Abstract
Purpose
Dose–volume histogram (DVH) measurements have been integrated into commercially available quality assurance systems to provide a metric for evaluating accuracy of delivery in addition to gamma analysis. We hypothesize that tumor control probability and normal tissue complication probability calculations can provide additional insight beyond conventional dose delivery verification methods.
Methods
A commercial quality assurance system was used to generate DVHs of treatment plan using the planning CT images and patient‐specific QA measurements on a phantom. Biological modeling was performed on the DVHs produced by both the treatment planning system and the quality assurance system.
Results
The complication‐free tumor control probability, P +, has been calculated for previously treated intensity modulated radiotherapy (IMRT) patients with diseases in the following sites: brain (−3.9% ± 5.8%), head‐neck (+4.8% ± 8.5%), lung (+7.8% ± 1.3%), pelvis (+7.1% ± 12.1%), and prostate (+0.5% ± 3.6%).
Conclusion
Dose measurements on a phantom can be used for pretreatment estimation of tumor control and normal tissue complication probabilities. Results in this study show how biological modeling can be used to provide additional insight about accuracy of delivery during pretreatment verification.
Keywords: IMRT QA, radiobiological QA, radiobiological verification, radiobiology
1. INTRODUCTION
Intensity‐modulated radiotherapy (IMRT) has improved modern radiation oncology.1, 2, 3, 4, 5 Compared to conventional beam delivery techniques, inverse planning results in unique fluence maps for each beam. Volumetric modulated arc therapy (VMAT) adds to the complexity with gantry rotation, dose rate variation, and motion of multi leaf collimators during treatment. Complexity demands that stringent patient specific quality assurance (QA) measures be implemented to ensure agreement between calculated treatment plans and delivered IMRT dose distributions. A widely adopted QA metric, called gamma index, was developed to evaluate and provide clinical confidence in IMRT treatment plans. The gamma index accounts for both differences in dose and geometry to quantify the agreement between calculated and measured dose.6, 7, 8, 9
The gamma index is used to provide confidence when evaluating accuracy of delivery; however, this analysis does not provide detailed dosimetric information about specific structures as well as hot or cold spots in the target.10, 11 Furthermore, studies have shown that gamma‐based analysis can be insensitive to detect errors or correlate dose errors in anatomic regions of interest.12, 13 Results derived from the usual individualized pretreatment QA tools have not been related with clinically relevant dosimetric errors on patient dose delivery. A more robust QA than the gamma index would be needed to quantify the clinical impact of dose measured prior to treatment in comparison to planned dose distribution, in addition to estimating the radiobiological implications of any dose differences.
A new approach for plan verification compares independently measured dose–volume histograms (DVHs) to that computed by a treatment planning system (TPS). There are commercially available solutions that incorporate dose measurements on phantom with the CT images of patient to compute pretreatment DVH. The capabilities of producing DVH of the Delta4DVH Anatomy 3D QA system (Scandidos, Uppsala), and both MapCHECK 2 and the ArcCHECK with 3DVH system (Sun Nuclear, Melbourne) have been evaluated in previous studies.14, 15, 16 New metrics for IMRT QA verification were explored in a study that utilized the COMPASS system (IBA Dosimetry, Bartlett, Tennessee) to incorporate pretreatment DVH into tumor control probability (TCP) and normal tissue complication probability (NTCP) models.17
TCP provides additional insight to plan quality as it is associated with the clinically observed tumor control rates. Similar association exists between NTCP and radiation‐induced toxicity to organs at risk (OAR). These radiobiological metrics offers accountability for the response of specific tissues to dose and dose per fraction, which is not considered in the gamma index.18 In previous studies, the complication‐free tumor control probability, P +, has demonstrated value in approximating complication rates of patients treated.19
The aim of this work is to demonstrate the value of incorporating P + as a pretreatment verification metric for IMRT plans.
2. MATERIALS AND METHODS
2.1. Patient cohort
Fifty‐four previously treated VMAT patient plans were used in this study. Prescribed doses and fraction schedules were dependent on the treatment site; however, no patients in the cohort received stereotactic body radiotherapy or radiosurgery. The patients were treated for 5 different anatomical sites consisting of 10 brain, 10 head‐neck, 10 lung, 14 pelvis, and 10 prostate patients. There was a variation in the dose, number of fractions, and modalities incorporated in the treatment plans (Table 1). No patients with extreme circumstances such as prosthetic implants or unusual physiological conditions were included in the study. All structures analyzed in this study are summarized in Table 1.
Table 1.
Summary of all structures used in TCP/NTCP comparison.
| Cohort | Dose range [Gy] | Fractions | Modality | Structures for TCP/NTCP comparison |
|---|---|---|---|---|
| Brain | 50.40‐60.00 | 28‐30 | 6X | Brain, brainstem, chiasm, Rt. optic nerve, Lt. optic nerve, PTV |
| Head‐neck | 30.00‐69.96 | 5‐35 | 6X | Rt. parotid, Lt. parotid, mandible, Rt. brachial plexus, Lt. brachial plexus, PTV |
| Lung | 30.00‐60.00 | 3‐30 | 6X, 10X | Esophagus, heart, lung, PTV |
| Pelvis | 34.20‐79.20 | 11‐28 | 6X, 10X | Bladder, rectum, sigmoid, bowel, penile bulb, PTV |
| Prostate | 45.00‐70.20 | 25‐30 | 10X | Bladder, rectum, sigmoid, penile bulb, PTV |
High‐risk and low‐risk PTV were used for head‐neck analysis.
Patient plans were planned in Pinnacle3 TPS (Philips, Koninklijke, Netherlands). The plans were delivered into the Octavius 4D phantom (PTW, Freiburg, Germany), and resulting measurements were compared to the planned dose using conventional gamma analysis. Two criteria used to test the data were dose difference/distance‐to‐agreement (DTA) of 3%/3 mm and 2%/2 mm normalized to 90% of max dose. The institutional criteria for all these plans were 90% pass rate for all pixels at 3%/3 mm normalized to 90% of max dose. 2%/2 mm criteria results are included to examine if simply tightening gamma criteria is indicative of accuracy of delivery.
Phantom measurements were used with the VeriSoft software ver 7.0 (PTW, Freiburg, Germany) to construct delivered DVH by scaling the measurements onto the patient CT set. The patient's DVHs that were originally computed in Pinnacle3 are then exported to perform radiobiological calculations (details below). The workflow is outlined in Fig. 1.
Figure 1.
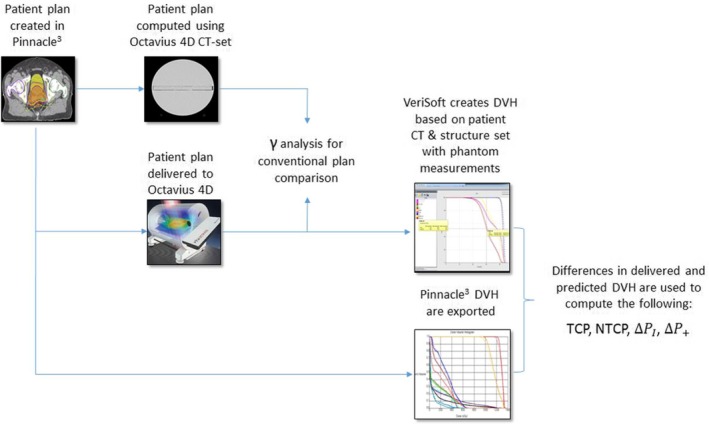
Steps of this study are shown in this workflow diagram.
