Abstract
Testosterone glucuronide (TG), androsterone glucuronide (AG), etiocholanolone glucuronide (EtioG) and dihydrotestosterone glucuronide (DHTG) are the major metabolites of testosterone (T), which are excreted in urine and bile. Glucuronides can be deconjugated to active androgen in gut lumen after biliary excretion, which in turn can affect physiological levels of androgens. The goal of this study was to quantitatively characterize the mechanisms by which TG, AG, EtioG and DHTG are eliminated from liver, intestine, and kidney utilizing relative expression factor (REF) approach. Using vesicular transport assay with recombinant human MRP2, MRP3, MRP4, MDR1 and BCRP, we first identified that TG, AG, EtioG, and DHTG were primarily substrates of MRP2 and MRP3, although lower levels of transport were also observed with MDR1 and BCRP vesicles. The transport kinetic analyses revealed higher intrinsic clearances of TG by MRP2 and MRP3 as compared to that of DHTG, AG, and EtioG. MRP3 exhibited higher affinity for the transport of the studied glucuronides than MRP2. We next quantified the protein abundances of these efflux transporters in vesicles and compared the same with pooled total membrane fractions isolated from human tissues by quantitative LC-MS/MS proteomics. The fractional contribution of individual transporters (ft) was estimated by proteomics-based physiological scaling factors, i.e., transporter abundance in whole tissue versus vesicles, and corrected for inside-out vesicles (determined by 5’-nucleotidase assay). The glucuronide metabolites of inactive androgens, AG and EtioG were preferentially transported by MRP3, whereas the glucuronides of active androgens, TG and DHTG were mainly transported by MRP2 in liver. Efflux by bile canalicular transport may indicate the potential role of enterohepatic recirculation in regulating the circulating active androgens after deconjugation in the gut. In intestine, MRP3 possibly contributes most to the efflux of these glucuronides. In kidney, all studied glucuronides seemed to be preferentially effluxed by MRP2 and MDR1 (for EtioG). These REF based analysis need to be confirmed with in vivo findings. Overall, characterization of the efflux mechanisms of T glucuronide metabolites is important for predicting the androgen disposition and interindividual variability, including drug-androgen interaction in humans. The mechanistic data can be extrapolated to other androgen relevant organs (e.g. prostate, testis and placenta) by integrating these data with quantitative tissue proteomics data.
Keywords: testosterone, glucuronides, efflux transporters, quantitative proteomics, vesicular transport
1. Introduction
Testosterone (T) is an adrenal steroid that is essential for sexual and reproductive development [1]. People with low T levels experience fatigue, depression, low sex drive, loss of muscle mass, diabetes, obesity, cardiovascular diseases, or osteoporosis [2]. T levels in men decline with increasing age starting from mid-30s and decrease 1.2% per year thereafter [2, 3]. In the United States, approximately 2.4 million males aged 40–69 years old suffer from hypogonadism, and 3% of men in their 40s and 3.8% of men in their 60s are under testosterone replacement therapy (TRT). The debate over the benefits and potential safety issues of TRT (e.g. cardiovascular risks, stroke, prostate cancer) continues over the past few decades [4–6]. Whether low endogenous T levels and the inconsistent TRT outcomes are associated with interindividual variability in the metabolism or transport of T are not fully characterized.
T is extensively metabolized by multiple enzymes such as UDP-glucuronosyltransferases (UGTs) [7], hydroxysteroid dehydrogenases (HSDs) [8], 5α-reductase [9] and sulfotransferases (SULTs) [10] in the liver and in extrahepatic organs and the majority of T is eliminated in urine as glucuronide metabolites. UGT2B17 and UGT2B15 are the major drug-metabolizing enzymes for T glucuronidation. Especially UGT2B17 is very selective and efficient towards testosterone, and it is highly expressed in intestine with increasing expression along the intestinal tract [11]. In humans, AG, TG, EtioG and the unconjugated androstenedione (AED) are detected as the major circulating T metabolites (Figure 1) [12]. Because of the high hydrophilicity of glucuronide conjugates, the cellular excretion of these glucuronides likely requires active efflux transporters for their elimination. The involvement of transporters as an effective clearance pathway have been reported for many endogenous and exogenous glucuronide conjugates. For example, estradiol-17β-glucuronide is a prominent substrate for multidrug resistance-associated proteins (MRP2, MRP3, and MRP4), breast cancer resistance protein (BCRP), and multidrug-resistance protein (MDR1) [13–16]. The glucuronide metabolite of antineoplastic drug irinotecan, SN-38 glucuronide, has been reported to be substrate of BCRP [17]. Among these efflux transporters, MRP2, BCRP, and MDR1 are expressed on the canalicular or apical membrane of hepatocytes, enterocytes, and proximal tubular cells, which excrete their substrates into bile, gastrointestinal (GI) lumen or urine for their elimination. MRP3 and MRP4 are expressed on the basolateral membrane of epithelial cells in liver, intestine, and kidney, which mediate the transport into blood circulation. Unlike estrogen, at present there is no information regarding the role of efflux transporters in the disposition of T glucuronide metabolites. Only one study [18] showed that TG could efficiently inhibit the ATP-dependent transport of estradiol-17β-glucuronide in rat canalicular membrane vesicles, which indirectly suggests that T glucuronide metabolites may also be substrates of ATP-dependent transporters such as MRPs, BCRP, and MDR1.
Figure 1.
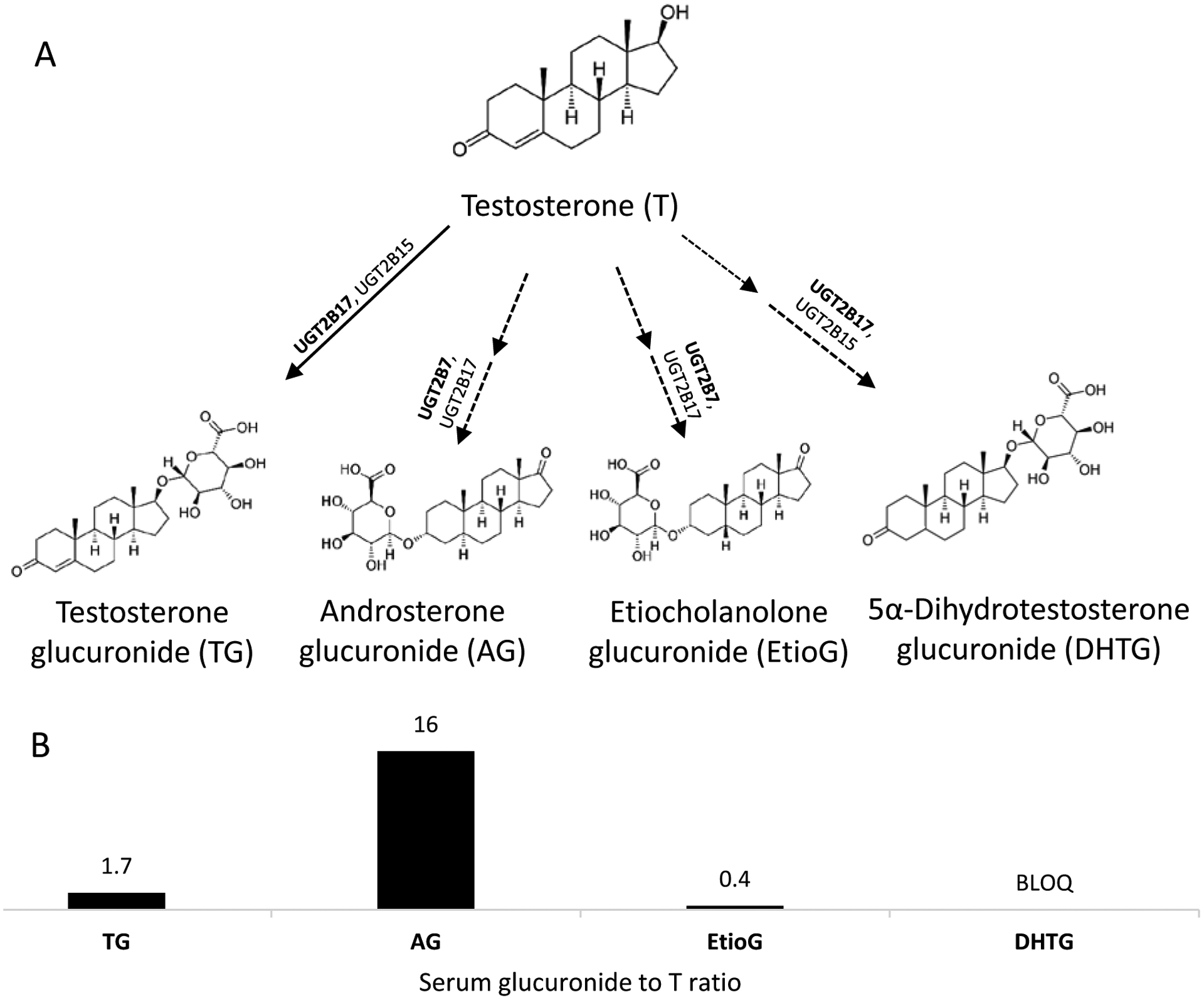
Glucuronidation pathways of testosterone (T) metabolism. A. T is glucuronidated by UGTs to form its primary (TG; solid arrow) and secondary (AG, EtioG, and DHTG; dotted arrows) metabolites. B. Fold-difference in the physiological serum concentration of respective metabolites as compared to T [12]. BLOQ, below limit of quantification. TG, testosterone glucuronide; AG, androsterone glucuronide; EtioG, etiocholanolone glucuronide; DHTG, dihydrotestosterone glucuronide.
