Abstract
Severe acute respiratory syndrome (SARS) is caused by a coronavirus (SARS-CoV) and has the potential to threaten global public health and socioeconomic stability. Evidence of antibody-dependent enhancement (ADE) of SARS-CoV infection in vitro and in non-human primates clouds the prospects for a safe vaccine. Using antibodies from SARS patients, we identified and characterized SARS-CoV B-cell peptide epitopes with disparate functions. In rhesus macaques, the spike glycoprotein peptides S471–503, S604–625 and S1164–1191 elicited antibodies that efficiently prevented infection in non-human primates. In contrast, peptide S597–603 induced antibodies that enhanced infection both in vitro and in non-human primates by using an epitope sequence-dependent (ESD) mechanism. This peptide exhibited a high level of serological reactivity (64%), which resulted from the additive responses of two tandem epitopes (S597–603 and S604–625), and a long-term human B-cell memory response with antisera from convalescent SARS patients. Thus, peptide-based vaccines against SARS-CoV could be engineered to avoid ADE via elimination of the S597–603 epitope. We provide herein an alternative strategy to prepare a safe and effective vaccine for ADE of viral infection by identifying and eliminating epitope sequence-dependent enhancement of viral infection.
Keywords: SARS-CoV, peptide, vaccine, antibody-dependent enhancement (ADE), epitope sequence-dependent (ESD) enhancement, B-cell peptide epitope
Graphical Abstract
Introduction
A novel severe acute respiratory syndrome coronavirus (SARS-CoV) was characterized in March 2003 after a global effort following the first epidemiological case in November 2002. 1 Ten years later (2012), a novel Middle East respiratory syndrome (MERS) coronavirus emerged, which was a beta-coronavirus, similar to SARS-CoV. 2 SARS is a deadly infectious disease and has the potential to seriously threaten public health and socioeconomic stability worldwide. 1 Serologic and genetic investigations indicate that SARS-CoV is of zoonotic origin, 3–4 with bats as the likely animal reservoir. Since identification of SARS-CoV in 2003, there have been major advances in understanding SARS-CoV genetics 1, 5–6 and molecular epidemiology 7 and in identification of host receptors 8, 9 and T-cell epitopes. 10, 11 Models in mice, ferrets, and monkeys have been established, each with particular strengths and weaknesses. 12 Vaccine candidates have included DNA, a live attenuated strain, recombinant proteins, inactivated whole virions and vector vaccines (13); however, none have been approved through clinical investigation.14 Safety concerns about a SARS-CoV vaccine have been raised, given the observation in vitro of antibody-dependent enhancement (ADE) of SARS-CoV infection that could, in theory, exacerbate disease. 15 Other observations include evidence of ADE reported here for the first time induced by an inactivated SARS-CoV vaccine in rhesus macaques (Fig 1) and by antisera from SARS patients (Table S1), as well as ADE in other coronavirus infections. 16–18
Figure 1. Observation of ADE induced by inactivated SARS-CoV vaccine in the lungs (H&E staining, 200×).
The monkeys were immunized intramuscularly with formalin-inactivated SARS-CoV virions and boosted on day 14 with the same dose of 1.0 mL/monkey (4 × 104 TCID50). The animals were then challenged with nasal cavity inoculation of SARS-CoV (1 × 106 TCID50 in 4.0 mL/monkey, PUMC01 SARS-CoV strain) 14 days after the boost. The animals were sacrificed 6 days after viral challenge, and lung tissues were sampled for general and pathological observations. Pathological examination procedures were as described in reference 37. Infected macaque: Lung interval broadened, visible macrophage infiltration with alveolar epithelial hyperplasia. ADE macaque: Lung interval broadened, lung interval fractured with large amounts of macrophage and lymphocyte infiltration, visible fibrin and protein-rich edema in alveolar cavity. Protected macaques: Lung interval slightly broadened, without visible abnormalities. The arrows indicate the lung lesions of infected and ADE animals.
ADE in vitro has been observed for many other viruses, including yellow fever virus, West Nile virus, human immunodeficiency virus (HIV), Ebola virus, respiratory syncytial virus (RSV), and influenza A virus. 19–21 HIV-induced ADE has been described 22–23, but the exact mechanism is still uncertain. 24 Among other examples of ADE, secondary infection with dengue virus of a heterologous serotype has been associated with an immunopathologic vascular leakage and hemorrhagic syndrome, Dengue Hemorrhagic Fever/Dengue Shock Syndrome (DHF/DSS). 25 Most descriptions of ADE relate to Fc-gamma receptor (FcγR) and/or complement components promoting viral uptake and virus replication pathways. 26–28 Goncalvez et al. reported that treatment of juvenile rhesus monkeys with the cross-reactive mAb 1A5, which recognizes the fusion loop in DII of dengue virus, enhanced DV4 viremia by several logs within 3–6 days of infection. 26 Furthermore, a 9-aa deletion at the N-terminus of the CH2 domain in the Fc region abrogated enhancement in vitro and in vivo, confirming that ADE occurred through an FcγR pathway for this class of antibodies. Another study analyzed the stoichiometric relationship between antibody-mediated neutralization and enhancement of West Nile virus (WNV) infection in cells expressing FcγR. 29 The results showed that there is an antibody occupancy threshold on the virion for neutralization or enhancement of virus infection. Strongly neutralizing DIII-lateral ridge-specific mAbs inhibit at lower occupancy, whereas weakly-neutralizing mAbs that bind distinct epitopes require much a higher mAb occupancy for neutralization. When mAb occupancy falls below the threshold for neutralization, ADE can occur. In subsequent studies, the same group showed that the level of antibody occupancy that promotes ADE in vivo is also modulated by the binding of C1q, which restricts ADE in an IgG subclass-specific manner.30 Based on these studies, many neutralizing antibodies may enhance infection in vitro in FcγR+ cells when their concentrations fall below a key occupancy threshold, and some Abs that poorly neutralize may strongly enhance over a wide dose-response range. However, for antibody isotypes that bind C1q avidly, much of the enhancing effect may be minimized in vivo.
Other studies suggest that FcγR-dependent ADE may not be the only mechanism for antibody enhancement of infection. Huang et al. 31 demonstrated ADE in vitro of an anti-prM mAb against DENV in cell lines that lacked expression of FcγR (e.g., baby hamster kidney cell line BHK-21 and murine fibroblast cell lineNIH3T3). Importantly, a peptide in the M3 region (CPFLKQNEPEDIDCW) of prM blocked this ADE in a dose-dependent manner. The antibody appeared to have dual specificity and bound to dengue virus virions and/or a cross-reactive HSP60 protein on the cell surface. Thus, ADE via non-FcγR- or complement-dependent mechanisms is plausible for DENV, yet poorly characterized in vitro and in vivo. Herein, we discovered that a peptide of the viral sequence simultaneously elicits the antibodies of disparate functions in protection and enhancement against SARS-CoV infection by the studies with host Vero E6 cells in vitro and in non-human primates.
Results
Observation of ADE induced by inactivated SARS-CoV vaccine in the lungs of macaques
First, we observed the ADE of SARS-CoV infection in lungs after immunization with the inactivated virus vaccine in macaques. The monkeys with or without the challenge of inactivated SARS-CoV vaccine were infected by nasal cavity inoculation of live SARS-CoV virions (Figure 1). Infected macaque showed the lung with broadened interval and visible macrophage infiltration with alveolar epithelial hyperplasia. However, ADE macaque after vaccination showed the lung with broadened interval, lung interval fractured with large amounts of macrophage and lymphocyte infiltration, visible fibrin and protein-rich edema in alveolar cavity. Protected macaques showed the lungs with slightly broadened intervals without visible abnormalities. The results implied that the simple vaccination with inactivated whole SARS-CoV virions might be ineffective for protection of virus infection.
Identification of highly immunodominant B-cell peptides of SARS-CoV in humans
Mapping highly immunoreactive human B-cell peptides by employing SARS patient antisera was carried out by a number of research groups. Consistent with previous reports, Jiang’s lab reported that the M1–131 and M132–162 peptides 32 from the membrane protein and the N153–178 and N362–412 peptides from the N protein 33 were highly reactive with all convalescent-phase test sera from SARS patients. The labs of Wu and Che collaboratively identified 3 conformational (amino acids 1–69, 68–213, and 337–422) and 3 linear (amino acids 1–69, 121–213, and 337–422) epitopes on the full-length N protein that were immunodominant. 34 Using a “split and mix” combinatorial strategy, we synthesized an overlapping peptide library spanning all four structural proteins of the SARS-CoV BJ01 strain, including the envelope (E), membrane (M), nucleocapsid (N) and spike (S). We screened this peptide library with antisera from convalescent SARS patients and identified dominant peptide epitopes recognized by anti-SARS IgG. 35 Further experiments in this paper indicate that three peptides in the S protein (S471–503, S604–625 and S1164–1191) were strongly bound by SARS-CoV-specific human IgG from 12.8%, 32.6% and 13.9%, respectively, of the 470 SARS patients (Table 1). Extension of the peptides (Table S2) to further characterize the epitopes’ N- and C-termini identified a new immunodominant peptide (S597–625), which was recognized by antisera from 64% of the patients (Table 1). The S597–625 peptide specifically reduced the titer of human IgG in convalescent sera from SARS patients who reacted with SARS-CoV viral lysates (Fig. 2A). We studied sequentially collected sera from 21 convalescent SARS patients whose sera (obtained 3 months after onset of infection) strongly reacted with the S597–625 peptide. The antisera from these patients continued to recognize the S597–625 at 12 months (12/21 [57.1%]), 18 months (7/21 [33.3%]) and 24 months (6/21 [28.6%]) after the onset of SARS (Fig. 2B), implying that the S597–625 peptide is a major immunodominant peptide in humans and elicits a long-term B-cell memory response after natural infection with SARS-CoV.
Table 1.
