Abstract
A rhodium-catalyzed direct insertion of ethylene into a relatively unstrained carbon–carbon bond in 1-indanones is reported, which provides a two-carbon ring expansion strategy for preparing seven-membered cyclic ketones. As many 1-indanones are commercially available and ethylene is inexpensive, this strategy simplifies synthesis of benzocycloheptenones that are valuable synthetic intermediates for bioactive compounds but challenging to prepare otherwise. In addition, the reaction is byproduct-free, redox neutral, and tolerant of a wide range of functional groups, which may have implications on unconventional strategic bond disconnec-tions for preparing complex cyclic molecules.
Ring expansion reactions of carbonyl compounds, such as Baeyer–Villiger oxidation, Beckmann rearrangement and various carbon-insertion reactions, are highly valuable transformations and have been frequently utilized in complex molecule syntheses.1 With a few exceptions, most direct ring expansion reactions could add only a one-atom unit to the existing structures. Compared to the well-established one- carbon homologation methods2 (Scheme 1a), limited approaches are known for direct two-carbon ring expansions of ketones.3 After an accidental discovery in 1974, Proctor elucidated that carbocyclic β-ketoesters could undergo a [2+2] cycloaddition with an activated alkyne, followed by an accelerated retro-4π cyclization, to give two-carbon extended products4 (Scheme 1b). Later, Kuninobu and Takai discovered a similar but efficient rhenium-catalyzed reaction for insertion of terminal alkynes with β-ketoesters,5 though the reaction was not suitable for preparing 7-membered rings. As a mechanistically related transformation, Caubere,6 and Stoltz,7 reported an intriguing two-carbon ring expansion method via benzyne insertion (Scheme 1b).
Scheme 1.
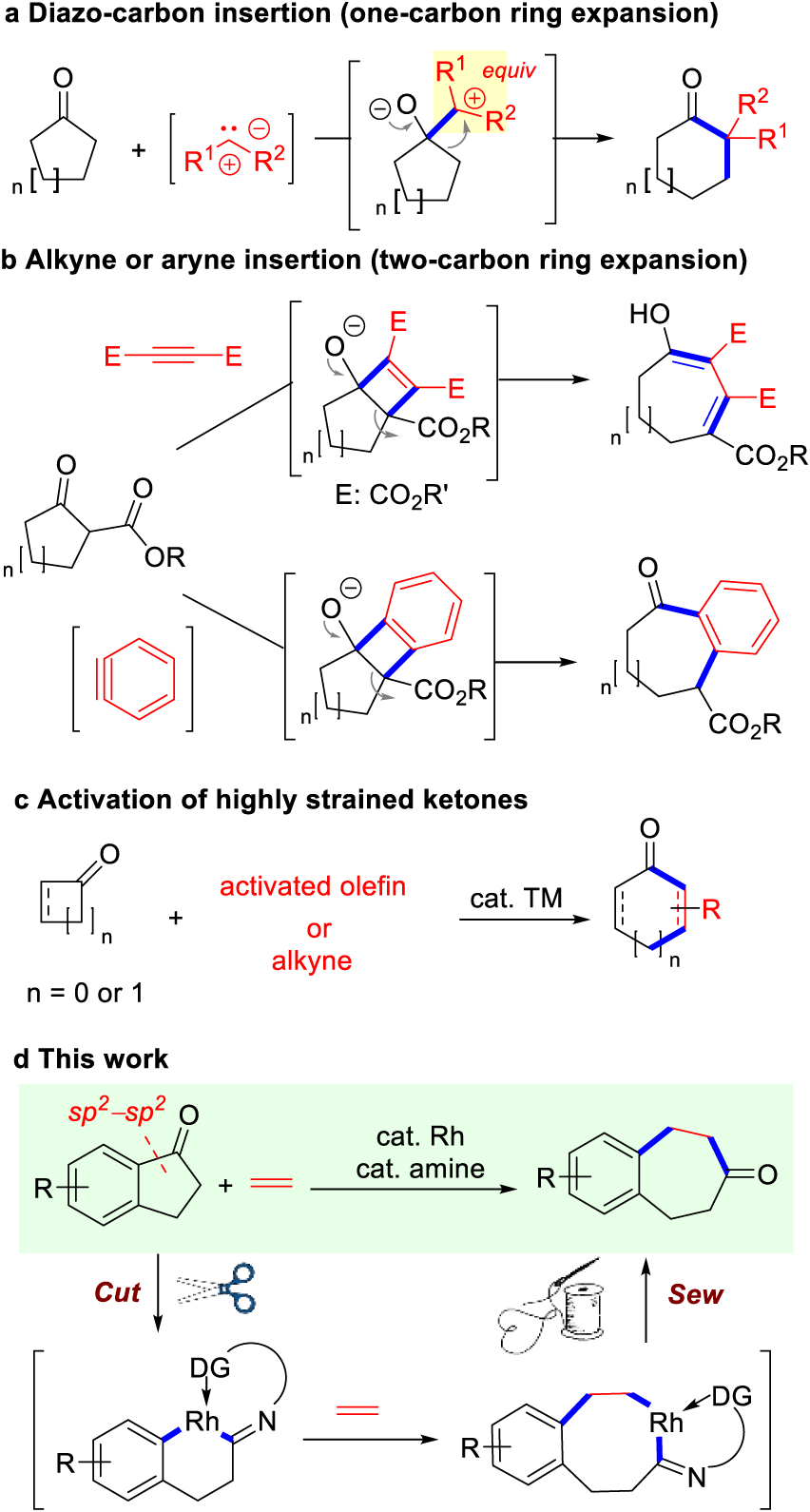
Representative Direct Methods for Ring Expansion of Cyclic Ketones
Alternatively, it could be attractive to directly insert a common unsaturated unit into a cyclic ketone through transition metal-catalyzed C–C activation,8–10 which should offer a straightforward and byproduct-free approach for multiatom ring expansions. The reaction involves oxidative addition of C–C bond to a low-valent transition metal,8b followed by 2π-insertion to give an enlarged metallocycle and C–C reductive elimination. Such a transformation, also known as a “cut-and-sew” process,9b has been extensively demonstrated in strained three- and four-membered ring systems (Scheme 1c).11 However, for unstrained systems,10 the scope of the process has been primarily limited to the use of polar C–CN bonds12 or some special intramolecular reactions.13 In addition, ethylene, as the most highly produced organic compound, may serve as an appealing two-carbon coupling partner; to the best of our knowledge, the “cut-and-sew” reaction using ethylene as a 2π unit has been elusive for either strained or unstrained systems. Moreover, the preference to break the stronger aryl–carbonyl bond (e.g., in 1-indanones), enabled by transition-metal catalysts, could offer complementary selectivity to the conventional1 or radical-mediated C–C cleavage reactions.14 Herein, we describe our preliminary development of a Rh-catalyzed two-carbon ring expansion of 1-indanones via insertion of ethylene into C–C bonds (Scheme 1d).
