Abstract
Background
The poor evidence base is a major problem for the German rehabilitation sector. This trial focused on testing the efficacy and benefit of inpatient medical rehabilitation compared to routine care in a single common entity, namely, chronic inflammatory bowel disease (IBD).
Methods
This pragmatic, multicenter, randomized controlled trial with a parallel group design included gainfully employed patients with IBD who were covered by one of four statutory health insurance providers. Patients in the intervention group were actively advised regarding options for rehabilitation and given support in applying for it; patients in the control group continued with the care they had been receiving before participation in the trial. The primary endpoint was social participation, and there were various secondary endpoints, including disease activity and sick days taken off from work. All parameters were assessed by questionnaire at the beginning of the trial and twelve months later. This was trial no. DRKS00009912 in the German clinical trials registry.
Results
In a complete case analysis, the intervention group (211 patients, of whom 112 underwent rehabilitation) did better than the control group (220 patients, of whom 15 underwent rehabilitation) in multiple respects. The reported limitation in social participation was reduced by 7.3 points in the intervention group and 2.9 points in the control group (p = 0.018; d = 0.23). Significant improvements were also seen in disease activity, vitality, health-related quality of life, and self-management, with effect sizes between 0.3 and 0.4. No benefit was seen in outcomes related to working capacity. Sensitivity analyses lent further support to the findings.
Conclusion
Rehabilitation research can be conducted with individually randomized, controlled trials. The findings of this trial indicate the absolute effectiveness of medically qualified rehabilitation for IBD patients, as well as its additional benefit compared to routine care.
Medical rehabilitation (MR) is a “service for participation.” In Germany, the DRV (Deutsche Rentenversicherung, German Pension Insurance) is its most important funding agency. The aim of MR is “to prevent impairment on the earning capacity of insured persons or early withdrawal from professional life or to integrate them in professional life as permanently as possible“ (section 9 subsection 1 of Book VI of the German Social Code [SGB VI]).A further aim of MR is to avert, eliminate, mitigate or compensate for and prevent progression of impairing chronic diseases (section 42 subsection 1, SGB IX).
Anyone wishing to participate in an MR program must submit an application themselves, which the attending physician accompanies with a form-based medical opinion. The DRV reviews whether certain medical and legal requirements are met. With an approval rate of 83%, the DRV funded about 832 936 inpatient MR services for adults in 2018 (1), including 3687 services for insured persons with chronic inflammatory bowel disease (IBD), such as Crohn’s disease (CD) and ulcerative colitis (UC). The mean rehabilitation duration was 23 days. The services were usually provided in specialized facilities located far away from the usual place of residence of the patient (2).
CD and UC belong to the group of disorders in which multiple areas are usually affected during periods of high disease activity (3). In addition to physical impairments, patients experience psychosocial problems, such as poor sex life, dietary restrictions, stress, or depression) (4). Such complex problems require an equally complex care approach. This is the strength of inpatient MR: It is provided by a multidisciplinary team, “all under one roof“. Besides medical specialists, the MR team comprises nursing staff, psychologists, physiotherapists, sports therapists, and occupational therapists, as well as nutritionists, social workers, and vocational counsellors.
The German system of inpatient MR is nearly unique; no other country has a directly comparable system in place (5). Thus, the increasing amount of data from studies conducted in other countries (6) does not do away with the question of the effectiveness and benefits of the German MR system. In 2014, the German Advisory Council on the Assessment of Developments in the Health Care System stated: “In conclusion, the lack of an evidence base is the core problem of the entire (national) rehabilitation sector. Studies on the absolute effectiveness of existing rehabilitation measures are lacking.“ (7)
In the following, we report results of a randomized controlled trial (MERCED study) evaluating the effectiveness of inpatient medical rehabilitation compared to the continuation of routine care close to where the patient lives (absolute effectiveness). Participants were working persons with chronic inflammatory bowel disease covered by social insurance. Since the study included a number of pension funds and health insurances as well as several rehabilitation clinics, statements on the general effectiveness of MR can be made. The study is based on a design that has been gradually optimized since 2006 (8– 11). It allows—to the best of our knowledge for the first time for this indication—to compare inpatient MR with the continuation of ongoing standard care. Our approach was pragmatic (12, 13) and—by involving a patient advisory board– also participatory (14).
The aim of the project is to reduce the deplored evidence deficit for an exemplary indication and, in doing so, contribute to the evidence base for MR in terms of methodology and content. At the same time, it is intended to demonstrate the repeatedly doubted feasibility of conducting a randomized controlled trial (RCT) on the absolute effectiveness of inpatient MR.
Methods
(For detailed information, please refer to the eMethods Section).
Study design
The study was funded by the German Research Foundation (reference no.: RA 314/13–1) and conducted as a pragmatic, multicenter, parallel group, randomized controlled trial with two points of measurement (T0, T1) at an interval of twelve months.
Inclusion criteria and recruitment
Working DRV-insured patients aged from 18 to 63 years, who were members of one of the four participating statutory health insurances, were included in this study. All of these patients had at least two reports of incapacity for work because of Crohn’s disease (ICD-10 K50) or ulcerative colitis (K51) in their health insurance billing data. Their respective health insurance mailed them the study information and invitation provided by us. Interested insured persons contacted the study administration in Lübeck which sent them detailed study information as well as a questionnaire to assure they met the inclusion criteria and to document the baseline situation. Invited persons were excluded if they lacked the subjective need for rehabilitation, if they lacked at least one complicating psychosocial problem (4), if they stated not being able to participate in a rehabilitation program in the near future, if they had undergone MR in the past 2 years, and if informed consent was not provided.
Randomization and rehabilitation application
The included insurees were 1:1 randomized to the two arms of the study. The intervention group (IG) was supported by the study administration in preparing the rehabilitation application with written material, access to an additional information website, and individual counseling by phone/email. Using their “option” (“Wunsch- und Wahlrecht” of section 9 SGB IX; the right to individual wishes and choice), they were asked to select one of the 7 rehabilitation facilities listed by the patient organization DCCV (Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung e. V., German Crohn’s Disease/Ulcerative Colitis Association). The standardized procedures for submitting a rehabilitation application, application review, application approval, and conduct of the rehabilitation program remained untouched.
The participants in the control group (CG) continued their previous treatment without any change. After the 12-month follow-up survey (T1), they received the study administration’s support offer.
Outcomes and measuring tools
The evaluation of the effectiveness of participation in medical rehabilitation was performed exclusively on the basis of patient-reported data from self-completion questionnaires. The primary outcome was the experienced limitation to participation in social life, measured using the index for measuring limitations in participation (IMET) (15, 16). Nine items are used to obtain data on limitations in various areas of everyday activities (including work, school, housework, leisure time, social relations) using numerical rating scales (0 = not impaired at all; 10 = completely impaired). The total score (0–90) was calculated based on these ratings.
In order to cover a broad range of potential rehabilitation effects, a total of 12 secondary outcomes were included and analyzed (table 1).
Table 1. Secondary outcomes.
| Outcome (time window) | Tool (reference) | Range/categories used |
| Disease activity (during the preceding 7 days) |
German Inflammatory Bowel Disease Activity Index (GIBDI) (17) |
0–18 |
| Self-monitoring and insight (current) |
Health Education Impact Questionnaire (heiQ) (18, 19) |
1–4 |
| Constructive attitudes (current) | Health Education Impact Questionnaire (heiQ) (18, 19) |
1–4 |
| Emotional well-being (current) | Health Education Impact Questionnaire (heiQ) (18, 19) |
1–4 |
| Psychological distress (during the preceding 2 weeks) |
Patient Health Questionnaire-4 (PHQ-4) (20) |
0–12 |
| Vitality (past 7 days) |
Subscale Short Form 36 (SF-36) (21) | 0–100 |
| Level of being informed about IBD (current) |
Individual item | 0–10 |
| Health-related quality of life (current) |
EuroQuol Visual Analogue Scale (EQ-VAS) (22) | 0–100 |
| Current state of health (compared to initial survey) |
Individual item | Much better/somewhat better/about as 12 months ago/somewhat worse/much worse |
| Employment status (current) |
Individual item | Working yes = working full-time, part-time or marginally employed |
| Days of incapacity for work (in the past 3 months) |
Individual item | 0–90 |
| Subjective employment prognosis (current) |
Subjective prognostic employment (SPE) scale (23) |
Unfavorable prognosis: score ≥ 2 |
IBD, inflammatory bowel disease
Data analysis
The primary analysis included all insured persons with data available for both points of measurement (complete case analysis, CCA). They were analyzed in the study arm to which they had been randomly allocated, regardless of their MR participation. Drop-out analyses were performed to estimate potential bias. A subgroup analysis compared IG participants who underwent an MR program during the study period with CG participants who did not undergo rehabilitation. During the sensitivity analysis for the primary outcome, data for all missing cases were imputed for an intention-to-treat (ITT) analysis, using various methods.
The significance level for the type 1 error was set at 5%. For the testing of the secondary outcomes, a Bonferroni correction was applied to adjust the significance level (24). For interval-scale outcomes, differences between T0 and T1 measurements were calculated for each participant and the mean differences (Δ T0-T1) between IG and CG were tested for significance using two-sided t-tests for independent samples. For nominal and ordinal data, the Chi-square test was used for significance testing. As effect sizes for continuous parameters, Cohen’s d was calculated, for dichotomous parameters odds ratios.
Patient advisory board
This participatory research project was supported by the patient organization DCCV e. V. and by an eight-member patient advisory board for the entire duration of the project. Its involvement ranged from stage 1 (consultation) to 3 (collaboration) of the 4-stage model described by Sweeney & Morgan (14); for example, the patient advisory board was involved in the selection of the outcomes.
Ethics Committee and registration
The study was approved by the Ethics Committee of the University of Lübeck (reference number 16–047 of 8 March 2016) and registered with the German Registry of Clinical Studies (DRKS00009912).
Results
Study sample
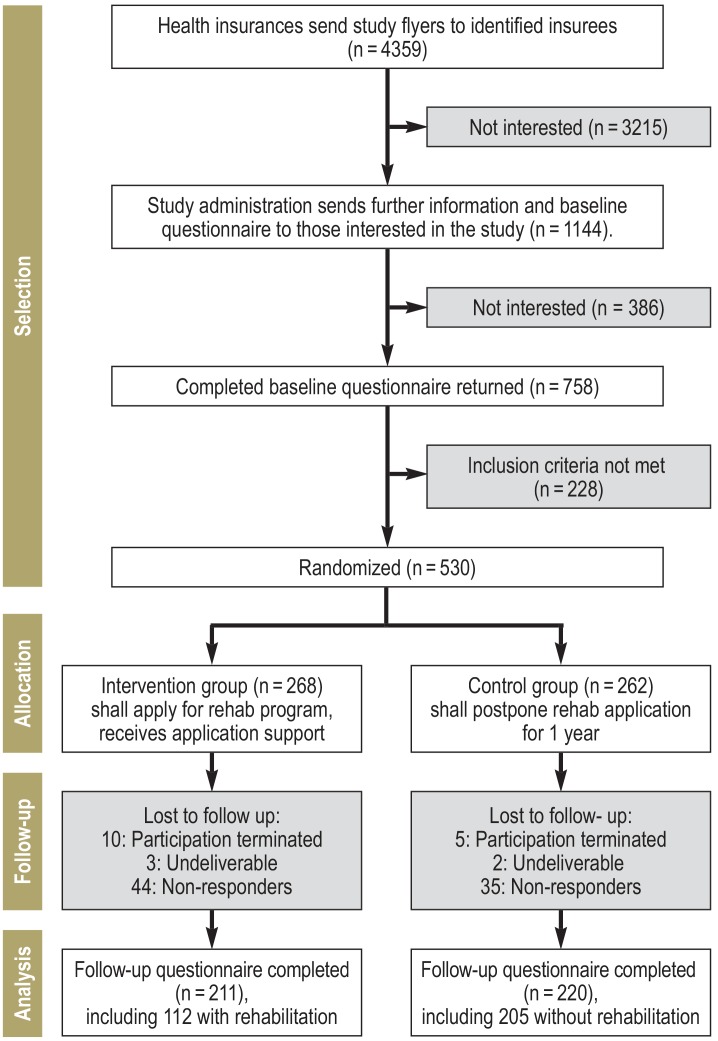
Of the 4359 insured persons informed about the study, 1144 requested detailed study information. 228 of the 758 insured persons willing to participate did not meet the inclusion criteria. 530 insured persons were included in the study and randomized to IG (268) or CG (262). The recruitment target was set at 300 patients in each study arm. Twelve months later, 431 study participants filled in the follow-up questionnaire. The drop-out rate for the interval between study inclusion and follow-up survey was 21.3% (57 of 268) and 16% (42 of 262) in the IG and the CG, respectively (p = 0.122) (figure).
