Abstract
Spleen samples from 292 wild carnivores from Colorado were screened for Bartonella infection. Bartonella DNA was detected in coyotes (Canis latrans) (28%), striped skunks (Mephitis mephitis) (23%), red foxes (Vulpes vulpes) (27%), and raccoons (Procyon lotor) (8%) but not in black bears (Ursus americanus), gray foxes (Urocyon cinereoargenteus), and mountain lions (Puma concolor). Two Bartonella species, B. vinsonii subsp. berkhoffii and B. rochalimae, were identified. All 10 infected striped skunks exclusively carried B. rochalimae while coyotes, red foxes, and raccoons could be infected with both Bartonella species. Five of seven infected coyotes carried B. v. berkhoffii whereas five of seven infected red foxes and 11 of 14 infected raccoons carried B. rochalimae. Further studies are needed to understand relationships between Bartonella species, wild carnivores, and their ectoparasites.
Keywords: Bartonella vinsonii subsp. berkhoffii, Bartonella rochalimae, Colorado, wild carnivores, wildlife diseases
INTRODUCTION
The bacteria of genus Bartonella are widely distributed among a variety of wild mammals and associated arthropod vectors. Bartonella species are usually host-specific, although the specificity may be observed at different taxonomic levels of host animals (Jardine et al. 2006; Bai et al. 2013). The question of host specificity is important in regard to the survival and persistence of Bartonella species (Dehio 2004; Chomel et al. 2009). Most Bartonella species persist subclinically within mammalian reservoirs while some may induce pathologic effects in incidental hosts—mainly demonstrated in humans, cats (Felts catus), and domestic dogs (Canis lupus familians; Dehio 2004).
Several studies have reported detection or isolation of Bartonella species in coyotes (Cams latrans), raccoons (Procyon lotor), gray foxes (Urocyon cinereoargenteus), island foxes (Urocyon littoralis), and other wildlife from multiple regions across the world, including the western US (Chang et al. 2000; Gerrikagoitia et al. 2012). Two Bartonella species, B. vinsonii subsp. berkhoffii and B. rochalimae, reported in wild mammals are also recognized as human pathogens. Bartonella vinsonii subsp. berkhoffii was originally isolated from a dog suffering infectious endocarditis (Kordick et al. 1996) and later was identified as a zoonotic agent causing endocarditis in a human patient (Roux et al. 2000). In California, B. v. berkhoffii was detected in coyotes and foxes, with higher prevalence in coyotes, suggesting that coyotes could be an important wildlife reservoir for B. v. berkhoffii (Chang et al. 2000).
Bartonella rochalimae was first described in a human patient exhibiting fever, myalgia, nausea, insomnia, mild anemia, and splenomegaly after returning to the US from Peru, where the patient experienced multiple insect bites (Eremeeva et al. 2007), This species was also reported in dogs with endocarditis (Henn et al. 2009b), gray foxes, raccoons, coyotes, and rats (Rattus norvégiens), with extremely high prevalence (42%) in red foxes (Henn et al. 2009a; Gundi et al. 2012). In addition, B. rochalimae was detected in fleas (Pulex simulans) collected on gray foxes (Gabriel et al. 2009), implying that the flea might be a vector for B. rochalimae.
Recognized as the main cause of cat scratch disease in humans (Chomel 2000), Bartonella henselae has been reported in wild felids, arctic foxes (Vulpes lagopus), mongooses (Herpestes auropunctatus), and palm civets (Paguma larvata) (Sato et al. 2013) in addition to the well-known domestic cat hosts (Chomel et al. 2002). Two more Bartonella species associated with domestic cats and recognized as human pathogens (B. clarridgeiae and B. koehlerae) have also been reported in wild animals (Kaewmongkol et al. 2011; Hwang and Gottdenker 2013). An isolate from the Japanese marten (Martes melampus) is closely related to Bartonella washoensis (Sato et al. 2012), a species commonly associated with sciurids and a source of human cardiac disease (Kosoy et al. 2003). Due to the zoonotic potential of various Bartonella species, information about the presence of Bartonella species in wild animal reservoirs is valuable from a public health standpoint.
Early investigations of Bartonella species in foxes, raccoons, and other carnivores were mostly based on detection of bacteria or bacterial DNA in animal blood. We report findings of Bartonella species in spleens of wild carnivores including foxes, coyotes, raccoons, and skunks from Colorado. Bartonella DNA has been detected in spleens of coyotes previously, although the prevalence was low (3/70 positive; Kehoe 2014). Our study provides additional evidence that spleen tissues can be used for Bartonella testing when blood is not available.