The transport of T glucuronide metabolites is also a critical process for enterohepatic circulation by serving as a distribution mechanism for T. Studies in humans using radioactive T suggested that most of biliary T metabolites were observed as glucuronides [19]. In the GI lumen, the glucuronide metabolites of T can be deconjugated by bacterial β-glucuronidases [20], leading to increased half-life of T. This phenomenon has been observed for several drug glucuronides such as SN-38 glucuronide [21]. Recent study also supports the role of microbiota in regulating the circulating T in mice, in which adult male mice with a normal microbiota had higher circulating levels of T compared to mice without gut microbiota, and transfer of cecal contents from male mice to female mice resulted in elevated circulating T in female mice [22]. In addition, endogenous β-glucuronidases have been found in several mammalian tissues such as placenta [23, 24], which can synthesize T from TG. Therefore, the intracellular androgen glucuronide metabolites can be considered as precursors of T and DHT (active) and A and Etio (inactive), and the altered expression of efflux transporters by factors such as genetic polymorphism [25], drug-drug interactions [26], diseases [27], age [28, 29], gender [30, 31], and ethnicity [32], may affect their disposition, leading to dysregulation of steroidal homeostasis. To test our hypotheses, we first identified the transporters that may contribute to the disposition of T glucuronide metabolites using in vitro vesicular assay. We then quantified the transporters that are expressed in human tissues including liver, intestine, and kidney using quantitative proteomics. Finally, we integrated the transporter abundance data in human tissue versus vesicles (using as scaling factors) to characterize the fractional contribution of each transporter (ft) in the disposition of major T glucuronide metabolites (Figure 2). Quantitative characterization of the roles of efflux transporters responsible for T glucuronide metabolites elimination will be important for 1) development of system-based pharmacokinetic model (PBPK) to describe fate of T and its metabolites; 2) predict drug-induced alteration of T disposition by the inhibitors and inducers of efflux transporters; 3) predict effects of genetic polymorphisms of efflux transporters on T homeostasis.
Figure 2.
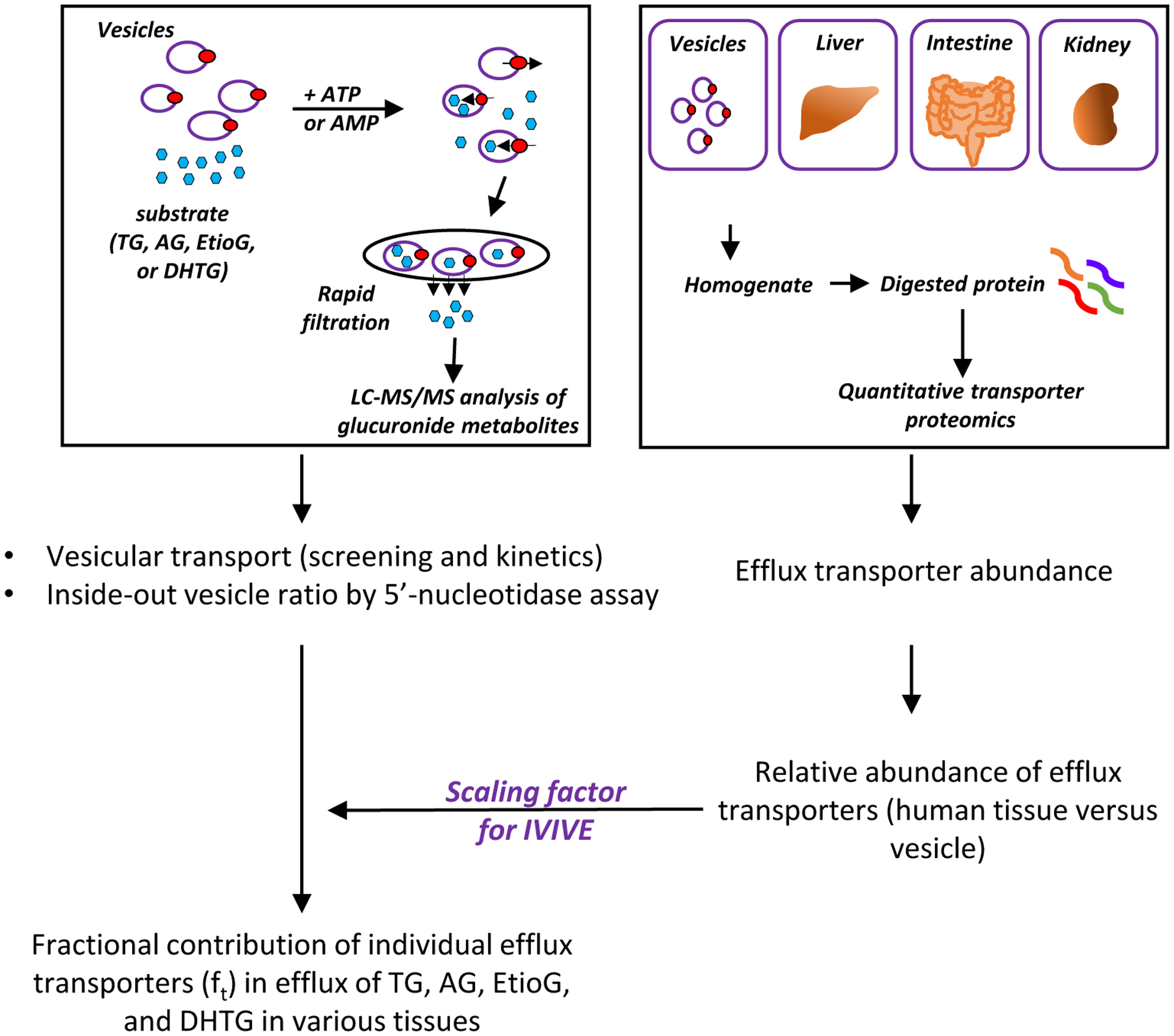
Schematic overview of experimental design. The transport of glucuronides into the membrane vesicles was performed by adding ATP, whereas AMP served as a negative control. The vesicles were then isolated on filter membranes and washed. The glucuronide substrates contained in the vesicles were quantified by a validated LC-MS/MS method [12]. The percentage of inside-out vesicles was characterized using 5’-nucleotidase assay. The protein abundances of efflux transporters in plasma membrane vesicles, human liver, intestine, and kidney were measured by quantitative LC-MS/MS proteomics. The relative abundance of efflux transporters in human tissue versus vesicles were used as scaling factors for in-vitro to in-vivo extrapolation (IVIVE) to characterize the fractional contribution of individual efflux transporters (ft) in efflux of TG, AG, EtioG, and DHTG from human liver, intestine and kidney.
2. Materials and methods
2.1. Chemicals and solvents
TG, AG, DHTG, and the corresponding deuterated stable-labeled internal standards TG-d3, AG-d4, and DHTG-d3 were purchased from Cerilliant corporation (Round Rock, TX). EtioG standard was purchased from Steraloids (Newport, RI). Membrane vesicle overexpressing the human BCRP, MDR1, MRP2, MRP3, and MRP4 were provided by Solvo Biotechnology (Budapest, Hungary). Adenosine 5’-triphosphate (ATP) disodium salt, adenosine 5’-monophosphate (AMP) monohydrate, glutathione, Tris-Base (Tris[hydroxymethyl]aminomethane), MgCl2, NaCl, Sucrose, MOPS (3-[N-Morpholino]propanesulfonic acid) were purchased from Sigma-Aldrich (St. Louis, MO). Bovine serum albumin (BSA), membrane protein extraction kit and trypsin digestion reagents were obtained from Thermo Fisher Scientific (Rockford, IL). The synthetic light peptide and heavy labeled peptides were purchased from New England Peptides (Boston, MA) and Thermo Fisher Scientific (Rockford, IL), respectively. Multiscreen™ HTS Vacuum Manifold and 96-well filter plates with class B glass fiber filters were purchased from EMD Millipore (Billerica, MA). Estradiol-17β-glucuronide (E2-17β-G), N-Methyl Quinidine (NMQ), dehydroepiandrosterone sulfate (DHEAS), and estrone-3-sulfate (E3S) were purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals and reagents, unless indicated otherwise, were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Functional characterization of vesicles overexpressing transporters
The activity of transporters expressed in the vesicles (MRP2, MRP3, MRP4, BCRP, or MDR1) was confirmed by incubating the vesicles with their specific probe substrates. Vesicular transport reactions were conducted as per reported protocols [33]. E2-17β-G was used as probe substrates for MRP2 (at 100 μM) and MRP3 (at 10 μM). N-Methyl Quinidine (NMQ) at 2 μM, dehydroepiandrosterone sulfate (DHEAS) at 0.5 μM, and estrone-3-sulfate (E3S) at 1 μM were used as probe substrates for MDR1, MRP4, and BCRP, respectively (Supplementary Table 1). The reactions were stopped with 200 μl of ice-cold termination buffer and rapid filtration on Millipore MSFBN6B10 filter plates (Merck Millipore, Billerica, MA, USA). Radioactivity of the filters was determined with a Microbeta scintillation counter (Perkin Elmer).