To explore the proposed ethylene-insertion reaction, unsubstituted 1-indanone (1a) was used as the model substrate, and the Jun’s ketimine directing mode8d,10b was employed for C–C activation. The reaction parameters, including different aminopyridines (serving as the temporary directing group), ligands, solvents, additives, temperature and pressure of ethylene, were carefully optimized (see Table S1). Ultimately, the desired benzocycloheptenone product 1b was obtained in 76% yield from 1-indanone (1a) and ethylene gas (100 psi) in the presence of 5 mol % [Rh(C2H4)2Cl]2, 10 mol % 1,3- bis(2,4,6-trimethylphenyl)imidazol-2-ylidene (IMes), 20 mol % p-toluenesulfonic acid monohydrate (TsOH H2O), 100 mol % 2-amino-3-picoline (DG-1) and 100 mol % H2O in tetrahy- drofuran (THF) (Table S1, entry 1). The only observable side product was the ketone α-C–H insertion product,15 2-ethyl-1- indanone (1c), which was formed in 8% yield. Interestingly, in the absence of the strong σ-donating IMes ligand, the reaction still afforded the desired product 1b in 60% yield, but gave a poorer selectivity with the α-alkylation product formed in 15% yield (Table S1, entry 2). The Rh catalyst, TsOH H2O and the DG-1 are all critical for this transformation, and no desired product can be produced without any of them (Table S1, entries 3–5). When decreasing DG-1 to 30 and 50 mol %, the seven-membered ring product 1b could still be obtained in 54% and 69% yield, respectively (Table S1, entries 6 and 7), suggesting that DG-1 exhibits some catalytic activity. Other temporary directing groups were found either less efficient or less selective (Table S1, entries 8–11), but it is worth noting that simple 2- aminopyridine DG-2 favors forming the ketone α-alkylation product (for more control experiments, see Supporting Information, Section 3).
With the optimized conditions in hand, the substrate scope of this ethylene-insertion reaction was then explored (Chart 1). First, C6-substituted 1-indanone substrates were tested. The electronic property of the 6-substituent only had a marginal influence on this reaction, as 1-indanones bearing either electron-donating (2a–7a) or -withdrawing groups (8a–15a) all gave comparable results to the model substrate 1a. A series of functional groups, including free phenol (6a), sulfonamide (7a), chloride (10a), ester (14a), methyl ketone (15a), silyl (16a) and free hydroxyl group (17a), are tolerated. Besides, substrates containing aryl bromide (11a) and boronate (18a), which are generally reactive moieties in transition-metal catalysis, still afforded the desired products in moderate yields. Notably, for the substrate that contains two ketone carbonyls (15a), C–C activation occurred exclusively at the indanone site. 1-Indanones bearing substituents at the 4- or 5-position exhibited similar reactivity, yielding the corresponding benzocycloheptenones in 52—75% yields (19a–25a). As expected, substitutions at the 7- position resulted in lower reactivity due to the increased steric hindrance around the carbonyl functionality (26a). In addition, several disubstituted 1-indanones (27a–31a) or naphthyl-fused cyclopentanone (32a) proved to be competent substrates, affording the desired seven-membered ring products in moderate to good yields.
