Abstract
Monocytes are circulating cells imperative to the response against pathogens. Upon infection, they are quickly recruited to the affected tissue where they can differentiate into specialized phagocytes and antigen-presenting cells. Additionally, monocytes play a vital role in chronic inflammation, where they can promote and enhance inflammation or induce its resolution. There are two major subsets of monocytes, “inflammatory” and “nonclassical,” which are believed to have distinct functions. In atherosclerosis, both types of monocytes are constantly recruited to lesions, where they contribute to plaque formation and atherosclerosis progression. Surprisingly, these cells can also be recruited to lesions and promote resolution of atherosclerosis. Tracking these cells in various disease stages may inform about the dynamic changes occurring in the inflamed and resolving tissues. In this chapter we will discuss methods for differential labeling of the two monocyte subsets in order to examine their dynamics in inflammation.
Keywords: Monocytes, Macrophages, Methods, Trafficking, Recruitment, Atherosclerosis resolution
1. Introduction
The discovery of phagocytes is mainly attributed to Ilya Metchnikoff, who received the Nobel Prize in 1908 for his findings. Metchnikoff discovered that a subtype of white blood cells that were recruited to sites of inflammation was able to engulf foreign bodies in starfish larva. The main observation was that these cells, which were termed “phagocytes,” mediate bacterial clearance through their capabilities to surround and kill other cells [1, 2].
Monocytes are a subset of phagocytes, which are released from bone marrow and extramedulla sites of hematopoiesis, patrol the blood, and can be rapidly recruited to sites of inflammation, such as infection or tissue damage. In tissues, monocytes may differentiate into macrophages and dendritic cells [3, 4]. All of the above mentioned cell types participate in diverse functions, including pathogen clearance, wound healing, removal of dead cells, and recruitment and activation of adaptive immune cells.
In the blood of mice and humans, monocytes represent 4–10% of the leukocytes and can be distinguished from other blood leukocytes through their expression of CD115 (CSF-1R) [5]. Classically, monocytes are divided into two major subsets, which have been described in multiple species and have distinct characteristics. In mice, classical monocytes are defined as CCR2+CX3CR1+Ly6chi and are commonly referred to as “inflammatorymonocytes,” whereas nonclassical monocytes are CCR2−CX3CR1++CCR5+Ly6clo [6]. In humans, the equivalent subpopulations are CD14++CD16− and CD14+CD16+, respectively [7].
The crucial roles of monocytes and monocyte-derived cells in homeostasis and inflammation prompted the interest in understanding their trafficking in vivo. Early studies used the intravenous injection of India ink, which was believed to be taken up primarily by monocytes. With that technique Ebert and Florey reported the recruitment of monocytes to sites of injury and their differentiation into macrophages in rabbit ears [8]. It was later shown that monocytes can be labeled with 3H-thymidine, a radioactive nucleotide that incorporates into newly synthesized DNA. In their kinetic studies, van Furth and Cohn demonstrated that 60–80% of blood monocytes can be radioactively labeled after either one intravenous or four intramuscular injections of 3H-thymidine in mice [9], with slightly different labeling kinetics; intravenous administration resulted in a peak of labeled blood monocytes after 48 h, whereas with intramuscular injections, labeling in the blood peaked 60 h postinjection.
Radiolabeling was also performed on leukocytes ex vivo, after which the cells were introduced back to the model animals. In one such study, labeled cells were introduced back into pigeons, to examine the origin of cells in the atherosclerotic plaque [10]. This study showed that most of the cells in early lesions are of monocyte/macrophage origin, whereas mature lesions are more complex and contain smooth muscle cells, extracellular matrix, and adaptive immune cells. Adoptive transfer of cells was also performed later without the need for radiolabeling but with the use of genetic differences between the transferred cells and the recipient animal [11]. For instance, monocytes of male mice were transferred to female recipients in order to quantify the recruitment of monocytes to atherosclerotic lesions using a polymerase chain reaction (PCR) to the Y-chromosome gene sry. In the past three decades, many other genetic-based models have been introduced to track leukocytes and monocytes in particular. The use of the congenic pan-leukocyte markers CD45.1 and CD45.2 enabled the tracking of adoptively transferred hematopoietic cells in the recipient mouse using PCR or flow cytometry [12].