2.2. Systems used
Plans were delivered using the same Novalis Tx LINAC (Varian Medical Systems, Palo Alto, California) to mitigate differences that could occur from plan delivery with different LINACs due to variations in treatment planning beam models or configurations.
Pinnacle3 TPS was used for calculating patient plans in addition to creating plans to be delivered to the phantom under the same conditions. A 2.5‐mm3 voxel grid resolution was used for TPS calculations. After completing calculation, DICOM (DCM) files consisting of plans, structures, dose, and CT image sets were exported to be used with VeriSoft DVH calculations later. Phantom DCM were created by importing plan configurations onto the CT study of an Octavius 4D phantom set that was previously acquired. The phantom DCM consisting of the plan's dose was exported to be used in gamma analysis.
An Octavius 1500D detector array was used with the four‐dimensional (4D) phantom. The phantom is cylindrical and is designed to rotate around its base in synchrony with the gantry rotation. An inclinometer, attached to the base of the gantry, sends information about its position to steer the rotation of the phantom's cylindrical body. There is a 200 ms measuring interval to ensure good correlation between dose data and gantry angles. This measuring interval combined with an inherent uncertainty of the gantry angle for the three‐dimensional (3D) dose reconstruction algorithm leads to a total uncertainty of ±1.2° for gantry speeds up to 360° per minute.20 The Octavius 1500 consists of 1405 0.06 cm3 vented cubic ion chambers (0.44 cm3 × 0.44 cm3 × 0.30 cm3) mounted beneath a 0.5 cm polystyrene build‐up layer. The ion chambers cover a 27 cm3 × 27 cm3 active area in a checkerboard pattern and are spread spatially with a center‐to‐center distance of 0.707 cm. Dose measurements were recorded and processed using VeriSoft. Once the measurements are collected, VeriSoft can reconstruct a 3D dose volume for comparison with the computed dose distribution in the phantom by the TPS. The reconstruction algorithm is based on percent depth dose (PDD) curves for different field sizes. First, the PDDs measured in water are converted to PDDs in the Octavius 4D phantom using the known relation of electron densities of water and phantom material. The PDD data are established at the time of the initial setup of the VeriSoft application during commissioning of the Octavius 4D system. PDD data for various field sizes ranging from 4 cm2 × 4 cm2 to 26 cm2 × 26 cm2 are entered into the system as part of the commissioning process.
At each gantry angle (parametrized as time), each detector of the panel measures a dose. For every gantry angle, the detector array measures a dose plane that is perpendicular to the incident beam. A ray through each detector of the panel is back‐projected to the source and the field size is determined through the irradiated detectors. During a measurement session, the effective field size is determined in real time by the software by integrating the position of the detectors that received radiation above a certain threshold. All dose points (measured and extrapolated at a given gantry angle) are summed over all gantry angles of the delivery to create a 3D dose distribution. Using the PDD appropriate to field sizes, the dose values along the ray lines that connect the irradiated detectors and the beam focus were reconstructed.20
VeriSoft allows the user to perform slice‐by‐slice two‐dimensional (2D) and 3D gamma index calculation, slice‐by‐slice comparison of the measured and computed dose distributions and dose profiles comparison. A volumetric 3D gamma index can be calculated for the entire 3D dose distribution, comparing the TPS calculations and the reconstructed 3D dose from the measurements. VeriSoft utilizes a pencil beam algorithm for computing dose and path length scaling to deal with inhomogeneity. This is less rigorous than the collapsed cone algorithm used in Pinnacle3 where kernel tilting is utilized to account for tissue inhomogeneity; however, it provides a tradeoff advantage in calculation speed which is beneficial during clinical time constraints.21, 22 VeriSoft imports patient structure sets from the initial TPS structure delineations and utilizes the 4D dose distributions from measurements to reconstruct patient DVH data. The version of the software (version 7.0) used in this study is unable to produce information for structures that do not entirely fit on the PTW 1500 matrix array surface area during dose acquisition. Figure 2 shows DVH produced with Pinnacle3 overlaid with VeriSoft's DVH for the patient.
Figure 2.
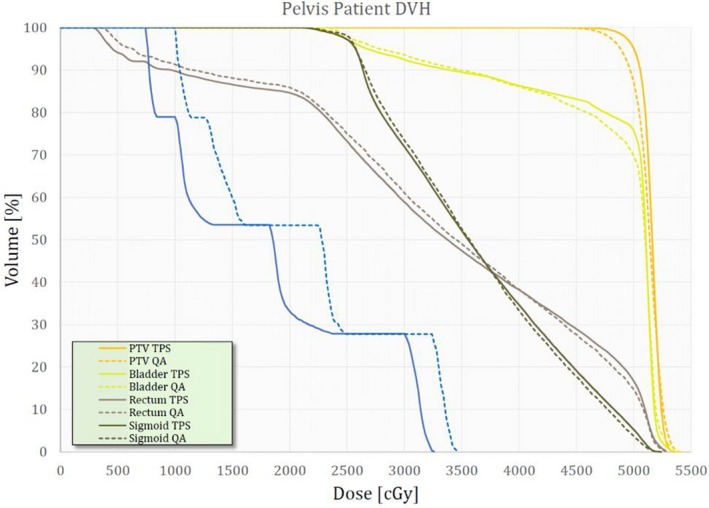
An example of a dose–volume histogram (DVH) set produced by Pinnacle3 and VeriSoft for a pelvis patient. The solid lines correspond to DVH from Pinnacle3 and the dashed lines correspond to DVH from VeriSoft.
2.3. TCP/NTCP modeling
A radiobiological evaluation was performed between the Pinnacle3 computed plans and the ones calculated from the phantom measurements. The DVH of organs represented in the plan pairs were used for estimation of radiobiological metrics. The formula that is used to calculate the response of each voxel or bin in a DVH for tumors and normal tissues is based on the Poisson model23, 24, 25:
| (1) |
where P(D) is the probability of response of a voxel irradiated by uniform irradiation of dose D, D 50 is the dose that induces response to 50% of the patients, and γ is the maximum normalized dose‐response gradient. In Eq. (1), the fractionation correction of dose is handled by using the quantity D 2 Gy, which is the equivalent dose at 2 Gy per fraction.26 The D 2Gy is calculated by the following expression:
| (2) |
where Dx Gy is the total dose when x Gy is the dose per fraction. To estimate normal tissue complications (NTCP) from nonuniform dose distributions, the relative seriality model was used:
| (3) |
The overall probability of injury, P I, for several OARs is expressed by the following equation24, 25:
| (4) |
where N organs is the total number of vital OARs, and NTCPj is the response probability of the organ j having the reference volume and been irradiated by a dose Di as given by Eq. (1). Furthermore, Δvi is the fractional subvolumes of the organ being irradiated, M is the total number of voxels or subvolumes in the considered organ, and s is relative seriality parameter of that organ. In tumors, for estimating tumor control probability from nonuniform dose distributions, the following model was used:
| (5) |
The overall probability of benefit, P B, can be quantified by the following expression:
| (6) |
where N tumors is the total number of tumors or targets involved in the clinical case. The effectiveness of different treatment plans were evaluated by the radiobiological concept of complication‐free tumor control probability, P +, which represents the probability of achieving tumor control without causing damage to normal tissues.27, 28
| (4) |
The radiobiological analysis was based on the DVH from Pinnacle3 and phantom measurements using the VeriSoft software. The corresponding TCP, NTCP, P I, and P + values were calculated. The difference in these values was obtained to estimate the expected clinical impact of the differences obtained by the dosimetric analysis. A detailed presentation of the software that was used for the radiobiological analysis can be found in the work by Su et al.29 Resulting TCP/NTCP calculated from doses reported by Pinnacle3 were used as reference values when comparing to TCP/NTCP calculations from doses reported by VeriSoft. Tables 2, 3, 4, 5 report the summary of the model parameter values used for the examined cancer cases.30, 31, 32, 33 D50 is the dose that is associated with the 50% response rate, γ is the maximum normalized value of the dose–response gradient, and s is the relative seriality parameter.18, 19, 34, 35
Table 2.