Chart 1. Scope of the Ethylene-Insertion Reactiona.
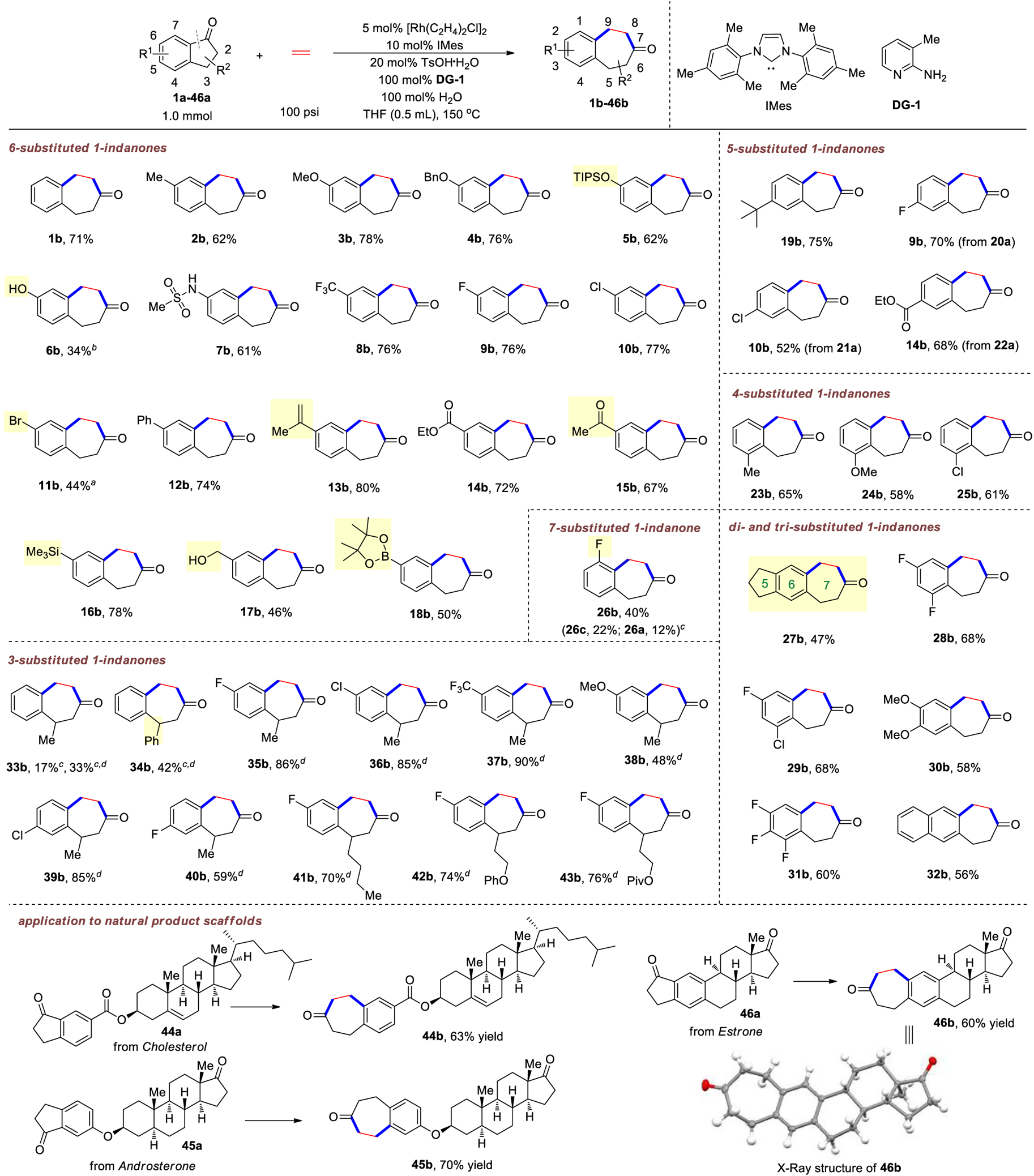
aUnless otherwise noted, all the reactions were carried out on 1.0 mmol scale in 72 h under the standard conditions and yields are of material isolated by silica gel chromatography. bThe reaction was carried out at 130 °C. cYields were determined by 1H nuclear magnetic resonance (NMR) using 1,1,2,2-tetrachloroethane as the internal standard. dIn the absence of IMes ligand and use of 50 mol % water. For details, see Supporting Information.