Figure.
Flowchart of the study procedure
The baseline characteristics of the analysis group are listed in Table 2.
eTable 2. Additional ITT analysis for the primary outcome (limitation in social participation) using various imputation methods.
| Imputation method | N (IG/CG) | ΔT0−T1IG | ΔT0−T1CG | p value |
ES [95% CI] |
| Last observation carried forward*1 | 267/261 | 5.77 (18.90) | 2.44 (15.85) | 0.029 | 0.19 [0.02; 0.36] |
| Best/worst scenario*2 Worst/best scenario*3 |
267/261 | 7.91 (18.66) 6.40 (18.71) | 2.93 (15.81) 4.09 (16.03) | 0.001 0.128 | 0.29 [0.12; 0.46] 0.13 [−0.04; 0.30] |
| Multiple imputation*4 | 268/262 | 7.29 (22.86) | 2.98 (18.90) | 0.017 | 0.21 [0.04; 0.38] |
| No imputation (CCA) | 210/219 | 7.33 (21.05) | 2.92 (17.31) | 0.018 | 0.23 [0.04; 0.42] |
*1 Baseline values replace missing values in the 12-month follow-up history.
*2 Best/worst scenario: Missing cases in der IG replaced by mean change in the IG responders who underwent rehabilitation (“best”: improvement of 10 points); missing cases in the CG replaced by mean change in the CG responders who did not undergo rehabilitation (“worst“: improvement of 3 points)
*3 Worst/best scenario: Missing cases in the IG replaced by mean change in the CG responders who did not undergo rehabilitation (“worst“: improvement by 3 points); missing cases in the CG replaced by mean change of the IG responders who underwent rehabilitation (“best“: improvement of 10 points)
*4 Multiple imputation using the Markov chain Monte Carlo method (MCMC), 50 imputations, 10 iterations, in the model all outcomes as well as age, sex, school education, and disease duration
CCA, complete case analysis; ES, effect size; IG, intervention group; ITT analysis, intention to treat analysis; CG, control group; CI, confidence interval; T0, baseline; T1, follow-up; Δ difference
In a drop-out analysis, the 99 drop-outs were compared with the 431 patients who participated in the follow-up survey. Only for the parameter “disease duration,” a significant difference was found: The disease duration among the drop-outs was 3 years shorter on average (etable 1).
eTable 1. Drop-out analysis—baseline survey data.
| Characteristics | Drop-outs (N = 99) | Completers (N = 431) | p value | |||
| Valid N | N (%)/M (SD) | Valid N | N (%)/M (SD) | |||
| Sex | Female | 99 | 65 (65.7%) | 431 | 246 (57.1%) | 0.118 |
| Age in years | 18–30 | 99 | 22 (22.2%) | 431 | 75 (17.4%) | 0.343 |
| 31–40 | 26 (26.3%) | 92 (21.3%) | ||||
| 41–50 | 24 (24.2%) | 116 (26.9%) | ||||
| 51–64 | 27 (27.3%) | 148 (34.3%) | ||||
| Employment status | Working full-time | 99 | 80 (80.8%) | 431 | 326 (75.6%) | 0.207 |
| School education (in years) | Up to 9 | 93 | 8 (8.6%) | 429 | 52 (12.1%) | 0.501 |
| 10–11 | 39 (41.9%) | 188 (43.8%) | ||||
| 12–13 | 46 (49.5%) | 189 (44.1%) | ||||
| Residential area | Core cities | 99 | 33 (33.3%) | 430 | 136 (31.6%) | 0.614 |
| Dense surrounding areas | 36 (36.4%) | 182 (41.3%) | ||||
| Rural surrounding areas | 14 (14.1%) | 60 (14.0%) | ||||
| Rural areas | 16 (16.2%) | 52 (12.1%) | ||||
| Diagnosis | Crohn’s disease | 99 | 59 (59.6%) | 431 | 229 (53.1%) | 0.418 |
| Ulcerative colitis | 37 (37.4%) | 192 (44.5%) | ||||
| Indeterminate colitis | 3 (3.0%) | 10 (2.3%) | ||||
| Disease duration | In years | 99 | 11.4 (9.1) | 426 | 14.0 (10.2) | 0.022 |
| Course of disease “during the preceeding years“ |
In remission | 99 | 11 (11.1%) | 423 | 53 (12.5%) | 0.148 |
| Alternating relapse/remission | 42 (42.4%) | 218 (51.5%) | ||||
| Constantly/increasingly active | 46 (46.5%) | 152 (35.9%) | ||||
| Disease activity during the past 7 days (GIBDI*) |
Remission (0–3) | 93 | 22 (23.7%) | 394 | 113 (28.7%) | 0.301 |
| Mild (4–7) | 44 (47.3%) | 187 (47.5%) | ||||
| Moderate (8–11) | 21 (22.6%) | 83 (21.1%) | ||||
| Severe (≥ 12) | 6 (6.5%) | 11 (2.8%) | ||||
| Surgery for IBD | Ever (yes) | 98 | 34 (34.7%) | 429 | 134 (31.2%) | 0.507 |
| Rehabilitation for IBD | Ever (yes) | 99 | 33 (33.3%) | 431 | 173 (40.1%) | 0.210 |
| Medication intake during the past 3 months(yes) | 5-aminosalicylates | 99 | 52 (52.5%) | 431 | 231 (53.6%) | 0.847 |
| Corticosteroids | 42 (42.4%) | 184 (42.7%) | 0.961 | |||
| Immunosuppressants | 26 (26.3%) | 115 (26.7%) | 0.932 | |||
| Biologic agents | 37 (37.4%) | 166 (38.5%) | 0.833 | |||
| None | 6 (6.1%) | 16 (3.7%) | 0.291 | |||
| Number of IBD-related specialist visits |
Past 12 months | 99 | 20.1 (18.2) | 431 | 18.6 (16.2) | 0.409 |
| Satisfaction with IBD care | 0–10 | 99 | 5.8 (2.5) | 430 | 6.3 (2.5) | 0.107 |
| Limitation in social participation (IMET) | 0–90 | 98 | 36.4 (18.6) | 430 | 33.8 (18.9) | 0.212 |
| Self-management (heiQ scales) (1–4) |
Self-monitoring and insight | 97 | 3.0 (0.4) | 422 | 3.0 (0.5) | 0.519 |
| Constructive attitudes | 99 | 3.1 (0.5) | 428 | 3.1 (0.5) | 0.824 | |
| Emotional well-being (the higher, the poorer) | 98 | 2.5 (0.7) | 429 | 2.4 (0.7) | 0.090 | |
| Level of being informed about IBD (NRS) | 0–10 | 99 | 6.3 (2.6) | 431 | 6.7 (2.4) | 0.217 |
| Current health-related quality of life (EQ-VAS) | 0–100 | 97 | 60.0 (18.8) | 429 | 62.0 (16.3) | 0.282 |
| Vitality (SF-36) | 0–100 | 98 | 32.7 (16.8) | 430 | 36.1 (17.5) | 0.081 |
| Psychological distress (PHQ-4) | 0–12 | 97 | 4.8 (2.6) | 428 | 4.4 (2.7) | 0.138 |
| Sick days taken off from work in the past 3 months | 0–90 | 97 | 13.3 (21.2) | 422 | 11.3 (17.1) | 0.325 |
| Negative subjective employment prognosis |
SPE ≥ 2 | 99 | 50 (50.5%) | 429 | 225 (52.4%) | 0.727 |
IFW, incapacity for work; IBD, inflammatory bowel disease; EQ-VAS, EuroQuol Visual Analogue Scale; GIBDI, German Inflammatory Bowel Disease Activity Index (*cannot be calculated for patients with stoma or indeterminate colitis); heiQ, Health Education Impact Questionnaire; IMET, index for measuring limitations of participation (“Index zur Messung von Einschränkungen der Teilhabe”); M, mean; N, number of valid cases; NRS, numerical rating scale; PHQ-4, Patient Health Questionnaire (short form with 4 items); SD, standard deviation; SF-36, Short Form 36 questionnaire; SPE, subjective prognostic employment scale
Application advice and rehabilitation participation
All participants in the IG received the written information pack; in addition, 28% (59 of 211) also made use of the website. 43% (90 of 211) approached the study administration directly with questions about the application process, either by phone or email.
112 (53.1%) of the IG members reported to have undergone a rehabilitation program which had been conducted in 81% in one of the 7 recommended rehabilitation facilities. The MR was performed about 4 months (median) after the baseline survey (interquartile range: 3–6 months).
In the CG, 15 patients (6.8%) had undergone MR between T0 and T1.
Complete case analysis for primary and secondary outcomes
The complete case analysis (N = 431) showed statistically significant advantages for the primary outcome in the IG (table 3): The experienced limitation in social participation was reduced in the IG by 7.3 (SD = 21.1), in the CG by 2.9 (SD = 17.3) points (p = 0.018); this corresponds to a small effect size of d = 0.23. A clinically relevant improvement by 10 points in the IMET was achieved by 43.8% and 32.1% in the IG and CG, respectively (p = 0.013). The statistically significant advantage in the primary outcome for the IG was confirmed by the additional ITT analysis (eMethods, eTable 2).
Table 3. Results of the complete case analysis (IG: N = 211; CG: N = 220).
