Abstract
Background
Cervical lymph node metastasis (LNM) is an independent risk factor for poor prognosis of papillary thyroid carcinoma (PTC), but the scope of PTC lateral neck dissection (LND) is controversial. Solitary lateral lymph node metastasis (SLNM) is a special type of PTC with lateral LNM. Currently, study on the preoperative clinical characteristics of SLNM has been seldomly reported. This study evaluated the preoperative characteristics for predicting the SLNM of PTC.
Methods
We included 391 patients diagnosed with PTC between May 2011 and July 2017. Among those patients, 44 had SLNM and 347 had multiple lateral neck node metastasis (MLNM). The clinicopathologic characteristics and other central lymph node metastasis risk factors were retrospectively analyzed.
Results
Univariate analysis revealed that age and tumor size (≤1 cm) were significantly correlated with SLNM. In ROC curve analysis, the optimal cutoff age of preoperative predictors for the prediction of SLNM was 46.5 years (AUC=0.623, 0.536–0.710). Besides, the frequency and mean number of CLNM was significantly less in the SLNM than MLNM group. The oval and round tumor shape and well-defined margin of the tumor were more common in the SLNM group (p =0.001; p=0.024, respectively). In addition, multivariate analysis revealed that age ≥47, capsular invasion, no extrathyroidal extension, with central lymph node metastases and irregular shape were independent SLNM predictors of PTCs (odds ratio 2.386, 0.173, 0.284, 0.239, 0.188; 95% CI 1.07–5.140, 0.058–0.840, 0.066–0.926, 0.091–0.437, 0.167–0.864, respectively).
Conclusion
This study supported that SLNM is more likely to happen in PTC patients with age ≥47 years, capsular invasion, no extrathyroidal extension, with central lymph node metastases and irregular shape. That denotes, selective single level neck dissection can be considered as an alternative to systemic lateral neck dissection in those patients.
Keywords: solitary lateral lymph node metastasis, papillary thyroid carcinoma, central lymph node dissection
Introduction
Thyroid cancer is one of the most common endocrine tumors, and its incidence is increasing gradually.1–4 Papillary thyroid carcinoma (PTC) is the most common pathological type of thyroid cancer, though with a low degree of malignancy, prone to cervical lymph node metastasis (LNM).5 Cervical LNM rate is approximately 30~80%, while, lateral LNM rate is up to 64.2%.6,7 Central lymph node metastasis (CLNM) has been proved to be an independent risk factor for local recurrence8–10 and decreased survival rate.11–13 At present, surgery is the main treatment for cervical LNM. As the pattern of clinical LNM varies,6,14,15 the scope of dissection is still controversial. A wide range of surgical dissection can lead to the risk of postoperative complications and reduce the quality of life after surgery, on the contrary, if the scope of lateral neck dissection (LND) is too small, postoperative recurrence will lead to the second operation. Therefore, it is necessary to analyze the characteristics of cervical lymph node metastasis for guiding surgery and preventing surgical complications.
Decisions about the scope of surgery are usually based on predictable patterns of LNM. Multiple lateral neck node (MLN) metastasis refer to the number of positive lymph nodes in the lateral regions more than two. LND including levels II to V is generally recommended for complete clearance of MLNM. However, there are different opinions on the dissection scope of solitary lateral neck node (SLN) metastasis. SLN metastasis is a special phenomenon in PTCs, in which patients have only one lateral lymph node metastasis regardless of central LNM or not. Therefore, various types of LND were proposed.16–18 If a large-scale LND is performed on a PTC patient with SLNM, it may result in increased postoperative complications, including secondary nerve injury, hypoparathyroidism, chylous leakage, skin numbness around the ear, neck and shoulder, and a decline in quality of life.19–21 It was expected that clinical features would be related to the SLN metastasis, and an attempt was made to find a relationship between clinical features and SLN metastasis.
For better distinction of SLNM and choices of surgical method in PTC patients, in our current analyses, 391 PTC patients were divided into SLNM and MLNM groups according to the number of LNM from postoperative histopathologic records, and identify the risk factors associated with SLNM in PTC by investigating preoperative clinicopathological and ultrasound clinical features retrospectively.
Methods
Ethics Statement
Approval to retrospectively review the medical records of patients was obtained from the Ethics Committee of Xiangya Hospital, Central South University and conducted according to the principles expressed in the Declaration of Helsinki. In addition to approving the study protocol, the Ethics Committee required neither patient approval nor informed consent for the review of records. Furthermore, we confirmed that the data related to this manuscript was anonymized.
