Abstract
The aim of the study was to assess the effects of a specific protocol, based on a focal muscle vibration, on mechanical parameters in an exercise composed of five repeated bouts of sprint interval tests (Wingate Anaerobic Tests, 10 seconds duration). Twenty-eight young male healthy subjects were randomized to two groups (VIB and CTRL). Peak power (PP), average peak between bouts (aP) and total exercise work (TW) were measured. In both groups, three different exercise sessions were carried out, interspersed by seven days: T0, T1 and T2. Between the baseline (T0) and T1, in the VIB group the intervention was administered on three successive days on quadriceps muscles, whereas a placebo administration was carried out in the CTRL group at the same time. At T1 (30 minutes after intervention) and T2 (7 days after) CTRL did not show any significant change, whereas VIB showed significant increases in PP (11.4%–9.3%), aP (6.6%–6.9%) and TW (5.7%–7.9%) with respect to T0. The results could be explained by an ameliorative agonist-antagonist balance, and this hypothesis is coherent with the literature. On the basis of the present findings, the investigated intervention might be usefully adopted to increase muscular power and endurance.
Keywords: Cycling exercise, Motor drive, Muscle work, Efficiency
INTRODUCTION
Muscle spindles constitute one of the major sensory inputs to plan and control motor execution. Many researchers have investigated the possibility to modify the human motor performance by mechanically acting on muscle spindle, in a non-invasive manner. Such an action is commonly carried out [1, 2] by applying a sinusoidal vibrating probe on the tendon or directly on the muscle (“focal vibration”), able to produce sequences of muscular stretches-shortenings [3], with a consequent sensorial inflow toward the central nervous system.
Various studies have investigated the optimal stimulus parameters to elicit CNS excitability, as stimulus frequency, amplitude and exposure duration, [1, 2, 4, 5, 6]. Focal stimulation with a frequency range of 75–120 Hz, a small vibration amplitude (<1 mm) and an adequate application time was found suitable to elicit plastic changes at the highest CNS levels [6]. Similar vibrating parameters were recognised by other authors able to change motor performance [7–19] and spatial perception [5, 20–22].
Several studies have analysed the effects of a specific intervention, called repeated muscle vibration (rMV), with focal stimulation characterized by a frequency of 100 Hz, an amplitude of 0.2–0.5 mm and lasting 30 minutes each day, during 3 consecutive days. In these studies, the authors proved that this intervention was able to persistently modify the activation interplay between the vibrated muscle and its antagonists and it was correlated with an increase of motor coordination in the joints and, therefore, with a likely articular performance increase 9, 16, 17]. In addition, the onset of rMV effects were recognised as soon as 60 minutes after the intervention ending [8, 9, 15, 16, 17]. On these bases, rMV was adopted in rehabilitation programmes, showing significant improvements in balance and force both in ageing [13, 15] and in an anterior cruciate ligament reconstruction [8] and in motor coordination in neurological deficit patients [10–12, 17, 19]. Likewise, healthy individuals, submitted to the same intervention, showed motor performance enhancements by means of mechanical and physiological parameters: muscle power [9, 15], muscular fatigue [14] and rate of force development [14]. It is notable as this latter one may be reasonably related to better muscular agonist-antagonist management, as also described in studies about rMV influence on CNS [16, 17]. All these results seem to suggest that rMV can induce an early motor drive reorganization and, therefore, to ameliorate muscular power supply and resistance.
Based on the literature, rMV effects were evaluated in neurophysiological, motor functional and exercise performance studies. In particular, neurophysiology studies have revealed a motor command re-arrangement involving central nervous structures. From an applicative point of view, rMV intervention was positively correlated with an increase in muscular power efficiency both in patients and in healthy subjects.
The Wingate Anaerobic Test (WAnT) is a standard test [23] simulating in the laboratory Sprint Interval Training (SIT), requiring very high mechanical and metabolic power levels and, when repeated more times with short rest periods, also demanding a high workout volume [24]. For these reasons, WAnT is largely adopted in sport sciences to test the anaerobic athletes’ quality and in sports requiring frequent activation bursts separated by short rest periods as well as basketball, football, rugby and sailing.
Finally, this study aimed to ascertain the effect of rMV on a very stressing exercise based on repeated bouts of the standard Wingate Anaerobic Test [223] in order to verify also the intervention efficacy based on validated parameters as well as peak power and external work.