| Outcome | Group | N | T0 | T1 | Δ T0–T1 | Difference ΔIG vs. ΔCG | |
| M (SD) | M (SD) | p value*1 | ES *2 [95% CI] | ||||
| Primary outcome | |||||||
| Limitation in social participation (IMET) (0–90) (higher scores = worse) |
IG | 210 | 34.68 (19.29) | 27.34 (20.39) | 7.33 (21.05) | 0.018 | 0.23 [0.04; 0.42] |
| CG | 218 | 32.79 (18.33) | 29.87 (20.37) | 2.92 (17.31) | |||
| Secondary outcomes | |||||||
| Disease activity during the preceeding 7 days (GIBDI*3) (0–18) (higher scores = worse) |
IG | 188 | 5.55 (2.83) | 4.17 (3.07) | 1.38 (3.00) | <0.001 | 0.37 [0.16; 0.57] |
| CG | 184 | 5.32 (2.89) | 5.05 (3.22) | 0.27 (3.06) | |||
| Self-monitoring and insight (heiQ scale) (1–4) (higher scores = better) |
IG | 208 | 2.96 (0.45) | 3.21 (0.41) | 0.25 (0.41) | 0.001 | 0.33 [0.14; 0.52] |
| CG | 210 | 3.01 (0.46) | 3.13 (0.45) | 0.12 (0.38) | |||
| Constructive attitudes (heiQ scale) (1–4) (higher scores = better) |
IG | 209 | 3.12 (0.51) | 3.31 (0.52) | 0.19 (0.49) | 0.022 | 0.23 [0.04; 0.42] |
| CG | 211 | 3.13 (0.54) | 3.21 (0.57) | 0.08 (0.47) | |||
| Emotional well-being (heiQ scale) (1–4)(higher scores = worse) |
IG | 205 | 2.38 (0.66) | 2.08 (0.66) | −0.30 (0.58) | 0.003 | 0.31 [0.11; 0.50] |
| CG | 215 | 2.37 (0.66) | 2.25 (0.64) | −0.12 (0.60) | |||
| Psychological distress (PHQ-4) (0–12) (higher scores = worse) |
IG | 211 | 4.44 (2.70) | 3.29 (2.61) | 1.15 (2.69) | 0.015 | 0.24 [0.05; 0.43] |
| CG | 216 | 4.28 (2.78) | 3.75 (2.73) | 0.53 (2.58) | |||
| Vitality (SF-36, subscale) (0–100) (higher scores = better) |
IG | 209 | 35.46 (16.87) | 45.05 (20.46) | −9.59 (19.75) | 0.001 | 0.33 [0.14; 0.52] |
| CG | 218 | 36.49 (18.07) | 39.86 (20.60) | −3.37 (18.25) | |||
| Level of being informed about IBD (NRS 0–10) |
IG | 210 | 6.56 (2.43) | 7.49 (2.33) | −0.92 (2.52) | 0.042 | 0.20 [0.01; 0.39] |
| CG | 219 | 6.78 (2.41) | 7.26 (2.46) | −0.48 (2.02) | |||
| Health-related quality of life (EQ-VAS) (0–100) (higher scores = better) |
IG | 209 | 60.76 (16.57) | 67.58 (17.47) | −6.82 (18.92) | 0.003 | 0.29 [0.10; 0.48] |
| CG | 219 | 63.12 (15.98) | 64.81 (18.48) | −1.69 (16.82) | |||
| Current state of health at T1 a lot/somewhat (compared to T0) better |
IG | 209 | 122 (58.4%) | <0.001 | OR = 2.7 [1.8; 3.9] |
||
| CG | 220 | 76 (34.5%) | |||||
| Employment status at T1 Working, yes | IG | 210 | 193 (91.9%) | 0.749 | OR = 0.9 [0.4; 1.8] |
||
| CG | 220 | 204 (92.7%) | |||||
| Negative subjective employment prognosis (SPE) at T1 Score ≥ 2 |
IG | 204 | 92 (45.1%) | 0.474 | OR = 0.7 [0.5; 1.1] |
||
| CG | 214 | 104 (48.6%) | |||||
| Number of sick days taken off from work*4 in the preceeding 3 months (0–90) |
IG | 188 | 11.67 (17.87) | 10.30 (21.09) | 1.37 (26.13) | 0.329 | 0.10 [−0.10; 0.30] |
| CG | 195 | 9.72 (15.15) | 10.77 (20.86) | −1.06 (22.33) | |||
*1 Non-adjusted; for secondary outcomes, only p values ≤ 0.004 are statistically significant after Bonferroni adjustment.
*2 Cohen’s d with denominator as mean pooled SD and corrected for different group sizes; positive d value = advantage IG, negative value = advantage CG
*3 GIBDI incalculable for stoma and indeterminate colitis
*4 Only persons working at T1
IBD, inflammatory bowel disease; EQ-VAS, EuroQuol Visual Analogue Scale; ES, effect size; GIBDI, German Inflammatory Bowel Disease Activity Index; heiQ, Health Education Impact Questionnaire; IG, intervention group; IMET, index for measuring restrictions of participation (“Index zur Messung von Einschränkungen der Teilhabe”); CI, confidence interval; CG, control group; M, mean; N, number of valid values; NRS, numerical rating scale; OR, odds ratio; PHQ-4, Patient Health Questionnaire (short form with 4 items); SD, standard deviation; SF-36, short form 36 questionnaire; SPE, subjective prognostic employment scale (without pensioners); T0, baseline; T1, follow-up
Likewise, in 6 of the 12 secondary outcomes, significant advantages for the IG were found after alpha-error adjustment (p-values ≤ 0.004). The largest effect size (d = 0.37) was demonstrated for disease activity (reduction in the GIBDI [German Inflammatory Bowel Disease Activity Index] by 1.4 and 0.3 points in the IG and CG, respectively; p<0.001); at the time of the follow-up survey, 51.2% and 36.1% of the patients were in remission in the IG and CG, respectively (GIBDI ≤ 3) (p = 0.002), including steroid-free remission: 41.1% versus 29.5% (p = 0.017). In 2 of the 3 documented self-management aspects, the IG showed greater improvements; similarly, the increase in vitality and health-related quality of life was more pronounced in the IG (effect sizes around 0.3). In the direct measurement of change at the time of the follow-up survey, 58% and 35% showed an improved health status in the IG and CG, respectively (p<0.001).
In the other secondary outcomes, differences between IG and CG were not significant, but more favorable developments were also apparent in the IG, also in terms of constructive attitudes, level of being informed, and mental stress. The differences in the work-related outcomes were non-significant and irrelevant from a social medicine perspective.
Subgroup analysis (rehabilitants in the IG versus non-rehabilitants in the CG)
In addition to the primary analysis, only rehabilitants of the IG (N = 112) were compared to the non-rehabilitants of the CG (N = 205). At baseline, the two groups did not differ from each other (etable 3). The rehabilitants of the IG achieved significantly better scores with larger (up to medium) effect sizes in all primary outcomes as well as all secondary outcomes, with the exception of work-related outcomes (etable 4).
eTable 3. Sample characteristics at baseline (subgroup analysis: rehabilitants in IG versus non-rehabilitants in CG).
| Characteristics | IG (N = 112) | CG (N = 205) | p value | |||
| Valid N | N (%)/M (SD) | Valid N | N (%)/M (SD) | |||
| Sex | Female | 112 | 66 (58.9%) | 205 | 11 (54.6%) | 0.461 |
| Age | 18–30 | 112 | 14 (12.5%) | 205 | 41 (20.0%) | 0.201 |
| 31–40 | 22 (19.6%) | 49 (23.9%) | ||||
| 41–50 | 36 (32.1%) | 53 (25.9%) | ||||
| 51–64 | 40 (35.7%) | 62 (30.2%) | ||||
| Employment status | Working, yes | 112 | 112 (100%) | 205 | 205 (100%) | 1 |
| Full-time | 85 (75.9%) | 160 (78.0%) | 0.536 | |||
| School education (in years) | Up to 9 | 112 | 12 (10.7%) | 203 | 24 (11.8%) | 0.746 |
| 10–11 | 53 (47.3%) | 87 (42.9%) | ||||
| 12–13 | 47 (42.0%) | 92 (45.3%) | ||||
| Residential region | Core cities | 112 | 36 (32.1%) | 204 | 69 (33.8%) | 0.978 |
| Dense surrounding areas | 46 (41.1%) | 83 (40.7%) | ||||
| Rural surrounding areas | 14 (12.5%) | 26 (12.7%) | ||||
| Rural areas | 16 (14.3%) | 26 (12.7%) | ||||
| Diagnosis | Crohn’s disease | 112 | 62 (55.4%) | 205 | 102 (49.8%) | 0.243 |
| Ulcerative colitis | 50 (44.6%) | 99 (48.3%) | ||||
| Indeterminate colitis | 0 (0.0%) | 4 (2.0%) | ||||
| Course of disease “during the preceeding years“ |
In remission | 109 | 9 (8.3%) | 202 | 26 (12.9%) | 0.436 |
| Alternating relapse/remission | 59 (54.1%) | 108 (53.5%) | ||||
| Increasing/continuous activity | 41 (37.6%) | 68 (33.7%) | ||||
| Disease duration | In years | 109 | 14.6 (10.3) | 205 | 13.1 (9.8) | 0.210 |
| Disease activity during the past 7 days (GIBDI*) |
None (0–3) | 104 | 27 (26.0%) | 189 | 57 (30.2%) | 0.877 |
| Mild (4–7) | 52 (50.0%) | 91 (48.1%) | ||||
| Moderate (8–11) | 23 (22.1%) | 37 (19.6%) | ||||
| Severe ≥ 12) | 2 (1.9%) | 4 (2.1%) | ||||
| Surgery for IBD | Ever (yes) | 112 | 34 (30.4%) | 205 | 63 (30.7%) | 0.945 |
| Rehabilitation for IBD | Ever (yes) | 112 | 39 (34.8%) | 205 | 74 (36.1%) | 0.821 |
| Medication intake during the preceeding 3 months (yes) |
5-aminosalicylates | 112 | 62 (55.4%) | 205 | 108 (52.7%) | 0.648 |
| Corticosteroids | 51 (45.5%) | 85 (41.5%) | 0.484 | |||
| Immunosuppressants | 23 (20.5%) | 58 (28.3%) | 0.130 | |||
| Biologic agents | 42 (37.5%) | 76 (37.1%) | 0.940 | |||
| None | 4 (3.6%) | 9 (4.4%) | 0.725 | |||
| IBD-related specialist visits | Past 12 months | 112 | 18.9 (13.0) | 205 | 17.7 (16.0) | 0.506 |
| Satisfaction with IBD care | NRS (0 = not satisfied at all; 10 =very satisfied) |
112 | 6.1 (2.6) | 205 | 6.3 (2.5) | 0.550 |
CCA, complete case analysis; IBD, inflammatory bowel disease; GIBDI. German Inflammatory Bowel Disease Activity Index (* cannot be calculated for patients with stoma or indeterminate colitis); IG, intervention group; CG, control group; M, mean; N, number of valid cases; NRS, numerical rating scale; SD, standard deviation
eTable 4. Results of the subgroup analysis (IG: 112 rehabilitants versus CG: 205 non-rehabilitants).
| Outcome | Group | N | T0 | T1 | Δ T0−T1 | Difference ΔIG vs. ΔCG | |
| M (SD) | M (SD) | p value*1 | ES*2 [95% CI] | ||||
| Primary outcome | |||||||
| Limitation in social participation (IMET) (0–90) (higher scores = worse) | IG | 112 | 35.37 (19.25) | 25.43 (20.13) | 9.94 (22.60) | 0.005 | 0.36 [0.13; 0.60] |
| CG | 203 | 32.44 (17.91) | 29.46 (19.97) | 2.98 (17.02) | |||
| Secondary outcomes | |||||||
| Disease activity (GIBDI*3) (0–18) (higher scores = worse) | IG | 101 | 5.54 (2.70) | 3.50 (2.69) | 2.04 (3.34) | <0.001 | 0.59 [0.34; 0.84] |
| CG | 173 | 5.15 (2.79) | 4.97 (3.20) | 0.17 (3.06) | |||
| Self-monitoring and insight (heiQ scale) (1–4) (higher scores = better) |
IG | 112 | 2.96 (0.39) | 3.25 (0.35) | 0.29 (0.40) | <0.001 | 0.44 [0.20; 0.67] |
| CG | 196 | 2.99 (0.47) | 3.11 (0.45) | 0.12 (0.38) | |||
| Constructive attitudes (heiQ scale) (1–4) (higher scores = better) |
IG | 112 | 3.12 (0.53) | 3.34 (0.48) | 0.22 (0.48) | 0.005 | 0.34 [0.10; 0.57] |
| CG | 197 | 3.14 (0.54) | 3.21 (0.57) | 0.06 (0.47) | |||
| Emotional well-being (heiQ scale) (1–4) (higher scores = worse) |
IG | 109 | 2.38 (0.67) | 1.99 (0.61) | −0.39 (0.64) | <0.001 | 0.47 [0.24; 0.71] |
| CG | 200 | 2.36 (0.65) | 2.26 (0.64) | −0.10 (0.60) | |||
| Psychological distress (PHQ-4) (0–12) (higher scores = worse) |
IG | 112 | 4.48 (2.79) | 2.82 (2.30) | 1.66 (2.77) | <0.001 | 0.47 [0.23; 0.70] |
| CG | 201 | 4.21 (2.72) | 3.78 (2.68) | 0.43 (2.55) | |||
| Vitality (SF-36) (0–100) (higher scores = better) |
IG | 112 | 35.05 (15.92) | 49.64 (19.45) | −14.60 (20.69) | <0.001 | 0.63 [0.40; 0.87] |
| CG | 204 | 36.74 (17.74) | 39.49 (20.53) | −2.75 (17.56) | |||
| Level of being informed about IBD (NRS 0–10) |
IG | 112 | 6.48 (2.31) | 7.81 (1.95) | −1.33 (2.55) | 0.002 | 0.41 [0.17; 0.64] |
| CG | 204 | 6.73 (2.37) | 7.17 (2.47) | −0.44 (1.98) | |||
| Health-related quality of life (EQ-VAS) (0–100) (higher scores = better) |
IG | 111 | 59.98 (16.65) | 71.40 (16.61) | −11.41 (19.46) | <0.001 | 0.56 [0.32; 0.79] |
| CG | 204 | 63.46 (15.76) | 65.03 (18.24) | −1.57 (16.73) | |||
| Current state of health a lot/somewhat at T1 (compared to T0) better |
IG | 112 | 82 (73.2%) | <0.001 | OR = 5.6 [3.4; 9.4] |
||
| CG | 205 | 67 (32.7%) | |||||
| Employment status at T1 Working, yes | IG | 112 | 101 (90.2%) | 0.345 | OR = 0.7 [0.3; 1.5] |
||
| CG | 205 | 191 (93.2%) | |||||
| Negative subjective employment prognosis (SPE) at T1 Score ≥ 2 |
IG | 107 | 41 (38.3%) | 0.080 | OR = 0.7 [0.4; 1.1] |
||
| CG | 201 | 98 (48.8%) | |||||
| Number of sick days taken off from work*4 in the past 3 months (0–90) |
IG | 99 | 12.32 (18.52) | 9.34 (20.44) | 2.98 (26.26) | 0.201 | 0.16 [−0.09; 0.41] |
| CG | 182 | 9.14 (14.28) | 9.75 (19.18) | −0.62 (20.14) | |||
*1 Non-adjusted; for secondary outcomes, only p values ≤ 0.004 are statistically significant after Bonferroni adjustment.
