Abstract
Background
Antibiotic treatment failure is common among patients with community-acquired pneumonia (CAP) who are managed in the outpatient setting and is associated with higher mortality and increased health care costs. This study’s objectives were to quantify the occurrence of antibiotic treatment failure (ATF) and to evaluate clinical and economic outcomes between CAP patients who experienced ATF relative to those who did not.
Methods
Retrospective analysis of the MarketScan Commercial & Medicare Supplemental Databases was performed, identifying patients ≥18 years old, with a pneumonia diagnosis in the outpatient setting, and who received a fluoroquinolone, macrolides, beta-lactam, or tetracycline. ATF was defined as any of the following events within 30 days of initial antibiotic: antibiotic refill, antibiotic switch, emergency room visit, or hospitalization. Outcomes included 30-day all-cause mortality and CAP-related health care costs.
Results
During the study period, 251 947 unique patients met inclusion criteria. The mean age was 52.2 years, and 47.7% were male. The majority of patients received a fluoroquinolone (44.4%) or macrolide (43.6%). Overall, 22.1% were classified as ATFs. Among 18–64-year-old patients, 21.2% experienced treatment failure, compared with 25.7% in those >65 years old. All-cause mortality was greater in the antibiotic failure group relative to the non–antibiotic failure group (18.1% vs 4.6%, respectively), and the differences in 30-day mortality between antibiotic failure groups increased as a function of age. Mean 30-day CAP-related health care costs were also higher in the patients who experienced treatment failure relative to those who did not ($2140 vs $54, respectively).
Conclusions
Treatment failure and poor outcomes from outpatient CAP are common with current guideline-concordant CAP therapies. Improvements in clinical management programs and therapeutic options are needed.
Keywords: antibiotic treatment failure, community-acquired pneumonia, outpatient
Despite notable advances in vaccine development and health care delivery, community-acquired pneumonia (CAP) continues to be a substantial cause of morbidity and mortality in the United States [1]. Pneumonia/influenza is the eighth leading cause of mortality among US adults, resulting in >60 000 deaths annually [2]. Treatment of CAP represents a financial burden on the US health care system [1, 3]. The American Lung Association estimates that annual direct medical costs linked to pneumonia exceed $17 billion [1]. Among the Medicare population, the burden of CAP costs was estimated to be $13 billion in 2008 [4]. As the number of at-risk individuals continues to grow with the aging population, the future impact of CAP in both financial and clinical terms is projected to grow [1, 5].
To date, many studies have quantified the clinical and economic outcomes associated with treating patients with CAP in the inpatient setting [3, 6–8]. However, few have evaluated the outcomes associated with managing patients with CAP in the outpatient setting [9, 10]. The National Health and Nutrition Examination Survey (NHANES III) estimated that there are ~4.5 million ambulatory visits per year for patients with CAP in the United States [11]. Antibiotic prescription data indicate that there were ~10 million CAP-related antibiotic prescriptions per year in the United Statesa between 1998 and 2009 [12–14]. The primary objective of the current analysis was to examine the clinical and economic outcomes associated with treating adult patients with CAP in the outpatient setting. As part of the study, we sought to define and quantify the occurrence of antibiotic treatment failure (ATF) in the outpatient setting and to assess its effect on mortality and health care costs. Given the increasing elderly population and known pneumonia risks in this group, we were also interested in evaluating consequences of ATF among patients aged ≥65 years compared with patients 18 to 64 years of age.
METHODS
Study Design and Population
A retrospective cohort analysis was conducted using the Truven Health Marketscan Commercial Claims and Encounters Database and Medicare Supplemental and Coordination Benefits Database for the period 2011–2015. The database contains >40 million individuals including >4 million Medicare patients. Patients were included in this analysis if they (1) were 18 years of age or older, (2) had an ICD-9 code for pneumonia (481, 482.x, 485.x, 486, and 487.0), (3) received and filled a single antibiotic prescription (monotherapy) within 3 days of diagnosis, (4) were not hospitalized within 2 days of the initial antibiotic prescription, (5) did not receive an antibiotic in the prior 30 days of initial antibiotic therapy, and (6) did not have a medical claim for a primary diagnosis of CAP in previous 30 days. Patients had to have had >12 months of continuous enrollment in a medical and pharmacy insurance benefit program.
