Abstract
Extracellular vesicles (EVs), including microvesicles and exosomes, are nano- to micron-sized vesicles, which may deliver bioactive cargos that include lipids, growth factors and their receptors, proteases, signaling molecules, as well as mRNA and non-coding RNA, released from the cell of origin, to target cells. EVs are released by all cell types and likely induced by mechanisms involved in oncogenic transformation, environmental stimulation, cellular activation, oxidative stress, or death. Ongoing studies investigate the molecular mechanisms and mediators of EVs-based intercellular communication at physiological and oncogenic conditions with the hope of using this information as a possible source for explaining physiological processes in addition to using them as therapeutic targets and disease biomarkers in a variety of diseases. A major limitation in this evolving discipline is the hardship and the lack of standardization for already challenging techniques to isolate EVs. Technical advances have been accomplished in the field of isolation with improving knowledge and emerging novel technologies, including ultracentrifugation, microfluidics, magnetic beads and filtration-based isolation methods. In this review, we will discuss the latest advances in methods of isolation methods and production of clinical grade EVs as well as their advantages and disadvantages, and the justification for their support and the challenges that they encounter.
Keywords: clinical grade EVs, exosomes, magnetic beads, microvesicles, sedimentation efficiency, ultracentrifugation
Introduction
Extracellular vesicles (EVs), including exosomes and microvesicles (MVs), are heterogeneous, membranous, cell-derived vesicles approximately 40–5000 nm in diameter that are released by a variety of cells into their microenvironment (Kalra et al., 2012; Momen-Heravi et al., 2012a,b). The terminologies used for naming EVs have changed tremendously over the last 10 years. First, isolated EVs were named based on the sample sources from which they were derived and their size. These concepts led to the emergence of various nomenclatures, such as oncosomes (exosomes derived from tumor cells), exosome-like vesicles, microparticles, apoptotic bodies, exosomes, prostasomes, microparticles, nanoparticles, microvesicles, and shedding microvesicles (Simpson and Mathivanan, 2012). Specifically, this confusion in terminology caused an ambiguity in isolation methods, where the words exosome and microvesicle were used more or less arbitrarily. For solving this problem, a consensus in the scientific community was achieved by categorizing EVs based on their mode of origin. Vesiculation events occur either at the plasma membrane, which leads to the formation of shedding microvesicles, or within endo-somal structures, which generates exosomes (Simpson and Mathivanan, 2012). EVs can be mainly categorized into three main classes, based on the mode of biogenesis: shedding microvesicles (originating from pitching of plasma membrane), exosomes [derived from multi-vesicular bodies (MVBs)], and apoptotic bodies (originating from apoptotic bulbs upon activation of apoptotic pathways) (Kalra et al., 2012) (Table 1).
Table 1.
Classification of extracellular vesicles (EVs) based on their mode of biogenesis.
| Type of EVs | Diameter/density | Origin | Common expressed markers |
|---|---|---|---|
| Ectosome or shedding microvesicles | 50–1000 nm | Outward budding of plasma membrane | Phosphatidylserin (PS) |
| Exosomes | 40–100 nm; density varies from 1.10 to 1.21 g/ml | Exocytosis from multivesicular bodies (MVBs) | Alix, TSG101, tetraspanins, and heat shock proteins |
| Apoptotic bodies | 50–5000nm | Programmed cell death or apoptosis | Annexin V/phosphatidylserin (PS) |
A growing body of studies is now focused on physiological and patho-physiological roles of EVs in cell-to-cell communication. EVs are suggested to contain bioactive molecules, including various proteins, microRNA (miRNA), and messenger RNA (mRNA), and have a lipid composition similar to those present on the plasma membranes of parent cells (Ratajczak et al., 2006). It has been shown that EVs can affect other cells via transfer of genetic cargo, transfer of receptors, and ultimately initiating pathways. Recent investigations revealed their role in immuno-responses (Dubyak, 2012), homeostasis (McKechnie et al., 2006), angiogenesis (Virgintino et al., 2012), thrombosis (Matzdorff et al., 1998), as well as tumor invasion and metastasis (Baj-Krzyworzeka et al., 2007; Luga et al., 2012; Peinado et al., 2012). The amount of EVs has been found to be elevated in certain disease states, including: malaria (Coltel et al., 2006); cardiovascular diseases (Azevedo et al., 2007); various types of cancer including glioblastoma, ovarian cancer, melanoma, and renal cancer (Meng et al., 2005; Lima et al., 2009; Balaj et al., 2011; Grange et al., 2011); auto-immune diseases like systemic lupus erythematosus and rheumatoid arthritis (Wan et al., 2008; Antwi-Baffour et al., 2010); as well as diabetes mellitus (Müller, 2012), and chronic renal failure (Gatti et al., 2011). In addition, as they carry cell-specific signatures, so the evaluation of EVs’ content may be used for early diagnosis for the above-mentioned conditions.
Given the natural ability of EVs (both exosomes and microvesicles) for the transport and intracellular delivery of bioactive micromolecules (Fais et al., 2012), they can be an attractive vehicle for the delivery of pharmaceutical proteins and nucleic acids, such as short interfering RNA (siRNA). Encapsulation of nucleic acid based therapeutics and proteins in endogenous transporting vesicles is a growing novel method to overcome most of these delivery issues. In particular, exosomes may be most appropriate for such delivery methods, because they are small (40–100 nm), relatively homogenous in size, and currently under intense investigation. Their size, 100 nm, is advantageous for their use as drug delivery systems, because this allows them to evade rapid clearance by the mononuclear phago-cyte system and enhances passage through fenestrations in the vessel wall, as might occur during inflammation.
EVs have been successfully isolated from cell culture conditioned medium (Balaj et al., 2011) and different body fluids including plasma (Ashcroft et al., 2012), serum (Dalton, 1975), saliva (Keller et al., 2011), amniotic fluid (Keller et al., 2011), breast milk (Hata et al., 2010), and urine (Wiggins et al., 1987). The gold standard and most commonly used protocol for EVs isolation/purification is differential centrifugation, which involves several centrifugation and ultracentrifugation steps, while protocols vary across users and this may lead to inconsistencies in recovery of EVs mainly because of different biofluid viscosity (Yuana et al., 2011; Momen-Heravi et al., 2012a). In some protocols, the first centrifugation steps can be replaced by microfiltration techniques (Théry et al., 2006), which may reduce isolation time and increase purity of isolated EVs. In addition, the last ultracentrifugation step can be followed by an extra purification step, such as a sucrose gradient centrifugation that provides a cleaner population of EVs without co-precipitation of EV-associated proteins and nucleic acid.