*2 Cohen’s d with denominator as mean pooled SD and corrected for different group sizes; positive d value = advantage IG, negative value = advantage CG
*3 GIBDI incalculable for stoma and indeterminate colitis.
*4 Excluded are those at T1 who are not working.
IBD, inflammatory bowel disease; EQ-VAS, EuroQuol Visual Analogue Scale; ES, effect size; GIBDI, German Inflammatory Bowel Disease Activity Index; heiQ, Health Education Impact Questionnaire; CG, control group; CI, confidence interval; IG, intervention group; IMET, index for measuring limitations of participation (“Index zur Messung von Einschränkungen der Teilhabe”); M, mean; N, number of valid values; NRS, numerical rating scale; OR, odds ratio under control of baseline distribution; PHQ-4, Patient Health Questionnaire (short form with 4 items); SD, standard deviation; SF-36, Short Form 36 questionnaire; SPE, subjective prognostic employment scale (without pensioners); T0, baseline; T1, follow-up
Utilization of healthcare services outside the rehabilitation sector
Patients in the IG and CG provided information about the utilization of the outpatient and inpatient healthcare sector before and during the study. No differences were found with regard to the type and frequency of consultation with specialists, the use of various medications and the use of rehabilitative services at the place of residence (etable 5).
eTable 5. Treatment outside the rehabilitation sector (complete cases).
| Characteristics | IG (N = 211) | CG (N = 220) | p value | |||
| Valid N | N (%)/M (SD) | Valid N | N (%)/M (SD) | |||
| Baseline data (T0) | ||||||
| Medication intakeduring the preceeding 3 months (yes) | 5-aminosalicylates | 211 | 114 (54.0%) | 220 | 117 (53.2%) | 0.860 |
| Corticosteroids | 92 (43.6%) | 92 (41.8%) | 0.708 | |||
| Immunosuppressants | 54 (25.6%) | 61 (27.7%) | 0.616 | |||
| Biologic agents | 82 (38.9%) | 84 (38.2%) | 0.885 | |||
| None | 6 (2.8%) | 10 (4.5%) | 0.350 | |||
| Surgery for IBD | In the past 12 months (yes) | 210 | 19 (9.1%) | 219 | 19 (8.6%) | 0.685 |
| Hospital stay for IBD | In the past 12 months (yes) | 211 | 71 (33.6%) | 220 | 65 (29.5%) | 0.359 |
| Specialist visits for IBD in the past 12 months | N contacts overall | 211 | 19.1 (16.0) | 220 | 18.1 (16.4) | 0.498 |
| N contacts gastroenterologist | 204 | 5.8 (5.9) | 209 | 6.1 (6.8) | 0.619 | |
| Use of non-medical treatments/ counseling for IBD (past 12 months) | 0 | 211 | 76 (36.0%) | 219 | 78 (35.6%) | 0.924 |
| 1–2 | 92 (43.6%) | 93 (42.5%) | ||||
| 3+ | 43 (20.4%) | 48 (21.9%) | ||||
| Follow-up data (T1) | ||||||
| Medication intakeduring the preceeding 3 months (yes) | 5-aminosalicylates | 210 | 99 (47.1%) | 218 | 99 (45.4%) | 0.720 |
| Corticosteroids | 58 (27.6%) | 68 (31.2%) | 0.417 | |||
| Immunosuppressants | 39 (18.6%) | 51 (23.4%) | 0.221 | |||
| Biologic agents | 88 (41.9%) | 90 (41.3%) | 0.896 | |||
| None | 10 (4.8%) | 14 (6.4%) | 0.455 | |||
| Surgery for IBD | Past 12 months (yes) | 208 | 19 (9.1%) | 217 | 12 (5.5%) | 0.153 |
| Hospital stay for IBD | Past 12 months (yes) | 210 | 44 (21.0%) | 217 | 41 (18.9%) | 0.594 |
| Specialist visits for IBD in the past 12 months | N contacts overall | 210 | 16.8 (18.7) | 220 | 16.7 (16.5) | 0.938 |
| N contacts gastroenterologist | 203 | 5.0 (5.5) | 210 | 5.4 (6.0) | 0.436 | |
| Use of non-medical treatments/ counseling for IBD (past 12 months) | 0 | 211 | 88 (41.7%) | 220 | 100 (45.5%) | 0.728 |
| 1–2 | 83 (39.3%) | 82 (37.3%) | ||||
| 3+ | 40 (19.0%) | 38 (17.3%) | ||||
IBD, inflammatory bowel disease; IG, intervention group; CG, control group; M, mean; N, number of valid values; SD, standard deviation; T0, baseline; T1, follow-up
Discussion
MERCED evaluated the effectiveness of inpatient MR for working persons with statutory health insurance suffering from IBD who at that time stated that they were in need of rehabilitation and prepared to undergo rehabilitation. The pragmatically designed RCT had no influence on the procedures of the rehabilitation funding authorities and the rehabilitation clinics.
Our analyses suggest that inpatient MR for this indication is of general and absolute effectiveness and benefit. The hypothesis of its specific effectiveness is supported by the finding that there were no differences between IG and CG patients’ utilization of other medical services, neither before nor after MR.
In the complete case analysis, the primary outcome and most secondary outcomes showed significantly higher levels of improvement in the IG compared to the CG. The effect sizes achieved were in the smaller range. Clinically relevant improvement in social participation and steroid-free remission were significantly more frequently observed in the IG compared to the CG. The subgroup analysis comparing rehabilitants (IG) with non-rehabilitants (CG) found numerically larger effect sizes; this also indicates specific effectiveness. Neither of the two analyses showed significant advantages for the IG in terms of the strictly socio-medical outcome parameters (employment status, subjective employment prognosis, number of sick days taken off from work). This finding will have to be addressed in a separate publication. It should be noted that staying in or returning to work is not the sole purpose of DRV-financed MR (25). In patients with IBD, MR appears to be most effective in terms of clinical and psychosocial outcomes.
Our study shows that randomized controlled trials can be conducted to evaluate the absolute general effectiveness of MR—contrary to opinions expressed for decades (26– 28). To this end, recruitment was carried out with full disclosure to the target persons before they submitted their application for rehabilitation. The EPRA study, which was carried out almost at the same time, used an alternative approach: It randomized asthma patients after their rehabilitation applications had been approved to either immediate or delayed start of rehabilitation (29). Due to strong rehabilitation preferences of the recruited patients, it can be assumed that such a waiting group design (30) only allows the evaluation of short-term effects of MR. The situation before submission of a rehabilitation application is different. Frequently, these IBD patients—and their physicians—express doubt about the effectiveness and sustainability of MR. By proactively approaching insured persons who had previously been selected by their health insurances and who continued to select themselves, participants were found who were prepared to postpone a rehabilitation application by one year.
Limitations and weaknesses of the MERCED study
In the MERCED study, the strategy of proactively approaching insured persons potentially in need of rehabilitation may have brought a patient group to MR which is not normally seen there. However, the fact that the patient population of the MERCED study is more similar in its characteristics to other samples recruited in rehabilitation clinics (31, 32) than to IBD samples from outpatient care (33, 34) supports the appropriateness of the approach (etable 6). Only in the area of school education is there an obvious difference: The proportion of patients with lower school education (max. 9 years) is smaller in the MERCED study. This most likely reflects the structure of the persons insured by the recruiting health insurances (35).
eTable 6. Comparison of the MERCED sample with other IBD study samples.
| Characteristics |
PROCED (4) N = 514 |
CEDnetz (34) N = 349 |
PACED (32) N = 537 |
CEDreha (31) N = 250 |
MERCED N = 431 |
| Baseline data from the year … | 2011 | 2013 | 2011 | 2013 | 2017 |
| Recruitment via … | 1 HI | Spec. practices | Rehab clinic | Rehab clinic | 4 KK |
| Sex (female) | 55% | 60% | 66% | 61% | 57% |
| Age in years | 42 | 43 | 43 | 45 | 43 |
| School education (max. 9 years) | 13% | 21% | 22% | 17% | 12% |
| Working | 80% | 80% | 84% | 84% | 100% |
| Diagnosis (UC/CD/IC) | 50/50/0% | 44/52/3% | 48/50/2% | 51/47/2% | 45/54/2% |
| Disease duration in years | 12.9 | 12.4 | 12.6 | 12.1 | 14.0 |
| Course of disease during the preceeding years – After relapses now remission – Alternating relapse/remission – Constant/increasing activity |
30% 46% 24% |
39% 39% 22% |
11% 49% 40% |
20% 42% 38% |
13% 52% 36% |
| Use of biologic agents | 8% | 37% | 29% | 29% | 39% |
| No rehabilitation in the past 4 years | 82% | 83% | n .r. | 80% | 79% |
| Satisfaction with IBD care (0 –10) | 6.9 | 8.1 | n .r. | 6.6 | 6.3 |
| Limitation in social participation (IMET) | 18.7 | 20.6 | n .r. | 34.9 | 33.8 |
| Disease activity (GIBDI*) | 3.5 | 3.2 | 4.6 | 4.4 | 5.4 |
| Health-related quality of life (EQ-VAS) | 70.5 | 71.6 | n .r. | 60.7 | 60.0 |
| Negative employment prognosis in working people (SPE ≥ 2) | 29% | 25% | n .r. | 57% | 51% |
| Level of being informed (0–10) | 7.1 | 8.1 | n .r. | 6.6 | 6.7 |
IBD, inflammatory bowel disease; IC, indeterminate colitis; UC, ulcerative colitis; EQ-VAS, EuroQuol Visual Analogue Scale; Spec. practices, gastroenterological specialist practices;
GIBDI, German Inflammatory Bowel Disease Activity Index (*cannot be calculated for patients with stoma or indeterminate colitis); IMET, index for measuring restrictions of participation (“Index zur Messung von Einschränkungen der Teilhabe”); HI, health insurance; CD, Crohn’s disease; N, number; n .r., not recorded; SPE, subjective prognostic employment scale
In the period between T0 and T1, about 20% of the randomized insured persons were lost to follow-up; these were not included in our complete case analysis. While no evidence of bias was found in the drop-out analysis, such bias cannot be excluded. A further weakness lies in the lack of clinical confirmation of the diagnosis. Instead, two incapacity for work certificates with matching ICD coding in the health insurance data and the self-declaration of a medically confirmed IBD were required for inclusion.
