Abstract
Background
Pressure ulcers (i.e. bedsores, pressure sores, pressure injuries, decubitus ulcers) are areas of localised damage to the skin and underlying tissue. They are common in the elderly and immobile, and costly in financial and human terms. Pressure‐relieving support surfaces (i.e. beds, mattresses, seat cushions etc) are used to help prevent ulcer development.
Objectives
This systematic review seeks to establish: (1) the extent to which pressure‐relieving support surfaces reduce the incidence of pressure ulcers compared with standard support surfaces, and, (2) their comparative effectiveness in ulcer prevention.
Search methods
In April 2015, for this fourth update we searched The Cochrane Wounds Group Specialised Register (searched 15 April 2015) which includes the results of regular searches of MEDLINE, EMBASE and CINAHL and The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 3).
Selection criteria
Randomised controlled trials (RCTs) and quasi‐randomised trials, published or unpublished, that assessed the effects of any support surface for prevention of pressure ulcers, in any patient group or setting which measured pressure ulcer incidence. Trials reporting only proxy outcomes (e.g. interface pressure) were excluded. Two review authors independently selected trials.
Data collection and analysis
Data were extracted by one review author and checked by another. Where appropriate, estimates from similar trials were pooled for meta‐analysis.
Main results
For this fourth update six new trials were included, bringing the total of included trials to 59.
Foam alternatives to standard hospital foam mattresses reduce the incidence of pressure ulcers in people at risk (RR 0.40 95% CI 0.21 to 0.74). The relative merits of alternating‐ and constant low‐pressure devices are unclear. One high‐quality trial suggested that alternating‐pressure mattresses may be more cost effective than alternating‐pressure overlays in a UK context.
Pressure‐relieving overlays on the operating table reduce postoperative pressure ulcer incidence, although two trials indicated that foam overlays caused adverse skin changes. Meta‐analysis of three trials suggest that Australian standard medical sheepskins prevent pressure ulcers (RR 0.56 95% CI 0.32 to 0.97).
Authors' conclusions
People at high risk of developing pressure ulcers should use higher‐specification foam mattresses rather than standard hospital foam mattresses. The relative merits of higher‐specification constant low‐pressure and alternating‐pressure support surfaces for preventing pressure ulcers are unclear, but alternating‐pressure mattresses may be more cost effective than alternating‐pressure overlays in a UK context. Medical grade sheepskins are associated with a decrease in pressure ulcer development. Organisations might consider the use of some forms of pressure relief for high risk patients in the operating theatre.
Keywords: Humans, Bedding and Linens, Beds, Beds/standards, Pressure Ulcer, Pressure Ulcer/prevention & control, Pressure Ulcer/therapy, Randomized Controlled Trials as Topic
Plain language summary
Can pressure ulcers be prevented by using different support surfaces?
Pressure ulcers (also called bed sores, pressure sores and pressure injuries) are ulcers on the skin caused by pressure or rubbing at the weight‐bearing, bony points of immobilised people (such as hips, heels and elbows). Different support surfaces (e.g. beds, mattresses, mattress overlays and cushions) aim to relieve pressure, and are used to cushion vulnerable parts of the body and distribute the surface pressure more evenly. The review found that people lying on ordinary foam mattresses are more likely to get pressure ulcers than those lying on a higher‐specification foam mattress. In addition the review also found that people who used sheepskin overlays on their mattress developed fewer pressure ulcers. While alternating‐pressure mattresses may be more cost effective than alternating‐pressure overlays, the evidence base regarding the merits of higher‐specification constant low‐pressure and alternating‐pressure support surfaces for preventing pressure ulcers is unclear. Rigorous research comparing different support surfaces is needed.
Background
Description of the condition
Pressure ulcers (also known as pressure injuries, pressure sores, decubitus ulcers and bed sores) are areas of localised damage to the skin and underlying tissue, believed to be caused by pressure, shear or friction (EPUAP‐NPUAP 2009). Pressure ulcers are more likely to occur in those who are seriously ill; neurologically compromised (e.g. individuals with spinal cord injuries (Elliot 1999)); have impaired mobility (Allman 1997; Berlowitz 1990; Berlowitz 1997; Bianchetti 1993; Henoch 2003; Livesley 2002); or who are immobile (including those wearing a prosthesis, body brace or plaster cast). Other risk factors include impaired nutrition (Banks 1998; Casey 1997; Casey 1998; Ek 1990; Henoch 2003; Livesley 2002); obesity (Gallagher 1997; Livesley 2002); poor posture, which puts extra pressure on bony prominences; or using equipment that does not provide appropriate pressure relief, such as seating or beds. Pressure ulcers particularly affect older people (Hefley 1990; Krainski 1992; Livesley 2002; Orlando 1998; Pase 1998; Ronda 2002; Spoelhof 2000; Thomas 2001; Waltman 1991); but have also been reported in pregnant women (Prior 2002). Pressure ulcers have also been associated with an increased incidence of infection, including osteomyelitis (Darouiche 1994; Livesley 2002).
The development of pressure ulcers is relatively common. A review of epidemiological studies in Europe, Canada and the USA described the reported prevalence of pressure ulcers in European hospitals as ranging from 8.3% to 23% (Kaltenhalter 2001). In the UK, the overall prevalence of pressure ulcers within care settings was 10.2%, with 59% of these being hospital‐acquired (Phillips 2009). In the USA and Canada, prevalence ranged from 12.3% in US health care facilities (VanGilder 2009), to 33% in patients in the community with spinal cord injury, and the overall estimate of pressure ulcer incidence in Canadian healthcare settings has been reported as 26% (Woodbury 2004). The presence of pressure ulcers has been associated with a two‐ to four‐fold increase in risk of death in older people in intensive care units, however, these findings were not adjusted for other prognostic factors (Bo 2003; Clough 1994; Thomas 1996). Based on the available European data, it has been estimated that between one‐in‐four and one‐in‐five patients within an acute hospital setting (i.e. neurology, intensive care unit (ICU), chronic and acute care units) will have had a pressure ulcer (Vanderwee 2007a). Estimates on pressure ulcer incidence and prevalence from hospital‐based studies vary widely according to the definition and grade of ulcer, the patient population and care setting. Within the community, the incidence rate within the UK ranges from 4.4% to 6.8%, and in the USA and Canada it is up to 16.5% (Kaltenhalter 2001).
The financial cost of treating ulcers in the UK varies from GBP 1,064 for a grade 1 ulcer to GBP 10,551 for a grade 4 ulcer, with total costs in the UK estimated as being GBP 1.4 to 2.1 billion annually, which is equivalent to 4% of the total National Health Service (NHS) expenditure (Bennett 2004). National prevalence and incidence data from the US, based on a 24 hour data collection period at each participating institution, indicate that the annual cost to the American health system of treating all hospital‐acquired pressure ulcers is between USD 2.2 and 3.6 billion (Whittington 2004). An Australian study of public hospitals in 2001‐2002 predicted a median of 95,695 cases of pressure ulcers with a median of 398,432 bed days lost, incurring median opportunity costs of AU$285 Million (Graves 2005).
Healthcare professionals attempt to prevent and treat pressure ulcers by using a variety of support surfaces with the aim of relieving pressure. These include ‐ but are not limited to ‐ mattresses, beds, overlays, cushions and chairs. A summary of the available support surfaces for pressure ulcer treatment is the subject of another Cochrane review (McInnes 2011).
Description of the intervention
The aim of pressure ulcer prevention strategies is to reduce either the magnitude, or duration, of pressure between a patient and his (or her) support surface (i.e. the interface pressure), or both. This may be achieved by regular manual repositioning (e.g. two‐hourly turning), or by using pressure‐relieving support surfaces such as cushions, mattress overlays, replacement mattresses or whole bed replacements, which are widely used in both institutional and non‐institutional settings. Often a combination of repositioning and support surface enhancement may be used. Support surfaces are used with the aim of redistributing pressure, reducing shearing forces and controlling the local microclimate. The cost of these interventions varies widely; from over GBP 30,000 for some bed replacements, to less than GBP 100 for some foam overlays. Information on the relative cost‐effectiveness of this equipment is needed to inform use.
How the intervention might work
Pressure‐relieving cushions, beds and mattresses either mould around the shape of the patient to distribute the patient's weight over a larger contact area (constant low‐pressure (CLP) devices); or vary the pressure beneath the patient mechanically, thus reducing the duration of the applied pressure (alternating‐pressure (AP) devices) (Bliss 1993). CLP devices (either overlays, mattresses or replacement beds) can be grouped according to their construction (foam, foam and air, foam and gel, profiled foam, hammocks, air suspension, water suspension and air‐particulate suspension/air‐fluidised). These devices fit, or mould, around the body so that the pressure is dispersed over a large area, and are mainly classified as being of a lower technological specification (i.e. "low‐tech"). By comparison, air‐fluidised beds, where warmed air circulates through fine ceramic beads covered by a permeable sheet, and low‐air‐loss beds, where patients are supported on a series of air sacs through which warmed air passes, are high‐specification (i.e. "high‐tech") CLP devices.
Alternating‐pressure devices generate alternating high and low interface pressures between body and support, usually by alternate inflation and deflation of air‐filled cells. Such devices are available as cushions, mattress overlays, and single‐or multi‐layer mattress replacements. These devices are classified as "high‐tech".
Other support surfaces, such as turning beds, turning frames, net beds, and turning/tilting beds move patients who are unable to turn themselves manually or automatically. Pressure ulcer prevention is often not the reason for using turning and tilting beds, which may be used in Intensive and Critical Care Units for other reasons, e.g. to promote chest drainage.
Why it is important to do this review
Research indicates that pressure ulcers represent a major burden of sickness and reduced quality of life for patients, their carers (Franks 1999; Franks 2002; New Reference; Hagelstein 1995), and their families (Benbow 1996; Elliot 1999). Often patients who develop pressure ulcers require prolonged and frequent contact with the healthcare system; and suffer much pain (Briggs 2013; Emflorgo 1999; Flock 2003; Freeman 2001; Healy 2003; Manfredi 2002), discomfort and inconvenience (Franks 1999).
The presence of a pressure ulcer creates a number of significant difficulties psychologically, physically and clinically to patients, carers and their families. Clinicians, working in a variety of clinical and non‐clinical settings, including primary care and acute trusts, also face challenges when providing holistic, person‐centred services for the assessment and treatment of pressure ulcers. These challenges include clinical decisions regarding methods of assessment, and which treatments to use on individuals with an existing pressure ulcer.
Healthcare professionals attempt to reduce the incidence of severe pressure ulcers by the identification of people at high risk, and the use of preventative strategies, such as the deployment of pressure‐relieving equipment. It is essential that initiatives are based on the best available clinical‐ and cost‐effectiveness evidence, and we have, therefore, undertaken a systematic review of the evidence for the effectiveness of pressure‐relieving support surfaces such as beds, mattresses, cushions, and repositioning interventions.
Objectives
This systematic review seeks to establish:
(1) the extent to which pressure‐relieving support surfaces reduce the incidence of pressure ulcers compared with standard support surfaces, and, (2) their comparative effectiveness in ulcer prevention.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised trials comparing support surfaces, and which measured the incidence of new pressure ulcers were included. Trials that only reported subjective measures of outcome (e.g. skin condition "better" or "worse") were excluded, as were trials that reported only proxy measures such as interface pressure. Trials were eligible for inclusion if they reported an objective, clinical, outcome measure such as incidence and severity of new pressure ulcers developed.
Types of participants
People receiving health care who were deemed to be at risk of developing pressure ulcers, in any setting. Some trials involved people who had existing pressure ulcers, however, only the incidence of new pressure ulcers was examined.
Types of interventions
Trials which evaluated the following interventions for preventing pressure ulcers were included:
1. "Low‐tech" CLP support surfaces
Standard foam mattresses.
Alternative foam mattresses/overlays (e.g. convoluted foam, cubed foam): these are conformable and aim to redistribute pressure over a larger contact area.
Gel‐filled mattresses/overlays: mode of action as above.
Fibre‐filled mattresses/overlays: mode of action as above.
Air‐filled mattresses/overlays: mode of action as above.
Water‐filled mattresses/overlays: mode of action as above.
Bead‐filled mattresses/overlays: mode of action as above.
Sheepskins: proposed mode of action unclear.
2. "High‐tech" support surfaces
Alternating‐pressure (AP) mattresses/overlays: patient lies on air‐filled sacs that inflate and deflate sequentially to relieve pressure at different anatomical sites for short periods; these may incorporate a pressure sensor.
Air‐fluidised beds: warmed air circulates through fine ceramic beads covered by a permeable sheet; allowing support over a larger contact area (CLP).
Low‐air‐loss beds: patients are supported on a series of air sacs through which warmed air passes (CLP).
3. Other support surfaces
Turning beds/frames: these work either by aiding manual repositioning of the patient, or by motor driven turning and tilting.
Operating table overlays: mode of action as for low‐tech CLP support surfaces (above)
Wheelchair cushions: either conforming cushions that reduce contact pressures by increasing surface area in contact, or mechanical cushions e.g. alternating pressure.
Limb protectors: pads and cushions of different forms to protect bony prominences.
Types of outcome measures
Primary outcomes
1. Incidence of new pressure ulcers
Many evaluations simply measure the pressure on different parts of the body in contact with the support surface (i.e. the interface pressure). This, however, is an intermediate, or surrogate, outcome measure with serious limitations as a proxy for a clinical outcome, since the process which leads to the development of a pressure ulcer almost certainly involves the complex interplay of several factors. In this review we have only considered trials that reported the clinical outcome measure of pressure ulcer incidence.
Some trials do not differentiate between those people who develop grade 1 ulcers (in which the skin is unbroken), and those who develop more severe ulcers. Trials that compare the incidence of pressure ulcers of grade 2 or greater are more likely to be reliable (see below for details of grading system), however, we included all trials irrespective of whether grade 1 ulcers were described separately.
2. Grades of new pressure ulcers
Various pressure ulcer severity classification systems are in use, including in trials of pressure relieving interventions. An example of a commonly‐used grading system is presented below; this has been adapted from the EPUAP‐NPUAP classification system (NPUAP‐EPUAP‐PPPIA 2014): Grade 1: persistent discolouration of the skin including non‐blanchable erythema; blue/purple/black discolouration. Grade 2: partial‐thickness skin loss involving epidermis and dermis. Grade 3: full‐thickness skin loss involving damage or necrosis of subcutaneous tissues, but not through the underlying fascia, and not extending to the underlying bone, tendon or joint capsule. Grade 4: full‐thickness skin loss with extensive destruction and tissue necrosis extending to the underlying bone, tendon or joint capsule.
Secondary outcomes
Costs of the devices.
Patient comfort.
Durability/longevity of the devices.
Acceptability of the devices for healthcare staff.
Quality of life.
Search methods for identification of studies
Electronic searches
For this fourth review update, the following databases were searched for reports of relevant RCTs:
Cochrane Wounds Group Specialized Register (Searched 15/04/15)
The Cochrane Central Register of Controlled Trials (CENTRAL) ‐ The Cochrane Library 2015, Issue 3
Ovid MEDLINE & Ovid MEDLINE ‐ In‐Process & Other Non‐Indexed Citations 2014 to April 14 2015
Ovid EMBASE ‐ 2014 to April 14 2015
EBSCO CINAHL ‐ 2014 to April 15 2015
We used the following search strategy in the Cochrane Central Register of Controlled Trials (CENTRAL): #1 MeSH descriptor: [Beds] explode all trees 274 #2 mattress*:ti,ab,kw 462 #3 cushion*:ti,ab,kw 190 #4 "foam" or transfoam:ti,ab,kw 940 #5 overlay*:ti,ab,kw 428 #6 "pad" or "pads":ti,ab,kw 1768 #7 "gel":ti,ab,kw 5698 #8 pressure next relie*:ti,ab,kw 125 #9 pressure next reduc*:ti,ab,kw 1596 #10 pressure next alleviat* 2 #11 "low pressure" near/2 device*:ti,ab,kw 4 #12 "low pressure" near/2 support:ti,ab,kw 4 #13 constant near/2 pressure:ti,ab,kw 139 #14 "static air":ti,ab,kw 3 #15 alternat* next pressure:ti,ab,kw 32 #16 air next suspension*:ti,ab,kw 3 #17 air next bag*:ti,ab,kw 2 #18 water next suspension*:ti,ab,kw 8 #19 elevation near/2 device*:ti,ab,kw 7 #20 (clinifloat or maxifloat or vaperm or therarest or sheepskin or hammock or "foot waffle" or silicore or pegasus or cairwave):ti,ab,kw 65 #21 (turn* or tilt*) next (bed* or frame*):ti,ab,kw 40 #22 kinetic next (therapy or table*):ti,ab,kw 23 #23 net next bed*:ti,ab,kw 5 #24 "positioning" or "repositioning":ti,ab,kw 2221 #25 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 13217 #26 MeSH descriptor: [Pressure Ulcer] explode all trees 579 #27 (pressure next (ulcer* or sore* or injur*)):ti,ab,kw 1004 #28 (decubitus next (ulcer* or sore*)):ti,ab,kw 84 #29 ((bed next sore*) or bedsore*):ti,ab,kw 39 #30 #26 or #27 or #28 or #29 1064 #31 #25 and #30 341
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 1, Appendix 2 and Appendix 3 respectively. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)(Lefebvre 2009). The EMBASE and CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2008). There was no restriction on the basis of the language in which the trial reports were written, nor publication status.
Searching other resources
Originally, experts in the field of wound care were contacted to enquire about potentially‐relevant ongoing, and recently published, trials. In addition, manufacturers of support surfaces were contacted for details of any trials they were conducting. This process was not productive, and so was not repeated for this update. However, reference lists within obtained reviews and papers were scrutinised in an effort to identify additional trials.
Data collection and analysis
Selection of studies
For this update the titles and abstracts of the search results were assessed for relevance independently by two review authors. Full copies of all potentially‐relevant trials were obtained. Decisions on final inclusion after retrieval of full papers was made by one review author and checked by a second; disagreements were resolved by discussion with a third review author. Rejected trials were checked by a third review author.
Data extraction and management
Two review authors extracted details of included trials independently using a pre‐prepared data extraction sheet. We resolved any disagreements over data by discussion, with referral to a third review author for adjudication if necessary. The following data were extracted from each trial:
Care setting.
Clear description of main interventions.
Key baseline variables by group, for example, age, sex, baseline risk of pressure ulcer development, baseline area of existing ulcers.
Description of the interventions and numbers of patients randomised to each intervention.
Description of any co‐interventions/standard care.
Duration and extent of follow‐up.
Acceptability and reliability of equipment within the clinical setting.
Description of inclusion and exclusion criteria used to derive the sample from the target population.
Description of a priori sample size calculation.
Incident ulcers described by severity grading as well as frequency (grade 1 ulcers are not breaks in the skin and are subject to more inter‐rater variation).
We included trials published in duplicate only once; we nominated a primary data source, although we reviewed secondary publications for additional data.
Assessment of risk of bias in included studies
Two review authors assessed each included trial independently using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2008). This tool addresses six specific domains, namely sequence generation; allocation concealment; blinding of either participants, or personnel or assessors, or any combination of the three; incomplete outcome data; selective outcome reporting and other issues (e.g. extreme baseline imbalance) (see Appendix 4 for details of criteria on which the judgements are based). Blinding and completeness of outcome data were assessed separately for each outcome. We completed a risk of bias table for each eligible trial. We discussed any disagreement amongst all review authors to achieve a consensus. We present a risk of bias summary figure, which summarises the risk of bias assessments for each included study (Figure 1). Evaluating the validity of each trial may assist the reader in interpreting and making conclusions about the trial.
1.
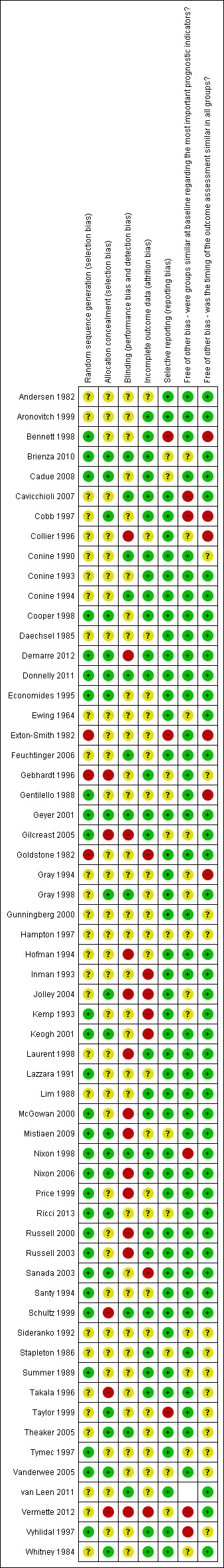
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Dealing with missing data
When a paper provided insufficient information for full data extraction, or if conflicting data were found, we approached trial authors for additional information. Where there were losses to follow‐up and a treatment effect existed we planned to test the robustness of the result to different assumptions in dealing with the missing data, for example assuming that all losses did not develop pressure ulcers.
Data synthesis
For each trial, we calculated risk ratio (RR) for categorical outcomes such as number of patients developing ulcers, with 95% confidence intervals (95% CI). The results were plotted on to graphs and individual study details are presented in the Characteristics of included studies. Where possible, Grade 1 pressure ulcers were reported separately from Grade 2 or higher pressure ulcers. Only the incidence of new pressure ulcers was reported in trials that included study participants with pre‐existing pressure ulcers.
Trials with similar patients, comparisons and outcomes were considered for pooled analysis. Where there was more than one trial comparing a similar device, statistical heterogeneity was assessed using I2 and tested for significance by use of the chi‐squared test. A value of I2 greater than 50% indicated substantial heterogeneity and was considered significant where p < 0.10 (Higgins 2003). In the absence of significant statistical heterogeneity, trials with similar comparisons where pooled using a random‐effect model. In the absence of significant statistical heterogeneity, trials with similar comparisons were pooled using a fixed‐effect model. Where pooling was inappropriate, the results of the trials were reported narratively.
For the purpose of meta‐analysis we assumed that the risk ratio remained constant for different lengths of follow‐up, hence studies were pooled if participants were followed‐up for different lengths of time. All statistical analysis were performed on RevMan 5.3 (RevMan 2014).
Results
Description of studies
Results of the search
The search for the fourth update of this review resulted in the inclusion of six new trials (Brienza 2010; Demarre 2012; Donnelly 2011; Ricci 2013; van Leen 2011; Vermette 2012). Four trials are classified as awaiting assessment; for two further information has been sought from trial authors (Allegretti 2008; Rafter 2011) and two trials are awaiting full text retrieval (Mastrangelo 2010; Mayer 2008). Eleven trials did not meet the inclusion criteria and were excluded (Bales 2012; Black 2012; Cassino 2013; Huang 2013; Jackson 2011; Nakahara 2012; Pham 2011a; Pham 2011b; Simonis 2012; Taccone 2009; Wu 2011) (see Characteristics of excluded studies table for reasons).
Included studies
The six new included trials brought the total number of included trials to 59 (Brienza 2010, Demarre 2012; Donnelly 2011; Ricci 2013; van Leen 2011; Vermette 2012) ) (see Characteristics of included studies and Table 1 which summarises some further aspects of study reporting quality). Thirty‐one trials involved participants without pre‐existing pressure ulcers (intact skin); ten trials included patients with ulcers greater than or equal to grade 1 at baseline; four trials did not specify the grading of the pre‐existing ulcers, and one trial only included people with grade 4 pressure ulcers. In 13 trials the baseline skin status of the participants was unclear.
1. Additional information on included studies.
| Trial | Clear inclusion & exclusion criteria | Sample size (arms) | A priori calculation | Grade 1 ulcer excluded | Intervention well documented |
| Andersen 1982 | Yes | 482 (3) | Yes | Yes | No |
| Aronovitch 1999 | Yes | 217 (2) | No | Yes | Yes |
| Bennett 1998 | Yes | 98 (2) | No | Yes | No |
| Brienza 2010 | Yes | 113/119 (2) | No | No | Yes |
| Cadue 2008 | Yes | 70/69 (2) | No | No | Yes |
| Cavicchioli 2007 | Yes | 170 (2) | No | No | Yes |
| Cobb 1997 | Yes | 123 (2) | No | No | Yes |
| Collier 1996 | No | 99 (9) | No | Not applicable | Yes |
| Conine 1990 | Yes | 187 (2) | No | Yes | No |
| Conine 1993 | Yes | 288 (2) | No | Yes | Yes |
| Conine 1994 | Yes | 163 (2) | No | Yes | Yes |
| Cooper 1998 | Yes | 100 (2) | No | Yes | Yes |
| Daechsel 1985 | Yes | 32 (2) | No | No | Yes |
| Demarre 2011 | Yes | 298/312 (2) | No | No | Yes |
| Donnelly 2011 | Yes | 120/119 (2) | No | No | Yes |
| Economides 1995 | Yes | 12 (2) | No | Yes | Yes |
| Ewing 1964 | No | 30 (2) | No | No | Yes |
| Exton‐Smith 1982 | Yes | 66 (2) | No | Yes | Yes |
| Feuchtinger 2006 | Yes | 175 (2) | Yes | No | Yes |
| Gebhardt 1996 | Yes | 43 (2) | No | Unclear | Yes |
| Gentilello 1988 | Yes | 65 (2) | No | No | Yes |
| Geyer 2001 | Yes | 32 (2) | No | Unclear | Yes |
| Gilcreast 2005 | Yes | 338 (2) | Yes | No | Yes |
| Goldstone 1982 | Yes | 75 (2) | No | No | Yes |
| Gray 1998 | Yes | 100 (2) | No | Yes | No |
| Gray 1994 | Yes | 170 (2) | No | Yes | Yes |
| Gunningberg 2000 | Yes | 101 (2) | Yes | Yes | Yes |
| Hampton 1997 | Yes | 75 (2) | No | No | Yes |
| Hofman 1994 | Yes | 44 (2) | Yes | Yes | Yes |
| Inman 1993 | Yes | 100 (2) | Yes | Yes | No |
| Jolley 2004 | Yes | 539 (2) | No | No | Yes |
| Kemp 1993 | Yes | 84 (2) | No | No | No |
| Keogh 2001 | Yes | 100 (2) | Yes | Yes | Yes |
| Laurent 1998 | Yes | 312 (4) | Yes | Yes | Yes |
| Lazzara 1991 | Yes | 74 (2) | No | Yes | No |
| Lim 1988 | Yes | 62 (2) | No | Yes | Yes |
| McGowan 2000 | Yes | 297 (2) | Yes | No | Yes |
| Mistiaen 2009 | Yes | 5434 (2) | Yes | No | Yes |
| Nixon 1998 | Yes | 446 (2) | Yes | Yes | Yes |
| Nixon 2006 | Yes | 1972 (2) | Yes | Yes | Yes |
| Price 1999 | Yes | 80 (2) | Yes | Yes | No |
| Ricci 2013 | Yes | 25 (2) | No | Yes | Yes |
| Russell 2000 | Yes | 198 (2) | No | No | Yes |
| Russell 2003 | Yes | 1166 (2) | Yes | No | Yes |
| Sanada 2003 | Yes | 103 (3) | Unclear | No | Yes |
| Santy 1994 | Yes | 505 (5) | Yes | No | Yes |
| Schultz 1999 | Yes | 413 (2) | Yes | No | No |
| Sideranko 1992 | Yes | 57 (3) | No | No | No |
| Stapleton 1986 | Yes | 100 (3) | No | Yes | No |
| Summer 1989 | Yes | 83 (2) | No | No | Yes |
| Takala 1996 | Yes | 40 (2) | Yes | Yes | Yes |
| Taylor 1999 | Yes | 44 (2) | Yes | No | Yes |
| Theaker 2005 | Yes | 62 (2) | Yes | Unclear | Yes |
| Tymec 1997 | Yes | 52 (2) | Yes | Yes | Yes |
| van Leen 2011 | No | 41/42 (2) | No | Yes | No |
| Vanderwee 2005 | Yes | 447 (2) | Yes | Yes | Yes |
| Vermette 2012 | No | 55 (2) | Yes | Unclear | Yes |
| Vyhlidal 1997 | Yes | 40 (2) | No | Yes | Yes |
| Whitney 1984 | No | 51 (2) | No | No | No |
Trial settings
Five trials evaluated different operating table surfaces (Aronovitch 1999; Feuchtinger 2006; Nixon 1998; Russell 2000; Schultz 1999); nine evaluated different surfaces in ICU (Cadue 2008; Gebhardt 1996; Gentilello 1988; Inman 1993; Laurent 1998; Sideranko 1992; Summer 1989; Takala 1996; Theaker 2005); eight trials confined their evaluation to orthopaedic patients (Cooper 1998; Exton‐Smith 1982; Goldstone 1982; Hofman 1994; McGowan 2000; Price 1999; Santy 1994; Stapleton 1986); and one involved both an Accident & Emergency and ward setting (Gunningberg 2000). Six trials were set in acute and extended care facilities (Conine 1990; Conine 1993; Conine 1994; Daechsel 1985; Donnelly 2011; Lim 1988); five trials were set in nursing homes (Brienza 2010; Geyer 2001; Lazzara 1991; Mistiaen 2009; van Leen 2011); and nine trials involved two or more different hospital wards (Bennett 1998; Cavicchioli 2007; Cobb 1997; Demarre 2012; Gray 1994; Kemp 1993; Russell 2003; Vanderwee 2005; Vermette 2012). Sixteen trials did not specify the trial setting (Andersen 1982; Collier 1996; Economides 1995; Ewing 1964; Gilcreast 2005; Gray 1998; Hampton 1997; Jolley 2004; Keogh 2001; Nixon 2006; Ricci 2013; Sanada 2003; Taylor 1999; Tymec 1997; Vyhlidal 1997; Whitney 1984).
Interventions
Twelve trials evaluated cushions; five evaluated the use of sheepskins; four looked at turning beds/tables; nineteen examined overlays; 28 looked at mattresses; three evaluated foam surfaces, two examined waffle surfaces and one examined the Heelift suspension boot. A number of trials evaluated multiple interventions.
Sample size
Small sample size was a major limitation of many of the trials; the median sample size was 98 (range 12 to 1171), and 21 trials reported an a priori sample size estimate.
Excluded studies
In total 70 studies were excluded from the review. Two were literature reviews (Heyneman 2009; Vanderwee 2008); nine studies reported insufficient information or data to allow a complete assessment and no further information was available through contact with the study authors (Barhyte 1995; Braniff‐Matthews 1997; Bliss 1995; Geelkerken 1994Holzgreve 1993; Neander 1996; Rafter 2011; Scott 1995; Zernike 1994); 24 trials did not report pressure ulcer incidence (Allen 1993; Ballard 1997; Brienza 2001; Cassino 2013; Colin 1996; deBoisblanc 1993; Della Valle 2001; Flam 1995; Gil Agudo 2009; Grindley 1996; Grisell 2008; Koo 1995; McMichael 2008; Pham 2011a; Pham 2011b; Rosenthal 1996; Scott 1999; Simonis 2012; Suarez 1995; Takala 1994; Turnage‐Carrier 2008; Wells 1984; Wild 1991; Zernike 1997); 16 studies did not use an eligible study design (Bales 2012; Black 2012; Bliss 1967; Büchner 1995; Chaloner 2000; Gray 2008; Gunningberg 1998; Huang 2013; Jackson 2011; Marchand 1993; Ooka 1995; Phillips 1999; Regan 1995; Reynolds 1994; Stoneberg 1986; Wu 2011); ten studies did not consider the intervention of interest, i.e. a support surface, (Defloor 1997; Defloor 2000; Defloor 2005; Huang 2009; Inman 1999; Jacksich 1997; Jesurum 1996; Nakahara 2012; Torra i Bou 2002; Vanderwee 2007) and nine studies did not meet the inclusion criteria for the review in other ways (Andrews 1989; Conine 1991; Fleischer 1997; Haalboom 1994; Hampton 1998; Hawkins 1997; Scott 2000; Thomas 1994; Timmons 2008).
Of the 24 studies which did not report pressure‐ulcer incidence, 14 recorded interface pressure as the primary outcome (Allen 1993; Brienza 2001; Della Valle 2001; Gil Agudo 2009; Grisell 2008; Koo 1995; McMichael 2008; Rosenthal 1996; Scott 1999; Suarez 1995; Takala 1994; Turnage‐Carrier 2008; Wells 1984; Wild 1991. Two reported comfort data (Ballard 1997; Grindley 1996); two reported a cost‐effective analysis (Pham 2011a; Pham 2011b); one reported healing data (Cassino 2013), and one reported hospital‐acquired pneumonia as a primary outcome and pressure ulcer incidence as a secondary outcome, but with no information as to whether the study was powered for secondary outcomes (Simonis 2012) (NB: the author has been contacted for further details). Other studies measured transcutaneous oxygen tension (Colin 1996); pneumonia (deBoisblanc 1993); skin temperature and moisture level (Flam 1995) and Zernike 1997 did not report the incidence of pressure ulcers
Risk of bias in included studies
Details of the risk of bias of each individual trial are included in Characteristics of included studies and shown in Figure 2 and Figure 1.
2.
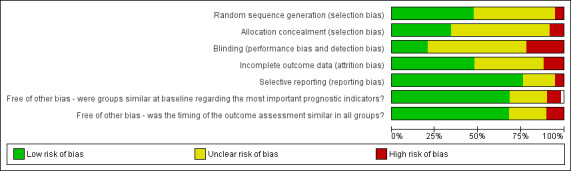
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
Allocation
The method of randomisation was unclear in 29 of the 59 (49%) included trials. Although the majority of trials reported patient eligibility criteria, just over a third of the reports gave information that indicated patients were allocated with concealed allocation (20 of the 59 trials or 34%).
Blinding
Blinded outcome assessment is rarely used in wound care trials, and this was the case in these evaluations of support surfaces. It can be difficult or impossible to disguise the surface that a patient is on for assessment of outcome, and patients are often too ill to be removed from their beds for assessment of their pressure areas. Nevertheless, some trials minimise bias in outcome assessment by having a second assessor and presenting inter‐rater reliability data, or by presenting photographic evidence of pressure area status which can then be assessed by an independent assessor blinded to treatment. Of the 59 RCTs in this review, we could be confident that blinded outcome assessment had been used in only twelve trials (20%).