The novelty of this study consists of an evaluation of the rMV effects in a standard test aimed to induce an exhaustive effort in both peak muscle power and endurance.
MATERIALS AND METHODS
Subjects
Participants were enrolled among college and PhD Sport Sciences students, regularly performing recreational aerobic physical activities, including cycling. Only subjects with no history of major neurological, musculoskeletal, cardiovascular diseases or recent musculoskeletal injuries were considered. Sample size was determined using G*Power 3.1 analysis (input parameters: f = 0.4, α = 0.05, β = 0.95, 2 group, 3 measurements, r repeated measures = 0.50, ε = 1) based on large effect sizes as inferred by previous studies using similar designs [9, 14, 15]. In the present study, 28 young healthy males (age 24 ± 3.0 years; height 172 ± 9.6 m; body mass 72 ± 12.5 kg) were randomized to two parallel groups of 14 individuals each: a VIB group (age 25 ± 3.6 years; height 173 ± 8.3 m; body mass 72 ± 11.0 kg) and a CTRL one (age 24 ± 2.3 years; height 172 ± 11.0 m; body mass 71 ± 14.2 kg). University of Cassino and Southern Lazio ethics committee approval was obtained for all study procedures. Detailed information about the study protocol and procedures was provided and written informed consent was obtained. The study conformed to the provisions of the Declaration of Helsinki.
Study design
The research was designed as a double-blind study. The VIB and the CTRL groups were respectively exposed to the rMV and to a placebo application. Treatments and measures were performed by different operators. Exercise involved five WAnT bouts and, in each one, peak power and work were measured; hence the exercise average peak and the total work were calculated. The same investigation was performed on the two groups at three times: at the baseline (T0), a week (T1) and two weeks (T2) after. Measurements were performed in the afternoon, at the same hour of the day, at least three hours after a light meal typically based on carbohydrates. During the experiment in the laboratory, temperature and humidity were adjusted to guarantee maximal comfort to the participants. The necessity of limiting, during the study period, the participants’ physical activity to the daily needs was clearly explained and stressed.
Procedures
About a week before the start, each subject visited the laboratory to get familiarized with the cycle-ergometer by performing light cycling exercise. Hence, three measurement sessions (trials) were performed, namely at T0 (baseline), T1 and T2, separated by a week from each other. For each trial, participants completed a series of five bouts of WAnT lasting 10 s, separated by 50 s of active recovery at 30 W, on a constant brake ergometer (894E AB, Monark Exercise, Vansbro, Sweden). The cycle-ergometer seat height was set to keep the knee flexed about 15° in the fully extended pedal position. Before the trial, participants completed a warm-up of 5 minutes at a rate of 60 RPM, and the workload was set to 1.0 kg. Just before starting the WAnT, each participant was given a workload, determined as 7.5% of body mass, placed on the basket [25]. A few seconds before every bout, participants quickly increased the cycling cadence to reach 150 RPM, when the basket dropped automatically. When the workload was applied to the flywheel, participants were required to achieve a maximal effort for 10 s. Verbal encouragement was given to each participant throughout the test.
Mechanical power output, expressed in W/kg, was determined as the product of the RPM and workload (i.e. load on the basket multiplied by a constant related to the bike architecture) as described by Rodio et al. [24], and scaled by body mass. Peak power (PP) was determined as the highest mechanical power among all bouts and, as expected, was reached on the first bout. Total exercise work (TW) represented the mechanical work during exercise over the recovery work, scaled by friction weight and was, consequently, expressed in RPM. Finally, average peak (aP) was defined as peak mean value among bouts scaled by friction weight, a parameter also expressed in RPM.