Peptides recognized by SARS-infected patients, relevant mouse mAbs and functional classification.
| Peptide | Sequence* | Serologic reactivity# | mAb | Isotype (Fc) | Binding affinity to peptide (Molar) | Functioζ |
|---|---|---|---|---|---|---|
|
| ||||||
| S471–503 | H-ALNCYWPLNDYG FYTTTGIGYQPYRV VVLSFEL-NH2 | 12.8% | 4E5 | IgG1/κ | 4.67 × 10−9 | Neu |
| 4A10 | IgG1/κ | 2.96 × 10−9 | Neu | |||
| 6G5 | IgG2b/κ | 5.82 × 10−9 | Neu | |||
|
| ||||||
| S604–625 | H-TDVSTAIHADQL TPAWRIYSTGC-NH2 | 32.6% | 11B1 | IgG2a/κ | 2.32 × 10−9 | Neu |
|
| ||||||
| S597–625 | H-LYQDVNCTDVST AIHADQLTPAWRIY STG-NH2 | 64.0% | 43–3-14 | IgG1/κ | 1.21 × 10−11 | ADE |
| 219–10-28 | IgG1/κ | 1.20 × 10−8 | ADE | |||
|
| ||||||
| Sl164–1191 | H-EIDRLNEVAKNL NESLIDLQELGKYE QYC-NH2 | 13.9% | 126–10 | IgG1/κ | 4.59 × 10−9 | Neu |
Red-, blue- and green-colored peptides represent the epitopes that were assembled into immunogenic peptides.
Neu (neutralizing) or ADE indicates the ability of the individual mAb to block or enhance, respectively, SARS-CoV infection of Vero E6 cells.
The reactivity of individual peptides with antisera from 470 convalescent SARS patients (Male: 192, Female: 278) was determined using an ELISA. Commercially available ELISA kits were used to confirm that 81% of the 470 antisera were positive against viral lysates as antigen (cutoff value: 0.1). The titer of each serum was averaged from duplicate wells.
Figure 2. Immunogenic specificity and long-term memory response of IgG from antisera of SARS patients to S597–625 peptide.
A. The reactivity of human IgG to SARS viral lysates (ELISA kit: Huada S20030004, Beijing, China) is specifically reduced in a dose-dependent manner by S597–625, but not by the hepatitis B virus (HBV) peptide MDIDPYKEFGATVELLSFLP. P223, P73, and P194 represent three antisera from convalescent SARS patients. Reactivity of human IgG with the S597–625 peptide was determined by an ELISA. Lysates (0.025 μg/well) were incubated overnight at room temperature and then blocked by 5% goat serum in PBS (125 μL) for 2 h. 10 μL of antiserum and 100 μL of PBS were further incubated for 30 min at 37°C. Proper amounts of goat anti-human IgG conjugated to HRP were used for detection of OD450 values. Each antiserum was used in duplicate, and the cutoff value was 0.1. B. The antisera of 21 convalescent SARS patients were collected and tested 3 (M3), 12 (M12), 18 (M18), and 24 (M24) months after onset of SARS-CoV infection. The antiserum of patient 9 collected 3 months after onset of infection was omitted due to insufficient sample quantity. The peptides were dissolved in a minimal volume of DMSO and then diluted to a final concentration of 10 μg/mL in carbonate buffer (pH=9.6). In total, 1.0 μg/well was used for capture antibodies from antiserum. The detailed procedure is the same as in Fig. 2A with a cutoff value of 0.1.
Epitope-specific antibodies of SARS-CoV exhibited enhancing or neutralizing functions in vitro.
To study the functional significance of immunodominant peptides of the S glycoprotein, we generated 23 monoclonal antibodies (mAbs, Table S3) by immunization of mice. Ten of these strongly bound to the SARS-CoV virion (Fig. 3A). Among them, 5 blocked SARS-CoV infection of Vero E6 cells (Fig. 3B), including mAb4E5, mAb4A10, and mAb6G5 against S471–503, mAb11B1 against S604–625, and mAb126-10 against S1164–1191.
Figure 3. Generated monoclonal antibodies bound to SARS-CoV virions and shared disparate functions.
A. The defined monoclonal antibodies bound to SARS-CoV virions of SARS-CoV PUMC01 strain (4 × 10−5 TCID50/mL) 46. The cutoff value was set to 0.1 (the average for binding of unrelated mAb-HIV-P27). B. Neutralization or enhancement of the SARS-CoV infection in Vero E6 cells by mAbs (1.0 μg/mL) was specifically reduced by the corresponding immunopeptide (0.1 μg/mL), but not by a hepatitis B virus (HBV) peptide (0.1 μg/mL). HBV peptide (LLDYQGMLPV) is an unrelated control peptide from an HBV surface protein. S597–625 and HBV peptide were preincubated with the corresponding mAbs or human antisera for 30 min at 4°C before function was tested. These experiments were performed in triplicate, and the data are presented as the mean ± standard deviation.
In contrast, two mAbs (mAb43-3-14 and mAb219-10-28) against S597–625, despite sharing some of the same isotypes and constant region configurations with neutralizing anti-peptide antibodies (IgG1/κ, IgG2a/κ, and IgG2b/κ; Table 1), markedly enhanced SARS-CoV infection of Vero E6 cells (Fig. 3B). This was particularly striking given that Vero E6 cells lack Fcγ receptors (FcγR), and the best-understood mechanism for ADE involves Fc-mediated internalization and replication of virus. mAb43-3-14- and mAb219-10-28-enhanced Vero E6 cell infection was reduced in a dose-dependent manner by S597–625, but not by an unrelated HBV peptide (Fig 4A, B), while ADE of human antisera from SARS-CoV-infected patients was also specifically blocked by the same peptide (Fig 4C, D), implying that peptide-based ADE occurred during the epidemic period of a SARS outbreak.
Figure 4. Antibody-dependent enhancement of SARS-CoV infection.
A, B, C, D: Enhancement of SARS-CoV infection by either mAbs (1.0 μg/mL) or human antisera (1:500) was reduced in a peptide dose-dependent manner. hW49 and hS101 represent two antisera from convalescent SARS patients 3 months after onset. HBV peptide (LLDYQGMLPV) is an unrelated control peptide from HBV surface protein. S597–625 and HBV peptide were preincubated with the corresponding mAb or human antisera for 30 min at 4 °C before function was tested. E. Alanine scanning mutagenesis of the S597–606 showed the minimum requirement (S597–603) for N-terminal binding of S597–625 to mAb43–3-14. This experiment was performed in triplicate, and the data are presented as the mean ± standard deviation.
An antibody-peptide binding ELISA indicated that mAb11B1 against S604–625 reacts with both S597–625 and S604–625, whereas mAb43-3-14 against S597–625 only bound to S597–625 (Fig. 4E). The data suggest that mAb11B1 was induced by S604–625 and is a consensus peptide of S597–625 and S604–625, whereas mAb43-3-14 was from the N-terminal peptide S597–603 (LYQDVNC) immunizing antigen. Synthesized peptides LYQDVN (S597–602), LYQDVNC (S597–603), and LYQDVNCT (S597–604) were all immunoreactive with the tested sera of 24 SARS-positive patients, further confirming that the S597–603 peptide is an immunodominant one in humans (Fig. S1). Alanine scanning mutagenesis of the S597–604 peptide demonstrated that L597, Y598, Q599, D600, and C603 are critical amino acid residues for interaction with mAb43-3-14 (Fig. 4E, Table S4). The S597–603 peptide lies close to the C-terminus of the SARS-CoV major receptor (ACE2)-binding domain (RBD) (8). We speculate that mAb43-3-14 may expose specific conformations that catalyze SARS-CoV attachment to and/or membrane fusion with target cells.
Neutralization or enhancement of SARS-CoV infection in non-human primates by epitope-specific antibodies
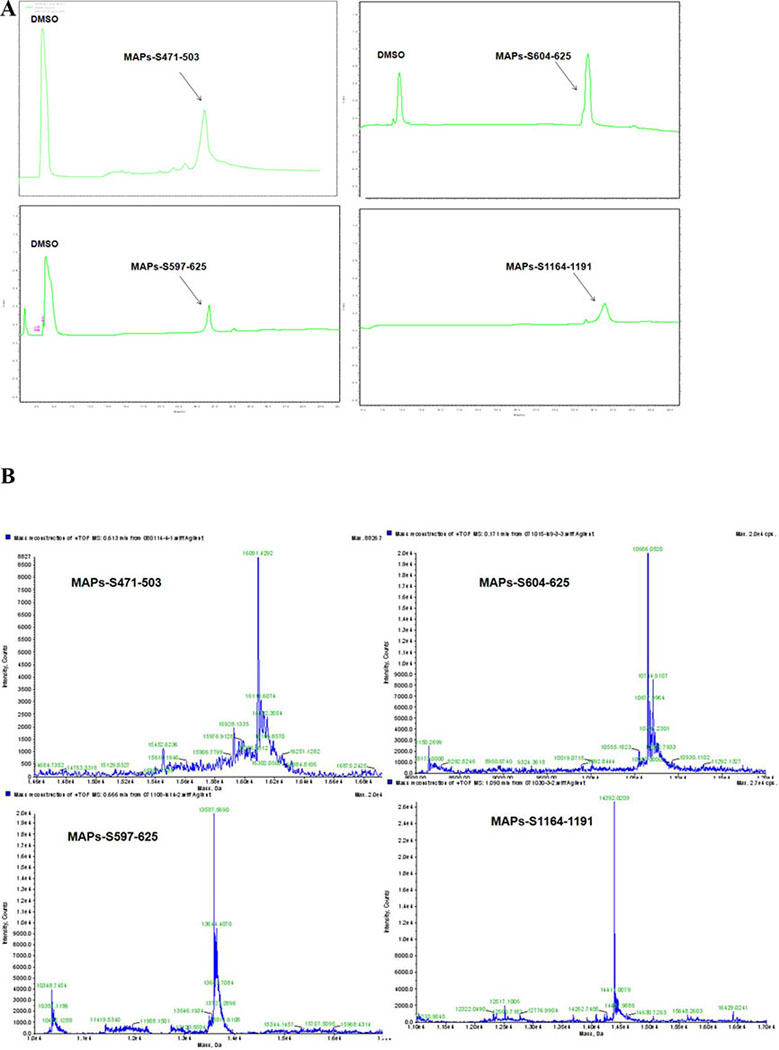
Low-molecular weight peptides behave like haptens, with relatively low immunogenicity. To make them more immunogenic, we chemically ligated 4 copies of each peptide to a lysine core [BrCH2CO-Lys2-(Lys)-β-Ala-CONH2(BrK2KA), Scheme 1] to prepare multiple antigen peptide system (MAP) 36 and purified them by preparative HPLC (Fig. 5A, Table S7). Molecular weight was confirmed by LC-MS/MS (Fig. 5B).