Incomplete outcome data
Assessment of whether incomplete outcome data had been adequately addressed in each trial involved examining whether reasons for attrition or exclusion were reported; whether there was re‐inclusion of participants; and whether the completeness of data for each main outcome was described. Twenty‐eight of the 59 trials reviewed (i.e. 47%) adequately addressed incomplete outcome data. Seven of the remaining trials did not address incomplete outcome data adequately, and, for the final 24 trials it was unclear or unstated. High attrition rates and lack of an intention‐to‐treat analysis were also common.
Selective reporting
For a trial to have demonstrated it was free of selective outcome reporting, a trial protocol stating all pre‐specified outcomes needed to have been reported, or, if the trial protocol was not available, clear inclusion of all expected outcomes (including pre‐specified outcomes) should have been evident. We were satisfied that 45 out of 59 (76%) of the trials were free of selective outcome reporting. Three trials were not free of selective outcome reporting due to: pre‐specified outcomes not being completely reported, incomplete reporting of outcomes, or reporting of outcomes that were not pre‐specified (Bennett 1998; Exton‐Smith 1982; Taylor 1999). For eleven trials, there was insufficient information to classify whether there was or was not selective outcome reporting (Cadue 2008; Gebhardt 1996; Gentilello 1988; Gilcreast 2005; Hampton 1997; Mistiaen 2009; Ricci 2013; Stapleton 1986; van Leen 2011; Vanderwee 2005; Vermette 2012). We cannot exclude the possibility that we have introduced some level of bias by excluding trials which did not report 'pressure ulcer outcomes', this issue will be explored in more detail in the next update.
Other potential sources of bias
Other potential sources of bias included assessing whether the timing of outcomes under investigation were similar in both groups, and whether the groups under investigation were similar at baseline regarding the most important prognostic indicators. Timing of outcomes under investigation were similar in both groups under investigation in 39 (66%) of the 59 trials. In trials of pressure ulcer prevention, it is extremely important for trialists to report the baseline comparability of the intervention groups for important variables such as baseline risk. Amongst the included trials, risk of pressure ulcer development was measured by a variety of tools including the Norton (Norton 1979), Waterlow (Waterlow 1985), Gosnell (Gosnell 1973) and Braden (Bergstrom 1998) scales. Some of the trials reviewed here did not present such baseline data, nor explain what the various cut‐offs for inclusion in the trials meant in terms of whether trial participants were at low, medium or high risk for the development of pressure ulcers. Baseline characteristics were similar between the groups under investigation in 41 (69%) of the 59 trials. Another shortcoming was that trial reports were unclear about whether grade 1 pressure ulcers were included in the trial sample or the analysis, or both.
Risk of bias was not used to weight the trials in the analysis using any statistical technique, however, methodological quality is discussed in relation to the interpretation of the results. Methodological flaws for each trial are presented in Characteristics of included studies.
Effects of interventions
How the results are presented and what the terms mean
Results of dichotomous variables are presented as risk ratio (RR) with 95% confidence intervals (CI). Risk ratio has been used rather than odds ratios as it is easier to interpret than odds ratios (Deeks 1998). Risk ratio is the pressure ulcer incidence rate in the experimental group divided by the incidence rate in the control group and indicates the likelihood of pressure ulcer development on an experimental device compared with a comparison device. As, by definition, the risk of an ulcer developing in the control group is one, then the relative risk reduction associated with using the experimental bed is one‐minus‐RR. The risk ratio indicates the relative benefit of a therapy, but not the actual benefit, i.e. it does not take into account the number of people who would have developed an ulcer anyway. The absolute risk reduction (ARR) can be calculated by subtracting the incidence rate in the experimental group from the incidence rate in the control group. The ARR tells us how much the reduction is due to the support surface itself, and its inverse is the number needed to treat, or NNT. Thus an incidence rate of 30% on a control mattress reduced to 15% with an experimental mattress translates into an ARR of 30‐15 = 15% or 0.15, and an NNT of seven, in other words seven patients would need to receive the experimental mattress to prevent the development of one additional pressure ulcer.
Methods for measuring secondary outcomes such as comfort, durability, reliability and acceptability were not well developed. Where data were presented they appear in the Characteristics of included studies, but were not incorporated in the analysis.
1. "Low‐tech" constant low‐pressure (CLP) supports
This section considers comparisons of standard foam hospital mattresses with other low specification (low‐tech), constant low‐pressure (CLP) supports. We regarded the following as low‐tech CLP: sheepskin, static air‐filled supports; water‐filled supports; contoured or textured foam supports; gel‐filled supports; bead‐filled supports; fibre‐filled supports, and alternative foam mattresses or overlays. It should be emphasised, however, that there is no international definition of what constitutes a standard foam hospital mattress, and, indeed, this changes over time within countries, and even within hospitals. Where a description of the standard was provided it is included in the Characteristics of included studies table. We have assumed that standard mattresses are likely to vary less within countries than between countries, and undertook subgroup analysis by country, although this was not pre‐specified.
1.1 Standard foam hospital mattress compared with other "low‐tech" CLP
Eight RCTs compared 'standard' mattresses or surfaces with "low‐tech" supports for the prevention of pressure ulcers (Andersen 1982; Collier 1996; Goldstone 1982; Gray 1994; Gunningberg 2000; Hofman 1994; Russell 2003; Santy 1994).
When compared with standard hospital mattresses, the incidence and severity of pressure ulcers in patients deemed to be high risk were significantly reduced when patients were placed on either the cubed foam mattress (Comfortex DeCube) (RR 0.34; 95% CI 0.14 to 0.85) (Hofman 1994); the bead‐filled mattress (Beaufort bead bed) (RR 0.32; 95% CI 0.14 to 0.76) (Goldstone 1982); the Softfoam mattress (RR 0.2; 95% CI 0.09 to 0.45) (Gray 1994); or the water‐filled mattress (RR 0.35; 95% CI 0.15 to 0.79) (Andersen 1982) (Analysis 1.1).
1.1. Analysis.
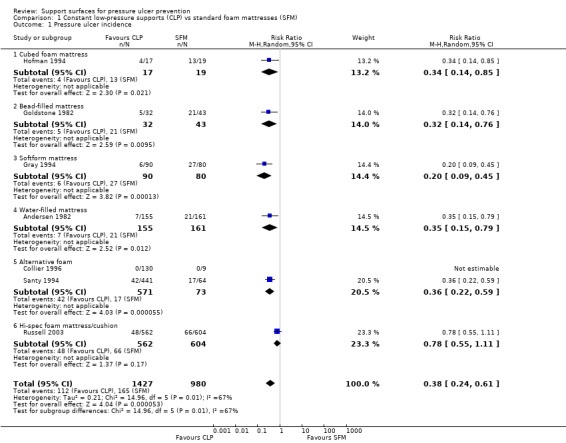
Comparison 1 Constant low‐pressure supports (CLP) vs standard foam mattresses (SFM), Outcome 1 Pressure ulcer incidence.
In an unpublished British trial of older people with hip fractures admitted to orthopaedic trauma wards, patients allocated to receive the then NHS standard foam mattress (manufactured by Relyon) experienced over three times the rate of pressure ulcers experienced by those using one of a number of foam alternatives (Clinifloat, Therarest, Transfoam and Vaperm) (RR 0.36; 95% CI 0.22 to 0.59) (Santy 1994). Another trial found a significant decrease in the incidence of grade 1 pressure ulcers from 26.3% to 19.9% (P value 0.0004), and a non‐significant decrease in the incidence of pressure ulcers grade 2 to 4 from 10.9% to 8.5% in patients allocated to the high‐specification foam mattress/cushion (CONFOR‐med) (RR 0.78; 95% CI 0.55 to 1.11) (Russell 2003). No patient developed a pressure ulcer in the Collier 1996 trial which involved a comparison of eight different foam mattresses (Reylon, Clinifloat, Omnifoam, Softform, STM5, Therarest, Transfoam and Vapourlux). The comparisons were considered too heterogeneous, and so we did not pool these seven trials (Analysis 1.1).
Gunningberg 2000 examined the effects of a viscoelastic foam trolley mattress and subsequent overlay on 101 patients with a suspected hip fracture in the Accident & Emergency (A&E) and ward setting. There was no significant difference in pressure ulcer incidence between those assigned a visco‐elastic foam trolley mattress on arrival in A&E followed by a viscoelastic foam overlay on the standard ward mattress (4/48, 8%) and those assigned a standard trolley mattress and then a standard hospital mattress on the ward (8/53, 15%).
The five trials comparing foam alternatives with the standard hospital foam mattress were pooled using a random‐effects model (I2 = 77%) (Collier 1996; Gray 1994; Hofman 1994; Russell 2003; Santy 1994). These trials were of mixed quality; they all provided evidence of allocation concealment, but none used blinded outcome assessment. To avoid double counting the control patients in the trials with more than two comparisons, and in the absence of major differences between the effects of different foams, the foam alternatives were pooled. This approach maintains the randomisation, but resulted in comparison groups of unequal size. This analysis yielded a pooled risk ratio of 0.40 (95% CI 0.21 to 0.74), or a relative reduction in pressure ulcer incidence of 60% (95% CI 26% to 79%) (Analysis 2.1). Concern regarding the heterogeneity in standard hospital mattress between these trials led us to undertake a separate meta analysis of UK‐based trials (where variation in the standard hospital mattress is likely to be lower). Pooling the four trials which compared alternative foam supports with standard foam mattresses in the UK resulted in the significant benefit of alternative foam over standard foam being maintained (RR 0.41; 95% CI 0.19 to 0.87) (Analysis 2.2) (Collier 1996; Gray 1994; Russell 2003; Santy 1994). However, the heterogeneity remained high (I2= 84%; P value 0.002), and Russell 2003 was removed as it was the only trial that clearly included grade 1 ulcers as incident ulcers, thereby potentially inflating its results compared with the other trials. This resulted in I2 being reduced to 39% (P value 0.20), and the results still favoured the alternative foam support over standard support (RR 0.29 95% CI 0.16 to 0.52). Therefore, foam alternatives to the standard hospital mattress significantly reduce the incidence of pressure ulcers in at‐risk patients, including patients with fractured neck of femur, when compared with the standard hospital foam.
2.1. Analysis.
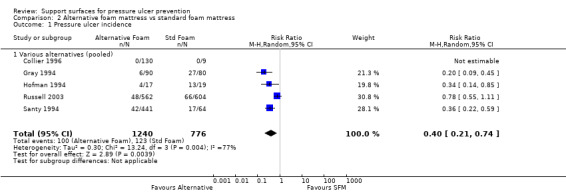
Comparison 2 Alternative foam mattress vs standard foam mattress, Outcome 1 Pressure ulcer incidence.
2.2. Analysis.
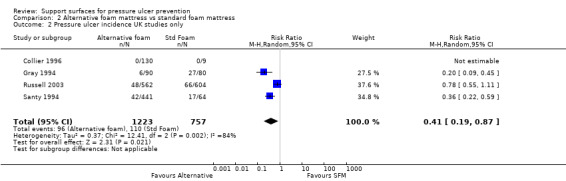
Comparison 2 Alternative foam mattress vs standard foam mattress, Outcome 2 Pressure ulcer incidence UK studies only.
1.2 Comparisons between alternative foam mattresses
This section covers results of head‐to‐head comparisons between high‐specification foam products (i.e. contoured foam, support surfaces comprising foam of different densities). Seven RCTs compared different foam mattresses (Analysis 3.1) (Collier 1996; Gray 1998; Kemp 1993; Ricci 2013; Santy 1994; van Leen 2011; Vyhlidal 1997).
3.1. Analysis.
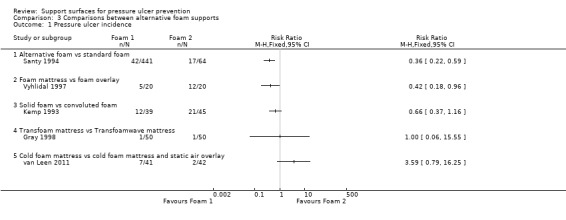
Comparison 3 Comparisons between alternative foam supports, Outcome 1 Pressure ulcer incidence.
No patients developed a pressure ulcer in the Collier 1996 trial, reported in the section above, which compared eight different foam mattresses. Santy 1994 and colleagues compared five alternative foam mattresses (Clinifloat, Vaperm, Therarest, Transfoam, NHS standard foam), and found significant reductions in pressure ulcer incidence associated with Clinifloat, Therarest, Vaperm and Transfoam compared with standard foam; and Vaperm compared with Clinifloat (RR 0.36; 95% CI 0.22 to 0.59). Vyhlidal 1997 compared a 4‐inch thick foam overlay (Iris 3000) with a foam and fibre mattress replacement (Maxifloat), and reported a significant reduction in pressure ulcer incidence with the mattress replacement (RR 0.42; 95% CI 0.18 to 0.96), however, this trial did not state the methods used for allocation concealment nor blinded outcome assessment clearly.
Kemp 1993 compared a convoluted foam overlay with a solid foam overlay in only 84 patients, and found no significant difference in pressure ulcer incidence rates, however, this may be a Type 2 error, as the small sample size may have precluded detection of a clinically important difference as statistically significant (RR 0.66; 95% CI 0.37 to 1.16). Gray 1998 compared the Transfoam and Transfoamwave foam mattresses, however, only one patient in each group (50 in each arm) developed an ulcer (Analysis 3.1).
No patient developed a pressure ulcer in the study by Ricci 2013 which compared the Airatext mattress overlay with the Akton mattress overlay and followed up patients for 28 days. However the sample size was small (25 in each group) and the study may have been at risk of Type 2 error.
Another trial compared standard cold foam mattress with a combination of standard cold foam mattress and static air overlay (van Leen 2011). No evidence of a difference was found (RR 3.59; 95% CI 0.79 to 16.25) (Analysis 3.1).
Summary: existing evidence is inadequate to guide choice between alternative foam mattresses.
1.3 Comparisons between "low‐tech" constant low‐pressure supports
This section covers head‐to‐head comparisons of the following types of support: foams; static air‐filled supports (including dry flotation); water‐filled supports; gel‐filled supports; silicore‐filled supports; heel elevators and sheepskins (Analysis 4.1). These devices and support surfaces feature particular or specialised technologies and therefore are considered in a separate category. [NB: 'Silicore' fibres are said to resist matting down and to provide insulation against heat or cold]
4.1. Analysis.
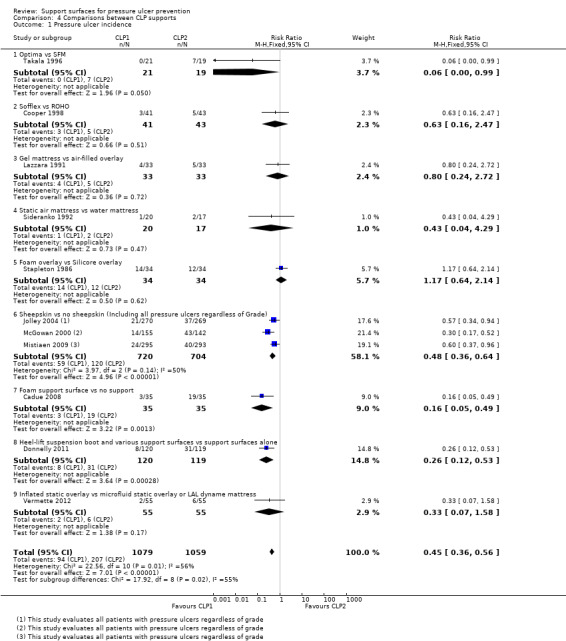
Comparison 4 Comparisons between CLP supports, Outcome 1 Pressure ulcer incidence.
Thirteen RCTs compared different "low‐tech" CLP devices (Cadue 2008; Cooper 1998; Donnelly 2011; Ewing 1964; Gilcreast 2005; Jolley 2004; Lazzara 1991; McGowan 2000; Sideranko 1992; Stapleton 1986; Takala 1996; Tymec 1997; Vermette 2012). Most of these trials were underpowered with, or without other methodological flaws.
Static air‐filled supports (including dry flotation); water‐filled supports; gel‐filled supports; silicore‐filled supports
A trial from Finland (Takala 1996), compared a constant low‐pressure mattress (Optima, Carital) ‐ that consists of 21 double air bags on a base ‐ with the standard hospital mattress and found that significantly more patients (37%) developed ulcers on the standard mattress than on the CLP mattress (on which nobody developed an ulcer) (RR 0.06; 95% CI 0 to 0.99). The report of this trial did not describe either allocation concealment or blinded outcome assessment.
Vermette 2012 compared the clinical and the cost effectiveness of an inflated overlay (inflated static overlay) with microfluid static overlay (allocated to 50 of the control group) or low‐air‐loss dynamic mattress with pulsation (allocated to 5 in the control group) for preventing pressure ulcers. There was no significant difference between groups (RR 0.33; 95%CI 0.07 to 1.58) (Analysis 4.1).
Vermette 2012, also conducted a cost‐effectiveness analysis and reported the total rental costs of the microfluid static overlay and the low‐air‐loss dynamic mattress with pulsation as $16,032 (USD) and the cost of the inflated static overlay (single purchase cost per patient) as $3,364 (USD). However, incremental cost effectiveness ratio data were not presented.
The remaining trials were all unique comparisons with low power (Cooper 1998; Lazzara 1991; Sideranko 1992; Stapleton 1986), and none found evidence of a difference between the surfaces tested (Analysis 4.1).
Heel devices
One trial (52 patients) compared a proprietary heel elevation device (Foot Waffle) comprising a vinyl boot with built‐in foot cradle, against elevation of the heels using a hospital pillow (Tymec 1997). The trial reported that more heel ulcers developed in the group using the Foot Waffle (n = 6) compared with the group using a hospital pillow) (n = 2) although this difference was not statistically significant, the number of people in each group was not clearly reported, and, therefore, data were not plotted.
Gilcreast 2005 assessed three heel pressure relief devices: a fleece cushion heel protector (the Bunny Boot); the egg‐crate heel lift positioner and the foot waffle air cushion. There was no evidence of a difference between the devices in terms of incidence of pressure ulcers (3/77 (4%) for the Bunny boot; 4/87 (4.6%) for the egg crate and 5/76 (6.6%) for the foot waffle). However, it was not clear from the trial whether the number of incident ulcers or number of participants with incident ulcers was being reported. Furthermore, the analysis of this trial was not by intention‐to‐treat, and 30% of data were not included in the analysis due, in part, to non‐compliance. Therefore this result is at high risk of bias.
Donnelly 2011 compared the Heelift suspension boot and pressure‐redistributing support surfaces with pressure‐redistributing support surface alone in a trial with 240 patients with hip fracture. There was a significant difference in pressure ulcer incidence rates favouring reduced incidence in the Heelift suspension boot group (RR 0.26; 95% CI 0.12 to 0.53). However, different pressure‐redistributing support surfaces allocated were allocated to both study groups (Pentaflex cut foam mattress, an AlphaXcell mattress overlay, an AutoExcel mattress overlay and Nimbus 3 alternating mattress), and these were allocated by ward nurses according to perceived need. (Analysis 4.1). The trial was stopped early on the basis of an interim analysis. Some patients reported that the boot was uncomfortable and hindered sleep.
Sheepskins
Four trials examined the effects of sheepskins on pressure ulcer incidence. The first, which compared the standard hospital mattress with, and without, sheepskin overlays (Ewing 1964), was considered too small and suffering from risk of bias to the extent that its results could not be regarded as valid. The second involved 297 orthopaedic patients (McGowan 2000), and found that pressure ulcer incidence was significantly reduced in those assigned an Australian medical sheepskin (RR for sheepskins relative to standard treatment was 0.30; 95% CI 0.17 to 0.52). The third, by Jolley 2004, was a trial on a mixed inpatient population of a metropolitan hospital comparing a sheepskin mattress overlay with ‘usual care’ that included repositioning and any other pressure‐relieving devices with, or without, "low‐tech" constant pressure relieving devices. It seems that analysis by intention‐to‐treat was not used, as 539 participants were randomised, but only 441 analysed. The trial stated that any patient whose risk increased to high, as measured by a Braden score of less than 12 for 48 hours, was no longer followed‐up. The rationale for this was not clear. The results, in terms of incidence of new pressure ulcers of grade 2 or above, were 12/218 (5.5%) for the sheepskin group and 20/223 (9%) for the ‘usual care’ group (reported denominators). A trial by Mistiaen 2009 investigated the use of an Australian medical sheepskin for use 48 hours after admission, compared with usual care. The 543 patients, mainly from aged care rehabilitation facilities, were followed‐up for 30 days. Pooling the trials by McGowan 2000; Jolley 2004 and Mistiaen 2009 using a random‐effects model, and including data for patients who developed pressure ulcers of any grade (including grade 1), showed there were fewer pressure ulcers among those allocated sheepskins (RR 0.48 95% CI 0.31 to 0.74) (Analysis 4.1). These three trials were then pooled using only data for patients with pressure ulcers grade 2 or above using a fixed‐effect analysis as the heterogeneity was low (I2 = 3%). The difference in risk of pressure ulceration was no longer statistically significant when grade 1 injury was excluded (RR 0.59 95% CI 0.33 to 1.05) (Analysis 4.2).
4.2. Analysis.
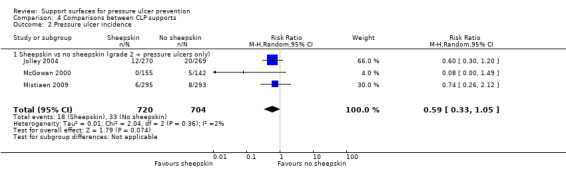
Comparison 4 Comparisons between CLP supports, Outcome 2 Pressure ulcer incidence.
Foam body support
One trial, with 70 intensive care unit participants (Cadue 2008), compared a foam body support plus usual care (half‐seated position, water mattress and preventative massage six times a day) with usual care alone for the prevention of heel ulcers. In total 8.6% (3/35) of participants in the support group developed heel ulcers (all grades) compared with 55.4% (19/35) in the control group, this was evidence of a difference in favour of the foam body support (RR 0.16 95% CI 0.05 to 0.49) (Analysis 4.1). This trial was at low or unclear risk of bias (unclear because we could not ascertain whether outcome assessment was blinded, nor whether there was risk of selective outcome reporting).
Summary: Foam alternatives to the standard hospital foam mattress reduce the incidence of pressure ulcers in people at risk, although one large trial found no difference between high‐specification foam mattress and use of standard mattress (Russell 2003). Three trials investigating the effectiveness of a specific sheepskin product in preventing pressure ulcers showed that sheepskin overlays are effective in reducing the incidence of pressure ulcers. While one trial of good quality showed a reduced incidence of pressure ulcers in the group allocated a heel suspension boot (Donnelly 2011), the lack of standardised co‐interventions and the lack of a standardised comparison (which consisted of variable pressure‐relieving support surfaces allocated by the ward nurses), makes it difficult to determine cause and effect. Other evidence about competing CLP devices did not show clear differences between the effectiveness of products.
2. "High‐tech" pressure supports
This section outlines three main groups of supports; alternating‐pressure (AP) supports, low‐air loss beds and air‐fluidised beds.
Alternating‐pressure supports
A variety of alternating‐pressure (AP) supports is used in hospital and community locations. The depth of the air‐cells, cell cycle time and mechanical robustness vary between devices, and these factors may be important in determining effectiveness. It is worth emphasising that most of the RCTs of AP supports did not describe the equipment being evaluated adequately, including the size of the air cells and cell cycle time.
Nineteen RCTs of AP supports for pressure ulcer prevention were identified: these included the following comparisons:
a) alternating‐pressure compared with standard hospital mattress ( two trials); b) alternating‐pressure compared with constant low‐pressure (11 trials) including:
static air;
water;
foam;
continuous low‐pressure;
silicore.
c) Comparison between different AP devices (six trials).
2.1 Alternating‐pressure compared with standard hospital mattress
Andersen 1982 reported that the use of alternating‐pressure surfaces significantly reduced the incidence of pressure ulcers compared with standard hospital mattresses. The report of this large trial, involving 482 patients who were defined by the authors as being at high‐risk of pressure ulcers, gave no indication that either allocation concealment or blinded outcome assessment had been used. In an underpowered and unblinded trial conducted on patients requiring head elevation, Sanada 2003 compared a single layer air cell overlay (the Air Doctor), a double‐layer cell overlay (the Tricell) (both with five‐minute alternating air pressure) and a standard hospital mattress (Paracare). In the Sanada trial, both the experimental groups and control group had a two‐hourly change of position and skin care. In the Air Doctor group 4/29 (13.8%) participants developed grade 2 pressure ulcers, in the Tricell group 1/26 (3.8%) participants developed grade 2 pressure ulcers; and in the standard hospital mattress group 6/27 (22%) participants developed grade 2 pressure ulcers. The number of grade 1 ulcers was also reported in the trial.The denominators are numbers presented by the authors after withdrawals and attrition, and the trial was not analysed by intention‐to‐treat (in that withdrawals were excluded from the analysis). For the purpose of meta‐analysis, this three‐armed trial was merged into two groups receiving AP overlay.
These two trials were pooled using a fixed‐effect model (I2 = 0%). There was a statistically significant reduction in development of pressure ulcers with the AP surface compared with the standard hospital mattress (RR 0.31; 95% CI 0.17 to 0.58), however, it should be recognised that these trials were at unclear or high risk of bias (Andersen 1982 was poorly reported for randomisation, allocation concealment and blinding and Sanada 2003 was at high risk of attrition bias) (Analysis 5.1).
5.1. Analysis.
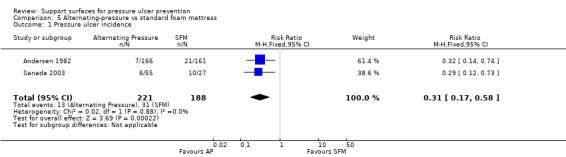
Comparison 5 Alternating‐pressure vs standard foam mattress, Outcome 1 Pressure ulcer incidence.
Summary: Results of two trials comparing AP devices with standard mattresses showed some evidence in favour of the AP support surfaces, however these trials were at high risk of bias.
2.2 Alternating‐pressure compared with constant low‐pressure
Eleven trials compared AP devices with various constant low‐pressure (CLP) devices, however, there was conflicting evidence regarding their relative effectiveness. A two‐armed trial compared a range of AP supports with a range of CLP supports in a range of specialties in acute care settings (Gebhardt 1996), and reported significantly more pressure ulcers in patients in the CLP group (34% compared with 13% in the AP group) (RR 0.38; 95% CI 0.22 to 0.66) (Analysis 6.1). This trial was difficult to interpret because of the wide variety of surfaces it used; there is currently insufficient evidence to support a 'class effect' for all alternating‐pressure devices and all constant low‐pressure devices.
6.1. Analysis.
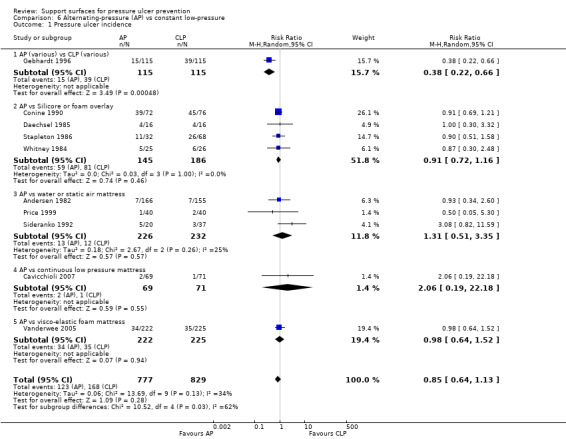
Comparison 6 Alternating‐pressure (AP) vs constant low‐pressure, Outcome 1 Pressure ulcer incidence.
In contrast, nine RCTs comparing different types of AP supports and a variety of CLP devices, such as the Silicore overlay (Conine 1990; Daechsel 1985; Stapleton 1986); a water mattress (Andersen 1982; Sideranko 1992); a foam pad (Stapleton 1986; Whitney 1984); and static air mattresses (Price 1999; Sideranko 1992); a visco‐elastic foam mattress (including four‐hourly turning and a sitting protocol with a cushion) (Vanderwee 2005); and CLP mode of the Hill‐Rom Duo mattress (Cavicchioli 2007); individually reported no difference in effectiveness, although some were too small to be able to detect clinically important differences as statistically significant. In the Vanderwee trial, a sub‐group analysis on the location of pressure ulcers reported that there were significantly more heel pressure ulcers in the control group using the viscoelastic mattress (P value 0.006 Fischer's exact test). The trial authors also noted that patients nursed on the experimental equipment (Huntleigh APAM, Alpha X‐cell) seemed to develop more severe ulcers (Analysis 6.1).
Four trials that compared AP with Silicore or foam overlays were pooled (Conine 1990; Daechsel 1985; Stapleton 1986; Whitney 1984). To avoid double counting of the patients in the AP arm of the Stapleton three‐arm trial, and in the absence of obvious heterogeneity in the outcomes for Silicore and foam, the Silicore and foam arms were pooled against the AP arm (maintaining the randomisation, avoiding double counting, but resulting in unequal comparison groups). Overall the pooled relative risk of pressure ulcer development for AP compared with Silicore or foam overlays (using a fixed‐effect model; I2 = 0%) was 0.91 (95% CI 0.72 to 1.16), indicating no evidence of a difference between Silicore or foam overlays and AP (Analysis 6.1).
The trials that compared AP with static water, or static air mattresses, were also considered together (Andersen 1982; Price 1999; Sideranko 1992). The Sideranko trial also had three comparison groups, and, for the purposes of the meta‐analysis, the water and static air arms of this trial were considered sufficiently similar to pool together against AP to avoid double counting of the AP patients. Pooling these three trials to answer the question of whether AP is associated with fewer incident ulcers than air‐ or water‐filled mattresses using a random‐effects model (I2 = 25%) yielded a pooled RR of 1.31 (95% CI 0.51 to 3.35), indicating no evidence of a difference (Analysis 6.1.3). It is worth emphasising, however, that some of these trials were small, and, even when pooled, were too underpowered to detect clinically important differences in effectiveness as statistically significant.
All nine RCTs comparing the various CLP devices and AP devices were pooled to try to determine whether AP is more effective than CLP in pressure ulcer prevention. Double counting was avoided for the Sideranko and Stapleton trials as before. In view of the different devices evaluated in the trials, the I2 of 34% and the Chi2 statistic of 13.69 (df = 9), a random‐effects model was applied. This yielded an overall relative risk of 0.85 (95% CI 0.64 to 1.13), which suggested no evidence of a difference between the rates of pressure ulcer incidence with AP compared with CLP (Analysis 6.1). Further trials are needed to determine whether the CLP and AP devices are associated with a clinically important difference in risk of pressure ulceration.
One trial used a complex factorial design to compare various combinations of standard, constant low‐pressure (Tempur) and alternating‐pressure (Nimbus) support in surgical intensive care patients intra‐ and post‐ICU. This trial (which involved only 75 to 80 patients in each group) did not identify any significant benefit associated with using alternating‐pressure in the ICU (Laurent 1998) (Analysis 7.1).
7.1. Analysis.
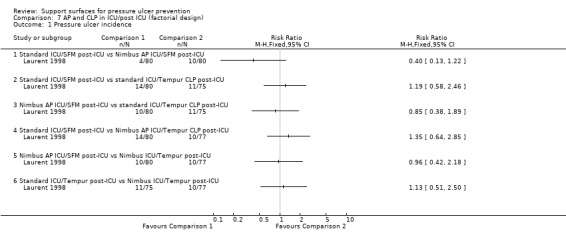
Comparison 7 AP and CLP in ICU/post ICU (factorial design), Outcome 1 Pressure ulcer incidence.
Summary: The relative merits of alternating‐ (AP) and constant low‐pressure (CLP) devices, and of the different AP devices for pressure ulcer prevention are unclear with most trials comparing AP with CLP devices and showing no significant difference between treatment groups. One large, high quality trial found no significant differences between an AP overlay with an AP mattress. However, the AP mattresses were associated with an 80% probability of reducing costs, due to a delay in pressure ulceration and reduced length of stay in hospital when they were used.
2.3 Comparisons between different alternating‐pressure devices
Six trials compared different alternating pressure devices. AP devices differ somewhat in structure, for example, the size of the inflatable air cells. One early trial of pressure ulcer prevention compared two large‐celled alternating‐pressure devices (Pegasus Airwave and the Large Cell Ripple ‐ similar except that the Airwave has two layers of cells) (Exton‐Smith 1982). The authors reported that the Airwave system was significantly more effective than the Large Cell Ripple in preventing and reducing severity of pressure ulcers in a high risk group of elderly patients. However, the allocation was not truly random, and an analysis which regarded losses to follow‐up as having not developed pressure ulcers did not show any evidence of a difference in the rate of pressure ulcers (16% versus 34%; P value > 0.05; Analysis 8.1).
8.1. Analysis.
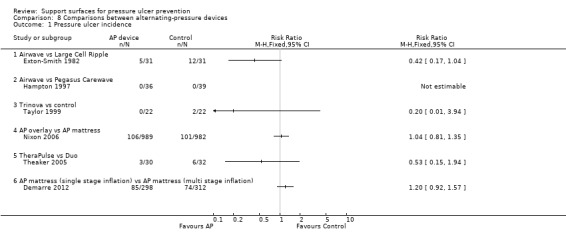
Comparison 8 Comparisons between alternating‐pressure devices, Outcome 1 Pressure ulcer incidence.
Hampton 1997 compared the Pegasus Airwave mattress with a new Cairwave Therapy system by the same manufacturer, in 75 patients. No patients developed an ulcer within the 20‐day follow‐up in either arm of this trial.
Taylor 1999 compared the Pegasus Trinova three‐cell alternating‐pressure air mattress plus a pressure redistributing cushion (intervention) with a two‐cell alternating‐pressure air mattress plus a pressure redistributing cushion (control). This trial was underpowered and so could not detect important differences (22 patients in each group), and, whilst two patients developed a superficial ulcer in the control group and none in the intervention group, there was no evidence of a difference between the two groups (RR 0.20; 95% CI 0.01 to 3.94) (Analysis 8.1).