Data Elements and Outcomes
Demographic elements included age at index date, sex, 12-month baseline comorbidities, 12-month baseline Charlson comorbidity index (CCI) score (overall and individual components), and antibiotic therapy. The primary outcome of interest was antibiotic treatment failure. Antibiotic treatment failure was defined as any of the following within 30 days of initial antibiotic: refill of initial antibiotic, switch to a new antibiotic, emergency room visit for CAP, and/or hospitalization for CAP. Secondary outcomes included 30-day all-cause mortality and CAP-related health care costs (medical and pharmacy) within the 30-day follow-up period from initial antibiotic prescription fill. Costs were inflated to 2015 US dollars.
Statistical Analysis Plan
The associations between 2 categorical variables were compared using the Fisher exact test. The Student t test was used to compare associations between categorical and continuous variables. Treatment failure outcome relationships were stratified by age (18–64 years vs ≥65 years) to assess for the presence of effect measure modification. All tests were 2-tailed, and a P value <.05 was deemed a priori to represent statistical significance. Given the nature of exploratory analysis, no adjustment was made for multiple comparisons. All data and analyses were performed using SAS, version 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
From a total of 890 784 patient charts reviewed, 257 165 (28.9%) adults (aged ≥18 years) met the criteria described above. The reasons for attrition of cases are shown in Table 1. The mean age of the study population (SD) was 52.2 (17.39) years, with 20.9% being aged ≥65 years. Overall 46.4% were male. The majority of patients were prescribed azithromycin (39.5%) or levofloxacin (36.9%).
Table 1.
Reasons for Attrition of Cases
| Target Population | No. | % |
|---|---|---|
| Patients with ICD9-diagnosis of CAP during the enrollment window (June 30, 2011–May 30, 2015) | ||
| Patients with ≥1 prescription fill for any of the 9 antibiotics of interest (amoxicillin, amoxicillin-clavulanate, ceftriaxone, azithromycin, clarithromycin, levofloxacin, moxifloxacin, ciprofloxacin, doxycycline) during the enrollment window within 3 days of CAP claim | 890 784 | 100.0 |
| Exclusion Criteria | No. | % |
| Patients without continuous enrollment in medical and pharmacy benefits for at least 12 months before the index date | 676 642 | 76.0 |
| Patients without continuous enrollment in medical and pharmacy benefits for a minimum of 30 days after the index date | 654 105 | 73.4 |
| Hospitalization for any reason within 2 days of index prescription claim | 618 326 | 69.4 |
| Patients with prescription fills for any antibiotic in the 30 days before diagnosis of CAP | 508 384 | 57.1 |
| Patients with medical claim containing a primary diagnosis of CAP in the 30 days before the first outpatient medical claim in the enrollment window | 498 408 | 56.0 |
| Patients with HMO enrollment | 424 577 | 47.7 |
| Patients on combination antibiotics | 413 801 | 46.5 |
| Sample size after applying all pre- and postindex eligibility requirements | 413 801 | 46.5 |
| Patients aged ≥18 years (final sample size) | 257 165 |
Of this population, 257 165 were aged ≥18 years and served as the primary focus of subsequent analysis.
Abbreviations: CAP, community-acquired pneumonia; HMO, Healthcare Maintenance Organization.
aSample size = 413 801 after all inclusion/exclusion criteria were met.
Baseline characteristics comparing patients who were 18–64 years and those aged ≥65 years are shown in Table 2. Comorbid conditions were significantly more common in older patients (≥65 years) compared with younger patients (18–64 years). The most frequent comorbidities seen in the younger population were chronic pulmonary disease (COPD; 14.6%), diabetes (9.4%), and asthma (8.7%). In patients aged ≥65 years, COPD was reported in 31.5%, arrythmias in 25.5%, and diabetes in 19.3%. Mean (SD) Charlson comorbidity index (CCI) scores were significantly higher in patients aged ≥65 years vs 18–64 years.
Table 2.