2.3. Vesicular transport assay
The vesicular transport assay was carried out in 96-well polystyrene plates at a final volume of 75 μl per sample as described before with a few modifications [13]. For each reaction, 25 μg of membrane vesicles were incubated in the presence of 4 mM AMP or ATP. The initial screening was conducted with 10 μM substrate at 37 °C for 1 min in the following assay buffers i) 40 mM MOPS-Tris (pH 7.0), 70 mM KCl, and 7.5 mM MgCl2 for MRP2, or ii) 10 mM Tris-HCl, 10 mM MgCl2, and 250 mM sucrose for BCRP, MDR1, MRP3 and MRP4. Glutathione (2 mM) was added to the incubation for MRP vesicles. The transport was stopped by the addition of 200 μl of cold wash buffer (40 mM MOPS-Tris, pH 7.0, 70 mM KCl) and were transferred to a 96-well filter plate. The filter plate was washed with 5 × 200 μl of ice-cold wash buffer under vacuum filtration. The substrate contained in the vesicles was eluted with 100 μl of 1:1 acetonitrile:0.2% formic acid containing corresponding deuterated internal standard and subjected to analysis by LC-MS/MS. The transport kinetic analyses were then carried out for the transporters that exhibited activity in the initial screening. The kinetic parameters for uptake of the glucuronide metabolites into vesicles were derived from assays conducted using substrate concentration range of 0.1 to 500 μM at 37 °C for 15 seconds, which represents linear uptake range (Supplemental Figure 1).
2.4. Measurement of the inside-out vesicle content
Because vesicle preparation generates both inside-out (active) and right-side-out (inactive) vesicles, % inside-out of vesicles was quantified to address this limitation by measuring 5’-nucleotidase activity based on the methods described before [34–36]. Briefly, 25 μg of membrane vesicles was incubated at 37 °C for 30 mins in 50 mM Tris-HCl (pH 7.4), 4 mM MgCl2, with or without 3 mM AMP or 0.3% Triton X-100. The phosphate released from AMP by 5’-nucleotidase was measured by malachite green system. The 5’-nucleotidase activity was measured at four conditions: a) incubating with both AMP and Triton X-100 (maximum activity); b) incubating with AMP only (activity in right-side-out vesicles); c) incubating with Triton X-100 only (background phosphate in the system); d) without incubation (background phosphate in the assay buffer). The percentage of inside-out vesicles was calculated as [(a - c) - (b - d)]/(a - c) * 100, where (a – c) represents the total background corrected activity of 5’-nucleotidase and (b – d) denotes the background corrected activity of the right-side-out vesicles only.
2.5. Quantification of efflux transporters in vesicles and human tissues using surrogate peptides and quantitative proteomics
The pooled total membrane fraction of human liver (n=36), kidney (n=21), and intestine (n=7) tissue samples were available in our lab from previous studies [29, 37, 38]. Total membrane protein concentration determined by bicinchoninic acid (BCA) assay (Pierce Biotechnology, Rockford, IL) was diluted to a working concentration of 2 mg/ml. For proteomics, 80 μl of human tissue membrane proteins (2 mg/ml) and 25 μg of vesicle (diluted to 80 μl) were incubated with 10 μl of dithiothreitol (250 mM), 30 μl of ammonium bicarbonate buffer (100 mM, pH 7.8), 20 μl of BSA (0.02 mg/ml), and 10 μl of human serum albumin (10 mg/ml) at 95°C for 10 mins. After cooling down to room temperature, 20 μl of iodoacetamide (500 mM) was added to the mixture and incubated at room temperature for 30 mins in the dark. To concentrate the sample, ice-cold methanol (0.5 ml), chloroform (0.1 ml), and water (0.4 ml) were added to each sample and thoroughly mixed by vortex. After centrifugation at 16,000 x g for 5 mins at 4°C, the pellet was washed once with ice-cold methanol (0.5 ml) and centrifuged at 8,000 x g for 5 mins at 4 °C. The pellet was resuspended with 60 μl of ammonium bicarbonate buffer (50 mM). Finally, the protein sample was digested with 20 μl of trypsin at 1:10 trypsin : protein ratio (w/w) and incubated for 16 h at 37°C with mixing at 300 rpm. The digestion reaction was quenched by 20 μl of chilled heavy internal standard (dissolved in 80% acetonitrile with 0.5% formic acid) and centrifuged at 4,000 x g for 5 mins at 4°C. For each sample, 5 μl of the supernatant was injected into the LC-MS/MS system for analysis. All samples were digested and processed in triplicate.
The surrogate peptides of BCRP, MDR1, MRP2, MRP3, and MRP4 were quantified in the digested samples using a validated LC-MS/MS method [37, 39]. Light peptides represent analytes and the corresponding heavy peptides were used as internal standards, as listed in Supplementary Table 2. The pooled total membrane sample isolated from liver tissue with known transporter abundance was used as a calibrator for estimation of abundances of individual transporters in vesicles. The calibration curve range and linearity were verified by serial dilutions of the studied transporter protein standards. The abundances of MDR1, MRP3, and MRP4 were measured by LC-MS/MS (SCIEX Triple Quadrupole 6500 system (Framingham, WA) coupled to an ACQUITY UPLC system (Waters Technologies, Milford, MA)). 5 μl of each sample was injected to the column (ACQUITY UPLC HSS T3 1.8 μm, C18 100A; 100 × 2.1 mm, Waters, Milford, MA). Quantification of BCRP and MRP2 was performed using Waters Xevo TQ-S tandem mass spectrometer coupled to an ACQUITY UPLC system (Waters, Hertfordshire, UK). The same column and method were used with a gradient mobile phase (0.3 ml/min) consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), as present in Supplementary Table 3. The LC-MS/MS data were analyzed by Skyline software.
2.6. LC-MS/MS analysis of glucuronide metabolites
The amounts of TG, AG, EtioG, or DHTG retained in the vesicles were quantified by LC-MS/MS using previously optimized parameters [12] with few modifications on the same SCIEX instrument discussed above (Supplementary Table 4).
2.7. Data analysis
The kinetic parameters of vesicular transport were obtained by fitting the Michaelis-Menten equation V = Vmax [S]/([S] + Km), where V is the velocity in pmol/min/mg protein, Vmax is maximal velocity, [S] is substrate concentration (μM), and Km is the Michaelis-Menten constant, using GraphPad Prism version 4.0 (La Jolla, CA). Intrinsic clearance of glucuronide transport in vesicles (Clint,vesicles, μl per min per pmol transporter) was estimated using Equation 1. Where the protein abundance of individual transporters in vesicles (Evesicles), and % of inside out vesicles were considered for normalizing the observed activity data (Table 1).
Table 1.