An important advance in the field was the generation of mice that allow fluorescent tagging of monocytic populations, such as the CX3CR1-GFP [13] and the CCR2-RFP [14] mice. The CX3CR1-GFP mouse is widely used and enables the tracking of all cells that express CX3CR1, among them monocytes. However, since CX3CR1 is frequently downregulated in monocytes that are differentiating into macrophages, another reporter mouse was recently described: the CD68-GFP mouse [15]. Additionally, a CX3CR1 fate-mapping mouse was generated, enabling the constitutive and, in a later development, conditional labeling of cells that had expressed CX3CR1 at any given time [16].
To research the trafficking of specific monocyte populations without the need for genetic manipulations or cell transfer, researchers have taken advantage of the innate properties of these cells to take up a wide variety of easily-labeled substances. One approach to fluorescently label phagocytes is via the administration of liposomes containing fluorescent dyes, such as DiI [17, 18]. Liposomes are bilayered phospholipid spheres that are phagocytosed by specialized cells, such as macrophages, dendritic cells, and monocytes. Encapsulation of a fluorescent dye in liposomes results in the fluorescent labeling of its ingesting cell, thus preferentially marking phagocytes.
The same cellular property is also utilized for the specific depletion of phagocytes, using liposomes loaded with clodronate [19], since phagocytes that ingest the clodronate-liposomes undergo apoptosis. Specifically, monocytes are eliminated from the circulation with maximal depletion at 18 h post intravenous injection of clodronate-liposomes. Following clodronate-liposome treatment, Ly6Chi cells begin to reappear in the blood after 2 days, while Ly6Clo monocytes reappear only after 7 days [17]. Sunderkötter and colleagues depleted all monocyte subsets with clodronate-liposomes and reinjected the mice with DiI-liposomes after 2 days, to preferentially label the newly formed Ly6Chi cells. With this method they have showed that Ly6Chi monocytes can differentiate into Ly6Clo cells.
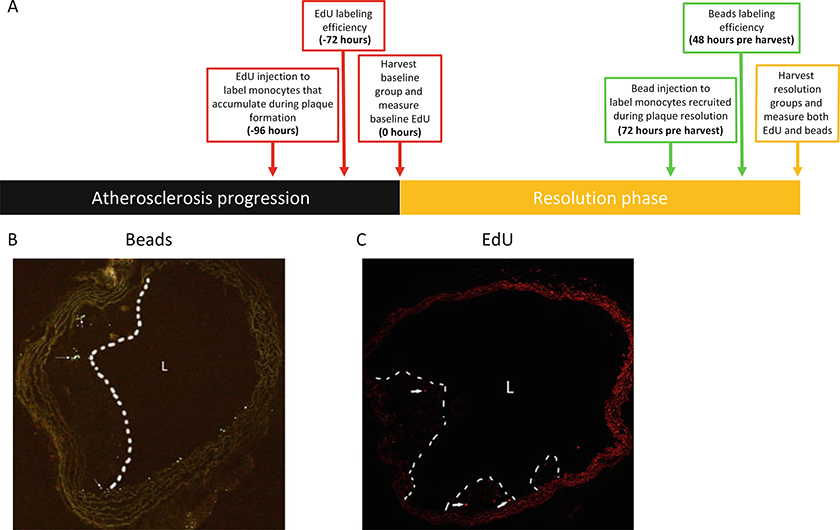
We and others have used fluorescent latex bead injection following monocyte depletion with clodronate-liposomes to mark circulating monocytes [6, 20]. Interestingly, administration of these inert latex beads without prior depletion of circulating monocytes results in differential labeling of Ly6Clo monocytes [21]. Hence, depending on the time point after clodronate depletion of monocytes, the fluorescent beads will label preferentially Ly6Chi or Ly6Clo monocyte subsets, and we and others have applied these protocols to track monocyte entry and the subsequent fate of monocyte-derived cells in atherosclerotic plaques [6, 22, 23] (Fig. 1a and 1b).
Fig. 1.
