Abstract
Although biosimilars offer cost savings in Canadian healthcare, uptake is low. We discuss the literature on international experiences with biosimilar adoption in the context of the Diffusion of Innovations model. We highlight potential challenges with biosimilar implementation and gaps in research needed to inform implementation efforts. We observe a lack of systematic description of implementation design and evaluation and a paucity of in-depth and engaged research to understand stakeholders' pragmatic considerations and the knowledge, messages and meanings that shape clinician and patient decisions to choose biosimilars.
Abstract
Bien que les biosimilaires permettent d'épargner des coûts pour les services de santé au Canada, leur adoption demeure faible. Nous commentons la littérature sur l'expérience internationale en matière d'adoption des biosimilaires au moyen du modèle de diffusion des innovations. Nous dégageons les défis potentiels quant à leur mise en œuvre et nous faisons état des lacunes en matière de recherche nécessaire pour éclairer les efforts de mise en œuvre. Nous observons un manque de description systématique des modèles de mise en œuvre et d'évaluation ainsi qu'une rareté de recherche approfondie pour comprendre les considérations pragmatiques des intervenants ainsi que les connaissances et les messages qui éclairent les décisions prises par les cliniciens et les patients au sujet des biosimilaires.
Background
Biosimilars, an economical alternative to traditional biologic treatment, offer exciting opportunities to improve access to treatment (Elek et al. 2017) and cost savings as Canadian healthcare costs rise. However, despite these potential benefits, biosimilar uptake is lower than expected (Health Canada 2017).
To increase biosimilar adoption, several countries instituted biosimilar prescription policies to increase biosimilar uptake by patients new to biologics and biosimilar switching by patients already taking biologics. In Canada, the provincial formularies of Alberta and British Columbia cover the infliximab biosimilar Inflectra for treatment-naïve patients (CADTH 2018), but neither province had a switching policy until 2019–2020. The Patented Medicines Prices Review Board (2017) forecast a potential rapidly growing market for biosimilars, with 13 biologic drugs losing Canadian market exclusivity by 2022. Canadian healthcare needs appropriate steps to reap benefits.
What can Canada learn from international experiences with biosimilar policy and implementation? The literature reflects barriers to biosimilar uptake in translating knowledge, changing prescribing practices, and adjusting drug administration logistics and the benefit/risk perception of patients, providers and payers (Calvo et al. 2018; Cohen et al. 2016; Peyrin-Biroulet et al. 2017). However, the published literature seldom explores in greater depth the key factors that implementation theory highlights. Implementation comprises active efforts to transition an innovation from adoption to routinization (Greenhalgh et al. 2004). Increasing biosimilar uptake and switching are complex implementation problems that benefit from implementation science. Despite the impact on access to biologics for patients and on cost-effectiveness in healthcare, surprisingly little is published on biosimilar implementation and uptake.
Greenhalgh et al. (2004) developed the Diffusion of Innovations model, a unifying conceptual model of factors and processes in spreading and sustaining innovations in health service organizations. The model is based on an extensive review of theoretical and empirical findings in the interdisciplinary literature (Figure 1). Here we discuss the literature on international experiences with biosimilar adoption in the context of Greenhalgh's model to highlight potential challenges with biosimilar implementation and gaps in research needed to inform implementation efforts.
Figure 1.
