Abstract
Advances in single-cell mass cytometry have increasingly improved highly multidimensional characterization of immune cell heterogeneity. The immunoassay multiplexing capacity relies on monoclonal antibodies labeled with stable heavy-metal isotopes. To date, a variety of rare-earth elements and noble and post-transition metal isotopes have been used in mass cytometry; nevertheless, the methods used for antibody conjugation differ because of the individual metal coordination chemistries and distinct stabilities of various metal cations. Herein, we provide three optimized protocols for conjugating monoclonal IgG antibodies with 48 high-purity heavy-metal isotopes: (i) 38 isotopes of lanthanides, 2 isotopes of indium, and 1 isotope of yttrium; (ii) 6 isotopes of palladium; and (iii) 1 isotope of bismuth. Bifunctional chelating agents containing coordinative ligands of monomeric DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) or polymeric pentetic acid (DTPA) were used to stably sequester isotopic cations in aqueous solutions and were subsequently coupled to IgG antibodies using site-specific biorthogonal reactions. Furthermore, quantification methods based on antibody inherent absorption at 280 nm and on extrinsic absorption at 562 nm after staining with bicinchoninic acid (BCA) are reported to determine metal-isotope-tagged antibodies. In addition, a freeze-drying procedure to prepare palladium isotopic mass tags is described. To demonstrate the utility, experiments using six palladium-tagged CD45 antibodies for barcoding assays of live immune cells in cytometry by time-of-flight (CyTOF) are described. Conjugation of pure isotopes of lanthanides, indium, or yttrium takes ~3.5 h. Conjugation of bismuth takes ~4 h. Preparation of palladium mass tags takes ~8 h. Conjugation of pure isotopes of palladium takes ~2.5 h. Antibody titration takes ~4 h.
Introduction
Elemental mass tags in CyTOF
Cytometric technology has facilitated the investigation of multiple features of heterogeneous biological systems at the single-cell level1,2. Mass cytometry, or CyTOF, has been regarded as the ‘next generation’ of flow cytometry because it uses metal-isotope-tagged antibodies (MitAbs) as reporting probes3–6. Using this advance, CyTOF overcomes the inherent limits of spectral overlap observed with conventional fluorescence-based cytometry and enables simultaneous measurements of >50 parameters in single cells7–12. In principle, mass cytometry originates from inductively coupled plasma TOF mass spectrometry (ICP-TOF-MS), which is broadly used for quantifying isotopic contents of elements having low ionization potentials in the periodic table13–15. To enable biomedical assays, elemental mass tags (EMTs) have been introduced for CyTOF to label biomolecular targets or probes of interest with specific elements or isotopes. A quantitative relationship is established between the concentration of targeted biomolecules and the intensity of the detection signal of the corresponding EMTs16–22.
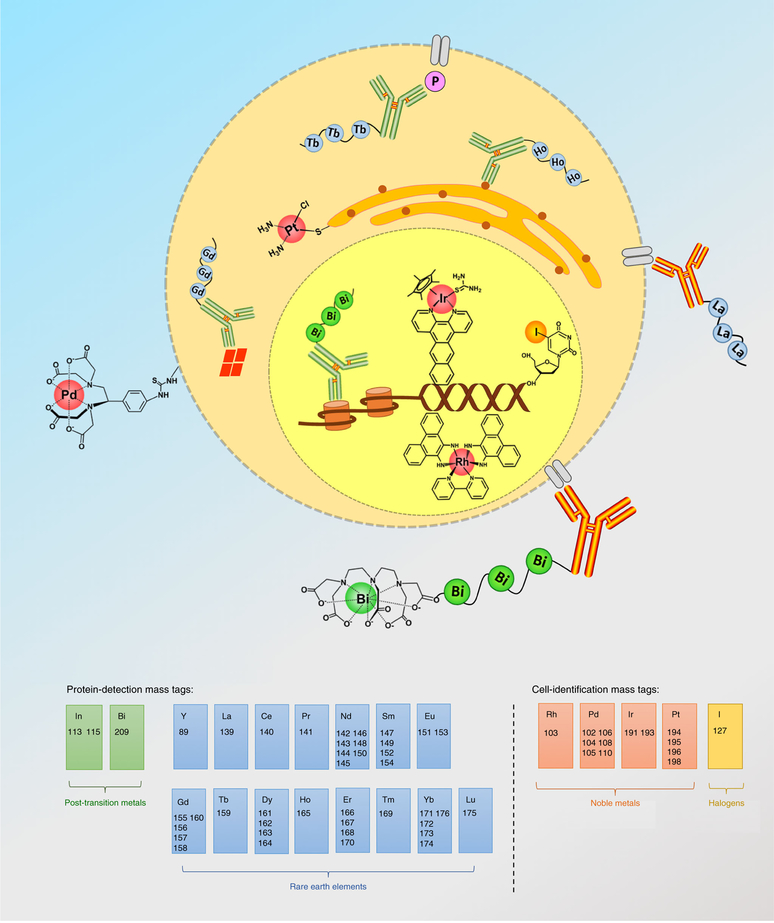
To minimize background signals, EMTs are generally composed of exogenous cellular elements such as noble and post-transition metals, rare-earth elements, and halogens, rather than the essential elements of endogenous cellular components such as sodium, potassium, calcium, copper, iron, and zinc. To date, the tagging elements and isotopes investigated in CyTOF have involved yttrium (Y)23,24, indium (In)4,25, the series of lanthanide elements (Ln, from La to Lu, except Pm)4,9,26, iodine (I)27, cadmium (Cd)4,21, tellurium (Te)28–30, silver (Ag)31, palladium (Pd)25,32,33, rhodium (Rh)34, iridium (Ir)34, platinum (Pt)35,36, ruthenium (Ru)37,38, osmium (Os)38, and bismuth (Bi)39,40. Basically, EMTs have two fundamental utilities: (i) measurement of the expressions of cellular proteins or their modifications and (ii) characterization of cell functions relating to viability35 and cell cycle27, as well as for high-throughput cell barcode32. Figure 1 illustrates the most widely used and commercially available EMTs in current CyTOF applications. The different utilities of EMTs are dependent on their chelation properties in antibody conjugation or biochemical functions in cell biology, such as incorporation of 5-iodo-2′-deoxyuridine (IdU) into the newly synthesized nucleic acids27. The metal isotopes used in protein-detection mass tags require substantial validation, as the presence of these tagged metallic cations may alter the specificity of antibody to recognize its antigen41.
Fig. 1 |. Elemental mass tags widely applied in CyTOF.
Elemental mass tags are typically classified as protein-detection mass tags or cell-identification mass tags according to their utilities in CyTOF analysis. To date, 56 stable heavy isotopes have been used in single-cell assays, coming from 15 rare-earth elements (Y, and from La to Lu, except Pm), four noble metals (Rh, Pd, Ir, and Pt), two post-transition metals (In and Bi), and one halogen (I).
Metal isotopes used in protein-detection mass tags
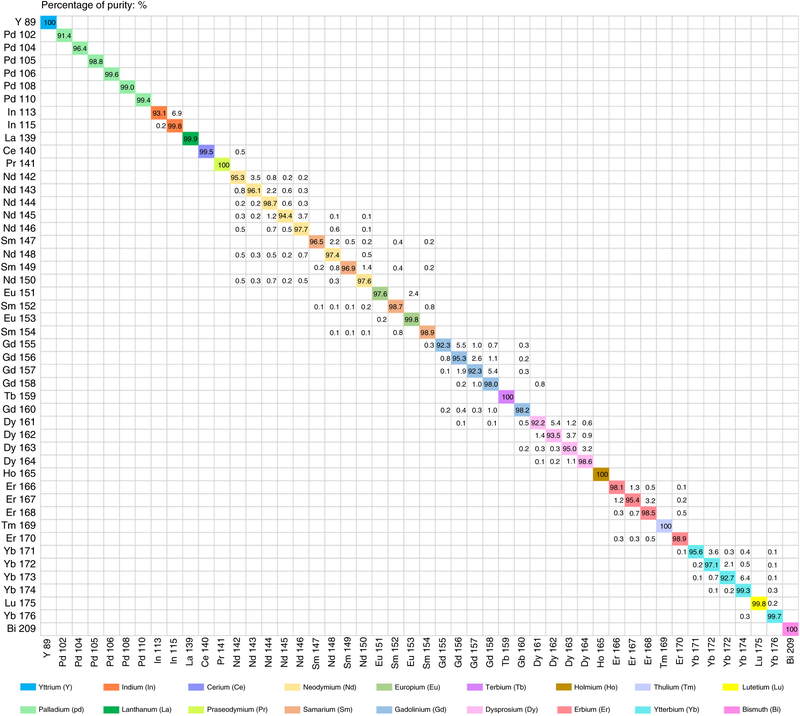
Although CyTOF has minimal overlap signals compared with fluorescence-based cytometry, the spillover effects still exist among different detection channels42. Therefore, the preparation of MitAbs must be evaluated for (i) impurities in the enriched isotopes; (ii) ‘M ± 1’ spillover between neighbor channels, referred as abundance sensitivity; and (iii) ‘M + 16’ spillover of oxide formation. To date, 48 isotopes coming from 18 heavy metals have been conjugated to monoclonal IgG antibodies and are widely used in single-cell CyTOF immunoassays. The isotopic purities of Y, Pd, In, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, and Bi, which are typically used in our lab (https://nolanlab.stanford.edu/cytofisotopes), are shown in Fig. 2. The purified isotopes are produced using electromagnetic separation by Trace Science International. Most of these isotopes are enriched by >95% and some are available at purities higher than 99%. There are six monoisotopic elements that show 100% abundance: 89Y, 141Pr, 159Tb, 165Ho, 169Tm, and 209Bi.
Fig. 2 |. Pure metal isotopes tagged in monoclonal antibodies.
Forty-eight stable metal isotopes of Y, Pd, In, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, and Bi have been used in protein-detection mass tags. The typical values of isotopic enrichment and spillover impurity are based on the product sheets from Trace Science International and early ICP-MS analytical measurements performed by DVS Sciences and our lab.
The series of lanthanides are the most widely used for EMTs because they have similar chemical properties and 38 stable isotopes that can be conjugated to antibodies using the same protocol. An issue with lanthanides is that oxide formation of the light lanthanides (from 139 to 160 amu) causes mass interferences with the heavy lanthanides (from 155 to 176 amu). This is referred to as the ‘M + 16’ effect. For example, 141Pr16O+ ions have spillover signal in the 157Gd+ detection channel. Table 1 shows the oxide species of light lanthanides and the relative ratios43 that result in spillover signals in the CyTOF detection channels of the heavy lanthanides. In CyTOF tuning procedures, radiofrequency power, flow speeds and makeup of nebulization gas, and positions of the sample torch and cone can be optimized so that the oxidation ratio of lanthanum (139La16O+/139La+), the most easily oxidized isotope, remains <3.0%3,18. Isotopes of 89Y, 102Pd, 104Pd, 105Pd, 106Pd, 108Pd, 110Pd, 113In, 115In, and 209Bi do not generate oxidation mass interferences with each other or with light or heavy lanthanides.
Table 1 |.
The oxides and their ratios of light lanthanides, affected CyTOF detection channels, and overlapped heavy lanthanide ions
| Light lanthanides | Heavy lanthanides | |||
|---|---|---|---|---|
| Interfering elements | One positive charge oxide ions | Ratios of oxides (%)a | Mass spectrum overlapped ions | Detection channels (m/z) |
| Lanthanum | 139La16O+ | ≤3.0 | 155Gd+ | 155 |
| Cerium | 140Ce16O+ | ≤3.0 | 156Gd+ | 156 |
| Praseodymium | 141Pr16O+ | ≤2.4 | 157Gd+ | 157 |
| Neodymium | 142Nd16O+ | ≤2.1 | 158Gd+ | 158 |
| 143Nd16O+ | 159Tb+ | 159 | ||
| 144Nd16O+ | 160Gd+ | 160 | ||
| 145Nd16O+ | 161Dy+ | 161 | ||
| 146Nd16O+ | 162Dy+ | 162 | ||
| 148Nd16O+ | 164Dy+ | 164 | ||
| 150Nd16O+ | 166Er+ | 166 | ||
| Samarium | 147Sm16O+ | ≤0.5 | 163Dy+ | 163 |
| 149Sm16O+ | 165Ho+ | 165 | ||
| 152Sm16O+ | 168Er+ | 168 | ||
| 154Sm16O+ | 170Er+ | 170 | ||
| Europium | 151Eu16O+ | ≤0.1 | 167Er+ | 167 |
| 153Eu16O+ | 169Tm+ | 169 | ||
| Gadolinium | 155Gd16O+ | ≤1.5 | 171Yb+ | 171 |
| 156Gd16O+ | 172Yb+ | 172 | ||
| 157Gd16O+ | 173Yb+ | 173 | ||
| 158Gd16O+ | 174Yb+ | 174 | ||
| 160Gd16O+ | 176Yb+ | 176 | ||
| Terbium | 159Tb16O+ | ≤1.2 | 175Lu+ | 175 |
The values are based on the published ratios of lanthanide oxides measured by inductively coupled plasma mass spectrometry analysis43.
