Abstract
Background
Thyroid nodules are very common in general medical practice, but rarely turn out to be a medullary thyroid carcinoma (MTC). Calcitonin is a sensitive tumour marker for the detection of MTC (basal calcitonin). Sometimes a stimulation test is used to improve specificity (stimulated calcitonin). Although the European Thyroid Association's guideline advocates calcitonin determination in people with thyroid nodules, the role of routine calcitonin testing in individuals with thyroid nodules is still questionable.
Objectives
The objective of this review was to determine the diagnostic accuracy of basal and/or stimulated calcitonin as a triage or add‐on test for detection of MTC in people with thyroid nodules.
Search methods
We searched CENTRAL, MEDLINE, Embase and Web of Science from inception to June 2018.
Selection criteria
We included all retrospective and prospective cohort studies in which all participants with thyroid nodules had undergone determination of basal calcitonin levels (and stimulated calcitonin, if performed).
Data collection and analysis
Two review authors independently scanned all retrieved records. We extracted data using a standard data extraction form. We assessed risk of bias and applicability using the QUADAS‐2 tool. Using the hierarchical summary receiver operating characteristic (HSROC) model, we estimated summary curves across different thresholds and also obtained summary estimates of sensitivity and specificity at a common threshold when possible.
Main results
In 16 studies, we identified 72,368 participants with nodular thyroid disease in whom routinely calcitonin testing was performed. All included studies performed the calcitonin test as a triage test. Median prevalence of MTC was 0.32%. Sensitivity in these studies ranged between 83% and 100% and specificity ranged between 94% and 100%.
An important limitation in 15 of the 16 studies (94%) was the absence of adequate reference standards and follow‐up in calcitonin‐negative participants. This resulted in a high risk of bias with regard to flow and timing in the methodological quality assessment.
At the median specificity of 96.6% from the included studies, the estimated sensitivity (95% confidence interval (CI)) from the summary curve was 99.7% ( 68.8% to 100%). For the median prevalence of MTC of 0.23%, the positive predictive value (PPV) for basal calcitonin testing at a threshold of 10 pg/mL was 7.7% (4.9% to 12.1%).
Summary estimates of sensitivity and specificity for the threshold of 10 pg/mL of basal calcitonin testing was 100% (95% CI 99.7 to 100) and 97.2% (95% CI 95.9 to 98.6), respectively. For combined basal and stimulated calcitonin testing, sensitivity ranged between 82% and 100% with specificity between 99% and 100%. The median specificity was 99.8% with an estimated sensitivity of 98.8% (95% CI 65.8 to 100) .
Authors' conclusions
Both basal and combined basal and stimulated calcitonin testing have a high sensitivity and specificity. However, this may be an overestimation due to high risk of bias in the use and choice of reference standard The value of routine testing in patients with thyroid nodules remains questionable, due to the low prevalence, which results in a low PPV of basal calcitonin testing. Whether routine calcitonin testing improves prognosis in MTC patients remains unclear.
Plain language summary
Calcitonin testing for detection of medullary thyroid cancer in patients with thyroid nodules
Review question
What is the value of the calcitonin test for the diagnosis of medullary thyroid cancer in people with a thyroid nodule?
Background
Thyroid nodules are very common in the general population. In some people this nodule turns out to be a medullary thyroid carcinoma, which is a rare tumour of the thyroid gland. Calcitonin is one of the hormones produced by the thyroid, but in a large proportion of patients with medullary thyroid cancer the calcitonin level is increased. It can therefore be used as a sensitive tumour marker. In certain cases the production of calcitonin by the tumour can be stimulated in a stimulation test, to differentiate more accurately between calcitonin production by the tumour or other causes. However, there is no consensus if calcitonin testing should be routinely used in all people who have a thyroid nodule. We evaluated the available literature to address the accuracy of calcitonin testing in people with thyroid nodules for detection of medullary thyroid carcinoma.
Study characteristics
We searched for evidence in the literature until June 2018 and identified a total of 16 studies. Studies were included if a routine calcitonin test (with or without the stimulation test) was performed in all included people with thyroid nodular disease.
Key results
In total 72,638 people with thyroid nodular disease were enrolled in the analysed studies, of which 187 had medullary thyroid carcinoma. Our findings indicate that both basal and stimulated calcitonin testing are able to detect nearly all people with medullary thyroid carcinoma. However, because medullary thyroid carcinoma is very rare in persons with a thyroid nodule, there is large chance that calcitonin levels are false positives (i.e. the test indicates the disease, whereas in fact there is none).
In practice this means that for every 10,000 persons with thyroid nodular disease, 23 persons will have medullary thyroid carcinoma. Of these, none will be missed using a basal calcitonin threshold of 10 pg/mL, while 280 people will have a false‐positive test result. This might lead to unnecessary surgery of the thyroid with the need for life‐long thyroid hormone supplementation and risk of complications. With the use of a stimulation test the chance of a false‐positive test result may be reduced, however due to lack of sufficient studies this could not be calculated.
Certainty of the evidence
The certainty of the evidence is importantly limited, because almost all studies did not report adequately on the outcome of people who had a negative calcitonin test. A number of patients who had medullary thyroid carcinoma were possibly not identified. The diagnostic accuracy can already be markedly affected when a small number of patients is missed because medullary thyroid carcinoma is very rare.
Conclusion
Based on the available literature, there is insufficient evidence for a routine calcitonin test in all people with a thyroid nodule. Further studies are needed, with also adequate reporting of the people who have a negative calcitonin test, to determine the role of the calcitonin test in people with thyroid nodules for detection of medullary thyroid carcinoma.
Summary of findings
Summary of findings'. 'Summary of findings table.
| Review question | What is the diagnostic accuracy of calcitonin testing in the detection of MTC in people with thyroid nodules? | |||||
| Population | People with thyroid nodular disease found by palpation or ultrasound. | |||||
| Setting | Mostly individuals referred to outpatient clinics. | |||||
| Index test | All serum tests used to determine basal and stimulated calcitonin. | |||||
| Importance | No consensus exists about routine calcitonin testing in the work‐up of people with thyroid nodules. | |||||
| Reference standard | Histopathological examination is considered the optimal reference standard, however this is a problem in people without an elevated calcitonin test, as surgery is performed very rarely. We therefore used an alternative clinical follow‐up for at least three years. | |||||
| Studies | We included all retrospective and prospective cohort studies in which all people with thyroid nodules had undergone determination of basal calcitonin levels (and stimulated calcitonin, if performed). | |||||
| Limitations | There was a poor reported follow‐up of calcitonin‐negative participants. Due to the small numbers of studies only for a few subgroups' summary measures could be calculated | |||||
| Test / subgroup |
Reported Sensitivity/specificity (range) |
Summary measures (95% CI) | No. of participants (studies) | Median prevalence (range) | Implications | Certainty of the evidence and comments |
| Basal calcitonin | ||||||
| Reported cut‐off value (range 4.6 ‐100 pg/mL) |
Sensitivity 83% to 100% Specificity 94% to 100% |
Median specificity: 96.6% Estimated sensitivity: 99.7% (68.8% to 100%) |
72,368 (16) | 0.32% (0% to 0.85%) |
There is a high median specificity across the studies with a high estimated sensitivity. However, due to the different thresholds it is difficult to make general implications | The reported cut‐off value included the cut‐off values of the calcitonin test reported by the different studies (range 4.6 pg/mL to 100 pg/mL). The reported follow‐up of calcitonin‐negative participants was poor, thereby limiting the interpretation of these results |
| Combined basal and stimulated calcitonin | ||||||
| Reported cut‐off value (range basal calcitonin 4.6 pg/mL to 35 pg/mL, range stimulated calcitonin 50 pg/mL to 100 pg/mL) |
Sensitivity 82% to 100% Specificity 99% to 100% |
Median specificity: 99.8% Estimated sensitivity: 98.8% (65.8% to 100%) |
69,702 (13) | 0.31% (0% to 0.85%) |
There is a high median specificity across the studies with a high estimated sensitivity. However due to the different thresholds it is difficult to make general implications | The reported cut‐off value included the cut‐off values of the basal and stimulated calcitonin test reported by the different studies (range basal calcitonin 4.6 pg/mL to 35 pg/mL; range stimulated calcitonin 50 pg/mL to 100 pg/mL) The reported follow‐up of calcitonin negative participants was poor, thereby limiting the interpretation of these results |
| Subgroup analysis | ||||||
| Cut‐off value | ||||||
| 10 pg/mL |
Sensitivity (92% to 100%) Specificity (94% to 99%) |
Sensitivity 100% (99.7% to 100%) Specificity 97.2% (95.9% to 98.6%) |
44,393 (10) | 0.23% (0% to 0.69%) |
With a prevalence of 0.23% in a population of 10,000 nodular thyroid disease patients, 23 people will have a MTC. Of these MTC patients none will be missed using a cut‐off value of 10 pg/mL, while 280 people will have a false‐positive test result | The reported follow‐up of calcitonin‐negative participants was poor, thereby limiting the interpretation of these results |
| Gender (basal calcitonin) | ||||||
| Female |
Sensitivity 96% to 100% Specificity 97% to 100% |
a | 14,858 (6) | 0.25% (0% to 0.51%) |
There is a high reported specificity and sensitivity across the studies. However due to the different thresholds it is difficult to make general implications | The reported cut‐off value included the cut‐off values of the calcitonin test reported by the different studies (range 4.6 pg/mL to 30 pg/mL). The reported follow‐up of calcitonin‐negative participants was poor, thereby limiting the interpretation of these results |
| Male |
Sensitivity 82% to 100% Specificity 92% to 100% |
a | 4339 (6) | 0.49% (0.23% to 1.78%) |
Most studies report a high specificity and sensitivity. However due to the different thresholds it is difficult to make general implications | The reported cut‐off value included the cut‐off values of the calcitonin test reported by the different studies (range 5 pg/mL to30 pg/mL). The reported follow‐up of calcitonin‐negative participants was poor, thereby limiting the interpretation of these results |
aCould not be assessed due to limited number of studies
CI: confidence interval; MTC: medullar thyroid cancer; pg/mL: picograms per millilitre;
Background
Thyroid nodules are very common in the general population, and they are found in 2.3% to 6.9% of all adults (Rallison 1991; Vander 1968; Wiest 1998). Ultrasound has an even higher detection rate of thyroid nodules (17% to 69%, Tan 1997). Thyroid nodules are more prevalent in women than in men (6.4% to 10% versus 1.5% to 2%) (Vander 1968; Vanderpump 1995) and the incidence increases with age (Mazzaferri 1993). Of all patients with thyroid nodules who undergo fine needle aspiration (FNA), approximately 7.7% to 12% have thyroid cancer and in 3.3% to 3.7% of these thyroid cancer patients medullary thyroid cancer (MTC) is diagnosed (Gilliland 1997; Hundahl 1998; Marqusee 2000; Nam‐Goong 2004; Papini 2002).
MTC is a neuro‐endocrine tumour originating from the parafollicular C‐cells. These C‐cells secrete calcitonin, a 32‐amino acid peptide, which can be used as a sensitive tumour marker. The 10‐year survival for MTC is about 75%, but the prognosis depends on the size of the primary tumour, the presence of nodal disease and distant metastases (de Groot 2006). The primary treatment for MTC is surgery, and consists of a total thyroidectomy with central compartment dissection and even a more extended lymph node dissection depending on the extent of the disease. Some patients develop irresectable recurrent disease, which limits the therapeutic options. Patients with progressive disease may benefit from newly developed targeted therapies (Ernani 2016; Hadoux 2016), although early diagnosis of MTC and adequate surgical treatment remain crucial for a favourable prognosis.
Calcitonin is elevated in virtually all MTC patients and therefore a very sensitive tumour marker, although MTC does not always produce calcitonin (Redding 2000; Wang 2008). On the other hand, hypercalcitoninaemia can also be caused by other conditions such as thyroiditis, sepsis, hypercalcaemia, hypergastrinaemia, other neuroendocrine tumours, chronic renal failure, chronic pulmonary disease, acute trauma, inhalation injury and pseudohypoparathyroidism (Baudin 1999; Machens 2000; Niccoli 1995; Vlaeminck‐Guillem 2001).
In the most recent guidelines of the American Thyroid Association (ATA), the diagnostic work‐up of a thyroid nodule consists, after history, physical examination and thyroid‐stimulating hormone (TSH) determination, of a diagnostic ultrasound and FNA when a suspicious nodule is seen on ultrasound. The role of calcitonin testing in the work‐up of thyroid nodules is unclear and there is still no clinical consensus on calcitonin testing (Haugen 2016). The ATA's revised evidence‐based guideline for MTC does not recommend for or against calcitonin determination, thereby giving the physicians the opportunity to consider the pro's and cons of calcitonin testing in their clinic. The AACE/ACE/AME guideline for work‐up of thyroid nodules does also not recommend for or against routine calcitonin testing, while the European Thyroid Association's consensus‐based guideline advocates calcitonin determination in all patients with thyroid nodules (Gharib 2016; Pacini 2006; Wells 2015). Based on these guidelines and several studies, routine calcitonin testing is practiced in multiple centres, while its use remains disputed.
Several studies have reported a high sensitivity and specificity of the calcitonin test. Because MTC is rare, a large group of patients has to be tested for the identification of one MTC patient. This increases the risk of a false‐positive test result. Accordingly, the positive predictive value (PPV) will be low, although some studies do report PPVs of up to 100% (Costante 2009). Furthermore, the cut‐off level of calcitonin has not yet been established and there are indications that different subgroups of patients need specific cut‐off points, since there are gender‐specific cut‐off levels (Machens 2009). Perhaps only a subset of patients should undergo calcitonin testing. It is also unclear whether calcitonin testing can contribute to longer overall survival or will increase the quality of life of MTC patients. Finally, to determine its role in the evaluation of thyroid nodules the cost‐effectiveness of calcitonin testing is also important (Borget 2007; Cheung 2008).
Role of calcitonin testing
There are several potential roles for calcitonin testing in the diagnostic work‐up of thyroid nodules (Figure 1). First, it can be used as a screening tool. Screening, however, implies that the entire healthy population will undergo determination of calcitonin, which is currently not clinically relevant. Therefore, we focus only on calcitonin testing in patients with thyroid nodules, detected through palpation or ultrasound. It can be performed in all patients with thyroid nodules at an early stage and before FNA (Figure 1: I). In this case the supposed sensitivity is very high but a great number of patients will have false‐positive results which might lead to unnecessary surgery. As FNA is also commonly used for diagnosing other types of thyroid cancer which do not secrete calcitonin, calcitonin testing as a replacement for FNA is irrational and clinically not relevant.
1.

The possible roles of calcitonin testing as triage (I), add‐on (II) or preoperative screening test (III) in the work‐up of thyroid nodules
Calcitonin testing can be used as an add‐on test following FNA in patients with suspicious or indeterminate cytology (Figure 1: II). In this case the number of false positives will be lower, but some MTC patients might be missed (when cytology is benign) with the risk that MTC in these patients will be diagnosed at a later stage or not at all. Calcitonin testing can also be used as a preoperative test in all patients who will undergo thyroid surgery (Figure 1: III). In that case not all MTC patients will be detected but the risk of patients who undergo an operation receiving too restricted surgery decreases. This form of calcitonin testing will not be included in this review as it is more focused on preoperative assessment of tumour type than on screening.
This review will address the value of calcitonin testing for diagnosing MTC in patients with thyroid nodules for the triage and add‐on roles of the calcitonin test. We want to give more insight into the different sensitivities and specificities for these different roles. By providing data on the diagnostic accuracy of the calcitonin test in light of the low prevalence of MTC in thyroid nodules, we want to contribute to the discussion on the role of the calcitonin test in patients with thyroid nodules.
Target condition being diagnosed
MTC in people with thyroid nodules.
Index test(s)
The available test for diagnosing MTC in thyroid nodules is the calcitonin assay. The former radioimmunoassays for calcitonin measurement recognised the monomeric and the dimeric form of calcitonin as well as its precursors, leading to false‐positive results. The more recent and most commonly used immunometric assays mainly recognise the mature, monomeric form of calcitonin. They rely on a 'sandwich' formation by two monoclonal or polyclonal antibodies recognising different epitopes on calcitonin (d'Herbomez 2007). However, limitations still exist in the calcitonin assays. If a one‐step assay is applied, in case of an extremely high calcitonin concentration, all the antibodies including the signal antibodies are saturated with the antigen, preventing a sandwich formation. Then, the antigen concentration measured may be falsely low (also known as the "high dose hook", (Leboeuf 2006)). Furthermore, also mainly in one‐step assays, the presence of heterophilic antibodies may give erroneously high results of calcitonin by cross‐linking the antibodies in the absence of calcitonin ( Bieglmayer 2002; Tommasi 2001). Very rarely "blocking" heterophilic antibodies are also able to produce false‐negative results (Preissner 2005). This problem is circumvented by assays that wash between incubation steps. Alternative methods for quantification, such as mass spectrometry may circumvent this problem as well, as was also shown for thyroglobulin (Netzel 2014). Furthermore, despite the World Health Organization international reference preparation for human calcitonin, differences exist between the same type of assays of different manufacturers, making it even more difficult to compare results from different studies and to establish an optimal cut‐off value (d'Herbomez 2007; Kratzsch 2011; Zanelli 1993). To improve the specificity of the calcitonin assay, calcitonin stimulation tests with pentagastrin or calcium are used (Wells 1978). These stimulation tests can distinguish calcitonin secreted by MTC from other sources of calcitonin (Samaan 1980), but there are some limitations. Stimulation with pentagastrin can induce unpleasant side effects, such as nausea, vomiting or skin rash (Ewers 1976). Furthermore, pentagastrin is not available in several countries. Calcium stimulation tests are better tolerated but are not routinely used although an increasing number of small studies have advocated the use of calcium (Colombo 2011; Doyle 2009; Kudo 2011). In this review we planned to perform a heterogeneity analysis on whether basal calcitonin, or both basal and stimulated calcitonin, were determined and also the type of stimulation test used.
Clinical pathway
Alternative test(s)
The alternative test for diagnosing MTC in patients with thyroid nodules is fine needle aspiration cytology (FNAC) with eventually immunohistochemical examination in suspicious lesions. FNAC is an accurate and cost‐effective method for evaluation of thyroid nodules, but the sensitivity for diagnosis of MTC is not optimal, ranging from 63% to 89% (Bugalho 2005; Chang 2005; Papaparaskeva 2000). The outcome of the FNAC in these studies resulted in surgery in 91% to 100% of patients. Although a large proportion of the patients received surgery despite incorrect FNAC results, this might be an inadequate test as MTC requires a different surgical approach than differentiated thyroid cancer. Other techniques, such as measuring calcitonin levels in washout fluids of fine needle aspirates can improve accuracy and are also recommended in the last update of the ATA guidelines when FNAC findings are inconclusive or suggestive of MTC (Abraham 2009; Boi 2007; Kudo 2007,Trimboli 2016; Wells 2015 ).
Rationale
A number of studies and reviews on this topic advocate calcitonin testing for detection of MTC. These studies are hard to compare, however, since they have different inclusion criteria and different cut‐off points for calcitonin levels. Moreover, there is no consensus between the American and European guidelines on thyroid nodules. Calcitonin testing in patients with thyroid nodules is associated with a high rate of false‐positive results and a low PPV. It has not been established that calcitonin testing reduces MTC‐related mortality in these patients. Cheung 2008 stated that calcitonin testing in the USA is cost‐effective at the same level as mammography screening and advocates calcitonin testing in subgroups of patients such as young men with larger thyroid nodules, but this also remains a matter of debate.
Objectives
The objective of this review was to determine the diagnostic accuracy of basal and stimulated calcitonin as a triage or add‐on test for detection of medullary thyroid cancer (MTC) in people with thyroid nodules.
Investigation of sources of heterogeneity
We planned to investigate several potential sources of heterogeneity, including differences in cut‐off values, assay types and different verification methods. We planned to evaluate a number of possible factors as source for heterogeneity.
Age.
Gender.
Nodules detected by palpation or ultrasound.
Nodule size.
Number of nodules.
Sonographic morphology of thyroid nodules.
Fine needle aspiration (FNA) procedures performed through ultrasound guidance versus palpation.
Basal versus stimulated calcitonin testing.
Type of stimulation test.
Methods
Criteria for considering studies for this review
Types of studies
We included all retrospective and prospective cohort studies in which all individuals with thyroid nodules had undergone determination of basal calcitonin levels and if performed stimulated calcitonin. We excluded cross‐sectional studies in which no histopathological examination or follow‐up of patients was performed.
Participants
We included participants with nodular thyroid disease (defined as solitary thyroid disease (toxic/non‐toxic), multinodular thyroid disease (toxic/non‐toxic), autonomously functioning thyroid nodule)) found by palpation or on ultrasound in whom calcitonin testing was performed. We distinguished between studies in which calcitonin testing was performed as a triage (before fine needle aspiration cytology (FNAC)) or as an add‐on test (after FNAC). We included participants with coexisting non‐nodular disease such as autoimmune thyroid disease (Graves' disease or Hashimoto's thyroiditis) and subacute thyroiditis. We excluded participants with only non‐nodular thyroid disease. If studies included both participants with nodular and non‐nodular disease, we included them only if it was possible to separate the calcitonin levels and surgical outcomes of these participant groups or if fewer than 10% of participants had non‐nodular disease. We excluded participants with known sporadic or familial MTC (multiple endocrine neoplasia type 2 (MEN2A/B), (familial medullary thyroid carcinoma (FMTC)) prior to calcitonin screening. We also excluded studies that included these participants and did not describe them separately.
Index tests
The index tests for this review included all serum assays used to determine basal and stimulated serum calcitonin levels.
Target conditions
The target condition was MTC.
Reference standards
We considered histopathological examination of the thyroid after surgery of all participants (even participants without elevated calcitonin levels) as the optimal clinical reference standard for diagnosis of MTC. In all of the studies, however, we encountered the problem of differential verification and only participants with (markedly) elevated calcitonin levels or people with suspicious cytology had histological verifications (although some patients underwent surgery for other reasons, e.g. mechanical complaints due to a multinodular goitre). We planned therefore to make use of other reference standards such as clinical follow‐up. We considered a follow‐up of at least three years as adequate as most clinically relevant MTCs will be identified at that time, while longer follow‐up carries the risk that MTC patients are diagnosed while not having the disease at the time of calcitonin testing. To determine whether standard of verification significantly influenced accuracy, we planned to include method of verification in the heterogeneity analysis.