Summary of the model parameter values for the brain group.
| Brain group | |||||
|---|---|---|---|---|---|
| Organs | D50 (Gy) | γ | α | α/β | Endpoint |
| PTV | 55.0 | 2.5 | Na | 10.0 | Control |
| Brain | 60.0 | 2.6 | 0.64 | 3.0 | Necrosis, infarction |
| Brainstem | 65.1 | 2.4 | 1.0 | 3.0 | Necrosis, infarction |
| Chiasm/optic nerve | 65.0 | 2.3 | 1.0 | 3.0 | Blindness |
| Spinal cord | 57.0 | 6.7 | 1.0 | 3.0 | Myelopathy |
Table 3.
Summary of the model parameter values for head‐neck group.
| Head‐Neck group | |||||
|---|---|---|---|---|---|
| Organs | D50 (Gy) | γ | α | α/β | Endpoint |
| PTV7000 | 51.0 | 7.5 | Na | 10.0 | Control |
| PTV5400 | 44.0 | 4.0 | Na | 10.0 | Control |
| Parotid gland | 46.0 | 1.8 | 0.01 | 3.0 | Xerostomia |
| Spinal cord | 57.0 | 6.7 | 1.0 | 3.0 | Myelopathy |
| Mandible | 70.3 | 3.8 | 1.0 | 3.0 | Marked limitation of joint function |
| Brachial Plexus | 75.1 | 2.8 | 8.4 | 3.0 | Nerve damage |
Table 4.
Summary of the model parameter values for the lung group.
| Lung group | |||||
|---|---|---|---|---|---|
| Organs | D50 (Gy) | γ | α | α/β | Endpoint |
| PTV | 49.2 | 1.0 | Na | 10.0 | Control |
| Esophagus | 68.0 | 2.8 | 3.4 | 3.0 | Clinical stricture/perforation |
| Heart | 70.7 | 0.96 | 1.0 | 3.0 | Cardiac mortality |
| Lung | 30.1 | 0.97 | 0.01 | 3.0 | Radiation pneumonitis |
| Spinal cord | 57.0 | 6.7 | 1.0 | 3.0 | Myelopathy |
Table 5.
Summary of the model parameter values for the pelvis and prostate groups.
| Pelvis and prostate group | |||||
|---|---|---|---|---|---|
| Organs | D50 (Gy) | γ | α | α/β | Endpoint |
| PTV7920 (prostate) | 63.0 | 5.0 | Na | 3.0 | Control |
| PTV6000 (pelvis) | 55.0 | 3.0 | Na | 3.0 | Control |
| Bladder | 80.0 | 3.0 | 0.3 | 3.0 | Symptomatic contracture |
| Rectum | 80.0 | 2.2 | 0.7 | 3.0 | Proctitis, necrosis, fistula, stenosis |
| Sigmoid | 80.0 | 2.2 | 0.7 | 3.0 | Ulceration |
| Bowel | 60.0 | 2.1 | 0.14 | 3.0 | Stenosis |
| Penile Bulb | 70.0 | 2.5 | 0.7 | 3.0 | Erectile Dysfunction |
| Femur head | 65.0 | 2.7 | 1.0 | 3.0 | Necrosis |
3. RESULTS
3.1. Gamma analysis
Table 6 summarizes the resulting values for gamma analysis of all cohorts. is the average 3D gamma score, is the uncertainty in gamma 3D scores, is the arithmetic mean, is the uncertainty of the arithmetic mean, is the mean of the medians, and is the uncertainty of the mean of medians. The column labeled ‘Range’ shows the minimum and maximum value of the gammas in the corresponding cohort.
Table 6.
Resulting values for gamma analysis of all cohorts.
| Site | Criteria | ϒ3Daverage (%) | Range (%) | σϒ3D (%) | μ‐arithmetic | σμ‐arithmetic | μmedian | σμ‐median |
|---|---|---|---|---|---|---|---|---|
| Brain | 3%/3 mm | 96.8 | 94.2‐99.5 | 1.6 | 0.64 | 0.04 | 0.69 | 0.03 |
| 2%/2 mm | 85.9 | 80.4‐89.8 | 3.5 | 0.81 | 0.08 | 0.76 | 0.04 | |
| H&N | 3%/3 mm | 92.8 | 88.8‐95.8 | 2.3 | 0.71 | 0.06 | 0.73 | 0.03 |
| 2%/2 mm | 78.0 | 72.0‐83.1 | 3.3 | 1.01 | 0.15 | 0.82 | 0.03 | |
| Lung | 3%/3 mm | 93.2 | 89.1‐96.1 | 2.5 | 0.72 | 0.05 | 0.74 | 0.03 |
| 2%/2 mm | 77.0 | 71.2‐81.0 | 3.9 | 1.03 | 0.17 | 0.84 | 0.03 | |
| Prostate | 3%/3 mm | 95.9 | 91.9‐97.9 | 2.3 | 0.64 | 0.05 | 0.69 | 0.04 |
| 2%/2 mm | 85.3 | 76.3‐90.0 | 4.8 | 0.82 | 0.09 | 0.77 | 0.05 | |
| Pelvis | 3%/3 mm | 96.5 | 94.9‐97.4 | 0.9 | 0.60 | 0.04 | 0.78 | 0.73 |
| 2%/2 mm | 86.8 | 81.8‐89.4 | 2.6 | 0.78 | 0.05 | 0.73 | 0.04 |
A histogram of values for all the cohorts is shown in Figs. 3 and 4 to visualize the data for both 3%/3 mm and 2%/2 mm criteria.
Figure 3.
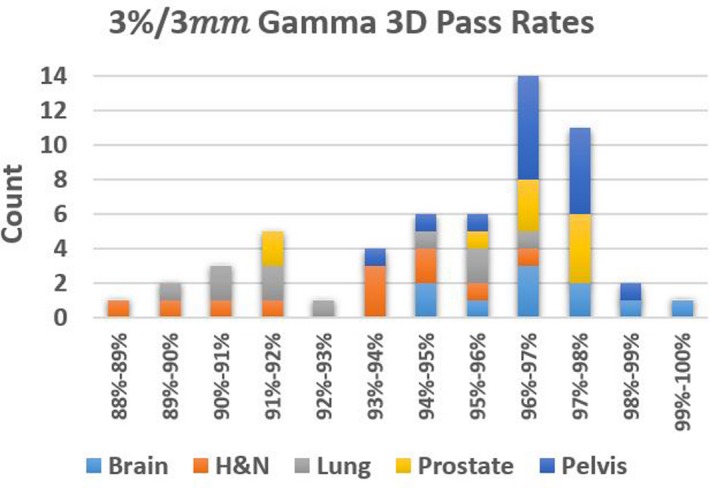
Histogram of Gamma 3D scores for all cohorts at 3%/3 mm tolerance.