Comparison of metal isotope chelation methods
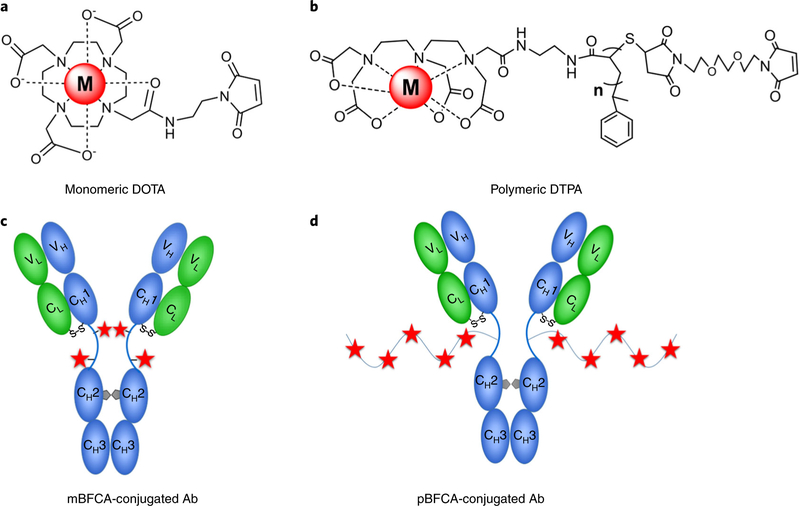
In order to site-specifically attach metal cations to an antibody, bifunctional chelating agents (BFCAs, Fig. 3a,b) are used. These agents consist of two functional groups: (i) the coordinative chelator, either DOTA or DTPA, which enables chelation of metal cations; and (ii) a maleimide-functionalized group, which enables coupling to sulfhydryl groups located in the hinge region between two heavy chains of the IgG antibody that is partially reduced under mild conditions. As some monoclonal antibodies have primary amine groups within the antigen-binding sites, sulfhydryl-reactive BFCAs have less effect on antigen-binding affinities of antibodies than do amine-reactive BFCAs. To increase the number of metal-chelating cations, polymeric BFCAs with multiple chelation sites along the backbone are developed44–46.
Fig. 3 |. Bifunctional chelating agents and corresponding conjugated antibodies.
a,b, Two types of bifunctional chelating agents (BFCAs) are complexed with tagging metal cations: monomeric DOTA (a) and polymeric DTPA (b). The selected antibody (Ab) is coupled with BFCAs via a biorthogonal reaction of maleimide moieties and sulfhydryl groups of cysteine residues in the hinge region between two heavy chains. c,d, Antibody conjugated with monomeric BFCAs (mBFCAs) (c) and antibody conjugated with polymeric BFCAs (pBFCAs) (d).
DOTA and DTPA are polyaminocarboxlyates with multiple oxygen and nitrogen donors that can form stable complexes with trivalent cations of Y, In, Ln, and Bi. The stability constant values (log K) of the complexes of DOTA and lanthanides in water are >22.8 (ref.47). It is important that the buffers used for chelating these cations be substantially different. Y3+, Ln3+, and Ln3+ cations are stable in ammonium acetate buffer (pH 5.5 to 6.0), whereas Bi3+ cations will precipitate in this buffer because of hydrolysis. The optimal condition for chelating Bi3+ cation to DTPA is placement in 2–5% (vol/vol) nitric acid. The complex of DTPA with Bi3+ cation has a distinct feature of the UV-visible absorption spectrum in the range from 250 to 300 nm, which interferes with protein absorption at 280 nm. As a result, although the same BFCA (Maxpar X8 polymer) is used, the antibody conjugation protocol and quantification method for Bi are different from those of Y, In, and Ln, as shown in Fig. 3d.
Polymeric BFCAs can label more metal cations to an antibody than monomeric BFCAs can; however, macromolecular structure and large stereoscopic volumes may affect antibody recognition ability of the antigen. It has been reported that antibodies conjugated with Pd2+ cations using polymeric BFCAs lose their immune specificities41. Conversely, the loss of antigen-binding specificity does not appear to be an issue when using monomeric BFCAs that have smaller molecular structures, e.g., isothiocyanobenzyl-EDTA (SCN-Bn-EDTA, molecular weight (MW) 439 Da) was successfully used to conjugate CD45 antibodies with palladium isotopic cations for barcoding assays of live cells25. A previous study of palladium complexes of aminopolycarboxylic ligands in aqueous solution reported that DTPA complex with Pd2+ is more stable (log K of 36.31) than EDTA complex with Pd2+ (log K of 23.60)48. Therefore, in this presented protocol, monomeric BFCA of maleimide-functionalized-DOTA was used to chelate Pd2+ isotopic cations and was conjugated to the monoclonal antibody, as illustrated in Fig. 3a,c.
Limitations of metal-isotope-tagged antibodies
The newest version of CyTOF (Helios) can simultaneously detect 135 channels with a mass range from 75 to 209 Da. However, the number of channels used to measure surface or intercellular proteins is currently <50, because of the lack of the available mass tags. Although nanoparticle-based mass tags such as metal-embedding nanocrystals, quantum dots, and polymer dots have been considered promising49–53, their routine usage in mass cytometry for single-cell immunoassays still requires more validation because of the inherent issue of nanoparticles, i.e., nonspecific physical absorption easily produces false-positive signals in negative-control cells. Furthermore, the large stereoscopic volumes of nanoparticles will result in inefficient diffusion through cell membranes.
At present, detection sensitivities in most of MitAbs are comparable to those of fluorochrome-tagged antibodies but are lower than those using high-sensitive fluorescent reporters such as phyco-erythrin (PE) dyes13. It is necessary to increase the number of tagged metal cations per antibody for a lower limit of detection. In addition, it should be noted that the protocols presented here are suitable only for IgG immunoglobulins. The protocols involve the reduction procedure of disulfide bonds, which may alter the abilities of IgE or IgM immunoglobulins that contain cross-linking disulfide bonds for their immunity structures.
Applications in single-cell CyTOF immunoassays
In mass cytometry, MitAbs have been widely used to detect cell surface, cytoplasmic, and nuclear proteins for immune phenotyping and signaling pathway profiling. The single-cell CyTOF-based investigations have been involved in the fields of basic and clinic immunology54–59, cancer cell biology60–63, stem cell biology64–66, pharmaceutical development67–69, and elemental biodistribution research70–73. The detection sensitivity of a particular MitAb is dependent on the ionization potential and the ion transmission efficiency of the tagged metal isotope. To obtain high signal-to-noise ratios, antibody panels are optimized to pair antibodies against different expression-level biomarkers with metal isotopes of different sensitivities23,74,75. Two general guidelines are followed. First, biomarkers expected to be of high abundance and thus readily detected (e.g., CD45, CD3, and CD20) are paired with low-sensitivity isotopes (89Y, 102–110Pd, 113In, and 115In) or medium-sensitivity isotopes (139–152Ln). Those biomarkers expected to be of low abundance or difficult to detect (e.g., CD56, p-STAT3, and interferon-γ (IFNγ)) are paired with high-sensitivity isotopes (153–176Ln and 209Bi). Second, care should be taken to avoid pairing a low-abundance biomarker with an isotope that has overlap with the oxide of an isotope conjugated to a high-abundance biomarker.
Experimental design
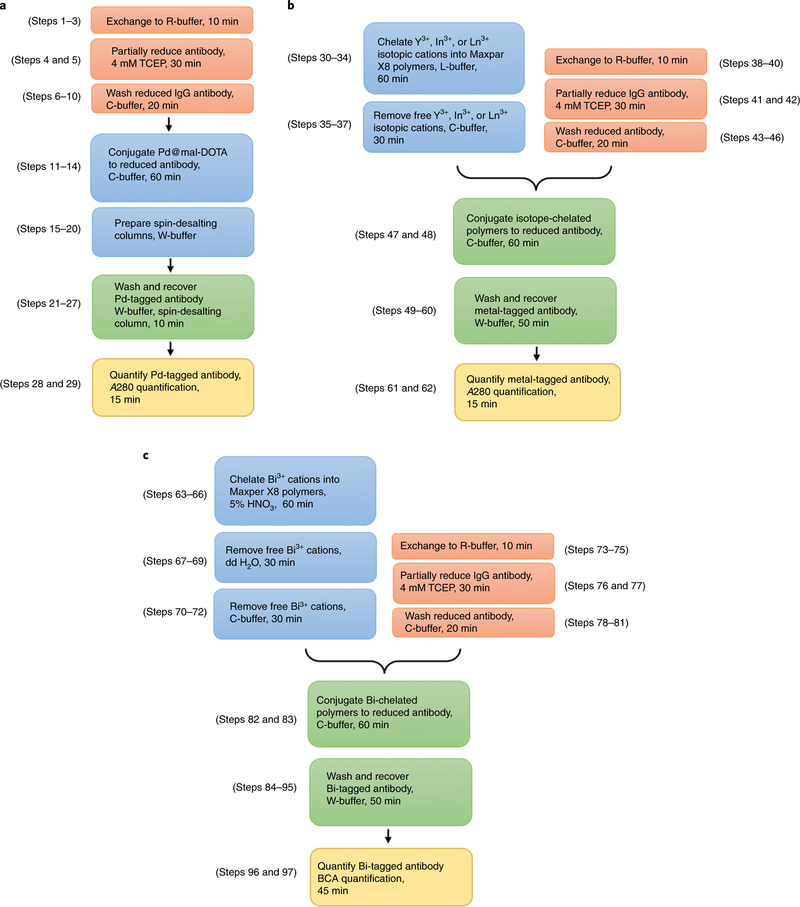
We describe three procedures for conjugation of metal mass tags to monoclonal antibodies for use in high-dimensional mass cytometry. These provide detailed descriptions of the preparation of metal isotopic mass tags, conjugation of IgG antibodies with 48 pure metal isotopes, and quantification methods for the conjugated antibodies. Equipment, including a lyophilizer and mass cytometers, is shown in Fig. 4. Procedures for single-cell immunoassays of CyTOF are also described.
Fig. 4 |. Materials and equipment.
a, Liquid nitrogen tank for snap freezing of the solutions of Pd@mal-DOTA complexes, PCR tubes in a 96-well metal block, dry ice for placing frozen sample PCR tubes, and Drierite desiccant for storing dried palladium mass tags to protect the from moisture. b, Lyophilizer and pump for ice-drying of palladium mass tags. c, CyTOF instruments of v1, v2, and v3 (Helios) used in our lab.
Antibody conjugation and quantification
Box 1 describes the preparation of monomeric BFCA mass tags containing six palladium isotopes using a freeze-drying process. The polymeric BFCA Maxpar X8 polymers were used to conjugate antibodies with the other 42 metal isotopes. Box 2 describes two quantification methods to determine the concentrations of conjugated antibodies. Sections 1, 2, and 3 in the Procedure describe the conjugation of IgG antibodies with metal isotopes of (i) palladium; (ii) lanthanide, yttrium, and indium; and (iii) bismuth, respectively. The timings for these conjugations are summarized in Fig. 5.
Box 1 |. Preparation of palladium isotopic mass tags ● Timing 8 h.
For the conjugation of monoclonal antibodies with six palladium isotopes, as described in Section 1 of the Procedure, mass tags of Pd@mal-DOTA are prepared using the materials and equipment shown in Fig. 4a,b.
Additional reagents
-
Isotope-enriched palladium nitrates with the following purities: 102Pd (91%), 104Pd (96%), 105Pd (98%), 106Pd (99%), 108Pd (99%), and 110Pd (99%) (Trace Sciences International)
! CAUTION Palladium nitrate is toxic. Avoid contact with skin and eyes.
Maleimido-mono-amide-DOTA (mal-DOTA; Macrocyclics, cat. no. B-272)
-
Hydrochloric acid (36.5–38.0% (wt/wt) HCl; Sigma, cat. no. H1758–100ML)
! CAUTION Hydrochloric acid is corrosive. Avoid contact with skin and eyes.
-
Liquid nitrogen
! CAUTION Liquid nitrogen has a boiling temperature of −196 °C at atmospheric pressure. Direct contact can freeze the skin, causing frostbite and cold burns. Protect the hands at all times with cryogenic gloves when handling with liquid nitrogen.
