm muscle SmO2 at baseline and during handgrip exercise and exercise recovery are presented in Figure 2 and Table 1. There were no significant differences (interaction effect for set of exercise by treatment) in SmO2min (p = 0.260), SmO2slope_1 (p = 0.819), SmO2slope_2 (p = 0.643), SmO2Ex (p = 0.459), SmO2Rex (p =0.141), tHbEx (p = 0.690) or tHbRec (p= 0.06). A trivial to small effect size was observed in SmO2min (set 1: d = 0.15; set 2: d = 0.52; set 3: d = 0.12), SmO2slope_1 (set 1: d = 0.14; set 2: d = 0.32; set 3: d = 0.04), SmO2slope_2 (set 1: d = 0.30; set 2: d = 0.17; set 3: d = 0.08), tHbEx (set 1: d = 0.14; set 2: d = 0.02; set 3: d = 0.01), and tHbRec (set 1: d = 0.38; set 2: d = 0.14; set 3: d =0.19).
Fig. 2.
Forearm muscle oxygen saturation (SmO2, %) during handgrip exercise after nutritional intervention.
Note: BG, beetroot-based gel; nitrate-depleted gel (PLA). Ex, minimum SmO2 value registered during exercise.
DISCUSSION
The aim of the present study was to investigate the effect of a single dose of high-nitrate beetroot-based gel on forearm muscle isometric strength and muscle oxygenation parameters in response to handgrip exercise. The main findings were that the high-nitrate BG supplementation: (i) attenuated the decline of handgrip strength after a fatiguing exercise; (ii) induced no changes in muscle oxygen saturation; and (iii) induced no changes in the time-to-fatigue during handgrip exercise.
It has been reported that forearm muscle is highly fatigued during bouts of combat sports; therefore, the ability to recover rapidly between combat bouts may be crucial to improving exercise performance [3]. The current study demonstrated that a single dose of nitrate supplementation improved ΔMVC after three bouts of exhaustive handgrip exercise in combat sport athletes. The results of the present study are in agreement with our previous study [22], which demonstrated that nitrate supplementation for 8 days was able to improve ΔMVC after three bouts of handgrip exercise in trained jiu-jitsu athletes. These beneficial effects of dietary nitrate supplementation on forearm muscle strength have also been observed in recreational, physically active subjects [12] and in older adults [23]. Collectively, the previous and present findings are particularly interesting since they suggest that beetroot-based supplementation improves muscle strength regardless of the period of supplementation (8 days vs. a single dose). This finding may be related to the fact that a slight increase in NO levels can regulate sarcoplasmic reticulum Ca2+ release during muscle contraction and muscle metabolism [7, 25, 27], which might improve muscle strength. Therefore, no additional effect would be observed with chronic supplementation.
Additionally, it is important to note that, in the present study, the assessment of changes in isometric muscle strength from baseline to 20 min after 3 sets of fatiguing handgrip exercise (ΔMVC) suggests that beetroot supplementation speeded up muscle strength recovery. Furthermore, although the ΔMVC was significantly different after BG ingestion (analysed by paired t-test), no significant changes in MVC at baseline and 20 min (analysed by two-way ANOVA test) after handgrip exercise in different nutritional intervention (PLA and BG) were observed. It was likely that the statistical test used to detect statistical differences between supplementation (PLA and BG) and time (baseline vs. 20 min after supplementation) may have somewhat influenced the detection of change in MVC after BG. However, a moderate effect size (d = 0.54) in MVC at 20 min after handgrip exercise in BG was found, indicating an improvement in muscle strength.
In contrast to the isometric muscle strength, ETF was not improved after acute BG intake, suggesting that the divergent effects of beetroot supplementation on exercise performance may be related to the type of muscle action involved in a given task (i.e. isometric vs. isotonic contraction). In line with the ETF findings, a previous study from our laboratory also failed to show improvement in ETF after 8 days of beetroot supplementation in trained jiu-jitsu athletes [22]. A recent meta-analysis showed that the exercise performance of individuals with higher fitness levels benefit less from nitrate supplementation than individuals with low fitness levels [16]. Porcelli et al. [28] reported that individuals with lower and moderate aerobic capacities performed a running 3-km time trial on a treadmill after dietary nitrate supplementation better than individuals with higher aerobic capacity. Although the mechanisms underlying the limited effect of dietary nitrate supplementation on exercise performance of trained individuals and/or athletes are still to be elucidated, there are factors that may explain the limited ergogenic effect of dietary nitrate supplementation in athletes. For instance, considering that trained athletes have higher energetic demand as compared to non-athlete individuals, a higher intake of dietary nitrate is also supposed to occur in this population and no additional beneficial effect with nitrate supplementation would be expected (inducing a “ceiling effect”) [29]. Corroborating this idea, a previous study demonstrated that there was no additional improvement in exercise tolerance after beetroot juice ingestion containing 16.8 as compared to 8.4 mmol of nitrate [26].