Scheme 1.
Synthesis of multiple antigen peptide systems (MAPS) by chemical ligation of a thiol nucleophilic substitution reaction. B-cell epitopes with a free -SH sequence (containing a Cys residue) were prepared directly. B-cell epitopes without a free -SH group were anchored by an additional Cysteine residue at the C-terminal (Fig S2). A four-branched, brominated, multiple-antigen core peptide (BrK2KA, Fig S3) via lysine two α-amino and side chain amino groups was prepared that finally conjugated with four copies of individual antigenic peptide.
Figure 5. Tetrameric forms of peptides were successfully synthesized on a branched lysine scaffold.
MAP-S471–503 = [(S471–503)4Lys]2Lys-β-Ala-CONH2; MAP-S604–625 = [(S604–625)4Lys]2Lys-β-Ala-CONH2; MAP-S597–625 = [(S597–625)4Lys]2 Lys-β-Ala-CONH2; MAP-S1164–1191 = [(S1164–1191)4Lys]2Lys-β-Ala-CONH2. RP-HPLC profiles (A) of the MAPs were analyzed by a Shimadzu LC-10AT analytical HPLC system with a C8 column (4.6 mm × 250 mm, 5 μm) and UV detection at 214 nm. The molecular weights (B) were determined by high resolution LC-MS in an Agilent LC/MSD TOF System and hypermass reconstruction of the raw MS data to a single charge.
We used these synthetic MAPs to vaccinate rhesus monkeys (Table S4) as follows: Vac1, control group (received 0.9% NaCl) of 4 macaques randomly assigned to 2 groups for day 2 or day 6 post-infection sacrifice; Vac2, MAP-S597–625 given to 6 macaques randomly assigned to 2 groups for day 2 or day 6 post-infection sacrifice; Vac3, a mixture of MAP-S471–503, MAP-S604–625 and MAP-S1164–1191 given to 6 macaques randomly assigned to 2 groups for day 2 or day 6 post-infection sacrifice; and Vac4, a mixture of MAP-S471–503, MAP-S597–625 and MAP-S1164–1191 given to 6 macaques randomly assigned to 2 groups for day 2 or day 6 post-infection sacrifice. The immunization protocol included one priming injection followed by 3 boosts at two-week intervals (Chart S1). Anti-peptide polyclonal Abs (monkey IgG) monitored by ELISA showed high-level responses to all immunized peptides after three boosts (Fig. 6 left).
Figure 6. Rhesus monkeys strongly responded to peptide-based vaccines (Table S5).
Vaccinations (A) were designed as Vac1 (0.9% NaCl) for the control group of four animals, Vac2 (MAPs-S597–625) for the enhancement of SARS-CoV infection in six experimental animals, Vac3 (MAPs-S471–503, MAPs-S604–625 and MAPs-S1164–1191) for the neutralization of SARS-CoV infection in six experimental animals, and Vac4 (MAPs-S471–503, MAPs-S597–625 and MAPs-S1164–1191) for the reduction of neutralizing ability in six experimental animals (Table S4). The average IgG levels against peptides in each vaccine group were monitored by ELISA on the day before injection or boost. IgG level (B) against S604–625 is an average of grouped macaques. The titer of anti-S604–625 IgG was greater than 1:106 in all relevant immunized monkey groups.
Antibodies (Fig 6A) against the four peptides in sera from Vac 1-immunized animals were at background level on all blood collection days. Vac2 gradually induced anti-S597–625 peptide antibodies after three boosts. Not surprisingly, both Vac 2 and Vac 4 immunizations elicited high levels of IgG against the S604–625 peptide because the vaccine components consisted of the S597–625 or S604–625 epitopes in these two animal groups. Anti-S604–625 and -S1164–1191 antibodies in the Vac 3-immunized animal group reached their highest levels before the second boost (28 days after the first injection). The average titer of anti-S604–625 IgG was greater than 1:106 in Vac3-immunized monkeys before SARS-CoV challenge and was 3-fold or 2-fold times that of Vac 2 or Vac 4, respectively. Interestingly, when Vac 3-vaccinated animals were challenged by SARS-CoV, the IgG level against S604–625 gradually decreased (Fig 6 B) on day 2 and day 6 post-infection. This is different from the Vac 2 and Vac 4 immunizations, after which IgG levels inexplicably increased on day 2 post-infection and subsequently decreased on day 6 post-infection. This observation implies that anti-S604–625 antibodies are strongly involved in the neutralization of SARS-CoV in monkeys and that the existing S597–603 epitope (in Vac 2 and Vac 4) may reduce the total titer of anti-S604–625 antibodies.
All immunized macaques were subsequently challenged with SARS-CoV (PUMC01 strain) via nasal inhalation on the 14th day after the last boost, as described. 37 At 2 and 6 days post-infection (DPI), the macaques were sacrificed (Chart S1, Table S7, S7). The data for lung damage and viral burden in these macaques are summarized in Table 2.
Table 2.
Summary of lung damage and viral burden of vaccinated macaques challenged by SARS-CoV.
| Gross lung pathology* | Infected cells in lung# | Viral burden& | ||||
|---|---|---|---|---|---|---|
| Day 2 | Day 6 | Day 2 | Day 6 | Day 2 | Day 6 | |
| Vac 1 | Grade IV | Grade IV | 13.5 ± 2.6 | 10.0 ± 3.0 | 141,000 | 136,000 |
| Vac 2 | Grade IV | Grade IV | 13.8 ± 2.1 | 9.8 ± 3.0 | 116,000 | 143,000 |
| Vac 3 | Grade I-II | Grade I-II | 7.4 ± 3.2 | 7.0 ± 2.9 | 6,200 | 7,300 |
| Vac 4 | Grade II-III | Grade II-III | 8.14 ±3.32 | 7.8 ± 2.91 | 6,835 | 31,254 |
Average gross lung pathology was determined by a standard in Table S8.
SARS-CoV-infected macrophages and alveolar epithelial cells in the vaccine groups were counted by using anti-peptide antibodies.
Quantitative viral burden analysis by quantitative RT-PCR (viral copies/mg lung tissue).
Gross lung pathology was evident at both 2 and 6 DPI in all groups. In mock-immunized macaques (Vac 1), the average pathology grade was grade IV (Fig. 7A, B; Table S5), with severe diffuse alveolar damage, including alveolar shrinking and rupture of elastic fibers. Massive macrophage infiltration was associated with fusion of alveolar septa. Pulmonary edema, fibrin, hemorrhage, and cellular debris contributed to the severe interstitial pneumonitis. Damage was similar (grade IV) for Vac 2, somewhat reduced (grade II-III) for Vac 4 and markedly diminished (grade I-II) for Vac 3. Immunohistochemistry using mAbs against the viral peptides revealed a large number of SARS-CoV-infected macrophages and alveolar epithelial cells in the Vac 1 group (Fig. 7C), averaging 13.5 ± 2.6 infected cells per 10 high-power fields (HPF) and 10.0 ± 3.0 infected cells per 10 HPF at 2 DPI and 6 DPI, respectively. Quantitative viral burden analysis by quantitative RT-PCR showed an average of 141,000 copies/mg lung tissue (2 DPI) and 136,000 copies/mg lung tissue (6 DPI) of SARS-CoV in the lungs of the Vac1 group.
Figure 7. Peptide-based vaccines neutralized or enhanced SARS-CoV infection of rhesus monkeys.