Recently, several alternative methods were introduced and utilized for isolation and purification of EVs, including antibody-coated magnetic beads, microfluidic devices, precipitation technologies (ExoQuick™), and filtration technologies. There is an urgent need for more efficient, reliable and reproducible EVs extraction methods, so that all downstream studies in the field of EVs can be more standardized and efficient. In this paper we will provide an overview of EV isolation methods, as well as some practical insights with advantages and disadvantages of each method.
Extracellular vesicle (EVs) isolation methods
Differential centrifugation
Differential centrifugation is considered a gold standard, and is the most common method to isolate EVs and is widely used to isolate them from body fluids and conditioned media. Although various protocols are available, generally it consists of multiple steps: first, a low speed spin (300 g for 10 min), which eliminates dead cells and bulky apoptotic debris, followed by higher speed spins, which varies among laboratories, from 1000 g to 20 000 g and eliminates larger vesicles and debris. A final high speed spin at 100 000 g precipitates EVs (Figure 1). The pellet of EVs is resuspended in phosphate buffered saline (PBS) and stored at −80°C for further characterization and analysis. For more purified EVs and eliminating contaminations, the pellet can be washed again in a large volume of PBS and centrifuged one last time at 100 000 g. This approach, however, is lengthy (4–5 h), requires an ultracentrifuge and results in a relatively low recovery of EVs (Momen-Heravi et al., 2012a), ranging from 5% to 25% of the starting EVs MHC class II concentration (Lamparski et al., 2002).
Figure 1. A typical ultracentrifugation protocol.
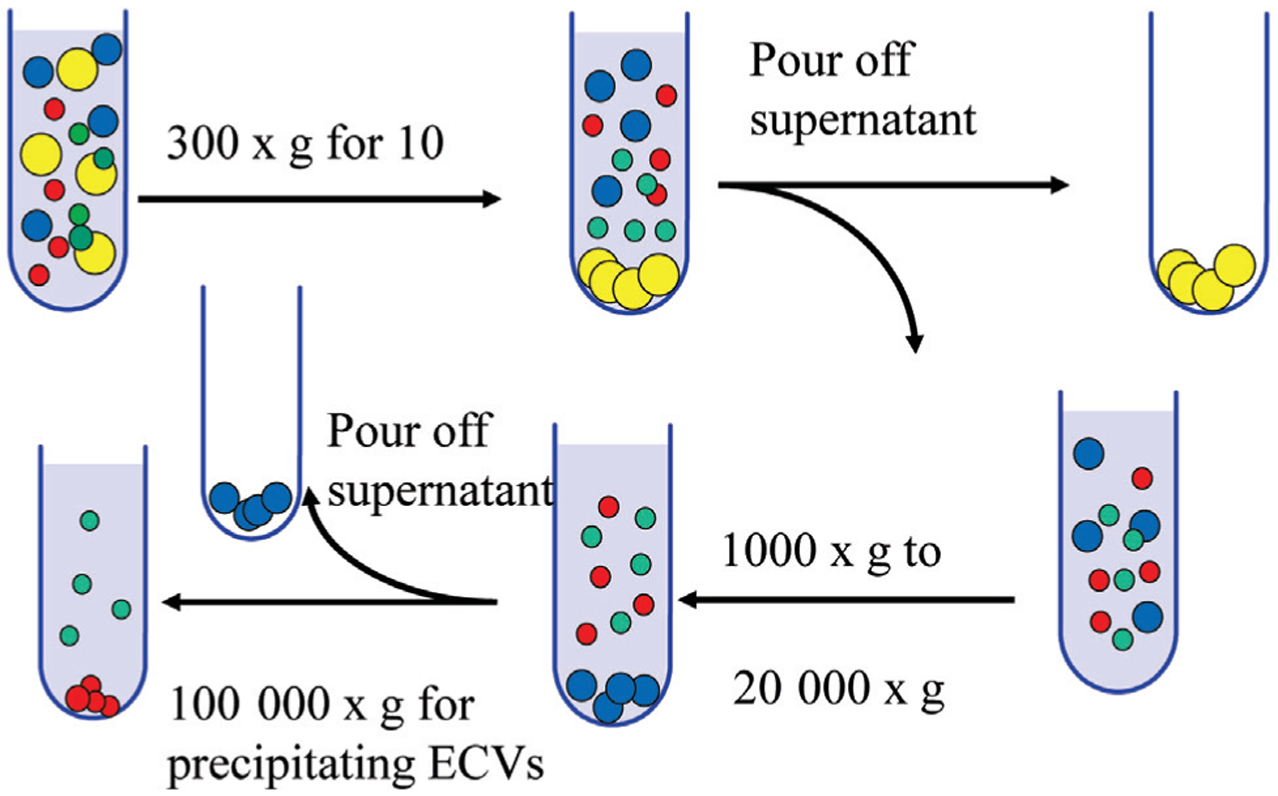
In consecutive rounds of centrifugation and pouring off, the RCF (g) and the centrifugation time are increased to pellet smaller particles. After each of the first three centrifugations, pellets that contain dead cells and cell debris are discarded, and the supernatant is kept for the next step. In contrast, after the 100 000 g centrifugations, pellets (containing EVs) are kept, and supernatants are discarded. The pellets are resuspended in phosphate buffered saline (PBS) for further analysis.
One of the most important factors in the determination of sedimentation efficiency of EVs in a differential centrifugation protocol is the clearing factor or k-factor of the rotor. The k-factor is a scale of the time taken for a particle to sediment through a particular medium. The value of the k-factor is determined by the maximum angular velocity (ω) of a centrifuge (in rad/s) and the minimum and maximum radius r of the rotor (Langer et al., 2003). It represents the relative sedimentation efficiency of a given centrifuge rotor at maximum rotation speed. K-factor can be utilized to predict the time t (in hours) required for sedimentation of a EVs using different rotors. The following formula represents the correlation between t, time (in hours); k-factor; and s, sedimentation coefficient (in Svedbergs):
Admittedly, the most efficient rotors have the lowest k-factor value and operate at a relatively high centrifugal force (RCF) or g, and have a low sedimentation path length. Comparing k-factors is a practical way of comparing the performance of different rotors and the following equation permits the calculation of the time required for EVs sedimentation in one rotor compared to another. The centrifugation times (t) and k-factors for two different rotors (1 and 2) are related by:
Another factor that should also be taken into account for increasing sedimentation stability is streaming, which affects both accuracy and resolution of sedimented EVs. Streaming, a factor that is related to Brownian motion of small particles through the suspending medium, is less considerable as the sedimentation path length of the rotor is reduced (Scott et al., 2005; Momen-Heravi et al., 2012a). So, low-angle fixed-angle rotors will provide better separations than swinging-bucket rotors. The drawback of using low-angle fixed-angle rotors is that the isolated pellets are less condensed and instead of compacting the pellet at the bottom of tube, it gets displaced through the wall of the tube.