In all cases, these were conditions and changes reported by the insured persons; health insurance data on incapacity for work were not available as originally planned.
For every participant, the second survey was conducted almost exactly twelve months after the first. The follow-up period after the end of medical rehabilitation, on the other hand, was not uniform in the IG. The median duration was 8 months (interquartile range: 6; 9). This is due to differences in the length of the interval between rehabilitation application and approval and start of rehabilitation.
Instead of the target of at least 60%, only 53% (112 of 211) of the IG patients participating until the end of the study completed a medical rehabilitation program (see Figure). It is therefore all the more remarkable that the analyses consistently indicated significant and relevant, albeit weak, advantages for the IG.
In summary, this pragmatic, randomized, standard care–controlled trial generated evidence on the effectiveness and additional benefit of inpatient MR in working persons with statutory health insurance suffering from IBD which we think is convincing. There is little doubt that the greater improvements observed in the IG can be attributed to MR. However, the mechanisms of action of this highly complex intervention cannot be determined with this study design. Also unanswered remains the question of the cost-effectiveness of MR. Further comparable studies on this and other indications should be conducted— including studies with controlled variation of the rehabilitation program.
Supplementary Material
eMethods section
eMethods
Study design
Since the aim of the MERCED study was to evaluate the effectiveness and benefits of medical rehabilitation (MR) under largely real-world health care conditions, it was designed as a pragmatic RCT. For classification of the chosen approach, the PRECIS-2 tool was used. This tool assesses in nine areas (including eligibility, recruitment, setting, flexibility of the intervention, primary outcome) how pragmatic (or explanatory) an RCT is (12, 13). The MERCED study attempted to increase the external validity and generalizability of the study results by involving members of various pension and health insurance funds, by not influencing the pension insurance’s review of the rehabilitation applications, and by offering rehabilitation in different rehabilitation facilities, which were also not influenced.
Study procedure
In order to recruit patients for this study, the 4 cooperating statutory health insurances (AOK Nordost, BARMER, Novitas BKK, Techniker Krankenkasse) sent out a total of more than 4000 study invitations in the first half of 2017 to insured persons with the following characteristics: working, age 18–63 years, at least two cases of incapacity for work in the past 12 months related to Crohn’s disease (MC) or ulcerative colitis (UC). Among other things, the study flyer described the role of the rehabilitative care sector and the aim of the study: “The primary aim of medical rehabilitation in a specialized clinic is to maintain or regain participation in all aspects of daily living. Those affected by illness have the opportunity there to learn how to better cope with their disease. Application is required to participate in a medical rehabilitation program. If you are employed, the costs will usually be covered by the German Pension Insurance. Many of those living with IBD wonder whether they would benefit from such a three-week rehabilitation program. To date, no study has specifically addressed this question. With the MERCED study, we aim to fill this gap.“
Those who were interested contacted the study administration directly at the University of Lübeck and were then provided with detailed written information explaining the aim and purpose of the study and the importance of randomization.
The section “What will happen in the two arms of the study“ stated: “As a member of group 1, you will receive a folder with detailed information and all documents for your rehabilitation application at the beginning of the study. If you need help, the study staff will assist you with the application process and offer telephone advice. However, the decision of the funding authority (German Pension Insurance) on your rehabilitation application will not be influenced by the MERCED study. Nevertheless, if your application is rejected, we will support you in filing an appeal. If you are assigned to Group 2, we will ask you to continue with your usual primary care and specialist treatments in medical practices or in hospital for the following twelve months. We will offer you our support in applying for a rehabilitation program only after twelve months, immediately after the second survey.“
Insurees wishing to participate in the study completed an 18-page questionnaire to collect baseline data on outcome measures and ensure fulfillment of the inclusion criteria. The questionnaire also covered treatments so far received for chronic inflammatory bowel disease (IBD). After twelve months, all participants in the study were sent a second questionnaire which, in addition to the outcome measures, included whether the person had undergone rehabilitation.
Selection of the inclusion criteria
In line with the study’s pragmatic approach, the inclusion criteria were designed to select study participants who showed the least possible difference from other patients utilizing the rehabilitative care sector. Since MR necessarily requires that the insured person submits an application, no insurees were included in the study who did not state to be in need of and willing to undergo rehabilitation. In order to make sure that an application of a study participant can be approved, insurance law requirements and criteria recommended by the German Pension Insurance (DRV) for socio-medical assessment of the need for rehabilitation in patients with IBD were also considered in the selection of the inclusion criteria (e1).
Assessment of disease activity
One of the secondary outcomes was disease activity. As in many other health services research studies on IBD, no physician-based rating of disease activity was available in the MERCED study. In order to still be able to provide insight into disease activity, the German Inflammatory Bowel Disease Activity Index (GIBDI), first presented in 2004 by a working group of the Competence Network Bowel Disease, was used. (e2). It exclusively relies on patient-reported questionnaire data and is available in 2 versions. The version designed for UC (GIBDIUC) comprises six items and covers the number of loose/liquid stools, general well-being, abdominal pain, blood in the stool, extraintestinal manifestations (in the form of joint, skin and eye involvement), and fever. The version for CD (GIBDICD) comprises seven items and covers numbers of liquid stools per day, general well-being, abdominal pain, extraintestinal manifestations (in the form of joint, skin and eye involvement), fever, fistulas (including anal abscess or fissures) as well as height and body weight. In both versions of the GIBDI, the score ranges from 0 to 18. Four categories are distinguished: remission (0–3), mild activity (4– 7), moderate activity (8– 11), strong activity (from 12). This score cannot be calculated for patients with stoma. An evaluation study found satisfactory agreement between the patient-based GIBD and physician-based disease activity indices (17).
Randomization
After study inclusion, 1:1 block randomization (block length 10) was performed, stratified by health insurance carrier. The randomization list was prepared by a study-independent scientist, using the software BiAs for Windows. It was impossible to blind study participants from knowledge of their allocation to IG or CG. Study staff responsible for the data analysis were not blinded.
No information about participation in the study was provided to the staff of the DRV who reviewed participants’ rehabilitation applications, nor to the staff of the rehabilitation clinics providing the rehabilitation programs.
Support with the rehabilitation application
Immediately following randomization, all members of the intervention group (IG) received an information pack by post. Besides general explanations, including recommendations on how to get medical rehabilitation, it comprised the current application forms with instructions for completion. Enclosed was an information sheet for the physician with the form for the medical findings report, a sample letter for the DRV with notes and sample sentences, as well as explanations on their right to individual wishes and choice together with a list of seven specialized IBD rehabilitation facilities (according to the patient organization DCCV, at least 150 IBD patients per year underwent MR in these facilities). These included the “Klinik am See” in Rüdersdorf, the “Klinik Föhrenkamp” in Mölln, the “Klinik Rosenberg” in Bad Driburg, the “Vitalisklinik” in Bad Hersfeld, the “Klinik Niederrhein” in Bad Neuenahr-Ahrweiler, the “Fachklinik Sonnenhof” in Waldachtal, and the “Klinik Hartwald” in Bad Brückenau.
All members of the IG were provided with access to an additional website containing the material from the information pack in electronic form as well as additional information about the medical rehabilitation procedure, household support and childcare during the rehabilitation stay, reimbursement of travel expenses, and co-payment exemption.
Individual counseling was available to IG members who requested it. This service was offered by two specially trained project team members who were introduced with photo and contact details (telephone and e-mail).
Beyond the above-mentioned application support, the standardized procedure for the submission and review of rehabilitation applications, approval, and conduct of the rehabilitation program was not influenced.
Following randomization, the participants in the control group (CG) continued their previous treatment without any change. They were free to apply for medical rehabilitation if they felt the need for it. After the 12-month follow-up, all of them also received the information pack and were granted access to the website and given the opportunity of a personal consultation.
Description of IBD care
In order to better understand the results on effectiveness in the healthcare context, information about the IBD care received was obtained at both points of measurement. Besides information on medication in the past three months, participants were asked about the frequency of IBD-related visits to specialist practices in the past 12 months (using a list of 16 medical specialties). Furthermore, the use of 20 different outpatient non-medical treatment and counseling services in the past 12 months was recorded (including nutritional counseling, stress management offerings). The global satisfaction with the IBD care received was evaluated using a numerical rating scale (0–10).
Participation in medical rehabilitation
At the 12-month follow-up, participants were asked to provide information about whether they had submitted a rehabilitation application and whether they had participated in a rehabilitation program in the period between the first and second survey. In addition to the name of the rehabilitation clinic visited, the respondents provided information about which treatments and/or consultations they had used during their rehabilitation program.
Calculation of sample size
Sample size planning was based on own study data (33, 34). These data showed changes over the course of one year of approximately 7 IMET points (SD = 20) for the primary outcome, “limitation of social participation“ (IMET score) (15, 16) in working insured persons regularly receiving treatment on an outpatient basis with subjective need for rehabilitation. A mean rehabilitation effect of half a standard deviation (difference in differential values between IG and CG: 10 points) was assumed. The estimate that 60% vs. 10% of participants in the IG and CG, respectively, would undergo rehabilitation, resulting in “dilution” of the expected effect, made it necessary to be able to detect a difference of 5 IMET points (effect size of 0.25) between IG and CG with a power of 80% and an alpha of 5%. Assuming 15% loss to follow-up, the calculated sample size for IG and CG is 300 persons each (calculation using BiAS 8.1) (e3).
Data analysis
Questionnaire data were entered in Access 2010 input masks and analyzed using the statistical software package IBM SPSS Version 22.
Imputation of missing cases was not included in the study protocol. The primary analysis was designed to evaluate the participants in both surveys independently of the reported (non-) participation in an MR program during the study period in the study arm assigned by randomization. In the study protocol, this analysis was referred to as the intention-to-treat (ITT) analysis. However, there are inconsistencies in the definition of the term ITT (e4). Stricter definitions require imputation of missing values before an ITT analysis can be performed (e5). To avoid misunderstandings, we decided to follow the narrower definition of ITT for the publication. Consequently, the planned primary analysis was referred to as the complete case analysis (CCA).
In order to estimate the influence of imputation strategies on the results, an ITT analysis was performed in addition to the CCA as part of a sensitivity analysis for the primary outcome. Missing cases were replaced using various imputation methods (e6).
The “last observation carried forward” (LOCF) method was used for direct replacement: a drop-out–related missing value in the follow-up survey was replaced by the corresponding value of the baseline measurement.
Another simple imputation strategy replaced the missing values in a worst/best or best/worst scenario. For the worst/best scenario, missing cases in the IG were replaced by the mean change observed in non-rehabilitants in the CG (worst) and the missing cases in the CG were replaced by the mean changes observed in the IG rehabilitants (best). For the best/worst scenario, the missing cases in the IG were replaced by the mean change observed in rehabilitants in the IG (best) and the missing cases in the CG were replaced by the mean change observed in non-rehabilitants in the CG (worst).
The Markov chain Monte Carlo (MCMC) method was used for multiple imputation. Based on sociodemographic data and the available outcomes, 50 different complete data sets were generated and combined into a pooled data set.
For the 12 secondary outcomes measures, an alpha-error adjustment using the Bonferroni correction was performed and the 5% significance level was divided by 12. The resulting adjusted significance level was 0.4% (24).
The t-test was used as a null-hypothesis significance test, irrespective of the result of the test for normal distribution of the continuous outcome measures. This test is considered to be particularly robust against violation of the normality assumption, especially with larger samples and differential values (e7, e8). Compared to the Mann–Whitney U test, a nonparametric statistical significance test, the t-test represents the more conservative testing approach.
Odds ratios were calculated as effect sizes for dichotomous outcomes; for continuous outcomes, Cohen’s d (Hedges’ g) was calculated in case of different sample sizes. For this purpose, differences between IG and CG in the mean changes between the two points of measurement are standardized based on the pooled standard deviation. This calculation was performed using the freeware of Psychometrika (https://www.psychometrica.de/effektstaerke.html) (e9).