Patients
Initially, 493 patients with pathologically confirmed LNM who underwent curative initial surgery with neck dissection for PTC between May 2011 and July 2017, were retrospectively screened. Patients with the following conditions were excluded: (1) other types of thyroid cancer; (2) a history of previous thyroidectomy; (3) previous neck radiotherapy history, patients without sufficient clinical and ultrasound data. SLNM of PTC was defined as patients who were confirmed having only one lateral lymph node metastasis. Only the data for which both preoperative ultrasound findings and postoperative pathologic reports were available were included. Finally, a cohort of 391 patients was analyzed. All patients underwent simultaneous total thyroidectomy (TT), bilateral central neck dissection (CND), and LND (at least from levels II to V). Among the study subjects, 44 (11.3%) patients were in the SLNM group, and 347 (88.8%) patients were in the MLNM group. All the participants in this study signed the written consent before surgery.
Clinicopathological Parameters
The preoperative sonographic and clinicopathological characteristics between two groups were analyzed. Preoperative clinical parameters included age, sex, number of tumors, capsular invasion, extrathyroidal extension, lymphocytic thyroiditis, TSH level, CLNM, number of CLNM. Chemiluminescence immunoassay was used to determine serum TSH, and the reference range was 0.27~4.2mIU/L. Ultrasound findings, including tumor size, lymphadenopathy, location, shape, margin, echogenicity, and calcification were evaluated. We defined the maximum diameter as tumor size, when patient was found with multiple foci, the dimension of the largest PTC lesion was recorded. Tumor location was classified as the superior, middle, or inferior third of the thyroid gland. Shape of the nodule was defined as having an irregular shape or oval and round. Margin was defined as poorly or well defined. Echogenicity was defined as hypoechogenic or other echogenic. In our study, other types of echogenicity included isoechoic, mixechoic and hyperechoic.
Statistical Analysis
Statistical software SPSS 22.0 was used to analyze the data. Continuous variables are presented as mean ± standard deviation. Pearson’s chi-squared test and the Student’s t‐test were used to compare the correlation between preoperative clinicopathological and ultrasound features of PTCs with lateral LNM. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimal cutoff points for patient age, tumor sizes and number of CLNM in the prediction of solitary and multiple lateral neck metastasis. Univariate analysis and multivariate analysis were performed by binary logistic regression to evaluate the predictors for SLNM in PTCs. All p-values were two-sided, and p-values of <0.05 were considered statistically significant.
Results
Clinicopathological and Sonographic Characteristics of Study Subjects
A total of 391 patients was analyzed, of which there were 124 (31.7%) male and 267 (68.3%) female, and their mean age was 36.92±12.88 years (range 9 to 77 years). The clinicopathological characteristics of the patients in two groups are presented in Table 1. The median age of SLNM group and MLNM group were 41.56±12.39 years and 36.22±12.56 years, with significant difference between the groups (p <0.05). In this study, 64 (16.4%) patients with PTC had skipped metastasis, and skipped LNMs were significantly more frequent in SLNM group than in MLNM group (43.2% versus 13.0%, p < 0.001). Besides, the mean number of central lymph node metastasis (CLNM) was lower in the SLNM than in the MLNM group (1.64±2.27 versus 3.84±4.64; p < 0.001).
Table 1.