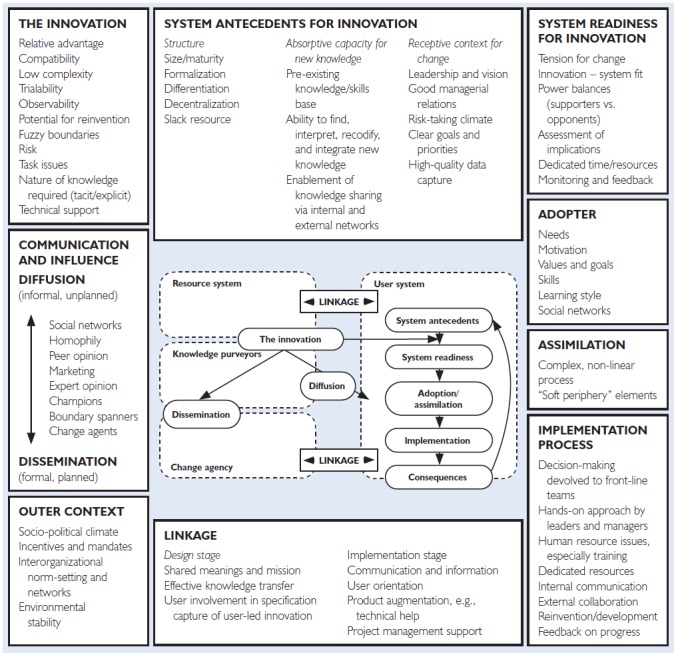
A conceptual model for Diffusion of Innovations in service organizations
Source: Reproduced with permission from Wiley
Diffusion of Innovations: An Implementation Model
Implementing innovations in healthcare requires stakeholders to change how they think and practise in specific situations. People are not passive about innovations; they appraise them, challenge them, experiment with them, find purpose or not in them, develop feelings about them, work around them and adapt them to fit situations and goals (Greenhalgh et al. 2004). These processes happen individually, across social and professional networks and within structural constraints.
Greenhalgh et al.'s model details aspects of context, such as the socio-political climate and system antecedents, that shape conditions in which organizations and individuals operate and interact with innovation. The model conceptualizes influences on innovation adoption and implementation of organizational and individual actors, factors and processes and linkages between them (Table 1). For example, innovation qualities such as relative advantage, compatibility, trialability, low risk and soft periphery impact implementation success. Diffusion and dissemination are affected by knowledge transfer through social networks, peer opinions and champions. The model outlines conditions that promote system readiness to take up and implement innovations, such as tension for change, assessment of implications, power, monitoring systems and innovations–system fit.
Table 1.
Diffusion of Innovations model definitions
| Select model terminology | Explanation |
|---|---|
| Innovation | Novel technology, behaviours aimed at improving health |
| Diffusion | Innovation spread by passive, informal and unplanned means |
| Readiness | Capacity of an organization or system to implement, including tension for change, clear implications, established systems to monitor impact and availability of resources |
| Adoption | Individual process from first contact with innovation to decision to adopt it |
| Implementation | Planned efforts to routinize an innovation within an organization and workflow |
| Relative advantage | Clear, unambiguous advantage (effectiveness or cost) |
| Compatibility | Aligned with adopter's values and needs |
| Trialability | Potential for adopter to experiment with the innovation |
| Low risk | Innovation has a low level of perceived risk and low uncertainty in outcomes |
| Adaptiveness of the soft periphery | Organizational structures, systems and tools required for implementation can be adapted according to contexts within different systems/organizations |
| Tension for change | Adopters perceive current situation as intolerable |
| Power balance | Balance between supporters and opponents of an innovation |
| Monitoring systems | Strategies and skills to monitor and evaluate impact of an innovation |
Innovations require individuals to adopt new behaviour or technologies. Adoption decisions are influenced by the individual needs, motivations, values and meanings they (and others) ascribe to the innovation and decisions for their organization or professional network. Once an adoption decision is made, the model suggests actions that help routinize and integrate the innovation into regular practice: dedicating resources, communicating and collaborating, engaging stakeholders early and widely in decision-making and planning, giving management support, motivating and building practitioner capacity.