Search methods for identification of studies
Electronic searches
We used the following sources for the identification of studies.
Cochrane Central Register of Controlled Trials via Cochrane Register of Studies Online (last searched 6 June 2018)
Ovid MEDLINE(R) <Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present> (last searched 6 June 2018)
Ovid Embase <1974 to 2018 June 04> (last searched 6 June 2018)
Science Citation Index Expanded via Web of Science (last searched 6 June 2018)
For detailed search strategies see Appendix 1. The Editorial Base of the Cochrane Metabolic and Endocrine Disorders Group provided support for generating the optimal search strategy. We used PubMed's 'My NCBI' (National Center for Biotechnology Information) email alert service for the identification of newly published studies using a basic search strategy (see Appendix 1). We excluded conference abstracts.
Searching other resources
We examined the references lists of relevant publications for additional studies. We searched in PubMed for related articles of relevant studies.
Data collection and analysis
Selection of studies
To determine the studies to be assessed further, two review authors (HHGV and JWBdG) independently scanned the abstract and/or title of every record retrieved. We investigated all potentially relevant references as full text. Any disagreements were resolved by a third review author (TPL). A PRISMA study flow diagram of study selection was made (Liberati 2009). We contacted all study authors with requests for additional data (Table 2).
1. Study author contact.
| Study ID | Recent contact details available | Request for information send | Response received? | Additional data available? |
| Rieu 1995 | No | No | NA | NA |
| Ozgen 1999 | Yes | Yes | No | NA |
| Hahm 2001 | Yes | Yes | No | NA |
| Hatzl‐Griesenhofer 2002 | Yes | Yes | Yes | Yes |
| Elisei 2004 | Yes | Yes | Yes | Yes |
| Karanikas 2004 | Yes | Yes | Yes | No |
| Vierhapper 2005 | Yes | Yes | No | NA |
| Papi 2006 | Yes | Yes | No | NA |
| Schuetz 2006 | Yes | Yes | Yes | No |
| Costante 2007 | Yes | Yes | Yes | Yes |
| Rink 2009 | Yes | Yes | Yes | Yes |
| Hasselgren 2010 | Yes | Yes | Yes | Yes |
| Herrmann 2010 | Yes | Yes | Yes | Yes |
| Schneider 2012 | Yes | Yes | No | NA |
| Giovanella 2012 | Yes | Yes | Yes | Yes |
| Grani 2012 | Yes | Yes | Yes | Yes |
Details of which study authors of the included studies were contacted and if additional data were available.
NA: not applicable.
Data extraction and management
We extracted data on study design and study population characteristics using a standard data extraction form (Table 3), in which we included the following items.
2. Data extraction form.
| Design | Study design: Inclusion criteria: Exclusion criteria: |
| Participant characteristics and setting | Number of participants: Number with NTD: Number with NTD and calcitonin testing: Sex (female%): Age (mean/SD): range: MTC: Type of thyroid nodules: Thyroid nodules detected by palpation or US: Nodule size: Number of nodules: Sonographic morphology of thyroid nodules: FNA procedures performed through ultrasound guidance or palpation: Setting: Country: |
| Index test | Index test: Calcitonin as a triage or add‐on test: Used calcitonin assay: Stimulated calcitonin performed: Indication stimulated calcitonin: Stimulative: Dose: Time: Reported and extracted cut‐off values Basal: Stimulated: |
| Reference standard | Target condition: Reference standards: Indication surgical treatment: Type of surgical treatment: Calcitonin negative (N) Number FNA: Number operated: Calcitonin positive (N) Number FNA: Number operated: |
| Flow and timing | Follow‐up calcitonin negative: Type: Duration: Follow‐up calcitonin positive: Type: Duration: |
FNA: fine‐needle aspiration; MTC: medullary thyroid carcinoma; NTD: nodular thyroid disease; SD: standard deviation; US: ultrasound.
Study design.
Included number of participants.
Inclusion and exclusion criteria.
General participant characteristics.
Type of calcitonin assay, reported cut‐off values (the cut‐off used in the study to define a positive test result) and extracted cut‐off values (see below).
Number of participants with nodular thyroid disease.
Number of participants with palpable nodules and/or nodules on ultrasound.
Number of participants who had undergone calcitonin testing and number of positive participants.
Number of participants operated and reason for operation.
Number of participants with known follow‐up and outcome of follow‐up.
Histological outcome of participants operated.
Number of participants with MTC.
For extraction of data, we used the reported cut‐off values and pre‐specified cut‐offs based on previous literature with different cut‐offs for basal and stimulated calcitonin levels. These cut‐off values were 10,15, 20, 30, 50 and 100 pg/mL for basal calcitonin levels and 100 pg/mL and 200 pg/mL for stimulated calcitonin levels.
Assessment of methodological quality
We assessed risk of bias and applicability using the QUADAS‐2 tool. We rated each of the four domains (participant selection, index test, reference standard, flow and timing) using the signalling questions as developed by the QUADAS‐2 group (Whiting 2011). The criteria for each signalling question are provided in Appendix 2. We scored all items in the QUADAS‐2 tool as ‘yes’, ‘no’ or ‘unclear', and used graphs to present the results of risk of bias and applicability for each domain.
Statistical analysis and data synthesis
We identified the true positives, false positives, true negatives and false negatives of each study, which we inserted in a 2 x 2 table and calculated test sensitivity and specificity with corresponding 95% confidence intervals (CIs). We entered the data into RevMan 5.3 (RevMan 2014), to present graphically coupled forest plots, showing the pairs of sensitivity and specificity of each study, for each threshold. We used SAS software for meta‐analysis. We obtained estimates of the expected operating points (sensitivity and specificity) using the hierarchical summary receiver operating characteristic (HSROC) model (Macaskill 2004). We used the HSROC model because different cut‐off values are reported in the included studies and this model also allows for calculation of summary estimates for a single threshold. If in a study no patients were identified for the disease group (or non‐diseased group), the analysis is still appropriate, since the analysis would treat the disease group (or non‐diseased group) as missing for that study. The non‐diseased group (or disease group) would still contribute to the meta‐analysis.
Investigations of heterogeneity
Depending on the number of included studies and available data, covariate's were added in the HSROC model, for investigation of possible sources of heterogeneity. Although we planned to investigate multiple sources of heterogeneity (cut‐off value, assay type, age, gender, detection method of nodules (palpation versus ultrasound), nodule size, number of nodules, morphology of nodules, FNA method and stimulated calcitonin testing), due to the limited number of studies we could only investigate cut‐off value, gender and stimulated calcitonin testing.
Sensitivity analyses
In order to explore the influence the risk of bias and applicability concerns of the included studies, we performed sensitivity analyses for the different domains of the QUADAS‐2 tool. For each domain of the risk of bias and applicability concerns we excluded studies when there was an unclear or high risk.
Results
Results of the search
We identified a total of 4092 unique records by our search in January 2012 and updated searches in June 2012, March 2013 and June 2018. An additional two records were identified by examining references list of relevant publications. Screening of all records resulted in 51 publications that were eligible for further evaluation. After assessment we excluded 26 studies ( 26 records), and six studies (seven records) are awaiting classification.In total, we included 16 studies in this review (Figure 2).
2.
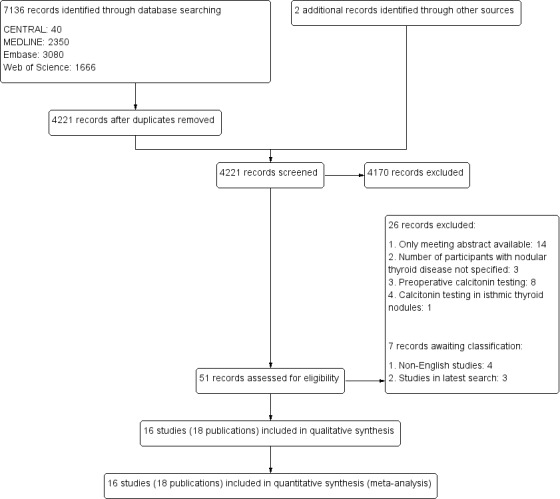
Study flow diagram for selection of studies (search until June 2018).
Included studies
Characteristics of the 16 included studies are shown in the table Characteristics of included studies. A total of 73,052 participants with nodular thyroid disease were included in these studies, of which 72,368 underwent basal calcitonin testing with or without stimulated calcitonin testing as shown in Table 4. A total of 187 medullary thyroid cancer (MTC) patients were identified. Three studies performed only basal calcitonin testing, whereas in 13 studies both basal and stimulated calcitonin testing was performed.
3. Overview of study populations.
| Study ID | (N) with nodular thyroid disease | (N) with calcitonin testing | (N) with positive basal calcitonin testing | (N) with stimulated calcitonin testing | (N) with positive stimulated calcitonin testing | (N) operated | (N) with follow‐up | (N) with MTC | MTC prevalence |
| Rieu 1995 | 469 | 469 | 4 | 4 | 4 | 15 | — | 4 | 0.85 |
| Ozgen 1999 | 773 | 773 | 4 | — | — | 175 | 3 | 4 | 0.52 |
| Hahm 2001 | 1448 | 1448 | 56 | 39 | 12 | 194 | — | 10 | 0.69 |
| Hatzl‐Griesenhofer 2002 | 3899 | 3899 | 230 | 157 | 30 | 39 | 43 | 12 | 0.31 |
| Elisei 2004 | 10,864 | 10,864 | 47 | 45 | 44 | 44 | 44 | 44 | 0.41 |
| Karanikas 2004 | 195 | 195 | 13 | 13 | 2 | 1 | 1 | 1 | 0.51 |
| Vierhapper 2005 | 10292 | 10,157 | 507 | 481 | 103 | 76 | 32 | 36 | 0.35 |
| Papi 2006 | 1474 | 1425 | 23 | 19 | 6 | 315 | — | 9 | 0.63 |
| Schuetz 2006 | 105 | 105 | 5 | 5 | 0 | 0 | — | 0 | 0 |
| Costante 2007 | 5817 | 5817 | 282 | 58 | 17 | 747 | 212 | 15 | 0.26 |
| Rink 2009 | 21,928 | 21,928 | 885 | 218 | 62 | 157 | 214 | 28 | 0.13 |
| Hasselgren 2010 | 959 | 702 | 39 | — | — | 246 | 702 | 6 | 0.85 |
| Herrmann 2010 | 1007 | 1007 | 17 | 16 | 4 | 5 | 12 | 2 | 0.20 |
| Schneider 2012 | 11,270 | 11,270 | 32 | 14 | 12 | 18 | 10 | 12 | 0.11 |
| Giovanella 2012 | 1479 | 1236 | 14 | 14 | 4 | 170 | 7 | 2 | 0.16 |
| Grani 2012 | 1073 | 1073 | 41 | — | — | 67 | — | 2 | 0.19 |
| Total | 73,052 | 72,368 | 2199 | 1083 | 300 | 2042 | 1280 | 187 | 0.26 |
—: denotes not reported; *Cross linkage with Danish Thyroid Cancer Database.
MTC: medullary thyroid cancer.
Calcitonin assays
An overview of the assay characteristics is provided in Table 5. Two studies used a radio immunometric assay (RIA) for determination of calcitonin, including one study which during the study period switched from an RIA assay to an immunoradiometric assay (IRMA). Five other studies used also an IRMA. Two of these five studies switched during the study period to a chemiluminescence assay (ICMA). The remaining nine studies used an ICMA assay. In conclusion, 13 studies used only one calcitonin assay during their study period, while three studies used two assays. One of these three studies that switched from an IRMA to an ICMA assay, used the ICMA assay only in 14 out of 702 participants, and we therefore regarded this as using an IRMA assay in further analyses (Hasselgren 2010). The other two studies that switched from calcitonin assay were not included in the covariate analysis regarding assay type. In total, calcitonin assays of nine different manufacturers were used (Table 5). Especially in the seven studies using a RIA or IRMA assay there was a large heterogeneity in manufacturers (N = 7); some studies used during the study period assays from two different producers. Within the nine studies using a ICMA assay, one study did not report the manufacturer (Grani 2012), while in the other studies an assay was used from one of two producers.
4. Assay characteristics.
| Study | Type of Assay | Manufacturer | Stimulation? | Reported basal cut‐off | Assay switching | Included in cut‐off covariate analysis (10 pg/mL) |
| Rieu 1995 | RIA (1989) IRMA (1990‐1993) |
Mallinckrodt Medical SA, France CIS‐Oris International, France |
Yes | 35 pg/mL 10 pg/mL |
Yes | No |
| Ozgen 1999 | RIA | Diagnostic System Laboratories Inc., US | No | 30 pg/mL | No | No |
| Hahm 2001 | IRMA | BioSource Europe S.A., Belgium | Yes | 10 pg/mL | No | Yes |
| Hatzl‐Griesenhofer 2002 | ICMA | Nichols Institute diagnostics, US | Yes | Females: 4.6 pg/mL Males: 11.5 pg/mL |
No | No |
| Elisei 2004 | IRMA | CIS, France | Yes | 20 pg/mL | No | No |
| Karanikas 2004 | ICMA | Nichols Institute Diagnostics, US | Yes | 10 pg/mL | No | Yes |
| Vierhapper 2005 | IRMA (1994‐1999) ICMA (1999‐2004) |
CIS‐biointernational, France Nichols Institute Diagnostics, US |
Yes | 10 pg/mL | Yes | Yes |
| Papi 2006 | ICMA | Nichols Institute diagnostics, US | Yes | 5 pg/mL | No | Yes |
| Schuetz 2006 | ICMA | Nichols Institute diagnostics, US | Yes | 10 pg/mL | No | No |
| Costante 2007 | ICMA | Nichols Institute diagnostics, US | Yes | 10 pg/mL | No | Yes |
| Rink 2009 | IRMA | IBL GmbH, Germany and Medipan GmbH, Germany |
Yes | 10 pg/mL | No | Yes |
| Hasselgren 2010 | IRMA ICMA |
MediLab A/S, Denmark Siemens Medical Solutions Diagnostics, Germany |
No | 100 pg/mL | Yes | No |
| Herrmann 2010 | ICMA | Siemens Healthcare Diagnostics, Germany | Yes | 10 pg/mL | No | Yes |
| Schneider 2012 | ICMA | Siemens Healthcare Diagnostics, Germany | Yes | 13 pg/mL | No | No |
| Giovanella 2012 | ICMA | Siemens Healthcare Diagnostics, Germany | Yes | 10 pg/mL | No | Yes |
| Grani 2012 | ICMA | Not reported | No | 10 pg/mL | No | Yes |
ICMA: immunochemiluminometric ASSAY; IRMA: immunoradiometric assay; pg/mL: picograms per millilitre; RIA: radioimmuno assay.
Verification method
Differential verification was present in all studies; all participants with a (highly) elevated basal, a stimulated calcitonin or both underwent surgery, while only a subset of participants with negative calcitonin tests had surgery. The percentage of calcitonin‐negative patients with reported surgery ranged between 0% and 33.6 %. The majority of these patients had benign thyroid disease. A smaller number of patients had thyroid cancer and in two studies MTC patients with a false‐negative calcitonin test were identified. In the study of Vierhapper 2005, two of the three participants with false‐negative test results were not operated on, but a hereditary MTC was suspected because of genetic testing (Vierhapper 2005). The study of Schneider 2012 identified two patients with a small MTC. Although histopathological examination in all patients with a negative calcitonin test is not feasible and, more importantly, not desirable, we considered clinical follow‐up of calcitonin‐negative participants as an appropriate alternative for detection of missed MTC patients. However, none of the included studies reported systematically on the clinical follow‐up of all of their calcitonin‐negative participants. Only in the study of Hasselgren 2010 was follow‐up performed that consisted of cross linkage with a national thyroid cancer database.
Calcitonin as triage or add‐on test
None of the studies included provided explicit information on the role of calcitonin testing in the diagnostic pathway of thyroid nodules. In nine studies authors described fine needle aspiration (FNA) in the materials and methods section as part of the diagnostic protocol. Most of these studies stated that surgery was indicated if basal or stimulated calcitonin was clearly elevated (e.g. greater than 100 pg/mL), regardless of the results of FNA. In these studies the role of calcitonin testing can be considered as a triage test in which participants with a positive calcitonin test are subjected to surgery, while participants with a negative calcitonin test require more diagnostic work‐up in the form of FNA. In all studies in which FNA was not described in the diagnostic protocol, calcitonin testing was performed in all included participants, independent of another diagnostic procedure, and constituted, if markedly elevated, an indication to perform surgery. Therefore, in these studies we regarded calcitonin testing also as a triage test. Hence, all studies were considered to perform the calcitonin test as a triage test.
Participant and study characteristics
Study authors described average or median age in 12 studies. The average age ranged between 42.1 and 56 years with ranges in individual studies from 8 to 97 years. Only one study reported the results of calcitonin testing specified in different age groups (Papi 2006). Fifteen studies provided Information on gender of the included participants, the average female percentage being 54.5% to 88.1%. Only in seven studies was detailed information on outcome given for both sexes. In nine studies information was available on whether thyroid nodules in the included participants were detected through palpation or ultrasonography (US); four studies included participants with thyroid nodules found by US and five studies included participants with thyroid nodules detected through US or palpation. With regard to nodule size, only one study provided information on summary measures of size for all the included participants (average nodules size 21.8 mm), although no detailed information was provided for participants with elevated calcitonin levels. No study presented information on the number of nodules or US morphology of all participants. In four studies information was given on whether FNA procedures were performed through palpation or US; in one study both techniques were performed, in the three others US‐guided FNA was performed.
Studies awaiting classification.
Three articles were written in non‐English languages. We contacted the study authors for information about the potential relevance of these studies. After translation we plan to assess these studies for inclusion in an update of our Cochrane Review. In the latest search we identified three other potentially relevant articles which will also be assessed for inclusion in an update of our Cochrane Review.
Excluded studies
In the Characteristics of excluded studies reasons for exclusion for 26 studies are shown. For 14 studies, only a conference abstract was available and no full‐text article was published. Eight studies reported on calcitonin testing in preoperative participants, which was not the topic of this review. One study reported on calcitonin testing in isthmic thyroid nodules; because this study group evaluated only nodules in a specific part of the thyroid, we excluded this study. Three studies did not specify the number of participants with nodular thyroid disease.
Methodological quality of included studies
In Figure 3 we showed the risk of bias and applicability concerns of the 16 included studies. In the domain 'Patient selection', one study scored high on the risk of bias as participants were included who showed evidence of growth during follow‐up examinations (Hatzl‐Griesenhofer 2002). This might have increased the rate of included patients with a malignancy. In all studies we scored the risk of bias for the conducting, or interpretation of the calcitonin test as low, because all studies used a pre‐specified threshold. The risk of bias with regard to the conducting or interpretation of the reference standard was unclear in all studies, as the reference standard was not described for most of the calcitonin‐negative participants. In some patients histological verification was obtained because the operation was carried out for other reasons, but in the patients who did not have thyroid surgery, almost no information on clinical follow‐up was provided. Due to this missing information about the reference standard, resulting in a verification bias, the risk of bias with regard to flow and timing was high in all studies except one (Hasselgren 2010). In this study using a cross linkage with a national thyroid cancer database we scored the risk as unclear. We had no applicability concerns for any of the included studies.
3.
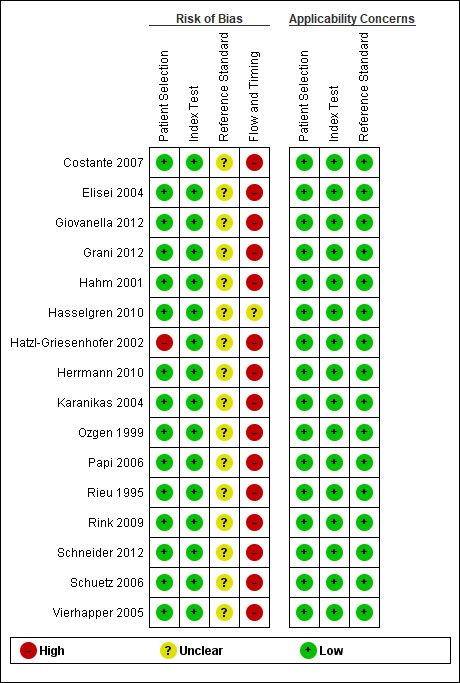
Methodological quality graph: risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study
Findings
The sensitivity of the reported basal calcitonin testing cut‐off (ranging from 4.6 pg/mL to 100 pg/mL) in the 15 included studies (n = 72,834) ranged from 83% to 100%, while the specificity ranged from 94% to 100% (Figure 4). In Schuetz 2006 we identified no MTC patients with nodular thyroid disease and could not calculate sensitivity. Figure 5 shows the summary ROC plot with the summary curve and 95% confidence and prediction regions of basal reported calcitonin cut‐off values. The median specificity of the included studies was 96.6% with an estimated sensitivity of 99.7% (95% CI 68.8 to 100).
4.
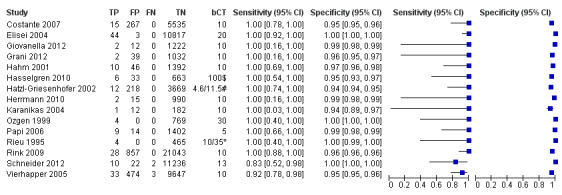
Forest plot showing sensitivity and specificity of reported basal calcitonin (bCT) cut‐off values (range 4.6 pg/mL to 100 pg/mL). (TP: true positive, FP: false positive, FN: false negative, TN: true negative, bCT: basal calcitonin). $Two different calcitonin assays were used during the study period, in which only 14 patients were measured with the second assay, therefore only the cut‐off value of 100 pg/mL was taken into account. # Gender‐specific cut‐off value was used (females: 4.6 pg/mL; males 11.5 pg/mL). * Two different assays with different cut‐off values were used during the study period.