Transport kinetics parameters (Km, Vmax and CLint, vesicles) for the transport of TG, AG, EtioG, or DHTG by MRP2, MRP3, BCRP, and MDR1. The kinetic parameters were derived from experimental data in Figure 4, after fitting the Michaelis-Menten equations. The 95% confidence intervals (CI) for the derived kinetic constants are presented in the parentheses.
| Transporter | Substrate | Km, μM (95% CI) | Vmax, pmol/min/mg (95% CI) | Proteomics and %inside-out normalized Clint, vesicles, μl/min/pmol transporter protein (95% CI) |
|---|---|---|---|---|
| MRP2 | TG | 109.6 (92.9 to 129.7) | 4767 (4499 to 5064) | 0.77 (0.70 to 0.86) |
| AG | 85.6 (66.0 to 111.3) | 2206 (2016 to 2422) | 0.46 (0.39 to 0.54) | |
| DHTG | 78.9 (66.6 to 93.6) | 3366 (3179 to 3570) | 0.76 (0.68 to 0.85) | |
| EtioG | 33.6 (26.0 to 43.5) | 1407 (1300 to 1527) | 0.75 (0.63 to 0.89) | |
| MRP3 | TG | 6.0 (4.3 to 8.4) | 1520 (1401 to 1658) | 1.82 (1.43 to 2.36) |
| AG | 1.0 (0.67 to 1.5) | 470.2 (445.3 to 496.3) | 3.38 (2.44 to 4.84) | |
| DHTG | 5.7 (3.8 to 8.5) | 986.2 (895.3 to 1095) | 1.26 (0.93 to 1.72) | |
| EtioG | 0.3 (0.22 to 0.40) | 382.3 (355.8 to 410) | 9.21 (7.47 to 11.47) | |
| MDR1 | EtioG | 53.4 (38.8 to 73.1) | 386.7 (348.6 to 429) | 0.54 (0.43 to 0.66) |
| BCRP | DHTG | 676.4 (368 to 1767) | 2470 (1729 to 4945) | 0.99 (0.76 to 1.27) |
| (1) |
The intrinsic clearance in human tissues (CLint, μl/min) was estimated using Equation 2, where Etissue, total is the transporter abundance (pmol of transporter per organ) in respective human tissue. Etissue, total was calculated by multiplying pmol transporter abundance per mg of protein with total membrane protein per gram of tissue (TM-PPGT, pmol protein per gram of tissue) and with tissue weight (kg). The TM-PPGT value was determined experimentally for human liver and kidney. For intestine, TM-PPGT was assumed similar to that in human liver (Supplementary Table 5).
| (2) |
The CLint for individual transporters was then used to estimate the total CL (Equation 3), which then allowed derivation of the fractional contribution (ft) of each transporter in glucuronide transport in vivo by Equation 4.
| (3) |
| (4) |
3. Results
3.1. Functional characterization of efflux transporters
As expected, MRP2- and MRP3-overexpressing vesicles showed ATP-dependent transport of the positive control (E2-17β-G) compared to control vesicles expressing empty vector (Supplementary Table 1). Similarly, NMQ, DHEAS, and E3S were used as positive controls for vesicles overexpressing MDR1, MRP4 and BCRP. No activity was observed in AMP-treated groups.
3.2. Characterization of inside-out (active) fraction of efflux transporters in vesicles
The percentage of inside-out vesicles using 5’-nucleotidase assay was observed to be 21~58%. (Supplementary Figure 2). The percent inside out vesicles in BCRP, MDR1, MRP2 were similar (30–40%), whereas it was significantly higher (58%) for MRP3 and lower (21%) for MRP4.
3.3. Protein abundance of efflux transporters in vesicles and human tissues
The protein abundances of MRP2, MRP3, MDR1, and BCRP in vesicles were 133.9, 239.4, 45.0, and 11.4 pmol/mg protein, respectively, which were exponentially higher than those in human tissues. In liver, MRP2 is the most abundant efflux transporter (1.45 pmol/mg protein), followed by MDR1, MRP3 and BCRP (0.53, 0.48, and 0.14 pmol/mg protein, respectively). Compared to the liver, human intestine has higher abundance of MRP3 (2.3-fold) and BCRP (1.8-fold), and lower abundance of MRP2 (0.62-fold). The expression of MDR1 in liver and intestine is similar. In kidney, MDR1 abundance is 4-fold greater than that in the liver, whereas MRP2 is comparable in liver and kidney. MRP3 and BCRP were below LLOQ in kidney (Supplementary Figure 3). Because MRP4 did not transport androgen glucuronides in the initial screening assay as discuss below, the protein abundance of MRP4 data is not presented.
3.4. ATP-dependent transport of glucuronide metabolites of testosterone
The initial screening results confirmed MRP2 and MRP3 as the main transporters involved in efflux of TG, EtioG and DHTG, whereas AG was primarily effluxed by MRP3 (Figure 3). EtioG and DHTG were also transported by MDR1 and BCRP, respectively. MRP4 was not involved in significant active transport of these glucuronides. Because the protein abundance of efflux transporters differs in the overexpressing vesicles, the transport activity of TG was re-tested with equal amount of transporter proteins (BCRP, MDR1, MRP2, and MRP3) to confirm the transport rate was not due to higher protein expression. Consistently, MRP2 and MRP3 showed higher transport rate toward TG, which was not observed for BCRP and MDR1 (Supplementary Figure 4). Comparing to the initial screening result as shown in Fig. 3A, the change of transport rate of TG is within the range from 6% to 35%.
Figure 3.
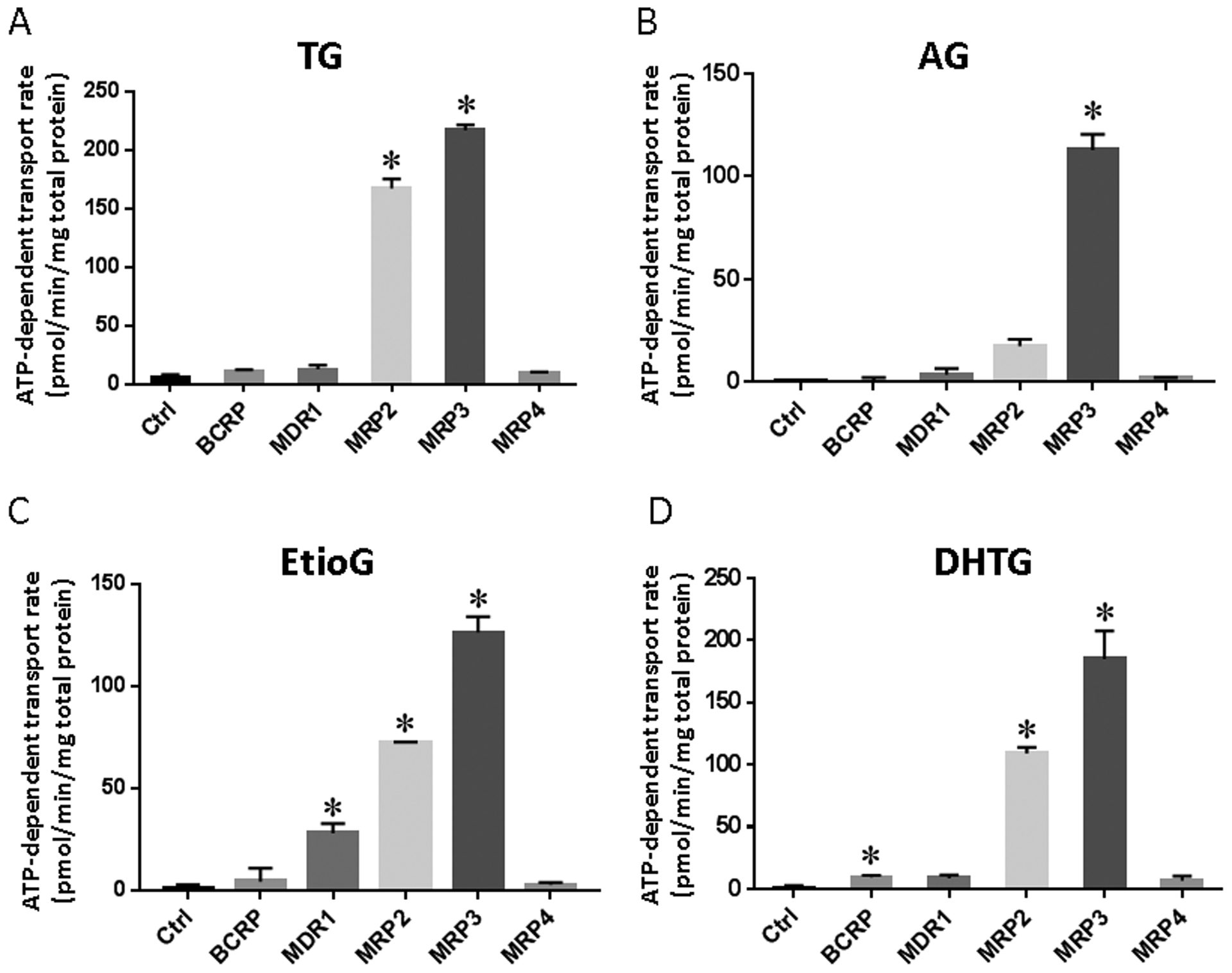
ATP-dependent transport rate of TG (A), AG (B), EtioG (C), and DHTG (D) in membrane vesicles overexpressing efflux transporters at 10 μM substrate concentration. The transport of substrates (TG, AG, EtioG, and DHTG) by BCRP, MDR1, MRP2, MRP3, and MRP4 was studied using 25 μg of total vesicle protein and 1 min incubation. Control vesicles (Ctrl) prepared using HEK293 cells expressing empty vector (mock) were used in all assays. Difference between the ATP and AMP groups (i.e., net ATP-dependent transport rate) was calculated and presented as means ± SD of triplicate samples. Asterisk (*) indicates statistically significant differences in net ATP-dependent transport rate between transporter-overexpressing and Ctrl vesicles by Student’s t-test (p < 0.05).
