Abstract
Objective
The study aimed to investigate the effects of intraoperative dexmedetomidine on postoperative sleep disturbance for different surgical patients and compare such effects between different dose of dexmedetomidine.
Methods
A total of 7418 patients undergoing nine types of non-cardiac major surgeries were retrospectively studied. Patients were separated into DEX (dexmedetomidine) or Non-DEX (Non-dexmedetomidine) groups based on the use of dexmedetomidine during surgery. The patients who reported they could not fall asleep during the night or woke up repeatedly during the most of the night at the day of the surgery and whose NRS were >6 were defined as cases with severe sleep disturbance. Propensity score matched analysis based on all preoperative baseline data was performed along with logistic regression analysis including different surgery types and dosage of dexmedetomidine use.
Results
In both of the unmatched cohort (OR, 0.49 [95% CI: 0.43–0.56]) and matched cohort (0.49 [95% CI: 0.42–0.58]), the DEX group had a significantly lower incidence of severe sleep disturbance than the Non-DEX group. In the subgroup analysis, for gynecological and urological surgery population, the ORs for DEX-group reached 0.21 (95% CI, 0.13–0.33; P<0.0001) and 0.30 (95% CI,0.19–0.47; P<0.0001), respectively. In addition, low-dose dexmedetomidine (0.2–0.4 μg·kg−1·h−1) showed the greatest effect with an odds ratio of 0.38 (95% CI: 0.31–0.44; P<0.0001), and the incidence of severe sleep disturbance in the low-dose group was significantly lower (11.5% vs. 17.7% vs. 16.5%, P<0.0001) than that in the medium- (0.4–0.6 μg·kg−1·h−1) and high-dose (0.6–0.8 μg·kg−1·h−1) groups.
Conclusion
Intraoperative dexmedetomidine use can significantly decrease the incidence of severe sleep disturbance on the day of surgery for patients undergoing non-cardiac major surgery, and the effects were most significant in patients receiving gynecological and urological surgery. Furthermore, low-dose dexmedetomidine (0.2–0.4 μg·kg−1·h−1) is most effective for prevention of postoperative sleep disturbance.
Keywords: dexmedetomidine, intraoperative use, postoperative sleep disturbance
Introduction
It is a common clinical problem for major surgery patients to experience severe postoperative sleep disturbance including sleep deprivation, disruption, and abnormal architecture, which could be caused by different factors such as anxiety, pain, or maladaptation to the ward environment.1–10 Postoperative sleep disturbance may worsen a patients’ physical condition by increasing the risk of postoperative delirium or cognitive dysfunction, and delaying recovery.3,4,10–12 Numerous attempts have been made to relieve severe sleep disturbances after surgery through eliminating noise and light in surgical wards with blinders or earplugs, the consolidation of patient care interactions.3,4,13–16 Pharmacological intervention such as short-acting non-benzodiazepine17,18 or multimodel analgesia19–21 is other methods used to improve postoperative sleep quality. However, less studies have attempted to use early intervention during the surgery to prevent postoperative sleep disturbance for surgery patients.
Dexmedetomidine, an α2 adrenoreceptor agonist, is usually used for perioperative sedation, the prevention of delirium and assistant analgesia.22–25 Continuous infusion of dexmedetomidine (0.1μg·kg−1·h−1) has been demonstrated to improve patients’ sleep quality in the intensive care unit;26 and one recent study15 has shown that continuous infusion with this dose dexmedetomidine at night can change critical care patients’ sleep structure and improve their sleep quality by increasing stage N2 sleep. Furthermore, one small sample size randomized trial27 showed that intraoperative use of dexmedetomidine (0.2–0.7μg·kg−1·h−1) could improve postoperative subjective sleep quality for the patients undergoing elective laparoscopic abdominal surgeries. These studies indicated that intraoperative use of dexmedetomidine has the potential to decrease the incidence of severe sleep disturbance after major surgery. However, because different types of surgery vary greatly and it is unknown whether intraoperative dexmedetomidine use still have the effects on the improvement of postoperative sleep quality for the patients undergoing other types of non-cardiac major surgery in real-world cohort. In addition, it is also unclear which intraoperative dose of dexmedetomidine is more effective on the improvement of postoperative sleep quality in clinical practice. Thus, based on the above analysis the current study retrospectively aimed to include a large sample size of patients undergoing nine types of non-cardiac major surgery based on a real-world clinical cohort to explore the following two questions, in order to provide better criteria about intraoperative use of dexmedetomidine for clinicians in clinical practice:
Is it common that intraoperative use of dexmedetomidine decreases the incidence of severe sleep disturbance for the patients undergoing all types of non-cardiac major surgery?
Which dose of intraoperative dexmedetomidine use is optimal in decreasing the incidence of severe sleep disturbance for the patients undergoing non-cardiac major surgery of nine types?
Methods
Patients
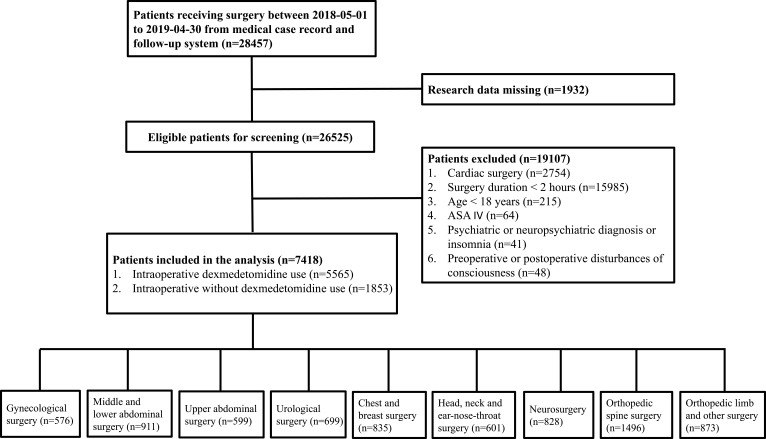
This study was designed as a single-center, retrospective cohort study, and the study was performed according to the STROBE guidelines.28 The study protocol was reviewed and approved by the Ethics Committee of The Second Affiliated Hospital of Army Medical University (Approved ID: 2019-096-01). The study was performed in accordance with Declaration of Helsinki and patient data were guaranteed confidentiality. Because the current study was designed as a retrospective study based on the hospital electronic medical record system, written informed consent was waived. A total of 28,457 patients receiving elective operation were acquired in the electronic medical record and follow-up system from May 1st 2018 to April 30th 2019. Due to missing data (n=1932), 26,525 patients were eventually enrolled (Figure 1). The inclusion criteria included ≥18 years of age, ASA (American Society of Anesthesiologists) grade I–III and time of operation ≥2 hrs. Patients were excluded if they met any of the following criteria: cardiac surgery; psychiatric or neuropsychiatric diagnosis or insomnia before surgery (See appendix List); the disturbances of consciousness. We classified the types of surgery according to the surgical site and population into the following categories: gynecological surgery; middle and lower abdominal surgery; upper abdominal surgery; urological surgery; chest and breast surgery; head, neck and ear-nose-throat surgery; neurosurgery; orthopedic spine surgery; orthopedic limb and other surgery. In the study the upper abdomen surgery includes Gallbladder surgery, Pancreatic surgery, Liver surgery, Exploratory surgery, Cholecystectomy and so on. And the middle and lower abdomen surgery include Gastro-intestinal surgery, Colon surgery, Rectal surgery, Exploratory surgery, Vulva surgery, Anal surgery, cystic resection and so on.