Figure 4.
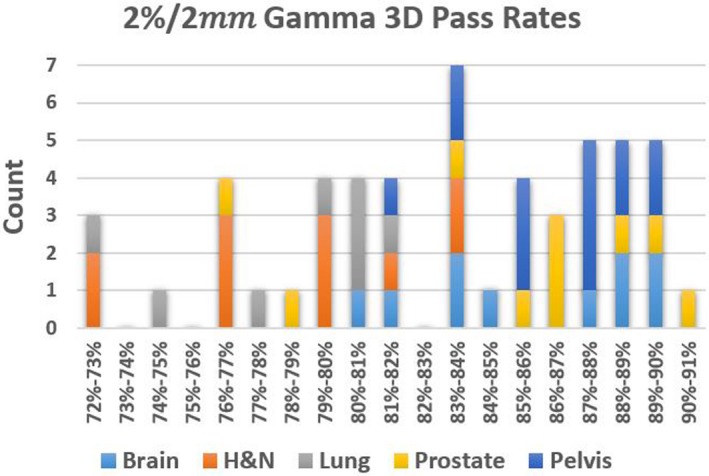
Histogram of Gamma 3D scores for all cohorts at 2%/2 mm tolerance.
3.2. TCP/NTCP results
Table 7 shows a comparison of mean ± standard deviation (stdev) of P + values reconstructed using Verisoft and that computed in Pinnacle3. All TCP and NTCP values were estimated and individual P + results can be found in Appendix A in Tables A1, A2, A3, A4, A5. A positive ΔP + value means that the delivered plan results in higher complication‐free tumor control than the TPS computed plan data which is desirable. In contrast, negative ΔP + values mean that the delivered plan results in lower complication‐free tumor control which indicates the delivered plan has poorer complication‐free tumor control than the TPS computed plan. Comparing the measured and computed dose distributions, 3D gamma values estimated using both 3%/3 mm and 2%/2 mm criteria are tabulated in Table 7.
Table 7.
Mean ± standard deviation of P + (Verisoft reconstructed), P_+ (Pinnacle computed) data and their differences are tabulated along with gamma pass rates (3%/3 mm; and 2%/2 mm) for the 5 treatment sites.
| Site | P + (Verisoft) | P + (Pinnacle3 TPS) | ΔP + | 3D gamma value (3%/3 mm) | 3D Gamma value (2%/2 mm) |
|---|---|---|---|---|---|
| Brain | 57.4 ± 6.8 | 61.3 ± 11.1 | −3.9 ± 5.8 | 96.8 ± 2.9 | 85.9 ± 5.6 |
| H&N | 44.3 ± 38.9 | 62.6 ± 31.5 | −18.2 ± 43.6 | 92.8 ± 2.1 | 78.0 ± 3.5 |
| Lung | 83.5 ± 16.3 | 75.7 ± 19.4 | 7.8 ± 18.3 | 93.2 ± 2.5 | 77.0 ± 4.2 |
| Prostate | 60.1 ± 25.3 | 53.0 ± 33.4 | 7.1 ± 12.1 | 95.9 ± 1.2 | 85.3 ± 2.6 |
| Pelvis | 67.1 ± 25.3 | 66.3 ± 27.5 | 0.8 ± 3.6 | 96.5 ± 2.3 | 86.8 ± 4.8 |
4. DISCUSSION
Gamma analysis performed on the five different treatment sites resulted in an average 3D gamma index of 95 ± 2% with 3%/3 mm tolerance and 82 ± 4% with 2%/2 mm tolerance. All the plans in this study except three passed our institutions evaluation criteria when using conventional gamma analysis. At 88.8%, 89.1%, and 89.4%, it is plausible that these were challenging cases and were accepted slightly below the clinical threshold.
It is pertinent to state here that while the P + values are based on the DVHs of target(s) and OARs, the gamma passing rates are determined across the dose grid volume on the entire CT dataset of the patient. It is natural to expect that high compliance among calculated and reconstructed DVHs (i.e., ΔP + ≈ 0) occur in regions where gamma passing rates are high (γ ≈ 100%). Figure 5 demonstrates the correlation between 3D gamma value (using 3%/3 mm criteria) and the absolute value of ΔP + on the brain cohort with a Pearson correlation coefficient, R2 = 0.64. Alternatively, significant differences between the measured and TPS computed doses can have big clinical impact if they coincide geometrically with critical structures that include targets, OARs. This exemplifies that gamma passing rates estimated for the entire volume does not provide region‐specific information of where a failure occurs, at which dose level or magnitude of dose error, and how clinically significant it could be.
Figure 5.
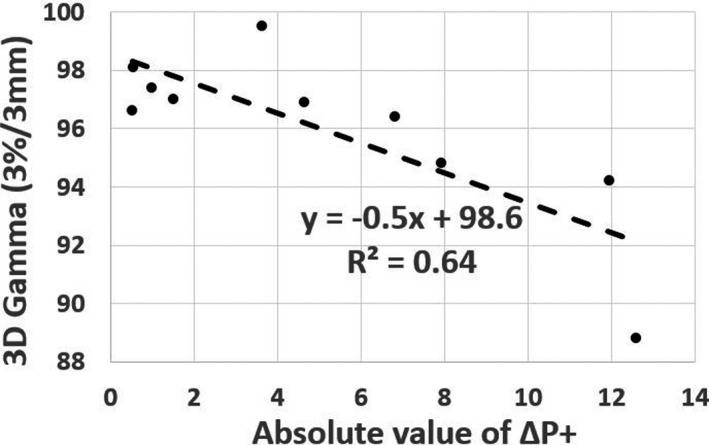
3D Gamma passing rates (3%/3 mm criteria) of brain cohort vs absolute value of ΔP +.
Patients 5 and 6 in the brain cohort as tabulated in Table A1 in Appendix A had 94.8% and 96.4% gamma pass rates with 3%/3 mm tolerance, respectively. The calculated ΔP + of the two plans was −7.94% and −6.82%, respectively, despite having clinically acceptable gamma pass rates. From Table A1, we can see that the measured dose predicts a reduction of TCP (PTV coverage) compared to the plan, ultimately resulting in a large negative difference.
Large reported differences in P I and P + values between the computed and delivered doses are observed in the head‐neck patients as tabulated in Table A2. It has been shown that dosimetric discrepancy of a few percent can have effects on TCP and NTCP.36, 37, 38, 39, 40, 41 This is also reflected in the low gamma passing rates of head‐neck plans. This may be attributed to poor spatial resolution of measurement against the computed dose in regions of steep dose falloff often witnessed in highly modulated head‐neck plans.
Patients 6 and 9 in the pelvis cohort resulted in ΔP + values of +15.11% and +12.35%, respectively. This is due to the lower NTCP of the delivered DVHs compared to the computed DVHs. The TCP calculated from the delivered DVHs was lower than that from TPS computed DVHs resulting in a positive ΔP + value (see Table A4). Similarly, the delivered dose to patient eight in the prostate cohort in Table A5 predicted lower TCP, resulting in a negative ΔP + of −6.31%.