In a recent study, we demonstrated (Momen-Heravi et al., 2012a,b) that viscosity has a significant correlation with the recovery of EVs. We evaluated sedimentation efficiency of different biofluids with different viscosities and reported less sedimentation efficiency for more viscous biofluid such as plasma, followed by serum. Pellet recovery for EVs in conditioned media, and spiked beads in PBS, had better recovery in fluids with lower viscosity. These results were confirmed when the samples were spiked with 100 nm polystyrene beads. The result of this study implicates that viscosity is an important parameter to consider when working with a biofluid where a lower viscous fluid yields more EVs in the pellet. Viscosity of different biofluids should be standardized and samples have to be diluted to reach similar viscosity values to use similar protocols. Otherwise, longer ultracentrifugation time and speed is needed for compensation of the viscosity.
Sucrose gradient centrifugation
One limitation in using differential centrifugation for isolating EVs is co-precipitation of protein aggregates, apoptotic bodies, or nucleosomal fragments, which may lead to less sample purity and less correctly bound proteins. One way to address these issues is to use a sucrose gradient, which separates vesicles based on their different flotation densities (Cantin et al., 2008). Figure 2 depicts differences between the amount of protein aggregate and purity of samples after sucrose gradient centrifugation in comparison with conventional deferential centrifugation via transmission electron microscopy (TEM) images. Exosomes, the finest sub-fraction of EVs, have floatation densities of 1.08–1.22 g/ml on sucrose gradients (Raposo et al., 1996). In comparison, vesicles purified from the endoplasmic reticulum float at 1.18–1.25 g/ml, and vesicles from the Golgi at 1.05–1.12 g/ml (Théry et al., 2006). Neither differential centrifugation nor sucrose gradient confer the ability to separate exosomes (40–100 nm) from viruses because of their similarities in density and size. Recently, a modified protocol involving separation on the iodixanol (optiprep™) gradient, was proposed. Utilizing the exosome marker acetylcholinesterase (AChE) and electron microscopy, it was demonstrated that the AChE-containing fraction (collecting at 8.4–12% iodixanol) included only exosomes and the AChE-free fraction (at 15.6%) contained only infectious virions (Cantin et al., 2008). The result of this study revealed that most EVs can be separated from HIV virions by Optiprep™ velocity.
Figure 2.
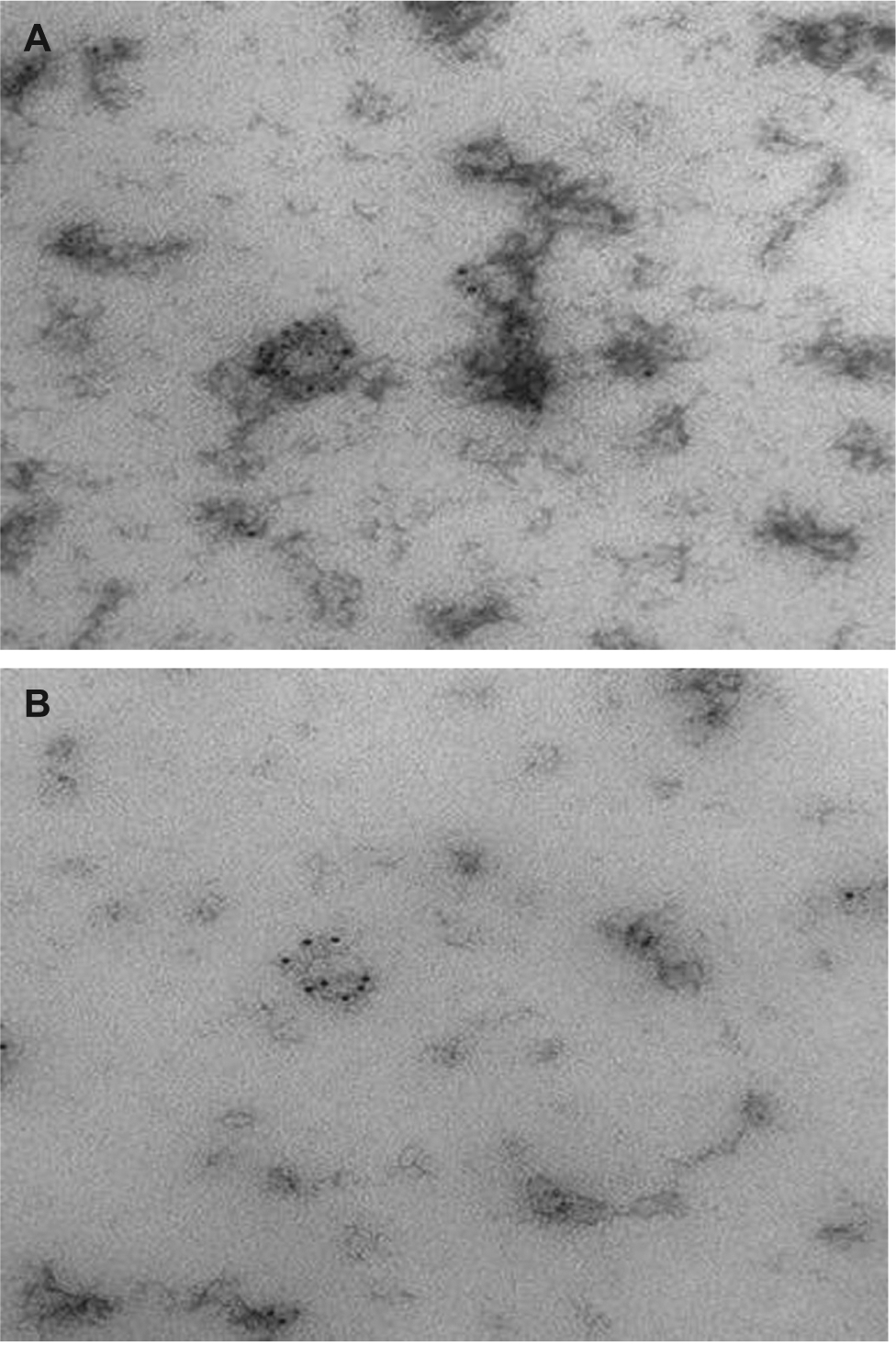
Transmission electron microscopy (TEM) characterization of human serum derived EVs isolated using (A) conventional differential centrifugation and (B) sucrose gradient centrifugation.(A) EVs isolated from human serum expressing CD63 transmembrane protein, which is believed to be exosome/microvesicles marker. There is more immuno-gold labeled protein aggregate in the background in this sample prepared via conventional differential centrifugation protocol. (B) EVs isolated from human serum expressing CD63 transmembrane, protein which is believed to be exosome/microvesicles marker. The background is neat, with minimal amount of protein aggregates after purification with sucrose gradient centrifugation.