Figure 1.
Study population inclusion summary.
Preoperative Data and Intraoperative Intervention
Demographic data, including sex, age, height, weight, BMI (body mass index), OSAHS (obstructive sleep apnea hypopnea syndrome), smoking and drinking status, were collected from the electronic medical record system. The ASA grade and the preoperative disorders of different systems (including cardiovascular, respiratory, urinary, digestive, nervous and hematological system) were recorded according to the patient’s chief complaint and preoperative examination. Anesthesia methods were divided into general anesthesia or non-general anesthesia, and surgical methods were divided into minimally invasive surgery and non-minimally invasive strategy. In addition, whether patients received postoperative patient-controlled intravenous analgesia provided by the anesthesiology department was also recorded.
After entering the operating room, all patients had routine vital sign monitoring including blood pressure, heart rate, pulse, and oxygen saturation. We collected the data of monitored vital signs for all patients when entering and leaving the operating room. In addition, for the primary aim of the study, patients were grouped according to dexmedetomidine use during surgery based on their medication records. In the hospital, the usage and dose of dexmedetomidine were determined by the anesthesiologist. Dexmedetomidine was uniformly administered by continuous intravenous pumping (0.2 to 0.8 μg·kg−1·h−1) after the induction of general anesthesia or after the non-general anesthesia was completed. The dosage of dexmedetomidine for each patient was calculated using the total amount of dexmedetomidine (μg) divided by weight (kg) and infusion time (hour). According to the calculated results patients were grouped into low- (0.2–0.4 μg·kg−1·h−1), medium- (0.4–0.6 μg·kg−1·h−1) and high-dose group (0.6–0.8 μg·kg−1·h−1).
Postoperative Follow-Up Data Collection
In the department of anesthesiology at The Second Affiliated Hospital of Army Medical University, a postoperative visiting team was established for surgery patients’ follow-up since April 2018. All surgery patients were followed from 08:00 to 10:00 (1st interview) and 16:00 to 18:00 (2nd interview) one day after surgery. Investigators used NRS (the numeric rating scale: an 11-point scale, where 0 indicates the best possible sleep and 10 indicates the worst possible sleep) and recorded the patient’s self-reports to assess subjective sleep quality of the patients on the night of surgery during the 1st interview. If the patients reported that they could not fall asleep during the night or woke up repeatedly during most of the night and the NRS of sleep quality >6, the patient was labeled as severe sleep disturbance. In addition, the investigator asked and recorded the cause of the patient’s sleep disturbance.
Investigators also evaluated the patients’ pain intensity (numeric rating scale score: “0” for painless and “10” for unbearable pain) at the 1st and 2nd interviews, and if the patient presented with a numeric rate scale score >3 within 24 hrs after the operation, they were classified as an inadequate analgesia case.29 In addition, the presence of nausea and vomiting, anal exsufflation, and whether patients could independently participate in off-bed activity were recorded for all surgery patients within 24 hrs after surgery.
Statistical Analysis
In the study incidence of severe postoperative sleep, disturbance was considered as the primary endpoint. In the preliminary analysis based on the patient population undergoing noncardiac major surgery from our hospital, we found that the incidence of severe postoperative sleep disturbance for Non-DEX patients was about 25%. We hypothesized that intraoperative DEX use can decrease the incidence by 10%. According to the design of comparison in rate difference between two groups, based on a significance level of 0.05, power of 0.99, the required minimum sample size for each group was determined to be 583 individuals using the sample size calculation software PASS, version 11.0 (NCSS, Kaysville, UT).
All data were assessed and analyzed using SPSS 22.0 and R statistical software by an experienced statistician. A two-sided P-value <0.05 was considered statistically significant. Continuous variables are presented as mean (standard deviation), and categorical variables are presented as counts (percentage). Patients were grouped according to dexmedetomidine use (DEX group and Non-DEX group) during the surgery. Based on 6.7% of missing data, we used traditional statistical methods and did not consider imputation techniques in the data analysis. The normal or skewed distributions of continuous variables were determined according to the Kolmogorov–Smirnov test, and Student’s t-tests or Mann–Whitney tests were accordingly performed to compare the difference between the DEX and Non-DEX group. The χ2 test was used to compare the categorical variables between the two groups. Relative risks with 95% confidence intervals were calculated for the postoperative outcomes.
Given the potential differences between the baseline data of the DEX and Non-DEX group, we also performed a propensity score analysis to further compare the effect of intraoperative dexmedetomidine use on postoperative sleep quality. Propensity score was calculated for all baseline variables: sex, age, height, weight, BMI, age group (≤45 years, 45–60 years, ≥60 years), BMI group (≤18.5 kg/m2, 18.5–24 kg/m2, ≥24 kg/m2), smoking (yes/no), drinking (yes/no), OSAHS (yes/no), ASA grade, surgery type, minimally invasive surgery (yes/no), general anesthesia (yes/no), with or without a cardiovascular system disorder, respiratory system disorder, urinary system disorder, digestive system disorder, nervous system disorder, hematological system disorder, and whether or not they were receiving patient-controlled intravenous analgesia. We performed matching using the 1:1 nearest neighbor method without replacement under a logit model, which yielded 1853 patients in the DEX group matched with 1853 patients in the Non-DEX group. Comparisons of baseline data and outcomes between the two groups were also performed as a non-matched cohort.