In another underpowered trial, Theaker 2005 examined two AP devices in an ICU setting. The KCI Therapulse, a stand‐alone unit that incorporates a mattress into a bed frame and uses optional pulsation technology and low‐air‐loss to reduce tissue interface pressure, and the Hill‐Rom Duo mattress (control), which is designed to lie directly on most standard hospital frames and uses either continuous or alternating low‐pressure modes. Details of the alternating cycle were not provided. Pressure ulcer incidence (restricted to grade 2 ulcers or greater) was 3/30 (10%) in the experimental group and 6/32 (19%) in the control group (no evidence of a difference).
In a large trial at low risk of bias, Nixon 2006 compared an AP overlay with an AP mattress for the primary outcome of incidence of pressure ulcers (grade 2 or above). An intention‐to‐treat analysis was conducted on data from 1971 participants (989 in the overlay group and 982 in the mattress group). One‐hundred and six (10.7%) people in the overlay group and 101 (10.3%) in the mattress group developed one or more new grade 2 pressure ulcers. The majority of incident ulcers were grade 2. There was no significant difference between the two groups in terms of development of a new pressure ulcer of grade 2 or greater (RR 1.04; 95% CI 0.81 to 1.35). More participants on the overlay requested a change to another device due to their dissatisfaction (23.3%), compared with patients allocated to the AP mattress (18.9%) (Analysis 8.1).
Nixon 2006 also conducted a full cost‐effectiveness analysis from the perspective of the UK NHS and Personal Social Service. Calculation of cost information was based on length of hospital stay and pressure‐relieving surface used. Benefits were measured as the number of pressure‐ulcer‐free days. In the base case analysis the mean cost per patient of the AP mattress was GBP 6509.73, and the mean cost per patient of the AP overlays was GBP 6793.33. The mattress cost on average GBP 283.6 less per patient, (95% CI, GBP 377.59 to GBP 976.79), and also conferred greater benefits (a delay in mean time to ulceration of 10.64 days (95% CI 24.40 to 3.09). Whilst neither the difference in costs nor benefits reached statistical significance, the assessment of uncertainty around the cost‐effectiveness decision indicated that, on average, AP mattresses were associated with an 80% probability of being a cost saving. This was because the mattress was associated with a delay in ulceration (measured by Kaplan Meier estimates), and reduced costs as a consequence of shorter length of hospital stay. The conclusions of the base case analysis was not altered when challenged in sensitivity analyses.
Demarre 2012 compared multistage versus single stage inflation and deflation cycle for alternating low pressure air mattresses to prevent pressure ulcers in a trial of 610 participants and found no difference in pressure ulcer incidence (RR 1.20; 95%CI 0.92 to 1.57). (Analysis 8.1).
Low‐air‐loss (LAL) beds
Three trials evaluated the use of low‐air‐loss beds. Such devices provide a flow of air that assists in controlling the microclimate of the patient's skin (NPUAP 2007).
2.4 Comparisons between LAL and other support surfaces
Inman 1993 reported that low‐air‐loss beds were more effective at decreasing the incidence of pressure ulcers in critically‐ill patients than a standard (but poorly described) ICU bed (RR 0.24; 95% CI 0.11 to 0.53) (Analysis 9.1).
9.1. Analysis.
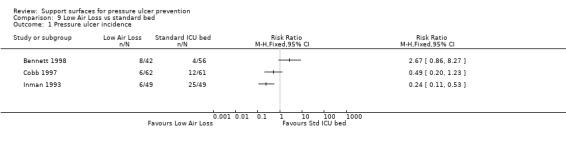
Comparison 9 Low Air Loss vs standard bed, Outcome 1 Pressure ulcer incidence.
A second trial of 98 participants, compared low‐air‐loss hydrotherapy (LAL‐hydro) with standard care (some patients received alternating‐pressure in this group); more patients developed ulcers of grade 2 ulcer or greater in the LAL‐hydro group (19%) than the standard care group (7%) though there was no evidence of a difference (Analysis 9.1) (Bennett 1998).
A third trial with 123 participants recruited from hospital wards and intensive care units compared a low‐air‐loss bed (KinAir) with a static air overlay in the prevention of pressure ulcers (Cobb 1997). Three people developed grade 1 ulcers on the low‐air‐loss bed (3/62) compared with one on the static air overlay (1/61). However, three people developed grade 2 ulcers on the low‐air‐loss bed (3/62) compared with 11 on the static air overlay (11/61). Comparing the incidence of all ulcers showed no evidence of a difference between the two groups (Analysis 9.1).
Cobb and Inman were pooled as they investigated LAL beds with alternatives in the ICU setting. This showed evidence of a difference in favour of the low‐air‐loss bed (RR 0.33; 95% CI 0.16 to 0.67) (random‐effects, I2 = 26% Analysis 9.2) (Cobb 1997; Inman 1993). Inman 1993 also reported that low‐air‐loss beds reduced the incidence of patients developing multiple pressure ulcers compared with the standard ICU mattress (RR 0.08 95% CI 0.01 to 0.62) (Analysis 9.3).
9.2. Analysis.
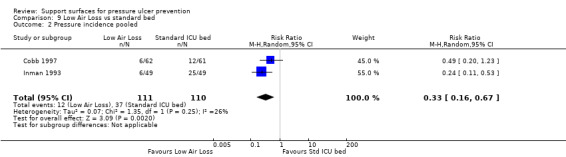
Comparison 9 Low Air Loss vs standard bed, Outcome 2 Pressure incidence pooled.
9.3. Analysis.
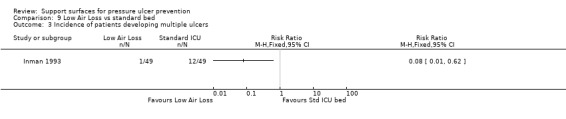
Comparison 9 Low Air Loss vs standard bed, Outcome 3 Incidence of patients developing multiple ulcers.
Air‐fluidised beds
2.5 Comparison between air‐fluidised bed and dry flotation mattress
One small trial that investigated 12 patients after plastic surgical repair of pressure ulcers showed no difference between an air‐fluidised bed and the Roho dry flotation mattress in postoperative tissue breakdown rates (Economides 1995) (Analysis 10.1).
10.1. Analysis.
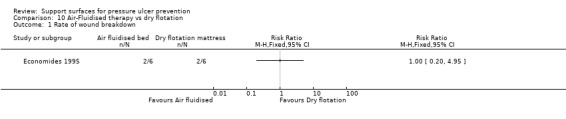
Comparison 10 Air‐Fluidised therapy vs dry flotation, Outcome 1 Rate of wound breakdown.
3. Other pressure supports
Other pressure supports included Kinetic turning tables, profiling beds, operating table overlays and seat cushions. Turning beds contain motors which constantly turn and tilt the patient. This includes kinetic beds and profiling beds. They are used in critical care settings, primarily to prevent pneumonia and atelectasis (collapsed lung). Operating table overlays are used as pressure relief during surgery.
Kinetic turning tables
3.1 Comparison between kinetic beds and conventional beds
Four RCTs were identified in a meta‐analysis of kinetic therapy (Choi 1992), however, full copies of only two of the individual trials could be obtained for this systematic review (Gentilello 1988; Summer 1989). These two trials evaluated kinetic bed against conventional beds. Sample sizes in all the trials were small, and no beneficial effect of kinetic therapy on incidence of pressure ulcers was detected (Analysis 11.1).
11.1. Analysis.
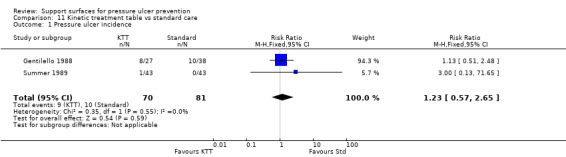
Comparison 11 Kinetic treatment table vs standard care, Outcome 1 Pressure ulcer incidence.
Profiling beds
3.2 Comparison between profiling bed and flat‐based bed
Keogh 2001 recruited 70 participants, and found that no pressure ulcers developed in either the group assigned to the profiling bed with a pressure‐reducing foam mattress or cushion combination or the group assigned to a flat‐based bed with a pressure‐relieving/redistributing foam mattress or cushion combination. Patients were followed‐up for five to 10 days, however, the extent of the follow‐up was difficult to ascertain
Operating table overlay
3.3 Comparison with viscoelastic polymer pad with standard table
Five RCTs evaluated different methods of pressure relief on the operating table. The first compared a viscoelastic polymer pad with a standard table (Nixon 1998), and found a relative reduction in the incidence of postoperative pressure ulcers of 47% associated with using the polymer pad for patients undergoing elective, major general, gynaecological or vascular surgery (supine or lithotomy) (RR 0.53; 95% CI 0.33 to 0.85) (Analysis 12.1). It is important to note that the majority of incident pressure ulcers were grade 1 (i.e. early ulcers with no break in the skin), and the length of follow‐up was eight days.
12.1. Analysis.
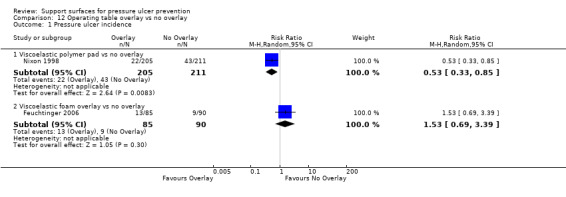
Comparison 12 Operating table overlay vs no overlay, Outcome 1 Pressure ulcer incidence.
Two further RCTs compared the Micropulse alternating system (applied both during surgery and postoperatively) with a gel pad during surgery and a standard mattress postoperatively. We pooled these two trials (I2 = 0%), and derived a pooled risk ratio (fixed‐effect) of 0.21 (95% CI 0.06 to 0.7) in favour of the Micropulse system (Aronovitch 1999; Russell 2000). It is not clear from these two trials whether the effect was due to the intra‐operative or the postoperative pressure relief, or both (Analysis 13.1).
13.1. Analysis.
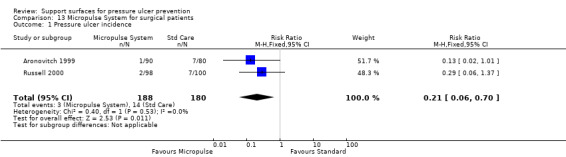
Comparison 13 Micropulse System for surgical patients, Outcome 1 Pressure ulcer incidence.
Schultz 1999 compared an operating theatre mattress overlay with usual care (which included padding as required, e.g. gel pads, foam mattresses). People in the overlay group were more likely to experience postoperative skin changes, and six patients in the overlay group developed ulcers of grade 2, or worse, compared with three people in the control group. No attempt was made to gather information on the patients' postoperative skin care. Details regarding stage of ulcer by group and of the unnamed product were sought unsuccessfully from the trial authors. In the absence of this information, the clinical importance of the findings is difficult to assess.
Gunningberg 2000 examined the effects of a viscoelastic foam trolley mattress and subsequent overlay on 101 patients with a suspected hip fracture in the A&E and ward setting, this trial is dealt with in the review in the section: 1.1 Standard foam hospital mattress compared with other low‐tech CLP.
Summary: Pressure‐relieving overlays on the operating table and in the postoperative period reduce the incidence of postoperative pressure ulcers, although there is some evidence that certain operating room overlays may result in postoperative skin changes.
3.4 Comparison of water‐filled warming mattress and thermoactive viscoelastic foam overlay with an operating theatre table with water‐filled warming mattress
Another trial compared an operating theatre table that included a water‐filled warming mattress and a 4‐cm thermoactive viscoelastic foam overlay, with an operating theatre table with water‐filled warming mattress only (Feuchtinger 2006). The trial was terminated before the full sample was recruited because more patients in the experimental group with the 4‐cm thermoactive viscoelastic foam overlay developed pressure ulcers (all were grades 1 to 2), with 15/85 (18%) in the experimental group and 10/90 (11%) in the control group. For grade 2 pressure ulcers only, there were two in the experimental group and one in the control group. There was no evidence of a difference between the two groups at the point at which the trial was terminated (Analysis 12.1).
Seat cushions
3.5 Comparisons between different cushions
Five RCTs compared different types of seating cushion for preventing pressure ulcers; one trial compared slab foam with bespoke contoured foam and found no difference between the groups (RR 1.06; 95% CI 0.75 to 1.49) (Lim 1988). The second trial compared contoured foam over a gel pad (Jay gel) plus a foam wheelchair cushion with a foam cushion alone in 141 people (Conine 1994), and found fewer ulcers in the gel pad plus cushion group, no evidence of a difference (RR 0.61; 95% CI 0.37 to 1.00). The third trial found no difference in pressure ulcer incidence between those assigned a slab foam cushion bevelled at the base and those assigned a contoured foam cushion with an area cut out to accommodate the patient's bottom (Conine 1993) (RR 1.00; 95% CI 0.81 to 1.18) ( Analysis 14.1). The fourth trial was a small pilot trial of 32 wheelchair‐users that compared a standard foam (eggcrate) cushion with a pressure‐reducing wheelchair cushion (Geyer 2001). The trial did not differentiate between patients with grade 1 ulcers or higher grades of ulcer. In total, 40% of participants on the pressure‐reducing cushion developed an ulcer (6/15) compared with 58.5% (10/17) on the foam cushion (RR 0.68; 95% 0.33 to 1.42); there was no evidence of a difference between the two groups (Analysis 14.1). The fifth trial (Brienza 2010) compared skin protection cushions with a segmented foam cushion in 232 wheelchair users. There was no evidence of a difference between the two groups in preventing pressure ulcers (RR0.60; 95%CI 0.31 to 1.17).(Analysis 14.1).
14.1. Analysis.
Comparison 14 Seat cushions, Outcome 1 Pressure ulcer incidence.
Summary: There is insufficient evidence to determine the value of seat cushions, various CLP devices and A&E trolley overlays as pressure ulcer prevention strategies.
Summary of results
Foam alternatives to the standard hospital foam mattress reduce the incidence of pressure ulcers in people at risk, although one large trial found no difference between high‐specification foam mattress and use of standard mattress (Russell 2003).
The relative merits of alternating‐ (AP) and constant low‐pressure (CLP) devices, and of the different AP devices for pressure ulcer prevention are unclear with most trials comparing AP with CLP devices and showing no significant difference between treatment groups. One large, high quality trial found no significant differences between an AP overlay with an AP mattress. However, the AP mattresses were associated with an 80% probability of reducing costs, due to a delay in pressure ulceration and reduced length of stay in hospital when they were used.
Results of two trials comparing AP devices with standard mattresses showed some evidence in favour of the AP support surfaces, however these trials were at high risk of bias.
Three trials investigating the effectiveness of a specific sheepskin product in preventing pressure ulcers showed that sheepskin overlays are effective in reducing the incidence of pressure ulcers. Other evidence about competing CLP devices did not show clear differences between the effectiveness of products.
Pressure‐relieving overlays on the operating table and in the postoperative period reduce the incidence of postoperative pressure ulcers, although there is some evidence that certain operating room overlays may result in postoperative skin changes.
There is insufficient evidence to determine the value of seat cushions, various CLP devices and A&E trolley overlays as pressure ulcer prevention strategies.
Discussion
The confidence with which we can draw firm conclusions from the trials detailed in this review is greatly tempered by (a) the poor quality of many of the trials; (b) the lack of replication of most comparisons; and (c) that the "standard" mattress is often not clearly defined. The clearest conclusion that can be drawn is that standard hospital mattresses have been consistently outperformed by a range of foam‐based, low‐pressure mattresses and overlays, and also by higher‐specification pressure‐relieving beds and mattresses in the prevention of pressure ulcers.
The application of this conclusion to current clinical practice is, however, hampered by the fact that the "standard" was poorly described in many of these trials, and what is standard varies by hospital, country and with time. This factor leads to major difficulties in interpretation of trial results and the importance of providing clear descriptions of all interventions in future trials cannot be overemphasised. In view of this, and because we thought there would be less variation within a country, a subgroup analysis of UK‐based trials was undertaken, which showed that the advantage of alternative foam was maintained.
Many of the trials reviewed did not provide convincing reassurance that manual repositioning was provided equally to each group of participants. This is a possible confounder, as care providers were not blinded to treatment allocation in any of the trials, and may have moved patients in one group more frequently if they perceived a particular mattress to be less effective. As experimental evidence of the effectiveness of manual repositioning is lacking, it is difficult to say what impact this has. In addition, in many trials the definitions of pressure ulcer free, low‐risk, moderate‐risk and high‐risk varied widely. Also, it is often difficult to ascertain whether trial participants with grade 1 ulcers have been accepted into the sample and included in the analyses, or not, and this needs to be taken into account when interpreting findings. Some of the included trials did recruit participants with pressure ulcers worse than grade 1 therefore only the incidence of new pressure ulcers was reported.
The results of three of the five trials evaluating the use of pressure‐relieving overlays on the operating table suggest that these are beneficial in reducing subsequent pressure ulcer incidence in high‐risk surgical patients. These three trials were of reasonable or good quality; in particular the Nixon 1998 trial was adequately powered, with allocation concealment and blinded outcome assessment lending further weight to the result. At present, the most effective means of pressure relief on the operating table is unclear; Nixon and colleagues found a gel‐filled overlay to be significantly better than a standard operating table, whilst a gel‐filled overlay on the operating table was less effective than an alternating‐pressure overlay intra‐ and postoperatively (the Micropulse system) in the other two trials (Aronovitch 1999; Russell 2000). The Micropulse trials were confounded by their provision of a standard mattress postoperatively in the gel overlay arm, and an alternating‐pressure overlay postoperatively in the Micropulse arm. Thus whilst there is clearly a reduction in pressure ulcer incidence associated with the alternating‐pressure system, it is not clear whether this is merely a result of better postoperative pressure relief. Two other trials showed that postoperative skin changes occurred as a result of different operating theatre overlays (Feuchtinger 2006; Schultz 1999), but the clinical importance of these results is difficult to determine in the absence of further details about pressure ulcer grading and products used.
Previously the evidence for different alternating‐pressure devices was unclear due to the poor quality and small size of existing trials. This review includes a large, robust trial which suggests that AP mattresses are clinically as effective as overlays, but likely to be more cost effective, and more acceptable to patients (Nixon 2006).
Trials published in the early 1980s found that water‐filled and bead‐filled mattresses were both associated with reductions in the incidence of pressure ulcers when compared with standard hospital mattresses, however, the products evaluated are no longer available.
There are tentative indications that four interventions may be harmful. Firstly, Tymec 1997 found that Foot Waffle heel elevators were associated with a trebling in the incidence of pressure ulcers, though this was not statistically significant and the trial was small (52 patients) (Tymec 1997). Secondly, Bennett 1998 evaluated low‐air‐loss hydrotherapy (LAL‐hydro) in a trial in which 19% LAL‐hydro patients developed ulcers compared with 7% of standard care patients, though again there was no evidence of a difference and the trial was underpowered (98 participants). Thirdly, Schultz 1999 investigated the effectiveness of an alternative foam overlay used in the operating theatre; the results suggested that patients placed on the intervention devices were significantly more likely to experience postoperative skin changes (i.e. mainly grade 1 pressure ulcers). It is difficult, however, to separate out the role of postoperative care and padding, which was used as a concomitant intervention, either of which may have caused the skin changes (mainly found on buttock and coccyx). Lastly Feuchtinger 2006 terminated the trial comparing an operating theatre table that included a water‐filled warming mattress and a 4‐cm thermoactive viscoelastic foam overlay with an operating theatre table with a water‐filled warming mattress only. The trial was terminated before the full sample was recruited because more patients ii the group receiving the 4‐cm thermoactive viscoelastic foam overlay developed pressure ulcers (all were grades 1 to 2). It is important to note, however, that two of the above trials did not provide clear information to indicate that the groups under investigation were similar at baseline for the most important prognostic factors (Bennett 1998; Tymec 1997).
Few comparisons have been replicated, and, as most of the completed trials were under‐powered there is little information from which to draw firm conclusions. For example, air‐fluidised therapy has only been compared with dry flotation as a prevention strategy, and low‐air‐loss only with standard care. There remain gaps in the knowledge base to which a rational research agenda could be addressed. It is always important to consider publication bias and its potential influence on the population of trials on a topic. Whilst equipment manufacturers appear to have contributed funding to many of the trials identified, it is difficult to see what the impact of this has been. For example, whilst bias in favour of positive results cannot be discounted, most of the trials published did not find any evidence of a difference. It is also important for the reader to be aware of the development of materials used in the production of support surfaces over the past 30 years, and how this may impact on the effectiveness of such devices. A systematic review of RCTs and quasi‐randomised trials investigating the prevention of heel pressure ulcers conducted by Junkin 2009 reported similar conclusions regarding the current state of the evidence, and the need for further rigorous research in this area.
Common methodological flaws which increase the risk of bias in trials investigating support surfaces include lack of allocation concealment, lack of baseline comparability, high attrition rates, lack of intention‐to‐treat analysis, lack of blind ‐ or independently verified ‐ outcome assessment. Specific to pressure ulcer intervention research, other flaws include failing to report on whether or not participants were free from pressure ulcers on trial entry, and providing an adequate definition for pressure ulcer status. These deficiencies further reduce the confidence with which we can regard many of the individual trial findings. It is, however, heartening that the recently included trials have improved reporting of some trial details to enable quality assessment. It is important to acknowledge that the different follow‐up times amongst the trials contribute to both clinical and statistical heterogeneity, and this needs to be taken into account when reading this review.
Future trials should continue to address these deficiencies and collect data on aspects of equipment performance such as reliability. It is hoped that future trials will be reported in line with current international standards for trial reporting (Moher 2001).
Authors' conclusions
Implications for practice.
For people at high risk of developing pressure ulcers, higher‐specification foam mattresses rather than standard hospital foam mattresses should be used, where possible. Organisations should consider the use of selected pressure relief devices for high risk patients in the operating theatre, as this is associated with a reduction in postoperative incidence of pressure ulcers. Medical grade sheepskins are associated with a decrease in pressure ulcer development. The relative merits of higher‐tech constant low‐pressure and alternating‐pressure for prevention are unclear, however, alternating‐pressure mattresses may be more cost effective than alternating‐pressure overlays in the UK context. Seat cushions have not been adequately evaluated.
Implications for research.
Independent, well‐designed, multi‐centre RCTs are needed to compare the clinical and cost‐effectiveness of different types of pressure‐relieving devices for patients at different levels of risk in a variety of settings. Particular gaps, include comparisons of:
(a) alternating‐pressure devices with other "high‐tech" equipment (such as low‐air‐loss and air‐fluidised beds) for prevention in very high risk groups; (b) alternating‐pressure devices with "lower‐tech" alternatives (such as different types of high‐specification foam mattresses and other constant low‐pressure devices).
The evaluation of alternating‐pressure devices is given emphasis as they are viewed as standard preventive interventions in some areas, but not others, and may vary widely in cost (from less than GBP 1000 to more than GBP 4,000).
Research is needed into valid and reliable methods of detecting early skin damage that is prognostic of pressure ulcer development, and of the impact of pressure ulcers on quality of life. Future research must address the methodological deficiencies associated with much of the research described in this review.
Patients should be truly randomised (with concealed allocation), trials should be of sufficient size to detect clinically‐important differences, and have clear criteria for measuring outcomes which, ideally, should be assessed without knowledge of the intervention received (blinded). Interventions under evaluation should be thoroughly and clearly described. Researchers should be encouraged to develop measures to assess patient experiences of pressure‐relieving equipment e.g. comfort. The trials should also have adequate follow‐up and appropriate statistical analysis. The CONSORT statement should be used as a guideline for reporting (Moher 2001).
Given the high costs associated with the prevention of pressure ulcers in general, and of pressure‐relieving surfaces specifically, emphasis should be given to robust economic evaluations to be conducted concurrently with trials.
What's new
| Date | Event | Description |
|---|---|---|
| 15 April 2015 | New citation required but conclusions have not changed | No change to conclusions. |
| 15 April 2015 | New search has been performed | Fourth update of review, new searches undertaken. Six new trials included (Brienza 2010; Demarre 2012; Donnelly 2011; Ricci 2013; van Leen 2011; Vermette 2012); and risk of bias assessment completed. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 20 December 2010 | New search has been performed | Third update of review, new searches undertaken. One new trial included; excluded list, pending assessment list and reference list updated. Risk of bias assessment completed. |
| 20 December 2010 | New citation required but conclusions have not changed | New author added to the review team. |
| 18 July 2008 | New citation required and conclusions have changed | Second update with the inclusion of 11 additional trials. |
| 18 July 2008 | New search has been performed | Second update of review. |
| 23 April 2008 | Amended | Converted to new review format. |
| 20 May 2004 | New citation required and conclusions have changed | First update (substantive amendment) published Issue 3, 2004. This review includes only trials which consider interventions which aim to prevent pressure ulcers. The title of the review has been changed to more accurately reflect the scope of the review. The original review: Beds, mattresses and cushions for preventing and treating pressure ulcers. Cullum N, Deeks J, Sheldon TA, Song F, Fletcher AW, has been substantially updated and now forms the basis of a prevention review and a separate treatment review. |
Notes
The original review: Beds, mattresses and cushions for preventing and treating pressure ulcers. Cullum N, Deeks J, Sheldon TA, Song F, Fletcher AW, has been substantially updated and now forms the basis of a prevention review and a separate treatment review. The review: Support surfaces for treating pressure ulcers is currently being updated. This review along with the updates: Support surfaces for pressure ulcer prevention has been prepared by McInnes E, Jammali‐Blasi A, Bell‐Syer SEM, Dumville JC, and Cullum NA and includes only trials which consider interventions which aim to prevent pressure ulcers. The title of the review has been changed to reflect the scope of the review more accurately.
Acknowledgements
The original review was commissioned by the UK NIHR HTA Programme (Cullum 2001). The authors are indebted to Julie Glanville, Centre for Reviews and Dissemination Information Service, for early assistance with the search, location and collection of the literature; to Trevor Sheldon, Alison Fletcher, Fujian Song and Jon Deeks who participated in early versions of this review. Early versions of this review have appeared as an Effective Healthcare Bulletin (Cullum 1995).
The authors would like to thank Janet Cuddigan, Margaret Harrison, David Margolis, Susan O'Meara, Gerben ter Riet and Gill Worthy who have peer reviewed at least one of the updates of this review and whose feedback contributed enormously to its final quality. The authors would like to acknowledge the contribution of Rosa Legood who made inclusion decisions, extracted data, assessed trial quality and contributed to the text for the first update and of Ruth Foxlee who undertook all database searches for the second and third updates. The authors would also like to thank Elizabeth Royle who copy edited the review update.
Appendices
Appendix 1. Ovid MEDLINE Search Strategy
1 exp Beds/ 2 mattress$.mp. 3 cushion$.mp. 4 (foam or transfoam).mp. 5 overlay$.mp. 6 (pad or pads).ti,ab. 7 gel.ti,ab. 8 pressure relie$.mp. 9 pressure reduc$.mp. 10 pressure alleviat$.mp. 11 (low pressure adj2 device$).mp. 12 (low pressure adj2 support).mp. 13 (constant adj2 pressure).mp. 14 static air.mp. 15 (alternat$ adj pressure).mp. 16 air suspension$.mp. 17 air bag$.mp. 18 water suspension$.mp. 19 (elevation adj2 device$).mp. 20 (clinifloat or maxifloat or vaperm or therarest or sheepskin or hammock or foot waffle or silicore or pegasus or cairwave).mp. 21 ((turn$ or tilt$) adj (bed$ or frame$)).mp. 22 (kinetic adj (therapy or table$)).mp. 23 net bed$.mp. 24 (positioning or repositioning).mp. 25 or/1‐24 26 exp Pressure Ulcer/ 27 (pressure adj (ulcer$ or sore$)).mp. 28 (decubitus adj (ulcer$ or sore$)).mp. 29 (bed adj (ulcer$ or sore$)).mp. 30 or/26‐29 31 25 and 30
Appendix 2. Ovid EMBASE Search Strategy
1 exp Bed/ 2 mattress$.mp. 3 cushion$.mp. 4 (foam or transfoam).mp. 5 overlay$.mp. 6 (pad or pads).ti,ab. 7 gel.ti,ab. 8 pressure relie$.mp. 9 pressure reduc$.mp. 10 pressure alleviat$.mp. 11 (low pressure adj2 device$).mp. 12 (low pressure adj2 support).mp. 13 (constant adj2 pressure).mp. 14 static air.mp. 15 (alternat$ adj pressure).mp. 16 air suspension$.mp. 17 air bag$.mp. 18 water suspension$.mp. 19 (elevation adj2 device$).mp. 20 (clinifloat or maxifloat or vaperm or therarest or sheepskin or hammock or foot waffle or silicore or pegasus or cairwave).mp. 21 ((turn$ or tilt$) adj (bed$ or frame$)).mp. 22 (kinetic adj (therapy or table$)).mp. 23 net bed$.mp. 24 (positioning or repositioning).mp. 25 or/1‐24 26 exp Decubitus/ 27 (pressure adj (ulcer$ or sore$)).mp. 28 (decubitus adj (ulcer$ or sore$)).mp. 29 (bed adj (ulcer$ or sore$)).mp. 30 or/26‐29 31 25 and 30
Appendix 3. EBSCO CINAHL Search Strategy
S29 S23 and S28 S28 S24 or S25 or S26 or S27 S27 TI decubitus or AB decubitus S26 TI ( bed sore* or bedsore* ) or AB ( bed sore* or bedsore* ) S25 TI ( pressure ulcer* or pressure sore* ) or AB ( pressure ulcer* or pressure sore* ) S24 (MH "Pressure Ulcer") S23 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 S22 TI ( positioning or repositioning ) or AB ( positioning or repositioning ) S21 TI net bed* or AB net bed* S20 TI ( kinetic therapy or kinetic table* ) or AB ( kinetic therapy or kinetic table* ) S19 TI ( turn* bed* or tilt* bed* ) or AB ( turn* frame* or tilt* frame* ) S18 TI ( clinifloat or maxifloat or vaperm or therarest or sheepskin or hammock or foot waffle or silicore or pegasus or cairwave ) or AB ( clinifloat or maxifloat or vaperm or therarest or sheepskin or hammock or foot waffle or silicore or pegasus or cairwave ) S17 TI elevation N2 device* or AB elevation N2 device* S16 TI water suspension or AB water suspension S15 TI air bag* or AB air bag* S14 TI air suspension or AB air suspension S13 TI alternat* pressure or AB alternat* pressure S12 TI static air or AB static air S11 TI constant N2 pressure or AB constant N2 pressure S10 TI low pressure N2 support or AB low pressure N2 support S9 TI low pressure N2 device* or AB low pressure N2 device* S8 TI pressure alleviat* or AB pressure alleviat* S7 TI pressure reduc* or AB pressure reduc* S6 TI pressure relie* or AB pressure relie* S5 TI ( overlay* or pad or pads or gel ) or AB ( overlay* or pad or pads or gel ) S4 TI ( foam or transfoam ) or AB ( foam or transfoam ) S3 TI ( mattress* or cushion* ) or AB ( mattress* or cushion* ) S2 (MH "Pillows and Cushions") S1 (MH "Beds and Mattresses+")
Appendix 4. Criteria for judgments for the sources of bias
1. Was the allocation sequence randomly generated?
Yes, low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
No, high risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of either Yes or No (as above) to be made.
2. Was the treatment allocation adequately concealed?
Yes, low risk of bias
Participants and investigators enrolling participants could not foresee assignment either because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
No, high risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, i.e. when allocation used: an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of either Yes or No to be made. This is usually the case if the method of concealment is not described, or is not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding was knowledge of the allocated interventions adequately prevented during the trial?
Yes, low risk of bias
Any one of the following:
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key trial personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key trial personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
No, high risk of bias
Any one of the following:
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key trial participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key trial personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Any one of the following:
Insufficient information to permit judgement of Yes or No to be made.
The trial did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Yes, low risk of bias
Any one of the following:
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
No, high risk of bias
Any one of the following:
Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
As‐treated analysis done with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following:
Insufficient reporting of attrition/exclusions to permit judgement of Yes or No (e.g. number randomised not stated, no reasons for missing data provided).
The trial did not address this outcome.
5. Are reports of the trial free of suggestion of selective outcome reporting?
Yes, low risk of bias
Any of the following:
The trial protocol is available and all of the pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way.
The trial protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon).
No, high risk of bias
Any one of the following:
Not all of the trial's pre‐specified primary outcomes reported.
One or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified.
One or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The trial report fails to include results for a key outcome that would be expected to have been reported for such a trial.
Unclear
Insufficient information to permit judgement of Yes or No to be made. It is likely that the majority of trials will fall into this category.
6. Other sources of potential bias:
Yes, low risk of bias
The trial appears to be free of other sources of bias.
No, high risk of bias
There is at least one important risk of bias. For example, the trial:
Had a potential source of bias related to the specific trial design used; or
Stopped early due to some data‐dependent process (including a formal‐stopping rule); or
Had extreme baseline imbalance; or
Has been claimed to have been fraudulent; or
Had some other problem.