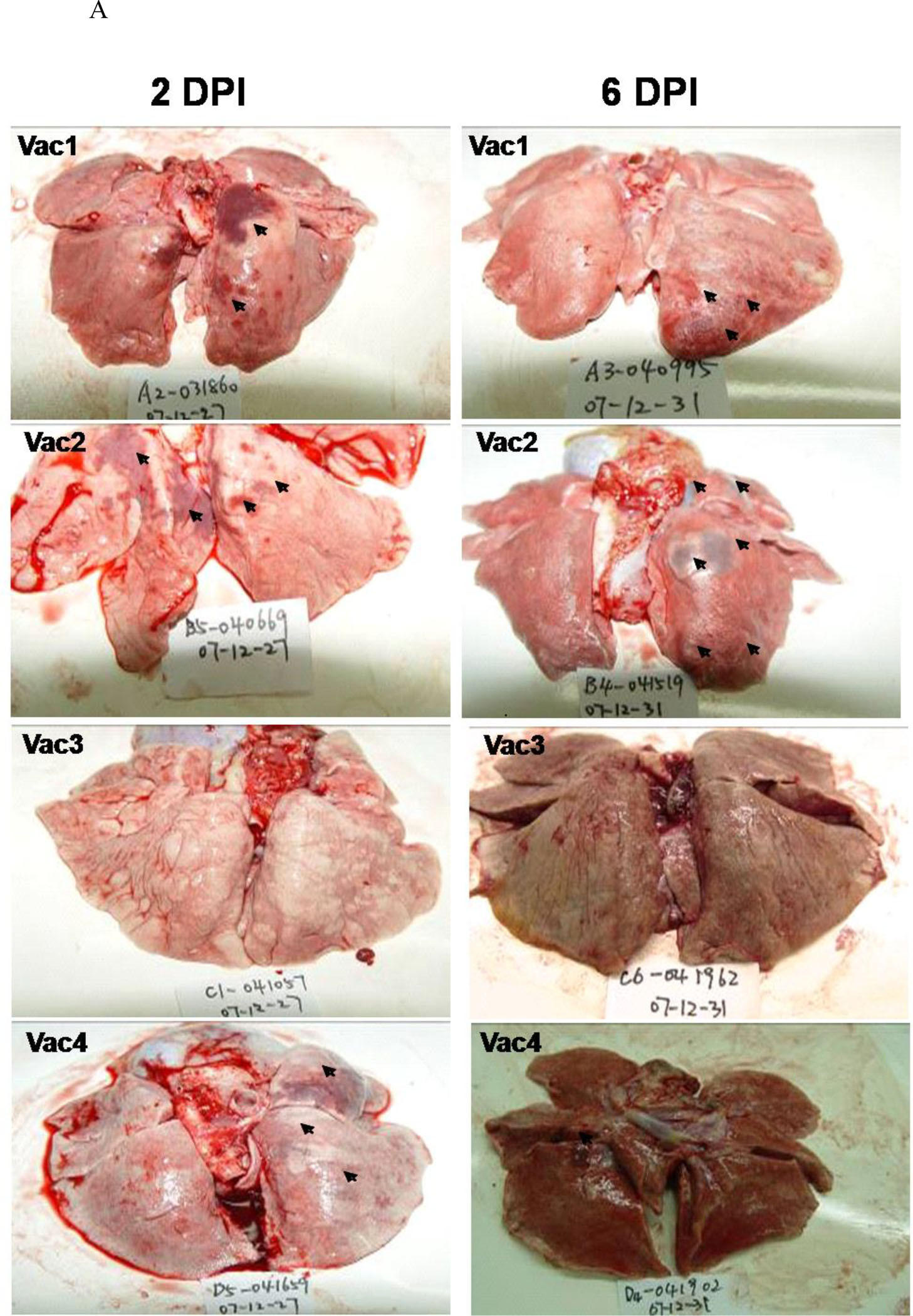
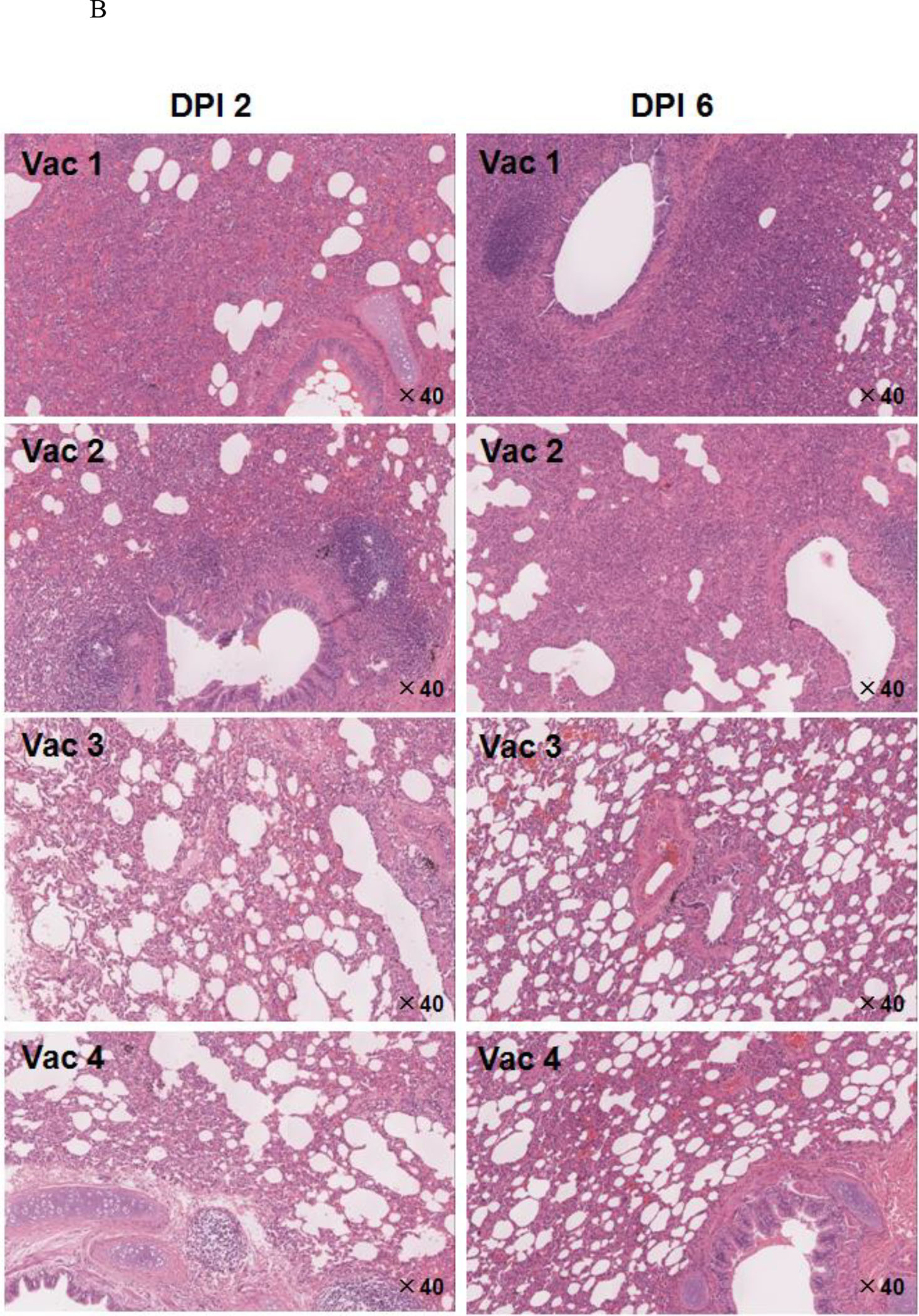
A. Pathologic changes in lung tissue. The arrows point to the areas that showed pathology. B. Histopathologic examination of macaque lung tissue. Lung damage was pathologically characterized as an average standard grade (table S5). C. Immunohistochemical staining of SARS-CoV-infected cells in lung tissue. Lung tissue sections were incubated with a mixture of mAb4E5, mAb11B1, and mAb9A6 (each at 0.01 μg/mL) and developed using horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (1:1,000 dilution, ZhongShan Inc., Guangzhou, PRC). SARS-CoV-positive cells show brown staining, which localizes in the cytoplasm of monocytes and pneumocytes (magnification 200×). Arrows indicate the lung lesions of animals.
Compared with the Vac1 group, the Vac2 group showed similar histology at 2 DPI, but the interstitial pneumonia was far worse at 6 DPI, characterized by massive numbers of inflammatory cells in hemorrhagic septa, with extensive exudation. SARS-CoV-infected cells in the lungs averaged 13.8 ± 2.1 per 10 HPF at 2 DPI and 9.8 ± 3.0 per 10 HPF at 6 DPI, whereas viral copies were 116,000/mg lung tissue and 143,000/mg lung tissue, respectively. Thus, even though S597–625 was immunodominant in patients, antigenic in monkeys and reactive with the neutralizing mAb 11B1, it was non-protective and even harmful when used as a vaccine.
In contrast, the immunized monkeys in the Vac3 group had a strongly increased ability to control SARS-CoV infection in association with induction of high levels of anti-S604–625 antibodies (Fig. 7E). At 2 DPI, hemorrhage in septa and elastic fibers of the alveolar wall were indicated by apparent inflammation in silver staining results. Alveolus septa broadening with increasing infiltration of inflammatory cells were recorded by 6 DPI, indicating that only early, acute diffuse alveolar damage occurred. SARS-CoV-infected lung cells in the Vac3 group averaged 7.4 ± 3.2 per 10 HPF (vs. Vac1 group: p < 0.01) and 7.0 ± 2.9 per 10 HPF (vs. Vac1 group: p < 0.01) at 2 DPI and 6 DPI, respectively. The SARS-CoV burden averaged 6,200 copies/mg lung tissue at 2 DPI and 7,300 copies/mg lung tissue at 6 DPI. This represents a ratio of 1:23 and 1:19 between the number of SARS-CoV copies in the Vac3 and Vac1 groups at 2 and 6 DPI, respectively.
For animals that received Vac 4, symptoms of acute diffuse alveolar damage were visible, including fusion of thick septa and ruptured elastic fibers of the alveoli. The average number of SARS-CoV infected cells in the lung tissues was 8.14 ± 3.32 per 10 HPF at 2 DPI (vs. Vac1 group: p < 0.01) and 7.8 ± 2.91 per 10 HPF at 6 DPI (vs. Vac1 group: p < 0.01). The SARS-CoV burden averaged 31,254 copies/mg.
For animals that received Vac 4, symptoms of acute diffuse alveolar damage were visible, including fusion of thick septa and ruptured elastic fibers of the alveoli. The average number of SARS-CoV infected cells in the lung tissues was 8.14 ± 3.32 per 10 HPF at 2 DPI (vs. Vac1 group: p < 0.01) and 7.8 ± 2.91 per 10 HPF at 6 DPI (vs. Vac1 group: p < 0.01). The SARS-CoV burden averaged of 31,254 copies/mg lung tissue at 6 DPI, i.e., 4.5 times the burden of 6,835 copies/mg lung tissue at 2 DPI. The Vac 4 group also showed a 4.3-fold increase to Vac 3 group (31,254 copies/mg vs. 7,300 copies/mg) of viral burden at 6 DPI, suggesting that the presence of IgG against S597–603 facilitated SARS-CoV infection of immunized macaques.
In a separate experiment focused on the phenomenon of ADE in macaques, rhesus monkeys were divided into six groups (n = 3 per group, table S9). Three groups were separately sacrificed at 2 DPI or 6 DPI; each time point included a control group and two groups that received the enhancing mAb43-3-14 at doses of 0.2 mg/kg or 1.8 mg/kg one day prior to challenge with the SARS-CoV PUMC01 strain. Gross pathologic changes were again recorded in the control group at a grade IV level (Fig. 8A, B). Although clear pathologic changes were observed in one of the three monkeys in the 0.2 mg/kg group, we concluded that previous treatment with a dose of 0.2 mg/kg of mAb43-3-14 did not, on average, significantly reduce or facilitate SARS-CoV infection. However, macaques treated with 1.8 mg/kg of mAb43–3-14 showed a marked increase in lung lesions. The lung lesion area in macaque D4-060060 (4.0 × 2.5 cm2) at 6 DPI was the largest in all 6 experimental groups. At 6 DPI, all lungs from the 1.8 mg/kg group showed larger areas of necrosis, severe sheets of septa fusion, necrotic lesions at the hemorrhagic septa and massive macrophage infiltration in the alveoli, indicating that the interstitial pneumonia was much more severe in the mAb43-3-14-treated group than in the control group (Fig. 8C). There were more SARS-CoV-infected cells in the lung tissue (12.5 ± 2.3 per 10 HPF at 2 DPI and 13.4 ± 2.6 per 10 HPF at 6 DPI, p < 0.01 vs. control group). The average SARS-CoV burden in the lungs was 911,000 copies/mg lung tissue at 2 DPI and 944,000 copies/mg lung tissue at 6 DPI, a 10-fold enhancement compared with the control group at 2 DPI and 14-fold at 6 DPI (Fig. 8D). This result further confirmed that enhancement of the SARS-CoV infection in macaques was directly related to antibodies against S597–603.