Detection of beads and EdU in atherosclerotic plaques. (a) Suggested labeling timing with EdU and beads for examining monocyte trafficking during the resolution of atherosclerosis (modified from ref. 23). EdU is injected 4–5 days prior to the induction of resolution, to mark cells recruited during plaque formation. Comparing the abundance of EdU-positive cells between baseline and the resolution group will characterize the subset of monocytes that are leaving the plaque. Beads are injected 2–3 days prior to harvest, to label monocytes that are newly recruited specifically during lesion resolution. (b, c) Frozen sections of aortic arches were imaged in the (b) 488 channel to visualize latex beads or in the (c) 647 channel to visualize EdU (see Note 1)
Differential labeling of Ly6chi monocytes can also be achieved by an approach that does not require clodronate treatment. Since their progenitors in the bone marrow are highly proliferative, this enables their labeling by compounds that intercalate into newly synthesized DNA, such as EdU or BrdU [24, 25] (Fig. 1c). Beginning at 6 h following BrdU treatment, labeled Ly6chi monocytes appear in the circulation and plateau at peak labeling 12–24 h posttreatment [25]. It should be noted that traditionally EdU and BrdU have been used to mark proliferating cells in tissues, highlighting the importance of the choice of the time point for analysis after injection of these nucleotide mimics. For example, if observations of macrophages in peripheral tissues, such as atherosclerotic plaques, are made within 6 h of injection, positively labeled macrophages will likely be the result of proliferation in situ. On the other hand, if the time point is later, positive cells in tissues will be the combination of newly recruited cells (with EdU or BrdU incorporation occurring in bone marrow precursors) and local proliferation. As noted in the laboratory procedures below, fortunately, it is relatively easy to determine if the observed labeling was from recruitment of circulating monocytes or from the proliferation of monocyte-derived cells in the tissue.
This chapter will discuss methods of monocyte labeling and subsequent cell tracking (entry into and disappearance from) atherosclerotic plaques, focusing on results we have published (e.g., [20, 23, 26]). Specifically, techniques of labeling Ly6chi or Ly6clo monocytes distinctly and the subsequent disappearance of macrophages derived from these cells (taken to indicate cell exit from plaques) will be presented. An illustration of the value of these methods is our recent demonstration that resolution of atherosclerosis, a process referred to often as regression, requires the recruitment of Ly6chi monocytes in a CCR2-dependent manner [22]. These studies employed one of the protocols described below for the dual labeling of circulating monocytes at different time points with latex beads and EdU in order to differentiate between monocyte recruitment into and macrophage disappearance from plaques after the resolution of atherosclerosis was induced [23].
The ability to track monocytes and cells derived from them has provided major insights into the study of the innate immune system in various contexts such as inflammatory diseases, including atherosclerosis, and the homeostatic regulation of tissue macrophages. The protocols described are relatively easy to implement, and we expect continued progress in their application by refinements in existing approaches as well as by the development of new ones.
Using the approach described herein, the EdU will label monocytes that will enter the plaque during the progression of the disease (Fig. 1a). This method allows the quantification of the EdU+ cell abundance in plaques, after which one can compare between baseline plaques (plaques not exposed to resolving conditions) and plaques that underwent atherosclerosis resolution. This comparison will indicate whether monocytes and cells derived from circulating monocytes (such as macrophages and dendritic cells) that were recruited during the progression phase had disappeared from the plaque during the resolution phase, as well as to quantitatively estimate the changes. Furthermore, with this method, because of when they are administered, fluorescent beads will label monocytes that are newly recruited to the plaque only in the phase of atherosclerosis resolution, which we know continues to occur even under conditions favorable to resolve plaques [27]. Importantly, alterations in the time of EdU or bead injection should be carefully considered, as discussed in the Introduction. For example, EdU injection <6 h before harvesting the mice will result in labeling of locally proliferating cells, rather than recruited monocytes, since labeled monocytes begin to appear in the blood 6 h after treatment [25]. Additional time would be required for the cells to circulate and extravagate to the affected tissue.
Though we are illustrating monocyte/macrophage trafficking methods in atherosclerosis, these methods may be applied to other inflammatory diseases such as arthritis and obesity, as well as to more general aspects of tissue macrophage homeostasis.
2. Materials
Clodronate-liposomes (see Note 2).
Insulin syringe, either 0.5 mL or 1 mL.
Sterile phosphate-buffered saline (PBS) 1×.
Fluoresbrite® YG Microspheres 1.00 μm.
Heparin-coated capillary tubes.
1.5 mL tubes.