We conducted a narrative review on biosimilar implementation literature and policy from the years 2012 to 2018. The review focused on the experiences, attitudes and barriers to introducing biosimilars to physicians, specialists, patients and caregivers and professional and patient organizations, as well as on policy documents and position papers reflecting governmental implementation strategies and experiences. We searched the databases Medline, Scopus, Google Scholar, EMBASE and PubMed using the keywords “biosimilar*,” and “subsequent entry biologic*,” along with “survey*,” “experience” and “perception.” Manual searches of various governmental health service providers (such as Health Canada, the U.S. Food and Drug Administration [FDA] and the National Health Service [NHS]) and professional groups that represent specialties in practice were conducted with an emphasis on biosimilar implementation policy and position papers with regard to this novel class of pharmaceuticals. Here we apply Greenhalgh et al.'s model to discuss components of the model where information is available and point out gaps in the literature that hinder a comprehensive assessment of implementation internationally.
Innovation
Increasing biosimilar uptake has a clear, observable advantage: biosimilars are less expensive than biologics, with no significantly different clinical results (Health Canada 2017), although this is contested to varying degrees depending on the available published evidence. This advantage can lead to improved access to biologics treatment and cost savings for healthcare systems or patients (Elek et al. 2017). Biosimilar introduction also has the potential for leveraging the adaptiveness of organizational structures, systems and tools required for implementation, which is an important innovation quality that supports implementation. For example, Scotland developed tools for education and adoption to fit the needs of individual adopters and user systems (Health Improvement Scotland 2018). These key features can support biosimilar implementation among adopters.
The advantages of biosimilars versus biologics are less clear when considering adopters' beliefs and values surrounding care. In Germany and Belgium, where biosimilars are accessible, physicians choose a biologic over a biosimilar for first-line therapy and prioritize treatment efficacy over cost-effectiveness (Sullivan et al. 2017; van Overbeeke et al. 2017). Controversies persist in specialist communities over biosimilar safety (Sullivan et al. 2017; van Overbeeke et al. 2017). The debate is fuelled by efforts of originator manufacturers to retain their market share and to question the efficacy and safety of biosimilars (Cassels 2017; Milne et al. 2017).
The potential for adopters to experiment with biosimilars (trialability of the innovation) without risk is limited and may thus pose a challenge for adoption that implementation efforts should consider. Patients must be screened for eligibility and treatment and may need complete re-evaluation. Biologics patients express concerns about medication changes (Peyrin-Biroulet 2017; Waller et al. 2017). When the clinical stakes are high due to the debilitating nature of the illness being treated, there is more anxiety about change. This is particularly true if after a complex course of illness a person is finally enjoying improved function and quality of life. Thus, switching from an effective treatment is naturally more fraught than a new initiation in a person who is suffering. Thus, clinicians and patients may be reluctant to trial biosimilars.
Diffusion of Biosimilar Information
Multiple modes influence how clinicians incorporate new knowledge into clinical practices. These include clinical practice guidelines, primary clinical studies, trusted opinion leaders, clinical colleagues, continuing professional education events, media and industry marketing, clinical experience and patient/family values and preferences (Gabbay and le May 2016). Through these influences, clinicians develop clinical mindlines (Gabbay and le May 2016), which guide daily practice. In the case of complex interventions such as biosimilar initiation and switching, the mindlines of whole clinical teams, including, most importantly, patients, need to evolve as changes happen to care delivery.
When regulatory agencies grant market approval for new biosimilars, they aim to affirm biosimilar safety and efficacy, raise awareness of biosimilars and share knowledge tailored to prescribers. The FDA (2018) took tangible steps in promoting biosimilar use in its Biosimilar Action Plan, with network formation initiatives to facilitate data sharing between the FDA and foreign regulatory agencies to streamline drug application approval processes for European-approved biosimilars.
Professional organizations and patient advocacy groups have widespread influence for members, but few champion biosimilar use. The literature that explores the diffusion of information, for example, how these organizations develop their statements, how their funding relationship to pharmaceutical companies affects their position and how patients and physicians are impacted by their messaging, is not clear. The American College of Rheumatology (2018) position statement affirms the need for cost-effective treatments but raises concerns about the diligence of studies used for market approval.
