Abstract
Background
Chronic pain is costly for patients and for the health care system. It negatively affects people's physical, emotional, social, and mental health. We conducted a health technology assessment of 10-kHz high-frequency spinal cord stimulation (SCS) in adults with chronic noncancer pain that was refractory to medical management, which included an evaluation of effectiveness, safety, cost-effectiveness, the budget impact of publicly funding 10-kHz high-frequency SCS, and patient preferences and values.
Methods
We performed a systematic literature search of the clinical evidence. We assessed the risk of bias of each included study using the Cochrane Risk of Bias and ROBINS-I tools and the quality of the body of evidence according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Working Group criteria. We performed a systematic economic literature search. We analyzed the 5-year budget impact of publicly funding 10-kHz high-frequency SCS in Ontario for adults with chronic noncancer pain who had already tried other available SCS therapies (up to 1.2 kHz). To contextualize the potential value of 10-kHz high-frequency SCS, we spoke with people who had chronic noncancer pain.
Results
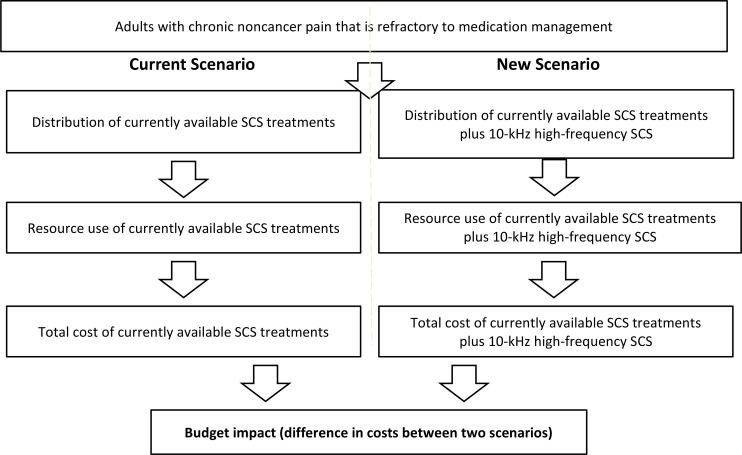
We included 5 studies (7 publications) in the clinical evidence review. Overall, 10-kHz high-frequency SCS likely provides reductions in pain intensity and functional disability, and improvements in quality of life in people with chronic noncancer pain (GRADE: Moderate). As well, patients may reduce their opioid consumption with 10-kHz high-frequency SCS (GRADE: Low). The two included economic evaluations found that 10-kHz high-frequency SCS was cost-saving compared with conventional SCS, but neither was applicable to the Ontario context. Owing to limited evidence about the effectiveness of 10-kHz high-frequency SCS in people who have first tried and failed SCS at lower frequencies (up to 1.2 kHz), we did not conduct a cost-effectiveness analysis comparing this pathway of care and 10-kHz high-frequency SCS for Ontario. Publicly funding 10-kHz high-frequency SCS (using the Freedom SCS system) in Ontario over the next 5 years would lead to a total net cost savings of $0.73 million (ranging from about $0.10 million in year 1 to about $0.21 million in year 5). However, if the province outsourced this therapy using the Senza HF10 SCS system, the total 5-year budget impact would be about $8.76 million. The people we spoke with who had chronic noncancer pain reported that their pain had a substantial negative impact on their activities and emotional well-being. Their direct knowledge of different pain therapies allowed them to provide context and comparisons when they discussed the impact of SCS on their chronic pain.
Conclusions
For adults with chronic noncancer pain that was refractory to medical management, 10-kHz high-frequency SCS was effective in relieving pain, reducing disability, and improving quality of life. Because there was limited evidence about the effectiveness of 10-kHz high-frequency SCS in people who had first tried and failed SCS at lower frequencies (up to 1.2 kHz), we were unable to determine whether 10-kHz high-frequency SCS is cost-effective in the Ontario context. We estimate that publicly funding 10-kHz high-frequency SCS in Ontario would result in cost savings of about $0.10 million to $0.21 million per year, for a potential total 5-year net cost savings of about $0.73 million. Although people with chronic noncancer pain knew little about SCS before they received it, they reported that it reduced their level of chronic pain, leading to improvements in function and their ability to perform activities of daily living.
OBJECTIVE
This health technology assessment evaluates the effectiveness, safety, cost-effectiveness, and budget impact of publicly funding 10-kHz high-frequency spinal cord stimulation (SCS) for adults with chronic noncancer pain that is refractory to medical management. It also evaluates the experiences, preferences, and values of people living with chronic noncancer pain.
BACKGROUND
Health Condition
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”1 Pain is considered to be chronic when episodes last over prolonged periods, usually longer than 3 or 6 months after an initial episode.2 Chronic pain is highly prevalent: 11% to 44% of Canadian adults experience it.1 Incidence rates for chronic pain based on the Canadian National Population Health Survey results for chronic pain were 6.0% to 8.7% for women and 4.8% to 6.1% for men.3 However, prevalence estimates are greatly affected by sampling methods, measurement, and definitions.4
Chronic pain may result from injury, infection, disease, or surgery—and some chronic pain may have no apparent cause. Chronic pain is a costly disease for patients and the health care system; it has a great impact on the lives of patients and their families, including on their relationships, lifestyles, and occupations.5–7 Chronic pain also negatively affects people's physical, emotional, social, and mental health.8–11
Clinical Need and Target Population
The diversity and complexity of chronic pain makes it extremely difficult to manage. Spinal cord stimulation has been used since the 1960s for diverse chronic pain populations.12–14 One population that commonly receives SCS is people who have chronic pain and have undergone unsuccessful spinal surgery; this is referred to as failed back surgery syndrome.15 Other common chronic pain syndromes for which SCS has been applied include complex regional pain syndrome, neuropathic pain (e.g., painful diabetic neuropathy), and ischemic pain syndromes (e.g., critical limb ischemia, refractory angina pectoris, and pain secondary to peripheral vascular disease).16 Because the pain pathways differ depending on the cause of the chronic pain, it is not generally known which patients with chronic pain would respond more favourably to SCS.
Current Treatment Options
Nonsurgical interventions are used to manage chronic pain, including physical and behavioural interventions (e.g., mindfulness-based stress reduction) and medications (called medical management; e.g., anti-inflammatories, muscle relaxants, gabapentinoids, antidepressants, and opioids).17–19 Spinal cord stimulation is typically recommended after medical management and/or physical and behavioral interventions have been unsuccessful. In such cases, SCS is used as a last resort or “rescue” option. As well, people would not be considered for SCS if they were candidates for surgery to correct spinal pathology. Spinal cord stimulation can be used independently or delivered as a component of a multimodal pain management program.
Spinal cord stimulation delivers electricity to spinal nerves to suppress pain signals. The technology, called a spinal cord stimulator, typically consists of several components: electrode leads, an implantable pulse generator (which is the battery for the system), extension cables (which connect the electrode leads to the pulse generator), and an external controller used to program the device. The pulse generator can be rechargeable or nonrechargeable and is implanted subcutaneously (under the skin) in the abdomen, buttock, or flank. A wireless pulse generator system also exists; it is worn externally and does not require implantation.20) The electrode leads of a spinal cord stimulator are thin, flexible, insulated wires that deliver the electrical stimulation generated by the implantable pulse generator. These leads are inserted in the epidural space (just outside the membrane that protects the spinal cord) and can be positioned there percutaneously (through the skin) under fluoroscopic guidance (x-ray) or by surgery. The overall procedure is minimally invasive when the electrode leads are placed percutaneously with a needle and a small incision is made for the pulse generator.
Once the spinal cord stimulator has been placed, a programmer (usually the physician) is needed to adjust the electrical settings. The external control system also has a patient controller interface device that gives patients some ability to fine-tune power levels or modify the default stimulation settings preset by the physician. Patients can adjust or customize settings depending on their pain experience, and they can shut off the device to avoid unwanted shocks. Some newer systems also allow patients to adjust settings using a mobile device, such as a smart phone or watch.
The basic unit of electrical stimulation is the pulse, which delivers a specific amount of current. This current stimulates the dorsal fibres, interfering with the transmission of pain signals to the brain. The parameters affecting this are the amplitude (the strength of the stimulation, measured in milliamperes) and the pulse width (the amount of time the stimulation lasts, measured in microseconds).21 The frequency or pulse rate is the number of electrical stimulations per second, measured in hertz (Hz). All of these parameters can be adjusted to optimize patients' pain management. Conventional SCS, also called low-frequency SCS, has been variably defined as frequencies of 30 to 200 Hz or 60 to 200 Hz.12 Generally, low-frequency SCS produces paresthesia and is associated with relatively low energy consumption. Paresthesia is a feeling of tingling or buzzing that people perceive in different ways. Some find the sensation uncomfortable or intolerable, while others are comforted by the sensation and feel more secure knowing that the device is working.
Treatment with SCS involves an initial trial period, typically of 1 to 2 weeks, during which the leads are placed and programming protocols are tested on a temporary pulse generator to determine patients' reactions and preferences. If the trial is successful—usually defined as a 50% or greater reduction in a patient's pain intensity over baseline—the pulse generator is implanted permanently.
Health Technology Under Review
High-frequency SCS is a new subtype of SCS for chronic pain that emits electrical pulses in kilohertz, exceeding the range of low-frequency SCS (i.e., is greater than 200 Hz). This higher frequency is beyond those that people can feel or sense. A stimulation frequency of 10,000 Hz (10 kHz) is the highest frequency currently delivered by SCS systems in clinical settings and is the focus of this assessment.
Similar to low-frequency SCS, a range of SCS models and designs are available for high-frequency SCS, offering different energy systems and programming, targets, delivery options, and patient programming adjustments. Like low-frequency SCS, the spinal-cord leads and pulse generators for high-frequency SCS can be implanted by interventional pain physicians (anesthesiologists), physical medicine and rehabilitation experts (physiatrists), or neurosurgeons. As well, an initial trial period is undertaken, and if successful (usually defined as a 50% or greater reduction in a patient's pain intensity over baseline) the pulse generator is implanted permanently.
Regulatory Information
Four companies have Health Canada regulatory approval for SCS devices that deliver a range of frequencies: Abbott Neuromodulation, Boston Scientific Corp., Medtronic Inc., and Stimwave Technologies Ltd.
The Senza HF10 SCS system (Nevro Corp., Menlo Park, CA) also delivers 10-kHz high-frequency SCS. It has patent-restricted regulatory approval to deliver a frequency stimulation range of 1.5 kHz to 10 kHz in other jurisdictions, including Europe, Australia, and the United States, but it does not have Health Canada regulatory approval and is not available in Canada. Nevro Corp. manages delivery of the treatment (e.g., the implant procedure) in manufacturer-developed and -supervised neuromodulation centres outside Canada.
As of November 2018, Stimwave had received regulatory approval in Canada to deliver 10-kHz high-frequency SCS. Stimwave's Freedom SCS system is a new type of SCS system (Freedom-4A and Freedom-8A; Stimwave Technologies Ltd., Pompano Beach, FL): the Freedom SCS system pulse generator is not implanted; instead, it wirelessly transmits electrical signals to the implanted epidural leads. The Freedom SCS system provides patients with three treatment modality options: tonic low-frequency, burst frequency, and 10-kHz high-frequency. This system has been approved by the United States Food and Drug Administration, the European Union (CE Mark), and Health Canada (written communication, Stimwave Technologies Ltd., November 2018).
Ontario Context
In 2005, the Ontario Health Technology Advisory Committee recommended increased access to low-frequency SCS as part of comprehensive pain management for chronic noncancer pain.22
Six designated centres of excellence in Ontario currently offer SCS (neuromodulation) for chronic pain: St. Michael's Hospital and the University Health Network in Toronto; Hamilton Health Sciences; London Health Sciences Centre; Kingston Health Sciences Centre; and the Ottawa Hospital.
At present, physicians who implant SCS devices include pain physicians (anesthesiologists), physical medicine and rehabilitation experts (physiatrists), and neurosurgeons. The SCS programs vary in their screening protocols, referral practices, implant procedures, device use, and patient follow-up. Few centres have program staff to assist in these procedures and cite lack of staff as a significant barrier to scaling up their programs (written communication, Ron Levy, MD, November 2018).
In 2018, approximately 200 SCS procedures were performed in Ontario (a population of approximately 13.4 million), mainly for chronic pain patients with back pain, failed back surgery syndrome, or complex regional pain syndrome. The centres of excellence performing the procedures have access to SCS devices with different designs and different energy systems: conventional low-frequency (≤ 200 Hz), moderate-frequency up to 1.2 kHz, and burst and multiwave platforms.
A patented 10-kHz SCS option (Nevro Corp.) is legally available only in other countries. As noted above, Stimwave Technologies Ltd. has obtained Health Canada regulatory approval for its wireless pulse generator device, which can deliver 10-kHz high-frequency SCS. As of November 2018, Stimwave's Freedom SCS systems have been marketed and used in at least one hospital in Ontario (written communications, Aaron Hong, MD, and Stimwave Technologies Ltd., November 2018).
This health technology assessment was requested by the Ontario Ministry of Health to meet the needs of people with chronic noncancer pain who may be eligible for 10-kHz high-frequency SCS as rescue therapy after being treated with all other therapies, including any other SCS system available in the province.
Expert Consultation
We engaged with experts in the specialty areas of interventional pain management, neurology, neurosurgery, and orthopedic surgery to help inform our understanding of the health technology and to contextualize the evidence. We also engaged with industry representatives to understand the technology.
PROSPERO Registration
This health technology assessment has been registered in PROSPERO, the international prospective register of systematic reviews (CRD42018109805), available at http://www.crd.york.ac.uk/PROSPERO/displayrecord.php?ID=CRD42018109805.
CLINICAL EVIDENCE
Research Question
What are the effectiveness and safety of 10-kHz high-frequency spinal cord stimulation (SCS) compared with other SCS strategies for the treatment of adults with chronic noncancer pain that is refractory to medical management?
Methods
Clinical Literature Search
We performed a clinical literature search on August 17, 2018, to retrieve studies published from inception until the search date. We used the Ovid interface in the following databases: MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, the Health Technology Assessment database, and the National Health Service Economic Evaluation Database (NHS EED).
A medical librarian developed the search strategies using controlled vocabulary (e.g., Medical Subject Headings) and relevant keywords. The final search strategy was peer-reviewed using the PRESS Checklist.23
We created database auto-alerts in MEDLINE and Embase and monitored for the duration of assessment period. We also performed a targeted grey literature search of health technology assessment agency websites as well as clinical trial and systematic review registries. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published from database inception until August 17, 2018
Randomized controlled trials, randomized crossover studies
Exclusion Criteria
Animal or in vitro studies
Systematic reviews, observational studies, case reports, editorials, letters, or commentaries
Expert reviews
Study protocol reports
Abstracts and conference proceedings
Participants
Adults (≥ 18 years) with chronic noncancer pain lasting 3 months or longer and refractory to medical management
Intervention
10-kHz high-frequency SCS
Comparator
Any other SCS modality (e.g., paresthesia or paresthesia-free SCS or alternative waveforms, such as burst)
Outcome Measures
-
Effectiveness
-
–
Pain: intensity, responders
-
–
Functional disability, physical activity, mobility, employment status
-
–
Medication use, reductions in opioid or other analgesic use
-
–
Patient satisfaction
-
–
Global Impression of Change
-
–
Sleep quality
-
–
Health-related quality of life, anxiety and depression
-
–
Safety: device/surgery-related postoperative and longer-term adverse events
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts, and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion.
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items using a data form to collect information on the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, study duration and years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, number of comparisons)
Outcomes (e.g., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], time points at which the outcomes were assessed)
We contacted study authors to provide clarification as needed.
Statistical Analysis
Meta-analysis was inappropriate in this review because of clinical, methodological, and statistical heterogeneity, so we undertook a narrative summary of the results.
Critical Appraisal of Evidence
We assessed risk of bias for randomized controlled trials using the Cochrane Risk of Bias Tool (Appendix 2).24 We assessed risk of bias for randomized crossover studies with validated outcome measures using the ROBINS-I measurement tool (Appendix 2).25
We evaluated the quality of the body of evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) Handbook.26 The body of evidence was assessed based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The overall rating reflects our certainty in the evidence.
Results
Clinical Literature Search
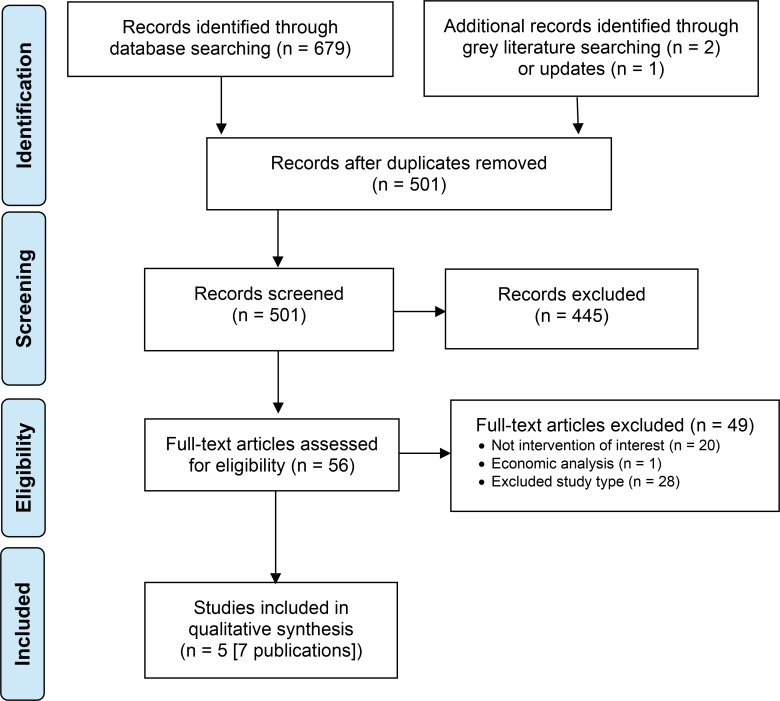
The database search of the clinical literature yielded 679 citations published from database inception to August 17, 2018. Three additional records were identified through grey literature search and database updates. We identified 5 studies (7 publications) that met our inclusion criteria. See Appendix 3 for a list of studies excluded after full-text review. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the clinical literature search.
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.27
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Characteristics of Included Studies
We included 5 studies (7 publications) in the clinical evidence review. Details of the 3 randomized controlled trials (5 publications) and 2 randomized crossover studies evaluating 10-kHz high-frequency SCS for chronic noncancer pain are provided in Appendix 4. All included trials evaluated 10-kHz high-frequency SCS for chronic noncancer pain, but they varied in their design and targeted populations. Some studies compared SCS protocols or waveforms with different devices in randomized chronic pain patient groups. Others evaluated different SCS protocols or waveforms using the same device within patients in a randomized crossover design.
Randomized Controlled Trials
Three randomized controlled trials28–30 compared 10-kHz high-frequency SCS with an alternate form of SCS.
The first study, the SENZA-RCT,30 was a multicentre, industry-sponsored trial designated as a pragmatic noninferiority trial comparing the Senza HF10 rechargeable SCS device with the Precision Plus (Boston Scientific) SCS, which provided conventional low-frequency (39 Hz–77 Hz) tonic paresthesia-based SCS stimulation. Both study groups used similar percutaneous leads with 8 contacts.30–32
The second study, by De Andres et al,29 was a single-site, non-industry-sponsored superiority trial comparing the Senza HF10 10-kHz high-frequency SCS with the Medtronic SureScan Restore Sensor (providing conventional low-frequency SCS). The study population included 60 patients: 29 randomized to receive 10-kHz high-frequency SCS and 31 to receive conventional SCS; 55 patients ultimately received a permanent implant and were followed up at 12 months.
The third study, called the SURF study,28 was also a multicentre industry-sponsored trial involving the Freedom SCS system (Stimwave Technologies Ltd.), a wireless device that produces several waveforms and frequencies up to 10 kHz. This study involved 99 patients with chronic back or back and leg pain, 51 randomized to 10-kHz high-frequency SCS and 48 to a comparator SCS protocol with the same wireless device. The comparator arm allowed patients to choose a preferred SCS programming option depending on their pain response, including tonic low frequency (20 Hz–200 Hz), burst (500 Hz), or 800 Hz–1,500 Hz moderate frequency. Patient preference for these options was mixed: 16 patients chose low-frequency, 13 chose burst, and 9 chose 800 Hz–1,500 Hz moderate frequency (written communication, Stimwave Technologies Ltd., February 2019). At the time of writing, 83 patients had reached 3-month follow-up, and 72 had reached 6-month follow-up.
The targeted pain population for the three randomized controlled trials differed with respect to pain chronicity and etiology. Patients were refractory to medical management for a minimum of 3 months in the SENZA-RCT study,30 a minimum of 6 months in the study by De Andres et al study,29 and 12 months in the SURF study.28 Although all trials recruited patients with chronic back and leg pain, the SENZA-RCT study did not specifically recruit patients with chronic pain following failed back surgery, but the study by De Andres et al and the SURF study did.
Each trial had a different primary outcome. In the SENZA-RCT study,30 the primary outcome was a composite of 3-month back pain responder rate (percentage with a ≥ 50% reduction in back-pain intensity over baseline according to a visual analogue scale [VAS]) and no stimulation-related neurological deficit. Outcomes were reported for 12 and 24 months' follow-up. The noninferiority design included a 10% noninferiority margin of difference in pain responder proportions between groups. The study by De Andres et al29 was a superiority trial, evaluating mean differences in global pain intensity (numeric rating scale pain score, scale of 0 to 10), neuropathic pain (painDETECT score33–35), and pain-related psychological variables in repeated measures over a 1-year follow-up. The SURF study28 was a noninferiority trial based on mean difference in 6-month back pain responder rate (percentage with ≥ 50% reduction in back-pain intensity pain score over baseline, VAS) between the study groups; it also included a 10% noninferiority margin for the difference.
In all three trials,28–30 the conversion rate for trial to permanent implant was high: 90% (171/189) in the SENZA-RCT study, 92% (55/60) in the study by De Andres et al, and 86% (85/99) in the SURF study.
Randomized Crossover Studies
Two studies36,37 involved randomized crossover study designs that compared different SCS protocols (Table 1).
Table 1:
Randomized Crossover Studies
| Author, Year | Crossover Comparisons | Device/Protocol | Trial Sample |
|---|---|---|---|
| Bocci et al, 201836 | 10-kHz (frequency 10 kHz; pulse width 30 μs; amplitude 0.1–13.0 mA) Burst SCS (burst complex of 5 spikes, with a pulse width of 1,000 μs per spike and a spike frequency per burst complex of 500 Hz) Conventional SCS (frequency 10–200 Hz; pulse width 1–1,000 μs; amplitude 0.1–18.0 mA) |
Unspecified IPG | N = 30; lower back pain with or without spine surgery |
| Thomson et al, 201837 | Range of SCS frequencies compared: 1, 4, 7, and 10 kHz | Precision (Boston Scientific) | N = 21; responded to low-frequency SCS, primary back pain and no recent spine surgery |
Abbreviations: FBSS, failed back surgery syndrome; IPG, implantable pulse generator; SCS, spinal cord stimulation.
In the study by Bocci et al,36 30 patients were randomized to either conventional SCS, 10-kHz high-frequency SCS, or burst SCS. The duration of treatment was at least 1 week, and there was a 2-day washout period before patients crossed over to receive the next treatment. There was no difference in the time to pain recurrence (approximately 10 minutes) among the SCS protocols.
The PROCO study by Thomson et al37 compared a range of frequencies (1 kHz to 10 kHz); pulse width and amplitude were titrated to optimize therapy. The primary study objective was to determine the reduction in pain intensity compared with baseline for frequencies from 1 kHz to 10 kHz. Patients underwent a 4-week trial of each stimulation frequency; their mean reduction in pain intensity for back, leg, and overall pain was determined from information in patient pain diaries. The washout period between treatments ranged from several hours to a day to allow pain to return to 80% of baseline levels before proceeding to the next treatment frequency.
Risk of Bias in the Included Studies
The Cochrane Risk of Bias Tool24 indicated an overall low to moderate risk of bias in the included studies (Appendix 2).
Pain Intensity
Randomized Controlled Trials
In the SENZA-RCT,30,31 the Senza HF10 was statistically noninferior and superior to conventional SCS at 3 months. The primary outcome was based on back pain responder status (a pain intensity VAS score decrease of ≥ 50% over baseline). The 3-month back pain responder rate was significantly higher for permanently implanted patients in the 10-kHz high-frequency SCS group than for the conventional SCS group (84.5% vs. 43.8%, P < .001); the 40.7% (95% CI 28.1%-54.4%) difference met the statistical criteria for noninferiority and superiority. The criteria for statistical noninferiority and superiority were met for all three analysis populations (intention-to-treat, per-protocol, and as-treated).
At 2 years, there were clinically relevant reductions (> 2 points) in mean back and leg pain scores in both study groups (Table 2). The back-pain responder rate remained higher for 10-kHz high-frequency SCS, with a 27.2 % (95% CI 10.1%-41.8%) difference between study groups: 76.5% for the Senza HF10 group versus 49.3% for the conventional SCS group. At 3 months, the back-pain remitter (i.e., low VAS pain scores ≤ 2.5) was also higher for the 10-kHz high-frequency SCS group than for the conventional SCS group (65.2% vs. 31.3%; a 34% difference). At 2 years, the back-pain remitter rate remained significantly higher for the 10-kHz high-frequency SCS group. Differences in remitter rates were statistically noninferior (P < .001) and superior (P = .003).
Table 2:
Pain Intensity at 24-Month Follow-Up, SENZA-RCT Study
| 10-kHz High-Frequency SCS | Conventional SCS | Difference | |
|---|---|---|---|
| Back Pain Intensity | |||
| VAS point decrease, mean ± SD | 5.0 ± 2.5 cm (66.9% ± 31.8%) |
3.2 ± 3.0 cm (41% ± 36.8%) |
— |
| Responder ratea | 76.5% | 49.3% | 27.2% (95% CI 10.1%–41.8%; P < .001) |
| Remitter rateb | 65.9% | 31% | 34.9% (95% CI 18.0%–49.0%; P NR) |
| Leg Pain Intensity | |||
| VAS point decrease, mean ± SD | 4.7 ± 2.8 cm (65.1% ± 36.0%) |
3.7 ± 3.0 cm (46.0% ± 40.4%) |
— |
| Responder ratea | 72.9% | 49.3% | 23.6% (95% CI; 5.9%–38.6%; P < .001) |
| Remitter rateb | 65.9% | 39.4% | 26.5% (95% CI; 8.0%–41.2%; P < .001) |
Abbreviations: CI, confidence interval; SCS, spinal cord stimulation; SD, standard deviation; VAS, visual analogue scale.
Responder: patient with a pain intensity VAS pain score decrease of ≥50% over baseline.
Remitter: pain intensity VAS pain score of ≤ 2.5.
Source: Kapural et al, 2016.31
The leg pain responder rate was also significantly higher for the 10-kHz high-frequency SCS group at 3 months (83.1% vs. 55.5%, P < .001) and at 2 years (72.9% vs. 49.3%, P < .001). The leg-pain remitter rate was also significantly higher for the 10-kHz high-frequency SCS at 3 months (76.4% vs. 37.5%, P < .001) and 2 years (65.9% vs. 39.4%, P < .001).
The study by De Andres et al29 did not meet the primary study objective of superiority for mean global NRS pain score reductions for the 10-kHz high-frequency SCS group compared with the conventional low frequency SCS group during 12-month follow-up. Mean global pain scores decreased significantly in both groups, but reductions overlapped between groups at all follow-up points (Table 3). Differences between groups at 12-month follow-up were not statistically significant (repeated-measures general linear model analysis; P = .560). Ratings on the painDETECT questionnaire also decreased in both groups, but the difference was not significant (P = .853).
Table 3:
Pain Intensity, De Andres et al
| Pain Intensity | 10-kHz High-Frequency SCS | Conventional SCS | Differencea |
|---|---|---|---|
| Global Pain, Numeric Rating Scale, Mean ± SD | |||
| Baseline | 7.50 ± 1.52 | 7.69 ± 1.27 | −0.19 |
| 3 months | 4.48 ± 2.14 | 5.10 ± 2.09 | −0.62 |
| 6 months | 5.98 ± 2.61 | 5.71 ± 2.09 | 0.27 |
| 12 months | 6.06 ± 2.13 | 5.86 ± 2.46 | 0.20 |
| Mean change, baseline to 12 months | 1.82 ± 2.45 | 1.44 ± 2.28 | — |
| Neuropathic Pain, painDETECT Questionnaire, Mean ± SD | |||
| Baseline | 16.35 ± 7.26 | 18.41 ± 6.90 | −2.05 |
| 3 months | 11.50 ± 7.14 | 13.45 ± 7.80 | −1.95 |
| 6 months | 12.35 ± 8.25 | 13.97 ± 8.62 | −1.62 |
| 12 months | 13.54 ± 8.53 | 14.89 ± 7.36 | −1.35 |
| Mean change, baseline to 12 months | 2.08 ± 6.77 | 3.14 ± 6.50 | — |
Abbreviations: SCS, spinal cord stimulation; SD, standard deviation.
Crude differences calculated for this health technology assessment.
Source: De Andres et al, 2017.29
The SURF study28 satisfied the primary study objective: the difference in proportion of mean VAS back-pain responders in the 10-kHz high-frequency SCS group and the comparator group (10%; 95% CI-6% to 25%) was statistically noninferior based on a 10% noninferiority margin, at 6 months. Pain responder proportions were high for both the 10-kHz high-frequency SCS group (92%) and the comparator SCS group (82%).
Findings for the primary outcome for both back and leg pain are detailed in Table 4. Both study groups experienced significant (P < .0001) reductions in mean back pain VAS intensity scores at 6-month follow-up: a mean 50-point decrease for the comparator SCS group and a mean 58-point decrease for the 10-kHz high-frequency SCS group. The proportion of back-pain remitters was higher for the 10-kHz high-frequency SCS group (84% vs. 47%), and the mean difference in proportion of remitters between the groups (37.2%; 95% CI 17% to 58%) supported statistical noninferiority and superiority for the 10-kHz high-frequency SCS group. At baseline, leg-pain intensity scores were lower than back-pain intensity scores, but both groups experienced statistically significant (P < .0001) reductions in mean leg-pain scores. Leg-pain responder and remitter rates were not reported.