Figure 8. mAb43-3-14 enhances SARS-CoV infection of rhesus monkeys.
A. Pathologic changes at 6 DPI. B. Histopathologic examination of macaque lung tissues. Lung damage was pathologically characterized as an average standard grade. Control group: grade IV, 0.2 mg/kg group: grade III–IV, and 1.8 mg/kg group: grade IV. C. Immunohistochemical staining of SARS-CoV-infected cells in lung tissue. The staining conditions were the same as in Fig. 6. D. SARS-CoV mRNA in infected monkey lung tissue was quantitatively analyzed from an average of three animals. The data are presented as the geometric mean ± standard deviation. *p < 0.05, **p < 0.01 vs. control group, Δp < 0.05, ΔΔp < 0.01 vs. 0.2 mg/kg group. Arrows indicate the lung lesions of animals.
Discussion
In this study, we reported for the first time that a SARS-CoV inactivated vaccine could induce ADE and lung pathology in experimental rhesus monkeys. Four antigenic peptides (S471–503, S604–625, S597–625 and S1164–1191) from the spike protein of SARS-CoV were identified by high cross-reactivity with a large number of antisera from convalescent SARS patients. Ten of 23 mAbs generated against these four peptides bound to the SARS-CoV virion (Fig. 3A), indicating that each peptide is exposed on the SARS-CoV surface. Among them, five mAbs against S471–503, S604–625, and S1164–1191, blocked SARS-CoV infection of Vero E6 cells (Fig. 3B), and two mAbs (mAb43-3-14 and mAb219-10-28) markedly enhanced SARS-CoV infection of Vero E6 cells. mAb43-3-14 alone significantly enhanced SARS-CoV infection at the tested high dose of 1.8 mg/kg in experimental rhesus monkeys, while Vac 3 (consisting of S471–503, S604–625, and S1164–1191 MAPs) successfully protected animals from SARS-CoV challenge and lung pathology. Alanine scanning mutagenesis indicated that L597, Y598, Q599, D600, and/or C603 are critical amino acid residues and that S597–603 is responsible for mAb43-3-14-induced enhancement, which may catalyze SARS-CoV attachment and/or membrane fusion with target cells by exposing specific conformations of the spike protein.
S471–503 is located at the virus RBD 8 and can block SARS-CoV infection of Vero E6 cells in vitro, 35 probably by interfering with high-affinity attachment of SARS virions to the human ACE2 receptor at residues T487 and N497 of the RBD. 38,39 The coronavirus spike proteins have been characterized as class I fusion proteins containing highly conserved heptad repeat regions (HR1 and HR2). 40 Thus, interfering with HR1 binding to HR2 has become a recent target for prevention of viral fusion and entry into target cells. S1164–1191 overlaps HR2 (1145–1184 aa) in the SARS-CoV spike glycoprotein. The mechanism by which anti-S1164–1191 antibodies reduce the efficiency of SARS-CoV fusion is likely to be associated with an exposed 5-turn α-helix conformation via HR2 binding to HR1 and an extended conformation formed by residues 1160–1177 and 1178–1184 of three antiparallel HR2 helixes in an oblique orientation surrounding a parallel, trimeric coiled-coil of three HR1 helices. 41 Binding of mAb581-39 to the SARS-CoV virion was the strongest among the mAbs (Fig. 3A), and it implies that S1164–1191 peptide may act as a major immunoantigen.
Severe lung injury occurred in one challenged rhesus monkey that had been immunized with an inactivated SARS-CoV vaccine (Fig. 1). Very strong serologic reactivity of S597–625 with antisera from 64.4% of convalescent SARS patients was observed; the response was to two tandem epitopes (S597–603 and S604–625), but each epitope generated antibodies with disparate functions regarding neutralization or enhancement of SARS-CoV infection. Thus, in persons infected by SARS-CoV, enhancing antibodies and neutralizing antibodies may partly counteract each other’s functions.
SARS-CoV’s ADE was also reported by other research laboratories. Using an HL-CZ human promonocyte cell line, Chen and Huang found that higher concentrations of antisera collected from SARS-CoV-infected patients facilitated SARS-CoV infection and induced higher levels of virus-induced apoptosis. They further demonstrated that this phenomenon occurred via anti-spike protein antibodies that mediated ADE, but not via anti-N protein antibodies. 42 Bruzzone and Jaume’s lab showed that anti-spike immune serum increased infection of human monocyte-derived macrophages by replication-competent SARS-CoV as well as by spike-pseudotyped lentiviral particles (SARS-CoVpp), although they did not clarify whether this enhancement was through the FcγRII pathway. 43 Jaume et al. reported that a recombinant, full-length spike protein trimer potentiated infection of human B cell lines, despite eliciting a neutralizing and protective immune response in rodents. The study demonstrated that anti-spike immune serum, while inhibiting viral entry in a permissive cell line, potentiated infection of immune cells by SARS-CoV spike-pseudotyped lentiviral particles, as well as by replication-competent SARS coronavirus. Jaume and collaborators proposed that ADE of SARS-CoV utilizes a novel cell entry mechanism into immune cells. 44
This study demonstrates for the first time that an antibody (mAb43-3-14) targeting a specific linear epitope (S597–603) of the SARS-CoV spike protein can mediate enhancement of virus infection both in vitro and in non-human primates via an epitope sequence-dependent mechanism. We also demonstrate that the viral peptides that induce protection from pathogenesis can be identified and distinguished from those inducing enhancement in primates. These findings reveal a new mechanism of virus evasion of host defense that potentially provides an alternative strategy to prepare safe and effective vaccines against ADE of virus infections.
Materials and Methods
Peptide Design and Synthesis
New peptides from the Spike glycoprotein were designed based on the information gained in the first round of immunopeptide discovery. 32
Peptides were synthesized manually by standard Fmoc chemistry protocols on Rink-amide MBHA resin (NovaBiochem, San Diego, USA, 0.44 mmol/g). All of the L-α-Fmoc-protected amino acids and coupling reagents (DIC and HOBt) were obtained from GL Biochem (Shanghai, China). All solvents were of analytical grade and used without further purification. The each amino acid assembly was completed by using 3 equivalents of L-α-Fmoc-protected amino acids and coupling reagents and the ninhydrin colorimetric test after each coupling was carried out to ensure no detectable amino remaining. In case that the peptide bearing beads were colorized (positive) by ninhydrin test, the free amino group was then blocked by reacting with 15% acetic anhydride in dichloromethane (DCM) (v:v) for 30 min. Crude peptides without Cys, Met, Arg and Trp residues were cleaved using a one-step TFA cleavage method under the following conditions: TFA (1.9mL), deionized water (50μL) and triethyl silane (50μL). For peptides containing Cys, Met, Arg or Trp residues, the following conditions were used: TFA (1.63mL), thioanisole (0.1mL), phenol (0.1g), deionized water (0.1mL), EDT (50μL) and triethyl silane (20μL). TFA solution was treated with ice-cooled diethyl ether to precipitate the peptide. The crude peptide was washed twice by ice-cooled diethyl ether and dried in the N2 flow condition.
In particular, peptide S471–503 was synthesized on SynPhase PA RAM D-series Lanterns (loading 8 μmol/lantern, Mimotopes, North Carolina, USA). The lanterns were treated with 20% piperidine in dimethyl formamide (DMF) for 15 min for deprotection. The general coupling conditions for lanterns utilized a solution of 80% DMF and 20% DCM, with reagents at the following concentrations: A.A. 120 mM, HOBt 144 mM, DIC 120 mM. For each lantern a minimum of 500 μL of the activated amino acid solution was required. Alternatively, bromophenol blue (5 μL/mL coupling solution, 5M) was used as an indicator to monitor the reaction. Simultaneous side chain deprotection and cleavage were carried out using 2.5 mL per lantern of a solution of 82.5% trifluoroacetic acid(TFA)/5% thioanisole/5% anisole/5% water/2.5% 1,2-ethanedithiol for 2 h.
All crude peptides were purified by HPLC to > 95% purity on a semipreparative RP C18 column (Zorbax, 300SB-C18, 9.4 × 250 mm. Agilent, Colorado, USA) eluting at 4 mL/min with a 0–20% CH3CN(buffer B) gradient in water (buffer A) containing 0.1% trifluoroacetic acid (TFA) over 5 min followed by a 20–65% CH3CN over 30 min. Pure peptides were identified by an SHIMADZU LC-10AT analytic HPLC system with a C8 column (4.6 mm × 250 mm, 5 μm) and Agilent LC-MSD TOF. (Fig S4. S604–625: calculated 2505.83 Da, observed 2505.19 Da; S1164–1191: calculated 3439.87 Da, observed 3438.70 Da; S597–625: calculated 3238.62 Da, observed 3238.54 Da; S471–503: calculated 3863.45 Da, observed 3864.20 Da.). The molecular weight of peptides was calculated by a Peptide Molecular Weight Calculator of Biopeptide Co., Inc. (https://www.biopeptide.com/PepCalc/).