In addition, subgroup analysis was performed according to the different surgery types the patients received. The presentation of severe postoperative sleep disturbance was considered a dependent outcome variable. Logistic regression analysis using enter model was performed and all baseline variables were included in the model. Odds ratios with 95% confidence intervals were calculated for dexmedetomidine use in the different population. Difference in incidences of severe postoperative sleep disturbance between patients with low-dose dexmedetomidine (0.2–0.4 μg·kg−1·h−1) and those with medium-dose (0.4–0.6 μg·kg−1·h−1) and high-dose (0.6–0.8 μg·kg−1·h−1) was compared using χ2 test. Logistic analysis with an enter model was also performed to explore the risk factors for severe postoperative sleep disturbance incidence. Dosage of dexmedetomidine (low-dose, medium-dose, high-dose and no dexmedetomidine use) and all the above baseline variables were included.
Results
Baseline Data
Demographic and preoperative data for all included patients are presented in Table 1. In the unmatched cohort, we identified 5565 patients in the DEX group and compared them with 1853 patients in the Non-DEX group (Figure 1). As shown in Table 1, a significant difference between DEX and Non-DEX groups was found in the percentage of males (50.0% vs. 43.0%; P<0.0001), age (52.61±13.83 vs. 53.61±14.12; P=0.008), smoking status (12.1% vs. 9.7%;P=0.005), drinking status (3.3% vs. 1.9%; P=0.002), respiratory system disorder (21.8% vs. 17.5%; P<0.0001), nervous system disorder (15.5% vs. 11.6%; P<0.0001), cardiovascular system disorder (24.6% vs. 27.4%; P=0.014), general anesthesia (91.1% vs. 95.0%; P<0.0001) and surgery type (P<0.0001). In the matched cohort, the analysis compared 3706 patients – 1853 patients in the DEX group and 1853 patients in the Non-DEX group (Table 1). There were no significant differences in most of the demographic and baseline data between the two groups. However, significant differences in the distributions of surgery type between the DEX and Non-DEX group were found (P=0.003).
Table 1.
Demographic and Clinical Characteristics at Baseline
| Unmatched Cohort | Matched Cohort | ||||||
|---|---|---|---|---|---|---|---|
| DEX Group (n= 5565) | Non-DEX (n=1853) | P value | DEX Group (n=1853) | Non-DEX (n=1853) | Standardized Differences | P value | |
| Male; n(%) | 2783(50.0) | 797(43.0) | <0.0001 | 795(42.9) | 797(43) | 0.002 | 0.974 |
| Age; year | 52.61±13.83 | 53.61±14.12 | 0.008 | 53.40±13.55 | 53.61±14.12 | 0.015 | 0.657 |
| Age group; n(%) | 0.414 | 0.543 | |||||
| ≤45 year | 970(17.4) | 311(16.8) | 287(15.5) | 311(16.8) | 0.035 | ||
| 45 to 60 year | 2682(48.2) | 874(47.2) | 880(47.5) | 874(47.2) | 0.007 | ||
| ≥60 year | 1913(34.4) | 688(36.0) | 686(37) | 668(36) | 0.020 | ||
| Height; cm | 159.71±15.54 | 159.13±13.42 | 0.153 | 159.74±8.30 | 159.83±8.24 | 0.011 | 0.750 |
| Weight; kg | 61.61±11.86 | 61.36±11.00 | 0.430 | 61.45±10.63 | 61.60±10.32 | 0.014 | 0.667 |
| BMI; kg/m2 | 23.9±3.5 | 24.1±3.4 | 0.178 | 24.04±3.50 | 24.07±3.41 | 0.010 | 0.772 |
| BMI group; n(%) | 0.142 | 0.265 | |||||
| ≤18.5 kg/m2 | 244(4.4) | 62(3.3) | 72(3.9) | 62(3.4) | 0.029 | ||
| 18.5 to 24 kg/m2 | 2712(48.7) | 921(49.7) | 875(47.2) | 921(49.7) | 0.050 | ||
| ≥24 kg/m2 | 2609(46.9) | 870(47.0) | 906(48.9) | 870(47) | 0.039 | ||
| Smoking status; n(%) | 672(12.1) | 179(9.7) | 0.005 | 189(10.2) | 179(9.7) | 0.018 | 0.621 |
| Drinking status; n(%) | 185(3.3) | 35(1.9) | 0.002 | 39(2.1) | 35(1.9) | 0.015 | 0.725 |
| OSAHS; n(%) | 322(5.8) | 86(4.6) | 0.061 | 84(4.5) | 86(4.6) | 0.005 | 0.937 |
| ASA grade; n(%) | 0.067 | 0.071 | |||||
| I | 28(0.5) | 17(0.9) | 6(0.3) | 17(0.9) | 0.076 | ||
| II | 3635(65.3) | 1177(63.5) | 1186(64) | 1177(63.5) | 0.010 | ||
| III | 1902(34.2) | 659(35.6) | 661(35.7) | 659(35.6) | 0.002 | ||
| Surgery type; n(%) | <0.0001 | 0.003 | |||||
| Gynecological surgery | 337(6.1) | 239(12.9) | 177(9.6) | 239(12.9) | 0.110 | ||
| Middle and lower abdominal surgery | 650(11.7) | 261(14.1) | 213(11.5) | 261(14.1) | 0.078 | ||
| Upper abdominal surgery | 454(8.2) | 145(7.8) | 161(8.7) | 145(7.8) | 0.031 | ||