Intervention
The VIB group, before T1, performed a vibratory treatment as described in several previous studies [8, 9, 14, 15]. Briefly, a mechanical stimulus was applied to both quadriceps muscles at the same time, near the common tendon insertion, on the distal end of the vastus medialis and the common tendon of the rectus and intermedius femoris, about 2 cm from the patella edge. Vibration was produced by an electromagnetic vibrator (Ling V203 Permanent Magnet Shaker, Ling Electronics, West Haven, CT, United States) driven by a digital sinusoidal signal generator. Peak-to-peak sinusoidal displacement amplitude was in a range of 0.2–0.5 mm. Amplitude was evaluated using a light X-Y infrared sensor detecting the displacement of an infrared light emitting diode placed orthogonally on the lateral face of the vibrator tip [15]. Vibration frequency was set at 100 Hz. rMV treatment was administered on three consecutive days. Each session (day) consisted of three applications lasting 10 minutes each, separated by about 2 resting minutes. During each vibratory session, the participants were supine and contracted their quadriceps. Based on previous experiments, specific contraction intensity during vibratory application did not appear to be important; hence the participants were asked to keep the popliteal cavum in contact with the bed [15]. None of the participants showed trouble in accomplishing such a condition, allowing the assessors to manually monitor the contraction presence and the vibratory involvement of the muscle body throughout the entire series of applications.
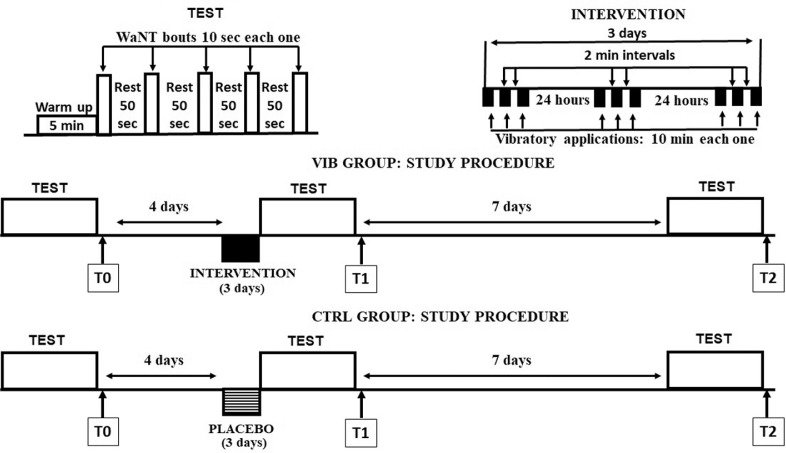
As stated, the CTRL group underwent a sham vibratory treatment. A placebo effect was achieved by placing the vibrator in the proximity of the skin on the extensor tendon, without touching the skin, whereas all the other treatment parameters were equal. In this condition the patients were submitted only to the weak vibrator buzzing during all three sessions. Figure 1 summarizes the time course relative to the exercise, intervention and measurements.
FIG. 1.
Experimental procedure. Upper row, left side, five WAnT bouts (open bars), preceded by a short warm up. Right side, intervention constituted by 9 vibratory applications (black bars) distributed throughout 3 consecutive days. Middle and lower rows, respectively, show the VIB and CTRL group study procedures.
Statistical analysis
Statistical analysis was performed using IBM SPSS, release 25 (IBM, Armonk, New York, USA), with the significance level set at p=0.05. The Kolmogorov-Smirnov normality test was performed to assess the normality distribution of the dependent variables PP, TW and aP in each group and for each of the three trials. This analysis also allowed us to verify the Gaussian distribution of the age and the anthropometric characteristics of VIB and CTRL groups.
According to the results of normality analysis, Student’s t-test for independent samples or the Mann-Whitney U test was used to determine the difference between the VIB and the CTRL groups at each time level, as well as to compare the two groups in terms of age, height and body mass (BM).
Moreover, if the normality test was satisfied, the mixed ANOVA statistical model was used to assess the interactions between the within-subjects factor (Time, 3 levels: T0, T1 and T2) and the between-subjects factor (Treatment, 2 levels: VIB and CTRL groups) on the dependent variables PP, TW and aP. Mauchly’s test of sphericity and Levene’s test of equality of error variances were performed, and the respective hypotheses were verified for each variable. If the mixed ANOVA evidenced significant differences in the time*treatment interaction, also the simple main effects were investigated for both factors. Specifically, the one-way repeated measures ANOVA and, if it was significant, a post hoc test using Bonferroni correction (p<0.017) were performed to investigate in which pairwise comparisons of the time levels the significant differences lay. If the normality test was not satisfied, the corresponding non-parametric analysis was performed.
Ethics
University of Cassino and Southern Lazio ethics committee approval was obtained for all study procedures. Detailed information about the study protocol and procedures were provided and written informed consent was obtained. The study conformed to the provisions of the Declaration of Helsinki.