Synthesis and Characterization Multiple Antigen Peptides (MAPs) 36
The branched lysine core (BrCH2CO-Lys2-(Lys)-β-Ala-CONH2) scaffold was synthesized manually by standard Fmoc chemistry protocols on 0.22 mmol (500 mg) of Rink-amide MBHA resin (NovaBiochem, 0.44 mmol/g) as described above. N-α-Fmoc-Lys (Fmoc) was used at the branch points. The Fmoc protected amino acids (5.0 equivalents) were assembled by using 5.0 equivalents of DIC coupling reagent and HOBt additive. The N-termini of the four branches of the lysine core were bromoacetylated on the resin with 10 equivalents of bromoacetic acid and DIC in DMF for 2 h. The bromoacetylated lysine core was cleaved from the resin after treatment with 5% H2O/95% TFA for 2 h. It was then precipitated with cold diethyl ether, lyophilized, and purified by HPLC on a semipreparative RP C18 column (Zorbax, 300SB-C18, 9.4 × 250 mm) eluting at 4 mL/min with a 0–20% CH3CN gradient in water containing 0.1% TFA over 5 min followed by a 20–40% CH3CN over 20 min. Pure lysine core (25% yield) was identified by Agilent LC-MSD TOF: calculated (MH+) 957.03 Da, observed 957.05 Da.
MAPs were prepared for immunizations using a modified chemical ligation protocol. 45 Ligation was achieved by mixing the lysine core and peptide in 500 μL solvent at certain pH value. The reaction was monitored by an analytical RP C8 column (Kromasil C8, The Nest Group, Inc., Southborough, MA, USA) under UV214 nm wavelength. After it appeared complete, the reaction was quenched by adding 0.05% TFA to adjust the pH to 7.0. The final product was then purified by semipreparative HPLC at a flow rate of 4 mL/min. The ligated peptide was lyophilized and characterized by an Agilent LC-MSD TOF. The preparative conditions of MAPs are listed in Table S10.
Detailed condition of each MAPs peptide are summarized. MAP-S471–597 (0.3mg BrK2KA reacted with 5.0mg S471–503 peptide): MeCN/H2O/DMSO (40/50/10=v/v/v) as mixture solvent, Na2CO3 adjusting pH=8.0, Sonication assisting reaction, 30% yield, HPLC preparation by a gradient of 0–25% MeCN against water for 5min, 25–40% MeCN for 50 min. MAP-S604–625(0.4mg BrK2KA reacted with 5.1mg S604–625 peptide): MeCN/H2O (25/75, v/v), Na2CO3 adjusting pH=7.8, 40% yield, HPLC preparation by a gradient of 0–25% MeCN against water for 5min, 25–40% MeCN for 50 min. MAP-S597–625(0.35mg BrK2KA reacted with 5.0mg S597–625 peptide): MeCN/H2O (50/50, v/v), Na2CO3 adjusting pH=8.5, 50%yield, HPLC preparation by a gradient of 0–25% MeCN against water for 5min, 25–40% MeCN for 50 min. MAP-S1164–1191(0.35mg BrK2KA reacted with 5.0mg S1164–1191 peptide): MeCN/H2O (50/50, v/v), Na2CO3 adjusting pH=8.5, 42%yield, HPLC preparation by a gradient of 0–35% MeCN against water for 5min, 35–40% MeCN for 50 min. Water and MeCN containing 0.1% TFA used for HPLC preparation were pre-prepared. The column and flow rate were Vydac 208TP510 and 4mL/min, respectively.
PEPscan (Peptide Screening) for Linear B-Cell Epitopes of SARS-CoV Recognized by Human Sera from SARS-CoV Recovered Patients
The studies of human sera to evaluate their levels of anti-SARS-CoV IgG that recognized the peptides were approved with an Expedited Review of Research Plan by the Institutional Review Board of Peking Union Medical College Hospital, Chinese Academy Medical Sciences & Peking Union Medical College (CAMS & PUMC). More than 500 antisera of SARS patients and 27 sera of healthy volunteers were involved in this study, which was conducted according to the guidelines for treatment of human subjects of National Institutes of Health, USA. Written Informed Consent was obtained from all study participants.
The PEPscan was carried out as described by Hu et al. 35 without major modification. The optical density was read at 450 and 630 nm in an optical density reader (Labsystem, Franklin, USA). The cutoff value was determined as twice the average value of the negative controls.
Binding of Mouse Monoclonal Antibodies to the SARS-CoV Virion
The SARS-CoV PUMC01 strain 46 was diluted with coating buffer to 4 × 10−5 TCID50/mL (bicarbonate/carbonate coating buffer, 0.05 M, pH 9.6). The virus was then coated on 96-well plates with 100 μL (4 × 10−4 TCID50) of virus per well for 12 h at 4 °C. The coating buffer was removed from the wells and 100 μL of blocking buffer was added per well and incubated for 12 h at 4 °C (2% BSA in PBS, pH 7.4. GIBCO, Carlsbad, USA). The plates were washed twice with PBS (pH 7.2). The mouse monoclonal antibodies against SARS-CoV and control mAb against HIV-P27 were diluted to 0.001 μg/μL in the blocking buffer, added to the wells separately and incubated for 1 h at 37 °C. The plates were washed twice with PBS and the secondary antibody (HRP-conjugated goat anti-mouse IgG, (GeneTex, Irvine, USA) diluted 1:1000 in blocking buffer) was added to each well and incubated for 1 h at 37 °C. The secondary antibody was removed and the plate was washed three times with PBS, then 100 μL of TMB substrate solution (Promega, Madison, WI, USA) was added to each well. After incubation for 30 min at room temperature, the absorbance of the test wells was read at 450 nm (A450).
Analysis of Neutralization Using a Neutral Red Staining (NRS)-based Assay
MAbs were serially diluted in DMEM culture medium. Human polyclonal antisera from convalescent SARS patients were diluted to 1:500 (v:v). The SARS-CoV PUMC01 strain was diluted with DMEM to 4 × 10−4 TCID50. The NRS neutralization assay was performed in 96-well flat-bottom plates. The setup is similar to that of the cytopathic effect (CPE)-based neutralization assay. After incubation of 50 μL (4 × 10−4 TCID50) of virus with 50 μL of antibodies or antisera in a total of 100 μL DMEM medium per well for 1 h at 37 °C, Vero E6 cells (2 × 105 cells in 100 μL, from Cell Culture Center of PUMC) were added to each well. On day five of infection, when more than 70% cells showed CPE in the viral control wells (with DMEM instead of antibody or antisera), the culture medium was removed from the test wells and 100 μL of 0.15% neutral red (Sigma, St. Louis, MO, USA) in DMEM medium was added to each well. After incubation for 1 h at 37 °C, the neutral red medium was removed, the plates were washed twice with PBS(pH 7.2) then 100 μL of acidified alcohol (1% acetic acid in 50% ethanol) was added to each well. After incubation for 30 min at room temperature, the absorbance of neutral red stained plates was read at 450 nm (A450).
Percent neutralization was determined by the formula: % neutralization = (A450 of antibody test wells − A450 of viral control wells)/(A450 of cell control wells − A450 of viral control wells) × 100.
Generation, purification and measurement the affinity of anti-peptide monoclonal antibodies
Ganp-transgenic mice were originally established in a C57BL/6 strain as described previously. 47 The mice were backcrossed with BALB/c mice for at least eight generations, and used for immunization to establish mAbs. All mice were maintained in specific pathogen-free conditions by the Center for Animal Resources and Development (CARD), Kumamoto University.
As immunogens, four peptides of S471–503, S604–625, S597–625, and S1164–1191 were synthesized and conjugated with keyhole limpet hemocyanin (KLH) by Operon Biotechnologies (Tokyo, Japan). One hundred micrograms of peptide-KLH emulsified in complete Freund’s adjuvant (CFA) was injected subcutaneously as a primary immunization and boosted after two weeks with incomplete Freund’s adjuvant (IFA). Sera of immunized mice were measured by ELISA using plates coated with free peptides. The mice with highest serum Ab titers were further immunized and four days later the spleen cells were obtained for polyethylene glycol-mediated cell fusion with mouse myeloma cell line X63 using standard procedures. 48 The fused cells were selected with HAT medium in microculture plates at a concentration of 2 × 104 cells/well in the presence of IL-6 (5 units/mL).
The mAbs binding to the immunizing peptide Ags were detected with HRP-conjugated goat anti-mouse IgG Ab (Zymed, South San Francisco, CA, USA) with the substrate OPD (Wako Chemicals, Osaka, Japan). The positive signals (A490 > 1.0) were selected and hybridomas were cloned by a limiting dilution method, adapted to serum-free media, and purified by protein G-Sepharose affinity chromatography (GE Healthcare, Buckinghamshire, UK). The purity of the antibodies was examined by SDS-PAGE and protein staining with Coomassie Brilliant Blue. The heavy and light chain isotypes were determined using an isotyping kit (BD Biosciences, Franklin Lakes, NJ, USA).
The affinity of the mAbs for peptides was determined by the BIAcore assay. 49 The on- and off-rate constants (kon and koff) for binding of the mAbs to S471–503, S604–625, S597–625, and S1164–1191 peptides (Table 1) were determined using the BIAcore system (BIAcore International AB, Uppsala, Sweden). The carboxyl-methylated dextran surface of the sensor chip was activated with N-ethyl-N′-(3-dietylaminopropyl)-carbodiimide and N-hydroxysuccinimide (EDC–EHS). 50 Each peptide was immobilized through the free thiol group of a cysteine residue that was deliberately placed at the N-terminus, by injection of 35 μL of a 20 μg/mL solution in 10 mM 2-(N-morpholino)ethanesulphonic acid buffer (pH = 6) to the EDC–NHS activated surface that had been reacted with 2-(2-pyridinyldithio)-ethaneamine. The excess disulfide groups were deactivated by the addition of cysteine. The mAbs were diluted in 10 mM Hepes (pH = 7.4), 150 mM NaCl, 3.4 mM EDTA, 0.05% (v/v) BIAcore surfactant P20 and injected over the immobilized antigen at a flow rate of 5 μL/min. The association was monitored by the increase in the refractive index of the sensor chip surface per unit time. The dissociation of the mAbs was monitored after the end of the association phase with a flow rate of 50 μL/min. Kinetic rate constants were calculated from the collected data using the Pharmacia Kinetics Evaluation software. 51 The kon was determined by measuring the rate of binding to the antigen at different protein concentrations.
Macaques experiments
Animals
Rhesus monkeys (3–4 years old, Table S4 and S6, from the Institute of Laboratory Animal Science) were examined according to the national microbiologic and parasite SPF standard and were determined free of anti-SARS antibody. The SPF monkeys were housed in an AAALAC-accredited facility before infection with SARS-CoV. The monkeys were then housed in a biosafety level 3 (BSL-3) facilities for infection experiments. All procedures were approved by the Animal Use and Care Committee of the Institute of Laboratory Animal Science, Peking Union Medical College (Animal Welfare Assurance NO. A5655-01, IACUC approval data: 09/24/2007).