Table 4:
Pain Intensity, SURF Study
| 10-kHz High-Frequency SCS | Comparator SCSa | Difference | |
|---|---|---|---|
| Back-Pain Intensity | |||
| VAS, baseline, mm | 75.8 ± 13.1 | 77.5 ± 9.9 | −1.7 |
| VAS, 6 months, mm | 17.8 ± 14.1 | 27.8 ± 23.2 | −10 |
| VAS change, points (%) | 58.0 (77) | 49.7 (64) | 8.30 |
| Responder rate, %b | 92 (95% CI 79.2–97.3) | 82 (95% CI 66.4–91.2) | 10.0 |
| Remitter rate, %c | 84 (95% CI 72.3–95.7) | 47 (95% CI 30.2–63.8) | 37.0 |
| Leg-Pain Intensity | |||
| VAS, baseline | 55.1 ± 27.2 | 61.5 ± 24.1 | −6.4 |
| VAS, 6 months | 13.3 ± 14.1 | 22.3 ± 24.4 | −9.0 |
| VAS change, points (%) | 41.8 (76) | 39.2 (64) | 2.6 |
| Responder rate, %b | NR | NR | — |
| Remitter rate, %c | NR | NR | — |
Abbreviations: CI, confidence interval; NR, not reported; SCS, spinal cord stimulation; VAS, visual analog scale.
Comparator SCS protocols included low-frequency, burst, and 1.2–1.5 kHz moderate-frequency.
Pain score >50% reduction over baseline.
Pain score ≤ 25 mm.
Source: Bolash et al, 2019.28
The overall GRADE assessment from the randomized controlled trials for pain intensity was moderate, rated down for risk of bias (Appendix 2).
Randomized Crossover Studies
In the study by Bocci et al,36 mean pain intensity was significantly reduced from baseline in all three treatments (three treatments as a group P < 0.0001; burst SCS 2.7 ± 3.3, P < .0012; conventional SCS 4.9 ± 6.0, P = .0049; 10-kHz high-frequency SCS 4.4 ± 4.1, P = .0012).
In the PROCO study by Thomson et al,37,38 all stimulation frequencies resulted in the same degree of reduction in pain intensity over baseline for back pain (P = .00002; Table 5). Leg-pain intensity (which was lower at baseline than back-pain intensity) also decreased at the same rate across stimulation frequencies (P = .003), as did overall global pain intensity (P = .0002).
Table 5:
Pain Intensity Reduction, Thomson et al
| SCS Frequency | |||||
|---|---|---|---|---|---|
| Pain Intensity, Mean ± SDa | Baseline | 1 kHz | 4 kHz | 7 kHz | 10 kHz |
| Back pain | 6.8 ± 0.3 | 3.2 ± 0.3 | 3.5 ± 0.3 | 3.2 ± 0.3 | 3.3 ± 0.4 |
| Leg pain | 5.5 ± 0.4 | 2.6 ± 0.4 | 2.7 ± 0.4 | 2.7± 0.4 | 2.9 ± 0.4 |
| Overall pain | 6.7 ± 0.3 | 3.2 ± 0.3 | 3.5 ± 0.3 | 3.2 ± 0.3 | 3.3 ± 0.4 |
The overall GRADE assessment for the outcome of pain intensity from the randomized crossover studies was low (rated down for risk of bias and imprecision; Appendix 2).
Functional Disability
All 3 RCTs reported on functional disability. In the SENZA-RCT,30,31 functional disability (measured by the Oswestry Disability Index [ODI]) was improved at 3 months in both groups by an average of 16.5 points for the 10-kHz high-frequency SCS group and 13 points for the conventional SCS group. At 24 months, there were substantial improvements in disability categories in both study groups: the proportion of patients with severe disability (ODI 40%–60%) or crippling back pain (ODI 60%–80%) declined (Table 6).
Table 6:
Functional Disability at Baseline and 24-Month Follow-Up, SENZA-RCT Study
| ODI Severity Categorya | 10-kHz High-Frequency SCS | Conventional SCS |
|---|---|---|
| Minimal disability, % | 0.0 | 0.0 |
| Moderate disability, % | 8.9 | 1.2 |
| Severe disability, % | 71.1 | 76.5 |
| Crippling back pain, % | 20.0 | 22.2 |
| Minimal disability, % | 23.5 | 9.9 |
| Moderate disability, % | 41.2 | 39.4 |
| Severe disability, % | 30.6 | 42.3 |
| Crippling back pain, % | 4.7 | 8.5 |
Abbreviations: ODI, Oswestry Disability Index; SCS, spinal cord stimulation.
The Oswestry Disability Index is scored from 0 to 100: minimal disability 0%–20%; moderate disability 20%–40%; severe disability 40%–60%; crippling back pain 60%–80%.
Source: Kapural et al, 2016.31
The study by De Andres et al29 reported a significant improvement in functional disability (evaluated using the ODI) in both groups compared with baseline (Table 7). The ODI disability scores improved by an average of 4 points; scores were not significantly different between groups at any follow-up point. However, a greater proportion of patients in the 10-kHz high-frequency SCS group achieved improvements in ODI scores at various thresholds of improvement on the ODI (9.5% more achieved ≥ 6.8 points, 15.3% more achieved ≥ 9.5 points, 13.9% more achieved ≥ 12.8 points, and 14.2% more achieved ≥ 15.0 points).
Table 7:
Functional Disability, De Andres et al
| 10-kHz High-Frequency SCS |
Conventional SCS | |
|---|---|---|
| Baseline | 27.00 ± 5.39 | 26.45 ± 5.85 |
| 3 months | 20.96 ± 7.56 | 21.93 ± 7.92 |
| 6 months | 21.85 ± 8.59 | 20.55 ± 8.32 |
| 12 months | 22.96 ± 7.06 | 22.07 ± 7.86 |
| Mean change, baseline to 12 months | 4.04 | 4.38 |
Abbreviations: NS, not statistically significant; ODI, Oswestry Disability Index; SCS, spinal cord stimulation.
Source: De Andres et al, 2017.29
The SURF study28 reported that at 6-month follow-up, mean ODI scores in both groups had improved over baseline (Table 8). Between-group differences in mean ODI scores were not significant (high-frequency SCS was noninferior to the comparator SCS protocols for disability with a noninferiority margin of 10%, P = 0.02).
Table 8:
Functional Disability ODI Scores, SURF Study
| 10-kHz High-Frequency SCS |
Comparator SCSa | |
|---|---|---|
| Baseline | 53 | 55 |
| 1 month | 29 | 33 |
| 3 months | 31 | 37 |
| 6 months | 29 | 31 |
| Mean change, baseline to 6 months (% change) | 24 (45) | 24 (44) |
Abbreviations: ODI, Oswestry Disability Index; SCS, spinal cord stimulation.
Comparator SCS protocols included low-frequency, burst, and 1.2–1.5 kHz moderate-frequency.
Source: Bolash et al, 2019.28
The overall GRADE assessment from the randomized controlled trials for functional disability was moderate, rated down for risk of bias (Appendix 2).
Opioid Use
Only the SENZA-RCT30,31 reported on opioid use. The majority of patients in each group were taking opioid analgesics at baseline (90.2% in the 10-kHz high-frequency SCS group and 86.2% in the conventional SCS group). At 12-month follow-up, 35.5% of patients in the 10-kHz high-frequency SCS group and 26.4% of patients in the conventional SCS group had decreased or eliminated their opioid use. In the 10-kHz high-frequency SCS group, the average morphine milligram equivalent decreased significantly (P = .014), from 112.7 ± 91 mg/day at baseline to 87.9 ± 85.2 mg/day—an 18.8% reduction. In the conventional SCS group, the average morphine milligram equivalent did not decline substantially over time (from 125.3 ± 150 to 118.0 ± 113.2 mg/day; 1% change). However, the variation in morphine milligram equivalent at baseline was significantly higher for the conventional SCS group.
The overall GRADE assessment from the randomized controlled trial for opioid use was low, rated down for risk of bias and imprecision (Appendix 2).
Patient Satisfaction
In the SENZA-RCT,30,31 patients rated satisfaction levels at 12 and 24 months (very satisfied, satisfied, not sure, dissatisfied, and very dissatisfied), and the majority of patients in both groups reported that they were “satisfied” or “very satisfied” (Table 9). Overall levels of patient satisfaction were significantly better for the 10-kHz high-frequency SCS group than for the conventional SCS group at 12 months (P = .01), but not at 24 months (P = .07).
Table 9:
Patient Satisfaction, SENZA-RCT Study
| Patient Satisfaction | 10-kHz High-Frequency SCS | Conventional SCS | Differencea |
|---|---|---|---|
| % Satisfied or Very Satisfied | |||
| 12 months | 83.1 | 78.5 | 4.6 |
| 24 months | 86.3 | 86.0 | 0.3 |
| % Dissatisfied or Very Dissatisfied | |||
| 12 months | 1.2 | 4.6 | −3.4 |
| 24 months | 1.3 | 3.5 | −2.2 |
The overall GRADE assessment for the outcome of patient satisfaction was moderate, rated down for risk of bias (Appendix 2).
Global Impression of Change
The SENZA-RCT30 reported on global impression of change. Patients and their physicians rated their global perceived assessment of change or patient recovery after 12 months and 24 months of follow-up. The self-reported assessment of change in this study was based on the Global Impression of Change scale, a 7-point Likert scale (1 representing no change or condition worse; 7 a great deal better).39–41 The rating scale did not allow for a separate measurement of worsening condition. Overall change was evaluated for activity limitations, symptoms, emotions, and overall quality of life related to the painful condition.
A greater proportion of patients in the 10-kHz high-frequency SCS group rated the change in their condition as “better” or “a great deal better” than the conventional SCS group at 12 months (56.8% vs. 37.6%) and at 24 months (63.5% vs. 36.6%; Table 10). As well, substantially more patients in the conventional SCS group reported minimal or no change in their condition than patients in the 10-kHz high-frequency SCS group at both 12 months (31.4% vs. 12.4%) and 24 months (30.9% vs. 20.0%). Overall ratings by patients were significantly better for those in the 10-kHz high-frequency SCS group than for the conventional SCS group at 12 months (P = .005) and 24 months (P = .004).
Table 10:
Patient and Physician Global Impression of Change, SENZA-RCT Study
| Patient Rating | Physician Rating | |||||
|---|---|---|---|---|---|---|
| Global Impression of Changea | 10-kHz High-Frequency SCS | Conventional SCS | Differenceb | 10-kHz High-Frequency SCS | Conventional SCS | Differenceb |
| % Who Rated Symptoms Better or A Great Deal Better | ||||||
| 12 months | 56.8 | 37.6 | 19.2 | 74.1 | 50.0 | 24.1 |
| 24 months | 63.5 | 36.6 | 26.9 | 68.6 | 48.6 | 20.0 |
| % Who Rated Symptoms Little Better, Almost the Same, or No Change | ||||||
| 12 months | 12.4 | 31.4 | −19.0 | 7.9 | 23.8 | −15.9 |
| 24 months | 20.0 | 30.9 | −10.9 | 14.0 | 25.7 | −11.7 |
Abbreviation: SCS, spinal cord stimulation.
Global Impression of Change is a 7-point Likert scale: a great deal better, better, moderately better, somewhat better, a little better, almost the same, no change.
Crude differences calculated for this health technology assessment.
Physician ratings of the improvement in patients' condition were similar to patient ratings: a greater proportion of patients in the 10-kHz high-frequency SCS group were judged to be “better” or “a great deal better,” and fewer patients had “little better” or “no change” at both follow-up points. Physician overall ratings were also significantly better for patients in the 10-kHz high-frequency SCS group than for the conventional SCS group at 12 months (P = .001) and 24 months (P = .002).
In the study by De Andres et al,29 patients and physicians completed the Global Impression of Change scale at 3, 6, and 12 months (Table 11). There were no significant differences in patient- or physician-reported change ratings between study groups at any follow-up point.
Table 11:
Global Impression of Change, De Andres et al
| Global Impression of Changea | 10-kHz High-Frequency SCS |
Conventional SCS | Differenceb |
|---|---|---|---|
| Patient Rating | |||
| 3 months | 2.35 ± 0.80 | 2.55 ± 0.87 | −0.2 |
| 6 months | 3.08 ± 1.55 | 3.00 ± 1.16 | 0.08 |
| 12 months | 3.31 ± 1.12 | 3.11 ± 1.42 | 0.2 |
| Physician Rating | |||
| 3 months | 1.62 ± 0.50 | 1.66 ± 0.67 | −0.04 |
| 6 months | 2.27 ± 1.25 | 1.93 ± 0.92 | 0.34 |
| 12 months | 2.23 ± 0.82 | 2.07 ± 1.12 | 0.16 |
Abbreviation: SCS, spinal cord stimulation.
Measured using a 7-point Likert scale: a great deal better, better, moderately better, somewhat better, a little better, almost the same, no change.
Crude differences calculated for this health technology assessment.
Source: De Andres et al, 2017.29
The SURF study28 reported that all patients demonstrated overall improvements in their symptoms, with a Global Impression of Change rating of 6 out of 7 (better and definite improvement). Change scores per study group were not reported.
The overall GRADE assessment from the randomized controlled trial for global impression of change was moderate, rated down for risk of bias (Appendix 2).
Sleep Quality
In the SENZA-RCT,30 the 10-kHz high-frequency SCS group reported greater improvements in sleep quality for 6 of the 7 subscales of the Pittsburgh Sleep Quality Index42,43: sleep quality, sleep latency, sleep duration, habitual sleep efficiency, use of sleep medications, and daytime dysfunction at 12 months (data not shown). At baseline, there was no difference between groups in the proportion of “good sleepers” and “poor sleepers” (“poor sleeper” was a global Pittsburgh score of 5 or higher). At 12 months there was a greater proportion of good sleepers in the 10-kHz high-frequency SCS group than in the conventional SCS group (P = .001; data not shown).
Mean sleep quality scores reported in the study by De Andres et al29 are summarized in Table 12 (see Appendix 5 for detailed longitudinal scores). Sleep quality was assessed using the Medical Outcomes Study-Sleep Scale, a 12-item questionnaire that provides a sleep problem index and six subscale scores; individual items are rated on a 6-point scale of “none of the time” to “all of the time.”44–46 The six subscales are somnolence (daytime sleepiness), sleep disturbance, sleep quantity (average number of hours slept per night), awake short of breath, snoring, and sleep adequacy.
Table 12:
Sleep Quality, De Andres et al
| MOS-SS Score, Mean ± SD | 10-kHz High-Frequency SCS |
Conventional SCS | Differencea |
|---|---|---|---|
| Somnolence | |||
| Baseline | 51.79 ± 23.04 | 53.10 ± 28.87 | −1.31 |
| Mean change, baseline to 12 months | −15.4 ± 21.65 | −10.0 ± 27.06 | — |
| Sleep Disturbance | |||
| Baseline | 29.66 ± 25.13 | 27.20 ± 25.24 | 2.46 |
| Mean change, baseline to 12 months | −11.59 ± 24.92 | −10.12 ± 27.95 | — |
| Sleep Quantity | |||
| Baseline | 5.25 ± 1.17 | 5.03 ± 1.37 | 0.22 |
| Mean change, baseline to 12 months | −0.48 ± 1.40 | −0.41 ± 1.63 | — |
| Awake Short of Breath | |||
| Baseline | 57.69 ± 31.15 | 57.93 ± 34.37 | −0.24 |
| Mean change, baseline to 12 months | −19.23 ± 35.09 | −7.07 ± 43.16 | — |
| Snoring | |||
| Baseline | 43.08 ± 38.65 | 34.48 ± 36.12 | 8.6 |
| Mean change, baseline to 12 months | −8.46 ± 31.07 | −5.71 ± 24.25 | — |
| Sleep Adequacy | |||
| Baseline | 27.42 ± 24.51 | 31.03 ± 26.23 | −3.61 |
| Mean change, baseline to 12 months | −12.58 ± 34.16 | −6.83 ± 38.63 | — |
Abbreviations: MOS-SS, Medical Outcomes Study-Sleep Scale; SCS, spinal cord stimulation; SD, standard deviation.
Crude differences calculated for this health technology assessment.
Source: De Andres et al, 2017.29
Overall, both study groups showed improved sleep scores for the 6 subscales. Improvements at 12-month follow-up included 10 to 15 points for somnolence, 10 to 12 points for sleep disturbance, and 7 to 13 points for sleep adequacy. The improvement in sleep quantity mean score represented a mean gain of 0.5 hours of sleep per night. However, none of the differences between the group mean change in any sleep subscore were statistically significant. In the SURF study,28 all patients reported that their sleep quality was improved (instrument not defined). Participants reported an average increase in nightly sleep duration of 1 hour, and a reduction in the number of awakenings from 3.7 to 2.11 (a 43% decrease) in the 10-kHz high-frequency SCS group and from 3.06 to 2.44 (a 20% decrease) in the comparator SCS group.
The overall GRADE assessment from the randomized controlled trials for sleep quality was moderate, rated down for risk of bias (Appendix 2).
Health-Related Quality of Life
In the SENZA-RCT,30 both groups showed improvements over baseline in their mental and physical composite subscores.47 The 12-month median improvement in physical health subscore over baseline was greater for the 10-kHz high-frequency SCS group (7.97 points, 95% CI 5.72–10.39) than for the conventional SCS group (6.20 points, 95% CI 3.70–8.78); results were similar for the median mental health subscore (3.77 points, 95% CI 0.13–7.53 vs. 2.10 points, 95% CI 1.26–5.55). The mental health subscores at baseline were higher (median score approximately 50) than the physical health subscores (median score approximately 30). Group differences were not significantly different for the physical or mental health subscores.
The study by De Andres et al29 assessed health-related quality of life using the SF-12 and reported separately for the physical and mental health subscales (see Appendix 5 for complete scores for the subdomains). The mean point differences at 6- and 12-month follow-up compared to baseline are outlined in Table 13. At 12 months, both groups showed significant improvements over baseline for all subdomains except vitality, where scores were unchanged from baseline. Social functioning scores were notably improved for patients in the 10-kHz high-frequency SCS group at 6 and 12 months but remained unchanged for the conventional SCS group. No between-group differences were statistically significant for any subdomain.
Table 13:
Health-Related Quality of Life, De Andres et al
| Mean Point Change From Baseline, 6 Months | Mean Point Change From Baseline, 12 Months | |||
|---|---|---|---|---|
| SF-12 Subscorea | 10-kHz High-Frequency SCS |
Conventional SCS | 10-kHz High-Frequency SCS |
Conventional SCS |
| Role physical | 14 | 5 | 13 | 5 |
| Bodily pain | 12 | 24 | 7 | 17 |
| General health | 12 | 16 | 9 | 20 |
| Vitality | 8 | 18 | 6 | 5 |
| Social functioning | 19 | 5 | 15 | 3 |
| Role emotional | 25 | 9 | 14 | 13 |
| Mental health | 9 | 15 | 6 | 10 |
Abbreviation: SCS, spinal cord stimulation.
Physical functioning score was not included because the scores for the 10-kHz high-frequency SCS group were zero at baseline.
Source: De Andres et al, 2017.29
The SURF study28 reported on the EQ-5D-5L health status measure, which is based on self-report of 5 health states (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression).48 Health value mean results increased by 21.2 points (60.3 to 81.5) for the 10-kHz high-frequency SCS group and by 26.6 points (51.8 to 78.4) for the comparator SCS group. Based on a summary grouping of health profiles in the EQ-5D-5L,49 a majority of patients in each study group had better health-related quality of life or health profiles that were better (health was better in at least one domain and no worse in any domain) after SCS (Table 14). A higher proportion of patients in the comparator SCS group had a health-related quality of life or health profile that was worse (health had worsened in one domain and not improved in any).
Table 14:
Health-Related Quality of Life, SURF Study
| EQ-5D-5L Health Profile Grouping, %a | 10-kHz High-Frequency SCS | Comparator SCSb | Combined |
|---|---|---|---|
| Better | 79 | 79 | 79 |
| Equal | 0 | 0 | 0 |
| Mixed | 18 | 9 | 14 |
| Worse | 3 | 12 | 7 |
Abbreviation: SCS, spinal cord stimulation.
EQ-5D-5L health profile groupings: better (at least 1 dimension better and no worse in any other); equal (health state exactly the same); mixed (better and worse on at least one dimension); worse (at least one dimension is worse and is no better on any other).
Comparator SCS protocols included low-frequency, burst, and 1.2–1.5 kHz moderate-frequency.
Source: Bolash et al, 2019.28
The overall GRADE assessment from the randomized controlled trials for health-related quality of life was moderate, rated down for risk of bias (Appendix 2).
Safety
The SENZA-RCT study30 reported adverse events for patients with predominant back pain and a pain etiology of failed back surgery syndrome, undergoing SCS, and followed for 24 months. The key safety outcome of the trial was the absence of a stimulation-related neurological deficit. This was the only study to report a standardized neurological assessment (motor, sensory, and reflex functions) in its follow-up. All adverse events were recorded, but major adverse events were not defined. The study was monitored by an independent data safety monitoring board consisting of a neurologist, an anesthesiologist, a neurosurgeon, and a biostatistician.
No stimulation-related neurological deficits were reported for either study group. Study-related major adverse event rates of 4.0% (4 patients) for the 10-kHz high-frequency SCS group and 7.2% (7 patients) for the conventional SCS group were not significantly different (P = .49). The most common major adverse event in each group was lead migration requiring surgical revision (3.0% in the 10-kHz high-frequency SCS group and 5.2% in the conventional SCS group) and wound complications (4.0% in the 10-kHz high-frequency SCS group and 3.1% in the conventional SCS group). Two patients died during the study, one in each group. The patient in the 10-kHz high-frequency SCS group died from a malignant hepatic tumour, and the patient in the conventional SCS group died from a myocardial infarction during the procedure. Minor or nonserious adverse events occurred more frequently and were also not significantly different between the trial arms (28% [28 patients] in the 10-kHz high-frequency SCS group and 33% [32 patients] in the conventional SCS group). The most common adverse event was implant site pain (11.9% of the 10-kHz high-frequency SCS group and 10.3% of conventional SCS group). Uncomfortable paresthesia was reported for 10.3% of the conventional SCS group and none of the patients in the 10-kHz high-frequency SCS group.
The study by De Andres et al29 also reported adverse events for patients with chronic back pain, all with failed back surgery syndrome, followed for 1 year. This study was a primary pain efficacy trial, and other outcomes, including safety, were secondary objectives. No infections, neurological deficits, or dysfunctions were reported for patients in either study arm. Implant site–related pain or infections were also not reported. Surgical revision for lead migration during the first year was the only complication reported, and was similar for the two study groups (3.4% [1 patient] in the 10-kHz high-frequency SCS group and 6.5% [2 patients] in the conventional SCS group). Uncomfortable paresthesia was not reported in either study group.
The SURF study28 reported adverse events for patients with failed back surgery syndrome, with chronic back or back/leg pain, followed for 6 months. Only one reported major adverse event was reported, in the comparator group: an infection at the incision site that required hospitalization. The overall minor adverse event rate was 26%: 22% in the 10-kHz high-frequency SCS group and 31% in the comparator group. Overall, 11 patients (11%) had complications that involved surgical revisions. The most common complication was lead migration (16%). Unintended or nontarget stimulation occurred in 4% of patients: 0% the 10-kHz high-frequency SCS group and 8% in the comparator group.
Discussion
We identified three randomized controlled trials28–30 exploring 10-kHz high-frequency SCS, two involving the Senza HF10 (a fully implanted and wired system) and one involving the Freedom SCS system (implanted epidural leads and a wireless pulse generator). The studies involving the Senza HF1029,30 compared patients randomized to 10-kHz high-frequency SCS or conventional SCS, delivered by different devices. In the SURF study,28 a wireless pulse generator device provided the 10-kHz high-frequency SCS to the investigational group and a mix of patient-selected stimulator protocols (LF, burst, or high-density) to the comparator group. All studies involved a more restricted chronic pain patient population, mainly those with back or back/leg pain who had failed previous back surgery. Follow-up in all trials was short-term: 2 years or less. The primary outcomes involved different criteria for noninferiority or superiority for 10-kHz high-frequency SCS.
The two trials conducted in the United States28,30 based their primary outcomes on back pain responder rates (≥ 50% reduction in back pain intensity), and both reported high pain responder rates for 10-kHz high-frequency SCS (85% and 92%). However, a very large difference in back-pain responder rates for the control groups (44% in SENZA-RCT and 82% in SURF) led to the superiority claim (41% mean difference) in the SENZA-RCT study and the noninferiority claim (10% mean difference) in the SURF study. Including only low-frequency SCS, the comparator arm created a greater difference in terms of benefit for 10-kHz high-frequency SCS than is seen when comparing to other forms of SCS currently available in clinical practice.
Fewer patients in both studies were classified as being pain remitters (having low or minimal back or leg pain scores).28,30 The difference in back pain remittance rates between study arms (10-kHz vs. comparator) was much closer for the two studies: a 35% (66% vs. 31%) mean remitter rate difference between groups in the SENZA-RCT study and a 37% (84% vs. 47%) mean remitter rate difference for the SURF study. Patients who underwent 10-kHz high-frequency SCS or conventional SCS were more likely to have their pain reduced than to have it eliminated, suggesting that patients should be counselled on the likelihood of these treatment outcomes.
The study by De Andres et al,29 conducted in Europe, reported overlapping mean pain intensity scores between the trial arms over a 12-month follow-up; pain responder rates were not reported. The mean pain score reductions after 10-kHz high-frequency SCS were significantly lower than those reported in the other randomized controlled trials. However, this was the only study to evaluate neuropathic pain separately; reductions in these scores overlapped between groups and were not significantly different at 12 months.
In all randomized controlled trials,28–30 secondary outcomes involving functional disability, psychological morbidity, and health-related quality of life (all evaluated using validated outcome measures) showed significant improvement. These results were consistent with initial and ongoing significant reductions in pain intensity with 10-kHz high-frequency SCS and conventional SCS.
Reductions in opioid use after 10-kHz high-frequency SCS was reported.30 The majority of patients in the randomized controlled trial had been using various analgesics, including high-dose opioids, for their chronic pain conditions. The daily mean morphine equivalent (MME) doses reported for patients in the studies were higher than the 90 MME/day, which is a high-risk dose that has been recommended to be considered carefully and prescribed mainly for cancer patients or those in palliative care.
In studies involving crossover designs where the same patient was randomly assigned to different SCS protocols or waveforms, individual patients' responses and preferences can be examined. In the study by Thomson et al,38 patients randomized to SCS with a range of frequencies for 3 weeks reported pain reductions and their frequency preference, which they maintained for 3 months after the randomization phase of the trial. Notably, reduction in pain intensity was similar over the 1-kHz to 10-kHz range, and preferences were reported for each frequency level, most preferring 1 kHz.
The significantly higher electrical dose for 10-kHz high-frequency SCS may also have implications for battery recharging and battery life, although the short-term follow-up of the included studies did not allow for an evaluation of battery life under real-life conditions of use. Battery recharging requirements, patient satisfaction with these requirements, and patients' device-programming adjustments were reported in the multinational Senza HF10 registry.38 Most patients reported daily recharging of up to an hour, and although most patients reported being satisfied with these requirements, 13% reported feeling either neutral or dissatisfied.
The common risks associated with conventional SCS—namely device/procedure-related complications such as implant site pocket pain and migration of the epidural leads—also occur with 10-kHz high-frequency SCS. In most cases, the effects of migrating leads were managed with reprogramming. Surgery or an additional procedure to reposition or replace epidural leads occurred infrequently. Migration of leads resulting in paralysis or nerve injury are potential complications, but they were not reported in any of the studies. No adverse neurological effects—specifically evaluated in several studies—were reported in any trials.
Infections occurred at incision sites, but deep infections such as epidural abscess occurred very infrequently. Although biological responses or reactions can occur to the materials in the epidural leads or pulse generators, or to any leaks of the implantable pulse generator, these complications were not reported, although clinical cohorts were small and follow-up was short-term. Pocket pain or pain at the implant site was one of the most common adverse events reported, but the cause of pain (e.g., infection or inflammatory reactions) was often not investigated or reported. The material of the epidural leads and pulse generator in the 10-kHz high-frequency SCS systems is similar to those used in other SCS systems, so additional risks because of biological or immune-related factors are not anticipated with 10-kHz high-frequency SCS. Again, however, the duration of follow-up was short—usually 2 years or less.
The main uncertainty related to the safety of 10-kHz high-frequency SCS relates to the longer-term use of continuous high electrical doses delivered to neural tissue. Dose calculations for 10-kHz high-frequency SCS versus moderate-frequency SCS (1 kHz, 4 kHz) indicated that the tissue dose is almost three times higher with 10-kHz high-frequency SCS. In long-term follow-up, however, it may be difficult to attribute adverse neurological events to 10-kHz SCS in chronic pain patient cohorts with ongoing spinal degenerative conditions that may be partially or mainly responsible for an altered or emerging pain of spinal complications.
Limitations
The comparisons in the randomized controlled trials all involved conventional low-frequency SCS as the active comparator. Although there are at least five sham-controlled trials for other SCS protocols for chronic pain,50–54 there are no trials of 10-kHz high-frequency SCS compared with a sham arm to evaluate placebo response.
The randomized controlled trials evaluating 10-kHz high-frequency SCS involved a more restricted patient population than used in other SCS trials. The generalizability of the findings (mainly involving those with back pain after failing back surgery) to other chronic pain populations may be limited. Although persistent postoperative pain is often neuropathic, few studies evaluated or reported this type of pain etiology in their study groups.
A 2-year follow-up does not provide evidence for the longer-term effectiveness of 10-kHz high-frequency SCS. It is also uncertain whether habituation or loss of efficacy would be a greater or less over time with continuous 10-kHz high-frequency SCS, than with conventional SCS or alternate waveforms. The short-term follow-up also limits any conclusions about the longevity of the pulse generator device, given the intensive charge load and frequent battery recharging required with 10-kHz high-frequency SCS. The longer-term safety and potential adverse effects of this high frequency on neural tissues are also unknown.