| Urological surgery | 561(10.1) | 138(7.4) | 167(9) | 138(7.4) | 0.057 | ||
| Chest and breast surgery | 684(12.3) | 151(8.1) | 187(10.1) | 151(8.1) | 0.068 | ||
| Head, neck and ENT surgery | 434(7.8) | 167(9.0) | 159(8.6) | 166(9) | 0.013 | ||
| Neurosurgery | 662(11.9) | 166(9.0) | 168(9.1) | 161(8.7) | 0.013 | ||
| Orthopedic spine surgery | 1106(19.9) | 390(21.0) | 431(23.3) | 396(21.4) | 0.045 | ||
| Orthopedic limb and other surgery | 677(12.2) | 196(10.6) | 190(10.3) | 196(10.6) | 0.011 | ||
| Minimally invasive surgery; n(%) | 1864(33.5) | 652(35.2) | 0.183 | 641(34.6) | 652(35.2) | 0.013 | 0.730 |
| General anesthesia; n(%) | 5071(91.1) | 1760(95.0) | <0.0001 | 1758(94.9) | 1760(95) | 0.005 | 0.940 |
| Cardiovascular system disorder; n(%) | 1367(24.6) | 508(27.4) | 0.014 | 496(26.8) | 508(27.4) | 0.015 | 0.684 |
| Respiratory system disorder; n(%) | 1213(21.8) | 325(17.5) | <0.0001 | 340(18.3) | 325(17.5) | 0.021 | 0.550 |
| Urinary system disorder; n(%) | 1046(18.8) | 378(20.4) | 0.129 | 373(20.1) | 378(20.4) | 0.007 | 0.870 |
| Digestive system disorder; n(%) | 1463(26.3) | 465(25.1) | 0.310 | 487(26.3) | 465(25.1) | 0.027 | 0.430 |
| Nervous system disorder; n(%) | 861(15.5) | 215(11.6) | <0.0001 | 198(10.7) | 215(11.6) | 0.029 | 0.404 |
| Hematological system disorder; n(%) | 241(4.3) | 73(3.9) | 0.469 | 92(5) | 73(3.9) | 0.050 | 0.152 |
| Received PCIA; n(%) | 2759(49.6) | 899(48.5) | 0.428 | 881(47.5) | 899(48.5) | 0.019 | 0.576 |
Abbreviations: BMI, Body Mass Index; ASA, American Society of Anesthesiologists; OSAHS, Obstructive sleep apnea hypopnea syndrome; ENT, ear-nose-throat; PCIA, patient controls intravenous analgesia.
Comparisons of Outcomes Between the DEX and Non-DEX Groups
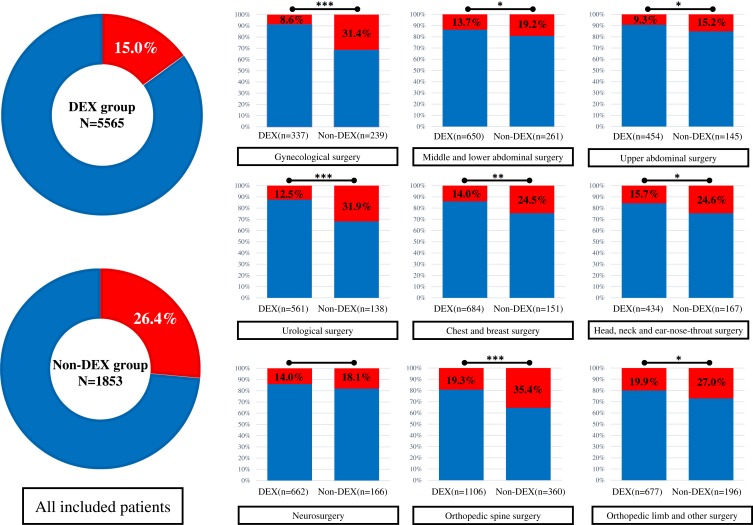
Intraoperative and postoperative data are presented in Table 2. In the unmatched cohort, the patients in the DEX group presented a significantly lower incidence of severe sleep disturbance on the day of surgery compared to patients in the Non-DEX group (15.0% vs. 26.4%; P<0.0001). The odds ratio for the DEX group was 0.49 (95% CI: 0.43 to 0.56) compared to the Non-DEX group. Fewer patients in the DEX group were able to participate in off-bed activity at 24 hrs after surgery compared to the Non-DEX group (8.6% vs. 13.2%; P<0.0001). In addition, no significant difference was found between the two groups with respect to surgery time, or postoperative nausea and vomiting, anal exsufflation, and inadequate analgesia at 24 hrs after surgery.
Table 2.
Intraoperative and Postoperative Clinical Outcomes
| Outcomes | Unmatched Cohort | Matched Cohort | ||||
|---|---|---|---|---|---|---|
| DEX Group (n= 5565) | Non-DEX (n=1853) | P value | DEX Group (n=1853) | Non-DEX (n=1853) | P value | |
| Surgery time; hour | 3.19±1.78 | 3.12±3.35 | 0.246 | 3.17±1.17 | 3.04±1.21 | 0.002 |
| Severe sleep disturbance at the day of the surgery; n(%) | 835 (15.0) | 490(26.4) | <0.0001 | 280(15.1) | 490(26.4) | <0.0001 |
| PONV during 24 hrs after surgery; n(%) | 537(9.6) | 192(10.4) | 0.373 | 184(9.9) | 192(10.4) | 0.703 |
| Off-bed activity at 24 hrs after surgery; n(%) | 478(8.6) | 245(13.2) | <0.0001 | 191(10.3) | 245(13.2) | 0.007 |
| Anal exsufflation at 24 hrs after surgery; n(%) | 1790(32.2) | 576(31.1) | 0.390 | 579(31.3) | 576(31.1) | 0.943 |
| Inadequate analgesia during 24 hrs after surgery; n(%) | 961(17.3) | 302(16.3) | 0.336 | 294(15.9) | 302(16.3) | 0.754 |
Abbreviation: PONV, Postoperative nausea and vomiting.
After matching, the results showed that patients in the DEX group also presented with a lower incidence of severe sleep disturbance on the day of surgery compared with the patients in the Non-DEX group (15.1% vs. 26.4%; P<0.0001) and the odds ratio for the DEX group was 0.49 (95% CI: 0.42 to 0.58). Less patients in the DEX group were able to participate in off-bed activity at 24 hrs after surgery compared to the Non-DEX group (10.3% vs. 13.2%; P=0.007). Surgery time in the DEX group was longer compared to the Non-DEX group (3.17±1.17 vs. 3.04±1.21; P=0.002). No difference was found in any other outcomes between the two groups.