RESULTS
The result of the normality test confirmed the normal distribution of all the data and allowed the use of parametric analysis. No significant differences were detected using the t-test between CTRL and VIB groups for age and anthropometric data, as well as in biomechanical parameters PP, TW and aP at T0. These latter results are reported in Table 1. PP values at T0, in both groups, classified our participants as “average” level with respect to muscular capacity and speed [26].
TABLE 1.
Anthropometric data and relative biomechanical parameters at baseline in CTRL and VIB groups. Data presented as mean and sd. No statistical differences were detected.
| AGE (Y) | HIGH (m) | BM (kg) | PP (W/kg) | TW (RPM) | aP (RPM) | |
|---|---|---|---|---|---|---|
| CTRL | 24±2.2 | 171 ±6.9 | 72 ±10.0 | 12.2 ±1.40 | 6930.7 ±695.44 | 168.4 ±11.4 |
| VIB | 25 ±3.6 | 173 ±7.3 | 75 ±10.9 | 12.1 ±1.23 | 6963.8 ±420.6 | 169.7 ±9.9 |
During and immediately after the intervention, no one expressed discomfort and there were no adverse or side effects and all subjects completed the sessions.
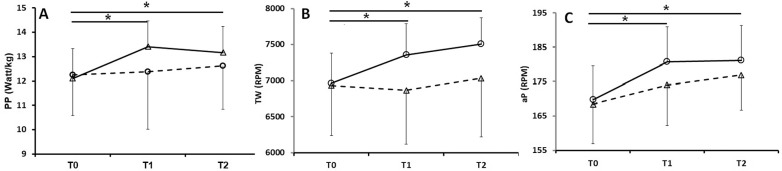
Figure 2 shows evaluated parameters values obtained at T0, T1 and T2. For the PP parameter, the mixed ANOVA analysis evidenced a significant time*treatment interaction (F(2,52)=3.923; p<0.05) and the repeated measures ANOVA showed significant differences only in the VIB group (F(2,26)=12.030; p<0.05). In detail, as evidenced in Figure 2, PP data showed a significant difference (p<0.017) in T1 and T2 with respect T0 only in the VIB group; no significant differences were detected between T1 and T2. Mean PP increment, with respect T0, in the VIB group was 11.4% and 9.3%, whereas in CTRL it was 0.5% and 3.2% at T1 and T2 respectively.
FIG. 2.
Peak power (A), total work (B) and average peak (C) values, measured at the experimental time T0, T1, T2, in VIB (triangles, solid line) and CTRL (circles, dashed line) groups (p<0.05).
With respect to muscular capacity and speed, PP data qualified the CTRL group as “average” or “above average”, respectively at T1 and T2, whereas at the same times the VIB group was found to be “excellent” [26].
A similar response behaviour was obtained in TW data: the mixed ANOVA evidenced a significant time*treatment interaction (F(2,52)=10.554; p<0.05) and the repeated measures ANOVA performed on the time factor showed significant differences exclusively in the VIB group (F(2,26)=62.058; p<0.05), in particular between T0 and T1 and between T0 and T2 (p<0.017). Mean TW increment, with respect T0, in the VIB group was 5.7% and 7.9%, whereas in CTRL it was 1.0% and 1.5% at T1 and T2 respectively.
Again, in aP data the mixed ANOVA evidenced a significant time*treatment interaction exclusively in the VIB group, (F(2,26)=30.054; p<0.05), in all the three pairwise comparisons (p<0.017). Mean aP increment, with respect T0, in the VIB group was 6.6% and 6.9%, whereas in CTRL it was 3.4% and 5.4% at T1 and T2 respectively.