Conclusions
For patients with chronic noncancer pain refractory to medical management, 10-kHz high-frequency SCS likely provides reductions in pain intensity and functional disability, and improvements in quality of life (GRADE: Moderate). Patients with chronic pain who were taking high levels of opioids may reduce their opioid consumption with 10-kHz high-frequency SCS (GRADE: Low). Patient treatment satisfaction and global impression of change likely improved with 10-kHz high-frequency SCS (GRADE: Moderate).
Randomized controlled trials supported the statistical noninferiority of 10-kHz high-frequency SCS to conventional SCS for pain responder rates at short-term follow-up, but the results were inconsistent with respect to the superiority of 10-kHz high-frequency SCS (GRADE: Moderate). In short-term follow-up, major adverse events were uncommon for both 10-kHz high-frequency SCS and conventional SCS. The short-term follow-up limited our ability to form conclusions about the longer-term effectiveness or safety of the continuous high-frequency electrical stimulation of neural tissue.
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of 10-kHz high-frequency spinal cord stimulation (SCS) compared with any other forms of SCS for the treatment of adults with chronic noncancer pain that is refractory to medical management?
Methods
Economic Literature Search
We performed an economic literature search on August 20, 2018, to retrieve studies published from database inception until the search date. To retrieve relevant studies, we developed a search using the clinical search strategy with an economic and costing filter applied.
We created database auto-alerts in MEDLINE and Embase and monitored them for the duration of the assessment period. We also performed a targeted grey literature search of health technology assessment agency websites, clinical trial and systematic review registries, and the Tufts Cost-Effectiveness Analysis Registry. See Clinical Literature Search, above, for further details on methods used. See Appendix 1 for our literature search strategies, including all search terms.
Eligibility Criteria
Studies
Inclusion Criteria
English-language full-text publications
Studies published from database inception until August 20, 2018
Cost-benefit analyses, cost-effectiveness analyses, cost-minimization analyses, cost–utility analyses, or cost–consequence analyses
Letters, conference abstracts, or commentaries reporting original study results
Exclusion Criteria
Narrative reviews, editorials, systematic reviews, study protocols, guidelines, or unpublished studies
Noncomparative costing studies or cost-of-illness studies
Population
Adults aged 18 years and older with chronic noncancer pain (e.g., failed back surgery syndrome or complex regional pain syndrome) who are refractory to medical management and potentially eligible for 10-kHz high-frequency SCS rescue therapy (e.g., Senza HF10 SCS treatment) after failing other SCS modalities
Studies in people with acute pain, cancer pain, major psychiatric comorbidity, or progressive disease were excluded
Interventions
10-kHz high-frequency SCS (e.g., Senza HF10)
Any other form of SCS (paresthesia or paresthesia-free frequency using tonic or burst waveforms) used as a standard of care or as a rescue therapy (a treatment of last resort for people who have failed all other treatment options)
Outcome Measures
Incremental costs
Incremental effectiveness (e.g., quality-adjusted life-years [QALYs], disability-adjusted life-years)
Incremental economic statistics such as incremental cost-effectiveness ratio (ICER) or incremental net benefit
Literature Screening
A single reviewer conducted an initial screening of titles and abstracts using DistillerSR55 and then obtained the full texts of studies that appeared eligible for review according to the inclusion criteria. A single reviewer then examined the full-text articles and selected studies eligible for inclusion. The reviewer also examined reference lists for any additional relevant studies not identified through the search. A second reviewer confirmed the study eligibility identified in the initial and full-text screening.
Data Extraction
We extracted relevant data on study characteristics and outcomes to collect information about the following:
Source (e.g., citation information, study type)
Methods (e.g., study design, analytic technique, perspective, time horizon, population, intervention[s], comparator[s])
Outcomes (e.g., health outcomes, costs, ICER[s])
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified quality appraisal checklist for economic evaluations originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom to inform the development of NICE's clinical guidelines.56 We modified the wording of the questions to remove references to guidelines and to make the questionnaire specific to Ontario. Next, we separated the checklist into two sections. In the first section, we assessed the applicability of each study to the research question (directly, partially, or not applicable). In the second section, we assessed the limitations (minor, potentially serious, or very serious) of the studies that we found to be directly applicable.
Results
Economic Literature Search
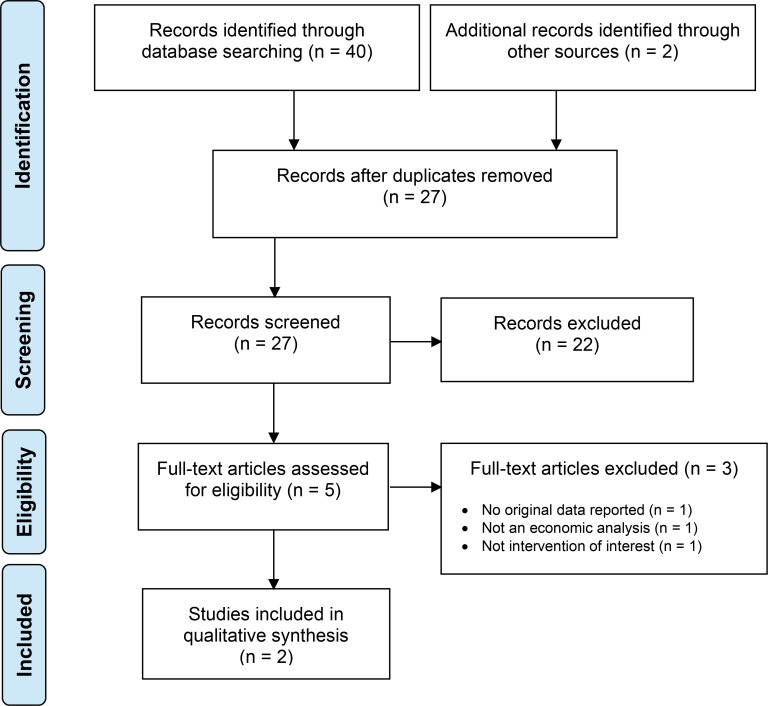
The economic literature search yielded 27 citations published from database inception until August 20, 2018, after removing duplicates. We identified 2 studies that met our inclusion criteria. See Appendix 6 for a list of studies excluded after full-text review. Figure 2 presents the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram for the economic literature search.
Figure 2: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al, 2009.27
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Overview of Included Economic Studies
We have summarized the results of the 2 included studies57 in Table 15. Neither study examined the cost-effectiveness of 10-kHz high-frequency SCS as a rescue therapy.
Table 15:
Results of Economic Literature Review—Summary
| Author, Year, Country of Publication | Analytic Technique, Study Design, Perspective, Time Horizon | Population | Intervention(s) and Comparator(s) | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | ||||
| NICE, 201958 United Kingdom (manufacturer's economic model submission) |
Type of economic analysis: CCA Study design: model-based economic study Perspective: NHS and PSS Time horizon: 15 years |
Adults with chronic paina Total: NA Mean age, y: NR Male, %: NR |
Intervention Senza HF10 SCS + CMM as required Comparators CNR-SCSb + CMM as required CR-SCSb + CMM as required |
Model outcomes based on utilities were not reported, to align with the NICE MTEP cost–consequence framework; reported outcomes of the model were costs only Clinical parametersc were derived largely from the SENZA-RCT study30,31 and informed transition probabilitiesd in the decision tree and Markov model |
Currency, cost year: £, 2016 Discount rate: 3.5% Total mean costs CNR-SCSb + CMM: £95,156 CR-SCSb + CMM: £92,192 Senza HF10 SCS + CMM: £87,400 Incremental costs Senza HF10 SCS vs. CR-SCSb: −£320/y or −£4,795 over 15 y Senza HF10 SCS vs. CNR-SCSb: −£500/y or −£7,755 over 15 y NICE determined that Senza HF10 SCS would accrue costs similar to low-frequency conventional SCS over 15 years, after taking into account an alternate estimate for the rate of unanticipated explantation61 |
Reference case Senza HF10 SCS vs. CR-SCSb: dominante Senza HF10 SCS vs. CNR-SCSb: dominante Sensitivity analyses PSA: Senza HF10 SCS vs. CR-SCS or CNR-SCS was cost-saving 73% or 74% of the time, respectively NICE determined that Senza HF10 SCS was approximately cost neutral compared with conventional SCS, when considering the new evidence for an alternate estimate of rate of unanticipated explantation61 |
| Annemans, 201459 United Kingdom |
Type of economic analysis: CUA Study design: model-based economic study Perspective: NHS Time horizon: 15 years |
Patients with chronic pain Total: NA Mean age, y: 49.7 Male, %: 45 |
Intervention Senza HF10 SCS Comparators CMM only Reoperation CNR-SCS CR-SCS |
Simulated cohort of 1,000 patients over 15 y Discount rate: 3.5% Intervention options vs. CNR-SCSf Total QALYs: CNR-SCS 4,647; CR-SCS 4,648; Senza HF10 SCS 5,151 Mean difference: CR-SCS vs. CNR-SCS 1; Senza HF10 SCS vs. CNR-SCS 504 Intervention options vs. CNR-SCSf Total QALYs: CR-SCS 4,439; CNR-SCS 4,648; Senza HF10 SCS 5,151 Mean difference: CNR-SCS vs. CR-SCS 209; Senza HF10 SCS vs. CR-SCS 712 |
Simulated cohort of 1,000 patients over 15 y Currency, cost year: £, NR Discount rate: 3.5% Intervention options vs. CNR-SCSg,h Total mean cost: CNR-SCS £92,392,857; CR-SCS £87,440,887; Senza HF10 SCS £86,417,656 Mean difference: SCS vs. CNR-SCS −£4,951,970; Senza HF10 SCS vs. CNR-SCS −£5,975,201 Intervention options vs. CR-SCSg,h Total mean cost: CR-SCS £92,561,091; CNR-SCS £87,440,887; Senza HF10 SCS £86,417,656 Mean difference: CNR-SCS vs. CR-SCS: −£5,120,204; Senza HF10 SCS vs. TR SCS: –£1,023,231 |
Intervention options vs. CNR-SCSj CR-SCS vs. CNR-SCS: dominantd Senza HF10 SCS vs. CNR-SCS: dominante Intervention options vs. CR-SCSjCNR-SCS vs. CR-SCS: dominant Senza HF10 SCS vs. CR-SCS: dominante One-way deterministic sensitivity analyses (Senza HF10 SCS vs. CMM) Driving parameters were device longevity (ICERs £700 to £6,500/QALY) and device cost (ICERs £0 to £1,300/QALY) Threshold analyses Senza HF10 SCS must achieve ≥60% responder rate (≥50% pain relief) at 6 months to remain dominante |
Abbreviations: CCA, cost–consequence analysis; CMM, conventional medical management; CNR-SCS; conventional nonrechargeable SCS; CRPS, complex regional pain syndrome; CR-SCS, conventional rechargeable SCS; CUA, cost–utility analysis; FBSS, failed back surgery syndrome; ICER, incremental cost-effectiveness ratio; MTEP, Medical Technologies Evaluation Programme; NA, not applicable; NHS, National Health Service; NICE, National Institute for Health and Care Excellence; NR, not reported; PSA, probabilistic sensitivity analyses; PSS, Personal Social Service; QALY, quality-adjusted life-year; SCS, spinal cord stimulation; VAS, visual analogue scale.
The target population was derived mainly from people with back and/or leg pain as a result of FBSS. Results of this study should not be extrapolated to people with neuropathic pain of the head, neck, or arm, or to people with CRPS.
Both CNR-SCS and CR-SCS were defined as low-frequency (up to 1.2 kHz).
Clinical parameters considered in model: pain scores (e.g., VAS score), duration of pain relief, patient satisfaction (e.g., relating to frequency of battery recharging), health-related quality of life, functional disability measures (e.g., disability index score, Oswestry Disability Index, and functional improvement, including ability to drive and perform work-related activities), opioid and other analgesic use, device-related adverse events, incidence of paresthesia, and reason for implant removal.
The transition probabilities informed by clinical parameters in the decision tree (initial 6 months) included probabilities of trial success leading to permanent implantation, probability of achieving optimal reduction in leg pain, and probability of nonserious complications; the transition probabilities informed by clinical parameters in the Markov model (beyond 6 months) included probability of nonserious adverse events (beyond 6 months) and probability of serious adverse events (i.e., ineffective pain control, intolerable paresthesia, and other adverse events, such as surgical site infections, or patient falls).
Dominant = lower cost and higher QALYs.
Reported reference case results also included mean and incremental QALYs for CMM and reoperation, not summarized in this table.
Reported reference case results also included mean and incremental costs for CMM and reoperation, not summarized in this table.
The cost of Senza HF10 SCS was assumed to be the same as for conventional rechargeable SCS, at £4,442 for the SCS trial procedure, £15,056 for the device, £1,720 for additional CMM as needed in first 6 months, £860 for additional CMM as needed per 3 months from the first year and onwards, £622 for implant-related complications, and £1,800 for device explantation (i.e., implantable pulse generator).
Reported reference case results also included ICERs for CMM and reoperation versus comparators, not summarized in this table.
The first study58 was a NICE medical technologies guidance on Senza HF10 (Nevro Corp., Menlo Park, CA). The guidance provided an analysis of the economic evidence, including an economic model developed by the manufacturer (Nevro Corp.) that assessed the cost-effectiveness of the Senza HF10 plus conventional medical management versus either conventional nonrechargeable or conventional rechargeable SCS plus conventional medical management. This economic model was developed from the perspective of the United Kingdom public payer for health care and social services (i.e., the National Health Service and Personal Social Service). It was an iteration of the model used in the study by Annemans et al (our second included study, below),59 but with updated clinical inputs from the comparative noninferiority SENZA-RCT study.30,31 The SENZA-RCT study compared the safety and efficacy of various SCS technologies in people with back and leg pain. Most of the study population was people with failed back surgery syndrome. The NICE medical technologies guidance reported costs in 2016 GBP and used a discount rate of 3.5% for both future benefits and costs.
In accordance with the NICE Medical Technologies Evaluation Programme methods guide,60 Nevro Corp. evaluated Senza HF10 SCS using a cost–consequence framework. The reference case results showed that the total mean costs per patient over 15 years for the Senza HF10 SCS, conventional nonrechargeable SCS, and conventional rechargeable SCS arms, were £87,400, £95,156, and £92,196, respectively. Therefore, in the economic model, Senza HF10 SCS was the least costly treatment. Compared with conventional nonrechargeable SCS and conventional rechargeable SCS, Senza HF10 SCS was associated with cost savings of £4,795 and £7,755, respectively.58 However, in its final analysis of the economic evidence,58 NICE determined that Senza SCS would accrue costs similar to conventional SCS (rechargeable or nonrechargeable) over 15 years, after taking into account an alternate estimate for the rate of unanticipated explantation provided by real-world data.61
The second study, by Annemans et al,59 assessed the cost-effectiveness of the Senza HF10 SCS compared with conventional medical management, reoperation, conventional nonrechargeable SCS, and conventional rechargeable SCS in people with failed back surgery syndrome. This study used a semi-Markov model, which included a decision tree that assessed the cost and health outcomes of treatment in the initial 6 months, followed by a Markov statetransition model with a time horizon of 15 years. The analytic perspective was the United Kingdom public health care payer (i.e., National Health Service); future benefits and costs (GBP) were discounted at 3.5%. Clinical inputs for the Senza HF10 SCS model parameters were obtained from the SENZA-EU observational study,62 which had a total population of 72 patients (including 57 people with failed back surgery syndrome) and reported results at 6 and 24 months. In the SENZA-EU study, the responder rate (i.e., the percentage of people with pain reduction ≥50%) for Senza HF10 SCS was 74% at 6 months and 71% at 24 months.62
Assuming equal costs for conventional rechargeable SCS and Senza HF10 SCS, the study by Annemans et al59 found that Senza HF10 SCS was dominant (i.e., generating more QALYs at a lower cost), amounting to cost savings of £5,975 per patient. Compared with conventional nonrechargeable SCS, Senza HF10 SCS was also dominant, amounting to cost savings of £1,023 per patient. Compared with conventional medical management and reoperation, Senza HF10 SCS was more expensive and more effective, with ICERs of £3,153 and £2,666 per QALY gained, respectively. Compared with all other treatment options, Senza HF10 SCS was either cost-saving or had an ICER lower than the reported willingness-to-pay value of £20,000. The results of both studies are summarized in Table 15.
Applicability and Limitations of the Included Studies
Appendix 7 provides the results of the quality appraisal checklists for economic evaluations applied to the included studies.
Both studies58,59 were deemed partially applicable to our research question.
First, the manufacturer's economic model in the NICE medical technologies guidance58 carried out a cost–consequence analysis using a United Kingdom public health care payer perspective. As a result, it was difficult to infer the cost-effectiveness of Senza HF10 SCS, because the study did not report utility-based outcomes. The study by Annemans et al59 was also conducted from a United Kingdom public health care payer perspective and also did not assess all SCS devices currently used in use in Ontario (e.g., SCS technologies using burst frequency stimulation).
Second, there were differences between our target population (adults with any type of chronic noncancer pain) and those of both included studies (people with chronic noncancer back or leg pain, specific to failed back surgery syndrome, failing appropriate conventional medical management, including lumbosacral spine surgery). Failed back surgery syndrome is one type of chronic pain condition.63 Our target population was also focused on patients with chronic pain who were eligible for rescue therapy after failing all previous lines of treatment, including conventional medical management (e.g., medications, physiotherapy, or psychological support) and other SCS modalities. In contrast, participants in the two included studies58,59 who received Senza HF10 SCS had not been previously treated with any type of SCS device.
Both the manufacturer's economic model in the NICE medical technologies guidance58 and the study by Annemans et al59 had potentially serious limitations.
For instance, the study by Annemans et al59 derived its clinical estimates for the effectiveness of Senza HF10 SCS from an observational study,62 whereas the manufacturer's economic model used estimates from a head-to-head comparative randomized controlled trial30,31 that was previously unavailable.
Furthermore, the cost–consequence framework used in the manufacturer's economic model may have some structural implications. First, because of simplifying assumptions, the model may not represent all possible outcomes of the strategies of interest.60,64 For instance, in the cost–utility model in the study by Annemans et al,59 a decision-tree model was used to simulate treated patients for the first 6 months, during which either optimal or suboptimal pain relief could have been achieved. In the long-term Markov model that followed, patients could transition to one of four health states: optimal pain relief, suboptimal pain relief, no pain relief, or death. In the NICE cost–consequence model, however, patients who achieved optimal or suboptimal pain relief upon entering the Markov model were only able to transition to one of two health states: no pain relief (i.e., device is explanted) or death.64 Second, the cost–consequence framework may not have adequately captured the potential costs associated with different degrees of pain, because the health-related utilities associated with the various pain states (optimal and suboptimal pain relief) were omitted.64
Finally, although we were able to ascertain the make and model of the devices used in the clinical trials that informed the model input parameters in both studies (Medtronic's Synergy informed the nonrechargeable SCS arm, and RestoreUltra systems informed the rechargeable SCS arm in the study by Annemans et al59; the rechargeable Boston Scientific PrecisionPlus system informed both conventional SCS arms in the manufacturer's economic model),30,31 it is important to note that these devices can be used to deliver frequencies from 1 Hz to 1.2 kHz; this range includes frequencies beyond conventional SCS. As such, it is not clear what frequency was applied for the conventional SCS arms compared to the Senza HF10 arm (which delivered a known frequency of 10 kHz).
Discussion
The two studies included in our economic literature review58,59 found that Senza HF10 SCS was either cost-saving or similar in costs to conventional SCS in adults with back and/or leg pain as a result of failed back surgery syndrome. Results from the study by Annemans et al59 demonstrated that Senza HF10 SCS was dominant compared to both conventional nonrechargeable and conventional rechargeable SCS, with greater health effects and cost savings of £5,975 and £1,023 per patient over 15 years, respectively.59 However, the NICE medical technology guidance58 determined that over 15 years, Senza HF10 SCS would accrue costs similar to conventional SCS (nonrechargeable or rechargeable).
Both studies58,59 made assumptions that could be considered conservative and may have underestimated the health effects reported in the reference case results. For instance, the manufacturer's economic model in the NICE medical technology guidance used effectiveness data on the reduction of leg pain from the SENZA-EU study, but Senza HF10 SCS may deliver a larger pain reduction for back pain.58 As well, both studies considered only the acquisition costs of the Senza HF10 system and the complication costs; they did not account for procedure costs, such as those associated with consultations, surgery, or hospital admissions, assuming that procedure costs were equivalent for all SCS devices. This was considered a conservative assumption, given that the Senza HF10 implantation procedure has a shorter duration than conventional SCS because paresthesia mapping is omitted, which may generate cost savings from reduced resource use related to hospital staff and operating room time.58,59 Although the reference case results differed between the two studies, these conservative assumptions may indicate that it is unlikely that Senza HF10 SCS will incur additional overall costs compared with conventional SCS.
However, it may be useful to have a better understanding of the average range of frequencies applied in the conventional SCS treatments in the included studies. Conventional SCS devices can be used to deliver a wide range of frequencies; theoretically, different frequencies may have different clinical effects (e.g., 3 Hz vs. 50 Hz vs. 1 kHz), and those may differ from 10 kHz.
Finally, unlike the focus of our included studies, our research question was focused on a subset of people with any type of chronic noncancer pain who had failed all other lines of treatment, including both conventional medical management (e.g., medications, physiotherapy, or psychological support) and other SCS modalities. For these reasons, the results from the study populations of our economic literature review could not be generalized to our target population.
Conclusions
We included two studies58,59 in our economic evidence review. One found that Senza HF10 SCS was cost-saving compared to both conventional nonrechargeable and conventional rechargeable SCS for people with failed back surgery syndrome, and the other found that it was similar in costs. Neither of these studies examined the cost-effectiveness of 10-kHz high-frequency SCS as rescue therapy after people had failed all other lines of treatment, including conventional medical management and other SCS modalities for the treatment of adults with chronic noncancer pain.
PRIMARY ECONOMIC EVALUATION
Several Canadian studies have shown favourable cost-effectiveness for conventional spinal cord stimulation (SCS) devices compared with medications for the treatment of chronic back and leg pain.65–67 These SCS systems can deliver paresthesia or paresthesia-free tonic or burst waveforms at frequencies of up to 1.2 kHz, and they are currently funded in Ontario for the treatment of adults with chronic noncancer pain, including failed back surgery syndrome, complex regional pain syndrome, or low back pain or neuropathic pain of certain origin (e.g., phantom/limb syndrome, spinal cord injury).68
Our review of the economic literature was focused on the cost-effectiveness of 10-kHz high-frequency SCS. Neither of the studies included in the review59,69 was directly applicable to our research question, which was focused on a subpopulation of people with chronic noncancer pain who would use 10-kHz high-frequency SCS as a rescue therapy after failing all other treatment options, including SCS modalities currently available in Ontario. The two included studies compared the Senza HF10 device with other standard treatments, predominantly in people with failed back surgery syndrome. The comparators were conventional tonic SCS modalities at unreported frequencies, reoperation, or conventional medical management. As well, these studies did not include all SCS devices currently available in Ontario, including burst SCS modalities or the 10-kHz high-frequency Freedom SCS system.
There is also limited evidence directly comparing 10-kHz high-frequency SCS using the Freedom SCS system with moderate-frequency SCS modalities (1 kHz and 1.2 kHz) that are used in Ontario for people with chronic noncancer pain in whom currently available SCS therapies have not been effective. As well, no published study has directly compared the effectiveness and cost-effectiveness of the Senza HF10 SCS and Freedom SCS system in people with chronic noncancer pain.
Because good-quality clinical and economic evidence is lacking for 10-kHz high-frequency SCS in managing chronic noncancer pain in people who have failed medication management and other SCS treatment options (including lower-frequency SCS, burst SCS, and moderate-frequency SCS [up to 1.2 kHz]), and because there is no access to the Senza HF10 system for people in Ontario, we did not pursue a primary economic evaluation.
BUDGET IMPACT ANALYSIS
Research Question
From the perspective of the Ontario Ministry of Health, what is the potential budget impact of publicly funding 10-kHz high-frequency spinal cord stimulation (SCS) for adults with chronic noncancer pain who are refractory to medical management and other currently available SCS modalities in Ontario?
Methods
Analytic Framework
We estimated the budget impact of publicly funding 10-kHz high-frequency SCS using the cost difference between two scenarios: (1) current clinical practice that includes available SCS modalities in Ontario used for treatment of adults with chronic noncancer pain (current scenario); and (2) use of 10-kHz high-frequency SCS as a rescue option after failing all other lines of therapy, including low-frequency or available moderate-frequency modalities (up to 1.2 kHz) for adults with chronic noncancer pain (new scenario). Figure 3 presents the budget impact model schematic. In this clinical pathway, therapy with 10-kHz high-frequency SCS is not considered to be the first-line treatment option. Therefore, people would be eligible for 10-kHz high-frequency SCS after they fail medication management and other currently available therapy with low- to moderate-frequency SCS (up to 1.2 kHz).
Figure 3: Budget Impact Model Schematic.
Abbreviation: SCS, spinal cord stimulation.
In the reference case analysis, the intervention of interest was 10-kHz high-frequency SCS using the Freedom SCS system. We made that choice because the Freedom SCS system (1) has 10-kHz high-frequency as one of its treatment modalities; (2) has obtained Health Canada approval; and (3) has been employed in clinical practice in a small subset of patients since November 2018 in Ontario (oral and written communications, Aaron Hong, MD, November and December 2018; written communication, Stimwave Technologies Ltd., November 2018).
In a scenario analysis, the intervention of interest was 10-kHz high-frequency SCS using the Senza HF10 system. Given that treatment with this device is accessible only at the Nevro Corp. supervised neuromodulation centres in the United States and parts of Europe, this scenario explored the budget impact of funding treatment with the Senza HF10 if it was outsourced to the United States and funded though the Ontario Ministry of Health Out-of-Country Prior Approval program (http://www.health.gov.on.ca/en/public/programs/ohip/outofcountry/prior_approval.aspx).
Key Assumptions
Based on expert consultation and information from the manufacturer, only a small number of people receive 10-kHz high-frequency SCS in the current scenario (written communication, Stimwave Technologies Ltd., November 2018).
Based on current clinical practice in Ontario and expert consultation, only people who fail all other treatment options available under the Ontario clinical pathway are considered eligible for 10-kHz high-frequency SCS as rescue therapy (oral and written communications, Anuj Bhatia, MD, PhD, and Aaron Hong, MD, November 2018). This subgroup remained small compared to all people with chronic noncancer pain who are eligible for SCS treatment in general.
Based on clinical evidence,29–31 rates of complications are similar between the SCS systems currently in use and the Senza HF10 10-kHz high-frequency SCS device. Therefore, we accounted for major complications only. We adjusted the complication rates associated with the Freedom SCS system because it uses a minimally invasive procedure and its power source is worn externally (written communication, Stimwave Technologies Ltd., November 2018). The Freedom SCS system batteries and pulse generator are not internally implanted, as they are for the other commonly used SCS devices or for the Senza HF10 (see Complication Costs, below, for more information).
Target Population
Spinal cord stimulation is recommended after medical management or physical interventions have failed to provide pain relief. Most people treated with SCS have failed back surgery syndrome, complex regional pain syndrome, or other neuropathic pain syndromes. In these people, 10-kHz high-frequency SCS is used as a treatment of last resort after they have failed all currently available SCS options, including those in the 1.2 kHz range.
The population of interest for this analysis was adults (age 18 years and older) with chronic noncancer pain who had failed medication management and SCS therapies currently available in Ontario, and became eligible for 10-kHz high-frequency SCS as a rescue option (with the Freedom SCS system or the Senza HF10).
Table 16 presents the overall number of cases funded for currently available SCS therapies over the last 2 years at the 6 centres of excellence in Ontario (written communication, Provincial Programs Branch, Ontario Ministry of Health, November 2018). We used these numbers to estimate the target populations for the current and future scenarios.
Table 16:
Cases Funded for Treatment With SCS Systems Currently Used in Ontario, 2016–2018
| 2017/18 | 2016/17 | |||
|---|---|---|---|---|
| Hospital | Funded Volume | Actually Reported | Funded Volume | Actually Reported |
| The Ottawa Hospital | 23 | 13 | 30 | 15 |
| Hamilton Health Sciences | 15 | 27 | 15 | 15 |
| London Health Sciences | 43 | 37 | 100 | 45 |
| St. Michael's Hospital | 50 | 48 | 50 | 0 |
| University Health Network | 40 | 42 | 40 | 32 |
| Kingston Health Sciences | 6 | 5 | 0 | 0 |
| Total | 177 | 172 | 235 | 107 |
Source: Data provided by the Provincial Programs Branch, Ontario Ministry of Health (written communication, November 2018).
Table 17 presents an estimate of the number of people currently funded for SCS in year 1 in Ontario. We conservatively assumed that the number of newly funded cases would represent an increase of approximately 5% per year (i.e., the volume of funded SCS cases increasing from 177 in year 1 to 215 in year 5).
Table 17:
Target Population: Reference Case Analysis
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
|---|---|---|---|---|---|---|
| Current Scenarioa | ||||||
| Current SCS (usual care) | 176 | 185 | 194 | 204 | 214 | 973 |
| 10-kHz high-frequency SCS | 1 | 1 | 1 | 1 | 1 | 5 |
| Total volume | 177 | 186 | 195 | 205 | 215 | 978 |
| Future Scenarioa,b | ||||||
| Current SCS (usual care) | 168 | 176 | 184 | 191 | 198 | 918 |
| 10-kHz high-frequency SCSb | 9 | 10 | 11 | 13 | 17 | 60 |
| Total volume | 177 | 186 | 195 | 205 | 215 | 978 |
Abbreviation: SCS, spinal cord stimulation.
In both scenarios, we made a conservative assumption of a 5% annual increase in the overall number of funded cases.
In the future scenario, we assumed that the people who received 10-kHz high-frequency SCS would increase with an uptake rate of 5% annually (5% in year 1 and 25% in year 5).
We confirmed that 10-kHz high-frequency SCS using the Freedom SCS system has recently been piloted in a few patients in Ontario (written communications, Aaron Hong, MD, and Stimwave Technologies Ltd., November and December 2019). Therefore, in the current scenario, we assumed that one patient per year received 10-kHz high-frequency SCS.