After examining the steric and electronic influence of the arene part on reactivity, the substitution effect at the aliphatic positions of 1-indanones was next investigated. 3-Methyl-1- indanone (33a) showed substantially reduced reactivity under the standard conditions; however, the yield could be improved in the absence of the IMes ligand with a reduced amount of water (50 mol %). Under these new reaction conditions, 3- phenyl 1-indanone (34a) afforded the desired product in 42% yield. Gratifyingly, the reaction efficiency was significantly improved when substrates containing an additional substituent on the benzene ring (35a–40a), though the exact reason is unclear. For example, 6-trifluoromethyl-3-methyl-1-indanone (37a) produced the desired product (37b) in 90% yield. Besides methyl and phenyl groups, other alkyl substituents at the 3- position were also tolerated (41b–43b). Unsurprisingly, substitution at the α-position (C2) of 1-indanones shut down the reactivity because the steric congestion around the ketone would inhibit forming the imine intermediate. Finally, this reaction can be applied to natural product-derived or tethered indanones. Indanone 44a with an ester-linked cholesterol and 45a with an ether-linked androsterone smoothly participated in this two-carbon ring expansion reaction. Similarly, starting from estrone-fused cyclopentanone 46a, a seven-membered ketone moiety can be efficiently introduced to give a unique 7–6–6–6–5 pentacyclic structure, which was unambiguously confirmed by X-ray crystallography.
From a practical viewpoint, the limits of the reaction condition, i.e., the lowest catalyst loading/temperature that could still afford good synthetic efficiency, were probed. To our delight, when decreasing the loading of the rhodium/IMes from 10 mol % to 3 mol % and decreasing DG-1 from 100 to 50 mol %, the reaction still worked well to give 85% conversion and 65% yield of product 9b (Scheme 2a, entry 2 vs entry 1). Using 5 mol % rhodium/IMes and 50 mol % DG-1, the reaction efficiency almost reached to the level of the original conditions (Scheme 2a, entry 3). Besides, when running at a lower temperature (130 °C), the reaction still proceeded smoothly even with 5 mol % rhodium/ligand (Scheme 2a, entry 4). The reaction is also scalable. On gram scales, good yields could still be obtained with 1-indanones bearing either an electron-donating or -with- drawing group, and DG-1 could be easily recycled (Scheme 2b). Moreover, synthesis of enantiomerically enriched 5-substituted benzocycloheptanone (Scheme 2c) could be achieved using chiral 1-indanone R-35a that was prepared in three steps from commercially available arylboronic acid 47 and α,β-unsaturated ester 48 via asymmetric conjugate addition.16 The slight erosion of enantioselectivity was likely due to the unproductive C–C activation at the C1–C2 position, which led to reversible β- hydrogen elimination.17