Clinical Characteristics by Age
| Age-Stratifieda | ||||||
|---|---|---|---|---|---|---|
| Total | 18–64 y | ≥65 y | ||||
| Clinical and Disease Characteristics | na = 408 360 | na = 199 190 | na = 52 757 | |||
| No. | % | No. | % | No. | % | |
| Pre-index (365-d) baseline comorbidities | ||||||
| Charlson comorbidity index, mean (SD) | 0.61 | 1.28 | 0.58 | 1.18 | 2.01 | 2.07 |
| Charlson comorbidity index | ||||||
| 0 | 281 757 | 69.0 | 135 981 | 68.3 | 14 373 | 27.2 |
| 1 | 73 606 | 18.0 | 37 954 | 19.1 | 12 035 | 22.8 |
| 2 | 24 462 | 6.0 | 13 959 | 7.0 | 9581 | 18.2 |
| 3+ | 28 535 | 7.0 | 11 296 | 5.7 | 16 768 | 31.8 |
| Individual CCI comorbidities | ||||||
| Chronic pulmonary disease | 69 067 | 16.90 | 29 063 | 14.5 | 16 635 | 31.50 |
| Cardiovascular disease | 42 808 | 10.5 | ||||
| Myocardial infarction | 4149 | 1.0 | 1673 | 0.8 | 2468 | 4.7 |
| Congestive heart failure | 12 120 | 3.0 | 3822 | 1.9 | 8153 | 15.5 |
| Peripheral vascular disease | 12 775 | 3.1 | 3853 | 1.9 | 8834 | 16.7 |
| Cerebrovascular disease | 13 764 | 3.4 | 4530 | 2.3 | 8904 | 16.9 |
| Diabetic disease | 38 044 | 9.30 | ||||
| Diabetes | 29 276 | 7.2 | 18 675 | 9.4 | 10 181 | 19.3 |
| Diabetes with chronic complications | 8768 | 2.1 | 4205 | 2.1 | 4549 | 8.6 |
| Dementia | 2762 | 7.0 | 126 | 0.1 | 2618 | 5.0 |
| Cancer | 14 898 | 3.6 | 6986 | 3.5 | 6968 | 3.5 |
| Renal disease | 8976 | 2.2 | 3172 | 1.6 | 5685 | 10.8 |
| Chronic kidney disease | 8880 | 2.2 | 3132 | 1.6 | 5631 | 10.7 |
| End-stage renal disease | 1019 | 0.2 | 513 | 0.3 | 493 | 0.9 |
| Mild liver disease | 7629 | 1.9 | 5774 | 2.9 | 1684 | 3.2 |
| Metastatic cancer | 2461 | 0.6 | 1347 | 0.7 | 1079 | 2.0 |
| Hemiplegia or paraplegia | 1835 | 0.4 | 657 | 0.3 | 550 | 1.0 |
| Peptic ulcer disease | 1813 | 0.4 | 1068 | 0.5 | 693 | 1.3 |
| AIDS | 600 | 0.1 | 573 | 0.3 | 22 | 0.0 |
| Moderate or severe liver disease | 429 | 0.1 | 279 | 0.1 | 132 | 0.3 |
| Other comorbidities | ||||||
| Asthma | 42 787 | 10.5 | 17 249 | 8.7 | 5362 | 10.2 |
| Arrhythmias | 22 906 | 5.6 | 8687 | 4.4 | 13 476 | 25.5 |
Data are presented as No. and % unless otherwise specified. All comparisons were statistically significant at P < .0001.
Abbreviations: CCI, Charlson comorbidity index; NEC, not elsewhere classified.
aAll clinical and disease characteristic analyses excluded patients with index drug (ceftriaxone and ciprofloxacin), due to low counts.
Table 3 shows the frequency of comorbidities by index antibiotic prescription class. A CCI score ≥2 was noted among 24.3% of those who received a fluoroquinolone or B-lactam, 23.0% of those receiving tetracycline, and 15.8% of those receiving a macrolide (P < .05), indicating that macrolide recipients represented a lower-mortality risk group. There were also differences in specific comorbidities by antibiotic class.
Table 3.