Subgroup Analysis
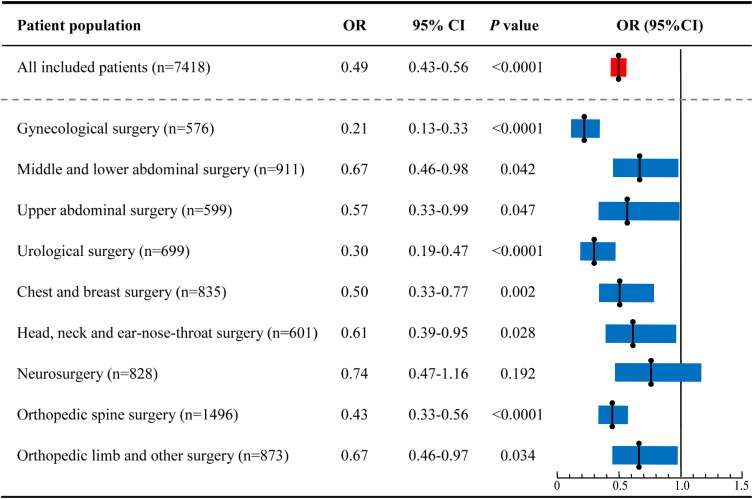
The incidence of severe sleep disturbance on the day of surgery for the different surgery populations is shown in Figure 2. The total incidence of severe sleep disturbance in the DEX group was lower than the Non-DEX group (15.0% vs. 26.4%; P<0.0001). In the subgroup analysis, for all patients except for those undergoing neurosurgery, the incidence of severe sleep disturbance in the DEX group was significantly lower than the Non-DEX group. We calculated the odds ratio for dexmedetomidine use on severe postoperative sleep disturbance for the different surgical populations using a logistic regression model including all baseline factors, and the results are shown in Figure 3. For gynecological surgery the odds ratio was 0.21 (95% CI: 0.13–0.33; P<0.0001), 0.67 for middle and lower abdominal surgery (95% CI:0.46–0.98; P=0.042), 0.57 for upper abdominal surgery (95% CI:0.33–0.99; P=0.047), 0.30 for urological surgery (95% CI:0.19–0.47; P<0.0001), 0.50 for chest and breast surgery (95% CI:0.33–0.77; P=0.002), 0.61 for head, neck, and ear-nose-throat surgery (95% CI:0.39–0.95; P=0.028), 0.43 for orthopedic spine surgery (95% CI:0.33–0.56; P<0.0001), and 0.67 for orthopedic limb and other surgeries (95% CI:0.46–0.97; P=0.034). For neurosurgery, the incidence of severe sleep disorder was 14.0% in the DEX group and 18.1% in the Non-DEX group with an odds ratio of 0.74 (95% CI:0.47–1.16; P=0.192).
Figure 2.
The incidence of severe sleep disturbance for different surgery population in DEX group and Non-DEX group. *P<0.05; **P<0.01; ***P<0.001.
Figure 3.
Effects of dexmedetomidine use on severe postoperative sleep disturbance for different surgical population. (Enter model logistic regression analysis performed for different surgical population, and demographic factors and clinical characteristics at baseline were also included in the model, and only in neurosurgery population dexmedetomidine use was not included in the final model).
Abbreviations: OR, odds ratio; CI, confidence interval.
Comparison the Effects Between Different Dosage of Dexmedetomidine
The logistic analysis for severe postoperative sleep disturbance is shown in Table 3. Dosage of dexmedetomidine, age group, BMI group, smoking, drinking, surgery type, digestive system disorder, and hematological system disorder was identified as factors affecting the incidence of severe postoperative sleep disturbance. For dexmedetomidine dosage 0.2–0.4μg·kg−1·h−1 the odds ratio was 0.38 (95% CI:0.31–0.44; P<0.0001), 0.59 for 0.4–0.6μg·kg−1·h−1 (95% CI:0.50–0.69; P<0.0001) and 0.57 for 0.6–0.8μg·kg−1·h−1 (95% CI:0.47–0.69; P<0.0001). The incidence of severe postoperative sleep disturbance in the low-dose group was 11.5% (249/2161), and it was lower than that in medium- (17.7%[367/2077]; P<0.0001) and high-dose group (16.5%[219/1327]; P<0.0001).
Table 3.
Logistic Analysis for Severe Postoperative Sleep Disturbance on the Day of Surgery
| Factors | Wald | P value | OR | 95% CI |
|---|---|---|---|---|
| Dosage group (Ref. Non-DEX) | 0.135.44 | <0.0001 | ||
| 0.2–0.4μg·kg−1·h−1 | 129.58 | <0.0001 | 0.38 | 0.31 to 0.44 |
| 0.4–0.6μg·kg−1·h−1 | 42.73 | <0.0001 | 0.59 | 0.50 to 0.69 |
| 0.6–0.8μg·kg−1·h−1 | 35.75 | <0.0001 | 0.57 | 0.47 to 0.69 |
| Sex (Ref. Female) | 4.73 | 0.303 | 0.86 | 0.75 to 0.99 |
| Age group(Ref. 45 to 60 year) | 7.72 | 0.021 | ||
| ≥60 year | 7.4 | 0.006 | 0.82 | 0.70 to 0.94 |
| ≤45 year | 0.02 | 0.895 | 0.99 | 0.83 to 1.18 |
| BMI group (Ref. 18.5 to 24 kg/m2) | 14.59 | 0.001 | ||
| ≤18.5 kg/m2 | 8.68 | 0.003 | 0.55 | 0.37 to 0.82 |
| ≥24 kg/m2 | 4.13 | 0.042 | 1.14 | 1.01 to 1.30 |
| Smoking status (Ref. Yes) | 4.65 | 0.031 | 0.79 | 0.63 to 0.98 |
| Drinking status (Ref. Yes) | 25.24 | <0.0001 | 8.41 | 3.66 to 19.30 |
| ASA grade (Ref. ASA III) | 9.65 | 0.008 | ||
| I | 9.35 | 0.002 | 2.72 | 1.43 to 5.16 |
| II | 1.53 | 0.216 | 1.11 | 0.94 to 1.30 |
| Surgery type (Ref. Middle and lower abdominal surgery) | 30.28 | <0.0001 | ||
| Gynecological surgery | 1.40 | 0.236 | 0.82 | 0.59 to 1.14 |
| Upper abdominal surgery | 1.84 | 0.175 | 0.79 | 0.56 to 1.11 |
| Urological surgery | 0.31 | 0.576 | 1.10 | 0.80 to 1.51 |
| Chest and breast surgery | 0.27 | 0.603 | 0.93 | 0.70 to 1.24 |
| Head, neck and ENT surgery | 1.29 | 0.255 | 0.84 | 0.61 to 1.14 |
| Neurosurgery | 1.68 | 0.195 | 0.79 | 0.55 to 1.13 |
| Orthopedic spine surgery | 3.74 | 0.053 | 1.35 | 1.00 to 1.82 |
| Orthopedic limb and other surgery | 2.78 | 0.96 | 1.31 | 0.95 to 1.80 |
| Minimally invasive surgery (Ref. Yes) | 2.46 | 0.117 | 1.14 | 0.97 to 1.34 |
| General anesthesia (Ref. Yes) | 1.00 | 0.317 | 0.87 | 0.67 to 1.14 |
| Cardiovascular system disorder (Ref. Yes) | 2.08 | 0.149 | 0.89 | 0.75 to 1.05 |
| Respiratory system disorder (Ref. Yes) | 0.99 | 0.320 | 1.09 | 0.92 to 1.31 |
| Urinary system disorder (Ref. Yes) | 0.03 | 0.873 | 1.02 | 0.84 to 1.22 |
| Digestive system disorder (Ref. Yes) | 19.56 | <0.0001 | 1.48 | 1.24 to 1.76 |
| Nervous system disorder (Ref. Yes) | 2.11 | 0.146 | 1.18 | 0.94 to 1.48 |
| Hematological system disorder (Ref. Yes) | 12.27 | <0.0001 | 2.10 | 1.39 to 3.18 |
| Received PCIA (Ref. Yes) | 0.43 | 0.511 | 1.07 | 0.88 to 1.30 |