Clinical relevance of effects
The minimal clinically important difference (MCID) for the primary outcome was defined as a change in IMET of ten points (= half a standard deviation) (e10). For the secondary outcome of disease activity, considered particularly important by the patient advisory board, a GIBDI score of less than 3 (= remission) was considered a clinically relevant event in the follow-up survey. The scientific advisory board tightened this criterion ex post by proposing to use steroid-free remission as a criterion for relevance.
Participatory research
Already in the study planning phase, the German Crohn’s Disease/Ulcerative Colitis Association (DCCV) e.V.—the largest patient organization for people living with IBD in Germany—was involved in the project and sent a member to the scientific project advisory board. Additional members of the scientific project advisory board included 4 rehabilitation scientists, two gastroenterologists, two clinical epidemiologists, and one DRV representative. Besides this scientific project advisory board, an eight-member patient advisory board supported the project over the entire study period. According to the 4-step model by Sweeney and Morgan, the intensity of involvement ranged from 1 (consultation) to 3 (collaboration) (14, e11). All project materials (e.g. flyers, cover letters, study information, reminders, questionnaires) were critically reviewed by the patient advisory board. The three-stage counseling plan for rehabilitation application support was jointly developed; the information pack used for this purpose and the information website were improved. The selection of the outcome measures was made in mutual agreement; the significance of the results was discussed in a separate meeting.
eResults
Review of inclusion criteria
758 insured persons sent a completed questionnaire to the study administration. These questionnaires were used to review the inclusion criteria. 228 persons were not included in the study. The reasons for this were (in the frequency of their occurrence):
119 of the 228 excluded persons (52%) did not meet more than one of the eligibility requirements.
Rehabilitation application, rehabilitation facility
Of the 211 participants in the IG responding to the follow-up survey, 76 reported that they had not submitted an application. Among the 135 applicants, there were two persons who had submitted their applications only a short while before the follow-up survey and had not yet received a decision. Nineteen applications were definitively rejected by the funding authority. At 14%, the rejection rate was below the general DRV rejection rate of 17% (2).
Of the 112 of the 211 IG participants who took part in medical rehabilitation during the observation period, 21 (19%) did not visit any of the facilities we recommended. Five of them had chosen another clinic for themselves, another five had not used their right of choice. Due to a lack of information, it remains unclear for which reasons on the part of the funding agency the wishes of the remaining eleven rehabilitants were not realized.
Additional ITT analysis for primary outcomes
The robustness of the positive results found with CCA for the limitation in social participation (primary outcome) was assessed using an ITT analysis.
The summary of the results of this analysis, using different imputation methods (such as LOCF or multiple imputation), confirms the observation of a statistically significant advantage of the IG over the CG (etable 2).
Lack of subjective rehabilitation need (74%)
No possibility to undergo rehabilitation in the near future (31%)
Rehabilitation participation in the past 2 years (28%)
Problem field assessment shows no current psychosocial problem field (27%)
Currently not working (8%)
Missing informed consent (3%)
No confirmation of IBD diagnosis (1%)
Age >63 years (1%)
Not DRV-insured (1%).
Table 2. Sample characteristics at baseline (complete cases: N = 431).
| Patient characteristics | Total | IG = 211 | CG = 220 | ||||
| valid N | n (%)/M (SD) | validN | n (%)/M (SD) | validN | n (%)/M (SD) | ||
| Sex | Female | 431 | 246 (57.1%) | 211 | 126 (59.7%) | 220 | 120 (54.5%) |
| Age in years | 18–30 | 431 | 75 (17.4%) | 211 | 33 (15.6%) | 220 | 42 (19.1%) |
| 31–40 | 92 (21.3%) | 40 (19.0%) | 52 (23.6%) | ||||
| 41–50 | 116 (26.9%) | 56 (26.5%) | 60 (27.3%) | ||||
| 51–64 | 148 (34.3%) | 82 (38.9%) | 66 (30.0%) | ||||
| Employment status | Working, yes | 431 | 431 (100%) | 211 | 211 (100%) | 220 | 220 (100%) |
| Full-time | 326 (75.6%) | 155 (73.5%) | 171 (77.7%) | ||||
| School education (years) | Up to 9 | 429 | 52 (12.1%) | 211 | 27 (12.8%) | 218 | 25 (11.5%) |
| 10–11 | 188 (43.8%) | 96 (45.5%) | 92 (42.2%) | ||||
| 12–13 | 189 (44.1%) | 88 (41.7%) | 101 (46.3%) | ||||
| Residential region | Core cities | 430 | 136 (31.6%) | 211 | 63 (29.9%) | 219 | 73 (33.3%) |
| Dense surrounding areas | 182 (42.3%) | 90 (42.7%) | 92 (42.0%) | ||||
| Rural surrounding areas | 60 (14.0%) | 32 (15.2%) | 28 (12.8%) | ||||
| Rural areas | 52 (12.1%) | 26 (12.3%) | 26 (11.9%) | ||||
| Diagnosis | Crohn’s disease | 431 | 229 (53.1%) | 211 | 120 (56.9%) | 220 | 109 (49.5%) |
| Ulcerative colitis | 192 (44.5%) | 86 (40.8%) | 106 (48.2%) | ||||
| Indeterminate colitis | 10 (2.3%) | 5 (2.4%) | 5 (2.3%) | ||||
| Disease duration | In years | 426 | 14.0 (10.2) | 206 | 15.0 (10.6) | 220 | 13.1 (9.7) |
| Course of disease “during the preceeding years“ | In remission | 423 | 53 (12.5%) | 207 | 25 (12.1%) | 216 | 28 (13.0%) |
| Alternating relapse/remission | 218 (51.5%) | 105 (50.7%) | 113 (52.3%) | ||||
| Increasing/continuous activity | 152 (35.9%) | 77 (37.2%) | 75 (34.7%) | ||||
| Disease activity during the past 7 days (GIBDI*) | None (0– 3) | 394 | 113 (28.7%) | 193 | 56 (29.0%) | 201 | 57 (28.4%) |
| Mild (4– 7) | 187 (47.5%) | 90 (46.6%) | 97 (48.3%) | ||||
| Moderate (8– 11) | 83 (21.1%) | 42 (21.8%) | 41 (20.4%) | ||||
| Severe (≥ 12) | 11 (2.8%) | 5 (2.6%) | 6 (3.0%) | ||||
| Surgery for IBD | Ever (yes) | 429 | 134 (31.2%) | 209 | 66 (31.6%) | 220 | 68 (30.9%) |
| Rehabilitation for IBD | Ever (yes) | 431 | 173 (40.1%) | 211 | 89 (42.2%) | 220 | 84 (38.2%) |
| Medication intake in the preceeding 3 months |
5-aminosalicylates | 431 | 231 (53.6%) | 211 | 114 (54.0%) | 220 | 117 (53.2%) |
| Corticosteroids | 184 (42.7%) | 92 (43.6%) | 92 (41.8%) | ||||
| Immunosuppressants | 115 (26.7%) | 54 (25.6%) | 61 (27.7%) | ||||
| Biologic agents | 166 (38.5%) | 82 (38.9%) | 84 (38.2%) | ||||
| None | 16 (3.7%) | 6 (2.8%) | 10 (4.5%) | ||||
| Number of IBD-related specialist visits | Past 12 months | 431 | 18.6 (16.2) | 211 | 19.1 (16.0) | 220 | 18.1 (16.4) |
| Satisfaction with IBD care | NRS (0 = not satisfied at all; 10 = very satisfied) | 430 | 6.3 (2.5) | 211 | 6.2 (2.4) | 219 | 6.3 (2.5) |
IBD, inflammatory bowel disease; GIBDI, German Inflammatory Bowel Disease Activity Index (*cannot be calculated for patients with stoma or indeterminate colitis); IG, intervention group; CG, control group; M, mean; N, number of valid cases; NRS, numerical rating scale; SD, standard deviation
Key messages.
In Germany, the practical feasibility, legal admissibility, and ethical justifiability of randomized controlled trials on the absolute effectiveness of inpatient medical rehabilitation has repeatedly been doubted. In 2014, the German Advisory Council on the Assessment of Developments in the Health Care System stressed the “lack of evidence base“ as a “fundamental problem“ in this service sector.
MERCED, a randomized controlled trial, removes these doubts and provides evidence for the effectiveness and benefits of an (on average) three-week inpatient medical rehabilitation for working persons with statutory health insurance living with chronic inflammatory bowel disease (IBD).
IBD patients, the majority of whom participated in medical rehabilitation at specialized IBD clinics, showed superior improvements in clinical and psychological outcomes after twelve months compared to IBD patients receiving standard care.
Medical rehabilitation seems to be a neglected health care option and should be included in IBD-specific guidelines.
Acknowledgments
Acknowledgement
We thank the statutory health insurances AOK-Nordost, BARMER, Novitas BKK, and TK as well as their insurees for their willingness to support this health services research project by participating in the study. Our thanks also go to the project and patient advisory boards for their helpful support of this study project.
Data sharing
Scientists who submit a methodologically useful analysis proposal may, after anonymization, be provided with individual participant data (including metadata) on which the results presented in this paper are based. Three months after publication, these data will be available for a period of 3 years. Interested researchers may contact the first author.
Footnotes
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Translated from the original German by Ralf Thoene, MD.