Red blood cell lysis buffer.
0.5 M EDTA pH 8.0.
Hank’s Balanced Salt Solution (HBSS) 10×.
Bovine serum albumin (BSA).
FACS buffer: 1× HBSS, 1% BSA, 0.2% 0.5 M EDTA pH 8.0.
PE/Cy7 anti-mouse CD45 antibody.
PE anti-mouse CD115 (CSF-1R) antibody.
FITC anti-mouse CD115 (CSF-1R) antibody.
APC anti-mouse Ly6c antibody.
EdU (5-ethynyl-2-deoxyuridine).
Click-iT™ Plus EdU Pacific Blue™ Flow Cytometry Assay Kit.
Click-iT Plus EdU staining solution: 87.5 μL PBS, 9 μL water, 1 μL 10× buffer additive, 2 μL CuSO4, 0.5 μL Pb azide.
Frozen section (see Note 3).
Poly-L-lysine-coated slides.
Slide holder.
Slide staining dish.
Click-iT™ Imaging Kit.
V2% paraformaldehyde in PBS.
4% paraformaldehyde in PBS.
EdU staining mix: 215 μL 1× reaction buffer, 10 μL CuSO4, 0.6 μL Alexa Fluor azide, 25 μL buffer additive.
Triton X-100.
DAPI diluted 1:10,000 in PBS.
ProLong Gold Antifade Reagent.
Coverslips.
Fluorescent microscope with capabilities of excitation at 441 nm emission and at 485 nm.
Confocal microscope with capabilities of excitation at 651 nm emission and at 667 nm.
3. Methods
3.1. Labeling Monocytes with Latex Beads (See Note 2)
Anesthetize a mouse with isoflurane.
Inject 250 μL of clodronate-liposomes intravenously.
Eighteen hours later, prepare fluorescent beads by diluting them 1:4 with PBS, under sterile conditions.
Anesthetize the mouse with isoflurane.
Inject 200 μL of the diluted beads retro-orbitally with an insulin syringe.
3.2. Measuring Latex Bead Incorporation in Blood Monocytes (See Note 4)
Twenty-four hours post-latex bead injection, harvest 50–80 μL of blood by tail-tipping using heparin-coated capillary tubes.
Collect the blood into a 1.5 mL tube containing 10 μL of 0.5 M EDTA pH 8.0.
Throughout the experiment keep cells on ice, in the dark.
Add 1.2 mL of 1× red blood cell lysis buffer. Incubate for 15 min.
While incubating, take 30 μL from each sample, and mix them in a new tube. These cells would be used for unstained and single-stained controls.
Spin down cells at 600 × g for 5 min.
Discard supernatant and resuspend in 1 mL FACS buffer. Dislodge the pellets after every wash, by gently pipetting.
Repeat steps 6 and 7 twice.
Divide the control cells into four: one unstained control and three for single antibodies.
Make antibody mix; dilute CD45-PE/Cy7, Ly6C-APC, and CD115-PE 1:100 in FACS buffer.
Add 100 μL of the antibody mix to each sample.
Incubate for 30 min.
Wash cells with 1.2 mL FACS buffer.
Spin down cells at 600 × g for 5 min.
Discard supernatant and transfer cells to FACS tubes.
Analyze single cells by flow cytometry (see Note 5).
3.3. Counting Latex Beads and Normalizing to Blood Monocyte Incorporation
After generating frozen sections [28], count beads in plaques using a fluorescent microscope (see Notes 6 and 7 and Fig. 2).