Scheme 2.
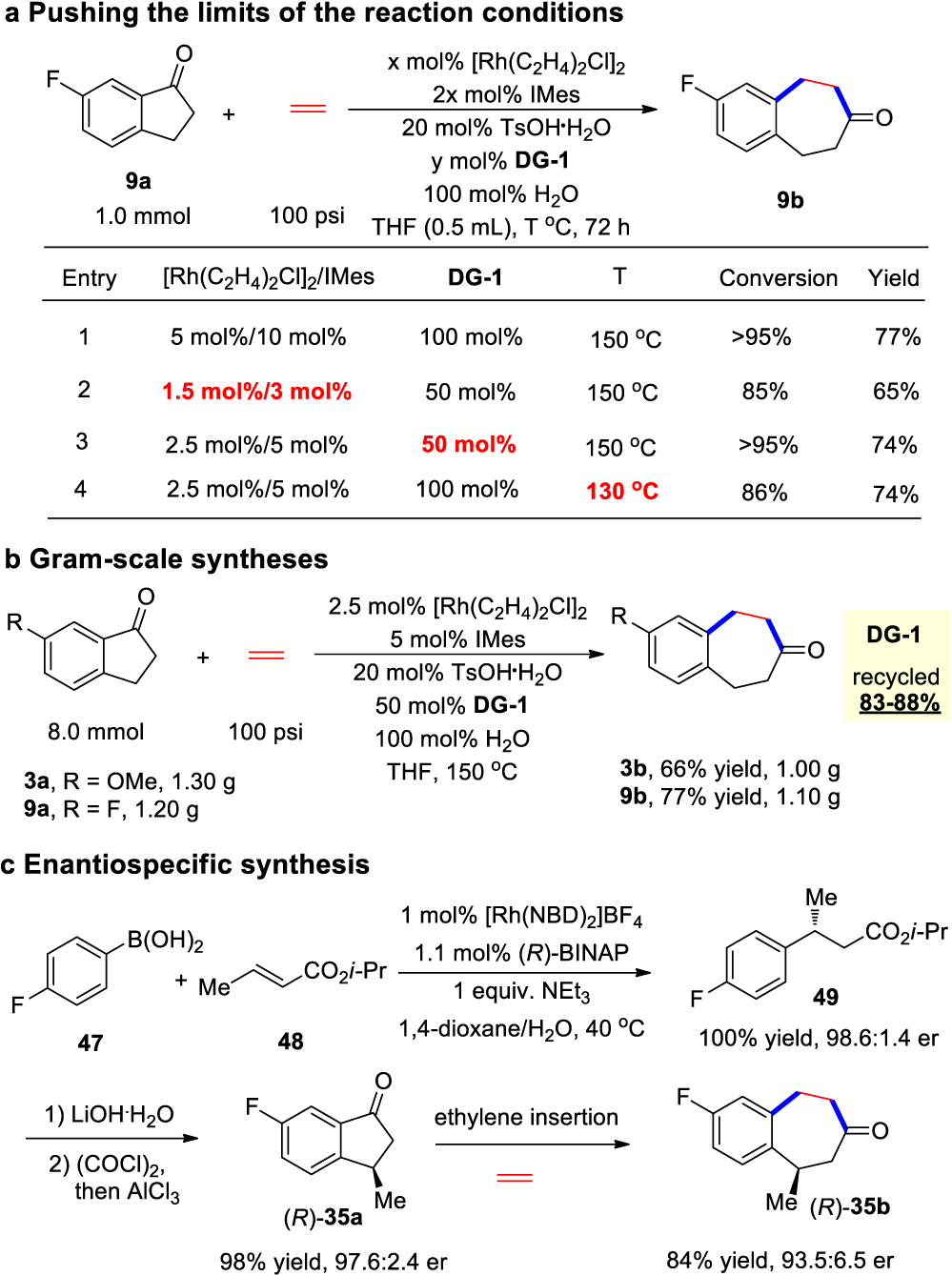
Synthetic Applications
Benzocycloheptenones have been frequently used in the synthesis of bioactive compounds that contain seven-membered rings; however, the conventional approaches for preparing benzocycloheptenones are often inefficient18–20 (Scheme 3). Thus, this two-carbon homologation approach could contribute to shortening the syntheses of those complex pharmaceutical agents. For example, the trifluoromethyl-substituted benzocy- cloheptanone (8b), prepared in a single step using this method, was the key intermediate in the synthesis of antiobesity agent 50 (Scheme 3a). As a comparison, the prior approach required five steps from 2-iodo-4-trifluoromethyl-aniline 51.18 In the second case, CEP-28122 is a highly potent and selective inhibitor of ALIK (anaplastic lymphoma kinase), showing promising antitumor activity in human cancers.19 One key structural motif is the benzocycloheptene moiety, which was synthesized from methoxyl-substituted benzocycloheptenone 24b. The previous synthesis of 24b used three steps from 1-methoxy- 2,3-dimethylbenzene 52 with a 33% overall yield.19 Now, 24b can be prepared straightforwardly via the “cut-and-sew” process from commercially available 4-methoxy-1-indanone 24a (Scheme 3b). The third example involves the synthesis of amine 53, which is a NMDA (N-methyl-D-aspartate) receptor for the potential treatment of various neurological disorders.20 The existing route prepared the key intermediate 10b in three steps from unsubstituted benzocycloheptenone 1b that is either very expensive or requires an additional two or three steps for preparation. In addition, the electrophilic aromatic substitution used in this synthetic route exhibited poor site-selectivity, leading to a low overall yield. Analogously, through the two- carbon homologation, compound 10b was made available in one-step from relatively inexpensive 6-chloro-1-indanone 10a (Scheme 3c). Moreover, simple transformations of the benzocycloheptanone moiety could afford a range of synthetic-cally useful scaffolds, and here symmetrical benzocycloheptenone 1b was used to avoid forming regioisomers. For instance, tetracyclic compound 54 was afforded in 88% yield via Fischer indole synthesis. Conjugated seven-membered enone 55 can be prepared in a good yield via ketone desaturation.21 Treatment with sodium azide under different conditions delivered either eight-membered lactam 56 (77% yield) or tetrazole-fused 6-8-5 tricycle 57 (75% yield), in which the structure of 57 was unambiguously confirmed by X-ray crystallography (Scheme 3d).
Scheme 3.