Agreement between measured and planned dose can be attributed to spatial resolution of detector panel, accuracy of Verisoft commissioning, dosimetric reconstruction accuracy, complexity of the treatment plan, etc. Large observed differences of TCP values should be investigated further by restricting cohorts to patients of identical pathology. For instance, forming a cohort of plans that only include targets that are superficial or deep seated can be considered in a future work.
There are precedence available in the literature. In a 2017 study on patient‐specific QA of IMRT plans, 2D gamma analysis alone could not assure radiobiological equivalence between planned and delivered dose.42 The authors concluded that radiobiological analysis in addition to physical dose comparison may provide adequate patient‐specific QA of IMRT plans. The variation in radiobiological metrics due to patient setup errors were simulated in a prostate study by Park et al.43 A radiobiological model‐based bioanatomical QA using TCP and NTCP provides feedback that cannot be evaluated by physical QA alone. In another study, Zhen et al studied the change in DVH, TCP, and NTCP metrics by intentional introduction of multileaf collimator (MLC) errors in 40 IMRT plans.44 By showing TCP and NTCP as both sensitive and specific metrics, the study concludes with a possibility of using changes in TCP (ΔTCP) and NTCP (ΔNTCP) as alternate QA metrics.
In an assessment study on the usefulness of biological metrics in patient‐specific QA, prostate VMAT plans of American Association of Physicists in Medicine (AAPM) Task group report 166 test cases were analyzed.45 In this analysis of two 3D verification systems, radiobiological parameters were incorporated into the individualized QA providing information complimentary to DVH metrics. Finally, Sumida et al's radiobiological gamma distribution was useful in identification of areas where dose is radio‐biologically different, not just physically different.46 The radiobiological gamma index (RGI) facilitates physician's understanding of the dose distribution from a clinical perspective. Our study results do not necessarily agree with Sumida et al that could be attributed to differences in device type, geometry, measurement and analysis techniques. While we used Octavius 1500D detector array to do cumulative dose analysis, Sumida et al had used per‐beam analysis on a MapCheck device. While the low doses from cumulative analysis could add up to be detectable by the device, the per‐beam analysis suppresses low dose for the individual beam. While Octavius 1500D array rotates with the gantry, MapCheck measurements are insensitive to gantry rotation due to on‐faus dose delivery. In addition, Sumida et al had used Niemierko's model to calculate TCP and NTCP, which was not utilized in this study.
TCP and NTCP models are based on some assumptions since they cannot account for all the involved biological and clinical mechanisms. For example, there are other factors such as chemotherapy, comorbidities that may impact dose response and consequently the determination of the model parameters of the different tumors and tissues. Additionally, there are uncertainties imposed by inaccuracies in patient imaging, treatment planning, patient setup and treatment delivery during radiotherapy. Consequently, the determined model parameters and the corresponding dose–response curves are characterized by confidence intervals. The results of this study depend on the accuracy of the radiobiological models and the parameters that describe the dose‐response relations of the different tumors and normal tissues. Most of those parameters have been derived from recently published clinical studies, where the confidence intervals have been reduced. For certain tissues, larger uncertainties are involved in the determination of their parameters. In those cases, the calculated TCP or NTCP values should be not seen as prediction of patient outcome but to assess uncertainty of the delivered dose.
5. CONCLUSION
Currently available commercial QA systems allow for users to compute DVHs using phantom measured dose, enabling more insightful QA methods to develop. This study demonstrated the ability to integrate TCP/NTCP modeling with cumulative DVHs produced with phantom measured dose. By incorporating TCP/NTCP models, the risk of a plan to result in injury by deviations in measured dose to normal tissues or tumor coverage can be assessed as a QA metric. Although there are no clinical requirements or literature‐based thresholds for maximum deviation allowed between TCP/NTCP values calculated for structures, guidelines can be determined for establishing thresholds. In certain cases, this can be useful for critical structures in patients that have pre‐existing pathologies to a structure or are approaching dose limits due to previous treatments. In cases showing considerable reductions in tumor control and increases in normal tissue complications, a replanning could be proposed giving more emphasis in the robustness of the plan especially regarding the degree of modulation used and the steepness of dose falloff around the target.
CONFLICT OF INTEREST
No conflicts of interest.
Appendix A. TCP–NTCP results
Table A1.
TCP/NTCP calculation results for brain patients.
| Patient | TCP (%) | NTCP (%) | P I (%) | P + (%) | ||||
|---|---|---|---|---|---|---|---|---|
| PTV | Brain | Brainstem | Chiasm | RON | LON | |||
| Brain: Pinnacle | ||||||||
| 1 | 79.6 | 0.1 | 0.6 | 1.0 | 0.6 | 0.0 | 2.2 | 77.8 |
| 2 | 69.2 | 0.3 | 1.4 | 2.2 | 0.0 | 7.8 | 11.4 | 61.4 |
| 3 | 69.6 | 6.7 | 1.1 | 0.0 | 0.0 | 0.0 | 7.8 | 64.2 |
| 4 | 67.2 | 1.5 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 | 66.2 |
| 5 | 74.8 | 7.9 | 0.0 | 0.0 | 0.0 | 0.0 | 7.9 | 68.9 |
| 6 | 74.7 | — | 0.0 | 0.0 | 0.0 | — | 0.0 | 74.7 |
| 7 | 73.9 | 21.7 | 0.0 | 7.9 | 3.7 | 1.9 | 31.9 | 50.4 |
| 8 | 74.8 | 11.5 | 0.5 | 3.9 | 11.5 | 0.0 | 25.1 | 56.0 |
| 9 | 74.6 | 6.5 | 28.0 | 5.4 | 0.0 | 0.0 | 36.3 | 47.5 |
| 10 | 74.7 | 0.2 | 0.0 | 27.8 | 3.4 | 11.5 | 38.3 | 46.0 |
| Brain: VeriSoft | ||||||||
| 1 | 65.5 | 0.0 | 0.0 | 0.4 | 0.1 | 0.0 | 0.5 | 65.2 |
| 2 | 63.8 | 0.2 | 0.6 | 0.3 | 0.0 | 2.0 | 2.9 | 61.9 |
| 3 | 62.3 | 4.0 | 0.4 | 0.0 | 0.0 | 0.0 | 4.5 | 59.5 |
| 4 | 54.7 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 54.2 |
| 5 | 63.6 | 4.2 | 0.0 | 0.0 | 0.0 | 0.0 | 4.2 | 60.9 |
| 6 | 67.9 | — | 0.0 | 0.0 | 0.0 | — | 0.0 | 67.9 |
| 7 | 69.0 | 17.8 | 0.0 | 8.6 | 3.3 | 1.7 | 28.5 | 49.4 |
| 8 | 67.8 | 8.0 | 0.4 | 2.2 | 6.8 | 0.0 | 16.5 | 56.6 |
| 9 | 63.8 | 3.5 | 19.2 | 1.5 | 0.0 | 0.0 | 23.2 | 49.0 |
| 10 | 75.2 | 0.1 | 0.0 | 22.7 | 2.5 | 12.2 | 33.9 | 49.7 |
LON, left optic nerve; RON, right optic nerve.
Table A2.