Dry ice
Drierite desiccant (VWR, cat. no. 22891–050)
Additional instruments
Lyophilizer (Labconco, model no. FreeZone 4.5)
Electrospray ionization mass spectrometer (Waters, model no. Micromass ZQ)
Procedure
Dissolve the isotope-enriched palladium nitrates to a 50 mM concentration in hydrochloric acid. Solutions will be dark brown and transparent. Store the solutions for up to 2 years at 4 °C.
-
Weigh out ~25 mg of solid mal-DOTA powder into a 2.0-mL Eppendorf tube. Add an appropriate volume of double-distilled water (ddH2O), then mix well to obtain a 25 mM mal-DOTA solution.
▲ ACRITICAL STEP The aqueous mal-DOTA solution is unstable. The solution should be prepared immediately before use.
Pipette 24 μL of 25 mM mal-DOTA solution and 10 μL of 50 mM palladium solution into a 0.2-mL PCR tube. The molar ratio of DOTA to Pd is 1.2:1. The slight excess of DOTA chelator is used to avoid free palladium cations in the solution.
Cap the tube and vortex to mix. Spin at 1,000g for 10 s at room temperature (RT, ~25°C) in a tabletop microcentrifuge.
Immediately snap-freeze the mixture of mal-DOTA and palladium in liquid nitrogen. Keep the PCR tube upright to ensure that the mixture remains at the bottom of the tube.
Place the frozen PCR tubes into a pre-cooled 96-well metal block sitting on dry ice.
Repeat steps 3–6 for each isotope of palladium to be used. All isotopes should be prepared as quickly as possible.
Replace the original caps of all the PCR tubes with caps pierced with a wide-gauge needle.
Lyophilize the mixtures for at least 6 h. This will yield brown or orange solids.
-
Replace the vented caps with intact caps and place the PCR tubes in a Ziploc bag containing 25 g of Drierite desiccant to protect from moisture.
■ PAUSE POINT The lyophilized mass tags can be stored for up to 2 years at −80 °C.
Dissolve the lyophilized mixtures in 50 μL of dimethyl sulfoxide (DMSO), yielding 10 mM solutions of Pd@mal-DOTA mass tags.
-
Divide the mass tags into aliquots of appropriate volume.
■ PAUSE POINT Palladium isotopic mass tags can be stored for up to 1 year at −20 °C.
? TROUBLESHOOTING
-
Analyze the palladium isotopic mass tags using ESI-MS in negative-ion mode.
▲ ACRITICAL STEP Because mal-DOTA is a triprotic acid and Pd@mal-DOTA is a monoprotic acid, the negative-ion ESI-MS mode should be used for all measurements.
Box 2 |. Quantification of metal-isotope-tagged antibodies ● Timing 1 h.
Two methods are described to quantify MitAbs: an assay based on absorbance at 280 nm (A280) (method A) and a bicinchoninic acid (BCA)-based assay (method B). The A280-based method does not require the generation of a standard curve. The molar extinction coefficient of a typical mammalian IgG antibody with a MW of 150,000 Da is 210,000 M/cm, which is equal to a percentage extinction coefficient of 13.7 for a 1% (wt/vol) (i.e., 10 mg/mL) IgG solution. This assay can be used to quantify the antibodies conjugated with the isotopes of lanthanides, indium, yttrium, and palladium, but not bismuth. The BCA-based method used for the quantification of Bi-tagged antibody requires staining with BCA dyes and measurement of UV-visible absorbance at 562 nm (A562). A calibration curve of A562 versus antibody concentration must be generated using IgG standards of known concentrations.
Additional reagents
BCA protein assay reagent A (Thermo Fisher Scientific, cat. no. 23225)
BCA protein assay reagent B (Thermo Fisher Scientific, cat. no. 23225)
IgG control antibody (1.0 mg; R&D Systems, cat. no. MAB004)
Reagent setup
BCA working solution. Combine 1,000 μL of BCA protein assay reagent A with 20 μL of BCA protein assay reagent B (50:1, reagent A/B), then mix well by vortexing. The solution should be freshly prepared.
IgG antibody standards. Dissolve 1.0 mg of lyophilized IgG control antibody in 1.0 mL of phosphate-buffered saline (PBS) to obtain a 1,000 μg/mL IgG antibody standard. Prepare a series of diluted IgG antibody standards at concentrations of 100, 200, 500, and 750 μg/mL. Store at 4 °C for up to 1 year.
Additional instruments
UV-visible spectrophotometer (Thermo Fisher Scientific, model no. NanoDrop 2000)
Method A (A280-based quantitative assay)
Zero the NanoDrop spectrophotometer with W-buffer.
Pipette 2.0 μL of Y-, Pd-, In-, or Ln-tagged antibody onto the measurement pedestal.
Measure the absorbance of each conjugated antibody at 280 nm (A280).
Calculate the concentration of the conjugated antibody using the average A280 value of three measurements. A concentration of 1,000 μg/mL corresponds to an A280 value of 1.37 absorbance units.
Method B (BCA-based quantitative assay)
Pipette 2.0 μL of each IgG antibody standard and Bi-tagged antibody into separate pre-labeled PCR tubes.
Add 30 μL of pre-prepared BCA working solution to each PCR tube and mix well by pipetting up and down slowly to avoid microbubbles.
Incubate the mixtures at 37 °C in a water bath for 30 min.
Cool all PCR tubes to RT.
Zero the NanoDrop spectrophotometer with ddH2O.
-
Measure the absorbance at 562 nm (A562) of all standards and Bi-tagged antibody within 10 min.
▲ CRITICAL STEP As the BCA assay does not reach a true end point, color development will slowly continue at RT. If all measurements of A562 are completed within 10 min, no substantial error will be introduced.
Plot the intensity of the A562 value of each IgG antibody standard versus its concentration to generate a calibration curve for the quantitative analysis.
-
Calculate the concentration of Bi-tagged antibody using the average A562 value from three measurements.
? TROUBLESHOOTING
Fig. 5 |. Timing for conjugating antibodies with different metal isotopes.
a, Steps for conjugation of palladium isotopic mass tags. b, Steps for conjugation of yttrium, indium, or lanthanide isotopic mass tags. c, Steps for conjugation of bismuth mass tag.
Characterization and applications of palladium isotopic mass tags
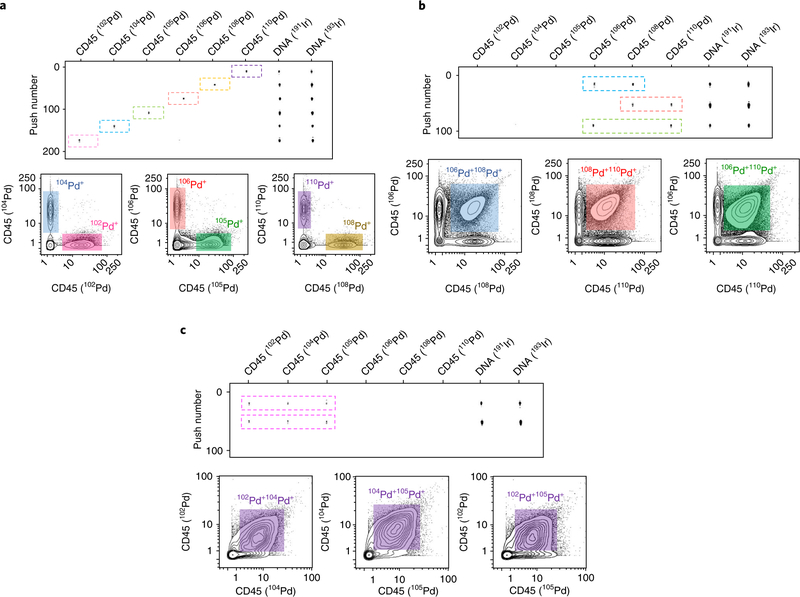
To characterize the synthesized palladium isotopic mass tags (Pd@mal-DOTA), consisting of palladium isotopic cations (Pd2+) and maleimido-mono-amide-DOTA (mal-DOTA), single quadrupole electrospray ionization mass spectrometry (ESI-MS) was used to identify the MW of each palladium isotopic complex, as shown in Fig. 6. Because palladium isotopes have low sensitivities in CyTOF and monomeric mal-DOTA is used to conjugate antibody, palladium isotopic mass tags are suitable for pairing with cellular biomarkers expressed at high levels, such as CD45 in immune cells. In mass cytometry, live-cell barcoding is very important for a reliable comparison between experimental samples; this high-throughput technology overcomes variations in cell staining and CyTOF measurements. Therefore, to demonstrate the utility of palladium isotopic mass tags, CD45 antibody was conjugated with 102Pd, 104Pd, 105Pd, 106Pd, 108Pd, and 110Pd, and was used for live Jurkat cell barcoding. In addition, three strategies for staining combinations were designed for multiplexed barcoding: (i) 6-choose-1, with 6 multiplexing; (ii) 6-choose-2, with 15 multiplexing; and (iii) 6-choose-3, with 20 multiplexing. The measurements of CyTOF ‘rain dots’ and analysis of biaxial contour plots are shown in Fig. 7.
Fig. 6 |. Mass spectra of palladium isotopic mass tags.
a–g, Negative-ion mode mass spectra of mal-DOTA, expected peak m/z = 525.2 (a), 102Pd@mal-DOTA, expected peak m/z = 625.2 (b), 104Pd@mal-DOTA, expected peak m/z = 627.2 (c), 105Pd@mal-DOTA, expected peak m/z = 628.2 (d), 106Pd@mal-DOTA, expected peak m/z = 629.2 (e), 108Pd@mal-DOTA, expected peak m/z = 631.2 (f), and 110Pd@mal-DOTA, expected peak m/z = 633.2 (g). MW, molecular weight.
Fig. 7 |. Barcoding live Jurkat cells using Pd-tagged CD45 antibodies.
Live Jurkat cells were barcoded using three strategies for combining CD45 antibodies tagged with six palladium isotopes. The top section of each panel shows the CyTOF measurements as ‘rain dots’. The bottom sections show the biaxial contour plots of the indicated isotopes. a, 6-choose-1 barcoding strategy: Jurkat cells were stained with each of the six Pd-tagged CD45 antibodies. b, 6-choose-2 barcoding strategy: Jurkat cells were stained with 106Pd- and 108Pd-tagged CD45 antibodies, 108Pd- and 110Pd-tagged CD45 antibodies, or 106Pd- and 110Pd-tagged CD45 antibodies. c, 6-choose-3 barcoding strategy: Jurkat cells were stained with 102Pd-, 104Pd-, and 105Pd-tagged CD45 antibodies.
Titrations of surface and intracellular antibodies
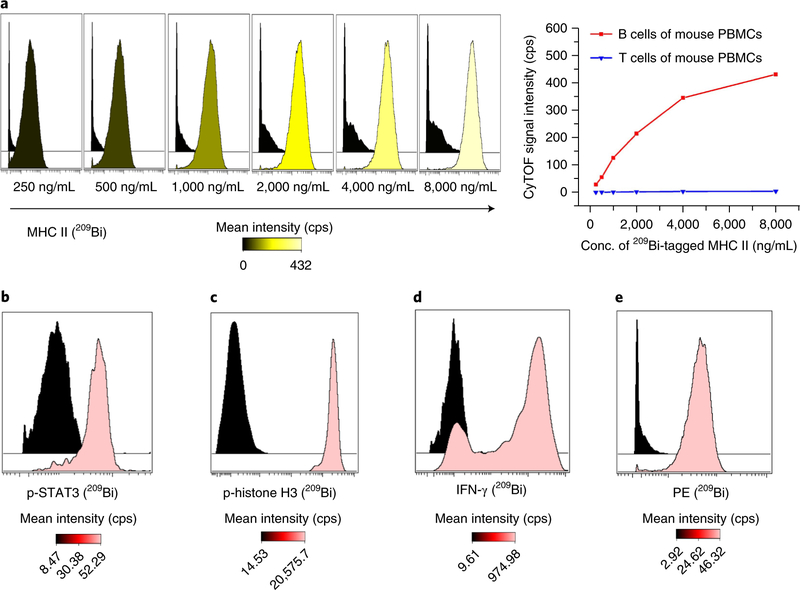
It is critical to choose appropriate cell populations as positive and negative controls to validate and titrate the conjugated antibodies. When using samples of human or mouse peripheral blood mononuclear cells (PBMCs), the positive and negative controls can be identified by gating the subset populations, e.g., to validate an antibody against human CD3, the positive control could be CD2+CD5+ human T cells and the negative control could be CD19+CD20+ human B cells, or to validate an antibody against mouse MHC class II, the positive control could be CD4−CD8−CD45R+ mouse B cells and the negative control could be CD4+or CD8+ mouse T cells. For antibodies against stimulation-inducible intracellular molecules (e.g., pSTAT3) or against secreted molecules (e.g., IFN-γ), appropriate stimulation conditions (e.g., interleukin (IL)-10, IL-12, or IL-18) are applied as positive controls, as shown in Fig. 8.