Furthermore, it has been demonstrated that nitrate and nitrite are present in human skeletal muscle and the baseline concentrations of these anions in skeletal muscle are higher than in plasma [30]. Therefore, improvements in exercise performance following dietary nitrate consumption may be mediated by skeletal muscle nitrate uptake, increased muscle nitrite concentration, and NO conversion from nitrite. However, inter-individual difference in muscle nitrate concentration has been observed [30], suggesting a relation to the variable expression of xanthine oxidoreductase (possibly involved in the reduction of nitrate to nitrite) and aldehyde oxidase (possibly involved in the reduction of nitrite to NO), as well as sialin (a protein involved in skeletal muscle nitrate uptake) [30]. These differences potentially compromise skeletal muscle NO bioavailability and may contribute to the differences in exercise performance observed following dietary nitrate consumption in trained individuals vs. athletes.
Another important factor is that the improved exercise performance observed after nitrate supplementation has been associated with an increased NO bioavailability, which may induce vasodilation and hence improve nutrient and oxygen delivery to the skeletal muscle. However, the high-intensity training programme in which combat sports athletes are engaged may induce physiological adaptations in the conduit artery (i.e. increased basal artery diameter), thereby reducing NO action on vasodilation during exercise [31]. Therefore, the effect of dietary nitrate supplementation on exercise performance in trained athletes may be annulled by exercise-induced artery adaptations.
Interestingly, there was no significant difference in the SmO2 parameters during handgrip exercise and exercise recovery after nitrate supplementation. In contrast, we have demonstrated increased SmO2 during exercise recovery in jiu-jitsu trained athletes [22] and older people [23] after 8 days and a single dose of a nitrate-rich beetroot-based gel, respectively. Papadopoulos et al. [12] demonstrated a significant increase in SmO2 and blood volume (total haemoglobin) during isometric handgrip exercise after a single dose of beetroot juice in physically active males. The NIRS-derived SmO2 allows for measuring the balance between the delivery and consumption of O2 by the muscle, which is related to vascular responsiveness and energy metabolism [32]. The effect of NO on muscle metabolism regulation and vascular responsiveness depends on its concentrations at the reaction site, the period of exposure, and type of vessel (conduit or resistance arteries) [7, 33]. Thus, it seems that trained athletes might need a longer period of exposure to dietary nitrate supplementation in order to change SmO2 during exercise and exercise recovery [26]. In contrast, recreational physically active subjects seem to improve the SmO2 after a single dose of dietary nitrate intake [12, 23].
One of the limitations of the present study was not evaluating nitrate and nitrite before and after beetroot supplementation. However, several studies have demonstrated a significant increase in plasma and urinary nitrate following a single dose of beetroot consumption [11, 23, 34]. For example, Coggan et al. [11] observed a 14.5-fold increase in plasma nitrate after beetroot consumption containing ~11 mmol of nitrate/dose. Oliveira et al. [23] demonstrated a 21-fold increase in urinary nitrate after BG supplementation containing ~12 mmol of nitrate/dose. Volino-Souza et al. [34] reported a 10-fold increase in urinary nitrate after beetroot consumption containing ~9 mmol of nitrate/dose. Thus, since a single dose of dietary nitrate containing ~12 mmol/dose was provided to the participants, increased plasma and/or urinary nitrate after a single dose of dietary nitrate would have been expected. Another limitation was regarding the compliance of nutritional supplementation restrictions by the participants; although all participants had been instructed not to take antioxidants, amino acids, and/or pre-workout supplements, there was no way to guarantee they had adhered to the nutritional and supplement restrictions.
CONCLUSIONS
A single dose of high-nitrate beetroot-based gel accelerated maximal forearm muscle isometric strength recovery 20 min after exhaustive handgrip exercise in recreational combat athletes. However, the exercise time until fatigue and muscle O2 saturation during exercise and exercise recovery were not improved. Therefore, a single dose of beetroot gel supplementation may be considered a good nutritional strategy to attenuate declining forearm muscle strength caused after several rounds of combat sports performed intensively or without adequate recovery time.
Conflict of interest statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript and the authors declare no conflict of interest.
Acknowledgements
The authors thank Ricky Toledano for revision of the English version of the manuscript. This work was supported by the Research Foundation of the State of Rio de Janeiro – FAPERJ (E-26/110.309/2014, E-26/202.905/2019 and E-26/203.308/2016) and the Brazilian National Council for Scientific and Technological Development – CNPq (442977/2014-0). Gustavo Oliveira and Mônica Volino-Souza acknowledge the financial support provided by CAPES (Brazil).