TCP/NTCP calculation results for head‐neck patients.
| Patient | TCP (%) | NTCP (%) | P I (%) | P + (%) | |||||
|---|---|---|---|---|---|---|---|---|---|
| PTV‐HR | PTV‐SR | Parotid‐R | Parotid‐L | Mandible | Brach‐Plex‐R | Brach‐Plex‐L | |||
| Head‐Neck: Pinnacle | |||||||||
| 1 | 95.9 | 98.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 94.4 |
| 2 | 100.0 | 98.9 | 1.3 | 24.3 | 25.9 | 29.3 | 35.5 | 74.8 | 25.0 |
| 3 | 95.0 | — | — | 0.0 | 0.2 | — | — | 0.2 | 94.7 |
| 4 | 100.0 | 98.7 | 0.0 | 0.0 | 20.8 | 0.0 | 2.6 | 22.8 | 76.2 |
| 5 | — | 97.7 | 0.0 | 0.0 | 1.0 | 55.6 | 1.6 | 56.7 | 42.3 |
| 6 | — | 96.4 | 0.0 | 0.0 | 2.9 | — | — | 2.9 | 93.6 |
| 7 | 100.0 | 99.7 | 0.0 | 0.0 | 3.2 | 41.1 | 20.5 | 54.7 | 45.2 |
| 8 | 100.0 | 99.8 | 0.0 | — | 2.6 | — | — | 2.6 | 97.2 |
| 9 | 100.0 | 99.3 | 0.0 | 0.0 | 0.5 | 28.5 | 49.0 | 63.8 | 36.0 |
| 10 | 100.0 | 99.1 | 0.0 | 0.0 | 1.6 | 60.4 | 44.7 | 78.4 | 21.4 |
| Head‐Neck: VeriSoft | |||||||||
| 1 | 100.0 | 94.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 94.0 |
| 2 | 99.8 | 94.0 | 0.1 | 3.3 | 12.0 | 35.5 | 22.4 | 57.4 | 39.9 |
| 3 | 57.5 | — | — | 0.0 | 0.1 | — | — | 0.0 | 0.0 |
| 4 | 99.9 | 96.5 | 0.0 | 0.0 | 10.1 | 2.6 | 1.3 | 13.5 | 83.4 |
| 5 | — | 92.6 | 0.0 | 0.0 | 0.1 | 1.6 | 0.0 | 1.7 | 0.0 |
| 6 | — | 98.3 | 0.0 | 0.0 | 0.6 | — | — | 0.6 | 0.0 |
| 7 | 100.0 | 98.9 | 0.0 | 0.0 | 0.5 | 20.5 | 17.8 | 34.9 | 64.3 |
| 8 | 100.0 | 99.2 | 0.0 | — | 0.3 | — | — | 0.3 | 98.9 |
| 9 | 100.0 | 98.2 | 0.0 | 0.0 | 0.0 | 49.0 | 43.7 | 71.3 | 28.2 |
| 10 | 100.0 | 97.7 | 0.0 | 0.0 | 0.3 | 44.7 | 35.4 | 64.4 | 34.7 |
HR, high risk; SR, standard risk.
Table A3.
TCP/NTCP calculation results for lung patients.
| Patient | TCP (%) | NTCP (%) | P I (%) | P + (%) | ||
|---|---|---|---|---|---|---|
| PTV | Esophagus | Heart | Lung | |||
| Lung: Pinnacle | ||||||
| 1 | 74.3 | — | 1.7 | — | 1.7 | 73.0 |
| 2 | 84.3 | 0.0 | 0.0 | 0.0 | 0.0 | 84.3 |
| 3 | 80.3 | — | — | — | 0.0 | 80.3 |
| 4 | 96.9 | — | — | — | 0.0 | 96.9 |
| 5 | 40.2 | 0.0 | — | — | 0.0 | 40.2 |
| 6 | 99.1 | 0.0 | 0.0 | — | 0.0 | 99.1 |
| 7 | 72.2 | — | — | — | 0.0 | 72.2 |
| 8 | 77.2 | 0.6 | 1.8 | 17.7 | 19.6 | 62.1 |
| 9 | 54.2 | — | — | — | 0.0 | 54.2 |
| 10 | 94.9 | 0.0 | — | — | 0.0 | 94.9 |
| Lung: VeriSoft | ||||||
| 1 | 76.5 | — | 1.8 | — | 1.8 | 75.1 |
| 2 | 90.7 | 0.0 | 0.0 | 0.0 | 0.0 | 90.7 |
| 3 | 91.1 | — | — | — | 0.0 | 91.1 |
| 4 | 96.8 | — | — | — | 0.0 | 96.8 |
| 5 | 98.3 | 0.0 | — | — | 0.0 | 98.3 |
| 6 | 97.5 | 0.0 | 0.0 | — | 0.0 | 97.5 |
| 7 | 76.8 | — | — | — | 0.0 | 76.8 |
| 8 | 78.7 | 0.2 | 1.3 | 14.3 | 15.5 | 66.5 |
| 9 | 49.0 | — | — | — | 0.0 | 49.0 |
| 10 | 93.3 | 0.0 | — | — | 0.0 | 93.3 |
Table A4.
TCP/NTCP calculation results for pelvis patients.
| Patient | TCP (%) | NTCP (%) | P I (%) | P + (%) | ||||
|---|---|---|---|---|---|---|---|---|
| PTV | Bladder | Rectum | Sigmoid | Bowel | Penile Bulb | |||
| Pelvis: Pinnacle | ||||||||
| 1 | 79.4 | 0.8 | 0.9 | 0.3 | — | 0.0 | 1.9 | 77.9 |
| 2 | 89.0 | 0.0 | 2.4 | 18.0 | — | 14.9 | 31.9 | 60.6 |
| 3 | 82.8 | 6.3 | 0.4 | 0.5 | 0.5 | 38.6 | 70.8 | 24.2 |
| 4 | 82.8 | 4.4 | 4.2 | 3.3 | — | — | 11.4 | 73.4 |
| 5 | 44.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 44.7 |
| 6 | 79.0 | 1.2 | 1.9 | 2.5 | — | 0.0 | 5.5 | 74.6 |
| 7 | 82.5 | 0.1 | 0.8 | 0.1 | — | 1.0 | 1.9 | 81.0 |
| 8 | 82.9 | 0.0 | 0.3 | 0.4 | — | 0.5 | 1.3 | 81.9 |
| 9 | 82.8 | 0.2 | 1.9 | 2.1 | 0.4 | — | 41.9 | 48.1 |
| 10 | 52.2 | 0.0 | 0.0 | 0.0 | — | 7.2 | 7.2 | 48.4 |
| 11 | 82.6 | 0.0 | 1.1 | 0.0 | 0.0 | 11.9 | 12.8 | 72.0 |
| 12 | 77.0 | 0.2 | 1.5 | 4.0 | 0.2 | — | 20.3 | 61.4 |
| 13 | 82.8 | 0.0 | 1.3 | 1.0 | 1.6 | 8.1 | 155.4 | —45.9 |
| 14 | 80.2 | — | — | 2.2 | 0.5 | — | 49.7 | 40.3 |
| Pelvis: VeriSoft | ||||||||
| 1 | 77.8 | 0.7 | 0.7 | 0.2 | — | 0.0 | 1.7 | 76.5 |
| 2 | 86.8 | 0.0 | 2.0 | 0.0 | — | 10.3 | 12.2 | 76.3 |
| 3 | 75.2 | 3.6 | 0.5 | 0.3 | 0.2 | 29.4 | 47.2 | 39.7 |
| 4 | 82.2 | 3.2 | 4.6 | 3.1 | — | — | 10.6 | 73.5 |
| 5 | 37.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 37.1 |
| 6 | 93.1 | 0.5 | 1.4 | 1.7 | — | 0.0 | 3.6 | 89.8 |
| 7 | 80.8 | 0.1 | 0.5 | 0.1 | — | 0.3 | 0.9 | 80.1 |
| 8 | 79.4 | 0.0 | 0.2 | 0.3 | — | 0.2 | 0.7 | 78.9 |
| 9 | 79.6 | 0.1 | 2.0 | 1.9 | 0.2 | — | 24.1 | 60.5 |
| 10 | 47.0 | 0.0 | 0.0 | — | — | 0.0 | 0.0 | 47.0 |
| 11 | 78.1 | 0.0 | 0.5 | 0.0 | 0.0 | 6.0 | 6.5 | 73.0 |
| 12 | 72.9 | 0.1 | 1.7 | 4.3 | 0.1 | — | 14.8 | 62.1 |
| 13 | 79.2 | 0.0 | 1.6 | 0.6 | 1.1 | 4.4 | 110.7 | —8.4 |
| 14 | 78.3 | — | — | 1.6 | 0.3 | — | 28.1 | 56.3 |
Table A5.