5.
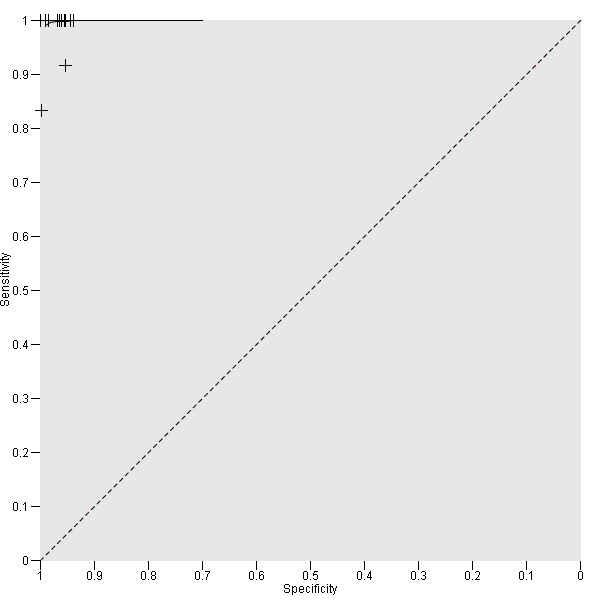
Summary ROC Plot showing the summary curve of basal reported calcitonin cut‐off values (range 4.6 pg/mL to 100 pg/mL)
Cut‐off value
We extracted data for several cut‐off values from the included studies, r anging from 10 pg/mL to 100 pg/mL. Summary estimates of sensitivity and specificity for the different cut‐off values of basal calcitonin other than 10 pg/mL could not be calculated due to limited number of studies. For the 10 pg/mL cut‐off analysis 10 studies were included (n = 44,393), six studies were excluded because no data were reported or could be extracted for this cut‐off value (Rieu 1995; Ozgen 1999; Hatzl‐Griesenhofer 2002; Elisei 2004; Hasselgren 2010; Schneider 2012). With the cut‐off value of 10 pg/mL the sensitivity of the individual studies ranged from 92% to 100%, while specificity ranged from 95% to 99%; summary estimates were 100% (95% CI 99.7 to 100) for sensitivity and 97.2% (95% CI 95.9 to 98.6) for specificity(Figure 6). With the highest cut‐off value of 100 pg/mL sensitivities ranged from 42% to 100% and specificity from 95% to 100% ( (Table 1).
6.

Forest plot showing sensitivity and specificity of basal calcitonin (bCT) cut‐off value of 10 pg/mL. (TP: true positive, FP: false positive, FN: false negative, TN: true negative, bCT: basal calcitonin)
Stimulated calcitonin
In13 studies (n = 69,702) the basal calcitonin test was combined with stimulated calcitonin. Eleven studies used a stimulated calcitonin threshold of 100 pg/mL. One study used a threshold of 60 pg/mL and one study used a gender‐specific threshold (50 pg/mL for females and 80 pg/mL for males). Sensitivity of individual studies ranged between 82% and 100% and specificity ranged from 99% to 100%. The median specificity in these studies was 99.8% with an estimated sensitivity of 98.8% (95% CI 65.8 to 100) (Figure 7; Table 1). Due to limited numbers of studies no summary estimates could be calculated for different cut‐off values.
7.
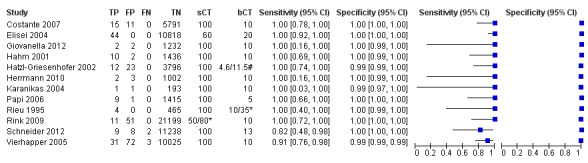
Forest plot showing sensitivity and specificity of basal and stimulated reported calcitonin testing cut‐off values (range basal calcitonin 4.6 pg/mL to 35 pg/mL, range stimulated calcitonin 50 pg/mL to 100 pg/mL). (TP: true positive, FP: false positive, FN: false negative, TN: true negative, bCT: basal calcitonin, sCT stimulated calcitonin).# Gender‐specific cut‐off value was used (females: 4.6 pg/mL; males 11.5 pg/mL). * Two different assays with different cut‐off values were used during the study period.
Gender
In Table 1 we report summary estimates for basal calcitonin of female and male subgroups. For women the sensitivity could be derived in six studies (n = 14,858) and ranged between 96% and 100% and the specificity ranged between 96% and 100%. For men the sensitivity could be derived in six studies (n = 4339) and ranged between 82% and 100% and the specificity ranged and between 90% and 100% (Figure 8). Only one study used gender‐specific basal calcitonin cut‐off values for all included participants (Hatzl‐Griesenhofer 2002). Another study which used two assays during the study period also had a gender‐specific cut‐off for the second assay, but this concerned only 14 participants (Hasselgren 2010). The study of Rink and colleagues used gender‐specific stimulated calcitonin cut‐off values (Rink 2009). Due to the limited number of studies we could not calculate summary estimates for different cut‐off values.
8.
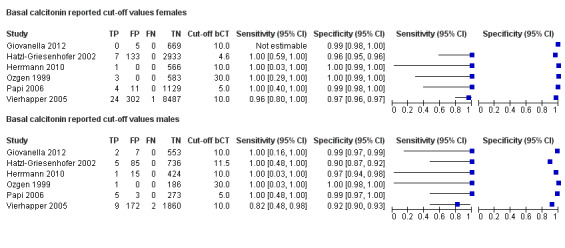
Forest plot of basal calcitonin testing reported cut‐off values for females and males (range basal calcitonin 4.6 pg/mL to 35 pg/mL, range stimulated calcitonin 50 pg/mL to 100 pg/mL). (TP: true positive, FP: false positive, FN: false negative, TN: true negative, bCT: basal calcitonin).
Other sources of heterogeneity
Due to the limited number of studies or limited reporting of studies no summary estimates could be calculated for the following sources of heterogeneity.
Age
Average, median age or both were described in 12 studies, but only one study reported the results of calcitonin testing specified in different age groups (Papi 2006). Mean age ranged between studies between 42.1 and 56 years and total range of age was between eight and 97 years (Characteristics of included studies). The study of Papi grouped patients in four different age categories (18 to 39 years; 40 to 49 years; 50 to 64 years; and > 65 years), and with inclusion of the MTC patients there was a significant increase of serum calcitonin concentration with age (2 pg/mL in patients 18 to 39 years versus 12.6 pg/mL in patients > 65 years). However, this significant correlation was lost when MTC patients were excluded (Papi 2006)
Detection method of thyroid nodules
In nine studies information was available on whether thyroid nodules in the included participants were detected through palpation or US; four studies included participants with thyroid nodules found by US and five studies included participants with thyroid nodules detected through US or palpation.
Nodule size
With regard to nodule size, one study (Papi 2006) reported detailed information on summary measures of size for all included participants. The average nodule size of all participants in this study was 21.8 mm (+ 4 mm; range 7 mm to 60 mm). The average nodule size of patients with elevated calcitonin was 19.4 mm (+ 2 mm; range 7 mm to 52 mm) versus 20.9 mm (+ 4.7 mm; range 7 mm to 44 mm) for patients with MTC. No correlation between nodule size and MTC was assessed. Three other studies provided also information of nodule size in all participants with an average nodule size of 14.66 mm (+ 7.5 mm) provided in one study (Grani 2012) and a range provided in the two other studies (which were 5 mm to 40 mm and 3 mm to 45 mm, respectively) (Costante 2007; Herrmann 2010). Four studies provided only information on nodule size of participants with elevated calcitonin levels (Giovanella 2012; Herrmann 2010; Rink 2009; Schuetz 2006), and four studies provided only information on nodule size of MTC patients (Elisei 2004; Hasselgren 2010; Schneider 2012; Vierhapper 2005) see Characteristics of included studies.
Number of nodules
None of the studies presented information on number of nodules of all participants. Three studies provided information on the number of participants with uni‐ or multinodular thyroid disease (Hasselgren 2010; Papi 2006; Rieu 1995). The percentage of patients with uninodular disease ranged between 27.2% and 38.2%, the percentage of patients with multinodular disease ranged between 51% and 69.5%. In these studies, no correlation was assessed between uni‐ or multinodularity and MTC.
Sonographic morphology of thyroid nodules
None of the studies presented information on US morphology of all participants.
Fine needle aspiration (FNA) method
In eight studies information was provided on whether FNA procedures were performed through palpation or US; in one study both techniques were performed, in six studies US‐guided FNA was used and in one study FNA procedures were performed by palpation.
Type of stimulation test
There was no heterogeneity in the type of stimulation test as all studies performing a stimulation test used the same type of stimulative (pentagastrin) and the same dose (0.5 µg/kg).
Sensitivity analysis
We planned to perform a sensitivity analysis with regard to methodological quality items scored with the QUADAS‐2 tool. However, no large differences were seen between studies regarding risk of bias There was only one study that had a high score with regard to the risk of bias on the domain of patient selection (Hatzl‐Griesenhofer 2002) as explained in the section Methodological quality of included studies. This study was already excluded in the cut‐off covariate analysis of 10 pg/mL, so no additional sensitivity analysis was performed. In the 'Risk of bias' domain with regard to the index test all the studies had a low risk, whereas the risk of bias associated with the reference standard and patient selection was assessed in all studies as unclear or high. Furthermore, in all studies there were low concerns of applicability.Therefore, no separate sensitivity analysis in these domains was performed.
Discussion
Summary of main results
In this review we included 16 studies for determination of the diagnostic accuracy of calcitonin testing in participants with thyroid nodules. Different thresholds were used, but median specificity across studies was high (96.6%) with a high estimated sensitivity (99.7%, (95% CI 68.8 to 100)). For a specific threshold of 10 pg/mL for basal calcitonin we also found high summary estimates of sensitivity 100% (99.7% to 100%) and specificity 97.2% (95.6% to 98.6%).
Strengths and weaknesses of the review
We evaluated the diagnostic accuracy of calcitonin testing for detection of medullary thyroid carcinoma (MTC) in patients with thyroid nodules with a comprehensive search of the literature and performing a formal diagnostic meta‐analysis.
One of the major limitations of this review is the lack of adequate reference standards for participants with a negative calcitonin test in 15 of the 16 included studies. This increases the risk that participants with a false‐negative calcitonin test are missed and the reported sensitivities are overestimated. In two of the included studies, MTC patients with a false‐negative calcitonin test were identified (Schneider 2012; Vierhapper 2005). One explanation may be that the histopathological examination focused more on the detection of MTC in (plasma) calcitonin‐negative thyroid nodules in the associated studies. In studies performing preoperative calcitonin testing, the rate of MTC patients with a false‐negative test ranged between 12.5% to 14.3% of all MTC patients identified (Chambon 2011; Niccoli 1997). Because the prevalence of MTC is low in people with thyroid nodules, even a small number of participants with a false‐negative calcitonin test can markedly affect sensitivity. The clinical relevance of these MTCs with a false‐negative test can be discussed as in most calcitonin testing studies these are patients with a micro‐MTC without nodal metastasis. However, reports also exist on MTC patients with more aggressive disease and undetectable calcitonin levels (Frank‐Raue 2013).
We assessed the risk of bias and applicability of the included studies using the QUADAS signalling questions. This gave rise to very similar results across all the domains of the risk of bias and applicability concerns for most of the studies, so a planned sensitivity analysis with regard to methodological quality was not performed. The risk of bias and applicability concern with regard to patient selection and the index test were considered low, which may be the result of our strict inclusion criteria. Most concerns existed with regard to the reference standard and the flow and timing of patients, because patients did not receive the same reference standard, and the information provided on the clinical follow‐up was absent in almost all studies. These concerns are, as mentioned above, a major limitation of this review.
Another limitation of this review is the small number of studies that could be included in final analyses. Due to this small number we could evaluate only for a few subgroups summary measures. Furthermore, we could not establish an optimal cut‐off point due to the heterogeneity of the used cut‐off levels in the different studies. This was further complicated by the large heterogeneity in assay types and manufacturers.
Other reports
Other reviews have been published on the value of calcitonin testing in the detection of MTC, although no systematic reviews were performed. Daniels 2011 provided an overview of 15 studies, but also included participants with preoperative calcitonin testing and multiple studies of one study group. He concluded that due to the large reservoir of undetected micro MTCs of uncertain malignant potential, and the unavailability of pentagastrin in the USA, calcitonin testing is not indicated in the USA and Canada (Daniels 2011). Costante 2009 evaluated 11 studies in their review and concluded that the question whether to routinely measure calcitonin remained unsolved because no evidence exists whether testing actually reduces MTC‐related mortality (Costante 2009).
Applicability of findings to the review question
This review provides summary estimates of sensitivity and specificity for basal and stimulated calcitonin. The role of calcitonin testing in the diagnostic evaluation in thyroid nodules remains unclear. The final purpose of calcitonin testing is to detect patients with MTC at an early stage, in which the chance of biochemical cure improves and thus the prognosis of patients. The findings of this review indicate that calcitonin testing is a very sensitive and specific test, but this has to be interpreted bearing in mind the low prevalence of MTC. The positive predictive value of calcitonin testing is therefore low, especially with lower cut‐off values. Although several conditions are known to cause increased calcitonin levels, still in a fairly large proportion of participants with elevated calcitonin levels MTC cannot be excluded. Repeated calcitonin testing and follow‐up in these participants is therefore warranted. A number of these persons will be operated without histological evidence of MTC. Some individuals will have C‐Cell hyperplasia, but the clinical relevance of this finding and its malignant potential remain unclear.
Cost‐effectiveness in health care becomes more and more important. In this review no formal cost‐effectiveness analysis was performed, so no validated statements can be made. However, Cheung 2008 performed a cost‐effectiveness analysis in which calcitonin testing was cost‐effective similar to colonoscopy and mammography screening (Cheung 2008). In their hypothetical model, several parameters had an important influence on cost‐effectiveness, such as specificity of the calcitonin test and prevalence of MTC. Cheung 2008used a cut‐off value of 50 pg/mL with a specificity of 98% in the baseline model, almost similar to the 96.6% median specificity we found of the basal calcitonin test. However, the MTC prevalence established in our review was 0.23%; one third of the prevalence used by Cheung and colleagues. With a three times lower prevalence, costs will also increase almost three times. In a review of autopsy studies, the prevalence of occult MTC was estimated to be 0.14% (Valle 2011). Although it is not known if all MTCs detected at autopsy are clinically irrelevant and could be detected through calcitonin screening, a proportion of these tumours will be, further lowering the prevalence of clinically relevant MTCs. Our findings with regard to sensitivity, specificity and MTC prevalence, applied to the cost‐effectiveness model of Cheung 2008, imply that basal calcitonin testing does not seem to be cost‐effective. The effects on cost‐effectiveness of a combined basal and stimulated calcitonin on cost‐effectiveness are more difficult to estimate. In their model Cheung 2008 gave a sensitivity and specificity for this combined approach of 80% and 98%, respectively while our summary estimates show both a higher sensitivity and specificity. However, it is likely that this model is influenced by the prevalence of MTC, decreasing cost‐effectiveness with lower prevalence.
Authors' conclusions
Implications for practice.
Calcitonin testing can be a sensitive and specific instrument for detecting medullary thyroid carcinoma (MTC) in thyroid nodules. However, due to the low prevalence of MTC, its role as a screening tool remains unclear. If we apply our findings from a basal calcitonin test with a cut‐off of 10 pg/mL to a population of 10,000 persons with a MTC prevalence of 0.23% (median prevalence of the included studies), 23 people will have MTC. All these 23 individuals will have an elevated basal calcitonin test, while 280 people without MTC also will have an elevated basal calcitonin. The positive predictive value (PPV) of the calcitonin test in this situation is 7.7% (95% CI 4.9 to 12.1). Surely not all persons with an elevated basal calcitonin will be operated but even if a cause of elevated calcitonin can be established in 90% of the individuals with a false‐positive test, the remaining 10% of people (N = 28) of the individuals might be operated unnecessarily. This operation results in life‐long thyroid hormone supplementation and also has a risk of recurrent laryngeal nerve damage and hypoparathyroidism. Increasing cut‐off values is likely to result in a higher specificity and thus a higher positive predictive value (PPV), but can come at the cost of patients with missed MTC, although we could not formally test this. Also, adding a stimulated calcitonin test is likely to increase specificity with very little effect on sensitivity, however we could not calculate a summary estimate for a specific cut‐of due to the limited number of studies.
The major reason to perform calcitonin testing is ultimately to improve prognosis of patients with MTC. The supposed value of calcitonin testing is the detection of patients with MTC at an earlier stage in which biochemical cure is still possible. However, to assess this, one has to know which of the patients with MTC would not have been detected through regular examinations (ultrasonography (US), fine needle aspiration (FNA), optional calcitonin or a combination thereof), and who of these detected individuals by calcitonin testing has or will develop a clinically relevant MTC. Some studies demonstrate that survival was significantly improved after introduction of routine calcitonin testing in people with thyroid nodules (Alevizaki 2012; Elisei 2004; Karga 2011). However, these studies have made their comparison with a historical group, and other factors might also have contributed to improved survival. These factors included, e.g. improved surgical treatment strategies and use of US. Furthermore, it is also interesting to note that MTC was supposedly detected at an earlier stage in these studies, but the age of the screened MTC patients was not lower compared to the participants that were not screened (Elisei 2004; Karga 2011). As MTC is considered to be a slow‐growing tumour which takes several years to become clinically evident, the fact that patients with MTC in the screened groups are of equal or even higher age, might indicate that additional MTC patients have been detected who would have otherwise had an indolent course of their disease. Another indication that otherwise undetected and possibly indolent patients with MTC are identified is the increased number of MTC patients detected in shorter periods in the calcitonin‐tested patients compared to the historical cohorts (Alevizaki 2012; Elisei 2004).
On the basis of the results of our review, there is insufficient evidence for a routine calcitonin test in all patients with a thyroid nodule. An alternative for routine testing is optional use and to perform the calcitonin test only on indication. These indications include: a suspicious clinical presentation, an inconclusive FNA result, or both. There may be a role for preoperative application for the calcitonin test, however this was not the scope of this review. The reported sensitivity of routine preoperative testing is lower compared to routine calcitonin testing in all participants. The lower sensitivity is likely the result from a difference in verification method; studies including only preoperative persons have histological verification in all calcitonin test‐negative participants, while studies including all participants have only histological confirmation in a limited number of calcitonin test‐negative participants (e.g. operated for other causes). The studies in which only a part of the patients will have histological verification are more likely to have a verification bias and are thus at risk to overestimate sensitivity. However, the sensitivity of preoperative calcitonin testing to detect MTC is reported to be higher than FNA cytology and can increase the rate of correct preoperative diagnoses (Bugalho 2005). This may result in more adequate initial surgical procedures which is of crucial importance in patients with MTC.
Implications for research.