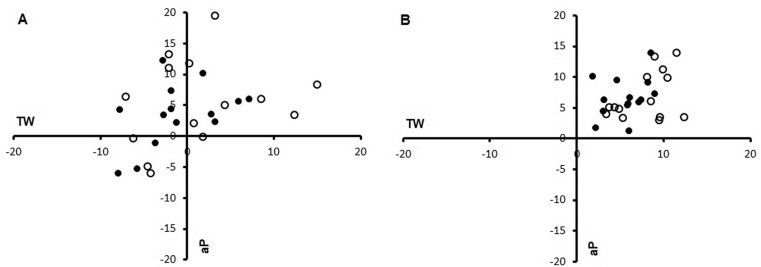
For each participant, immediate evidence of the rMV effect on TW and aP was graphically evidenced in Figure 3A and 3B, where individual percentage variations with respect to baseline (T0) were reported in a bi-dimensional graph. As evident in Figure 3, CTRL group data (panel A) were scattered around the origin mainly in the first and fourth quadrants. In contrast, in the VIB group (panel B), data showed in all participants a clear shift towards the first quadrant, indicating an increase in all performance indexes.
FIG. 3.
Two-dimensional plot showing total work (abscissa) and average peak (ordinate) percentage variations in CTRL (panel A) and VIB (panel B) groups. T1 vs T0: black circles; T2 vs T0: white circles.
DISCUSSION
This study was aimed at assessing the effects of a specific intervention, based on a focal vibration, on some biomechanical parameters, during an exercise composed of a sequence of five WAnT bouts, each lasting 10 seconds. The main results are the statistical increases of the peak power and total mechanical work in the VIB group both immediately after the intervention lasting at least a week whereas no differences were obtained in the CTRL group. This exercise requires very high mechanical power levels, without a complete recovery, noticeably stressing metabolic anaerobic and aerobic pathways [24]. Biomechanical parameters analysed were PP, representing the highest power exerted among bouts, i.e. a measure of neuromuscular system to speedily generate the highest force levels, aP representing the mean value of PP in each bout, i.e. the neuromuscular system capacity to respond to repetitive high stressing requests and TW as total mechanical work in all bouts. The biomechanical parameters were evaluated at three different times (T0, T1 and T2) separated from each other by seven days, between T0 and T1, the intervention was administered only in the VIB group (see details in Figure 1).
With respect to the baseline (T0), the results showed a significant post-intervention (T1) increase of exercise PP (~10%), aP (~7%), and TW (~8%), only in the VIB group, persisting at least 7 days after, while no significant variations were detected in the CTRL group, as summarized by Figures 2 and 3. PP after rMV, at T1 and T2, allowed the VIB group participants to be classified as “excellent”, being at T0 “average” relating the muscular capacity and speed [26]. The CTRL group showed, at T0 and T1, the same classification (“average”) while at T2 it reached “above average” [26], even if no statistically significant differences were detected.
This finding is very interesting because it evidences a clear change in muscular activation before and after rMV. Indeed, muscular power is related to strength and motor task velocity to reach an intensity level (power=strength*velocity) and an increase of this may be due to an increase in force production, velocity or both. Since improvements were evidenced as early as 30 minutes after treatment ending (T1) and, considering that both groups were submitted to the same exercise, it appears that improvements in the VIB group are likely related to the sole intervention. In this respect, it seems unlikely that rMV induced structural modifications (i.e. hypertrophy), since the time interval between the intervention ending and the T1 evaluations was too short (30 minutes or less). Moreover, the peak-to-peak amplitude vibratory displacement was too small (also in relation to quadriceps muscle fibre length) to generate local muscular damage, and VIB subjects never reported soreness or movement impairments during or after rMV. The exclusion of the above considered possibilities and the early onset of the effects, confirmed by other authors [8, 9, 15, 16, 17], suggest an adaptation by the central nervous system as a response to the intervention, as also reviewed by Souron et al. about a variety of effects induced by focal vibrations [6]. In addition, it is noteworthy that transcranial magnetic stimulation evidenced a direct long lasting rMV action on cortical motor control circuits [16, 17] with consequent sensorimotor rearrangement [27, 16, 17] and enhancement of motor performance [17]. Marconi’s studies [16, 17] reported significant short interval intracortical inhibition increase in the agonist and a parallel decrease in the antagonist, following rMV. In the general sense, intracortical inhibition is considered as a mechanism that improves the muscle selection and reduces superfluous co-contractions, thereby improving the efficiency of the motor task [28, 29]. Other authors [7, 9–13, 14, 15, 18, 19] have evidenced improved motor function, probable expression of ameliorated muscular coordination, supported by better agonist-antagonist activation after rMV. Consequently, present PP enhancement might be justified by a different motor unit recruitment and agonist-antagonist interplay as consequence of modified motor control management. The rMV effects’ persistence from T1 to T2 is another important issue. This result confirms previous observations; several studies adopting rMV have reported persisting effects in healthy individuals: until at 14 days [14, 16] , 90 [15] and 240 [8] days Marconi et al. [16, 17] suggested that rMV induces a form of long-term potentiation, a well-known physiological mechanism, which may also be consolidated and maintained by a spontaneous enhancement of individual daily activities [15] or, probably, also enhanced by specific training.