Comorbidities by Index Prescription Antibiotic Class
| Clinical Characteristics by Index Drug Class—Age ≥18 y | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Index Drug Class–Stratified | ||||||||||
| Total | Beta-lactam | Macrolide | Fluoroquinolone | Tetracycline | ||||||
| Clinical and Disease Characteristics | na = 251 947 | na = 16 526 | na = 112 054 | na = 109 179 | na = 14 188 | |||||
| No. | % | No. | % | No. | % | No. | % | No. | % | |
| Pre-index (365-d) baseline comorbidities | ||||||||||
| Charlson comorbidity index, mean (SD) | 0.88 | 1.53 | 1.04 | 1.68 | 0.69 | 1.32 | 1.04 | 1.66 | 0.98 | 1.59 |
| Charlson comorbidity index | ||||||||||
| 0 | 150 354 | 59.7 | 9103 | 55.1 | 73 649 | 65.7 | 59 663 | 54.6 | 7939 | 56.0 |
| 1 | 49 989 | 19.8 | 3418 | 20.7 | 20 571 | 18.4 | 23 012 | 21.2 | 2988 | 21.1 |
| 2 | 23 540 | 9.3 | 1650 | 10.0 | 8790 | 7.8 | 11 671 | 10.7 | 1429 | 10.1 |
| 3+ | 28 064 | 11.1 | 2355 | 14.3 | 9044 | 8.1 | 14 833 | 13.6 | 1832 | 12.9 |
| Individual CCI comorbidities | ||||||||||
| Chronic pulmonary disease | 45 698 | 18.1 | 3436 | 20.8 | 17 139 | 15.3 | 22 134 | 20.3 | 2989 | 21.1 |
| Cardiovascular disease | 42 237 | 16.7 | ||||||||
| Cerebrovascular disease | 13 434 | 5.3 | 1090 | 6.6 | 4494 | 4.0 | 6995 | 6.4 | 855 | 6.0 |
| Peripheral vascular disease | 12 687 | 5.0 | 1013 | 6.1 | 4151 | 3.7 | 6666 | 6.1 | 857 | 6.0 |
| Congestive heart failure | 11 975 | 4.8 | 1087 | 6.6 | 3726 | 3.3 | 6289 | 5.8 | 873 | 6.2 |
| Myocardial infarction | 4141 | 1.6 | 337 | 2.0 | 1318 | 1.2 | 2173 | 2.0 | 313 | 2.2 |
| Diabetic disease | 37 610 | 14.5 | ||||||||
| Diabetes | 28 856 | 11.0 | 2146 | 13.0 | 10 550 | 9.4 | 14 368 | 13.2 | 1792 | 12.6 |
| Diabetes with chronic complications | 8754 | 3.5 | 642 | 3.9 | 3050 | 2.7 | 4528 | 4.1 | 534 | 3.8 |
| Cancer | 14 571 | 5.8 | 1083 | 6.6 | 5261 | 4.7 | 7352 | 6.7 | 875 | 6.2 |
| Renal disease | 8857 | 3.5 | 739 | 4.5 | 2974 | 2.7 | 4519 | 4.1 | 625 | 4.4 |
| Chronic kidney disease | 8763 | 3.5 | 731 | 4.4 | 2948 | 2.6 | 4463 | 4.1 | 621 | 4.4 |
| Mild liver disease | 7458 | 3.0 | 574 | 3.5 | 2773 | 2.5 | 3680 | 3.4 | 431 | 3.0 |
| Dementia | 2744 | 1.1 | 242 | 1.5 | 696 | 0.6 | 1636 | 1.5 | 170 | 1.2 |
| Metastatic cancer | 2426 | 1.0 | 204 | 1.2 | 683 | 0.6 | 1429 | 1.3 | 110 | 0.8 |
| Peptic ulcer disease | 1761 | 0.7 | 150 | 0.9 | 653 | 0.6 | 841 | 0.8 | 117 | 0.8 |
| Hemiplegia or paraplegia | 1207 | 0.5 | 138 | 0.8 | 424 | 0.4 | 575 | 0.5 | 70 | 0.5 |
| End-stage renal disease | 1006 | 0.4 | 84 | 0.5 | 331 | 0.3 | 527 | 0.5 | 64 | 0.5 |
| AIDSb | 595 | 0.2 | 31 | 0.2 | 229 | 0.2 | 309 | 0.3 | 26 | 0.2 |
| Moderate or severe liver disease | 411 | 0.2 | 28 | 0.2 | 128 | 0.1 | 230 | 0.2 | 25 | 6.2 |
| Other comorbidities | ||||||||||
| Asthma | 22 611 | 9.0 | 1597 | 9.7 | 9383 | 8.4 | 10 185 | 9.3 | 1446 | 10.2 |
| Arrhythmias | 22 163 | 8.8 | 1977 | 12.0 | 7588 | 6.8 | 10 896 | 10.0 | 1702 | 12.0 |
Data are presented as No. and % unless otherwise specified.
Abbreviations: CCI, Charlson comorbidity index; NEC, not elsewhere classified.
aAll clinical and disease characteristic analyses excluded patients with index drug (ceftriaxone and ciprofloxacin), due to low sample numbers.
bAll comparisons were statistically significant at P < .0001, except AIDS, which was .0004.