Unclear
There may be a risk of bias, but there is either:
Insufficient information to assess whether an important risk of bias exists; or
Insufficient rationale or evidence that an identified problem will introduce bias
Data and analyses
Comparison 1. Constant low‐pressure supports (CLP) vs standard foam mattresses (SFM).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 7 | 2407 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.24, 0.61] |
| 1.1 Cubed foam mattress | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.14, 0.85] |
| 1.2 Bead‐filled mattress | 1 | 75 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.14, 0.76] |
| 1.3 Softform mattress | 1 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.09, 0.45] |
| 1.4 Water‐filled mattress | 1 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.15, 0.79] |
| 1.5 Alternative foam | 2 | 644 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.22, 0.59] |
| 1.6 Hi‐spec foam mattress/cushion | 1 | 1166 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.55, 1.11] |
Comparison 2. Alternative foam mattress vs standard foam mattress.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 5 | 2016 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.74] |
| 1.1 Various alternatives (pooled) | 5 | 2016 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.74] |
| 2 Pressure ulcer incidence UK studies only | 4 | 1980 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.19, 0.87] |
Comparison 3. Comparisons between alternative foam supports.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Alternative foam vs standard foam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Foam mattress vs foam overlay | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Solid foam vs convoluted foam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Transfoam mattress vs Transfoamwave mattress | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Cold foam mattress vs cold foam mattress and static air overlay | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Comparisons between CLP supports.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 11 | 2138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.36, 0.56] |
| 1.1 Optima vs SFM | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.00, 0.99] |
| 1.2 Sofflex vs ROHO | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.16, 2.47] |
| 1.3 Gel mattress vs air‐filled overlay | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.24, 2.72] |
| 1.4 Static air mattress vs water mattress | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 4.29] |
| 1.5 Foam overlay vs Silicore overlay | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.64, 2.14] |
| 1.6 Sheepskin vs no sheepskin (Including all pressure ulcers regardless of Grade) | 3 | 1424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.36, 0.64] |
| 1.7 Foam support surface vs no support | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.49] |
| 1.8 Heel‐lift suspension boot and various support surfaces vs support surfaces alone | 1 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.12, 0.53] |
| 1.9 Inflated static overlay vs microfluid static overlay or LAL dyname mattress | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.07, 1.58] |
| 2 Pressure ulcer incidence | 3 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
| 2.1 Sheepskin vs no sheepskin (grade 2 + pressure ulcers only) | 3 | 1424 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.05] |
Comparison 5. Alternating‐pressure vs standard foam mattress.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.17, 0.58] |
Comparison 6. Alternating‐pressure (AP) vs constant low‐pressure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 10 | 1606 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 1.1 AP (various) vs CLP (various) | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.22, 0.66] |
| 1.2 AP vs Silicore or foam overlay | 4 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.72, 1.16] |
| 1.3 AP vs water or static air mattress | 3 | 458 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.51, 3.35] |
| 1.4 AP vs continuous low pressure mattress | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.19, 22.18] |
| 1.5 AP vs visco‐elastic foam mattress | 1 | 447 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.64, 1.52] |
Comparison 7. AP and CLP in ICU/post ICU (factorial design).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Standard ICU/SFM post‐ICU vs Nimbus AP ICU/SFM post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Standard ICU/SFM post‐ICU vs standard ICU/Tempur CLP post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Nimbus AP ICU/SFM post‐ICU vs standard ICU/Tempur CLP post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Standard ICU/SFM post‐ICU vs Nimbus AP ICU/Tempur CLP post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Nimbus AP ICU/SFM post‐ICU vs Nimbus ICU/Tempur post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Standard ICU/Tempur post‐ICU vs Nimbus ICU/Tempur post‐ICU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Comparisons between alternating‐pressure devices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Airwave vs Large Cell Ripple | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Airwave vs Pegasus Carewave | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Trinova vs control | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 AP overlay vs AP mattress | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 TheraPulse vs Duo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 AP mattress (single stage inflation) vs AP mattress (multi stage inflation) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Low Air Loss vs standard bed.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Pressure incidence pooled | 2 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.16, 0.67] |
| 3 Incidence of patients developing multiple ulcers | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Air‐Fluidised therapy vs dry flotation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate of wound breakdown | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Kinetic treatment table vs standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.57, 2.65] |
Comparison 12. Operating table overlay vs no overlay.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Viscoelastic polymer pad vs no overlay | 1 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.33, 0.85] |
| 1.2 Viscoelastic foam overlay vs no overlay | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.69, 3.39] |
Comparison 13. Micropulse System for surgical patients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 2 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.70] |
Comparison 14. Seat cushions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pressure ulcer incidence | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Slab foam v Bespoke contoured foam | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Jay Gel Cushion v Foam | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Pressure reducing cushion v Standard foam cushion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Skin protection cushion with segmented foam cushion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersen 1982.
| Methods | RCT with 10 day follow‐up. Method of allocation unclear. | |
| Participants | Patients in acute setting at high risk of pressure ulcer development (Andersen scale), and without existing pressure ulcers. | |
| Interventions | 1. Standard hospital mattress (n = 161). 2. Alternating air mattress (AP) (n = 166). 3. Water‐filled mattress (air mattress for camping filled with water) (n = 155). | |
| Outcomes | Incidence of pressure ulcers (skin examined on alternate days): 1. Standard mattress: 13.0% (21/161). 2. Alternating mattress: 4.2% (7/166). 3. Water mattress: 4.5% (7/155). | |
| Notes | 118 out of 600 selected patients dropped out during first 24 h. A priori sample size calculation. AP easily punctures and in this study was not always set at optimum pressure. Water bed is heavy and time‐consuming to fill. Patients more satisfied with ordinary bed: complained of the noise and pressure changes of AP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients "were allotted to one of the three group". Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only participant drop‐out pre‐randomisation reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | “The distribution showed no significant difference between the three groups according to age, sex, body weight, or risk score”. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Observation took place on alternate days for 10 days. |
Aronovitch 1999.
| Methods | Quasi‐randomised trial with 7 day follow‐up. | |
| Participants | > 18 y; free of pressure ulcers; undergoing elective surgery under GA, of > 3 h operative time. No significant differences between groups for age, sex, race, weight, height, smoking status at baseline, but patients in conventional management group were at greater risk of pressure ulcer development as defined by Knoll score. | |
| Interventions | 1. AP system intra and postoperatively (MicroPulse) (n = 112). Micropulse is thin pad with over 2,500 small air cells in rows; 50% cells inflated at any time. 2. Conventional management (n = 105): consisted of use of a gel pad in the operating room and a replacement mattress postoperatively. | |
| Outcomes | Occurance of pressure ulcer within 7 days of surgery: number/size/grade of ulcers on each postoperative day: 1. MicroPulse system 1% (1/90), however, ulcer was due to a foreign body and considered "not related to the bed". 2. Conventional management 9% (7/80) (7 patients developed 11 pressure ulcers; the stage of 6 of these could not be determined because of eschar). Grade 1: 1; Grade 2: 4. |
|
| Notes | 1. MicroPulse system: device was inadvertently turned off during treatments of 4 patients. 4 patients asked to withdraw for various unreported reasons. 3 patients withdrew due to back pain. 12 patients assigned to this group were placed on another surface postoperatively for reasons unrelated to the surface. 2. Conventional management: 6 patients were placed on the MicroPulse postoperatively. Analysis was on an ITT basis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quasi‐randomised: "randomisation was performed by week rather than by patient to decrease protocol error". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons/numbers for attrition/exclusions reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | It was stated, however, that all data were not available for all patients. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Outcomes assessed on days 1, 4 and 7. |
Bennett 1998.
| Methods | RCT with 60‐day follow‐up. Median length of follow‐up (days): Group 1: 4 (1‐60). Group 2: 6 (1‐62) P value <0.017. | |
| Participants | Acute and long‐term care patients incontinent of urine and/or faeces, in bed >16 h/day, with pressure ulcers grade 2 or below (or none). If urinary catheter present, this was removed in the LAL group (not control group). Most common diagnoses: sepsis; malignancy; fractured neck of femur; hypovolaemia; dementia. | |
| Interventions | Group 1. Low‐air‐loss Hydrotherapy (LAL) (n = 42) Clensicair (SSI/Hill Rom). Permeable fast drying filter sheet over low‐air‐loss cushions (circulating air). Urine collection device integral to bed. Group 2. Standard care (n = 56) comprised standard bed or foam, air, alternating‐pressure mattresses. Skin care not standardised. | |
| Outcomes | Number of patients who developed any kind of skin lesion more than 1 day after enrolment: Group 1: 64% (27/42); Group 2: 18% (10/56). Number of patients who developed pressure ulcers Grade 2‐4: Group 1: 19% (8/42); Group 2: 7% (4/56) P value 0.11; NS. Number of patients with non‐blanchable erythema (Grade 1): Group 1: 14% (6/42); Group 2: 0/56 P value 0.008. Only 26 ulcers present on enrolment, and only 3 were Grades 3 or 4, so no healing data presented. | |
| Notes | The first 68 patients were discounted, and a further 26 out of 116 withdrew. No ITT analysis. Nurses received special extra training for the LAL bed. LAL patients were interviewed about satisfaction, control patients were not. There were many nurse complaints about the LAL; firmly held belief that it was associated with more ulceration. Two subjects in the LAL group developed hypothermia. Findings may not relate to subsequent products developed since. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of subject to low‐air‐loss hydrotherapy or standard care was done by unblocked allocation using a table of random numbers stratified by pressure sore and by setting". |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Shown in Table 2 and reported in text. |
| Selective reporting (reporting bias) | High risk | "Because too few patients with pressure sores at enrolment were enrolled long enough to have changes in pressure sore size, grade, or status, no data on change in pressure sores present at enrolment are presented herein". |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | "There were no statistically significant differences in enrolment characteristics between the two groups". |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | High risk | "For all subjects, the study treatment period commenced on the day of enrolment and continued until withdrawal of consent, discharge from the hospital, transfer to a critical care unit from a medical‐surgical ward or to the acute hospital from the chronic hospital ward, death, cessation of incontinence, bed use less than 16 hours per day, enrolment for more than 60 days, or end of the overall study". |
Brienza 2010.
| Methods | RCT, with a 6 month follow up or until pressure ulcer incident Allocation as follows: A research team member independent of those with participant contact prepared a 1:1 allocation randomization scheme stratifying according to clinical facility. |
|
| Participants | 232 nursing home residence, aged >65 years, Braden score <18, combine Braden activity and Mobility subscale score <5, no ischial pressure ulcers, using a wheelchair >6 hours/day and able to accommodate seating and positioning needs. Set in 12 nursing homes in the greater Pittsburgh area. | |
| Interventions | Group 1: Skin Protection Cushion (SPC) ‐ a commercially available cushion with an incontinence cover. Cushions were selected from a group of three an air, viscous fluid and foam, or gel and foam cushion Group 2: Segmented Foam Cushion (SFC) ‐ a crosscut, 7.6‐cm thick SFC a fitted incontinence cover, and a solid seat insert. Each participant received a new, properly fitted wheelchair. Two models were used: the Guardian Escort or the Breezy Ultra 4. |
|
| Outcomes | Incidence of ischial tuberosities (IT) ulcers: 1: 1/113 (0.9%) 2: 8/119 (6.7%) P= 0.4 Incidence of combine IT and sacral ulcers: 1: 12/113 (10.6%) 2: 21/119 (17.6%) P = 0.14 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomized blocks of varying length (containing random permutations of the two treatment combinations) were used for randomization.” Sufficient evidence that offsite allocation occurred. |
| Allocation concealment (selection bias) | Low risk | “… keeping clinical center staff masked as to the treatment the next participant was to receive”. Sufficient evidence that offsite allocation occurred. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | “the research staff members who performed outcome measures were masked to treatment group assignment.” All the identifying labels were removed from the cushions and the same colour and style incontinence covers to achieve this objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure one. All reasons/numbers for attrition/exclusions reported. |
| Selective reporting (reporting bias) | Unclear risk | Data on PU incidence provided but not on interface pressure. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | "No statistically significant difference found between the two groups, except for ambulation”. No mention of how they adjusted for this difference in the analysis. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | "The research team’s skin assessor (a research nurse trained in detecting and staging pressure ulcers; MK) who was masked to the treatment assignment performed weekly skin and risk assessments (Braden score)”. Timing of the outcome assessment therefore appears same in each group. |
Cadue 2008.
| Methods | RCT with maximum follow‐up 30 days. | |
| Participants | Patients in an intensive care setting with a Waterlow Score >10, no existing heel pressure ulcers, ≥18 y or over. Participants seemed generally matched at baseline. | |
| Interventions | 1. Foam body support and standard pressure prevention protocol (half‐seated position, water mattress preventative massage 6 times/day) (n = 35). 2. Standard pressure ulcer protocol (as above) (n = 35). | |
| Outcomes | Number of participants developing non‐blanching pressure ulcer or worse on the heel: 1. Foam body support 8.6% (3/35); 2. Usual care 55.4% (19/35). | |
| Notes | Full paper not available in English. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | From English summary. Quote: “a randomisation table was used to allocate 70 patients into 2 groups”. The two groups were formed randomly by following a randomisation table. |
| Allocation concealment (selection bias) | Low risk | Quote: “envelope cachetee” translated as sealed envelope. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | “le masseur‐kinesitherapeute et l'infirmiere” translated to:the physiotherapist and nurse assessed the stage of the lesion daily ‐ but it is not clear if they were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70 patients were included, 35 in each group. Table 2 presents the principle results and notes that “n = 35” which has been interpreted that data were presented on 35 patients in each group. No mention was found of any withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | The judgement has been recorded due to the difficulty in making this assessment in a trial that has been published in French and partially translated. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | “a l'inclusion il n'existait pas de difference significative entre les 2 groupes au niveau du risque theorique de developper des escarres ni au niveau des principaux facteurs connus pour favoriser la survenue d'escarres”, was translated to: at inclusion there was no significant difference between the 2 groups in the theoretical risk of developing pressure ulcers or any of the main factors known to contribute to the occurrence of bedsores. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | The physiotherapist and nurse assessed the stage of the lesion daily? it is assumed this was done for both experimental and control groups. |
Cavicchioli 2007.
| Methods | RCT with follow‐up of 2 weeks. | |
| Participants | Acute and long‐term care participants deemed at risk of pressure ulceration (Braden score < 17 activity or mobility sub‐scales < 3 respectively). Patients had an expected admission of at least 2 weeks. Patients could have 1 grade 1 pressure ulcer at baseline, but were excluded if they had more; or the ulcer was grade 2 or above. Baseline balance for age, sex and Braden score in the randomised groups. | |
| Interventions | 1. High‐tech (Duo 2, Hill Rom) mattress on alternating low‐pressure setting (n = 86). 2. High‐tech (Duo 2, Hill Rom) mattress on continuous low‐pressure setting (n = 84). | |
| Outcomes | Number of participants with Incidence pressure ulcer (blinded outcome assessment at study end):
Grade 1:
1. Alternating low‐pressure 1% (1/69);
2. Continuous low‐pressure 0/71. Grade 2: 1. Alternating low‐pressure 1% (1/69); 2. Continuous low‐pressure 1% (1/71). |
|
| Notes | This was a 3‐armed study. There was a 2‐armed RCT, as described, and a control group (standard mattress), which was not formed by randomisation and not included here. Blinded outcome assessment was conducted for the randomised groups. Follow up figures were: 1. 69 (4 deaths, 8 participants discharged before final assessment, and 5 classed as not having completed the study due to non‐concordance); 2. 71 (5 deaths, 4 discharged and 4 classed as non‐concordant). Not ITT. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants not randomly allocated to the 3 groups from same pool of patients. Controls from another hospital. Only patients in high tech groups appeared to be randomised "by means of a sealed envelope". |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | External observer was blinded to which treatment mattress was in use. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for attrition and exclusion reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | High risk | "The two treatments groups were assessed as at greater risk of pressure ulceration than the control group both at baseline (p <0.001) and the study end (p <0.005)." |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | 2‐week study period with assessments taking place at the beginning and end of the study. |
Cobb 1997.
| Methods | RCT with 40‐day follow‐up. | |
| Participants | Recruitment in hospital wards and intensive care units. Participants > 18 y of age, ≤ 290 pounds, without pre‐existing pressure ulcer, an expected length of stay of 1‐2 weeks and considered at “high risk” on the basis of the Braden Scale. Patients allocated through the selection of a treatment card by an independent nurse. Some baseline imbalance observed with older participants; more participants with co‐morbidities in the KinAir group. | |
| Interventions | 1. Low loss air bed (KinAir Bed) (n = 62). 2. Static air mattress overlay (EHOB waffle) (n = 61). | |
| Outcomes | Number of participants with incidence pressure ulcer (ICU participants assessed daily, ward patients assessed every 48 h):
Grade 1
1. KinAir Bed 5% (3/62);
2. EHOB waffle 2% (1/61). Grade 2. 1. KinAir Bed 5% (3/62); 2. EHOB waffle 18% (11/61) Eschar 1. KinAir Bed 3% (2/62); 2. EHOB waffle 0/61. |
|
| Notes | No higher grades reported. No loss to follow‐up reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were placed into one of the study groups by random selection of a treatment card". Method of randomisation unclear. |
| Allocation concealment (selection bias) | Low risk | The use of an independent nurse picking a treatment card. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No numbers/reasons given for exclusions/attrition. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | High risk | EHOB waffle group had more participants in younger age bracket; KinAir group had more with diabetes and cancer. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | High risk | "Patients in the ICUs had skin assessments daily and those on the wards were assessed every 48 hours". |
Collier 1996.
| Methods | RCT comparing 8 different foam mattresses; length of follow‐up not clear but patients assessed weekly. Allocation as follows: mattresses assigned to beds and coded numerically with only the principal investigator and ward link nurse aware of identity of each mattress. Mattresses then allocated to patients "as available". | |
| Participants | Patients on a general medical ward; no further details given. | |
| Interventions | Comparison of 8 foam mattresses: 1. New Standard Hospital Mattress (Relyon) (130 mm) (n = 9). 2. Clinifloat (n = 11). 3. Omnifoam (n = 11). 4. Softform (n = 12). 5. STM5 (n = 10). 6. Therarest (n = 13). 7. Transfoam (n = 10). 8. Vapourlux (n = 14). | |
| Outcomes | Incidence of pressure ulcers. Patients assessed at least weekly throughout hospital stay. No patient developed a pressure ulcer of any grade during whole study. | |
| Notes | 9 patients allocated the Cyclone mattress, however, this group was withdrawn from the study at manufacturer's request and data not presented. All mattresses assessed for "grounding", deterioration of cover and contamination of inner foam core, interface pressures. No "grounding" of any mattresses during the evaluation period; softening of the centre of the foam base in Standard and Omnifoam mattresses on completion of study (detected using a "fist test" of unknown reliability). All mattress covers remained intact and inner foam protected. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only information provided: “Mattresses were randomly allocated to patients on admission as available”. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | “Only the principal investigator and the ward link nurse knew the identification of each mattress”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 patients missing from data in Table 2 as their treatment, Cyclone mattress, was removed during the evaluation process at the request of the manufacturer. No other raw data presented in the paper to evaluate if incomplete outcome data addressed. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Not reported. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | High risk | "Frequency of assessment was determined by each patient's condition, but in all cases was conducted at least weekly throughout their period in hospital". |
Conine 1990.
| Methods | Sequential RCT with 3‐month follow‐up. Method of allocation unclear. | |
| Participants | Patients with chronic neurological diseases aged 18‐55 y with no evidence of skin breakdown for at least 2 weeks prior to the study. Patients in the 2 groups were well matched at baseline for key variables e.g. Norton score; sex; age; underweight/overweight; diagnoses; years as a wheelchair user; history of previous pressure ulcers; incontinence. Setting extended care facility for chronic neurological conditions. | |
| Interventions | 1. Alternating‐pressure overlay (n = 72); 10‐cm air cells. Cycle time not reported, nor the make of overlay. 2. Silicore (Spenco) overlay (n = 76); siliconised hollow fibres in waterproofed cotton placed over standard hospital mattress (spring or foam). All patients received usual care including 2‐3 hourly turning; daily bed baths; weekly bath/shower; use of heel, ankle and other protectors. | |
| Outcomes | Incidence of pressure ulcers (including grade 1). Pressure ulcer status was checked by another researcher blind to the study. Inter‐rater reliability high. Included grade 1 ulcers: 1. Alternating air overlay: 54% (39/72); 2. Spenco overlay: 59% (45/76). The alternating air overlay group had a slightly lower than average 'Exton‐Smith severity score' (1.59 vs 1.69); a shorter than average healing duration (25 days vs 29 days); NS. | |
| Notes | Alternating air overlay needed frequent monitoring and expensive prolonged repairs. Reported that patients sank into the Silicore overlay and found it difficult to move. Patients complained of build‐up of bad odour, instability (especially Silicore), and noise of the alternating‐pressure motor. High dropout rate due to discomfort. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only information given: a modified sequential clinical trial as described by Pocock (1981) was used to assign subjects randomly to one of the two mattress groups of 20. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | "The Norton's scale was administered by a blind experienced occupation therapist who was external to the institution" and "The research nurse...was responsible for the assessment of all outcome measures. She was not associated with the institution and was not informed about the study". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | As shown in Table 1. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No statistically significant differences between the 2 groups as shown in Table II. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Timing not specified. |
Conine 1993.
| Methods | Trial with 3‐month follow‐up. | |
| Participants | Extended care patients > 60 y; free of skin breakdown for at least 2 weeks prior to study; considered to be at high risk of pressure ulcers; sitting in wheelchair for a minimum of 4 consecutive h; free of any progressive disease which could lead to bed confinement. | |
| Interventions | 1. Slab cushion bevelled at base to prevent seat sling (n = 144). 2. Contoured foam cushion with a posterior cut out in the area of ischial tuberosities and an anterior ischial bar (n = 144). | |
| Outcomes | 1. Slab cushion 68% (85/125); 2. Contoured foam cushion 68% (84/123). | |
| Notes | No ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation unclear, only information given: "the patients were entered into the trial in sequential groups of 40, 20 on each cushion type". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | “The Exton‐Smith scale was used weekly by a blinded research assistant who was a registered nurse (RN)”, but, “A sore was declared to be healed by the patient’s primary nurse with the joint agreement of the research RN” ‐ unclear if the primary nurse was blinded to treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Shown in Table 3. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | “No significant differences were found between the slab and contoured groups in the reasons for drop‐outs or between the group characteristics of the 248 remaining patients”. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | It is reported that the occupational therapist conducted monthly checks for change in status. The checking of ulcers was carried out 30 minutes after returning to bed by the patient's primary nurse with the joint agreement of the research RN. |
Conine 1994.
| Methods | RCT of 2 wheelchair cushions with 3‐month follow‐up. Method of randomisation unclear as patients were described as "randomly allocated by the principal investigator". | |
| Participants | Elderly patients (mean age 82 y) in an extended care hospital deemed at high risk of pressure ulcers (Norton Score ≤ 14); sitting in a wheelchair for minimum of 4 consecutive h/day; free of progressive disease likely to confine to bed. Excluded if diabetic, had peripheral vascular disease; confined to bed for more than 120 consecutive h (except if to heal a pressure ulcer). There were no statistically significant differences between groups at baseline for Norton scores; age; hours in bed/day; sex; diagnosis; sensory loss; history of previous ulcers; weight; nutritional status; oedema; incontinence; hours in wheelchair/day. | |
| Interventions | 1. Jay cushion (n = 68); the Jay cushion is a contoured urethane foam base over gel pad. 2. Foam cushion (n = 73); 30 kg/m3 density foam bevelled at the bottom to prevent sling effect. Both cushions fitted with identical Jay air‐exchange covers of knitted polyester. Patients assigned to their specific wheelchairs by a seating specialist according to a local policy unaffected by the trial. | |
| Outcomes | 1. Jay Cushion 25% (17/68); 2. Foam Cushion 41% (30/73). Pressure ulcer incidence data presented as number of ulcers and number of affected patients for all grades of ulcer, but only as number of ulcers by grade (and there were cases of multiple ulcers on the same patient). Therefore impossible to present the incidence data as number of patients affected by ulcers of grade 2 or above. | |
| Notes | 13% attrition; not analysed by ITT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Qualified patients were randomly assigned to either foam or Jay cushions in groups of 40 by the principal investigator" Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | "The principal investigator was blind to all data" and "A research assistant, an experienced registered nurse (RN), examined the patients weekly, blind, and classified the status of any skin lesions”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Shown in Table 3. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | "No statistically significant differences were found between groups". |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Weekly for 3 months. |
Cooper 1998.
| Methods | RCT with 7‐day follow‐up. Allocation by consecutively‐numbered, sealed, opaque envelopes. | |
| Participants | 100 patients > 65 y, with no pressure ulcers, from 3, 24‐bedded mixed emergency orthopaedic trauma wards. All patients at risk of pressure ulcers with Waterlow Risk scores of ≥15. Baseline variables similar for each group (age, sex, mobility, Waterlow scores). | |
| Interventions | 1. Dry flotation mattress (Roho) (n = 49) (data supplied for only 43). 2. Dry flotation mattress (Sofflex) (n = 51) (data supplied for only 41). | |
| Outcomes | Grade 2 ulcers and above: 1. Roho mattress: 5% (2/43); Sofflex mattress: 2% (1/51). Grade 1 ulcers: 1. Roho mattress: 12% (5/43); 2. Sofflex mattress 5% (2/41). | |
| Notes | Roho mattress: 79% patients found it comfortable or very comfortable, 5 found it uncomfortable. Sofflex mattress: 90% patients found it comfortable or very comfortable. Staff had difficulty setting the level of inflation correctly; this can now be done automatically. 16% attrition; no ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects were then randomly allocated to one of two types of mattress using consecutively numbered sealed opaque envelopes". |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. Reasons for attrition reported: death, change in care circumstances, transferred and discharged, however, not specified for each intervention group. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | As seen in Table 1. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | 24 h post admission and at 7 days. |
Daechsel 1985.
| Methods | RCT with 3‐month follow‐up. Method of allocation unclear. | |
| Participants | 32 patients with chronic neurological conditions in a long term care hospital. All aged 19‐60 y, free from skin breakdown on entry, considered at high risk of pressure ulcers. | |
| Interventions | 1. Alternating‐pressure mattress (Gaymar Inc) (n = 16). 2. Silicore overlay (JW Westman Inc) (n = 16). | |
| Outcomes | Included grade 1 ulcers: 1. Alternating overlay: 25% (4/16); 2. Spenco overlay: 25% (4/16). No statistically significant differences were found between the 2 groups with regard to location and severity of pressure ulcers. | |
| Notes | 100% follow‐up. Patients' satisfaction was similar for both devices. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "All qualified subjects were entered into the trial for a period of three months and all were randomly assigned to one of the two types of mattress". Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons/numbers for exclusions/attrition given. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | “Statistical tests of significance indicated that the groups were comparable on the factors that are considered to be associated with the development of DU”. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Daily observations and weekly skin checks over 3 months. |
Demarre 2012.
| Methods | RCT, with a 14 day follow up. Allocation as follows: Mattresses were allocated by the ward nurse contacting the research (24h telephone accessibility). The ward nurse then received a number of the type of allocated mattress (first available on the computer generated list. |
|
| Participants | 610 patients at risk for pressure ulcer development as measured by the Braden Scale (less than 17 were considered at risk), over 18 years old, weighed 30 – 160kg and had an admission of greater than or equal to 3 days. Setting 25 wards from five Belgian hospitals of which 8 were geriatric wards and 17 were medical wards (6 neurology, 3 rehabilitation, 2 cardiology, 1 dermatology, 1 pneumology, 1 oncology and 1 chronic care). | |
| Interventions | Group 1: Alternating low pressure air mattress (ALPAM) with multi‐stage inflation and deflation of air cells Group 2: ALPAM with single stage inflation and deflation of air cells Co‐intervention: Seating protocol using static air cushion |
|
| Outcomes | Incidence of pressure ulcers (including stage 1): Group 1: 68/298 (22.8%) Group 2: 56/312 (17.9%) Incidence of pressure ulcers (excluding stage 1): Group 1: 17/298 (5.7%) Group 2: 18/312 (5.8%) P= 0.97 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “random allocation sequence was based on a computer generated list of random numbers”. Sufficient evidence that this was done rigorously. |
| Allocation concealment (selection bias) | Low risk | “[The patient] were assigned to one of the mattresses by [the ward nurses] contacting the researcher (24 h telephone accessibility). The ward nurse received a number indicated which type of allocated mattress should be allocated (first available on the computer generated list)”. Sufficient evidence that offsite allocation occurred. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | “ Study could not be blinded” p.g. 419 “Data analysis was not blinded” pg.420. However,it was unclear if participants and personnel were able to tell the difference between mattresses (single cell vs mutli‐cell). “No information was provided to the ward nurses about the differences between the experimental and control study device.” Pg. 419‐420. Both devices were presented as alternating pressure air mattresses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes were reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Table 2. No statistically significant differences found. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | “Daily skin assessment was performed by the ward nurses in each patient in the morning”. Timing of the outcome assessment therefore appears same in each group. |
Donnelly 2011.
| Methods | RCT, with daily follow up until discharge. Median length of follow‐up (days): Group 1: 12.18 days Group 2: 10.78 days Allocation as follows: patients were allocated to either the intervention group (heel elevation) or the control group (standard care), according to a computer‐generated block randomisation schedule (in permuted blocks of 20). |
|
| Participants | 239 patients in a fracture trauma unit of a major tertiary referral centre, aged > 65, with a hip fracture in the previous 48 hours | |
| Interventions | Group 1: Heel elevation: using the HeeLift Suspension Boot (n=120) Group 2: Standard Care: (not specifically described) (n=119) All patients were nursed on pressure‐redistributing support surfaces. These included the Pentaflex cut foam mattress, an AlphaXcell mattress overlay, an AutoExcel mattress overlay and the Nimbus 3 alternating mattress (ArjoHuntleigh); all are standard pressure‐redistributing support surfaces used within the clinical setting. For pragmatic reasons, mattress type was determined by ward nurses according to perceived need. Their choice, which varied between a cut foam mattress and an alternating mattress, was recorded and analysed as a covariate. |
|
| Outcomes | Included Grade 1 ulcers 1: heel elevation 7% (8/120) 2. Standard care 26% (31/119) |
|
| Notes | Patients poor concordance noted Attrition unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were allocated ...according to a computer generated block randomisation schedule". Sufficient evidence that this was done rigorously |
| Allocation concealment (selection bias) | Low risk | “In order to assure allocation concealment the randomisation schedule was held and managed by a senior research nurse not directly involved in the study”. Sufficient evidence that offsite allocation occurred |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | Tissue viability nurse who viewed photographs of suspected pressure damage, as well as intact pressure points was asked to categorise images using the NPUAP scale and was blinded to the subject’s history, results of skin assessments and the group to which the subject had been assigned (pg.312). Tissue viability nurse was unconnected with the study. The outcome assessments were based on photographs. However, nil blinding of personnel and participants due to high visibility of the intervention. Heelift suspension boot cannot be concealed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes were reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Pressure points were inspected daily, for all patients during their admission period for signs of tissue discolouration/ulceration‐ pg “10.78 days, experimental: 12.18 days”. Timing of the outcome assessment therefore appears same in each group. |
Economides 1995.
| Methods | RCT with 2‐week follow‐up. Allocation by sealed envelope. | |
| Participants | 12 patients with grade 4 pressure ulcers needing myocutaneous flap closure. 10/12 participants paraplegic or quadriplegic. Groups appeared broadly comparable at baseline, except the Roho group seem to have slightly better nutritional status (not tested for significance). | |
| Interventions | 1. Roho dry flotation mattress (n = 6) ‐ bed overlay consisting of 720 air cells that conform to the body to provide maximum support area and a "floating" environment. 2. Air‐fluidised Clinitron bed (n = 6) ‐ ceramic microspheres through which warm pressurised air is blown, covered by a polyester sheet. The bed forms a dry‐fluid environment on which the patient floats, thus distributing body weight away from bony prominences. | |
| Outcomes | Wound breakdown: 33% (2/6) on Roho vs 33% (2/6) on Clinitron. No significant difference between 2 support surfaces in the prevention of flap breakdown in the immediate postoperative period. | |
| Notes | Do not appear to have had any withdrawals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were assigned to a support surface by using a table of random numbers". |
| Allocation concealment (selection bias) | Low risk | "The names of the two support surfaces were placed in envelopes that were sealed and numbered sequentially". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons/numbers for exclusions/attrition reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Table 1. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Daily assessments for 2 weeks. |
Ewing 1964.
| Methods | RCT with 6‐month follow‐up. Mode of allocation unclear ‐ reported as random selection. | |
| Participants | Elderly patients, average age 72.5 y, confined to bed, with reduced mobility in legs due to neurological disorder, fixed joints, or peripheral vascular disease. No baseline data given and baseline comparability not described. Setting was geriatric unit of a convalescent hospital. | |
| Interventions | 1. Sheepskins adjusted so that both legs were supported on the woolly fleece (n = 18). 2. Control, without sheepskins (n = 18). All were submitted to the same 4‐hourly routine skin care involving washing, drying, powdering, light massage of pressure areas, bed cradle. | |
| Outcomes | The study was too small and poorly designed to detect a difference. No reports of withdrawals. Outcomes not clearly described or reported in terms of numerator and denominator. Reports incidence of pressure ulcers areas of 'reddened' skin. Grading of outcomes not done. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The patients were studied for a period of six months, and were allotted to a 'treated' or a 'control' group by random selection'". Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons/numbers for attrition/exclusions reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | No patient demographics given. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | At the end of the study period ‐ at six months |
Exton‐Smith 1982.
| Methods | Trial with 2‐week follow‐up. Allocation by alternation, and, where surface of choice was not available, patients were given an available surface. | |
| Participants | Newly‐admitted geriatric patients, with fractured neck of femur, and long‐stay patients; without pressure ulcers of grade 2 or greater. Norton score <14. Patients were matched in pairs for sex and Norton score. Where a match was not possible, the Airwave patient was matched with a Large Cell Ripple patient with a higher risk score. Groups appear well matched at baseline. | |
| Interventions | 1. Pegasus Airwave system (AWS) (n = 31) 2 layers of air cells; pressure alternated by deflating every 3rd cell in a 7.5 minute cycle. Mattress ventilated by pinholes through which air passes to keep patient's skin dry. 2. Large Cell Ripple (LCR) mattress (n = 31) large cell ripple not described. | |
| Outcomes | Grade 2 ulcer or greater: 1. AWS: 16% (5/31); 2. LCR: 39% (12/31). | |
| Notes | During the trial period, no breakdowns with AWS, 10 breakdowns on LCR, 4 patients withdrawn; 94% follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were alternately allocated the AWS or the LCM unless the appropriate mattress was not available: in that case the patient was allocated the mattress not in use". Proper randomisation not completed. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons/numbers for exclusions/attrition reported. |
| Selective reporting (reporting bias) | High risk | Not all of the study’s pre‐specified primary outcomes were reported i.e. the reliability and acceptability of both types of apparatus. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | "There was no significant difference between the two groups". |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | High risk | "Each patient remained on the allocated regimen for 2 weeks unless he died or was discharge from hospital, or the clinic score rose to 17 or more". |
Feuchtinger 2006.
| Methods | RCT with 5‐day follow‐up (postoperative). | |
| Participants | Recruitment from a Department of Cardiovascular Surgery. Eligible patients > 18 y, scheduled for cardiac surgery with extracorporal circulation. Not required to be free of pressure ulcers; 4 patients had grade 1 pressure ulcers as they went into surgery. Participants well matched at baseline. | |
| Interventions | 1. Operating table with waterfilled warming mattress and a 4‐cm thermoactive viscoelastic foam overlay (Thermo) (n = 85). 2. Standard OR table configuration (OR table with waterfilled warming mattress) (n = 90). | |
| Outcomes | Number of participants with incidence pressure ulcer (assessed day 1, 3 and 5 postoperatively; blinded outcome assessment):
Grade 1 ulcers postoperative days 0‐5:
1. Thermo 15.3% (13/85);
2. Standard 10% (9/90). Grade 2 ulcers postoperative day 0‐5: 1. Thermo 2.4% (2/85); 2. Standard 1% (1/90). |
|
| Notes | No higher grades of ulcers reported. No participant loss reported. The study was stopped after interim analysis due to the 11.1% total incidence in the standard group compared with 17.6% in the treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Included patients were randomised to either the standard operating table configuration or the test configuration". Method of randomisation unclear. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | “The postoperative nurses who assess the skin condition were unaware of the patient assignment”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No numbers/reasons given for exclusions/attrition. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | "Ninety paired assessments were undertaken for the inter‐rater reliability assessment”. No statistically significant differences were found. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Day 1, 3 and 5 postoperatively. |
Gebhardt 1996.
| Methods | Trial allocation by hospital number. Two systems: patients were automatically placed on the low‐cost mattress within the allocated system. Patients who deteriorated or experienced persisted erythema were transferred to a medium‐cost mattress. If deterioration continued they were placed on the highest‐cost mattress, or transferred to the alternate group, if appropriate. | |
| Participants | Patients in ICU with a Norton score <13, who had been in the unit for < 3 days, with no pressure ulcers. | |
| Interventions | 1. Alternating‐pressure air mattresses (shallow small cell overlays, medium depth large cell overlays, deep mattresses and deep pulsating low‐air‐loss beds) (n = 23). 2. Constant low‐pressure supports (fibre overlays, foam mattresses/overlays, static air overlays, gel overlay, water overlay, bead overlay, low‐air‐loss mattresses, static air overlay, low‐air‐loss beds and air‐fluidised bead beds) (n = 20). | |
| Outcomes | 1. Support provided.