MATERIALS AND METHODS
Sample collection for this study was approved by the US Department of Agriculture, National Wildlife Research Center Quality Assurance Unit, and authorized by a Colorado Parks and Wildlife permit.
Study sites and sample collection
Spleen samples from 292 wild carnivores were mostly collected in Larimer, Boulder, and Weld counties of Colorado during 2013 and 2015 during the enhanced rabies surveillance. Carcasses were sourced from a subset of public health rabies submissions (pet/human contact cases) and supplemented with no-contact cases from county health departments, Colorado Parks and Wildlife, and local humane societies or wildlife rehabilitation centers. For no-contact cases, sampling was targeted toward animals with neurologic symptoms that had been humanely euthanized by local authorities or animals that were found dead. Necropsy was performed on carcasses of 44 striped skunks (Mephitis mephitis), 186 raccoons, 25 coyotes, 26 red foxes, one gray fox, seven black bears, and three mountain lions (Table 1), and spleens were removed and stored in individual containers. Instruments used for necropsy were cold-sterilized and autoclaved before use. Following necropsy, samples were stored at −80 C until testing.
Table 1.
Detection and identification of Bartonella species by 16S–23S internal transcribed spacer (ITS) in wild carnivores, Colorado, August 2013 and June 2015.
| Common names | Species | No. Tested | No. Positive | Prevalence (%) | B. berkhoffii (proportion %) | B. rochalimae (proportion %) |
|---|---|---|---|---|---|---|
| Coyote | Canis latrans | 25 | 7 | 28 | 5 (71) | 2 (29) |
| Striped skunk | Mephitis mephitis | 44 | 10 | 23 | 10 (100) | |
| Raccoon | Procyon lotor | 186 | 14 | 8 | 3 (21) | 11 (79) |
| Mountain lion | Puma concolor | 3 | 0 | 0 | —a | — |
| Gray fox | Urocyon cinereoargenteus | 1 | 0 | 0 | — | — |
| Black bear | Ursus americanus | 7 | 0 | 0 | — | — |
| Red fox | Vulpes vulpes | 26 | 7 | 27 | 2 (29) | 5 (71) |
| Total | 292 | 38 | 13 | 10 (26) | 28 (74) |
Dash = not applicable.
DNA extraction and PCR detection
The spleens were homogenized using Bullet Blender® Gold homogenizer (Next Advance, Averill Park, New York, USA) following the spleen protocol provided by the manufacturer. The homogenates were then used for DNA extraction using QIAxtractor (Qiagen, Valencia, California, USA) following the tissue protocol provided by the manufacturer. For initial screening of Bartonella DNA, we performed a PCR assay targeting the 16S–23S internal transcribed spacer (ITS) by using Bartonella-specific primers 325s (CTT CAG ATG ATG ATC CCA AGC CTT CTG GCG) and HOOas (GAA CCG ACG ACC CCC TGC TTG CAA AGC A). Any ITS-positive samples were further analyzed by two additional genes, citrate synthase gene (gitA) and cell division gene (ftsZ), using a nested PCR platform. Two pairs of specific primers were applied for each target (e.g., primers CS443F (GCT ATG TCT GCA TTC TAT CA; Jardine et al. 2005) and CS1210R (GAT CYT CAA TCA TTT CTT TCC A; Gundi et al. 2012) as the outer set and Bhcs.781p (GGG GAC CAG CTC ATG GTG G) and Bhcs.ll37n (AAT GCA AAA AGA ACA GTA AAC A; Norman et al. 1995) as the inner set for git A; primers Bfpl (ATT AAT CTG CAY CGG CCA GA) and Bfp2 (ACV GAD ACA CGA ATA ACA CC; Zeaiter et al. 2002) were used as the outer set and the newly designed primers R83 (ATA TCG CGG AAT TGA AGC C); and L83 (CGC ATA GAA GTA TCA TCC C) as the inner set for ftsZ. Positive and negative controls were included in each PCR run to evaluate the presence of appropriately sized amplicons and contamination, respectively. Only samples with PCR products that resulted in clear sequence information were considered as positive for further analysis.