References
- 1.Deutsche Rentenversicherung (eds.) Deutsche Rentenversicherung Bund. Berlin: 2019. Statistik der DRV. Rehabilitation 2018. Band 216. [Google Scholar]
- 2.Deutsche Rentenversicherung. H. Heenemann GmbH & Co. KG. Berlin: 2018. Reha-Bericht 2018. [Google Scholar]
- 3.Raspe H. Chronische Erkrankungen. Definition und Verständnis. Bundesgesundheitsbl. 2011;54:4–8. doi: 10.1007/s00103-010-1180-2. [DOI] [PubMed] [Google Scholar]
- 4.Hüppe A, Langbrandtner J, Raspe H. Komplexe psychosoziale Problemlagen bei Morbus Crohn und Colitis ulcerosa - Fragebogengestütztes Assessment als erster Schritt zur Aktivierung von Patientinnen und Patienten. Z Gastroenterol. 2013;51:257–270. doi: 10.1055/s-0032-1325354. [DOI] [PubMed] [Google Scholar]
- 5.Mittag O, Welti F. Medizinische Rehabilitation im europäischen Vergleich und Auswirkungen des europäischen Rechts auf die deutsche Rehabilitation. Bundesgesundheitsbl. 2017;60:378–385. doi: 10.1007/s00103-017-2516-y. [DOI] [PubMed] [Google Scholar]
- 6.Negrini S, Levack W, Gimigliano F, Arienti C, Villafane JH, Kiekens C. The struggle for evidence in physical and rehabilitation medicine: publication rate of randomized controlled trials and systematic reviews is growing more than in other therapeutic fields. Am J Phys Med Rehabil. 2019;98:258–265. doi: 10.1097/PHM.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 7.Sachverständigenrat (SVR) Gesundheitswesen (eds.) Hans Huber. Bern: 2014. Bedarfsgerechte Versorgung - Perspektiven für ländliche Regionen und ausgewählte Leistungsbereiche. [Google Scholar]
- 8.Hüppe A, Glaser-Möller N, Raspe H. Trägerübergreifendes Projekt zur Früherkennung von Rehabilitationsbedarf bei Versicherten mit muskuloskelettalen Beschwerden durch Auswertung von Arbeitsunfähigkeitsdaten: Ergebnisse einer randomisierten, kontrollierten Evaluationsstudie. Gesundheitswesen. 2006;68:347–356. doi: 10.1055/s-2006-926870. [DOI] [PubMed] [Google Scholar]
- 9.Hüppe A, Parow D, Raspe H. Wirksamkeit und Nutzen eines Screeningverfahrens zur Identifikation von rehabilitationsbedürftigen Personen mit Diabetes mellitus Typ 2: Eine randomisierte, kontrollierte Evaluationsstudie unter Versicherten der Hamburg Münchener Krankenkasse. Gesundheitswesen. 2008;70:590–599. doi: 10.1055/s-0028-1086005. [DOI] [PubMed] [Google Scholar]
- 10.Mittag O, Döbler A, Pollmann H, Farin-Glattacker E, Raspe H. Praktikabilität und Nutzen eines aktiven Screenings auf Rehabedarf mit anschließender schriftlicher Beratung zur Rehaantragstellung bei AOK-Versicherten im Disease-Management-Programm Diabetes Typ 2 (PARTID-Studie) Rehabilitation. 2014;53:313–320. doi: 10.1055/s-0034-1370984. [DOI] [PubMed] [Google Scholar]
- 11.Schlademann S, Hüppe A, Raspe H. Ergebnisse einer randomisierten kontrollierten Studie zur Akzeptanz und zu Outcomes einer Beratung auf stationäre medizinische Rehabilitation unter erwerbstätigen GKV-Versicherten mit rheumatoider Arthritis. Gesundheitswesen. 2007;69:325–335. doi: 10.1055/s-2007-982519. [DOI] [PubMed] [Google Scholar]
- 12.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 13.Loudon K, Trewek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350 doi: 10.1136/bmj.h2147. h2147. [DOI] [PubMed] [Google Scholar]
- 14.Kirschning S, Pimmer V, Matzat J, Brüggemann S, Buschmann-Steinhage R. Beteiligung Betroffener an der Forschung. Rehabilitation. 2012;51:12–20. doi: 10.1055/s-0032-1327728. [DOI] [PubMed] [Google Scholar]
- 15.Deck R. Begel J, Wirtz M, Zwingmann C, editors. IMET Index zur Messung von Einschränkungen der Teilhabe Diagnostische Verfahren in der Rehabilitation. Göttingen: Hogrefe. 2008:372–374. [Google Scholar]
- 16.Deck R, Walther A, Staupendahl A, Katalinic A. Einschränkung der Teilhabe in der Bevölkerung - Normdaten für den IMET auf der Basis eines Bevölkerungssurveys in Norddeutschland. Rehabilitation. 2015;54:402–408. doi: 10.1055/s-0035-1559670. [DOI] [PubMed] [Google Scholar]
- 17.Hüppe A, Langbrandtner J, Häuser W, Raspe H, Bokemeyer B. Validation of the „German Inflammatory Bowel Disease Activity Index (GIBDI)“: an instrument for patient-based disease activity assessment in Crohn‘s Disease and Ulcerative Colitis. Z Gastroenterol. 2018;56:1267–1275. doi: 10.1055/a-0605-4080. [DOI] [PubMed] [Google Scholar]
- 18.Schuler M, Musekamp G, Faller H, et al. Assessment of proximal outcomes of self-management programs: translation and psychometric evaluation of a german version of the health education impact questionnaire (heiQ) Qual Life Res. 2013;22:1391–1403. doi: 10.1007/s11136-012-0268-6. [DOI] [PubMed] [Google Scholar]
- 19.Osborne RH, Elsworth GR, Whitfield K. The health education impact questionnaire (heiQ): an outcomes and evaluation measure for patient education and self-management interventions for people with chronic conditions. Patient Educ Couns. 2007;66:192–201. doi: 10.1016/j.pec.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Löwe B, Wahl I, Rose M, et al. A 4 item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010;122:86–95. doi: 10.1016/j.jad.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Morfeld M, Kirchberger I, Bullinger M. SF-36 Fragebogen zum Gesundheitszustand: deutsche Version des Short Form-36 Health Survey. Göttingen: Hogrefe. 2011 [Google Scholar]
- 22.The EuroQol Group. EuroQol - a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 23.Mittag O, Raspe H. Eine kurze Skala zur Messung der subjektiven Prognose der Erwerbstätigkeit: Ergebnisse einer Untersuchung an 4279 Mitgliedern der gesetzlichen Arbeiterrentenversicherung zu Reliabilität (Guttman-Skalierung) und Validität der Skala. Rehabilitation. 2003;42:169–174. doi: 10.1055/s-2003-40095. [DOI] [PubMed] [Google Scholar]
- 24.Victor A, Elsäßer A, Hommel G, Blettner M. Judging a plethora of p-values: how to contend with the problem of multiple testing—part 10 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2010;107:50–56. doi: 10.3238/arztebl.2009.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buschmann-Steinhage R, Brüggemann S. Veränderungstrends in der medizinischen Rehabilitation der gesetzlichen Rentenversicherung. Bundesgesundheitsbl - Gesundheitsforsch - Gesundheitsschutz. 2011;54:404–410. doi: 10.1007/s00103-011-1240-2. [DOI] [PubMed] [Google Scholar]
- 26.Beck M, Löffler H, editors. Rehabilitation heute: die Rehastudie Baden. Karlsruhe. G. Braun. 1984 [Google Scholar]
- 27.Gerdes N, Weidemann H, Jäckel WH, editors. Die Protos-Studie. Ergebnisqualität stationärer Rehabilitation in 15 Kliniken. Darmstadt: Steinkopff. 2000 [Google Scholar]
- 28.Haaf HG. DRV-Bund. Berlin: 2009. Reha-Erfolg - Ist die Reha überhaupt wirksam? In: Ergebnisqualität in der medizinischen Rehabilitation der Rentenversicherung; 33 pp. [Google Scholar]
- 29.Schultz K, Seidl H, Jelusic D, et al. Effectiveness of pulmonary rehabilitation for patients with asthma: study protocol of a randomized controlled trial (EPRA) BMC Pulm Med. 2017;17 doi: 10.1186/s12890-017-0389-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jäckel WH, Cziske R, Gerdes N, Jacobi E. Überprüfung der Wirksamkeit stationärer Rehabilitationsmaßnahmen bei Patienten mit chronischen Rückenschmerzen: eine prospektive, randomisierte, kontrollierte Studie. Rehabiltiation. 1990;29:129–133. [PubMed] [Google Scholar]
- 31.Hüppe A, Steimann G, Janotta M, et al. Auf dem Prüfstand: stationäre medizinische Rehabilitation bei chronisch entzündlichen Darmerkrankungen. Rehabilitation. 2016;55:248–255. doi: 10.1055/s-0042-109590. [DOI] [PubMed] [Google Scholar]
- 32.Reusch A, Weiland R, Gerlich C, et al. Self-management education for rehabilitation inpatients suffering from inflammatory bowel disease: a cluster-randomized controlled trial. Health Educ Res. 2016;31:782–791. doi: 10.1093/her/cyw042. [DOI] [PubMed] [Google Scholar]
- 33.Hüppe A, Langbrandtner J, Raspe H. Inviting patients with inflammatory bowel disease to active involvement in their own care: a randomized controlled trial. Inflamm Bowel Dis. 2014;20:1057–1069. doi: 10.1097/MIB.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 34.Langbrandtner J, Hüppe A, Jessen P, et al. Quality of care in inflammatory bowel disease: results of a prospective controlled cohort study in Germany (NETIBD) Clin Exp Gastroenterol. 2017;10:215–227. doi: 10.2147/CEG.S135346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoffmann F, Koller D. Verschiedene Regionen, verschiedene Versichertenpopulationen? Soziodemografische und gesundheitsbezogene Unterschiede zwischen Krankenkassen. Gesundheitswesen. 2017;79:e1–e9. doi: 10.1055/s-0035-1564074. [DOI] [PubMed] [Google Scholar]
- E1.Deutsche Rentenversicherung. Leitlinien für die sozialmedizinische Begutachtung für Stoffwechsel und gastroenterologische Erkrankungen sowie Adipositas. 2011. https://deutsche-rentenversicherung.de/SharedDocs/Downloads/DE/Experten/infos_fuer_aerzte/begutachtung/leitlinien_rehabeduerftigkeit_stoffwechsel_langfassung_pdf.pdf;jsession id=FE2BCAAED5D7A05E405C6C2AE6773B09.delivery2-7-replication?__blob=publicationFile&v=1 (last accessed on 12 November 2019) [Google Scholar]
- E2.Janke KH, Raible A, Bauer M, et al. Questions on life satisfaction (FLZM) inflammatory bowel disease. Int J Colorectal Dis. 2004;19:343–353. doi: 10.1007/s00384-003-0522-z. [DOI] [PubMed] [Google Scholar]
- E3.Ackermann H. BiAS für Windows. https://www.bias-online.de/ (last accessed on 12 November 2019) [Google Scholar]
- E4.Alshurafa M, Briel M, Akl EA, et al. Inconsistent definitions for intention-to-treat in relation to missing outcome data: systematic review of the methods literature. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049163. e49163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Kabisch M, Ruckes C, Seibert-Grafe M, et al. Randomized controlled trials: part 17 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2011;108:663–668. doi: 10.3238/arztebl.2011.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E6.Jakobsen JC, Gluud C, Wetterslev J, Winkel P. When and how should multiple imputation be used for handling missing data in randomised clinical trials - a practical guide with flowcharts. BMC Med Res Methodol. 2017;17 doi: 10.1186/s12874-017-0442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Edgell SE, Noon SM. Effect of violation of normality on the t test of the correlation coefficient. Psychological Bulletin. 1984;95:576–583. [Google Scholar]
- E8.Bortz J, Schuster C. Statistik für Human und Sozialwissenschaftler. Berlin: Springer. 2010 [Google Scholar]
- E9.Lenhard W, Lenhard A. Calculation of effect sizes. https://www.psychometrica.de/effect_size.html (last accessed on 12 November 2019) [Google Scholar]
- E10.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- E11.Farin-Glattacker E, Kirschning S, Meyer T, Buschmann-Steinhage R. Partizipation an der Forschung - eine Matrix zur Orientierung Stellungnahme des Ausschuss „Reha-Forschung“ der Deutschen Vereinigung für Rehabilitation (DVfR) und der Deutschen Gesellschaft für Rehabilitationswissenschaften (DGRW) www.dvfr.de/fileadmin/user_upload/DVfR/Downloads/Fachausschuesse/Forschung/Partizipation_an_der_Forschung_%E2%80%93_ eine_Matrix_zur_Orientierung.pdf (last accessed on 12 November 2019) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods section
eMethods
Study design
Since the aim of the MERCED study was to evaluate the effectiveness and benefits of medical rehabilitation (MR) under largely real-world health care conditions, it was designed as a pragmatic RCT. For classification of the chosen approach, the PRECIS-2 tool was used. This tool assesses in nine areas (including eligibility, recruitment, setting, flexibility of the intervention, primary outcome) how pragmatic (or explanatory) an RCT is (12, 13). The MERCED study attempted to increase the external validity and generalizability of the study results by involving members of various pension and health insurance funds, by not influencing the pension insurance’s review of the rehabilitation applications, and by offering rehabilitation in different rehabilitation facilities, which were also not influenced.
Study procedure
In order to recruit patients for this study, the 4 cooperating statutory health insurances (AOK Nordost, BARMER, Novitas BKK, Techniker Krankenkasse) sent out a total of more than 4000 study invitations in the first half of 2017 to insured persons with the following characteristics: working, age 18–63 years, at least two cases of incapacity for work in the past 12 months related to Crohn’s disease (MC) or ulcerative colitis (UC). Among other things, the study flyer described the role of the rehabilitative care sector and the aim of the study: “The primary aim of medical rehabilitation in a specialized clinic is to maintain or regain participation in all aspects of daily living. Those affected by illness have the opportunity there to learn how to better cope with their disease. Application is required to participate in a medical rehabilitation program. If you are employed, the costs will usually be covered by the German Pension Insurance. Many of those living with IBD wonder whether they would benefit from such a three-week rehabilitation program. To date, no study has specifically addressed this question. With the MERCED study, we aim to fill this gap.“
Those who were interested contacted the study administration directly at the University of Lübeck and were then provided with detailed written information explaining the aim and purpose of the study and the importance of randomization.