Abbreviations: OR, odds ratio; CI, confidence interval; Ref., Reference; BMI, Body Mass Index; ASA, American Society of Anesthesiologists; ENT, ear-nose-throat; PCIA, patient controls intravenous analgesia.
Discussion
To the best of our knowledge, this is the first real-world cohort study to evaluate the role of intraoperative dexmedetomidine use to improve postoperative sleep quality for patients undergoing non-cardiac major surgery. Here, we reported the following findings. First, intraoperative use of dexmedetomidine was shown to decrease the incidence of severe sleep disturbance on the day of surgery in patients after undergoing non-cardiac major surgery. Second, the improved sleep quality was observed in almost all types of surgery, especially in gynecological and urological. Third, the effects are most significant in patients receiving low-dose dexmedetomidine (0.2–0.4 μg·kg−1·h−1) during the surgery compared to medium- and high-dose.
The current study demonstrated that intraoperative use of dexmedetomidine could decrease the incidence of severe sleep disturbance in patients undergoing non-cardiac major surgery. Based on this real-world cohort we enrolled a large sample size population including different anesthesia methods and surgery types. Furthermore, we also performed a propensity score and subgroup analysis based on different surgery types to validate the findings. All analyses uniformly showed that intraoperative dexmedetomidine use had the same tendency to improve postoperative sleep quality. Accordingly, we concluded that intraoperative use of dexmedetomidine could significantly decrease the incidence of severe sleep disturbance in patients after undergoing non-cardiac major surgery, and this finding may be extended to routine clinical practice.
However, it should be noted that the effects of intraoperative dexmedetomidine use for sleep quality improvement differed for different surgery types based on the subgroup analysis. The most significant effects were observed in the patients receiving gynecological, urological and orthopedic spine surgery with odds ratios of 0.21, 0.30 and 0.43, respectively. The clear effects may be attributed to the higher incidence of postoperative sleep disturbance in female, urological and orthopedic spine surgery patients (all incidences of severe postoperative sleep disturbance in the Non-DEX group for these surgery patients were more than 30%). Conversely, we found that there was no statistically significant difference in the incidence of sleep disturbance between the DEX and Non-DEX group in neurosurgery patients (the incidence of severe postoperative sleep disturbance in the Non-DEX group was approximately 18%). However, while there was no statistical difference, a trend towards improved sleep quality was observed. Thus, the effects of intraoperative use of dexmedetomidine on sleep quality improvement in neurosurgery patients should not be denied based on the current study.
As we know, postoperative pain was one of the most common causes affecting postoperative sleep quality, and this is reported in many previous studies.19–21,30 However, we observed that intraoperative use of dexmedetomidine had no effect on improving postoperative pain, and the incidence of inadequate postoperative analgesia was similar in the DEX and Non-DEX group. Thus, we speculated that postoperative sleep quality improvement induced by intraoperative use of dexmedetomidine might be due to other causes rather than reducing postoperative pain. Some studies31,32 have indicated that the intraoperative use of dexmedetomidine may provide better sedation effects. In addition, one recent study15 has shown that dexmedetomidine (0.1 μg·kg−1·h−1) improves postoperative sleep quality through increasing stage N2 sleep, decreasing stage N1 sleep and avoiding the sleep structure disorder of patients. In the current study, the dosage of intraoperative dexmedetomidine reached 0.2–0.8 μg·kg−1·h−1. Considering the characteristics of long metabolism time and the positive correlation between time and medication duration of dexmedetomidine,33–35 these findings support that the effects of dexmedetomidine might still exist when patients have left the operating room and could, therefore, improve the sleep quality of patients after surgery.
In clinical practice, the intraoperative use of dexmedetomidine was mainly used to assist in sedation, maintaining hemodynamic stability and preventing the patient’s postoperative delirium.22,36–38 However, there was currently a lack of clinical evidence for the intraoperative use of dexmedetomidine in the nine types of non-cardiac major surgery to affect postoperative sleep disturbance. Clinicians seemed to have not noticed and considered this problem in their actual work. Therefore, it could be said that our research project was equivalent to a double-blind study. At the same time, the results of the study showed that intraoperative use of dexmedetomidine did improve the quality of postoperative sleep, reflecting the “Do not treat but cure” effect of dexmedetomidine on improvement of sleep quality. In other words, intraoperative use of dexmedetomidine may have a “pretreatment” effect on the improvement of postoperative sleep quality.