TCP/NTCP calculation results for prostate patients.
| Patient | TCP (%) | NTCP (%) | P I (%) | P + (%) | |||
|---|---|---|---|---|---|---|---|
| PTV | Bladder | Rectum | Sigmoid | Penile Bulb | |||
| Prostate: Pinnacle | |||||||
| 1 | 95.3 | 1.4 | 0.4 | 0.8 | 0.5 | 3.1 | 92.3 |
| 2 | 97.5 | 0.6 | 3.9 | 21.9 | 3.1 | 27.7 | 70.5 |
| 3 | 96.0 | 2.7 | 15.7 | 34.2 | 5.7 | 49.1 | 48.9 |
| 4 | 98.0 | 0.2 | 4.5 | 0.0 | 0.0 | 4.7 | 93.4 |
| 5 | 97.2 | 2.5 | 5.4 | — | — | 7.7 | 89.7 |
| 6 | 97.3 | 3.3 | 3.4 | — | 48.9 | 52.3 | 46.5 |
| 7 | 97.4 | 2.9 | 7.8 | 12.5 | 3.9 | 24.7 | 73.3 |
| 8 | 96.0 | 1.7 | 4.5 | 0.0 | 0.0 | 6.2 | 90.1 |
| 9 | 99.9 | 36.9 | 18.3 | 36.0 | 70.0 | 90.1 | 9.9 |
| 10 | 97.4 | 8.2 | 9.6 | 26.2 | 18.5 | 50.1 | 48.6 |
| Prostate: VeriSoft | |||||||
| 1 | 92.2 | 1.0 | 0.3 | 1.0 | 0.2 | 2.5 | 89.9 |
| 2 | 95.3 | 0.3 | 3.3 | 18.7 | 2.8 | 23.8 | 72.6 |
| 3 | 93.3 | 2.0 | 12.4 | 30.6 | 2.7 | 42.0 | 54.1 |
| 4 | 94.0 | 0.0 | 3.2 | 0.0 | 0.0 | 3.2 | 91.0 |
| 5 | 93.3 | 0.5 | 3.1 | — | — | 3.6 | 90.0 |
| 6 | 96.2 | 2.1 | 3.3 | — | 44.5 | 47.5 | 50.5 |
| 7 | 95.7 | 1.3 | 8.5 | 9.0 | 3.2 | 20.4 | 76.2 |
| 8 | 86.2 | 0.4 | 2.4 | 0.0 | 0.0 | 2.7 | 83.8 |
| 9 | 99.7 | 31.4 | 14.9 | 31.1 | 71.3 | 88.5 | 11.5 |
| 10 | 95.0 | 4.3 | 10.6 | 23.0 | 17.2 | 45.5 | 51.8 |
REFERENCES
- 1. Webb S. Intensity‐Modulated Radiation Therapy. Bristol, UK: Institute of Physics Pub.; 2001. http://www.crcnetbase.com/isbn/9781420034110. Accessed April 14, 2017. [Google Scholar]
- 2. Webb S. Optimisation of conformal radiotherapy dose distributions by simulated annealing. Phys Med Biol. 1989;34(10):1349–1370. [DOI] [PubMed] [Google Scholar]
- 3. Mackie TR. History of tomotherapy. Phys Med Biol. 2006;51(13):R427–R453. [DOI] [PubMed] [Google Scholar]
- 4. Bortfeld TR, Kahler DL, Waldron TJ, Boyer AL. X‐ray field compensation with multileaf collimators. Int J Radiat Oncol. 1994;28(3):723–730. [DOI] [PubMed] [Google Scholar]
- 5. Spirou SV, Chui CS. Generation of arbitrary intensity profiles by dynamic jaws or multileaf collimators. Med Phys. 1994;21(7):1031–1041. [DOI] [PubMed] [Google Scholar]
- 6. Van Dyk J, Barnett RB, Cygler JE, Shragge PC. Commissioning and quality assurance of treatment planning computers. Int J Radiat Oncol Biol Phys. 1993;26(2):261–273. [DOI] [PubMed] [Google Scholar]
- 7. Hogstrom KR, Mills MD, Meyer JA, et al. Dosimetric evaluation of a pencil‐beam algorithm for electrons employing a two‐dimensional heterogeneity correction. Int J Radiat Oncol Biol Phys. 1984;10(4):561–569. [DOI] [PubMed] [Google Scholar]
- 8. Harms WB, Low DA, Wong JW, Purdy JA. A software tool for the quantitative evaluation of 3D dose calculation algorithms. Med Phys. 1998;25(10):1830–1836. [DOI] [PubMed] [Google Scholar]
- 9. Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys. 1998;25(5):656–661. [DOI] [PubMed] [Google Scholar]
- 10. Zhen H, Nelms BE, Tomé WA. Moving from gamma passing rates to patient DVH‐based QA metrics in pretreatment dose QA: from passing rates to DVH‐based QA metrics. Med Phys. 2011;38(10):5477–5489. [DOI] [PubMed] [Google Scholar]
- 11. Ma T, Podgorsak MB, Kumaraswamy LK. Accuracy of one algorithm used to modify a planned DVH with data from actual dose delivery. J Appl Clin Med Phys. 2016;17(5):273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nelms BE, Zhen H, Tomé WA. Per‐beam, planar IMRT QA passing rates do not predict clinically relevant patient dose errors: predicative power of conventional IMRT QA. Med Phys. 2011;38(2):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steers JM, Fraass BA. IMRT QA: selecting gamma criteria based on error detection sensitivity. Med Phys. 2016;43(4):1982–1994. [DOI] [PubMed] [Google Scholar]
- 14. Hauri P, Verlaan S, Graydon S, Ahnen L, Klöck S, Lang S. Clinical evaluation of an anatomy‐based patient specific quality assurance system. J Appl Clin Med Phys. 2014;15(2):181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nelms BE, Opp D, Robinson J, et al. Measurement‐guided 4D dose reconstruction on a patient: measurement‐guided 4D dose reconstruction. Med Phys. 2012;39(7Part1):4228–4238. [DOI] [PubMed] [Google Scholar]
- 16. Kruszyna M, Adamczyk M. SU‐E‐T‐634: Pre‐verification of FFF prostate VMAT plans with Gamma Method and DVHs reconstructed based on measurements with 2D‐ARRAY (PTW 1500) and OCTAVIUS 4D. Med Phys. 2015;42(6Part22):3482–3482. [Google Scholar]
- 17. Garcia‐Romero A, Hernandez‐Vitoria A, Millan‐Cebrian E, Alba‐Escorihuela V, Serrano‐Zabaleta S, Ortega‐Pardina P. On the new metrics for IMRT QA verification. Med Phys. 2016;43(11):6058–6071. [DOI] [PubMed] [Google Scholar]
- 18. Petrou E, Narayanasamy G, Lavdas E, et al. Evaluation of the generalized gamma as a tool for treatment planning optimization. Int J Cancer Ther Oncol. 2014;2(4):020418. [Google Scholar]
- 19. Warkentin BJ, Stavrev P, Stavreva N, Field C. Fallone BG. A TCP‐NTCP estimation module using DVHs and known radiobiological models and parameter sets. J Appl Clin Med Phys. 2004;5(1):50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Allgaier B, Schule E, Würfel J.Dose reconstruction in the OCTAVIUS 4 D phantom and in the patient without using dose information from the TPS. 2013. pdfs.semanticscholar.org/4b76/bb19cde7c5a97300737dbb57fe53460d7f67.pdf?_ga=2.66053550.1006090449.1581343471-245750488.1581343471. Accessed February 07, 2020.