REFERENCES
- 1.Bonitch-Góngora JG, Bonitch-Domínguez JG, Padial P, Feriche B. The effect of lactate concentration on the handgrip strength during judo bouts. J Strength Cond Res. 2012;26(7):1863–1871. doi: 10.1519/JSC.0b013e318238ebac. [DOI] [PubMed] [Google Scholar]
- 2.Andreato VL, Esteves JVDC, Julio UF, Panissa VLG, Hardt F, Moraes SMF, Miranda ML, Pastório JJ, Pastório EJ, Branco BHM, Franchini E. Psychological, physiological, performance and perceptive responses to brazilian jiu-jitsu combats. Kinesiology. 2014;46(1):44–52. [Google Scholar]
- 3.Andreato LV, Julio UF, Panissa VL, Esteves JV, Hardt F, de Moraes SM, de Souza CO, Franchini E. Brazilian Jiu-Jitsu Simulated Competition Part I: Metabolic, Hormonal, Cellular Damage, and Heart Rate Responses. J Strength Cond Res. 2015;29(9):2538–2549. doi: 10.1519/JSC.0000000000000918. [DOI] [PubMed] [Google Scholar]
- 4.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 5.Wan JJ, Qin Z, Wang PY, Sun Y, Liu X. Muscle fatigue: general understanding and treatment. Exp Mol Med. 2017;49(10):e384. doi: 10.1038/emm.2017.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7(2):156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 7.Dai Z, Wu Z, Yang Y, Wang J, Satterfield MC, Meininger CJ, Bazer FW, Wu G. Nitric oxide and energy metabolism in mammals. Biofactors. 2013;39(4):383–391. doi: 10.1002/biof.1099. [DOI] [PubMed] [Google Scholar]
- 8.Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, Smith L, Golden M, Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med. 1995;1(6):546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 9.Affourtit C, Bailey SJ, Jones AM, Smallwood MJ, Winyard PG. On the mechanism by which dietary nitrate improves human skeletal muscle function. Front Physiol. 2015;6:211. doi: 10.3389/fphys.2015.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191(1):59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 11.Coggan AR, Leibowitz JL, Spearie CA, Kadkhodayan A, Thomas DP, Ramamurthy S. Acute Dietary Nitrate Intake Improves Muscle Contractile Function in Patients With Heart Failure: A Double-Blind, Placebo-Controlled, Randomized Trial. Circ Heart Fail. 2015;8(5):914–920. doi: 10.1161/CIRCHEARTFAILURE.115.002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papadopoulos S, Dipla K, Triantafyllou A, Nikolaidis MG, Kyparos A, Touplikioti P, Vrabas IS, Zafeiridis A. A Beetroot Increases Muscle Performance and Oxygenation During Sustained Isometric Exercise, but Does Not Alter Muscle Oxidative Efficiency and Microvascular Reactivity at Rest. J Am Coll Nutr. 2018;37(5):361–372. doi: 10.1080/07315724.2017.1401497. [DOI] [PubMed] [Google Scholar]
- 13.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011;13(2):149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Breese BC, McNarry MA, Marwood S, Blackwell JR, Bailey SJ, Jones AM. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2013;305(12):R1441–R1450. doi: 10.1152/ajpregu.00295.2013. [DOI] [PubMed] [Google Scholar]
- 15.Aucouturier J, Boissière J, Pawlak-Chaouch M, Cuvelier G, Gamelin FX. Effect of dietary nitrate supplementation on tolerance to supramaximal intensity intermittent exercise. Nitric Oxide. 2015;49:16–25. doi: 10.1016/j.niox.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Campos HO, Drummond LR, Rodrigues QT, Machado FSM, Pires W, Wanner SP, Coimbra CC. Nitrate supplementation improves physical performance specifically in non-athletes during prolonged open-ended tests: a systematic review and meta-analysis. Br J Nutr. 2018;119(6):636–657. doi: 10.1017/S0007114518000132. [DOI] [PubMed] [Google Scholar]
- 17.Cermak NM, Gibala MJ, Van Loon LJ. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int J Sport Nutr Exerc Metab. 2012;22(1):64–71. doi: 10.1123/ijsnem.22.1.64. [DOI] [PubMed] [Google Scholar]
- 18.Muggeridge DJ, Howe CC, Spendiff O, Pedlar C, James PE, Easton C. The effects of a single dose of concentrated beetroot juice on performance in trained flatwater kayakers. Int J Sport Nutr Exerc Metab. 2013;23(5):498–506. doi: 10.1123/ijsnem.23.5.498. [DOI] [PubMed] [Google Scholar]