The European League Against Rheumatism's (2018) position statement on biosimilars educates patients and emphasizes the need to strengthen patient input in policy formation. Some groups position themselves more strongly; Crohn's and Colitis Canada (n.d.) initiated a patient-driven letter-writing campaign to government about “forced switching.” It is crucial for implementation researchers and policy makers to reflect on how information and messaging reaches clinicians, teams and patients and how this diffusion of knowledge impacts adopters' attitudes to the introduction of innovations. These important influences on physician practice and patient decisions are little explored in the available literature.
Readiness
Greenhalgh et al.'s model (2004) defines the readiness of a clinical setting to implement innovations as arising from a perceived intolerable current situation (tension for change), clear implications of adopting an innovation to change the current situation, systems to monitor impact and resource availability to support innovation implementation.
The literature suggests a lack of tension to adopt biosimilars and little enthusiasm for biosimilars. A survey of Belgian rheumatology specialists and patients suggests significant adopter indifference to biosimilar approval (van Overbeeke et al. 2017). Rheumatologists perceived more risk with biosimilars because of a lack of clinical trials in specific indications. The literature repeatedly establishes the need to have more comprehensive data available to inform clinical decisions (Cohen et al. 2016; van Overbeeke et al. 2017) and to have monitoring systems in place to evaluate patient outcomes (Calvo et al. 2018; Health Improvement Scotland 2018).
Adopting biosimilars and facilitating switching require dedicated financial and human resources. These processes require increased clinic hours and extra staff and infrastructure to facilitate the switch and to collect data for monitoring patient outcomes. Determining organizational readiness to implement biosimilars requires a thorough evaluation of the clinical setting. Engaged management and front-line staff can develop locally suitable solutions to improve readiness. For example, Norway's initial policy to automatically switch patients from originator to biosimilar infliximab failed to attain the anticipated market share. This led to a concerted effort to consult stakeholders to develop a targeted educational campaign to promote biosimilar use and adjust pricing. This resulted in Norway's two approved biosimilars at the time reaching a majority market share (Institute of Health Economics 2016). The key to this more successful strategy to encourage the use of biosimilars was understanding organizational readiness and involving stakeholders in developing targeted strategies for biosimilar implementation.
Adoption by Individuals
Individual decisions to adopt and routinize an innovation are impacted by their needs, motivations, values, meanings ascribed to the innovation and decisions of their organization or patient/professional network. Relationship quality between adopters and organizations developing policy impacts the meaning attached to innovation (Greenhalgh et al. 2004).
Surveys and focus groups show patient concerns about clinical stability and treatment affordability with regard to biologics treatment and biosimilar switching. A focus group organized by five prominent Canadian patient organizations indicated that switching to a different biologic or biosimilar is perceived as potentially disruptive (The Arthritis Society 2017). Surveys also indicate that patients lack knowledge about biosimilars and trust their physician's decision (Peyrin-Biroulet et al. 2017; van Overbeeke et al. 2017).
In 2015, the Government of the Netherlands framed biosimilar adoption as policies “for securing the affordability and accessibility of expensive medicines” (Government of the Netherlands 2015). This coincides with patient concerns about the affordability of biologic treatments. Where patients do not directly pay for drugs, policy efforts may tap into adopters' altruistic attitudes on collectively reducing health-associated costs. Messaging from government, professional and patient organizations, industry and media shapes meaning for adopters and fuels debates over cost savings, evidence quality, risks and safety (Cassels 2017; Rowland 2019). Divergent messaging poses challenges for adopters in making decisions about adopting biosimilars.