In the future scenario, we also assumed that a relatively small volume of people would be treated with 10-kHz high-frequency SCS as rescue therapy. The rationale for this decision (supported by clinical opinion and the literature70) was that the percentage of people who fail treatment with currently available SCS options in Ontario (making them eligible for 10-kHz high-frequency SCS) is approximately 5% to 7% (written and oral communications, Anuj Bhatia, MD, PhD, and Aaron Hong, MD, November 2018). We also assumed that the initial percentage of people eligible for 10-kHz high-frequency SCS would increase at a rate of 5% per year. As such, the total number of people receiving 10-kHz high-frequency SCS was estimated to be about 60 over the next 5 years (from 9 in year 1, rising to 17 in year 5). We considered a larger volume of people eligible for 10-kHz high-frequency SCS in a scenario analysis.
Current Intervention Mix
Most of the SCS devices currently available in Ontario have 3 major components: an implantable pulse generator (IPG), electrode leads (varying number depending on the type of device), and extension cables. The IPG is the battery of the SCS system and can be rechargeable or nonrechargeable. It is implanted subcutaneously in the abdomen or the buttock. The electrode leads are insulated wires that deliver electrical stimulation generated by the IPG. They are inserted into the epidural space of the spinal canal. Finally, the extension cables connect the electrode leads to the IPG.16
Conventional SCS generated electrical stimulation at frequencies of 40 Hz to 100 Hz produce paresthesia—a tingling or buzzing sensation intended to modulate pain. However, people perceive paresthesia differently: some find it uncomfortable or intolerable, and others find it comforting.16 Newer SCS devices generate electrical stimulation at higher frequencies (in the kHz range) and provide pain relief without paresthesia; these include the burst SCS (which generates 40 Hz of electrical stimulation with 5 spikes of 500 Hz), and the Senza HF10 and Freedom SCS system (which generate 10-kHz high-frequency electrical stimulation). As previously mentioned, the Senza HF10 does not have Health Canada regulatory approval and is not currently available in Canada.
The Freedom SCS systems (Freedom 4A and Freedom 8A) provide tonic, burst, and 10-kHz high-frequency SCS, and is the only 10-kHz technology to have the majority of its components approved by Health Canada. Its wireless system is minimally invasive; it does not require implantation of the batteries and IPG, and the transmitter is worn externally (written communication, Stimwave Technologies Ltd., November 2018; product information is also available at http://stimwave.com/mobile/products/). The Freedom SCS system uses electrodes (implanted epidurally or subcutaneously) with a built-in receiver that communicates wirelessly through the skin with a patient-worn transmitter and battery.71 The system consists of a stimulator (called the “Freedom Stimulator”) and a transmitter (wearable antenna assembly) to power the device. The transmitter provides the power and stimulation parameters using a proprietary amplitude and pulse-width modulation scheme.71 A wing anchor (e.g., SandShark) is used to fix the devices in the desired location, and once this is done the anchor is placed over the device and punctures the tissue.71 The Freedom SCS system does not require a surgical procedure to implant an IPG subcutaneously; implantation is done in one step and under ambulatory care, which reduces patient hospital stay and operating room time (written communication, Stimwave Technologies Ltd., November 2018). A few Ontario hospitals have piloted this SCS system since the end of November 2018 (written communications, Aaron Hong, MD, and Stimwave Technologies Ltd., December 2018).
In Ontario, SCS devices are available that deliver low-frequency, paresthesia-free, moderate-frequency tonic stimulation up to 1.2 kHz, as well as burst stimulation. Table 18 lists the SCS devices considered in the budget impact analysis, including the Senza HF10 SCS. Information about market share is proprietary; based on consultations with industry and experts, we made a simplifying assumption that most SCS devices had equal market share throughout the centres of excellence in Ontario, while the burst SCS (Abbott) was provided mostly in one centre of excellence.
Table 18:
Spinal Cord Stimulation Devices Considered in the Budget Impact Analysis
| SCS Device | Manufacturer |
|---|---|
| SCS Systems Used in Ontario (Frequencies up to 1.2 kHz) | |
| Precision Montage MRI Spinal Cord Stimulator System, Precision Spectra Spinal Cord Stimulator System (rechargeable, tonic and burst modalities) | Boston Scientific Inc. |
| Precision Novi (nonrechargeable, tonic and burst modalities) | Boston Scientific Inc. |
| RestoreSensor SureScan MRI neurostimulator (rechargeable) | Medtronic Inc. |
| Intellis implantable neurostimulator (rechargeable) | Medtronic Inc. |
| Itrel 3 system (nonrechargeable)a | Medtronic Inc. |
| Proclaim Elite (Proclaim 5/Proclaim 7; nonrechargeable, burst) | Abbott |
| Prodigy MRI IPG with burst (rechargeable, burst) | Abbott |
| SCS System Newly Available in Ontario (10-kHz High-Frequency as 1 of 3 Modalities) | |
| Freedom 4A/Freedom 8A (external batteries; wireless; neurostimulator; 3 modalities in 1 device: tonic, burst, and 10-kHz high-frequency) | Stimwave Technologies Ltd. |
| SCS System Not Available in Ontario (10 kHz High-Frequency Only) | |
| Senza HF10 | Nevro Corp. |
Abbreviation: IPG, implantable pulse generator, MRI, magnetic resonance imaging; SCS, spinal cord stimulation.
To be replaced by the new Restore SCS systems by December 2019 (oral communication, Medtronic Inc., November 2018).
Sources: Information received from the manufacturers (oral and written communications, Boston Scientific Inc., Medtronic Inc., Abbott, and Stimwave Technologies Ltd., November 2018 to February 2019).
Uptake of the New Intervention, Future Intervention Mix, and Market Effects
We conservatively assumed that the number of newly funded cases would represent an increase of approximately 5% per year. This analysis accounted for combined additional costs associated with 10-kHz high-frequency SCS for certain eligible patients and the cost of usual care with conventional SCS for the rest of the population. We assumed no substantial changes in market share over the next 5 years.
We included a separate scenario analysis to examine replacement of the Freedom SCS system with the Senza HF10, funded via the Out-of-Country Prior Approval program because there is no access to this device or a Nevro Corp. centre in Ontario.
Resources and Costs
Procedure Costs
In this section, we describe our approach to estimating the costs of the SCS procedure, including the cost of complications and resource use, as well as the cost of the SCS devices considered in the treatment of people with chronic noncancer pain (defined by specific CCI procedure codes and ICD-10 codes). We reported all costs in 2018 Canadian dollars. Where 2018 costs were unavailable (i.e., costs of some complications), we used the health care component of the Consumer Price Index72 to adjust to 2018 Canadian dollars.
We estimated the costs related to the following procedures and complications of SCS with current and 10-kHz high-frequency SCS systems: moderate-frequency SCS with rechargeable systems currently used in Ontario and 10-kHz high-frequency SCS with the Freedom SCS system (written communication, Stimwave Technologies Ltd., November 2018).
We estimated the costs of SCS in Ontario using data from Ontario sources and published literature when Ontario data were not available. We obtained the fees for professional visits, procedures, and consultations from the Ontario Schedule of Benefits for Physician Services.73 We obtained hospitalization costs associated with SCS treatment in an acute patient setting or in an ambulatory or 1-day surgery setting from the Ontario Case Costing Initiative74 database of the Ontario Ministry of Health. We used the following ICD-10 codes to identify people with failed back surgery syndrome and complex regional pain syndrome as exemplars of our target population: M96.1, M89.00-02, and R52.1. We combined the ICD-10 codes with CCI procedure codes that are linked to SCS implant procedures: 1.AX.53.LA-DV,1.AX.54.LA-DV, 1.YY.53.LA-DV, 1.AX.54.JA-DV, 1.AX.55, 1.YY.84, and 1.YY.55.
Table 19 presents costs and resource use for the trial and implant stages of the SCS procedure, as well as for follow-up care:
Table 19:
Estimated Annual Average Procedure Costs for SCS Systems Used in Ontario—Trial, Permanent Implantation, and Follow-up Care (Usual Care)
| Resource Item | Unit/Frequency | Cost Per Visit, $a | Data Source/Explanation |
|---|---|---|---|
| Trial | |||
| Preprocedure consultation, anesthesiologist | 1 | 106.15b | Schedule of Benefits (A015)73 |
| (pain doctor) or neurosurgeon | 160.00b | Schedule of Benefits (A935)73 | |
| Preprocedure consultation, average | 1 | 142.05b | Crude estimate accounting for variability in clinical practice |
| MRI, professional fee | 1 | 73.00 | Schedule of Benefits (X421)73 |
| MRI, procedure cost | 1 | 972.00 | OCCI 2016, ambulatory74 |
| MRI, average cost estimate, given that 80% of patients have MRI scans at consultation | — | 209.00 | Estimate (written communication, Aaron Hong, MD, December 2018) |
| X-ray, professional fee | 1 | 53.55 | Schedule of Benefits (X032)73 |
| X-ray, procedure cost | 1 | 76.00 | OCCI 2016, ambulatory74 |
| X-ray, total cost | — | 129.55 | — |
| SCS trial | 1 | 9,367.00 | OCCI 2016, ambulatory74 |
| Physician fees, anesthesiologist (pain doctor, including inserter fee for permanent neurosurgeon | 1 | 306.00b | Schedule of Benefits (Z942)73 |
| 739.50b,c | Schedule of Benefits (Z941A–Z943A)73 | ||
| 816.00b | Schedule of Benefits (N530)73 | ||
| Average cost, physician fees | 1 | 769.25b | Crude estimate accounting for variability in clinical practice |
| Anesthesiologist (sedation) | 6 | 51.95 | Schedule of Benefits (Z942)73 |
| Total average cost for trial procedured | — | 10,892.12 | Estimate accounting for permanent implant (high conversion rate) during the trial stage |
| Permanent Implantation | |||
| Procedure cost, inpatient or ambulatory | 1 | 17,566.00 | OCCI 2016, acute inpatient74 |
| 9,367.00 | OCCI 2016, ambulatory74 | ||
| Procedure cost, averagee | 1 | 15,926.20 | Crude estimate accounting for variability in clinical practice |
| Physician fees, pain doctor, inserter or neurosurgeon | 1 | 739.50 | Schedule of Benefits (Z941A-943A)73 |
| 510.00 | Schedule of Benefits (N563)73 | ||
| Physician fees, averagef | 1 | 555.90 | Crude estimate accounting for fees for permanent implant |
| Anesthesiologist (sedation) | 6 | 51.95 | Schedule of Benefits (Z942)73 |
| Total average cost for permanent implant procedureg | — | 10,552.10 | Estimate accounting for variations in conversion rate between centres |
| Follow-up Care | |||
| Consultation, reprogramming | 3 | 102.00 | Schedule of Benefits (Z943A)73 |
| Neuromodulation nurse | NA | NA | Covered by current bundle payment |
| Total cost for follow-up care | — | 306.00 | — |
| Overall Average Cost | $21,750.23 | Estimate accounting for permanent implant (high conversion rate) during the trial stageg | |
Abbreviation: SCS, spinal cord stimulation; MRI, magnetic resonance imaging; NA, not applicable; OCCI, Ontario Case Costing Initiative.
All costs are reported in 2018 Canadian dollars.
We assumed that in two of the six centres of excellence, the consultation and procedure would be done by pain doctors (written and oral communications, Aaron Hong, MD, and Anuj Bhatia, MD, PhD, November to December 2018). The preprocedure consultation fee for pain doctors was $106.15 and for neurosurgeons was $160, for an average of $142.05 for all centres of excellence.
Accounts for permanent implant inserter fees done in one centre of excellence (written communication, Aaron Hong, MD, December 2018).
Total costs adjusted for the fact that 5 centres of excellence proceed with permanent implant procedure, computed as follows: [($142.05 + $209.00 + $129.55 + $9,367.00 + $769.25 + $311.70) × 5 + ($142.05 + $209.00 + $129.55 + $9,367.00 + $769.25 + $311.70) × 0.98] ÷ 6 = $10,892.12.
Accounts for variability in practice and does not double-count the procedure cost for one centre of excellence where the SCS trial and permanent implantation were done at the same time with a high conversion rate, computed as follows: ($17,566.00 × 4 + $9,367.00) ÷ 5 = $15,926.20.
Accounts for variability in practice and does not double-count, computed as follows: ($739.50 + $510 × 4) ÷ 5 = $555.90.
Adjusted for the fact that five centres of excellence proceed with permanent implant procedure, computed as follows: [(5 × 0.75 × ($15,926.20 + $555.90 + $311.70) + (1 × 0.02 × ($15,926.20 + $555.90 + $311.70)] ÷ 6 = $10,552.10.
Professional fees (e.g., anesthesiologist or neurosurgeon, radiologist)
-
SCS procedure: depending on the centre of excellence, the SCS procedure could be done in an acute inpatient setting or in a 1-day surgery/ambulatory care setting. We estimated procedure costs using two sources of data reported in the Ontario Case Costing Initiative database (written communication, Ontario Ministry of Health, Provincial Programs Branch, November 2018):
-
∘
Acute inpatient SCS procedure costs (including nursing time; operating room, interventional radiology suite or intensive care unit; magnetic resonance imaging [MRI] technician time; MRI; x-ray; additional support from social worker/psychologist and physiotherapist; overhead costs as ascertained though the bundle for SCS, and as costed using one or all of the following CCI procedure codes): 1.AX.53.DA-DV; 1.AX.53.LA-DV; 1.YY.53.LA-DV; 1.AX.54.JA-DV; 1.AX.54.LA-DV; 1.YY.54.LA-DV
-
∘
Ambulatory or day surgery SCS procedure costs (costed using one or all of the following CCI procedure codes): 1.AX.53.DA-DV; 1.AX.53.LA-DV; 1.YY.53.LA-DV; 1.AX.54.JA-DV; 1.AX.54.LA-DV; 1.YY.54.LA-DV
-
∘
The cost of the SCS procedure may vary between centres of excellence because of differences in clinical practice and implementation strategies. For instance, at two centres, SCS treatment is a 2-day procedure with minimal use of operating room time (written communication, Aaron Hong, MD, and Anuj Bhatia, MD, PhD, November 2018). In one of these centres, the stimulation trial is occasionally bypassed because of a conversion (or trial success) rate of over 95%, and SCS devices are permanently implanted as a one-step procedure (written communication, Aaron Hong, MD, November and December 2018). For the remaining centres in Ontario, we assumed a conversion rate of 75% (as a midpoint of the rates suggested in the literature75,76) and accounted for the costs of both the trial and implantation phases of the SCS treatment. In some of these centres, the implantation of SCS devices (trial and permanent implant procedures) is done by a neurosurgeon, and the procedure may account for a hospital stay of approximately 5 days (oral and written communications, Aaron Hong, MD, and Anuj Bhatia, MD, PhD, November 2018). Our cost calculations approximated and accommodated between-practice differences in costs driven by the duration of hospital stay. In addition, because there may be changes in the types of devices available over the next 5 years, we accounted for the cost of newer currently available rechargeable SCS systems that are more expensive (compared with nonrechargeable SCS systems) but would have a longer battery lifespan and require less operating room time.
Table 20 presents costs and resource use for the SCS procedure using the Freedom SCS system. Owing to the specifics of the Freedom SCS system, we assumed that the operating room and other related procedure costs were done as a one-step ambulatory procedure (written communication, Stimwave Technologies Ltd., November 2018).
Table 20:
Estimated Annual Procedure Average Costs for the Freedom SCS System—Trial, Permanent Implantation, and Follow-up Care (Intervention, Future Scenario)
| Resource Item | Unit/Frequency | Cost Per Visit, $a | Data Source/Explanation |
|---|---|---|---|
| Trial/Permanent Implantation | |||
| Preprocedure consultation, anesthesiologist (pain doctor) or neurosurgeon | 1 | 106.15b 160.00b |
Schedule of Benefits (A015)73 Schedule of Benefits (A935)73 |
| Preprocedure consultation, average | 1 | 142.05b | Crude estimate accounting for variability in clinical practice |
| MRI, professional fee | 1 | 73.00 | Schedule of Benefits (X421)73 |
| MRI, procedure cost | 1 | 972.00 | OCCI 2016, ambulatory74 |
| MRI, average cost estimate, given that 80% of patients have MRI scans at consultation | — | 209.00 | Estimate (written communication, Aaron Hong, MD, December 2018) |
| X-ray, professional fee | 1 | 53.55 | Schedule of Benefits (X032)73 |
| X-ray, procedure cost | 1 | 76.00 | OCCI 2016, ambulatory74 |
| X-ray, total cost | — | 129.55 | — |
| SCS one-step procedure | 1 | 9,367.00 | OCCI 2016, ambulatory74 |
| Physician fees, anesthesiologist (pain doctor, including inserter fee for permanent trial) or neurosurgeon | 1 | 306.00b 739.50b,c 816.00b |
Schedule of Benefits (Z942)73 Schedule of Benefits (Z941A–Z943A)73 Schedule of Benefits (N530)73 |
| Average cost, physician fees | 1 | 892.50b | Crude estimate accounting for variability in clinical practice |
| Anesthesiologist (sedation) | 6 | 51.95 | Schedule of Benefits (Z942)73 |
| Total average cost for SCS procedure | 11,051.80d | Estimate accounting for trial and permanent implant | |
| Follow-up Care | |||
| Procedure cost, inpatient or ambulatory | NA | NA | OCCI 2016, acute inpatient74 OCCI 2016, ambulatory74 |
| Physician fees, pain doctor or neurosurgeon | 1 | 306.00 | Schedule of Benefits (Z942)73 |
| Reprogramming, first year | 3 | 102.00 | Schedule of Benefits (Z943A)73 |
| Neuromodulation nurse | NA | NA | Covered by current bundle payment |
| Total cost for follow-up care | — | 612.00 | — |
| Overall Average Cost | 11,663.80 | Estimate accounting for permanent implant (high conversion rate) during the trial stage | |
Abbreviation: SCS, spinal cord stimulation; MRI, magnetic resonance imaging; NA, not applicable; OCCI, the Ontario Case Costing Initiative.
All costs are reported in 2018 Canadian dollars.
We assumed that in two of the six centres of excellence, the consultation and procedure would be done by pain doctors (written and oral communications, Aaron Hong, MD, and Anuj Bhatia, MD, PhD, November to December 2018). The preprocedure consultation fee for pain doctors was $106.15 and for neurosurgeons was $160, for an average of $142.05 for all centres of excellence.
Accounts for permanent implant inserter fees done in one centre of excellence (written communication, Aaron Hong, MD, December 2018).
Total costs adjusted for the fact that 5 centres of excellence proceed with permanent implant procedure, computed as follows: $142.05 + $209.00 + $129.55 + $9,367.00 + $892.50 + $311.70 = $11,051.80.
Complication Costs
Table 21 describes the costs associated with managing complications resulting from SCS implantation. This analysis included only major, severe complications that require an inpatient stay or reoperation, with or without explanation of the device (written and oral communications, Aaron Hong, MD and Anuj Bhatia, MD, PhD, November 2018).
Table 21:
Probabilities and Costs of Common Severe Complications Related to SCS (Including 10-kHz High-Frequency SCS)
| Resource Item | Probability | Total Cost, $a | Data Source |
|---|---|---|---|
| Biological Complications | |||
| Pocket pain with infectionb | 0.06b | — | Van Buyten et al, 201362 |
| Deep infection with hospitalizationb | 0.048b | — | Van Buyten et al, 201362 |
| Initial consult | — | 58.25 | Schedule of Benefits (CO46)73 |
| Inpatient, hospitalization (3-day hospital stay) | — | 4,635.71 | Kumar and Bishop, 200965 |
| Operation, explanation | — | 306.00 | Schedule of Benefits (N531)73 |
| Additional consult | — | 58.25 | Schedule of Benefits (CO43)73 |
| Intravenous antibiotic therapy | — | 1,069.35 | Kumar and Bishop, 200965 |
| Home care nurse | — | 3,136.12 | Kumar and Bishop, 200965 |
| Total cost (deep infection)b | — | 9,263.68c | — |
| Hardware-Related Complications | |||
| Lead fracture or lead migration requiring explanation | 0.072d | 6,901.17 | Van Buyten et al, 201362; Deer et al, 201416 Reoperation, lead replacement (Kumar and Bishop, 200965) |
| Device malfunction and removal | 0.02 | 7,285.36 | Van Buyten et al, 201761 Reoperation, device replacement (Kumar and Bishop, 200965) |
All costs are reported in 2018 Canadian dollars.
The costs of pocket pain with infection or deep infection were assumed to be the same.
Given the specific design of the Freedom SCS system, the pocket pain and infection complication rates were not applicable and were not accounted for in the estimates of the procedure costs related to this device.
Based on clinical trial evidence,29–31 we assumed the same rate of complications for SCS systems in current use and for the Senza HF10 system. We based the frequency of complications on the findings of our clinical review and on the annual cost of complications on the literature65 and the Ontario Schedule of Benefits.73 The Freedom SCS system pulse generator is not implanted internally, so the pocket pain and infection complications requiring hospital care can be avoided (written communication, Stimwave Technologies Ltd., November 2018). Consequently, we did not consider biological complication costs in the future scenario including 10-kHz high-frequency SCS delivered by the Freedom SCS system.
Based on data from Table 21, we estimated an average per-patient total complication cost of approximately $1,643.07 for currently available SCS treatment (usual care) and $642.59 for 10-kHz high-frequency SCS treatment with the Freedom SCS system.
Device Costs
Table 22 presents estimates of the SCS device costs for the current and future scenarios.
Table 22:
SCS Device Costs
| Overall Cost, $a | Source | |||
|---|---|---|---|---|
| Devices Currently Available in Ontariob | ||||
| Rechargeable tonic wave, Boston Scientific Inc. | 27,000.00c | Boston Scientific Inc. (average based on provided list prices, January 2019) | ||
| Rechargeable tonic wave, Medtronic Inc. | 19,995.00c | Medtronic Inc. (average based on provided list prices, January 2019) | ||
| Burst wave systems, Abbott | 29,300.00d | Estimate (written communication, Aaron Hong, MD, November 2018) | ||
| Overall estimated average cost | 24,464.58e | Crude estimate accounting for variability in practice | ||
| Device Newly Available in Ontario (Next 5 Years; i.e., Freedom 8A) | ||||
| Freedom 8A (3 modalities: tonic, burst, and 10-kHz high-frequency) | 22,350.00f | Stimwave Technologies Ltd. (estimated from list price; written communication, November 2018) | ||
| Device Not Available in Ontario | ||||
| Senza HF10 SCS system | 207,066.86g,h | Estimate (written communication, MOH OOC-PA, June 2018) | ||
Abbreviations: MOH, Ontario Ministry of Health; OOC-PA, Out-of-Country Prior Approval program; SCS, spinal cord stimulation.
All costs are reported in 2018 Canadian dollars.
See Table 18 for the specific trade names of SCS systems used in Ontario; the list prices are proprietary and are not shown in this table. We have estimated an average device cost by manufacturer, and have used those costs to estimate an overall average for SCS devices available in Ontario.
Information received from the manufacturers (oral and written communications, Boston Scientific Inc., Medtronic Inc., November 2018 through January 2019). The prices of an average rechargeable SCS device (by Boston Scientific Inc.) include the IPG, the implantable leads typically used with these systems, surgical anchors and other surgical tools that are often used during the procedure, and patient accessories like remote controls, or chargers, if applicable (written communication, Boston Scientific Inc., January 2019).
Information received during expert consultation (oral and written communications, Aaron Hong, MD, November 2018 through January 2019).
A crude average estimate of the cost of a SCS device was computed as follows: [($27,000 × 0.5 + $19,995 ×0.5) × 5 + (1 × $29,300)]/6 = $24,464.58.
The cost of the Freedom SCS system was based on list price estimates provided for Freedom 8A; it represents an actual cost of the technology and not the cost of future innovations; the cost includes the following cost components: device including 2 stimulators and system bundle, SandShark injectable anchor system, replacement charger, touchy needle 6“ (14 gauge), guidewire 3 pack, waist belt and peripheral band (written communication, Stimwave Technologies Ltd., November 2018).
The outsourcing treatment for the Senza HF10 SCS system was estimated at US$154,000 (2018 dollars; written communication, Out-of-Country Prior Approval program, Ontario Ministry of Health, June 2018).
Considered in a scenario analysis.
It is unclear whether the currently available per-procedure funding for SCS includes the cost of the SCS device. In practice, this cost may be indirectly covered by public funding through hospital global budgets. As such, we accounted for device costs separately when generating our estimate of the total costs of usual care with SCS.
Manufacturers provided the list prices of the devices used in Ontario. However, because this information is proprietary and confidential, we developed an estimated average price for the rechargeable devices currently in use (oral and written communications, Boston Scientific Inc., January 2019; oral and written communication, Medtronic Inc. January 2019).
We estimated the average price of the Freedom SCS system based on the list price of the Freedom 4A and 8A SCS devices (written communication, Stimwave Technologies Ltd., November 2018).
The list price for Ontario of the Senza HF10 is unknown; we based the approximate cost on communication with the Ontario Ministry of Health Out-of-Country Prior Approval program (written communication, Ontario Ministry of Health, June 2018). The estimate in Table 22 represents the cost of treatment, including the trial, implantation procedure, and follow-up care. We converted costs from the corresponding estimate in 2018 dollars using the published exchange rate on December 19, 2018 (USD:CAD = 1.345; www.xe.com/currencyconverter/convert/).
Total Costs, Reference Case Analysis
Based on the information in Tables 19, 20, and 22, we estimated average annual per-case costs for SCS treatment with devices currently used in Ontario and 10-kHz high-frequency SCS with the Freedom SCS system, excluding the cost of complications:
SCS therapy with currently available devices: $46,215, including a crude estimate of the device cost (approximately $24,465)
SCS therapy with the Freedom SCS system: $34,014, including device cost (approximately $22,350)
The calculations above do not include complication costs (adjusted for the probability of each complication, Table 21). Total annual per-person cost estimates including complication costs are presented below in Table 23.
Table 23:
Total Annual Per-Person Costs of SCS
| Estimated Average Costs | Currently Available SCS, $a | 10-kHz High-Frequency SCS, $a |
|---|---|---|
| SCS device | 24,465 | 22,350 |
| SCS procedure | 21,750 | 11,664 |
| SCS complications | 1,643 | 643 |
| Average annual total per-person cost | 47,858 | 34,657 |
Abbreviation: SCS, spinal cord stimulation.
All costs are reported in 2018 Canadian dollars.
Table 24 presents the total costs associated with SCS treatment in the future and current scenarios over the next 5 years. We calculated these estimates by applying the costing data (Tables 19 to 23) to our target population estimate (Table 17).
Table 24:
Cost Estimates Over 5 Years—Current and Future Scenarios
| Current Scenario, $a | Future Scenario, $a | |||||
|---|---|---|---|---|---|---|
| Year | 10-kHz High-Frequencyb | Currently Available SCSc | Total | 10-kHz High-Frequencyb | Currently Available SCSc | Total |
| Year 1 | 34,656 | 8,420,948 | 8,455,604 | 306,709 | 8,045,354 | 8,352,064 |
| Year 2 | 34,656 | 8,844,388 | 8,879,044 | 337,380 | 8,426,450 | 8,763,830 |
| Year 3 | 34,656 | 9,288,999 | 9,323,656 | 387,987 | 8,801,194 | 9,189,181 |
| Year 4 | 34,656 | 9,755,842 | 9,790,498 | 465,584 | 9,160,906 | 9,626,491 |
| Year 5 | 34,656 | 10,246,026 | 10,280,682 | 581,980 | 9,490,395 | 10,072,376 |
Note: Results may appear inexact due to rounding.
All costs are reported in 2018 Canadian dollars.
10-kHz high-frequency SCS therapy (i.e., costs of device, procedures and complications) with the Freedom SCS system.
The cost of device included in the current scenario accounts for all devices: [($27,000 × 0.5 + $19,995 × 0.5) × 5 + (0.99 × $29,300 + 0.01 × $22,350)] ÷ 6 = $24,453.
Analysis
We conducted a reference case and sensitivity analyses.
The reference case analysis examined the budget impact as the difference in total costs between the current and future scenarios. We estimated the cost of the future scenario by combining the costs of usual care and 10-kHz high-frequency SCS with the Freedom SCS system (Table 23). This analysis also generated an average cost of SCS for the target population in Ontario, which may help with future financial planning, because it accounts for the costs of novel rechargeable (tonic moderate-frequency and burst) SCS devices that may be covered by hospital global budgets.
Our sensitivity analysis included five scenarios:
Scenario 1: to estimate changes in the budget impact if 10-kHz high-frequency SCS were provided using the Senza HF10, which is not currently accessible in Ontario. We estimated the average annual cost of outsourcing SCS therapy (including the device, procedure, and follow-up costs) with the Senza HF10 to be approximately $207,066 (Table 22)
Scenario 2: to estimate changes in the budget impact as a result of increased volumes for SCS treatment with usual care and 10-kHz high-frequency SCS using the Freedom SCS system, compared with the target population estimate used in the reference case analysis. The capacity of major centres in Ontario for SCS treatment may be 150 to 200 patients per year (for a total of 1,200 patients in the first year; written communication, Aaron Hong, MD, December 2018). We based our estimates of the target population eligible for 10-kHz high-frequency SCS on the same assumptions. The final estimates are presented in Table 25.
Scenario 3: to estimate changes in the budget impact if we assumed a 25% manufacturer discount on the total cost of the SCS devices (i.e., average estimated cost of currently available SCS devices: $18,349; average estimated cost of the 10-kHz high-frequency SCS device: $16,762). The discounted rate for certain number of devices may enable the provision of SCS therapy to more people in need of this treatment.
Scenario 4: to estimate changes in the budget impact if we assumed only incremental costs associated with 10-kHz high-frequency SCS in the target population eligible for the rescue therapy (i.e., the “no SCS treatment” scenario). We assumed that patients might use the 10-kHz option directly; in that case, the current scenario would be associated with no costs. This option would help us estimate the incremental cost of the 10-kHz high-frequency SCS with the Freedom SCS system. We conducted two analyses within this scenario related to the target population: Scenario 4a was based on the current volume of patients (Table 17), and Scenario 4b was based on a higher volume of patients (Table 25).