- 19.Boorsma RK, Whitfield J, Spriet LL. Beetroot juice supplementation does not improve performance of elite 1500-m runners. Med Sci Sports Exerc. 2014;46(12):2326–2334. doi: 10.1249/MSS.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 20.MacLeod KE, Nugent SF, Barr SI, Koehle MS, Sporer BC, MacInnis MJ. Acute Beetroot Juice Supplementation Does Not Improve Cycling Performance in Normoxia or Moderate Hypoxia. Int J Sport Nutr Exerc Metab. 2015;25:359–366. doi: 10.1123/ijsnem.2014-0129. [DOI] [PubMed] [Google Scholar]
- 21.Lowings S, Shannon OM, Deighton K, Matu J, Barlow MJ. Effect of Dietary Nitrate Supplementation on Swimming Performance in Trained Swimmers. Int J Sport Nutr Exerc Metab. 2017;27(4):377–384. doi: 10.1123/ijsnem.2016-0251. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira GV, Nascimento LADD, Volino-Souza M, Mesquita JS, Alvares TS. Beetroot-based gel supplementation improves handgrip strength and forearm muscle O2 saturation but not exercise tolerance and blood volume in jiu-jitsu athletes. Appl Physiol Nutr Metab. 2018;43(9):920–927. doi: 10.1139/apnm-2017-0828. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira GV, Morgado M, Conte-Junior CA, Alvares TS. Acute effect of dietary nitrate on forearm muscle oxygenation, blood volume and strength in older adults: A randomized clinical trial. PLoS One. 2017;12(11):1–15. doi: 10.1371/journal.pone.0188893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgado M, de Oliveira GV, Vasconcellos J, Monteiro ML, Conte-Junior C, Pierucci AP, Alvares TS. Development of a beetroot-based nutritional gel containing high content of bioaccessible dietary nitrate and antioxidants. Int J Food Sci Nutr. 2016;67(2):153–160. doi: 10.3109/09637486.2016.1147531. [DOI] [PubMed] [Google Scholar]
- 25.Fulford J, Winyard PG, Vanhatalo A, Bailey SJ, Blackwell JR, Jones AM. Influence of dietary nitrate supplementation on human skeletal muscle metabolism and force production during maximum voluntary contractions. Pflugers Arch. 2013;465(4):517–528. doi: 10.1007/s00424-013-1220-5. [DOI] [PubMed] [Google Scholar]
- 26.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. J Appl Physiol (1985) 2013;115(3):325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]
- 27.Hoon MW, Fornusek C, Chapman PG, Johnson NA. The effect of nitrate supplementation on muscle contraction in healthy adults. Eur J Sport Sci. 2015;15(8):712–719. doi: 10.1080/17461391.2015.1053418. [DOI] [PubMed] [Google Scholar]
- 28.Porcelli S, Ramaglia M, Bellistri G, Pavei G, Pugliese L, Montorsi M, Rasica L, Marzorati M. Aerobic Fitness Affects the Exercise Performance Responses to Nitrate Supplementation. Med Sci Sports Exerc. 2015;47:1643–1651. doi: 10.1249/MSS.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 29.Jonvik KL, Nyakayiru J, van Loon LJ, Verdijk LB. Can elite athletes benefit from dietary nitrate supplementation? J Appl Physiol (1985) 2015;119(6):759–761. doi: 10.1152/japplphysiol.00232.2015. [DOI] [PubMed] [Google Scholar]
- 30.Wylie LJ, Park JW, Vanhatalo A, Kadach S, Black MI, Stoyanov Z, Schechter AN, Jones AM, Piknova B. Human skeletal muscle nitrate store: influence of dietary nitrate supplementation and exercise. J Physiol. 2019 doi: 10.1113/JP278076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green DJ, Rowley N, Spence A, Carter H, Whyte G, George K, Naylor LH, Cable NT, Dawson EA, J Thijssen DH. Why isn’t flow-mediated dilation enhanced in athletes? Med Sci Sports Exerc. 2013;45(1):75–82. doi: 10.1249/MSS.0b013e318269affe. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari M, Wei Q, Carraresi L, De Blasi RA, Zaccanti G. Time-resolved spectroscopy of the human forearm. J PhotochemPhotobiol B. 1992;16(2):141–153. doi: 10.1016/1011-1344(92)80005-g. [DOI] [PubMed] [Google Scholar]
- 33.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Volino-Souza M, de Oliveira GV, Alvares TS. A single dose of beetroot juice improves endothelial function but not tissue oxygenation in pregnant women: a randomised clinical trial. Br J Nutr. 2018;120(9):1006–1013. doi: 10.1017/S0007114518002441. [DOI] [PubMed] [Google Scholar]