Use the following equation to calculate the corrected bead per section:
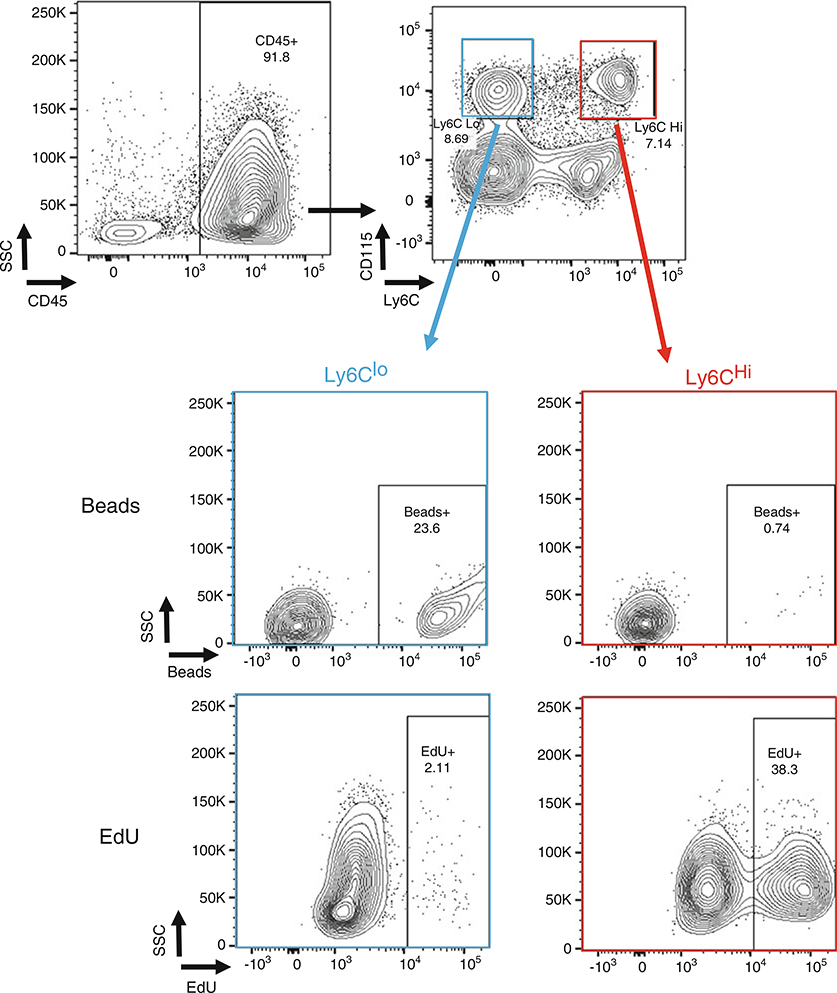
Fig. 2.
Differential labeling of Ly6Chi and Ly6Clo monocytes with EdU and fluorescent beads, respectively. Flow cytometry plots of blood cells harvested from mice injected either with fluorescent beads or EdU 24 h prior to measurement. Ly6Chi (red) and Ly6Clo (blue) were gated from all CD45+ cells and analyzed for beads and EdU incorporation
3.4. Labeling Ly6chi Monocytes with EdU and Measuring Incorporation in Blood Monocytes (See Notes 8 and 9)
Inject 250 μL of 1× EdU intraperitoneally.
Twenty-four hours post-EdU injection, harvest 50–80 μL of blood by tail-tipping using heparin-coated capillary tubes.
Proceed protocol as in Subheading 3.2 steps 3–8.
Divide the control cells into five: one unstained control, three for single antibodies, and one for EdU only control (see Note 10).
Add 100 μL Click-iT® fixative or 2% paraformaldehyde. Incubate for 15 min at room temperature.
Wash twice by adding 1 mL FACS buffer, gently pipette up and down, and centrifuge at 600 × g for 5 min. Discard the supernatant.
Add 100 μL perm/wash reagent. Incubate 15 min on ice.
Centrifuge at 1000 × g for 5 min. All centrifugation steps from here onward are at 1000 × g.
Prepare the EdU staining solution (see Note 11).
Remove all the supernatant carefully with a 200 μL pipette.
Add 100 μL of the staining solution, and mix gently by pipetting up and down. Incubate for 30 min at room temperature, in the dark.
Wash cells twice by adding 1 mL perm/wash reagent and centrifuging for 5 min. Discard supernatant.
Prepare antibody mix; dilute CD45-PE/Cy7, Ly6C-APC, and CD115-FITC 1:100 in perm/wash reagent.
Add 100 μL of the antibody mix. Incubate on ice, in the dark, for 30 min.
Wash with 1 mL FACS buffer, centrifuge for 5 min, and discard the supernatant.
Transfer cells to FACS tubes using 150 μL FACS buffer.
Analyze single cells using flow cytometry.
3.5. EdU Staining in Plaques
Generate frozen sections [28].
Thaw frozen sections at room temperature for 5 min. All incubation steps are performed at room temperature.