Current data also suggest that gaps of knowledge and uncertainty regarding biosimilars exist among physicians. Researchers who conducted a survey of US-based physicians and specialists concluded that there is a “significant need for evidence-based education” on biosimilars in the areas of understanding bioequivalence and differentiating between biologics and biosimilars (Cohen et al. 2016). Programs such as the FDA's Biosimilar Action Plan are designed to address these shortcomings by enhancing the resources available to prescribers regarding the approval of biosimilars (FDA 2018). In other surveys, European gastroenterologists and rheumatologists expressed doubts over the safety of switching patients to biosimilars (Sullivan et al. 2017; van Overbeeke et al. 2017). As a result of the uncertainty, in 2017, the European Medicines Agency and the European Commission published a guide for healthcare professionals on the benefits of biosimilars, unequivocally saying that there are “no differences” in the expected safety and efficacy between biologics and biosimilars.
Physicians and patients together negotiate knowledge, risk and implications. A recent survey of switched patients mentioned the lack of support from clinicians as a factor that negatively affected their switching experience (Attipoe et al. 2018). Health Improvement Scotland (2018) addressed this potential challenge by providing information letters to patients on biosimilars and treatment implications, preparing patients for physician encounters.
As an innovation, biosimilars cannot be assessed in isolation from their mode of administration and associated barriers and supports. In Canada, some biologics are linked to industry-sponsored patient support programs for drug access and delivery, such as Pfizer Inc.'s patient support program for their infliximab biosimilar, Inflectra (Pfizer Canada 2019). These programs are valued by patients and may drive an individual prescription decision (The Arthritis Society 2017).
Authoritative measures, such as Norway's initial adoption regulations, may boost initial implementation but not long-term routinization. Mandated (must-do's) adoption and switching may be the deciding factor for individual adoption. However, without stakeholder engagement and understanding of local context, mandated actions risk underestimating organizational capacity for the complex logistics of biosimilar implementation.
Implementation
Key to implementation are the features of the innovation, diffusion and dissemination, system and organization readiness and adoption.
In 2017, NHS England published the Commissioning Framework for Biological Medicines (Including Biosimilar Medicines), which is, along with Scotland's (Health Improvement Scotland 2018), one of a few examples of biosimilar implementation found in the literature. The framework entrusted more than 150 Clinical Commissioning Groups (CCGs), bodies tasked with commissioning health services for regional NHS organizations, in England to engage stakeholders to gauge concerns and identify the cost/benefit of biosimilar uptake. CCGs and adopters then assess readiness and tailor implementation strategies for specific regions. The goal is 90% uptake by patients who have never before been treated with biologics within three months of framework enactment and 80% switching for existing patients within 12 months. Strategies include interprofessional clinical teams and tool kits to identify and educate patients eligible for switching. Financial incentives to providers fund educational programs and staff for extra clinic hours but are expected to cease over time as the numbers of patients eligible to switch fall.
The framework builds readiness by concerted support for innovation and adopter ability to monitor and evaluate patient outcomes, with CCG time and analytical resources dedicated to working with providers. NHS England recommends assessing the consequences of biosimilar implementation through pharmacovigilance and monitoring: reporting adverse drug reactions and observing financial benefits, clinical outcomes, patient perspectives and unexpected adoption challenges. Data are not yet published on the success of these measures.
Discussion
We reviewed the literature on the international experience with biosimilar implementation in the context of Greenhalgh et al.'s Diffusion of Innovations model. Using surveys, policy documents and position statements, we discussed biosimilar introduction as innovation, its diffusion process, challenges with clinical readiness and factors facilitating or hindering individual adoption.
Most information is from surveys on adopter perceptions and knowledge of biosimilars, which do not adequately explore the complex processes and circumstances that have an impact on patients, physicians and teams of allied healthcare providers (nurses, pharmacists) and healthcare organizations, as well as professional and patient associations' knowledge, meanings and practical challenges that shape clinician and patient decisions on biosimilars. There is little literature on the impact of relationships between patients, clinicians, organizations, industry and governing bodies on biosimilar implementation. Beyond examples from the UK and Scotland, the literature is scarce on implementation design and process evaluation.