This review shows that the diagnostic accuracy of calcitonin testing in MTC is high. However, this conclusion is based on studies in which the MTC prevalence in calcitonin‐negative participants might have been underestimated, due to lack of (reporting) adequate reference standards in calcitonin‐negative participants. Future studies should therefore report more accurately on the follow‐up of calcitonin‐negative participants, to ensure that no MTC patients are missed, and also to provide more information on the clinical behavior of these tumours. Furthermore, accurate reporting of assay type and manufacturer is crucial for establishing optimal cut‐off points for diagnosis of MTC. Also, the role of the calcitonin test, being a triage or add‐on test next to FNA ( including measurement of calcitonin in washout fluids) should be further evaluated. Furthermore, reporting results for subgroups may also identify subgroups with a higher MTC prevalence in which calcitonin testing can be more cost‐effective.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (via Cochrane Register of Studies Online) |
| #1 MeSH descriptor Thyroid neoplasms explode all trees #2 MeSH descriptor Goiter, Nodular explode all trees #3 ((thyroid adj6 neoplas*) or (thyroid adj6 cancer) or (thyroid adj6 carcinoma*) or (thyroid adj6 macrocarcinoma*) or (thyroid adj6 microcarcinoma*)):TI,AB,KY #4 ((thyreoid adj6 neoplas*) or (thyreoid adj6 cancer) or (thyreoid adj6 carcinoma*) or (thyreoid adj6 macrocarcinoma*) or (thyreoid adj6 microcarcinoma*)):TI,AB,KY #5 ((goiter adj6 neoplas*) or (goiter adj6 carcinoma*) or (goiter adj6 cancer) or (goiter adj6 macrocarcinoma*) or (goiter adj6 microcarcinoma*)):TI,AB,KY #6 ((goitre adj6 neoplas*) or (goitre adj6 cancer) or (goitre adj6 carcinoma*) or (goitre adj6 macrocarcinoma*) or (goitre adj6 microcarcinoma*)):TI,AB,KY #7 ((thyroid adj6 tumour*) or (thyreoid adj6 tumour*) or (goiter adj6 tumour*) or (goitre adj6 tumour*)):TI,AB,KY #8 ((multinodul* adj6 thyroid*) or (multinodul* adj6 thyreoid*) or (multinodul* adj6 goiter) or (multinodul* adj6 goitre)):TI,AB,KY #9 ((multi‐nodul* adj6 thyroid*) or (multi‐nodul* adj6 thyreoid*) or (multi‐nodul* adj6 goiter) or (multi‐nodul* adj6 goitre)):TI,AB,KY #10 ((nodul* adj6 thyroid*) or (nodul* adj6 thyreod*) or (nodul* adj6 goiter) or (nodul* adj6 goitre)):TI,AB,KY #11 ((medullary adj6 thyroid*) or (medullary adj6 thyreoid*)):TI,AB,KY #12 MTC:TI,AB,KY #13 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor Carcinoma, Medullary explode all trees #15 MeSH descriptor Neoplasms, ductal, lobular, and medullary explode all trees #16 (medullary adj6 carcinom*):TI,AB,KY #17 ((neoplasm* adj3 ductal) or (neoplasm* adj3 lobular) or (neoplasm* adj3 medullary)):TI,AB,KY #18 #14 or #15 or #16 or #17 #19 (thyroid* or thyreoid* or goiter or goitre):TI,AB,KY #20 #18 and #19 #21 #13 or #20 #22 MeSH descriptor Calcitonin explode all trees #23 (calcitrin* or calcitonin* or thyrocalcitonin*):TI,AB,KY #24 (#22 or #23) #25 MeSH descriptor diagnostic tests, routine explode all trees #26 MeSH descriptor Biopsy, fine‐needle explode all trees #27 MeSH descriptor Diagnostic techniques, endocrine explode all trees #28 MeSH descriptor Magnetic resonance Imaging explode all trees #29 MeSH descriptor Ultrasonography explode all trees #30 MeSH descriptor Biomarkers explode all trees #31 MeSH descriptor Carcinoembryonic antigen explode all trees #32 MeSH descriptor diagnostic imaging explode all trees #33 MeSH descriptor Pentagastrin #34 MeSH descriptor Immunoassay explode all trees #35 MeSH descriptor Carcinoma, Medullary explode all trees with qualifiers DI #36 MeSH descriptor limit of detection explode all trees #37 MeSH descriptor sensitivity and specificity explode all trees #38 MeSH descriptor roc curve explode all trees #39 MeSH descriptor predictive value of tests explode all trees #40 MeSH descriptor reproducibility of results explode all trees #41 (diagnos* or screen* or detect*) #42 (sensitivity or specificity) #43 (accuracy or precision) #44 (validat* or validity or cross‐validat*) #45 (pretest* or pre‐test* or posttest* or post‐test*) #46 (likelihood and ratio*) #47 (predic* or chemiluminescen*) #48 (receiver and operating and characteristic*) #49 ((ROC adj6 analy*) or (ROC adj6 curve*)) #50 ((tumour adj6 marker*) or (tumor adj6 marker*) or (biological adj6 marker*)) #51 (RIA or IRMA or ILMA) #52 ((calcitonin and test*) or (pentagastrin and test*)) #53 (PET‐CT or PET) #54 (fine and needle and aspiration*) #55 (MRI or FNA or FNAC or FNAB) #56 (cytology or immunhistochem* or ultrasonograph* or echograph*) #57 (imaging and technique) #58 (genetic and screen*) #59 #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 #60 #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 #61 #21 and #24 and #60 |
| MEDLINE (Ovid) |
| 1. exp Thyroid Neoplasms/ 2. exp Goiter nodular/ 3. ((thyroid* or thyreoid* or goiter or goitre) adj6 (neoplas* or cancer or carcinoma* or macrocarcinoma* or microcarcinoma* or tumo?r*)).tw,ot. 4. ((nodul* or multinodul* or multi‐nodul*) adj6 (thyroid* or thyreoid* or goiter or goitre)).tw,ot. 5. ((thyroid* or thyreoid* goiter or goitre) adj6 (multinodul* or multi nodul*)).tw,ot. 6. (medullary adj6 (thyroid* or thyreoid*)).tw,ot. 7. MTC*.ab. 8. or/1‐7 9. exp Carcinoma, medullary/ 10. exp "Neoplasms, Ductal, Lobular, and Medullary"/ 11. (medullary adj6 carcinom*).tw,ot. 12. (neoplasm* adj3 (ductal or lobular or medullary)).tw,ot. 13. or/9‐12 14. (thyroid* or thyreoid* or goiter or goitre).tw,ot. 15. 13 and 14 16. 8 or 15 17. Calcitonin/ 18. (calcitrin* or calcitonin* or thyrocalcitonin).tw,ot. 19. 9007‐12‐9.rn. 20. or/17‐19 21. exp Diagnostic Tests, Routine/ 22. exp Biopsy, Fine‐Needle/ 23. exp Diagnostic Techniques, Endocrine/ 24. exp Magnetic Resonance Imaging/ 25. exp Ultrasonography/ 26. exp Biomarkers/ 27. exp Carcinoembryonic antigen/ 28. exp Diagnostic Imaging/ 29. exp Pentagastrin/ 30. exp Immunoassay/ 31. Carcinoma, medullary/di [Diagnosis] 32. exp Limit of Detection/ or (exp "Sensitivity and Specificity"/) or exp ROC Curve/ or exp Predictive Value of Tests/ or exp Reproducibility of Results/ 33. (diagnostic or diagnosis).tw,ot. 34. (screen* or detect*).tw,ot. 35. (sensitivity or specificity).tw,ot. 36. (accuracy or precision).tw,ot. 37. (validat* or validity or cross‐validat*).tw,ot. 38. ((pre‐test* or pretest* or post‐test* or posttest*) adj probability).tw,ot. 39. likelihood ratio*.tw,ot. 40. (predictive or prediction*).tw,ot. 41. receiver operating characteristic*.tw,ot. 42. (ROC adj6 (analy* or curve*)).tw,ot. 43. chemiluminescen*.tw,ot. 44. ((tumo?r or biological) adj6 marker*).tw,ot. 45. diagnos*.tw,ot. 46. (RIA or IRMA or ILMA).tw,ot. 47. calcitonin* test*.tw,ot. 48. PET.mp. 49. RET.mp. 50. fine needle aspiration*.tw,ot. 51. pentagastrin‐test*.tw,ot. 52. (MRI or FNA or FNAC or FNAB).tw,ot. 53. (cytology or immunohistochem* or ultrasonograph* or echograph*).tw,ot. 54. CEA.tw,ot. 55. imaging technique*.tw,ot. 56. genetic screening.tw,ot. 57. or/21‐56 58. 16 and 20 and 57 59. (animals not (animals and humans)).sh. 60. 58 not 59 |
| Embase (Ovid) |
| 1 thyroid tumor/ 2 exp thyroid cancer/ 3 exp thyroid nodule/ 4 exp thyroid medullary carcinoma/ 5 exp nodular goiter/ 6 ((thyroid* or thyreoid* or goiter or goitre) adj6 (neoplas* or cancer or carcinoma* or macrocarcinoma* or microcarcinoma* or tumo?r)).tw,ot. 7 ((thyroid* or thyreoid*) adj6 medullary carcinoma*).tw,ot. 8 ((nodul* or multinodul* or multi‐nodul*) adj3 (thyroid* or thyreoid* or goiter or goitre)).tw,ot. 9 MTC*.tw,ot. 10 or/1‐9 11 exp calcitonin/ 12 (calcitrin* or calcitonin* or thyrocalcitonin*).tw,ot. 13 9007‐12‐9.rn. 14 or/11‐13 15 exp diagnostic test/ 16 exp needle biopsy/ 17 exp endocrine system examination/ 18 exp nuclear magnetic resonance imaging/ 19 exp echography/ 20 exp biological marker/ 21 exp carcinoembryonic antigen/ 22 exp diagnostic imaging/ 23 exp immunoassay/ 24 exp chemiluminescent/ 25 exp "sensitivity and specificity"/ 26 exp receiver operating characteristic/ 27 exp predictive value/ 28 pentagastrin test.mp. 29 exp medullary carcinoma/di [Diagnosis] 30 exp reproducibility/ 31 ((tumo?r or biological) adj6 marker*).tw,ot. 32 diagnos*.tw,ot. 33 (sensitivity or specificity).tw,ot. 34 (accuracy or precision).tw,ot. 35 (validat* or validity or cross‐validat*).tw,ot. 36 ((pre‐test* or pretest* or post‐test* or posttest*) adj probability).tw,ot. 37 likelihood ratio*.tw,ot. 38 (predict* or chemiluminescen*).tw,ot. 39 (RIA or IRMA or ILMA).tw,ot. 40 ((calcitonin* or pentagastrin*) adj6 test*).tw,ot. 41 (screen* or detect*).tw,ot. 42 receiver operating characteristic*.tw,ot. 43 (ROC adj6 (analy* or curve*)).tw,ot. 44 PET‐CT.mp. 45 ((tumo?r or biological) adj6 marker*).tw,ot. 46 RET.mp. 47 fine needle aspiration*.tw,ot. 48 (MRI or FNA or FNAC or FNAB).tw,ot. 49 (cytology or immunhistochem* or ultrasonograph* or echograph*).tw,ot. 50 CEA.tw,ot. 51 imaging technique*.tw,ot. 52 genetic screening.tw,ot. 53 or/15‐38 54 10 and 14 and 53 |
| Science Citation Index Expanded (Web of Science) |
| #1 TS=((thyroid* tumor*) OR (thyroid* cancer) OR (thyroid* neoplas*) OR (thyroid* carcinoma*) OR (thyroid* microcarcinoma*) OR (thyroid* macrocarcinoma) OR (thyroid* medullary carcinoma*) OR (thyroid* nodul*) OR (thyroid* multinodul*) OR (nodul* goiter*)) #2 TS=((thyreoid* tumor*) OR (thyreoid* cancer) OR (thyreoid* neoplas*) OR (thyreoid* carcinoma*) OR (thyreoid* microcarcinoma*) OR (thyreoid* macrocarcinoma) OR (thyreoid* medullary carcinoma*) OR (thyreoid* nodul*) OR (thyreoid* multinodul*) OR (nodul* goiter*) OR (MTC)) #3 TS=((calcitonin*) OR (calcitrin*) OR (thyrocalcitonin*)) #4 #1 OR #2 #5 #3 AND #4 #6 TS=((diagnostic test*) OR (needle biopsy) OR (endocrine examination*) OR (echograph*) OR (ultrasonograph*) OR (magnetic resonance imaging) OR (MRI) OR (biological marker*) OR (diagnostic imaging) OR (immunoassay) OR (chemiluminescent*) OR (tumor marker*) OR (carcinoembryonic antigen) OR (CEA) OR (diagn*) OR (RIA) OR (IRMA) OR (ILMA) OR (PET) OR (pentagastrin test*) OR (calcitonin test*) OR (screen*) OR (sensitivity) OR (specificity) OR (validat*) OR (validity) OR (roc curve) OR (reproducibility) OR (FNA) OR (genetic screen*)) #7 TS=((carcioembryonic antigen) OR (CEA) OR (pentagastrin test*) OR (diagnos*) OR (calcitonin* test*) OR (screen*) OR (detect*) OR (RIA) OR (IRMA) OR (ILMA) OR (PET‐CT) OR (RET)) #8 TS=((FNAC) OR (FNAB) OR (cytology) OR (immunhistochem*) OR (imaging technique*)) #9 #6 OR #7 OR #8 #10 #5 AND #9 #11 TS=(animal*) #12 #10 NOT #11 |
| 'My NCBI' alert service |
| ("thyroid nodule"[MeSH Terms] OR ("thyroid"[All Fields] AND "nodule"[All Fields]) OR "thyroid nodule"[All Fields]) AND ("calcitonin"[MeSH Terms] OR "calcitonin"[All Fields]) |
Appendix 2. QUADAS‐2 signalling questions for bias
| Domain | Yes | Unclear | No |
| Patient selection | |||
| 1. Consecutive or random sample enrolled? | A consecutive or random sample of patients was enrolled in the study. | It is unclear whether a consecutive or random sample of patients was enrolled in the study. | There was no consecutive or random sample included in the study (e.g. only patients already suspected of (medullary) thyroid malignancy and patients with high risk for (familial) MTC. |
| 2. Case control design avoided? | There was no case control design. | It is unclear if there was a case control design | There was a case control design |
| 3. Inappropriate exclusions avoided? | There are no patients inappropriate excluded (e.g. patients with suspicious US, who will already be operated on) | It is unclear if there was avoidance of inappropriate exclusions | There is inappropriate exclusion of patients (e.g. exclusion of patients with high risk of malignancy) |
| Index test | |||
|
1. Index test results interpreted without knowledge results reference standard? |
This item will be omitted as only studies are included in which the reference standard (histopathological examination) is performed after calcitonin testing. Furthermore calcitonin testing is a objective test (although interpretation depends on the threshold, but this will be assessed in the next item) | ||
| 2. Pre‐specified threshold? | There was a pre‐specified calcitonin cut‐off level. | It is unclear if there was a pre‐specified cut‐off level | There was no pre‐specified calcitonin cut‐ff level. |
| Reference standard | |||
|
1. Reference standard likely to correctly classify target condition? |
In patiens receiving surgery there is adequate histopathological examination of thyroid tissue. In patients receiving follow‐up, there is at least three years follow‐up years including at least one US examination and if indicated FNAC. |
In patients receiving surgery it's unclear how histopathological examination is performed. In patients receiving follow‐up the time and protocol for follow‐up is unclear |
In patients receiving surgery histopathological examination is not adequate. In patients without surgery, follow‐up is to short or does not include at least one US examination and FNAC. |
|
2. Reference standard results interpreted without knowledge results index test? |
The outcome assessor of histopathological and follow‐up results was not aware of calcitonin testing results | It is not clear if the outcome assessor of histopathological and follow‐up results was aware of calcitonin testing results | The outcome assessor of histopathological and follow‐up results was aware of calcitonin testing results |
| Flow and timing | |||
| 1. Appropriate interval between index test and reference standard? | Time between calcitonin testing and histopathological examination is < 3 months | It is unclear what the time period between reference standard and index test is. | Time between calcitonin testing and histopathological exceeds 3 months. |
| 2. All patients received reference standard? | All patients received surgery, and patients who did not receive surgery have clinical follow‐up of at least three years. | It is not clear if the whole sample did receive surgery or follow‐up. | Only a selected subset of the patients received or surgery or not all patients have clinical follow‐up. |
| 3. Patients received same reference standard? | All patients were operated and histopathological examination of the thyroid was performed. | It is not clear if all patients were operated and received histopathological examination. | Not all patients were operated or histopathological examination was not performed in all patients. |
| 4. All patients included in the analysis? | All patients enrolled were included in the analysis | It is not clear if all patients were included in the analysis. | Not all patients enrolled were included in the analysis (e.g. patients lost to follow‐up) |
MTC: medullary thyroid carcinoma; US: ultrasonography.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
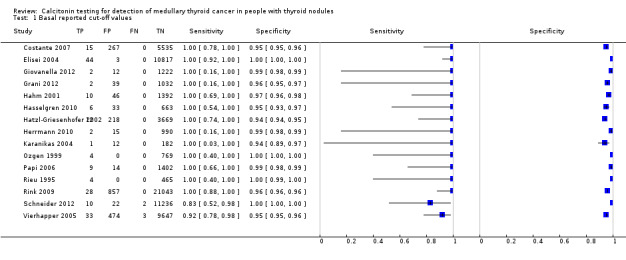
Basal reported cut‐off values.
2. Test.
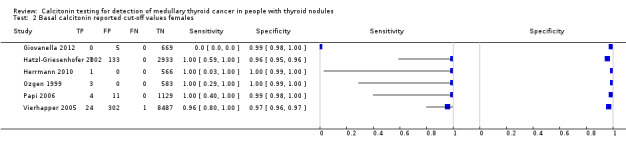
Basal calcitonin reported cut‐off values females.
3. Test.
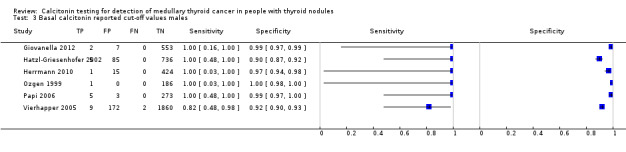
Basal calcitonin reported cut‐off values males.
4. Test.
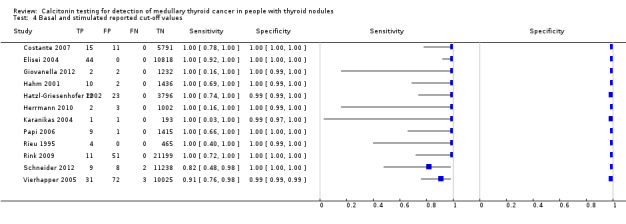
Basal and stimulated reported cut‐off values.
5. Test.
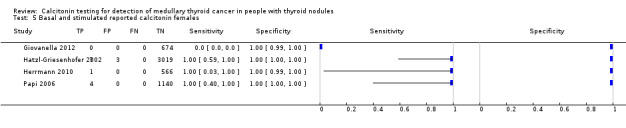
Basal and stimulated reported calcitonin females.
6. Test.
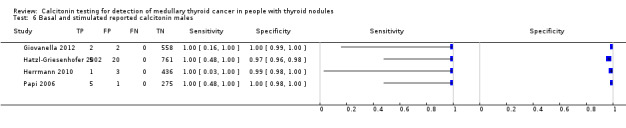
Basal and stimulated reported calcitonin males.
7. Test.
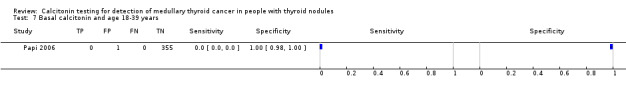
Basal calcitonin and age 18‐39 years.
8. Test.

Basal calcitonin and age 40‐49 years.
9. Test.

Basal calcitonin and age 50‐64 years.
10. Test.

Basal calcitonin and age > 65 years.
11. Test.
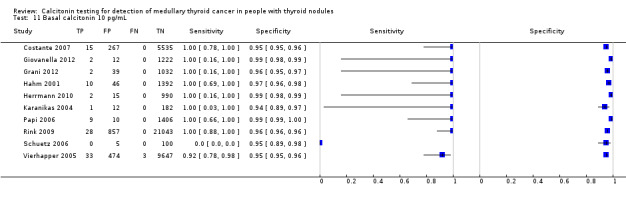
Basal calcitonin 10 pg/mL.
12. Test.
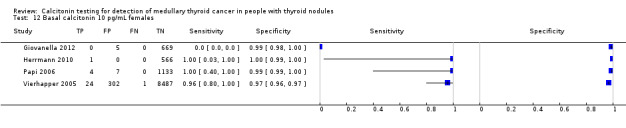
Basal calcitonin 10 pg/mL females.
13. Test.
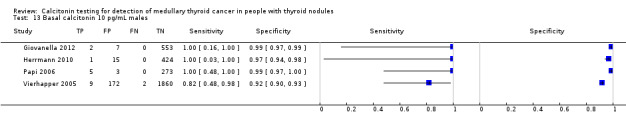
Basal calcitonin 10 pg/mL males.
14. Test.
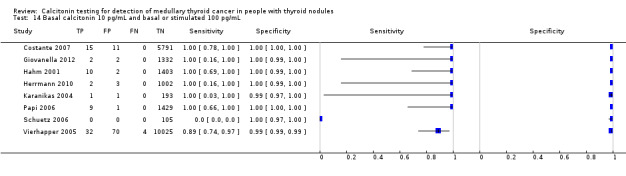
Basal calcitonin 10 pg/mL and basal or stimulated 100 pg/mL.
15. Test.
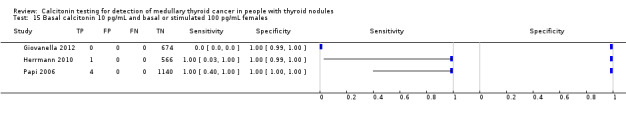
Basal calcitonin 10 pg/mL and basal or stimulated 100 pg/mL females.
16. Test.
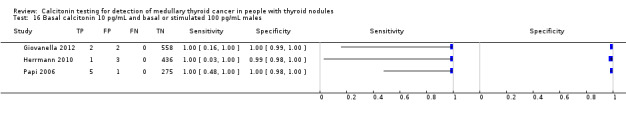
Basal calcitonin 10 pg/mL and basal or stimulated 100 pg/mL males.
17. Test.
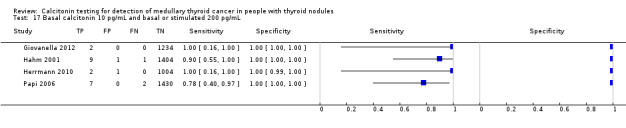
Basal calcitonin 10 pg/mL and basal or stimulated 200 pg/mL.
18. Test.
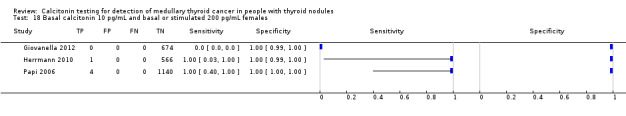
Basal calcitonin 10 pg/mL and basal or stimulated 200 pg/mL females.
19. Test.
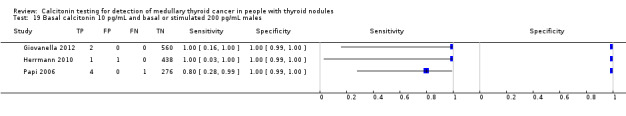
Basal calcitonin 10 pg/mL and basal or stimulated 200 pg/mL males.
20. Test.
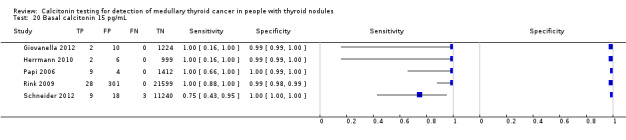
Basal calcitonin 15 pg/mL.
21. Test.
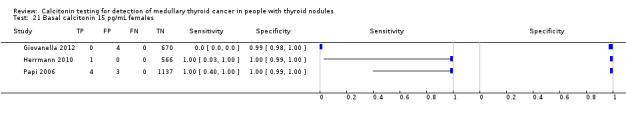
Basal calcitonin 15 pg/mL females.
22. Test.
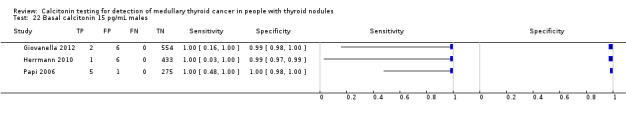
Basal calcitonin 15 pg/mL males.
23. Test.
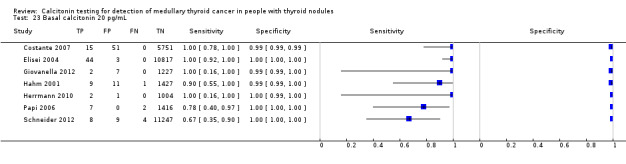
Basal calcitonin 20 pg/mL.
24. Test.
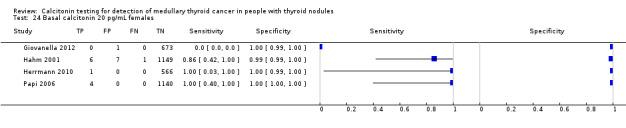
Basal calcitonin 20 pg/mL females.