2. Pressure ulcer development: Alternating pressure group (n=0 participants), Constant low‐pressure group (n=8 participants of which grade 2 (n=4), grade 3 (n=2), both grade 2 and 3 (n=2))
3. Cost: Low cost (less than £500) n=22 (n=1shallow small cell overlay, n=5 fibre overlays, n=4 medium‐depth large‐cell overlays, n=6 foam mattresses/overlays, n=3 static air overlays, n=1 gel overlay, n=1 water overlay, n=1 bead overlay) Medium cost (£500‐ £5000) n=4 (n=2 deep mattresses, n=1 low‐air‐loss mattresses, n=1 static air overlays) High cost (Greater than £5000) n=6 (n=2 deep pulsating low‐air‐loss beds, n=2 low‐air‐loss beds, n=2 air fluidised bead beds) |
|
| Notes | No ITT analysis. Mechanical unreliability and poor management of alternating‐pressure supports was a problem. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients allocated "according to the final digit of their hospital number (even to alternating pressure, odd to constant low pressure supports)." |
| Allocation concealment (selection bias) | High risk | Patients allocated "according to the final digit of their hospital number (even to alternating pressure, odd to constant low pressure supports)." |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for exclusion/attrition given. |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified aims not reported. Incidence of pressure ulcers as well as mean cost per type of mattress provided. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | As shown in Table 2. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | “Patients were visited four times weekly by the research nurse”, however, “patients were taken out of the trial after three months, or if their condition improved so that they were no longer at risk of developing pressure sores, if they were discharged or transferred to another ward or hospital, or if they died”. |
Gentilello 1988.
| Methods | RCT, duration of follow up unclear. Trial primarily not a pressure ulcer trial, but of kinetic treatment tables used to prevent chest infection in immobile patients. | |
| Participants | Critically ill patients in surgical ICU immobilised because of head injury, spinal injuries or traction. Groups well matched at baseline for demographic and pulmonary risk factors; patients in the conventional bed group had higher incidence of cigarette smoking. | |
| Interventions | 1. Kinetic Treatment Table (KTT) (n = 27): rotates through an arc of 124o every 7 minutes. Nurses instructed to leave bed rotating except when vital signs were being recorded and treatments being given. If a patient developed a serious complication as result of KTT, they were moved onto a conventional bed. 2. Conventional beds (n = 38): patients turned in conventional fashion every 2 h. Patients who developed a chest infection, where positioning was thought to be a factor, were moved onto a KTT. | |
| Outcomes | Primary outcomes were: Incidence of pulmonary complications. Other outcomes measured included Incidence of pressure ulcers: KTT 30%; Conventional beds: 26%. | |
| Notes | 1 patient withdrew and was not included in the analysis. No raw data provided for incidence of pressure ulcers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed by drawing a randomizing card”. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Only reported that the physician in charge of interpreting X‐rays was blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reasons/numbers for attrition/exclusion reported. |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | "Patients in the control and experimental groups were similar for most demographic variables". |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | High risk | "Patients were evaluated daily. The active study period started with randomisation and ended when the patient was allowed out of bed, died or was discharged from the SICU". |
Geyer 2001.
| Methods | Pilot RCT with 12‐month follow‐up. | |
| Participants | Recruitment in nursing homes (for the elderly). Eligible patients were wheelchair users aged > 65 y at risk of developing pressure ulcers (Braden score ≤ 18); with a combined Barden activity and mobility sub‐scale of ≤ 5; no pressure ulcers on their sitting surface; and tolerant of daily wheelchair sitting for ≥ 6 h in the ETAC twin wheelchair (body weight required to be < 250 lb). Participants well matched at baseline for age, initial Braden score, sex. | |
| Interventions | 1. Pressure‐reducing wheelchair cushion (n = 15). No single make of cushion specified, rather this could be selected by the nurse from a group of cushions based on the participants' clinical status. Further details about cushion design were not provided. 2. Standard foam (eggcrate) cushion (Bioclinic Standard, Sunrise Medical) (n = 17). | |
| Outcomes | Number of participants with Incidence pressure ulcer (weekly assessment; blinded outcome assessment): Grade not reported (all grades): 1. Pressure‐reducing cushion 40% (6/15); 2. Foam cushion 58.5% (10/17). | |
| Notes | Seating assessments were performed in both groups throughout the study. 1. 1 participant died, 3 lost to follow‐up. 2. 1 participant died, 2 lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Random treatment assignments with a 1‐to‐1 scheme were generated prior to the start of the study by a separate research team member who was not involved in executing the trial”. |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered sealed envelopes containing the treatment assignment were prepared". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | "Nursing staff members performing the outcomes measurements were blinded to the treatment group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons/numbers for attrition/exclusion provided. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Table 4 in the study report. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Weekly patient assessments. |
Gilcreast 2005.
| Methods | RCT of heel ulcers: follow‐up period unclear. | |
| Participants | Recruitment from military tertiary‐care academic medical centres. Eligible patients were at moderate or high risk of pressure ulcer development (Braden score ≤ 14). Patients with hip surgery were excluded, as were patients anticipated to be admitted for < 72 h, and those with pre‐existing heel pressure ulcers. Limited baseline information presented. There was baseline imbalance in sex. | |
| Interventions | 1. Bunny Boot (fleece) high cushion heel protector. 2. Egg crate heel lift positioner (holds the foot suspended above the bed surface with heel through a window). 3. Foot waffle air cushion (felt coated plastic inflatable plastic pillow that encircles the foot). | |
| Outcomes | Pressure ulcer incidence (did not stratify by grade; baseline numbers not available and unclear whether the unit was number of ulcers or number of patients): 1. Bunny Boot 4% (3/77); 2. Egg crate 5% (4/87); 3. Foot waffle 7% (5/76). | |
| Notes | 69% of participant were in ICU. Of the initial 338 patients, only 240 had follow‐up data, given as n in outcomes. Not clear how the 338 were distributed among the three groups. 53 not included, as did not wear the devices for at least 48 h; 45 not included as they were non‐compliant. No ITT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of cards. |
| Allocation concealment (selection bias) | High risk | Inadequate (non‐numbered envelopes) . |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | "The 1 nurse was performing all research tasks and was not blinded to the device to which the participant was assigned". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reasons/numbers for attrition/exclusions reported. |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified outcomes reported, however, raw numbers of participants unclear. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Only differences in gender distribution reported. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Daily assessments. |
Goldstone 1982.
| Methods | Patients randomised alternately on arrival in A&E to 1 of 2 alternative surfaces. Follow‐up not clear. | |
| Participants | Patients (> 60 y) with femur fracture. (Mean Norton score 13). Groups comparable at baseline for age and Norton score. | |
| Interventions | 1. Beaufort bead bed system (includes bead‐filled mattress on A&E trolley; bead‐filled operating table overlay; bead‐filled sacral cushion for operating table; bead‐filled boots to protect heels on operating table (n = 32). 2. Standard supports in A&E, operating theatre, ward (n = 43). | |
| Outcomes | Grading of ulcers not given.
Beaufort bead bed system: 16%;
Standard surface: 49%. Maximum width of broken skin (mean): Beaufort bead bed system: 6.4 mm; Standard surface: 29.5 mm. |
|
| Notes | Patients in the Beaufort bead bed group who were incontinent of urine (numbers not given) were catheterised, however, this did not seem to be the same for the control group. Patients who were removed from the Beaufort bed standard surfaces for any reason not included in analysis. Number of withdrawals unclear; no ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | “Patients...were assigned alternately (from a random start) either to the Beaufort system or to the existing ‘standard’ surfaces as encountered on trolleys, beds, surgical tables etc”. |
| Allocation concealment (selection bias) | Unclear risk | See above ‐ not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Patients who were later found to have suffered no fracture, or who requested to be removed from the Beaufort system for any reason, or who died before reaching the post operative ward are excluded from the analysis". |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | “The two groups were well matched on a variety of criteria on admission”. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Gray 1994.
| Methods | RCT with 10‐day follow‐up. Allocation by sealed envelope. | |
| Participants | Patients from orthopaedic trauma, vascular and medical oncology units without breaks in the skin (Waterlow score > 15). Groups well matched at baseline for age, sex and Waterlow score. | |
| Interventions | 1. Softfoam mattress (n = 90). 2. Standard 130 mm NHS foam mattress (n = 80). | |
| Outcomes | Incidence of pressure ulcers. Skin condition assessed at 5 and 10 days; presumably assessor not blind to treatment group. Grade 2 or greater ulcer: Softform: 7%; Standard: 34%. Rate of transfer to dynamic support surface: 19% in standard group vs 2% in Softform group. | |
| Notes | Impossible to calculate attrition rate, as incidence reported as % only and unclear what the denominator was. Nurses were more positive, and patients gave higher comfort scores to Softform mattress. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The subjects were then "randomly allocated to one of the two types of mattress using unmarked envelopes". |
| Allocation concealment (selection bias) | Unclear risk | The subjects were then "randomly allocated to one of the two types of mattress using unmarked envelopes". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | No mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers not reported post‐baseline. Might have been issues as the discussion notes that: "A number of patients were excluded from the study because the Waterlow score awarded by the ward staff differed greatly from that of the researcher". Not clear if this exclusion was post‐randomisation. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | More patients in orthopaedic and vascular specialities in the treatment arm. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | High risk | "...were assessed for deterioration in skin condition at 5 and 10 days respectively...." |
Gray 1998.
| Methods | Trial with follow‐up of 10 days. | |
| Participants | Patients admitted to a District General Hospital for bed‐rest or surgery, with intact skin, no other skin abnormalities, no terminal illness, weight <160 kg. Mean Waterlow score on admission: Group 1: 14 (3.6); Group 2: 13 (2.5). | |
| Interventions | 1. Transfoam mattress (n = 50). 2. Transfoamwave (n = 50) (both foam). | |
| Outcomes | 1. 1x grade 4 ulcer. 2. 1x grade 2 ulcer. | |
| Notes | 95% follow‐up; ITT analysis. Length of stay, pressure ulcer incidence. Comfort not specified (and only in treatment arm). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Subjects were selected from the admissions using serially numbered, sealed opaque envelopes and allocated to either a study mattress... or a non ‐study mattress.......This form of randomisation ensured that staff were not able to choose which patients be allocated to the study mattress". |
| Allocation concealment (selection bias) | Low risk | "Subjects were selected from the admissions using serially numbered, sealed opaque envelopes and allocated to either a study mattress... or a non ‐study mattress.......This form of randomisation ensured that staff were not able to choose which patients be allocated to the study mattress". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | "Subjects were reviewed at 5 and 10 days post admission. Observations of the skin were made and any pressure sores documented; these observations were confirmed blindly by the ward link nurse". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers not reported post‐baseline. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Only data for the treatment arm were provided. People were randomised to a non‐study treatment, but were not followed‐up. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Subjects were reviewed at 5 and 10 days post admission. |
Gunningberg 2000.
| Methods | RCT with follow‐up until discharge, or 14 days postoperatively. | |
| Participants | Patients admitted with a suspected hip fracture via an A&E department. Participants were > 65 y and did not have pressure ulcers. | |
| Interventions | 1. 10 cm visco‐elastic foam mattress on arrival in A&E, and visco‐elastic foam overlay on standard ward mattress (n = 48). 2. Standard A&E trolley mattress and ward mattress (n = 53). | |
| Outcomes | Grade 2 to 4 incidence:1. 8.3% (4/48); 2. 15% (8/53). Pressure ulcer incidence (all grades):1. 25% (12/48);2. 32% (17/53). Mean comfort rating:1. 4.2;2. 4.0 All results NS. |
|
| Notes | Only 44 participants completed the comfort questionnaire. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only details of process provided state, "On arrival to A and E patients with a suspected hip fracture were randomised to an experimental or control group with concealed allocation". |
| Allocation concealment (selection bias) | Unclear risk | "On arrival to A and E patients with a suspected hip fracture were randomised to an experimental or control group with concealed allocation". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Main outcome not blinded, but study authors undertook blinded outcome assessment as a ‘process check’ on a sub‐set. "25% Pus....in 13 patients were photographed during the study. The ulcers in these photos were graded by an expert nurse..who was blinded to treatment..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Difficult to tell if 18 people were excluded before or after randomisation. Outcomes reported for 101 patients: “This study...included 119 patients aged over 65 years with a hip fracture....Eighteen were excluded because they died, did not have skin assessment on arrival, were admitted with PUs. Of the remaining 101 patients 48 and 53 were allocated to the experimental and control groups respectively”. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Similar. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | "The pressure ulcer nurse on the ward usually performed the assessments on the 4th day postoperatively and at discharge". |
Hampton 1997.
| Methods | RCT, but method of allocation not described. Duration of follow‐up to a maximum of 20 days. | |
| Participants | Very little detail; average age 77 y. No data regarding baseline status of patients presented in published paper, therefore, impossible to judge baseline comparability. Only limited information obtained on request: number of patients at high‐very high risk Airwave group = 31; number of patients at high‐very high risk Cairwave group = 27. Mean age: Airwave group = 79 y; Cairwave group = 75 y. | |
| Interventions | 1. Alternating‐pressure (Cairwave System) (n = 36): 3‐cell, 7.5 minute cycle. Manufacturers claim that zero pressure achieved for more than 20% of the cycle. 2. Alternating‐pressure (Airwave System) (n = 39): cells arranged in sets of 3 and inflated in waves. 7.5 minute cycle; zero pressure said to be applied for 15% of the time. | |
| Outcomes | Incidence of pressure ulcers. No patient in this study developed a pressure ulcer. | |
| Notes | Attrition unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Report states, "randomised controlled trial", but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | States, "patients were allocated to the Cairwave Therapy System during the randomised controlled trial", but no further information was given. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | No report of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | No access to study protocol. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | No information provided. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | No information provided. |
Hofman 1994.
| Methods | RCT with 2‐week follow‐up. Patients randomised in blocks of 6 but method of randomisation not described. | |
| Participants | Patients with a femoral‐neck fracture and risk score > 8 (Dutch consensus scale). Excluded patients with pressure ulcers of grade 2 or greater on admission. Groups were similar at baseline for pressure ulcer risk; haemoglobin; total serum protein and serum albumin. | |
| Interventions | 1. Cubed foam mattress (Comfortex DeCube mattress) (n = 21) ‐ allows removal of small cubes of foam from beneath bony prominences. 2. Standard hospital mattress (n = 23) ‐ standard polypropylene SG40 hospital foam mattress. Both groups were treated according to the Dutch consensus protocol for the prevention of pressure ulcers. | |
| Outcomes | Incidence of ulcers of grade 2 or greater at 2 weeks. Outcome assessment not blind to treatment group. Patients were examined 1 and 2 weeks after surgery by 2 independent observers; disagreement resolved by a 3rd observer.
Grade 2 or greater ulcers:
Comfortex DeCube: 24% (4/17);
Standard: 68% (13/19). Maximum pressure ulcer gradings were significantly higher for the standard mattress than the DeCube mattress at 1 and 2 weeks. |
|
| Notes | 78% follow‐up. No ITT analysis. DeCube mattress was not always used correctly, and its size was not optimal for all patients. A priori sample size calculation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Each group of 6 consecutively admitted patients was randomly divided into 3 patients nursed preoperatively and postoperatively on the standard Vredestein polyproleen SG 40 hospital mattress (Vredestein, Netherlands) and 3 nursed on the comfotex DeCube" |
| Allocation concealment (selection bias) | Unclear risk | See above, only description of the randomisation process in paper. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | “The study was not blinded with respect to observer or nurse”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 46 patients randomised, 2 were excluded due to the randomisation not being performed correctly (no further details) both in control group. By week 1, 1 patient had left each group (1 death, 1 discharge). By 2 weeks post randomisation, 4 patients in each group had been discharged or died. It is not totally clear but seems that only those remaining (n = 17 compared with 19) were included in the 2 week analysis. |
| Selective reporting (reporting bias) | Low risk | Main outcome of interest was occurrence of pressure ulcers and this was recorded. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Age and length of hospital stay balanced. More medial fractures in control group and 24% male in treatment group compared with 4% in control group. Not sure, though, how these would be linked to outcome and cause bias. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Patients were examined 1 and 2 weeks after surgery. |
Inman 1993.
| Methods | RCT with an average of 17 days' follow‐up. Method of allocation unclear. | |
| Participants | Patients > 17 y with an Acute Physiology and Chronic Health Evaluation (APACHE II) score > 15 who had an expected ICU stay of > 3 days. | |
| Interventions | 1. Low‐air‐loss beds (n = 49). 2. Standard ICU bed (n = 49); patients rotated every 2 h. | |
| Outcomes | Incidence of pressure ulcers reported in the trial both as ulcers per patient and patients with ulcers. We have only extracted the incidence of patients developing ulcers.
Grade 2 or greater ulcers:
Low‐air‐loss beds: 12% (6/49);
Standard ICU bed: 51% (25/49). Patients with multiple pressure ulcers: Low‐air‐loss beds: 2% (1/49); Standard ICU bed: 24% (12/49). |
|
| Notes | A priori sample size calculation. 98/100 randomised participants completed the study, 1 lost from each group as did not stay in ICU for 3 days; neither developed an ulcer. No ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "100 consecutive patients were randomly assigned to receive treatment with either the air suspension bed or a standardised ICU bed". |
| Allocation concealment (selection bias) | Unclear risk | See above. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Blinding not mentioned in the description of outcome assessment. Not explicit that anyone was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 100 randomised, 98 analysed. One patient from each group was excluded post‐randomisation. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Groups similar at baseline in age and reason for admission. More men in control compared with standard group (59% vs 45%). |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Timing of assessment daily in both groups. |
Jolley 2004.
| Methods | RCT with unclear follow‐up period, mean bed days observed/participant Group 1: 7 days, and Group 2: 7.9 days. | |
| Participants | Participants recruited from a single hospital, and had to be at low to moderate risk of developing a pressure ulcer and > 18 y. Patients were excluded if they had no risk or high risk (as more complex interventions required), if they had any pre‐existing ulcers, had an expected length of stay of < 48 h or had darkly‐pigmented skin (justified by authors as making grade 1 ulcers difficult to detect). Participants well matched at baseline for age, sex, mean pressure ulcer risk score. |
|
| Interventions | 1. Sheepskin mattress overlay: leather‐backed with a dense, uniform 25 mm wool pile. Used as a partial mattress overlay. Pressure points that were not covered by sheepskin were protected by a second sheepskin, or specific sheepskin elbow and heel protectors. Overlays were changed 3 times a week (unless required). Received usual care including repositioning (n = 270). 2. Usual care as determined by ward staff. Included repositioning and any other PRD or prevention strategy with/without low‐tech constant pressure relieving devices (n = 269). | |
| Outcomes | Number of participants with incidence pressure ulcer (daily assessment; unblinded outcome assessment):
All ulcers (grade 1 and 2; no grade 3 or 4 recorded)
1. Sheepskin: 10% (21/218);
2. Usual care: 17% (37/223). Total number of ulcers: 1. Sheepskin: 27; 2. Usual care: 58. Total number of incident grade 2 ulcers: 1. Sheepskin: 12; 2. Usual care: 20. |
|
| Notes | Whilst 270 were allocated to the sheepskin and 269 to control; only 218 and 223 received their allocated treatment and are included in the analysis. Not ITT. "Any patient whose risk increased to high (Braden score <12) for 48 h was no longer followed up for pressure‐ulcer endpoints." Authors did not say why. Of the 218 participants in the sheepskin group 2 died, 7 became high risk (treatment change), 14 requested withdrawal, 6 had ward staff intervention and 11 changed treatment for other reasons. Of the 223 control participants 5 died, 1 became high risk, 8 requested withdrawal, 5 had ward staff intervention and 10 changed treatments for other reasons. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Shuffling cards(?): "Patients were randomly allocated to receive.......using numbered cards in individually sealed opaque envelopes; blocks of 16 envelopes (eight of each group) were shuffled before use". |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly allocated to receive.......using numbered cards in individually sealed opaque envelopes; blocks of 16 envelopes (eight of each group) were shuffled before use". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | "As it was logistically impossible to blind patients, ward staff and research nurses to the treatment group this was an open‐label, unblinded trial". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "539....were randomly allocated. Of these, 441 received the allocated intervention. All 441 were followed up to the endpoints...". Data for 441 not 539. Is a per protocol analysis. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Baseline data for 441 participants and not the 539 randomised. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | "Research nurse assessed each participant daily for pressure ulcer risk.....and skin integrity". |
Kemp 1993.
| Methods | RCT with 1‐month follow‐up. Allocation by random‐number table. | |
| Participants | Inclusion criteria: > 65 y, inpatients, with a Braden Score of ≤16. Age ranged from 65‐98 y, 58 women, 26 men. Recruited from general medicine, acute geriatric medicine and long term care. All patients free from pressure ulcers on admission. Groups similar for important variables at baseline. | |
| Interventions | 1. Convoluted foam overlay (CF), 3 or 4 inches thick (n = 45). 2. Solid foam overlay (SF) 4 inches thick, sculptured (n = 39). | |
| Outcomes | Incidence of pressure ulcers assessed by Research Nurse presumably not blinded to intervention. Included grade 1 ulcers: CF: 47%; SF: 31%. | |
| Notes | All patients appear to have completed the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "..a random number table was used to assign study participants to...." |
| Allocation concealment (selection bias) | Unclear risk | not clearly reported |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | not clearly reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "..45 patients were assigned to the CF group and 39 to the SF group......" "...33 (39%) patients developed a total of 57 pressure ulcers...." |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Similar for Braden score, age, mobility, but these figures were not presented for all those randomised. Treatment group were lighter, 118.51 lb vs 129.46 lb when all participants included. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | "Research nurses assessed each patient’s skin and completed a Braden scale every Monday, Wednesday and Friday for 1 month or until discharge...". |
Keogh 2001.
| Methods | RCT with follow‐up of 5‐10 days. | |
| Participants | Patients from 2 surgical and 2 medical wards: > 18 y; Waterlow score of 15‐25; tissue damage no greater than grade 1. | |
| Interventions | 1. Profiling bed with a pressure reducing foam mattress/cushion (n = 50). 2. Flat‐based bed with a pressure relieving/redistributing mattress/cushion (n = 50). | |
| Outcomes | Number of pressure ulcers developed: 1. 0/35; 2. 0/35. Healing of existing grade 1 ulcers: 1. 100% (4/4); 2. 20% (2/10). | |
| Notes | Extent of follow‐up difficult to ascertain. No difference between the groups in terms of transferring in and out of bed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The block design randomisation code was computer generated by an independent statistician using blocks of eight". |
| Allocation concealment (selection bias) | Low risk | "The allocation for each patient was placed in sealed opaque envelopes that were numbered sequentially. The patient and researcher were not aware of allocation until after recruitment". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Pressure ulcer incidence. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "A total of 100 patients were recruited into the study. Data were incomplete for 30 of these patients. All 100 patients were included in an intention‐to‐treat analysis in respect of pressure ulcer incidence". |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Imbalance in male to female ratio (M:F 20:30 in control and 35:15 in treatment). Balanced on initial nutritional assessment score BMI, age, mobility score. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | "Waterlow scores were assessed and pressure areas observed daily". |
Laurent 1998.
| Methods | RCT with factorial design. 2 pressure‐relieving mattresses used either in ICU (alternating‐pressure), or in post‐ICU hospitalisation (constant low‐pressure), or in combination, and compared in each case with the standard surface. Randomised "by blocks" ‐ method of allocation unclear. | |
| Participants | Adults over 15 y of age, admitted for major cardiovascular surgery, hospital stay likely to be at least 5 days, with a period on ICU. Little data provided regarding baseline comparability. | |
| Interventions | 2 X 2 factorial design: 1: Standard mattress in ICU; standard mattress postoperatively (n = 80). 2: Nimbus (AP) in ICU; standard mattress postoperatively (n = 80). 3: Standard mattress in ICU; Tempur (CLP) postoperatively (n = 75). 4: Nimbus in ICU; Tempur postoperatively (n = 77). | |
| Outcomes | Incidence of ulcers of grade 2 or above (partial‐ or full‐thickness skin loss and worse): Group 1: 18% (14/80); Group 2: 13% (10/80); Group 3: 15% (11/75); Group 4: 13% (10/77). NS. | |
| Notes | A priori sample size calculation. No reports of withdrawals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Exact randomisation procedure not reported: "patients were randomised among four groups" and "patients were randomised by blocks". |
| Allocation concealment (selection bias) | Unclear risk | See above ‐ not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | Blinding discussed as follows: “given the kind of material tested, blinding was not possible”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data available for all participants enrolled in study (no attrition). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | “There was no imbalance of characteristics, risk factors, or surgical procedures between the groups”. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Lazzara 1991.
| Methods | RCT (allocation by random‐number tables) in elderly nursing home population with 6‐month follow‐up. | |
| Participants | Nursing home residents at risk of pressure ulcers (Norton score > 15). 9/66 subjects had pressure ulcers on entry to the study. | |
| Interventions | 1. Air‐filled (SofCare) overlay (33 randomised; 2 ulcer on admission; 10/31 developed a new one). 2. Gel mattress (33 randomised; 7 ulcer on admission; 8/26 developed a new one). | |
| Outcomes | Grade 2 or greater ulcers: 1. Air overlay: 16% (5/31); 2. Gel mattress: 15% (4/26). | |
| Notes | Interventions not well described. Of the 74 who entered the study, only those who participated for 4‐6 months were included in the analysis (total of 66). 19 patients died and were excluded from the analysis, but these might be at highest risk. It was difficult to maintain inflation of the air overlay; it also punctured easily. During the trial, 110 air overlays were used for 76 patients. Gel mattress was heavy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Blinding not reported: “patients in both study groups were assessed by the same researcher for the presence of pressure ulcer development over areas of bony prominence”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Nineteen participants died during the 6‐month study; individuals participating for 4‐6 months were included in the data analysis, although exact numbers included were not reported. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No important baseline differences. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Lim 1988.
| Methods | RCT with 5‐month follow‐up. Patients were "randomly assigned" but method of allocation not described. | |
| Participants | 62 residents of an extended care facility; aged ≥ 60; free of pressure ulcers but at high risk of developing an ulcer (Norton score ≤ 14); using a wheelchair for ≥3 h/day; without progressive disease or confined to bed. Groups well matched at baseline for sex, age, weight, Norton score, primary diagnosis, sensory status, time spent in wheelchair, and mobility. | |
| Interventions | 1. Foam slab cushion (2.5 cm medium density foam glued to 5 cm firm chipped foam) (n = 26). 2. Contoured foam cushion (same foam as above; cut into a customised shape to relieve pressure on ischial tuberosities) (n = 26). Both cushions fitted with identical snug fitting covers of knitted polyester. | |
| Outcomes | Included grade 1 ulcers: 1. Slab foam: 73% (19/26); 2. Contoured foam: 69% (18/26). Mean severity score was 1.9 in the slab and 1.7 in the contoured (P value > 0.05), and the mean healing duration was 6.2 weeks in the slab and 5.4 weeks in the contoured group (P value > 0.05). | |
| Notes | 84% follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Exact randomisation procedure not described: "qualifying consenting subjects were randomly assigned to one of the two cushions for a period of 5 months". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | “The incidence, location, severity, and healing time of DU were determined weekly by another occupational therapist, a research assistant, who was from outside of the facility and was not knowledgeable of the Norton’s score of the subjects”; assessments taken a half‐hour after participants returned to bed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 participants reported as dropouts with reasons given; no ITT analysis conducted, but attrition within 20% limit of total recruited sample. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No important baseline differences. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
McGowan 2000.
| Methods | RCT. Discharge from hospital, transfer to a rehabilitation ward. | |
| Participants | Orthopaedic patients aged ≥ 60; assessed as being at low or moderate risk of pressure ulcer development by Braden scale; intact skin; anticipated LOS > 48 h. | |
| Interventions | 1. Standard hospital mattress, sheet and an Australian Medical Sheepskin overlay; sheepskin heel and elbow protectors as required (n = 155). 2. Standard hospital mattress, sheet with or without other low tech constant pressure devices as required (n = 142). Sheepskins were changed as required (at least every 3 days) | |
| Outcomes | 1. Sheepskin:9% (4/155) (21 ulcers) 7 participants developed 1 ulcer; 7 developed 2, all grade I. 2. Control: 30% (43/142) (67 ulcers) 25 participants developed 1 ulcer; 7 developed 2; 11 developed 3. 4 ulcers were grade II, 1 grade IV. Comfort rated significantly greater in experimental group. Limb protectors difficult to keep in place. | |
| Notes | 1 patient from each group withdrew prior to data collection. 6 patients in experimental group withdrew because sheepskin too hot or irritable; 7 in control group withdrew plus 3 in experimental group due to protocol violations (no ITT). Patients in experimental group rated comfort significantly higher than controls (P value < 0.0001). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated, "patients were randomly allocated (using sealed envelopes) by research nurses to receive one of two interventions". |
| Allocation concealment (selection bias) | Unclear risk | Not reported, although sequence generation based on sealed envelopes (see above). |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | “Blinded outcome assessments were not possible because the support surfaces could not be disguised and patients could not be moved off the bed for assessment of their pressure ulcers”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals from study reported with reasons given; and “data collected for patients up until the time of withdrawal has been included in the analysis with the exception of five controls and two patients from the experimental group for whom study participation time was not available”. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No important baseline differences. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Mistiaen 2009.
| Methods | RCT with 30‐day follow‐up. | |
| Participants | Patients recruited from aged care facility (predominantly rehabilitation department) and rehabilitation centre. Grade 1 pressure ulcers included in the sample. | |
| Interventions | 1. Australian medical sheepskin within 48 h of admission on the patient's bed. Application in wheelchair recommended and under heels permitted. (Hi‐temp, urine resistant, size XXL mattress) (n = 271).
2. Usual care (n = 272). Cointerventions: usual intervention for prevention of pressure ulcers in study settings. |
|
| Outcomes | Number and grade of pressure ulcers developed. 1. Grade 1 = 18, Grade 2 = 6, Grade 3 = 0, Grade 4 = 0. 2. Grade 1 = 32, Grade 2 = 6, Grade 3 = 2, Grade 4 = 0. | |
| Notes | ITT analyses performed. Sample size calculation performed, however, not included in this paper (included in published protocol). 33% of intervention group believed the sheepskin to be too warm, and thus the trial was stopped early in these patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Truly random methods of randomisation used. |
| Allocation concealment (selection bias) | Low risk | Adequate methods of allocation concealment used. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | No blinding on patients, clinicians, outcome assessors. Unclear/unstated blinding of data analysts. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported. |
| Selective reporting (reporting bias) | Unclear risk | Not reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Groups were well matched. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Nixon 1998.
| Methods | RCT with 8‐day follow‐up. Telephone randomisation (i.e. full allocation concealment) stratified by centre, and age. | |
| Participants | Patients aged ≥ 55 y, admitted for elective major general, gynaecological or vascular surgery in supine or lithotomy position and free of preoperative pressure damage greater than grade 1. Groups well matched at baseline for age, sex, Braden score, type of surgery, duration of surgery, length of preoperative stay, proportion of time hypotensive during surgery. | |
| Interventions | 1. Dry visco‐elastic polymer pad on operating table (n = 222). 2. Standard operating theatre table mattress plus Gamgee heel support (n = 224). | |
| Outcomes | Incidence and severity of pressure ulcers: Overall incidence of pressure ulcers of 16% (65/416): 1. Dry visco‐elastic polymer pad on operating table: 11% (22/205); 2. Standard mattress: 20% (43/211); P value 0.01, OR = 0.46; 95% CI 0.26‐0.82. 56/65 episodes of skin damage were conversions from grade 0 to grade 1 ulcers. 4/65 grade 0 to grade 2A conversions. 5/65 grade 0 to grade 2B conversions. These data were not broken down by group. | |
| Notes | A priori sample size calculation. 133 paired assessments by 94 nurses for pre‐study inter‐rater reliability assessments undertaken. Disagreement in only 2.2% assessments, and only 2 disagreements related to differentiating between grade 1 and grade 2a ulcers (the remainder were grade 0 and grade 1). The majority were associated with heel assessments. For the recovery and ward area assessments, there were discrepant assessments in only 8.5% cases. Sensitivity analysis assessing the impact of this level of misclassification on the overall result determined that the overall difference between the mattresses remained. Main endpoint data reported for 416 patients; incomplete data for 30 patients (lost forms 3; incomplete postoperative skin assessment 27). The patients with incomplete data were not reported by group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation by centre and age: "a telephone randomisation schedule was developed within random permuted blocks of 6, with a run‐in of 8"; age as 55‐69 and 70 or over. |
| Allocation concealment (selection bias) | Low risk | Randomisation managed by the Northern and Yorkshire Clinical Trials and Research Unit. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | The Data Monitoring Committee and statistician were blind to treatment allocation; “the record pertaining to the intra‐operative randomised mattress allocation remained separate from the main data collection pro forma to maintain the blind” . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses conducted. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | High risk | Standard mattress group: longer length of operation, longer pre‐operative stay, more time in hypotensive state than dry polymer pad group. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Nixon 2006.
| Methods | RCT with 30‐day follow‐up twice weekly, and a further 30‐day follow‐up once weekly. | |
| Participants | Recruited from 11 hospitals. Patients admitted as acute or elective cases. Eligible patients aged ≥ 55, expected to stay for at least 7 days, with either limited activity or mobility (Braden scale activity and mobility score of 1 or 2), or an existing pressure ulcer of grade 2. Elective surgical participants without limited activity or mobility were eligible if the mean LOS for surgery was at least 7 days and they were expected to have Braden scale activity and mobility scores of 1 or 2 for at least 3 days postoperatively. Exclusion criteria: grade 3 or worse pressure ulcer on admission, planned admission to ICU after surgery, admitted to hospital more than 4 days before surgery, slept at night in a chair, weighted > 140 kg or < 45 kg (as per mattress specifications). Participants were well matched at baseline. |
|
| Interventions | 1. Alternating‐pressure overlay (n = 990): alternating cell height minimum 8.5cm, max 12.25cm; cell cycle time 7.5‐30 minutes.