Scenario 5: to estimate changes in the budget impact if we assumed that permanent implant was done as an ambulatory procedure (i.e., shorter hospital stay), resulting in lower overall procedure costs with currently available SCS devices. In this analysis, the overall procedure costs for the current scenario would decrease to $17,629 from the $21,750 estimated for the reference case analysis. This would result in lower total costs for currently available SCS of $43,725 (compared to $47,858 in the reference case analysis, Table 23).
Table 25:
Target Population for Budget Impact Scenario Analyses
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
|---|---|---|---|---|---|---|
| Current Scenarioa | ||||||
| Current SCS (usual care) | 1,199 | 1,259 | 1,322 | 1,388 | 1,458 | 6,626 |
| 10-kHz high-frequency SCS | 1 | 1 | 1 | 1 | 1 | 5 |
| Total volume | 1,200 | 1,260 | 1,323 | 1,389 | 1,459 | 6,631 |
| Future Scenarioa,b | ||||||
| Current SCS (usual care) | 1,140 | 1,194 | 1,247 | 1,298 | 1,345 | 6,224 |
| 10-kHz high-frequency SCS | 60 | 66 | 76 | 91 | 114 | 407 |
| Total volume | 1,200 | 1,260 | 1,323 | 1,389 | 1,459 | 6,631 |
Abbreviation: SCS, spinal cord stimulation.
In both scenarios, we made a conservative assumption of a 5% annual increase in the overall number of funded cases.
In the future scenario, we assumed that the people who received 10-kHz high-frequency SCS would increase with an uptake rate of 5% annually (5% in year 1 and 25% in year 5).
In all five scenario analyses, we assumed the same rate of uptake for SCS interventions as in the reference case analysis (5% per year). In scenarios 2 to 5, we assumed that 10-kHz high-frequency SCS used the Freedom SCS system. The size of the target population remained the same (Table 17) in scenarios 1, 3, 4, and 5, although, as mentioned above, we conducted an additional analysis in scenario 4 that considered a higher volume of people eligible for treatment with the 10-kHz high-frequency Freedom SCS system.
We conducted all analyses using Excel Office 365 (Microsoft; Redmond, WA).
Results
Reference Case
Table 26 presents the results of publicly funding the 10-kHz high-frequency SCS (Freedom SCS system) for adults with chronic noncancer pain in whom currently available SCS therapies have not been effective. See Table 23 for the data used to calculate the budget impact.
Table 26:
Budget Impact Analysis Results—Reference Case
| Total Budget Impacta | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Current scenario | $8,455,604 | $8,879,044 | $9,323,656 | $9,790,498 | $10,280,682 | $46,729,484 |
| Future scenario | $8,352,064 | $8,763,830 | $9,189,181 $ | 9,626,491 | $10,072,376 | $46,003,942 |
| Budget impactb | −$103,541 | −$115,214 | −$134,474 | −$164,007 | −$208,306 | −$725,543 |
Note: Results may appear inexact due to rounding.
All costs are reported in 2018 Canadian dollars.
Negative budget impact estimates indicate cost savings.
Adopting 10-kHz high-frequency SCS with the Freedom SCS system at a 5% uptake rate in year 1, increasing to 25% in year 5, would lead to cost savings of about $103,541 in year 1 to about $208,306 in year 5. We estimated total net budget savings of $725,543 over the next 5 years.
Based on our estimates of the total 5-year costs for the current scenario (for 978 people receiving SCS), we estimated an average annual per-case cost of SCS in the current scenario of approximately $47,779. For the future scenario (which accounted for use of the 10-kHz high-frequency SCS with the Freedom SCS system), the annual per-case cost was approximately $47,037. The estimated per-case net budget saving was approximately $13,192 (given that an additional 55 cases would receive SCS with the 10-kHz high-frequency Freedom SCS system).
Sensitivity Analysis
The findings for the scenario analyses are presented in Table 27. In all but two scenarios, 10-kHz high-frequency SCS with the Freedom SCS system remained cost saving compared with usual care. However, 10-kHz high-frequency SCS was associated with incremental costs if it was delivered using the Senza HF10 (i.e., outsourced out of country, Scenario 1), or if we assumed that no additional costs would be incurred for currently available SCS treatment in Ontario (Scenario 4). In the latter case, the estimated per-case budget impact would be about $31,773 with 10-kHz high-frequency SCS.
Table 27:
Results of the Budget Impact Sensitivity Analysis
| Total Budget Impacta | ||||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Total | |
| Reference Case | ||||||
| Budget impact | −$103,541 | −$115,214 | −$134,474 | −$164,007 | −$208,306 | −$725,543 |
| Scenario 1: 10-kHz High-Frequency SCS With the Senza HF10b | ||||||
| Current scenario | $8,628,015 | $9,051,454 | $9,496,066 | $9,962,908 | $10,453,093 | $47,591,537 |
| Future scenario | $9,877,896 | $10,442,246 | $11,119,360 | $11,942,705 | $12,967,643 | $56,349,850 |
| Budget impact | $1,249,881 | $1,390,792 | $1,623,293 | $1,979,796 | $2,514,550 | $8,758,313 |
| Scenario 2: Larger Volume of Patients Eligible for 10-kHz High-Frequency SCS With the Freedom SCS Systemc | ||||||
| Current scenario | $57,402,364 | $60,273,141 | $63,287,458 | $66,452,490 | $69,775,774 | $317,191,228 |
| Future scenario | $56,624,159 | $59,415,798 | $62,299,534 | $65,264,344 | $68,287,294 | $311,891,129 |
| Budget impact | −$778,204 | −$857,344 | −$987,924 | −$1,188,147 | −$1,488,481 | −$5,300,099 |
| Scenario 3: Manufacturer Discountd | ||||||
| Current scenario | $7,374,085 | $7,743,422 | $8,131,227 | $8,538,421 | $8,965,975 | $40,753,130 |
| Future scenario | $7,274,671 | $7,632,801 | $8,002,112 | $8,380,951 | $8,765,972 | $40,056,507 |
| Budget impact | −$99,414 | −$110,621 | −$129,114 | −$157,470 | −$200,003 | −$696,622 |
| Scenario 4a: “No SCS Treatment” Current Scenario, Reference Case Target Populatione | ||||||
| Current scenario | $34,656 | $34,656 | $34,656 | $34,656 | $34,656 | $173,282 |
| Future scenario | $306,709 | $337,380 | $387,987 | $465,584 | $581,980 | $2,079,641 |
| Budget impact | $272,053 | $302,724 | $353,331 | $430,928 | $547,324 | $1,906,359 |
| Scenario 4b: “No SCS Treatment” Current Scenario, Larger Population Volumee | ||||||
| Current scenario | $34,656 | $34,656 | $34,656 | $34,656 | $34,656 | $173,282 |
| Future scenario | $2,079,383 | $2,287,322 | $2,630,420 | $3,156,504 | $3,945,630 | $14,099,260 |
| Budget impact | $2,044,727 | $2,252,665 | $2,595,764 | $3,121,848 | $3,910,974 | $13,925,978 |
| Scenario 5: Reduced Procedure Cost, Usual Care | ||||||
| Current scenario | $7,730,244 | $8,117,210 | $8,523,524 | $8,950,153 | $9,398,114 | $42,719,246 |
| Future scenario | $7,659,056 | $8,037,996 | $8,431,068 | $8,837,392 | $9,254,896 | $42,220,409 |
| Budget impact | −$71,188 | −$79,214 | −$92,456 | −$112,761 | −$143,218 | −$498,837 |
Note: Results may appear inexact due to rounding.
All costs are reported in 2018 Canadian dollars.
10-kHz high-frequency SCS with the Senza HF10 system outsourced via the Ontario Ministry of Health Out-of-Country Prior Approval program.
Larger volume of people eligible for 10-kHz high-frequency SCS with the Freedom SCS system.
A negotiated financial arrangement with the manufacturer.
A “do nothing” current scenario: 4a with reference case target volume and 4b with larger volume as estimated for scenario 2.
Discussion
We conducted a budget impact analysis to explore adopting 10-kHz high-frequency SCS as rescue therapy for adults with chronic noncancer pain in whom previous SCS therapies were not effective. Compared to the 2005 Health Quality Ontario report68 on SCS for neuropathic pain, the current budget impact analysis provides updated estimates of the cost of usual care for people with chronic noncancer pain, including a variety of new, more expensive rechargeable SCS systems that are currently used in Ontario.
Assuming a 5% increase in access per year, an additional 55 people would receive 10-kHz high-frequency SCS using the Freedom SCS system over the next 5 years. This corresponds to a net budget cost savings of approximately $0.73 million. The estimated cost savings are related to the potentially lower procedure and complication costs with the Freedom SCS system compared to SCS systems currently in use, and to the relatively high list-price costs of the newer rechargeable SCS systems. However, if the province were to outsource and fund 10-kHz high-frequency SCS to the United States through the Ontario Ministry of Health Out-of-Country Prior Approval program using the Senza HF10 SCS system, the 5-year additional cost burden would be about $8.76 million.
Our sensitivity analysis explored changes in the net budget impact estimates if the capacity for 10-kHz high-frequency SCS were to increase, and larger volumes of patients were to be funded for both currently available SCS and 10-kHz high-frequency SCS (Freedom SCS system). If the total volume of patients in need of rescue therapy increased over 5 years about 6.7 times (e.g., from 60 in the reference case to 407), the 5-year cost savings would be higher (about $5.3 million).
Because there is a cost assigned to the 10-kHz high-frequency SCS procedure in Ontario (Freedom SCS system), we conducted a separate scenario analysis that assumed no cost incurred for currently available SCS treatment (so called “No SCS treatment” scenario). In this case, assuming the reference case target number, the province could expect to pay about $1.9 million over the next 5 years.
In general, SCS treatment in people with chronic pain refractory to medications might appear expensive. However, a study by Farber et al77 compared health care utilization for conventional SCS compared to other management (e.g., medication) in adults with failed back surgery syndrome and found that SCS treatment might lead to a short-term increase in costs in the first year, but that subsequent annual cumulative costs significantly decreased in the 9 years following implantation.77 In addition, a recently published study75 has suggested that the cost of SCS implantation could be reduced. The authors found that considerable cost savings could be achieved by adopting an implantation strategy without a screening trial. In Ontario, a few clinicians have already adopted this approach of proceeding with the permanent implantation in one step because of high SCS trial success rates, reducing time in the operating room and decreasing hospital stays (written communication, Aaron Hong, MD, December 2018). If this approach is widely adopted in Ontario, it would be possible to increase patient access to SCS options for those who would benefit from currently available SCS and for those who would benefit from 10-kHz high-frequency SCS only.
Strengths and Limitations
Owing to the lack of access to the Senza HF10 SCS system in Canada and to limited clinical evidence on the effectiveness of 10-kHz high-frequency SCS as rescue therapy in people with chronic noncancer pain who have failed all currently available SCS modalities, we did not conduct a cost–utility analysis. However, the budget impact analysis did consist of comprehensive costing assessments of usual care (including currently available rechargeable SCS systems) and the new intervention of interest (10-kHz high-frequency SCS).
We based our estimates on all available data and made assumptions based on insights gained from expert consultation. We accounted for variations in practice between the centres of excellence that provide SCS treatment across Ontario; however, this information is not published or available from a publicly funded open-source registration system. In our analysis, the total costs associated with SCS treatment included the estimated average cost of the device, the cost of health care utilization, the cost of trial and permanent implant procedures, and the cost of major complications. However, the estimated total costs and net budget savings could have been overestimated for at least two reasons: first, some of the data we used in our calculations represented approximations (for instance, the actual costs of the devices currently used in Ontario are protected by proprietary laws); and second, current SCS trial and implant procedures with current SCS therapies may be done more efficiently (e.g., as one-step procedure) in all centres of excellence, which would reduce the cost of SCS therapy in general.
Given that the longevity and battery life are more than 9 years for rechargeable SCS devices, we did not account for maintenance costs (or amortization). However, a Canadian study estimated that the amortization and maintenance costs account for an additional 18% of the total per-patient costs for each actively managed patient.65
We estimated our target population assuming that a very small percentage of people may require 10-kHz high-frequency SCS; however, we considered larger volumes in our scenario analyses.
Finally, we estimated the impact of funding 10-kHz high-frequency SCS in Ontario assuming that no additional costs would be incurred for usual care. These analyses may be helpful to further plan the capacity expansion for SCS treatment in Ontario.
Conclusions
We estimate that publicly funding 10-kHz high-frequency SCS (delivered using the Freedom SCS system) for treatment of adults with chronic noncancer pain in whom currently available SCS therapies were not effective would be associated with a total net cost savings of $0.73 million over the next 5 years ($0.10 million in year 1 to $0.21 million in year 5). If the province funded the outsourcing of 10-kHz high-frequency SCS therapy using the Senza HF10 through the Out-of-Country Prior Approval program, the 5-year budget impact would be $8.76 million ($1.25 million in year 1 to $2.51 million in year 5).
PATIENT PREFERENCES AND VALUES
Objective
The objective of this analysis was to explore the underlying preferences, values, needs, and priorities of those who have lived experience with chronic noncancer pain. The treatment focus was 10-kHz high-frequency spinal cord stimulation (SCS).
Background
Exploring patient preferences and values provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat the health condition. It includes the impact of the condition and its treatment on the person with the health condition, their family and other caregivers, and the person's personal environment. Engagement also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., outcomes important to those with lived experience that are not reflected in the literature).78–80 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, preferences, priorities, and values of those with lived experience in Ontario are not often adequately explored in published literature, we speak directly with people who live with a given health condition, including those with experience with the intervention we are exploring.
Methods
Partnership Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of people with chronic noncancer pain and those of their families and other caregivers. We engaged people via confidential one-on-one interviews over the phone.
We used a qualitative interview, as this method of engagement allowed us to explore the meaning of central themes in the experiences of people with chronic noncancer pain, as well as those of their families and caregivers.81 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our choice of an interview methodology.
We also compared our results with consultation comments obtained for a health technology assessment by the National Institute for Health and Care Excellence (NICE) on the Senza system, delivering 10-kHz high-frequency SCS to treat chronic neuropathic pain, published in January 2019 (NICE HTA).58 The NICE HTA examines 10-kHz high-frequency SCS, and our participants discussed SCS at other frequencies. However, despite these differences, there was great overlap between patient comments in the NICE HTA and in our direct interviews. Where appropriate, we highlighted those complementary comments.
Participant Outreach
We used an approach called purposive sampling,82–85 which involves actively reaching out to people with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations, including the Ontario Pain Foundation and the Chronic Pain Association of Canada, to spread the word about this engagement activity and to contact people with chronic noncancer pain, family members, and caregivers, including those with experience of SCS.
Inclusion Criteria
We sought to speak with people who had chronic noncancer pain, or to the family members or caregivers of these people. The causes of participants' chronic pain varied. While 10-kHz high-frequency SCS has only recently become available in Ontario, moderate-frequency SCS is already in use, so we sought to speak with people who had direct experience with these devices, although use of SCS was not a requirement for participation.
Exclusion Criteria
Children with chronic pain were outside of the scope of this health technology assessment.
Participants
For this project, we spoke with 13 adults who lived with chronic noncancer pain living in Ontario. Of these, 11 had received moderate-frequency SCS, and two were aware of SCS as a potential treatment option. One participant was specifically waiting for a 10-kHz high-frequency SCS device to become available in Ontario. Participants were mainly from the Greater Toronto Area, but also included people from Ottawa and the Kingston area.
Approach
At the beginning of the interview, we explained the role of our organization, the purpose of this health technology assessment, the risks of participation, and how participants' personal health information would be protected. We gave this information to participants both verbally and in a letter of information (Appendix 8). We then obtained participants' verbal consent before starting the interview. With participants' consent, we audio-recorded and then transcribed the interviews.
Interviews lasted approximately 15 to 30 minutes. The interview was semistructured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.86 Questions focused on the impact of chronic noncancer pain on quality of life, people's experiences with treatments for chronic pain, and their decision-making values and experiences. Where applicable, we spoke about their perceptions of the benefits and limitations of SCS. See Appendix 9 for our interview guide.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze interview transcripts. The grounded-theory approach allowed us to organize and compare information on experiences across participants. This method consists of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.87,88 We used the qualitative data analysis software program NVivo89 to identify and interpret patterns in the data. The patterns we identified allowed us to highlight the impact of chronic noncancer pain and treatments on the patients, family members, and caregivers we interviewed.
Results
Chronic Noncancer Pain
Impact
Chronic noncancer pain can have a range of sources and ultimate causes; the path and progression of chronic pain is often unique to an individual. The participants reported many different types of lived experience with chronic pain. Some had been burdened with pain for many years, while others had lived with it for a shorter time. For some, the pain developed following a traumatic injury, and for others, it was a symptom of an underlying (noncancer) disease or the side effect of a surgical treatment.
Second pitch, he got fouled, tipped back, hit me just below the knee and just smashed me. Broke the lower part of my leg. So I had multiple surgeries and multiple physio-type stuff. I was given a pain specialist forever and ever, who helped, but it only worked so long.
I was diagnosed with dermatofibroma glaucoma in 2013 … So I had a little lump, and we thought it was a cyst. It ends up it was cancer. So I had a big resection, and I had a lot of post-op complications after that. And I never really healed very well: I ended up with chronic pain.
In all cases, however, participants clearly spoke of the huge impact that chronic pain could have on their daily lives. As the pain progressed, many participants spoke of the increasing effects it had on their ability to perform daily activities. The most commonly reported challenges were with leg movement: walking, standing upright, or maintaining balance. Sensation loss and lack of flexibility or movement in the legs and feet were also commonly reported.
I don't tolerate drugs of any kind. The pain seems to be getting worse, because I can't sit for a long time, I can't stand for any length of time, I can't walk without a cane.
I don't think people understand just how significant—how debilitating—pain can be.
My back pain progressed, and I then started getting sciatic nerve pain through my bum and down my leg, which I was coping with for a while, and then I was getting foot drop in my right foot. It was only on the right side. [I was] having a lot of mobility issues, and the pain was getting really severe.
I couldn't flex it. I couldn't point my toe. My foot was constantly stuck in one position because even the slightest movement and I would be in tears, just completely unable [to move it].
Such reflections on the impact of pain were not restricted to our interviews with people in Ontario. In the NICE HTA, several participants shared their experiences with chronic noncancer pain58:
I had had six spinal operations and was on huge amounts of opioids and [medications], struggling to work full-time and having given up any hobbies being in tears most days. My mixed and neuropathic pain never scored less than a nine on any day and could not sleep longer than 2–4 hours.
Often, these restrictions on movement led to a severe decrease in participants' ability to take part in everyday activities, including social interactions and employment opportunities. One participant talked about how his long career in the Canadian Armed Forces ended because of the movement restrictions caused by his pain. These restrictions on employment could also lead to financial hardship and extreme stress in participants' daily lives.
My return to work failed, and the doctor forced me to go back to school, so I was fired and lost all my benefits. After about 6 weeks, I was back in psychosis and in hospital. But now I had no income, no home, no health benefits … and [I was] still in pain.
I volunteer [at the zoo, and I've been doing that] for 15 to 17 years. But I got so I could get to the zoo and from the zoo, but I couldn't walk the zoo. For years I did school tours, taking school kids around, depending on what grade they were in and what the science part of it was, but I couldn't do tours anymore.
I was in the process of being screened to take a command position in Afghanistan, and I failed the screening because I couldn't lie in a prone position for an extended period of time with a weapon … I was released because I was incapable of what the military calls meeting the basic universality of service requirements to be a soldier.
Several participants also spoke of the emotional impact of living in constant pain. The pain often affected interactions with family members, causing emotional turmoil. The daily burden was emotionally difficult, and several participants talked about their depression and their desperation to address and treat their pain.
The fact was that I needed my life back. I was bedbound. I couldn't walk downstairs in my house without having pain. I've got a 6-year-old … she was littler at the time, and I couldn't even play with my daughter.
Well, it depresses you. I mean, I wasn't a depressed person, but I'm only 68 and I have grandkids and stuff like that, and I thought, “Crap. There has to be something I can do.” I did the physio. I really tried to do everything I could to beat it, with no luck at all. So it was affecting my personal relationships. I was constantly unhappy, because I was hurting so bad. Yeah, it was difficult. Like I say, I'm not that type of person normally, but it was starting to wear me down.
My husband is amazing. He's fully supportive, but it wears on your marriage too, you know, and I just wanted my life back …
Treatments
Almost all of the participants we interviewed had direct lived experience with SCS, a treatment only offered after other treatments have failed. Because of this, participants had a great deal of experience with other treatments for their chronic pain, including pharmacotherapy, acupuncture, massage, injections, physiotherapy, chiropractic support, and others.
I've done swimming. I've done the exercise, the core strengthening. I've done acupuncture in the past. I've done chiropractic. I've done physiotherapy. That's sort of the gamut. I've done some cognitive behavioural therapy in terms of pain management on my own. I've tried meditation and breathing techniques in terms of trying to deal with the pain management.
I was seeing a pain specialist. He did injections and they helped, but first I was going every 3 weeks, and every 2 weeks, and then I was going every week.
I was in so much pain. They were having to put me on so many medications that I couldn't really be me, because my memory was going because of the pain meds they had me on. Just general symptoms from that—nothing really unusual for pain medication, but I couldn't live my life.
Participants felt that existing treatments were ultimately ineffective in relieving their chronic pain, but this was the opinion of those we interviewed and does not reflect the efficacy of existing treatments in everyone with chronic noncancer pain. However, those we interviewed reported great frustration as a result of trying to find an effective treatment for their chronic pain. They noted that a given therapy would often work for a short time, but its efficacy would decrease the more it was used. Often participants spent a great deal of time and money in their attempts to find relief from their chronic pain.
It was so much money for those therapies and stuff, especially while you're on medical leave and you don't have any money coming into the house.
It was very stressful, but especially when I kept on getting my hopes up for each new procedure they sent me for. I was like, “OK, this is going to be the one, this is going to be what fixes it, I'm going to be able to start doing my therapies and get back to work and start being me again.”
And I kept on getting disappointed and disappointed and disappointed after every single thing. It was kind of like OK, if this doesn't work for me what do I do?
This desperation for an effective treatment often led people to explore further alternatives and was typically how these people first learned of SCS.
Spinal Cord Stimulation
Information
Participants typically reported that they were unaware of SCS as a treatment option until most other therapies had failed to manage their chronic pain. They often discovered it through health care practitioners who spoke of it as a treatment of “last resort.” Occasionally, participants reported that they learned about SCS from other people or from their own searches. For this reason, participants reported that they often did not know much about SCS beforehand, or that the information they had turned out to be incorrect.
[The pain specialist] explained it to me, and it was funny because I went to my GP [general practitioner] and I did my homework. I asked her; she'd never heard of it either. Yeah, I don't know why we don't know more about it.
So I had heard the name. I did Google it, just to try to be informed about what they were talking about, so when they were saying things I wasn't completely lost, but it was never something that I really wanted. I didn't want to have to go for surgery, especially because of the fact that I regularly have a very, very active job.
Yeah, so I did a little bit of reading online. There isn't a whole lot out there. What is out there is very different from what I experienced. I thought it was a big long surgery through the spine and all that sort of thing, so I read a little bit before I actually went to see [the doctor] …
Participants who received SCS reported that their expectations for this type of treatment were moderate. Participants spoke of years of failed therapies; often they viewed SCS as the last in a long line of treatments that did not control their chronic pain. They did not necessarily expect SCS to work better than previous therapies. Participants reported that health care providers were often realistic about what they should expect from the device and its effect on their chronic pain.
When I was having the last one done [the doctor] said, “Don't give up hope on everything.” He said, “There is something else,” and he explained to me about the spinal cord stimulator. So I really had nothing to lose at this point.
Well, I have my hopes, and then I have what I'm trying to keep as realistic expectations, just based off of where I was. If my realistic expectation is even if I have to walk with a cane for the rest of my life, I'll take that over being in a wheelchair, like leaps and bounds.
I was told I wasn't going to get another surgery, the scarring was going to be worse than the fix of the surgery … and there really weren't a heck of a lot of other options, so … my understanding was that this was my last hope to try and mitigate the pain.
Expectations of 10-kHz high-frequency SCS were not mentioned by patients in the NICE HTA.
Participants who had not received SCS also noted that there was not a great deal of information about the device and procedure. However, these people had larger concerns about the long-term effects of the device and scarring that made them hesitate to use the device.
My concern has to do with complications and problems arising out of that: what about the scarring?
I probably will never try it … Well, maybe if I was a little bit older and less concerned about the consequences.
Barriers
Participants reported that the main barriers to accessing SCS were not knowing about the procedure and wait times. As reported above, participants were often unaware of SCS until after they had tried many other treatments. Once they were aware of SCS, participants reported waiting up to a year to see a surgical specialist for an SCS implant.
From what I've heard, I was actually in fairly quickly, compared to other people I've talked to with my condition. Some of them have waited as long as 3 years to be able to get in to see [the doctor]. Within a year I got in, so I am beyond grateful for that. I got it fairly quickly … but it felt like an eternity.
I was told the waiting list was at least a year to get a consult. I got it within a few months, which was amazing. And the whole team there is phenomenal.
Most of the participants we interviewed lived in southern and eastern Ontario and were able to access surgical specialists in Toronto and Kingston. Perhaps because of this geographical bias, few participants said that location was a barrier to accessing SCS. One participant who lived in Ottawa spoke of having to travel to Toronto for the surgery and device adjustment but did not indicate that this was particularly burdensome. We expect that participants living in Northern Ontario or more rural areas would raise geographical access to SCS as a barrier to treatment, although we were unable to confirm this through direct interviews.
Surgical Procedure
Participants noted that implantation of the SCS device was fairly simple and not overly burdensome. They reported that the device was installed but kept external for a certain amount of time while it was calibrated and adjusted to ensure good pain relief. Once pain relief had been established, the device was permanently implanted below the skin a few days later via a simple surgical procedure. Participants mentioned pain and stiffness associated with the surgery, but nothing unexpected or concerning.
It was done in two surgeries … They put the leads in and then the little stimulator boxes on the outside, because they want to make sure that you're really going to have some benefit before they permanently implant it.
And then I went back a week later and had the implant. I mean it's nothing more than you'd expect. There's discomfort from surgery. Nothing, really. You were in there from probably total 6 or 8 hours each time.
Often, participants also followed up with a technician to make adjustments to the device settings. These adjustments allowed for increased pain relief or changed how the pain was managed, depending on the particular needs of the patient. Participants reported that the ability to change the frequency and adjust the level of the device was seen as beneficial.
And then follow-up visits … The programmer is there every time you go back, and he's made adjustments. He tweaked it. He could move the leads or whatever he does with this magic box. So they've adjusted it, and then I also have the ability to turn up the intensity if I find I'm starting to get any symptoms. I can make it stronger.
Impact
Participants reported that they felt the effect of SCS fairly quickly. Before permanently implanting the device, physicians wanted to make sure that the device was working effectively to manage their chronic pain. Typically, participants reported that they felt a positive effect within a week; several stated that their pain relief was almost immediate.
As soon as I woke up [from] the initial implant, the guy was there that activated it all … and I was still kind of groggy. He said, “Now you do have freezing in there, but let's stand up and see what you feel.“ It was almost 90% gone when I woke up. It was just unreal, totally unbelievable.
I noticed it because it takes a little time for the actual stimulation to kick in. It's not like it was put in and I felt instant relief. You're uncomfortable from the pain from your surgery. It takes a couple of days to get over that sort of shocky stuff, but I found within a day I noticed a difference.
Participants clearly expressed that SCS provided not only relief from the physical pain but also an emotional lift, easing the burden of dealing with chronic pain. Even though the pain was often not completely eliminated, a reduction in pain could be emotionally impactful for participants.
I was still amazed at how well it worked, just because … I had been [unable] to put a sock on for so long, and within the first day or two of being able to not only put a sock on, but being able to put a shoe on, was … I will say, the first time I managed to put a sock on without pain, I did cry.
I still have a fair amount of pain … Especially my big toe on my right foot, my toes are numb. I'm down to … a good 50% of the pain is relieved, but that's … Hallelujah.
This sentiment was mirrored in the comments in the NICE HTA. Participants reflected on the fact that their pain was not completely eliminated, but the device was nevertheless a huge benefit for engaging in daily activities.58
While the original operation killed off nerves in my leg and foot, and I do walk with a stick, I can actually work full-time and enjoy life again … It was hoped that I would gain about 85% relief, but, while this was not to be, the level of relief I get is more than I thought it would be.
Participants expressed their appreciation for being able to return to many of the activities they had been forced to curtail because of their chronic pain. These activities could be small, such as performing household tasks, or large, such as returning to a career and regular employment.
I'm back to doing almost everything that I did before … I still have arthritic pain, but the things that were prevented by this thing in my leg, it pretty much is gone. Everything.
It was like being born again. It was just so much improved. It's not the be all and end all. [That] is getting rid of all the pain and getting rid of the numbness, but I'm up, I walk, I talk, I waddle like a duck. I volunteered at the zoo for years; I got to the point where I couldn't do it. Now I do it. So I'm a quasi-happy camper.
Pain-wise in my leg, even though I still have a bit of pain there, I'm not walking with my cane. It's still leaps and bounds better than where I was, so even though it's not 100%, it's still for quality of life. I have more quality; I can do things I wasn't able to do before.
Challenges
Participants were asked about any unexpected side effects or challenges from implantation of the SCS device. In response, participants spoke mainly of discomfort because of the fact that the device stays in the lower back. Participants reported that it took time to get used to, and that it could make sitting down slightly awkward and uncomfortable. For those who chose to have a rechargeable battery in their device, the time required to charge it was a slight challenge. These challenges were mentioned in the NICE HTA as well.58 Participants were clear, however, that these were small issues and did not detract from the positive impact of the device on their daily lives.
I'm totally used to it. I can lie on it. I can do anything. If I had a complaint about it, the only thing I would say is [that in] the model I got, you have to charge [the battery] by conduction with this little docking thing. That's a little bit of a pain, but you get used to it. It's about every 5 days. It takes about 2 hours to charge it, but [that's] a small price to pay.
Not really. If you could make the batteries thinner. So if I sit back in a wooden chair, I'm not being uncomfortable. I sit with a slouch.