Fix with 4% paraformaldehyde solution for 1 h.
Wash slides twice, by placing the slide holder apparatus in a bath containing 3% BSA in PBS. All washing steps are performed using 3% BSA in PBS for 5 min.
Permeabilize with 0.5% Triton X-100 diluted in PBS for 1 h.
Wash slides twice.
Prepare EdU staining mix.
Cover the sections with 250 μL EdU staining mix, and incubate for 1 h, protected from light.
Wash slides with PBS for 5 min.
Incubate with 5 μg/mL DAPI for 5 min.
Wash slides twice with PBS for 5 min.
Put 1 drop of ProLong Gold Antifade Reagent in the middle of the slide, and place a coverslip on the slide.
Visualize the tissue using a confocal microscope.
Normalize the counted events to the EdU incorporation in blood monocytes, using the equation in Subheading 3.3 step 2.
Acknowledgments
We thank the current and past trainees in the Fisher lab for their contributions to establishing the protocols described in this article and applying them productively to the study of atherosclerosis progression and resolution. We thank Michela L. Garabedian for her contribution in the preparation of this chapter. Current relevant research in the Fisher lab is supported by grants from the NIH (HL127930, 129433, 131481, 131478), the US Department of Defense (W81XWH-16-1-0374), and the American Heart Association (18POST34080390).
Footnotes
As noted in the Introduction, using the labeling method with the fluorescent latex beads and EdU at different time points, it is possible to investigate the recruitment versus disappearance of monocyte-derived cells during the resolution of atherosclerosis [23]. This can be achieved by injecting mice with EdU 3–5 days before the resolution of atherosclerosis is induced [29–31] and injection of beads 1–3 days before mice in a resolving environment (e.g., after lowering of plasma lipids) are harvested. An example of an experimental design to implement this approach is summarized in a figure modified from our recent paper [23] and reproduced in Fig. 1a.
For labeling of Ly6chi monocytes, use this protocol; however, for labeling of Ly6clo monocytes, start the protocol from step 3.
When harvesting, place the aortic root or arch in molds containing OCT, perpendicular to the mold base, and quickly freeze by placing over dry ice. Using a cryostat, generate 6 μm slices, and place them on poly-l-lysine-coated slides.
It is crucial to measure the incorporation of beads and EdU in blood monocytes, since there can be significant mouse-to-mouse variations. The percentage of monocytes that had incorporated these materials would be used as a normalization factor for each mouse individually (details in Subheading 3.3).
Be mindful that the beads are extremely bright and may leak into other channels. For the flow cytometry analysis, we typically use the BD Bioscience LSRII cytometer.
No additional steps are required for bead visualization. It is recommended to count the beads before staining the slides for morphometric analysis.
For bead counting use the GFP/FITC channel of a fluorescent microscope. We typically use the Leica DM4000B microscope.
Labeling with BrdU instead of EdU will produce similar results; however, staining protocols for BrdU are more complicated, and thus we tend to use EdU.
Since EdU and BrdU label proliferating cells, an analysis of dividing cells in the target tissue is required (such as Ki67 staining), to confirm that EdU intercalated into recruited monocytes and not to locally proliferating cells. Furthermore, it may also be necessary to perform co-localization immune-histochemical studies with cell type-specific antibodies or to use such antibodies in flow cytometric analyses after aortic digestion.
An additional control is required (fluorescent minus one), since the EdU staining increases the background noise. The ideal control is a blood of a mouse that has not been injected with EdU. An alternative control will lack CuSO4—a key component for the fluorescent reaction.
Prepare the EdU reaction mix not more than 15 min before use, and add its component in the mentioned order.
For EdU-positive cell counting, use the 647 channel of a confocal microscope. We typically use the Leica TCS SP5 microscope.