Clinicopathological Characteristics of Study Subjects
| Clinical Characteristics | Total (n=391) | SLNM Group (n=44) | MLNM Group (n=347) | p valuea |
|---|---|---|---|---|
| Age (9~77 years) | 36.92±12.88 | 41.56±12.39 | 36.22±12.56 | 0.008b |
| Sex | ||||
| Male | 124(31.7%) | 17(38.6%) | 107(30.8%) | 0.295 |
| Female | 267(68.3%) | 27(61.4%) | 240(69.2%) | |
| Number of tumors | ||||
| Solitary | 246(62.9%) | 33(75%) | 213(61.4%) | 0.078 |
| Multifocality | 145(37.1%) | 11(25%) | 134(38.6%) | |
| Tumor size (0.1~9.7 cm) | 2.14±1.31 | 2.08±1.48 | 2.23±1.29 | 0.461b |
| >1cm | 326(83.4%) | 32(72.3%) | 294(84.7%) | 0.044 |
| ≤1cm | 65(16.6%) | 12(27.3%) | 53(15.3%) | |
| Capsular invasion | ||||
| Present | 129(33%) | 12(27.3%) | 117(33.7%) | 0.392 |
| Absent | 262(67%) | 32(72.3%) | 230(66.3%) | |
| Extrathyroidal extension | ||||
| Present | 115(29.4%) | 15(34.1%) | 100(28.8%) | 0.470 |
| Absent | 276(70.6%) | 29(65.9%) | 247(71.2%) | |
| Lymphocytic thyroiditis | ||||
| Present | 146(37.3%) | 15(34.1%) | 131(37.8%) | 0.636 |
| Absent | 245(62.7%) | 29(65.9%) | 216(62.2%) | |
| TSH level | ||||
| High | 59(15.1%) | 6(13.6%) | 53(15.3%) | 0.658 |
| Normal | 322(82.4%) | 36(81.8%) | 286(82.4%) | |
| Low | 10(2.5%) | 2(4.6%) | 8(2.3%) | |
| Number of CLNM | 2.37±2.27 | 1.64±2.27 | 3.84±4.64 | 0.000b |
| CLNM | ||||
| Present | 327(83.6%) | 25(56.8%) | 302(87.0%) | 0.000 |
| Absent | 64(16.4%) | 19(43.2%) | 45(13.0%) |
Notes: aPearson’s chi-squared test was adopted; bThe Student’s t‐test was adopted.
Abbreviations: SLNM, solitary lateral neck node metastasis; MLNM, multiple lateral neck node metastasis; TSH, thyroid stimulating hormone; CLNM, central lymph node metastasis.
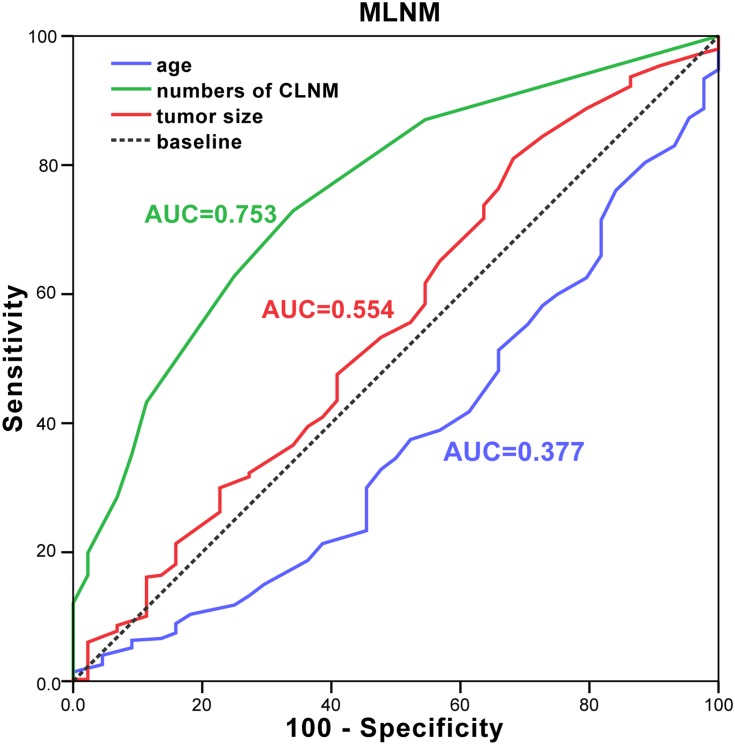
The analysis of preoperative sonographic features is listed in Table 2. In this study, ultrasonography (US) did not find a single lymph node enlargement in the lateral cervical region. And the difference in lymphadenopathy on neck US between the two groups was not statistically significant (p>0.05). In receiver operating characteristic (ROC) curve analysis, we found tumor size would be a well predictor for MLNM. The optimal cutoff tumor sizes in MLNM group were 1.15 cm (area under curve (AUC) =0.554, 0.459–0.648) (Figure 1). While, we found that papillary thyroid microcarcinoma (PTMC) (tumor sizes≤1 cm) was more common in the SLNM group than in the MLNM group (p = 0.044). An oval and round tumor shape was more common in the SLNM group (63.6%) than in the MLNM group (37.5%, p =0.001). The well-defined margin of the tumor was also significantly more frequent in the SLNM group (63.6% versus 45.5%, p=0.024). No significant differences in location and calcification of the tumor were found between two groups.
Table 2.