The only niggle ever commented on is the fact that the Senza has to be charged either daily or every other day, which is significantly more frequent than low-frequency traditional SCS, but most members agree that this is a small price to pay for the improvement in quality of life, and the recharging just becomes part of your daily routine.
You know, it's wireless recharging, so there's the downside of having to find 3 hours a week to sit in a chair so I can put this bloody thing around my waist and recharge. My kids think it's pretty funny, but in the greater scheme of things, no, I've never had any malfunctions or whatever. The battery lasts a ridiculous amount of time.
One participant reported that implantation of the SCS device led to an infection, and the device had to be removed and eventually replaced. This participant reported that the second device did not work as well, but they were hoping that further adjustments would lead to increased pain relief. The NICE HTA contained comments on potential side effects with conventional low-frequency SCS that were resolved with use of the high-frequency device.58
In 2010 she was fitted with a low-frequency unit, but this was not as useful as hoped. While it did alleviate some pain (notably period pain), the side effects were both paraesthesia and leg paralysis in certain positions … In 2015 she was offered a trial of the high-frequency [device]. She was in hospital for a week in Feb[ruary] 2016, testing the system, and the benefit was immediate.
Perceptions
Participants were asked about how they perceived the potential impact and use of 10-kHz high-frequency SCS. Generally, participants were unaware of the type of device they were using, or the frequency it used. When the basics of the 10-kHz high-frequency SCS device were explained to them, participants were generally supportive, feeling that if the device was as effective as their current device, then it would be of benefit. One participant, who was waiting for the 10-kHz high-frequency SCS device to become available, was hopeful that it would have the kind of effect she had heard high-frequency devices could have on pain.
Well, if it works as well as this one [high-frequency SCS], then that would be good.
I appreciate you keeping me up to date as to what's going on so that I can live with hope rather than the pain that I'm in.
Discussion
All participants had lived experience with chronic noncancer pain for a number of years and were able to speak about its effect on their lives. They reported substantial negative effects on their activities and emotional well-being.
Because most of the participants had had chronic pain for a long time, they were able to speak extensively about their experience with different treatments, including pharmacotherapy, physiotherapy, chiropractic services, and acupuncture. Their direct knowledge of different therapies allowed them to provide context and comparisons when they discussed the impact of SCS on their chronic pain.
The nature of participant recruitment that meant a selection bias existed: participants' chronic pain had not been adequately managed with any of the other treatments. Almost all participants had received SCS at frequencies lower than 10 kHz. This is not reflective of the overall efficacy of different treatments for chronic pain in the general population.
As well, participants were generally located in urban areas close to surgical centres where they could access SCS. We did not hear from participants with chronic pain who lived in rural or remote locations, including Northern Ontario, and who experienced geographical barriers in accessing SCS.
Conclusions
Participants reported that chronic noncancer pain had a substantial negative impact on their daily activities and emotional well-being. Participants knew little about SCS before they received it, but they reported that it reduced their level of chronic pain, leading to improvements in function and their ability to perform activities of daily living.
CONCLUSIONS OF THE HEALTH TECHNOLOGY ASSESSMENT
For patients with chronic noncancer pain, 10-kHz high-frequency spinal cord stimulation (SCS) likely provides reductions in pain intensity and functional disability, and improvements in quality of life (GRADE: Moderate). Randomized controlled trials supported the statistical noninferiority of 10-kHz high-frequency SCS to conventional SCS for pain responder rates in the short term, but the results were inconsistent with respect to the superiority of 10-kHz high-frequency SCS (GRADE: Moderate). Patients with chronic pain who were taking high levels of opioids may reduce their opioid consumption after 10-kHz high-frequency SCS (GRADE: low). The longest follow-up with 10-kHz high-frequency SCS was 2 years, limiting our ability to form conclusions about its longer-term effectiveness or safety.
We identified two studies in our economic evidence review. Both found that Senza HF10 SCS was cost-saving compared to both conventional nonrechargeable and conventional rechargeable SCS for people with failed back surgery syndrome. However, neither of these studies was directly applicable to the Ontario context.
Because of a lack of good-quality clinical and economic evidence for 10-kHz high-frequency SCS in managing chronic noncancer pain in people who have failed currently available SCS treatment options (such as burst or tonic moderate-frequency SCS in kHz ranges), we did not pursue a primary economic evaluation.
Publicly funding 10-kHz high-frequency SCS for the treatment of adults with chronic noncancer pain who have failed currently available SCS therapies would be associated with a total net cost savings of $0.73 million over the next 5 years. If the province funded the outsourcing of 10-kHz high-frequency SCS therapy using the Senza HF10 SCS system through the Out-of-Country Prior Approval program, the 5-year budget impact would be $8.76 million.
Although participants knew little about SCS before they received it, they reported that it reduced their level of chronic pain, leading to improvements in function and their ability to perform activities of daily living.
Acknowledgments
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The lead clinical epidemiologist was Gaylene Pron, the secondary clinical epidemiologist was Kristen McMartin, the lead health economist was Olga Gajic-Veljanoski, the secondary health economist was Shawn Xie, the health economics associate was Jennifer Guo, the patient and public partnership analyst was David Wells, and the medical librarian was Corinne Holubowich.
The medical editor was Jeanne McKane. Others involved in the development and production of this report were Harrison Heft, Claude Soulodre, Kara Cowan, Kathryn Schwarz, Sarah McDowell, Vivian Ng, Andrée Mitchell, Nancy Sikich, Amy Lang, and Irfan Dhalla.
We are grateful to the following individuals for lending their expertise to the development of this report: Anuj Bhatia (Anesthesia Chronic Pain Clinical Services and Neuromodulation for Pain Program, Toronto Western Hospital); Philip Chan (Chronic Pain Clinic, Department of Anesthesia, McMaster University); Aaron Hong (Chronic Pain Medicine and Regional Anesthesia and Neuromodulation, St. Michael's Hospital); Keith MacDougall (Department of Neurosurgery, London Health Sciences Centre); and William W. L. Wong (School of Pharmacy, University of Waterloo).
We also thank our lived experience participants who generously gave their time to share their stories with us for this report.
The statements, conclusions, and views expressed in this report do not necessarily represent the views of those we consulted.
ABBREVIATIONS
- CI
Confidence interval
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- IPG
Implantable pulse generator
- NICE
National Institute for Health and Care Excellence
- NRS
Numeric rating scale
- ODI
Oswestry Disability Index
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
Quality-adjusted life-year
- SCS
Spinal cord stimulation
- SD
Standard deviation
- VAS
Visual analogue scale
GLOSSARY
- Adverse event
An adverse event is any unexpected problem that happens during or as a result of treatment, regardless of the cause or severity.
- Budget impact analysis
A budget impact analysis estimates the financial impact of adopting a new health care intervention on the current budget (i.e., its affordability). It is based on predictions of how changes in the intervention mix impact the level of health care spending for a specific population. Budget impact analyses are typically conducted for a short-term period (e.g., 5 years). The budget impact, sometimes referred to as the net budget impact, is the estimated cost difference between the current scenario (i.e., the anticipated amount of spending for a specific population without using the new intervention) and the new scenario (i.e., the anticipated amount of spending for a specific population following the introduction of the new intervention).
- Cost-effective
A health care intervention is considered cost-effective when it provides additional benefits, compared with relevant alternatives, at an additional cost that is acceptable to a decision-maker based on the maximum willingness-to-pay value.
- Cost–utility analysis
A cost–utility analysis is a type of economic evaluation used to compare the benefits of two or more health care interventions with their costs. The benefits are measured using quality-adjusted life-years (QALYs), which capture both the quality and quantity of life. In a cost–utility analysis, the main outcome measure is the incremental cost per quality-adjusted life-year gained.
- Deterministic sensitivity analysis
Deterministic sensitivity analysis is an approach used to explore uncertainty in the results of an economic evaluation by varying parameter values to observe the potential impact on the cost-effectiveness of the health care intervention of interest. One-way sensitivity analysis accounts for uncertainty in parameter values one at a time, whereas multiway sensitivity analysis accounts for uncertainty in a combination of parameter values simultaneously.
- Discounting
Discounting is a method used in economic evaluations to adjust for the differential timing of the costs incurred and the benefits generated by a health care intervention over time. Discounting reflects the concept of positive time preference, whereby future costs and benefits are reduced to reflect their present value. The health technology assessments conducted by Ontario Health (Quality) use an annual discount rate of 1.5% for both future costs and future benefits.
- Dominant
A health care intervention is considered dominant when it is more effective and less costly than its comparator(s).
- Dorsal fibres
Within the peripheral nervous system, the dorsal fibres are long, slender projections of neurons (nerve cells). In spinal cord stimulation, the electrical current stimulates the dorsal fibres, interfering with the transmission of pain signals to the brain.
- Health-related quality of life
Health-related quality of life is a measure of the impact of a health care intervention on a person's health; it includes the dimensions of physiology, function, social life, cognition, emotions, sleep and rest, energy and vitality, health perception, and general life satisfaction.
- Health state
A health state is a particular status of health (e.g., sick, well, dead). A health state is associated with some amount of benefit and may be associated with specific costs. Benefit is captured through individual or societal preferences for the time spent in each health state and is expressed in quality-adjusted weights called utility values. In a Markov model, a finite number of mutually exclusive health states are used to represent discrete states of health.
- Incremental cost
An incremental cost is the additional cost, typically per person, of a health care intervention versus a comparator.
- Incremental cost-effectiveness ratio (ICER)
The incremental cost-effectiveness ratio (ICER) is a summary measure that indicates, for a given health care intervention, how much more a consumer must pay to get an additional unit of benefit relative to an alternative intervention. It is obtained by dividing the incremental cost by the incremental effectiveness. Incremental cost-effectiveness ratios are typically presented as the cost per life-year gained or the cost per quality-adjusted life-year gained.
- Markov model
A Markov model is a type of decision-analytic model used in economic evaluations to estimate the costs and health outcomes (e.g., quality-adjusted life-years gained) associated with using a particular health care intervention. Markov models are useful for clinical problems that involve events of interest that may recur over time (e.g., stroke). A Markov model consists of mutually exclusive, exhaustive health states. Patients remain in a given health state for a certain period of time before moving to another health state based on transition probabilities. The health states and events modelled may be associated with specific costs and health outcomes.
- Ministry of Health perspective
The perspective adopted in economic evaluations determines the types of cost and health benefit to include. Ontario Health (Quality) develops health technology assessment reports from the perspective of the Ontario Ministry of Health. This perspective includes all costs and health benefits attributable to the Ministry of Health, such as treatment costs (e.g., drugs, administration, monitoring, hospital stays) and costs associated with managing adverse events caused by treatments. This perspective does not include out-of-pocket costs incurred by patients related to obtaining care (e.g., transportation) or loss of productivity (e.g., absenteeism).
- One-way sensitivity analysis
A one-way sensitivity analysis is used to explore uncertainty in the results of an economic evaluation. It is done by varying one model input (i.e., a parameter) at a time between its minimum and maximum values to observe the potential impact on the cost-effectiveness of the health care intervention of interest.
- Paresthesia
Paresthesia is a physical sensation of tingling (“pins and needles”), chilling, burning, or numbness. It occurs when sustained pressure is placed on a nerve and is sometimes experienced by people undergoing spinal cord stimulation.
- Peripheral nervous system
The human nervous system coordinates the body's actions by transmitting signals to and from different parts of the body. The nervous system consists of the central nervous system and the peripheral nervous system. The peripheral nervous system sends information from the brain and spinal cord to the rest of the body. In spinal cord stimulation, the electrical current stimulates the dorsal fibres of the peripheral nervous system, interfering with the transmission of pain signals to the brain.
- Probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA) is used in economic models to explore uncertainty in several parameters simultaneously. It is done using Monte Carlo simulation. Model inputs are defined as a distribution of possible values. In each iteration, model inputs are obtained by randomly sampling from each distribution, and a single estimate of cost and effectiveness is generated. This process is repeated many times (e.g., 10,000 times) to estimate the number of times (i.e., the probability) that the health care intervention of interest is cost-effective.
- Quality-adjusted life-year (QALY)
The quality-adjusted life-year (QALY) is a generic health outcome measure commonly used in cost–utility analyses to reflect the quantity and quality of life-years lived. The life-years lived are adjusted for quality of life using individual or societal preferences (i.e., utility values) for being in a particular health state. One year of perfect health is represented by one quality-adjusted life-year.
- Reference case
The reference case is a preferred set of methods and principles that provide the guidelines for economic evaluations. Its purpose is to standardize the approach of conducting and reporting economic evaluations, so that results can be compared across studies.
- Scenario analysis
A scenario analysis is used to explore uncertainty in the results of an economic evaluation. It is done by observing the potential impact of different scenarios on the cost-effectiveness of a health care intervention. Scenario analyses include varying structural assumptions from the reference case.
- Sensitivity analysis
Every economic evaluation contains some degree of uncertainty, and results can vary depending on the values taken by key parameters and the assumptions made. Sensitivity analysis allows these factors to be varied and shows the impact of these variations on the results of the evaluation. There are various types of sensitivity analysis, including deterministic, probabilistic, and scenario.
- Time horizon
In economic evaluations, the time horizon is the time frame over which costs and benefits are examined and calculated. The relevant time horizon is chosen based on the nature of the disease and health care intervention being assessed, as well as the purpose of the analysis. For instance, a lifetime horizon would be chosen to capture the long-term health and cost consequences over a patient's lifetime.
- Utility
Utilities are values that represent people's preferences for various health states. Typically, utility values are anchored at 0 (death) and 1 (perfect health). In some scoring systems, a negative utility value indicates a state of health valued as being worse than death. Utility values can be aggregated over time to derive quality-adjusted life-years, a common outcome measure in economic evaluations.
- Willingness-to-pay value
A willingness-to-pay value is the monetary value a health care consumer is willing to pay for added health benefits. When conducting a cost–utility analysis, the willingness-to-pay value represents the cost a consumer is willing to pay for an additional quality-adjusted life-year. If the incremental cost-effectiveness ratio is less than the willingness-to-pay value, the health care intervention of interest is considered cost-effective. If the incremental cost-effectiveness ratio is more than the willingness-to-pay value, the intervention is considered not to be cost-effective.
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Search date: August 17, 2018
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, CRD Health Technology Assessment Database, and NHS Economic Evaluation Database
Database: EBM Reviews = Cochrane Central Register of Controlled Trials <July 2018>, EBM Reviews = Cochrane Database of Systematic Reviews <2005 to August 15, 2018>, EBM Reviews = Health Technology Assessment <4th Quarter 2016>, EBM Reviews = NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 33>, Ovid MEDLINE(R) ALL <1946 to August 16, 2018>
Search Strategy:
-
1
Spinal Cord Stimulation/ (6290)
-
2
exp Spinal Cord/ (177559)
-
3
Electric Stimulation Therapy/ (21685)
-
4
Electric Stimulation/ (164601)
-
5
3 or 4 (185259)
-
6
2 and 5 (11261)
-
7
(((spinal cord∗ or spine or spines or column∗ or sc or epidur∗) adj3 (stimulat∗ or electrostimulat∗)) or SCS).ti,ab,kf. (26206)
-
8
or/1,6-7 (37074)
-
9
(HF10∗ or HF 10∗).ti,ab,kf. (750)
-
10
(high frequenc∗ or highfrequenc∗).ti,ab,kf. (178709)
-
11
(10-kHz or 10khz or 10 kilohertz or 10kilohertz or 10 kilo-hertz or 10kilo-hertz or 10,000 hz or 10,000hz or 10000 hz or 10000hz or 10,000 hertz or 10,000hertz or 10000 hertz or 10000hertz).ti,ab,kf. (4246)
-
12
(nevro∗ or senza∗).ti,ab,kf. (617)
-
13
or/9-12 (183212)
-
14
8 and 13 (1118)
-
15
(HF SCS or HF10 SCS).ti,ab,kf. (217)
-
16
or/14-15 (1158)
-
17
exp Animals/ not Humans/ (15182018)
-
18
16 not 17 (451)
-
19
limit 18 to english language [Limit not valid in CDSR; records were retained] (412)
-
20
19 use medall,cctr,coch,clhta,cleed (236)
-
21
spinal cord stimulation/ (6290)
-
22
exp spinal cord/ (177559)
-
23
electrotherapy/ (21770)
-
24
electrostimulation/ (58606)
-
25
or/23-24 (80328)
-
26
22 and 25 (3974)
-
27
(((spinal cord∗ or spine or spines or column∗ or sc or epidur∗) adj3 (stimulat∗ or electrostimulat∗)) or SCS).tw,kw,dv. (26448)
-
28
or/21,26-27 (30587)
-
29
(HF10∗ or HF 10∗).tw,kw,dv. (774)
-
30
(high frequenc∗ or highfrequenc∗).tw,kw,dv. (179575)
-
31
(10-kHz or 10khz or 10 kilohertz or 10kilohertz or 10 kilo-hertz or 10kilo-hertz or 10,000 hz or 10,000hz or 10000 hz or 10000hz or 10,000 hertz or 10,000hertz or 10000 hertz or 10000hertz).tw,kw,dv. (4249)
-
32
(nevro∗ or senza∗).tw,kw,dv. (639)
-
33
or/29-32 (184108)
-
34
28 and 33 (975)
-
35
exp spinal cord/ (177559)
-
36
high-frequency electrotherapy/ (31)
-
37
35 and 36 (0)
-
38
(HF SCS or HF10 SCS).tw,kw,dv. (217)
-
39
or/34,37-38 (1015)
-
40
(exp animal/ or nonhuman/) not exp human/ (9784507)
-
41
39 not 40 (710)
-
42
limit 41 to english language [Limit not valid in CDSR; records were retained] (670)
-
43
42 use emez (443)
-
44
20 or 43 (679)
-
45
44 use medall (166)
-
46
44 use emez (443)
-
47
44 use coch (0)
-
48
44 use cctr (70)
-
49
44 use clhta (0)
-
50
44 use cleed (0)
-
51
remove duplicates from 44 (512)
Economic Evidence Search
Search date: August 20, 2018
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, and National Health Service (NHS) Economic Evaluation Database
Database: EBM Reviews = Cochrane Central Register of Controlled Trials <July 2018>, EBM Reviews = Cochrane Database of Systematic Reviews <2005 to August 15, 2018>, EBM Reviews = Health Technology Assessment <4th Quarter 2016>, EBM Reviews = NHS Economic Evaluation Database <1st Quarter 2016>, Embase <1980 to 2018 Week 34>, Ovid MEDLINE(R) ALL <1946 to August 17, 2018>
Search Strategy:
-
1
Spinal Cord Stimulation/ (6305)
-
2
exp Spinal Cord/ (177636)
-
3
Electric Stimulation Therapy/ (21697)
-
4
Electric Stimulation/ (164659)
-
5
3 or 4 (185328)
-
6
2 and 5 (11263)
-
7
(((spinal cord∗ or spine or spines or column∗ or sc or epidur∗) adj3 (stimulat∗ or electrostimulat∗)) or SCS).ti,ab,kf. (26239)
-
8
or/1,6-7 (37113)
-
9
(HF10∗ or HF 10∗).ti,ab,kf. (750)
-
10
(high frequenc∗ or highfrequenc∗).ti,ab,kf. (178833)
-
11
(10 khz or 10khz or 10 kilohertz or 10kilohertz or 10 kilo-hertz or 10kilo-hertz or 10,000 hz or 10,000hz or 10000 hz or 10000hz or 10,000 hertz or 10,000hertz or 10000 hertz or 10000hertz).ti,ab,kf. (4254)
-
12
(nevro∗ or senza∗).ti,ab,kf. (617)
-
13
or/9-12 (183344)
-
14
8 and 13 (1122)
-
15
(HF SCS or HF10 SCS).ti,ab,kf. (219)
-
16
or/14-15 (1163)
-
17
economics/ (247973)
-
18
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (753035)
-
19
economics.fs. (408785)
-
20
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).ti,ab,kf. (792268)
-
21
exp “costs and cost analysis”/ (537727)
-
22
(cost or costs or costing or costly).ti. (239361)
-
23
cost effective∗.ti,ab,kf. (287142)
-
24
(cost∗ adj2 (util∗ or efficacy∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kf. (188964)
-
25
models, economic/ (11540)
-
26
markov chains/ or monte carlo method/ (72519)
-
27
(decision adj1 (tree∗ or analy∗ or model∗)).ti,ab,kf. (36834)
-
28
(markov or markow or monte carlo).ti,ab,kf. (116391)
-
29
quality-adjusted life years/ (34537)
-
30
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).ti,ab,kf. (61762)
-
31
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).ti,ab,kf. (100234)
-
32
or/17-31 (2314716)
-
33
16 and 32 (42)
-
34
33 use medall,coch,cctr,clhta (25)
-
35
16 use cleed (0)
-
36
or/34-35 (25)
-
37
limit 36 to english language [Limit not valid in CDSR; records were retained] (22)
-
38
spinal cord stimulation/ (6305)
-
39
exp spinal cord/ (177636)
-
40
electrotherapy/ (21782)
-
41
electrostimulation/ (58662)
-
42
or/40-41 (80395)
-
43
39 and 42 (3976)
-
44
(((spinal cord∗ or spine or spines or column∗ or sc or epidur∗) adj3 (stimulat∗ or electrostimulat∗)) or SCS).tw,kw,dv. (26481)
-
45
or/38,43-44 (30624)
-
46
(HF10∗ or HF 10∗).tw,kw,dv. (774)
-
47
(high frequenc∗ or highfrequenc∗).tw,kw,dv. (179700)
-
48
(10 khz or 10khz or 10 kilohertz or 10kilohertz or 10 kilo-hertz or 10kilo-hertz or 10,000 hz or 10,000hz or 10000 hz or 10000hz or 10,000 hertz or 10,000hertz or 10000 hertz or 10000hertz).tw,kw,dv. (4257)
-
49
(nevro∗ or senza∗).tw,kw,dv. (639)
-
50
or/46-49 (184241)
-
51
45 and 50 (978)
-
52
exp spinal cord/ (177636)
-
53
high frequency electrotherapy/ (31)
-
54
52 and 53 (0)
-
55
(HF SCS or HF10 SCS).tw,kw,dv. (219)
-
56
or/51,54-55 (1019)
-
57
Economics/ (247973)
-
58
Health Economics/ or Pharmacoeconomics/ or Drug Cost/ or Drug Formulary/ (118618)
-
59
Economic Aspect/ or exp Economic Evaluation/ (418461)
-
60
(econom∗ or price or prices or pricing or priced or discount∗ or expenditure∗ or budget∗ or pharmacoeconomic∗ or pharmaco-economic∗).tw,kw. (816063)
-
61
exp “Cost”/ (537727)
-
62
(cost or costs or costing or costly).ti. (239361)
-
63
cost effective∗.tw,kw. (298018)
-
64
(cost∗ adj2 (util∗ or efficac∗ or benefit∗ or minimi∗ or analy∗ or saving∗ or estimate∗ or allocation or control or sharing or instrument∗ or technolog∗)).ab,kw. (196399)
-
65
Monte Carlo Method/ (58044)
-
66
(decision adj1 (tree∗ or analy∗ or model∗)).tw,kw. (40534)
-
67
(markov or markow or monte carlo).tw,kw. (121207)
-
68
Quality-Adjusted Life Years/ (34537)
-
69
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw,kw. (65507)
-
70
((adjusted adj1 (quality or life)) or (willing∗ adj2 pay) or sensitivity analys∗s).tw,kw. (119678)
-
71
or/57-70 (1974687)
-
72
56 and 71 (48)
-
73
72 use emez (18)
-
74
limit 73 to english language [Limit not valid in CDSR; records were retained] (18)
-
75
37 or 74 (40)
-
76
75 use medall (15)
-
77
75 use emez (18)
-
78
75 use coch (0)
-
79
75 use cctr (7)
-
80
75 use clhta (0)
-
81
75 use cleed (0)
-
82
remove duplicates from 75 (25)
Grey Literature Search
Performed: July 16–20, 2018
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, ClinicalTrials.gov, PROSPERO, EUnetHTA, Tufts CEA Registry
Keywords used:
HF10, HF 10, spinal cord stimulation, SCS, stimulation, high frequency, 10 khz, 10 kilohertz, 10,000 hz, senza, neuromodulation
Results (included in PRISMA): 2
Ongoing clinical trials (ClinicalTrials.gov): 14
Ongoing HTAs (PROSPERO/EUnetHTA): 2
Appendix 2: Critical Appraisal of Clinical Evidence
Table A1:
Risk of Biasa Among Randomized Trials (Cochrane Risk of Bias Tool)
| Author, Year | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|
| Amirdelfan et al, 201832 | Low risk | Low risk | Moderate riskb | Low risk | Low risk | — |
| Bocci et al, 201836 | Low risk | Low risk | Moderate riskb | Low risk | Low risk | — |
| Bolash et al, 201928 | Low risk | Low risk | Moderate riskb | Low risk | Low risk | — |
| De Andres et al, 201729 | Low risk | Low risk | Moderate riskb | Low risk | Low risk | — |
| Kapural et al, 2015, 201630,31 | Low risk | Low risk | Moderate riskb | Low risk | Low risk | — |
| Thomson et al, 201838 | Low risk | Low risk | Moderate riskb | Low risk | Low risk | — |
Possible risk of bias levels: low, high, and unclear.
Patients and investigators were not blinded; this may have posed a risk of bias to the main study outcome.
Table A2:
GRADE Evidence Profile for Comparison of 10-kHz High-Frequency SCS Versus Conventional SCS for Chronic Noncancer Pain—Randomized Controlled Trials
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Pain Intensity | |||||||
| 3 (RCTs)28–30 | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕ ⊕ ⊕ Moderate |
| Functional Disability | |||||||
| 3 (RCTs)28–30 | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕ ⊕ ⊕ Moderate |
| Opioid Use | |||||||
| 1 (RCT)28 | Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | NA | ⊕ ⊕ Low |
| Patient Satisfaction | |||||||
| 2 (RCTs)28,30 | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕ ⊕ ⊕ Moderate |
| Global Impression of Change | |||||||
| 3 (RCTs)28–30 | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕ ⊕ ⊕ Moderate |
| Sleep Quality | |||||||
| 2 (RCTs)28,30 | Serious limitations (−1)a | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕ ⊕ ⊕ Moderate |
| Health-Related Quality of Life | |||||||
| 3 (RCTs)28–30 | Serious limitations (−1)a | Serious limitations | No serious limitations | No serious limitations | Undetected | NA | ⊕ ⊕ ⊕ Moderate |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial; SCS, spinal cord stimulation.
Blinding of patients and investigators was not done.
Only one trial reported on opioid use, and doses were extremely variable.
Table A3:
GRADE Evidence Profile for 10-kHz High-Frequency SCS for Chronic Noncancer Pain—Crossover Trials
| Number of Studies (Design) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Pain Intensity, Randomized Crossover, kHz Range | |||||||
| 1 (randomized crossover)36 | Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | NA | ⊕ ⊕ Low |
| Pain Intensity, Randomized Crossover, Low-Frequency Versus Burst Versus 10-kHz High-Frequency SCS | |||||||
| 1 (randomized crossover)38 | Serious limitations (−1)a | No serious limitations | No serious limitations | Serious limitations (−1)b | Undetected | NA | ⊕ ⊕ Low |
Abbreviations: GRADE, Grading of Recommendations Assessment, Development, and Evaluation; SCS, spinal cord stimulation.
Blinding of patients and investigators was not done.
Only one trial, and that involved a small study sample.