MRP3 exhibited higher affinity (lower Km values) for the transport of TG, AG, EtioG, and DHTG ranging from 0.3 to 6.0 μM as compared to MRP2, which showed Km values of 33.6–109.6 μM, respectively (Table 1). Similarly, affinity of EtioG for MDR1 was weaker than MRP3 and MRP2 (Km, MDR1, 53.4 μM) (Figure 4C and Table 1). Poorer affinity of DHTG for BCRP (as compared to MRPs) was also observed as shown by its non-saturable kinetics (Fig. 4D). When differences in protein abundances were not observed, MRP2 and MRP3 exhibited higher apparent Vmax,vesicles for TG, which was followed by DHTG, AG, and EtioG (Figure 4A and B).
Figure 4.
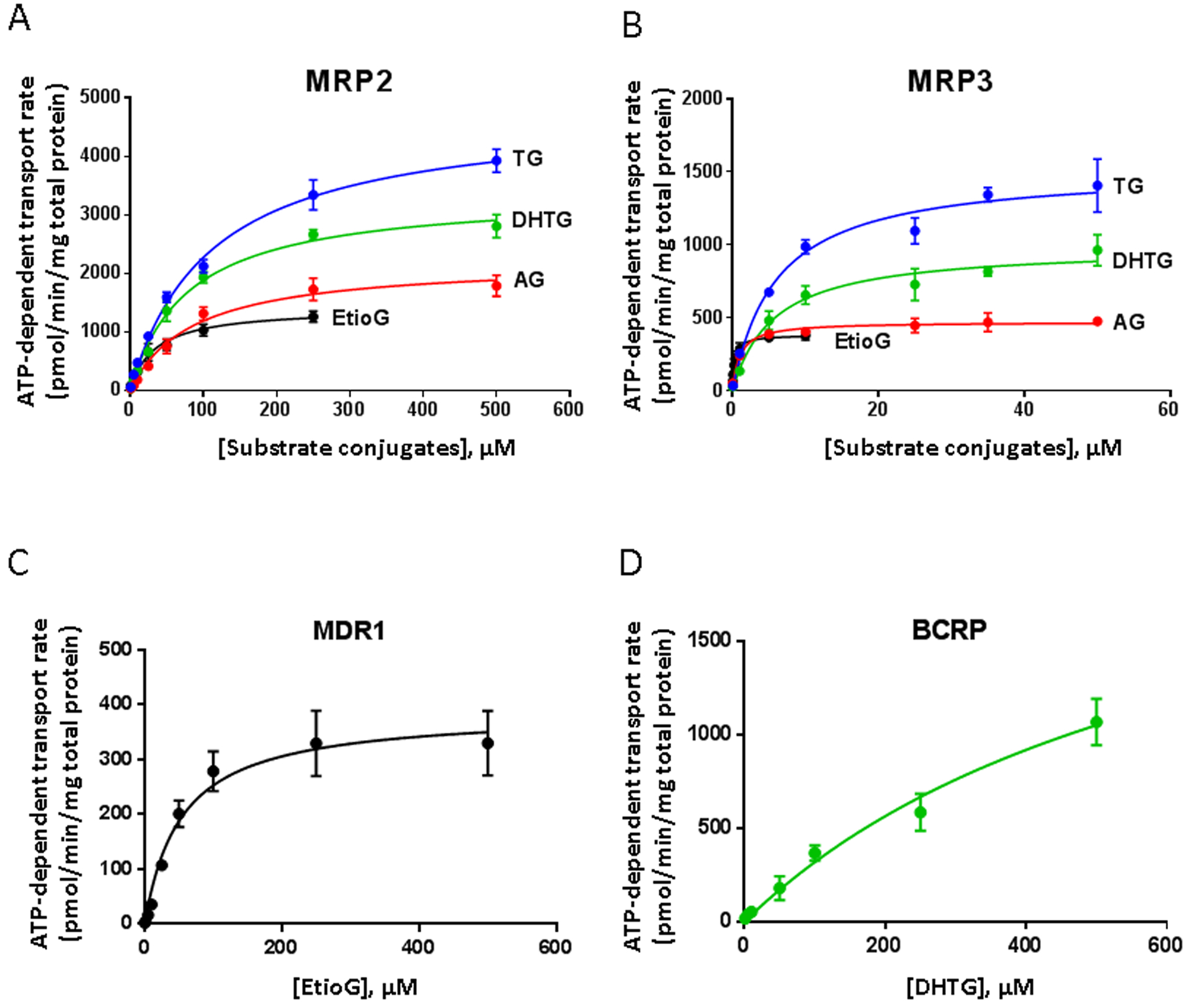
ATP-dependent transport kinetics of glucuronide metabolites of T by efflux transporters. MRP2 (A) and MRP3 (B) mediated transport kinetics of TG (blue), DHTG (green), AG (red), and EtioG (black). MDR1 (C) mediated transport kinetics of EtioG and BCRP (D) mediated transport kinetics of DHTG. The kinetic experiments were conducted at various concentrations with 25 μg vesicle proteins for 15 seconds. Differences between the ATP and AMP groups (net ATP-dependent transport rates) were calculated and Michaelis-Menten equation (ν = Vmax*[S]/([S]+Km)) is fitted to the data. The kinetic constants (Vmax and Km with the 95% CI) for the studied glucuronide metabolites and transporters are presented in Table 1.
Although Km is independent of protein abundance, apparent Vmax,vesicles is directly proportional to the protein levels (Eq. 1). Because transporter protein is abundant and variable in vesicles, it is critical to normalize the Vmax and Km derived apparent clearance value by the transporter protein abundance. Similarly, because vesicle preparation could contain both inside-out and right-side out vesicles, the apparent clearance was also corrected for % of inside-out vesicles to generate Clint,vesicles data (μl per min per pmol transporter protein) (Table 1). The normalized Clint,MRP3 for TG was 2.4-fold higher than that of CLint,MRP2. Similarly, the normalized Clint,MRP3 for AG was 7.4-fold higher than that of Clint,MRP2. For DHTG, the normalized CLint,MRP3 was highest, which was 1.7- and 1.3-fold greater than CLint,MRP2 and CL int,BCRP, respectively. Whereas for EtioG, CLint,MRP3 was highest, which was 12.4- and 17.2-fold higher than that of CLint,MRP2 and CLint,MDR1, respectively (Table 1).
3.5. Fractional contribution of individual transporters to CLint based on tissue transporter abundances
When normalized to transporter abundance in tissues, MRP3 showed highest intrinsic clearance in liver towards glucuronide transport as compared to other studied transporters in following order EtioG≥AG>>TG>DHTG (ft, 0.76 to 0.33). MRP2 was the second most important transporter with intrinsic clearance greatest for DHTG and TG followed by AG≥EtioG (Figure 5A). About 56–60% of TG and DHTG were effluxed by MRP2, whereas AG and DHTG were preferentially transported by MRP3 (~70%) in the liver. Similarly, when corrected for the transporter abundance in intestine, MRP3 contributed most to the efflux of EtioG (91%), AG (90%), TG (74%), and DHTG (60%), while MRP2 contributed 26~29% to the efflux of TG and DHTG, with minor contribution for AG (10%) and EtioG (6%). Because MRP3 abundance in kidney is small (which was considered equivalent to the lower limit of detection), almost all the studied glucuronides were effluxed preferentially by MRP2, with the ft value ranging from 0.4 to 0.92. Higher expression of MDR1 in kidney resulted in significant contribution of MDR1 in EtioG efflux in kidney (43%). (Figure 5A).
Figure 5.