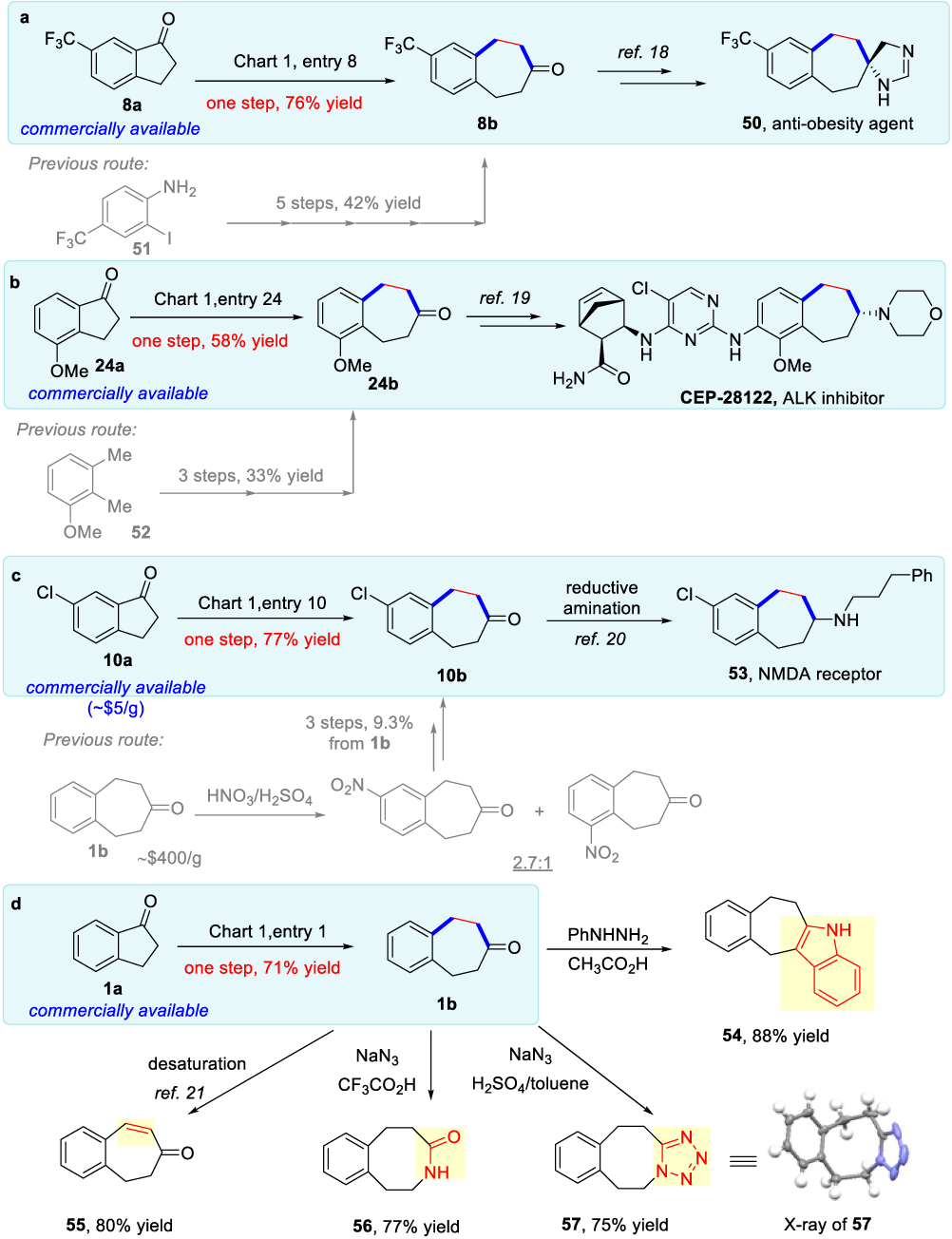
Application Potentials in the Syntheses of Bioactive Molecules
In summary, we disclose a two-carbon ring expansion method that inserts ethylene into relatively unstrained C–C bonds in 1- indanones, which offers a straightforward but strategically distinct approach for preparing benzocycloheptenones. The reaction is chemoselective, scalable and redox-neutral, which could be used to simplify the syntheses of benzocycloheptene-derived bioactive compounds. Efforts on extending the reaction scope to other cyclic ketones and unsaturated coupling partners,22 as well as detailed mechanistic studies, are ongoing.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by NIGMS (R01GM109054). We thank Mr. Ki-Young Yoon for X-ray crystallography and Dr. Jun Zhu for checking the experiment. Chiral Technologies is acknowledged for their generous donation of chiral high performance liquid chromatography columns. We also thank Umicore AG & Co. KG for generous donation of rhodium salts.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b07445.
Experimental procedures; crystallographic data; spectral data (PDF)
Crystallographic data of 46b (CIF)
Crystallographic data of 57 (CIF)
The authors declare no competing financial interest.