Sonographic Characteristics of Study Subjects
| Clinical Characteristics | Total (n=391) | SLNM Group (n=44) | MLNM Group (n=347) | p valuea |
|---|---|---|---|---|
| Lymphadenopathy on neck US | ||||
| Present | 353(90.3%) | 41(93.2%) | 312(91.0%) | 0.491 |
| Absent | 38(9.7%) | 3(6.8%) | 35(9.0%) | |
| Location of the tumor on neck US | ||||
| Superior lobe | 125(32%) | 15(34.1%) | 110(31.7%) | 0.869 |
| Middle lobe | 146(37.3%) | 17(38.6%) | 129(37.2%) | |
| Inferior lobe | 120(30.7%) | 12(27.3%) | 108(31.1%) | |
| Shape of the tumor on neck US | ||||
| Irregular shape | 233(59.6%) | 16(36.4%) | 217(62.5%) | 0.001 |
| Oval and round | 158(40.4%) | 28(63.6%) | 130(37.5%) | |
| Margin of the tumor on neck US | ||||
| Poorly defined | 205(52.4%) | 16(36.4%) | 189(54.5%) | 0.024 |
| Well defined | 186(47.6%) | 28(63.6%) | 158(45.5%) | |
| Echogenicity of the tumor on neck US | ||||
| Hypoechogenic | 260(66.5%) | 29(65.9%) | 231(66.6%) | 0.930 |
| Other echogenic | 131(33.5%) | 15(34.1%) | 116(33.4%) | |
| Calcification of the tumor on neck US | ||||
| Present | 376(96.2%) | 43(97.7%) | 333(96.0%) | 0.567 |
| Absent | 15(3.8%) | 1(2.3%) | 14(4.0%) |
Note: aPearson’s chi-squared test was adopted.
Abbreviations: SLNM, solitary lateral neck node metastasis; MLNM, multiple lateral neck node metastasis; US, ultrasonography.
Figure 1.
ROC curve for age (blue line), tumor sizes (red line) and number of CLNM (green line) in the prediction of MLNM.
Abbreviations: ROC, receiver operating characteristic; MLNM, multiple lateral neck node metastasis; CLNM, central lymph node metastasis; AUC, area under curve.
Optimal Cutoff of Preoperative Predictors for the Prediction of SLNM and MLNM
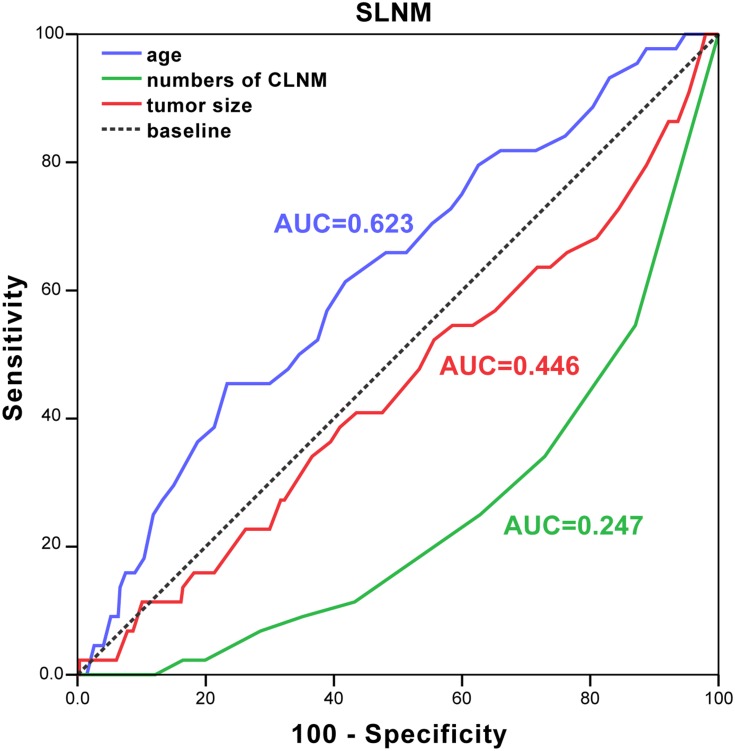
In order to evaluate the value of age, numbers of CLNM and tumor size in predicting SLNM or MLNM, ROC curve analysis was calculated. In our results, the area under the curve of age in SLNM group was 0.623 (the optimal cutoff age was 46.5 years), indicating that the accuracy of the test was good, which means the age≥47 was a well predictor of SLNM (Figure 2). The cutoff value of the numbers of CLNM predictors for MLNM was defined as 1.5 (AUC=0.753, 0.681–0.826), and the optimal cutoff tumor size in MLNM was 1.15 cm (AUC=0.554, 0.459–0.648) (Figure 1). PTC patients with two or more central lymph node metastasis and tumor size ≥1.15 cm was at high risk for MLNM.