Antibiotic treatment failure was observed in 22.1% of patients. The most common type of treatment failure was change to a new antibiotic prescription. Treatment failure by index prescription drug class and age group is shown in Table 4. Antibiotic treatment failure occurred in 21.2% of persons in the younger group and 25.7% in the older group (P < .05). The specific reason(s) that defined ATF were broadly similar between age groups, but a higher occurrence of hospitalization was observed in patients aged ≥65 years compared with the 18–64-year-old group (9.5% vs 4.1%, respectively; P < .05). In both age groups, change to a new antibiotic was the most common type of ATF, accounting for 64.0% in the older group and 72.1% in the younger group. Antibiotic refill accounted for 20.1% and 22.3% of ATF for the younger and older age groups, respectively. Assessment of ATF by initial drug class prescribed showed that patients treated with fluoroquinolones had a higher rate of ED visits or hospitalization, 4.1% and 12.2% among those aged ≥65 years and 3.9% and 6% among those aged 18–64 years (Table 4).
Table 4.
ATF by Age and Drug Class Category
| All Patients | Beta-lactam | Macrolide | Fluoroquinolone | Tetracycline | ||
|---|---|---|---|---|---|---|
| Age Group | Index Failure Type | n = 199 190 | n = 12 516 | n = 93 770 | n = 82 144 | n = 10 760 |
| 18–64 y | Composite treatment failure, % | 21.2 | 25.6 | 21.1 | 20.5 | 21.8 |
| Treatment failure type,a % | ||||||
| Refill index antibiotic | 20.1 | 11.1 | 19.6 | 24.0 | 8.2 | |
| Change to new antibiotic prescription | 72.1 | 81.9 | 74.6 | 65.7 | 84.2 | |
| CAP hospitalization | 4.1 | 4.4 | 2.5 | 6.0 | 3.4 | |
| CAP ER visit | 3.2 | 2.5 | 2.8 | 3.9 | 3.7 | |
| Age Group | Index Failure Type | All Patients | Beta-lactam | Macrolide | Fluoroquinolone | Tetracycline |
| n = 52 757 | n = 4010 | n = 18 284 | n = 27 035 | n = 3428 | ||
| ≥65 y | Composite treatment failure, % | 25.7 | 27.1 | 26.6 | 24.9 | 26.0 |
| Treatment failure type,b % | ||||||
| Refill index antibiotic | 22.3 | 18.9 | 21.1 | 24.8 | 15.2 | |
| Change to new antibiotic prescription | 64 | 70.3 | 68.9 | 58.4 | 71.8 | |
| CAP hospitalization | 9.5 | 7.6 | 6.4 | 12.2 | 9.6 | |
| CAP ER visitd | 3.5 | 2.9 | 3.0 | 4.1 | 2.7 |
All treatment failure analyses excluded patients with index drug (ceftriaxone and ciprofloxacin), due to low sample numbers.
Abbreviations: ATF, antibiotic treatment failure; CAP, community-acquired pneumonia; ER, emergency room.
aA total of 404 patients (0.5%) had treatment failures, defined by having both a refill and a fill for a new agent on the same day.
bA total of 216 patients (0.5%) with treatment failures were defined by having both a refill and a fill for a new agent on the same day.
cA total of 82 patients (0.6%) with treatment failures were defined by having both a refill and a fill for a new agent on the same day.
dAll comparisons were statistically significant with a P value <.0001, except for CAP ER visit among the population aged ≥65 years (P = .0036).
eAdjusted failure rates.
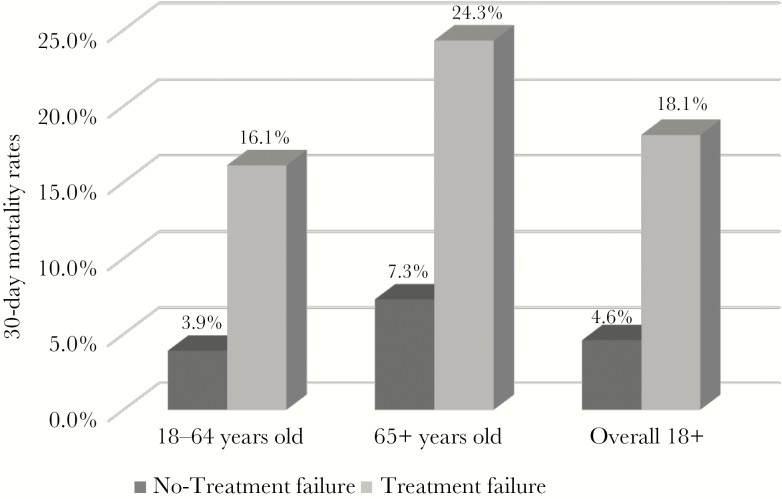
Mortality within 30 days of index treatment occurred in 7.6% of all adult patients (P < .05). Among patients classified as ATF, the mortality rate was 18.1%, compared with 4.6% in the antibiotic success cohort (P < .05). In the age group ≥65 years, 24.3% of ATF patients died, compared with 7.3% of non-ATF patients (P < .05) (Figure 1).