Greenhalgh et al.'s model suggests that linkages between change agents, knowledge purveyors, resources systems and user systems are decisive for innovation diffusion and implementation. Linkages build on networks, collaboration and relationships for understanding adopter readiness, creating shared meanings, transferring knowledge effectively and making decisions that align with adopter needs and values. Implementation can fail if relationships and bidirectional communication are not attended to. Crucial are effective stakeholder engagement and qualitative methods that better capture influences on patients' and on physicians' and teams' (biologics nurses, pharmacists) perceptions, resource constraints and decision-making early in policy and implementation design. Such engagement and research could help establish common ground and develop trust and shared language on the benefits and implications of introducing biosimilars, as well as uncover situations that could pose challenges for implementation.
The literature reveals that much of this sense-making and meaning-making around biosimilars is left to physician–patient encounters. Ideally, knowledge about biosimilar treatment and its implications for each patient are collaboratively negotiated during clinical conversations. This requires supporting providers and patients with consistent evidence-informed messaging, the time and resources for meaningful conversations and collaboratively created tools that satisfy their needs.
In the international experience, we observe a “disconnect” between organizations wishing to implement biosimilars into practice, adopters who must routinize the innovation and the research available to inform implementation. This disconnect goes beyond adopter values around biosimilars to understanding necessary resources that sustain biosimilar implementation in daily practice.
Evolving Context – An Addendum (January 1, 2020).
In the original article we reported that although Alberta and British Columbia are covering biosimilar medications as part of their provincial formularies, neither had instituted a switching policy. This is no longer the case.
Residents of British Columbia who are on an originator biologic being reimbursed by the provincial PharmaCare program are now required to switch to the biosimilar alternative as part of a two-phase initiative broken down by indication. The first phase (from May 27, 2019 to November 25, 2019) included patients who were taking the originator biologic for indications that included ankylosing spondylitis and rheumatoid arthritis. The second phase (from September 5, 2019 to March 5, 2020) was for patients who were taking the originator biologic for ulcerative colitis or Crohn's disease (Government of British Columbia 2019). Over the course of the two phases, the biologic would still be covered for the specified indication, however, the expectation is that patients and prescribers would work together to ensure the transition deadlines would be met (Government of British Columbia 2019).
In Alberta, however, all patients on one of the biologics with an approved biosimilar being covered by the provincial program are required to switch by June 30, 2020, regardless of indication (Alberta Blue Cross 2019).
Acknowledgements
We would like to acknowledge our funders for this project, the Institute for Health Economics, Edmonton, Canada, as well as the Office for Lifelong Learning/The Physician Learning Program at the University of Alberta, Canada. The authors express their thanks to Research Coordinator, Melanie Heatherington, for her support. We thank Wiley Publishing, Inc., for their permission to reproduce the figure of the Conceptual Model for the Diffusion of Innovations.
Contributor Information
Danial Khan, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, AB.
Thea Luig, Social Sciences Lead, The Office of Lifelong Learning and the Physician Learning Program, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB.
Dianne Mosher, Professor of Medicine and Associate Dean, Strategic Partnerships and Community Engagement, Cumming School of Medicine, University of Calgary, Calgary, AB.
Denise Campbell-Scherer, Professor of Family Medicine and Associate Dean, The Office of Lifelong Learning and Physician Learning Program, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB.
References
- Alberta Blue Cross. 2019. “Government-Sponsored Biosimilar Initiative.” Retrieved December 27, 2019. <https://www.ab.bluecross.ca/government-plan/biosimilar-initiative.php>.
- American College of Rheumatology. 2018. American College of Rheumatology Position Statement: Biosimilars. Retrieved August 12, 2018. <https://www.rheumatology.org/Portals/0/Files/Biosimilars-Position-Statement.pdf>.