Sequencing and identification of Bartonella species
Amplicons of appropriate size compared to the positive controls were further identified by sequencing. All Bartonella-positive PCR products from any of the three targets were purified using a QIAquick PCR Purification Kit (Qiagen) and then sequenced in both directions with the same primers which were used for the initial PCR screening (inner primers for git A and ftsZ). Using Lasergene Versionl2 (DNASTAR, Madison, Wisconsin, USA), Bartonella sequences from all samples were compared among themselves and with other known Bartonella species or genotypes that were available from GenBank. Newly identified sequence variants were submitted to GenBank.
RESULTS
Molecular detection
Using PCR assays targeting ITS we detected Bartonella DNA in 38 of 292 samples (13%). We detected Bartonella DNA at varying prevalences in coyotes, striped skunks, raccoons, and red foxes but not in black bears, gray foxes, or mountain lions (Table 1). Among 38 ITS-positive samples, 36 were also positive by gItA and 33 samples were positive by flsZ. Overall, 32 samples were Bartonella-positive by all three targets, and six samples were positive by two targets. All amplicons were considered positive only after confirmation as Bartonella species by sequencing of the targeted genes.
Identification of Bartonella species
Sequencing analysis of all three targets demonstrated that the Bartonella species detected in the carnivores were either B. v. berkhoffii or B. rochaliniae. Six ITS genetic variants were identified among the 38 positive samples with three variants of B. v. berkhoffii in 10 samples and three variants of B. rochaliniae in 28 samples (Fig. 1). Four variants were identical to previously published sequences (DQ059763, DQ059765, DQ676487, and DQ676491) identified in dogs with valvular endocarditis (Maggi et al. 2006; Henn et al. 2009). One variant from each group that had at least one nucleotide mismatch with previously published sequences were submitted to GenBank (accessions KU292576 and KU292577).
Figure 1.
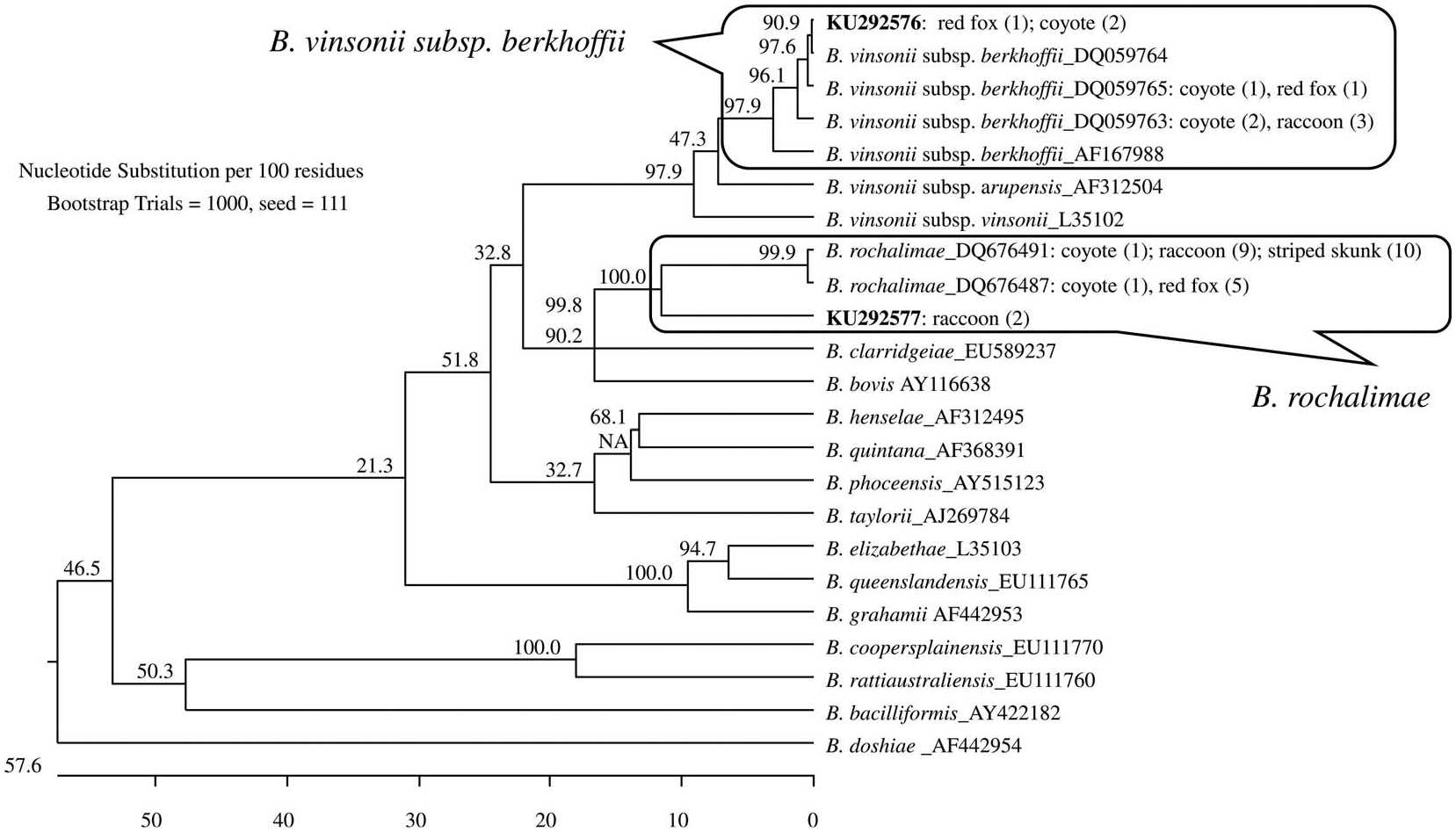
Phylogenetic relationships between the Bartonella genotypes detected in carnivores from Colorado and some reference Bartonella species based on sequencing analysis of 16S–23S internal transcribed spacer (ITS). Six Bartonella genotypes were identified in the carnivores. Each genotype is indicated by its GenBank accession number and is followed by the common names of carnivores and number of identical DNA obtained from the host species in parenthesis. Newly identified genotypes are indicated in bold. The genotypes belonged to either Bartonella vinsonii subsp. berkhojfii or Bartonella rochalimae (clades within boxes). The phylogenetic tree was constructed by the neighbor-joining method, and bootstrap values were calculated with 1,000 replicates.