25. Test.
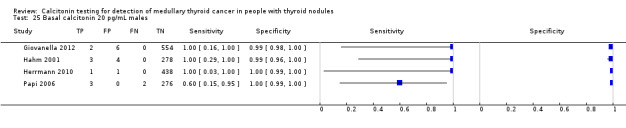
Basal calcitonin 20 pg/mL males.
26. Test.
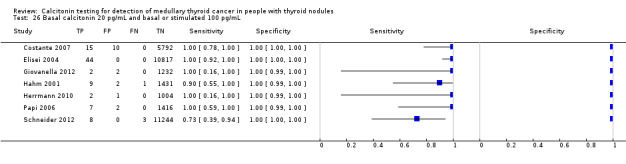
Basal calcitonin 20 pg/mL and basal or stimulated 100 pg/mL.
27. Test.
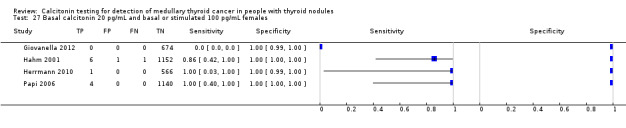
Basal calcitonin 20 pg/mL and basal or stimulated 100 pg/mL females.
28. Test.
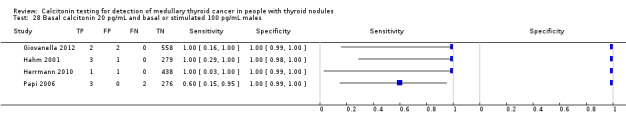
Basal calcitonin 20 pg/mL and basal or stimulated 100 pg/mL males.
29. Test.
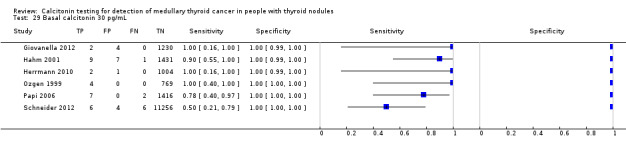
Basal calcitonin 30 pg/mL.
30. Test.
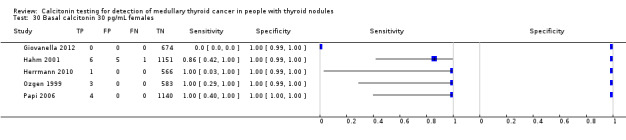
Basal calcitonin 30 pg/mL females.
31. Test.
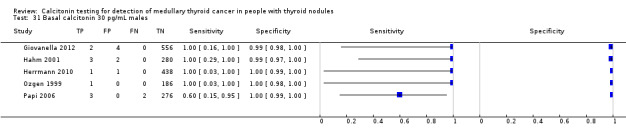
Basal calcitonin 30 pg/mL males.
32. Test.
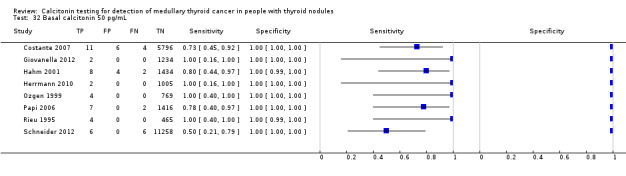
Basal calcitonin 50 pg/mL.
33. Test.
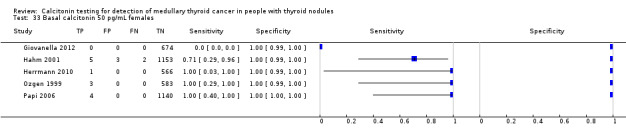
Basal calcitonin 50 pg/mL females.
34. Test.
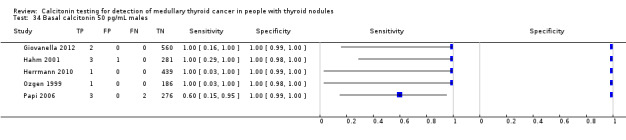
Basal calcitonin 50 pg/mL males.
35. Test.
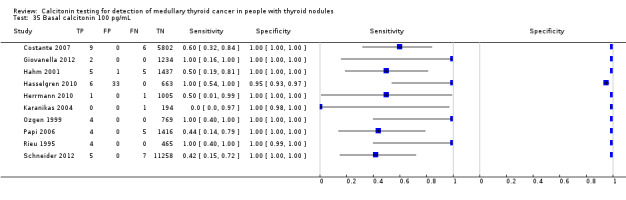
Basal calcitonin 100 pg/mL.
36. Test.
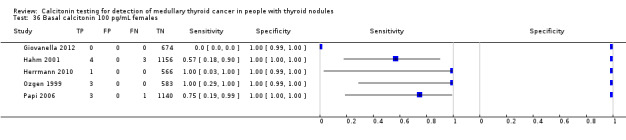
Basal calcitonin 100 pg/mL females.
37. Test.
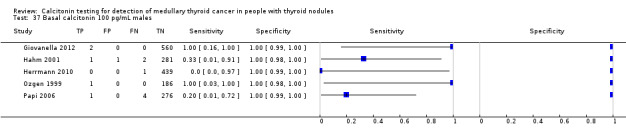
Basal calcitonin 100 pg/mL males.
Characteristics of studies
Characteristics of included studies [author‐defined order]
Rieu 1995.
| Study characteristics | |||
| Patient sampling |
Design: prospective cohort study Inclusion criteria: participants with thyroid nodules detected by clinical examination or with abnormal TSH levels or both Exclusion criteria: — |
||
| Patient characteristics and setting |
Number of participants: 657 participants Number with NTD: 469 participants Number with NTD and calcitonin testing: 469 participants Sex (female%)(N): 88.1% (579/657); only reported of whole study population Age (year, mean/SD): 45, SD: ‐ range: 15‐87 years; only reported for the whole study population MTC: 4 participants Type of thyroid nodules: non toxic uninodular goitre (N = 136), autonomously functioning thyroid nodule (N = 14), non toxic multinodular goitre (N = 224), toxic multinodular goitre (N = 15), nodular Hashimoto thyroiditis (N = 53), nodular Graves' disease (N = 25), nodular formation related to subacute thyroiditis in a hyperthyroid phase (N = 2). Thyroid nodules detected by palpation or US: both, number not specified Nodule size: — Number of nodules: uninodular (N = 150), multinodular (N = 239), ‐ (N = 80) Sonographic morphology of thyroid nodules: a localised thyroid area was considered nodular when it had a distinctive rim at two different incidences. When this rim was absent, a thyroid area with round or oval patterns, and also echogenicity different from that of the surrounding normal tissue, was classified as nodular FNA procedures performed through ultrasound guidance or palpation: — Setting: — Country: France |
||
| Index tests |
Index test: basal and stimulated calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: 01‐1989 – 12‐1989, RIA (Mallinckrodt Medical SA, Evry, France) 01‐1990 – 12‐1993: IRMA (CIS‐Oris International, Saint Quentin en Yvelins, France) Stimulated calcitonin: yes Indication: participants with basal serum CT above normal range Stimulative: pentagastrin Dose: 0.5 ug/kg Time: 0, 3, 5, 15 and 30 minutes after injection Reported and extracted cut‐off values Basal: reported: RIA: 35 ng/L, IRMA 10 ng/L; extracted 50 ng/L; 100 ng/L Stimulated: reported: 100 ng/L; extracted 100 ng/L. |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNA in participants whose nodular formation was accessible to such a procedure, in selected participants histopathological examination after surgery Indication surgical treatment: participants with increased basal serum CT values, pentagastrin stimulated CT levels > 100 pg/L, regardless of FNAC result, FNAC suggestive of thyroid carcinoma (N = 7), compressive goitre (N = 4) Type of surgical treatment: for suspected MTC total thyroidectomy and central neck dissection. Bilateral modified neck dissection was performed only in the presence of evident lymph node involvement in one or both sides. For all other types of thyroid carcinoma, total thyroidectomy and lymph node dissection on the side of the thyroid nodule(s) or bilaterally (as appropriate) Calcitonin negative (N = 465) Number FNAC: — Number operated: 11 participants Calcitonin positive: (N = 4) Number FNAC: 4 participants Number operated: 4 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: — Type: — Duration: — |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: — Publication status: full article |
||
| Stated aim of study | Quote from publication: "to asses the prevalence of sporadic MTC in nodular and non‐nodular thyroid diseases by routine basal serum CT measurements" | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Ozgen 1999.
| Study characteristics | |||
| Patient sampling |
Design: prospective study Inclusion criteria: participants with nodular goitre Exclusion criteria: participants with previously known medullary carcinoma and their relatives |
||
| Patient characteristics and setting |
Number of participants: 773 participants Clinical features: participants with nodular goitre Number with NTD: 773 participants Number with NTD and calcitonin testing: 773 participants Sex (female%)(N): 75.8% (586) Age (mean/SD): range: 42.1 years, range 17‐78 Number with MTC: 4 participants Type of thyroid nodules: multinodular goitre (nontoxic/toxic), solitary (nontoxic/toxic) thyroid nodules Thyroid nodules detected by palpation or US: both; number not specified Nodule size: — Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: both (US guidance in nonpalpable nodules), number not specified Setting: outpatient clinic Country: Turkey |
||
| Index tests |
Index test: basal calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: commercial kit; DSL‐5200 Ultrasensitive calcitonin RIA kit, Diagnostic System Laboratories Inc., Webster, Tx. Sensitivity: — Stimulated calcitonin: no Indication: — Stimulative: — Dose: — Time: — Reported and extracted cut‐off values Basal: reported: 30 pg/mL; extracted:30 pg/mL, 50 pg/mL, 100 pg/mL Stimulated: — |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAB and in selected cases histopathological examination after surgery Indication surgical treatment: malignant or suspicious results in FNAB, or elevated calcitonin levels regardless of the result of FNAB Type of surgical treatment: thyroid surgery Calcitonin negative (N = 669) Number FNAB: 669 participants Number operated: 171 participants Calcitonin positive: (N = 4) Number FNAB: 4 participants Number operated: 4 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: described for 3 MTC participants Type: basal and stimulated calcitonin levels Duration: 14‐18 months |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: — Publication status: full article |
||
| Stated aim of study | Quote from publication: "To identify MTC by screening patients who have thyroid nodules with basal calcitonin measurements and to determine whether basal serum calcitonin measurement should be a part of the routine evaluation of a nodular goitre." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Hahm 2001.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: participants with nodular thyroid disease Exclusion criteria: — |
||
| Patient characteristics and setting |
Number of participants: 1448 participants Number with NTD: 1448 participants Number with NTD and calcitonin testing: 1448 participants Sex (female%)(N): 80,3% (1163) Age (mean/SD): 46 years Range: 14‐86 years Number with MTC: 10 participants Type of thyroid nodules: — Thyroid nodules detected by palpation or US: — Nodule size: — Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: — Setting: Thyroid clinic of Samsung Medical Center Country: Korea |
||
| Index tests |
Index test: basal and stimulated calcitonin Used calcitonin assay: two‐site immunoradiometric assay commercial kit (MED‐GENIX CT‐US.‐IRMA kit) BioSource Europe S.A., Belgium Sensitivity: 0.8 pg/mL Stimulated calcitonin: yes Indication: reported for 39 participants: basal calcitonin > 10 pg/mL (N = 23), family members of MEN2 or MTC participants (N = 14) and FNAC findings suspicious for MTC (N = 2) Stimulative: pentagastrin (Peptavlon, Ayerst Laboratories Ind. Philadelphia, PA) Dose: 0.5 ug/kg body weight Time: just before, 2, 5, and 10 minutes Reported and extracted cut‐off values Basal: reported: 10 pg/mL (N = 56); extracted: 10, 20, 30, 50, 100 pg/mL Stimulated: reported: 100 pg/mL; extracted: 100, 200 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAC in all participants with palpable or visible thyroid nodule by US, in selected participants histopathological examination after surgery Indication surgical treatment: abnormal findings suspicious of malignancy by FNAC, participants who had basal or stimulated calcitonin concentrations of more than 100 pg/mL Type: thyroidectomy Calcitonin negative (N = 1392 participants) Number FNAC: — Number operated: 169 participants Calcitonin positive: (N = 56 participants) Number FNAC: 55 participants Number operated: 25 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: — Type: Duration: |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: other funding Publication status: peer review journal/journal supplement/full article/conference paper/other) |
||
| Stated aim of study | Quote from publication: "To evaluate the usefulness of routine measurement of serum calcitonin concentration in patients with nodular thyroid diseases, and to identify the validity of pentagastrin simulation test and FNAC " | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Hatzl‐Griesenhofer 2002.
| Study characteristics | |||
| Patient sampling |
Design: retrospective cohort study Inclusion criteria: participants with nodular thyroid disease or with evidence of nodular growth in follow‐up examinations Exclusion criteria: additional author information: no participants excluded |
||
| Patient characteristics and setting |
Number of participants: 3899 participants Number with NTD: 3899 participants Number with NTD and calcitonin testing: 3899 participants Sex (female%)(N): 78,8% (3073) Age (mean/SD): 54.6 (+11.2) years range: 6‐90 years Number with MTC: 12 participants Type of thyroid nodules: additional author information: most solitary nodules. Thyroid nodules detected by palpation or US: — Nodule size: — Number of nodules: — Sonographic morphology of thyroid nodules: only reported for MTC participants FNA procedures performed through ultrasound guidance or palpation: additional author information: palpation. Setting: outpatient ward of the department of Nuclear Medicine and Endocrinology of the General Hospital Linz Country: Austria |
||
| Index tests |
Index test: basal and stimulated calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: a two‐site chemiluminescence immunoassay, Nichols institute diagnostics Sensitivity: 0.7 pg/mL Stimulated calcitonin: yes Indication: participants with slightly or moderately elevated normal calcitonin levels (< 80 pg/mL) Stimulative: pentagastrin, Peptavlon, Zeneca, Vienna Dose: 0.5 ug/kg Time: before, 2, 5, and 8 minutes Reported and extracted cut‐off values Basal: reported: females: 4.6 pg/mL; males 11.5 pg/mL; extracted: females: 4.6 pg/mL; males 11.5 pg/mL Stimulated: reported:100 pg/mL (males and females); extracted: 100 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: histological evaluation in participants referred to surgery Indication surgical treatment: markedly elevated bCT and positive pentagastrin stimulation tests Type: hemithyroidectomy, (sub)total thyroidectomy, node dissection in selected participants Calcitonin negative ( N = 3669 participants) Number FNA: — Number operated: — Calcitonin positive: (N = 230 participants) Number FNA: — Number operated: 39 participants (?) |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: (1) participants with pathological stimulation test in which the consultant for internal medicine dissuaded against surgery because of elevated risks (N = 2). (2) participants with elevated basal calcitonin levels who declined pentagastrin stimulation (N = 41). Type: (1) ultrasound and determination of basal and stimulated calcitonin (2) clinical and biochemical follow up Number with follow‐up: 43 participants Duration: — |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: — Publication status: full article |
||
| Stated aim of study | Quote from publication: "To evaluate retrospectively the results of routine calcitonin measurements in patients with nodular thyroid disease" | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Elisei 2004.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: participants with nodular thyroid disease; additional author information: presence of at least one thyroid nodule. Exclusion criteria: — |
||
| Patient characteristics and setting |
Number of participants: 10864 participants Number with NTD: 10864 participants Number with NTD and calcitonin testing: 10864 participants Sex (female%)(N): 81.4% (8692). Age (mean/SD): 49 years SD ‐ Range: 12‐82 years MTC: 44 participants Type of thyroid nodules: single nodules, nontoxic multinodular goitre, autonomous functioning thyroid nodules, and autoimmune thyroid disease associated with distinct cold nodules; additional author information: 28 MTC patiens multinodular goitre, 16 MTC participants uninodular goitre Thyroid nodules detected by palpation or US: additional author information: US was performed in all cases. Nodule size: additional author information: in MTC participants cancer nodules ranged from 0.8 to 6 cm. Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: additional author information: ultrasound guidance Setting: — Country: Italy |
||
| Index tests |
Index test: basal and stimulated calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: solid phase 2 site immunoradiometric assay (ELSA‐hCT, CIS, Gif Sur Yvette, France) Sensitivity: 14 pg/mL Stimulated calcitonin: yes Indication: participants with detectable levels of basal CTand twice confirmed Stimulative: pentagastrin Dose: 0.5 ug/kg Time: before, 2, 5, 15 and 30 minutes Reported and extracted cut‐off values Basal: reported: 20 pg/mL; extracted: 20 pg/mL Stimulated: reported 60 pg/mL; extracted: 60, 100 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAC in nodules > 1 cm or in nodules < 1 cm with suspicious aspects at neck US, in selected participants histopathological examination after surgery Indication surgical treatment: participants with elevated bCT levels (confirmed by abnormal Pg‐stimulated CT levels) regardless of the results of FNAC and in those with FNAC suspicious of malignancy independently from the results of serum CT Type: total thyroidectomy and dissection of the central neck compartment Calcitonin negative (N = 10817) Number FNAC: — Number operated: — Calcitonin positive: (N = 47) Number FNAC: 47 participants Number operated: 44 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: only reported of MTC participants Type: — Number with follow‐up: 44 participants Duration: mean 6.2 + 2.5 years (range, 3‐10 years) |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: full article |
||
| Stated aim of study | Quote from publication: "To asses whether we could confirm the results of our preliminary study of 1991 and to compare the outcome of patients diagnosed by serum CT measurement with that of a historical group of MTC patients diagnosed and treated before the introduction of serum CT screening". | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Karanikas 2004.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: participants referred for the work‐up of various suspected thyroid disorders Exclusion criteria: — |
||
| Patient characteristics and setting |
Number of participants: 414 participants Number with NTD: 195 participants Number with NTD and calcitonin testing: 195 participants Sex (female%)(N): 79.5% (329) NB only reported for whole study population Age (mean/SD): 56 years; SD ‐ range: 18‐88 years, NB only reported for whole study population MTC: 1 participant (1 MTC patient in non‐nodular study population) Type of thyroid nodules: Uni and multinodular disease Thyroid nodules detected by palpation or US: — Nodule size: only thyroid size for participants with elevated CT; volume 21 + 10 mL Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: — Setting: outpatient department Country: Austria |
||
| Index tests |
Calcitonin as a triage or add‐on test: — Index test: basal and stimulated calcitonin. Used calcitonin assay: commercial assay by a Nichols Advantage Chemiluminescence System (Nichols Institute Diagnostics, San Juan Capistrano, Ca, USA) Sensitivity: 1 pg/mL Stimulated calcitonin: yes Indication: basal serum CT equal to or exceeding 10 pg/mL. Stimulative: pentagastrin Injection BP, Cambridge Laboratories, Tyne & Wear, UK Dose: 0.5 ug/kg Time: before, 2 and 5 minutes after injection Reported and extracted cut‐off values Basal: reported: 10 pg/mL; extracted: 10 pg/mL, 100 pg/mL Stimulated: reported: 100 pg/mL (abnormal), 500 pg/mL (pathological); extracted 100 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: in selected participants histological examination after total thyroidectomy, follow‐up Indication surgical treatment: participants with abnormal and pathological PG tests Type of surgical treatment: total thyroidectomy and lymph node dissection along both recurrent nerves in participants Calcitonin negative (N = 182) Number FNAC: — Number operated: — Calcitonin positive: (N = 13) Number FNAC: — Number operated: 1 patient |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: only for MTC patient Type: basal and stimulated CT Number with follow‐up: 1 Duration: — |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: — Publication status: full article |
||
| Stated aim of study | Quote from publication: "To compare the distribution and relevance of elevated CT levels in referrals with nonneoplastic and neoplastic thyroid disease (...)" | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Vierhapper 2005.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: participants with suspected thyroid disorders Exclusion criteria: participants with a known elevation of hCT |
||
| Patient characteristics and setting |
Number of participants: 25,669 participants Number with NTD: 10,292 participants Number with NTD and calcitonin testing: 10,157 participants Sex (female%)(N): 79.9% (8114) Age (mean/SD): ‐ range: — MTC: 36 participants Type of thyroid nodules: — Thyroid nodules detected by palpation or US: both Nodule size: only reported of MTC participants Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: — Setting: thyroid outpatient clinic Country: Austria |
||
| Index tests |
Index test: basal and stimulated calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: 1994‐1999: a commercially available immunoradiometric assay (CIS‐biointernational, Gif‐Sur‐Yvette, France) 1999‐2004: Acridinium‐ester‐labeled chemiluminescent immunoassay, running on the ‘Advantage’ auto‐analyser (Nichols Institute Diagnostics, US) Sensitivity: — Stimulated calcitonin: yes Indication: all participants with basal hCT> 10.0 pg/mL Stimulative: pentagastrin (CambridgeLaboratories, Wallsend, UK) Dose: 0.5 ug/kg Time: prior to, 2, 5 and 10 minutes Reported and extracted cut‐off values Basal: reported: 10 pg/mL extracted: 10 pg/mL Stimulated: reported 100 pg/mL extracted: 100 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAB, in selected participants histopathological examination after surgery, follow‐up Indication surgical treatment: elevated basal and/or pentagastrin stimulated serum concentrations of hCT(>100 pg/mL) or cytological findings Type: In participants with elevated basal and/or stimulated calcitonin a total thyroidectomy was performed. Routinely both recurrent nerves were dissected carefully and a systematic microdissection of the central lymph node compartment. If MTC was documented intraoperatively a systematic bilateral microdissection of the lateral lymph node compartments was added Calcitonin negative (N = 9650) Number FNA: — Number operated: at least 1 participant Calcitonin positive: (N = 507) Number FNAB: 15 participants (only reported for MTC patients) Number operated: 75 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: only reported of MTC patients Type: stimulated hCTs Number with follow‐up: 32 participants Duration: 1‐9 years |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: full article |
||
| Stated aim of study | Quote from publication: — | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Papi 2006.