REFERENCES
- (1).For recent reviews of ring expansion reactions, see:; (a) Hesse M. Ring Enlargement in Organic Chemistry; VCH: Weinheim, Germany, 1991. [Google Scholar]; (b) Roxburgh CJ Syntheses of Medium Sized Rings by Ring Expansion Reactions. Tetrahedron 1993, 49, 10749–10784. [Google Scholar]; (c) Kant-orowski EJ; Kurth MJ Expansion to Seven-membered Rings. Tetrahedron 2000, 56, 4317–4353. [Google Scholar]; (d) Donald JR; Unsworth WP Ring-expansion Reactions in the Synthesis of Macrocycles and Medium-sized Rings. Chem. - Eur. J 2017, 23, 8780–8799. [DOI] [PubMed] [Google Scholar]
- (2).For a recent review, see:; Candeias NR; Paterna R; Gois PMP Homologation Reaction of Ketones with Diazo Compounds. Chem. Rev 2016, 116, 2937–2981. [DOI] [PubMed] [Google Scholar]
- (3).Dowd P; Zhang W Free Radical-mediated Ring Expansion and Related Annulations. Chem. Rev 1993, 93, 2091–2115. [Google Scholar]
- (4).(a) Lennon M; McLean A; McWatt I; Proctor GR Azabenzocycloheptenones. Some Substitution Reactions in Tetrahy-dro-l-benzazepin-5-ones. J. Chem. Soc., Perkin Trans 1 1974, 1828–1833. [Google Scholar]; (b) Frew AJ; Proctor GR Ring-expansion of Carbocyclic π-Ketoesters with Acetylenic Esters. J. Chem. Soc., Perkin Trans 1 1980, 1245–1250. [Google Scholar]
- (5).Kuninobu Y; Kawata A; Takai K Efficient Catalytic Insertion of Acetylenes into a Carbon–carbon Single bond of Nonstrained Cyclic Compounds under Mild Conditions. J. Am. Chem. Soc 2006, 128, 11368–11369. [DOI] [PubMed] [Google Scholar]
- (6).Caubere P; Guillaumet G; Mourad MS Synthese Generale de Benzocyclenones Non Substituees; Mecanisme de Condensation du Benzyne Surles Enolates de Cetones. Tetrahedron 1973, 29, 1857–1863. [Google Scholar]
- (7).Tambar UK; Stoltz BM The Direct Acyl-alkylation of Arynes. J.Am. Chem. Soc 2005, 127, 5340–5341. [DOI] [PubMed] [Google Scholar]
- (8).(a) Rybtchinski B; Milstein D Metal Insertion into C–C Bonds in Solution. Angew. Chem., Int. Ed 1999, 38, 870–883. [DOI] [PubMed] [Google Scholar]; (b) Souillart L; Cramer N Catalytic C–C bond Activations via Oxidative Addition to Transition Metals. Chem. Rev 2015, 115, 9410–9464. [DOI] [PubMed] [Google Scholar]; (c) Murakami M; Ishida N Potential of Metal-catalyzed C–C Single Bond Cleavage for Organic Synthesis. J. Am. Chem. Soc 2016, 138, 13759–13769. [DOI] [PubMed] [Google Scholar]; (d) Kim D-S; Park W-J; Jun C-H Metal–organic Cooperative Catalysis in C–H and C–C Bond Activation. Chem. Rev 2017, 117, 8977–9015. [DOI] [PubMed] [Google Scholar]
- (9).(a) Fumagalli G; Stanton S; Bower JF Recent Methodologies That Exploit C–C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes. Chem. Rev 2017, 117, 9404–9432. [DOI] [PubMed] [Google Scholar]; (b) Chen P; Billett B; Tsukamoto T; Dong G “Cut and Sew” Transformations via Transition-metal-catalyzed Carbon–Carbon Bond Activation. ACS Catal 2017, 7, 1340–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Murakami M; Amii H; Ito Y Selective Activation of Carbon–Carbon Bonds next to a Carbonyl Group. Nature 1994, 370, 540–541. [Google Scholar]; (b) Jun C-H; Lee H Catalytic Carbon–Carbon Bond Activation of Unstrained Ketone by Soluble Transition-Metal Complex. J. Am. Chem. Soc 1999, 121, 880–881. [Google Scholar]; (c) Chen F; Wang T; Jiao N Recent Advances in Transition-metal-catalyzed Functionalization of Unstrained Carbon–Carbon Bonds. Chem. Rev 2014, 114, 8613–8661. [DOI] [PubMed] [Google Scholar]; (d) Song F; Gou T; Wang B-Q; Shi Z-J Catalytic Activations of Unstrained C–C Bond Involving Organo-metallic Intermediates. Chem. Soc. Rev 2018, 47, 7078–97115. [DOI] [PubMed] [Google Scholar]