Figure 1.
Mortality by treatment failure and age. Among adults aged ≥18 who fail treatment for community-acquired pneumonia (CAP), 18.1% die within the next 30 days compared with 4.6% of nontreatment failures (P < .0001). Among the elderly (ie, ≥65 years), 24.3% of patients with CAP who fail treatment die within the subsequent 30 days compared with 7.3% of nontreatment failures (P < .0001).
The total mean (SD) 30-day CAP-related health care costs for those classified as non-ATF was $54 ($252). For those who experienced treatment failure, the total mean (SD) health care cost was $2140 ($15 145) (Table 5).
Table 5.
Breakdown of Health Care Costs by Treatment Failure: 30-Day Follow-up
| No Treatment Failure | Treatment Failure | |||
|---|---|---|---|---|
| n = 196 206 | n = 55 741 | |||
| Mean, $ | SD, $ | Mean, $ | SD, $ | |
| Total costs | 54 | 252 | 2140 | 15 145 |
| Total medical | 54 | 251 | 2099 | 15 145 |
| Total inpatient | - | - | 1860 | 15 059 |
| Total outpatient | 54 | 251 | 239 | 935 |
| Total pharmacy | 0 | 6 | 41 | 185 |
DISCUSSION
This retrospective analysis of a large-community commercial health care database offers important insights into the outcomes of adult patients with CAP who received their initial care in the outpatient setting. Treatment failure, objectively defined as an antibiotic refill, antibiotic switch, or ED/hospital visit within 30 days of the initial antibiotic prescription, occurred in >20% of patients with CAP who received their care in the outpatient setting. Among those aged ≥65 years, treatment failure was found to occur in 1 in 4 patients. Although the incidence of treatment failure observed in this study appears to be high, it is nearly identical to 2 previous studies that have evaluated the occurrence of treatment failure among CAP patients in the outpatient setting [9, 10]. Hess and colleagues [9], using a nearly identical definition of treatment failure as in our study, reported that ~1 in 5 patients with an outpatient diagnosis of CAP experienced treatment failure. Similarly, Ye et al. [10] reported that unadjusted rates of treatment failure (similar definition to our study) were 21.1% and 22.7% for CAP patients who received levofloxacin or macrolide, respectively, in the outpatient setting.
The poor patient outcomes observed with those who experienced ATF are both clinically relevant and highly concerning. Even though the overwhelming majority of treatment failure classifications were a result of a new or refill prescription, 30-day mortality among patients who experienced treatment failure was 16.1% among those aged 18–64 years and 24.3% among those aged >65 years (Figure 1), compared with 3.9% and 7.3%, respectively, in the absence of treatment failure. Total 30-day health care costs were also substantially higher among those who experienced a treatment failure. There were variations in the total 30-day health care costs associated with each treatment failure classification. Not surprisingly, the average 30-day health care costs were highest for those whose initial treatment failure type for index therapy was hospital admission. However, an antibiotic refill or switch as the initial treatment failure type for index therapy was found to result in 30-day health care costs, on average, in excess of $2000. The major cost driver across all 4 treatment failure classifications was medical costs; pharmacy only constituted a small proportion of total 30-day health care costs.
One of the strengths of our investigation is that we can be fairly certain that the treating physician for each of our study patients believed they were treating a case of outpatient CAP, evidenced by the pneumonia billing diagnosis code and prescription of a CAP-appropriate antibiotic. Despite the fact that most patients were treated with American Thoracic Society/Infectious Diseases Society of America guideline–appropriate antibiotics, the observed rates of ATF- and ATF-associated patient mortality were unacceptably high. One potential explanation for the observed outcomes could be that the initial CAP diagnosis was inappropriate, and the clinician missed, for example, an underlying cardiac problem. Continued focus on the differential diagnosis of respiratory symptoms combined with improvements in laboratory diagnostics, including expanding use of procalcitonin measurements, may be helpful to identify missed noninfectious diagnoses in these patients [15].