Figure 2.
ROC curve for age (blue line), tumor sizes (red line) and number of CLNM (green line) in the prediction of SLNM.
Abbreviations: ROC, receiver operating characteristic; CLNM, central lymph node metastasis; AUC, area under curve; SLNM, solitary lateral neck metastasis.
Multivariate Analysis of Risk Factors Related to Solitary Lateral Neck Metastasis
To define the predictors of SLNM of PTCs, we performed binary logistic regression analyses with clinicopathological and US features (Table 3). We found that age ≥47 years was significant predictors of SLNM (OR=2.386, p=0.026). However, capsular invasion, no extrathyroidal extension, with central lymph node metastases and irregular shape were statistically significant differences (OR=0.220, p=0.027; OR=0.247, p=0.038; OR=0.200, p=0.000 and OR=0.380, p=0.021, respectively). As can be seen from the results, these factors were all protective factors for SLN metastasis in PTC patients. In other words, they were risk factors of PTC patients with MLNM and in the presence of these factors, lateral regional lymph node dissection should be performed.
Table 3.
Multivariate Analysis of Risk Factors Related to Solitary Lateral Neck Metastasis
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Patients’ age ≥47 years | 2.386 | 1.07–5.140 | 0.026 |
| Male gender | 1.828 | 0.858–3.896 | 0.118 |
| Tumor size < 1cm | 0.463 | 0.168–1.272 | 0.135 |
| Capsular invasion | 0.220 | 0.058–0.840 | 0.027 |
| No extrathyroidal extension | 0.247 | 0.066–0.926 | 0.038 |
| Lymphocytic thyroiditis | 1.240 | 0.569–2.702 | 0.588 |
| With central lymph node metastases | 0.200 | 0.091–0.437 | 0.000 |
| Lymphadenopathy of the tumor on neck US | 1.966 | 0.485–7.969 | 0.344 |
| Irregular shape | 0.380 | 0.167–0.864 | 0.021 |
| Poorly defined margin of the tumor | 0.653 | 0.283–1.507 | 0.318 |
Note: Variables with statistical significance were shown in bold.
Abbreviations: US, ultrasonography; OR, odds ratio; CI, confidence interval.
Discussion
Our study is the first time to evaluate preoperative sonographic and clinicopathological characteristics for predicting SLNM in PTC patients. We found that age ≥47 years, capsular invasion, no extrathyroidal extension, with central lymph node metastasis and irregular shape were significantly associated with SLNM. Multivariate analysis showed that patients’ age ≥47 years was the risk of SLNM. Nevertheless, capsular invasion, no extrathyroidal extension, with central lymph node metastasis and irregular shape were identified as protective factors for SLNM in PTC patients. In the absence of the above characteristics, there was a high possibility of SLNM.
The effect of age on lateral LNM remained contradictory. Age under 45 years has been reported to be associated with lateral LNM,22 while, age ≥45 years was considered to be an independent risk factor for LNM in the PTC with lateral lymph node metastasis.23,24 In this study, age ≥47 years was considered as a strong marker for predicting SLNM.
Studies have shown that extrathyroidal extension is a risk factor for lateral LNM.5,25 However, the absence of capsular invasion could also be a predictor of SLNM.26 Similarly, we found in this study that when the tumor invaded the capsule but no extrathyroidal extension was present, the risk of SLM metastasis was reduced. Hence, PTC with SLN metastasis may be in a special biological state. Therefore, preoperative evaluation of the lateral cervical lymph nodes should be conducted carefully, and selective single regional lymph node dissection should be considered.