2. Alternating‐pressure mattress (n = 982): alternating cell height min 19.6cms, max 29.4cms; cell cycle time 7.5‐30 minutes. Intervention was allocated within 24 hrs of admission. |
|
| Outcomes | Number of participants with incidence pressure ulcer grade 2 and above (unblinded outcome assessment):
1. Overlay: 11% (106/989);
2. Mattress: 10% (101/982). Patient acceptability: requests for mattress change: 1. Overlay: 23% (230/989); 2. Mattress: 19% (186/982). Healing of existing pressure ulcers: 1. Overlay: 34% (20/59); 2. Mattress: 35% (19/54). Cost of treatment (GBP): 1. Overlay: Sterling 6793.33; 2. Mattress: Sterling 6509.73. Mean difference in time to pressure ulcer (grade 2 or higher) development (days). Participants in mattress group took 10.64 days longer to develop pressure ulcer than overlay group. |
|
| Notes | 1 participant was recruited to the trial twice (group 1) and was excluded from analysis. Factors that had a significant effect on the proportion of people developing a new pressure ulcer were admission for an acute condition, the presence of a wound skin trauma or non‐blanching erythema on any site at baseline, age, haemoglobin level and diabetes. The authors stated that differences in health benefits and total costs for hospital stay between alternating‐pressure mattresses and alternating‐pressure overlays were not statistically significant. However, a cost effectiveness acceptability curve indicated that on average alternating‐pressure mattresses were associated with an 80% probability of cost saving compared with alternating‐pressure overlays. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using a computer‐generated algorithm. |
| Allocation concealment (selection bias) | Low risk | "To maintain allocation concealment, the minimisation algorithm and subsequent treatment assignment was provided through an independent, central, secure 24‐hour randomisation automated telephone service by the Clinical Trials Research Unit (CTRU), University of Leeds". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | Stated, “owing to the nature of the mattresses under investigation, it was not possible to mask the randomised intervention to the patients participating in the trial, ward nursing staff or the CRNs conducting the skin assessments”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No important baseline differences. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Price 1999.
| Methods | RCT with follow‐up 14 days postoperatively. | |
| Participants | Patients with fractured neck of femur and Medley score of > 25 (very high risk), aged over 60 y. | |
| Interventions | 1. Repose system (low‐pressure inflatable mattress and cushion in polyurethane material) (n = 40). 2. Nimbus III dynamic flotation plus TransCell cushion (n = 40): all other care standard best practice, including regular repositioning. | |
| Outcomes | 1. Repose system: at admission 14/40 has pressure ulcers; preoperatively, 7/36; at 7 days: 6/32; at 14 days: 5/24. 2. Nimbus III: at admission had pressure ulcers, 13/40; preoperatively, 8/37; at 7 days: 5/31; at 14 days: 4/26. | |
| Notes | 80 patients randomised; 50 featured in final analysis (assessed 14 days post‐operatively) i.e. 38% attrition. Patients with pressure ulcers recruited. Difficult to ascertain how many of those with existing pressure ulcer included in 7‐day and 14‐day follow up assessments (see Table 4 of paper) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stated, "a concealed computer‐generated list was used to randomise eligible consecutive consenting patients to one of the support systems". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | “Patients were not assessed blindly as it was considered that displacement for examination would cause excessive discomfort”. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “No patient was excluded from the analyses. In many patients the data were incomplete, but they have been included in the analyses for those time points where data are present”; data from 50 (out of 80 patients) only analysed for final assessment. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No statistically significant differences on prognostic indicators at baseline between groups. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Ricci 2013.
| Methods | RCT, with a 4 week follow up. Allocation as follows: Patients were randomized according to a computer generated pre‐defined assignment list in sealed envelopes to use a standard mattress plus either three‐dimensional or viscoelastic overlay. |
|
| Participants | 50 patients of both genders, aged 65 years and over, with a Braden score between 9‐13 and Norton score between 7‐11 and pressure ulcer stage 0 or 1 with an expected hospital stay greater than 4 weeks. Setting two long term care units with a total of 150 beds. | |
| Interventions | Group 1: Aiartex (Herniamesh® Sri )– new CE‐marked three‐dimensional anti‐decubitus mattress overlay made flame retardant polyester Group 2: Akton – a commercially available viscoelastic mattress overlay for the prevention of pressure ulcers development All patients received repositioned every 2 hours, alternating lateral (30°) and supine position. |
|
| Outcomes | Primary Outcome: Incidence of Pressure ulcers at 28 days (excluding grade 1): Group 1: 0/25 Group 2: 0/25 Secondary Outcome: Patient subjective safety and tolerability: Group 1: ‐ Good: 20/25 (80%) ‐ Excellent: 5/25 (20%) Group 2: ‐ Good: 24/25 (96%) ‐ Excellent: 1/25 (4%) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated pre‐defined assignment list used. |
| Allocation concealment (selection bias) | Low risk | “Patients were randomized according to a computer generated pre‐defined assignment list in sealed envelopes to use a standard mattress plus either three dimensional or viscoelastic overlay.” Don’t mention if this was performed off site or who was contacted. It does not also state who accessed these sealed envelopes. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | One medical operator was responsible for enrolling patients but no further information provided. Blinding of participants and personnel was not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear as no information reported regarding loss to follow up etc |
| Selective reporting (reporting bias) | Unclear risk | Nil data provided regarding pain scores. Does report pain outcomes in narrative forms however no figures are provided. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | “No significant difference between groups between the two groups of patients”, No statistically significant difference found between the two groups. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Patient’s conditions were then re‐assessed at days 7, 14, 21 and day 28. Based on the above quote it is reasonable to assume, even though they don’t say, that both timing of the intervention and control groups occurred at similar timing. |
Russell 2000.
| Methods | RCT with 7‐day follow‐up. Randomisation using sealed opaque envelopes. | |
| Participants | Patients aged ≥ 18 y; undergoing scheduled cardiothoracic surgery under GA; surgery of at least 4 h duration; free of pressure ulcers. Both groups comparable at baseline for pressure ulcer risk (modified Knoll); history of previous ulceration; disease status; sex; age; weight; height. | |
| Interventions | 1. MicroPulse system in the OR and postoperatively (n = 98). 2. Conventional care (gel pad in OR, standard mattress postoperatively) (n = 100). | |
| Outcomes | Incidence and severity of pressure ulcers:
1. MicroPulse system: 2%* (2/98);
2. Conventional management: 7% (7/100 patients developed 10 ulcers).
Grade of ulcers: 1. MicroPulse system: grade 2 = 22; 2. Conventional management: grade 1 = 2; grade 2 = 5; grade 3 = 3* *1/2 discounted by original authors from their analysis as thought to occur for reasons "not related to the use of the MicroPulse system"! |
|
| Notes | No equipment‐related adverse events were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was done blindly by using a sealed opaque envelope that contained the randomisation information (i.e., multi‐cell pulsating dynamic mattress system vs. conventional management)." |
| Allocation concealment (selection bias) | Unclear risk | Not reported, although sequence generation based on sealed envelopes (see above). |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | Immediate post‐surgical assessment described, therefore, patients likely to be using mattresses at time, so blinding of outcome assessors not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis conducted. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No statistically significant baseline differences. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Russell 2003.
| Methods | RCT. Median days in study presented by group by hospital (3 hospitals). For the experimental group median days ranged from: 8‐14; control group 9‐17. Central allocation at trials office/pharmacy, sequentially‐numbered or coded vials. | |
| Participants | Elderly acute, orthopaedic and rehabilitation wards; > 65 y; Waterlow score of 15‐20. | |
| Interventions | 1. Visco‐polymer energy absorbing foam mattress (CONFOR‐Med)/cushion combination (n = 562). 2. Standard mattress/cushion combination (n = 604). | |
| Outcomes | Development of non‐blanching erythema or worse (including with and without blanching erythema on admission to trial)
1. CONFOR‐Med: 19.9% (110/562);
2. Standard mattress: 26.3% (161/604); P value 0.005. Development of non‐blanching erythema or worse: 1. CONFOR‐Med: 8.5% (48/562); 2. Standard mattress: 10.9% (66/604). NS. Data for ulcers of grades > 1 not presented separately. |
|
| Notes | Patient comfort scores non significant. No adverse events reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Different randomisation procedure for sites 1 and 2 from site 3: “equipment allocation at 2 sites was made by converting random numbers...on a 50:50 basis (0‐0.5 and 0.5‐1.0). At site 3, trial numbers were allocated sequentially and the patient chose from 1 of 2 opaque envelopes. No blocking or stratification was used at any site”. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | “Because the data collection team examined participants at bedside and the experimental mattress surface is distinctive, data collection could not be blinded”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “Participants who died were included in all statistical analyses”; ITT analysis conducted on all randomised patients (excluding 2 where protocol violations had occurred). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No statistically significant differences on prognostic indicators at baseline between groups. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Sanada 2003.
| Methods | RCT: duration of follow‐up not reported. | |
| Participants | Recruitment from a single acute care unit. Eligile patients had a Braden score of ≤ 16, were bed bound, free of pressure ulcers before the start of the study, and required head elevation. Exclusion criteria not discussed. Baseline variables were generally balanced. | |
| Interventions | 1. Double‐layer air cell overlay (Tricell) (n = 37): two layers consisting of 24 narrow cylinder air cells.
2. Single‐layer air cell overlay (Air doctor) (n = 36): single layer consisting of 20 round air cells.
In both overlays the pressure was alternated between cells at 5‐minute intervals. 3. Standard hospital mattress (Paracare) (n = 35). All groups had change of body position every 2 h, and special skin care to guard against friction and sheer. Nutritional intervention was given where required. |
|
| Outcomes | Number of participants with incidence pressure ulcer (daily assessment). All ulcers were grade 1 or 2.
Grade 1 ulcers:
1. Double‐layer: 0/26;
2. Single‐layer: 3% (1/29);
3. Standard mattress: 15% (4/27). Grade 2 ulcers: 1. Double‐layer: 4% (1/26); 2. Single‐layer: 14% (4/29); 3. Standard mattress: 22% (6/27). |
|
| Notes | Numbers included in study analysis were 26 for the double‐layer group (2 discontinued, 2 deaths, 7 head elevation ≤ 30 degrees); 29 for the single‐layer group (1 mattress malfunction, 2 deaths, 2 head elevation ≤ 30 degrees); and 27 for the standard mattress group (1 death, 7 head elevation ≤ 30 degrees). No ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The subjects were randomly allocated to the groups by sequentially‐labelled sealed envelopes”. |
| Allocation concealment (selection bias) | Low risk | Following randomisation, “after baseline assessment, the registered nurses opened the envelopes that indicated which surface each subject would be treated on”. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 41 patients withdrew from trial; no ITT analysis conducted. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No statistically significant differences on prognostic indicators at baseline between groups. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Santy 1994.
| Methods | RCT with 14‐day follow‐up. Allocation by random‐number tables; degree of allocation concealment unclear. | |
| Participants | Patients aged > 55 y with hip fracture, with or without pressure ulcers. Excluded: those with a pressure ulcer of grade 3 or 4 at entry. Patients in each group were well matched for age and Waterlow score at baseline. | |
| Interventions | Results for Group 2 (NHS contract surface ‐ standard foam): 17/64 Results for Groups 1, 3, 4 and 5, alternating foam combined) 42/441 | |
| Outcomes | Rates of removal from study due to skin deterioration: 1. Clinifloat: 9%; 2. NHS contract: 27%; 3. Transfoam: 10%; 4. Therarest: 11%; 5. Vaperm: 8%. | |
| Notes | 9% attrition. At interim analysis, Clinifloat and NHS contract mattresses were removed from the study; Clinifloat due to superior performance, and the NHS mattress due to high rates of pressure ulcer development. This explains why there were fewer patients on these surfaces. Omnifoam mattress showed foam collapse after 6 weeks and were withdrawn from use and replaced with Vaperm mattresses. Problems with mattress cover found on 2 Therarest mattresses, 3 Transfoam mattress covers, and 3 times with the Clinifloat mattress. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number tables used. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Skin assessments undertaken by research nurse; patient unlikely to be removed from mattress for assessment, although not explicitly reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patient removal numbers reported; attrition within reasonable limits (20% of total participants recruited at baseline). |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Mean age and Waterlow scores reported as well‐matched across different mattress groups. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Schultz 1999.
| Methods | RCT with 6‐day follow‐up. | |
| Participants | Patients admitted for surgery lasting at least 2 h in lithotomy position, aged ≥18; admitted with intact skin. | |
| Interventions | 1. Experimental mattress overlay in operating room made of foam with a 25% indentation load deflection (ILD) of 30 lb and density of 1.3 cubic feet (n = 206). 2. Usual care (padding as required, including gel pads, foam mattresses, ring cushions (donuts) etc) (n = 207). | |
| Outcomes | 1. Experimental operating room mattress overlay: 27% (55/206); 6 people had ulcers of grade 2 or more. 2. Usual care: 16% (34/207); 3 people had ulcers of grade 2 or more. | |
| Notes | Experimental product caused postoperative skin changes. Authors contacted for more information relating to grade of ulcer by group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐numbers tables used. |
| Allocation concealment (selection bias) | High risk | Patients randomly assigned for consideration in study from operating room schedule, then screened by nurses or primary investigator against inclusion/exclusion criteria before randomisation to experimental or control group. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | “Beginning on the day after surgery an continuing for 6 days, 2 research assistants, blinded to the study group of the patient, examined the skin over the bony prominences of each patient for any evidence of skin changes”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No important baseline differences. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | No other concerns. |
Sideranko 1992.
| Methods | RCT with mean follow‐up of 9.4 days. Method of randomisation not reported though said to be "random". | |
| Participants | Adult, surgical ICU patients: Surgical ICU stay > 48 h, without existing skin breakdown on admission. Groups broadly similar at baseline, although water mattress group appeared to be heavier and had fewer days in ICU (significance of these differences unclear). | |
| Interventions | 1. Alternating air mattress: 1.5‐inch thick Lapidus Airfloat System (n = 20). 2. Static air mattress: 4‐inch thick Gay Mar Sof Care (n = 20). 3. Water mattress: 4‐inch thick Lotus PXM 3666 (n = 17). | |
| Outcomes | Grade of ulcers not reported. 1. Alternating air mattress: 25% (5/20); 2. Static air mattress: 5% (1/20); 3. Water mattress: 12% (2/17). | |
| Notes | The trial was primarily about interface pressure and patient position, therefore, there was relatively little detail about the incidence part of the study, and no description of co‐interventions. No withdrawals reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not explicitly reported, "...randomly assigned...". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. Patients and carers would note have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals reported. 57 patients were enrolled in the study but no numbers were provided in the results text or tables, except to say that 8 subjects (14% of the total sample) developed pressure ulcers. |
| Selective reporting (reporting bias) | Low risk | Pressure measurement and development of pressure ulcers were described as the outcomes of interest (with interface pressure and patient position being the main outcomes of interest) and these were both reported in the results section. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | “Demographic information from patients’ charts describing patient age, sex, height, and weight upon admission were records” but the data were not provided in the results section. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
Stapleton 1986.
| Methods | Quasi RCT: allocation by means of alternation. Duration of follow‐up unclear. | |
| Participants | Female elderly patients with fractured neck of femur, without existing pressure ulcers, Norton score 14 or less. Baseline data presented and groups well matched for age and Norton score. | |
| Interventions | 1. Large Cell Ripple (Talley) (n = 32). 2. Polyether foam pad 2 feet x 2 feet x 3‐inch thickness (n = 34). 3. Spenco pad (n = 34). | |
| Outcomes | Ulcers of grade 2 or greater:
1. Large Cell Ripple: 34% (11/32);
2. Polyether foam pad: 41% (14/34);
3. Spenco pad: 35% (12/34). Grade 3 and greater: 1. Large Cell Ripple: 0% (0/32); 2. Foam pad: 24% (8/34); 3. Spenco pad: 6% (2/34). |
|
| Notes | 45 Large Cell Ripple mattresses required 50 motor repairs and 90 material repairs during 12‐ month study. Patients did not like the feel of the ripples. No mention of withdrawals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation for first 2 groups, but not for subsequent groups: “patients for the first two groups were selected by lottery, and thereafter patients were allocated to each group systematically, in rotation”; total numbers for the first 2 groups were not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Out of 100 patients recruited, “two patients allocated to the Ripple pad were lost to ward transfer”. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes not pre‐specified. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | No baseline differences on mean age and Norton scores (the presence of existing pressure ulcers was an exclusion criterion for the study). |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Inclusion criteria stated female patients only. |
Summer 1989.
| Methods | RCT: duration of follow‐up unclear. Randomisation by random sequences of letters corresponding to treatment groups, however, level of concealment unclear. | |
| Participants | Patients admitted to the ICU in diagnostic groups, namely: sepsis‐sepsis syndrome/pneumonia; respiratory failure; drug overdose; metabolic coma; stroke/neuromuscular disease; adult respiratory distress syndrome. Groups comparable at baseline for APACHE score; condition of pressure area at baseline not discussed. | |
| Interventions | 1. Kinetic Treatment Table (n = 43) 7 feet x 3 feet padded, vinyl‐covered platform on central rotating pivot which turns through an arc every 1.7 seconds. Reported to be of value in respiratory failure. 2. Routine 2‐hourly turning on conventional beds (n = 43). | |
| Outcomes | 1 patient developed small facial ulcer on Kinetic Treatment Table; none on conventional beds. | |
| Notes | 3/86 (3%) patients lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random sequences of 30 letters (K for KTT and C for control) were supplied using standard tables of random numbers for each of the six groups...". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | The study nurse collecting APACHE score data was not involved in patient management or triage decisions, but there is no indication that outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83 patients were analysed as 5 separate groups, but later in results 11/86 were diagnosed independently by infection control surveillance. It would appear that 3/86 were not analysed, but this was only 3.5% so well within conventional limits. Reasons for drop‐outs not given. |
| Selective reporting (reporting bias) | Low risk | “...(9) development of new decubitus ulcers”. Results section: “No patient developed a classic decubitus ulcer during the entire period.” And later “...one patient developed a small facial ulcer related to pressure from a padded support of the kinetic table...”. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | There was no significant difference in the initial mean APACHE‐II score between all individuals placed on KTT...and the manually turned subjects... but it is not clear if this is at baseline or throughout the study. No other baseline data provided and no Table of characteristics shown. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
Takala 1996.
| Methods | RCT with 14‐day follow‐up. Randomisation influenced by mattress availability, therefore, not concealed. | |
| Participants | Non‐trauma patients admitted to ICU expected to stay > 5 days. Treatment groups similar at baseline, however, not compared for degree of pressure ulcer risk. | |
| Interventions | 1. Carital Optima (n = 21): constant low pressure mattress comprising 21 double air bags on a base. 2. Standard hospital foam mattress (n = 19): 10 cm thick foam density 35 kg/m3. | |
| Outcomes | 1. No ulcers. 2. 37% (7/19) patients developed a total of 13 ulcers. P value < 0.005. 9 ulcers were grade 1A (erythema), 4 were grade 1B (superficial and limited to the dermis). | |
| Notes | 40% withdrawals; ITT analysis undertaken. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not explicitly reported, "...randomly assigned..." Later the authors talk about "...each block of four patients that completed treatment". This may refer to block randomisation but it is not clear. |
| Allocation concealment (selection bias) | High risk | Randomisation influenced by mattress availability, therefore, allocation not concealed. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | This study was not blinded, since the severity of illness of the patients precluded their transfer for evaluation of the skin condition by a blinded reviewer, and the type of mattress in the bed could not be blinded but further on note that ...all sore areas were measured and photographed for independent verification of severity... It would appear that perhaps some outcome assessment was perhaps blinded but this is still unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An ITT was performed but there were significant losses ‐ “Ten patients were randomised but not treated due to either early discharge or death...” and, “Six patients randomised on the pressure‐relieving mattress were included only in the intention‐to‐treat analysis, since the start of treatment was delayed due to mattress non‐availability...” No discussion of how the trialists handled the missing data. |
| Selective reporting (reporting bias) | Low risk | Methods: ...primary outcome variable (pressure sore formation)... All outcomes reported related to pressure ulcer formation. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Table 1 (from study report) indicates that patient characteristics were well balanced, e.g. age, clinically infected and APACHE score. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
Taylor 1999.
| Methods | RCT ‐ length of follow up ‐ discharge from hospital or death. | |
| Participants | Hospital inpatients aged 16 or over, with intact skin, requiring a pressure‐relieving support. | |
| Interventions | 1. Alternating‐pressure mattress with pressure‐redistributing cushion (Pegasus Trinova) (n = 22). 2. Alternative alternating‐pressure system (unnamed) with pressure‐redistributing cushion (control) (n = 22). | |
| Outcomes | 1. TriNova: 0/22; 2. Control: 9% (2/22) (both ulcers superficial). | |
| Notes | Study underpowered. Data relating to comfort were not reported for control group.
Nurse rating of acceptability: 1. TriNova: good to very good n = 15; acceptable n = 1; 2. Control: good to very good n = 9; acceptable n = 11. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract states "...randomised controlled trial". No further details reported. |
| Allocation concealment (selection bias) | Low risk | "Upon recruitment, the data collector opened the next opaque envelope in sequence...". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. Patients and nurses would not have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement regarding drop‐outs/withdrawals. “Forty‐four subjects were recruited to the study over a 5‐month period, with equal numbers of subjects allocated to the two mattress groups”. “...eighteen (81.8%) of the 22 patients allocated to the Trinova completed the comfort questionnaire...” but comfort data were not reported for the control group, so losses in that group, and the way in which they compared to the intervention group, remain unknown. This is a loss of 20% overall which is acceptable but there is no indication that there was an ITT analysis. |
| Selective reporting (reporting bias) | High risk | "..the primary end point rests with measuring differences in comfort and acceptance, while the secondary objective of the study is to measure clinical outcomes of a group of patients vulnerable to pressure sore development." Although comfort data were not reported for the control group, but only for the intervention group. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Table 2 (from study report) indicates that patient characteristics were well balanced, e.g. age, weight and Waterlow score. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
Theaker 2005.
| Methods | RCT: follow‐up for 2 weeks after discharge from ICU. | |
| Participants | Recruitment from an ICU. Eligible participants were deemed at high risk of pressure ulcer development (from a set of 5 predetermined factors; details not provided, but reference given), and aged ≥18 y. Patients with pressure ulcers on admission were excluded. Baseline data presented by outcome, so difficult to assess. | |
| Interventions | 1. KCI TheraPulse bed (n = 30). 2. Hill‐Rom Duo mattress (n = 32). No further details provided about the devices. | |
| Outcomes | Number of participants with incidence pressure ulcer (assessed every 8 h; blinded outcome assessment*); all grades (not given by group, reported that most were grade 2 with one grade 3): 1. TheraPulse: 10% (3/30); 2. Duo:19% (6/32). 8/9 ulcers were heel ulcers. | |
| Notes | Participant lost not mentioned. * Trial is described as unblinded, but the methods described blinded outcome assessment with photographs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients "...were randomly assigned..." "Selection of an unmarked envelope from a pile of envelopes by staff unconnected with the study formed the randomisation process". Describes an adequate concealment of allocation sequence, but not how the sequence was generated. |
| Allocation concealment (selection bias) | Low risk | "Selection of an unmarked envelope from a pile of envelopes by staff unconnected with the study formed the randomisation process". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | This was an unblinded, randomised prospective trial, but it appears that outcome assessment was blinded for the primary outcome: For the study purposes, the digital photographs were anonymised and analysed subsequently by two independent Tissue Viability Nurses for confirmation of the existence of a pressure ulcer and assessment of severity. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No statement made regarding withdrawals. |
| Selective reporting (reporting bias) | Low risk | The only outcome mentioned in the methods section was pressure ulcer development : "Patients were assessed once every 8 h for pressure sore development”. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | "There were no significant differences in age, sex, Apache score or length of stay...". |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
Tymec 1997.
| Methods | RCT. | |
| Participants | 52 patients admitted to selected nursing units of a large hospital with a Braden score of <16 (risk); intact skin on heels. 23 women and 29 men aged 27‐90 y, mean age 66.6 ± 16.5 y. Mean Braden score on admission 11.8; 21 patients with respiratory conditions; 6 with cancer; 5 with stroke. | |
| Interventions | Factorial design evaluating effect of heel elevation device plus positioning and order of positioning.
1. Foot Waffle (FDA approved, non‐abrasive vinyl boot with built‐in foot cradle and inflated air chamber).
2. Hospital pillow under both legs from below knee to the Achilles tendon. Unclear how many patients in each group. |
|
| Outcomes | Number of pressure ulcers developed:
1. Foot Waffle: 6.
2. Hospital pillow: 2. Denominators unclear. |
|
| Notes | There did not appear to be any losses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to either pillow or Foot Waffle was undertaken "...using a block randomised list and the patient's position order "...was determined by a coin toss". |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | The blinding of outcome assessment was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 52 patients (23 women and 29 men) in the study, but nowhere was the number/group reported. 8/52 patients developed grade 1 pressure ulcers and were removed from the study, so it would appear that the 52 participants were followed‐up. |
| Selective reporting (reporting bias) | Low risk | Occurrence of a pressure ulcer, mean survival time (i.e. time until one occurred), and mean interface pressures were reported. These are all meaningful outcomes. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | No table of characteristics provided. Methods section gives characteristics for the sample overall, but not by group. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
van Leen 2011.
| Methods | RCT, with a 6 month follow up. Method of allocation unclear. |
|
| Participants | 83 patients aged >65 years, Norton score between 5‐12 and no pressure ulcers in the previous months. Set in a single centre nursing home in De Naaldhorst (Netherlands). | |
| Interventions | Group 1: Standard 15 cm cold foam mattress with a static air overlay mattress Group 2: Standard 15 cm cold foam mattress alone All patients sat on a static air pillow following the institutional PUPP when out of bed. |
|
| Outcomes | Number of patients with a pressure ulcer (Did not include grade 1): 1: 2/42 (4.8%) 2: 7/41 (17.1%) |
|
| Notes | Attrition unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “ Randomization into two groups was performed after informed consent using numbered envelopes”. No information on this aspect of randomisation process other than to say randomization occurred using numbered envelopes. How they generated the sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | “ Randomization into two groups was performed after informed consent using numbered envelopes”. Potential for tampering as it is does not state that the envelopes were opaque. No mention of offsite third party randomisation keeping the allocation concealed. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Low risk | “Weekly inspection of the skin to assess the possible occurrence of a skin lesion was done by an independent nurse”. No further information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of patients excluded prior to the study and number of patients excluded after randomisation is unstated. There is no flow chart of recruitment and retention to clarify this. |
| Selective reporting (reporting bias) | Low risk | All of the study's pre‐specified outcomes were reported. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | "A weekly inspection f the skin to assess the possible occurrence of a skin lesion”. This is reasonable evidence to assume that the timing of the outcome assessments were the same in each group. |
Vanderwee 2005.
| Methods | RCT. | |
| Participants | Recruitment from 19 surgical, internal medicine or geriatric hospital wards. Eligible patients at risk of developing pressure ulcer (Braden score < 17); or had at least 1 grade 1 ulcer; aged ≥18 y, with expected hospital stay of > 3 days; not contraindicated for turning. Particpants excluded if had a grade 2 or worse pressure ulcer, or weighed > 140 kg. Participants well balanced at baseline. | |
| Interventions | 1. APAM (Alpha X‐cell, Huntleigh Healthcare): generates alternating high and low interface pressure between the body and support by alternating inflation and deflation. Sitting protocol with air cushion (Airtech, Huntleigh), with no turning protocol (n = 222). 2. Visco‐elastic foam mattress (Tempur, Tempur‐World). Sitting protocol with air cushion (Airtech, Huntleigh). Turning every 4 h (n = 225). | |
| Outcomes | Number of participants with incidence pressure ulcer (assessed daily by ward nurse; grade 1 excluded): Grade 2 to 4 pressure ulcers (NS): 1. APAM: 15.3% (34/222); 26 grade 2; 8 grade 3 or 4. 2. Visco: 15.6% (35/225); 33 grade 2; 2 grade 3 or 4. | |
| Notes | No significant difference in incidence of pressure ulcers (grades 2‐4) between the groups. There were significantly more heel pressure ulcers in the control group (P value 0.006). However, authors noted that patients nursed on an APAM seemed to develop more severe pressure ulcers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomisation tables generated with the SPSS 10 software package...". |
| Allocation concealment (selection bias) | Low risk | "Serially numbered closed envelopes were made for each participating ward". |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | A random sample of patients was observed at unexpected moments by both the researcher and the data nurse. In addition a data nurse was responsible for the follow‐up of the study on each ward. So the researchers and data nurse were probably not blinded to allocation because they were at the patients' bedsides. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs/withdrawals not reported. Flow chart showed 447 patients enrolled in total, 297 assessed by Braden and 150 by non‐blanchable erythema (NBE). Numbers in Table 2 (from study report) match these. |
| Selective reporting (reporting bias) | Unclear risk | Assessment were designed to detect skin changes; used NBE and Braden scale. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Low risk | Patients well balanced at baseline, e.g. Braden score and age. Since the groups were similar in all characteristics except medical specialty, this variable was adjusted for in the analysis. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
Vermette 2012.
| Methods | RCT, with a maximum of a 14 day follow up. Median length of follow‐up (days): Group 1: 9.2 days (+4.8) Group 2: 9.9 days (+4.3) Method of allocation unclear. |
|
| Participants | 110 participants at moderate or high risk of developing a pressure ulcer (Braden score <14), over 18 years, weighing <300 pounds and with no pre‐existing ski lesions. Setting an acute care 257 bed facility, which included medical, surgical, active geriatric and ICU wards. | |
| Interventions | Group 1: ISO (Inflated static overlay) – Waffle overlay Group 2: MSO (microfluid static overlay) – RIK (for those weighing < 200 lbs and Braden score > 14) OR LALDM (low‐air‐loss dynamic mattress) – TheraKair (for those weighing between 200 and 300lb and Braden score > 14) All patients received standard care which included 2 hrly positioning schedule + preventative measures including moisturizing the sacrum, position, minimizing head elevation, avoiding bony prominence massage, use of side lying position and pillows to keep feet and ankles off the mattress. |
|
| Outcomes | Unsure if incidence of pressure ulcers included grade 1. Group 1: 2/55 (4%) Group 2: 5/55 (11%) P= 0.2706 Incidence of pressure ulcers (in those using MSO): Group 1: 2/55 (4%) Group 2: 6/50 (12%) |
|
| Notes | Dual control interventions Measurement of comfort and dichotomous results relating to comfort in tables 2 and 3 are questionable as it is unclear what cut‐off was used to indicate comfort. Skin assessment was assessed only 3 days a week (Mon, wed, fri): ‐ There is also the possibility that skin changes that occurred on days the research nurse was not performing a skin assessment were not detected as promptly. The possibility of an increase in the Braden score on those days also cannot be eliminated. These changes might have influenced the number of days of participation in the study for some subjects. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Participants were randomly assigned a rented surface....done by draw by research nurse and witnessed by the subject”. No information on this aspect of randomisation process other than to say opaque envelopes available for research nurse. |
| Allocation concealment (selection bias) | High risk | “drawn by research nurse using an opaque envelope and the subject witnessing the draw”. Research nurse did recruitment and allocation. Potential for tampering even though it is stated that the subjects witnessed the draw. |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | High risk | “Blinding was not obtained for the patient, the clinical staff, or the research evaluator because the surfaces were visible” p.g 209. Not possible due to high visibility of the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The number of patients excluded prior to the study and number of patients excluded after randomisation is unstated. Unclear whether the people who ended the study early were included. ITT analysis would presume this was the case however there is no flow chart of recruitment and retention to clarify this. |
| Selective reporting (reporting bias) | Unclear risk | “The rented surfaces for the subjects in the control group were allocated according to the subject’s weight (MSO < 200lb) or needed edema management (LALDM with a Gore‐Tex cove to control humidity)". From the above quote it is identified that the control contained two different interventions. Table 3 shows that authors removed, for unknown reasons, LALDM from one analysis. However table 2 shows full data. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | High risk | |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | 3x a week for a maximum of 14 days. This is reasonable evidence to assume that the timing of the outcome assessments were the same in each group. |
Vyhlidal 1997.
| Methods | RCT with 10‐21 day follow‐up. Allocation to surfaces achieved by investigator drawing assignment out of a hat, therefore, extent of concealment inadequate. | |
| Participants | Patients newly admitted to a skilled nursing facility; estimated stay ≥ 10 days; free of pressure ulcers but at risk (Braden score <18 with sub‐scale score of < 3 in sensory perception, mobility or activity levels). Diagnoses: musculoskeletal 45%; cardiovascular 27.5%; neurological 12.4%; others 15%. Patients in the MAXIFLOAT group were younger, though not significantly so. Braden Scale scores (risk of pressure ulcer development) similar between groups at baseline. Patients in the MAXIFLOAT group were significantly heavier and stayed on the mattress longer than the IRIS group. | |
| Interventions | 1. IRIS 3000: 4‐inch thick foam overlay with dimpled surface (n = 20). 2. MAXIFLOAT: mattress replacement in 5 sections (n = 20). The mattress has a water/bacteria repellent top cover; is made of 1.5‐inch thick antimicrobial foam with a centre core of cut foam; has a non‐removable polyester fibre heel pillow and a water/bacteria‐proof bottom cover. Subjects in both groups received standards of care according to the protocols of the organisation. | |
| Outcomes | All grades of ulcer: 1. IRIS 3000: 60% (12/20); Grade 1: 25% (4/20); Grade 2: 40% (8/20). 2. MAXIFLOAT: 25% (5/20); Grade 1: 10% (2/20); Grade 2: 15% (3/20). P value 0.025. Time to ulcer: 1. IRIS 3000: 6.5 days; 2. MAXIFLOAT: 9.2 days (NS). | |
| Notes | No record of any withdrawals. The IRIS 3000 is an overlay which goes on an existing mattress resulting (in the trial) in a bed height of 29 inches. 1 participant refused the IRIS because of the height of the bed. IRIS is lighter at 6.9 lb than the MAXIFLOAT (25 lb) and easier to manipulate, however, the latter is still lighter than standard hospital mattress (48 lb). IRIS can be sent home with patient. IRIS costs USD 38 compared to USD 260 for MAXIFLOAT. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...subjects were randomly assigned by research interviewer by drawing assignment out of a hat". "...randomly assigned by lot by the investigator...". |
| Allocation concealment (selection bias) | Unclear risk | No information about what was drawn out of the hat (as above). |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | "....skin assessments and vital signs were performed...by a research team member". Probably not the research interviewer but it was not clear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No statement regarding withdrawals. There were 20 patients per group and it was reported that, " ...17 subjects developed pressure ulcers, 12 of the 20 in the Iris 3000 group and 5 of the 20 in the MAXIFLOAT group." |
| Selective reporting (reporting bias) | Low risk | “purpose of this study was to compare the incidence of pressure ulcers in 40 newly admitted...”. Outcomes discussed were number of participants developing pressure ulcers, and average number of days to pressure ulcer development, but there was no mention in the methods of what the trialists intended to measure, only that “...skin assessments and vital signs were performed...”. |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | High risk | "Subjects in the MAXIFLOAT group were significantly heavier..." "the MAXIFLOAT group also stayed on the mattress longer.." Text states both differences were statistically significant. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Unclear risk | Not reported. |
Whitney 1984.
| Methods | RCT with 8‐day follow‐up. Method of allocation not reported; patients were "selected at random" for each group. | |
| Participants | Patients on medical‐surgical units who were in bed for 20 h/day. Most patients had relatively little skin breakdown. Ages ranged from 19‐91 y; mean 63.2 y. Majority of patients were confused, lethargic, stuporous. Only 39% classed as mentally alert. Baseline data were not presented. | |
| Interventions | 1. Alternating‐pressure mattress (n = 25): consisted of 134 3‐inch diameter air cells. 3‐minute cycle.