In addition, we also found that that low-dose use of dexmedetomidine (0.2–0.4 μg·kg−1·h−1) showed the greatest improvement in postoperative sleep quality compared to the medium-dose (0.4–0.6 μg·kg−1·h−1) and high-dose (0.6–0.8 μg·kg−1·h−1). One other previous study3 has examined the effect of oxycodone combined with two different dose of dexmedetomidine (2.4 μg·kg−1 vs. 4.8 μg·kg−1) on postoperative sleep quality and also showed that using low-dose dexmedetomidine (2.4 μg·kg−1) after surgery produced the better effects on the improvement of postoperative sleep quality comparing to the high-dose dexmedetomidine (4.8 μg·kg−1). This further supported that low-dose dexmedetomidine use (0.2–0.4 μg·kg−1·h−1) may be optimally recommended for the treatment of postoperative sleep disturbance.
The following limitations should be considered when interpreting our study results. First, this study estimates an average therapeutic effect of intraoperative dexmedetomidine use from a single center and retrospective cohort study, which has its own inherent limitations, e.g., we were able to exclude only diagnosed patients with preoperative insomnia. Thus, a well-designed randomized controlled trial is needed to validate the current finding definitively. Second, the intraoperative use dosage of dexmedetomidine may be different for patients according to different anesthetists and patients may receive different therapies after surgery, which may lead to deviations in results. Finally, the sample size of subgroup analysis based on different surgery types was limited though the overall sample size enrolled in our study was relatively large. Thus, the effects of intraoperative dexmedetomidine use in postoperative sleep quality improvement for different surgery patients need to be further confirmed with larger sample sizes.
Conclusion
Intraoperative use of dexmedetomidine can improve postoperative sleep quality and significantly decrease the incidence of severe sleep disturbance on the day of surgery for patients who have undergone non-cardiac major surgery. The effects of improvement in postoperative sleep quality are the most pronounced in patients undergoing gynecological and urological surgeries. Furthermore, low-dose dexmedetomidine (0.2–0.4 μg·kg−1·h−1) during the surgery has the most significant sleep improvement effect.
Funding Statement
The study was supported by National Key Research and Development Project (No. 2018YFC0117200), and Clinical Research Projects of Second Affiliated Hospital, Army Medical University (No. 2015YLC09 and 2016YLC10).
Abbreviations
DEX, dexmedetomidine; Non-DEX, Non-dexmedetomidine; BMI, Body Mass Index; OSAHS, Obstructive sleep apnea hypopnea syndrome; ASA, American Society of Anesthesiologists; ENT, ear-nose-throat; PCIA, patient controls intravenous analgesia; PONV, Postoperative nausea and vomiting; OR, odds ratio; CI, confidence interval; Ref., Reference.
Data Sharing Statement
The dataset in the current study is available from the corresponding author (email: lh78553@163.com) on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Madsen MT, Rosenberg J, Gogenur I. Actigraphy for measurement of sleep and sleep-wake rhythms in relation to surgery. J Clin Sleep Med. 2013;9(4):387–394. doi: 10.5664/jcsm.2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gogenur I, Wildschiotz G, Rosenberg J. Circadian distribution of sleep phases after major abdominal surgery. Br J Anaesth. 2008;100(1):45–49. doi: 10.1093/bja/aem340 [DOI] [PubMed] [Google Scholar]
- 3.Jiang Z, Zhou G, Song Q, et al. Effect of intravenous oxycodone in combination with different doses of dexmedetomdine on sleep quality and visceral pain in patients after abdominal surgery: a Randomized Study. Clin J Pain. 2018;34(12):1126–1132. doi: 10.1097/AJP.0000000000000645 [DOI] [PubMed] [Google Scholar]
- 4.Su X, Wang DX. Improve postoperative sleep: what can we do? Curr Opin Anaesthesiol. 2018;31(1):83–88. doi: 10.1097/ACO.0000000000000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 2014;18(3):273–282. doi: 10.1016/j.smrv.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Cronin AJ, Keifer JC, Davies MF, King TS, Bixler EO. Postoperative sleep disturbance: influences of opioids and pain in humans. Sleep. 2001;24(1):39–44. doi: 10.1093/sleep/24.1.39 [DOI] [PubMed] [Google Scholar]
- 7.Hillman DR. Postoperative sleep disturbances: understanding and emerging therapies. Adv Anesth. 2017;35(1):1–24. doi: 10.1016/j.aan.2017.07.001 [DOI] [PubMed] [Google Scholar]
- 8.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed). 1985;290(6474):1029–1032. doi: 10.1136/bmj.290.6474.1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes NM, Nield LE, Popel N, et al. Symptoms of disturbed sleep predict major adverse cardiac events after percutaneous coronary intervention. Can J Cardiol. 2014;30(1):118–124. doi: 10.1016/j.cjca.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 10.Kjolhede P, Langstrom P, Nilsson P, Wodlin NB, Nilsson L. The impact of quality of sleep on recovery from fast-track abdominal hysterectomy. J Clin Sleep Med. 2012;8(4):395–402. doi: 10.5664/jcsm.2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans JL, Nadler JW, Preud’homme XA, et al. Pilot prospective study of post-surgery sleep and EEG predictors of post-operative delirium. Clin Neurophysiol. 2017;128(8):1421–1425. doi: 10.1016/j.clinph.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 12.Johns MW, Large AA, Masterton JP, Dudley HA. Sleep and delirium after open heart surgery. Br J Surg. 1974;61(5):377–381. doi: 10.1002/bjs.1800610513 [DOI] [PubMed] [Google Scholar]