LNM is a risk factor for recurrence in PTC.27,28 Lymph node dissection can significantly improve prognosis, so lymph nodes should be carefully evaluated before surgery. At present, there is still no clear suggestion on the scope of lateral neck dissection. Emerging study has attempted to evaluate preoperative sonographic and clinicopathological characteristics predicting SLNM of PTC. Some studies reported that tumor size, location and the number of central LNM were risk factors for lateral LNM.5,29 When the positive number of central lymph node metastasis were two or more, the metastatic rate of lateral LNM was 70.0 ~ 93.3%.30 In this analysis, the ROC curve showed that the optimal cutoff value of central LNM was 1.5. This result is consistent with many previous studies. However, in the current series, location was not found to be associated with the number of lateral LNM. Whereas, without central metastases, the lateral LNM can also occur in PTC patients, known as skip metastasis.27 This study found that skip metastasis was more common in patients with SLNM than in patients with MLNM, supported by previous reports.26,31 ROC curve analysis determined that 1.15 cm was an optimal tumor size cutoff for predicting MLNM.
The preoperative US features of PTC patients were also evaluated, and their clinical significance for predicting SLNM was analyzed. Oval and round shape and well-defined tumor were associated with SLN metastasis. These findings suggest that differences in the US findings may reflect different pathological properties in PTC patients with MLNM. The multivariate analysis showed that irregular shape was a protective factor for SLNM. On the contrary, its presence suggested a high risk of MLNM.
According to our results, low-risk PTC characteristics in terms of small primary tumor size and no extrathyroidal extension were shown in patients with SLNM. Low-risk patients carry a lower risk of cancer mortality and recurrence.28,32 Extensive dissection in these patients will increase the risk of complications, for instance, accessory nerve injury, chyle leakage, skin numbness around the ear, neck and shoulder.33–36 When performing lymph node dissection, it is necessary to make clear the scope of dissection, grasp the clear anatomical hierarchy, and protect the vascular branches and nerves. Therefore, in order to ensure the surgical benefit of patients, single regional or several sub-regional lymph node dissections is feasible for PTC patients with SLNM.
This study has some notable limitations, including those inherent to its retrospective design. For patients with occult metastases, we did not perform preventive LND, so we could not determine the true incidence of SLNM. Although preoperative lymph node status was evaluated, we were unable to determine whether prophylactic lateral neck dissection improved patient outcome.26 In this study, there was no comparison of ultrasonic characteristics of lymph node, which could be further studied in the future. Despite these limitations, the main advantage of this study is that we evaluated the preoperative characteristics of SLNM in order to decide on whom to perform selective single regional LND, which could provide the basis for the future prediction model.
Conclusion
The results of our study indicate that age≥47 years, capsular invasion, no extrathyroidal extension, with central lymph node metastases and irregular shape were protective factors for SLNM in PTC patients. Selective single regional or several sub-regional LND can be considered in PTC patients with SLNM, whom with small primary tumor size (≤1 cm) and no extrathyroidal extension were in low risk.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81974423), the Key Research and Development Programme of Hunan Province (2019SK2031) and the Xiangya Hospital Clinical Research Fund (2016L05).
Abbreviations
LNM, lymph node metastasis; PTC, papillary thyroid carcinoma; SLNM, solitary lateral lymph node metastasis; MLNM, multiple lateral neck node metastasis; CLNM, central lymph node metastasis; SLN, solitary lateral lymph node; MLN, multiple lateral neck node; LND, lateral neck dissection; TT, total thyroidectomy; CND, central neck dissection; CI, confidence interval; US, ultrasonography; OR, odds ratio; ROC, receiver operating characteristic; AUC, area under curve; PTMC, papillary thyroid microcarcinoma; TSH, thyroid stimulating hormone.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi: 10.1016/S0140-6736(16)30172-6 [DOI] [PubMed] [Google Scholar]