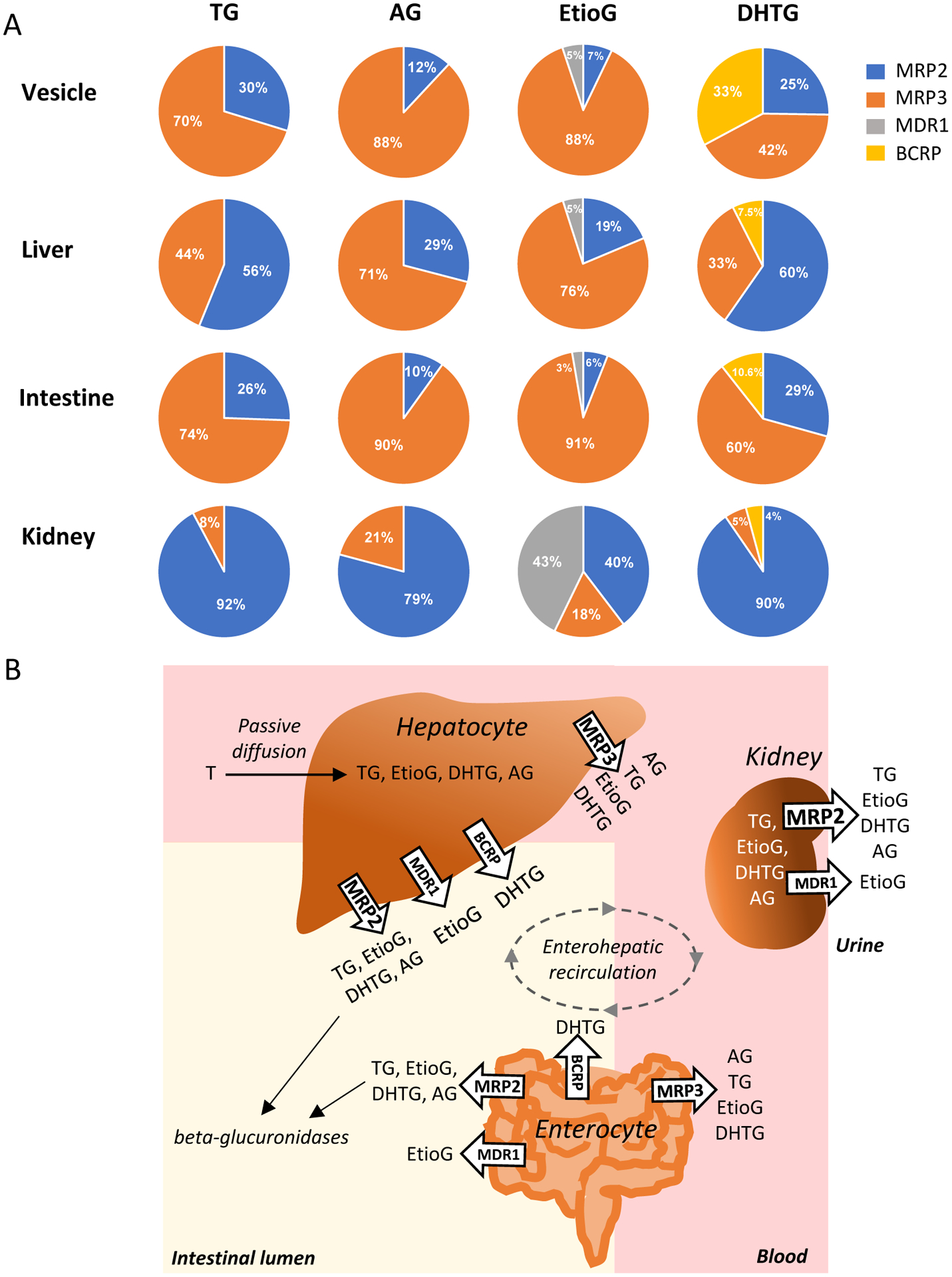
A. Observed and extrapolated fractional contribution (ft) of individual efflux transporters to the clearance of TG, AG, EtioG, and DHTG in membrane vesicles, and in human tissues after integrating the scaling factor. The individual scaling factors were derived from the relative abundance of efflux transporters in human liver, intestine and kidney versus in vesicles as shown in Supplemental Table 5. B. A proposed disposition model of glucuronide metabolites of testosterone (T) in liver, intestine and kidney. MRP3 is expressed in basolateral or sinusoidal side, whereas MRP2, MDR1 and BCRP are expressed in apical or canalicular side. MRP3 and MRP2 are more important in the transport of testosterone glucuronides in human liver, intestine and kidney.
4. Discussion
To our knowledge, the present study is the first to identify and confirm that MRP3 and MRP2 are the major transporters for the efflux of TG, AG, EtioG, and DHTG, whereas MDR1 and BCRP also play roles in the efflux of EtioG and DHTG, respectively. Because the abundances of these transporters differ across tissues, we proposed a novel proteomics-based IVIVE method that may enable us in estimating contribution of individual transporters in the efflux of steroids and drugs in any tissue. Accordingly, this study is also the first to characterize the fractional contribution of individual transporter (ft) to the efflux of T glucuronide metabolites in individual tissues. Because these transporters are localized in either the apical (facing lumen) or basolateral (facing blood) membranes in hepatocytes, enterocytes, and proximal tubular cells, ft characterization is important to elucidate the fraction of intracellular glucuronides that is excreted into lumen versus blood (Figure 5B).
While the efflux from intestine, liver and kidney results in decreased tissue glucuronide concentration, the apical transporters allow elimination of these substrates from the body whereas the basolateral transporters lead to increased plasma concentration of these metabolites. So, depending on the tissue protein abundance of transporters and their localization, we estimated that the majority of TG was transported by MRP3 to blood from enterocytes, and a substantial fraction of TG can be transported by MRP2 into GI lumen and proximal tubule lumen. Human liver and intestine (but not kidney) can produce TG (from T) as both UGT2B17 and UGT2B15 are expressed in these first-pass organs. However, more TG formation can be expected in intestine due to higher UGT2B17 abundance, which increases along the GI tract [7]. ~60% MRP2 mediated efflux of TG and DHTG explains the significant biliary elimination of the radioactive dose of T after non-oral administration [19]. Similarly, MRP3 mediated sinusoidal or basolateral efflux of TG is consistent with high circulating levels of TG explaining that TG is rapidly distributed into blood after its formation in intestine and liver during the first-pass [12]. The circulating TG can be actively taken up by kidneys [40], which is then secreted by MRP2 leading to high urinary excretion of TG as reported before [41].
MRP3 was estimated to be the major transporter responsible for AG efflux from the liver and intestine (Figure 5A). AG is one of the major circulating metabolites of T. AG formation is catalyzed by UGT2B7 (major) and UGT2B17 (minor). Because UGT2B7 is expressed in the liver, intestine and kidney [42], all three organs can produce AG. Recently, we have shown that the plasma levels of AG and TG increased ~390- and 85-fold compared to T, respectively after 800 mg oral administration [12]. The higher levels of circulating AG compared to T and TG corroborate with greater role of sinusoidal and basolateral MRP3 efflux for AG.
Based on the protein abundance data [42] and in vitro activity using recombinant UGT isoforms [12], liver, intestine and kidney can produce EtioG from Etio by UGT2B7 and UGT2B17, whereas DHTG is formed in liver and intestine by UGT2B17 and UGT2B15. Based on the data presented here, the majority of EtioG that are formed in liver and intestine are excreted into blood, whereas DHTG that is formed in the liver can be excreted either into blood (33%) or GI lumen (67.5%). DHTG that are formed in the intestine are mainly excreted into blood. The MRP3-mediated transport of EtioG supports its high plasma levels [12]. In the kidney, MRP2 is the major transporter that effluxes TG, AG, EtioG and DHTG into urine, and MDR1 also contributes significantly to the efflux of EtioG there. In general, the high expression of MRP2 and MDR1 in kidney indicates unidirectional efflux of these glucuronides into urine. Although BCRP has small contribution for DHTG efflux, high expression of this transporter in endocrine organs such as placenta [43, 44], prostate [45, 46] and testis [47, 48] can lead to increased clinical significance in these tissues. Notably, genetic polymorphism in BCRP (ABCG2 c.421C > A) which is prevalent in Asian population [49] can influence intracellular concentrations of the most potent androgen, DHTG.
The Km and CLint,vesicles data as well as IVIVE approach presented here can be employed to predict ft for these glucuronides in different tissues. It is interesting that in contrast to MRP2, MRP3 has a higher affinity towards all the studied glucuronides, as demonstrated by the lower Km values (Table 1). This is in line with the literature that reports higher affinity of MRP3 for glucuronide substrates than MRP2, although they both usually share similar substrate specificity [50]. These findings suggest that MRP3 can lead to non-linear PK of its substrates by saturation at higher substrate concentrations. No clear effect of MRP2 and MRP3 knockout is shown on androgen disposition, whereas MRP4 knockout mice are shown to be associated with age-dependent decrease in T production [62]. However, human MRP4 was not found to be directly involved in efflux of any of the tested glucuronides in this study.
The important role of MRP2 in transport of active androgens (TG and DHTG) indicates the potential clinical importance of enterohepatic recirculation in regulating the circulating T and DHT after deconjugation in the GI lumen. The apparent longer half-life and multiple peak phenomenon observed in T PK [12] support this phenomenon. The evidences of enterohepatic recirculation of T have been shown in mice [22], where compared to mice without gut microbiota, mice with a normal microbiota showed higher circulating T, and transfer of cecal contents from male mice to female mice resulted in higher circulating T in female mice.
The novel findings from this study have significant importance towards reproductive, physical and psychological development, which are regulated by sex hormones like T and DHT. During fetal development, T is synthesized by Leydig cells, leading to the development of male sexual characteristics. A small amount of T is also produced by placenta [51] and the expression of MRP2 and MRP3 in placenta [52–54] suggest they could play important roles in regulating androgens in the fetal circulation. This is particularly relevant as glucuronidases that are capable of regenerating unconjugated androgens from the glucuronides, are also expressed in the placenta [24]. High levels of T exposure during fetal development could cause low birth weights, fetal growth retardation, insulin resistance and metabolic defects [55–59]. Further, integrating the mechanistic transport data with tissue-specific transporter abundance (using quantitative proteomics) can help predict and prevent the adverse reactions caused by excess androgen in other androgen relevant tissues (e.g. placenta, testis, and prostate).