- 21. Ahnesjö A. Collapsed cone convolution of radiant energy for photon dose calculation in heterogeneous media: photon dose calculation. Med Phys. 1989;16(4):577–592. [DOI] [PubMed] [Google Scholar]
- 22. Papanikolaou N, Stathakis S. Dose‐calculation algorithms in the context of inhomogeneity corrections for high energy photon beams: Inhomogeneity corrections for photon beams. Med Phys. 2009;36(10):4765–4775. [DOI] [PubMed] [Google Scholar]
- 23. Agren A, Brahme A, Turesson I. Optimization of uncomplicated control for head and neck tumors. Int J Radiat Oncol Biol Phys. 1990;19(4):1077–1085. [DOI] [PubMed] [Google Scholar]
- 24. Källman P, Agren A, Brahme A. Tumour and normal tissue responses to fractionated non‐uniform dose delivery. Int J Radiat Biol. 1992;62(2):249–262. [DOI] [PubMed] [Google Scholar]
- 25. Lind BK, Mavroidis P. Optimization of the dose level for a given treatment plan to maximize the complication‐free tumor cure. Acta Oncol. 1999;38(6):787–798. [DOI] [PubMed] [Google Scholar]
- 26. Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 27. Källman P, Lind BK, Brahme A. An algorithm for maximizing the probability of complication‐free tumour control in radiation therapy. Phys Med Biol. 1992;37(4):871–890. [DOI] [PubMed] [Google Scholar]
- 28. Mavroidis P, Lind BK, Brahme A. Biologically effective uniform dose (D) for specification, report and comparison of dose response relations and treatment plans. Phys Med Biol. 2001;46(10):2607–2630. [DOI] [PubMed] [Google Scholar]
- 29. Su F‐C, Mavroidis P, Shi C, Ferreira BC, Papanikolaou N. A graphic user interface toolkit for specification, report and comparison of dose–response relations and treatment plans using the biologically effective uniform dose. Comput Methods Programs Biomed. 2010;100(1):69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cronqvist A‐KÅ. Quantification of the Response of Heterogeneous Tumours and Organized Normal Tissues to Fractionated Radiotherapy. Edsbruk, Sweden: Akademitryck; 1995. [Google Scholar]
- 31. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol. 1991;21(1):109–122. [DOI] [PubMed] [Google Scholar]
- 32. Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose‐volume effects in the heart. Int J Radiat Oncol. 2010;76(3):S77–S85. [DOI] [PubMed] [Google Scholar]
- 33. Eriksson F, Gagliardi G, Liedberg A, et al. Long‐term cardiac mortality following radiation therapy for Hodgkin's disease: analysis with the relative seriality model. Radiother Oncol. 2000;55(2):153–162. [DOI] [PubMed] [Google Scholar]
- 34. Zelefsky MJ, Whitmore WF. Long‐term results of retropubic permanent 125iodine implantation of the prostate for clinically localized prostatic cancer. J Urol. 1997;158(1):23–29; discussion 29–30. [DOI] [PubMed] [Google Scholar]
- 35. Lohia S, Rajapurkar M, Nguyen SA, Sharma AK, Gillespie MB, Day TA. A comparison of outcomes using intensity‐modulated radiation therapy and 3‐dimensional conformal radiation therapy in treatment of oropharyngeal cancer. JAMA Otolaryngol Neck Surg. 2014;140(4):331. [DOI] [PubMed] [Google Scholar]
- 36. Boyer AL, Schultheiss T. Effects of dosimetric and clinical uncertainty on complication‐free local tumor control. Radiother Oncol. 1988;11(1):65–71. [DOI] [PubMed] [Google Scholar]
- 37. AAPM Radiation Therapy Committee , Task Group No. 65 , American Association of Physicists in Medicine . Tissue Inhomogeneity Corrections for Megalovoltage Photon Beams: Report of Task Group No. 65 of the Radiation Therapy Committee of the American Association of Physicists in Medicine. Madison, WI: Medical Physics Publishing; 2004. [Google Scholar]
- 38. Calvo OI, Gutiérrez AN, Stathakis S, Esquivel C, Papanikolaou N. On the quantification of the dosimetric accuracy of collapsed cone convolution superposition (CCCS) algorithm for small lung volumes using IMRT. J Appl Clin Med Phys. 2012;13(3):43–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones AO, Das IJ. Comparison of inhomogeneity correction algorithms in small photon fields: small photon field corrections. Med Phys. 2005;32(3):766–776. [DOI] [PubMed] [Google Scholar]
- 40. Khan FM, Gibbons JP. Khan's the Physics of Radiation Therapy, 5th edn Philadelphia, PA: Lippincott Williams & Wilkins/Wolters Kluwer; 2014. [Google Scholar]
- 41. Arumugam S, Xing A, Young T, Thwaites D, Holloway L. Three dimensional dose verification of VMAT plans using the Octavius 4D dosimetric system. J Phys Conf Ser. 2015;573:012081. [Google Scholar]
- 42. Paudel NR, Narayanasamy G, Han EY, et al. Dosimetric and radiobiological comparison for quality assurance of IMRT and VMAT plans. J Appl Clin Med Phys. 2017;18(5):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Park JY, Lee JW, Chung JB, et al. Radiobiological model‐based bio‐anatomical quality assurance in intensity‐modulated radiation therapy for prostate cancer. J Radiat Res. 2012;53(6):978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhen H, Nelms BE, Tomé WA. On the use of biomathematical models in patient‐specific IMRT dose QA. Med Phys. 2013;40(7):071702. [DOI] [PubMed] [Google Scholar]
- 45. Clemente‐Gutiérrez F, Pérez‐Vara C, Clavo‐Herranz MH, López‐Carrizosa C, Pérez‐Regadera J, Ibáñez‐Villoslada C. Assessment of radiobiological metrics applied to patient‐specific QA process of VMAT prostate treatments. J Appl Clin Med Phys. 2016;17(2):341–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sumida I, Yamaguchi H, Kizaki H, et al. Novel radiobiological gamma index for evaluation of 3‐dimensional predicted dose distribution. Int J Rad Oncol Biol Phys. 2015;92(4):779–786. [DOI] [PubMed] [Google Scholar]