- 2.Zhang K, Liu J, Li C, Peng X, Li H, Li Z. Identification and validation of potential target genes in papillary thyroid cancer. Eur J Pharmacol. 2019;843:217–225. doi: 10.1016/j.ejphar.2018.11.026 [DOI] [PubMed] [Google Scholar]
- 3.Xia F, Wang W, Jiang B, Chen Y, Li X. DNA methylation-mediated silencing of miR-204 is a potential prognostic marker for papillary thyroid carcinoma. Cancer Manag Res. 2019;11:1249–1262. doi: 10.2147/CMAR.S184566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang P, Mao LF, Zhang ZP, et al. Down-regulated miR-125a-5p promotes the reprogramming of glucose metabolism and cell malignancy by increasing levels of CD147 in thyroid cancer. Thyroid. 2018;28(5):613–623. doi: 10.1089/thy.2017.0401 [DOI] [PubMed] [Google Scholar]
- 5.Feng JW, Qin AC, Ye J, Pan H, Jiang Y, Qu Z. Predictive factors for lateral lymph node metastasis and skip metastasis in papillary thyroid carcinoma. Endocr Pathol. 2019:1–10. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, Gong Y, Yan S, Zhu J, Li Z, Gong R. Risk factors for level V lymph node metastases in solitary papillary thyroid carcinoma with clinically lateral lymph node metastases. Cancer Med. 2016;5(8):2161–2168. doi: 10.1002/cam4.2016.5.issue-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King JM, Corbitt C, Miller FR. Management of lateral cervical metastases in papillary thyroid cancer: patterns of lymph node distribution. Ear Nose Throat J. 2011;90(8):386–389. doi: 10.1177/014556131109000814 [DOI] [PubMed] [Google Scholar]
- 8.Lee YC, Na SY, Park GC, Han JH, Kim SW, Eun YG. Occult lymph node metastasis and risk of regional recurrence in papillary thyroid cancer after bilateral prophylactic central neck dissection: a multi-institutional study. Surgery. 2017;161(2):465–471. doi: 10.1016/j.surg.2016.07.031 [DOI] [PubMed] [Google Scholar]
- 9.Kim MJ, Lee SG, Kim K, et al. Current trends in the features of male thyroid cancer: retrospective evaluation of their prognostic value. Medicine. 2019;98(19):e15559. doi: 10.1097/MD.0000000000015559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh YJ, Kwon H, Kim SJ, et al. Factors affecting the locoregional recurrence of conventional papillary thyroid carcinoma after surgery: a retrospective analysis of 3381 patients. Ann Surg Oncol. 2015;22(11):3543–3549. [DOI] [PubMed] [Google Scholar]
- 11.Molteni G, Bonali M, Mattioli F, et al. Central compartment revision surgery for persistent or recurrent thyroid carcinoma: analysis of survival and complication rate. Eur Arch Otorhinolaryngol. 2019;276(2):551–557. doi: 10.1007/s00405-018-5239-2 [DOI] [PubMed] [Google Scholar]
- 12.Randolph GW, Duh QY, Heller KS, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22(11):1144–1152. doi: 10.1089/thy.2012.0043 [DOI] [PubMed] [Google Scholar]
- 13.Aboelnaga EM, Ahmed RA. Difference between papillary and follicular thyroid carcinoma outcomes: an experience from Egyptian institution. Cancer Biol Med. 2015;12(1):53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roh JL, Kim JM, Park CI. Lateral cervical lymph node metastases from papillary thyroid carcinoma: pattern of nodal metastases and optimal strategy for neck dissection. Ann Surg Oncol. 2008;15(4):1177–1182. doi: 10.1245/s10434-008-9813-5 [DOI] [PubMed] [Google Scholar]
- 15.Takada H, Kikumori T, Imai T, Sawaki M, Shibata A, Kiuchi T. Patterns of lymph node metastases in papillary thyroid carcinoma: results from consecutive bilateral cervical lymph node dissection. World J Surg. 2011;35(7):1560–1566. doi: 10.1007/s00268-011-1133-4 [DOI] [PubMed] [Google Scholar]
- 16.Kang BC, Roh JL, Lee JH, et al. Candidates for limited lateral neck dissection among patients with metastatic papillary thyroid carcinoma. World J Surg. 2014;38(4):863–871. doi: 10.1007/s00268-013-2361-6 [DOI] [PubMed] [Google Scholar]
- 17.Bocca E, Pignataro O, Oldini C, Cappa C. Functional neck dissection: an evaluation and review of 843 cases. Laryngoscope. 1984;94(7):942–945. doi: 10.1288/00005537-198407000-00015 [DOI] [PubMed] [Google Scholar]