Appendix 3: Selected Excluded Studies—Clinical Evidence
For transparency, we provide a list of studies that readers might have expected to see in the clinical evidence review but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary Reason for Exclusion |
|---|---|
| Abejon D, Rueda P, Vallejo R. Threshold evolution as an analysis of the different pulse frequencies in rechargeable systems for spinal cord stimulation. Neuromodulation. 2016;19(3):276-82. | Not 10-kHz high-frequency SCS (1,200 Hz) |
| Ahmadi SA, Vesper J, Schu S, Slotty PJ. High-frequency spinal cord stimulation in surgery-naive patients: a prospective single-center study. Neuromodulation. 2017;20(4):348-53. | Wrong study design |
| Al-Kaisy A, Palmisani S, Smith T, Harris S, Pang D. The use of 10-kilohertz spinal cord stimulation in a cohort of patients with chronic neuropathic limb pain refractory to medical management. Neuromodulation. 2015;18(1):18-23. | Wrong study design |
| Al-Kaisy A, Palmisani S, Smith TE, Pang D, Lam K, Burgoyne W, et al. 10 kHz high-frequency spinal cord stimulation for chronic axial low back pain in patients with no history of spinal surgery: a preliminary, prospective, open label and proof-of-concept study. | Wrong study design |
| Al-Kaisy A, Palmisani S, Smith TE, Carganillo R, Houghton R, Pang D, et al. Long-term improvements in chronic axial low back pain patients without previous spinal surgery: a cohort analysis of 10-kHz high-frequency spinal cord stimulation over 36 months. Pain Med. 2018;19(6):1219-26. | Wrong study design |
| Al-Kaisy A, Van Buyten JP, Smet I, Palmisani S, Pang D, Smith T. Sustained effectiveness of 10khz high-frequency spinal cord stimulation for patients with chronic, low back pain: 24-month results of a prospective multicenter study. Pain Med. 2014;15(3):347-54. | Wrong study design |
| Al-Kaisy A, Palmisani S, Pang D, Sanderson K, Wesley S, Tan Y, et al. Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS Frequency Study). Neuromodulation. 2018;21(5):457-65. | Not 10-kHz high-frequency SCS (maximum 5,882 Hz) |
| Annemans L, Van Buyten JP, Smith T, Al-Kaisy A. Cost effectiveness of a novel 10-kHz high-frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). J Long Term Eff Med Implants. 2014;24(2-3):173-84. | Cost-effectiveness study |
| Arcioni R, Palmisani S, Mercieri M, Vano V, Tigano S, Smith T, et al. Cervical 10 kHz spinal cord stimulation in the management of chronic, medically refractory migraine: a prospective, open-label, exploratory study. Eur J Pain. 2016;20(1):70-8. | Wrong study design |
| Bennett DS, Alo KM, Oakley J, Feler CA, Hagen J. Spinal cord stimulation for complex regional pain syndrome I [RSD]: a retrospective multicenter experience from 1995 to 1998 of 101 patients. Neuromodulation. 1999;2(3):202-10. | Not 10-kHz high-frequency SCS (250 Hz, lead positioning) |
| Billet B, Hanssens K, De Coster O, Nagels W, Weiner RL, Wynendaele R, et al. Wireless high-frequency dorsal root ganglion stimulation for chronic low back pain: a pilot study. Acta Anaesthesiol Scand. 2018. | Wrong study design |
| Crapanzano JT, Harrison-Bernard LM, Jones MR, Kaye AD, Richter EO, Potash MN. High frequency spinal cord stimulation for complex regional pain syndrome: a case report. Pain Physician. 2017;20(1):E177-82. | Wrong study design |
| De Carolis G, Paroli M, Tollapi L, Doust MW, Burgher AH, Yu C, et al. Paresthesia-independence: an assessment of technical factors related to 10-kHz paresthesia-free spinal cord stimulation. Pain Physician. 2017;20(4):331-41. | Technical investigation of paresthesia and high-frequency SCS |
| De Ridder D, Lenders MWPM, De Vos CC, Dijkstra-Scholten C, Wolters R, Vancamp T, et al. A 2-center comparative study on tonic versus burst spinal cord stimulation: amount of responders and amount of pain suppression. Clin J Pain. 2015;31(5):433-7. | Not 10-kHz high-frequency SCS (burst and conventional frequency) |
| Edelbroek C, Terheggen M. High-frequency spinal cord stimulation and pregnancy: a case report. Neuromodulation. 2015;18(8):757-8. | Wrong study design |
| Falowski SM. An observational case series of spinal cord stimulation waveforms visualized on intraoperative neuromonitoring. Neuromodulation. 2019;22(2):219-28. | Intraoperative findings |
| Haider N, Ligham D, Quave B, Harum KE, Garcia EA, Gilmore CA, et al. Spinal cord stimulation (SCS) trial outcomes after conversion to a multiple waveform SCS system. Neuromodulation. 2018;21(5):504-7. | Wrong study design |
| Harandi S, Kapural L. Four-extremity neurostimulation using two cervical octapolar leads and high frequency of 10 kHz. Pain Pract. 2018;18(2):269-72. | Wrong study design |
| Herschkowitz D, Kubias J. Wireless peripheral nerve stimulation for complex regional pain syndrome type i of the upper extremity: A case illustration introducing a novel technology. Scand J Pain. 2018;18(3):555-60. | Peripheral nerve, not SCS |
| Kalmar Z, Kovacs N, Balas I, Perlaki G, Plozer E, Orsi G, et al. Effects of spinal cord stimulation on heart rate variability in patients with chronic pain. Ideggyogyaszati Szemle. 2013;66(3-4):102-6. | Not 10-kHz high-frequency SCS (200 Hz) |
| Kinfe TM, Pintea B, Link C, Roeske S, Guresir E, Guresir A, et al. High frequency (10 kHz) or burst spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: preliminary data from a prospective observational study. Neuromodulation. 2016;19(3):268-75. | Wrong study design |
| Kissoon NR, Hoelzer BC, Martin DP, Lamer TJ. High-frequency spinal cord stimulation in a patient with an implanted cardiac device. Pain Pract. 2017;17(4):558-63. | Wrong study design |
| Kriek N, Groeneweg JG, Stronks DL, Huygen FJ. Comparison of tonic spinal cord stimulation, high-frequency and burst stimulation in patients with complex regional pain syndrome: a double-blind, randomised placebo controlled trial. BMC Musculoskelet Disord. 2015;16(222). | Not 10-kHz high-frequency SCS (1,200 Hz) |
| Kriek N, Schreurs MWJ, Groeneweg JG, Dik WA, Tjiang GCH, Gultuna I, et al. spinal cord stimulation in patients with complex regional pain syndrome: a possible target for immunomodulation? Neuromodulation. 2018;21(1):77-86. | Not 10-kHz high-frequency SCS (burst and 1,200 Hz) |
| Kumar V, Prusik J, Lin Y, Hwang R, Feustel P, Pilitsis JG. Efficacy of alternating conventional stimulation and high-frequency stimulation in improving spinal cord stimulation outcomes: a pilot study. Neuromodulation. 2018;21(5):466-471. | Not 10-kHz high-frequency SCS (260 Hz) |
| Kumpulainen T, Ronty H, Koivukangas J. Management of patients with pain. Ann Clin Res. 1986;18(Suppl. 47):97-101. | Not 10-kHz high-frequency SCS (1,200 Hz) |
| Lambru G, Trimboli M, Palmisani S, Smith T, Al-Kaisy A. Safety and efficacy of cervical 10 kHz spinal cord stimulation in chronic refractory primary headaches: a retrospective case series. J Headache Pain. 2016;17(1):66. | Wrong study design |
| McAuley J, van Groningen R, Green C. Spinal cord stimulation for intractable pain following limb amputation. Neuromodulation. 2013;16(6):530-6; discussion 6. | Not 10-kHz high-frequency SCS (100 Hz) |
| Miyazaki Y, Koike H, Akane A, Shibata Y, Nishiwaki K, Sobue G. Spinal cord stimulation markedly ameliorated refractory neuropathic pain in transthyretin Val30Met familial amyloid polyneuropathy. Amyloid. 2011;18(2):87-90. | Not 10-kHz high-frequency SCS (high-frequency not reported) |
| Muhammad S, Roeske S, Chaudhry SR, Kinfe TM. Burst or high-frequency (10 kHz) spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: one year comparative data. Neuromodulation. 2017;20(7):661-7. | Wrong study design |
| Noori S, Mehta N. Management of medically refractory central poststroke pain using high-frequency spinal cord stimulation at 10 kHz. Neuromodulation. 2018;21(8):823-5. | Wrong study design |
| North JM, Hong KSJ, Cho PY. Clinical outcomes of 1 kHz subperception spinal cord stimulation in implanted patients with failed paresthesia-based stimulation: results of a prospective randomized controlled trial. Neuromodulation. 2016;19(7):731-7. | Not 10-kHz high-frequency SCS (1,000 Hz) |
| Owusu S, Huynh A, Gruenthal E, Prusik J, Owusu-Sarpong S, Cherala R, et al. Prospective evaluation of patient usage of above and below threshold waveforms with conventional spinal cord stimulation devices. Neuromodulation. 2017;20(6):567-74. | Not 10-kHz high-frequency SCS (1,200 Hz) |
| Perruchoud C, Eldabe S, Batterham AM, Madzinga G, Brookes M, Durrer A, et al. Analgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled study. Neuromodulation. 2013;16(4):363-9. | Not 10-kHz high-frequency SCS (5,000 Hz) |
| Rapcan R, Mlaka J, Venglarcik M, Vinklerova V, Gajdos M, Illes R. High-frequency spinal cord stimulation. Bratisl Lek Listy. 2015;116(6):354-6. | Wrong study design |
| Reddy CG, Dalm BD, Flouty OE, Gillies GT, Howard MA, Brennan TJ. Comparison of conventional and kilohertz frequency epidural stimulation in patients undergoing trialing for spinal cord stimulation: clinical considerations. World Neurosurg. 2016;88:586-91. | Wrong study design |
| Russo M, Van Buyten JP. 10-kHz High-frequency SCS therapy: a clinical summary. Pain Med. 2015;16(5):934-42. | Clinical review |
| Russo M, Verrills P, Mitchell B, Salmon J, Barnard A, Santarelli D. High frequency spinal cord stimulation at 10 khz for the treatment of chronic pain: 6-month Australian clinical experience. Pain Physician. 2016;19(4):267-80. | Wrong study design |
| Shamji MF, Rodriguez J, Shcharinsky A, Paul D. High rates of undiagnosed psychological distress exist in a referral population for spinal cord stimulation in the management of chronic pain. Neuromodulation. 2016;19(4):414-21. | Not 10-kHz high-frequency SCS |
| Simopoulos T, Yong RJ, Gill JS. Treatment of chronic refractory neuropathic pelvic pain with high-frequency 10-kilohertz spinal cord stimulation. Pain Pract. 2018;18(6):805-9. | Wrong study design |
| Sitzman BT, Kapural L, Yu G, Doust MW. Long-term outcomes of predominant leg pain and predominant back pain cohorts from a multicentre randomized controlled pivotal trial (SENZA-RCT) Comparing 10-kHz high-frequency and conventional low-frequency spinal cord stimulation. Neurotherapeutics. 2016;13 (3):654-5. | Abstract |
| Smith H, Youn Y, Pilitsis JG. Successful use of high-frequency spinal cord stimulation following conventional treatment failure. Stereotact Funct Neurosurg. 2015;93(3):190-3. | Not 10-kHz high-frequency SCS (1,200 Hz) |
| Steinbach K, Bettstetter H, Link C. High-frequency spinal cord stimulation at 10 kHz for the treatment of chronic neuropathic pain after a II-III degree burn. Pain Med. 2017;18(9):1826-8. | Wrong study design |
| Tiede J, Brown L, Gekht G, Vallejo R, Yearwood T, Morgan D. Novel spinal cord stimulation parameters in patients with predominant back pain. Neuromodulation. 2013;16(4):370-5. | Wrong study design |
| Torre-Amione G, Alo K, Estep JD, Valderrabano M, Khalil N, Farazi TG, et al. Spinal cord stimulation is safe and feasible in patients with advanced heart failure: early clinical experience. Eur J Heart Fail. 2014;16(7):788-95. | Not 10-kHz high-frequency SCS (50 Hz) |
| Van Buyten JP, Wille F, Smet I, Wensing C, Breel J, Karst E, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642-9. | Wrong study design |
| Weiner RL, Yeung A, Montes Garcia C, Tyler Perryman L, Speck B. Treatment of FBSS low back pain with a novel percutaneous DRG wireless stimulator: pilot and feasibility study. Pain Med. 2016;17(10):1911-6. | Not 10-kHz high-frequency SCS (1,500 Hz) |
| Youn Y, Smith H, Morris B, Argoff C, Pilitsis JG. The effect of high-frequency stimulation on sensory thresholds in chronic pain patients. Stereotact Funct Neurosurg. 2015;93(5):355-9. | Not 10-kHz high-frequency SCS (1,200 Hz) |
Abbreviation: SCS, spinal cord stimulation.
Appendix 4: Summary of Included Studies—Clinical Evidence Review
Table A4:
Comparative Trials Evaluating 10-kHz High-Frequency SCS for Chronic Noncancer Pain
| Author, Year Country Sites | Study Design | Patient Eligibility Criteria | Conversion Rate: Trial to Permanent Implant | Primary Outcome | Length of Follow-Up Outcomes |
|---|---|---|---|---|---|
| Randomized Controlled Trials | |||||
| Bolash et al, 201928 United States 7 sites |
Prospective RCT comparing 2 SCS protocols with the same device: conventional (0-1,500 Hz) and 10-kHz high-frequency with the Freedom SCS system external PG | Chronic pain of trunk or limbs, refractory to conservative therapy for a minimum of 3 months | Screened: n = 241 Randomized: n = 198 Implant trial: n = 189 Permanent implant: n = 171 |
Primary 6-month efficacy ≥50% reduction back pain over baseline | 12 months Back pain intensity, leg pain intensity opioid use, GAF, ODI, patient satisfaction, adverse events |
| De Andres et al, 201729 Spain 1 site |
Prospective superiority RCT comparing 2 rechargeable SCS systems: Senza HF10 (Nevro Corp.) and Surescan RestoreSensor (Medtronic) | One or more back surgeries followed by FBSS (chronic pain of the back and/or limbs refractory to conservative therapy for at least 6 months) | Screened: n = 78 Randomized: n = 60 Implant trial: n = 60 Permanent implant: n = 55 |
≥50% reduction in pain intensity NRS | 12 months ODI, PDQ, SF-12, HAD, MOS-SS, patient GIC, adverse outcomes |
| Kapural et al, 201530 Kapural et al,201631 Amirdelfan et al, 201832 United States 10 sites |
Prospective pragmatic multicentre noninferiority trial comparing 2 rechargeable SCS systems: Senza HF10 (Nevro Corp.) and Precision Plus (Boston Scientific) | Chronic back and/or limb pain refractory to conservative therapy for at least 3 months | Screened: n = 241 Randomized: n = 198 Implant trial: n = 189 Implant success: n = 171 |
3-month composite outcome of safety (no stimulation-related neurological deficit) and efficacy (percentage reporting ≥50% reduction in back pain VAS score) | 12 months Back pain intensity VAS, leg pain intensity VAS, analgesic medication, GAF, ODI, patient satisfaction adverse events |
| 12 months SF-12, GIC, ODI, GAF, SF-MPQ-2, sleep quality (PSQI), reliance on programmer |
|||||
| 24 months Back pain intensity VAS, leg pain intensity VAS, responders (≥50% reduction VAS), remitters (VAS ≤ 2.5), ODI, analgesic medication, patient/physician GIC and satisfaction, adverse events |
|||||
| Randomized Crossover Trials | |||||
| Bocci et al, 201836 Italy 1 site |
Prospective crossover trial comparing 3 SCS protocols: conventional (10 Hz to 200 Hz), burst and 10-kHz high-frequency | Chronic lower back pain with or without surgery with neuropathic or mixed low-back pain spreading to both legs and implanted with a SCS for >3 months | NR | Electrophysiologic changes during 3 SCS protocols | Duration to pain recurrence, pain intensity |
| Thomson et al, 201838 United Kingdom 4 sites |
Prospective, multicentre double-blind crossover trial (PROCO) comparing effects of SCS protocols at various kHz levels (1, 4, 7, and 10 kHz) with the Precision SCS (Boston Scientific) | Persistent or recurrent low back pain with or without lesser leg pain for at least 3 months prior to screening and 3 months of unsuccessful pain management and no back surgery in previous 6 months | Screened: n = 39 Implanted: n = 34 Included: n = 34 1-week paresthesia SCS trial to ensure usual care, those unresponsive (<50% pain reduction) to the paresthesia trial underwent a 1-week 10-kHz SCS trial |
3-month mean low back pain reduction over baseline across kHz frequency | 3 months Leg pain, overall pain relief, ODI, EQ-5D-5L, PSQI, patient GIC |
Abbreviations: BDI, Beck Depression Inventory; EQ-5D-5L, 5-layer ED-5D quality-of-life questionnaire; FBSS, failed back surgery syndrome; GAF, Global Assessment of Function; GIC, Global Impression of Change; HAD, Hospital Anxiety and Depression scale; MOS-SS, Medical Outcomes Study Sleep Scale; NRS, numeric rating scale; ODI, Oswestry Disability Index; PDQ, painDETECT questionnaire; PSQI, Pittsburgh Sleep Quality Index; RCT, randomized controlled trial; SCS, spinal cord stimulation; SF-12, SF-12 Health Questionnaire; SF-MPQ-2, Short Form McGill Pain Questionnaire; VAS, visual analogue scale.
Appendix 5: Additional Findings—Clinical Evidence Review
Table A5:
Pain Intensity, SENZA-RCT Study
| Outcome | 10-kHz High-Frequency SCS | Conventional SCS | Differencea | Relative Risk (95% CI) |
|---|---|---|---|---|
| Back Pain Intensity, VAS, Mean ± SD | ||||
| Baseline | 7.4 ± 1.2 | 7.8 ± 1.2 | 0.33 | — |
| 3 months | NR | NR | — | — |
| 6 months | NR | NR | — | — |
| 24 months | 2.4 ± 2.3 | 4.5 ± 2.9 | 5.0 ± 2.5 | — |
| Back Pain Responder, %b | ||||
| 3 months | 84.3 | 43.8 | 40.5 | 1.9 (1.4–2.5) |
| 6 months | 76.4 | 51.9 | 24.5 | 1.5 (1.2–1.9) |
| 12 months | 78.7 | 51.3 | 27.4 | 1.5 (1.2–1.9) |
| 24 months | 76.5 | 49.3 | 27.2 | — |
| Back Pain Remitter, %c | ||||
| 3 months | 65.2 | 31.3 | 33.9 | 2.1 (1.4–3.0) |
| 6 months | 59.6 | 36.7 | 22.9 | 1.6 (1.1–2.3) |
| 12 months | 68.5 | 36.3 | 32.2 | 1.9 (1.3–2.7) |
| 24 months | 65.9 | 31.0 | 27.2 | — |
| Leg Pain Intensity, VAS, Mean ± SD | ||||
| Baseline | 7.1 ± 1.5 | 7.6 ± 1.4 | 0.34 | — |
| 3 months | NR | NR | — | — |
| 6 months | NR | NR | — | — |
| 12 months | 2.1 | 3.8 | 1.7 | — |
| 24 months | 2.4 ± 2.5 | 3.9 ± 2.8 | 1.5 | — |
| Leg Pain Responder, %b | ||||
| 3 months | 83.1 | 55.0 | 28.1 | 1.5 (1.2–1.9) |
| 6 months | 80.9 | 54.4 | 26.5 | 1.5 (1.2–1.9) |
| 12 months | 78.7 | 51.3 | 27.4 | 1.5 (1.2–2.0) |
| 24 months | 72.9 | 49.3 | 23.6 | — |
| Leg Pain Remitter, %c | ||||
| 3 months | 76.4 | 37.5 | 38.9 | 2.0 (1.5–2.8) |
| 6 months | 68.6 | 44.3 | 24.3 | 1.5 (1.2–2.0) |
| 12 months | 67.4 | 42.5 | 24.9 | 1.6 (1.2–2.1) |
| 24 months | 65.9 | 30.4 | 35.5 | — |
Abbreviations: SCS, spinal cord stimulation; VAS, visual analogue scale.
Crude differences calculated for this health technology assessment.
Pain responder when pain intensity decreases ≥50% over baseline.
Pain remitter when pain intensity score ≤ 2.5.
Source: Kapural et al, 2015.30
Table A6:
Functional Disability, SENZA-RCT Study
| ODI Severity, % | 10-kHz High-Frequency SCS | Conventional SCS | Differencea | |
|---|---|---|---|---|
| Baseline | Minimal | 0.0 | 0.0 | 0.0 |
| Moderate | 8.9 | 1.2 | 7.7 | |
| Severe | 71.1 | 76.5 | −5.4 | |
| Crippling | 20.0 | 22.2 | −2.2 | |
| 3 months | Minimal | 16.9 | 6.2 | 10.7 |
| Moderate | 51.7 | 45.7 | 6.0 | |
| Severe | 28.1 | 45.7 | −17.6 | |
| Crippling | 3.4 | 2.5 | 0.9 | |
| 6 months | Minimal | 16.9 | 11.1 | 5.8 |
| Moderate | 46.1 | 33.3 | 12.8 | |
| Severe | 31.5 | 50.6 | −19.1 | |
| Crippling | 5.6 | 4.9 | −0.7 | |
| 12 months | Minimal | 16.9 | 8.6 | 8.1 |
| Moderate | 46.1 | 37.0 | 9.1 | |
| Severe | 34.8 | 44.4 | −9.6 | |
| Crippling | 2.2 | 9.9 | −7.7 | |
| 24 months | Minimal | 23.5 | 9.9 | 13.6 |
| Moderate | 41.2 | 39.4 | 1.8 | |
| Severe | 30.6 | 42.3 | −11.7 | |
| Crippling | 4.7 | 8.5 | −3.8 |
Abbreviations: ODI, Oswestry Disability Index; SCS, spinal cord stimulation.
Crude differences calculated for this health technology assessment.
Source: Kapural et al, 2015.30
Table A7:
Global Impression of Change and Treatment Satisfaction, SENZA-RCT Study
| 10-kHz High-Frequency SCS | Conventional SCS | Differencea | ||
|---|---|---|---|---|
| Global Impression of Change, % | ||||
| Baseline | No symptoms | 0.0 | 0.0 | 0 |
| Minimal symptoms | 6.7 | 9.9 | −3.2 | |
| Transient symptoms | 17.8 | 19.8 | −2.0 | |
| Mild symptoms | 37.8 | 35.8 | 2.0 | |
| Moderate symptoms | 26.7 | 27.2 | −0.5 | |
| Serious symptoms | 11.1 | 6.2 | 4.9 | |
| Some impairment | 0.0 | 1.2 | −1.2 | |
| 3 months | No symptoms | 6.7 | 4.9 | 1.8 |
| Minimal symptoms | 27.0 | 23.5 | 3.5 | |
| Transient symptoms | 27.0 | 22.2 | 4.8 | |
| Mild symptoms | 29.2 | 29.6 | −0.4 | |
| Moderate symptoms | 7.9 | 16.0 | −8.1 | |
| Serious symptoms | 1.1 | 3.7 | −2.6 | |
| Some impairment | 1.1 | 0.0 | 1.1 | |
| 6 months | No symptoms | 14.6 | 7.4 | 7.2 |
| Minimal symptoms | 28.1 | 27.2 | 0.9 | |
| Transient symptoms | 23.6 | 25.9 | −2.3 | |
| Mild symptoms | 24.7 | 24.7 | 0 | |
| Moderate symptoms | 6.7 | 12.3 | −5.6 | |
| Serious symptoms | 2.2 | 2.5 | −0.3 | |
| Some impairment | 0.0 | 0.0 | 0 | |
| 12 months | No symptoms | 19.1 | 13.6 | 5.5 |
| Minimal symptoms | 24.7 | 22.2 | 2.5 | |
| Transient symptoms | 27.0 | 23.5 | 3.5 | |
| Mild symptoms | 18.0 | 23.5 | −5.5 | |
| Moderate symptoms | 7.9 | 13.6 | −5.7 | |
| Serious symptoms | 3.4 | 3.7 | −0.3 | |
| Some impairment | 0.0 | 0.0 | 0 | |
| Global Impression of Change, Patient | ||||
| 12 months | Great deal better | 29.5 | 21.3 | 6.2 |
| Better | 27.3 | 16.3 | 11.0 | |
| Moderately better | 21.6 | 22.5 | −0.9 | |
| Somewhat better | 9.1 | 8.8 | 0.3 | |
| Little better | 4.5 | 8.8 | −4.3 | |
| Almost the same | 3.4 | 6.3 | −2.9 | |
| No change | 4.5 | 16.3 | −11.8 | |
| 24 months | Great deal better | 34.1 | 21.1 | 13.0 |
| Better | 29.4 | 15.5 | 13.9 | |
| Moderately better | 11.8 | 19.7 | −7.9 | |
| Somewhat better | 4.7 | 12.7 | −8.0 | |
| Little better | 2.4 | 5.6 | −3.2 | |
| Almost the same | 8.2 | 4.2 | 4.0 | |
| No change | 9.4 | 21.1 | −11.7 | |
| Global Impression of Change, Physician | ||||
| 12 months | Great deal better | 39.3 | 25.0 | 14.3 |
| Better | 34.8 | 25.0 | 9.8 | |
| Moderately better | 11.2 | 16.3 | −5.1 | |
| Somewhat better | 6.7 | 10.0 | −3.3 | |
| Little better | 0.0 | 3.8 | −3.8 | |
| Almost the same | 3.4 | 5.0 | −1.6 | |
| No change | 4.5 | 15 | −10.5 | |
| 24 months | Great deal better | 40.7 | 20.0 | 20.7 |
| Better | 27.9 | 28.6 | −0.7 | |
| Moderately better | 9.3 | 12.9 | −3.6 | |
| Somewhat better | 8.1 | 12.9 | −4.8 | |
| Little better | 2.3 | 1.4 | 0.9 | |
| Almost the same | 1.2 | 1.4 | −0.2 | |
| No change | 10.5 | 22.9 | −12.4 | |
| Patient Satisfaction, % | ||||
| 3 months | Very satisfied | 54.1 | 33.8 | 20.3 |
| Satisfied | 29.4 | 43.2 | −13.8 | |
| Not sure | 14.1 | 17.6 | −3.5 | |
| Dissatisfied | 1.2 | 2.7 | −1.5 | |
| Very dissatisfied | 1.2 | 2.7 | 1.5 | |
| 6 months | Very satisfied | NR | NR | — |
| Satisfied | NR | NR | — | |
| Not sure | NR | NR | — | |
| Dissatisfied | NR | NR | — | |
| Very dissatisfied | NR | NR | — | |
| 12 months | Very satisfied | 55.4 | 32.3 | 23.1 |
| Satisfied | 27.7 | 46.2 | −18.5 | |
| Not sure | 15.7 | 16.9 | −1.2 | |
| Dissatisfied | 1.2 | 3.1 | −1.9 | |
| Very dissatisfied | 0.0 | 1.5 | −1.5 | |
| 24 months | Very satisfied | 60.0 | 40.4 | 19.6 |
| Satisfied | 26.3 | 45.6 | −19.3 | |
| Not sure | 10.0 | 10.5 | −0.5 | |
| Dissatisfied | 1.3 | 3.5 | −2.2 | |
| Very dissatisfied | 0.0 | 0.0 | 0 | |
Abbreviation: SCS, spinal cord stimulation.
Crude differences calculated for this health technology assessment.
Source: Kapural et al, 2015.30
Table A8:
Health-Related Quality of Life and Sleep Quality, De Andres et al
| 10-kHz High-Frequency SCS | Conventional SCS | Differencea | |
|---|---|---|---|
| SF-12 Mean ± SD | |||
| Baseline | |||
| Physical functioning | 0.0 ± 0.00 | 13.79 ± 29.57 | — |
| Role physical | 14.42 ± 18.94 | 18.97 ± 21.81 | −4.55 |
| Bodily pain | 25.96 ± 20.59 | 20.69 ± 24.15 | −5.27 |
| General health | 18.27 ± 26.06 | 18.10 ± 17.55 | 0.17 |
| Vitality | 20.00 ± 18.76 | 22.07 ± 25.27 | −2.07 |
| Social functioning | 36.15 ± 29.40 | 51.03 ± 33.20 | −14.88 |
| Role emotional | 36.54 ± 48.08 | 41.38 ± 50.12 | −4.84 |
| Mental health | 42.69 ± 20.70 | 38.28 ± 24.79 | 4.41 |
| 12 Months | |||
| Physical functioning | 23.08 ± 40.57 | 18.75 ± 33.76 | 4.33 |
| Role physical | 28.37 ± 27.28 | 25.00 ± 28.87 | 3.37 |
| Bodily pain | 32.69 ± 27.17 | 37.50 ± 32.98 | −4.81 |
| General health | 26.92 ± 6.38 | 38.39 ± 22.03 | −11.47 |
| Vitality | 26.15 ± 26.39 | 27.14 ± 33.65 | −0.99 |
| Social functioning | 51.15 ± 29.44 | 53.93 ± 32.58 | −2.78 |
| Role emotional | 50.00 ± 50.99 | 54.46 ± 48.14 | −4.46 |
| Mental health | 48.46 ± 24.77 | 49.64 ± 24.26 | −1.18 |
| HAD, Anxiety, Mean ± SD | |||
| Baseline | 10.31 ± 4.03 | 10.72 ± 4.60 | −0.41 |
| 3 months | 7.46 ± 4.12 | 8.07 ± 4.54 | −0.61 |
| 6 months | 8.35 ± 5.18 | 8.24 ± 5.37 | 0.11 |
| 12 months | 8.69 ± 5.08 | 8.54 ± 5.67 | 0.15 |
| Mean change, 12 months to baseline | 1.62 | 2.18 | — |
| HAD, Depression, Mean ± SD | |||
| Baseline | 8.96 ± 4.04 | 9.45 ± 4.31 | −0.49 |
| 3 months | 5.69 ± 3.51 | 6.34 ± 4.05 | −0.65 |
| 6 months | 6.69 ± 4.86 | 7.03 ± 5.52 | −0.34 |
| 12 months | 8.19 ± 5.00 | 7.21 ± 4.97 | 0.98 |
| Mean change, 12 months to baseline | 0.77 | 2.20 | — |
| MOS-SS, Mean ± SD | |||
| Somnolence | |||
| Baseline | 51.79 ± 23.04 | 53.10 ± 28.87 | −1.31 |
| 6 months | 58.21 ± 24.19 | 62.99 ± 18.05 | −4.78 |
| 12 months | 67.18 ± 19.95 | 63.10 ± 24.98 | 4.08 |
| Mean change, 12 months to baseline | −15.4 | −10.0 | — |
| Sleep Disturbance | |||
| Baseline | 29.66 ± 25.13 | 27.20 ± 25.24 | −2.46 |
| 6 months | 45.82 ± 29.56 | 39.31 ± 24.98 | 6.51 |
| 12 months | 41.25 ± 25.95 | 37.32 ± 29.71 | 3.93 |
| Mean change, 12 months to baseline | −11.59 | −10.12 | — |
| Sleep Quantity | |||
| Baseline | 5.25 ± 1.17 | 5.03 ± 1.37 | 0.22 |
| 6 months | 5.79 ± 1.54 | 5.57 ± 1.29 | 0.22 |
| 12 months | 5.73 ± 1.11 | 5.48 ± 1.54 | 0.25 |
| Mean change, 12 months to baseline | −0.48 | −0.45 | — |
| Awake, Short of Breath | |||
| Baseline | 57.69 ± 31.15 | 57.93 ± 34.37 | −0.24 |
| 6 months | 73.85 ± 24.50 | 68.28 ± 31.86 | 5.57 |
| 12 months | 76.92 ± 29.77 | 65.00 ± 31.09 | 14.92 |
| Mean change, 12 months to baseline | −19.23 | −7.07 | — |
| Snoring | |||
| Baseline | 43.08 ± 38.65 | 34.48 ± 36.12 | 8.6 |
| 6 months | 51.54 ± 38.02 | 45.52 ± 36.99 | 6.02 |
| 12 months | 51.54 ± 41.63 | 41.43 ± 35.25 | 10.11 |
| Mean change, 12 months to baseline | −8.46 | −6.95 | — |
| Sleep Adequacy | |||
| Baseline | 27.42 ± 24.51 | 31.03 ± 26.23 | −3.61 |
| 6 months | 46.92 ± 32.59 | 42.07 ± 33.21 | 4.85 |
| 12 months | 40.00 ± 29.53 | 37.86 ± 32.01 | 2.14 |
| Mean change, 12 months to baseline | −12.58 | −6.83 | — |
Abbreviation: HAD, Hospital Anxiety and Depression; MOS-SS, Medical Outcomes Study Sleep Scale; SCS, spinal cord stimulation; SD, standard deviation; SF-12, SF-12 health-related quality of life questionnaire.