| Study characteristics | |||
| Patient sampling |
Design: cohort study of consecutive participants Inclusion criteria: (1) male & female participants > 18 years (2) palpable nodules (3) non‐palpable nodules > 10 mm (4) non palpable nodule < 10 mm with malignant features on US Exclusion criteria: (1) participants < 18 years (2) non palpable nodules < 10 mm without malignant US features (3) participants previously evaluated for nodular goitre by FNA and/or CT measurement (4) participants reporting familial history of MEN and participants with known MTC (5) participants with hyper‐ and hypothyroidism without thyroid nodules (6) participants in follow‐up for thyroid disease (7) participants not confirmed as having thyroid diseases |
||
| Patient characteristics and setting |
Number of participants: 1474 participants Number with NTD: 1474 participants Number with NTD and calcitonin testing: 1425 participants Sex (female%)(N): 80% (1144) Age (mean/SD): 49.6(+ 6.8) years range: 18‐91 years MTC: 9 participants Type of thyroid nodules: euthyroid nodular thyroid disease (N = 1369), hypothyroid nodular thyroid disease (N = 32), sub acute de Quervain's thyroiditis (N = 1), Graves' disease (N = 2), toxic nodular goitre (N = 21). Multinodular disease (N = 1024), uninodular disease (N = 401) Thyroid nodules detected by palpation or US: both Nodule size: 21.8 + 4 mm Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: ultrasound Setting: — Country: Italy |
||
| Index tests |
Index test: basal and stimulated calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: two‐site chemiluminescence assay, Nichols institute diagnostics, San Juan Capristano, CA92675, USA) Sensitivity: 1 pg/mL Stimulated calcitonin: yes Indication: when basal serum CT concentrations exceeded 5 pg/mL, but not exceeded 100 pg/mL Stimulative: pentagastrin (Cambridge laboratories, Walsend, Tyne and Wear, NE289NX) Dose: 0.5 ug/kg Time: before, 2, 5 and 10 minutes after injection Reported and extracted cut‐off values Basal: reported: 5 pg/mL extracted: 5, 10, 15, 20, 30, 50,100 pg/mL Stimulated: reported: 100 pg/mL extracted: 100, 200 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAC and in selected participants, histopathological examination after surgical treatment Indication surgical treatment: basal serum CT concentrations > 5 pg/mL < 100 pg/mL and Pg‐stimulated CT levels > 100 pg/mL or basal serum CT concentrations > 100 pg/mL; participants with a suspicious or repeatedly non‐diagnostic FNAC and participants with a benign FNAC and compressive symptoms Type: in participants with benign FNAC and compressive symptoms a lobectomy or near‐total thyroidectomy. In participants with suspicious or non‐diagnostic FNAC, surgical extent depended on histological examination of frozen sections. In participants with malignancy other than MTC a total thyroidectomy and in case of lymph node involvement a lymphadenectomy. In MTC patients a total thyroidectomy and a systematic microdissection of both central neck and bilateral neck compartments. Contralateral lymph node dissection was omitted in MTC patients with a unilateral thyroid tumour and no ipsilateral and central lymph node involvement Calcitonin negative (N = 1402) Number FNAC: 1402 participants Number operated: 292 participants Calcitonin positive: (N = 23) Number FNAC: 23 participants Number operated: 23 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: — Type: — Duration: — |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: — Publication status: full article |
||
| Stated aim of study | Quote from publication: "To assess the prevalence of hypercalcitoninemia and MTC in NTD patients, to compare the ability of CT measurement and fine needle aspiration cytology (FNAC) to predict MTC, to identify age groups of NTD patients who should be better candidates than others to undergo routine measurement of CT." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Schuetz 2006.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: participants with Hashimoto's thyroiditis with documented positivity for TPOAb, Negativity for anti‐TSH receptor antibodies and thyroid ultrasound imaging suggestive of a chronic thyroiditis Exclusion criteria: — |
||
| Patient characteristics and setting |
Number of participants: 568 participants Number with NTD: 105 participants Number with NTD and calcitonin testing: 105 participants Sex (female%)(N): 88.0% (500) NB only reported for whole study population Age (mean/SD): 55 + ‐ range: 18‐88 years, NB only reported for whole study population MTC: 0 participants (1 MTC patient in non‐nodular study population) Type of thyroid nodules: — Thyroid nodules detected by palpation or US: US Nodule size: Only reported for participants with elevated calcitonin (13 + 5 mm; range 4.5‐17 mm) Number of nodules: — Sonographic morphology of thyroid nodules: only reported for participants with elevated calcitonin; all nodules were hypoechoic/circumscribable FNA procedures performed through ultrasound guidance or palpation: — Setting: outpatient department Country: Austria |
||
| Index tests |
Calcitonin as a triage or add‐on test: — Index test: basal and stimulated calcitonin Used calcitonin assay: commercial assay by a Nichols Advantage Chemiluminescence System (Nichols Institute Diagnostics, San Juan Capistrano, Ca, USA) Sensitivity: 1 pg/mL Stimulated calcitonin: yes Indication: basal serum CT equal to/or exceeded 10 pg/mL Stimulative: pentagastrin Injection BP, Cambridge Laboratories, Tyne & Wear, UK Dose: 0.5 ug/kg Time: before, 2,3 and 5 minutes after injection Reported and extracted cut‐off values Basal: reported: 10 pg/mL; extracted: 10 pg/mL Stimulated: reported: 100 pg/mL; extracted 100 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: in selected participants histological examination after total thyroidectomy, follow‐up Indication surgical treatment: participants with abnormal and pathological PG tests Type of surgical treatment: total thyroidectomy and lymph node dissection along both recurrent nerves in participants with a abnormal PG tests and an additional lateral lymph node dissection in participants with a pathological PG test or intraoperatively verified MTC Calcitonin negative (N = 100) Number FNAC: — Number operated: — Calcitonin positive: (N = 5) Number FNAC: — Number operated: 0 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: — Type: — Number with follow‐up: ‐ Duration: ‐ |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: — Publication status: full article |
||
| Stated aim of study | Quote from publication: "To evaluate the relevance of routine CT measurements for detection of MTC or its pre malignant associated conditions (micro MTC and neoplastic C cell hyperplasia) in HT patients." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Costante 2007.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: patients diagnosed with thyroid nodules. Exclusion criteria: renal failure, persistent or recurrent MTC, or a family history of MTC. |
||
| Patient characteristics and setting |
Number of participants: 5817 participants Number with NTD: 5817 participants Number with NTD and calcitonin testing: 5817 participants Sex (female%)(N): 80.9% (4706) Age (mean/SD): 49.7 + 16.6 years range: 11‐72 years MTC: 15 participants Type of thyroid nodules: euthyroid nodular/multinodular goitre (N = 4894), Hashimoto's thyroiditis with nodules (N = 436), autonomously functioning thyroid nodules (N = 276), toxic nodular goitre ( N = 211). Thyroid nodules detected by palpation or US: — Nodule size: additional author information: patients with calcitonin testing: 3‐45 mm, participants with MTC: 4‐32 mm Number of nodules: additional author information; only information of participants with MTC: 4 solitary nodules; 11 multinodular Sonographic morphology of thyroid nodules: additional author information: only information of participants with MTC: 14 hypo‐echoic, 1 iso‐echoic, 4 microcalcifications, 6 peripheral vascularisation, 4 central vascularisation FNA procedures performed through ultrasound guidance or palpation: additional author information: US guidance Setting: a national healthcare system hospital (outpatient and inpatient) sectors Country: Italy |
||
| Index tests |
Index test: basal and stimulated calcitonin Calcitonin as a triage or add‐on test: ‐ Used calcitonin assay: chemiluminescence assay (Nichols advantage Calcitonin Chemiluminesence assay, San Juan Capistrano, CA) Sensitivity: 1 pg/mL Stimulated calcitonin: yes Indication: basal calcitonin levels > 20 pg/mL and < 100 pg/mL Stimulative: pentagastrin Dose: 0.5 ug/kg Time: 2 and 5 minutes after iv injection Reported and extracted cut‐off values Basal: 10 pg/mL, 20 pg/mL, 50 pg/mL and 100 pg/mL; extracted 10, 20, 50 and 100 pg/mL Stimulated: 100 pg/mL; extracted: 100 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAB in participants with non autonomous nodules exceeding 10 mm in diameter; in selected participants histological examination after surgery, follow‐up Indication surgical treatment: 1) FNAB indicative or suggestive of thyroid malignancy, 2) multinodular autonomous or toxic goitres, 3) large euthyroid goitres causing compression, 4) serum CT levels (basal or PG stimulated) more than 100 pg/mL Type: — Calcitonin negative (N = 5535) Number FNAB: — Number operated: 723 participants Calcitonin positive: (N = 282) Number FNAB: — Number operated: 24 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: ‐; author information: no new MTC case in participants from this series has been recorded at the participating centres. Type: — Duration: — Follow‐up calcitonin positive: in participants with basal CT between 10‐100 pg/mL and negative Pg testing Type: basal CT in participants with basal CT between 10‐20 pg/mL; yearly stimulated CT in participants with basal CT between 20‐100 pg/mL Number with follow‐up: 212 participants Duration: 2‐4 years |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: full article |
||
| Stated aim of study | Quote from publication: "To evaluate the diagnostic accuracy of systematic CT measurement in non‐multiple endocrine neoplasia type 2 patients with nodular thyroid disease." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Rink 2009.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: nodular thyroid disease diagnosed by high‐resolution US Exclusion criteria: additional author information: all participants without thyroid nodules |
||
| Patient characteristics and setting |
Number of participants: 21928 participants Number with NTD: 21928 participants Number with NTD and calcitonin testing: 21928 participants Sex (female%)(N): 76.9% (16857) Age (mean/SD): ‐ range: 8‐97 years MTC: 28 participants Type of thyroid nodules: additional author information; reported for 376 participants with elevated calcitonin level in whom no other reason for the increased calcitonin level could be identified. Thyroid nodules detected by palpation or US: US additional author information; reported for 376 participants with elevated calcitonin level in whom no other reason for the increased calcitonin level could be identified Nodule size: only reported for MTC participants additional author information; reported for 376 participants with elevated calcitonin level in whom no other reason for the increased calcitonin level could be identified Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: — Setting: four sites in central Germany Country: Germany |
||
| Index tests |
Index test: basal and stimulated calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: radioimmunoassays calcitonin‐IRMA (IBL GmbH, Hamburg, Germany) and Calcitonin‐IRMA magnum (Medipan GmbH, Dahlewith/Berlin, Germany) Sensitivity: 0.7 ng/L (IBL), 1.5 ng/L (Medipan) Stimulated calcitonin: yes Indication: if basal CT > 10 ng/L and if renal insufficiency as well as proton pump inhibitor medication could be ruled out Stimulative: pentagastrin (pentagastrin injection BP, Cambridge Laboratiries Ltd., Tyne & Wear, England) Dose: 0.5 ug/kg Time: — Reported and extracted cut‐off values Basal: reported: 10 ng/L; extracted: 10, 15 ng/L Stimulated: reported males: 80 ng/L, females 50 ng/L, extracted 50, 80 ng/L |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: in selected participants histopathological examination after thyroid surgery, follow‐up Indication surgical treatment: an abnormal PGT, a basal CT exceeding 30 ng/L, increasing basal or progressive morphologic alterations during follow‐up Type: — Calcitonin negative: (N = 21073) Number FNA: — Number operated: — Calcitonin positive: (N = 855) Number FNA: — Number operated: 157 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: in participants not having surgery and without renal insufficiency Type: ultrasound and determination of basal CT Number with follow‐up: 214 participants Duration: mean observation 21 months, median 17 months, range 3‐87 months |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: non‐commercial Publication status: full article |
||
| Stated aim of study | Quote from publication: "the calculation and validation of upper limits for basal and stimulated pCT‐Cs in a large patient population with nodular thyroid disease, to distinguish between the subgroups with and without MTC." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Hasselgren 2010.
| Study characteristics | |||
| Patient sampling |
Design: retrospective study Inclusion criteria: participants with non‐toxic nodular goitre Exclusion criteria: participants with missing data or with wrongly registered diagnosis |
||
| Patient characteristics and setting |
Number of participants: 959 participants Number with NTD: 959 participants Number with NTD and calcitonin testing: 702 participants Sex (female%)(N): 84.6% (811) NB of whole study population (N = 959) Age (mean/SD): mean ‐; median 49 years range: 13‐93 years NB of whole study population (N = 959) MTC: 6 participants Type of thyroid nodules: non toxic nodular goitre; solitary nodule (N = 366), multinodular goitre (N = 492), thyroid cyst (N = 76) Thyroid nodules detected by palpation or US: ‐ additional author information: all participants had one or more nodules on ultrasound Nodule size: only reported of MTC participants Number of nodules: — Sonographic morphology of thyroid nodules: — FNA procedures performed through ultrasound guidance or palpation: ultrasound Setting: secondary/tertiary referral centre Country: Denmark |
||
| Index tests |
Index test: basal calcitonin Calcitonin as a triage or add‐on test: — Used calcitonin assay: (1) double‐antibody radioimmunoassay technique (MediLab A/S, Copenhagen, Denmark) (N =668) (2) solid‐phase, enzyme‐labelled, 2‐site chemiluminescent immunometric principle (Immulite 2000, Calcitonin, Siemens Medical Solutions Diagnostics, Erlangen, Germany) (N = 14) Sensitivity: ‐ (1 and 2) Stimulated calcitonin: no Indication: — Stimulative: — Dose: — Time: — Reported and extracted cut‐off values Basal: reported 0.10 ug/L (= 100 ng/L(pg/mL), 0.20 ug/L, 0.50 ug/L). Stimulated: — |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAB, in selected participants histopathological examination after surgery, cross‐linkage with the Danish Thyroid Cancer Database Indication surgical treatment: based on the composition of the following variables: (1) clinical evaluation including age, comorbidity, thyroid size, suspicion of malignancy; (2) sonographic appearance; (3) result of FNAB; (4) result of serum calcitonin measurement; (5) patient preference Type: — Calcitonin negative: (N = 663) Number FNAB: — Number operated: 223 participants Calcitonin positive: (N = 39) Number FNAB: — Number operated: 23 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: all participants Type: cross linkage with Danish Thyroid Cancer Database Number with follow‐up: 663 participants Duration: median follow‐up 7 years (range 3‐10 years) (whole study population). Follow‐up calcitonin positive: all participants Type: cross linkage with Danish Thyroid Cancer Database Number with follow‐up: 39 participants Duration: median follow‐up 7 years (range 3‐10 years) (whole study population) |
||
| Comparative | |||
| Publication details | Language of publication: English Funding: — Publication status: full article | ||
| Stated aim of study | Quote from publication: "To estimate the validity of serum calcitonin for detection of MTC in a consecutive population of patients with nontoxic nodular goitre, living in a mild to moderate iodine‐deficient area." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | Yes | ||
| Unclear | |||
Herrmann 2010.
| Study characteristics | |||
| Patient sampling |
Design: retrospective cohort study Inclusion criteria: participants with nodular thyroid disease found by sonography, living in central Germany (an area with endemic goitre due to previous iodine deficiency) Exclusion criteria: known elevation of hCt, Graves' disease, autoimmune thyroid disease |
||
| Patient characteristics and setting |
Number of participants: 1007 participants Number with NTD: 1007 participants Number with NTD and calcitonin testing: 1007 participants Sex (female%)(N): 56.3% (567) Age (mean/SD): 55 + 14, range: — MTC: 2 participants Type of thyroid nodules: additional author information: only available participants with elevated bCT Thyroid nodules detected by palpation or US: US Nodule size: additional author information: patients with calcitonin testing: between 5 mm to 40 mm Number of nodules: additional author information: only available participants with elevated bCT Sonographic morphology of thyroid nodules: additional author information: only available participants with elevated bCT FNA procedures performed through ultrasound guidance or palpation: additional author information: only available participants with elevated bCT Setting: Division of Endocrinology, Technology Center Bochum, Germany Country: Germany |
||
| Index tests |
Index test: basal and stimulated hCT Calcitonin as a triage or add‐on test: — Used calcitonin assay: solid‐phase, enzyme labelled, two site chemiluminescent assay, with the Immulite 2000, (Siemens Immulite 2000, Munich, Germany) Sensitivity: — Stimulated calcitonin: Yes Indication: basal CT > 10 and < 100 pg/mL Stimulative: pentagastrin (Peptavlon; Laboratoires SERB, Paris, France) Dose: 0.5 ug/kg bodyweight Time: 2 and 5 minutes after injection Reported and extracted cut‐off values Basal: 10 pg/mL; extracted: 10, 15, 20, 30, 50 and 100 pg/mL Stimulated: 100 pg/mL; extracted:100 and 200 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAB, in selected participants histopathological examination after surgery, follow‐up Indication surgical treatment: elevated stimulated hCT > 100 pg/mL Type: total thyroidectomy, with careful dissection of both recurrent nerves and a systematic microdissection of the central lymph node compartments along both nerves from the upper thoracic outlet up to the larynx Calcitonin negative (N = 990) Number FNAC: — Number operated: — Calcitonin positive: (N = 17) Number FNAB: 2 participants Number operated: 5 participants |
||
| Flow and timing |
Calcitonin negative: — Type: — Duration: — Calcitonin positive: participants with stimulated hCT < 100 pg/mL Type: re‐testing Number with follow‐up: 12 participants Duration: — |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: other funding Publication status: full article |
||
| Stated aim of study | Quote from publication: "Shedding further light on the hCT measurement and its testing after pentagastrin stimulation in patients with thyroid nodule disease." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Schneider 2012.
| Study characteristics | |||
| Patient sampling |
Design: cohort study Inclusion criteria: participants diagnosed with thyroid nodules > 2 mm based on the spatial resolution of ultrasound equipment Exclusion criteria: participants referred with elevated or previously determined CT values, MTC or a family history of MTC, renal insufficiency, bacterial infection, alcohol abuse, proton‐pump inhibitor therapy, Graves'disease or autoimmune thyroid disease |
||
| Patient characteristics and setting |
Number of participants: 11270 participants Number with NTD: 11,270 participants Number with NTD and calcitonin testing: 11,270 participants Sex (female%)(N): — Age (mean/SD): — range: — MTC: 12 participants Type of thyroid nodules: — Thyroid nodules detected by palpation or US: ultrasound Nodule size: only reported of MTC and PTC participants Number of nodules: — Sonographic morphology of thyroid nodules: only reported of participants with elevated basal CT levels FNA procedures performed through ultrasound guidance or palpation: — Setting: — Country: Germany |
||
| Index tests |
Calcitonin as a triage or add‐on test: — Index test: basal and stimulated calcitonin Used calcitonin assay: solid‐phase, enzyme labelled, two‐site chemiluminescent assay with Immulite 2000 (Siemens Immulite 2000, Munich, Germany) Sensitivity: — Stimulated calcitonin: yes Indication: bCT > 13 and < 100 pg/mL (if PG was available and the participants physical condition allowed testing) Stimulative: pentagastrin (pentagastrin injection BP, Ireland, UK) Dose: 0.5 ug/kg bodyweight Time: 2 and 5 minutes after injection Reported and extracted cut‐off values Basal: reported 13 pg/mL; extracted: 13, 15, 20, 30, 50, 100 pg/mL Stimulated: reported 100 pg/mL; extracted: 100, 200 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: histopathological examination after thyroid surgery, follow‐up Indication surgical treatment: 1) basal CT > 100 pg/mL, PG‐stimulated CT > 100 pg/mL, 3) suspicious thyroid nodules based on the participants history, sonography (hypoechogenicity, irregular margins, microcalcifications) or scintigraphy (cold nodules have been consistently associated with malignancy) Type: — Calcitonin negative (N = 11238) Number FNAC: — Number operated: — Calcitonin positive: (N = 32) Number FNAC: — Number operated: 18 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: participants with no sCT > 100 pg/mL, participants with no sCT performed Type: repeated bCT testing Number with follow‐up: 10 participants Duration: mean 7 months |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: no conflicts of interest Publication status: full article |
||
| Stated aim of study | Quote from publication: "The positive predictive value (PPV) of a slightly elevated basal calcitonin for the diagnosis of medullary thyroid cancer is still under debate." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Giovanella 2012.
| Study characteristics | |||
| Patient sampling |
Design: prospective cohort study Inclusion criteria: participants with nodular thyroid disease Exclusion criteria: pulmonary or pancreatic tumours, kidney failure, Graves' disease, autonomously functioning thyroid nodules, autoimmune thyroid diseases, sepsis, alcohol abuse, smoking or the use of proton‐pump inhibitor therapy in the last month |
||
| Patient characteristics and setting |
Number of participants: 1236 participants Number with NTD: 1236 participants Number with NTD and calcitonin testing: 1236 participants Sex (female%)(N): 54.5% (674) Age (mean/SD): 53 + 17 years range: — MTC: 2 participants Type of thyroid nodules: — Thyroid nodules detected by palpation or US: additional author information: both: 587 participants palpable nodules; 649 participants nodules on US. Nodule size: additional author information: available for participants with elevated bCT Number of nodules: — Sonographic morphology of thyroid nodules: additional author information; available for participants with elevated bCT FNA procedures performed through ultrasound guidance or palpation: additional author information: FNA procedures performed through US. Setting: — Country: Switzerland |
||
| Index tests |
Calcitonin as a triage or add‐on test: — Index test: basal and stimulated calcitonin Used calcitonin assay: Immulite 2000XPi platform (Siemens Healthcare Diagnostics, Erlangen, Germany) Sensitivity: 2 pg/mL Stimulated calcitonin: yes Indication: participants with true high CT value Stimulative: pentagastrin (pentagastrin injection BP, Cambridge Laboratories, Wallsend, UK) Dose: 0.5 ug/kg Time: 2 and 5 minutes after injection Reported and extracted cut‐off values Basal: reported: 10 pg/mL; extracted: 10, 15, 20, 30, 50, 100 pg/mL Stimulated: reported 100 pg/mL; extracted: 100, 200 pg/mL |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAC, in selected participants histopathological examination after surgery, follow‐up Indication surgical treatment: basal and/or stimulated CT levels > 100 pg/mL, participants with indeterminate, suspicious or malignant cytological outcome Typeof surgical treatment: thyroidectomy with bilateral dissection of the central compartment (in participants with basal and/or stimulated CT levels > 100 pg/mL) Calcitonin negative (N = 1222) Number FNAC: — Number operated: additional author information: data available in 854 cases, 163 participants operated. Calcitonin positive: (N = 14) Number FNAC: 14 participants Number operated: 7 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: additional author information : N = 385 Type: — Duration: — Follow‐up calcitonin positive: participants with benign cytology Type: US, basal CT and PCT Number with follow‐up: 7 participants Duration: 24‐35 months |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: other funding Publication status: full article |
||
| Stated aim of study | Quote from publication: "To prospectively evaluate the role of routine PCT measurement in detecting MTC among patients with thyroid nodules, and to assess the potential improvement provided by adding PCT to increased basal CT." | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | No | ||
| High | |||
Grani 2012.
| Study characteristics | |||
| Patient sampling |
Design: additional author information: cross‐sectional, retrospective observational study. Inclusion criteria: at least one discrete nodular lesion of the thyroid or a multinodular goitre and were referred to our institution to undergo FNAC because of clinical or ultrasonographic suspicion (irregular margins, micro calcifications, and chaotic pattern vascularisation) Exclusion criteria: additional author information: No exclusion criteria were applied. |
||
| Patient characteristics and setting |
Number of participants: 1073 participants Number with NTD: 1073 participants Number with NTD and calcitonin testing: 1073 participants Sex (female%)(N): 83.2% (893) Age (mean/SD): 55.7 + 13.4 years range: — MTC: 2 participants Type of thyroid nodules: one discrete nodular lesion of the thyroid or a multinodular goitre with clinical or ultrasonographic suspicion (irregular margins, microcalcifications, and chaotic pattern vascularisation) Thyroid nodules detected by palpation or US: both additional author information: all participants had nodules on ultrasound, the prevalence of palpable nodules was not recorded. Nodule size: additional author information: mean nodule volume 1.49 mL, maximum diameter was 14.66 mm + 7.45 mm Number of nodules: additional author information: only available for MTC participants. Sonographic morphology of thyroid nodules: irregular margins, microcalcifications, and chaotic pattern vascularisation FNA procedures performed through ultrasound guidance or palpation: ultrasonography Setting: The Thyroid Center of Sapienza University of Rome Country: Italy |
||
| Index tests |
Calcitonin as a triage or add‐on test: — Index test: basal calcitonin Used calcitonin assay: automated two‐site immuno chemiluminometric assay Sensitivity: 2 pg/mL Stimulated calcitonin: no Indication: — Stimulative: — Dose: — Time: — Reported and extracted cut‐off values Basal: reported: 10 pg/mL; extracted: 10 pg/mL Stimulated: — |
||
| Target condition and reference standard(s) |
Target condition: MTC Reference standards: FNAC, in selected participants histological examination after total thyroidectomy, follow‐up Indication surgical treatment: — Type of surgical treatment: total thyroidectomy Calcitonin negative: (N = 1032) Number FNAC: 1032 participants Number operated: additional author information: N = 64 Calcitonin positive: (N = 41) Number FNAC: 41 participants Number operated: 3 participants |
||
| Flow and timing |
Follow‐up calcitonin negative: — Type: — Duration: — Follow‐up calcitonin positive: participants with unexplained hypercalcitonaemia Type: basal CT Number with follow‐up: 34 participants Duration: 12‐36 months |
||
| Comparative | |||
| Publication details |
Language of publication: English Funding: other funding Publication status: full article |
||
| Stated aim of study | Quote from publication: "To evaluate the basal CT values in patients with and without thyroid autoimmunity" | ||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Did all patients receive the reference standard? | Yes | ||
| High | |||
—: denotes not reported.