In addition, characterization of the efflux mechanism of androgen glucuronides is of clinical significance for the treatment of androgen-related diseases. Androgens are key drivers of prostate cancer, resulting in therapies primarily focused on androgen deprivation in the form of surgical or chemical castration [60]. However, the duration of improvement is highly variable, and the prostate cancer relapses in majority of patients within 12 to 18 months of therapy [61]. Recently, the enhancement of androgen glucuronidation by UGTs is becoming a new approach for therapy [61]. As the intracellular glucuronide concentration is regulated by efflux transporters and endogenous glucuronidases, the identification of transporters in the present study is applicable in predicting androgen homeostasis in prostate.
In summary, the intrinsic Vmax and Km values of TG, AG, EtioG, and DHTG can be used in PBPK modeling to predict the drug-drug interactions, and the effect of genetic polymorphism on testosterone metabolism. Besides androgen disposition modeling, the IVIVE methodology proposed in this study can be extended to other glucuronides, sulfate conjugates, drugs, and xenobiotics. Considering that enterohepatic recirculation can be mediated by microbiome, glucuronidation can be considered as a distribution mechanism instead of elimination mechanism.
Supplementary Material
Acknowledgements
We would like to thank Dr. Joe Zolnerciks from SOLVO Biotechnology for advice on the vesicle experiment and suggestions on this manuscript, Professor Jashvant D. Unadkat from School of Pharmacy (University of Washington) for advice on this work, and Matthew Karasu from the School of Pharmacy (University of Washington) for help with the reagent purchase.
Funding was provided by the University of Washington Department of Pharmaceutics and Amgen Inc, as well as grants to Prasad (R01HD081299) from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), a branch of the National Institutes of Health. Majority of this work is funded by Amgen and Solvo Biotechnology provided the transporter expressing vesicles for transporter study. Gupta is an employee of Amgen and Gáborik and Kis are the employees of Solvo Biotechnology.
References
- [1].Traish A, Testosterone therapy in men with testosterone deficiency: Are we beyond the point of no return?, Investig Clin Urol, 57 (2016) 384–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Allan CA, McLachlan RI, Age-related changes in testosterone and the role of replacement therapy in older men, Clin Endocrinol (Oxf), 60 (2004) 653–670. [DOI] [PubMed] [Google Scholar]
- [3].Gray A, Feldman HA, McKinlay JB, Longcope C, Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study, J Clin Endocrinol Metab, 73 (1991) 1016–1025. [DOI] [PubMed] [Google Scholar]
- [4].Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS, Trends in androgen prescribing in the United States, 2001 to 2011, JAMA Intern Med, 173 (2013) 1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore A Longitudinal Study of, Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging, J Clin Endocrinol Metab, 86 (2001) 724–731. [DOI] [PubMed] [Google Scholar]
- [6].Shoskes JJ, Wilson MK, Spinner ML, Pharmacology of testosterone replacement therapy preparations, Transl Androl Urol, 5 (2016) 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang H, Basit A, Busch D, Yabut K, Bhatt DK, Drozdzik M, Ostrowski M, Li A, Collins C, Oswald S, Prasad B, Quantitative characterization of UDP-glucuronosyltransferase 2B17 in human liver and intestine and its role in testosterone first-pass metabolism, Biochem Pharmacol, 156 (2018) 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Belanger A, The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology, Steroids, 62 (1997) 148–158. [DOI] [PubMed] [Google Scholar]
- [9].Randall VA, Role of 5 alpha-reductase in health and disease, Baillieres Clin Endocrinol Metab, 8 (1994) 405–431. [DOI] [PubMed] [Google Scholar]
- [10].Sanchez-Guijo A, Neunzig J, Gerber A, Oji V, Hartmann MF, Schuppe HC, Traupe H, Bernhardt R, Wudy SA, Role of steroid sulfatase in steroid homeostasis and characterization of the sulfated steroid pathway: Evidence from steroid sulfatase deficiency, Mol Cell Endocrinol, 437 (2016) 142–153. [DOI] [PubMed] [Google Scholar]
- [11].Bao BY, Chuang BF, Wang Q, Sartor O, Balk SP, Brown M, Kantoff PW, Lee GS, Androgen receptor mediates the expression of UDP-glucuronosyltransferase 2 B15 and B17 genes, Prostate, 68 (2008) 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Basit A, Amory JK, Prasad B, Effect of Dose and 5alpha-Reductase Inhibition on the Circulating Testosterone Metabolite Profile of Men Administered Oral Testosterone, Clin Transl Sci, 11 (2018) 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jarvinen E, Deng F, Kidron H, Finel M, Efflux transport of estrogen glucuronides by human MRP2, MRP3, MRP4 and BCRP, J Steroid Biochem Mol Biol, 178 (2018) 99–107. [DOI] [PubMed] [Google Scholar]
- [14].Zamek-Gliszczynski MJ, Hoffmaster KA, Nezasa K, Tallman MN, Brouwer KL, Integration of hepatic drug transporters and phase II metabolizing enzymes: mechanisms of hepatic excretion of sulfate, glucuronide, and glutathione metabolites, Eur J Pharm Sci, 27 (2006) 447–486. [DOI] [PubMed] [Google Scholar]
- [15].Huang L, Hoffman T, Vore M, Adenosine triphosphate-dependent transport of estradiol-17beta(beta-D-glucuronide) in membrane vesicles by MDR1 expressed in insect cells, Hepatology, 28 (1998) 1371–1377. [DOI] [PubMed] [Google Scholar]
- [16].Gerk PM, Li W, Megaraj V, Vore M, Human multidrug resistance protein 2 transports the therapeutic bile salt tauroursodeoxycholate, J Pharmacol Exp Ther, 320 (2007) 893–899. [DOI] [PubMed] [Google Scholar]
- [17].Mao Q, Unadkat JD, Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update, AAPS J, 17 (2015) 65–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Vore M, Hoffman T, Gosland M, ATP-dependent transport of beta-estradiol 17-(beta-D-glucuronide) in rat canalicular membrane vesicles, Am J Physiol, 271 (1996) G791–798. [DOI] [PubMed] [Google Scholar]
- [19].Sandberg AA, Slaunwhite WR Jr., Metabolism of 4-C14-testosterone in human subjects. I. Distribution in bile, blood, feces and urine, J Clin Invest, 35 (1956) 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Graef V, Furuya E, Nishikaze O, Hydrolysis of steroid glucuronides with beta-glucuronidase preparations from bovine liver, Helix pomatia, and E. coli, Clin Chem, 23 (1977) 532–535. [PubMed] [Google Scholar]
- [21].Takasuna K, Hagiwara T, Hirohashi M, Kato M, Nomura M, Nagai E, Yokoi T, Kamataki T, Inhibition of intestinal microflora beta-glucuronidase modifies the distribution of the active metabolite of the antitumor agent, irinotecan hydrochloride (CPT-11) in rats, Cancer Chemother Pharmacol, 42 (1998) 280–286. [DOI] [PubMed] [Google Scholar]
- [22].Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS, Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity, Science, 339 (2013) 1084–1088. [DOI] [PubMed] [Google Scholar]
- [23].Oleson L, Court MH, Effect of the beta-glucuronidase inhibitor saccharolactone on glucuronidation by human tissue microsomes and recombinant UDP-glucuronosyltransferases, J Pharm Pharmacol, 60 (2008) 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Contractor SF, Shane B, Purification and characterization of lysosomal -glucuronidase from human placenta, Biochem J, 128 (1972) 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ieiri I, Higuchi S, Sugiyama Y, Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs, Expert Opin Drug Metab Toxicol, 5 (2009) 703–729. [DOI] [PubMed] [Google Scholar]
- [26].Lund M, Petersen TS, Dalhoff KP, Clinical Implications of P-Glycoprotein Modulation in Drug-Drug Interactions, Drugs, 77 (2017) 859–883. [DOI] [PubMed] [Google Scholar]
- [27].Atilano-Roque A, Roda G, Fogueri U, Kiser JJ, Joy MS, Effect of Disease Pathologies on Transporter Expression and Function, J Clin Pharmacol, 56 Suppl 7 (2016) S205–221. [DOI] [PubMed] [Google Scholar]