References
- 1.Gordon S (2008) Elie metchnikoff: father of natural immunity. Eur J Immunol 38 (12):3257–3264 [DOI] [PubMed] [Google Scholar]
- 2.Gordon S (2007) The macrophage: past, present and future. Eur J Immunol 37(Suppl 1): S9–S17 [DOI] [PubMed] [Google Scholar]
- 3.Ginhoux F, Jung S (2014) Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 14 (6):392–404 [DOI] [PubMed] [Google Scholar]
- 4.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K (2010) Development of monocytes, macrophages, and dendritic cells. Science 327(5966):656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ (2011) Monocyte trafficking in acute and chronic inflammation. Trends Immunol 32 (10):470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J et al. (2007) Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest 117(1):185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19(1):71–82 [DOI] [PubMed] [Google Scholar]
- 8.Ebert RH HW F (1939) The extravascular development of the monocyte observed in vivo. Br J Exp Pathol 20(4):342–356 [Google Scholar]
- 9.van Furth R, Cohn ZA (1968) The origin and kinetics of mononuclear phagocytes. J Exp Med 128(3):415–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis JC, Taylor RG, WG J (1985) Foam cell characteristics in coronary arteries and aortas of white Carneau pigeons with moderate hypercholesterolemia. Ann N Y Acad Sci 454:91–100 [DOI] [PubMed] [Google Scholar]
- 11.Steinberg D, Khoo JC, Glass CK, Palinski W, Almazan F (1997) A new approach to determining the rates of recruitment of circulating leukocytes into tissues: application to the measurement of leukocyte recruitment into atherosclerotic lesions. Proc Natl Acad Sci U S A 94 (8):4040–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyse EA (1977) The increasing value of congenic mice in biomedical research. Lab Anim Sci 27(5 Pt 2):771–781 [PubMed] [Google Scholar]
- 13.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A et al. (2000) Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol 20 (11):4106–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL et al. (2010) Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS One 5(10):e13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iqbal AJ, McNeill E, Kapellos TS, Regan-Komito D, Norman S, Burd S et al. (2014) Human CD68 promoter GFP transgenic mice allow analysis of monocyte to macrophage differentiation in vivo. Blood 124(15):e33–e44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M et al. (2013) Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38 (1):79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA et al. (2004) Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172(7):4410–4417 [DOI] [PubMed] [Google Scholar]
- 18.Leenen PJ, Radosevic K, Voerman JS, Salomon B, van Rooijen N, Klatzmann D et al. (1998) Heterogeneity of mouse spleen dendritic cells: in vivo phagocytic activity, expression of macrophage markers, and subpopulation turnover. J Immunol 160 (5):2166–2173 [PubMed] [Google Scholar]
- 19.Van Rooijen N, Sanders A (1994) Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods 174 (1–2):83–93 [DOI] [PubMed] [Google Scholar]
- 20.Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J et al. (2011) HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci U S A 108(17):7166–7171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ginhoux F, Tacke F, Angeli V, Bogunovic M, Loubeau M, Dai XM et al. (2006) Langerhans cells arise from monocytes in vivo. Nat Immunol 7(3):265–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahman K, Vengrenyuk Y, Ramsey SA, Vila NR, Girgis NM, Liu J et al. (2017) Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest 127(8):2904–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bradfield PF, Menon A, Miljkovic-Licina M, Lee BP, Fischer N, Fish RJ et al. (2016) Divergent JAM-C expression accelerates monocyte-derived cell exit from atherosclerotic plaques. PLoS One 11(7):e0159679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jongstra-Bilen J, Haidari M, Zhu SN, Chen M, Guha D, Cybulsky MI (2006) Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med 203(9):2073–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu SN, Chen M, Jongstra-Bilen J, Cybulsky MI (2009) GM-CSF regulates intimal cell proliferation in nascent atherosclerotic lesions. J Exp Med 206(10):2141–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L et al. (2011) Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123(9):989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ (2004) Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci U S A 101 (32):11779–11784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menon P, Fisher EA (2015) Immunostaining of macrophages, endothelial cells, and smooth muscle cells in the atherosclerotic mouse aorta. Methods Mol Biol 1339:131–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewing B, Fisher EA (2012) Preclinical mouse models and methods for the discovery of the causes and treatments of atherosclerosis. Expert Opin Drug Discov 7(3):207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peled M, Nishi H, Weinstock A, Barrett TJ, Zhou F, Quezada A et al. (2017) A wild-type mouse-based model for the regression of inflammation in atherosclerosis. PLoS One 12 (3):e0173975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Basu D, Hu Y, Huggins LA, Mullick AE, Graham MJ, Wietecha T et al. (2018) Novel reversible model of atherosclerosis and regression using oligonucleotide regulation of the LDL receptor. Circ Res 122(4):560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]