(b/h/s) CT: (basal/serum/stimulated) calcitonin; FNA: fine needle aspiration; FNAB: fine needle aspiration biopsy; FNAC: fine needle aspiration cytology; IRMA: immunoradiometric assay; MEN2: multiple endocrine neoplasia type 2; MTC: medullary thyroid carcinoma; NTD: nodular thyroid disease; PCT: procalcitonin; PG: pentagastrin; pg/mL: picograms per millilitre; RIA: radio immunometric assay; SD: standard deviation;TSH: thyroid‐stimulating hormone; US: ultrasonography.
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Lepage 1992 | Only meeting abstract available |
| Niccoli 1997 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Kaserer 1998 | Included participants with thyroid disease; number of participants with nodular thyroid disease not specified. (partially) same study population as Vierhapper 2005 |
| Mariss 2001 | abstrOnly meeting abstract available |
| Iacobone 2002 | Included participants with thyroid disease; number of participants with nodular thyroid disease not specified |
| Mirallie 2004 | Included participants with thyroid disease; number of participants with nodular thyroid disease not specified |
| Gibelin 2005 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Papi 2010 | Calcitonin testing in isthmic thyroid nodules |
| Lipp 2011 | Only meeting abstract available |
| Chambon 2011 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Marui 2012 | Only meeting abstract available |
| Wiedemann 2012 | Only meeting abstract available |
| Zaplatnikov 2012 | Only meeting abstract available |
| Ubl 2013 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Rosario 2013 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Krebs 2014 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Ubl 2014 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Bostico 2015 | Only meeting abstract available |
| Reyes 2015 | Evaluation of preoperative calcitonin determination (not part of this review) |
| Simon 2015 | Only meeting abstract available |
| Cavallo 2015 | Only meeting abstract available |
| Simeakis 2016 | Only meeting abstract available |
| Storani 2016 | Only meeting abstract available |
| Sukhov 2017 | Only meeting abstract available; (partially) same study population as Wiedemann 2012 |
| Sukhov 2018 | Only meeting abstract available; (partially) same study population as Wiedemann 2012 |
Characteristics of studies awaiting classification [ordered by study ID]
Giovanella 2018.
| Study characteristics | |||
| Patient sampling | 2705 participants | ||
| Patient characteristics and setting | Patients with thyroid nodules | ||
| Index tests | Serum procalcitonin and serum calcitonin (basal and stimulated) in patients with elevated procalcitonin or patients who had a surgical indication | ||
| Target condition and reference standard(s) | Target condition: medullary thyroid carcinoma Reference standard: histopathological examination |
||
| Flow and timing | Follow‐up procalcitonin‐positive participants: surgery in 8 participants, follow‐up in 1 participant Follow‐up procalcitonin‐negative participants: surgery in 369 participants |
||
| Comparative | |||
| Notes | |||
Henry 1995.
| Study characteristics | |||
| Patient sampling | 2975 participants | ||
| Patient characteristics and setting | Patients seen for thyroid exploration | ||
| Index tests | Calcitonin testing (basal and stimulated) | ||
| Target condition and reference standard(s) | Target condition: medullary thyroid carcinoma Reference standard: histopathological examination and follow‐up |
||
| Flow and timing | Follow‐up calcitonin‐positive participants: operation in 34 participants, follow‐up in 14 participants Follow‐up calcitonin‐negative participants: operation in 1446 of 2927 participants |
||
| Comparative | |||
| Notes | |||
López‐Guzmán 2002.
| Study characteristics | |||
| Patient sampling | 907 participants | ||
| Patient characteristics and setting | Patients with nodular thyroid disease (nontoxic multinodular goitre; nontoxic uninodular goitre; toxic multinodular goitre; autonomously functioning thyroid nodule; nodular Hashimoto thyroiditis; nodular Graves' disease; nodular subacute thyroiditis) | ||
| Index tests | Calcitonin testing (basal and stimulated) | ||
| Target condition and reference standard(s) | Target condition: medullary thyroid carcinoma Reference standard: histopathological examination |
||
| Flow and timing | Follow‐up calcitonin‐positive participants: operation in 6 participants Follow‐up calcitonin‐negative participants: — |
||
| Comparative | |||
| Notes | |||
Rosario 2016.
| Study characteristics | |||
| Patient sampling | 1023 participants | ||
| Patient characteristics and setting | Patients with nodular thyroid disease without an indication for FNA or with benign cytology, and without an indication for surgery | ||
| Index tests | Calcitonin testing (basal and stimulated) | ||
| Target condition and reference standard(s) | Target condition: medullary thyroid carcinoma Reference standard: histopathological examination and follow‐up |
||
| Flow and timing | Follow‐up calcitonin‐positive participants: operation in 17 participants, follow‐up in 5 participants Follow‐up calcitonin‐negative participants: — |
||
| Comparative | |||
| Notes | |||
Shong 1996.
| Study characteristics | |||
| Patient sampling | 1048 participants | ||
| Patient characteristics and setting | Patients with thyroid nodules | ||
| Index tests | Calcitonin testing (basal and stimulated) | ||
| Target condition and reference standard(s) | Target condition: medullary thyroid carcinoma Reference standard: histopathological examination |
||
| Flow and timing | Follow‐up calcitonin‐positive participants: operation in 3 participants Follow‐up calcitonin‐negative participants: — |
||
| Comparative | |||
| Notes | |||
Turk 2017.
| Study characteristics | |||
| Patient sampling | 640 participants | ||
| Patient characteristics and setting | Patients with nodular thyroid disease | ||
| Index tests | Calcitonin testing (basal and stimulated) | ||
| Target condition and reference standard(s) | Target condition: medullary thyroid carcinoma Reference standard: histopathological examination and follow‐up. |
||
| Flow and timing | Follow‐up calcitonin‐positive participants: surgery in 19 participants, follow‐up in 4 participants Follow‐up calcitonin‐negative participants: — |
||
| Comparative | |||
| Notes | |||
FNA: fine needle aspiration.
Differences between protocol and review
No separate meta‐analyses for calcitonin testing as a triage or as an add‐on test was performed because all included studies were considered as performing calcitonin testing as a triage test. A large part of planned heterogeneity analyses could not be performed due to limited reporting of studies and small number of studies.
Contributions of authors
Hans HG Verbeek (HHGV): protocol draft, search strategy development, trial selection, data extraction, data analysis, data interpretation and review draft. Jan Willem B de Groot (JWBdG): protocol draft, search strategy development, trial selection, data extraction, data analysis, data interpretation and review draft. Wim J Sluiter (WJS): protocol draft, data extraction, data analysis, data interpretation and review draft. Anneke C Muller Kobold (ACMK): protocol draft, data interpretation and review draft. Edwin R van den Heuvel (ERH): data analysis, data interpretation and review draft John TM Plukker (JTMP): protocol draft, data interpretation and review draft. Thera P Links (TPL): protocol draft, search strategy development, trial selection, data extraction, data analysis, data interpretation and review draft.
Sources of support
Internal sources
University Medical Center Groningen’s Graduate School of Medical Sciences (GUIDE), Netherlands.
External sources
No sources of support supplied
Declarations of interest
HHGV: none known. JWBdG: received personal fees for advisory roles from Bristol‐Myers Squibb (BMS), Merck Sharp & Dohme (MSD), Pierre Fabre and Shire. WJS: none known. ACMK: none known. ERH: none known. JTMP: none known. TPL: none known.
New
References
References to studies included in this review
Costante 2007 {published data only}
- Costante G, Meringolo D, Durante C, Bianchi D, Nocera M, Tumino S, et al. Predictive value of serum calcitonin levels for preoperative diagnosis of medullary thyroid carcinoma in a cohort of 5817 consecutive patients with thyroid nodules. Journal of Clinical Endocrinology and Metabolism 2007;92(2):450‐5. [DOI] [PubMed] [Google Scholar]
Elisei 2004 {published data only}
- Elisei R, Bottici V, Luchetti F, Coscio G, Romei C, Grasso L, et al. Impact of routine measurement of serum calcitonin on the diagnosis and outcome of medullary thyroid cancer: experience in 10,864 patients with nodular thyroid disorders. Journal of Clinical Endocrinology and Metabolism 2004;89(1):163‐8. [DOI] [PubMed] [Google Scholar]
- Pacini F, Fontanelli M, Fugazzola L, Elisei R, Romei C, Coscio G, et al. Routine measurement of serum calcitonin in nodular thyroid diseases allows the preoperative diagnosis of unsuspected sporadic medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 1994;78(4):826‐9. [DOI] [PubMed] [Google Scholar]
Giovanella 2012 {published data only}
- Giovanella L, Verburg F A, Imperiali M, Valabrega S, Trimboli P, Ceriani L. Comparison of serum calcitonin and procalcitonin in detecting medullary thyroid carcinoma among patients with thyroid nodules. Clinical Chemistry and Laboratory Medicine: CCLM / FESCC 2012;50:1‐5. [DOI] [PubMed] [Google Scholar]
Grani 2012 {published data only}
- Grani G, Nesca A, Sordo M, Calvanese A, Carbotta G, Bianchini M, et al. Interpretation of serum calcitonin in patients with chronic autoimmune thyroiditis. Endocrine‐related Cancer 2012;19(3):345‐9. [PUBMED: 22399011] [DOI] [PubMed] [Google Scholar]
Hahm 2001 {published data only}
- Hahm JR, Lee MS, Min YK, Lee MK, Kim KW, Nam S J, et al. Routine measurement of serum calcitonin is useful for early detection of medullary thyroid carcinoma in patients with nodular thyroid diseases. Thyroid: official journal of the American Thyroid Association 2001;11(1):73‐80. [DOI] [PubMed] [Google Scholar]
Hasselgren 2010 {published data only}
- Hasselgren M, Hegedus L, Godballe C, Bonnema S J. Benefit of measuring basal serum calcitonin to detect medullary thyroid carcinoma in a Danish population with a high prevalence of thyroid nodules. Head & Neck 2010;32(5):612‐8. [DOI] [PubMed] [Google Scholar]
Hatzl‐Griesenhofer 2002 {published data only}
- Hatzl‐Griesenhofer M, Pichler R, Bogner S, Wolfl S, Weinhausel A, Huber H, et al. Results of calcitonin screening in a Central Upper Austrian Region. Journal of Endocrine Genetics 2002;3(2):75‐85. [Google Scholar]
Herrmann 2010 {published data only}
- Herrmann B L, Schmid KW, Goerges R, Kemen M, Mann K. Calcitonin screening and pentagastrin testing: predictive value for the diagnosis of medullary carcinoma in nodular thyroid disease. European Journal of Endocrinology / European Federation of Endocrine Societies 2010;162(6):1141‐5. [DOI] [PubMed] [Google Scholar]
Karanikas 2004 {published data only}
- Karanikas G, Moameni A, Poetzi C, Zettinig G, Kaserer K, Bieglmayer C, et al. Frequency and relevance of elevated calcitonin levels in patients with neoplastic and nonneoplastic thyroid disease and in healthy subjects. Journal of Clinical Endocrinology and Metabolism 2004;89(2):515‐9. [DOI] [PubMed] [Google Scholar]
Ozgen 1999 {published data only}
- Ozgen AG, Hamulu F, Bayraktar F, Yilmaz C, Tuzun M, Yetkin E, et al. Evaluation of routine basal serum calcitonin measurement for early diagnosis of medullary thyroid carcinoma in seven hundred seventy‐three patients with nodular goitre. Thyroid : official journal of the American Thyroid Association 1999;9(6):579‐82. [DOI] [PubMed] [Google Scholar]
Papi 2006 {published data only}
- Papi G, Corsello SM, Cioni K, Pizzini AM, Corrado S, Carapezzi C, et al. Value of routine measurement of serum calcitonin concentrations in patients with nodular thyroid disease: A multicenter study. Journal of Endocrinological Investigation 2006;29(5):427‐37. [DOI] [PubMed] [Google Scholar]
Rieu 1995 {published data only}
- Rieu M, Lame MC, Richard A, Lissak B, Sambort B, Vuong‐Ngoc P, et al. Prevalence of sporadic medullary thyroid carcinoma: the importance of routine measurement of serum calcitonin in the diagnostic evaluation of thyroid nodules. Clinical Endocrinology 1995;42(5):453‐60. [DOI] [PubMed] [Google Scholar]
Rink 2009 {published data only}
- Rink T, Truong PN, Schroth HJ, Diener J, Zimny M, Grunwald F. Calculation and validation of a plasma calcitonin limit for early detection of medullary thyroid carcinoma in nodular thyroid disease. Thyroid : official journal of the American Thyroid Association 2009;19(4):327‐32. [DOI] [PubMed] [Google Scholar]
Schneider 2012 {published data only}
- Schneider C, Kobe C, Schmidt M, Kahraman D, Malchau G, Faust M, et al. Calcitonin screening in patients with thyroid nodules. Diagnostic value. Nuklearmedizin. Nuclear Medicine 2012;51(6):228‐33. [DOI] [PubMed] [Google Scholar]
Schuetz 2006 {published data only}
- Schuetz M, Beheshti M, Oezer S, Novotny C, Paul M, Hofmann A, et al. Calcitonin measurements for early detection of medullary thyroid carcinoma or its premalignant conditions in Hashimoto's thyroiditis. Anticancer Research 2006;26(1B):723‐7. [PubMed] [Google Scholar]
Vierhapper 2005 {published data only}
- Vierhapper H, Niederle B, Bieglmayer C, Kaserer K, Baumgartner‐Parzer S. Early diagnosis and curative therapy of medullary thyroid carcinoma by routine measurement of serum calcitonin in patients with thyroid disorders. Thyroid: official journal of the American Thyroid Association 2005;15(11):1267‐72. [DOI] [PubMed] [Google Scholar]
- Vierhapper H, Raber W, Bieglmayer C, Kaserer K, Weinhausl A, Niederle B. Routine measurement of plasma calcitonin in nodular thyroid diseases. TJournal of Clinical Endocrinology and Metabolism 1997;82(5):1589‐93. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bostico 2015 {published data only}
- Bostico ST, Storani M, Subies F, Musich M. Routine serum calcitonin measurement in thyroid nodular disease in a series of 724 patients. Thyroid 2015;25:A257. [PubMed] [Google Scholar]
Cavallo 2015 {published data only}
- Cavallo AC, Brenzoni P, Iorcansky S, Lencioni M, San Roman A, Guerrieri J, et al. Usefulness of routine measurement of basal serum calcitonin in nodular thyroid disease for the detection of medullary thyroid carcinoma. Thyroid 2015;25:A260. [Google Scholar]
Chambon 2011 {published data only}
- Chambon G, Alovisetti C, Idoux‐Louche C, Reynaud C, Rodier M, Guedj AM, et al. The use of preoperative routine measurement of basal serum thyrocalcitonin in candidates for thyroidectomy due to nodular thyroid disorders: results from 2733 consecutive patients. Journal of Clinical Endocrinology and Metabolism 2011;96(1):75‐81. [DOI] [PubMed] [Google Scholar]
Gibelin 2005 {published data only}
- Gibelin H, Essique D, Jones C, Levillain P, Marechaud R, Kraimps JL. Increased calcitonin level in thyroid nodules without medullary carcinoma. British Journal of Surgery 2005;92(5):574‐8. [DOI] [PubMed] [Google Scholar]
Iacobone 2002 {published data only}
- Iacobone M, Niccoli‐Sire P, Sebag F, Micco C, Henry JF. Can sporadic medullary thyroid carcinoma be biochemically predicted? Prospective analysis of 66 operated patients with elevated serum calcitonin levels. World Journal of Surgery 2002;26(8):886‐90. [DOI] [PubMed] [Google Scholar]
Kaserer 1998 {published data only}
- Kaserer K, Scheuba C, Neuhold N, Weinhausel A, Vierhapper H, Haas O A, et al. C‐cell hyperplasia and medullary thyroid carcinoma in patients routinely screened for serum calcitonin. American Journal of Surgical Pathology 1998;22(6):722‐8. [DOI] [PubMed] [Google Scholar]
Krebs 2014 {published data only}
- Krebs M. Refining calcium test for the diagnosis of medullary thyroid cancer: Cutoffs, procedures, and safety. Austrian Journal of Clinical Endocrinology and Metabolism 2014;7(3):106. [DOI] [PubMed] [Google Scholar]
Lepage 1992 {published data only}
- Lepage M, Bounaud MP, Breton I, Bouinpineau MH, Fieuzal S, Kraimps JL, et al. Calcitonin determination in assessment of cold thyroid‐nodules ‐ prospective‐study on 195 patients. Revue De Medecine Interne 1992;13:S364. [Google Scholar]
Lipp 2011 {published data only}
- Lipp RW, Grohs S, Mader J, Obermaier‐Pitsch B, Pieber T, Piswanger‐Soelkner J. Routine calcitonin screening for medullary thyroid cancer detection in an Austrian population. European Journal of Nuclear Medicine and Molecular Imaging 2011;38:S100. [Google Scholar]
Mariss 2001 {published data only}
- Mariss P, Kammeier A, Thermann M, Emrich D, Raute‐Kreinsen U. Calcitonine screening in patients with nodular goitre for detection of medullary thyroid cancer. European Journal of Nuclear Medicine 2001; Vol. 28, issue 8:1043.