The section “What will happen in the two arms of the study“ stated: “As a member of group 1, you will receive a folder with detailed information and all documents for your rehabilitation application at the beginning of the study. If you need help, the study staff will assist you with the application process and offer telephone advice. However, the decision of the funding authority (German Pension Insurance) on your rehabilitation application will not be influenced by the MERCED study. Nevertheless, if your application is rejected, we will support you in filing an appeal. If you are assigned to Group 2, we will ask you to continue with your usual primary care and specialist treatments in medical practices or in hospital for the following twelve months. We will offer you our support in applying for a rehabilitation program only after twelve months, immediately after the second survey.“
Insurees wishing to participate in the study completed an 18-page questionnaire to collect baseline data on outcome measures and ensure fulfillment of the inclusion criteria. The questionnaire also covered treatments so far received for chronic inflammatory bowel disease (IBD). After twelve months, all participants in the study were sent a second questionnaire which, in addition to the outcome measures, included whether the person had undergone rehabilitation.
Selection of the inclusion criteria
In line with the study’s pragmatic approach, the inclusion criteria were designed to select study participants who showed the least possible difference from other patients utilizing the rehabilitative care sector. Since MR necessarily requires that the insured person submits an application, no insurees were included in the study who did not state to be in need of and willing to undergo rehabilitation. In order to make sure that an application of a study participant can be approved, insurance law requirements and criteria recommended by the German Pension Insurance (DRV) for socio-medical assessment of the need for rehabilitation in patients with IBD were also considered in the selection of the inclusion criteria (e1).
Assessment of disease activity
One of the secondary outcomes was disease activity. As in many other health services research studies on IBD, no physician-based rating of disease activity was available in the MERCED study. In order to still be able to provide insight into disease activity, the German Inflammatory Bowel Disease Activity Index (GIBDI), first presented in 2004 by a working group of the Competence Network Bowel Disease, was used. (e2). It exclusively relies on patient-reported questionnaire data and is available in 2 versions. The version designed for UC (GIBDIUC) comprises six items and covers the number of loose/liquid stools, general well-being, abdominal pain, blood in the stool, extraintestinal manifestations (in the form of joint, skin and eye involvement), and fever. The version for CD (GIBDICD) comprises seven items and covers numbers of liquid stools per day, general well-being, abdominal pain, extraintestinal manifestations (in the form of joint, skin and eye involvement), fever, fistulas (including anal abscess or fissures) as well as height and body weight. In both versions of the GIBDI, the score ranges from 0 to 18. Four categories are distinguished: remission (0–3), mild activity (4– 7), moderate activity (8– 11), strong activity (from 12). This score cannot be calculated for patients with stoma. An evaluation study found satisfactory agreement between the patient-based GIBD and physician-based disease activity indices (17).
Randomization
After study inclusion, 1:1 block randomization (block length 10) was performed, stratified by health insurance carrier. The randomization list was prepared by a study-independent scientist, using the software BiAs for Windows. It was impossible to blind study participants from knowledge of their allocation to IG or CG. Study staff responsible for the data analysis were not blinded.
No information about participation in the study was provided to the staff of the DRV who reviewed participants’ rehabilitation applications, nor to the staff of the rehabilitation clinics providing the rehabilitation programs.
Support with the rehabilitation application
Immediately following randomization, all members of the intervention group (IG) received an information pack by post. Besides general explanations, including recommendations on how to get medical rehabilitation, it comprised the current application forms with instructions for completion. Enclosed was an information sheet for the physician with the form for the medical findings report, a sample letter for the DRV with notes and sample sentences, as well as explanations on their right to individual wishes and choice together with a list of seven specialized IBD rehabilitation facilities (according to the patient organization DCCV, at least 150 IBD patients per year underwent MR in these facilities). These included the “Klinik am See” in Rüdersdorf, the “Klinik Föhrenkamp” in Mölln, the “Klinik Rosenberg” in Bad Driburg, the “Vitalisklinik” in Bad Hersfeld, the “Klinik Niederrhein” in Bad Neuenahr-Ahrweiler, the “Fachklinik Sonnenhof” in Waldachtal, and the “Klinik Hartwald” in Bad Brückenau.
All members of the IG were provided with access to an additional website containing the material from the information pack in electronic form as well as additional information about the medical rehabilitation procedure, household support and childcare during the rehabilitation stay, reimbursement of travel expenses, and co-payment exemption.
Individual counseling was available to IG members who requested it. This service was offered by two specially trained project team members who were introduced with photo and contact details (telephone and e-mail).
Beyond the above-mentioned application support, the standardized procedure for the submission and review of rehabilitation applications, approval, and conduct of the rehabilitation program was not influenced.
Following randomization, the participants in the control group (CG) continued their previous treatment without any change. They were free to apply for medical rehabilitation if they felt the need for it. After the 12-month follow-up, all of them also received the information pack and were granted access to the website and given the opportunity of a personal consultation.
Description of IBD care
In order to better understand the results on effectiveness in the healthcare context, information about the IBD care received was obtained at both points of measurement. Besides information on medication in the past three months, participants were asked about the frequency of IBD-related visits to specialist practices in the past 12 months (using a list of 16 medical specialties). Furthermore, the use of 20 different outpatient non-medical treatment and counseling services in the past 12 months was recorded (including nutritional counseling, stress management offerings). The global satisfaction with the IBD care received was evaluated using a numerical rating scale (0–10).
Participation in medical rehabilitation
At the 12-month follow-up, participants were asked to provide information about whether they had submitted a rehabilitation application and whether they had participated in a rehabilitation program in the period between the first and second survey. In addition to the name of the rehabilitation clinic visited, the respondents provided information about which treatments and/or consultations they had used during their rehabilitation program.
Calculation of sample size
Sample size planning was based on own study data (33, 34). These data showed changes over the course of one year of approximately 7 IMET points (SD = 20) for the primary outcome, “limitation of social participation“ (IMET score) (15, 16) in working insured persons regularly receiving treatment on an outpatient basis with subjective need for rehabilitation. A mean rehabilitation effect of half a standard deviation (difference in differential values between IG and CG: 10 points) was assumed. The estimate that 60% vs. 10% of participants in the IG and CG, respectively, would undergo rehabilitation, resulting in “dilution” of the expected effect, made it necessary to be able to detect a difference of 5 IMET points (effect size of 0.25) between IG and CG with a power of 80% and an alpha of 5%. Assuming 15% loss to follow-up, the calculated sample size for IG and CG is 300 persons each (calculation using BiAS 8.1) (e3).
Data analysis
Questionnaire data were entered in Access 2010 input masks and analyzed using the statistical software package IBM SPSS Version 22.
Imputation of missing cases was not included in the study protocol. The primary analysis was designed to evaluate the participants in both surveys independently of the reported (non-) participation in an MR program during the study period in the study arm assigned by randomization. In the study protocol, this analysis was referred to as the intention-to-treat (ITT) analysis. However, there are inconsistencies in the definition of the term ITT (e4). Stricter definitions require imputation of missing values before an ITT analysis can be performed (e5). To avoid misunderstandings, we decided to follow the narrower definition of ITT for the publication. Consequently, the planned primary analysis was referred to as the complete case analysis (CCA).
In order to estimate the influence of imputation strategies on the results, an ITT analysis was performed in addition to the CCA as part of a sensitivity analysis for the primary outcome. Missing cases were replaced using various imputation methods (e6).
The “last observation carried forward” (LOCF) method was used for direct replacement: a drop-out–related missing value in the follow-up survey was replaced by the corresponding value of the baseline measurement.
Another simple imputation strategy replaced the missing values in a worst/best or best/worst scenario. For the worst/best scenario, missing cases in the IG were replaced by the mean change observed in non-rehabilitants in the CG (worst) and the missing cases in the CG were replaced by the mean changes observed in the IG rehabilitants (best). For the best/worst scenario, the missing cases in the IG were replaced by the mean change observed in rehabilitants in the IG (best) and the missing cases in the CG were replaced by the mean change observed in non-rehabilitants in the CG (worst).
The Markov chain Monte Carlo (MCMC) method was used for multiple imputation. Based on sociodemographic data and the available outcomes, 50 different complete data sets were generated and combined into a pooled data set.
For the 12 secondary outcomes measures, an alpha-error adjustment using the Bonferroni correction was performed and the 5% significance level was divided by 12. The resulting adjusted significance level was 0.4% (24).
The t-test was used as a null-hypothesis significance test, irrespective of the result of the test for normal distribution of the continuous outcome measures. This test is considered to be particularly robust against violation of the normality assumption, especially with larger samples and differential values (e7, e8). Compared to the Mann–Whitney U test, a nonparametric statistical significance test, the t-test represents the more conservative testing approach.
Odds ratios were calculated as effect sizes for dichotomous outcomes; for continuous outcomes, Cohen’s d (Hedges’ g) was calculated in case of different sample sizes. For this purpose, differences between IG and CG in the mean changes between the two points of measurement are standardized based on the pooled standard deviation. This calculation was performed using the freeware of Psychometrika (https://www.psychometrica.de/effektstaerke.html) (e9).
Clinical relevance of effects
The minimal clinically important difference (MCID) for the primary outcome was defined as a change in IMET of ten points (= half a standard deviation) (e10). For the secondary outcome of disease activity, considered particularly important by the patient advisory board, a GIBDI score of less than 3 (= remission) was considered a clinically relevant event in the follow-up survey. The scientific advisory board tightened this criterion ex post by proposing to use steroid-free remission as a criterion for relevance.
Participatory research
Already in the study planning phase, the German Crohn’s Disease/Ulcerative Colitis Association (DCCV) e.V.—the largest patient organization for people living with IBD in Germany—was involved in the project and sent a member to the scientific project advisory board. Additional members of the scientific project advisory board included 4 rehabilitation scientists, two gastroenterologists, two clinical epidemiologists, and one DRV representative. Besides this scientific project advisory board, an eight-member patient advisory board supported the project over the entire study period. According to the 4-step model by Sweeney and Morgan, the intensity of involvement ranged from 1 (consultation) to 3 (collaboration) (14, e11). All project materials (e.g. flyers, cover letters, study information, reminders, questionnaires) were critically reviewed by the patient advisory board. The three-stage counseling plan for rehabilitation application support was jointly developed; the information pack used for this purpose and the information website were improved. The selection of the outcome measures was made in mutual agreement; the significance of the results was discussed in a separate meeting.
eResults
Review of inclusion criteria
758 insured persons sent a completed questionnaire to the study administration. These questionnaires were used to review the inclusion criteria. 228 persons were not included in the study. The reasons for this were (in the frequency of their occurrence):
119 of the 228 excluded persons (52%) did not meet more than one of the eligibility requirements.
Rehabilitation application, rehabilitation facility
Of the 211 participants in the IG responding to the follow-up survey, 76 reported that they had not submitted an application. Among the 135 applicants, there were two persons who had submitted their applications only a short while before the follow-up survey and had not yet received a decision. Nineteen applications were definitively rejected by the funding authority. At 14%, the rejection rate was below the general DRV rejection rate of 17% (2).
Of the 112 of the 211 IG participants who took part in medical rehabilitation during the observation period, 21 (19%) did not visit any of the facilities we recommended. Five of them had chosen another clinic for themselves, another five had not used their right of choice. Due to a lack of information, it remains unclear for which reasons on the part of the funding agency the wishes of the remaining eleven rehabilitants were not realized.
Additional ITT analysis for primary outcomes
The robustness of the positive results found with CCA for the limitation in social participation (primary outcome) was assessed using an ITT analysis.
The summary of the results of this analysis, using different imputation methods (such as LOCF or multiple imputation), confirms the observation of a statistically significant advantage of the IG over the CG (etable 2).
Lack of subjective rehabilitation need (74%)
No possibility to undergo rehabilitation in the near future (31%)
Rehabilitation participation in the past 2 years (28%)
Problem field assessment shows no current psychosocial problem field (27%)
Currently not working (8%)
Missing informed consent (3%)
No confirmation of IBD diagnosis (1%)
Age >63 years (1%)
Not DRV-insured (1%).