- (11).For examples on two-carbon ring expansion via transition metal-catalyzed C–C activation of highly strained ketones with activated olefins or alkynes, see:; (a) Kondo T; Nakamura A; Okada T; Suzuki N; Wada K; Mitsudo T.-a Ruthenium-catalyzed Reconstructive Synthesis of Cyclopentenones by Unusual Coupling of Cyclobutenediones with Alkenes Involving Carbon–Carbon Bond Cleavage. J.Am. Chem. Soc 2000, 122, 6319–6320. [Google Scholar]; (b) Kondo T; Taguchi Y; Kaneko Y; Niimi M; Mitsudo T.-a Ru- and Rh-catalyzed C–C Bond Cleavage of Cyclobutenones: Reconstructive and Selective Synthesis of 2-Pyranones, Cyclopentenes, and Cyclohexanones. Angew. Chem., Int. Ed 2004, 43, 5369–5372. [DOI] [PubMed] [Google Scholar]; (c) Kondo T; Niimi M; Nomura M; Wada K; Mitsudo T.-a Rhodium-catalyzed Rapid Synthesis of Substituted Phenols from Cyclobutenones and Alkynes or Alkenes via C–C Bond Cleavage. Tetrahedron Lett. 2007, 48, 2837–2839. [Google Scholar]; (d) Juliá-Hernández F; Ziadi A; Nishimura A; Martin R Nickel-catalyzed Chemo-, Regio- and Diastereoselective Bond Formation through Proximal C–C Cleavage of Benzocyclobutenones. Angew. Chem, Int. Ed 2015, 54, 9537–9541. [DOI] [PubMed] [Google Scholar]
- (12).Tobisu M; Chatani N Catalytic Reactions Involving the Cleavage of Carbon–Cyano and Carbon–Carbon Triple Bonds. Chem. Soc. Rev 2008, 37, 300–307. [DOI] [PubMed] [Google Scholar]
- (13).Though not ring expansion reactions, some related examples are:; (a) Dreis AM; Douglas CJ Catalytic Carbon–Carbon σ Bond Activation: An Intramolecular Carbo-acylation Reaction with Acylqui-nolines. J. Am. Chem. Soc 2009, 131, 412–413. [DOI] [PubMed] [Google Scholar]; (b) Wentzel MT; Reddy VJ; Hyster TK; Douglas CJ Chemoselectivity in Catalytic C–C and C–H Bond Activation: Controlling Intermolecular Carboacylation and Hydroarylation of Alkenes. Angew. Chem. Int. Ed 2009, 48, 6121–6123. [DOI] [PubMed] [Google Scholar]; (c) Rong Z-Q; Lim HN; Dong G Intramolecular Acetyl Transfer to Olefins via Catalytic C–C Bond Activation of Unstrained Ketones. Angew. Chem. Int. Ed 2018, 57, 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Hu A; Chen Y; Guo J-J; Yu N; An Q; Zuo Z Cerium-Catalyzed Formal Cycloaddition of Cycloalkanols with Alkenes through Dual Photoexcitation. J. Am. Chem. Soc 2018, 140, 13580–13585. [DOI] [PubMed] [Google Scholar]; (b) Zhao K; Yamashita K; Carpenter JE; Sherwood TC; Ewing WR; Cheng PTW; Knowles RR Catalytic Ring Expansions of Cyclic Alcohols Enabled by Proton-Coupled Electron Transfer. J.Am. Chem. Soc 2019, 141, 8752–8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Mo F; Dong G Regioselective Ketone α-Alkylation with Simple Olefins via Dual Activation. Science 2014, 345, 68–72. [DOI] [PubMed] [Google Scholar]
- (16).Itooka R; Iguchi Y; Miyaura N Rhodium-catalyzed 1,4-Addition of Arylboronic Acids to α, β-Unsaturated Carbonyl Compounds: Large Accelerating Effects of Bases and Ligands. J. Org. Chem 2003, 68, 6000–6004. [DOI] [PubMed] [Google Scholar]
- (17).Xia Y; Lu G; Liu P; Dong G Catalytic Activation of Carbon-Carbon Bonds in Cyclopentanones. Nature 2016, 539, 546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Boussard M-F; Guette JP; Wierzbicki M; Beal P; Fournier J; Boulanger M; Della-Zuana O; Duhault J Preparation and Pharmacological Profile of 2-Trifluoromethyl-benzo(8,9)-1,3-diaza-spiro(4,6)-undeca-2,8-diene and Its Enantiomers As New Anti-obesity Agents. Arzneim. Forsch 2000, 50, 1084–1092. [DOI] [PubMed] [Google Scholar]
- (19).Gingrich DE; Lisko JG; Curry MA; Cheng M; Quail M; Lu L; Wan W; Albom MS; Angeles TS; Aimone LD; Haltiwanger RC; Wells-Knecht K; Ott GR; Ghose AK; Ator MA; Ruggeri B; Dorsey BD Discovery of an Orally Efficacious Inhibitor of Anaplastic Lymphoma Kinase. J. Med. Chem 2012, 55, 4580–4593. [DOI] [PubMed] [Google Scholar]
- (20).Gawaskar S; Temme L; Schreiber JA; Schepmann D; Bonifazi A; Robaa D; Sippl W; Strutz-Seebohm N; Seebohm G; Wunsch B Design, Synthesis, Pharmacological Evaluation and Docking Studies of Glun2b-Selective NMDA Receptor Antagonists with a Benzo[7]annulen-7-amine Scaffold. ChemMedChem 2017, 12, 1212–1222. [DOI] [PubMed] [Google Scholar]
- (21).Albrecht S; Al-Lakkis-Wehbe M; Orsini A; Defoin A; Pale P; Salomon E; Tarnus C; Weibel J-M Amino-benzosuberone: A Novel Warhead for Selective Inhibition of Human Aminopeptidase-N/CD13. Bioorg. Med. Chem 2011, 19, 1434–1449. [DOI] [PubMed] [Google Scholar]
- (22).Use of nonethylene olefins or saturated cyclopentanones is challenging at this initial stage, likely due to a slow migratory insertion step.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