Marui 2012 {published data only}
- Marui S, Danilovic DS, Camargo RY, Lando VS, Knobel M, Batista MC. [The utility of serum calcitonin measurement in thyroid nodule evaluation: experience of a single university hospital in Sao Paulo, Brazil]. Endocrine Reviews 2012;33(Suppl 1). [DOI: 10.1093/edrv/33.supp.1] [DOI] [Google Scholar]
Mirallie 2004 {published data only}
- Mirallie E, Iacobone M, Sebag F, Henry JF. Results of surgical treatment of sporadic medullary thyroid carcinoma following routine measurement of serum calcitonin. European Journal of Surgical Oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2004;30(7):790‐5. [DOI] [PubMed] [Google Scholar]
Niccoli 1997 {published data only}
- Niccoli P, Wion‐Barbot N, Caron P, Henry JF, Micco C, Saint Andre JP, et al. Interest of routine measurement of serum calcitonin: study in a large series of thyroidectomized patients. the French medullary study group. Journal of Clinical Endocrinology and Metabolism 1997;82(2):338‐41. [DOI] [PubMed] [Google Scholar]
Papi 2010 {published data only}
- Papi G, Rossi G, Corsello SM, Corrado S, Fadda G, Donato C, et al. Nodular disease and parafollicular C‐cell distribution: results from a prospective and retrospective clinico‐pathological study on the thyroid isthmus. European Journal of Endocrinology / European Federation of Endocrine Societies 2010;162(1):137‐43. [PUBMED: 19793761] [DOI] [PubMed] [Google Scholar]
Reyes 2015 {published data only}
- Reyes A, Zunino A, Garcia Roel V, Ilera V, Viale F, Dios A, et al. Our experience in serum calcitonin before thyroid surgery. Thyroid 2015;25:A264. [Google Scholar]
Rosario 2013 {published data only}
- Rosario PW, Penna GC, Brandao K, Souza BE. Usefulness of preoperative serum calcitonin in patients with nodular thyroid disease without suspicious history or cytology for medullary thyroid carcinoma. Arquivos Brasileiros de Endocrinologia e Metabologia 2013;57(4):312‐6. [DOI] [PubMed] [Google Scholar]
Simeakis 2016 {published data only}
- Simeakis G, Patinioti I, Mitropoulou M, Anagnostou E, Sapounas S, Zapanti E, et al. Association of serum calcitonin levels with multinodular thyroid disease: 10‐year single center experience. European Thyroid Journal 2016;5:129. [Google Scholar]
Simon 2015 {published data only}
- Simon D, Beindorf M, Kornely E, Farahati J, Gorges R. Calcitonin screening of nodular goiter in routine diagnostic procedures‐Analysis of more than 12,000 cases. Langenbeck's Archives of Surgery 2015;400(7):859‐60. [Google Scholar]
Storani 2016 {published data only}
- Storani ME, Bostico ST, Subies FH, Musich M, Sabelli V, Lopez F, et al. [Routine serum calcitonin measurement in thyroid nodular disease in a series of 1021 patients]. Endocrine Reviews 2016;37(Suppl 1). [DOI: 10.1093/edrv/37.supp.1] [DOI] [Google Scholar]
Sukhov 2017 {published data only}
- Sukhov V, Kirichenko P, Marin A, Wiedemann W, Zaplatnikov K. Serum calcitonin increase‐guided evaluation of MTC in patients with multinodal goiter and correlation with Tc99m‐DMSA (V) scintigraphy. European Journal of Nuclear Medicine and Molecular Imaging 2017;44(2):S653. [Google Scholar]
Sukhov 2018 {published data only}
- Sukhov VY, Zaplatnikow K. Serum calcitonin increase‐guided evaluation of MTC in patients with multinodal goiter. Nuklearmedizin 2018;57(2):A81‐2. [Google Scholar]
Ubl 2013 {published data only}
- Ubl P, Marculescu R, Scheuba C, Niederle B, Li S. Comparison of pentagastrin and calcium stimulation test for preoperative diagnosis of medullary thyroid carcinoma in patients with nodular goiter. European Journal of Nuclear Medicine and Molecular Imaging 2013;40:S355. [Google Scholar]
Ubl 2014 {published data only}
- Ubl P, Niederle B, Scheuba C, Krebs M, Gessl A, Hacker M, et al. Comparison of pentagastrin and calcium stimulation test for preoperative diagnosis of medullary thyroid carcinoma. European Journal of Nuclear Medicine and Molecular Imaging 2014;41:S459. [Google Scholar]
Wiedemann 2012 {published data only}
- Wiedemann W, Soukhov V, Zaplatnikov K. Serum calcitonin increase‐guided evaluation of MTC in patients with multinodal goiter. European Thyroid Journal 2012;1:139. [Google Scholar]
Zaplatnikov 2012 {published data only}
- Zaplatnikov K, Soukhov V. Nuclear medicine methods and prognostic significance of calcitonin and pentagastrin test at diagnosis of sporadic MTC. European Journal of Nuclear Medicine and Molecular Imaging 2012;39:S594. [Google Scholar]
References to studies awaiting assessment
Giovanella 2018 {published data only}
- Giovanella L, Imperiali M, Piccardo A, Taborelli M, Verburg FA, Daurizio F, et al. Procalcitonin measurement to screen medullary thyroid carcinoma: a prospective evaluation in a series of 2705 patients with thyroid nodules. European Journal of Clinical Investigation 2018;48(6):e12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 1995 {published data only}
- Henry JF, Denizot A, Niccoli P, Gramatica L, Conte‐Devolx B, Micco C. Preoperative diagnosis of sporadic medullary thyroid carcinoma. Interest of routine measurement of serum calcitonin [Depistage des cancers medullaires sporadiques de la thyroide par le dosage systematique de la calcitonine]. Lyon Chirurgical 1995;91(6):467‐72. [Google Scholar]
- Henry JF, Denizot A, Puccini M, Niccoli P, Conte‐Devolx B, Fie Micco C. Early diagnosis of sporadic medullary cancer of the thyroid: contribution of routine calcitonin assay [Diagnostic precoce des cancers medullaires sporadiques de la thyroide: Interet du dosage systematique de la calcitonine]. Presse Medicale 1996;25(33):1583‐8. [PubMed] [Google Scholar]
López‐Guzmán 2002 {published data only}
- López‐Guzmán A, Escola CA, Andia VM, Arranz A, Garcia B, Campo AG. Routine calcitonin measurement in nodular thyroid disease [Determinacion sistematica de calcitonina en la enfermedad nodular tiroidea]. Endocrinologia y Nutricion 2002;49(7):222‐6. [Google Scholar]
Rosario 2016 {published data only}
- Rosario PW, Calsolari MR. Usefulness of serum calcitonin in patients without a suspicious history of medullary thyroid carcinoma and with thyroid nodules without an indication for fine‐needle aspiration or with benign cytology. Hormone and Metabolic Research 2016;48(6):372‐6. [DOI] [PubMed] [Google Scholar]
Shong 1996 {published data only}
- Shong YK, Choi CS, Park HY, Cho BY. Clinical significance of routine measurement of serum calcitonin in Korean patients with thyroid nodules as a screening test of sporadic thyroid medullary carcinoma. Journal of Korean Society of Endocrinology 1996;11:11‐7. [Google Scholar]
Turk 2017 {published data only}
- Turk Y, Makay O, Ozdemir M, Ertunc G, Demir B, Icoz G, et al. PMC5378556; Routine calcitonin measurement in nodular thyroid disease management: is it worthwhile?. Annals of Surgical Treatment and Research 2017;92(4):173‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abraham 2009
- Abraham D, Gault PM, Hunt J, Bentz J. Calcitonin estimation in neck lymph node fine‐needle aspirate fluid prevents misinterpretation of cytology in patients with metastatic medullary thyroid cancer. Thyroid 2009 Sep;19(9):1015‐6. [DOI] [PubMed] [Google Scholar]
Alevizaki 2012
- Alevizaki M, Saltiki K, Rentziou G, Papathoma A, Sarika L, Vasileiou V, et al. Medullary thyroid carcinoma: the influence of policy changing in clinical characteristics and disease progression. European journal of Endocrinology / European Federation of Endocrine Societies 2012;167(6):799‐808. [PUBMED: 22989468] [DOI] [PubMed] [Google Scholar]
Baudin 1999
- Baudin E, Bidart JM, Rougier P, Lazar V, Ruffie P, Ropers J, et al. Screening for multiple endocrine neoplasia type 1 and hormonal production in apparently sporadic neuroendocrine tumors. Journal of Clinical Endocrinology and Metabolism 1999;84(1):69‐75. [DOI] [PubMed] [Google Scholar]
Bieglmayer 2002
- Bieglmayer C, Niederle B, Vierhapper H. Interference causes false high calcitonin levels with a commercial assay. Journal of Endocrinological Investigation 2002;25(2):197. [DOI] [PubMed] [Google Scholar]
Boi 2007
- Boi F, Maurelli I, Pinna G, Atzeni F, Piga M, Lai ML, et al. Calcitonin measurement in wash‐out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 2007;92(6):2115‐8. [DOI] [PubMed] [Google Scholar]
Borget 2007
- Borget I, Pouvourville G, Schlumberger M. Editorial: Calcitonin determination in patients with nodular thyroid disease. Journal of Clinical Endocrinology and Metabolism 2007;92(2):425‐7. [DOI] [PubMed] [Google Scholar]
Bugalho 2005
- Bugalho MJ, Santos JR, Sobrinho L. Preoperative diagnosis of medullary thyroid carcinoma: fine needle aspiration cytology as compared with serum calcitonin measurement. Journal of Surgical Oncology 2005;91(1):56‐60. [DOI] [PubMed] [Google Scholar]
Chang 2005
- Chang TC, Wu SL, Hsiao YL. Medullary thyroid carcinoma: pitfalls in diagnosis by fine needle aspiration cytology and relationship of cytomorphology to RET proto‐oncogene mutations. Acta Cytologica 2005;49(5):477‐82. [DOI] [PubMed] [Google Scholar]
Cheung 2008
- Cheung K, Roman SA, Wang TS, Walker HD, Sosa JA. Calcitonin measurement in the evaluation of thyroid nodules in the united states: a cost‐effectiveness and decision analysis. Journal of Clinical Endocrinology and Metabolism 2008;93(6):2173‐80. [DOI] [PubMed] [Google Scholar]
Colombo 2011
- Colombo C, Verga U, Mian C, Ferrero S, Perrino M, Vicentini L, et al. Comparison of calcium and pentagastrin tests for the diagnosis and follow‐up of medullary thyroid cancer. Journal of Clinical Endocrinology and Metabolism 2012;97(3):905‐13. [PUBMED: 22170709] [DOI] [PubMed] [Google Scholar]
Costante 2009
- Costante G, Durante C, Francis Z, Schlumberger M, Filetti S. Determination of calcitonin levels in C‐cell disease: clinical interest and potential pitfalls. Nature Clinical Rractice. Endocrinology & Metabolism 2009;5(1):35‐44. [DOI] [PubMed] [Google Scholar]
d'Herbomez 2007
- d'Herbomez M, Caron P, Bauters C, Do Cao C, Schlienger JL, Sapin R, et al. Reference range of serum calcitonin levels in humans: influence of calcitonin assays, sex, age, and cigarette smoking. European Journal of Endocrinology / European Federation of Endocrine Societies 2007;157(6):749‐55. [PUBMED: 18057382] [DOI] [PubMed] [Google Scholar]
Daniels 2011
- Daniels GH. Screening for medullary thyroid carcinoma with serum calcitonin measurements in patients with thyroid nodules in the United States and Canada. Thyroid : official journal of the American Thyroid Association 2011;21(11):1199‐207. [PUBMED: 21936671] [DOI] [PubMed] [Google Scholar]
de Groot 2006
- Groot JW, Plukker JT, Wolffenbuttel BH, Wiggers T, Sluiter WJ, Links TP. Determinants of life expectancy in medullary thyroid cancer: age does not matter. Clinical Endocrinology 2006;65(6):729‐36. [DOI] [PubMed] [Google Scholar]
Doyle 2009
- Doyle P, Duren C, Nerlich K, Verburg FA, Grelle I, Jahn H, et al. Potency and tolerance of calcitonin stimulation with high‐dose calcium versus pentagastrin in normal adults. Journal of Clinical Endocrinology and Metabolism 2009;94(8):2970‐4. [PUBMED: 19491231] [DOI] [PubMed] [Google Scholar]
Ernani 2016
- Ernani V, Kumar M, Chen AY, Owonikoko TK. Systemic treatment and management approaches for medullary thyroid cancer. Cancer Treatment Reviews 2016;50:89‐98. [PUBMED: 27664392] [DOI] [PubMed] [Google Scholar]
Ewers 1976
- Ewers HR, Brouwers HP, Merguet P, Hengstebeck W. Side effects after stimulation of gastric secretion with pentagastrin. Medizinische Klinik 1976;71(1):19‐23. [PubMed] [Google Scholar]
Frank‐Raue 2013
- Frank‐Raue K, Machens A, Leidig‐Bruckner G, Rondot S, Haag C, Schulze E, et al. Prevalence and clinical spectrum of nonsecretory medullary thyroid carcinoma in a series of 839 patients with sporadic medullary thyroid carcinoma. Thyroid: official journal of the American Thyroid Association 2013;23(3):294‐300. [PUBMED: 22946486] [DOI] [PubMed] [Google Scholar]
Gharib 2016
- Gharib H, Papini E, Garber JR, Duick DS, Harrell RM, Hegedus L, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules‐‐2016 Update. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2016;22(5):622‐39. [PUBMED: 27167915] [DOI] [PubMed] [Google Scholar]
Gilliland 1997
- Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population‐based study of 15,698 cases from the surveillance, epidemiology and end results (SEER) program 1973‐1991. Cancer 1997;79(3):564‐73. [DOI] [PubMed] [Google Scholar]
Hadoux 2016
- Hadoux J, Pacini F, Tuttle RM, Schlumberger M. Management of advanced medullary thyroid cancer. Lancet. Diabetes & Endocrinology 2016;4(1):64‐71. [PUBMED: 26608066] [DOI] [PubMed] [Google Scholar]
Haugen 2016
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid: official journal of the American Thyroid Association 2016;26(1):1‐133. [PUBMED: 26462967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hundahl 1998
- Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A national cancer data base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985‐1995. Cancer 1998;83(12):2638‐48. [DOI] [PubMed] [Google Scholar]
Karga 2011
- Karga H, Giagourta I, Papaioannou G, Doumouchtsis K, Polymeris A, Thanou S, et al. Changes in risk factors and tumor node metastasis stage of sporadic medullary thyroid carcinoma over 41 years, before and after the routine measurements of serum calcitonin. Metabolism: Clinical and Experimental 2011;60(5):604‐8. [PUBMED: 20667564] [DOI] [PubMed] [Google Scholar]
Kratzsch 2011
- Kratzsch J, Petzold A, Raue F, Reinhardt W, Brocker‐Preuss M, Gorges R, et al. Basal and stimulated calcitonin and procalcitonin by various assays in patients with and without medullary thyroid cancer. Clinical Chemistry 2011;57(3):467‐74. [PUBMED: 21159900] [DOI] [PubMed] [Google Scholar]
Kudo 2007
- Kudo T, Miyauchi A, Ito Y, Takamura Y, Amino N, Hirokawa M. Diagnosis of medullary thyroid carcinoma by calcitonin measurement in fine‐needle aspiration biopsy specimens. Thyroid 2007;17(7):635‐8. [DOI] [PubMed] [Google Scholar]
Kudo 2011
- Kudo T, Miyauchi A, Ito Y, Yabuta T, Inoue H, Higashiyama T, et al. Serum calcitonin levels with calcium loading tests before and after total thyroidectomy in patients with thyroid diseases other than medullary thyroid carcinoma. Endocrine Journal 2011;58(3):217‐21. [PUBMED: 21358115] [DOI] [PubMed] [Google Scholar]
Leboeuf 2006
- Leboeuf R, Langlois MF, Martin M, Ahnadi CE, Fink GD. "Hook effect" in calcitonin immunoradiometric assay in patients with metastatic medullary thyroid carcinoma: case report and review of the literature. Journal of Clinical Endocrinology and Metabolism 2006;91(2):361‐4. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLOS Medicine 1999;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2004
- Macaskill P. Empirical Bayes estimates generated in a hierarchical summary ROC analysis agreed closely with those of a full Bayesian analysis. Journal of Clinical Epidemiology 2004;57(9):925‐32. [PUBMED: 15504635] [DOI] [PubMed] [Google Scholar]
Machens 2000
- Machens A, Haedecke J, Holzhausen HJ, Thomusch O, Schneyer U, Dralle H. Differential diagnosis of calcitonin‐secreting neuroendocrine carcinoma of the foregut by pentagastrin stimulation. Langenbecks Archives of Surgery 2000;385(6):398‐401. [DOI] [PubMed] [Google Scholar]
Machens 2009
- Machens A, Hoffmann F, Sekulla C, Dralle H. Importance of gender‐specific calcitonin thresholds in screening for occult sporadic medullary thyroid cancer. Endocrine‐related cancer 2009;16(4):1291‐8. [DOI] [PubMed] [Google Scholar]
Marqusee 2000
- Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES, et al. Usefulness of ultrasonography in the management of nodular thyroid disease. Annals of Internal Medicine 2000;133(9):696‐700. [DOI] [PubMed] [Google Scholar]
Mazzaferri 1993
- Mazzaferri EL. Management of a solitary thyroid nodule. New England Journal of Medicine 1993;328(8):553‐9. [DOI] [PubMed] [Google Scholar]
Nam‐Goong 2004
- Nam‐Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography‐guided fine‐needle aspiration of thyroid incidentaloma: correlation with pathological findings. Clinical Endocrinology 2004;60(1):21‐8. [DOI] [PubMed] [Google Scholar]
Netzel 2014
Niccoli 1995
- Niccoli P, Brunet P, Roubicek C, Roux F, Baudin E, Lejeune PJ, et al. Abnormal calcitonin basal levels and pentagastrin response in patients with chronic renal failure on maintenance hemodialysis. European Journal of Endocrinology / European Federation of Endocrine Societies 1995;132(1):75‐81. [DOI] [PubMed] [Google Scholar]
Pacini 2006
- Pacini F, Schlumberger M, Dralle H, Elisei R, Smit JW, Wiersinga W (European Thyroid Cancer Taskforce). European consensus for the management of patients with differentiated thyroid carcinoma of the follicular epithelium. European Journal of Endocrinology / European Federation of Endocrine Societies 2006;154(6):787‐803. [DOI] [PubMed] [Google Scholar]
Papaparaskeva 2000
- Papaparaskeva K, Nagel H, Droese M. Cytologic diagnosis of medullary carcinoma of the thyroid gland. Diagnostic Cytopathology 2000;22(6):351‐8. [DOI] [PubMed] [Google Scholar]
Papini 2002
- Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: Predictive value of ultrasound and color‐doppler features. Journal of Clinical Endocrinology and Metabolism 2002;87(5):1941‐6. [DOI] [PubMed] [Google Scholar]
Preissner 2005
- Preissner CM, Dodge LA, O'Kane DJ, Singh RJ, Grebe SK. Prevalence of heterophilic antibody interference in eight automated tumor marker immunoassays. Clinical Chemistry 2005;51(1):208‐10. [PUBMED: 15613712] [DOI] [PubMed] [Google Scholar]
Rallison 1991
- Rallison ML, Dobyns BM, Meikle AW, Bishop M, Lyon JL, Stevens W. Natural history of thyroid abnormalities: prevalence, incidence, and regression of thyroid disease in adolescents and young adults. American Journal of Medicine 1991;91(4):363‐70. [DOI] [PubMed] [Google Scholar]
Redding 2000
- Redding AH, Levine SN, Fowler MR. Normal preoperative calcitonin levels do not always exclude medullary thyroid carcinoma in patients with large palpable thyroid masses. Thyroid 2000;10(10):919‐22. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Samaan 1980
- Samaan NA, Castillo S, Schultz PN, Khalil KG, Johnston DA. Serum calcitonin after pentagastrin stimulation in patients with bronchogenic and breast cancer compared to that in patients with medullary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 1980;51(2):237‐41. [DOI] [PubMed] [Google Scholar]
Tan 1997
- Tan GH, Gharib H. Thyroid incidentalomas: management approaches to nonpalpable nodules discovered incidentally on thyroid imaging. Annals of Internal Medicine 1997;126(3):226‐31. [DOI] [PubMed] [Google Scholar]
Tommasi 2001
- Tommasi M, Brocchi A, Cappellini A, Raspanti S, Mannelli M. False serum calcitonin high levels using a non‐competitive two‐site IRMA. Journal of Endocrinological Investigation 2001 May;24(5):356‐60. [DOI] [PubMed] [Google Scholar]
Trimboli 2016
- Trimboli P, Guidobaldi L, Bongiovanni M, Crescenzi A, Alevizaki M, Giovanella L. Use of fine‐needle aspirate calcitonin to detect medullary thyroid carcinoma: a systematic review. Diagnostic Cytopathology 2016;44(1):45‐51. [PUBMED: 26481456] [DOI] [PubMed] [Google Scholar]
Valle 2011
- Valle LA, Kloos RT. The prevalence of occult medullary thyroid carcinoma at autopsy. Journal of Clinical Endocrinology and Metabolism 2011;96(1):E109‐13. [PUBMED: 20943788] [DOI] [PubMed] [Google Scholar]
Vander 1968
- Vander JB, Gasston EA, Dawber TR. The significance of nontoxic thyroid nodules. Final report of a 15‐year study of the incidence of thyroid malignancy. Annals of Internal Medicine 1968;69(3):537‐40. [DOI] [PubMed] [Google Scholar]
Vanderpump 1995
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, Clark F, et al. The incidence of thyroid disorders in the community: A twenty‐year follow‐up of the Whickham survey. Clinical Endocrinology 1995;43(1):55‐68. [DOI] [PubMed] [Google Scholar]
Vlaeminck‐Guillem 2001
- Vlaeminck‐Guillem V, D'herbomez M, Pigny P, Fayard A, Bauters C, Decoulx M, et al. Pseudohypoparathyroidism Ia and Hypercalcitoninemia. Journal of Clinical Endocrinology and Metabolism 2001;86(7):3091‐6. [DOI] [PubMed] [Google Scholar]
Wang 2008
- Wang TS, Ocal IT, Sosa JA, Cox H, Roman S. Medullary thyroid carcinoma without marked elevation of calcitonin: A diagnostic and surveillance dilemma. Thyroid 2008;18(8):889‐94. [DOI] [PubMed] [Google Scholar]
Wells 1978
- Wells SA Jr, Baylin SB, Linehan WM, Farrell RE, Cox EB, Cooper CW. Provocative agents and the diagnosis of medullary carcinoma of the thyroid gland. Annals of Surgery 1978;188(2):139‐41. [PUBMED: 686877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2015
- Wells SA Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid: official journal of the American Thyroid Association 2015;25(6):567‐610. [PUBMED: 25810047] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]
Wiest 1998
- Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RJ, et al. Thyroid palpation versus high resolution thyroid ultrasonography in the detection of nodules. Journal of Ultrasound in Medicine 1998;17(8):487‐96. [DOI] [PubMed] [Google Scholar]
Zanelli 1993
- Zanelli JM, Gaines‐Das RE, Corran P. Establishment of the second international standards for porcine and human calcitonins: report of the international collaborative study. Acta Endocrinologica 1993;128(5):443‐50. [PUBMED: 8317192] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Verbeek 2012
- Verbeek HH, Groot JW, Sluiter WJ, Muller Kobold AC, Plukker JT, Links TP. Calcitonin testing for detection of medullary thyroid cancer in patients with thyroid nodules. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010159] [DOI] [PMC free article] [PubMed] [Google Scholar]