Microfiltration technologies
Although filtration techniques by themselves have been relatively recently introduced to isolate EVs, in some differential centrifugation protocols filtration is used in combination with ultracentrifugation instead of first and second spins of regular differential centrifugation protocol. This filtration step will eliminate dead cells, apoptotic bulbs and large debris while keeping small membranes for further purification by ultracentrifugation.
Cheruvanky et al., 2007 demonstrated that urinary EVs can be rapidly enriched from human urine using a nano-membrane concentrator. Their approach was able to enrich exosomal/microvesicles proteins from small urine volumes (0.5 ml). Applying this method, some proteins, such as annexin V, NSE, and PODXL, did not attach to the nano-membrane and were readily recovered. However, some other EVs proteins such as AQP2 and TSG101 attached to the nano-membrane and could not be recovered from the retentate in a great extent.
Recently Merchant et al. (2010) proposed a microfiltration isolation method, using low protein-binding size exclusion filters for isolation of urinary biomarkers. They utilized hydrophilized polyvinylidene difluoride membrane to easily isolate EVs from fresh urine samples. Liquid chromatography-mass spectrometry immuno-blot analysis, and electron microscopy were used for validation and assessing the efficacy of the microfiltration method. They reported equivalent enrichment of EVs proteomes with reduced co-purification of abundant urinary proteins in comparison with other standard methods of ECV isolation, including ultracentrifugation and nano-filtration.
Although filtration technologies are improving quickly, they face several challenges, such as the lack of EVs condensation, co-purifying abundant proteins with EVs isolation, contamination of isolated EVs, and trapping of EVs in nano- or micro-pores. Therefore, isolation conditions must be optimized for maximal recovery of EVs and a more pure isolation/enrichment.
Antibody-coated magnetic beads
Proteomic studies characterizing the molecular composition of EVs have revealed the presence of both ubiquitous and cell-specific proteins that can be used as markers for EVs immune-isolation. Table 2 depicts several more abundant proteins found in EVs as listed on ExoCarta (Mathivanan and Simpson, 2009). The sub-population of interest can be extracted based on differentiating protein markers by means of immune-magnetic beads (Tauro et al., 2012) (Figure 3). The tetraspanin protein family is most prevalently associated with EVs and in particular, CD9, CD63, CD81, and CD82 are found in EVs from nearly any cell type (Théry et al., 2002).
Table 2.
Statistics of the ten most abundant proteins that are identified in extracellular vesicles (EVs).
| Protein symbol | Protein name | Times identified in the literature | Identified in EVs (exosomes/microvesicles) derived from the following tissue/cell type |
|---|---|---|---|
| HSPA8 | heat shock 70 kDa protein 8 | 52 | B cells, bladder cancer cells, breast milk, colorectal cancer cells, melanoma, malignant ascites, mesenchymal stem cells, nasopharyngeal carcinoma cells, nasopharyngeal carcinoma cells, prostate cancer cells, saliva, serum, urine |
| CD9 | CD9 Molecule | 50 | Amniotic fluid, B cells, bladder cancer cells, bone marrow cells, breast milk, colorectal cancer cells, dendritic cells, mast cells, ovarian cancer cells, plasma, pancreatic adenocarcinoma cells, prostate cancer cells, saliva, serum, T-Cell, urine |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 48 | Cortical neurones, hepatocytes, reticulocytes, pancreatic adenocarcinoma |
| ACTB | Actin, β | 43 | B cells, bladder cancer cells, breast milk, colorectal cancer cells, melanoma cells, mesenchymal stem cells, prostate cancer cells, saliva, tracheobronchial cells, urine |
| CD63 | CD63 Molecule | 41 | B cells, bladder cancer cells, breast milk, bronchoalveolar lavage fluid, colorectal cancer cells, dentritic cells, intestinal epithelial cells, mast cells, melanoma cells, mesenchymal stem cells, mesothelioma cells, pancreatic adenocarcinoma cells, plasma, platelets, saliva, stomach cancer cells, T cells, tracheobronchial cells, urine |
| CD81 | CD81 Molecule | 39 | Ascites, B cells, bladder cancer cells, breast milk, colorectal cancer cells, dendritic cells, malignant ascites, malignant pleural effusions, mast cells, melanoma cells, mesenchymal stem cells, plasma, prostate cancer cells, saliva, T cells, tabecular meshwork cells, trophoblast cells, urine |
| ANXA2 | Annexin A2 | 37 | Aqueous humor, B cells, Bladder cancer cells, breast milk, colorectal cancer cells, melanoma cells, mesenchymal stem cells, mesothelioma cells, prostate cancer cells, saliva, tabecular meshwork cells, tracheobronchial cells, urine |
| ENO1 | Enolase 1, (alpha) | 36 | B cells, bladder cancer cells, breast milk, colorectal cancer cells, intestinal epithelial cells, melanoma cells, mesenchymal stem cells, mesothelioma cells, prostate cancer cells, saliva, urine |
| HSP90AA1 | Heat shock protein 90 kDa alpha (cytosolic), class A member 1 | 34 | B cell, bladder cancer cells, colorectal cancer cells, intestinal epithelial cells, malignant pleural effusions, mesenchymal stem cells, mesothelioma cells, prostate cancer cells, saliva, urine |
| EEF1A1 | Eukaryotic translation elongation factor 1 alpha 1 | 34 | B cells, bladder cancer cells, breast milk, colorectal cancer cells, mesenchymal stem cells, prostate cancer cells, saliva, urine |
Figure 3. Antibody-coated magnetic beads.