2. Convoluted foam pad (Eggcrate) (n = 26). Patients in both groups were turned every 2 h. |
|
| Outcomes | Changes in skin condition did not differ significantly between patients using the alternating‐pressure air mattress and the foam mattress (better: 20% vs 19%; same: 60% vs 58%; worse 20% vs 23%). | |
| Notes | 4 patients died. Analysis by ITT. Alternating‐pressure mattress: pump maintenance was costly, patients objected to the movement. The alternating mattress was more easily cleaned and retained its original properties over several weeks compared to the foam, which compressed and flattened. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "26 were selected at random and placed in the foam mattress group, 25 in the AP mattress group...". |
| Allocation concealment (selection bias) | Low risk | "Upon recruitment, the data collector opened the next opaque envelope in sequence..." . |
| Blinding (performance bias and detection bias) Pressure ulcer incidence | Unclear risk | Not reported. Patients and nurses would not have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No statement regarding drop‐outs/withdrawals, but there were 51 patients in the study and Table 3 (from study report) indicates that data for all of these were included (25 +26 = 51). |
| Selective reporting (reporting bias) | Low risk | The study was conducted to determine "...which mattress is the best choice for pressure sore prevention and under which circumstances". |
| Free of other bias ‐ were groups similar at baseline regarding the most important prognostic indicators? | Unclear risk | Not reported. Patient characteristics described for the group as a whole, not by mattress group. |
| Free of other bias ‐ was the timing of the outcome assessment similar in all groups? | Low risk | Description of outcome assessment seems to indicate all patients were treated the same, "Risk factors and skin assessment scores were recorded three times each week". It is noted that, "In most cases patients were assessed by two investigators as a team and occasionally by only one...", but that would not impact on timing of assessment. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allen 1993 | No clinical outcomes, only interface pressure recorded. |
| Andrews 1989 | Did not fulfil study design criteria. |
| Bales 2012 | This is a quasi‐randomised study. The method of randomisation is unclear and insufficiently described: “The sample was randomized by alternating the application of each intervention when the patients were admitted to the unit”. It appears that sequence generation took place by a rule based on admission time and therefore there is a high risk of bias. |
| Ballard 1997 | Data recorded were comfort data; no pressure ulcer outcomes. |
| Barhyte 1995 | Did not fulfil study design criteria. No data presented. |
| Berthe 2007 | Did not fulfil study design criteria. Was an RCT however unclear evidence to adequately assess risk of bias. |
| Black 2012 | This study was a prospective, observational study with no randomisation involved. |
| Bliss 1967 | Did not fulfil study design criteria. Patients were recruited to the trial on the basis of their risk score. |
| Bliss 1995 | Whilst 8 surfaces were evaluated in this prospective trial, not all surfaces were in the trial at the same time, therefore, the surfaces were not truly compared with one another contemporaneously. Furthermore, it was possible for patients to be re‐randomised back into the study, which occurred frequently, with a total of 457 mattress trials reported for only 238 patients. The data were not presented by patient; only by mattress trial. Duplicate citation of Bliss 1994 (Conference abstract). |
| Braniff‐Matthews 1997 | Healing and prevention outcome data were not separated. |
| Brienza 2001 | Study of pressure measurement. |
| Büchner 1995 | Did not fulfil study design criteria. Criteria for anti‐decubitus management not reported and decided by nurses. Number of pillows provided to third arm of the study was limited and not given to all participants. |
| Cassino 2013 | Healing outcomes |
| Chaloner 2000 | Did not fulfil study design criteria, randomisation corrupted, authors reported that randomisation was compromised on the basis of bed availability. |
| Colin 1996 | No clinical outcomes recorded; only measurements taken were for transcutaneous oxygen tension. |
| Conine 1991 | Did not fulfil study design criteria. |
| deBoisblanc 1993 | Outcome incidence of pneumonia, no pressure ulcer outcomes. |
| Defloor 1997 | Compared turning. |
| Defloor 2000 | Did not compare surfaces. |
| Defloor 2005 | Compared turning. |
| Della Valle 2001 | Outcome of interface pressure. |
| Flam 1995 | Outcome skin temperature and skin moisture level, no pressure ulcer outcomes. |
| Fleischer 1997 | Did not fulfil study design criteria. |
| Geelkerken 1994 | Did not fulfil study design criteria. No data presented. |
| Gil Agudo 2009 | Outcome measure of interface pressure. |
| Gray 2008 | Not an RCT, but a clinical audit. |
| Grindley 1996 | Patients were crossed over between intervention groups at 3 days. Outcome used was the assessment of patient comfort. |
| Grisell 2008 | Outcome measure of interface pressure. |
| Gunningberg 1998 | Did not fulfil study design criteria. Study of risk calculation rather than prevention. |
| Haalboom 1994 | Did not fulfil study design criteria. |
| Hampton 1998 | Did not fulfil study design criteria. |
| Hawkins 1997 | Did not fulfil study design criteria. |
| Heyneman 2009 | Meta‐analysis of 2 previously published RCTs (Vanderwee 2005; Vanderwee 2007). Vanderwee 2005 already included in this review. Vanderwee 2007 excluded as it is a turning trial. |
| Holzgreve 1993 | Full paper unavailable. Insufficient information to assess. |
| Huang 2009 | Evaluated dressings. |
| Huang 2013 | Meta ‐analysis of surgical – related Pressure ulcers |
| Inman 1999 | Comparison of a bed rental versus a bed purchase strategy, not a comparison of surfaces. |
| Jacksich 1997 | Did not fulfil study design criteria. |
| Jackson 2011 | No evidence of randomisation |
| Jesurum 1996 | Did not fulfil study design criteria. |
| Koo 1995 | Did not fulfil study design criteria, study of interface pressure in healthy volunteers. |
| Marchand 1993 | Did not fulfil study design criteria, was a retrospective chart audit. |
| McMichael 2008 | Outcome measure of interface pressure. |
| Nakahara 2012 | Examines pressure ulcer healing only |
| Neander 1996 | Paper in German ‐ translator stated it was not an RCT. There were no data on how the decision to include patients in the control and intervention groups was made. |
| Ooka 1995 | Did not fulfil study design criteria, convenience sample used. |
| Pham 2011a | A costing study only |
| Pham 2011b | A costing study only |
| Phillips 1999 | N of 1 trial design, only one participant in the trial |
| Regan 1995 | This study reported an audit of pressure ulcer incidence after implementation of a comprehensive pressure ulcer policy; it is not a prospective RCT. |
| Reynolds 1994 | Did not fulfil study design criteria. |
| Rosenthal 1996 | Did not fulfil study design criteria. Outcome measure of interface pressure. |
| Scott 1995 | Insufficient information available to make a decision. |
| Scott 1999 | No clinical outcomes, healthy volunteer study of interface pressures. |
| Scott 2000 | Not an RCT of beds and mattresses. |
| Simonis 2012 | Hospital‐acquired pneumonia is primary outcome. Author contacted regarding whether study powered for secondary outcome of pressure injury incidence. |
| Stoneberg 1986 | Historical control group. |
| Suarez 1995 | Controlled clinical trial which recorded only pressure measurements |
| Taccone 2009 | Not investigating support surfaces |
| Takala 1994 | Not an RCT, outcome measure of interface pressure. |
| Thomas 1994 | Did not fulfil study design criteria. |
| Timmons 2008 | Did not fulfil study design criteria. Review of a product not a trial. |
| Torra i Bou 2002 | Evaluated dressings. |
| Turnage‐Carrier 2008 | Outcome measure of interface pressure. |
| Vanderwee 2007 | Compared turning. |
| Vanderwee 2008 | Literature review of previously conducted studies. |
| Wells 1984 | Only recorded interface pressure measurements. |
| Wild 1991 | Interface pressure measurements. |
| Wu 2011 | Study states that it is a clinical trial and no evidence of randomization is provided at any point in the paper. |
| Zernike 1997 | Incidence of pressure ulcers not reported |
| Zernike 1994 | Unable to assess due to information in research paper. Email address provided was no longer valid and we were unable to find other contact details. |
Characteristics of studies awaiting assessment [ordered by study ID]
Allegretti 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Dissertation. Author contacted and advised us to wait for publication. |
Mastrangelo 2010.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Rerun searches but could not identify study. Awaiting full text retrieval |
Mayer 2008.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Rerun searches but could not identify the study. Awaiting full text retrieval |
Rafter 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Email sent to author, requesting more information about the trial. Nil response to date |
Abbreviations
> = more than ≥ = greater than or equal to < = less than ≤ = less than or equal to A&E = Accident and Emergency department AP = alternating pressure AWS = airwave system BMI = body mass index CF = convoluted foam CRN = clinical research nurse FDA = Food and Drug Administration GA = general anaesthetic h = hour(s) ICU = intensive care unit ITT = intention‐to‐treat analysis LAL = low air loss LCR = large cell ripple LOS = length of stay n = number in sample/group NBE = non‐blanchable erythema NS = not statistically significant OR = odds ratio PRD = pressure reducing device SF = solid foam vs = versus y = year(s)
Contributions of authors
NC conceived the original idea, wrote the protocol, extracted and analysed the data and drafted the original review, contributed to the updates and is responsible for the final edit. EMcI made inclusion decisions, extracted data, assessed trial quality, undertook analyses and contributed to the text for all updates. SBS undertook searching, inclusion decisions, analysis, contributed text for all updates, addressed the copy editor feedback and edited the updates. JD made inclusion decisions, extracted data, assessed trial quality, undertook analyses and contributed to the text for the second and third updates and agreed the fourth update. AJ‐B made inclusion decisions, extracted data, updated the pending assessment of trials list, updated the background section, undertook analyses and contributed to the text for the third and fourth updates. VM made inclusion decisions, extracted data, updated the pending assessment of trials list and contributed to the text for the fourth updates.
Sources of support
Internal sources
Department of Health Sciences, University of York, UK.
School of Nursing, Midwifery and Social Work, University of Manchester, UK.
External sources
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Incentive funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health (all versions), UK.
NHS Health Technology Assessment Programme (original review), UK.
National Institute of Clinical Excellence Guidelines Programme (first update), UK.
Nursing Research Institute SV&MHS and Australian Catholic University, Sydney (third update), Australia.
Declarations of interest
Nicky Cullum was the Principal Investigator in the PRESSURE Trial, one of the trials included in this review (Nixon 2006), however, she was not involved in the data extraction or analysis for this trial. Kinetic Concepts Inc (KCI) supplied (free of charge) three VAC therapy units and starter packs for use in a pilot RCT of negative pressure wound therapy for pressure ulcers. They also provided product training, support and access to the KCI 24hr advice service for clinical and technical queries. However KCI had no input into the design, conduct, analysis or reporting of that research or this review which is not concerned with this technology. E McInnes: no interests declared. A Jammali‐Blasi: no interests declared. SEM Bell‐Syer: no interests declared. JC Dumville: no interests declared. V Middleton: was funded as research assistant by Nursing Research Institute (Australian Catholic University) to assist with this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Andersen 1982 {published data only}
- Andersen KE, Jensen O, Kvorning SA, Bach E. Decubitus prophylaxis: a prospective trial on the efficiency of alternating pressure air mattresses and water mattresses. Acta Dermato‐Venereologica (Stockholm) 1982;63(3):227‐30. [PubMed] [Google Scholar]
Aronovitch 1999 {published data only}
- Aronovitch SA, Wilber M, Slezak S, Martin T, Utter D. A comparative study of an alternating air mattress for the prevention of pressure ulcers in surgical patients. Ostomy/Wound Management 1999;45(3):34‐40, 42‐4. [PubMed] [Google Scholar]
Bennett 1998 {published data only}
- Bennett RG, Baran PJ, DeVone LV, Bacetti H, Kristo B, Tayback M, et al. Low airloss hydrotherapy versus standard care for incontinent hospitalized patients [see comments]. Journal of the American Geriatrics Society 1998;46(5):569‐76. [DOI] [PubMed] [Google Scholar]
Brienza 2010 {published data only}
- Brienza D, Kelsey S, Karg P, Allegretti A, Olson M, Schmeler M, Zanca J, Geyer MJ, Kusturiss M, Holm M. A randomized clinical trial on preventing pressure ulcers with wheelchair seat cushions. Journal of the American Geriatrics Society 2010;58(12):2308‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cadue 2008 {published data only}
- Cadue J‐F, Karolewicz S, Tardy C, Barrault C, Robert R, Pourrat O. Prevention of heel pressure sores with a foam body‐support device. A randomized controlled trial in a medical intensive care unit [Efficacite de supports anatomiques en mousse pour la prevention des escarres de talons]. Presse Medicale 2008;37(1 Pt 1):30‐6. [DOI] [PubMed] [Google Scholar]
Cavicchioli 2007 {published data only}
- Cavicchioli A, Carella G. Clinical effectiveness of a low‐tech versus high‐tech pressure redistributing mattress. Journal of Wound Care 2007;16(7):285‐9. [DOI] [PubMed] [Google Scholar]
Cobb 1997 {published data only}
- Cobb GA, Yoder LH, Warren JB. Pressure ulcers: patient outcomes on a KinAir bed or EHOB waffle mattress. TriService Nursing Research Program (TSNRP). Bethesda, Maryland, USA, 1997.
Collier 1996 {published data only}
- Collier ME. Pressure‐reducing mattresses. Journal of Wound Care 1996;5(5):207‐11. [DOI] [PubMed] [Google Scholar]
Conine 1990 {published data only}
- Conine TA, Daechsel D, Choi AK, Lau MS. Costs and acceptability of two special overlays for the prevention of pressure sores. Rehabilitation Nursing 1990;15(3):133‐7. [DOI] [PubMed] [Google Scholar]
- Conine TA, Daechsel D, Lau MS. The role of alternating air and silicore overlays in preventing decubitus ulcers. International Journal of Rehabilitation Research 1990;13(1):57‐65. [DOI] [PubMed] [Google Scholar]
Conine 1993 {published data only}
- Conine TA, Daeschel D, Hershler C. Pressure sore prophylaxis in elderly patients using slab foam or customised contoured foam wheelchair cushions. Occupational Therapy Journal of Research 1993;13(2):101‐16. [Google Scholar]
Conine 1994 {published data only}
- Conine TA, Hershler C, Daechsel D, Peel C, Pearson A. Pressure sore prophylaxis in elderly patients using polyurethane foam or Jay wheelchair cushions. International Journal of Rehabilitation Research 1994;17(2):123‐37. [DOI] [PubMed] [Google Scholar]
Cooper 1998 {published and unpublished data}
- Cooper PJ, Gray DG, Mollison J. A randomised controlled trial of two pressure reducing surfaces. Journal of Wound Care 1998;7(8):374‐6. [DOI] [PubMed] [Google Scholar]
Daechsel 1985 {published data only}
- Daechsel D, Conine TA. Special mattresses: effectiveness in preventing decubitus ulcers in chronic neurologic patients. Archives of Physical Medicine and Rehabilitation 1985;66(4):246‐8. [DOI] [PubMed] [Google Scholar]
Demarre 2012 {published data only}
- Demarré L, Beeckman D, Vanderwee K, Defloor T, Grypdonck M, Verhaeghe S. Multi‐stage versus single‐stage inflation and deflation cycle for alternating low pressure air mattresses to prevent pressure ulcers in hospitalised patients: A randomised‐controlled clinical trial. International Journal of Nursing Studies 2012;49(4):416‐426. [DOI] [PubMed] [Google Scholar]
Donnelly 2011 {published data only}
- Donnelly J, Winder J, Kernohan WG, Stevenson M. An RCT to determine the effect of a heel elevation device in pressure ulcer prevention post‐hip fracture. Journal of Wound Care 2011;20(7):309‐318. [DOI] [PubMed] [Google Scholar]
Economides 1995 {published data only}
- Economides NG, Skoutakis VA, Carter CA, Smith VH. Evaluation of the effectiveness of two support surfaces following myocutaneous flap surgery. Advances in Wound Care 1995;8(1):49‐53. [PubMed] [Google Scholar]
Ewing 1964 {published data only}
- Ewing MR, Garrow C, Presley TA, Ashley C, Kinsella NM. Further experiences in the use of sheep skins as an aid in nursing. The Australian Nurses' Journal 1964;Sept:215‐9. [Google Scholar]
Exton‐Smith 1982 {published data only}
- Exton‐Smith AN, Overstall PW, Wedgewood J, Wallace G. Use of the 'air wave system' to prevent pressure sores in hospital. The Lancet 1982;1(8284):1288‐90. [DOI] [PubMed] [Google Scholar]
Feuchtinger 2006 {published data only}
- Feuchtinger J. Preventing decubitus ulcer in heart surgery interventions: visco‐elastic foam layer on the operating room table — a study [Viskoelastische schaumstoffauflage auf dem operationstisch — eine studie]. Pflege Zeitschrift 2006;59(8):498‐501. [PubMed] [Google Scholar]
- Feuchtinger J, Bie R, Dassen T, Halfens R. A 4‐cm thermoactive viscoelastic foam pad on the operating room table to prevent pressure ulcer during cardiac surgery. Journal of Clinical Nursing 2006;15(2):162‐7. [DOI] [PubMed] [Google Scholar]
Gebhardt 1996 {published data only}
- Gebhardt K. A randomized trial of alternating pressure (AP) and constant low pressure (CLP) supports for the prevention of pressure sores. Journal of Tissue Viability 1994;4(3):93. [Google Scholar]
- Gebhardt KS, Bliss MR, Winwright PL. A randomised controlled trial to compare the efficacy of alternating and constant low pressure supports for preventing pressure sores in an intensive care unit. Personal Communication 1994.
- Gebhardt KS, Bliss MR, Winwright PL, Thomas J. Pressure relieving supports in an ICU. Journal of Wound Care 1996;5(3):116‐21. [DOI] [PubMed] [Google Scholar]
Gentilello 1988 {published data only}
- Gentilello L, Thompson DA, Tonnesen AS, Hernandez D, Kapadia AS, Allen SJ, et al. Effect of a rotating bed on the incidence of pulmonary complications in critically ill patients. Critical Care Medicine 1988;16(8):783‐6. [DOI] [PubMed] [Google Scholar]
Geyer 2001 {published data only}
- Geyer MJ, Brienza DM, Karg P, Trefler E, Kelsey S. A randomized control trial to evaluate pressure‐reducing seat cushions for elderly wheelchair users. Advances in Skin and Wound Care 2001;14(3):120‐9. [DOI] [PubMed] [Google Scholar]
Gilcreast 2005 {published data only}
- Gilcreast DM, Warren JB, Yoder LH, Clark JJ, Wilson JA, Mays MZ. Research comparing three heel ulcer‐prevention devices. Journal of Wound, Ostomy and Continence Nursing 2005;32(2):112‐20. [DOI] [PubMed] [Google Scholar]
Goldstone 1982 {published data only}
- Goldstone L, Norris M, O'Reilly M, White J. A clinical trial of a bead bed system for the prevention of pressure sores in elderly orthopaedic patients. Journal of Advanced Nursing 1982;7(6):545‐8. [DOI] [PubMed] [Google Scholar]
Gray 1994 {published data only}
- Gray D. A randomised clinical trial of two foam mattresses. Aberdeen Royal Hospitals NHS Trust.
- Gray D. A randomised controlled trial of two foam mattresses. Journal of Tissue Viability 1994;4(3):92. [Google Scholar]
- Gray DG, Campbell M. A randomized clinical trial of two types of foam mattresses. Journal of Tissue Viability 1994;4(4):128‐32. [Google Scholar]
- Gray DG, Cooper PJ, Campbell M. A study of the performance of a pressure reducing foam mattress after three years of use. Journal of Tissue Viability 1998;8(3):9‐13. [DOI] [PubMed] [Google Scholar]
Gray 1998 {published data only}
- Gray D, Smith M. A randomized controlled trial of two pressure‐reducing foam mattresses. European Wound Management Association Conference; 1998 November; Harrogate, UK. 1998:4.
Gunningberg 2000 {published data only}
- Gunningberg L, Lindholm C, Carlsson M, Sjoden P‐O. Effect of visco‐elastic foam mattresses on the development of pressure ulcers in patients with hip fractures. Journal of Wound Care 2000;9(10):455‐60. [DOI] [PubMed] [Google Scholar]
Hampton 1997 {published and unpublished data}
- Hampton S. Evaluation of the new Cairwave Therapy System in one hospital trust. British Journal of Nursing 1997;6(3):167‐70. [DOI] [PubMed] [Google Scholar]
Hofman 1994 {published data only}
- Hofman A, Geelkerken RH, Wille J, Hamming JJ, Hermans J, Breslau PJ. Pressure sores and pressure‐decreasing mattresses: controlled clinical trial. Lancet 1994;343(8897):568‐71. [DOI] [PubMed] [Google Scholar]
Inman 1993 {published data only}
- Inman KJ, Sibbald WJ, Rutledge FS, Clark BJ. Clinical utility and cost‐effectiveness of an air suspension bed in the prevention of pressure ulcers. JAMA 1993;269(9):1139‐43. [PubMed] [Google Scholar]
Jolley 2004 {published data only}
- Jolley DJ, Wright R, McGowan S, Hickey MB, Campbell DA, Sinclair RD, et al. Preventing pressure ulcers with the Australian Medical Sheepskin: an open‐label randomised controlled trial. Medical Journal of Australia 2004;180(7):324‐7. [DOI] [PubMed] [Google Scholar]
Kemp 1993 {published data only}
- Kemp MG, Kopanke D, Tordecilla L, Fogg L, Shott S, Matthiesen V, et al. The role of support surfaces and patient attributes in preventing pressure ulcers in elderly patients. Research in Nursing and Health 1993;16(2):89‐96. [DOI] [PubMed] [Google Scholar]
Keogh 2001 {published data only}
- Dealey C, Keogh A. A randomised controlled trial comparing the effect of using an electric profiling bed with standard hospital bed for patients at high risk of pressure sore development. 10th Conference of the European Wound Management Association; 2000 18‐20 May; Stockholm, Sweden. 2000:30.
- Keogh A, Dealey C. Profiling beds versus standard hospital beds: effects on pressure ulcer incidence outcomes. Journal of Wound Care 2001;10(2):15‐9. [DOI] [PubMed] [Google Scholar]
Laurent 1998 {unpublished data only}
- Laurent S. Effectiveness of pressure decreasing mattresses in cardiovascular surgery patients: a controlled clinical trial. 3rd European Conference for Nurse Managers, 1997 Oct; Brussels, Belgium. Brussels, 1998.
Lazzara 1991 {published data only}
- Lazzara DJ, Buschmann MBT. Prevention of pressure ulcers in elderly nursing home residents: are special support surfaces the answer?. Decubitus 1991;4(4):42‐6. [PubMed] [Google Scholar]
Lim 1988 {published data only}
- Lim R, Sirett R, Conine TA, Daechsel D. Clinical trial of foam cushions in the prevention of decubitis ulcers in elderly patients. Journal of Rehabilitation Research 1988;25(2):19‐26. [PubMed] [Google Scholar]
McGowan 2000 {published data only}
- McGowan S, Montgomery K, Jolley D, Wright R. [The role of sheepskins in preventing pressure ulcers in elderly orthopaedic patients]. First World Wound Healing Congress; 2000, 10‐13 September; Melbourne, Australia. 2000:108.
- McGowan S, Montgomery K, Jolley D, Wright R. The role of sheepskins in preventing pressure ulcers in elderly orthopaedic patients. Primary Intention 2000;8(4):1‐8. [Google Scholar]
Mistiaen 2009 {published data only}
- Mistiaen P, Francke A, Achterberg W, Ament A, Halfens R, Huizinga J. Australian Medical Sheepskin is effective for the prevention of pressure ulcers [Australische Medische Schapenvacht effectief bij de preventie van stuitdecubitus]. Tijdschrift voor Ouderengeneeskunde 2009;5:186‐90. [Google Scholar]
Nixon 1998 {published data only}
- Bridel‐Nixon J, McElveney D, Brown J, Mason S. [Findings from a double‐triangular sequential‐design randomized clinical trial of a dry polymer pad]. European Wound Management Association Conference; 1997, 27‐29 April; Milan, Italy. London: Macmillan Magazines, 1997:20‐1.
- Bridel‐Nixon J, McElveney D, Brown J, Mason S. A randomized controlled trial using a double‐triangular sequential design: methodology and management issues. European Wound Management Association Conference; 1997, 27‐29 April; Milan, Italy. London: Macmillan Magazines, 1997:65‐6.
- Nixon J, McElvenny D, Mason S, Brown J, Bond S. A sequential randomised controlled trial comparing a dry visco‐elastic polymer pad and standard operating table mattress in the prevention of postoperative pressure sores. International Journal of Nursing Studies 1998;35(4):193‐203. [DOI] [PubMed] [Google Scholar]
Nixon 2006 {published data only}
- Iglesias C, Nixon J, Cranny G, Nelson EA, Hawkins K, Phillips A, et al. Pressure relieving support surfaces (PRESSURE) trial: cost effectiveness analysis. BMJ 2006;332(7555):1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J, Cranny G, Iglesias C, Nelson EA, Hawkins K, Phillips A, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;332(7555):1413‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al. Pressure relieving support surfaces: A randomised evaluation. Health Technology Assessment (Winchester, England) 2006;10(22):iii‐101. [DOI] [PubMed] [Google Scholar]
Price 1999 {published data only}
- Price P, Bale S, Newcombe R, Harding K. Challenging the pressure sore paradigm. Journal of Wound Care 1999;8(4):187‐90. [DOI] [PubMed] [Google Scholar]
Ricci 2013 {published data only}
- Ricci E, Roberto C, Ippolito A, Bianco A, Scalise MT. A new pressure‐relieving mattress overlay. European Wound Management Association Journal 2013;13(1):27‐32. [Google Scholar]
Russell 2000 {published and unpublished data}
- Dunlop V. Preliminary results of a randomised controlled study of a pressure ulcer prevention system. Advances in Wound Care 1998;11(3 suppl 1):14. [PubMed] [Google Scholar]
- Lichtenstein S. A 7 day comparative randomized parallel single centre study to determine the safety and efficacy of the Micropulse system for the prevention of pressure ulcers. Micropulse 1997.
- Russell JA, Lichtenstein SL. Randomised controlled trial to determine the safety and efficacy of a multi‐cell pulsating dynamic mattress system in the prevention of pressure ulcers in patients undergoing cardiovascular surgery. Ostomy/Wound Management 2000;46(2):46‐51, 54‐5. [PubMed] [Google Scholar]
Russell 2003 {published data only}
- Russell LJ, Reynolds TM, Park C, Rithalia S, Gonsalkorale M, Birch J, et al. Randomised clinical trial comparing CONFOR‐Med and standard hospital mattresses: results of the prevention of pressure ulcers study (PPUS‐1). Advances in Skin and Wound Care 2003;16(6):317‐27. [DOI] [PubMed] [Google Scholar]
Sanada 2003 {published data only}
- Matsui Y, Miyake S, KawasakiT, Konya C, Sugama J, Sanada H. Randomized controlled trial of a two layer type air cell mattress in the prevention of pressure ulcers. Japanese Journal of Pressure Ulcers 2001;3(3):331‐7. [Google Scholar]
- Sanada H, Sugama J, Matsui Y, Konya C, Kitagawa A, Okuwa M, et al. Randomised controlled trial to evaluate a new double‐layer air‐cell overlay for elderly patients requiring head elevation. Journal of Tissue Viability. 2003;13(3):112‐4, 116, 118 passim. [DOI] [PubMed] [Google Scholar]
Santy 1994 {published data only}
- Santy JE, Butler MK, Whyman JD. A comparison study of 6 types of hospital mattress to determine which most effectively reduces the incidence of pressure sores in elderly patients with hip fractures in a District General Hospital. Report to Northern & Yorkshire Regional Health Authority. 1994.