The observed mortality for patients with ATF provides strong evidence that patients with CAP who do not appear to be responding to treatment should not simply receive an antibiotic refill or new antibiotic prescription. Patients diagnosed with CAP who are failing treatment represent a high-risk group worthy of additional attention and resource allocation. Ideally, a uniform treatment approach or clinical pathway should be incorporated into practice for CAP patients in the outpatient setting, and it should include specific recommendations for patients who are not responsive to initial therapy. The potential care plan options will vary by treatment setting but may include early hospitalization, admission to skilled nursing facilities, utilization of home health nursing, or repeated physician visits. Procalcitonin testing may again play a role in patient triage, as elevated levels have been shown to predict progression to mechanical ventilation [16]. Clinicians practicing in urgent care centers or other settings where longitudinal care can be challenging may have to think creatively to mitigate the subsequent outcomes from CAP observed in our study. Similar to the implementation of any standardized clinical protocol, the outcomes should be assessed on a continual basis to ensure the protocol’s adequacy. As part of the triage process of outpatients with CAP, clinicians should be aware of the mortality risk in patients with CAP who do not appear to be responding to initial treatment and do their best to mitigate those risks to the patient.
This study has several limitations that should be noted when attempting to interpret the findings. Diagnosis codes and pharmacy data were used to identify patients with CAP in the outpatient setting. Laboratory and other electronic medical record information were not available. Due to the nature of the database, we were thus unable to ascertain the infecting organism or antibiotic resistance pattern for CAP. We also were unable to determine the reason(s) for which patients received a new prescription (eg, lack of response to or adverse effect of index treatment) or required a refill or elucidate the reasons for subsequent outpatient and inpatient care among these CAP patients. We also did not capture the exact day in relation to the initial prescription when antibiotic switches and new refills occurred; we only documented if it occurred within 30 days of initial prescription. Also, we did not capture duration of antibiotic therapy for the initial or subsequent prescriptions. The database did not document which antibiotics patients received in the “antibiotic switch” failure group. The database provided only incidence of patient mortality. It did not provide code-specific cause of death. Despite these limitations, we believe that the objective clinical findings of our study support that our data are clinically meaningful. Although we were not able to assess clinical response through chart review, the objective measure of treatment failure employed in this study minimized any subjective biases that may have resulted from assessing and interpreting observational data. More importantly, there were tangible differences in outcomes, including 30-day mortality, between patients who experienced treatment failure relative to those who did not, indicating that the treatment failure end point employed in this study was reasonable.
We did not attempt a formal comparison of treatment failure by index drug received. The primary objective of this analysis was to quantify the clinical and economic outcomes associated with treating adult patients with CAP in the outpatient setting by treatment failure and age. Although the sample size was conducive for a comparison of index treatments received, we recognize that such comparisons could be spurious due to the lack of available baseline clinical and laboratory data and consequent lack of severity-of-illness scores. Moreover, we identified clear evidence for prescribing bias in our data. As an example, fluoroquinolones were typically reserved for the more severely ill patients with CAP, and this was reflected in the finding that patients who received fluoroquinolones had a greater frequency of individual CCI comorbidities at baseline relative to the other groups. It was also supported by the observation that patients who received fluoroquinolones had the highest incidence of treatment failure due to 30-day hospitalization. Although propensity score analyses are often used to facilitate treatment comparisons when there are notable baseline differences, it is well established that multivariate analyses cannot remedy selection biases like prescribing bias, when only a limited number of baseline covariates are available. Further comparator studies, preferably randomized nonregistration trials, are needed to define the best outpatient therapies for adult patients with CAP that confer the lowest rates of treatment failure.
Finally, it is unclear if the outcomes observed in this study are due to treatment, the patients’ underlying comorbidities, severity of illness at presentation, invading pathogen, or a combination of factors. We only included outcomes if they were CAP-related. Almost 40% of the study participants in our analysis had multiple comorbidities, and data clearly show that chronic disease comorbidities significantly affect the outcomes of CAP patients [17–20]. As the infecting pathogen and susceptibility profile are also known to modify outcomes of CAP, attempts should be made to apply new rapid molecular diagnostic tools to help elucidate the causative organism(s) in future outpatient therapy studies for CAP.