- 13.Xie H, Kang J, Mills GH. Clinical review: the impact of noise on patients’ sleep and the effectiveness of noise reduction strategies in intensive care units. Crit Care. 2009;13(2):208. doi: 10.1186/cc7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Guen M, Nicolas-Robin A, Lebard C, Arnulf I, Langeron O. Earplugs and eye masks vs routine care prevent sleep impairment in post-anaesthesia care unit: a randomized study. Br J Anaesth. 2014;112(1):89–95. doi: 10.1093/bja/aet304 [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Cui F, Zhang C, et al. Low-dose dexmedetomidine improves sleep quality pattern in elderly patients after noncardiac surgery in the intensive care unit. Anesthesiology. 2016;125(5):979–991. doi: 10.1097/ALN.0000000000001325 [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg-Adamsen S, Kehlet H, Dodds C, Rosenberg J. Postoperative sleep disturbances: mechanisms and clinical implications. Br J Anaesth. 1996;76(4):552–559. doi: 10.1093/bja/76.4.552 [DOI] [PubMed] [Google Scholar]
- 17.Krenk L, Jennum P, Kehlet H. Postoperative sleep disturbances after zolpidem treatment in fast-track hip and knee replacement. J Clin Sleep Med. 2014;10(3):321–326. doi: 10.5664/jcsm.3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemmer B. The sleep-wake cycle and sleeping pills. Physiol Behav. 2007;90(2–3):285–293. doi: 10.1016/j.physbeh.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 19.Dolan R, Huh J, Tiwari N, Sproat T, Camilleri-Brennan J. A prospective analysis of sleep deprivation and disturbance in surgical patients. Ann Med Surg (Lond). 2016;6:1–5. doi: 10.1016/j.amsu.2015.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knill RL, Moote CA, Skinner MI, Rose EA. Anesthesia with abdominal surgery leads to intense REM sleep during the first postoperative week. Anesthesiology. 1990;73(1):52–61. doi: 10.1097/00000542-199007000-00009 [DOI] [PubMed] [Google Scholar]
- 21.Celik S, Oztekin D, Akyolcu N, Issever H. Sleep disturbance: the patient care activities applied at the night shift in the intensive care unit. J Clin Nurs. 2005;14(1):102–106. doi: 10.1111/j.1365-2702.2004.01010.x [DOI] [PubMed] [Google Scholar]
- 22.Deiner S, Luo X, Lin HM, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg. 2017;152(8):e171505. doi: 10.1001/jamasurg.2017.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim D, Jeong JS, Park H, et al. Postoperative pain control after the use of dexmedetomidine and propofol to sedate patients undergoing ankle surgery under spinal anesthesia: a randomized controlled trial. J Pain Res. 2019;12:1479–1487. doi: 10.2147/JPR.S195745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du Z, Zhang XY, Qu SQ, et al. The comparison of dexmedetomidine and midazolam premedication on postoperative anxiety in children for hernia repair surgery: a randomized controlled trail. Paediatr Anaesth. 2019. doi: 10.1111/pan.13667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skrobik Y, Duprey MS, Hill NS, et al. Low-dose nocturnal dexmedetomidine prevents ICU delirium. a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2018;197(9):1147–1156. doi: 10.1164/rccm.201710-1995OC [DOI] [PubMed] [Google Scholar]
- 26.Su X, Meng Z, Wu X, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–1902. doi: 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]
- 27.Song B, Li Y, Teng X, et al. The effect of intraoperative use of dexmedetomidine during the daytime operation vs the nighttime operation on postoperative sleep quality and pain under general anesthesia. Nat Sci Sleep. 2019;11:207–215. doi: 10.2147/NSS.S225041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenach JC, Kheterpal S, Houle TT. Reporting of observational research in anesthesiology: the importance of the analysis plan. Anesthesiology. 2016;124(5):998–1000. doi: 10.1097/ALN.0000000000001072 [DOI] [PubMed] [Google Scholar]
- 29.Gerbershagen HJ, Rothaug J, Kalkman CJ, et al. Determination of moderate-to-severe postoperative pain on numeric rating scale: a cut-off point analysis four different methods. Br J Anaesth. 2011;107(4):4. doi: 10.1093/bja/aer195 [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Tang R, Zhang R, Jiang Y, Liu Y. Effects of dexmedetomidine administered for postoperative analgesia on sleep quality in patients undergoing abdominal hysterectomy. J Clin Anesth. 2017;36:118–122. doi: 10.1016/j.jclinane.2016.10.022 [DOI] [PubMed] [Google Scholar]
- 31.Arcangeli A, D’Alo C, Gaspari R. Dexmedetomidine use in general anaesthesia. Curr Drug Targets. 2009;10(8):687–695. doi: 10.2174/138945009788982423 [DOI] [PubMed] [Google Scholar]
- 32.Dutta A, Sethi N, Sood J, et al. The effect of dexmedetomidine on propofol requirements during anesthesia administered by bispectral index-guided closed-loop anesthesia delivery system: a Randomized Controlled Study. Anesth Analg. 2019;129(1):84–91. doi: 10.1213/ANE.0000000000003470 [DOI] [PubMed] [Google Scholar]
- 33.Anttila M, Penttila J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003;56(6):691–693. doi: 10.1046/j.1365-2125.2003.01944.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weerink M, Struys M, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913. doi: 10.1007/s40262-017-0507-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Kim BH, Lim K, et al. Pharmacokinetics and pharmacodynamics of intravenous dexmedetomidine in healthy Korean subjects. J Clin Pharm Ther. 2012;37(6):698–703. doi: 10.1111/j.1365-2710.2012.01357.x [DOI] [PubMed] [Google Scholar]
- 36.Wang HM, Shi XY, Qin XR, Zhou JL, Xia YF. Comparison of dexmedetomidine and propofol for conscious sedation in inguinal hernia repair: a prospective, randomized, controlled trial. J Int Med Res. 2017;45(2):533–539. doi: 10.1177/0300060516688408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsujikawa S, Ikeshita K. Low-dose dexmedetomidine provides hemodynamics stabilization during emergence and recovery from general anesthesia in patients undergoing carotid endarterectomy: a randomized double-blind, placebo-controlled trial. J Anesth. 2019;33(2):266–272. doi: 10.1007/s00540-019-02612-w [DOI] [PubMed] [Google Scholar]
- 38.Wu F, Duan H, Xie Y. Preventive effects of dexmedetomidine on renal dysfunction and hemodynamic stability in malignant obstructive jaundice patients during peri-operative period. Med Sci Monit. 2019;25:6782–6787. doi: 10.12659/MSM.916329 [DOI] [PMC free article] [PubMed] [Google Scholar]