Fig. 8 |. Titrations of various 209Bi-tagged antibodies.
a, Titration histograms of 209Bi-tagged MHC class II antibody with mouse PBMCs and titration curves in B cells and T cells. b–e, Titration histograms at the optimal concentrations. b, 209Bi-tagged p-STAT3 antibody of 1,000 ng/mL with CD33+CD14+ monocytes in human PBMCs with stimulation of 100 ng/mL IL-10 as positive control and without stimulation as negative control. c, 209Bi-tagged p-histone H3 antibody of 2,000 ng/mL with mesodermal progenitors derived from human embryonic stem cells on day 6 of differentiation as positive control and cardiomyocytes derived from human embryonic stem cells as negative control. d, 209Bi-tagged IFN-γ antibody of 1,000 ng/mL with natural killer cells with stimulation of 50 ng/mL IL-12 and IL-18 as positive control and without stimulation as negative control. e, 209Bi-tagged anti-PE antibody of 1,000 ng/mL with PE-conjugated CD3-antibody-stained T cells and B cells in human PBMCs as positive and negative controls, respectively. cps, counts per second.
Comparisons of bismuth- and lanthanide-tagged antibodies
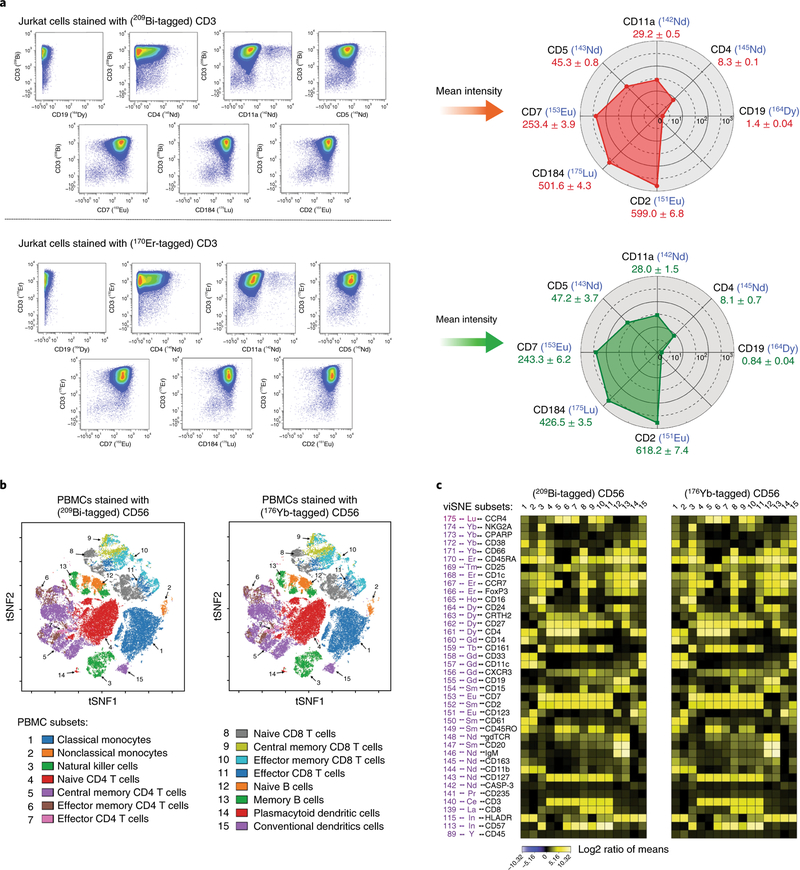
To validate the measurement accuracy of bismuth-tagged antibody in high-dimensional CyTOF assays, the same antibodies were conjugated with both bismuth and lanthanide mass tags: (i) human anti-CD3 tagged with 209Bi and 170Er, (ii) human anti-CD56 tagged with 209Bi and 176Yb, and (iii) mouse anti-MHC class II tagged with 209Bi and 176Yb. Experiments were carried out in Jurkat cells, human PBMCs, and immune cells of mouse organs. The comparisons of the analysis results using biaxial scatter plots, heat maps, viSNE plots, and scaffold maps were performed as shown in Figs. 9 and 10.
Fig. 9 |. Comparisons of bismuth- and lanthanide-tagged antibodies in single-cell assays.
a, Left, Comparable biaxial scatter plots of CD3 versus CD19, CD4, CD11a, CD5, CD7, CD184, and CD2 in Jurkat cells stained with either 209Bi- or 170Er-tagged CD3. a, Right, Data analysis from plots at left, depicted in radar charts of the mean intensities of the seven markers. b, Comparable immunophenotypic gating as shown by viSNE plots of data from human PMBCs stained for 40 markers and either 209Bi- or 176Yb-tagged CD56. Fifteen cell subsets were identified (gating hierarchy described in Supplementary Fig. 2). c, Data from b depicted with heat maps. There is a strong correlation between data collected using 209Bi- and 176Yb-tagged CD56 (Pearson correlation, r = 0.991, P < 0.00001, Supplementary Fig. 1b).
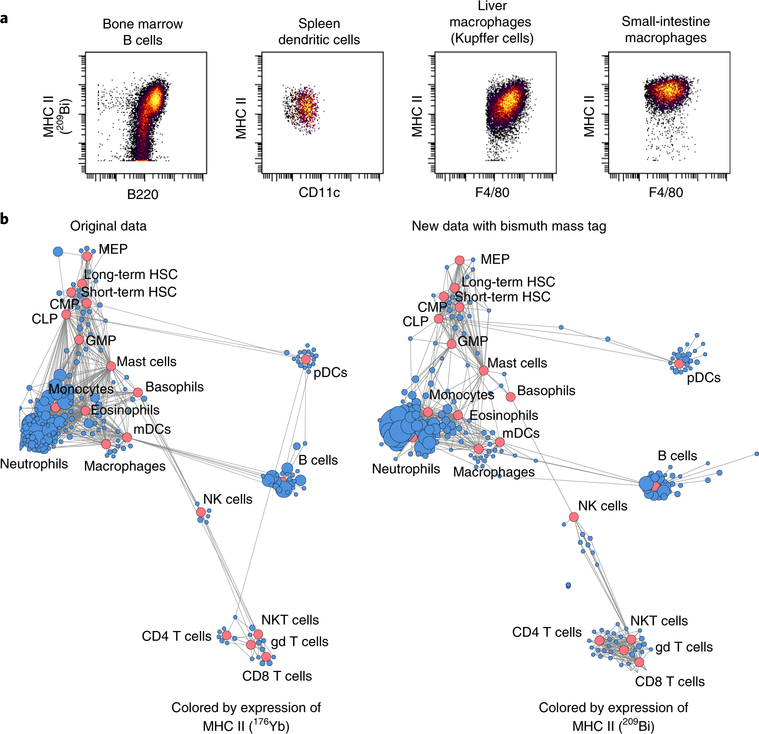
Fig. 10 |. Application of 209Bi-tagged MHC class II antibody in systematic immune analysis.
a, Measurements of expression of MHC class II in different mouse organ cells. b, Comparison of 209Bi- and 176Yb-tagged MHC class II antibodies in scaffold maps, revealing immune organization of the bone marrow for C57BL/6 mice. Red nodes denote landmark manually gated cell populations; blue nodes present unsupervised cell clusters from the same data. Data in left panel of b from ref.9. gd T cells, γδ T cells; HSC, hematopoietic stem cell; mDCs, myeloid dendritic cells; NK, natural killer; NKT, natural killer T; pDCs, plasmacytoid dendritic cells.
Materials
Reagents
Palladium conjugation
Tris-(2-carboxyethyl)phosphine hydrochloride (TCEP; Thermo Fisher Scientific, cat. no. 77720)
C-buffer for antibody conjugation (Maxpar Labeling Kits; Fluidigm)
W-buffer for washing conjugated antibodies (Maxpar Labeling Kits; Fluidigm)
Antibody stabilizer (Candor Biosciences, cat. no. 131050)
Zeba 7-kDa 0.5-mL spin desalting columns (Thermo Fisher Scientific, cat. no. 89883)
Lanthanide, yttrium, and indium conjugation
Tris-(2-carboxyethyl)phosphine hydrochloride (TCEP; Thermo Fisher Scientific, cat. no. 77720)
Maxpar X8 polymer (Maxpar Labeling Kits; Fluidigm)
L-buffer for chelation of polymers (Maxpar Labeling Kits; Fluidigm)
R-buffer for partial reduction of antibodies (Maxpar Labeling Kits; Fluidigm)
C-buffer for antibody conjugation (Maxpar Labeling Kits; Fluidigm)
W-buffer for washing conjugated antibodies (Maxpar Labeling Kits; Fluidigm)
Antibody stabilizer (Candor Biosciences, cat. no. 131050)
Isotope-enriched lanthanide (III) solution (Fluidigm, catalog number varies by isotope)
Isotope-enriched lanthanide (III) nitrates or chlorides (Trace Sciences International)
Yttrium (III) nitrate tetrahydrate (Sigma-Aldrich, cat. no. 217239–10G)
Isotope-enriched indium (III) chlorides (Trace Sciences International)
Bismuth conjugation
Tris-(2-carboxyethyl)phosphine hydrochloride (TCEP; Thermo Fisher Scientific, cat. no. 77720)
Maxpar X8 polymer (Maxpar Labeling Kits; Fluidigm)
R-buffer for partial reduction of antibodies (Maxpar Labeling Kits; Fluidigm)
C-buffer for antibody conjugation (Maxpar Labeling Kits; Fluidigm)
W-buffer for washing conjugated antibodies (Maxpar Labeling Kits; Fluidigm)
Antibody stabilizer (Candor Biosciences, cat. no. 131050)
Nitric acid (HNO3; Sigma-Aldrich, cat. no. 225711)
Bismuth (III) nitrate pentahydrate (Sigma-Aldrich, cat. no. 254150)
Purified antibodies
100 μg of IgG antibody in low-sodium azide buffer (carrier-free) with specificity of choice, such as purified anti-human CD45 antibody (BD Biosciences, cat. no. 555480); purified anti-human CD3 antibody (BioLegend, cat. no. 300416); purified anti-human p-STAT3 antibody (BD Biosciences, cat. no. 612357); purified anti-human p-histone H3 antibody (BioLegend, cat. no. 650802); purified anti-human IFN-γ antibody (BD Biosciences, cat. no. 554698); or purified anti-mouse MHC class II antibody (BioLegend, cat. no. 107637). We provide a list of antibodies used in CyTOF assays in Supplementary Table 1. Store according to the manufacturer’s instructions. ▲ CRITICAL The antibodies must be free of cysteine-containing carrier proteins (e.g., BSA) for efficient conjugation, as the carrier may compete for maleimide-functionalized groups. If a carrier is present, antibody cleanup kits (e.g., Abcam BSA Removal Kit, cat. no. ab173231; Melon Gel IgG Spin Purification Kit, Thermo Fisher Scientific, cat. no. 45206) can be used for antibody purification.
Cell assays
Jurkat Clone E6–1 human T lymphocyte cell line (ATCC, cat. no. TIB-152). Human and mouse PBMCs are isolated from whole blood using Ficoll-Paque density gradient centrifugation. Cell suspensions from tissues or organs are prepared as previously described9,39. For a reliable CyTOF analysis, at least 1.0 × 106 cells per sample and two replicates are required. ! CAUTION The cell lines used in your research should be regularly checked to ensure that they are authentic and are not infected with mycoplasma. ! CAUTION All experiments involving animal or human samples should adhere to the relevant governmental and institutional ethics guidelines and regulations. Informed consent should be obtained from donors of human blood or tissue. All of our animal studies were performed in accordance with the investigators’ protocols approved by the Stanford University institutional animal care and use committee.
RPMI 1640 cell culture medium (Life Technologies, cat. no. 21870–092)
FBS (Thermo Fisher Scientific, cat. no. SH3007003)
Penicillin-streptomycin (10,000 U/mL; Thermo Fisher Scientific, cat. no. 15140122)
L-glutamine (100x; Thermo Fisher Scientific, cat. no. 25030081)
Recombinant human IL-10 (BD Biosciences, cat. no. 554611)
Recombinant human IL-12 (Peprotech, cat. no. 200–12)
Recombinant human IL-18 (R&D Systems, cat. no. B001–5)
Brefeldin A solution (1,000x; eBioscience, cat. no. 00-4506-51)
Monensin solution (1,000x; eBioscience, cat. no. 00-4505-51)
Ficoll-Paque plus density gradient medium (Thermo Fisher Scientific, cat. no. 45-001-749)
Iridium Ir191/Ir193 DNA intercalator (Fluidigm, cat. no. 201192A)
16% (wt/vol) Paraformaldehyde (PFA, electron microscopy grade; Electron Microscopy Sciences, cat. no. 15710) ! CAUTION PFA is an irritant. Avoid contact with skin and eyes. Avoid inhalation.