Variant KU292577, detected only in two raccoons, was clustered with the group of sequences closely related to B. rochaliniae but more distant from others (Fig. 1). Except for this variant, other variants were found in carnivore hosts of different species (Fig. 1), demonstrating potential host-switching. Noticeably, all sequences derived from striped skunks were identical to variant DQ67649 (B. rochaliniae); nevertheless, this variant was also detected in coyotes and raccoons. The majority of infected coyotes carried B. v. berkhoffii while most infected red foxes and raccoons carried B. rochaliniae (Table 1).
Analysis of gItA and ftsZ sequences from positive samples consistently supported identification of Bartonella species based on the ITS phylogeny. Specifically, seven unique variants were identified based on gItA sequences, with three (accessions U28075, KU292567, and KU292568) belonging to B. v. herkhoffii and the other four (DQ683195, KU292569-KU292571) to B. rochalimae; seven unique variants were identified based on ftsZ sequences, with three (CP003124, KU292572, and KU292573) belonging to B. v. herkhoffii and four (DQ676490, FN645461, KU292574, and KU292575) to B. rochalimae. Similar to ITS phylogeny, gItA and ftsZ showed that the strain infecting the two raccoons (ITS variant KU292577) belonged to B. rochalimae but was much more distant in the phylogenetic relationship to other variants (3.8% versus 0.3% by gItA; 5.4% versus 0.3% by flsZ).
Human or domestic animal exposure
Among the 38 Bartonella-positive animals, six (15%) were involved in human or domestic animal exposures whereas 32 were animals which had been collected in the absence of known human or domestic animal contact. The six contact cases included three striped skunks with reported contact with dogs (n=2) or livestock (n=1), a raccoon with reported dog contact, and two coyotes with reported human (n=2) and dog (n=1) contact. Both B. v. herkhoffii and B. rochalimae were identified in these animals (B. v. herkhoffii in the two coyotes and B. rochalimae in the striped skunks and the raccoon.
DISCUSSION
Using a molecular approach to test Bartonella infections in spleen tissues of carnivores from Colorado, our results demonstrated prevalences of these bacteria are comparable with previous reports based on surveys of blood samples (Chang et al. 2000; Henn et al. 2009a). This suggests spleen tissues can be alternative materials for Bartonella investigation when blood is not available. The high prevalence of Bartonella observed in coyotes, red foxes, and striped skunks suggests that these carnivores may be natural reservoirs of Bartonella species. The negative results in mountain lions, black bears, or gray foxes may reflect small sample sizes (n=1–7) from these species, especially considering the high prevalence of Bartonella species observed in gray foxes elsewhere (Henn et al. 2009a).