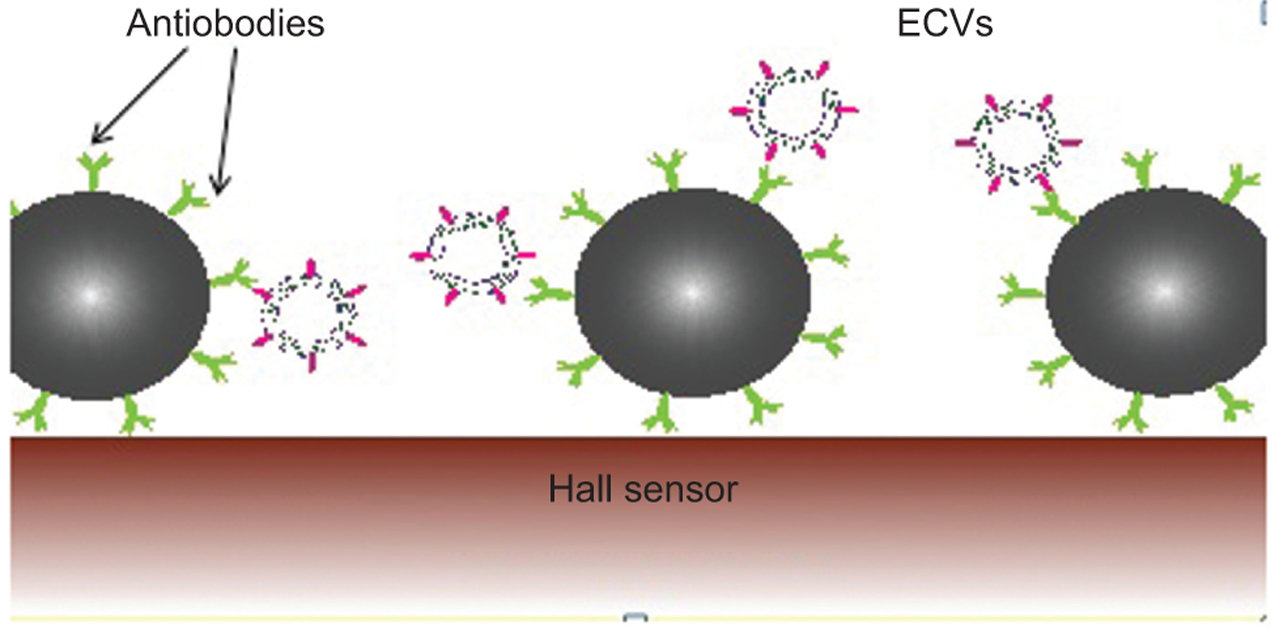
This illustration demonstrates how antigens of extracellular vesicles (EVs) bind to the antibodies of coated magnetic beads, which also bind the magnetic sensors, called Hall sensors, on the surface of the chip.
Antibody-coated magnetic bead isolation of EVs for antigen presenting cells has been shown by Clayton et al. (2001). EVs of tumor origin were isolated by Koga et al. (2005) using adherence to magnetic beads coated with antibodies against tumor-associated marker HER2. Taylor and Gercel-Taylor (2008) isolated circulating tumor-derived EpCAM-positive EVs using anti-EpCAM magnetic beads. A proteomic analysis study conducted by Mathivanan et al. (2009) targeted the colon epithelial cell-specific A33 antigen for immune-affinity capture of A33-containing EVs. They noted that while ultracentrifuged EVs contained a mixture of molecular signatures, the bead prepared EVs had other molecular signatures distinctive of their cellular origin (Mathivanan et al., 2009). Using a proteomic label-free spectral count strategy, Tauro et al. (2012) examined EVs-specific marker enrichment in LIM1863 colorectal carcinoma cells isolated by three different techniques. In comparison to differential centrifugation and density gradient separation, they found immune-affinity capture as the most effective strategy for isolation of EVs (Tauro et al., 2012).
Using antibody-coated magnetic beads against specific antigens for immune-isolation of exosomes offers the advantage of flow cytometric, immune-blot, and electron microscopy analysis of bead-EVs complexes (Théry et al., 2006). However, it is important to note this method is not suited for large sample volumes and captured EVs may not retain functionality even if successfully eluted from the bead surface (Théry et al., 2006).
In a recent study, Yoo et al. developed a direct extraction method for microRNAs of EVs. After isolation of EVs from human serum by immuno-affinity magnetic beads, microRNAs were directly isolated by mixing beads with a lysis solution consisting of a nonionic detergent and NaCl, and heating without further purification. They reported quantitatively comparable RNA content with conventional immuno-captured EVs (Yoo et al., 2012).
Another platform adopting this concept is ExoTEST™ (HANSABIOMED, Tallinn, Estonia), a platform consisting of ELISA plates pre-coated with proprietary exosomal antibodies enabling specific capture of exosomes from different biofluids and culture supernatants. This platform enables capturing of all exosomes or selectively sub-populations of exosomes. It is then possible to characterize and quantitate the exosomal proteins by means of detecting antibodies against exosome-associated antigens, either common to all exosomes, or specific to certain cell types or cell conditions.
Although using antibody-coated magnetic beads is a promising method for specific isolation and characterization of EVs, it is not intended for isolation of large amounts of EVs. In that case, pre-concentration of samples and prior centrifugations should be considered to reduce the sample volume.
Microfluidic devices
Microfluidics is the study and manipulation of fluid flow at the microscale. At small scales, the mechanics of fluid flow are dominated by frictional forces rather than kinetic forces. This offers unique options for control of separation, reaction and measurement processes that are unavailable at the macroscale, as well as presenting unique challenges. The use of microfluidic devices can offer significant advantages by reducing material costs, energy consumption and sample sizes, while increasing through-put and permitting multiplexing for many familiar laboratory processes. Microfluidic processes and devices typically exhibit characteristic dimensions between 100 nanometers and several hundred micrometers, large surface area-to-volume ratios and low Reynolds numbers, holding them firmly within the laminar flow regime.
Lab-on-chip devices are useful techniques in clinical care for medical diagnosis and blood tests. These miniaturized devices allow for small volumes of sample, shorter processing times, improved sensitivity and eventually reduced clinical care costs. Microfluidic devices operate by means of specific binding of EVs to antibody-coated surfaces. The biofluid of interest is then loaded on a pump that slowly pushes fluid through the chip, allowing targeting isolation of EVs.
Microfluidic devices, like the one developed by Chen et al, offer the advantage of single-step capture from serum as opposed to the multi-step procedures found in antibody-coated magnetic bead isolation (Chen et al., 2010). Moreover, their device allows direct lysis of EVs on the chip for subsequent protein or nucleic acid extraction. This is a relatively efficient way of purifying EVs, although it is not applicable to a large amount of samples. More recently, Ashcroft et al. (2012) developed a microfluidic flow cell to isolate EVs presenting the CD41 antigen and subsequently analyzed their size distribution by atomic force microscopy (AFM). This microfluidic device demonstrated direct capture of EVs from dilute blood plasma thereby avoiding potential quantitative and phenotypic discrepancies associated with pre-processing of EVs – conditions that have yet to be standardized. Their isolation method ultimately increased AFM sensitivity by lessening AFM scanning time because of the increased concentration of captured EVs on the mica surface of their flow cells (Ashcroft et al. 2012).
Another highly sensitive and rapid method of isolating EVs has been described in Shao et al., which makes use of micro-nuclear magnetic resonance (μNMR) (Shao et al., 2012). This technique employs labeling of EVs with the antibody of interest that has been coupled to magnetic nanoparticles, which are then quantified by a μNMR. Using this technique, authors were successful in separating tumor EVs from the normal host cell-derived EVs and were also able to detect and monitor changes on tumor specific biomarkers on circulating EVs (Shao et al., 2012).