In conclusion, 1 in 5 adults with CAP who were treated in the outpatient setting with a guideline-concordant antibiotic experienced treatment failure. Patients who experienced treatment failure had greater 30-day mortality and higher 30-day CAP-related health care costs. The deleterious outcomes associated with treatment failure were more pronounced in patients’ aged >65 years vs those aged 18–64 years. Collectively, our findings indicate significant shortcomings in the current treatment approach to managing patients with CAP in the outpatient setting, especially among those who are not responsive to initial therapy. The results of this study also identify great opportunities to improve clinical management plans, including appropriate allocation of health care resources, to avoid adverse outcomes for patients with CAP.
Acknowledgments
The authors would like to thank N. Theriault and P. Blumberg for scientific and editorial assistance.
Financial support. This work was funded by Cempra Pharmaceuticals, Chapel Hill, NC 29407.
Conflicts of interest. T.P.L. is a consultant to Melinta Therapeutics, Paratek Pharmaceuticals, and Nabriva. P.C. was an employee of Cempra Pharmaceuticals. G.T. was an employee of Cempra Pharmaceuticals and is a consultant to Melinta Therapeutics, Summit plc, KBP Biosciences, Paratek Pharmaceuticals, and GSK. D.M. received research grant support and served as a scientific advisor to Cempra Pharmaceuticals. J.M. served as a consultant to Cempra Pharmaceuticals for the current manuscript. J.M. is also a consultant for Achaogen, Allergan, Cempra, Melinta, Menarini, and Thermo Fisher Scientific. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. File TM Jr, Marrie TJ. Burden of community-acquired pneumonia in North American adults. Postgrad Med 2010; 122:130–41. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, National Center for Health Statistics. Pneumonia. Available at: cdc.gov/nchs/fastats/pneumonia.htm. Published 2017 Accessed 27 November 2017.
- 3. Sato R, Gomez Rey G, Nelson S, Pinsky B. Community-acquired pneumonia episode costs by age and risk in commercially insured US adults aged ≥50 years. Appl Health Econ Health Policy 2013; 11:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yu H, Rubin J, Dunning S, et al. Clinical and economic burden of community-acquired pneumonia in the Medicare fee-for-service population. J Am Geriatr Soc 2012; 60:2137–43. [DOI] [PubMed] [Google Scholar]
- 5. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown JD, Harnett J, Chambers R, Sato R. The relative burden of community-acquired pneumonia hospitalizations in older adults: a retrospective observational study in the United States. BMC Geriatr 2018; 18:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McLaughlin JM, Johnson MH, Kagan SA, Baer SL. Clinical and economic burden of community-acquired pneumonia in the Veterans Health Administration, 2011: a retrospective cohort study. Infection 2015; 43:671–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jain S, Self WH, Wunderink RG, et al. ; CDC EPIC Study Team Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015; 373:415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hess G, Hill JW, Raut MK, et al. Comparative antibiotic failure rates in the treatment of community-acquired pneumonia: results from a claims analysis. Adv Ther 2010; 27:743–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ye X, Sikirica V, Schein JR, et al. Treatment failure rates and health care utilization and costs among patients with community-acquired pneumonia treated with levofloxacin or macrolides in an outpatient setting: a retrospective claims database analysis. Clin Ther 2008; 30:358–71. [DOI] [PubMed] [Google Scholar]
- 11. Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 13 2011; (169):1–38. [PubMed] [Google Scholar]
- 12. Niederman MS, McCombs JS, Unger AN, et al. The cost of treating community-acquired pneumonia. Clin Ther 1998; 20:820–37. [DOI] [PubMed] [Google Scholar]
- 13. National Ambulatory Medical Care Survey 1993–2015 Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Datasets/NAMCS. Accessed July 2019.
- 14. National Hospital Ambulatory Medical Care Survey 1992–2016 Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Datasets/NHAMCS. Accessed July 2019.
- 15. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2017; 10:CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Self WH, Grijalva CG, Williams DJ, et al. Procalcitonin as an early marker of the need for invasive respiratory or vasopressor support in adults with community-acquired pneumonia. Chest 2016; 150:819–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lim WS, Baudouin SV, George RC, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax 2009; 64(Suppl 3):iii1–55. [DOI] [PubMed] [Google Scholar]
- 18. Corrales-Medina VF, Suh KN, Rose G, et al. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med 2011; 8:e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kornum JB, Thomsen RW, Riis A, et al. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care 2008; 31:1541–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Polsky D, Bonafede M, Suaya JA. Comorbidities as a driver of the excess costs of community-acquired pneumonia in U.S. commercially-insured working age adults. BMC Health Serv Res 2012; 12:379. [DOI] [PMC free article] [PubMed] [Google Scholar]