All positive striped skunks were exclusively infected with identical strains of B. rochalimae, which indicates a likely specific relationship between striped skunks and a particular strain of B. rochalimae. Further, the fairly high prevalence suggests that striped skunks can be a reservoir of B. rochalimae in addition to previously demonstrated species such as gray and red foxes (Henn et al. 2007). Unlike striped skunks, coyotes and red foxes showed little specificity to a particular Bartonella species but are the preferable reservoirs of B. v. berkhoffii and B. rochalimae, respectively. These results are consistent with findings from California and other areas (Chang et al. 2000; Henn et al. 2009a). Raccoons are not very susceptible to Bartonella infection but may harbor multiple Bartonella species. Hwang and Gottdenker (2013) reported detection of B. henselae and B. koehlerae in raccoons in Georgia, USA. These observations may suggest that the raccoon is a general reservoir of Bartonella species and can serve as an alternative host in maintaining Bartonella infections. Finally, a unique variant only found in raccoons suggests the variant might represent a separate subspecies of B. rochalimae. Confirmatory studies are needed.
Similarly to other investigators, we did not observe a specific relationship between Bartonella species and the carnivore species studied, with the exception of striped skunks and B. rochalimae. As Bartonella species are presumably transmitted by ectoparasites, the observations on distribution of Bartonella species in carnivores could be improved from learning differences in composition of ectoparasites associated with mammalian hosts. Studying ectoparasites of carnivores could help to estimate the role of arthropods in the transmission of Bartonella species and other vector-borne diseases and to understand how ectoparasite specificity may contribute to Bartonella-wild mammal associations.
Urbanization of natural areas has been associated with the current apparent emergence of infectious diseases. Carnivores are among the species that adapt well to urban and periurban environments, facilitating cross-species disease transmission with domestic animals, and potentially with their owners, as mechanical dispersers of infected ectoparasites. In our study, several infected animals (15%) had a history of human or dog exposures. Such information may suggest a common source from where humans or domestic animals contacted B. v. berkhoffii and B. rochalimae.
ACKNOWLEDGMENTS
We thank Ivy LeVan, Nikki Crider, Samantha Eaton, Tara Rigg, Chad Wickham, Darren Wostenberg, Dennis Kohler, Jennifer Kanine, Molly Diefenbach, and Shylo Johnson for assistance with sample collection. We thank Laura Tappen Collar for assistance in performing laboratory tests. We thank staff with the Larimer County, Weld County, and Boulder County Public Health Departments and also the Larimer Humane Society, Longmont Humane Society, Boulder Valley Humane Society, and Greenwood Wildlife Rehabilitation Center.
LITERATURE CITED
- Bai Y, Malania L, Castillo DA, Moran D, Boonmar S, Chanlun A, Suksawat F, Maruyama S, Knobel D, Kosoy M. 2013. Global distribution of Bartonella infections in domestic bovine and characterization of Bartonella bovis strains using multi-locus sequence typing. PLoS ONE 8:e80894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Kasten RW, Chomel BB, Simpson DC, Hew CM, Kordick DL, Heller R, Piemont Y, Breitschwerdt EB. 2000. Coyotes (Cams latrans) as the reservoir for a human pathogenic Bartonella sp.: Molecular epidemiology of Bartonella vinsonii subsp. berkhoffii infection in coyotes from central coastal California. J Clin Microbiol 38:4193–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel BB. 2000. Cat-scratch disease. Rev Sci Tech 19: 136–150. [DOI] [PubMed] [Google Scholar]
- Chomel BB, Boulouis HJ, Breitschwerdt EB, Kasten RW, Vayssier-Taussat M, Birtles RJ, Koehler JE, Dehio C. 2009. Ecological fitness and strategies of adaptation of Bartonella species to their hosts and vectors. Vet Res 40:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomel BB, Boulouis HJ, Petersen H, Kasten RW, Yamamoto K, Chang CC, Gandoin C, Bouillin C, Hew CM. 2002. Prevalence of Bartonella infection in domestic cats in Denmark. Vet Res 33:205–213. [DOI] [PubMed] [Google Scholar]