ExoQuick™
Several biotechnology companies are currently working on developing quick and easy ways to isolate EVs from biological fluids and one such example is ExoQuick™ (System Biosciences, Mountain View, CA, USA). This kit overcomes the need for long differential centrifugation by adding a mix to samples of interest and then allowing EVs to precipitate by an overnight incubation step. Taylor et al. (2011) compared the efficacy of different extraction methods including the ExoQuick precipitation approach, chromatography, ultracentrifugation, and DynaBeads. They showed that precipitation of EVs via ExoQuick™ resulted in a higher yield of miRNA with greater purity and quantity than other techniques, while this precipitation approach isolates EVs in general and does not exhibit specificity for the originating cell (Taylor et al., 2011). However, further studies are necessary to confirm the applicability of this product to EVs purification. In a recent study, the highest yield of EVs was achieved using ultracentrifugation with ExoQuick™ precipitation, whereas higher quality EVs isolation with intact morpho-logical structures was achieved by ultracentrifugation with density gradient centrifugation (Yamada et al., 2012).
Production of clinical grade EVs
EVs are promising tools for development of vaccine against infectious agents (Ellis et al., 2010) and cancer (Zitvogel et al., 1998). Vesicles are produced by gram-negative and gram-positive bacteria and contain many of the bacterial products recognized by the host immune system during infection. EVs released by bacteria are known as outer-membrane vesicles (OMVs) and are secreted naturally by the bacteria in the growth medium or can be induced by treating the bacterial pellet with a detergent as, for example, sodium deoxycholate (Frasch et al., 2001).
Immunization with bacterial EVs successfully protected mice against infections with Burkholderia pseudomallei (Nieves et al., 2011), Vibrio cholerae (Bishop et al., 2012), Shigella flexneri (Camacho et al., 2011), Streptococcus pneumoniae (Muralinath et al., 2011), Salmonella typhimurium (Alaniz et al., 2007), Borrelia burgdorferi (Whitmire and Garon, 1993), Flavobacterium (Aoki et al., 2007) and Neisseria meningitides (Van de Waterbeemd et al., 2012), indicating that EVs can be used as antigen delivery systems to generate effective immune responses. Efficacy of artificially-generated EVs against serotype B meningococcal disease has been demonstrated for humans in Cuba, Norway, Chile, Brazil and New Zealand (Bjune et al., 1991; Martin et al., 1998; Tappero et al., 1999; Holst et al., 2009). Several protocols are used to isolate EVs from prokaryotes: bacteria are grown in LB-medium to late exponential phase. Cells are then removed by centrifugation at 4500 g. The supernatant is passed first through a 0.45 μm and then a 0.22 μm pore-size filters to remove cells and protein aggregates. The EVs are pelleted by ultracentrifugation at 140 000 g. The EVs are resuspended in 3% sucrose to avoid aggregation and finally EVs are sterile-filtered through a 0.22 um filter, the vesicles can be stored at −80 C (Frasch et al., 2001).
Mature dendritic cells (DCs) produce EVs able to trigger a potent immune activation, resulting in tumor elimination. As EVs are stable and easy to modified artificially, EVs derived from autologous mature DC or ascites of tumor-bearing patients have been vaccinated into patients with non-small cell lung cancer (Escudier et al., 2005), melanoma (Morse et al., 2005), ovarian cancer (Navabi et al., 2005), colorectal cancer (Dai et al., 2008) and glioma (Bu et al., 2011). Results from the clinical trials show that EV are well tolerated and have few side effects. Several protocols have therefore been developed in order to isolate EVs from large volume of culture supernatants and under good manufacturing practice. Lamparski et al., describe a protocol for the reproducible isolation of EVs from DCs pulsed with tumor antigens. This method has the advantage of the unique properties of EVs: size and density and combine ultrafiltration followed by ultracentrifugation on a 30% sucrose cushion, allowing rapid isolation of EVs in 4–6 h. The percentage recovery ranged from 40% to 50% based on the exosomes MHC Class II concentration of the starting clarified supernatant. Ps are isolated from patients and the monocytes are differentiated in vitro into DCs. The EVs are then collected from the culture supernatant (1–4 l), which is first clarified through a 0.8 μm filter. The clarified supernatant is then concentrated by ultrafiltration through a 500-kDa exclusion limit membrane under a constant transmembrane pressure concentrating the EVs up to 40 times and reducing the amount of protein aggregates. The concentrated EVs are diafiltered five times with an equal volume of PBS to further reduce the amount of soluble protein. After loading into centrifuge tubes on a 30% sucrose cushion, the samples are ultracentrifuged at 100 000 g. The EVs-sucrose density cushions are pooled and diafiltered into the formulation buffer. In contrast to differential ultracentrifugation, the diafiltration provide the advantage to eliminate the formation of EV aggregates. For clinical administration, the EVs are sterile-filtered through a 0.22 μm Sterivex-GV capsule filter (Lamparski et al., 2002).
Acknowledgments:
This work was conducted, at least in part, through the Harvard Catalyst Laboratory for Innovative Translational Technologies (HC-LITT) with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health.
Contributor Information
Fatemeh Momen-Heravi, Laboratory for Innovative Translational Technologies, Harvard Medical School, 77 Louis Pasteur Avenue, Harvard Medical School, Boston, MA 02115, USA.
Leonora Balaj, Department of Neurology and Radiology, Massachusetts General Hospital, 149 13th Street, Charlestown, MA 02129, USA.
Sara Alian, Biopolymers Facility, Harvard Medical School, 77 Louis Pasteur Avenue, Boston, MA 02115, USA.
Pierre-Yves Mantel, Department of Immunology and Infectious Diseases, Harvard School of Public Health, 665 Huntington Avenue, Boston, MA 02120, USA.
Allison E. Halleck, Laboratory for Innovative Translational Technologies, Harvard Medical School, 77 Louis Pasteur Avenue, Harvard Medical School, Boston, MA 02115, USA
Alexander J. Trachtenberg, Laboratory for Innovative Translational Technologies, Harvard Medical School, 77 Louis Pasteur Avenue, Harvard Medical School, Boston, MA 02115, USA
Cesar E. Soria, University of New Mexico, 1 University Blvd NE, Albuquerque, NM 87131, USA
Shanice Oquin, University of North Texas, 1155 Union Circle, Denton, TX 76203, USA.
Christina M. Bonebreak, Harvard School of Dental Medicine, 188 Longwood Avenue, Boston, MA 02115, USA
Elif Saracoglu, Faculty of Dentistry, Istanbul University, Turgut Özal Caddesi (Millet Cd.), 34390 Istanbul, Turkey.