Saponin (Sigma-Aldrich, cat. no. S-7900)
Methanol (Thermo Fisher Scientific, cat. no. A412–4) ! CAUTION Methanol is flammable. Keep away from heat. Avoid contact with skin and eyes. Avoid inhalation.
Dimethyl sulfoxide (DMSO; Sigma-Aldrich, cat. no. D2650)
BSA fraction V (Sigma-Aldrich, cat. no. A-2153)
Sodium azide (NaN3; Sigma-Aldrich, cat. no. S-8032) ! CAUTION Sodium azide is highly toxic. Avoid contact with skin, eyes, and clothing. This compound is fatal if swallowed.
Sodium phosphate dibasic heptahydrate (Sigma-Aldrich, cat. no. S9390)
Potassium phosphate monobasic anhydrous (Sigma-Aldrich, cat. no. P0662)
Potassium chloride (Thermo Fisher Scientific, cat. no. P330)
Sodium chloride (Thermo Fisher Scientific, cat. no. S271)
Equipment
Tabletop microcentrifuge (Eppendorf, model no. 5424)
Tabletop refrigerated swinging-bucket centrifuge (Beckman Coulter, model no. Allegra 6R)
Aspirator (VP Scientific, cat. no. VP 177A-1)
Screw-top Eppendorf tubes (Thermo Fisher Scientific, cat. no. 02-707-353)
Polystyrene round-bottom test tubes with 35-μ™ filter cap (FACS tubes; Thermo Fisher Scientific, cat. no. 08-771-23)
Automated cell counter (Bio-Rad, model no. TC20)
Digital-control water bath (Thermo Fisher Scientific, cat. no. 15-462-5Q)
Mass cytometers (CyTOF version 1, CyTOF version 2, and CyTOF version 3 (Helios); Fluidigm)
3-kDa Amicon Ultra 0.5-mL centrifugal filter column (EMD Millipore, cat. no. UFC500396)
50-kDa Amicon Ultra 0.5-mL centrifugal filter column (EMD Millipore, cat. no. UFC505096)
Syringe filter with 200-nm nylon membrane (Pall, cat. no. 4550)
Reagent setup
5.0% (vol/vol) HNO3 solution
Mix 5 mL of high-purity concentrated HNO3 with 95 mL of ddH2O. Store for up to 2 years at RT. ! CAUTION HNO3 is very corrosive. Avoid contact with skin, eyes, and clothing.
100 mM Ammonium acetate solution, pH 5.5
Dissolve 7.7 g of ammonium acetate in 995 mL of ddH2O and add 1.03 mL of glacial acetic acid.
50 mM Lanthanide isotope solutions
Dissolve ~25 mg of lanthanide nitrate or chloride in an appropriate volume of L-buffer or in 100 mM ammonium acetate solution to obtain a 50 mM 139–176Ln3+ solution. Mix gently by pipetting up and down using filter tips. Store at 4 °C for up to 2 years.
50 mM Yttrium solution
Dissolve ~10 mg of 89Y(NO3)3•4H2O in an appropriate volume of L-buffer or in 100 mM ammonium acetate solution to obtain a 50 mM 89Y3+ solution. Mix gently by pipetting up and down using filter tips. Store at 4 °C for up to 2 years.
50 mM Indium isotope solutions
Dissolve ~10 mg of 113InCl3 and 115InCl3 individually in an appropriate volume of L-buffer or 100 mM ammonium acetate solution to obtain 50 mM 113In3+ and 115In3+ solutions. Mix gently by pipetting up and down using filter tips. Store at 4 °C for up to 2 years.
50 mM Bismuth solution
Dissolve ~25 mg of 209Bi(NO3)3•5H2O in an appropriate volume of 5.0% (vol/vol) HNO3 to obtain a 50 mM 209Bi3+ solution. Mix gently by pipetting up and down using filter tips. Store at 4 °C for up to 2 years. ▲ CRITICAL STEP The concentration of HNO3 should not be <5.0% (vol/vol); a lower concentration of HNO3 may result in precipitation of bismuth in the following steps.
10× PBS
Dissolve 320 g of sodium chloride, 8 g of potassium chloride, 46 g of sodium phosphate dibasic heptahydrate, 8 g of potassium phosphate monobasic (anhydrous) in 3 L of ddH2O, adjust to pH 7.4 with sodium hydroxide, and bring to a volume of 4 L. Store at RT for up to 6 months. ! CAUTION Use high-purity reagents to avoid metal contamination from common lab chemicals.
PBS
Dilute 10x PBS at a 1:10 ratio with ddH2O. Store at RT for up to 6 months.
Cell staining medium
Dissolve appropriate amounts of BSA and NaN3 to achieve concentrations of 0.5% (wt/vol) and 0.02% (wt/vol), respectively, in PBS. Store at 4 °C for up to 6 months.
Cell culture medium
Add 50 mL of FBS, 5 mL of penicillin-streptomycin, and 5 mL of L-glutamine to 500 mL of RPMI 1640 medium. Store at 4 °C for up to 6 months.
Iridium Ir191/Ir193 DNA intercalator solution
Mix 1 mL of PBS with 100 μL of 16% (wt/vol) PFA solution. For methanol-permeabilized cells, add 0.25 μL of 500 μM iridium DNA intercalator (1:4,000); for non-permeabilized cells, add 0.40 μL of 500 μM iridium DNA intercalator (1:2,500). ! CAUTION Iridium compounds are highly toxic. Avoid contact with skin, eyes, and clothing.
4 mM TCEP solution for antibody partial reduction
Mix 8 μL of 500 mM TCEP stock with 992 μL of R-buffer; vortex briefly.
Antibody stabilization solution
Add 0.1% (vol/vol) NaN3 to antibody stabilizer. Store it at 4 °C for up to 1 year.
Procedure
▲ CRITICAL The Procedure has two main parts: the first contains three sections describing three individual protocols for antibody conjugation; the second has one section describing cell staining procedures to validate and titrate the conjugated antibodies. Section 1 starts at Step 1 and describes the conjugation of reduced antibodies with Pd@mal-DOTA mass tags that are prepared as described in Box 1. Section 2 starts at Step 30 and describes the chelation of Y3+, In3+, or Ln3+ cations into Maxpar X8 polymers, and the conjugation with reduced antibodies. Section 3 starts at Step 63 and describes the chelation of Bi3+ cations into Maxpar X8 polymers, and the conjugation with reduced antibodies. Section 4 describes brief procedures and instructions for staining cells with different types of conjugated antibodies (i.e., against stable surface and intracellular molecules and against stimulation-induced signaling molecules and cytokines).
Section 1: conjugation of antibody with palladium isotopic mass tags
Partial reduction of antibody ● Timing ~1 h
-
1
Add 100 μg of antibody stock to a 50-kDa 0.5-mL centrifugal filter column.
▲ CRITICAL STEP The filter columns are designed to hold up to 500 μL of solution. If 100 μg of antibody requires addition of >200 μL of solution, pre-concentrate the antibody first in the same filter column by spinning at 12,000g for 10 min at RT.
-
2
Add 300 μL of R-buffer to the column to bring it a final volume of 500 μL.
-
3
Spin the column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
4
Add 100 μL of 4 mM TCEP solution to the antibody in the filter column, mix thoroughly by pipetting up and down using filter tips.
-
5
Incubate the mixture at 37 °C for 30 min in a water bath.
▲ CRITICAL STEP The antibody should not be incubated in 4 mM TCEP solution for >30 min; a longer incubation may result in the reduction of disulfide bonds that are necessary for the structural integrity of the antibody.
-
6
Add 300 μL of C-buffer to the partially reduced antibody in the filter column to stop the reaction.
-
7
Spin the column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
8
Add 400 μL of C-buffer to the partially reduced antibody in the filter column.
-
9
Spin the column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
▲ CRITICAL STEP The second wash step is necessary to remove excess TCEP solution.
-
10
Resuspend the partially reduced antibody in 100 μL of C-buffer. Mix by pipetting up and down using filter tips.
Conjugation of the reduced antibody with Pd@mal-DOTA mass tags ● Timing ~1 h
-
11
Retrieve one PCR tube of 10 mM Pd@mal-DOTA mass tags (prepared as described in Box 1) with the desired isotope (102Pd, 104Pd, 105Pd, 106Pd, 108Pd, or 110Pd).
-
12
Warm the tube to RT. Vortex briefly and spin at 1,000g for 10 s in a microcentrifuge to ensure that the reagent is at the bottom of the tube.
-
13
Add 1 μL of 10 mM Pd@mal-DOTA to the antibody solution (from Step 10).
-
14
Mix the solution thoroughly by pipetting up and down using filter tips. Incubate the mixture at 37 °C for 1 h in a water bath.
-
15
During the incubation, prepare three Zeba 7-kDa 0.5-mL spin desalting columns, remove the columns’ closures and loosen the caps. Place each column in a 2.0-mL collection tube.
-
16
Spin at 1,500g for 1 min at RT to remove the storage solution.
-
17
Make a mark on the side of each column where the compacted resin is slanted upward. Place the columns in the microcentrifuge with the mark facing outward in all subsequent centrifugation steps.
-
18
Add 300 μL of W-buffer on top of the resin bed, spin at 1,500g for 1 min at RT, and discard the column flow-through.
-
19
Add 300 μL of W-buffer on top of the resin bed, spin at 1,500g for 1 min at RT, and discard the column flow-through.
-
20
Put these three columns into new 2.0-mL collection tubes marked W-1, W-2, and W-3, respectively.
Washing and recovering the Pd-tagged antibody ● Timing ~10 min
-
21
Transfer the solution of conjugated antibody (from Step 14) to the top of the compact resin bed of the tube marked W-1.
-
22
Spin at 1,500g for 2 min at RT to collect the flow-through. Discard the desalting column after use.
-
23
Transfer the flow-through of W-1 to the top of the compact resin bed of the tube marked W-2.
-
24
Spin at 1,500g for 2 min at RT to collect the flow-through. Discard the desalting column after use.
-
25
Transfer the flow-through of W-2 to the top of the compact resin bed of the tube marked W-3.
-
26
Spin at 1,500g for 2 min at RT to collect the flow-through. Discard the desalting column after use.
-
27
Transfer the flow-through of W-3 to a new screw-top tube. Label it with the name of the antibody and the palladium isotope.
■ PAUSE POINT The conjugated antibody can be stored at 4 °C for up to 1 month.
? TROUBLESHOOTING
Quantification of the Pd-tagged antibody ● Timing ~15 min
-
28
Determine the concentration of the Pd-tagged antibody using method A, which is described in Box 2.
-
29
Dilute the Pd-tagged antibody to an appropriate concentration (0.2 mg/mL is recommended) in antibody stabilization solution.
■ PAUSE POINT Store at 4 °C for up to 6 months.
Section 2: conjugation of antibody with yttrium, indium, or lanthanide isotopes
Chelation of trivalent isotopic cations (Y3+, In3+, or Ln3+) into Maxpar X8 polymers ● Timing ~1 h
-
30
Retrieve one tube (~200 μg) of lyophilized Maxpar X8 polymers, this is sufficient to conjugate 100 μg of IgG antibody. Spin the tube at 1,000g for 10 s at RT in a microcentrifuge to ensure that the reagent is at the bottom of the tube.
-
31
Dissolve the polymers in 95 μL of L-buffer. Mix gently by pipetting up and down.
-
32
Add 5 μL of 50 mM Y3+, In3+, or Ln3+ isotopic cations (in L-buffer or 100 mM ammonium acetate buffer, pH 5.5) to the polymer solution for a final concentration of 2.5 mM. Mix gently by pipetting up and down using filter tips.
-
33
Incubate the mixture at RT for 1 h. Mix approximately every 20 min by pipetting up and down using filter tips.
-
34
After 30 min, start Step 38 (partially reduce the IgG antibody) to ensure that the metal-isotope-chelated polymers and partially reduced antibody will be ready at the same time. If additional steps are required to pre-concentrate the antibody, this step should begin 10 min earlier.
▲ CRITICAL STEP The maleimide-functionalized groups of Maxpar X8 polymers hydrolyze in aqueous solutions; the reduction of the disulfide bonds of antibodies is reversible in aqueous solutions. To obtain efficient antibody conjugation, make sure that the chelated Maxpar X8 polymers and the reduced antibody are ready at same time.