- 18.Adelstein D, Gillison ML, Pfister DG, et al. NCCN guidelines insights: head and neck cancers, version 2.2017. J Natl Compr Canc Netw. 2017;15(6):761–770. doi: 10.6004/jnccn.2017.0101 [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Meng C, Ouyang Q, Xie J, Li X. Magnesemia: an independent risk factor of hypocalcemia after thyroidectomy. Cancer Manag Res. 2019;11:8135–8144. doi: 10.2147/CMAR.S218179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deepak CA, Sarvadnya JJ, Sabitha KS. Variant anatomy of internal jugular vein branching. Ann Maxillofac Surg. 2015;5(2):284–286. doi: 10.4103/2231-0746.175751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Xia F, Meng C, Zhang Z, Bai N, Li X. Prediction of permanent hypoparathyroidism by parathyroid hormone and serum calcium 24h after thyroidectomy. Am J Otolaryngol. 2018;39(6):746–750. doi: 10.1016/j.amjoto.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 22.Back K, Kim JS, Kim JH, Choe JH. Superior located papillary thyroid microcarcinoma is a risk factor for lateral lymph node metastasis. Ann Surg Oncol. 2019;26(12):3992–4001. doi: 10.1245/s10434-019-07587-2 [DOI] [PubMed] [Google Scholar]
- 23.Zhao W, Chen S, Hou X, Liao Q, Chen G, Zhao Y. Predictive factors of lateral lymph node metastasis in papillary thyroid microcarcinoma. Pathol Oncol Res. 2019;25(3):1245–1251. doi: 10.1007/s12253-018-0511-8 [DOI] [PubMed] [Google Scholar]
- 24.Gong Y, Yang J, Yan S, et al. Pattern of and clinicopathologic risk factors for lateral lymph node metastases in papillary thyroid carcinoma patients with lateral cervical lymphadenopathy. Medicine. 2018;97(36):e12263. doi: 10.1097/MD.0000000000012263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain NK, Mostoufi-Moab S, Hawkes CP, et al. Extrathyroidal extension is an important predictor of regional lymph node metastasis in pediatric differentiated thyroid cancer. Thyroid. 2019. doi: 10.1089/thy.2019.0229 [DOI] [PubMed] [Google Scholar]
- 26.Kim SM, Chun KW, Chang HJ, et al. Solitary lateral neck node metastasis in papillary thyroid carcinoma. World J Surg Oncol. 2014;12:109. doi: 10.1186/1477-7819-12-109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie X, Tan Z, Ge M. Skip metastasis in papillary thyroid carcinoma is difficult to predict in clinical practice. BMC Cancer. 2017;17(1):702. doi: 10.1186/s12885-017-3698-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong W, Horiuchi K, Tokumitsu H, et al. Time-varying pattern of mortality and recurrence from papillary thyroid cancer: lessons from a long-term follow-up. Thyroid. 2019;29(6):802–808. doi: 10.1089/thy.2018.0128 [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Xiao C, Chen J, et al. Risk factor analysis for predicting cervical lymph node metastasis in papillary thyroid carcinoma: a study of 966 patients. BMC Cancer. 2019;19(1):622. doi: 10.1186/s12885-019-5835-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao GZ, Gao L. Central lymph node metastasis: is it a reliable indicator of lateral node involvement in papillary thyroid carcinoma? World J Surg. 2010;34(2):237–241. doi: 10.1007/s00268-009-0347-1 [DOI] [PubMed] [Google Scholar]
- 31.Park JH, Lee YS, Kim BW, Chang HS, Park CS. Skip lateral neck node metastases in papillary thyroid carcinoma. World J Surg. 2012;36(4):743–747. doi: 10.1007/s00268-012-1476-5 [DOI] [PubMed] [Google Scholar]
- 32.Sugitani I, Fujimoto Y, Yamada K, Yamamoto N. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J Surg. 2008;32(11):2494–2502. doi: 10.1007/s00268-008-9711-9 [DOI] [PubMed] [Google Scholar]
- 33.Ruggiero R, Gubitosi A, Conzo G, et al. Sutureless thyroidectomy. Int J Surg. 2014;12(Suppl 1):S189–S193. doi: 10.1016/j.ijsu.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 34.Medas F, Tuveri M, Canu GL, Erdas E, Calo PG. Complications after reoperative thyroid surgery: retrospective evaluation of 152 consecutive cases. Updates Surg. 2019;71(4):705–710. doi: 10.1007/s13304-019-00647-y [DOI] [PubMed] [Google Scholar]
- 35.Park I, Her N, Choe JH, Kim JS, Kim JH. Management of chyle leakage after thyroidectomy, cervical lymph node dissection, in patients with thyroid cancer. Head Neck. 2018;40(1):7–15. doi: 10.1002/hed.24852 [DOI] [PubMed] [Google Scholar]
- 36.Won HR, Chang JW, Kang YE, Kang JY, Koo BS. Optimal extent of lateral neck dissection for well-differentiated thyroid carcinoma with metastatic lateral neck lymph nodes: a systematic review and meta-analysis. Oral Oncol. 2018;87:117–125. doi: 10.1016/j.oraloncology.2018.10.035 [DOI] [PubMed] [Google Scholar]