- Attipoe L., Patel S., Brit R., Crooks J., Hunt K., Grigoriou A. 2018. What Factors Predict Good Patient Experiences of Switching from Reference Etanercept to an Etanercept Biosimilar in a South West London General Hospital? Rheumatology 57(3 Suppl): iii63. 10.1093/rheumatology/key075.285. [Google Scholar]
- Calvo B., Martinez-Gorostiaga J., Echevarria E. 2018. The Surge in Biosimilars: Considerations for Effective Pharmacovigilance and EU Regulation. Therapeutic Advances in Drug Safety 9(10): 601–08. 10.1177/2042098618790442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Agency for Drugs and Technologies in Health (CADTH). 2018. Biosimilars – Regulatory, Health Technology Assessment, Reimbursement Trends, and Market Outlook. Ottawa, ON: CADTH; Retrieved August 2, 2018. <https://cadth.ca/sites/default/files/pdf/ES0317_biosimilars.pdf>. [Google Scholar]
- Cassels A. 2017, January 9 Why Biosimilars Should Be Interchangeable with Biologics. Clinical Pharmacist. Retrieved April 17, 2019. <https://www.pharmaceutical-journal.com/opinion/insight/why-biosimilars-should-be-interchangeable-with-biologics/20202121.article?firstPass=false>.
- Cohen H., Beydoun D., Chein D., Lessor T., McCabe D., Muenzberg M. et al. 2016. Awareness, Knowledge, and Perceptions of Biosimilars among Specialty Physicians. Advances in Therapy 33(12): 2160–72. 10.1007/s12325-016-0431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crohn's and Colitis Canada. (n.d.). The Issue: Forced Switching. Retrieved August 15, 2018. <http://action.crohnsandcolitis.ca/forced-switching>.
- Elek P., Harsányi A., Zelei T., Csetneki K., Kaló Z. 2017. Policy Objective of Generic Medicines from the Investment Perspective: The Case of Clopidogrel. Health Policy 121(5): 558–65. [DOI] [PubMed] [Google Scholar]
- European League Against Rheumatism. 2018, August Biosimilars – Position Paper: Updating Position Statement from the European League Against Rheumatism (EULAR) Standing Committee of People with Arthritis/Rheumatism in Europe (PARE). Retrieved August 2, 2018. <https://www.eular.org/myUploadData/files/biosimilars_paper_updated_2018_09_14_dw.pdf>.
- European Medicines Agency and the European Commission. 2017. Biosimilars in the EU: Information Guide for Healthcare Professionals. Retrieved August 4, 2018. <https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf>.
- Gabbay J., le May A. 2016. Mindlines: Making Sense of Evidence in Practice. British Journal of General Practice 66(649): 402–03. 10.3399/bjgp16X686221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Government of British Columbia. 2019. “Biosimilars Initiative for Patients.” Retrieved December 27, 2019. <https://www2.gov.bc.ca/gov/content/health/health-drug-coverage/pharmacare-for-bc-residents/what-we-cover/drug-coverage/biosimilars-initiative-patients>.
- Government of the Netherlands. 2015, December Appendix 2: Complete Set of Regulations for Securing the Affordability and Accessibility of Expensive Medicines. Retrieved August 12, 2018. <https://www.government.nl/binaries/government/documents/publications/2016/03/07/appendix-2-complete-set-of-regulations-for-securing-the-affordability-and-accessibility-of-expensive-medicines/appendix-2-set-of-regulations.pdf>.
- Greenhalgh T., Robert G., Macfarlane F., Bate P., Kyriakidou O. 2004. Diffusion of Innovations in Service Organizations: Systematic Review and Recommendations. The Milbank Quarterly 82(4): 581–629. 10.1111/j.0887-378X.2004.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. 2017. Health Canada's 2017 Biosimilars Workshop: Summary Report. Retrieved August 14, 2018. <https://www.canada.ca/en/health-canada/services/drugs-health-products/biologics-radiopharmaceuticals-genetic-therapies/applications-submissions/guidance-documents/biosimilars-workshop.html>.