Crude differences calculated for this health technology assessment.
Source: De Andres et al, 2017.29
Table A9:
Safety Outcomes, 10-kHz SCS Studies for Chronic Pain
| Author, Year Country Sites | Study Size Patient Mean Age Pain Etiology | Follow-Up Study Design | Leads and Placement | Adverse Neurological Events | Adverse Events | Major Adverse Eventsa |
|---|---|---|---|---|---|---|
| Kapural et al, 201530 Kapural et al, 201631 United States 11 sites |
10-kHz high-frequency SCS n = 101 54.6 ± 12.4 y Back and leg pain with (87%) or without prior back surgery |
3 months, 24 months RCT |
Percutaneous epidural placement of 2 leads with anchoring to the supraspinous ligaments |
No stimulation-related neurological deficits | Implant site pain (11.9%) | Lead migration requiring surgical revision (3%) Wound complications (Ne = 5 Np = 4; 4%) Paresis (Ne = 1, Np = 1; 1%) |
|
Conventional SCS n = 97 55.2 ± 13.4 y Back and leg pain with (86%) or without prior back surgery |
No stimulation-related neurological deficits | Implant site pain (10.3%) Uncomfortable paresthesia (11.3%) |
Lead migration requiring surgical revision (5.2%) Wound complications (Ne = 3 Np = 3; 3.1%) Arrhythmia (Ne = 1, Np = 1; 1%) Cardiac arrest (Ne = 1, Np = 1; 1%) Extradural abscess (Ne = 1, Np = 1; 1%) Intracranial hypotension (Ne = 1, Np = 1; 1%) Post lumbar puncture syndrome (Ne = 1, Np = 1; 1%) |
|||
| De Andres et al, 201729 Spain 1 site |
10-kHz high-frequency SCS n = 26 51.6 ± 9.3 y FBSS (100%) |
12 months RCT |
Percutaneous epidural placement of 2 leads with 8-contact electrodes and anchoring to the supraspinous ligaments | No stimulation-related neurological deficits | Lead migration (Ne = 4; 10.3%) | Lead migration with replacement (Ne = 1; 3.4%) |
|
Conventional SCS n = 29 53.8 ± 11.5 y FBSS (100%) |
No stimulation-related neurological deficits | — | Lead migration with replacement (Ne = 2; 6.5%) |
|||
| Bolash et al, 201928 United States 7 sites |
10-kHz high-frequency SCS n = 50 58.5 ± 12 y |
6 months RCT | Percutaneous epidural lead placement with 8-contact electrodes and external wireless IPG | No stimulation-related neurological deficits | Lead migration 12% Lead breakage 2% Loss of stimulation 4% Unintended stimulation 0 Redness/drainage 4% Unintended stimulation 0 Redness/drainage 4% Incisional pain 0 Other minor 8% |
|
|
Comparator SCS (low-frequency, burst, 1.2–1.5 kHz) n = 49 58.2 ± 12 y FBSS (100%) |
No stimulation-related neurological deficits | Lead migration 20% Lead breakage 2% Loss of stimulation 6% Unintended stimulation 8% Redness/drainage 4% Unintended stimulation 0 Redness/drainage 0 Incisional pain 6% Other minor 8% |
Serious infection 1% |
Abbreviations: FBSS, failed back surgery syndrome; IPG, implant pulse generator; Ne, number of events; Np, number of patients; RCT, randomized controlled trial; SUNA, short-lasting unilateral neuralgiform headache attacks with autonomic symptoms.
Appendix 6: Selected Excluded Studies—Economic Evidence
For transparency, we provide a list of studies that readers might have expected to see in the economic evidence review but that did not meet the inclusion criteria, along with the primary reason for exclusion.
| Citation | Primary Reason for Exclusion |
|---|---|
| Mekhail N. The horizon for painful neuropathies. Pain Pract. 2018;18(Suppl 1):22. | No original data reported |
| Baranidharan G, Titterington J. Recent advances in spinal cord stimulation for pain treatment. Pain Manag. 2016;6(6):581-9. | Not an economic analysis (cost–utility, cost-effectiveness, cost–benefit, cost–consequence, or cost-minimization) |
| Dettori JR. Spinal cord stimulation assessing signals for update [Internet]. Olympia (WA): Washington State Health Care Authority; 2016 [cited 2019 Jan]. Available from: https://www.hca.wa.gov/assets/program/Spinal%20Cord%20Stimulation%20Signals%20for%20Update%208-29-16.pdf | Does not include economic analyses of the intervention of interest |
Appendix 7: Results of Applicability and Limitation Checklists for Studies Included in the Economic Literature Review
Table A10:
Assessment of the Applicability of Studies Evaluating the Cost-Effectiveness of 10-kHz High-Frequency SCS
| Author, Year, Country of Publication | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system studied sufficiently similar to Ontario? | Were the perspectives clearly stated? If yes, what were they? | Are all direct effects included? Are all other effects included where they are material? | Are all future costs and outcomes discounted? If yes, at what rate? | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall Judgmenta |
|---|---|---|---|---|---|---|---|---|---|
| NICE, 201769 United Kingdom | Partially | Yes | Partially | Yes: NHS and PSS | Partially | Yes: 3.5% | No | NA | Partially applicable |
| Annemans, 201459 United Kingdom | Partially | Yes | Yes | Yes: NHS | Partially | Yes: 3.5% | Yes | NA | Partially applicable |
Abbreviations: NHS, National Health Service; NICE, National Institute for Health and Care Excellence; PSS, Personal Social Services.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Overall judgment may be “directly applicable,” “partially applicable,” or “not applicable.”
Table A11:
Assessment of the Limitations of Studies Evaluating the Cost-Effectiveness of 10-kHz High-Frequency SCS
| Author, Year, Country of Publication | Does the model structure adequately reflect the nature of the health condition under evaluation? | Is the time horizon sufficiently long to reflect all important differences in costs and outcomes? | Are all important and relevant health outcomes included? | Are the clinical inputsa obtained from the best available sources? | Do the clinical inputsa match the estimates contained in the clinical sources? | Are all important and relevant (direct) costs included in the analysis? | Are the estimates of resource use obtained from the best available sources? | Are the unit costs of resources obtained from the best available sources? | Is an appropriate incremental analysis presented, or can it be calculated from the reported data? | Are all important and uncertain parameters subjected to appropriate sensitivity analysis? | Is there a potential conflict of interest? | Overall Judgmentb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NICE, 201769 United Kingdom | Partially | Yes | Yes | Yes | Yes | Partially | Yes | Yes | No | Yes | No | Potentially serious limitations |
| Annemans, 201459 United Kingdom | Partially | Yes | Yes | No | Yes | Partially | Yes | Yes | Yes | Yes | Unclear | Potentially serious limitations |
Abbreviation: NICE, National Institute for Health and Care Excellence.
Note: Response options for all items were “yes,” “partially,” “no,” “unclear,” and “NA” (not applicable).
Clinical inputs include relative treatment effects, natural history, and utilities.
Overall judgment may be “minor limitations,” “potentially serious limitations,” or very serious limitations.“
Appendix 8: Letter of Informationa
LETTER OF INFORMATION
Health Quality Ontario is conducting a review of ultra-high-frequency spinal cord stimulation for the treatment of noncancer chronic pain. The purpose is to understand whether these devices should be more broadly funded in Ontario.
An important part of this review involves speaking to patients and families of those who may be impacted by chronic pain. Our goal is to make sure the experiences of patients and caregivers are considered in the funding recommendations for this device.
WHAT DO YOU NEED FROM ME?
-
✓
20–40 minutes of your time for a phone or in-person interview to share your story
-
✓
Permission to audio= (not video-) record the interview
WHAT YOUR PARTICIPATION INVOLVES
If you agree to share your experiences, you will be asked to have an interview with Health Quality Ontario staff. The interview will likely last 20-40 minutes. It will be held in a private location or over the telephone. With your consent, the interview will be audio-taped. The interviewer will ask you questions about you or your loved one's condition and your perspectives about chronic pain and spinal cord stimulation.
Participation is voluntary. You may refuse to participate, refuse to answer any questions or withdraw before your interview. Withdrawal will in no way affect the care you receive.
CONFIDENTIALITY
All information collected for the review will be kept confidential and privacy will be protected except as required by law. The results of this review will be published, however no identifying information will be released or published. Any records containing information from your interview will be stored securely.
RISKS TO PARTICIPATION:
There are no known physical risks to participating. Some participants may experience discomfort or anxiety after speaking about their lived experience. If this is the case, please contact any staff.
If you are interested in participating, please contact Health Quality Ontario staff:
Abbreviation: SCS = spinal cord stimulation.
Note: This letter refers to “ultra-high frequency SCS,” meaning 10-kHz high-frequency SCS. The people in our final interview sample had experience with low= and moderate-frequency SCS, but not 10-kHz high-frequency SCS.
Health Quality Ontario is now the Quality business unit at Ontario Health.
Appendix 9: Interview Guideb
Interview for HF 10 Nerve Stimulation
Intro
Explain HQO purpose, HTA process, and purpose of interview History of chronic pain = diagnosis and background (general only)
Lived Experience
Day-to-day routine
What is the impact on quality of life?
Impact on loved-ones/caregivers, work, etc.?
Therapies
What current therapies/treatments are used and their impact?
Is accessibility to therapies/treatments an issue (are you able to take advantage of all potential therapies?)
Expectations of current therapies?
Spinal Cord Nerve Stimulation
Any experience with spinal cord stimulation therapy?
Information surrounding this therapy (or other nerve stimulation)?
Decision-making for treatment. Was it difficult to weigh potential risks/benefits?
Expectations
Description of the procedure
Result, impact, change in quality of life
Health Quality Ontario is now the Quality business unit at Ontario Health.
Author contributions
This report was developed by a multidisciplinary team from the Quality business unit at Ontario Health. The lead clinical epidemiologist was Gaylene Pron, the secondary clinical epidemiologist was Kristen McMartin, the lead health economist was Olga Gajic-Veljanoski, the secondary health economist was Shawn Xie, the health economics associate was Jennifer Guo, the patient and public partnership analyst was David Wells, and the medical librarian was Corinne Holubowich.
KEY MESSAGES
What Is This Health Technology Assessment About?
Chronic pain is pain that lasts for a long time, usually more than 3 months. People may develop chronic pain because of an injury, an infection, a disease, or a surgery—or there may be no obvious reason for the pain. Chronic pain has a negative effect on people's physical, emotional, social, and mental health.
People may try a range of treatment options to manage their chronic pain, including physiotherapy, mindfulness practices, and medications. Spinal cord stimulation is typically recommended if these options do not work to relieve a person's pain. It delivers low-voltage electricity to the nerves in the spine to suppress pain signals. Conventional spinal cord stimulation uses lower frequencies (30 to 200 Hz), but it can cause paresthesia, a feeling of tingling or buzzing that some find uncomfortable. High-frequency spinal cord stimulation is a relatively new form of the treatment that delivers stimulation at higher frequencies, beyond what people can feel or sense. A stimulation frequency of 10 kHz is the highest frequency currently delivered by spinal cord stimulation systems in clinical settings and is the focus of this assessment.
This health technology assessment looked at how safe and effective 10-kHz high-frequency spinal cord stimulation is for adults with chronic noncancer pain that does not respond to medical management. It also looked at the budget impact of publicly funding 10-kHz high-frequency spinal cord stimulation in adults who have not found relief with medication or other types of spinal cord stimulation. Finally, it looked at the experiences, preferences, and values of people with chronic noncancer pain.
What Did This Health Technology Assessment Find?
10-kHz high-frequency spinal cord stimulation reduced people's pain, decreased their disability, and improved their quality of life.
We did not have enough evidence to determine whether 10-kHz high-frequency spinal cord stimulation was cost-effective for adults who had first tried currently available SCS at lower frequencies (up to 1.2 kHz). Publicly funding 10-kHz high-frequency spinal cord stimulation for adults who have not found relief with medication or other types of spinal cord stimulation would save about $0.73 million over the next 5 years in Ontario.
People with chronic noncancer pain said that their pain affected their ability to do daily activities and affected their emotional well-being. They said that spinal cord stimulation reduced their pain, allowing them to function better.
Contributor Information
Ontario Health (Quality):
Gaylene Pron, Kristen McMartin, Olga Gajic-Veljanoski, Shawn Xie, Jennifer Guo, David Wells, and Corinne Holubowich
About Us
This health technology assessment was produced by the Quality business unit at Ontario Health, the government agency that when fully established will be responsible for ensuring all Ontarians receive high-quality health care where and when they need it.
For more information about Ontario Health, visit ontariohealth.ca.
REFERENCES
- (1).Tunks ER, Crook J, Weir R. Epidemiology of chronic pain with psychological comorbidity: prevalence, risk, course, and prognosis. Can J Psychiatry. 2008;53(4):224–34. [DOI] [PubMed] [Google Scholar]
- (2).Birse TM, Lander J. Prevalence of chronic pain. Can J Public Health. 1998;89(2):129–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Reitsma M, Tranmer JE, Buchanan DM, VanDenKerkhof EG. The epidemiology of chronic pain in Canadian men and women between 1994 and 2007: longitudinal results of the National Population Health Survey. Pain Res Manag. 2012;17(3):166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Van Den Kerkhof EG, Hopman WM, Towheed TE, Anastassiades TP, Goldstein DH. The impact of sampling and measurement on the prevalence of self-reported pain in Canada. Pain Res Manag. 2003;8(3):157–63. [DOI] [PubMed] [Google Scholar]
- (5).Moulin DE, Clark AJ, Speechley M, Morley-Forster PK. Chronic pain in Canada–prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manag. 2002;7(4):179–84. [DOI] [PubMed] [Google Scholar]
- (6).Parthan A, Evans CJ, Le K. Chronic low back pain: epidemiology, economic burden and patient-reported outcomes in the USA. Expert Rev Pharmacoecon Outcomes Res. 2006;6(3):359–69. [DOI] [PubMed] [Google Scholar]
- (7).Reid KJ, Harker J, Bala MM, Truyers C, Kellen E, Bekkering GE, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–62. [DOI] [PubMed] [Google Scholar]
- (8).Currie SR, Wang J. Chronic back pain and major depression in the general Canadian population. Pain. 2004;107(1-2):54–60. [DOI] [PubMed] [Google Scholar]
- (9).Hooten WM. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc. 2016;91(7):955–70. [DOI] [PubMed] [Google Scholar]
- (10).van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology: where do lifestyle factors fit in? Br J Pain. 2013;7(4):209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013;111(1):13–8. [DOI] [PubMed] [Google Scholar]
- (12).Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. 2018;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Song JJ, Popescu A, Bell RL. Present and potential use of spinal cord stimulation to control chronic pain. Pain Physician. 2014;17(3):235–46. [PubMed] [Google Scholar]
- (14).Wong SSC, Chan CW, Cheung CW. Spinal cord stimulation for chronic non-cancer pain: a review of current evidence and practice. Hong Kong Med J. 2017;23(5):517–23. [DOI] [PubMed] [Google Scholar]
- (15).Scalone L, Zucco F, Lavano A, Costantini A, De Rose M, Poli P, et al. Benefits in pain perception, ability function and health-related quality of life in patients with failed back surgery syndrome undergoing spinal cord stimulation in a clinical practice setting. Health Qual Life Outcomes. 2018;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17(6):515–50. [DOI] [PubMed] [Google Scholar]
- (17).Bouhassira D, Attal N. Emerging therapies for neuropathic pain: new molecules or new indications for old treatments? Pain. 2018;159(3):576–82. [DOI] [PubMed] [Google Scholar]
- (18).Boulanger A, Clark AJ, Squire P, Cui E, Horbay GL. Chronic pain in Canada: have we improved our management of chronic noncancer pain? Pain Res Manag. 2007;12(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3:17002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Yearwood T, Perryman L. Peripheral neurostimulation with a microsize wireless stimulator. Prog Neurol Surg. 2016;29:168–91. [DOI] [PubMed] [Google Scholar]
- (21).Miller JP, Eldabe S, Buchser E, Johanek LM, Guan Y, Linderoth B. Parameters of spinal cord stimulation and their role in electrical charge delivery: a review. Neuromodulation. 2016;19(4):373–84. [DOI] [PubMed] [Google Scholar]
- (22).Health Quality Ontario. OHTAC recommendation: spinal cord stimulation for the management of neuropathic pain [Internet]. Toronto (ON): Queen's Printer for Ontario; 2005. [cited 2019 Mar 5]. Available from: http://www.ontla.on.ca/library/repository/mon/10000/253081.pdf [Google Scholar]
- (23).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–6. [DOI] [PubMed] [Google Scholar]
- (24).Higgins JP, Altman DG, G⊘tzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. Br Med J. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Guyatt GH, Alonso-Coello P, Vandvik PO. Experience with GRADE. J Clin Epidemiol. 2012;65(12):1243–4. [DOI] [PubMed] [Google Scholar]
- (27).Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(6):e1000097. [PMC free article] [PubMed] [Google Scholar]
- (28).Bolash R, Creamer DO, Rauck R, Vahedifar P, Calodney A, Fox I, et al. Wireless high frequency spinal cord stimulation (10 kHz) compared to multi-waveform low frequency spinal cord stimulation in the management of chronic pain in failed back surgery syndrome subjects: preliminary results of a multicenter prospective randomized controlled study. Pain Med. 2019;0(0):1–9. [DOI] [PubMed] [Google Scholar]
- (29).De Andres J, Monsalve-Dolz V, Fabregat-Cid G, Villanueva-Perez V, Harutyunyan A, Asensio-Samper JM, et al. Prospective, randomized blind effect-on-outcome study of conventional vs high-frequency spinal cord stimulation in patients with pain and disability due to failed back surgery syndrome. Pain Med. 2017;18(12):2401–21. [DOI] [PubMed] [Google Scholar]
- (30).Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, et al. Novel 10-kHz high-frequency therapy (HF10 therapy) is superior to traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: the SENZA-RCT randomized controlled trial. Anesthesiology. 2015;123(4):851–60. [DOI] [PubMed] [Google Scholar]
- (31).Kapural L, Yu C, Doust MW, Gliner BE, Vallejo R, Sitzman BT, et al. Comparison of 10-kHz High-frequency and traditional low-frequency spinal cord stimulation for the treatment of chronic back and leg pain: 24-month results from a multicenter, randomized, controlled pivotal trial. Neurosurgery. 2016;79(5):667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Amirdelfan K, Yu C, Doust MW, Gliner BE, Morgan DM, Kapural L, et al. Long-term quality of life improvement for chronic intractable back and leg pain patients using spinal cord stimulation: 12-month results from the SENZA-RCT. Qual Life Res. 2018;27(8):2035–44. [DOI] [PubMed] [Google Scholar]
- (33).Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–20. [DOI] [PubMed] [Google Scholar]
- (34).Nikaido T, Sumitani M, Sekiguchi M, Konno S. The Spine painDETECT questionnaire: development and validation of a screening tool for neuropathic pain caused by spinal disorders. PLoS One. 2018;13(3):e0193987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Tampin B, Bohne T, Callan M, Kvia M, Melsom Myhre A, Neoh EC, et al. Reliability of the English version of the painDETECT questionnaire. Curr Med Res Opin. 2017;33(4):741–8. [DOI] [PubMed] [Google Scholar]
- (36).Bocci T, De Carolis G, Paroli M, Barloscio D, Parenti L, Tollapi L, et al. Neurophysiological comparison among tonic, high frequency, and burst spinal cord stimulation: novel insights into spinal and brain mechanisms of action. Neuromodulation. 2018;21(5):480–8. [DOI] [PubMed] [Google Scholar]
- (37).Thomson S, Tavakkoli Zadeh M, Love-Jones S, Patel N, Bains A, Gu W, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation (SCS): Final results from the proco randomised controlled trial: A multicentre, double-blind, crossover study. Pain Pract. 2018;18 (Supplement 1):33. [Google Scholar]
- (38).Thomson SJ, Tavakkolizadeh M, Love-Jones S, Patel NK, Gu JW, Bains A, et al. Effects of rate on analgesia in kilohertz frequency spinal cord stimulation: results of the PROCO randomized controlled trial. Neuromodulation. 2018;21(1):67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient's view of change as a clinical outcome measure. JAMA. 1999;282(12):1157–62. [DOI] [PubMed] [Google Scholar]
- (40).Kamper SJ, Ostelo RW, Knol DL, Maher CG, de Vet HC, Hancock MJ. Global Perceived Effect scales provided reliable assessments of health transition in people with musculoskeletal disorders, but ratings are strongly influenced by current status. J Clin Epidemiol. 2010;63(7):760–6. [DOI] [PubMed] [Google Scholar]
- (41).Schmitt J, Di Fabio RP. The validity of prospective and retrospective global change criterion measures. Arch Phys Med Rehabil. 2005;86(12):2270–6. [DOI] [PubMed] [Google Scholar]
- (42).Manzar MD, BaHammam AS, Hameed UA, Spence DW, Pandi-Perumal SR, Moscovitch A, et al. Dimensionality of the Pittsburgh Sleep Quality Index: a systematic review. Health Qual Life Outcomes. 2018;16(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Sitasuwan T, Bussaratid S, Ruttanaumpawan P, Chotinaiwattarakul W. Reliability and validity of the Thai version of the Pittsburgh Sleep Quality Index. J Med Assoc Thai. 2014;97(Suppl 3):S57-67. [PubMed] [Google Scholar]
- (44).Allen RP, Kosinski M, Hill-Zabala CE, Calloway MO. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep). Sleep Med. 2009;10(5):531–9. [DOI] [PubMed] [Google Scholar]
- (45).Rejas J, Ribera MV, Ruiz M, Masrramon X. Psychometric properties of the MOS (Medical Outcomes Study) Sleep Scale in patients with neuropathic pain. Eur J Pain. 2007;11(3):329–40. [DOI] [PubMed] [Google Scholar]
- (46).Viala-Danten M, Martin S, Guillemin I, Hays RD. Evaluation of the reliability and validity of the Medical Outcomes Study sleep scale in patients with painful diabetic peripheral neuropathy during an international clinical trial. Health Qual Life Outcomes. 2008;6:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Busija L, Pausenberger E, Haines TP, Haymes S, Buchbinder R, Osborne RH. Adult measures of general health and health-related quality of life: Medical Outcomes Study Short Form 36-Item (SF-36) and Short Form 12-Item (SF-12) Health Surveys, Nottingham Health Profile (NHP), Sickness Impact Profile (SIP), Medical Outcomes Study Short Form 6D (SF-6D), Health Utilities Index Mark 3 (HUI3), Quality of Well-Being Scale (QWB), and Assessment of Quality of Life (AQoL). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S383-412. [DOI] [PubMed] [Google Scholar]
- (48).Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Devlin NJ, Parkin D, Browne J. Patient-reported outcome measures in the NHS: new methods for analysing and reporting EQ-5D data. Health Econ. 2010;19(8):886–905. [DOI] [PubMed] [Google Scholar]
- (50).Al-Kaisy A, Palmisani S, Pang D, Sanderson K, Wesley S, Tan Y, et al. Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS frequency study). Neuromodulation. 2018;21(5):457–65. [DOI] [PubMed] [Google Scholar]
- (51).De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S. Burst spinal cord stimulation for limb and back pain. World Neurosurg. 2013;80(5):642–9.e1. [DOI] [PubMed] [Google Scholar]
- (52).Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain. 2017;21(3):507–19. [DOI] [PubMed] [Google Scholar]
- (53).Perruchoud C, Eldabe S, Batterham AM, Madzinga G, Brookes M, Durrer A, et al. Analgesic efficacy of high-frequency spinal cord stimulation: a randomized double-blind placebo-controlled study. Neuromodulation. 2013;16(4):363–9. [DOI] [PubMed] [Google Scholar]
- (54).Schu S, Slotty PJ, Bara G, von Knop M, Edgar D, Vesper J. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation. 2014;17(5):443–50. [DOI] [PubMed] [Google Scholar]
- (55).DistillerSR systematic review software. Evidence Partners. Ottawa (ON). Available at: https://www.evidencepartners.com/products/distillersr-systematic-review-software/.
- (56).National Institute for Health and Care Excellence. Methods for the development of NICE public health guidance. 3rd ed. London (UK): The Institute; 2012. Appendix I, quality appraisal checklist—economic evaluations. [PubMed] [Google Scholar]
- (57).!!! INVALID CITATION !!!.
- (58).National Institute for Health and Care Excellence. Senza spinal cord stimulation system for delivering HF10 therapy to treat chronic neuropathic pain [Internet]. London: The Institute; 2019. [cited 2019 Apr]. Available from: https://www.nice.org.uk/guidance/mtg41/resources/senza-spinal-cord-stimulation-system-for-delivering-hf10-therapy-to-treat-chronic-neuropathic-pain-pdf-64372050739141 [Google Scholar]
- (59).Annemans L, Van Buyten JP, Smith T, Al-Kaisy A. Cost effectiveness of a novel 10 kHz high-frequency spinal cord stimulation system in patients with failed back surgery syndrome (FBSS). J Long Term Eff Med Implants. 2014;24(2-3):173–83. [DOI] [PubMed] [Google Scholar]
- (60).National Institute for Health and Care Excellence. Medical technologies evaluation programme methods guide [Internet]. London: The Institute; 2017. [cited 2019 Jan]. Available from: https://www.nice.org.uk/process/pmg33/chapter/introduction [PubMed] [Google Scholar]
- (61).Van Buyten JP, Wille F, Smet I, Wensing C, Breel J, Karst E, et al. Therapy-related explants after spinal cord stimulation: results of an international retrospective chart review study. Neuromodulation. 2017;20(7):642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Van Buyten JP, Al-Kaisy A, Smet I, Palmisani S, Smith T. High-frequency spinal cord stimulation for the treatment of chronic back pain patients: results of a prospective multicenter European clinical study. Neuromodulation. 2013;16(1):59–65. [DOI] [PubMed] [Google Scholar]
- (63).Thomson S. Failed back surgery syndrome = definition, epidemiology and demographics. Br J Pain. 2013;7(1):56–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Newcastle and York External Assessment Centre. Advice on Senza HF10 SCS consultation comments. York (UK): York Health Economics Consortium; 2018. [Google Scholar]
- (65).Kumar K, Bishop S. Financial impact of spinal cord stimulation on the healthcare budget: a comparative analysis of costs in Canada and the United States. J Neurosurg Spine. 2009;10(6):564–73. [DOI] [PubMed] [Google Scholar]
- (66).Kumar K, Malik S, Demeria D. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: cost-effectiveness analysis. Neurosurgery. 2002;51(1):106–15. [DOI] [PubMed] [Google Scholar]
- (67).Kumar K, Rizvi S. Cost-effectiveness of spinal cord stimulation therapy in management of chronic pain. Pain Med. 2013;14(11):1631–49. [DOI] [PubMed] [Google Scholar]
- (68).Health Quality Ontario. Spinal cord stimulation for neuropathic pain: an evidence-based analysis. Ont Health Technol Assess Ser. 2005;5(4):1–78. [PMC free article] [PubMed] [Google Scholar]
- (69).Willits I, Cole H, Arber M, Craig J, Sims A. Senza spinal cord stimulation (SCS) system for the treatment of chronic pain. London: National Institute for Health and Care Excellence; 2017. [Google Scholar]
- (70).Dettori JR. Spinal cord stimulation assessing signals for update [Internet]. Olympia (WA): Washington State Health Care Authority; 2016. [cited 2019 Jan]. Available from: https://www.hca.wa.gov/assets/program/Spinal%20Cord%20Stimulation%20Signals%20for%20Update%208-29-16.pdf [Google Scholar]
- (71).Weiner RL, Yeung A, Montes Garcia C, Tyler Perryman L, Speck B. Treatment of FBSS low back pain with a novel percutaneous DRG wireless stimulator: pilot and feasibility study. Pain Med. 2016;17(10):1911–6. [DOI] [PubMed] [Google Scholar]
- (72).Statistics Canada. Table 18-10-0004-01: Consumer price index, monthly, not seasonally adjusted [Internet]. Ottawa (ON): Statistics Canada; 2018. [cited 2018 Apr 30]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=3260020 [Google Scholar]
- (73).Ministry of Health and Long-Term Care. Schedule of benefits: physician services under the Health Insurance Act [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2019 Jan]. Available from: http://www.health.gov.on.ca/en/pro/programs/ohip/sob/physserv/sob_master20160401.pdf [Google Scholar]
- (74).Ontario Case Costing Initiative (OCCI). OCCI Costing Analysis Tool 2016/2017 [Internet]. Toronto: Ministry of Health and Long-Term Care; 2016. [cited 2019 Apr 10]. Available from: https://hsimi.ca/occp/occpreports/ [Google Scholar]
- (75).Duarte RV, Thomson S. Trial versus no trial of spinal cord stimulation for chronic neuropathic pain: cost analysis in United Kingdom National Health Service. Neuromodulation. 2019;22(2):208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Huang KT, Martin J, Marky A, Chagoya G, Hatef J, Hazzard MA, et al. A national survey of spinal cord stimulation trial-to-permanent conversion rates. Neuromodulation. 2015;18(2):133–9. [DOI] [PubMed] [Google Scholar]
- (77).Farber SH, Han JL, Elsamadicy AA, Hussaini Q, Yang S, Pagadala P, et al. Long-term cost utility of spinal cord stimulation in patients with failed back surgery syndrome. Pain Physician. 2017;20(6):E797-E805. [PMC free article] [PubMed] [Google Scholar]
- (78).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (79).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (80).Ontario Health Technology Advisory Committee Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015 Apr. [cited 2018 Apr 30]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (81).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage; 1996. [Google Scholar]
- (82).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage; 1999. p. 33–45. [Google Scholar]
- (83).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods. Thousand Oaks (CA): Sage; 1994. p. 23–41. [Google Scholar]
- (84).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage; 2002. [Google Scholar]
- (85).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage; 1998. [Google Scholar]
- (86).Health Technology Assessment International. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2018 Apr 30]. Available from: http://www.htai.org/fileadmin/HTAi_Files/ISG/PatientInvolvement/v2_files/Resource/PCISG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (87).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (88).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage; 1994. p. 273–85. [Google Scholar]
- (89).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]