Removal of free Y3+, In3+, or Ln3+ isotopic cations ● Timing ~30 min
-
35
After 1-h incubation, add 300 μL of C-buffer to the isotope chelation mixture (from Step 33) for a final volume of 400 μL. Transfer the solution to a 3-kDa MWCO filter column.
-
36
Spin the column at 14,000g for 30 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
37
Re-suspend Y3+, In3+, or Ln3+ isotope-chelated polymers in 200 μL of C-buffer. Gently pipette to mix and rinse the walls of the filter column to increase the recovery of the polymers.
Partial reduction of the IgG antibody ● Timing ~1 h
-
38
Add 100 μg of antibody stock to a 50-kDa 0.5-mL centrifugal filter column.
▲ CRITICAL STEP The filter columns are designed to hold up to 500 μL of solution. If 100 μg of antibody requires addition of >200 μL of solution, pre-concentrate the antibody first in the same filter column by spinning at 12,000g for 10 min at RT.
-
39
Add 300 μL of R-buffer to the column to bring a final volume of 500 μL.
-
40
Spin the column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
41
Add 100 μL of 4 mM TCEP solution to the antibody in the filter column and mix thoroughly by pipetting up and down using filter tips.
-
42
Incubate the mixture at 37 °C for 30 min in a water bath.
▲ CRITICAL STEP The antibody should not be incubated in 4 mM TCEP solution for >30 min; a longer incubation may result in the reduction of disulfide bonds that are necessary for the structural integrity of the antibody.
-
43
Add 300 μL of C-buffer to the partially reduced antibody in the filter column to stop the reaction.
-
44
Spin the column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
45
Add 400 μL of C-buffer to the partially reduced antibody in the filter column.
-
46
Spin the column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
▲ CRITICAL STEP The second wash step is necessary to remove excess TCEP solution.
Conjugation of the reduced antibody with metal-isotope-chelated polymers ● Timing ~1 h
-
47
Transfer the isotope-chelated polymers suspended in C-buffer (from Step 37) to the partially reduced antibody in the 50-kDa MWCO filter column. Mix thoroughly by pipetting up and down using filter tips.
-
48
Incubate the mixture at 37 °C for 1 h in a water bath.
Washing and recovering the metal-isotope-tagged antibody ● Timing ~50 min
-
49
Add 200 μL of W-buffer to the filter column. Mix thoroughly by pipetting up and down using filter tips.
-
50
Spin the filter column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
51
Add 400 μL of W-buffer to the filter column. Mix thoroughly by pipetting up and down using filter tips.
-
52
Spin the filter column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
53
Repeat Steps 51 and 52 two more times.
-
54
Add 50 μL of W-buffer to the filter column. Pipette to mix and rinse the walls of the filter thoroughly.
-
55
Invert the filter column into a new collection tube.
-
56
Spin the inverted filter and collection tube assembly at 2,000g for 2 min at RT.
-
57
Gently remove the filter column from the collection tube. Add an additional 50 μL of W-buffer to the filter column. Pipette to mix and rinse the walls of the filter thoroughly.
-
58
Invert the filter column into the same collection tube.
-
59
Spin the assembly at 2,000g for 2 min at RT.
-
60
Transfer the MitAb solution to a new screw-top tube.
■ PAUSE POINT The conjugated antibody can be stored at 4 °C for up to 1 month.
Quantification of the metal-isotope-tagged antibody ● Timing ~15 min
-
61
Determine the concentration of Y-, In-, or Ln-tagged antibody using method A, which is described in Box 2.
? TROUBLESHOOTING
-
62
Dilute the conjugated antibody to an appropriate concentration (0.2 mg/mL is recommended) in antibody stabilization solution.
▲ CRITICAL STEP Ideally, the antibody should be diluted such that 1–2 μL is sufficient for a 100-μL cell-staining reaction. This facilitates the creation of low-volume staining cocktails.
■ PAUSE POINT Store at 4 °C for up to 6 months.
Section 3: conjugation of antibody with bismuth
Chelation of 209Bi3+ cations into Maxpar X8 polymers ● Timing ~1 h
-
63
Retrieve one tube (~200 μg) of lyophilized Maxpar X8 polymers; this is sufficient to conjugate 100 μg of IgG antibody. Spin at 1,000g for 10 s at RT in a microcentrifuge to ensure that the reagent is at the bottom of the tube.
-
64
Dissolve the polymers in 95 μL of 5.0% (vol/vol) HNO3. Mix gently by pipetting up and down.
▲ CRITICAL STEP Do not dissolve the polymers in other solutions such as ddH2O or L-buffer because these solvents will result in the precipitation of 209Bi3+ cations.
-
65
Add 5 μL of 50 mM 209Bi3+ solution (in 5.0% (vol/vol) HNO3) to the polymer solution. The final concentration of 209Bi3+ cations will be 2.5 mM. Gently mix by pipetting up and down using filter tips.
-
66
Incubate the mixture at RT for 1 h. Mix approximately every 20 min by pipetting up and down using filter tips.
Removal of free 209Bi3+ cations ● Timing ~1 h
-
67
Add 300 μL of ddH2O to the bismuth chelation mixture for a final volume of 400 μL. Transfer the solution to a 3-kDa MWCO filter column.
▲ CRITICAL STEP Do not add other solutions, such as PBS, R-buffer, or C-buffer, because these solvents may result in the precipitation of free 209Bi3+ cations.
-
68
Spin the filter column at 14,000g for 30 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
69
When centrifugation starts, begin Step 73 (partial reduction of IgG antibody) to ensure that 209Bi-chelated polymers and reduced antibody will be ready at the same time. If additional steps are required to pre-concentrate the antibody, this step should begin 10 min earlier.
▲ CRITICAL STEP The maleimide-functionalized groups of Maxpar X8 polymers hydrolyze in aqueous solutions; the reduction of the disulfide bonds of the antibodies is reversible in aqueous solutions. To obtain efficient antibody conjugation, make sure the chelated Maxpar X8 polymers and the reduced antibody are ready at same time.
-
70
Add 400 μL of C-buffer to the filter column (from Step 68).
? TROUBLESHOOTING
-
71
Spin the filter column at 14,000g for 30 min at RT. The final volume should not be >20 μL; discard the column flow-through.
▲ CRITICAL STEP The second wash in C-buffer is important for removing the excess 209Bi3+ cations and adjusting the pH value for antibody conjugation.
-
72
Resuspend the 209Bi-chelated polymers in 200 μL of C-buffer. Gently pipette to mix and rinse the walls of the filter column to increase the recovery of the polymers.
Partial reduction of the IgG antibody ● Timing ~1 h
-
73
Add 100 μg of antibody stock to a 50-kDa 0.5-mL centrifugal filter column.
▲ CRITICAL STEP The filter columns are designed to hold up to 500 μL of solution. If 100 μg of antibody requires addition of >200 μL of solution, pre-concentrate the antibody first in the same filter column by spinning at 12,000g for 10 min at RT.
-
74
Add 300 μL of R-buffer to the column to bring it a final volume of 500 μL.
-
75
Spin at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
76
Add 100 μL of 4 mM TCEP solution to the antibody in the filter column and mix thoroughly by pipetting up and down using filter tips.
-
77
Incubate the mixture at 37 °C for 30 min in a water bath.
▲ CRITICAL STEP The antibody should not be incubated in 4 mM TCEP solution for >30 min; a longer incubation may result in the reduction of the disulfide bonds that are necessary for the structural integrity of the antibody.
-
78
Add 300 μL of C-buffer to the partially reduced antibody in the filter column to stop the reaction.
-
79
Spin at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
80
Add 400 μL of C-buffer to the partially reduced antibody in the filter column.
-
81
Spin at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
▲ CRITICAL STEP The second wash step is necessary to remove excess TCEP solution.
Conjugation of the reduced antibody with 209Bi-chelated polymers ● Timing ~1 h
-
82
Transfer the 209Bi-chelated polymers suspended in C-buffer (from Step 72) to the partially reduced antibody in the 50-kDa MWCO filter column. Mix thoroughly by pipetting up and down using filter tips.
-
83
Incubate the mixture at 37 °C for 1 h in a water bath.
Washing and recovering the 209Bi-tagged antibody ● Timing ~50 min
-
84
Add 200 μL of W-buffer to the filter column. Mix thoroughly by pipetting up and down using filter tips.
-
85
Spin the filter column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
86
Add 400 μL of W-buffer to the filter column. Mix thoroughly by pipetting up and down using filter tips.
-
87
Spin the filter column at 12,000g for 10 min at RT. The final volume should not be >20 μL; discard the column flow-through.
-
88
Repeat Steps 86 and 87 two more times.
-
89
Add 50 μL of W-buffer to the filter column. Pipette to mix and rinse the walls of the filter thoroughly.
-
90
Invert the filter column into a new collection tube.
-
91
Spin the inverted filter and collection tube assembly at 2,000g for 2 min at RT.
-
92
Gently remove the filter column from collection tube. Add an additional 50 μL of W-buffer to the filter column. Pipette to mix and rinse the walls of the filter thoroughly.
-
93
Invert the filter column into the same collection tube.
-
94
Spin the assembly at 2,000g for 2 min at RT.
-
95
Transfer the solution of 209Bi-tagged antibody to a new screw-top tube.
■ PAUSE POINT The conjugated antibody can be stored at 4 °C for up to 1 month.
Quantification of the 209Bi-tagged antibody ● Timing ~45 min
-
96
Determine the concentration of 209Bi-tagged antibody using the method B, described in Box 2.
-
97
Dilute the 209Bi-tagged antibody to an appropriate concentration (0.2 mg/mL is recommended) in antibody stabilization solution.
■ PAUSE POINT Store at 4 °C for up to 6 months.
Part 4
Cell staining for validation and titration of metal-isotope-tagged antibodies ● Timing ~45 min
-
98Identify the positive- and negative-control cell populations for the conjugated antibody. The differential selection of populations and stimulation procedures are described below: option A is for antibodies against stable surface or intracellular molecules (e.g., CD45, CD3, MHC II, and p-histone H3); option B is for antibodies against stimulation-inducible intracellular molecules or modifications (e.g., pSTAT3); and option C is for antibodies against secreted molecules (e.g., IFN-γ).
- Antibodies against a stable surface or intracellular molecule
- Add 1 mL of cell culture medium or cell staining medium at 37 °C to FACS tubes.
- For live-cell barcoding with Pd-tagged CD45 antibodies (Pd-CD45), 1.0 × 106 Jurkat cells are added to each FACS tube and pelleted by centrifuging at 500g for 5 min at RT. In the strategy of 6-choose-1, six FACS tubes should be prepared, each containing one antibody of 100 μL of 2 μg/ml Pd-CD45; in the strategy of 6-choose-2, three FACS tubes should be prepared, each containing two antibodies of 50 μL of 2 μg/ml Pd-CD45; in the strategy of 6-choose-3, one FACS tube should be prepared containing three antibodies of 50 μL of 1.5 μg/ml Pd-CD45.
- Incubate the cells for 30 min at RT with gentle agitation.
- Wash the cells twice using 4 mL of cell staining medium by centrifuging at 500g for 5 min at RT.
- Antibody against an induced intracellular molecule (e.g., pSTAT3)
- Add 1 mL of the cell culture medium at 37 °C to FACS tubes labeled ‘stimulated’ and ‘unstimulated’. To obtain reliable results, at least two replicates are required.
- Add 10 × 106 human PBMCs to each tube.
- Add stimulation molecules such as IL-10 to the tubes labeled ‘stimulated’ to a final concentration of 100 ng/mL; add an equal volume of cell culture medium to the tubes labeled ‘unstimulated’.
-
Incubate the cells at 37 °C for 15 min.▲ CRITICAL STEP Cellular-signaling events are very time sensitive. Be ready to proceed to Step 99 immediately.▲ CRITICAL STEP The stimulation should result in selective induction of the activated form of the signaling molecule of interest. Additional information on antibody targets and appropriate stimulation is provided in previous work76.
- Antibody against a secreted intracellular cytokine (e.g., IFN-γ)
- Add 1 mL of cell culture medium at 37 °C to FACS tubes labeled ‘stimulated’ and ‘unstimulated’. To obtain reliable results, at least two replicates are required.
- Add 10 × 106 human PBMCs to each tube.
- Add a stimulation cocktail, such as a combination of IL-12 and IL-18, to final concentrations of 50 ng/mL to the tubes labeled ‘stimulated’; add an equal volume of cell culture medium to the tubes labeled ‘unstimulated’.
- Add the secretion inhibitors Brefeldin A and Monensin to each tube; 1,000x stocks should be added at a dilution of 1:1,000.