Johan Skog, Exosome Diagnostic Inc., 3960 Broadway, New York, NY 10032, USA.
Winston Patrick Kuo, Harvard Catalyst Laboratory for Innovative Translational Technologies, Harvard Medical School, Boston, MA 02115, USA; and Department of Developmental Biology, Harvard School of Dental Medicine, Boston, MA 02115, USA.
References
- Alaniz RC, Deatherage BL, Lara JC, and Cookson BT (2007). Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol 179, 7692–7701. [DOI] [PubMed] [Google Scholar]
- Antwi-Baffour S, Kholia S, Aryee YK, Ansa-Addo EA, Stratton D, Lange S, and Inal JM (2010). Human plasma membrane-derived vesicles inhibit the phagocytosis of apoptotic cells – possible role in SLE. Biochem. Biophys. Res. Commun 398, 278–283. [DOI] [PubMed] [Google Scholar]
- Aoki M, Kondo M, Nakatsuka Y, Kawai K, and Oshima S (2007). Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine 25, 561–569. [DOI] [PubMed] [Google Scholar]
- Ashcroft BA, de Sonneville J, Yuana Y, Osanto S, Bertina R, Kuil ME, and Oosterkamp TH (2012). Determination of the size distribution of blood microparticles directly in plasma using atomic force microscopy and microfluidics. Biomed. Microdevices 14, 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo LC, Pedro MA, and Laurindo FR (2007). Circulating microparticles as therapeutic targets in cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov 2, 41–51. [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M, Szatanek R, Weglarczyk K, Baran J, and Zembala M (2007). Tumour-derived microvesicles modulate biological activity of human monocytes. Immunol. Lett 113, 76–82. [DOI] [PubMed] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, and Skog J (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun 2, 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AL, Tarique AA, Patimalla B, Calderwood SB, Qadri F, and Camilli A (2012). Immunization of mice with Vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission. J. Infect. Dis 205, 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjune G, Høiby EA, Grønnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nøkleby H, and Rosenqvist E (1991). Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet 338, 1093–1096. [DOI] [PubMed] [Google Scholar]
- Bu N, Wu H, Sun B, Zhang G, Zhan S, Zhang R, and Zhou L (2011). Exosome-loaded dendritic cells elicit tumor-specific CD8+ cytotoxic T cells in patients with glioma. J. Neurooncol 104, 659–667. [DOI] [PubMed] [Google Scholar]
- Camacho AI, de Souza J, Sánchez-Gómez S, Pardo-Ros M, Irache JM, and Gamazo C (2011). Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine 29, 8222–8229. [DOI] [PubMed] [Google Scholar]
- Cantin R, Diou J, Bélanger D, Tremblay AM, and Gilbert C (2008). Discrimination between exosomes and HIV-1: purification of both vesicles from cell-free supernatants. J. Immunol. Methods 338, 21–30. [DOI] [PubMed] [Google Scholar]
- Chen C, Skog J, Hsu C, Lessard RT, Balaj L, Wurdinger T, Carter BS, Breakefield XO, Toner M, and Irimia D (2010). Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab. Chip 10, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, and Star RA (2007). Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am. J. Physiol. Renal Physiol 292, 1657–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton A, Court J, Navabi H, Adams M, Mason MD, Hobot JA, Newman GR, and Jasani B (2001). Analysis of antigen presenting cell derived exosomes, based on immune-magnetic isolation and flow cytometry. J. Immunol. Methods 247, 163–174. [DOI] [PubMed] [Google Scholar]
- Coltel N, Combes V, Wassmer SC, Chimini G, and Grau GE (2006). Cell vesiculation and immunopathology: implications in cerebral malaria. Microbes Infect. 8, 2305–2316. [DOI] [PubMed] [Google Scholar]
- Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, and Li G (2008). Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol. Ther 16, 782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton AJ (1975). Microvesicles and vesicles of multivesicular bodies versus “virus-like” particles. J. Natl. Cancer Inst 54, 1137–1148. [DOI] [PubMed] [Google Scholar]
- Dubyak GR (2012). P2X7 receptor regulation of non-classical secretion from immune effector cells. Cell Microbiol. 14, 1697–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis TN, Leiman SA, and Kuehn MJ (2010). Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun 78, 3822–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudier B, Dorval T, Chaput N, André F, Caby M-P, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, et al. (2005). Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J. Transl. Med 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S, Logozzi M, Lugini L, Federici C, Azzarito T, Zarovni N, and Chiesi A (2012). Exosomes: the ideal nanovectors for biodelivery. Biol. Chem 394, 1–15. [DOI] [PubMed] [Google Scholar]
- Frasch CE, van Alphen L, Holst J, Poolman JT, and Rosenqvist E (2001). Outer membrane protein vesicle vaccines for meningococcal disease. Methods Mol. Med 66, 81–107. [DOI] [PubMed] [Google Scholar]
- Gatti S, Bruno S, Deregibus MC, Sordi A, Cantaluppi V, Tetta C, and Camussi G (2011). Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant 26, 1474–1483. [DOI] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, and Camussi G (2011). Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 71, 5346–5356. [DOI] [PubMed] [Google Scholar]
- Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, and Aoki N (2010). Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem. Biophys. Res. Commun 396, 528–533. [DOI] [PubMed] [Google Scholar]
- Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, and Rosenqvist E (2009). Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 27 (Suppl) 2, B3–12. [DOI] [PubMed] [Google Scholar]
- Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, et al. (2012). Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 10, e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Ridinger J, Rupp AK, Janssen JW, and Altevogt P (2011). Body fluid derived exosomes as a novel template for clinical diagnostics. J. Transl. Med 9, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Matsumoto K, Akiyoshi T, Kubo M, Yamanaka N, Tasaki A, Nakashima H, Nakamura M, Kuroki S, Tanaka M, et al. (2005). Purification, characterization and biological significance of tumor-derived exosomes. Anticancer Res. 25, 3703–3708. [PubMed] [Google Scholar]
- Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, and Le Pecq JB (2002). Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 270, 211–226. [DOI] [PubMed] [Google Scholar]
- Langer K, Balthasar S, Vogel V, Dinauer N, von Briesen H, and Schubert D (2003). Optimization of the preparation process for human serum albumin (HSA) nanoparticles. Int. J. Pharm 257, 169–180. [DOI] [PubMed] [Google Scholar]
- Lima LG, Chammas R, Monteiro RQ, Moreira ME, and Barcinski MA (2009). Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 8, 168–175. [DOI] [PubMed] [Google Scholar]
- Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, and Wrana JL (2012). Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell 21, 1542–1556. [DOI] [PubMed] [Google Scholar]
- Martin DR, Walker SJ, Baker MG, and Lennon DR (1998). New Zealand epidemic of meningococcal disease identified by a strain with phenotype B:4:P1.4. J. Infect. Dis 177, 497–500. [DOI] [PubMed] [Google Scholar]
- Mathivanan S and Simpson RJ (2009). ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 9, 4997–5000. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Lim JWE, Tauro BJ, Ji H, Moritz RL, and Simpson RJ (2009). Proteomic analysis of A33 immunoaffinity-purified exosomes released from the human colon tumor cell line LIM1215 reveals a tissue-specific protein signature. Mol. Cell. Proteomics 9 2, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzdorff AC, Berchner D, Kühnel G, Kemkes-Matthes B, Pralle H, and Voss R (1998). Relative and absolute changes of activated platelets, microparticles and platelet aggregates after activation in vitro. Haemostasis 28, 277–288. [DOI] [PubMed] [Google Scholar]
- McKechnie NM, King BC, Fletcher E, and Braun G (2006). Fas-ligand is stored in secretory lysosomes of ocular barrier epithelia and released with microvesicles. Exp. Eye Res 83, 304–314. [DOI] [PubMed] [Google Scholar]
- Meng Y, Kang S, and Fishman DA (2005). Lysophosphatidic acid stimulates fas ligand microvesicle release from ovarian cancer cells. Cancer Immunol. Immunother 54, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant ML, Powell DW, Wilkey DW, Cummins TD, Deegens JK, Rood IM, McAfee KJ, Fleischer C, Klein E, and Klein JB (2010). Microfiltration isolation of human urinary exosomes for characterization by MS. Proteomics Clin. Appl 4, 84–96. [DOI] [PubMed] [Google Scholar]
- Momen-Heravi F, Balaj L, Alian S, Trachtenberg AJ, Hochberg FH, Skog J, and Kuo WP (2012a). Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 3, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen-Heravi F, Balaj L, Alian S, Tigges J, Toxavidis V, Ericsson M, Distel RJ, Ivanov AR, Skog J, and Kuo WP (2012b). Alternative methods for characterization of extracellular vesicles. Front Physiol. 3, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, et al. (2005). A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller G (2012). Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab. Syndr. Obes 5, 247–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralinath M, Kuehn MJ, Roland KL, and Curtiss R (2011). Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae. Infect. Immun 79, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navabi H, Croston D, Hobot J, Clayton A, Zitvogel L, Jasani B, Bailey-Wood R, Wilson K, Tabi Z, Mason MD, et al. (2005). Preparation of human ovarian cancer ascites-derived exosomes for a clinical trial. Blood Cells Mol. Dis 35, 149–152. [DOI] [PubMed] [Google Scholar]
- Nieves W, Asakrah S, Qazi O, Brown KA, Kurtz J, Aucoin DP, McLachlan JB, Roy CJ, and Morici LA (2011). A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine. 29, 8381–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med 18, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, and Geuze HJ (1996). B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med 183, 1161–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, and Ratajczak MZ (2006). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Harding E, and Rowe AJ (2005). Analytical ultracentrifugation: techniques and methods. (Cambridge, UK: The Royal Society of Chemistry; ), pp. 273–276. [Google Scholar]
- Shao H, Min C, Issadore D, Liong M, Yoon T, Weissleder R, and Lee H (2012). Magnetic nanoparticles and microNMR for diagnostic applications. Theranostics 2, 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RJ and Mathivanan S (2012). Extracellular microvesicles: the need for internationally recognised nomenclature and stringent purification criteria. J. Proteomics Bioinform 5, ii–ii. DOI: 10.4172/jpb.10000e10. [DOI] [Google Scholar]
- Tappero JW, Lagos R, Ballesteros AM, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Høiby EA, Holst J, et al. (1999). Immunogenicity of 2 serogroup B outer-membrane protein meningococcal vaccines: a randomized controlled trial in Chile. J. Am. Med. Assoc 281, 1520–1527. [DOI] [PubMed] [Google Scholar]
- Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivinan S, Scott AM, and Simpson RJ (2012). Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 56, 293–304. [DOI] [PubMed] [Google Scholar]
- Taylor DD and Gercel-Taylor C (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol 110, 13–21. [DOI] [PubMed] [Google Scholar]
- Taylor DD, Zacharias W, and Gercel-Taylor C (2011). Exosome isolation for proteomic analyses and RNA profiling. Methods Mol. Biol 728, 235–246. [DOI] [PubMed] [Google Scholar]
- Théry C, Zitvogel L, and Amigorena S (2002). Exosomes: Composition, Biogenesis, and Function. Nature Rev. 2, 569–578. [DOI] [PubMed] [Google Scholar]
- Théry C, Amigorena S, Raposo G, and Clayton A (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol Chapter 3, Unit 3.22. DOI: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- Van de Waterbeemd B, Streefland M, van Keulen L, van den Ijssel J, de Haan A, Eppink MH, and van der Pol LA (2012). Identification and optimization of critical process parameters for the production of NOMV vaccine against Neisseria meningitidis. Vaccine 30, 3683–3690. [DOI] [PubMed] [Google Scholar]
- Virgintino D, Rizzi M, Errede M, Strippoli M, Girolamo F, Bertossi M, and Roncali L (2012). Plasma membrane-derived microvesicles released from tip endothelial cells during vascular sprouting. Angiogenesis 15, 761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S, Zhou Z, Duan B, and Morel L (2008). Direct B cell stimulation by dendritic cells in a mouse model of lupus. Arthritis Rheum. 58, 1741–1750. [DOI] [PubMed] [Google Scholar]
- Whitmire WM and Garon CF (1993). Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect. Immun 61, 1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins R, Glatfelter A, Kshirsagar B, and Beals T (1987). Lipid microvesicles and their association with procoagulant activity in urine and glomeruli of rabbits with nephrotoxic nephritis. Lab. Invest 56, 264–272. [PubMed] [Google Scholar]
- Yamada T, Inoshima Y, Matsuda T, and Ishiguro N (2012). Comparison of methods for isolating exosomes from bovine milk. J. Vet. Med. Sci 74, 1523–1525. [DOI] [PubMed] [Google Scholar]
- Yoo CE, Kim G, Kim M, Park D, Kang HJ, Lee M, Huh N (2012). A direct extraction method for microRNAs from exosomes captured by immunoaffinity beads. Anal. Biochem 431, 96–98. [DOI] [PubMed] [Google Scholar]
- Yuana Y, Bertina RM, and Osanto S (2011). Pre-analytical and analytical issues in the analysis of blood microparticles. Thromb. Haemost 105, 396–408. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, and Amigorena S (1998). Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med 4, 594–600. [DOI] [PubMed] [Google Scholar]