- [28].Brouwer KL, Aleksunes LM, Brandys B, Giacoia GP, Knipp G, Lukacova V, Meibohm B, Nigam SK, Rieder M, de Wildt SN, Pediatric Transporter Working G, Human Ontogeny of Drug Transporters: Review and Recommendations of the Pediatric Transporter Working Group, Clin Pharmacol Ther, 98 (2015) 266–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Prasad B, Gaedigk A, Vrana M, Gaedigk R, Leeder JS, Salphati L, Chu X, Xiao G, Hop C, Evers R, Gan L, Unadkat JD, Ontogeny of Hepatic Drug Transporters as Quantified by LC-MS/MS Proteomics, Clin Pharmacol Ther, 100 (2016) 362–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bebawy M, Chetty M, Gender differences in p-glycoprotein expression and function: effects on drug disposition and outcome, Curr Drug Metab, 10 (2009) 322–328. [DOI] [PubMed] [Google Scholar]
- [31].Morris ME, Lee HJ, Predko LM, Gender differences in the membrane transport of endogenous and exogenous compounds, Pharmacol Rev, 55 (2003) 229–240. [DOI] [PubMed] [Google Scholar]
- [32].Joseph S, Nicolson TJ, Hammons G, Word B, Green-Knox B, Lyn-Cook B, Expression of drug transporters in human kidney: impact of sex, age, and ethnicity, Biol Sex Differ, 6 (2015) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heredi-Szabo K, Kis E, Krajcsi P, The vesicular transport assay: validated in vitro methods to study drug-mediated inhibition of canalicular efflux transporters ABCB11/BSEP and ABCC2/MRP2, Curr Protoc Toxicol, Chapter 23 (2012) Unit 23 24. [DOI] [PubMed] [Google Scholar]
- [34].Meszaros P, Klappe K, Hummel I, Hoekstra D, Kok JW, Function of MRP1/ABCC1 is not dependent on cholesterol or cholesterol-stabilized lipid rafts, Biochem J, 437 (2011) 483–491. [DOI] [PubMed] [Google Scholar]
- [35].Grinstein S, Cohen S, Measurement of sidedness of isolated plasma-membrane vesicles: quantitation of actin exposure by DNase I inactivation, Anal Biochem, 130 (1983) 151–157. [DOI] [PubMed] [Google Scholar]
- [36].Meszaros P, Hoekstra D, Kok JW, The toolbox of vesicle sidedness determination, Anal Biochem, 429 (2012) 89–91. [DOI] [PubMed] [Google Scholar]
- [37].Prasad B, Johnson K, Billington S, Lee C, Chung GW, Brown CD, Kelly EJ, Himmelfarb J, Unadkat JD, Abundance of Drug Transporters in the Human Kidney Cortex as Quantified by Quantitative Targeted Proteomics, Drug Metab Dispos, 44 (2016) 1920–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Drozdzik M, Groer C, Penski J, Lapczuk J, Ostrowski M, Lai Y, Prasad B, Unadkat JD, Siegmund W, Oswald S, Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine, Mol Pharm, 11 (2014) 3547–3555. [DOI] [PubMed] [Google Scholar]
- [39].Wang L, Prasad B, Salphati L, Chu X, Gupta A, Hop CE, Evers R, Unadkat JD, Interspecies variability in expression of hepatobiliary transporters across human, dog, monkey, and rat as determined by quantitative proteomics, Drug Metab Dispos, 43 (2015) 367–374. [DOI] [PubMed] [Google Scholar]
- [40].Sweeney DE, Vallon V, Rieg T, Wu W, Gallegos TF, Nigam SK, Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney, Mol Pharmacol, 80 (2011) 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Leder BZ, Catlin DH, Longcope C, Ahrens B, Schoenfeld DA, Finkelstein JS, Metabolism of orally administered androstenedione in young men, J Clin Endocrinol Metab, 86 (2001) 3654–3658. [DOI] [PubMed] [Google Scholar]
- [42].Knights KM, Rowland A, Miners JO, Renal drug metabolism in humans: the potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT), Br J Clin Pharmacol, 76 (2013) 587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ceckova-Novotna M, Pavek P, Staud F, P-glycoprotein in the placenta: expression, localization, regulation and function, Reprod Toxicol, 22 (2006) 400–410. [DOI] [PubMed] [Google Scholar]
- [44].Szilagyi JT, Vetrano AM, Laskin JD, Aleksunes LM, Localization of the placental BCRP/ABCG2 transporter to lipid rafts: Role for cholesterol in mediating efflux activity, Placenta, 55 (2017) 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sabnis NG, Miller A, Titus MA, Huss WJ, The Efflux Transporter ABCG2 Maintains Prostate Stem Cells, Mol Cancer Res, 15 (2017) 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Saupe M, Rauschenberger L, Preuss M, Oswald S, Fussek S, Zimmermann U, Walther R, Knabbe C, Burchardt M, Stope MB, Differential expression of the multidrug resistance 1 (MDR1) protein in prostate cancer cells is independent from anticancer drug treatment and Y box binding protein 1 (YB-1) activity, World J Urol, 33 (2015) 1481–1486. [DOI] [PubMed] [Google Scholar]
- [47].Qian X, Cheng YH, Mruk DD, Cheng CY, Breast cancer resistance protein (Bcrp) and the testis--an unexpected turn of events, Asian J Androl, 15 (2013) 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Su L, Jenardhanan P, Mruk DD, Mathur PP, Cheng YH, Mok KW, Bonanomi M, Silvestrini B, Cheng CY, Role of P-glycoprotein at the blood-testis barrier on adjudin distribution in the testis: a revisit of recent data, Adv Exp Med Biol, 763 (2012) 318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tanaka Y, Kitamura Y, Maeda K, Sugiyama Y, Quantitative Analysis of the ABCG2 c.421C>A Polymorphism Effect on In Vivo Transport Activity of Breast Cancer Resistance Protein (BCRP) Using an Intestinal Absorption Model, J Pharm Sci, 104 (2015) 3039–3048. [DOI] [PubMed] [Google Scholar]
- [50].Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B, Differential modulation of the human liver conjugate transporters MRP2 and MRP3 by bile acids and organic anions, J Biol Chem, 278 (2003) 23529–23537. [DOI] [PubMed] [Google Scholar]
- [51].Miller WL, Auchus RJ, The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders, Endocr Rev, 32 (2011) 81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Collier AC, Ganley NA, Tingle MD, Blumenstein M, Marvin KW, Paxton JW, Mitchell MD, Keelan JA, UDP-glucuronosyltransferase activity, expression and cellular localization in human placenta at term, Biochem Pharmacol, 63 (2002) 409–419. [DOI] [PubMed] [Google Scholar]
- [53].Meyer zu Schwabedissen HE, Jedlitschky G, Gratz M, Haenisch S, Linnemann K, Fusch C, Cascorbi I, Kroemer HK, Variable expression of MRP2 (ABCC2) in human placenta: influence of gestational age and cellular differentiation, Drug Metab Dispos, 33 (2005) 896–904. [DOI] [PubMed] [Google Scholar]
- [54].Joshi AA, Vaidya SS, St-Pierre MV, Mikheev AM, Desino KE, Nyandege AN, Audus KL, Unadkat JD, Gerk PM, Placental ABC Transporters: Biological Impact and Pharmaceutical Significance, Pharm Res, 33 (2016) 2847–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wolf CJ, Hotchkiss A, Ostby JS, LeBlanc GA, Gray LE Jr., Effects of prenatal testosterone propionate on the sexual development of male and female rats: a dose-response study, Toxicol Sci, 65 (2002) 71–86. [DOI] [PubMed] [Google Scholar]
- [56].Bruns CM, Baum ST, Colman RJ, Eisner JR, Kemnitz JW, Weindruch R, Abbott DH, Insulin resistance and impaired insulin secretion in prenatally androgenized male rhesus monkeys, J Clin Endocrinol Metab, 89 (2004) 6218–6223. [DOI] [PubMed] [Google Scholar]
- [57].Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V, Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep, Endocrinology, 145 (2004) 790–798. [DOI] [PubMed] [Google Scholar]
- [58].Crespi EJ, Steckler TL, Mohankumar PS, Padmanabhan V, Prenatal exposure to excess testosterone modifies the developmental trajectory of the insulin-like growth factor system in female sheep, J Physiol, 572 (2006) 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Smith AS, Birnie AK, French JA, Maternal androgen levels during pregnancy are associated with early-life growth in Geoffroy’s marmosets, Callithrix geoffroyi, Gen Comp Endocrinol, 166 (2010) 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gomella LG, Effective testosterone suppression for prostate cancer: is there a best castration therapy?, Rev Urol, 11 (2009) 52–60. [PMC free article] [PubMed] [Google Scholar]
- [61].Grosse L, Paquet S, Caron P, Fazli L, Rennie PS, Belanger A, Barbier O, Androgen glucuronidation: an unexpected target for androgen deprivation therapy, with prognosis and diagnostic implications, Cancer Res, 73 (2013) 6963–6971. [DOI] [PubMed] [Google Scholar]
- [62].Morgan JA, Cheepala SB, Wang Y, Neale G, Adachi M, Nachagari D, Leggas M, Zhao W, Boyd K, Venkataramanan R, Schuetz JD, Deregulated hepatic metabolism exacerbates impaired testosterone production in Mrp4-deficient mice, J Biol Chem, 287 (2012) 14456–14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