Challenges with SARS-CoV
Monkey challenges were performed in a BSL-3 facility. 37 Briefly, the animals were anaesthetized with ketamine (0.5 mL/kg, i.m.). The TCID50 10–6 (4.0 mL/monkey) of the stock PUMC01 strain virus was sprayed into the nasal cavity of the animals.
Pathologic Examination
The lung tissues were sampled from the euthanized animals. Organs were examined grossly and photos were taken to record the lung damage.
Samples of 5–10 g lung tissues were collected from each lung and frozen at −80 °C. The remaining tissues were fixed in 10% formaldehyde for one week in the BSL-3 laboratory. The tissues were then processed in the regular pathology laboratory by dehydration, embedding, and sectioning.
For hematoxylin and eosin (H&E) staining, the sections were sequentially treated with xylene I 10 min; xylene II 10 min; 100% alcohol 5 min; 95% alcohol 5 min; 90% alcohol 5 min; 80% alcohol 5 min; 70% alcohol 5 min and PBS 5 min. The sections were then stained in Hematoxylin 5 min; washed with tap water; double-distilled water 5 sec; PBS 5 min; 95% alcohol 30 sec; eosin 30 sec; 95% alcohol 5 sec; 100 % alcohol I 5 sec; 100% alcohol II 5 sec; xylene I 5 min; xylene II 5 min; xylene III 5 min; and mounted with mounting buffer (ZhongShan Inc., Guangzhou, China).
Immunohistochemical Analysis of the Infected Lung Tissues
A mixture of the mouse monoclonal antibodies mAb-4E5 (0.01 μg/mL), mAb-11B1 (0.01 μg/mL) and mAb-9A6 (0.01 μg/mL) was used as primary antibody for the immunohistochemical analysis of the infected lung tissues. Briefly, tissue sections were treated with the primary antibody at 4 °C overnight, washed three times with PBS, incubated with HRP-goat-anti-mouse IgG (1:1000, ZhongShan Inc., PRC) at 37 °C for 30 min, then washed three times with PBS. The DAB substrate (1:50) was added and incubated at 37 °C for 30 min and then stained with hematoxylin for 3 min.
RT-PCR for analysis of the SARS-CoV burden
The tissues from cranial, caudal lobes of the left lung and cranial, middle, caudal lobes of the right lung from each animal were sampled and pooled up together and the total RNA were isolated from the lung tissues using Trizol (Invitrogen, USA). 100 mg of lung tissue was mixed with 1 mL of Trizol extraction buffer and homogenized for 60 seconds (Homogenizer, IKA, German). The RNA wan then extracted with chloroform and precipitated with isopropanol. RNA was dissolved in 50 μL of DEPC water and the RNA quality was verified by electrophoresis on agarose gel.
For the reverse transcription, 5 μg total RNA was reverse transcribed with a high-capacity cDNA kit (RecertAid™ First Strand cDNA Synthesis Kit (Fermentas, Glen Burnie, Maryland, USA) with random hexamer primers. The reaction was performed at 42 °C for 60 min, and terminated at 70 °C for 10 min.
Two microliters of reverse transcription mixture were used as template. The primers for SARS-CoV were 5′-ATGAATTACCAAGTCAATGGTTAC-3′ and 5′-CATAACCAGTCGGTACAGCTAC-3′. The PCR was normalized against monkey glyceraldehyde phosphate dehydrogenase (GAPDH) (NM002046). The GAPDH primers were 5′-TCAACAGCGACACCCACTC-3′ and 5′-CTTCCTCTTGTGCTCTTGCTG-3′ (product size 201 bp). The PCR was performed with a PCR kit (Taq DNA polymerase, Fermentas). The PCR products were detected by 1.6% agarose gel electrophoresis.
Reaction Conditions: 10× Taq buffer (2 μL), 2 mM dNTP(2 μL), Forward primer (20 μM, 0.4 μL), Reverse primer (20 μM, 0.4 μL), Taq DNA polymerase(0.8 μL), 25 mM MgCl2(2 μL), Template DNA (cDNA, 2 μL), H2O(9.4 μL), step (1): 94 °C for 3 min, step (2): 94 °C for 30 sec, 55 °C for 30 sec, 72 °C for 30 sec, for 26 cycles, then 72 °C for 10 min.
qRT-PCR for analysis of the SARS-CoV burden
The SARS-CoV fragment was prepared by RT-PCR. A 240bp fragment of SARS-CoV was amplified and separated by 1.6% agarose electrophoresis. The DNA fragment was recovered from the gel with a gel extraction kit (MinElute Gel Extraction Kit, QIAGEN Stanford Valencia, CA, USA). The recovered DNA was measured and adjusted to concentrations of 1 × 106 copies/μL, 1 × 105 copies/μL, 1 × 104 copies/μL, 1 × 103 copies/μL, 1 × 102 copies/μL and 1 × 101 copies/μL, which were used to generate a standard curve. This known copy number standard curve was used to calculate the copy number of SARS-CoV mRNA in the infected lung tissues.
Real-time PCR for the infected samples was performed under the same conditions used for standard curves with the Roche Light Cycler following the instructions for the SYBR Green Realtime PCR Master Mix (Toyobo, Osaka, Japan).
The copy number of the virus was detected as follows. Briefly, 2 μL of the reverse transcription mixture from step (1) was used as template. The primers for SARS-CoV were (5′-ATGAATTACCAAGTCAATGGTTAC-3′) and (5′-CATAACCAGTCGGTACAGCTAC-3′). The reaction was continued for 40 cycles under the conditions: 2 × QuantiTect SYBR Green PCR(10 μL), Forward primer (10 μM, 1 μL), Reverse primer (10 μM, 1 μL), RNase-free water(3 μL), Template DNA (cDNA, 5 μL), Total volume(20 μL), Step (1): 94 °C for 3 min, step (2): 94 °C for 15 sec, 55 °C for 30 sec, 72 °C for 20 sec, for 40 cycles then move to the melt curve procedure.
Immunization of Monkeys
The peptide and/or peptide mixtures were dissolved as 480 μg/mL of each peptide in 0.9% NaCl and then formulated with an equal volume of Freund’s Complete Adjuvant (FCA) (Sigma, St. Louis, MO 63178, USA) according to the manufacturer’s instructions. The injected dose of vaccine was 0.5 mL/4kg/monkey (120 μg/4 kg/monkey) given through intramuscular (i.m.) injections at four sites on the arms and legs.
Rhesus monkeys were boosted at two, four and six weeks after the first injection with the same dose of peptides and volume of vaccine as for the first injection, but formulated in an equal volume of Freund’s incomplete adjuvant (IFA) (Sigma).
Venous blood samples (2 mL/each) were collected every seven days from each monkey. The sera were isolated by centrifugation at 2000 rpm and immediately stored at −80 °C.
Antipeptidic IgG were detected by ELISA. Free peptides were diluted in carbonate buffer (pH 9.6) to a final concentration of 10 μg/mL. 100 μL of the peptide solution was added to 96-well polystyrene high BIND microplates (Corning Life Science, Santa Clara, CA, USA) for coating overnight at 4 °C. The wells of plates were then washed three times with PBS-Tween buffer (10 mM phosphate buffer, 150 mM NaCl, 0.05% Tween 20, pH 7.2) and subsequently blocked with 3% BSA in PBS-Tween buffer for additional 1 h at 37 °C. After thoroughly washing the wells three times with PBS-Tween buffer, the antigen-coated microplates were incubated with diluted monkey sera at room temperature for 1 h. After washing with PBS-Tween buffer three times, bound antibodies were detected with HRP-conjugated goat anti-monkey IgG (1:2000 v:v, Gene Tex). After three washes with PBS-Tween, a TMB substrate solution (Promega, Madison, WI, USA) was added and the color developed for 20 min before the reaction was stopped with 100 μL of 0.2M H2SO4. The absorbance at 450 nm was read using a microtiter plate reader.
Macaques treated by mAb43-3-14
Eighteen rhesus monkeys in six groups (table S6, 3–4 years old) were examined according to the national microbiologic and parasite SPF standard and were verified free of anti-SARS antibody. mAb43-3-14 was adjusted to a concentration of 0.4 mg/mL and 3.6 mg/mL with 0.9% NaCl, respectively. A dose of 0.2 mg/kg and 1.8 mg/kg of the antibody given by intravenous (i.v.) injection was used to treat the different groups. The same volume of 0.9% NaCl was used for the control group. The procedures were approved by the Animal Use and Care Committee of the Institute of Laboratory Animal Science, Peking Union Medical College (Animal Welfare Assurance NO. A5655-01, IACUC approval data: 09/24/2007).
Statistical analysis
All data were analyzed using Prism software (GraphPad software). For neutralization assays and ELISA, mean and standard deviation were used to determine significance. For qRT-PCR, geometric mean and standard deviation were used to determine virus burden.
Supplementary Material
Acknowledgments
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are grateful to Professor Carl F. Nathan and Dr. Michael S. Diamond for their kind discussion and manuscript preparation. We thank Dr. Baoxing Fan for his serological evaluation of the large number of SARS-CoV convalescent antisera. The project described in this article was supported by the National Institute of Allergy and Infectious Diseases (U01AI061092). The study was also partially supported for preparation of the monoclonal antibodies by contract research for MEXT for Emerging and Reemerging Infectious Diseases and Promotion of Fundamental Studies in Health Sciences (04–2) of the NIBIO and National Natural Science Foundation of China (No. 90713045).
Footnotes
The authors declare no competing financial interest.
This information is available free of charge via the Internet at http://pubs.acs.org/.
ASSOCIATED CONTENT
* Supporting Information
This information is available free of charge via the Internet at http://pubs.acs.org/. Neutralization and enhancement of SARS-CoV infection of Vero E6 cells in the presence of human antisera of convalescent SARS patients, design and synthesized new peptides, generated antipeptide mAbs, monkeys for peptide vaccine immunization against SARS-CoV, Pathologic classification of the severity of the lung damage in SARS-CoV-infected rhesus macaques, the conditions for preparation of multiple antigen peptides were attached (PDF).