Schultz 1999 {published data only}
- Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN Journal 1999;70(3):434, 437‐40, 443‐9. [DOI] [PubMed] [Google Scholar]
- Schultz AA. Study results: prediction and prevention of pressure ulcers in surgical patients. Advances in Wound Care 1998;11(3 Suppl):11. [PubMed] [Google Scholar]
Sideranko 1992 {published data only}
- Sideranko S, Quinn A. Burns K, Froman RD. Effects of position and mattress overlay on sacral and heel pressures in a clinical population. Research in Nursing and Health 1992;15(4):245‐51. [DOI] [PubMed] [Google Scholar]
Stapleton 1986 {published data only}
- Stapleton M. Preventing pressure sores ‐‐ an evaluation of three products... foam, ripple pads, and Spenco pads. Geriatric Nursing (London, England) 1986;6(2):23‐5. [PubMed] [Google Scholar]
Summer 1989 {published data only}
- Summer WR, Curry P, Haponikm EF, Nelson S, Elston R. Continuous mechanical turning of intensive care unit patients shortens length of stay in some diagnostic‐related groups. Journal of Critical Care 1989;4:45‐53. [Google Scholar]
Takala 1996 {published data only}
- Takala J, Varmavuo S, Soppi E. Prevention of pressure sores in acute respiratory failure: a randomised controlled trial. Clinical Intensive Care 1996;7(5):228‐35. [Google Scholar]
Taylor 1999 {published data only}
- Taylor L. Evaluating the Pegasus Trinova: a data hierarchy approach. British Journal of Nursing 1999;8(12):771‐8. [DOI] [PubMed] [Google Scholar]
Theaker 2005 {published data only}
- Theaker C, Kuper M, Soni N. Pressure ulcer prevention in intensive care ‐ a randomised control trial of two pressure‐relieving devices. Anaesthesia 2005;60(4):395‐9. [DOI] [PubMed] [Google Scholar]
Tymec 1997 {published data only}
- Tymec AC, Pieper B, Vollman K. A comparison of two pressure relieving devices on the prevention of heel pressure ulcers. Advances in Wound Care 1997;10(1):39‐44. [PubMed] [Google Scholar]
Vanderwee 2005 {published data only}
- Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age and Ageing 2005;34(3):261‐7. [DOI] [PubMed] [Google Scholar]
van Leen 2011 {published data only}
- Leen M, Hovius S, Neyens J, Halfens R, Schols J. Pressure relief, cold foam or static air? A single center, prospective, controlled randomized clinical trial in a dutch nursing home. Journal of TIssue Viability 2011;20:30‐34. [DOI] [PubMed] [Google Scholar]
Vermette 2012 {published data only}
- Vermette S, Reeves I, Lemaire J. Cost effectiveness of an air‐inflated static overlay for pressure ulcer prevention: A randomized controlled trial. WOUNDS 2012;24(8):207. [PubMed] [Google Scholar]
Vyhlidal 1997 {published data only}
- Vyhlidal SK, Moxness D, Bosak KS, Meter FG, Bergstrom N. Mattress replacement or foam overlay? A prospective study on the incidence of pressure ulcers. Applied Nursing Research 1997;10(3):111‐20. [DOI] [PubMed] [Google Scholar]
Whitney 1984 {published data only}
- Whitney JD, Fellows BJ, Larson E. Do mattresses make a difference?. Journal of Gerontological Nursing 1984;10(9):20‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allen 1993 {published data only}
- Allen V, Ryan DW, Murray A. Potential for bed sores due to high pressures: influence of body sites, body position, and mattress design. British Journal of Clinical Practice 1993;47(4):195‐7. [PubMed] [Google Scholar]
Andrews 1989 {published data only}
- Andrews J, Balai R. The prevention and treatment of pressure sores by use of pressure distributing mattresses. Care Science and Practice 89;7(3):72‐6. [PubMed] [Google Scholar]
Bales 2012 {published data only}
- Bales I. A comparison between the use of intravenous bags and the Heelift suspension boot to prevent pressure ulcers in orthopedic patients. Advances in Skin & Wound Care 2012;25(3):125‐131. [DOI] [PubMed] [Google Scholar]
Ballard 1997 {published data only}
- Ballard K. Pressure‐relief mattresses and patient comfort. Professional Nurse 1997;13(1):27‐32. [PubMed] [Google Scholar]
Barhyte 1995 {published data only}
- Barhyte DY, McCance L, Valenta A, Tatenhove J, Walker MS, Bethea S. Selection of a standard hospital mattress: data‐based decision making. Journal of Wound, Ostomy and Continence Nursing 1995;22(6):267‐70. [DOI] [PubMed] [Google Scholar]
Berthe 2007 {published data only}
- Berthe JV, Bustillo A, Melot C, Fontaine S. Does a foamy‐block mattress system prevent pressure sores? A prospective randomised clinical trial in 1729 patients. Acta Chirurgica Belgica 2007;107(2):155‐61. [PubMed] [Google Scholar]
Black 2012 {published data only}
- Black J, Berke C, Urzendowski G. Pressure ulcer incidence and progression in critically ill subjects: Influence of low air loss mattress versus a powered air pressure redistribution mattress. Journal of Wound Ostomy & Continence Nursing 2012;39(3):267‐273. [DOI] [PubMed] [Google Scholar]
Bliss 1967 {published data only}
- Bliss MR, McLaren R, Exton Smith AN. Preventing pressure sores in hospital: controlled trial of a large‐celled ripple mattress. British Medical Journal 1967;1(537):394‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss MR, McLaren R, Exton‐Smith AN. Mattresses for preventing pressure sores in geriatric patients. Medical Bulletin of the Ministry of Health 1966; Vol. 25:238‐68. [PubMed]
Bliss 1995 {published data only}
- Bliss MR. Preventing pressure sores in elderly patients ‐ a comparison of seven mattress overlays. Age and Ageing 1995;24:297‐302. [DOI] [PubMed] [Google Scholar]
- Bliss MR. Randomised controlled trial of seven pressure relieving mattress overlays for preventing pressure sores in elderly patients. Conference of the Tissue Viability Society; 1994; Harrogate, UK. 1994:5.
Braniff‐Matthews 1997 {published data only}
- Braniff‐Matthews C, Rhodes J. Prevention versus treatment: long term management of care. New Approaches to the Management of Chronic Wounds. 1997:27‐9.
Brienza 2001 {published data only}
- Brienza DM, Karg PE, Geyer MJ, Kelsey S, Trefler E. The relationship between pressure ulcer incidence and buttock‐seat cushion interface pressure in at‐risk elderly wheelchair users. Archives of Physical Medicine and Rehabilitation 2001;82(4):529‐33. [DOI] [PubMed] [Google Scholar]
- Geyer MJ, Brienza D, Karg P, Kelsey S, Trefler E. Are commercial seat cushions efficacious in preventing pressure ulcers in the at‐risk, elderly nursing home population?. The 13th Annual Symposium on Advanced Wound Care and 10th Annual Medical Research Forum on Wound Repair. USA: HMP Communications, LLC, 2000.
Büchner 1995 {published data only}
- Büchner T. Comparison of 3 decubitus prevention measures stemming from the same principle ‐ with reference to cost‐benefit analysis. Krankenpflege 1995;33(6):248‐52. [PubMed] [Google Scholar]
Cassino 2013 {published data only}
- Cassino R, Ippolito AM, Cuffaro C, Corsi A, Ricci E. A controlled, randomised study on the efficacy of two overlays in the treatment of decubitus ulcers. Minerva chirurgica 2013;68(1):105‐116. [PubMed] [Google Scholar]
Chaloner 2000 {published data only}
- Chaloner D. A prospective, controlled comparison between two dynamic alternating mattress replacement systems within a community setting. 9th European Conference on Advances in Wound Management; 1999, 9‐11 November; Harrogate UK. 2000:9‐11.
Colin 1996 {published data only}
- Colin D, Loyant R, Abraham P, Saumet JL. Changes in sacral transcutaneous oxygen tension in the evaluation of different mattresses in the prevention of pressure ulcers. Advances in Wound Care 1996;9(1):25‐8. [PubMed] [Google Scholar]
Conine 1991 {published data only}
- Conine TA, Hershler C. Effectiveness: a neglected dimension in the assessment of rehabilitation devices and equipment. International Journal of Rehabilitation Research 1991;14(2):117‐22. [PubMed] [Google Scholar]
deBoisblanc 1993 {published data only}
- deBoisblanc BP, Castro M, Everret B, Grender J, Walker CD, Summer WR. Effect of air‐supported, continuous, postural oscillation on the risk of early ICU pneumonia in non‐traumatic clinical illness. Chest 1993;103(5):1543‐7. [DOI] [PubMed] [Google Scholar]
Defloor 1997 {published data only}
- Defloor T. The effect of position and mattress on the development of pressure sores. Verpleegkunde 1997;12(3):140‐9. [PubMed] [Google Scholar]
Defloor 2000 {published data only}
- Defloor T. The effect of position and mattress on interface pressure. Applied Nursing Research 2000;13(1):2‐11. [DOI] [PubMed] [Google Scholar]
Defloor 2005 {published data only}
- Defloor T. Less frequent turning intervals and yet less pressure ulcers [Wisselhounding minder frequent en toch minder decubitus]. Tijdschrift voor Gerontologie en Geriatrie 2001;32:174‐7. [PubMed] [Google Scholar]
- Defloor T, Bacquer D, Grypdonck MHF. The effect of various combinations of turning and pressure reducing devices on the incidence of pressure ulcers. International Journal of Nursing Studies 2005;42:37‐46. [DOI] [PubMed] [Google Scholar]
Della Valle 2001 {published data only}
- Della Valla AG, Salonia‐Ruzo P, Peterson MGE, Salvati EA, Sharrock NE. Inflatable pillows as axillary support devices during surgery performed in the lateral decubitus position under epidural anesthesia. Anesthesia and Analgesia 2001;93(5):1338‐43. [DOI] [PubMed] [Google Scholar]
Flam 1995 {published data only}
- Flam E, Isayeva E, Kipervas Y, Shklyarevsky V, Raab L. Skin temperature and moisture management with a low air loss surface. Ostomy Wound Management 1995;41(9):50‐6. [PubMed] [Google Scholar]
Fleischer 1997 {published data only}
- Fleischer I, Bryant D. Evaluating replacement mattresses. Nurse Management 1997;28(8):38‐42. [PubMed] [Google Scholar]
Geelkerken 1994 {published data only}
- Geelkerken RH, Breslau PJ, Hermans J, Wille J, Hofman A, Hamming JJ. Anti‐decubitus mattress (letter; comment). Nederlands Tijdschrift voor Geneeskunde 1994;138(36):1834. [PubMed] [Google Scholar]
Gil Agudo 2009 {published data only}
- Gil‐Agudo A, Peña‐González A, Ama‐Espinosa A, Pérez‐Rizo E, Díaz‐Domínguez E, Sánchez‐Ramos A. Comparative study of pressure distribution at the user‐cushion interface with different cushions in a population with spinal cord injury. Clinical Biomechanics 2009;24(7):558‐63. [DOI] [PubMed] [Google Scholar]
Gray 2008 {published data only}
- Gray D, Cooper P, Bertram M, Duguid K, Pirie G. A clinical audit of the Softform Premier Active[trademark] mattress in two acute care of the elderly wards. Wounds UK 2008;4(4):124‐8. [Google Scholar]
Grindley 1996 {published data only}
- Grindley A, Acres J. Alternating pressure mattresses: comfort and quality of sleep. British Journal of Nursing 1996;5(21):1303‐10. [DOI] [PubMed] [Google Scholar]
Grisell 2008 {published data only}
- Grisell M, Place HM. Face tissue pressure in prone positioning: a comparison of three face pillows while in the prone position for spinal surgery. Spine 2008;33(26):2938‐41. [DOI] [PubMed] [Google Scholar]
Gunningberg 1998 {published data only}
- Gunningberg L, Lindholm C, Carlsson M, Sjoden PO. Patients with hip fracture: risk for pressure ulcers. A prospective, controlled study of 124 patients in Uppsala, Sweden. European Wound Management Association and Journal of Wound Care Autumn Conference; 1998 November; Harrogate, UK. 1998:10.
Haalboom 1994 {published data only}
- Haalboom J R. Anti‐decubitus mattresses. Nederlands Tijdschrift voor Geneeskunde 1994;138(26):1309‐10. [PubMed] [Google Scholar]
Hampton 1998 {published data only}
- Hampton S. Can electric beds aid pressure sore prevention in hospitals?. British Journal of Nursing 1998;7(17):1010‐7. [DOI] [PubMed] [Google Scholar]
Hawkins 1997 {published data only}
- Hawkins JE. The effectiveness of pressure‐reducing table pads as an intervention to reduce the risk of intraoperatively acquired pressure sores. Military Medicine 1997;162:759‐61. [PubMed] [Google Scholar]
Heyneman 2009 {published data only}
- Heyneman A, Vanderwee K, Grypdonck M, Defloor T. Effectiveness of two cushions in the prevention of heel pressure ulcers. Worldviews on Evidence‐Based Nursing 2009;6(2):114‐20. [DOI] [PubMed] [Google Scholar]
Holzgreve 1993 {published data only}
- Holzgreve A, Waldner M, Waldner PW, Hohlbach G. Bed sore prophylaxis in patients with chronic arterial occlusive disease with a new thermoactive pressure alternating mattress. Langenbecks Archiv fur Chirurgie 1993;Suppl Kongessbericht:1117. [Google Scholar]
Huang 2009 {published data only}
- Huang TT, Tseng CE, Lee TM, Yeh JY, Lai YY. Preventing pressure sores of the nasal ala after nasotracheal tube intubation: from animal model to clinical application. Journal of Oral and Maxillofacial Surgery 2009;67(3):543‐51. [DOI] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang HY, Chen HL, Xu XJ. Pressure‐redistribution surfaces for prevention of surgery‐related pressure ulcers: A meta‐analysis. Ostomy/Wound Management 2013;59(4):36‐48. [PubMed] [Google Scholar]
Inman 1999 {published data only}
- Inman KJ, Dymock K, Fysh N, Robbins B, Rutledge FS, Sibbald WJ. Pressure ulcer prevention: a randomized controlled trial of 2 risk‐directed strategies for patient surface assignment. Advances in Wound Care 1999;12(2):72‐80. [PubMed] [Google Scholar]
Jacksich 1997 {published data only}
- Jacksich BB. Pressure ulcer prevalence and prevention of nosocomial development: one hospital's experience. Ostomy/Wound Management 1997;43(3):32‐4, 36, 38‐40. [PubMed] [Google Scholar]
Jackson 2011 {published data only}
- Jackson M, McKenney T, Drumm J, Merrick B, LeMaster T, VanGilder C. Pressure ulcer prevention in high‐risk postoperative cardiovascular patients. Critical Care Nurse 2011;31(4):44‐53. [DOI] [PubMed] [Google Scholar]
Jesurum 1996 {published data only}
- Jesurum J, Joseph K, Davis JM. Balloons, beds and breakdown. Effects of low air loss therapy on the development of pressure ulcers in cardiovascular surgical patients with intra‐aortic balloon pump support. Critical Care Nursing Clinics of North America 1996;8(4):423‐40. [PubMed] [Google Scholar]
Koo 1995 {published data only}
- Koo TK, Mak AF, Lee YL. Evaluation of an active seating system for pressure relief. Assistive Technology 1995;7(2):119‐28. [DOI] [PubMed] [Google Scholar]
Marchand 1993 {published data only}
- Marchand AC, Lidowski H. Reassessment of the use of genuine sheepskin for pressure ulcer prevention and treatment. Decubitus 1993;6(1):44‐7. [PubMed] [Google Scholar]
McMichael 2008 {published data only}
- McMichael JC, Place HM. Face tissue pressures in prone positioning: a comparison of 3 pillows. Journal of Spinal Disorders and Techniques 2008;21(7):508‐13. [DOI] [PubMed] [Google Scholar]
Nakahara 2012 {published data only}
- Nakahara R, Ohto S, Kato H. Treating pressure ulcers using alternating pressure replacement mattresses. 4th congress of the world union of wound healing societies; september2‐6. Yokohama, Japan, 2012.
Neander 1996 {published data only}
- Neander KD, Michels S, Bering F, Rich A, Merseburg M. Effects of soft bedding on body perception and posture. Pflege 1996;9(4):293‐9. [PubMed] [Google Scholar]
Ooka 1995 {published data only}
- Ooka M, Kemp MG, McMyn R, Shott S. Evaluation of three types of support surfaces for preventing pressure ulcers in patients in a surgical intensive care unit. Journal of Wound Ostomy Continence Nursing 1995;22(6):271‐9. [DOI] [PubMed] [Google Scholar]
Pham 2011a {published data only}
- Pham B, Stern A, Chen W, Sander B, John‐Baptiste A, Thein HH, Gomes T, Wodchis WP, Bayoumi A, Machado M, Carcone S, Krahn M. Preventing pressure ulcers in long‐term care: A cost‐effectiveness analysis. Archives of Internal Medicine 2011;171(20):1839‐47.. [DOI] [PubMed] [Google Scholar]
Pham 2011b {published data only}
- Pham B, Teague L, Mahoney J, Goodman L, Paulden M, Poss J, Li J, Ieraci L, Carcone S, Krahn M. Early prevention of pressure ulcers among elderly patients admitted through emergency departments: a cost‐effectiveness analysis. Annals of emergency medicine 2011;58(5):468‐478. e3. [DOI] [PubMed] [Google Scholar]
Phillips 1999 {published data only}
- Phillips L. Providing correct pressure‐relieving devices for optimum outcome. British Journal of Nursing 1999;8(21):1447‐52. [DOI] [PubMed] [Google Scholar]
Regan 1995 {published data only}
- Regan MB, Byers BH, Mayrovitz HN. Efficacy of a comprehensive pressure ulcer prevention programme in an extended care facility. Advances in Wound Care 1995;8(3):51‐5. [PubMed] [Google Scholar]
Reynolds 1994 {published data only}
- Reynolds A, Suarez C. Pressure‐reducing capability of Conforma II mattress overlay. Advances in Wound Care 1994;7:36‐40. [PubMed] [Google Scholar]
Rosenthal 1996 {published data only}
- Rosenthal MJ, Felton RM, Hileman DL, Lee M, Friedman M. A wheelchair cushion designed to redistribute sites of sitting pressure. Archives of Physical Medicine and Rehabilitation 1996;77:278‐82. [DOI] [PubMed] [Google Scholar]
Scott 1995 {published data only}
- Scott F. Easing the pressure for hip fracture patients. Nursing Times 1995;91(29):30‐1. [PubMed] [Google Scholar]
Scott 1999 {published data only}
- Scott EM, Baker EA, Kelly PJ, Stoddard EJ, Leaper DJ. Measurement of interface pressures in the evaluation of operating theatre mattresses. Journal of Wound Care 1999;8:437‐41. [DOI] [PubMed] [Google Scholar]
Scott 2000 {published data only}
- Scott EM. The prevention of pressure ulcers in the operating department. Journal of Wound Care 2000;9(1):18‐21. [DOI] [PubMed] [Google Scholar]
Simonis 2012 {published data only}
- Simonis G, Steiding K, Schaefer K, Rauwolf T, Strasser RH. A prospective, randomized trial of continuous lateral rotation (“kinetic therapy”) in patients with cardiogenic shock. Clinical Research in Cardiology 2012;101(12):955‐962. [DOI] [PubMed] [Google Scholar]
Stoneberg 1986 {published data only}
- Stoneberg C, Pitcock N, Myton C. Pressure sores in the homebound: one solution. American Journal of Nursing 1986;April:426‐8. [PubMed] [Google Scholar]
Suarez 1995 {published data only}
- Suarez CH, Reynolds A. Pressure reduction with a hospitalized population using a mattress overlay. Ostomy Wound Management 1995;41:58‐60, 62‐3. [PubMed] [Google Scholar]
Taccone 2009 {published data only}
- Taccone P, Polli F, Mietto C, Gattinoni L. Effects of a new rotational device on complication rate during prone ventilation in patients with acute respiratory distress syndrome: A retrospective analysis. Intensive Care Medicine 2009;36(4):585‐99. [Google Scholar]
Takala 1994 {published data only}
- Takala J, Soini HO, Soppi E, Kataja M, Olkkonen K. Is it possible to reduce the risk factors of pressure sores by means of special mattresses?. Duodecim 1994;110(4):407‐14. [PubMed] [Google Scholar]
Thomas 1994 {published data only}
- Thomas J. Support surface evaluation methodological and statistical considerations. Journal of Tissue Viability 1994;4(3):93. [Google Scholar]
Timmons 2008 {published data only}
- Timmons J, Bertram M. The use of micro‐stimulation in pressure ulcer prevention. Wounds UK 2008;4(4):120‐3. [Google Scholar]
Torra i Bou 2002 {published data only}
- Torra i Bou JE, Rueda López J, Camañes G, Herrero Narváez E, Blanco Blanco J, Martínez‐Esparza EH, et al. Heel pressure ulcers. Comparative study between heel protective bandage and hydrocellular dressing with special form for the heel. Revista de Enfermeria 2002;25(5):50‐6. [PubMed] [Google Scholar]
Turnage‐Carrier 2008 {published data only}
- Turnage‐Carrier C, McLane KM, Gregurich MA. Interface pressure comparison of healthy premature infants with various neonatal surfaces. Advances in Neonatal Care 2008;8(3):176‐84. [DOI] [PubMed] [Google Scholar]
Vanderwee 2007 {published data only}
- Vanderwee K, Grypdonck MH, Bacquer D, Defloor T. Effectiveness of turning with unequal time intervals on the incidence of pressure ulcer lesions. Journal of Advanced Nursing 2007;57(1):59‐68. [DOI] [PubMed] [Google Scholar]
Vanderwee 2008 {published data only}
- Vanderwee K, Grypdonck M, Defloor T. Alternating pressure air mattresses as prevention for pressure ulcers: a literature review. International Journal of Nursing Studies 2008;45(5):784‐801. [DOI] [PubMed] [Google Scholar]
Wells 1984 {published data only}
- Wells P, Geden E. Paraplegic body‐support pressure on convoluted foam, waterbed, and standard mattresses. Research in Nursing and Health 1984;7(2):127‐33. [DOI] [PubMed] [Google Scholar]
Wild 1991 {published data only}
- Wild D. Body pressures and bed surfaces. Nursing Standard 1991;5(27):23, 25‐7. [DOI] [PubMed] [Google Scholar]
Wu 2011 {published data only}
- Wu T, Wang ST, Lin PC, Liu CL, Chao YFC. Effects of using a high‐density foam pad versus a viscoelastic polymer pad on the incidence of pressure ulcer development during spinal surgery. Biological Research for Nursing 2011;13(4):419‐424. [DOI] [PubMed] [Google Scholar]
Zernike 1994 {published data only}
- Zernike W. Preventing heel pressure sores: a comparison of heel pressure relieving devices. Journal of Clinical Nursing 1994;3(6):375‐80. [DOI] [PubMed] [Google Scholar]
Zernike 1997 {published data only}
- Zernike W. Heel pressure relieving devices how effective are they?. Australian Journal of Advanced Nursing 1997;14(4):12‐9. [PubMed] [Google Scholar]
References to studies awaiting assessment
Allegretti 2008 {unpublished data only}
- Allegretti A. Factors associated with clinical decisions and pressure ulcer development in long term care residents. University of Pittsburgh.
Mastrangelo 2010 {published data only}
- Mastrangelo D, Farina E, Gallicchio V, Anna D, Bresadola F, FArina MA. Initial analyses of the prospective study randomized on the anti‐decubitis lesion mattress cover. SAWC Spring: The Symposium on Advanced Wounds Care and the Wound Healing Society; 2010, April 17‐20; Orlando, Florida. 2010:S32, Abstract CR‐053.
Mayer 2008 {published data only}
- Mayer H, Schröder G, Osterbrink J. Clinical evaluation of the efficiency of the micro‐stimulation‐system thevo‐activ: Final report [Klinische Evaluation der Wirksamkeit des MiS Micro‐Stimulations‐Systems Thevo‐Activ]. Florida International University 2008.
Rafter 2011 {published data only}
- Rafter L. Evaluation of patient outcomes: pressure ulcer prevention mattresses. British Journal of Nursing 2011;20(11):32. [DOI] [PubMed] [Google Scholar]
Additional references
Allman 1997
- Allman RM. Pressure ulcer prevalence, incidence, risk factors, and impact. Clinical Geriatric Medicine 1997;13(3):421‐36. [PubMed] [Google Scholar]
Banks 1998
- Banks V. Nutrition and pressure area management. Journal of Wound Care 1998;7(6):318‐9. [DOI] [PubMed] [Google Scholar]
Benbow 1996
- Benbow M. Pressure sore guidelines: patient/carer involvement and education. British Journal of Nursing 1996;5(3):182,184‐7. [DOI] [PubMed] [Google Scholar]
Bennett 2004
- Bennett G, Dealey C, Posnett J. The cost of pressure ulcers in the UK. Age and Ageing 2004;33(3):230‐5. [DOI] [PubMed] [Google Scholar]
Bergstrom 1998
- Bergstrom, N, Braden, B, Champagne, M, Kemp, M, & Ruby, E. Predicting pressure ulcer risk: a multisite study of the predictive validity of the Braden Scale. Nursing Research 1998;47(5):261‐269. [DOI] [PubMed] [Google Scholar]
Berlowitz 1990
- Berlowitz DR, Wilikin SVB. Risk factors for pressure sores: a comparison of cross‐sectional and cohort derived data. Journal of the American Geriatrics Society 1990;37(11):1043‐50. [DOI] [PubMed] [Google Scholar]
Berlowitz 1997
- Berlowitz DR, Brandeis GH, Anderson J, Brand HK. Predictors of pressure ulcer healing among long‐term care residents. Journal of the American Geriatrics Society 1997;45(1):30‐4. [DOI] [PubMed] [Google Scholar]
Bianchetti 1993
- Bianchetti A, Zanetti O, Rozzini R, Trabucchi M. Risk factors for the development of pressure sores in hospitalized elderly patients: results of a prospective study. Archives of Gerontology and Geriatrics 1993;16(3):225‐32. [DOI] [PubMed] [Google Scholar]
Bliss 1993
- Bliss MR, Thomas JM. Clinical trials with budgetary implications: establishing randomised trials of pressure‐relieving aids. Professional Nurse 1993;8(5):292‐6. [PubMed] [Google Scholar]
Bo 2003
- Bo M, Massaia M, Raspo S, Bosco F, Cena P, Molaschi M, et al. Predictive factors of in‐hospital mortality in older patients admitted to a medical intensive care unit. Journal of American Geriatrics Society 2003;51(4):529‐33. [DOI] [PubMed] [Google Scholar]
Briggs 2013
- Briggs M, Collinson M, Wilson L, Rivers C, McGinnis E, Dealey C, Brown J, et al. The prevalence of pain at pressure areas and pressure ulcers in hospitalised patients. BMC Nursing 2013;12(1):19‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Casey 1997
- Casey G. Nutrition helps wound healing. Nursing New Zealand 1997;3(11):19. [PubMed] [Google Scholar]
Casey 1998
- Casey G. The importance of nutrition in wound healing. Nursing Standard 1998;13(3):51‐2. [DOI] [PubMed] [Google Scholar]
Choi 1992
- Choi SC, Nelson LD. Kinetic therapy in critically ill patients: combined results based on meta‐analysis. Journal of Critical Care 1992;7:57‐62. [Google Scholar]
Clough 1994
- Clough NP. The cost of pressure area management in an intensive care unit. Journal of Wound Care 1994;3:33‐5. [DOI] [PubMed] [Google Scholar]
Darouiche 1994
- Darouiche RO, Landon GC, Klima M, Musher DM, Markowski J. Osteomyelitis associated with pressure sores. Archives of Internal Medicine 1994;154(7):753‐8. [PubMed] [Google Scholar]
Deeks 1998
- Deeks J. Odds ratios should be used only in case control studies and logistic regression analyses. BMJ 1998;317:1155‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ek 1990
- Ek AC, Larsson J, Schenck H, Thorslund S, Unosson M, Bjurulf P. The correlation between energy, malnutrition and clinical outcome in an elderly hospital population. Clinical Nutrition 1990;9(4):185‐9. [DOI] [PubMed] [Google Scholar]
Elliot 1999
- Elliott TR, Shewchuk RM, Scott Richards J. Caregiver social problem‐solving abilities and family member adjustment to recent‐onset physical disabilities. Rehabilitation Psychology 1999;44(1):104‐23. [Google Scholar]
Emflorgo 1999
- Emflorgo CA. The assessment and treatment of wound pain. Journal of Wound Care 1999;8(8):384‐5. [DOI] [PubMed] [Google Scholar]
EPUAP‐NPUAP 2009
- European Pressure Ulcer Advisory Panel and National Pressure Ulcer Advisory Panel. Prevention and treatment of pressure ulcers: quick reference guide. Washington DC: National Pressure Ulcer Advisory Panel 2009.
Flock 2003
- Flock P. Pilot study to determine the effectiveness of diamorphine gel to control pressure ulcer pain. Journal of Pain and Symptom Management 2003;25(6):547‐54. [DOI] [PubMed] [Google Scholar]
Franks 1999
- Franks PJ, Morfatt CJ. Focus on wound management: Quality of life issue in chronic wound management. British Journal of Community Nursing 1999;4(6):283‐4. [Google Scholar]
Franks 2002
- Franks PJ, Winterburg JH, Moffatt CJ. Health‐related quality of life and pressure ulceration assessment in patients treated in the community. Wound Repair and Regeneration 2002;10(3):133‐40. [DOI] [PubMed] [Google Scholar]
Freeman 2001
- Freeman K, Smyth C, Dallam L, Jackson B. Pain measurement scales: a comparison of the visual analogue and faces rating scales in measuring pressure ulcer pain (comment). Journal of Wound, Ostomy and Continence Nursing 2001;28(6):290‐6. [DOI] [PubMed] [Google Scholar]
Gallagher 1997
- Gallagher SM. Morbid obesity: a chronic disease with an impact on wound and related problems. Ostomy/Wound Management 1997;43(5):18‐24,26‐7. [PubMed] [Google Scholar]
Gosnell 1973
- Gosnell DJ. An assessment tool to identify pressure sores. Nursing Research 1973;22:55‐59. [PubMed] [Google Scholar]
Graves 2005
- Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infection Control and Hospital Epidemiology 2005;26(3):293‐7. [DOI] [PubMed] [Google Scholar]
Hagelstein 1995
- Hagelstein SM, Banks V. Maintaining quality of life for elderly patients in residential care. Journal of Wound Care 1995;8(4):347‐8. [DOI] [PubMed] [Google Scholar]
Healy 2003
- Healy M. Wound care and pain. World of Irish Nursing 2003;11(5):39‐40. [Google Scholar]
Hefley 1990
- Hefley ML, Radcliffe J. The 1988‐1989 decubitus study. Can a standardization of treatment be set for the elderly patient with decubiti ulcers stage III?. Hawaii Medical Journal 1999;49(9):346, 349‐50. [PubMed] [Google Scholar]
Henoch 2003
- Henoch I, Gustafsson M. Pressure ulcers in palliative care:development of a hospice pressure ulcer risk assessment scale. International Journal of Palliative Nursing 2003 2003;9(11):474–84. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Junkin 2009
- Junkin J, Gray M. Are pressure redistribution surfaces or heel protection devices effective for preventing heel pressure ulcers?. Journal of Wound Ostomy Continence Nursing 2009;36(6):602‐8. [DOI] [PubMed] [Google Scholar]
Kaltenhalter 2001
- Kaltenhaler E, Whitfield, Walters SJ, Akehurst RL, Paisley S. UK, USA and Canada: how do their pressure ulcer prevalence and incidence data compare?. Journal of Wound Care 2001;10(1):530‐5. [DOI] [PubMed] [Google Scholar]
Krainski 1992
- Krainski MM. Pressure ulcers and the elderly. Ostomy Wound Management 1992;38(5):22. [PubMed] [Google Scholar]
Lefebvre 2009
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Livesley 2002
- Livesley NJ, Chow AW. Infected pressure ulcers in elderly individuals. Clinical infectious diseases 2002;35:1390–6. [DOI] [PubMed] [Google Scholar]
Manfredi 2002
- Manfredi PL, Breuer B, Meier DE, Libow L. Pain assessment in the elderly with severe dementia. Journal of Pain and Symptom Management 2002;25(1):48‐52. [DOI] [PubMed] [Google Scholar]
McInnes 2011
- McInnes E, Dumville JC, Jammali‐Blasi A, Bell‐Syer SEM. Support surfaces for treating pressure ulcers. Cochrane Database of Systematic Reviews 2011, Issue Issue 12. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. CONSORT group. JAMA 2001;285(15):1987‐91. [DOI] [PubMed] [Google Scholar]
Norton 1979
- Norton D, McLaren R, Exton‐Smith A.N. An Investigation of Geriatric Problems in Hospital. 3rd Edition. London: Churchill Livingstone, 1979. [Google Scholar]
NPUAP 2007
- National Pressure Ulcer Advisory Panel (NPUAP). Support Surface Standards Initiative. Terms and Definitions Related to Support Surfaces. www.npuap.org/NPUAP_S3I_TD.pdf 2007.
NPUAP‐EPUAP‐PPPIA 2014
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. International NPUAP/EPUAP pressure ulcer classification system. In: Emily Haesler editor(s). Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Perth, Australia: Cambridge Media, 2014:11‐13. [Google Scholar]
Orlando 1998
- Orlando PL. Pressure ulcer management in the geriatric patient. Annals of Pharmacotherapy 1998;32(11):1221‐7. [DOI] [PubMed] [Google Scholar]
Pase 1998
- Pase MN, Hoffman RG. Selection and use of risk assessment tools and treatment protocol in extended care facilities in the southwest. Journal of Wound Ostomy and Continence Nursing 1998;25(1):44‐50. [DOI] [PubMed] [Google Scholar]
Phillips 2009
- Phillips L, Buttery J. Exploring pressure ulcer prevalence and preventative care. Nursing Times 2009;105(16):34‐6. [PubMed] [Google Scholar]
Prior 2002
- Prior J. The pressure is on: midwives and decubitus ulcers. RCM Midwives Journal 2002;5(5):196‐200. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration, 2014. Review Manager (RevMan). The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, Version 5.3.
Ronda 2002
- Ronda L, Falce RL. Skin care in older people. Primary Health Care 2002;12(7):51‐7. [Google Scholar]
SIGN 2008
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. http://www.sign.ac.uk/methodology/filters.html#random (accessed 28 February 2008).
Spoelhof 2000
- Spoelhof GD. Management of pressure ulcers in the nursing home. Annals of Long Term Care 2000;8(8):69‐77. [Google Scholar]
Thomas 1996
- Thomas DR, Goode PS, Tarquine PH, Allman RM. Hospital acquired pressure ulcers and risk of death. Journal of American Geriatrics Society 1996;44:1435‐40. [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas DR. Improving outcomes of pressure ulcers with nutritional interventions: a review of the evidence. Nutrition 2001;17:121‐5. [DOI] [PubMed] [Google Scholar]
Vanderwee 2007a
- Vanderwee K, Clark M, Dealey C, Gunningberg L, Defloor T. Pressure ulcer prevalence in Europe: a pilot study. Journal of Evaluation in Clinical Practice 2007;13(2):227‐35. [DOI] [PubMed] [Google Scholar]
VanGilder 2009
- VanGilder C, Amlung S, Harrison P, Meyer S. Results of the 2008‐2009 International Pressure Ulcer Prevalence Survey and a 3‐year, acute care, unit‐specific analysis. Ostomy/Wound Management 2009;55(11):39‐45. [PubMed] [Google Scholar]
Waltman 1991
- Waltman NL, Bergstrom N, Armstrong N, Norvell K, Braden B. Nutritional status, pressure sores, and mortality in elderly patients with cancer. Oncology Nursing Forum 1991;18(5):867‐73. [PubMed] [Google Scholar]
Waterlow 1985
- Waterlow J. A risk assessment card. Nursing Times 1985;81(49):51‐5. [PubMed] [Google Scholar]
Whittington 2004
- Whittington K, Briones R. National prevalence and incidence study: 6‐year sequential acute care data. Advances in Skin and Wound Care 2004;17(9):490‐5. [DOI] [PubMed] [Google Scholar]
Woodbury 2004
- Woodbury M, Houghton P. Prevalence of pressure ulcers in Canadian healthcare settings. Ostomy/Wound Management 2004;50(10):22‐4,26,28,30,32,34,36‐8. [PubMed] [Google Scholar]
References to other published versions of this review
Cullum 1995
- Cullum NA, Deeks JJ, Fletcher AW, Sheldon TA, Song F. Preventing and treating pressure sores. Quality in Health Care 1995;4:289‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cullum 2001
- Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) Beds: (6) Compression: (7) Laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technology Assessment 2001;5(9):1‐221. [DOI] [PubMed] [Google Scholar]
Cullum 2003
- Cullum N, Nelson EA, Nixon JE. Pressure Sores. Clinical Evidence. Vol. 9, London: BMJ Publishing Group, June 2003:408‐9. [Google Scholar]