-
Incubate the cells at 37 °C for 15 min.▲ CRITICAL STEP The stimulation should result in selective induction of the activated form of the molecule of interest. Additional information on secreted cytokines and appropriate stimulation can be found in previous work21.
Fixing and permeabilization of the cells ● Timing ~30 min
-
99
Add 100 μL of 16% (wt/vol) PFA to each FACS tube and pipette up and down thoroughly. Incubate for 10 min at RT.
-
100
Wash the cells twice using 4 mL of cell staining medium by centrifuging at 500g for 5 min at RT.
-
101
Add 1 mL of −20 °C methanol to each of the disrupted cell pellets. Vortex to mix and incubate on ice for 10 min.
■ PAUSE POINT Store at −80 °C for up to 1 month.
Immune staining with the titrated antibodies ● Timing ~1 h
-
102
For antibody titrations, wash the cells twice using 4 mL of cell staining medium by centrifuging at 500g for 5 min at RT.
-
103
Aspirate the supernatant and leave the cells in 300 μL of residual volume, then divide the cells into six new FACS tubes.
-
104
Prepare a six-step and twofold serial dilution of the conjugated antibodies. Add 50 μL of the diluted antibodies to each 50 μL of cell sample. The final titration concentrations of the conjugated antibodies range from 250 to 8,000 ng/mL.
-
105
Incubate the cells for 30 min at RT with gentle agitation.
-
106
Wash the cells twice using 4 mL of cell staining medium by centrifuging at 500g for 5 min at RT.
Staining of the cells in DNA intercalator solution ● Timing ~30 min
-
107
Add 1 mL of Ir DNA intercalator solution to each FACS tube and mix thoroughly. Incubate for at least 20 min at RT.
■ PAUSE POINT Cells can be stored in DNA intercalator solution for up to 3 d at 4 °C before acquisition on a mass cytometer.
-
108
Wash the cells twice using 4 mL of cell staining medium by centrifuging at 500g for 5 min at RT.
-
109
Wash the cells using 4 mL of ddH2O by centrifuging at 500g for 5 min at RT and then resuspend the cells in ddH2O at a concentration of 1–2 × 106 cells per mL.
-
110
Filter the cells through a 35-μm filter cap and measure each tube using the mass cytometer (illustrated in Fig. 4c). Collect cell events at a rate of 500–1,000 cells per s.
Troubleshooting
Troubleshooting advice can be found in Table 2.
Table 2 |.
Troubleshooting table
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| Box 1, step 12 | Inefficient conjugation of mass tags to antibodies | Maleimide-functionalized groups lose activities | Minimize the number of freeze-thaw cycles that the palladium isotopic mass tags undergo |
| Box 2, step 8 | Absorbance at 562 nm is variable or does not increase with antibody concentration | Working solution and antibody are not mixed well | Mix thoroughly by pipetting up and down, and make sure that all the liquid is at the bottom of tube; incubate with gentle shaking |
| 27 | Inefficient washing by desalting columns | Improper sample loading | Load the sample directly into the center of the resin bed and carefully touch the pipette tip to the resin to expel the antibody sample |
| 61 | Lower recovery of antibody conjugates (<50%) | Precipitation induced by excess metal cations or denaturation due to reduction | Ensure complete washes of the polymer and metal cations with post-centrifugation filter volumes of <20 μL. Ensure that reduction time is not >30 min |
| 61 | Higher recovery of antibody conjugates (>95%) | Carrier proteins | Check manufacturer specifications for carrier proteins (BSA, gelatin, or other protein stabilizer). Obtain carrier-free stock or purify the antibody away from the carrier |
| Improper antibody starting amount | Measure the concentration of the starting antibody stock by A280; ensure that the conjugation amount is 100 μg | ||
| 70 | White flocculent precipitate observed | The pH exchange causes the precipitation of bismuth | Redissolve the precipitate in 5.0–10.0% (vol/vol) HNO3 solution. Mix by vigorous pipetting up and down. Filter the solution through a syringe filter with a 200-nm nylon membrane. Wash the polymers again in 0.5% (vol/vol) HNO3 solution again |
Timing
Section 1, conjugation of antibody with palladium isotopic mass tags
Steps 1–10, partial reduction of the IgG antibody (Fig. 5a): ~1 h
Steps 11–20, conjugation of the reduced antibody with Pd@mal-DOTA mass tags: ~1 h
Steps 21–27, washing and recovering the Pd-tagged antibody: ~10 min
Steps 28 and 29, quantification of the Pd-tagged antibody: ~15 min
Section 2, conjugation of antibody with yttrium, indium, or lanthanide isotopes
Steps 30–34, chelation of Y3+, In3+, or Ln3+ isotopic cations to Maxpar X8 polymers (Fig. 5b): ~1 h
Steps 35–37, removal of the free Y3+, In3+, or Ln3+ isotopic cations: ~30 min
Steps 38–46, partial reduction of the IgG antibody: ~1 h
Steps 47 and 48, conjugation of the reduced antibody with metal-isotope-chelated polymer: ~1 h
Steps 49–60, washing and recovery of the MitAb: ~50 min
Steps 61 and 62, quantification of the MitAb: ~15 min
Section 3, conjugation of antibody with bismuth
Steps 63–66, chelation of 209Bi3+ cations to Maxpar X8 polymers (Fig. 5c): ~1 h
Steps 67–72, removal of the free 209Bi3+ cations: ~1 h
Steps 73–81, partial reduction of the IgG antibody: ~1 h
Steps 82 and 83, conjugation of the reduced antibody with 209Bi-chelated polymers: ~1 h
Steps 84–95, washing and recovery of the 209Bi-tagged antibody: ~50 min
Steps 96 and 97, quantification of the 209Bi-tagged antibody: ~45 min
Section 4, cell staining for validation and titration of metal-isotope-tagged antibodies
Step 98, preparation of the cell samples: ~45 min
Steps 99–101, fixing and permeabilization of the cells: ~30 min
Steps 102–106, immune staining with the titrated antibodies: ~1 h
Steps 107–110, staining of the cells in DNA intercalator solution: ~30 min
Box 1, preparation of palladium isotopic mass tags: 8 h
Box 2, quantification of metal-isotope-tagged antibodies: 1 h
Anticipated results
Characterization of palladium isotopic mass tags
Six Pd@mal-DOTA mass tags were synthesized and measured using ESI-MS, according to the procedures described in Box 1. The chemical formula of mal-DOTA is C22H34N6O9, and its theoretical MW is 526.23; therefore, the main mass peak at an m/z value of 525.2 in negative-ion ESI-MS was identified as representing the single-charge negative molecular ions [M-1]− of mal-DOTA (Fig. 6a). In the Pd@mal-DOTA complexes, two hydrogen ions of mal-DOTA are replaced by the coordinated Pd2+ cation; thus, the expected mass for Pd@mal-DOTA is the sum of the atomic weight of the palladium isotope and the MW of mal-DOTA minus 3 Da (i.e., M-3 + Pd). For example, as shown in Fig. 6b, the main mass peak of 102Pd@mal-DOTA is at the m/z value of 625.2, as expected for [M-3 + 102]− (i.e., 526.2 − 3 + 102 = 625.2). The mass spectra of 104Pd@mal-DOTA, 105Pd@mal-DOTA, 106Pd@mal-DOTA, 108Pd@mal-DOTA, and 110Pd@mal-DOTA are shown in Fig. 6c–g, respectively.
Cell barcoding assays using Pd-tagged CD45 antibodies
Multiplexed barcoding of live Jurkat cells was carried out using CD45 antibodies conjugated with palladium isotopes as described in Section 1 of the Procedure. Three different barcoding strategies are demonstrated. For the 6-choose-1 strategy, Fig. 7a shows the CyTOF measurements as ‘rain dots’ of each barcode of Pd-tagged CD45 and biaxial contour plots comparing pairs of barcoding channels. These data indicate that each barcoded cell sample was effectively distinguished and that the double-channel-positive populations were rare. For the 6-choose-2 strategy, 106Pd- and 108Pd-tagged CD45, 108Pd- and 110Pd-tagged CD45, or 106Pd- and 110Pd-tagged CD45 were used to stain Jurkat cells. Both ‘rain dots’ and biaxial contours demonstrate that there are three expected double-positive populations, as shown in Fig. 7b. For the 6-choose-3 strategy, 102Pd-, 104Pd-, and 105Pd-tagged CD45 antibodies were used to stain Jurkat cells. The expected triple-positive populations were observed, as shown in Fig. 7c. In principle, by using the 6-choose-1 strategy, six samples can be measured simultaneously. The strategies of 6-choose-2 and 6-choose-3 enable multiplexing of 15 and 20, respectively. It should be taken into consideration that, because of saturation of cell immune staining, the higher multiplexing will result in the lower signal intensity in each barcoding channel and consequentially might decrease the recovery of cell debarcoding.
Titrations of various 209Bi-tagged antibodies
Antibody titration is an important experiment use to validate and examine the specificity and affinity of the conjugated antibody. It is used to determine the optimal concentration of antibody for cell staining. As an example of how to do this, a wide variety of antibodies against stable surface and intracellular molecules and against stimulation-induced signaling molecules and cytokines have been successfully conjugated to bismuth mass tags, as described in Section 3 of the Procedure. Titration experiments were performed in positive- and negative-control cells to determine the maximum ratio of positive signal to background, which is referred to as the optimal concentration. As shown in Fig. 8a,209Bi-tagged MHC class II antibody was incubated with mouse PBMCs at concentrations ranging from 250 to 8,000 ng/mL in two fold dilutions. The titration curves were generated by analysis of B cells, which express MHC class II antigens, and T cells, which do not. Figure 8b,c show the titration histograms of intracellular phosphorylation antibodies of 209Bi-tagged phospho-STAT3(Tyr705) and 209Bi-tagged phospho-histone H3(Ser28) at their optimal concentrations. Figure 8d shows the titration histogram of the intracellular cytokine antibody of 209Bi-tagged IFN-γ at the optimal concentration in stimulated and unstimulated natural killer cells. Figure 8e shows the titration histogram of secondary antibody of 209Bi-tagged anti-PE at the optimal concentration in PE-conjugated CD3 antibody-stained T cells and B cells of human PBMCs. These results indicate that the bismuth conjugation protocol is suitable for various types of antibodies used in mass cytometry.
Comparisons of bismuth- and lanthanide-tagged antibodies in high-dimensional assays
By following the protocol described in Section 2 of the Procedure, a number of antibodies have been conjugated with yttrium, indium, and lanthanide isotopic mass tags (Supplementary Table 1). In high-dimensional CyTOF assays, bismuth- and lanthanide-tagged antibodies should provide comparable results without the isotope crosstalk. To demonstrate this, the same antibodies were conjugated with both bismuth and lanthanide mass tags: (i) human anti-CD3 tagged with 209Bi and 170Er, (ii) human anti-CD56 tagged with 209Bi and 176Yb, and (iii) mouse anti-MHC class II tagged with 209Bi and 176Yb. Three separate comparisons were carried out in Jurkat cells, human PBMCs, and immune cells of mouse organs.
First, Jurkat cells were stained with lanthanide-tagged antibodies against CD19, CD4, CD11a, CD5, CD7, CD184, and CD2, and either 209Bi-tagged CD3 or 170Er-tagged CD3. As the results in biaxial scatter plots (Fig. 9a, left) and radar charts (Fig. 9a, right) show, bismuth- and lanthanide-tagged CD3 provided comparable values of CD3 expression in Jurkat cells, and mean intensities of other compared CD markers had a strong correlation (Pearson correlation, r = 0.997, P < 0.00001, Supplementary Fig. 1a). Second, human PBMCs were then stained with 37 antibodies against surface markers and three intercellular markers (Supplementary Table 1), and either 209Bi-tagged CD56 or 176Yb-tagged CD56. Fifteen immune cell subpopulations were manually gated (Supplementary Fig. 2). As the results in viSNE (visualization of t-distributed stochastic neighbor embedding) plots (Fig. 9b) and heat maps (Fig. 9c) show, there are highly comparable immunophenotypic clustering and strong correlations between data collected using 209Bi-tagged CD56 and 176Yb-tagged CD56 (Pearson correlation, r = 0.991, P < 0.00001, Supplementary Fig. 1b). Third, comparison of 209Bi- and 176Yb-tagged MHC class II antibodies in scaffold maps revealing the immune organization of bone marrow for C57BL/6 mice are shown in Fig. 10.
Supplementary Material
Acknowledgements
We thank G. Jager for administrative support and A. Jager for mass cytometry quality control and instrument maintenance. We thank J. Ramunas, S.-Y. Chen, V.D. Gonzalez, and E.R. Zunder for valuable discussions. This work was supported by grants from the US National Institutes of Health (U19 AI057229, 1U19AI100627, R01CA184968, R33 CA183654, and R33 CA183692), US National Heart, Lung, and Blood Institute (N01-HV-00242), US Department of Defense (OC110674 and 11491122), Bill & Melinda Gates Foundation (OPP1113682), and Food and Drug Administration (HHSF223201210194C).