An exercise including a series of WAnT can be defined as intermittent periods of intense exercise separated by periods of recovery [30] and is a potent stimulus to increase maximum aerobic capacity [31]; for these reasons it is largely adopted as interval training exercise. In this exercise, aP is representative of the muscular capacity to produce and maintain the power levels, counteracting the fatigue phenomenon induced by the exercise progression. The observed aP increase, in the VIB group after rMV, may be interpreted as more efficient neuromuscular functionality consequent to a minor antagonist muscular activation, as supported in Marconi’s research [16, 17], in turn reducing the antagonist brake effect. In our study we observed, in the VIB group, TW increments of about 6 and 8% with respect to the baseline while in the CTRL group they oscillated between -1 and 1%. Also, in the case of TW results, they could be a direct consequence of a rebalance between internal and external work.
There were remarkable amplitude increments in the measured parameters (ranging between 5.7% and 11.4%), which were obtained without any form of physical training. It implies that rMV might constitute a booster if applied at the beginning of physical training, as also suggested in a recent study on a regional-level volleyball team, in which, at the end of the season, the VIB group improved the jump height by up to 26%, compared to the CTRL with up to 11% [9].
Limitations
The study, while promising, has some intrinsic limitations. The intervention effects were analysed on a massive exhaustive exercise requiring a very high cardiovascular and muscular effort. Because of this, the present study necessarily involved healthy and collaborative subjects. In other categories a different exercise might be required. Moreover, an electromyographic analysis might be useful to evidence the interplay between agonist and antagonist muscles during exercise, and hence to confirm the hypothesis.
CONCLUSIONS
In conclusion, the present results showed more efficient muscular power generation and a higher workout, as evidenced by PP and TW parameters, in a series of exercise sprints interspersed with rest periods when the rMV protocol was applied. Moreover, the effects were recognized significantly immediately after the intervention termination and lasted a week without additional conditioning. The amplitude and long-term persistence of the effects of the intervention versus the application time form the premises for its application in sports and the rehabilitative field.
Practical Applications
The mechanical parameter enhancements, evidenced in this exercise, suggest that rMV application could be positively extended from a rehabilitative field, as certified by the literature, to sport sciences. The rMV, associated with traditional training protocols, might increase performances in sports requiring high-intensity intermittent motor tasks, e.g. rugby, soccer, volleyball, basketball, futsal, sailboat match racing and hockey, as well as in training protocols adopting sprint internal training or high intensity interval training [32]. Moreover, such potentialities may be applied for faster athlete rehabilitation after an injury [8, 33].
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors are grateful to Dr. Franco Esposito for English revision.
Conflict of interest declaration
The authors have no conflict of interests.