References
- 1.Hilgenfeld R, Peiris M (2013) From SARS to MERS: 10 years of research on highly pathogenic human coronavirus, Antiviral Res. 100(1): 286–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR et al. (2003) Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348: 1967–1976. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Shi Z, Yu M, Ren W, Smith C et al. (2003) Bats are natural reservoirs of SARS-like coronaviruses. Science 310: 676–679. [DOI] [PubMed] [Google Scholar]
- 4.Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL et al. (2003) Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302: 276–278. [DOI] [PubMed] [Google Scholar]
- 5.Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A et al. (2003) The Genome sequence of the SARS-associated coronavirus. Science 300: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 6.Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R et al. (2003) Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science 300: 1394–1399. [DOI] [PubMed] [Google Scholar]
- 7.The Chinese SARS molecular epidemiology consortium. (2004). Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 303: 1666–1669. [DOI] [PubMed] [Google Scholar]
- 8.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK et al. (2004) Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 426: 450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffers SA, Tusell SM, Gillim-Ross L, Hemmila EM, Achenbach JE et al. (2004) CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. USA 101: 15748–15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang B, Chen H, Jiang X, Zhang M, Wan T et al. (2004) Identification of an HLA-A*0201-restricted CD8+ T-cell epitope SSp-1 of SARS-CoV spike protein. Blood 104: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YD, Sin WY, Xu GB, Yang HH, Wong TY et al. (2004) T-cell epitopes in severe acute respiratory syndrome (SARS) coronavirus spike protein elicit a specific T-cell immune response in patients who recover from SARS. J. Virol. 78: 5612–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts A, Wood J, Subbarao K, Ferguson M, Wood D et al. (2006) Animal models and antibody assays for evaluating candidate SARS vaccines: summary of a technical meeting 25–26 August 2005, London, UK. Vaccine 24: 7056–7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillim-Ross L and Subbarao K (2006) Emerging respiratory viruses: challenges and vaccine strategies. Clin. Microbiol. Rev. 19: 614–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham RL, Donaldson EF, Baric RS (2013) A decade after SARS: strategies for controlling emerging coronaviruses, Nature Review Microbiology, Nat Rev Microbiol. 11(12): 836–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang ZY, Werner HC, Kong WP, Leung K, Traggiai E et al. (2005) Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. USA 102: 797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dandekar AA and Perlman S (2005) Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 5: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Subbarao K and Roberts A (2006) Is there an ideal animal model for SARS? Trends Microbiol. 14: 299–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor DR (2006) Obstacles and advances in SARS vaccine development. Vaccine 24: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takada A and Kawaoka Y (2003) Antibody-dependent enhancement of viral infection: molecular mechanisms and in vivo implications. Rev. Med. Virol. 13: 387–398. [DOI] [PubMed] [Google Scholar]
- 20.Tirado SMC and Yoon KJ (2003) Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 16: 69–86. [DOI] [PubMed] [Google Scholar]
- 21.Gorlani A, Forthal DN(2013) Antibody-dependent enhancement and the risk of HIV infection. Curr HIV Res. 11(5):421–6. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan N, Sun Y, Binley J, Lee J, Barbas CF 3rd, Parren PW, Burton DR, Sodroski J (1998) Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J Virol. 72(8): 6332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillon C, Schutten M, Boers PH, Gruters RA, Osterhaus AD(2002) Antibody-mediated enhancement of human immunodeficiency virus type 1 infectivity is determined by the structure of gp120 and depends on modulation of the gp120-CCR5 interaction. J Virol. 76(6): 2827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorlani A, Forthal DN (2013) Antibody-dependent enhancement and the risk of HIV infection. Curr HIV Res. 11(5): 421–6. [DOI] [PubMed] [Google Scholar]
- 25.Halstead SB (2007) Dengue. Lancet 370: 1644–1652. [DOI] [PubMed] [Google Scholar]
- 26.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ (2007) Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 104: 9422–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierson TC, Xu Q, Nelson S, Oliphant T, Grant E et al. (2007) The Stoichiometry of Antibody-Mediated Neutralization and Enhancement of West Nile Virus Infection. Cell Host & Microbe 1: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S (2015) Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. 268(1): 340–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierson TC, Sánchez MD, Puffer BA, Ahmed AA, Geiss BJ, Valentine LE, Altamura LA, Diamond MS, Doms RW (2006) A rapid and quantitative assay for measuring antibody-mediated neutralization of West Nile virus infection. Virology 346(1): 53–65. [DOI] [PubMed] [Google Scholar]
- 30.Mehlhop E, Ansarah-Sobrinho C, Johnson S, Engle M, Fremont DH, Pierson TC, Diamond MS (2007) Complement protein C1q inhibits antibody-dependent enhancement of flavivirus infection in an IgG subclass-specific manner. Cell Host Microbe. 2(6): 417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang KJ, Yang YC, Lin YS, Huang JH, Lin HS, Yeh TM, Chen SH, Liu CC, and Lei HY (2006) The dual-specific binding of dengue virus and target cells for the antibody-dependent enhancement of dengue virus infection, J Immunol. 176, 2825–2832. [DOI] [PubMed] [Google Scholar]
- 32.He YX, Zhou Y, Siddiqui P, Niu J and Jiang SB(2005) Identification of Immunodominant Epitopes on the Membrane Protein of the Severe Acute Respiratory Syndrome-Associated Coronavirus, J Clin Microbiol. 43(8): 3718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He YX, Zhou Y, Wu H, Kou ZH, Liu SW and Jiang SB (2004) Mapping of Antigenic Sites on the Nucleocapsid Protein of the Severe Acute Respiratory Syndrome Coronavirus, J Clin Microbiol. 42(11): 5309–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang YF, Wan Y, Qiu, Li. W, Zhou J, Ni B, Guo B, Zou Q, Zou LY, Zhou W, Jia ZC, Che XY, and Wu YZ (2005) Comprehensive Antibody Epitope Mapping of the Nucleocapsid Protein of Severe Acute Respiratory Syndrome (SARS) Coronavirus: Insight into the Humoral Immunity of SARS. Clin Chem. 51(8):1382–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu H, Li L, Kao RY, Kou B, Wang Z, et al. (2005) Screening and identification of linear B-cell epitopes and entry-blocking peptide of severe acute respiratory syndrome (SARS)-associated coronavirus using synthetic overlapping peptide library. J. Comb. Chem. 7: 648–656. [DOI] [PubMed] [Google Scholar]
- 36.Tam JP (1988) Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. USA 85: 5409–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin C, Wang J, Wei Q, She M, Marasco WA et al. (2005) An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus. J. Pathol. 206: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong SK, Li W, Moore MJ, Choe H, Farzan M (2004) A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 279: 3197–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W, Zhang C, Sui J, Kuhn JH, Moore M,et al J. (2005) Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 24: 1634–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu Y, Zhu J, Liu Y, Lou Z, Yuan F, et al. (2004) Characterization of the heptad repeat regions, HR1 and HR2, and design of a fusion core structure model of the spike protein from severe acute respiratory syndrome (SARS) coronavirus. Biochemistry 43: 14064–71. [DOI] [PubMed] [Google Scholar]
- 41.Xu Y, Lou Z, Liu Y, Pang H, Tien P et al. (2004) Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J. Biol. Chem. 279: 49414–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang SF, Tseng SP, Yen CH, Yang JY, Tsao CH, Shen CW, Chen KH, Liu FT, Liu WT, Chen YM, Huang JC (2014) Antibody-dependent SARS coronavirus infection is mediated by antibodies against spike proteins Biochem Biophys Res Commun. 451(2): 208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yip MS, Leung NH, Cheung CY, Li PH, Lee HH, Daëron M, Peiris JS, Bruzzone R, Jaume M (2014) Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus, Virol J. 11:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaume M, Yip MS, Cheung CY, Leung HL, Li PH, Kien F, Dutry I, Callendret B, Escriou N, Altmeyer R, Nal B, Daëron M, Bruzzone R, Peiris JS (2011) Anti-Severe Acute Respiratory Syndrome Coronavirus Spike Antibodies Trigger Infection of Human Immune Cells via a pH- and Cysteine Protease-Independent FcγR Pathwa. J Virol. 85(20):10582–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dawson PE, Muir TW, Clark-Lewis I and Kent SB (1994) Synthesis of proteins by native chemical ligation. Science 266: 776–779. [DOI] [PubMed] [Google Scholar]
- 46.Ruan YJ, Wei CL, Ee AL, Vega VB, Thoreau H, Su ST, Chia JM, Ng P, Chiu KP, Lim L, Zhang T, Peng CK, Lin EO, Lee NM, Yee SL, Ng LF, Chee RE, Stanton LW, Long PM, Liu ET (2003) Comparative full-length genome sequence analysis of 14 SARS coronavirus isolates and common mutations associated with putative origins of infection. Lancet 361(9371): 1779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi N, Kimura T, Matsushita S, Fujimura S, Shibata J et al. (2005) Generation of High-Affinity Antibody against T Cell-Dependent Antigen in the Ganp Gene-Transgenic Mouse. J. Immunol. 174: 4485–4494. [DOI] [PubMed] [Google Scholar]
- 48.Kuwahara K, Yoshida M, Kondo E, Sakata A, Watanabe Y et al. (2000) A novel nuclear phosphoprotein, GANP, is up-regulated in centrocytes of the germinal center and associated with MCM3, a protein essential for DNA replication. Blood 95: 2321–2328. [PubMed] [Google Scholar]
- 49.Jönsson U (1991) Real-time biospecific interaction analysis using surface plasmon resonance and sensor chip technology. BioTechniques 11: 620–627. [PubMed] [Google Scholar]
- 50.Johnsson B, Löfas S and Lindquist G (1991) Immobilization of proteins to a carboxymethyldextran-modified gold surface for biospecific interaction analysis in surface plasmon resonance sensors. Anal. Biochem. 198: 268–277. [DOI] [PubMed] [Google Scholar]
- 51.Karlsson R, Michaelsson A and Mattsson L (1991) Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J. Immunol. Methods 145, 229–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.