- Health Improvement Scotland. 2018, March Biosimilar Medicines: A National Prescribing Framework. Retrieved August 2, 2018. <http://www.healthcareimprovementscotland.org/his/idoc.ashx?docid=93faeca2-1f4d-4ffc-a41f-7a17909ae236&version=-1>.
- Institute of Health Economics. 2016. Towards an Alberta Approach for Biosimilar Reimbursement – Summary Report of the IHE Biosimilars Forum October 6, 2016. Retrieved July 20, 2018. <https://www.ihe.ca/download/towards_an_alberta_approach_for_biosimilar_reimbursement.pdf>.
- Milne V., Tepper J., Taylor M. 2017, November 13 Do Drug Funding Decisions Need PR? Healthy Debate. Retrieved April 17, 2019. <https://healthydebate.ca/2017/11/topic/drug-funding-advertisements>.
- National Health Service (NHS) England. 2017. Commissioning Framework for Biological Medicines (Including Biosimilar Medicines). Retrieved August 2, 2018. <https://www.england.nhs.uk/wp-content/uploads/2017/09/biosimilar-medicines-commissioning-framework.pdf>.
- Patented Medicines Prices Review Board. 2017. Potential Savings from Biosimilars in Canada. Retrieved July 30, 2018. <https://www.pmprb-cepmb.gc.ca/view.asp?ccid=1304>.
- Peyrin-Biroulet L., Lönnfors S., Roblin X., Danese S., Avedano L. 2017. Patient Perspectives on Biosimilars: A Survey by the European Federation of Crohn's and Ulcerative Colitis Associations. Journal of Crohn's and Colitis 11(1): 128–33. 10.1093/ecco-jcc/jjw138. [DOI] [PubMed] [Google Scholar]
- Pfizer Canada. 2019. Inflectra. Retrieved June 1, 2019. <https://www.inflectra.ca/>.
- Rowland C. 2019, January 9 ‘Marketers Are Having a Field Day’: Patients Stuck in Corporate Fight against Generic Drugs. The Washington Post. Retrieved April 17, 2019. <https://www.washingtonpost.com/business/economy/drugmakers-alleged-scare-tactics-may-hold-back-competition/2019/01/09/612ac994-046d-11e9-9122-82e98f91ee6f_story.html?noredirect=on&utm_term=.5cc17a924c19>.
- Sullivan E., Piercy J., Waller J., Black C.M., Kachroo S. 2017. Assessing Gastroenterologist and Patient Acceptance of Biosimilars in Ulcerative Colitis and Crohn's Disease across Germany. PLoS One 12(4): e0175826. 10.1371/journal.pone.0175826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Arthritis Society. 2017, March Biosimilar Focus Group Project. Retrieved August 6, 2018. <https://arthritis.ca/AS/media/pdf/About%20Arthritis/TAS_FocusGroupReport_Final_ENG_V5.pdf>.
- U.S. Food and Drug Administration (FDA). 2018, July Biosimilars Action Plan: Balancing Innovation and Competition. Retrieved August 15, 2018. <https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/UCM613761.pdf>.
- van Overbeeke E., De Beleyr B., de Hoon J., Westhovens R., Huys I. 2017. Perception of Originator Biologics and Biosimilars: A Survey among Belgian Rheumatoid Arthritis Patients and Rheumatologists. BioDrugs 31(5): 447–59. 10.1007/s40259-017-0244-3. [DOI] [PubMed] [Google Scholar]
- Waller J., Sullivan E., Piercy J., Black C.M., Kachroo S. 2017. Assessing Physician and Patient Acceptance of Infliximab Biosimilars in Rheumatoid Arthritis, Ankylosing Spondyloarthritis and Psoriatic Arthritis across Germany. Patient Preference and Adherence 11: 519–30. 10.2147/PPA.S129333. [DOI] [PMC free article] [PubMed] [Google Scholar]