REFERENCES
- 1.Fallon JB, Macefield VG. Vibration sensitivity of human muscle spindle and Golgi tendon organs. Muscle Nerve. 2007;36(1):21–29. doi: 10.1002/mus.20796. [DOI] [PubMed] [Google Scholar]
- 2.Matthews PB, Watson JD. Action of vibration on the response of cat muscle spindle Ia afferents to low frequency sinusoidal stretching. J Physiol. 1981;317:365–381. doi: 10.1113/jphysiol.1981.sp013830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. London: Edward Arnold; 1972. [Google Scholar]
- 4.Fattorini L, Tirabasso A, Lunghi A. https://www.scopus.com/authid/detail.uri?authorId=56533525000&eid=2-s2.0-84960323236Di Giovanni R, Sacco F, Marchetti E. Muscular synchronization and hand-arm fatigue. Int J Ind Ergon. 2017;62:13–16. [Google Scholar]
- 5.Pettorossi VE, Panichi R, Botti FM, Biscarini A, Filippi GM, Schieppati M. Long-lasting effects of neck muscle vibration and contraction on self-motion perception of vestibular origin. Clin Neurophysiol. 2015;126(10):1886–1900. doi: 10.1016/j.clinph.2015.02.057. [DOI] [PubMed] [Google Scholar]
- 6.Rosenkranz K, Rothwell JC. The effect of sensory input and attention on the sensorimotor organization of the hand area of the human motor cortex. J Physiol. 2004;561:307–332. doi: 10.1113/jphysiol.2004.069328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardigò LP, Iacono AD, Zagatto AM, Bragazzi NL, Kuvacic G, Bellafiore M, Padulo J. Vibration effect on ball score test in international vs. national level table tennis. Biol Sport. 2018;35(4):329–334. doi: 10.5114/biolsport.2018.78051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunetti O, Filippi GM, Liti A, Panichi R, Roscini M, Pettorossi VE, Cerulli G. Improvement of posture stability by vibratory stimulation following anterior cruciate ligament (ACL) reconstruction. Knee Surg Sports Traumatol Arthrosc. 2006;14(11):1180–1187. doi: 10.1007/s00167-006-0101-2. [DOI] [PubMed] [Google Scholar]
- 9.Brunetti O, Botti FM, Roscini M, Brunetti A, Panichi R, Filippi GM, Biscarini A, Pettorossi VE. Focal vibration of quadriceps muscle enhances leg power and decreases knee joint laxity in female volleyball players. J Sports Med Phys Fitness. 2012;52(6):596–660. [PubMed] [Google Scholar]
- 10.Caliandro F, Celletti C, Padua L, Minciotti I, Russo G, Granata G, La Torre G, Granieri E, Camerota F. Focal muscle vibration in the treatment of upper limb spasticity: a pilot randomized controlled trial in patients with chronic stroke. Arch Phys Med Rehabil. 2012;93(9):1656–1661. doi: 10.1016/j.apmr.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Camerota F, Celletti C, Di Sipio E, De Fino C, Simbolotti C, Germanotta M, Mirabella M, Padua L, Nociti V. Focal muscle vibration, an effective rehabilitative approach in severe gait impairment due to multiple sclerosis. J Neurol Sci. 2017;15:33–39. doi: 10.1016/j.jns.2016.11.025. 372. [DOI] [PubMed] [Google Scholar]
- 12.Celletti C, Camerota F. Preliminary evidence of focal muscle vibration effects on spasticity due to cerebral palsy in a small sample of Italian children. Clin Ter. 2011;162(5):125–128. [PubMed] [Google Scholar]
- 13.Celletti C, Fattorini L, Camerota F, Ricciardi D, La Torre G, Landi F, Filippi GM. Focal muscle vibration as a possible intervention to prevent falls in elderly women: a pragmatic randomized controlled trial. Aging Clin Exp Res. 2015;27(6):857–863. doi: 10.1007/s40520-015-0356-x. [DOI] [PubMed] [Google Scholar]
- 14.Fattorini L, Ferraresi A, Rodio A, Azzena GB, Filippi GM. Motor performance changes induced by muscle vibration. Eur J Appl Physiol. 2006;98(1):79–87. doi: 10.1007/s00421-006-0250-5. [DOI] [PubMed] [Google Scholar]
- 15.Filippi GM, Brunetti O, Botti FM, Panichi R, Roscini M, Camerota F, Cesari M, Pettorossi VE. Improvement of stance control and muscle performance induced by focal muscle vibration in young-elderly women: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90(12):2019–2025. doi: 10.1016/j.apmr.2009.08.139. [DOI] [PubMed] [Google Scholar]