- Dehio C 2004. Molecular and cellular basis of Bartonella pathogenesis. Annu Rev Microbiol 58:365–390. [DOI] [PubMed] [Google Scholar]
- Eremeeva ME, Gerns HL, Lydy SL, Goo JS, Ryan ET, Mathew SS, Ferraro MJ, Holden JM, Nicholson WL, Dasch GA, et al. 2007. Bacteremia, fever, and splenomegaly caused by a newly recognized Bartonella species. N Engl J Med 356:2381–2387. [DOI] [PubMed] [Google Scholar]
- Gabriel MW, Henn J, Foley JE, Brown RN, Kasten RW, Foley P, Chomel BB. 2009. Zoonotic Bartonella species in fleas collected on gray foxes (Urocyon cinereoargenteus). Vector Borne Zoonotic Dis 9:597–602. [DOI] [PubMed] [Google Scholar]
- Gerrikagoitia X, Gil H, García-Esteban C, Anda P, Juste RA, Barrai M. 2012. Presence of Bartonella species in wild carnivores of northern Spain. Appl Environ Microbiol 78:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundi VA, Billeter SA, Rood MP, Kosoy MY. 2012. Bartonella spp. in rats and zoonoses, Los Angeles, California, USA. Emerg Infect Dis 18:631–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn JB, Chomel BB, Boulouis HJ, Kasten RW, Murray WJ, Bar-Gal GK, King R, Courreau JF, Baneth G. 2009a. Bartonella rochalimae in raccoons, coyotes, and red foxes. Emerg Infect Dis 15:1984–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn JB, Gabriel MW, Kasten RW, Brown RN, Koehler JE, MacDonald KA, Kittleson MD, Thomas WP, Chomel BB. 2009b. Infective endocarditis in a dog and the phylogenetic relationship of the associated “Bartonella rochalimae” strain with isolates from dogs, gray foxes, and a human. J Clin Microbiol 47: 787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J, Gottdenker NL. 2013. Bartonella species in raccoons and feral cats, Georgia, USA. Emnerg Infect Dis 19:1167–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine C, McColl D, Wobeser G, Leighton FA. 2006. Diversity of Bartonella genotypes in Richardson’s ground squirrel populations. Vector Borne Zoonotic Dis 6:395–403. [DOI] [PubMed] [Google Scholar]
- Kaewmongkol G, Kaewmongkol S, Fleming PA, Adams PJ, Ryan U, Irwin PJ, Fenwick SG. 2011. Zoonotic Bartonella species in fleas and blood from red foxes in Australia. Vector Borne Zoonotic Dis 11:1549–1553. [DOI] [PubMed] [Google Scholar]
- Kehoe SP, Chomel BB, Stuckey MJ, Kasten RW, Balakrishnan N, Sacks BN, Breitschwerdt EB. 2014. Zoonotic Bartonella species in cardiac valves of healthy coyotes, California, USA. Emerg Infect Dis 20:2133–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosoy M, Murray M, Gilmore RD Jr, Bai Y, Gage KL. 2003. Bartonella strains from ground squirrels are identical to Bartonella washoensis isolated from a human patient. J Clin Microbiol 41:645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordick DL, Swaminathan B, Greene CE, Wilson KH, Whitney AM, O’Connor S, Hollis DG, Matar GM, Steigerwalt AG, Malcolm GB, et al. 1996. Bartonella vinsonii subsp. berkhoffii subsp. nov., isolated from dogs; Bartonella vinsonii subsp. vinsonii; and emended description of Bartonella vinsonii. Int J Syst Bacteriol 46:704–709. [DOI] [PubMed] [Google Scholar]
- Roux V, Eykyn SJ, Wyllie S, Raoult D. 2000. Bartonella vinsonii subsp. berkhoffii as an agent of afebrile blood culture-negative endocarditis in a human. J Clin Microbiol 38:1698–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Kabeya H, Miura T, Suzuki K, Bai Y, Kosoy M, Sentsui H, Kariwa H, Maruyama S. 2012. Isolation and phylogenetic analysis of Bartonella species from wild carnivores of the suborder Caniformia in Japan. Vet Microbiol 161:130–136. [DOI] [PubMed] [Google Scholar]
- Sato S, Kabeya H, Shigematsu Y, Sentsui H, Une Y, Minami M, Murata K, Ogura G, Maruyama S. 2013. Small Indian mongooses and masked palm civets serve as new reservoirs of Bartonella henselae and potential sources of infection for humans. Clin Microbiol Infect 19:1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]