Footnotes
Competing interests
G.P.N. declares that he had a personal financial interest in Fluidigm, the manufacturer of the mass cytometer used in this study, for the duration of this project. The remaining authors declare no competing interests.
Supplementary information is available for this paper at https://doi.org/10.1038/s41596-018-0016-7.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
1. Han, G. et al. Cytometry A 91, 1150–1163 (2017): https://doi.org/10.1002/cyto.a.23283
2. Porpiglia, E. et al. Nat. Cell Biol. 19, 558–567 (2017): https://doi.org/10.1038/ncb3507
References
- 1.Robinson JP & Roederer M Flow cytometry strikes gold. Science 350, 739–740 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Kling J Measure for measure. Nature 518, 439–443 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Bandura DR et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal. Chem 81, 6813–6822 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Bendall SC et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janes MR & Rommel C Next-generation flow cytometry. Nat. Biotechnol 29, 602–604 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Chen JH, Pelka K & Hacohen N Heavy metal enlightens tumor immunity. Cell 169, 567–569 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amir ED et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotechnol 31, 545–552 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjornson ZB, Nolan GP & Fantl WJ Single-cell mass cytometry for analysis of immune system functional states. Curr. Opin. Immunol 25, 484–494 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spitzer MH et al. An interactive reference framework for modeling a dynamic immune system. Science 349, 12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anchang B et al. Visualization and cellular hierarchy inference of single-cell data using SPADE. Nat. Protoc 11, 1264–1279 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Newell EW & Cheng Y Mass cytometry: blessed with the curse of dimensionality. Nat. Immunol 17, 890–895 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Spitzer MH & Nolan GP Mass cytometry: single cells, many features. Cell 165, 780–791 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bendall SC, Nolan GP, Roederer M & Chattopadhyay PK A deep profiler’s guide to cytometry. Trends Immunol. 33, 323–332 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanner SD, Baranov VI, Ornatsky OI, Bandura DR & George TC An introduction to mass cytometry: fundamentals and applications. Cancer Immunol. Immunother 62, 955–965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tricot S et al. Evaluating the efficiency of isotope transmission for improved panel design and a comparison of the detection sensitivities of mass cytometer instruments. Cytometry A 87A, 357–368 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Baranov VI, Quinn Z, Bandura DR & Tanner SD A sensitive and quantitative element-tagged immunoassay with ICPMS detection. Anal. Chem 74, 1629–1636 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Zhang ZY, Yu BB, Shi JJ & Zhang XR Application of the biological conjugate between antibody and colloid Au nanoparticles as analyte to inductively coupled plasma mass spectrometry. Anal. Chem 74, 96–99 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Ornatsky OI et al. Development of analytical methods for multiplex bio-assay with inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom 23, 463–469 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han G, Xing Z, Dong Y, Zhang S & Zhang X One-step homogeneous DNA assay with single-nanoparticle detection. Angew. Chem. Int. Ed. Engl 50, 3462–3465 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Han G, Zhang S, Xing Z & Zhang X Absolute and relative quantification of multiplex DNA assays based on an elemental labeling strategy. Angew. Chem. Int. Ed. Engl 52, 1466–1471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newell EW, Sigal N, Bendall SC, Nolan GP & Davis MM Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8(+) T cell phenotypes. Immunity 36, 142–152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newell EW et al. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat. Biotechnol 31, 623–629 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi C et al. Mass cytometry panel optimization through the designed distribution of signal interference. Cytometry A 91A, 39–47 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Lavin Y et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169, 750–765 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mei HE, Leipold MD, Schulz AR, Chester C & Maecker HT Barcoding of live human peripheral blood mononuclear cells for multiplexed mass cytometry. J. Immunol 194, 2022–2031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodenmiller B et al. Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nat. Biotechnol 30, 858–867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Behbehani GK, Bendall SC, Clutter MR, Fantl WJ & Nolan GP Single-cell mass cytometry adapted to measurements of the cell cycle. Cytometry A 81A, 552–566 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edgar LJ et al. Isotopologous organotellurium probes reveal dynamic hypoxia in vivo with cellular resolution. Angew. Chem. Int. Ed. Engl 55, 13159–13163 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Edgar LJ et al. Identification of hypoxic cells using an organotellurium tag compatible with mass cytometry. Angew. Chem. Int. Ed. Engl 53, 11473–11477 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Park H, Edgar LJ, Lumba MA, Willis LM & Nitz M Organotellurium scaffolds for mass cytometry reagent development. Org. Biomol. Chem 13, 7027–7033 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Schulz AR, Stanislawiak S, Baumgart S, Gruetzkau A & Mei HE Silver nanoparticles for the detection of cell surface antigens in mass cytometry. Cytometry A 91A, 25–33 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Zunder ER et al. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat. Protoc 10, 316–333 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behbehani GK et al. Transient partial permeabilization with saponin enables cellular barcoding prior to surface marker staining. Cytometry A 85A, 1011–1019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ornatsky OI et al. Study of cell antigens and intracellular DNA by identification of element-containing labels and metallointercalators using inductively coupled plasma mass spectrometry. Anal. Chem 80, 2539–2547 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Fienberg HG, Simonds EF, Fantl WJ, Nolan GP & Bodenmiller B A platinum-based covalent viability reagent for single-cell mass cytometry. Cytometry A 81A, 467–475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy RL, Mak DH, Burks JK & Barton MC Rapid monoisotopic cisplatin based barcoding for multiplexed mass cytometry. Sci. Rep 7, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern AD, Rahman AH & Birtwistle MR Cell size assays for mass cytometry. Cytometry A 91A, 14–24 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Catena R, Ozcan A, Zivanovic N & Bodenmiller B Enhanced multiplexing in mass cytometry using osmium and ruthenium tetroxide species. Cytometry A 89A, 491–497 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Spitzer MH et al. Systemic immunity is required for effective cancer immunotherapy. Cell 168, 1–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han G et al. Atomic mass tag of bismuth-209 for increasing the immunoassay multiplexingcapacity of mass cytometry. Cytometry A 91A, 1150–1163 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majonis D, Ornatsky O, Kinach R & Winnik MA Curious results with palladium- and platinum-carrying polymers in mass cytometry bioassays and an unexpected application as a dead cell stain. Biomacromolecules 12, 3997–4010 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Chevrier S et al. Compensation of signal spillover in suspension and imaging mass cytometry. Cell Syst. 6, 612–620 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dulski P Interferences of oxide, hydroxide and chloride analyte species in the determination of rare-earth elements in geological samples by inductively-coupled plasma-mass spectrometry. Fresenius J. Anal. Chem 350, 194–203 (1994). [Google Scholar]
- 44.Liu P et al. Metal-chelating polymers (MCPs) with zwitterionic pendant groups complexed to trastuzumab exhibit decreased liver accumulation compared to polyanionic MCP immunoconjugates. Biomacromolecules 16, 3613–3623 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Majonis D et al. Synthesis of a functional metal-chelating polymer and steps toward quantitative mass cytometry bioassays. Anal. Chem 82, 8961–8969 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lou X et al. Polymer-based elemental tags for sensitive Bioassays. Angew. Chem. Int. Ed. Engl 46, 6111–6114 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwarz G, Mueller L, Beck S & Linscheid MW DOTA based metal labels for protein quantification: a review. J. Anal. At. Spectrom 29, 221–233 (2014). [Google Scholar]
- 48.De Stefano C, Gianguzza A, Pettignano A & Sammartano S Palladium(II) complexes of aminopolycarboxylic ligands in aqueous solution. J. Chem. Eng. Data 56, 4759–4771 (2011). [Google Scholar]
- 49.Pichaandi J et al. Liposome-encapsulated NaLnF(4) nanoparticles for mass cytometry: evaluating nonspecific binding to cells. Chem. Mater 29, 4980–4990 (2017). [Google Scholar]
- 50.Tong L, Lu E, Pichaandi J, Zhao GY & Winnik MA Synthesis of uniform NaLnF(4) (Ln: Sm to Ho) nanoparticles for mass cytometry. J. Phys. Chem. C 120, 6269–6280 (2016). [Google Scholar]
- 51.Zhao GY, Tong L, Cao PP, Nitz M & Winnik MA Functional PEG-PAMAM-tetraphosphonate capped NaLnF(4) nanoparticles and their colloidal stability in phosphate buffer. Langmuir 30, 6980–6989 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu E, Pichaandi J, Arnett LP, Tong L & Winnik MA Influence of Lu3+ doping on the crystal structure of uniform small (5 and 13 nm) NaLnF(4) upconverting nanocrystals. J. Phys. Chem. C 121, 18178–18185 (2017). [Google Scholar]
- 53.Wu X et al. Lanthanide-coordinated semiconducting polymer dots that function for both flow cytometry and mass cytometry. Angew. Chem. Int. Ed. Engl 56, 14908–14912 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Gorman WE et al. Mass cytometry identifies a distinct monocyte cytokine signature shared by clinically heterogeneous pediatric SLE patients. J. Autoimmun 81, 74–89 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lun ATL, Richard AC & Marioni JC Testing for differential abundance in mass cytometry data. Nat. Methods 14, 707–709 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diggins KE, Greenplate AR, Leelatian N, Wogsland CE & Irish JM Characterizing cell subsets using marker enrichment modeling. Nat. Methods 14, 275–278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng J et al. The transcriptional coactivator TAZ regulates reciprocal differentiation of T(H)17 cells and T-reg cells. Nat. Immunol 18, 800–812 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Chevrier S et al. An immune atlas of clear cell renal cell carcinoma. Cell 169, 736–749 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.See P et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science 356, aag3009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leelatian N et al. Single cell analysis of human tissues and solid tumors with mass cytometry. Cytometry B Clin. Cytom 92, 68–78 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgart S, Peddinghaus A, Schulte-Wrede U, Mei HE & Gruetzkau A OMIP-034: comprehensive immune phenotyping of human peripheral leukocytes by mass cytometry for monitoring immunomodulatory therapies. Cytometry A 91A, 34–38 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Baca Q, Cosma A, Nolan G & Gaudilliere B The road ahead: implementing mass cytometry in clinical studies, one cell at a time. Cytometry B Clin. Cytom 92, 10–11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Unen V et al. Mass cytometry of the human mucosal immune system identifies tissue- and disease-associated immune subsets. Immunity 44, 1227–1239 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Zunder ER, Lujan E, Goltsev Y, Wernig M & Nolan GP A continuous molecular roadmap to iPSC reprogramming through progression analysis of single-cell mass cytometry. Cell Stem Cell 16, 323–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lujan E et al. Early reprogramming regulators identified by prospective isolation and mass cytometry. Nature 521, 352–356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lakshmikanth T et al. Mass cytometry and topological data analysis reveal immune parameters associated with complications after allogeneic stem cell transplantation. Cell Rep. 20, 2238–2250 (2017). [DOI] [PubMed] [Google Scholar]
- 67.Atkuri KR, Stevens JC & Neubert H Mass cytometry: a highly multiplexed single-cell technology for advancing drug development. Drug Metab. Dispos 43, 227–233 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Wang YJ et al. Single-cell mass cytometry analysis of the human endocrine pancreas. Cell Metab. 24, 616–626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nassar AF, Wisnewski AV & Raddassi K Progress in automation of mass cytometry barcoding for drug development. Bioanalysis 8, 1429–1435 (2016). [DOI] [PubMed] [Google Scholar]
- 70.Yang YSS et al. High-throughput quantitation of inorganic nanoparticle biodistribution at the single-cell level using mass cytometry. Nat. Commun 8, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guo YT, Baumgart S, Stark HJ, Harms H & Muller S Mass cytometry for detection of silver at the bacterial single cell level. Front. Microbiol 8, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ivask A et al. Single cell level quantification of nanoparticle-cell interactions using mass cytometry. Anal. Chem 89, 8228–8232 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Rahman AH, Lavin Y, Kobayashi S, Leader A & Merad M High-dimensional single cell mapping of cerium distribution in the lung immune microenviroment of an active smoker. Cytometry B Clin. Cytom 10.1002/cyto.b.21545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Diggins KE, Ferrell PB & Irish JM Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data. Methods 82, 55–63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kleinsteuber K et al. Standardization and quality control for high-dimensional mass cytometry studies of human samples. Cytometry A 89A, 903–913 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krutzik PO, Trejo A, Schulz KR & Nolan GP Phospho flow cytometry methods for the analysis of kinase signaling in cell lines and primary human blood samples. Methods Mol. Biol 699, 179–202 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.