- 16.Marconi B, Filippi GM, Koch G, Pecchioli C, Salerno S, Don R, Camerota F, Saraceni VM, Caltagirone C. Long-term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J Neurol Sci. 2008;275(12):51–59. doi: 10.1016/j.jns.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 17.Marconi B, Filippi GM, Koch G, Giacobbe V, Pecchioli C, Versace V, Camerota F, Saraceni VM, Caltagirone C. Long-term effects on cortical excitability and motor recovery induced by repeated muscle vibration in chronic stroke patients. Neurorehabil Neural Repair. 2011;25(1):48–60. doi: 10.1177/1545968310376757. [DOI] [PubMed] [Google Scholar]
- 18.Padulo J, Di Giminiani R, Dello Iacono A, Zagatto AM, Migliaccio GM, Grgantov Z, Ardigò LP. Lower Arm Muscle Activation during Indirect-Localized Vibration: The Influence of Skill Levels When Applying Different Acceleration Loads. Front Physiol. 2016;16(7):242. doi: 10.3389/fphys.2016.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pazzaglia C, Camerota F, Germanotta M, Di Sipio E, Celletti C, Padua L. Efficacy of focal mechanic vibration treatment on balance in Charcot-Marie-Tooth 1A disease: a pilot study. J Neurol. 2016;263:1434–1441. doi: 10.1007/s00415-016-8157-5. [DOI] [PubMed] [Google Scholar]
- 20.Johannsen L, Ackermann H, Karnath HO. Lasting amelioration of spatial neglect by treatment with neck muscle vibration even without concurrent training. J Rehabil Med. 2003;35(6):249–253. doi: 10.1080/16501970310009972. [DOI] [PubMed] [Google Scholar]
- 21.Kerkhoff G. Modulation and rehabilitation of spatial neglect by sensory stimulation. Prog Brain Res. 2003;142:257–71. doi: 10.1016/S0079-6123(03)42018-9. [DOI] [PubMed] [Google Scholar]
- 22.Schindler I, Kerkhoff G. Convergent and divergent effects of neck proprioceptive and visual motion stimulation on visual space processing in neglect. Neuropsychologia. 2004;42(9):1149–1155. doi: 10.1016/j.neuropsychologia.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Bar-Or O. The Wingate anaerobic test a update on methodology, reliability and validity. Sports Med. 1987;4(6):381–394. doi: 10.2165/00007256-198704060-00001. [DOI] [PubMed] [Google Scholar]
- 24.Rodio A, Quattrini FM, Fattorini L, Egidi F, Faiola F, Pittiglio GC. Power output and metabolic response in multiple Wingate tests performed with arms. Med Sport. 2008;61:21–28. [Google Scholar]
- 25.Inbar O, Bar-Or O, Skinner JS. The Wingate Anaerobic Test. 6th ed. Champaign, Ill: Human Kinetics; 1996. [Google Scholar]
- 26.Zupan MF, Arata AW, Dawson LH, Wile AL, Paynz TL, Hannon ME. Wingate Anaerobic Test Peak Power and Anaerobic Capacity Classification for Male and Female Intercollegiate Athletes. J Strength Cond Res. 2009;23(9):2598–2604. doi: 10.1519/JSC.0b013e3181b1b21b. [DOI] [PubMed] [Google Scholar]
- 27.Lopez S, Del Percio C, Marinozzi F, Celletti C, Suppa A, Ferri R, Staltari E, Camerota F, Babiloni C. Electroencephalographic sensorimotor rhythms are modulated in the acute phase following focal vibration in healthy subjects. Neuroscience. 2017;352:236–248. doi: 10.1016/j.neuroscience.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Dai W, Pi YL, Ni Z, Tan XY, Zhang J, Wu Y. Maintenance of balance between motor cortical excitation and inhibition after long-term training. Neuroscience. 2016;336:114–122. doi: 10.1016/j.neuroscience.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 29.Stinear CM, Byblow W.D. Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol. 2003;89(4):2014–2020. doi: 10.1152/jn.00925.2002. [DOI] [PubMed] [Google Scholar]
- 30.Fox EL, Bartels RL, Billings CE, Mathews DK, Bason R, Webb WM. Intensity and distance of interval training programs and changes in aerobic power. Med Sci Sports. 1973;5:18–22. [PubMed] [Google Scholar]
- 31.MacDougall JD, Hicks AL, MacDonald JR, McKelvie RS, Green HJ, Smith KM. Muscle performance and enzymatic adaptations to sprint interval training. J Appl Physiol. 1998;84:2138–2142. doi: 10.1152/jappl.1998.84.6.2138. [DOI] [PubMed] [Google Scholar]
- 32.MacInnis MJ, Gibala MJ. Physiological adaptations to interval training and the role of exercise intensity. J Physiol. 2017;595(9):2915–2930. doi: 10.1113/JP273196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz D, Morrison S. Current concepts in periodization of strength and conditioning for the sports physical therapist. Int J Sports Phys Ther. 2015;10(6):734–747. [PMC free article] [PubMed] [Google Scholar]